Abstract
Honey is a complex foodstuff found in nature which is used without any processing. Honey has been in use in medicine as well as raw food since ancient times. Essentially, it is a blend of sugars especially fructose and glucose. The objectives of the study were to determine major sugar composition as well as pesticides contamination in honey samples. Further, Hydroxy-methyl-furfuraldehyde (HMF) level was also determined to ascertain the freshness of honey samples. A total of 14 samples were collected from local market and tested for fructose, glucose, sucrose, HMF and organochlorine pesticides using HPLC and GC–MS techniques respectively. The total sugars in the 14 honey samples were found ranging between 50.26 and 74.74 g/100 g of honey. The chromatographic results showed the presence of the sugars like fructose and glucose in all honey samples. The honey sample SH–11 was found to contain the highest amount of fructose (40.63%). On the other hand, the lowest amount of fructose with 29.08% was observed in SH–7. The HPLC analysis also revealed the presence of sucrose in two samples but under the permissible limit. The average ratio of fructose to glucose in these honey samples was 1.3. None of the sample has ratio below 1.0 indicating lesser chances for honey to crystallize on storage. Out of 14 honey samples, 13 samples were found negative for the presence of any of the 63 pesticides tested. Only sample No. 13, was found to contain 15.95 ppb hexachlorobenzene per kilogram of honey. The HMF was not detected in four samples but in remaining samples it was well below the maximum permissible limit. No pesticide and sugar adulteration was observed in any of the honey samples. The honeys collected from Saudi Arabian markets were found to confirm the standards set by the regional and international standardization organization, the GSO and Codex Alimentarius Commission respectively.
Keywords: Honey, HMF, Pesticides, Sugars, Fructose, Glucose
1. Introduction
Honey is a natural sweet substance produced by honey bees from the flower nectar, which the bees collect and transform by combining with specific substances of their own, deposit, dehydrate, store and leave in the honey comb to ripen and mature (Codex Alimentarius Commission, 2001). It is a complex natural foodstuff and undoubtedly the lone sweetener that is used without any processing. Honey has been in use in medicine as well as raw food since ancient times. Essentially, it is a blend of sugars especially fructose and glucose. Additionally, very small amounts of several compounds such as enzymatic and non-enzymatic antioxidants, glucose oxidase, catalase, ascorbic acid, flavonoids, phenolic acids, carotenoid derivatives, organic acids, amino acids, proteins and volatile compounds are also present in honey (Aljadi and Kamaruddin, 2004, Gheldof et al., 2002, Lachman et al., 2010). The concentration of these compounds is determined by numerous elements like natural conditions during the assortment of the crude material, sorts of blooms, assortment of honey bees, climatic conditions, etc. (da Silva et al., 2016, Escuredo et al., 2014). The cardioprotective, anti-carcinogenic, antimicrobial, immune stimulant and anti-inflammatory properties of honey have been credited to the presence of different types antioxidants (Küçük et al., 2007, Liu et al., 2013, McKibben and Engeseth, 2002).
Food adulterations are deceitful activities within the food industry, which is defined as intentional addition or substitution cheaper food materials to get maximum economic benefit. (Spink and Moyer, 2011). Malpractices in food sector have serious effects on society that reflects on economic, social as well as health aspects of the people. Honey has been placed at sixth position among the most adulterated food (Izquierdo et al., 2019). Increased demand for honey in European Union has led to its import from other regions of the world. A large number of these imported honey have been found either adulterated or deceitfully labeled. The regional and international standardization organization such as GSO and Codex Alimentarius Commission have laid down the quality standards for honey are listed in Table 1.
Table 1.
Physical and chemical specifications of honey compositions according to the GSO standards.
| Honey composition | Specification |
|---|---|
| Hydroxylmethylfurfural (HMF) | Not more than 60 mg/kg |
| Total reducing sugar | Not less than 60% |
| Fructose | 27–44.3% |
| Glucose | 22–40.7% |
| Sucrose | Not more than 5% |
| Fructose/Glucose ratio | Not less than 1% |
| Heavy metals and other additives (arsenic, lead, mercury, pesticide, etc.) | Absent or not to exceed maximum levels allowed |
| pH | 3.24–6.1 |
The above-mentioned facts have lead the researchers and law enforcing agencies to develop newer and sophisticated methods to identify the adulterations in honey and other food materials (Guler et al., 2007). Different methods have been created to investigate diverse, unique and contaminated biochemical substance in honey (Ohmenhaeuser et al., 2013). As the chemical compositions of honey and different syrups are very similar to each other, it is highly difficult to distinguish between them. To prevail over this issue, techniques like nuclear magnetic resonance, high performance liquid chromatography, etc. have been applied to identify adulterant in honey samples (Ohmenhaeuser et al., 2013, de Ribeiro et al., 2014, Wang et al., 2015). But major drawbacks with these approaches are high costs and time consuming as well as expert personnel are required to handle such sophisticated instruments, which make them difficult to apply for routine applications.
The aim of this study was to establish testing and affirm the use of Gas chromatography (GC) coupled with mass spectrometry (MS) as an effective method that can be used to detect toxins or pesticides in honey. The resulting knowledge of these toxins may be used to promote awareness among beekeepers so that best practices can be applied in this important sector of agriculture.
2. Materials and methods
2.1. Sample collection
The samples of honey were obtained from different cities of Saudi Arabia. In total 14 samples were collected as arranged in Table 2. All honey samples were collected in 2018.
Table 2.
Major sugar components and HMF in tested honey samples as determined by HPLC.
| Sample Number | Sample Code | Sugar content (%) |
Fructose/glucose (Ratio) | HMF (mg/kg) | |||
|---|---|---|---|---|---|---|---|
| Total | Fructose | Glucose | Sucrose | ||||
| 1. | SH–1 | 59.25 | 33.49 | 25.77 | − | 1.3 | ND |
| 2. | SH–2 | 62.62 | 35.84 | 26.78 | − | 1.3 | 0.478 |
| 3. | SH–3 | 63.46 | 31.72 | 28.79 | 2.95 | 1.1 | 12.47 |
| 4. | SH–4 | 56.44 | 33.02 | 23.43 | − | 1.4 | 1.00 |
| 5. | SH–5 | 60.06 | 32.85 | 27.20 | − | 1.2 | 0.641 |
| 6. | SH–6 | 63.84 | 36.67 | 27.17 | − | 1.3 | 0.541 |
| 7. | SH–7 | 50.26 | 29.08 | 21.18 | − | 1.4 | 2.47 |
| 8. | SH–8 | 52.99 | 32.97 | 20.02 | − | 1.6 | 10.7 |
| 9. | SH–9 | 63.81 | 36.34 | 27.47 | − | 1.3 | ND |
| 10. | SH–10 | 62.22 | 34.91 | 27.31 | − | 1.3 | ND |
| 11. | SH–11 | 74.74 | 40.63 | 34.10 | − | 1.2 | 2.94 |
| 12. | SH–12 | 66.96 | 33.48 | 30.63 | 2.85 | 1.1 | 28.97 |
| 13. | SH–13 | 68.47 | 39.48 | 28.99 | − | 1.4 | 1.3 |
| 14. | SH–14 | 61.21 | 36.38 | 24.83 | − | 1.5 | ND |
| Average | 61.88 | 34.77 | 26.70 | 0.41 | 1.3 | 4.39 | |
2.2. Sugar analysis in honey samples
Determination of sugar in honey by HPLC was carried out according to the method described earlier (Aljohar et al., 2018). Briefly, standard solutions of fructose (2 g%), glucose (2 g%), and sucrose (0.5 g%) were prepared in distilled water. The working sugar mixture solution was prepared by transferring 1 ml of each of standard solution of the three sugars to 10 ml volumetric flask and then the final volume was completed with distilled water. Sample preparation was carried out from the corresponding honey sample by dissolving 2.5 g of sample in 25 ml de-ionized water in a beaker. The resultant solution was filtered through 0.045 μm nylon filter in a 50 ml volumetric flask. The suitable volume of solution then transferred in HPLC vial. HPLC analysis was performed using liquid chromatography coupled with Refractive index detector (LC-RID) and data handling system was Software OpenLab (LC-RID). Separation was carried out using ZORBEX carbohydrate column 150 × 4.6 mm column. Chromatographic analysis was performed with 20:80 volume/volume mixture of distilled water and acetonitrile as mobile phase. The 10 μl sample was injected with a flow rate of 1.5 ml/min. The temperature of column was maintained at 27 °C during the whole run.
Sugar identification in honey samples was done by comparing the resulting chromatograms with those obtained from standard sugar mixture. Peak identification was performed through retention time matching. Quantitative determination of each sugar was accomplished by relating the peak area obtained for a particular sugar in the examined samples to that of standard sugar solutions.
2.3. Pesticides analysis in honey samples
The preparation of honey samples for GC–MS analysis was done according to the method described earlier (Rissato et al., 2004). In short, 10 g honey samples were weighed separately in an Erlenmeyer flask mixed with 5 ml water and homogenized by shaking, to reduce its viscosity and facilitate its handling. The samples were then mixed with 50 ml of acetonitrile and agitated for 20 min. The organic phase was separated by centrifugation at 2500g, for 10 min, the supernatant was collected and the residue was re-extracted with 40 ml of fresh solvent. The two portions collected were combined and the solvent was evaporated in a rotary evaporator, under reduced pressure at 65 °C and air dried. Finally, the residue was dissolved in 5 ml of ethyl acetate and filtered through 0.045 μm nylon filter.
Pesticides analysis in honey was carried using Gas Chromatography coupled with Mass spectrometry. Analysis was done on the Agilent 5977B GC/MSD (Santa Clara, United States). The HP-5 ms column was used which has very low bleed characteristics that are ideal for GC/MS. The column HP-5 ms has excellent inertness for active compounds both acidic and basic and improved signal-to-noise ratio for better sensitivity and mass spectral integrity. Carrier gas used was helium and the flow rate of 24.2 ml/min was maintained during the run. Conditions: initial temperature 50 °C increased at 25 °C/min to 150 °C, held for 1 min, increased at 3 °C/min to 200 °C, held for 1 min and 8 °C/min to 290 °C being held for 10 min at 280 °C.
2.4. Detection of Hydroxy-methyl-furfuraldehyde (HMF)
The HMF detection and its level in honey samples were carried out using HPLC technique. To prepare the samples for the detection of HMF, 2.5 g of honey was dissolved in 25 ml de-ionized water in a 50 ml beaker. The resultant solution was filtered through 0.45 μm filter, transferred to 50 ml volumetric flask and additional water was added up to the mark. The analytical standard and sample preparations were separately injected in equal volumes (5 μl), the chromatograms were recorded for the retention times of major peak and the peak response was measured as peak areas. HPLC analysis was performed using liquid chromatography coupled with a diode-array detector (DAD). Separation was carried out using Shimadzu SHIMPACK VP-ODS 4.6 × 150 mm, 5 μm RP column. Chromatographic analysis was performed with methanol and distilled water mixture (10:90 v/v) as mobile phase. The volume of 5 μl was injected with a flow rate of 1.2 ml/min. Run time was 10 min. During the run, temperature of column was maintained at 30 °C.
3. Results and discussion
3.1. Analysis of sugars in honey samples
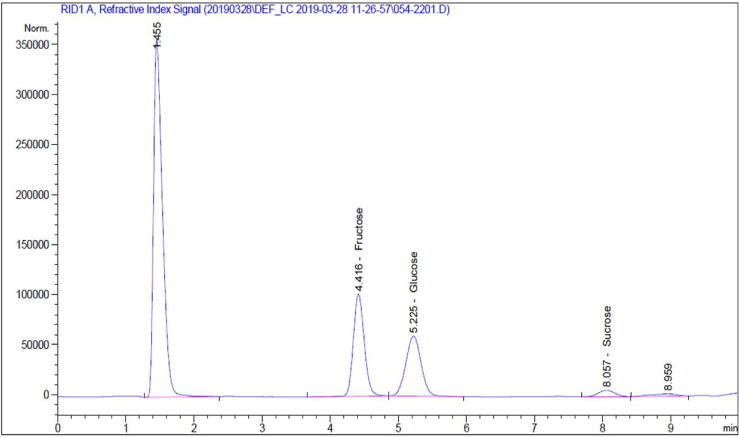
The determination of different sugar contents is employed to distinguish pure honey from adulterated ones. One of the major characteristics of honey is that fructose is present in higher amount than glucose. Approximately, about 40% is fructose and 30% glucose in honey, but this can be changed depending upon the storage time and temperature among other factors. The sucrose is also present in low amount ideally not more than 5% except honey from few specific plants (Aljohar et al., 2018). The total sugars in the 14 honey samples were analyzed and have been found in the range of 50.26–74.74 g/100 g of honey i.e., 50.26–74.74% (Table 2). The chromatographic results showed the presence of the sugars like fructose and glucose in all honey samples. Total sugar content was highest in sample SH–11 (74.74%) whereas sample SH–7 was found to contain lowest amount of total sugar (50.26%) amongst all the samples. The amount of fructose in each sample was found to be higher than the amount of glucose, which is a characteristic of natural honey. The honey sample SH–11 was found to contain the highest amount of fructose (40.63%). On the other hand, the lowest amount of fructose with 29.08% was observed in SH–7. Amongst all the honey samples, the least amount of glucose was found to contain in SH–8. The HPLC analysis of tested honey samples also revealed the presence of sucrose in two samples (SH–3 and SH–12). These values are all bellow the 5% mass ratio limit for sucrose that is allowed in unadulterated honey. Based on the data presented, the 14 honey samples do not appear to be adulterated with cheaper sweeteners. A representative HPLC chromatogram of sugars is depicted in Fig. 1.
Fig. 1.
Representative HPLC chromatogram of fructose, glucose and sucrose.
Ratio of fructose and glucose was also typical for honey. The more glucose a honey has, the faster it tends to crystallize. In honey, the ratio of fructose to glucose ideally should range from 0.9 to 1.35. A fructose to glucose ratio below 1.0 leads to faster honey crystallization whereas crystallization become slower when this ratio is more than 1.0 (Aljohar et al., 2018, Draiaia et al., 2015, El Sohaimy et al., 2015). In the present study, the average ratio of fructose to glucose was 1.3. Moreover, none of the sample has ratio below 1.0 which indicates the lesser chances for honey crystallization. The malpractice of mixing honey with maple, corn, sugar cane syrups should be detected as per the recommendations of the reference organization (Codex Alimentarius Commission, 2001). In our study, we found only 2 samples to contain sucrose that too less than 5% limit set by GCC Standardization Organization (GSO, 2014). Usually pure honey has low amount of sucrose because of the presence of enzyme invertase in honey which breakdown the sucrose. Therefore, sucrose adulteration in honey can be suspected by the presence of higher sucrose content i.e., higher than 8 percent (El Sohaimy et al., 2015, Rybak-Chmielewska, 2007, Siddiqui et al., 2017). In our study, the average amount of total sugar was 61.88 g in 100 g of honey which is higher than the GSO standards. Four samples namely SH–1, SH–4, SH–7, SH–8 were found to contain less than 60% total sugar limit according to the GSO standards. However, majority of samples were at par according to the GSO standards with respect to the total sugar content and were consistent with previous researches from the Arabian Gulf region (Al-Farsi et al., 2018, Aljohar et al., 2018, El Sohaimy et al., 2015). On the other hand, avocado and acacia honey from Spain and Czech Republic has been reported to contain more than 80% sugar content (Juan-Borrás et al., 2014, Rodríguez et al., 2019).
3.2. Pesticides analysis in honey samples
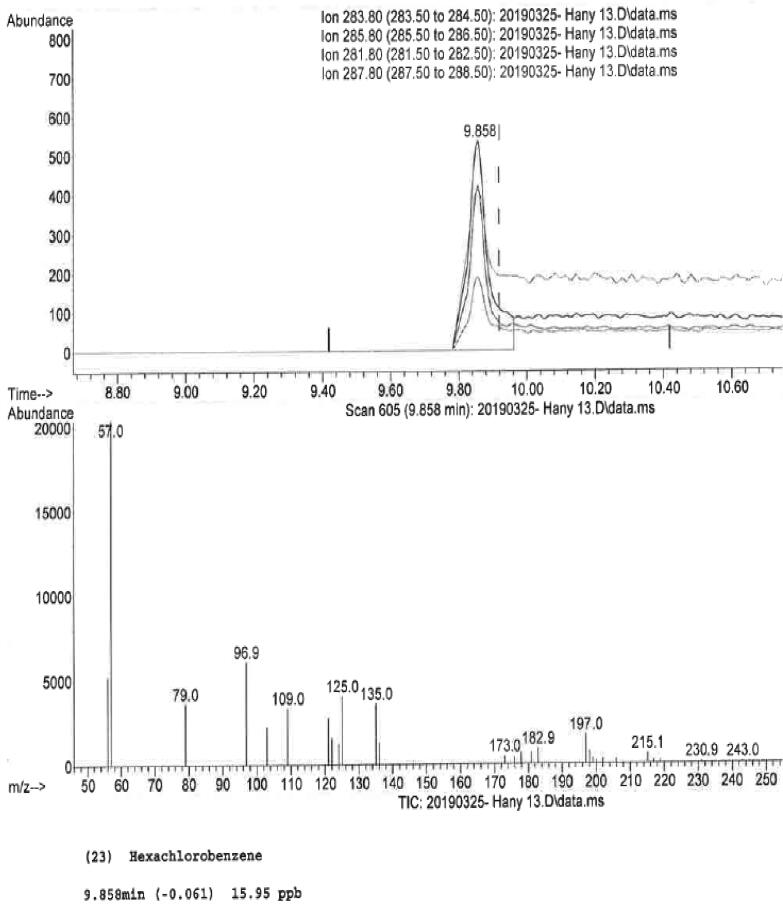
Honey bees are very sensitive creatures that work in harmony with their surrounding environment and therefore they get exposed to all types of harmful pollutions. The exposure of bees to different types of pollutants and toxins in the environment results in the form of contamination of their honey with pollutants and toxins. The presence of contaminants in honey poses a risk to public health. On the other hand, beekeeping is a source of revenue to many; standards are set to ensure the quality of the product specially to combat adulteration. Out of 14 honey samples, 13 samples were found negative for the presence of any of the 63 pesticides tested. Only sample No. 13 (SH–13), which was employed as control showed the peak at 9.858 min indicating the presence of hexachlorobenzene or HCB (Fig. 2). Its amount in the sample was estimated to be 15.95 ppb (0.01595 mg/kg of honey). The organochlorine pesticides are among the most dangerous environmental contaminants which are widely used in agricultural practices. These pesticides are known to cause mutations in DNA besides other damages to different cell organelles (Panseri et al., 2014). The presence of pesticides in honey has been widely reported via the pesticide utilization in agriculture (Bargańska et al., 2013, Panseri et al., 2014, Ravoet et al., 2015). The presence of pesticides in materials such as nectar, pollen, plant exudates or bee products like honey, royal jelly, etc. decreases quality. The pesticides may get entry into the honey through contamination of air, water, plants and soil and finally into the beehive by the bees. Moreover, using insecticides, fungicides, and acaricides control bee diseases in bee keeping practices also pollute honey directly (Amendola et al., 2011). In recent past there has been a sharp decline in honeybee populations around the world. It has been widely believed that the major reason for this decline in honeybee population is their exposure to pesticides (Goulson et al., 2015). Earlier studies have reported the occurrence of organochlorine pesticides in honey from Spain and Portugal (Blasco et al., 2004). A study from Italy has reported 94% of 72 honey samples were contaminated with at least one of the pesticides. Analysis of results revealed that pesticide contaminated honey was originated from apple orchards where pesticide use is common. Those honey samples that were collected from mountains dedicated for organic farming were found to be free from pesticides contamination (Panseri et al., 2014). Another study has reported the presence of pesticides in 29% of 45 honey samples collected from different apiaries in Northern Poland (Bargańska et al., 2013). In our study, of total 14 honey samples, we have found only one sample contaminated with organochlorine pesticide (HCB). In Saudi Arabia most of the land is both desert or arid where very little agriculture is practiced, hence environmental contamination of pesticides is least expected, and this has been reflected in our results on honey.
Fig. 2.
GC–MS chromatogram showing hexacholorobenzene in sample SH–13.
3.3. Detection of HMF
Permissible limit of HMF in honey samples is different in different countries. It is usually higher in hot tropical countries. The maximum permissible limit of HMF is 60 mg/kg in Saudi Arabia as per the country’s standards. The chromatographic results for the four honey samples i.e., sample SH–1, SH–9, SH–10 and SH–14 failed to detect any amount of HMF (Table 2; Fig. 3–B). In remaining samples of honey, the HMF levels were found to be well below the maximum permissible limit indicating the freshness of the honey samples as well as suggest that these honey samples were not heated (Table 2). The presence of HMF can be seen at time 7.8 min in chromatogram (Fig. 3A). 5-Hydroxymethylfurfural is a furanic compound which forms as an intermediate in the Maillard Reaction (Ames, 1992). Additionally, HMF is also produced in a process called caramelization which is direct drying out of sugars under acidic conditions (Kroh, 1994). Concentration of HMF also increases with the increase in length of storage time (Capuano and Fogliano, 2011, Fallico et al., 2004). Fresh and unheated honey either contains very small amount of HMF or does not contain it at all. It implies that low HMF values guarantee that the honey is practically unaltered (Bogdanov et al., 2004, Escriche et al., 2008). In international trade the maximum HMF level allowed is 40 mg/kg. However, in the case of honey of declared origin from countries or regions with tropical ambient temperatures the HMF content shall not be more than 80 mg/kg (Codex Alimentarius Commission, 2019). A study from Pakistan confirmed that the storage of honey increases its HMF level as they found higher HMF level in branded honey samples compared with fresh honeys (Sajid et al., 2020). In an earlier study on Saudi honey, 3 out of total 13 samples were found to contain very high amount of HMF. The researchers concluded that these samples were either stored for longer duration or over-heated. However, HMF level in remaining 10 samples was under the permissible limits (Alqarni et al., 2016). The presence of very low amount of HMF in our honey samples suggests that the honeys were fresh, not heated and free from adulteration of other syrup.
Fig. 3.
Representative HPLC chromatograms of (A) sample containing HMF and (B) sample without HMF.
4. Conclusion
Honey is widely used in the Middle Eastern countries owing to its beneficial properties as well as religious importance since it has been described as curing agent in the holy book of Islam. The honeys collected from Saudi Arabian markets were found to confirm the standards set by the GSO and Codex Alimentarius Commission. The HPLC analysis revealed the presence of sucrose in two samples only that too under permissible limit. All samples have fructose to glucose ratio above 1.0 which is suggestive of lesser chances for honey to crystallize on storage. Only one sample was found to contain traces of single pesticide (HCB) though tested for the presence of 63 different pesticides. The presence of very low amount of HMF in our honey samples suggests that the honey samples were fresh, not heated and free from adulteration of other syrup.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgment
The authors are thankful to the owner of Mnahel Al-rushed, Mr. Abdullah Al-Zahrani for providing the honey samples.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Peer review under responsibility of King Saud University.
References
- Al-Farsi M., Al-Belushi S., Al-Amri A., Al-Hadhrami A., Al-Rusheidi M., Al-Alawi A. Quality evaluation of Omani honey. Food Chem. 2018;262:162–167. doi: 10.1016/j.foodchem.2018.04.104. [DOI] [PubMed] [Google Scholar]
- Aljadi A.M., Kamaruddin M.Y. Evaluation of the phenolic contents and antioxidant capacities of two Malaysian floral honeys. Food Chem. 2004;85:513–518. doi: 10.1016/S0308-8146(02)00596-4. [DOI] [Google Scholar]
- Aljohar H.I., Maher H.M., Albaqami J., Al-Mehaizie M., Orfali Rawan, Orfali Razan, Alrubia S. Physical and chemical screening of honey samples available in the Saudi market: An important aspect in the authentication process and quality assessment. Saudi Pharmaceut. J. 2018;26:932–942. doi: 10.1016/j.jsps.2018.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alqarni A.S., Owayss A.A., Mahmoud A.A. Physicochemical characteristics, total phenols and pigments of national and international honeys in Saudi Arabia. Arabian J. Chem. 2016;9:114–120. doi: 10.1016/j.arabjc.2012.11.013. [DOI] [Google Scholar]
- Amendola G., Pelosi P., Dommarco R. Solid-phase extraction for multi-residue analysis of pesticides in honey. J. Environ. Sci. Health B. 2011;46:24–34. doi: 10.1080/03601234.2010.515170. [DOI] [PubMed] [Google Scholar]
- Ames J.M. The maillard reaction. In: Hudson B.J.F., editor. Biochemistry of Food Proteins. Springer; US, Boston, MA: 1992. pp. 99–153. [DOI] [Google Scholar]
- Bargańska Ż., Ślebioda M., Namieśnik J. Pesticide residues levels in honey from apiaries located of Northern Poland. Food Control. 2013;31:196–201. doi: 10.1016/j.foodcont.2012.09.049. [DOI] [Google Scholar]
- Blasco C., Lino C.M., Picó Y., Pena A., Font G., Silveira M.I.N. Determination of organochlorine pesticide residues in honey from the central zone of Portugal and the Valencian community of Spain. J. Chromatogr. A. 2004;1049:155–160. doi: 10.1016/j.chroma.2004.07.049. [DOI] [PubMed] [Google Scholar]
- Bogdanov S., Ruoff K., Persano Oddo L. Physico-chemical methods for the characterisation of unifloral honeys: a review. Apidologie. 2004;35:S4–S17. doi: 10.1051/apido:2004047. [DOI] [Google Scholar]
- Capuano E., Fogliano V. Acrylamide and 5-hydroxymethylfurfural (HMF): A review on metabolism, toxicity, occurrence in food and mitigation strategies. LWT - Food Sci. Technol. 2011;44:793–810. doi: 10.1016/j.lwt.2010.11.002. [DOI] [Google Scholar]
- da Silva P.M., Gauche C., Gonzaga L.V., Costa A.C.O., Fett R. Honey: Chemical composition, stability and authenticity. Food Chem. 2016;196:309–323. doi: 10.1016/j.foodchem.2015.09.051. [DOI] [PubMed] [Google Scholar]
- Codex Alimentarius Commission Draft revised standard for standard for honey (at step 10 of the Codex procedure) Alinorm. 2001;01(25):19–26. [Google Scholar]
- Codex Alimentarius Commission, 2019. Draft amended standard for standard for honey. CXS 12 – 1981, Amended in 2019 pp. 1–9.
- Draiaia R., Dainese N., Borin A., Manzinello C., Gallina A., Mutinelli F. Physicochemical parameters and antibiotics residuals in Algerian honey. Afr. J. Biotechnol. 2015;14 doi: 10.5897/AJB2015.14456. 1242–1251–1251. [DOI] [Google Scholar]
- El Sohaimy S.A., Masry S.H.D., Shehata M.G. Physicochemical characteristics of honey from different origins. Ann. Agric. Sci. 2015;60:279–287. doi: 10.1016/j.aoas.2015.10.015. [DOI] [Google Scholar]
- Escriche I., Visquert M., Carot J.M., Domenech E., Fito P. Effect of honey thermal conditions on hydroxymethylfurfural content prior to pasteurization. Food Sci. Technol. Int. 2008;14:29–35. doi: 10.1177/1082013208094580. [DOI] [Google Scholar]
- Escuredo O., Dobre I., Fernández-González M., Seijo M.C. Contribution of botanical origin and sugar composition of honeys on the crystallization phenomenon. Food Chem. 2014;149:84–90. doi: 10.1016/j.foodchem.2013.10.097. [DOI] [PubMed] [Google Scholar]
- Fallico B., Zappalà M., Arena E., Verzera A. Effects of conditioning on HMF content in unifloral honeys. Food Chem. 2004;85:305–313. doi: 10.1016/j.foodchem.2003.07.010. [DOI] [Google Scholar]
- Gheldof N., Wang X.-H., Engeseth N.J. Identification and quantification of antioxidant components of honeys from various floral sources. J. Agric. Food. Chem. 2002;50:5870–5877. doi: 10.1021/jf0256135. [DOI] [PubMed] [Google Scholar]
- Goulson D., Nicholls E., Botías C., Rotheray E.L. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science. 2015;347 doi: 10.1126/science.1255957. [DOI] [PubMed] [Google Scholar]
- Guler A., Bakan A., Nisbet C., Yavuz O. Determination of important biochemical properties of honey to discriminate pure and adulterated honey with sucrose (Saccharum officinarum L.) syrup. Food Chem. 2007;105:1119–1125. doi: 10.1016/j.foodchem.2007.02.024. [DOI] [Google Scholar]
- GCC Standardization Organization (GSO), 2014. GSO 5/FDS Final Draft for Honey. pp. 1–12.
- Izquierdo M., Lastra-Mejías M., González-Flores E., Cancilla J.C., Pérez M., Torrecilla J.S. Convolutional decoding of thermographic images to locate and quantify honey adulterations. Talanta. 2019;120500 doi: 10.1016/j.talanta.2019.120500. [DOI] [PubMed] [Google Scholar]
- Juan-Borrás M., Domenech E., Hellebrandova M., Escriche I. Effect of country origin on physicochemical, sugar and volatile composition of acacia, sunflower and tilia honeys. Food Res. Int., Authenticity, Typicality, Traceability Intrinsic Quality Food Products. 2014;60:86–94. doi: 10.1016/j.foodres.2013.11.045. [DOI] [Google Scholar]
- Kroh L.W. Caramelisation in food and beverages. Food Chem., Interactions Beverages. 1994;51:373–379. doi: 10.1016/0308-8146(94)90188-0. [DOI] [Google Scholar]
- Küçük M., Kolaylı S., Karaoğlu Ş., Ulusoy E., Baltacı C., Candan F. Biological activities and chemical composition of three honeys of different types from Anatolia. Food Chem. 2007;100:526–534. doi: 10.1016/j.foodchem.2005.10.010. [DOI] [Google Scholar]
- Lachman J., Orsák M., Hejtmánková A., Kovářová E. Evaluation of antioxidant activity and total phenolics of selected Czech honeys. LWT - Food Sci. Technol. 2010;43:52–58. doi: 10.1016/j.lwt.2009.06.008. [DOI] [Google Scholar]
- Liu J.-R., Ye Y.-L., Lin T.-Y., Wang Y.-W., Peng C.-C. Effect of floral sources on the antioxidant, antimicrobial, and anti-inflammatory activities of honeys in Taiwan. Food Chem. 2013;139:938–943. doi: 10.1016/j.foodchem.2013.02.015. [DOI] [PubMed] [Google Scholar]
- McKibben J., Engeseth N.J. Honey as a protective agent against lipid oxidation in ground turkey. J. Agric. Food Chem. 2002;50:592–595. doi: 10.1021/jf010820a. [DOI] [PubMed] [Google Scholar]
- Ohmenhaeuser, M., Monakhova, Y.B., Kuballa, T., Lachenmeier, D.W., 2013. Qualitative and Quantitative Control of Honeys Using NMR Spectroscopy and Chemometrics [WWW Document]. International Scholarly Research Notices. https://doi.org/10.1155/2013/825318.
- Panseri S., Catalano A., Giorgi A., Arioli F., Procopio A., Britti D., Chiesa L.M. Occurrence of pesticide residues in Italian honey from different areas in relation to its potential contamination sources. Food Control. 2014;38:150–156. doi: 10.1016/j.foodcont.2013.10.024. [DOI] [Google Scholar]
- Ravoet J., Reybroeck W., de Graaf D.C. Pesticides for apicultural and/or agricultural application found in Belgian honey bee wax combs. Bull. Environ. Contam. Toxicol. 2015;94:543–548. doi: 10.1007/s00128-015-1511-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro, R. de O.R., Mársico, E.T., Carneiro, C. da S., Monteiro, M.L.G., Júnior, C.C., Jesus, E.F.O. de, 2014. Detection of honey adulteration of high fructose corn syrup by Low Field Nuclear Magnetic Resonance (LF 1H NMR). J. Food Eng. 135, 39–43. https://doi.org/10.1016/j.jfoodeng.2014.03.009.
- Rissato S.R., Galhiane M.S., Knoll F.R.N., Apon B.M. Supercritical fluid extraction for pesticide multiresidue analysis in honey: determination by gas chromatography with electron-capture and mass spectrometry detection. J. Chromatogr. A. 2004;1048:153–159. [PubMed] [Google Scholar]
- Rodríguez I., Cámara-Martos F., Flores J.M., Serrano S. Spanish avocado (Persea americana Mill.) honey: Authentication based on its composition criteria, mineral content and sensory attributes. LWT. 2019;111:561–572. doi: 10.1016/j.lwt.2019.05.068. [DOI] [Google Scholar]
- Rybak-Chmielewska H. High Performance Liquid Chromatography (Hplc) Study of Sugar Composition in Some Kinds of Natural Honey and Winter Stores Processed by Bees from Starch Syrup. J. Apicultural Sci. 2007;51:23–38. [Google Scholar]
- Sajid M., Yamin M., Asad F., Yaqub S., Ahmad S., Mubarik M.A.M.S., Ahmad B., Ahmad W., Qamer S. Comparative study of physio-chemical analysis of fresh and branded honeys from Pakistan. Saudi J. Biol. Sci. 2020;27:173–176. doi: 10.1016/j.sjbs.2019.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui, A.J., Musharraf, S.G., Choudhary, M.I., Rahman, A.-, 2017. Application of analytical methods in authentication and adulteration of honey. Food Chem. 217, 687–698. https://doi.org/10.1016/j.foodchem.2016.09.001 [DOI] [PubMed]
- Spink J., Moyer D.C. Defining the public health threat of food fraud. J. Food Sci. 2011;76:R157–R163. doi: 10.1111/j.1750-3841.2011.02417.x. [DOI] [PubMed] [Google Scholar]
- Wang S., Guo Q., Wang L., Lin L., Shi H., Cao H., Cao B. Detection of honey adulteration with starch syrup by high performance liquid chromatography. Food Chem. 2015;172:669–674. doi: 10.1016/j.foodchem.2014.09.044. [DOI] [PubMed] [Google Scholar]