Abstract
This study evaluates the antimicrobial effects of ethanolic extract of five herbal plants; Guava (Psidium guajava), Sage (Salvia officinalis), Rhamnus (Ziziphusspina Christi), Mulberry (Morusalba L.), and Olive (Oleaeuropaea L) leaves against several microbial population representing Gram positive, Gram negative and Mollicutes; S. aureus, E. coli, Pasteurella multocida, B. cereus, Salmonella Enteritidis and M. gallisepticum using standard agar disc diffusion technique and minimal inhibitory concentration (MIC). Different extracts reveal variable results against the microorganism under study. All extracts have no antibacterial potency for Mycoplasma gallisepticum except Psidium guajava. The results of minimal inhibitory concentration (MIC) and Minimum bactericidal concentration (MBC) of the extracts against the six bacteria ranged from 625 to 5000 μg/ml. The used herbal extract could inhibit the selected microorganism under study with variable minimal inhibitory concentration (MIC) and minimum bactericidal concentration (MBC).
Keywords: Antimicrobial effect, Herbal extract, Gram positive, Gram negative, Mycoplasma gallisepticum
1. Introduction
Infectious diseases are still a major health concern accounting for 41% of the global disease burden measured in terms of Disability – Adjusted Life Years (DALYS) (Noah and Fidas, 2000). One of the main causes of this problem is the widespread of acquired bacterial resistance to antibiotics in such a way that the world is facing today, a serious threat to global public health (Chopra, 2000) in the form of not only epidemics, but also pandemics of antibiotic resistance (Chanda et al., 2010, Osman et al., 2012). Due to this problem of resistance against antibiotics, attention is now being shifted towards biologically active components isolated from plant species communing used as herbal medicine, as they may produce a new potent source of antibacterial and antifungal activities (Maiyo et al., 2010, Erfan and Marouf, 2019). The antimicrobial properties of plants related to their ability to give several secondary metabolites of relatively complex structures possessing antimicrobial activities (Matasyoh et al., 2009, Souza et al., 2005).
Guava (Pisidium guajava L.) is a fruit plant belonging to the family Myttacease, guava leaves, roots, and used for prevention and treatment of diarrhea (Lutterodt, 1989, Alnieida et al., 1995), guava also showed a significant antibacterial activity against food borne diarrhea causing bacteria which are Staphylococcus species, Shigella species. and Pseudomonas species. (Alnieida et al., 1995, Jaiarj et al., 1999). Guava is also used as anti-inflammatory and antiseptic as well as treatment of diabetes, hypertension, pain fever, respiratory disorders, gastroenteritis, diarrhea and dysentery (Gutierrez et al., 2008).
Sage (Salvia officinalis L.) from family Lamiaceae is aromatic plant used in traditional medicine for treating many ailments as inflammation of mouth and throat (Baricevic et al., 2001) and used as antimutagenic and anticarcenogenic agent (Craig, 1999, Simic et al., 2000). It is also employed as diuretic, tonic, local styptic, antiseptic, antifungal and spasmodic pain relief (Loannides, 2002).
Rhamnus (Ziziphusspina Christi) is a plant belongs to family Rhamnaceae, its extracts showed antiviral, antifungal and antibacterial activities and used in the Egyptian folk medicine for treatment of several diseases including gastrointestinal tract ailments, diabetes and diarrhea (Shahat et al., 2001).
Mulberry (Moursalba L) belongs to Family Moreaceac which used to treat fever and headache, Mulberry can help treat chronic diseases of digestive tract and improve appetite. It is also used in treating chronic gastritis and hepatitis. It is helpful in Cough, dyspepsia, oedema and oligurea (Sunil and Ammani, 2009). Recent studies have shown M. alba has antioxidant, antibacterial, antiviral and anti-inflammatory properties (El-Beshbishy et al., 2006).
Olive tree leaves (Oleaeuropaea L.) is widely tested for its pharmacological use, where the leaves are important for their secondary metabolites such as secoiridoid compounds which are responsible for hypotensive activity (Hansen et al., 1996) and hypoglycemic activity (Gonzalez et al., 1992). Several reports have shown that plant has capacity to lower blood pressure (Samuelsson, 1951), relieve arrhythmia and prevent intestinal muscle spasm (Garcia et al., 2000).
The present work designed to investigate the antimicrobial effects of ethanolic extract of five herbal plants, namely: Guava (Psidium guajava), Sage (Salvia officinalis), Rhamnus (Ziziphusspina Christi), Mulberry (Morusalba L.), and olive (Oleaeuropaeal L.) against several microbial strains (S. aureus, E. coli, Pasteurella multocida, B. cereus, Salmonella Enteritidis and Mycoplasma gallisepticum) which are incriminated in different diseases among livestock animals and birds.
2. Materials and method
2.1. Plant material
In the investigation, the whole plant of Guava (Psidium guajava), Sage (Salvia officinalis), Rhamnus (Ziziphusspina Christi), Mulberry (Morusalba L.), and olive (Olea europaea L) leaves were collected from field market of Egypt. The plants are air dried in an oven at 40 °C for 48 h. Two hundred and fifty grams of dried powdered plant sample was extracted by one liter of ethanol 70% at 30 °C for 48 h and filtering through Whatman No. 4 filter paper. The plants were extracted by rotatory evaporation at 50 °C till complete dryness occurs. The total extract was dissolved in water in a concentration of 500 mg/ml and stored at −20 °C for further use.
2.2. Test organisms
Six types of microorganisms were investigated; Gram positive (S. aureus and B. cereus), Gram negative (E. coli, Pasteurella multocida, Salmonella Entritidis) and Mollicutes (Mycoplasma gallisepticum). All organisms obtained and well identified onto Microbiology Department, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt.
2.3. Antimicrobial activity
The antimicrobial potentiality of the above mentioned plants ethanolic extracts were determined by standard agar disc diffusion technique and minimal inhibitory concentration (MIC) in accordance with the Clinical and Laboratory Standards Institute (CLSI, 2015) against the tested microorganisms. Mycoplasma gallisepticum was determined in accordance with (Hannan, 2000).
2.3.1. Disc diffusion method
Pure colonies of each microorganism were suspended in sterile saline until a turbidity match McFarland tube number 0.5 (1.5 × 108 CFU/ ml). A loopful from each adjusted organisms were swabbed onto Muller Hinton agar (Difco Laboratories). Sterile paper discs (6 mm in diameter) were impregnated with 100 μl of each 10%, 25%, 50%, 75% and 100% diluted extracts then dried at 100 °C for two hours in hot air oven and were dispensed onto the surface of the inoculated agar plate. The plates were then incubated according to growth requirement of each organism. Each sample was tested in triplicates and antibacterial activity was evaluated by measuring and recorded the zones of inhibition in mm (including the 6 mm disk). A parallel analysis study with commercial antimicrobial agents included Amoxicillin-Clavulinic (10 μg), Ampicillin (10 μg), Vancomycin (30 μg), Lincomycin (30 μg), Gentamicin (10 μg), Streptomycin (10 μg) and Tetracycline (30 μg) was conducted in order to compare their antimicrobial potency with the plant extracts.
2.3.2. Minimal inhibitory concentration (MIC)
In dilution technique, two fold serial dilutions of the extracts were prepared in concentrations ranging from 625 to 10,000 μglmI. From each dilution of each extract, one milliliter of was mixed with 9 ml of Muller Hinton Agar. Ten microliter of each standardized broth cultures (1.5 × 108 CFU/ml) was cultivated on the surface of the plates containing various concentrations of the extracts. The plates were then incubated according to growth requirement of each organism and observed for any visible bacterial growth. MIC was the lowest concentration of extract that resulted in no visible growth on the surface of the agar.
2.3.3. Minimum bactericidal concentration (MBC)
Blocks of agar plates showing no growth after MIC tests were inoculate to fresh nutrient broth (acting as the recovery medium) for the determination of the MBC. The broths were incubated according to growth requirement of each organism. The absence of turbidity in the recovery medium was evidence of bactericidal activity.
2.4. Statistical analysis
The inhibition zones were calculated as means ± SD. The significance was evaluated by analysis of variance (ANOVA) using Microsoft Excel program. Significant differences in the data were established at the 5% level of significance.
3. Results
The ethanolic extracts of the used plants under study reveal that the extract can be a better antimicrobial agent in comparison with the result of commercial antimicrobial agents used in the work.
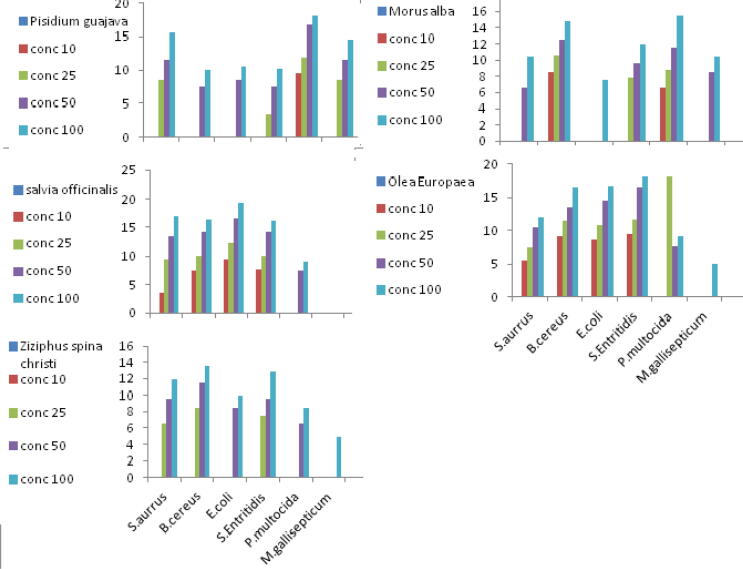
In Disk diffusion technique, the extracts of Salvia officinalis and Psidium guajava have the ability to inhibit S. aureus growth, while the extract of Olea europaea and Morus alba have antibacterial potency against B. cereus. Salvia officinalis and Olea europaea have the ability to stop E. coli growth. Moreover, Olea europaea and Salvia officinalis extracts can inhibit the growth of Salmonella Entritidis. The plate of Pasteurella multocida show inhibition zone with Psidium guajava and Morus alba. All extracts have no antibacterial potency for Mycoplasma gallisepticum except Psidium guajava. The diameter of inhibition zone in correlation to concentration of extracts and types of organism is listed in Table 1 and demonstrated in Fig. 1.
Table 1.
Antimicrobial activity of examined plant extracts on six microorganism using disk diffusion method.
| Organism | Inhibition zone of extract (mm) |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Psidiumguajava |
Salvia officinalis |
Ziziphusspinachristi |
Morus alba |
OleaEuropaea |
||||||||||||||||
| 10% | 25% | 50% | 100% | 10% | 25% | 50% | 100% | 10% | 25% | 50% | 100% | 10% | 25% | 50% | 100% | 10% | 25% | 50% | 100% | |
| S. aureus | – | 8.42 ± 0.41 | 11.5 ± 0.31 | 15.62 ± 1.15 | 3.5 ± 0.32 | 9.45 ± 0.35 | 13.5 ± 0.25 | 17.05 ± 1.05 | – | 6.5 ± 0.4 | 9.5 ± 0.25 | 11.82 ± 2.5 | – | – | 6.5 ± 0.55 | 10.5 ± 1.15 | 5.5 ± 0.05 | 7.42 ± 0.4 | 10.5 ± 0.25 | 12.02 ± 2.05 |
| B. cereus | – | – | 7.5 ± 0.25 | 10.05 ± 0.15 | 7.5 ± 0.32 | 10.05 ± 0.25 | 14.2 ± 0.05 | 16.45 ± 1.05 | – | 8.5 ± 0.25 | 11.5 ± 0.45 | 13.52 ± 2.1 | 8.5 ± 0.55 | 10.6 ± 0.05 | 12.5 ± 1.55 | 14.75 ± 0.15 | 9.1 ± 0.15 | 11.52 ± 0.07 | 13.5 ± 0.45 | 16.62 ± 1.05 |
| E. coli | – | – | 8.5 ± 0.35 | 10.55 ± 0.15 | 9.5 ± 1.32 | 12.25 ± 1.05 | 16.5 ± 0.75 | 19.25 ± 0.65 | – | – | 8.5 ± 0.15 | 10.02 ± 0.05 | – | – | – | 7.5 ± 0.15 | 8.5 ± 1.05 | 10.72 ± 1.04 | 14.5 ± 0.75 | 16.72 ± 0.55 |
| S. Enteritidis | – | 3.42 ± 1.4 | 7.5 ± 0.71 | 10.12 ± 0.55 | 7.75 ± 0.52 | 10.05 ± 0.35 | 14.5 ± 0.15 | 16.25 ± 0.75 | – | 7.5 ± 0.4 | 9.5 ± 0.25 | 12.82 ± 2.5 | – | 7.75 ± 0.25 | 9.6 ± 0.15 | 12.02 ± 0.05 | 9.5 ± 0.55 | 11.72 ± 0.45 | 16.5 ± 0.35 | 18.02 ± 0.05 |
| P. multocida | 9.57 ± 1.55 | 11.92 ± 1.45 | 16.75 ± 1.35 | 18.02 ± 0.95 | – | – | 7.5 ± 0.25 | 9.05 ± 1.05 | – | – | 6.5 ± 0.25 | 8.52 ± 2.5 | 6.5 ± 0.75 | 8.72 ± 0.54 | 11.55 ± 0.41 | 15.42 ± 0.15 | – | 3.52 ± 0.49 | 7.5 ± 0.25 | 9.12 ± 0.05 |
| M. gallisepticum | – | 8.5 ± 0.98 | 11.54 ± 0.61 | 14.62 ± 0.55 | – | – | – | – | – | – | – | 4.82 ± 2.5 | – | – | 8.5 ± 0.55 | 10.5 ± 1.15 | – | – | – | 5.02 ± 2.05 |
Fig. 1.
Antimicrobial activity of examined plant extracts on six microorganism using disk diffusion method.
All commercial antimicrobial agents admitted to the work reveal a verity of sensitivity and resistance against the used microorganism. Lincomycin is the only one that has killing action on all used organisms as described in Table 2.
Table 2.
Antimicrobial activity of commercial antimicrobial agentson six microorganism using disk diffusion method:
| organism | Inhibition zone of extract (mm) |
||||||
|---|---|---|---|---|---|---|---|
| Amoxicillin-Clavulinic(10 μg) | Ampicillin(10 μg) | Vancomycin (30 μg) | Lincomycin(30 μg) | Gentamicin(10 μg) | Streptomycin(10 μg) | Tetracycline(30 μg) | |
| S. aureus | 25 ± 0.71 (S) | 13.0 ± 0.4 (R) | 17.0 ± 0.4(S) | 27.0 ± 0.65 (S) | 10.0 ± 0.23 (R) | 12.0 ± 1.15 (R) | 11.0 ± 0.71 (R) |
| B. cereus | 23.0 ± 0.45 (S) | 11.0 ± 1.25 (R) | 18.0 ± 1.15 (S) | 25.0 ± 0.02 (S) | 10.0 ± 0.54 (R) | 10.0 ± 0.71 (R) | 17.0 ± 0.49 (I) |
| E. coli | 17.0 ± 1.15 (I) | 10.0 ± 0.71 (R) | 19.0 ± 0.25 (S) | 21.0 ± 0.25 (S) | 21.0 ± 0.4 (S) | 15.0 ± 0.75 (I) | 10.0 ± 1.15 (R) |
| S. Enteritidis | 16.0 ± 0.25 (I) | 9.0 ± 1.15 (R) | 19.0 ± 0.75 (S) | 20.0 ± 1.15 (S) | 22.0 ± 0.69 (S) | 19.0 ± 0.4 (S) | 13.0 ± 0.36 (S) |
| P. multocida | 23.0 ± 1.05 (S) | 8.0 ± 0.25 (R) | 10.0 ± 0.55 (R) | 17.0 ± 0.4 (I) | 9.0 ± 0.71 (R) | 11.0 ± 0.25 (R) | 18.0 ± 0.25 (I) |
| M. gallisepticum | 15 ± 1.15 (I) | 10.0 ± 0.85 (R) | 11.0 ± 1.25 (R) | 21.0 ± 0.89 (S) | 8.0 ± 0.46 (R) | 11.0 ± 1.25 (R) | 16.0 ± 0.4 (I) |
S = Sensitive I = Intermediate R = Resistant.
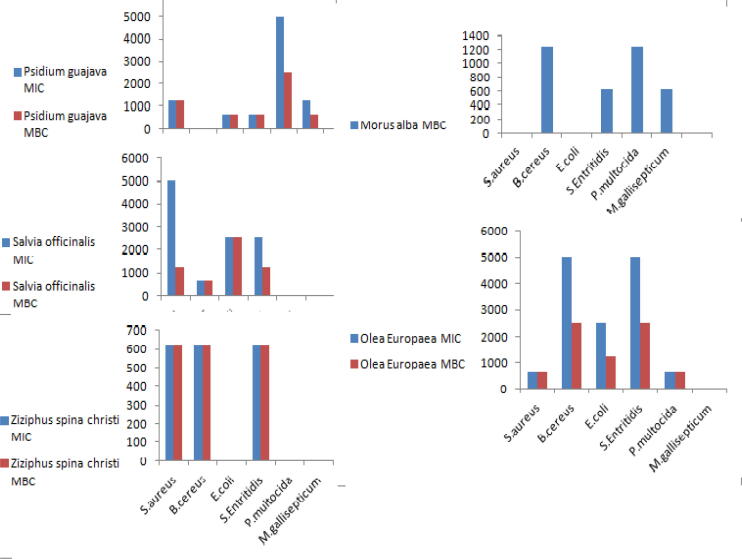
The minimal inhibitory concentration (MIC) and Minimum bactericidal concentration (MBC) of the extracts against the six bacteria ranged from 625 to 5000 μglmI. S. aureus reveals MIC value of 1250, 5000, 625 and 625 μglmI for Psidium guajava, Salvia officinalis, Ziziphusspina christi and Olea europaea L, while MBC value of the extracts for S. aureus is 1250, 1250, 625 and 625 μglmI in the same order. The MIC value for B. cereus at examination of Salvia officinalis, Ziziphusspina christi, Morus alba and Olea europaea L is 625, 625, 2500 and 5000 μglmI, while the MBC value for the same organism and the same extracts is 625, 625, 1250 and 2500 μglmI. The MIC and MBC of Psidium guajava at examination against E. coli, Salmonella Enteritidis, Pasteurella multocida and Mycoplasma gallisepticum are 625, 625; 625, 625; 5000, 2500 and 1250, 625 μglmI respectively. The MIC and MBC of Salvia officinalis for E. coli and Salmonella Entritidis are 2500, 2500; 2500, 1250 μglmI, while The MIC and MBC of Ziziphusspina christi are 625 and 625 μglmI for Salmonella Entritidis in the same order, the MIC and MBC value for Morus alba against Salmonella Entritidis, P. multocida and M. gallisepticum are 625; 1250 and 625 μglmI. Finally the MIC and MBC of Olea europaeal against E. coli, Salmonella Entritidis and Pasteurella multocida are 250, 1250; 5000, 2500 and 625, 625 μglmI (Table 3) and demonstrated in Fig. 2.
Table 3.
Minimal inhibitory concentration (MIC) and Minimum bactericidal concentration (MBC) of examined plant extracts on six microorganism:
| Organism |
Psidiumguajava |
Salvia officinalis |
Ziziphusspinachristi |
Morus alba |
OleaEuropaea |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| MIC (μglmI) | MBC (μglmI) | MIC (μglmI) | MBC (μglmI) | MIC (μglmI) | MBC (μglmI) | MIC (μglmI) | MBC (μglmI) | MIC (μglmI) | MBC (μglmI) | |
| S. aureus | 1250.00 | 1250.00 | 5000.00 | 1250.00 | 625.00 | 625.00 | – | – | 625.00 | 625.00 |
| B. cereus | – | – | 625.00 | 625.00 | 625.00 | 625.00 | 2500.00 | 1250.00 | 5000.00 | 2500.00 |
| E. coli | 625.00 | 625.00 | 2500.00 | 2500.00 | – | – | – | – | 2500.00 | 1250.00 |
| S. Enteritidis | 625.00 | 625.00 | 2500.00 | 1250.00 | 625.00 | 625.00 | 625.00 | 625.00 | 5000.00 | 2500.00 |
| P. multocida | 5000.00 | 2500.00 | – | – | – | – | 1250.00 | 1250.00 | 625.00 | 625.00 |
| M. gallisepticum | 1250.00 | 625.00 | – | – | – | – | 625.00 | 625.00 | – | – |
Fig. 2.
Minimal inhibitory concentration (MIC) and Minimum bactericidal concentration (MBC) of examined plant extracts on six microorganism.
4. Discussion
Phytochemical screening revealed exist several classes of secondary metabolites such as alkaloids, polyphenols, flavonoids, anthraguinones, coumarins, saponins, tannins, triterpenes and steroids. Several molecules are active on pathogenic microorganisms (Cowan, 1999, Awouafack et al., 2013, Tsopmo et al., 2013, Erfan and Marouf, 2019). Exist such metabolites in the tested plant extracts can give a preliminary explanation on their antimicrobial antibacterial activities. Differences were observed in the antibacterial activities of the extracts. These could be due to the differences in their chemical composition as well as in the mechanism of action of their bioactive constituents (Cowan, 1999). All the extracts are rich in secondary metabolites; however, activity does not depend only exist secondary metabolites in the plant extracts, but also on their concentration and the possible interaction with other components (Dzotam et al., 2016). The antibacterial effect for tannins attributed to its ability to react with proteins to form stable water-insoluble components since bacterial cell wall made from proteins (Dangoggo et al., 2012). In addition, it may bind to proline – rich protein and interfere with the protein synthesis (Shimada, 2006), also the antimicrobial activity of saponins rendered to cause leakage of proteins and certain enzymes from cell (detergent like properties). The antibacterial effect of alkaloids due to its ability to interchelate with DNA of both Gram positive and negative bacteria and interfere with cell division (Bukar et al., 2015), while flavonoids activity is due to their ability to bind with intracellular and soluble proteins and to bind with bacterial cell walls and the antibacterial properties of steroids are due to complex with membrane lipids and exerts its action by causing leakage (Marjorie, 1999). The problem of microbial resistance is growing by time and the outlook for the use of antimicrobial drugs in the future is still uncertain. Therefore, actions must be taken to face this problem and to introduce the antibacterial activity of medicinal plants (Cowan, 1999, Nostro et al., 2000, Osman et al., 2013, Osman et al., 2014a, Osman et al., 2014b, Osman et al., 2014c; Wagdy et al., 2016; Abdel Halium et al., 2019, Mohamed et al., 2019, Ibrahim et al., 2019). In this study the antibacterial findings of Psidium guajava extract at different concentration have that able to inhibit the growth of S. aureus, Pasteruella multocida and Mycoplasma gallisepticum that correlated with the findings of Concalves et al. (2008), who studied guava extracts against food born pathogen and spoilage bacteria that due to the phenolic components which make them effective against the tested microorganisms. This result confirmed by Malaviya and Mishra (2011) and these observations matched also with that of the findings of Hogue et al. (2007) who showed the antibacterial activity of guava leaf extracts based on how the phenolic components act particularly flavonoids. These observations also agreed with Ismail et al. (2012) who exhibited the antibacterial activity of guava against food borne pathogens. According to the study the extracts of Ziziphusspina christi of all parts act as antibacterial agent against the tested pathogenic bacterial strains. This activity attributed to exist tannins and leucocyanidin (Rizk et al., 1993), also due to exist other active components like saponins, flavonoids, steroids, glycosides (Lee, 2006, Lam, 2007). This result agreed with results recorded by El-kamali and Mahjoub 2009. Who recorded the seed extracts was effective only against three bacterial strains. The investigation also agreed with Al-Mutairi et al. (2016) who recorded that ethanol and methanol leaves extracts also were effective against most tested strains. The antibacterial activity of S. officinalis extract against S. aureus, E. coli and Salmonella Enteritidis, confirming results already reported by Velckovic et al. (2003) who found that sage ethanol extract possesses antibacterial activity against S. aureus, B. subtilis, E. coli and Salmonella Enteritidis. Lai and Roy (2004) also reported that sage thanolic extract is effective against B. cereus, S. aureus and Vibrio parahaemolyticus. Availability of some compounds like α-pienene, camphor, 1,8-cineole, borneol in salvia officinalis shows that antibacterial effect attributed to these compounds (Knoblochet al., 1989). Investigation of Morus alba extract show antimicrobial potency against B. cereus and Pasteurella multocida, of the same with Sunil and Ammani (2009) who found that Morus alba ethanolic extract showed antibacterial activity against 15 bacterial and fungal spp. and also confirmed by Mohamed et al. (2013) who recorded that the most active extracts observed by M. alba against Gram positive and Gram negative bacteria. That activity may due to the plant is rich in phyto constituent slike; tannins, phytosterols, sitosterols, saponins, anthroquinones, glycosides and Oleanolic Compounds (Chen et al., 2005). The present results showed a good inhibitory effects olive extract on pathogenic bacteria. Many studies confirm the positive role of olive extracts in inhibitory pathogenic bacteria. Such as, Markinet al. (2003) who reported that water extract of olive leaf killed E. coli, P. aeruginosa S. aureus and K. pnebdmonia and B. subtilis. In another study, Korukluoglu et al. (2010) investigated the effect of the extraction solvent on the antimicrobial efficiency of S. aureus, E. coli, Salmonella Enteritidis, Salmonella Typhimurium, they also recorded that ethanol olive extracts showed the highest antimicrobial activity against E. coli and Salmonella Enteritidis. Papers about the antimicrobial properties of olive refer to compounds obtained from olive fruit particularly hydroxytyrosol and oleuropein (Bisignano et al., 1999).
5. Conclusion
The present investigation reveals a real solution for antimicrobial resistance by using a natural herbal extracts. The five above mentioned plant extracts have a powerful antimicrobial activity that inhibit or at less stop the growth of six microbial populations of different bacterial categories; so those extract may be used as food additives and food preservatives to control such microbial population and conserve human and animal health.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the Deanship of Scientific Research at King Saud University through the research group project No.: RG-162.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdel Halium M.M., Salib F.A., Marouf S.A., Abdel Massieh E.S. Isolation and molecular characterization of Mycoplasma species in sheep and goats in Egypt. Vet. World. 2019;12(5):664–670. doi: 10.14202/vetworld.2019.664-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Mutairi M.H., Ali S., Aly S.M., Aldebasi Y. Antibacterial activity of sider (ziziphusspina – Christi), leaves extract against selected pathogenic bacteria. Europ. J. Pharma. Med. Res. 2016;3:138–144. [Google Scholar]
- Alnieida C.C., Karnikowski M.G., Flieto R., Baldis-serotto B. Analysis of antidiarrheal effect of plants used in popular medicine. Rev. Saudepublica. 1995;29:428–433. doi: 10.1590/s0034-89101995000600002. [DOI] [PubMed] [Google Scholar]
- Awouafack M.D., Tane P., Kuete V., Eloff J.N. Medicinal Plant Research in Africa. Elsevier; 2013. Sesquiterpenes from the medicinal plants of Africa; pp. 33–103. [Google Scholar]
- Baricevic D., Sasa S., Della Loggia R., Tubaro A., Simonovska B., Krasna A., Zupancic A. Topical anti-inflammatory activity of salvia officinalis L. leaves: the relevance of urosolic acid. J. Ethnopharmacol. 2001;75:125–132. doi: 10.1016/s0378-8741(00)00396-2. [DOI] [PubMed] [Google Scholar]
- Bukar A.M., Kyari M.Z., Gwaski P.A., Gudusu M., Kuburi F.S., Abadam Y.I. Evaluation of phytochemical and potential antibacterial activity of ziziphusspina-christi against some medically important pathogenic bacteria. J. Pharmacogen. Phytochem. 2015;3(5):98–101. [Google Scholar]
- Chanda S.Y., Daravalia M.K., Rakholiya K. Fruit and vegetable peels – strong natural source of antimicrobics. Curr. Res., Technol. Educat. Topic Appl. Microbiol. Microbial Biotech. 2010;444:450. [Google Scholar]
- Chen C.C., Liu L.K., Hsu J.D., Huang H.P., Yang M.Y., Wang C.J. Mulberry extract inhibits the development of atherosclerosis in cholesterol fed rabbits. Food Chem. 2005;91:601–607. [Google Scholar]
- Chopra I. Drugs for the superbugs. Microbiol. Today. 2000;27:4–6. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI), 2015. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fifth Informational Supplement, 35(3), 1–236.
- Concalves F.A., Andrade Neto M., Bezerra J.N.S., Macrae A., Sousa O.V., FontelesFilho A.A., Vierira R.H. Antibacterial activity of guava, pisidiumguajva Linnaeus, Leaf extracts on diarrhea – causing enteric bacteriaisolated from Seabob shrimps. Xiphopenaeuskroyeri (Heller). Rev. Inst. Med. Trop. S. Paulo. 2008;50(1):11–15. doi: 10.1590/s0036-46652008000100003. [DOI] [PubMed] [Google Scholar]
- Cowan M.M. Plant products as antimicrobial agents. Clin. Microbial. Rev. 1999;12(4):564–582. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig J.W. Health – promoting properties of common herbs. Am. J. Clin. Nutr. 1999;70(Suppl.):491S–499S. doi: 10.1093/ajcn/70.3.491s. [DOI] [PubMed] [Google Scholar]
- Dangoggo S.M., Hassan L.G., Sadig I.S., Manga S.B. Phytochemical analysis and antibacterial screening of leaves of diospyrosespiliformis and ziziphusspina-christi. J. Chem. Eng. 2012;1(1):31–37. [Google Scholar]
- Dzotam J.K., Touani F.K., Kuete V. Antibacterial and antibiotic – modifying activities of three food plants (xanthosomamafaffa. Lam., Moringaoleifera (L.). Schott and passifloraeduilissims) against multidrug resistant (MDR) Gram – negative bacteria. BMC Complement. Altern. Med. 2016;16(1):9. doi: 10.1186/s12906-016-0990-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Beshbishy H.A., Singab A.N., Sinkkonen J., Pihlaja K. Hypolipidemic and antioxidant effects of Morusalba L. (Egyptian Mulberry) root and bark fractions supplementation in cholesterol fed rats. Life Sci. 2006;78:2724–2733. doi: 10.1016/j.lfs.2005.10.010. [DOI] [PubMed] [Google Scholar]
- El-Kamali H.H., Mahjoub S.A. Antibacterial activity of francoeuriacrispa, pulicariaundulatat, ziziphusspina- Christi and cucurbitapepo against seven standard pathogenic bacteria. Ethnobot. Leaflets. 2009;13:722–733. [Google Scholar]
- Erfan A.M., Marouf S. Cinnamon oil downregulates virulence genes of poultry respiratory bacterial agents and revealed significant bacterial inhibition: An in vitro perspective. Vet. World. 2019;12(11):1707–1715. doi: 10.14202/vetworld.2019.1707-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia O.B., Castillo J., Lorente J., Ortuno A., Del-Rio J.A. Antioxidant activity of phenolics extracted from oleaeuropaea L. leaves. Food Chem. 2000;68:457–462. [Google Scholar]
- Gonzalez M., Zarauelo A., Gamez M.J., Utrilla M.P., Jimenez J., Osuna I. Hypoglycemic activity of olive leaf. Planta Med. 1992;58:513–515. doi: 10.1055/s-2006-961538. [DOI] [PubMed] [Google Scholar]
- Gutierrez R.P., Mitchell S., Solis R.V. Pisidiumguajava: A review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2008;117:1–27. doi: 10.1016/j.jep.2008.01.025. [DOI] [PubMed] [Google Scholar]
- Hansen K., Adsersen A., Christensen B.S., Brooegger S., Rosendal J.S., Nyman U., Wagner Smitt U. Isolation of an angiotensin converting enzyme (ACE) inhibitor from oleaeuropaea and olealancea. Phytomed. 1996;2:319–324. doi: 10.1016/S0944-7113(96)80076-6. [DOI] [PubMed] [Google Scholar]
- Hannan P.C.T. Guidelines and recommendations for antimicrobial minimum inhibitory concentration (MIC) testing against veterinary mycoplasma species. International Research Program on Comparative Mycoplasmol. Vet. Res. 2000;31(4):373–395. doi: 10.1051/vetres:2000100. [DOI] [PubMed] [Google Scholar]
- Hogue M.M., Bari M.L., Inatsu Y., Vijay K.J., Kawamoto S. Antibacterial activity of Guava (Pisidiumguajava L.) and Neem (Azadirachtaidica A Juss.) extracts Against Foodbrone Pathogens and Spoilage Bacteria. 2007;4(4):481–488. doi: 10.1089/fpd.2007.0040. [DOI] [PubMed] [Google Scholar]
- Ibrahim W.A., Marouf S.A., Erfan A.M., Nasef S.A., El Jakee J.K. The occurrence of disinfectant and antibiotic-resistant genes in Escherichia coli isolated from chickens in Egypt. Vet. World. 2019;12(1):141–145. doi: 10.14202/vetworld.2019.141-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail M., Minhas P.S., Khanum F., Sahnan V.M., Sowmya C. Antibacterial (Activity of leaves extracts of Guava (PisidiumGuajava) Int. J. Res. Pharmaceut. Biomed. Sci. 2012;3(1):1–2. [Google Scholar]
- Jaiarj P., Khoohaswan P., Wongkraing Y., Peungricha P., Suriyawong P., Sumalsaraya M.L., Ruangsom-boon M.L. Anticough and antimicrobial activities of pisidiumguajava: Linn. Leaf Extracts. J. Ethnopharmacol. 1999;67:203–212. doi: 10.1016/s0378-8741(99)00022-7. [DOI] [PubMed] [Google Scholar]
- Knobloch K., Pauli A., Iberi B. J. Essen. Oil Res. 1989;1:119–128. [Google Scholar]
- Korukluoglu M., Sahan Y., Yigit A., Ozer E.T., Gucer S. J. Food Process. Preserv. 2010;34:383–396. [Google Scholar]
- Lam K.S. New aspects of natural products in drug discovery. Trends Microbiol. 2007;15(6):279–289. doi: 10.1016/j.tim.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Lai P.K., Roy J. Antimicrobial and chemopreventive properties of herbs and spices. Curr. Med. Chem. 2004;11(11):1451–1460. doi: 10.2174/0929867043365107. [DOI] [PubMed] [Google Scholar]
- Lee C.P. Who’s in the business of saving lives? J. Med. Philos. 2006;31:465–482. doi: 10.1080/03605310600912667. [DOI] [PubMed] [Google Scholar]
- Loannides C. Pharmacokinetic interactions between herbal remedies and medicinal drugs. Xenobiotica. 2002;32(6):451–478. doi: 10.1080/00498250210124147. [DOI] [PubMed] [Google Scholar]
- Lutterodt G.D. Inhibition of gastrointestinal release of acetylcholine by querctin as a possible mode of action of pisidiumguajava leaf extracts in the treatment of acute diarrhoeal diseases. J. Ethnopharmacol. 1989;25:235–247. doi: 10.1016/0378-8741(89)90030-5. [DOI] [PubMed] [Google Scholar]
- Maiyo Z., Ngure R., Matasyoh J., Chepkorir R. Phytochemical Constituents and antimicrobial activity of leaf extracts of three Amaranthus plant species. Afr. J. Biotechnol. 2010;9(21):3178–3182. [Google Scholar]
- Malaviya A., Mishra N. Antimicrobial activity of tropical fruits. Biological forum. Int. J. 2011;3(1):1–4. [Google Scholar]
- Marjorie C. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999;12:564–582. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markin D., Duek L., BerdiceVsky I. Mycoser. 2003;46:132–136. doi: 10.1046/j.1439-0507.2003.00859.x. [DOI] [PubMed] [Google Scholar]
- Matasyoh J., Maiyo Z., Ngure R., Chepkorir R. Chemical composition and antimicrobial activity of the essential oilofCoriandrumsativum. Food Chem. 2009;113(2):526–529. [Google Scholar]
- Mohamed Z., Hussein M., Yousry A., Abdel-EL Wahab G. Biological activity of extracts from Morus alba L., Albizzialebbeck (L) Benth. and CasuarinaglaucaSieber against the growth of some pathogenic bacteria. Int. J. Agric. Food Res. 2013;2(1):9–22. [Google Scholar]
- Mohamed S.A., Marouf S., Erfan A., El-Jakee, Ashgan M.H., Turki M.D., Saleh A.K., Ihab M.M. Risk factors associated with E. coli causing neonatal calf diarrhea. Saudi J. Biolog. Sci. 2019;26:1084–1088. doi: 10.1016/j.sjbs.2018.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noah D., Fidas G. National Intelligence Council; Washington DC: 2000. The Global Infectious Disease Threat and its Implications for the United States. [PubMed] [Google Scholar]
- Nostro A., Germano M.D., Angelo V., Marino A., Cannatell M.A. Extraction method and bioautography for evaluation of medical plant antimicrobial activity. Lett. Appl. Microbiol. 2000;30:379. doi: 10.1046/j.1472-765x.2000.00731.x. [DOI] [PubMed] [Google Scholar]
- Osman K.M., Marouf S.H., Samir A., AlAtfeehy N. The prevalence of multidrug resistance of various numbers of antimicrobial classes, multiple resistance patterns, and distribution of Salmonella isolates from human and avian clinical cases of diarrheoa. J. Chemother. 2012;24(5):300–304. doi: 10.1179/112000912X13418499354968. [DOI] [PubMed] [Google Scholar]
- Osman K.M., Marouf S.H., AlAtfeehy N. Antimicrobial resistance and virulence-associated genes of Salmonella enterica subsp. Enterica serotypes Muenster, Florian, Omuna, and Noya strains isolated from clinically diarrheic humans in Egypt. Microbial Drug Resist. 2013;(5):19370–19377. doi: 10.1089/mdr.2012.0151. [DOI] [PubMed] [Google Scholar]
- Osman K.M., Marouf S.H., Erfan A., AlAtfeehy N. Salmonella enterica in imported and domestic day-old turkey poults in Egypt: repertoire of virulence genes and their antimicrobial resistance profiles. Rev. Sci. Tech. Off. Int. Epiz. 2014;33(3):1017–1026. doi: 10.20506/rst.33.3.2338. [DOI] [PubMed] [Google Scholar]
- Osman K.M., Marouf S.H., Mehana O.A., AlAtfeehy N. Salmonella enterica serotypes isolated from squabs reveal multidrug resistance and a distinct pathogenicity gene repertoire. Rev. Sci. Tech. Off. Int. Epiz. 2014;33(3):997–1006. doi: 10.20506/rst.33.3.2336. [DOI] [PubMed] [Google Scholar]
- Osman K.M., Marouf S.H., Zolnikov T.R., AlAtfeehy N. Isolation and characterization of Salmonella enterica in day-old ducklings in Egypt. Pathogens Global Health. 2014;108(1):37–47. doi: 10.1179/2047773213Y.0000000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson G. The blood pressure lowering factory in leaves of oleaeuropaea. Farmacevtisk Revy. 1951;15:229–239. [Google Scholar]
- Shahat A.A., Pieters L., Apers S., Nazeif N.M., Abdel-Azim N.S., Berghe D.V., Vlietinck A.J. Chemical and biological investigations on zizphusspina – Christi L. Phytother. Res. 2001;15(7):593–597. doi: 10.1002/ptr.883. [DOI] [PubMed] [Google Scholar]
- Shimada T. Salivary proteins as a defense against dietary tannins. J. Chem. Ecol. 2006;32(6):1149–1163. doi: 10.1007/s10886-006-9077-0. [DOI] [PubMed] [Google Scholar]
- Simic D., Knezevic-Vukcevic J., Vukovic-Gacic B. Proc. of the First Conf. on Med. Arom. Plants South. Europ. Coun. 2000. Prospects in using medicinal and aromatic plants in cancer prevention; pp. 97–104. [Google Scholar]
- Souza E.L.D., Lima E.D.O., Freire K.L., Sousa C.D. Inhibitory action of some essential oils and phytochemicals on the growth of various moulds isolated from foods. Brazil. Arch Biol. Tech. 2005;48(2):245–250. [Google Scholar]
- Sunil K., Ammani K. Antimicrobial activity of Morusalba L. Asian J. Bio Sci. 2009;4(1):124–126. [Google Scholar]
- Tsopmo A., Awah F.M., Kuete V. Medicinal Plant Research in Africa. Elsevier; 2013. Lignans and stilbenes from African medicinal plants; pp. 435–478. [Google Scholar]
- Velckovic D., Randselovic N., Ristic M. Chemical constituents and antimicrobial activity of the ethanol extracts obtained from flower, leaf and stem of satviaofficinalis L. J. Serb. Chem. 2003;68(1):17–24. [Google Scholar]