Abstract
As one of the most serious threats to human being, cancer is hard to be treated when metastasis happens. What's worse, there are few identified targets of metastasis for drug development. Therefore, it is important to develop strategies to prevent metastasis or treat existed metastasis. This review focuses on the procedure of metastasis, and first summarizes the targeting delivery strategies, including primary tumor targeting drug delivery, tumor metastasis targeting drug delivery and hijacking circulation cells. Then, as a promising treatment, the application of immunotherapy in tumor metastasis treatment is introduced, and strategies that stimulating immune response are reviewed, including chemotherapy, photothermal therapy, photodynamic therapy, ferroptosis, sonodynamic therapy, and nanovaccines. Finally, the challenges and perspective about nanoparticle-enabled tumor metastasis treatment are discussed.
KEY WORDS: Nanoparticles, Tumor metastasis, Tumor targeting drug delivery, Immunotherapy, Photodynamic therapy, Ferroptosis, Nanovaccines, Stimulating immune response
Graphical abstract
The review systemically summarizes the strategies of delivering drugs to tumor or tumor metastasis for treatment. Immunotherapy, especially other treatment mode-stimulated immunotherapy, is reviewed for tumor metastasis treatment.
1. Introduction
Cancer is one of the most serious threats to human being. In the past decade, although the 5-year survival of cancer-bearing patients is increased, patients with metastasis don't share the same trend1. The development of various drugs could more effectively manage the tumor, but little attention is paid on the targets about metastasis, and there is even overt scepticism in pharmaceutical industry and academy about the drugging metastasis1. Compared with primary tumor, tumor metastasis is hard to be surgically resected or treated by drugs because of their widely distribution, small size and high heterogeneity2. Therefore, it is urgent to develop strategies to prevent or treat tumor metastasis.
Tumor metastasis starts when the tumor mass is small. However, it is not easy for cancer cells to depart from its primary site and further form a distant metastatic colonization. Multiple factors will be involved for the migration of cancer cells. First, when a tumor mass grow up exceed to 1 mm in diameter, the secretion of proangiogenic and antiangiogenic factors will be out of balance, which induces vascularization and establishes a complex capillary network3. Then, the secretion of a series of enzymes, such as cathepsins and matrix metalloproteinase4, 5, 6, promotes the invasion and migration of cancer cells into surrounding tissues and the circulatory system. Once cancer cells enter the lymphatic or blood vessels, they will become circulating tumor cells (CTCs). The metastatic colonization process is very inefficient, less than 0.01% of CTCs actually survives and forms macrometastases at secondary sites after exposing of immune defenses, shear forces, and oxidative stress of the circulation7,8. Platelets give assistance to CTCs for survival by building a partial physical barrier9, 10, 11. Furthermore, contacting with macrophages can stimulate extravasation of associated CTCs in lungs, brain or other organs by releasing kinds of cytokines (e.g., sialyltransferase enzyme)10. Meanwhile, organs with rich and tortuous capillaries, like liver, lungs, brain and bone provide the ability for CTCs survival, contribute to a high rate of tumor metastases formation12. In addition, tumor exosome integrins also plays an important role in determine organotropic metastasis (e.g., α6β4 and α6β1 were associated with lung metastasis)13, 14, 15. Then, after CTCs lodging in the capillary beds of distant organs, they need to extravasate into the parenchyma, adapt to the new microenvironment and finally proliferate to become a new micrometastasis16, 17, 18.
Based on the whole process, drugs could intervene in any steps of the procedure to inhibit tumor metastasis2. And they could directly induce apoptosis of tumor cells in metastasis when the metastasis forms. However, specific delivery the drugs to their targets, no matter in primary tumor, blood circulation, or metastatic site, is a great challenge for researchers. The development of nanotechnology provides a powerful tool to deliver the drugs to a specific tissue or cells with high efficiency, which could greatly improve the therapeutic effect and reduce side effect19,20. What's more, the nanoparticles could be designed to deliver several drugs for combinational therapy, which is a trend in tumor treatment21. Except directly inducing tumor cell apoptosis, immunotherapy could employ the immune system to kill tumor cells in blood circulation or metastatic site. However, the immune response is tolerated in tumor-bearing patients22. To improve the immunotherapy outcome, various nanoparticles are developed to stimulate the antitumor immune response, which have shown better antitumor effect when combined with immunotherapy23.
This review focuses on the nanoparticle-based strategies for the management of tumor metastasis. According to the aim of various nanoparticles, the strategies are divided into cancer targeting drug delivery and stimulating antitumor immunity response. At the end of the review, the problems and challenges are also discussed in tumor metastasis management.
2. Cancer targeting drug delivery
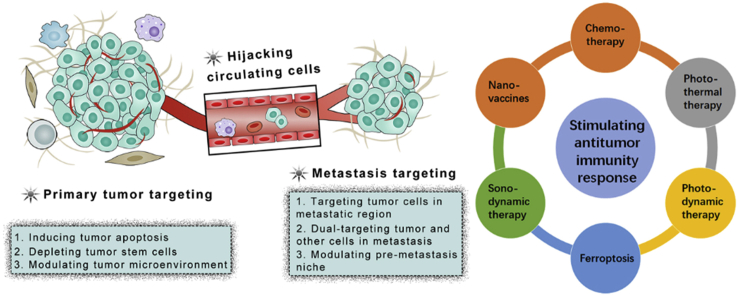
Tumor have specific properties that could be used for targeting drug delivery, among which, enhanced permeability and retention (EPR) effect is the most widely used rationale24. Furthermore, the tumor cells and tumor stroma cells display distinct surface markers due their quick proliferation and altered metabolism pathways. Therefore, these markers could serve as targets for targeting drug delivery. Traditionally, ligands of these markers are decorated onto the surface of nanoparticles, which could bind with makers on tumor or stroma cells and trigger internalization, improving their distribution in tumor cells20,25. According to the location of the tumor cells, we could divide the strategies into primary cancer targeting drug delivery and cancer metastasis-targeting drug delivery (Fig. 1 and Table 126, 27, 28, 29, 30, 31, 32, 33).
Figure 1.
Targeting drug delivery strategies for tumor metastasis treatment.
Table 1.
Targeting drug delivery strategies for tumor metastasis treatment.
| Category | Strategy | Example | Ref. |
|---|---|---|---|
| Primary cancer-targeting drug delivery | Targeting tumor cells | Paclitaxel (PTX)- and indocyanine green (ICG)-loaded cationic bovine serum albumin protected gold nanocluster (AuNC@CBSA) and hyaluronic acid (HA)-fabricated nanoparticles (AuNC@CBSA@HA) showed good breast cancer targeting capacity, which could induce tumor cell apoptosis and inhibit metastasis. | 26 |
| Targeting Cancer stem cells (CSCs) | Norcantharidin diacid form (NCTD)-loaded RGD modified nanoparticles (RGD-LPH-NCTD) could repress β-catenin pathway to reduce the number of CSC in breast cancer cells and inhibit metastasis. | 27 | |
| Modulating tumor microenvironment | Intraperitoneal injection of temsirolimus to inhibit HIF1 expression and prevent metastasis in combination with combretastatin A4-loaded PLGA nanoparticles. | 28 | |
| Cancer metastasis-targeting drug delivery | Targeting metastasis site | Zoledronic acid (ZOL)-decorated mesoporous silica nanoparticles-coated gold nanorods (Au@MSNs-ZOL) could effectively targeting bone metastasis because of the high affinity of ZOL with bone mass. | 29 |
| Dual-targeting tumor and other cells in metastasis | Folic acid and alendronate-modified PLGA nanoparticles could dual-target bone mass and tumor cells in bone metastasis. | 30 | |
| Modulating pre-metastasis niche | S100A4 siRNA-loaded exosome could downregulate the expression of S100A4 in lung fibroblast, which significantly reduce breast cancer lung metastasis. | 31 | |
| Hijacking circulating cells | Hijacking platelet | P-Selectin-modified redox-responsive paclitaxel-loaded micelle (PSN-PEG-SS-PTX4) could bind with activated platelet in circulation and then target to lung metastasis. | 32 |
| Hijacking neutrophil | Ppa-loaded albumin nanoparticles could be internalized by activated neutrophil and then target tumor with the assistance of TA99 antibody | 33 |
2.1. Primary cancer targeting drug delivery
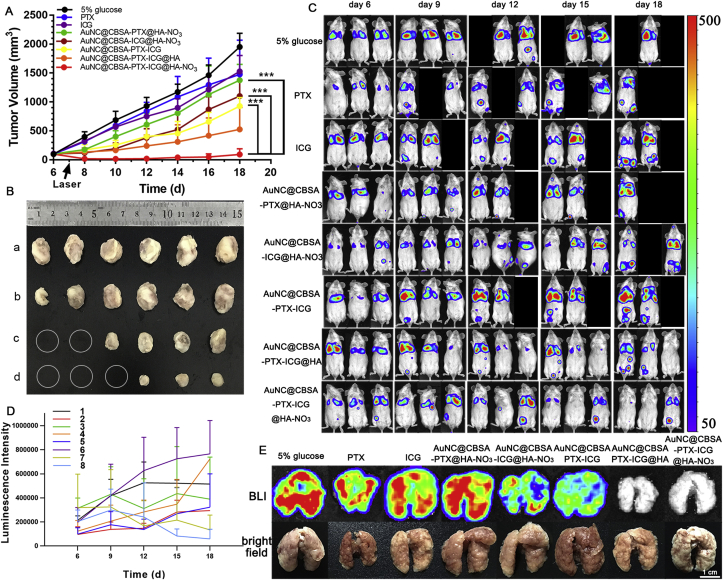
Although the review focuses on the treatment of tumor metastasis, many studies showed successfully prohibiting tumor metastasis by inhibiting primary cancer growth or inducing cancer cells apoptosis. For example, our group developed a cationic bovine serum albumin-protected gold nanocluster (AuNC@CBSA) and hyaluronic acid (HA) fabricated nanoparticles (AuNC@CBSA@HA) for breast cancer targeting drug delivery26,34, owing to the specific interaction between HA and tumor cell-overexpressed CD44 receptor35. Compared with AuNC@CBSA, AuNC@CBSA@HA showed higher distribution in subcutaneous breast cancer. After decorating with nitric oxide (NO) donor, which could improve tumor blood supply and enhance tumor penetration36, the tumor accumulation of AuNC@CBSA@HA-NO3 was further enhanced. Consequently, paclitaxel (PTX) and indocyanine green (ICG)-loaded nanoparticles could greatly inhibit primary tumor growth. The tumor inhibition rate was as high as 95.3%. As a result, 88.4% of lung metastasis was prohibited (Fig. 2). Similarly, co-administration of iRGD with nanoparticles could further improve tumor accumulation of nanoparticles, thus resulting in better antitumor effect37. Additionally, the reduced size could facilitate the nanoparticles transport from tumor to lymph nodes, which may treat lymph metastasis. For example, the 100 nm iCluster could distribute in tumor and reduce the size to 5 nm38. As a result, the iCluster showed 4.6-fold higher distribution in lymph nodes than the particles which did not shrink their size. Consequently, the number of lung metastatic nodules was reduced from 50 to 5. These studies indicated the effective depleting primary tumor cells and/or tumor cells in lymph nodes could greatly inhibit tumor metastasis.
Figure 2.
Anti-tumor effect in vivo. (A) Tumor volumes of mice treated with different formulations. (B) Tumors imaged by digital camera. (a) Control, (b) AuNC@CBSA-PTX-ICG, (c) AuNC@CBSA-PTX-ICG@HA, (d) AuNC@CBSA-PTX-ICG@HA-NO3. The circles represent the tumors of mice were eliminated. (C) Lung metastasis growth inhibition measured by BLI. Different colors in color scale represents different luminescence counts. The absences represent the dead mice. (D) The growth of metastasis along the timeline semi-quantified by luminescence intensity. (1) 5% glucose, (2) PTX, (3) ICG, (4) AuNC@CBSA-PTX@HA-NO3, (5) AuNC@CBSA-ICG@HA-NO3, (6) AuNC@CBSA-PTX-ICG, (7) AuNC@CBSA-PTX-ICG@HA, (8) AuNC@CBSA-PTX-ICG@HA-NO3. (E) BLI and morphology under the natural light of ex vivo lungs. P < 0.05, P < 0.01 and P < 0.001 were considered as significant difference (∗, ∗∗ and ∗∗∗, respectively). Reproduced with permission from Ref. 34. Copyright © 2018, Elsevier Inc.
Cancer stem cells (CSCs) are a group of cells with highly self-renewal potential, which are responding to tumor recurrence and metastasis39. Reducing CSC-liker property or targeting depletion of CSC can effectively kill primary tumor and inhibit metastasis. The Wnt/β-catenin pathway is an important pathway that involved in the generation and maintenance of CSCs40, which are highly activated in triple negative breast cancer41. Norcantharidin diacid form (NCTD) could repress β-catenin pathway to inhibit cancer cell proliferation42, so Li et al.27 encapsulated NCTD into Arg-Gly-Asp (RGD)-modified lipid-polymer hybrid (LPH) nanoparticle (RGD-LPH-NCTD) due to the binding affinity of RGD to integrin α5 on breast cancer cells. In cell growth clonogenic assay, one dose of RGD-LPH-NCTD significantly reduced the clonogenic number of breast cancer cells, which was much better than free NCTD and LPH-NCTD that without RGD modification. According to serum-free culture tumorigenic sphere formation assay, RGD-LPH-NCTD greatly reduced the number of tumorigenic suspension spheres, indicating that the RGD-LPH-NCTD could reduce the number of CSC in breast cancer cells. As expected, the treatment considerably reduced expression of active (non-phospho-β-catenin) and total β-catenin protein in breast cancers, suggesting the RGD-LPH-NCTD reduced breast cancer cells CSC-like property through β-catenin pathway. In vivo, the RGD-LPH-NCTD significantly inhibited primary breast cancer growth and its lung metastasis. Alternatively, Liu et al.43 conjugated CD20 antibody, an antibody of marker of lung CSC, onto silica-based magnetic nanoparticles (CD20-HSPI&Fe3O4@SiNPs) to target lung CSC and deplete them by chemotherapy and photothermal therapy. The particles showed higher uptake in lung CSCs, and could effectively deplete lung metastasis by intravenous injection. Yin et al.44 developed RNA nanoparticles that carried an RNA aptamer for CD133 targeting and anti-microRNA (miR) for miRNA21 inhibition. The particles showed higher uptake in breast CSCs because of the interaction between aptamer and CD133 on the surface of breast CSC, and several miR21-targeted tumor suppressor genes, such as PTEN and PDCD, were upregulated to induce more apoptosis. Additionally, chemotherapy may promote the stemness of tumor cells through cyclooxygenase-2 (COX-2)-mediated pathway, so co-delivery chemotherapeutics with COX-2 inhibitor celecoxib showed good antitumor effect and low metastasis45. These studies demonstrated that targeting CSC, no matter reducing its CSC-like property or its number, can be used as an effective strategy for tumor metastasis inhibition or treatment.
However, the targeting capacity of ligand-decorated nanoparticles is influenced by the protein corona, which is formed as soon as the particles are introduced into biological fluid46,47. To overcome the shortages, many biomimetic nanoparticles were constructed by various cell membranes, whole cells and exosomes. There are many reviews that have well overviewed the development of biomimetic nanoparticles48, 49, 50, 51, so we would not describe them in detail in this review. Overall, biomimetic nanoparticles are effective in delivering various cargoes to tumor, expanding their antitumor capacity and metastasis inhibition.
The tumor metastasis has several pathways, while some drugs could not inhibit tumor metastasis, although they could kill primary tumors. Therefore, combination with different agents is an attractive direction. Di-2-pyridylketone-4,4-dimethyl-3-thiosemicarbazone (Dp44mT), a thiosemicarbazone chelator, has high Fe-binding affinity and membrane permeability, which could inhibit tumor cell migration52. Ding et al.53 coloaded Dp44mT and cisplatin into nanoparticles for tumor targeting therapy and metastasis inhibition. In vitro, the A549 cells without treatment showed 71% wound closure, demonstrating their high wound healing ability and metastasis potential. Dp44mT contained nanoparticles greatly reduced the wound closure to 13%, while cisplatin treatment did not show any significant influence on wound healing. As expected, the Dp44mT and cisplatin-coloaded nanoparticles greatly reduced the expression of hypoxia inducible factor-1 (HIF1) and vascular endothelial growth factor (VEGF), which participate the tumor invasion and migration. However, cisplatin treatment did not influence them. Consequently, the in vivo lung metastasis after treatment was significantly inhibited.
The tumor microenvironment may promote metastasis, such as hypoxia and leaky vascular system, making tumor microenvironment modulation a promising strategy in primary tumor treatment and metastasis inhibition54,55. Hypoxia contributes to tumor resistance and metastasis, and several treatment strategies, such as radiotherapy, phototherapy and vascular disrupting agents, could elevate hypoxia microenvironment56. Temsirolimus is a mammalian target of the rapamycin (mTOR) inhibitor that can inhibit HIF1 and VEGF expression57. To attenuate the hypoxia microenvironment, combretastatin A4 (a vascular disrupting agent)-loaded PLGA nanoparticles was combined with intraperitoneal injection of temsirolimus28. In vitro, 4T1 cells treated with temsirolimus significantly reduced the expression of HIF1 as determined by Western blot. In vivo, although combretastatin A4-loaded nanoparticles treatment decreased primary tumor growth, the expression of HIF1α in tumor elevated to 2.3-fold, and the relative necrotic area increased to 17-fold compared to PBS treatment. However, after co-treatment with temsirolimus, the expression of HIF1α significantly reduced. As a result, the lung metastasis rate reduced from 46% to 18% for nanoparticles treatment and co-treatment respectively. C−X–C motif chemokine ligand 12 (CXCL12) that secreted by cancer associated fibroblasts (CAF) could promote tumor cell migration, survival and proliferation. Therefore, delivery CXCL12 siRNA to prostate cancer could effectively reduce the metastasis in many organs, such as liver, spleen and kidney58. These studies demonstrated that modulating tumor microenvironment is a promising strategy for cancer metastasis prevention and treatment.
2.2. Cancer metastasis-targeting drug delivery
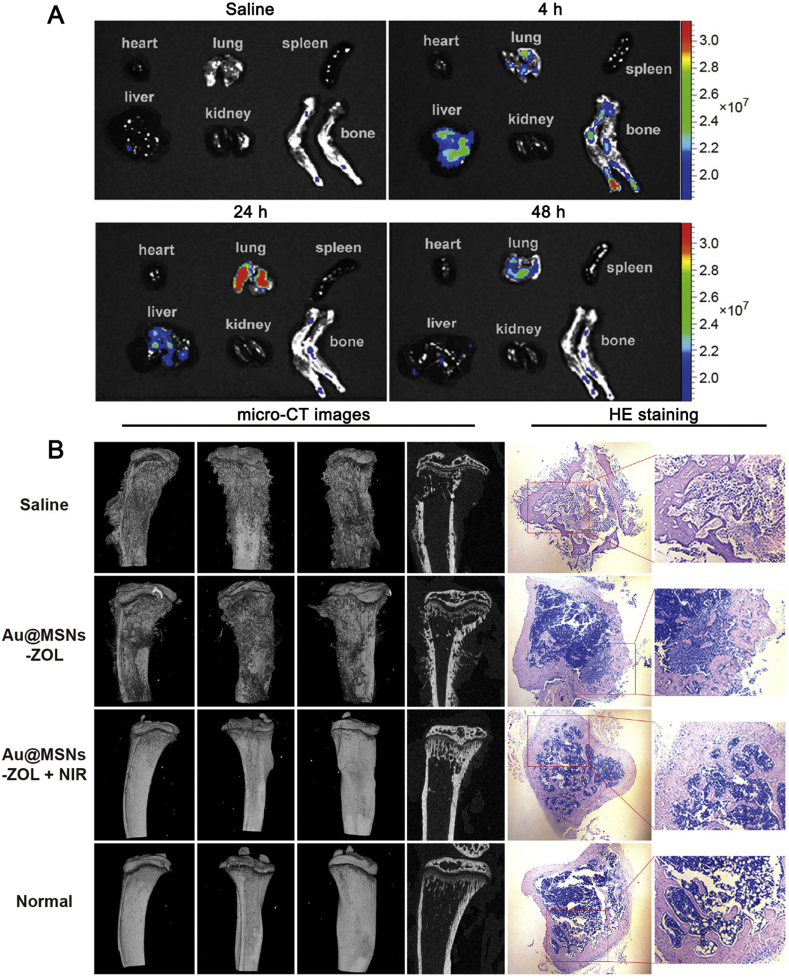
Different tissue metastasis has different characteristics. Bone metastasis did not only reduce patient survival time, but also greatly decrease the quality of life due to pain and clinical complications59. Bisphosphonates are widely used in bone metastasis targeting drug delivery and treatment because of their high affinity with bone mass. Zoledronic acid (ZOL), a nitrogen containing bisphosphonate, could not only induce tumor cell apoptosis, reduce vascular endothelial growth factor level, inhibit farnesyl pyrophosphate synthase which leads to the loos of osteoclast, but also has strong affinity with bone and could drive nanoparticles to bone metastasis60. Sun et al.29 conjugated zoledronic acid (ZOL) a third-generation bisphosphonate, onto mesoporous silica nanoparticles coated gold nanorods (Au@MSNs-ZOL) for breast cancer bone metastasis targeting and combinational therapy. After intravenous injection, the fluorescent imaging showed that Au@MSNs-ZOL could accumulate in the leg region, with intensity higher than unmodified nanoparticles (Au@MSNs, Fig. 3A). Consequently, after 10 min laser irradiation, the temperature reached 46.3 °C, while the Au@MSNs group only reached 34.1 °C. Therefore, the Au@MSNs-ZOL could be used for bone metastasis photothermal therapy. After intraosseous injection of MDA-MB231 breast cancer cells, the tumor grew very quickly, and could reach over 13,000 mm3 on Day 35. However, the tumor volume greatly reduced to 21 mm3 if treating the tumor-bearing mice with Au@MSNs-ZOL+ laser for four times from Days 4 to 28, suggesting the Au@MSNs-ZOL+ laser could effectively suppress growth of bone metastasis. What's more, the bone pain was also decreased which was determined by the leg lifting time. According to the CT results (Fig. 3B), the osteolysis on the surfaces and inside of the tumor-bearing tibias was greatly attenuated after the treatment with Au@MSNs-ZOL+ laser, which was similar to the normal bone.
Figure 3.
A, Representative fluorescence images of ex vivo heart, lung, spleen, liver, kidney, and bone at 4, 24, and 48 h after intravenous injection of Au@MSNs-ZOL-FITC. The colored scale represents the fluorescence signal to quantify the amount of Au@MSNs-ZOL-FITC. B, CT images of tibias from different angles and micrographs of H&E-stained tibias (right) at the end of the treatment period (35 days). Reproduced with permission from Ref. 29. Copyright © 2019, American Chemical Society.
Similarly, alendronate, another commonly used bisphosphonate, was anchored onto polyamidoamine (PAMAM) dendrimer for bone metastasis targeting delivery of docetaxel (DTX@ALN-PAMAM)61. The DTX@ALN-PAMAM significantly reduced the size of bone metastasis as determined by micro-CT, and the number of paw lifting was also considerably reduced. However, as discussed in section 2.1, the targeting capacity of ligand modification would be influenced by the protein corona, which could reduce blood circulation time of nanoparticles62. To minimize the side effect and maximum the targeting capacity, Vanderburgh et al.30 optimized the density of alendronate on the surface of nanoparticles. Although 100%-functionalized alendronate showed highest bone affinity, the blood circulation time was greatly reduced. To balance the bone binding and blood circulation, biodistribution ratio of bone-to-liver was introduced. It showed that 10% alendronate modification generated highest biodistribution ratio. Consequently, after loading with small molecule Gli2 inhibitor, the alendronate-modified nanoparticles decreased 3-fold of tumor-associated bone lesion area and increased 2.5-fold of bone volume fraction in the tibiae of the mice. These studies demonstrated that the nanoparticles decorated with bisphosphonate could effectively target bone metastasis, inhibit tumor growth and attenuate the complications. Additionally, the bisphosphonates only improved affinity with bone tissue rather than tumor cells in bone metastasis. Therefore, folic acid was anchored onto alendronate-modified PTX-loaded poly(lactic-co-glycolic acid, PLGA) nanoparticles for dual targeting delivery63. In vitro, the nanoparticles, AFTPNs, showed higher 4T1 breast cancer cell uptake than ATPNs, which did not have folic acid modification. In vivo, the tumor-bearing mice treated with PTX-loaded AFTPNs displayed lower tumor growth rate and longer survival time than those of ATPNs. These results indicated the dual targeting delivery to improve tumor cellular uptake is beneficial for bone metastasis treatment.
Brain metastasis is hard to be treat due to restriction by blood‒brain barrier (BBB)64. Traditional chemotherapeutics cannot distribute into brain tumor and metastasis, resulting in a very short survival time of patients (about 2.3–7.1 months for breast cancer brain metastasis)65. Therefore, developing targeting drug delivery systems is important for brain metastasis management20. To target the breast cancer brain metastasis, Zhou et al.66 modified gene delivery nanoparticles with AMD3100, a small molecule antagonist of CXCR4 that is overexpressed in breast cancer brain metastasis67. The AMD3100-modified nanoparticles showed higher accumulation in brain metastasis than the PEGylated nanoparticles as determined by in vivo fluorescent imaging. After encapsulated with artificial gene, proMel, the AMD3100-modified nanoparticles could effectively deliver the gene to brain metastasis, resulting in the expression of secretory promelittin protein. The promelittin could be cleaved by tumor overexpressed MMP-2 and release cytolytic melittin to induce tumor cell apoptosis. But in normal tissue, the promelittin could not be activated due to low level of MMP-2, enabling good safety of the drug delivery system. As a result, the luciferase signals from brain metastasis of the nanoparticles-treated mice were significantly reduced, and the survival was significantly increased. Over 50% mice were still alive at Day 60, while all mice of control groups died in 45 days.
Except functionalization nanoparticles with metastasis targeting ligand, biomimetic nanoparticles could also be utilized for metastasis targeting drug delivery due to their specific interaction with tumor metastasis cells68. Platelet is known to bind with metastatic tumor cells and circulation tumor cells through interaction between P-selectin and CD44 or other receptors69. Therefore, Ye et al.70 coated PLGA nanoparticles with platelet membrane (PMNPs) for breast cancer lymphatic metastasis targeting therapy. Due to the highly expressed CD44 on MDA-MB-231 breast cancer cells, the PMNPs showed higher cellular uptake than uncoated nanoparticles. In vivo, to mimic the circulation tumor cells, MDA-MB-231 cells were intravenously injected and the lung micrometastases were calculated. In saline-treated mice, many lung micrometastases were observed, while treatment with doxorubicin- and ICG-loaded PLGA nanoparticles only slightly decreased the number of lung micrometastases. Promisingly, treatment with drug-loaded PMNPs significantly reduced the micrometastases number, indicating the platelet membrane coating could improve the adhesion to circulation tumor cells and eliminate them, thus reducing the lung metastasis.
In many lethal metastasis cases, there are multiple metastasis nodules which infiltrated grow with normal cells. Especially at late stage, these metastasis nodules are hard to be surgically resected, and chemotherapy or radiotherapy may disrupt normal cells and harm functions of these organs71. Therefore, targeting the metastasis with tumor cell specific toxicity is a promising strategy. To treat liver metastasis, Huo et al.72 developed a photosensitizer (Ce6)-loaded triphenyl phosphonium (TPP) mesoporous silica nanoparticle (MSN) for mitochondria targeting photodynamic therapy and a nuclear localization sequence (NLS) decorated W18O49 nanoparticle (WONPs) for nucleus targeting photothermal therapy. Then the mitochondria-targeting MSNs [Mito(T)] were conjugated with nucleus-targeting WONPs [Nuc(T)] through cathepsin B cleavable peptide [Mito(T)-pep-Nuc(T)]. In hepatocytes, due to the low level of cathepsin B, the Mito(T)-pep-Nuc(T) would be kept intact, and the laser irradiation-generated singlet oxygen by Mito(T) could be consumed by WONPs and changed them into photothermally inert WO3, thus abolishing their photothermal capacity. Therefore, Mito(T)-pep-Nuc(T) had good safety in normal hepatocytes. But in cancer cells of liver metastasis, the overexpressed cathepsin B could cleave the peptide and release Mito(T) and Nuc(T), which could then target to mitochondria and nucleus, respectively. Upon laser irradiation, the singlet oxygen by Mito(T) and photothermal capacity of Nuc(T) could destroy both organelles, resulting in apoptosis of tumor cells. This study provided an interesting strategy that could overcome the unintended toxicity to normal cells and improve the treatment specificity.
The formation of metastasis depends not only conditions in primary tumor, but also the microenvironment in the distant organs, called pre-metastasis niche, so it may inhibit tumor metastasis by targeting the pre-metastasis niches and altering their microenvironment73. Upregulation of S100A4 could form the pre-metastasis niches and establish a microenvironment that can promote breast cancer cells seed and proliferation74. Additionally, it showed the exosome from cancer cells could target the pre-metastasis niches-enriched lung13. Therefore, Zhao et al.31 conjugated S100A4 siRNA with cationic bovine serum albumin (CBSA) and then coated them with exosome membrane for targeting modulation of pre-metastasis niches in lung. In vitro, the particles, CBSA/siS100A4@exosome, obviously downregulated the expression of S100A4 in mouse embryonic lung fibroblast, which was better than free siRNA and siRNA-loaded lipofectamine 2000. Furthermore, the particles could reduce the migration of breast cancer cells. In vivo, the CBSA/siS100A4@exosome showed higher accumulation in lung than siRNA-loaded liposomes. Consequently, the index of lung weight of CBSA/siRNA/exosome treatment was the lowest among all the groups, while the number of metastatic nodules decreased from about 37 in saline group to 4, which was much lower than other siRNA groups. The study demonstrated the exosome coating could effectively target pre-metastasis niches in lung and modulate the metastasis microenvironment by downregulating S100A4, representing an effective strategy for cancer metastasis inhibition.
2.3. Hijacking circulation cells
In the blood, there are several kinds of cells that involved in the metastasis of cancer. For example, platelet can be activated by tumor cells through intercellular adhesion molecules, and help tumor cells disassociate from primary tumor and migrate to distant organs69. Therefore, the activated platelet shows with targeting ability to primary tumor, circulating tumor cells and cancer metastasis. To hijack the activated platelets for cancer metastasis targeting, Zhang et al.32 modified P-selectin targeting peptide (PSN) onto a redox-responsive paclitaxel-loaded micelle (PSN-PEG-SS-PTX4). After activated by adenosine diphosphate, the whole blood was incubated with BODIPY-labeled PSN micelles, and significant higher fluorescence was observed compared with the unmodified micelles, while the fluorescence was greatly reduced if pre-incubation with free PSN, indicating the PSN modification indeed improved binding with activated platelet. After binding with platelet, the PSN micelles showed higher MDA-MB-231 cell uptake and cytotoxicity than the unmodified micelles. In vivo, the PSN micelles could target not only primary breast tumor but also its lung metastasis as determined by both in vivo fluorescent imaging and confocal microscopy. After three times treatment with PSN-PEG-SS-PTX4 micelles, there was almost no tumor signal in lung, which was greatly lower than PTX-loaded unmodified micelles and free drugs-treated groups. The study showed that hijacking platelet was a promising strategy to target and treat both primary tumor and its metastasis with a single formulation.
Other cells, such as circulating leukocytes, can be actively recruited in tumor or metastasis, which can be used for targeting drug delivery. The denatured albumin nanoparticles could be taken up by activated neutrophils in blood circulation. So, Chu et al.33 loaded pyropheophorbide-a (Ppa), a photosensitizer, into albumin nanoparticles, and co-injected them with TA99 antibody. The TA99 antibody could activate neutrophils and improve internalization of albumin nanoparticles in blood circulation. At 24 h post-injection, the percentage of nanoparticle-labeled neutrophils in co-injection group was 6.2%, while the number was only 0.7% in nanoparticles only group. Consequently, the distribution of nanoparticles in tumor was greatly increased. Similarly, in vitro anchoring nanoparticles onto or into leukocytes could also target tumor or metastasis site75. However, few studies used the strategy for in vivo hijacking cells to improve tumor metastasis treatment.
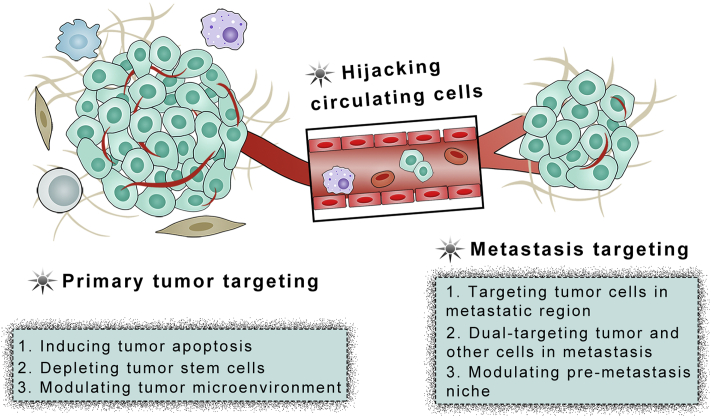
3. Stimulating antitumor immunity response
Cancer immunotherapy has gained great attention in the recent years, and the 2018 Nobel Prize in Physiology or Medicine was awarded to researchers in the field of cancer immunotherapy. Clinically, most immunotherapy agents are immune checkpoint blockade (ICB) antibodies, which could block the immune checkpoint interaction between cytotoxic T cells and tumor cells to recover the antitumor function of cytotoxic T cells76. Although several ICB antibodies are approved for clinical use, the response rate is low. A main reason is the immunological tolerance of tumors, such as low immunogenicity, poor antigen presentation efficiency and low T cell infiltration 77. Except ICB, there are other metabolic immune surveillance pathways, such as the indoleamine 2,3-dioxygenase (IDO-1) pathway78. The IDO catalyzes the oxidative catabolism of tryptophan (Trp) to kynurenine (Kyn), which prevent sufficient T-cell priming and impair CD8+ T cells survival. Therefore, stimulating antitumor immunity response may settle the immunological tolerance and improve treatment of cancer metastasis (Fig. 4).
Figure 4.
Strategies to stimulate antitumor immunity response for tumor metastasis treatment.
3.1. Chemotherapy-enhanced immunotherapy
Several chemotherapeutics, such as doxorubicin (DOX) and oxaliplatin, are demonstrated with ability of inducing immunity caused death (ICD). ICD could release tumor associated antigens, which could distribute into draining lymph node to stimulate immune response or be captured by macrophages in tumor for antigen presenting. Therefore, delivery chemotherapeutics to tumor could stimulate immunity response and treat cancer metastasis. However, the immunity response is pretty low due to the immunosuppressive conditions in tumor. So, many researches combined the chemotherapy-induced ICD with ICB to further boost the immunotherapy. Our group reported a dimer-7-ethyl-10-hydroxycamptothecin (d-SN38) and dimer-lonidamine (d-LND)-coloaded bilirubin nanoparticles (SL@BRNPs) to improve the immunotherapy of PD-L1 antibody79. With the assistance of iRGD peptide, the SL@BRNPs could actively distribute primary breast cancer, and induce robust antitumor effect and immune response. The CD3+CD8+ T cells in SL@BRNPs/iRGD+anti-PD-L1 group were much higher than controls, while CD4+Foxp3 cells were greatly reduced. Consequently, the lung metastasis in SL@BRNPs/iRGD+anti-PD-L1 group almost disappeared, indicating an excellent lung metastasis treatment capacity. Similarly, Kuai et al.80 reported a kind of nanodiscs to deliver DOX into tumor, which could boost the antitumor immune response and enhance the PD-L1 antibody treatment outcome. Folinic acid, 5-fluorouracil and oxaliplatin, is a standard treatment for colorectal cancer patients. Nanoparticles loaded with these three drugs successfully inhibited liver metastasis of colorectal cancer when combined with anti-PD-L1 treatment81. These studies indicated chemotherapy could serve as an effective strategy to boost antitumor immune response and enhanced ICB treatment outcome.
Combination chemotherapy with IDO pathway inhibitor could enhance the immunotherapy to prevent or treat cancer metastasis. Indoximod (IND) is an IDO inhibitor, Lu et al.82 conjugate IND with lipid to form prodrug, which could be used to prepare liposomes and encapsulate DOX. In vitro, the DOX induced certain expression of calreticulin (CRT) in 4T1 cells; in vivo, subcutaneous injection of dying 4T1 cells caused by DOX incubation could considerably suppress tumor growth, indicating the DOX could cause ICD and induce antitumor immune response. The IND and DOX dual-loaded liposome, DOX/IND-liposome, showed a blood half-life (t1/2) of 3.3 h, which was significantly longer than free drugs. In tumor, the percent of injected IND (dose/g tissue) of DOX/IND-liposome was 9.6%, which was greatly higher than free IND (0.5%), suggesting the liposomes could deliver more drugs to tumor. As a result, the DOX/IND-liposome could suppress the tumor growth, and inhibit lung metastasis when further combined with PD-L1 antibody.
Combination chemotherapy with immune adjuvant could stimulate strong antitumor immunotherapy to treat tumor metastasis. CpG ODN is a Toll-like receptor 9 (TLR9) agonist that is widely used in cancer immunotherapy to improve antitumor immune response. Liu et al.83 developed nanovaccine by self-assembly of CpG, antigenic peptide and cationic polymeric nanoparticles, and then they loaded the nanovaccine with nanomedicine into hydrogel, while the nanomedicine encapsulated curcumin to induce cancer cell apoptosis. The nanomedicine-contained hydrogel successfully delayed the growth of primary tumor, and reduced the lung metastasis. After evaluating the mechanism, results showed the combination therapy promoted higher level of infiltration of CD8+ T cells in the tumor and spleen compared with nanomedicine or nanovaccine. The serum cytokine level also demonstrated the combination therapy stimulated higher level of TNF-α and IL-6. The study demonstrated combination immune adjuvant with chemotherapy could promote stronger antitumor immune response, which is a promising strategy for tumor metastasis treatment or prevention.
3.2. Photothermal therapy-enhanced immunotherapy
Photothermal therapy could stimulate antitumor immune response by producing tumor-related antigens. Similar to chemotherapy, the tumor related antigens could stimulate immune response. In a preliminary clinical study, laser irradiation of tumor after indocyanine green (ICG) injection could deplete not only the primary tumor but the non-treatment lesions84, indicating the generation of antitumor immune response. Therefore, several photothermal agents and nanoparticles were developed to combine with immunotherapy for tumor metastasis treatment.
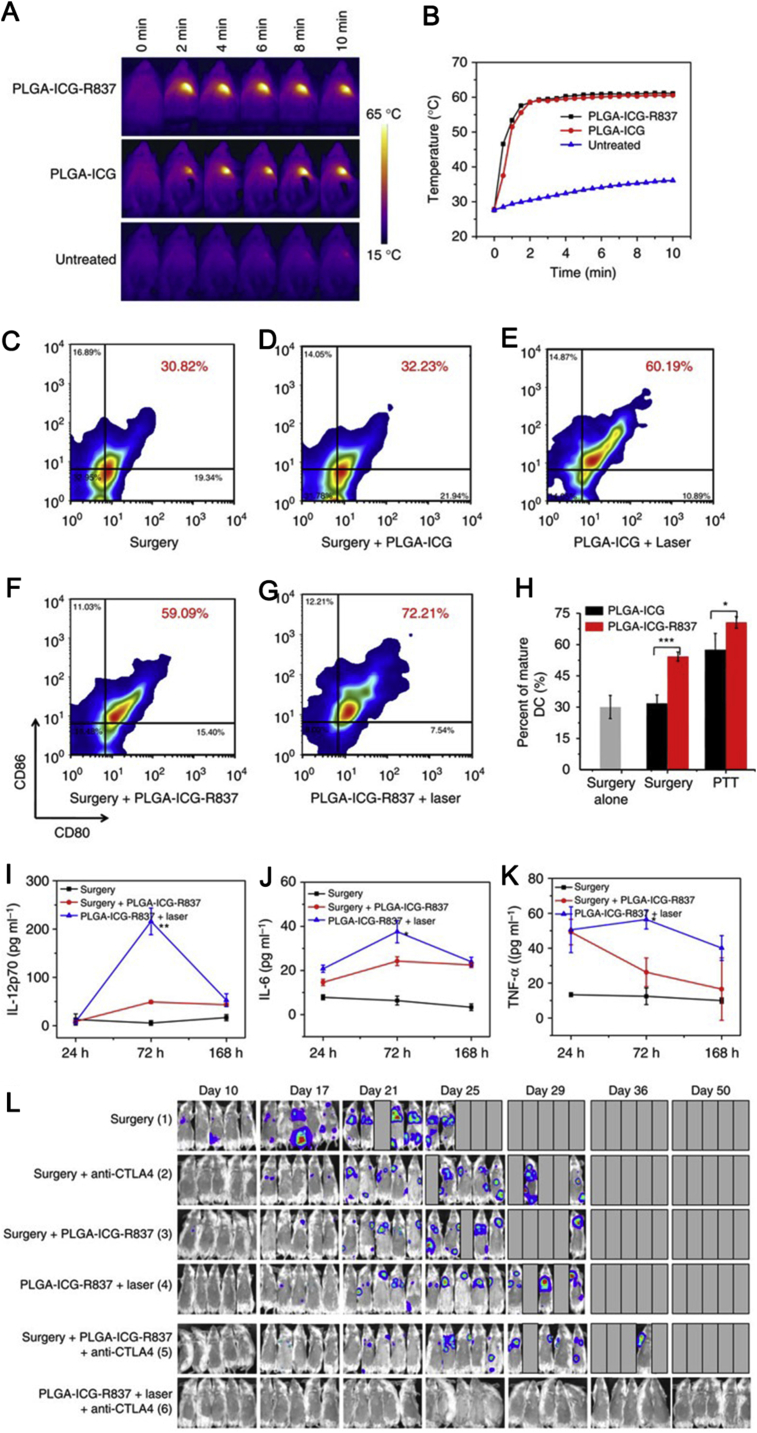
Although there are several studies showing that the photothermal therapy could inhibit tumor metastasis85, the antitumor immune response is still restricted by the immunosuppressive tumor microenvironment. So, combination photothermal therapy with checkpoint blockade is a promising strategy. Chen et al.86 developed a kind of ICG and imiquimod (R837) dual-loaded PLGA nanoparticles (PLGA-ICG-R837) to treat tumor metastasis in combination with anti-cytotoxic T-lymphocyte antigen-4 (CTLA4). In vitro, after incubated with PLGA-ICG-R837, the bone-marrow-derived DCs were maturated in a higher level than the free R837 treatment. In vivo, after subcutaneous injection of PLGA-ICG-R837, the percentage of CD11c+CD80+CD86+-matured DCs increased to 45%, which was significantly higher than saline (28%), PLGA-ICG (30%), and free R837 (35%), indicating the combination of photothermal agent and Toll-like-receptor-7 agonist could elevate immunity response. After intratumor injection of PLGA-ICG-R837 and laser irradiation, the temperature of tumor quickly increased to about 60 °C (Fig. 5A and B), which was high enough to ablate tumor and release tumor-associated antigens. Three days after photothermal therapy, the tumor-draining lymph nodes showed 72% maturation of dendritic cells (DC, Fig. 5C–H), which was significantly higher than nanoparticles without R837 or other treatment. Additionally, the pro-inflammatory cytokines, such as IL12p70, IL-6, and TNF-α, were secreted with higher level than other groups (Fig. 5I–K). These results indicated the photothermal therapy with adjuvant could stimulate stronger antitumor immune response. Consequently, PLGA-ICG-R837 could suppress the growth of primary tumor, distant tumor and tumor metastasis. However, it could not completely deplete the tumor due to the immunosuppressive condition. In combination with CTLA-4, the PLGA-ICG-R837 almost depleted the tumor completely. At Day 50 since treatment, no lung metastasis was observed, while mice in other groups died due to the serious lung metastasis (Fig. 5L). Similarly, polydopamine loaded with resiquimod (R848) also showed effective liver and lung metastasis inhibition when combination with PD-1 antibody, and the tumor-infiltrated CD3+CD8+ T cells greatly increased87. These studies indicated the photothermal therapy with immune adjuvant could stimulate robust antitumor response due to the effective release of tumor associated antigen and successful antigen presenting, which could considerably elevate anti-metastasis capacity of ICB.
Figure 5.
Immune responses and lung metastasis treatment of PLGA-ICG-R837. (A) IR thermal images of 4T1-tumour-bearing mice injected with PLGA-ICG-R837, PLGA-ICG or PBS under the 808 nm laser (0.5 W cm−2) irradiation. (B) The tumour temperature changes based on IR thermal imaging date in a. (C–H) DC maturation induced by PLGA-ICG-R837-based PTT on mice-bearing 4T1 tumours (gated on CD11c + DC cells). Cells in the tumour-draining lymph nodes were collected 72 h after various treatments for assessment by flow cytometry after staining with CD11c, CD80 and CD86. (I–K) Cytokine levels in sera from mice isolated at 24, 72 and 168 h post different treatments (surgery, surgery and s.c. injection of PLGA-ICG-R837, i.t. injection of PLGA-ICG-R837 and PTT). (L) Invivo bioluminescence images to track the spreading and growth of i.v. injected fLuc-4T1 cancer cells in different groups of mice after the cancer cells after various treatments to eliminate their primary tumours. Three mice were measured in each group in (A–K). Data are presented as the mean ± SEM. P values were calculated by Tukey's post-hoc test (***P < 0.001, **P < 0.01 or *P < 0.05). For (I–K), P values were determined between the second group (Surgery + PLGA-ICG-R837) and the third group (PLGA-ICG-R837+laser). Reproduced with permission from Ref. 86. Copyright © 2016, Nature Springer Inc.
Combination photothermal therapy with chemotherapy could further boost the immunotherapy. A polypyrrole-loaded camptothecin (CPT)-conjugated HA nanoparticle (P@CH) was developed for tumor targeting combinational therapy88. The P@CH showed high tumor accumulation due to the overexpressed CD44 receptors on 4T1 cells. With laser irradiation, the temperature of tumor could achieve 48.4 °C, which could effectively deplete primary tumor with assistance of camptothecin. The tumor volume of combinational therapy was significantly lower than mice that received monotherapy. When further combined with anti-PD-L1 antibody, the primary tumor was completely depleted, and the lung metastasis could not be observed, which was much better than anti-PD-L1 antibody treatment, indicating the combination could enhance the immunotherapy of ICB.
3.3. Photodynamic therapy-enhanced immunotherapy
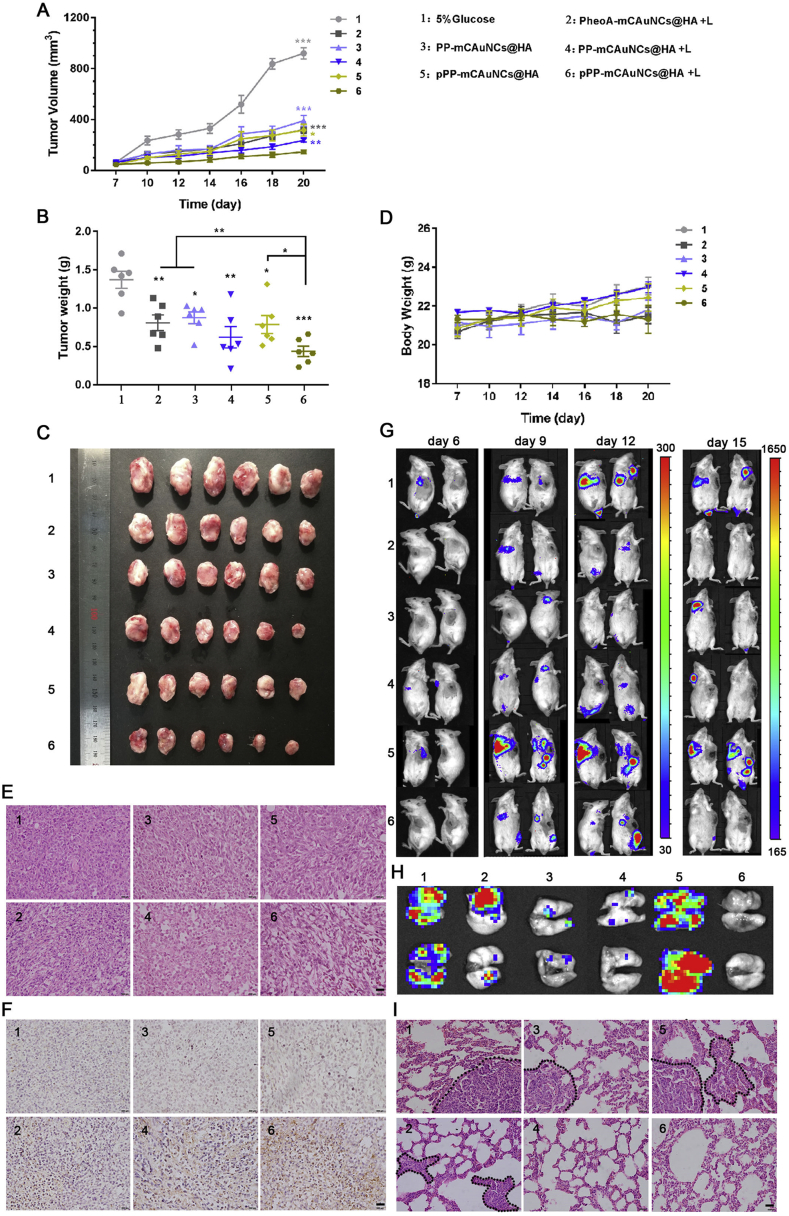
Photodynamic therapy could induce ICD because of the generation of ROS, such as singlet oxygen. The ROS-induced cell death could lead the release of damage-associated molecular pattern proteins (DAMPs), which is the characteristic of ICD and could stimulate tumor-specific antigen presenting and antitumor immune response. In our group, we designed a hyaluronidase-responsive size-changeable nanoparticles (pPP-mCAuNCs@HA) with blood red cell membrane coating to deliver photosensitizer pheophorbide A, chemotherapeutic PTX, and anti-PD-L1 peptide dPPA89,90. The pPP-mCAuNCs@HA could respond to overexpressed hyaluronidase in tumor to decrease the particle size, so the small particles could penetrate into deep tumor and deliver drug to tumor homogenously. After laser irradiation, the particles could produce high concentration of ROS. Consequently, the surface exposure of CRT and secretion level of HMGB1 was greatly elevated, much higher than those without laser irradiation. In vivo, the percentages of CD3+CD8+ T cells of pPP-mCAuNCs@HA with laser irradiation was about 2.47-fold higher than the groups without laser irradiation, which clearly demonstrated the photodynamic therapy could stimulate antitumor immune response. As a result, the pPP-mCAuNCs@HA with laser irradiation greatly inhibited the primary tumor growth, and the tumor inhibition rate achieved 84.2%. What's more, the lung metastasis was greatly inhibited, and no obvious lung metastasis was observed (Fig. 6). Similarly, chlorin e6 (Ce6)-loaded shape changeable nanoparticles could improve the accumulation of Ce6 and chemotherapeutics, thus stimulating strong antitumor immune response, which could suppress the lung metastasis of breast cancer91,92.
Figure 6.
In vivo anti-tumor effect. (A) Tumor growth curves after treated with different preparations, Symbol on each column represents statistic difference comparing with pPP-mCAuNCs@HA + laser group (n = 6). (B) Tumor weights at the end points, symbol on each column represents statistic difference comparing with 5% glucose group. (C) Photographs of tumors after treated for 4 times. (D) Body weight changes of 4T1 tumor bearing mice during the therapeutic process. H&E (E) and TUNEL (F) staining of tumor slices of different treatment groups. (G) Inhibition of lung metastasis detected through bioluminescence imaging. (H) Bioluminescence of ex vivo lungs. (I) H&E staining of ex vivo lungs in lung metastasis 4T1-tumor bearing mice. Scale bar represents 500 μm. Reproduced with permission from Ref. 89. Copyright © 2019, Elsevier Inc.
The effective photodynamic therapy requires sufficient oxygen in tumor to generate ROS. However, in the deep tumor, especially hypoxia, the oxygen level is low, thus restricting ROS generation. Therefore, delivering oxygen or generating oxygen in situ could effectively promote photodynamic therapy. Hemoglobin is the endogenous carrier of oxygen, which was hybridized with albumin to deliver both oxygen and Ce6 (C@HPOC)93. The nanoparticles induced higher intracellular ROS level and more cell apoptosis in hypoxic condition. In vivo, the infiltration of CD8+ T cells was improved, resulting in better inhibition of lung metastasis. The nanoenzymes, such as MnO2-based nanoparticles, could catalyze H2O2 to O2, improving oxygen level in hypoxic tumor. Therefore, delivering nanoenzymes to tumor could also enhance the photodynamic therapy and the following anti-metastasis94.
The photodynamic therapy could combine with IDO inhibitor for boosting immune response. Liu et al.95 conjugated Ppa with lipid through disulfate bond, which could response to tumor overexpressed GSH and release the Ppa. Then an IDO inhibitor, NLG-8189, was loaded into the redox activatable liposomes (RAL) for tumor targeting therapy. The Ppa in RAL was greatly quenched due to homofluorescence resonance energy transfer, while the fluorescence activation ratio was 149-fold higher with the incubation of 10 mmol/L GSH, indicating the redox activation capacity of RAL could provide the Ppa with site specific phototoxicity. In cellular level, RAL treatment with laser irradiation greatly enhanced the expression of CRT, reduced the intracellular ATP and HMGB1, and elevated the release of extracellular HMGB1 and ATP, suggesting its great potential in inducing ICD. In vivo, the blood t1/2 increased from 1.17 h of free IND to 8.23 h of RAL, and the area under curves at 24 h was 3.8-fold higher than that of free IND. Consequently, the intratumor concentration of ARL was 11-fold higher than that of free IND. As a result, the IND-loaded ARL significantly suppressed primary tumor growth, improved survival time and induced effective antitumor metastasis. The percentage of CD3+CD8+ T cells in the tumor was 2-fold higher than the PBS. Similarly, our group loaded Ce6 with IND by size-reducible nanoparticles, which could greatly suppress the lung metastasis of breast cancer96. These studies suggested the co-delivery IDO inhibitor with photosensitizer could stimulate robust antitumor immune response, which contributed to the good antitumor metastasis capacity.
3.4. Ferroptosis-enhanced immunotherapy
Ferroptosis is a recently identified format that could inhibit tumor growth by an iron-dependent irreversible accumulation of lipid peroxides, which is caused by elevating ROS level and attenuated activity of glutathione peroxidase 497,98. Therefore, it may also induce mild immunogenicity. Jiang et al.99 loaded sulfasalazine (SAS) magnetic nanoparticles (Fe3O4) and coated them with platelet membranes (Fe3O4-SAS@PLT) for tumor ferroptosis and enhancing PD-1 blockade therapy. SAS could inhibit uptake of cysteine and work together with Fe3O4 to induce synergistical ferroptosis at low concentration, while platelet membrane could drive the nanoparticles to tumor metastasis with high efficiency. The ferroptosis inhibitor ferrostatin-1 and iron chelator deferoxamine significantly decreased 4T1 cytotoxicity caused by Fe3O4-SAS@PLT. Similarly, Fe3O4-SAS@PLT considerably elevated cellular ROS level, while ferrostatin-1 and deferoxamine could decrease it. These results indicated the cytotoxicity of Fe3O4-SAS@PLT involved in ferroptosis. According to detailed evaluation, the main ROS induced by the particles was hydroxyl radical, and it could induce high lipid peroxidation level in 4T1 cells. Furthermore, the Fe3O4-SAS@PLT could deplete GSH through inhibiting cystine transportation by glutamate-cystine antiporter system Xc− transporter. After coculture the immature DCs with Fe3O4-SAS@PLT treated 4T1 cells, the maturity of DCs was significantly higher than control group (CD86+/CD80+, gated on CD11c+ cells, 82.8% versus 1.1%), while ferrostatin-1 and deferoxamine coculture reduced the ratio to 16.9% and 21.0%, respectively, indicating the Fe3O4-SAS@PLT-induced ferroptosis could effectively stimulate antitumor immune response. In vivo, Fe3O4-SAS@PLT showed higher accumulation in lung metastasis than Fe3O4-SAS without platelet membrane coating. The level of CD86+/CD80+ positive mature DCs in draining lymph elevated significantly in Fe3O4-SAS@PLT treated group than other groups. As expected, the lung metastasis in Fe3O4-SAS@PLT-treated group was significantly reduced, while combination with PD-1 antibody could completely inhibit lung metastasis. These studies demonstrated that the ferroptosis could effective prime immune response and enhance cancer metastasis immunotherapy.
Similarly, other ferroptosis agents also could inhibit or treat metastasis in combination with ICB. Folic acid-modified FePt/MoS2 multifunctional nanoparticles could effectively deplete primary tumor because of FePt-enabled Fenton reaction and MoS2-enabled photothermal therapy100. Then, with the help of CpG, the systemic immune response was successfully stimulated, which could erase tumor metastasis in combination with anti-CTLA-4 antibody. These studies demonstrated the combination therapy could provide benefit for metastasis management.
3.5. Sonodynamic therapy-enhanced immunotherapy
Sonodynamic therapy is a new ultrasound-based therapeutic modality that activates the sonosensitizers to generate ROS, which may induce tumor cell ICD and stimulate immune response101. A sonosensitizer, Ce6, was grafted onto chondroitin sulfate, and docetaxel was also decorated onto the material by adipic dihydrazide bond, and then the drugs-conjugated materials could self-assemble to nanoparticles for tumor targeting delivery due to the recognition of chondroitin sulfate with CD44 receptors on tumor cells102. The particles could inhibit tumor growth due to combination of chemotherapy with ROS generated by sonodynamic therapy. The enhanced ROS led to release of cytochrome C and the expression of cleaved caspase-9. What's more, the immune response was stimulated as determined by the enhanced CTL percentage in tumor tissue and INF-γ level, which successfully inhibit lung metastasis of melanoma. The targeting capacity could be further improved by membrane coating. Zhao et al.103 coated Ce6 and tirapazamine dual-loaded nanoparticles with platelet membrane. The particles showed good tumor accumulation, and enhanced antitumor metastasis due to the sonodynamic therapy and chemotherapy.
Effective antitumor response also requires effective antigen presenting. Therefore, combination the sonodynamic therapy with immune adjuvant could further boost the immunotherapy. Yue et al.104 loaded imiquimod (R837), a Toll-like receptor-7 (TLR7) agonist, with a sonosensitizer hematoporphyrin monomethyl ether (HMME) into liposomes for combinational therapy. The HMME/R837@Lip significantly enhanced intracellular ROS production under ultrasound irradiation, while R837 did not influence the generation of ROS. The ROS generated by ultrasound irradiation induced obvious 4T1 cell apoptosis and produce antigens. When co-culturing the treated cells with immature DCs, the percentage of CD80+CD86+ DCs significantly increased, which was higher than the treatment without R837, indicating the immune adjuvant could promote antigen presenting and DC maturation. Consequently, the HMME/R837@Lip treatment triggered highest level of secreted cytokines, such as IL-6 and TNF-α. Similarly, in vivo sonodynamic therapy with HMME/R837@Lip stimulated 26.8% DC maturation, while HMME@Lip only stimulated 14.9%. Therefore, the sonodynamic therapy significantly suppressed the growth of both primary and distant tumors and lung metastasis, while the combination with PD-L1 antibody could first boost the antitumor effect. In the tumor after treatment with HMME/R837@Lip, percentage of CD8+ cells increased from 6.7% of PBS group to 18.6%, percentage of CD4+Foxp3+ effective T cells increased, while percentage of CD4+Foxp3− regulatory T cells decreased, suggesting the combinational therapy effectively boosted antitumor immune response and attenuated the immunosuppressive environment of tumor.
3.6. Nanovaccines-enhanced immunotherapy
The aim of the above strategies is to induce ICD and release tumor specific antigens, which could stimulate antitumor immune response. However, the effective antitumor immune response requires the effective delivery of antigens to DCs and successful antigen presentation. Therefore, nanovaccines are developed to address these requirements. Min et al.105 developed a kind of antigen-capturing nanoparticles (AC-NPs) by poly(lactic-co-glycolic acid, PLGA). The PLGA AC-NPs could bind with antigens through ono-covalent hydrophobic–hydrophobic interaction. In vitro, after incubation with B16F10 melanoma cell lysates, PLGA AC-NPs captured the more proteins that AC-NPs with amine, maleimide or methoxypolyethylene glycol (mPEG) surfaces. Notably, the captured proteins contained a number of DAMPs, such as HMGB1. In combination with radiotherapy, the PLGA AC-NPs significantly improved immunotherapy and abscopal effect. After mechanism evaluation, it was found that the PLGA AC-NPs could drain from tumor to lymph nodes and accumulate at higher concentration in the antigen-presenting dendritic cells (CD11c+), macrophages and B-cells than control nanoparticles. Since the PLGA AC-NPs could activate more CD8+ T cells, a higher concentration of tumor infiltrated CD8+ T cells was observed in the abscopal tumor, resulting in better tumor depletion. Similarly, Lynn et al.106 directly loaded peptide neoantigens in nanoparticles (SNP-7/8a) to stimulate antigen specific immunity. The SNP-7/8a was detected with higher level in the draining lymph nodes (DLNs) compared with peptide solution. Consequently, the SNP-7/8a stimulated over 35% of antigen-positive CD11c+ DC in the DLNs, while the peptide solution stimulated less than 1%. After intravenous or subcutaneous injection, the SNP-7/8a could stimulate a robust antitumor immune response and enhance the tumor regression.
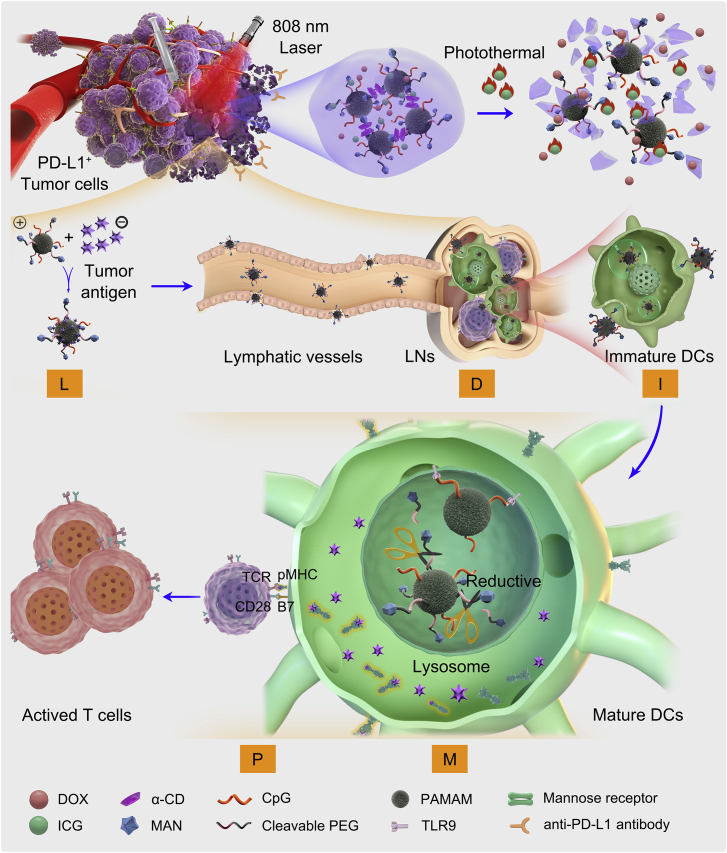
However, an effective tumor nanovaccine should not only load neoantigen, drain to lymph nodes and be internalized by DCs, but also maturate DCs for co-stimulatory molecules expression and present peptide-MHC-I complexes to T cells, which called LDIMP cascade for short. Our group designed a dendrimer-loaded α-cyclodextrin (CD)-based hydrogel to meet the requirement of LDIMP cascade (Fig. 7)107. In the system, PAMAM with small size was conjugated with CpG as adjuvant and reductive-cleavable 4-aminophenyl-α-d-mannopyranoside (MAN) to improve lymph targeting and DC internalization (CpG-P-ss-M). After loading the CpG-P-ss-M, DOX and ICG into the hydrogel, it was intratumorally injected. After laser irradiation, the photothermal therapy of ICG could induce ICD and sustained release CpG-P-ss-M and DOX. DOX could also induce ICD to assistant ICG for long term release of tumor-associated antigens. The released CpG-P-ss-M could adsorb the tumor associated antigens with high efficiency, and then drain to lymph nodes due to its small size. The MAN on the surface of CpG-P-ss-M could significantly improve DC uptake by 2 folds compared with the particles without MAN modification. Furthermore, the disulfate bond was cleaved in endosomes of DC to facilitate endosome escape of CpG-P. With the assistance of CpG, the particles could effectively maturate DCs and then present the complex to T cells. Consequently, the system induced highest percentages of CD80+ and CD86+ DCs. On a postoperative B16-OVA tumor model, the system greatly prolonged median survival time and prevented lung metastasis. It showed higher frequencies of OVA-specific IFN-γ secreting CTLs in both spleens and lymph nodes, which is responsible for the better lung metastasis inhibition capacity. The study demonstrated that rationally designed nanovaccines to meet the requirement of LDIMP cascade could greatly improve anti-metastasis prevention.
Figure 7.
LDIMP process. (A) Fabrication of the integrated regimen and the release process of CpG-P-ss-M. (B) Simplified mechanism of CpG-P-ss-M-mediated DCs maturation for cancer immunotherapy. Letters LDIMP in green frame represent: loading tumor-specific antigens by DDS, draining to LNs, internalization by DCs, DCs maturation for co-stimulatory molecules expression and presenting peptide-MHC-I complexes to T cells, respectively. Reproduced with permission from Ref. 107. Copyright © 2020, Nature Springer Inc.
4. Conclusion and perspective
Tumor metastasis is a critical challenge for tumor treatment. To overcome the challenge, many strategies are developed to induce tumor cell apoptosis, no matter whether they are the tumor cells located in primary site, blood circulation or metastatic site. Except the tumor cells, the tumor microenvironment and tumor stroma cells also facilitate tumor metastasis. Therefore, strategies are developed to block the pro-metastasis microenvironment and niches or reverse the pro-metastasis phenotype of tumor stroma cells. Encouraging by the quickly development of immunotherapy, cancer immunotherapy is considered as a powerful strategy to manage tumor metastasis. However, the cancer treatment by ICB is restricted by low immune response level. So, we summarized various methods to stimulate the immunotherapy by other therapy strategies, such as chemotherapy, photothermal therapy, and photodynamic therapy, etc.
Although promising results are obtained in the research, there are several aspects should be taken into consideration. Firstly, the tumor metastasis is a complex procedure that was involved in many pathways. Blocking a certain pathway may alter other pathways and make the anti-metastasis treatment failed. It is important to carefully evaluate the influence of a certain treatment strategy on various related pathways. Secondly, stimulating antitumor immune response could produce tumor antigen specific T cells and release cytokines. However, the most serious side effect of immunotherapy is cytokine storms, and there are limited studies that provided resolutions to prevent cytokine storms during stimulating immune response. Thirdly, there is a trend to design multifunctional nanoparticles for the treatment of tumor and metastasis. Although various functions elevate cancer treatment outcome, the poor repeatability and complex preparation procedure shadow their application. Researchers should pay attention on developing simple but multifunctional nanoparticles, which may have better clinical translation potential.
Overall, great improvement has been made in tumor metastasis treatment. A better understanding about the tumor metastasis mechanism will further improve the treatment outcome.
Acknowledgments
The work was supported by National Natural Science Foundation of China (81961138009), and 111 Project (B18035, China).
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Huile Gao, Email: gaohuile@scu.edu.cn, gaohuilescu@163.com.
Jiang Hu, Email: hujiang8711@163.com.
Author contributions
Huile Gao and Jiang Hu designed the review. Wei Zhang searched references and wrote the manuscript with assistance of Fei Wang, Chuan Hu, and Yang Zhou. Huile Gao revised the manuscript. All of the authors have read and approved the final manuscript.
Conflicts of interest
The authors have no conflicts of interest to declare.
References
- 1.Steeg P.S. Targeting metastasis. Nat Rev Canc. 2016;16:201–218. doi: 10.1038/nrc.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang F., Zhao Z., Sun B., Chen Q., Sun J., He Z. Nanotherapeutics for antimetastatic treatment. Trends Cancer. 2020;6:645–659. doi: 10.1016/j.trecan.2020.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Nagy J.A., Dvorak H.F. Heterogeneity of the tumor vasculature: the need for new tumor blood vessel type-specific targets. Clin Exp Metastasis. 2012;29:657–662. doi: 10.1007/s10585-012-9500-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liotta L.A. Tumor invasion and metastases—role of the extracellular matrix: Rhoads Memorial Award lecture. Canc Res. 1986;46:1–7. [PubMed] [Google Scholar]
- 5.Quail D.F., Joyce J.A. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang D., Feng F., Liu R., Zhu W., Yao L. Mechanochemistry in cancer cell metastasis. Chin Chem Lett. 2019;30:7–14. [Google Scholar]
- 7.Cristofanilli M., Budd G.T., Ellis M.J., Stopeck A., Matera J., Miller M.C. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–791. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 8.Fein M.R., Egeblad M. Caught in the act: revealing the metastatic process by live imaging. Dis Model Mech. 2013;6:580–593. doi: 10.1242/dmm.009282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rachidi S., Metelli A., Riesenberg B., Wu B.X., Nelson M.H., Wallace C. Platelets subvert T cell immunity against cancer via GARP–TGFbeta axis. Sci Immunol. 2017;2 doi: 10.1126/sciimmunol.aai7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Labelle M., Begum S., Hynes R.O. Direct signaling between platelets and cancer cells induces an epithelial–mesenchymal-like transition and promotes metastasis. Canc Cell. 2011;20:576–590. doi: 10.1016/j.ccr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haemmerle M., Stone R.L., Menter D.G., Afshar-Kharghan V., Sood A.K. The platelet lifeline to cancer: challenges and opportunities. Canc Cell. 2018;33:965–983. doi: 10.1016/j.ccell.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eckhardt B.L., Francis P.A., Parker B.S., Anderson R.L. Strategies for the discovery and development of therapies for metastatic breast cancer. Nat Rev Drug Discov. 2012;11:479–497. doi: 10.1038/nrd2372. [DOI] [PubMed] [Google Scholar]
- 13.Hoshino A., Costa-Silva B., Shen T.L., Rodrigues G., Hashimoto A., Tesic M.M. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peinado H., Aleckovic M., Lavotshkin S., Matei I., Costa-Silva B., Moreno-Bueno G. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18:883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hernandez L., Magalhaes M.A., Coniglio S.J., Condeelis J.S., Segall J.E. Opposing roles of CXCR4 and CXCR7 in breast cancer metastasis. Breast Cancer Res. 2011;13:R128. doi: 10.1186/bcr3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fidler I.J., Kripke M.L. The challenge of targeting metastasis. Canc Metastasis Rev. 2015;34:635–641. doi: 10.1007/s10555-015-9586-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joyce J.A., Pollard J.W. Microenvironmental regulation of metastasis. Nat Rev Canc. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta G.P., Massague J. Cancer metastasis: building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 19.van der Meel R., Sulheim E., Shi Y., Kiessling F., Mulder W., Lammers T. Smart cancer nanomedicine. Nat Nanotechnol. 2019;14:1007–1017. doi: 10.1038/s41565-019-0567-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao H. Perspectives on dual targeting delivery systems for brain tumors. J Neuroimmune Pharmacol. 2017;12:6–16. doi: 10.1007/s11481-016-9687-4. [DOI] [PubMed] [Google Scholar]
- 21.Li C., Wang J., Wang Y., Gao H., Wei G., Huang Y. Recent progress in drug delivery. Acta Pharm Sin B. 2019;9:1145–1162. doi: 10.1016/j.apsb.2019.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalbasi A., Ribas A. Tumour-intrinsic resistance to immune checkpoint blockade. Nat Rev Immunol. 2020;20:25–39. doi: 10.1038/s41577-019-0218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldberg M.S. Improving cancer immunotherapy through nanotechnology. Nat Rev Canc. 2019;19:587–602. doi: 10.1038/s41568-019-0186-9. [DOI] [PubMed] [Google Scholar]
- 24.Shi J., Kantoff P.W., Wooster R., Farokhzad O.C. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Canc. 2017;17:20–37. doi: 10.1038/nrc.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang C., Wu W., Li R., Qiu W., Zhuang Z., Cheng S. Peptide-based multifunctional nanomaterials for tumor imaging and therapy. Adv Funct Mater. 2018;28 180449250. [Google Scholar]
- 26.Liu R., Hu C., Yang Y., Zhang J., Gao H. Theranostic nanoparticles with tumor-specific enzyme-triggered size reduction and drug release to perform photothermal therapy for breast cancer treatment. Acta Pharm Sin B. 2019;9:410–420. doi: 10.1016/j.apsb.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y., Xiao Y., Lin H.P., Reichel D., Bae Y., Lee E.Y. In vivo beta-catenin attenuation by the integrin alpha5-targeting nano-delivery strategy suppresses triple negative breast cancer stemness and metastasis. Biomaterials. 2019;188:160–172. doi: 10.1016/j.biomaterials.2018.10.019. [DOI] [PubMed] [Google Scholar]
- 28.Yu H., Shen N., Bao Y., Chen L., Tang Z. Tumor regression and potentiation of polymeric vascular disrupting therapy through reprogramming of a hypoxia microenvironment with temsirolimus. Biomater Sci. 2020;8:325–332. doi: 10.1039/c9bm01398a. [DOI] [PubMed] [Google Scholar]
- 29.Sun W., Ge K., Jin Y., Han Y., Zhang H., Zhou G. Bone-targeted nanoplatform combining zoledronate and photothermal therapy to treat breast cancer bone metastasis. ACS Nano. 2019;13:7556–7567. doi: 10.1021/acsnano.9b00097. [DOI] [PubMed] [Google Scholar]
- 30.Vanderburgh J., Hill J.L., Gupta M.K., Kwakwa K.A., Wang S.K., Moyer K. Tuning ligand density to optimize pharmacokinetics of targeted nanoparticles for dual protection against tumor-Induced bone destruction. ACS Nano. 2020;14:311–327. doi: 10.1021/acsnano.9b04571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao L., Gu C., Gan Y., Shao L., Chen H., Zhu H. Exosome-mediated siRNA delivery to suppress postoperative breast cancer metastasis. J Control Release. 2020;318:1–15. doi: 10.1016/j.jconrel.2019.12.005. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y., Zhu X., Chen X., Chen Q., Zhou W., Guo Q. Activated platelets-targeting micelles with controlled drug release for effective treatment of primary and metastatic triple negative breast cancer. Adv Funct Mater. 2019;29:180662013. [Google Scholar]
- 33.Chu D., Zhao Q., Yu J., Zhang F., Zhang H., Wang Z. Nanoparticle targeting of neutrophils for improved cancer immunotherapy. Adv Healthc Mater. 2016;5:1088–1093. doi: 10.1002/adhm.201500998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu R., Xiao W., Hu C., Xie R., Gao H. Theranostic size-reducible and no donor conjugated gold nanocluster fabricated hyaluronic acid nanoparticle with optimal size for combinational treatment of breast cancer and lung metastasis. J Control Release. 2018;278:127–139. doi: 10.1016/j.jconrel.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 35.Luo Z., Dai Y., Gao H. Development and application of hyaluronic acid in tumor targeting drug delivery. Acta Pharm Sin B. 2019;9:1099–1112. doi: 10.1016/j.apsb.2019.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qin L., Gao H. The application of nitric oxide delivery in nanoparticle-based tumor targeting drug delivery and treatment. Asian J Pharm Sci. 2019;14:380–390. doi: 10.1016/j.ajps.2018.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu C., Yang X., Liu R., Ruan S., Zhou Y., Xiao W. Coadministration of iRGD with multistage responsive nanoparticles enhanced tumor targeting and penetration abilities for breast cancer therapy. ACS Appl Mater Interfaces. 2018;10:22571–22579. doi: 10.1021/acsami.8b04847. [DOI] [PubMed] [Google Scholar]
- 38.Liu J., Li H.J., Luo Y.L., Xu C.F., Du X.J., Du J.Z. Enhanced primary tumor penetration facilitates nanoparticle draining into lymph nodes after systemic injection for tumor metastasis inhibition. ACS Nano. 2019;13:8648–8658. doi: 10.1021/acsnano.9b03472. [DOI] [PubMed] [Google Scholar]
- 39.Yang L., Shi P., Zhao G., Xu J., Peng W., Zhang J. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct Target Ther. 2020;5:8. doi: 10.1038/s41392-020-0110-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nusse R., Clevers H. Wnt/beta-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169:985–999. doi: 10.1016/j.cell.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 41.Pohl S.G., Brook N., Agostino M., Arfuso F., Kumar A.P., Dharmarajan A. Wnt signaling in triple-negative breast cancer. Oncogenesis. 2017;6 doi: 10.1038/oncsis.2017.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang W.J., Wu M.Y., Shen M., Zhi Q., Liu Z.Y., Gong F.R. Cantharidin and norcantharidin impair stemness of pancreatic cancer cells by repressing the beta-catenin pathway and strengthen the cytotoxicity of gemcitabine and erlotinib. Int J Oncol. 2015;47:1912–1922. doi: 10.3892/ijo.2015.3156. [DOI] [PubMed] [Google Scholar]
- 43.Liu D., Hong Y., Li Y., Hu C., Yip T.C., Yu W.K. Targeted destruction of cancer stem cells using multifunctional magnetic nanoparticles that enable combined hyperthermia and chemotherapy. Theranostics. 2020;10:1181–1196. doi: 10.7150/thno.38989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yin H., Xiong G., Guo S., Xu C., Xu R., Guo P. Delivery of anti-miRNA for triple-negative breast cancer therapy using RNA nanoparticles targeting stem cell marker CD133. Mol Ther. 2019;27:1252–1261. doi: 10.1016/j.ymthe.2019.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu J., Chang B., Li Q., Xu L., Liu X., Wang G. Redox-responsive dual drug delivery nanosystem suppresses cancer repopulation by abrogating doxorubicin-promoted cancer stemness, metastasis, and drug resistance. Adv Sci. 2019;6:1801987. doi: 10.1002/advs.201801987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiao W., Gao H. The impact of protein corona on the behavior and targeting capability of nanoparticle-based delivery system. Int J Pharm. 2018;552:328–339. doi: 10.1016/j.ijpharm.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 47.Xiao W., Xiong J., Zhang S., Xiong Y., Zhang H., Gao H. Influence of ligands property and particle size of gold nanoparticles on the protein adsorption and corresponding targeting ability. Int J Pharm. 2018;538:105–111. doi: 10.1016/j.ijpharm.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 48.Xia Q., Zhang Y., Li Z., Hou X., Feng N. Red blood cell membrane-camouflaged nanoparticles: a novel drug delivery system for antitumor application. Acta Pharm Sin B. 2019;9:675–689. doi: 10.1016/j.apsb.2019.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li R., He Y., Zhang S., Qin J., Wang J. Cell membrane-based nanoparticles: a new biomimetic platform for tumor diagnosis and treatment. Acta Pharm Sin B. 2018;8:14–22. doi: 10.1016/j.apsb.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fernandez-Trillo F., Grover L.M., Stephenson-Brown A., Harrison P., Mendes P.M. Vesicles in nature and the laboratory: elucidation of their biological properties and synthesis of increasingly complex synthetic vesicles. Angew Chem Int Ed Engl. 2017;56:3142–3160. doi: 10.1002/anie.201607825. [DOI] [PubMed] [Google Scholar]
- 51.Zhou Y., Chen X., Cao J., Gao H. Overcoming the biological barriers in the tumor microenvironment for improving drug delivery and efficacy. J Mater Chem B. 2020;8:6765–6781. doi: 10.1039/d0tb00649a. [DOI] [PubMed] [Google Scholar]
- 52.Wang J., Yin D., Xie C., Zheng T., Liang Y., Hong X. The iron chelator Dp44mT inhibits hepatocellular carcinoma metastasis via N-Myc downstream-regulated gene 2 (NDRG2)/gp130/STAT3 pathway. Oncotarget. 2014;5:8478–8491. doi: 10.18632/oncotarget.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ding F., Zhang L., Chen H., Song H., Chen S., Xiao H. Enhancing the chemotherapeutic efficacy of platinum prodrug nanoparticles and inhibiting cancer metastasis by targeting iron homeostasis. Nanoscale Horiz. 2020;5:999–1015. doi: 10.1039/d0nh00148a. [DOI] [PubMed] [Google Scholar]
- 54.Yang S., Gao H. Nanoparticles for modulating tumor microenvironment to improve drug delivery and tumor therapy. Pharmacol Res. 2017;126:97–108. doi: 10.1016/j.phrs.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 55.Gao H. Shaping tumor microenvironment for improving nanoparticle delivery. Curr Drug Metabol. 2016;17:731–736. doi: 10.2174/1389200217666160630203600. [DOI] [PubMed] [Google Scholar]
- 56.Guan X. Cancer metastases: challenges and opportunities. Acta Pharm Sin B. 2015;5:402–418. doi: 10.1016/j.apsb.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Del B.D., Ciuffreda L., Trisciuoglio D., Desideri M., Cognetti F., Zupi G. Antiangiogenic potential of the Mammalian target of rapamycin inhibitor temsirolimus. Canc Res. 2006;66:5549–5554. doi: 10.1158/0008-5472.CAN-05-2825. [DOI] [PubMed] [Google Scholar]
- 58.Lang J., Zhao X., Qi Y., Zhang Y., Han X., Ding Y. Reshaping prostate tumor microenvironment to suppress metastasis via cancer-associated fibroblast inactivation with peptide-assembly-based nanosystem. ACS Nano. 2019;13:12357–12371. doi: 10.1021/acsnano.9b04857. [DOI] [PubMed] [Google Scholar]
- 59.Kawatani M., Osada H. Osteoclast-targeting small molecules for the treatment of neoplastic bone metastases. Canc Sci. 2009;100:1999–2005. doi: 10.1111/j.1349-7006.2009.01294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ramanlal C.K., Kumar A., Megraj K.V., Ukawala M., Manjappa A.S., Mishra A.K. Bone metastasis targeting: a novel approach to reach bone using Zoledronate anchored PLGA nanoparticle as carrier system loaded with docetaxel. J Control Release. 2012;158:470–478. doi: 10.1016/j.jconrel.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 61.Bai S.B., Cheng Y., Liu D.Z., Ji Q.F., Liu M., Zhang B.L. Bone-targeted PAMAM nanoparticle to treat bone metastases of lung cancer. Nanomedicine. 2020;15:833–849. doi: 10.2217/nnm-2020-0024. [DOI] [PubMed] [Google Scholar]
- 62.Gao H., He Q. The interaction of nanoparticles with plasma proteins and the consequent influence on nanoparticles behavior. Expet Opin Drug Deliv. 2014;11:409–420. doi: 10.1517/17425247.2014.877442. [DOI] [PubMed] [Google Scholar]
- 63.Chen S.H., Liu T.I., Chuang C.L., Chen H.H., Chiang W.H., Chiu H.C. Alendronate/folic acid-decorated polymeric nanoparticles for hierarchically targetable chemotherapy against bone metastatic breast cancer. J Mater Chem B. 2020;8:3789–3800. doi: 10.1039/d0tb00046a. [DOI] [PubMed] [Google Scholar]
- 64.Gao H. Progress and perspectives on targeting nanoparticles for brain drug delivery. Acta Pharm Sin B. 2016;6:268–286. doi: 10.1016/j.apsb.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Steeg P.S., Camphausen K.A., Smith Q.R. Brain metastases as preventive and therapeutic targets. Nat Rev Canc. 2011;11:352–363. doi: 10.1038/nrc3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou Y., Zhang S., Chen Z., Bao Y., Chen A.T., Sheu W.C. Targeted delivery of secretory promelittin via novel poly(lactone-co-beta-amino ester) nanoparticles for treatment of breast cancer brain metastases. Adv Sci. 2020;7:1901866. doi: 10.1002/advs.201901866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mukherjee D., Zhao J. The Role of chemokine receptor CXCR4 in breast cancer metastasis. Am J Cancer Res. 2013;3:46–57. [PMC free article] [PubMed] [Google Scholar]
- 68.Huang Y., Gao X., Chen J. Leukocyte-derived biomimetic nanoparticulate drug delivery systems for cancer therapy. Acta Pharm Sin B. 2018;8:4–13. doi: 10.1016/j.apsb.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gay L.J., Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Canc. 2011;11:123–134. doi: 10.1038/nrc3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ye H., Wang K., Wang M., Liu R., Song H., Li N. Bioinspired nanoplatelets for chemo-photothermal therapy of breast cancer metastasis inhibition. Biomaterials. 2019;206:1–12. doi: 10.1016/j.biomaterials.2019.03.024. [DOI] [PubMed] [Google Scholar]
- 71.Lo S.S., Moffatt-Bruce S.D., Dawson L.A., Schwarz R.E., Teh B.S., Mayr N.A. The role of local therapy in the management of lung and liver oligometastases. Nat Rev Clin Oncol. 2011;8:405–416. doi: 10.1038/nrclinonc.2011.75. [DOI] [PubMed] [Google Scholar]
- 72.Huo D., Zhu J., Chen G., Chen Q., Zhang C., Luo X. Eradication of unresectable liver metastasis through induction of tumour specific energy depletion. Nat Commun. 2019;10:3051. doi: 10.1038/s41467-019-11082-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Peinado H., Zhang H., Matei I.R., Costa-Silva B., Hoshino A., Rodrigues G. Pre-metastatic niches: organ-specific homes for metastases. Nat Rev Canc. 2017;17:302–317. doi: 10.1038/nrc.2017.6. [DOI] [PubMed] [Google Scholar]
- 74.Mishra S.K., Siddique H.R., Saleem M. S100A4 calcium-binding protein is key player in tumor progression and metastasis: preclinical and clinical evidence. Canc Metastasis Rev. 2012;31:163–172. doi: 10.1007/s10555-011-9338-4. [DOI] [PubMed] [Google Scholar]
- 75.Dong X., Chu D., Wang Z. Leukocyte-mediated delivery of nanotherapeutics in inflammatory and tumor sites. Theranostics. 2017;7:751–763. doi: 10.7150/thno.18069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ribas A., Wolchok J.D. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350–1355. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gong N., Zhang Y., Zhang Z., Li X., Liang X. Functional nanomaterials optimized to circumvent tumor immunological tolerance. Adv Funct Mater. 2019;29:18060873. [Google Scholar]
- 78.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Canc. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 79.Yang X., Hu C., Tong F., Liu R., Zhou Y., Qin L. Tumor microenvironment-responsive dual drug dimer-loaded PEGylated bilirubin nanoparticles for improved drug delivery and enhanced immune-chemotherapy of breast cancer. Adv Funct Mater. 2019;29:1901896. [Google Scholar]
- 80.Kuai R., Yuan W., Son S., Nam J., Xu Y., Fan Y. Elimination of established tumors with nanodisc-based combination chemoimmunotherapy. Sci Adv. 2018;4:o1736. doi: 10.1126/sciadv.aao1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Guo J., Yu Z., Das M., Huang L. Nano codelivery of oxaliplatin and folinic acid achieves synergistic chemo-immunotherapy with 5-fluorouracil for colorectal cancer and liver metastasis. ACS Nano. 2020;14:5075–5089. doi: 10.1021/acsnano.0c01676. [DOI] [PubMed] [Google Scholar]
- 82.Lu J., Liu X., Liao Y.P., Wang X., Ahmed A., Jiang W. Breast cancer chemo-immunotherapy through liposomal delivery of an immunogenic cell death stimulus plus interference in the IDO-1 pathway. ACS Nano. 2018;12:11041–11061. doi: 10.1021/acsnano.8b05189. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 83.Liu X., Feng Z., Wang C., Su Q., Song H., Zhang C. Co-localized delivery of nanomedicine and nanovaccine augments the postoperative cancer immunotherapy by amplifying T-cell responses. Biomaterials. 2020;230:119649. doi: 10.1016/j.biomaterials.2019.119649. [DOI] [PubMed] [Google Scholar]
- 84.Li X., Naylor M.F., Le H., Nordquist R.E., Teague T.K., Howard C.A. Clinical effects of in situ photoimmunotherapy on late-stage melanoma patients: a preliminary study. Canc Biol Ther. 2010;10:1081–1087. doi: 10.4161/cbt.10.11.13434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hu C., Cun X., Ruan S., Liu R., Xiao W., Yang X. Enzyme-triggered size shrink and laser-enhanced NO release nanoparticles for deep tumor penetration and combination therapy. Biomaterials. 2018;168:64–75. doi: 10.1016/j.biomaterials.2018.03.046. [DOI] [PubMed] [Google Scholar]
- 86.Chen Q., Xu L., Liang C., Wang C., Peng R., Liu Z. Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nat Commun. 2016;7:13193. doi: 10.1038/ncomms13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lu Q., Qi S., Li P., Yang L., Yang S., Wang Y. Photothermally activatable PDA immune nanomedicine combined with PD-L1 checkpoint blockade for antimetastatic cancer photoimmunotherapy. J Mater Chem B. 2019;7:2499–2511. doi: 10.1039/c9tb00089e. [DOI] [PubMed] [Google Scholar]
- 88.Sun W., Du Y., Liang X., Yu C., Fang J., Lu W. Synergistic triple-combination therapy with hyaluronic acid-shelled PPy/CPT nanoparticles results in tumor regression and prevents tumor recurrence and metastasis in 4T1 breast cancer. Biomaterials. 2019;217:119264. doi: 10.1016/j.biomaterials.2019.119264. [DOI] [PubMed] [Google Scholar]
- 89.Yu W., He X., Yang Z., Yang X., Xiao W., Liu R. Sequentially responsive biomimetic nanoparticles with optimal size in combination with checkpoint blockade for cascade synergetic treatment of breast cancer and lung metastasis. Biomaterials. 2019;217:119309. doi: 10.1016/j.biomaterials.2019.119309. [DOI] [PubMed] [Google Scholar]
- 90.Yu W., Liu R., Zhou Y., Gao H. Size-tunable strategies for a tumor targeted drug delivery system. ACS Cent Sci. 2020;6:100–116. doi: 10.1021/acscentsci.9b01139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu R., An Y., Jia W., Wang Y., Wu Y., Zhen Y. Macrophage-mimic shape changeable nanomedicine retained in tumor for multimodal therapy of breast cancer. J Control Release. 2020;321:589–601. doi: 10.1016/j.jconrel.2020.02.043. [DOI] [PubMed] [Google Scholar]
- 92.Liu R., Yu M., Yang X., Umeshappa C.S., Hu C., Yu W. Linear chimeric triblock molecules self-assembled micelles with controllably transformable property to enhance tumor retention for chemo-photodynamic therapy of breast cancer. Adv Funct Mater. 2019;29:1808462. [Google Scholar]
- 93.Chen Z., Liu L., Liang R., Luo Z., He H., Wu Z. Bioinspired hybrid protein oxygen nanocarrier amplified photodynamic therapy for eliciting anti-tumor immunity and abscopal effect. ACS Nano. 2018;12:8633–8645. doi: 10.1021/acsnano.8b04371. [DOI] [PubMed] [Google Scholar]
- 94.Gao F., Wu J., Gao H., Hu X., Liu L., Midgley A.C. Hypoxia-tropic nanozymes as oxygen generators for tumor-favoring theranostics. Biomaterials. 2020;230:119635. doi: 10.1016/j.biomaterials.2019.119635. [DOI] [PubMed] [Google Scholar]
- 95.Liu D., Chen B., Mo Y., Wang Z., Qi T., Zhang Q. Redox-activated porphyrin-based liposome remote-loaded with indoleamine 2,3-dioxygenase (IDO) inhibitor for synergistic photoimmunotherapy through induction of immunogenic cell death and blockage of IDO pathway. Nano Lett. 2019;19:6964–6976. doi: 10.1021/acs.nanolett.9b02306. [DOI] [PubMed] [Google Scholar]
- 96.Hu C., Lei T., Wang Y., Cao J., Yang X., Qin L. Phagocyte-membrane-coated and laser-responsive nanoparticles control primary and metastatic cancer by inducing anti-tumor immunity. Biomaterials. 2020;217:120159. doi: 10.1016/j.biomaterials.2020.120159. [DOI] [PubMed] [Google Scholar]
- 97.Dixon S.J., Lemberg K.M., Lamprecht M.R., Skouta R., Zaitsev E.M., Gleason C.E. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang S., Liao H., Li F., Ling D. A mini-review and perspective on ferroptosis-inducing strategies in cancer therapy. Chin Chem Lett. 2019;30:847–852. [Google Scholar]
- 99.Jiang Q., Wang K., Zhang X., Ouyang B., Liu H., Pang Z. Platelet membrane-camouflaged magnetic nanoparticles for ferroptosis-enhanced cancer immunotherapy. Small. 2020 doi: 10.1002/smll.202001704. [DOI] [PubMed] [Google Scholar]
- 100.Zhang D., Cui P., Dai Z., Yang B., Yao X., Liu Q. Tumor microenvironment responsive FePt/MoS2 nanocomposites with chemotherapy and photothermal therapy for enhancing cancer immunotherapy. Nanoscale. 2019;11:19912–19922. doi: 10.1039/c9nr05684j. [DOI] [PubMed] [Google Scholar]
- 101.Pan X., Wang H., Wang S., Sun X., Wang L., Wang W. Sonodynamic therapy (SDT): a novel strategy for cancer nanotheranostics. Sci China Life Sci. 2018;61:415–426. doi: 10.1007/s11427-017-9262-x. [DOI] [PubMed] [Google Scholar]
- 102.Liu M., Khan A.R., Ji J., Lin G., Zhao X., Zhai G. Crosslinked self-assembled nanoparticles for chemo-sonodynamic combination therapy favoring antitumor, antimetastasis management and immune responses. J Control Release. 2018;290:150–164. doi: 10.1016/j.jconrel.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 103.Zhao H., Zhao B., Li L., Ding K., Xiao H., Zheng C. Biomimetic decoy inhibits tumor growth and lung metastasis by reversing the drawbacks of sonodynamic therapy. Adv Healthc Mater. 2020;9 doi: 10.1002/adhm.201901335. [DOI] [PubMed] [Google Scholar]
- 104.Yue W., Chen L., Yu L., Zhou B., Yin H., Ren W. Checkpoint blockade and nanosonosensitizer-augmented noninvasive sonodynamic therapy combination reduces tumour growth and metastases in mice. Nat Commun. 2019;10:2025. doi: 10.1038/s41467-019-09760-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Min Y., Roche K.C., Tian S., Eblan M.J., McKinnon K.P., Caster J.M. Antigen-capturing nanoparticles improve the abscopal effect and cancer immunotherapy. Nat Nanotechnol. 2017;12:877–882. doi: 10.1038/nnano.2017.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lynn G.M., Sedlik C., Baharom F., Zhu Y., Ramirez-Valdez R.A., Coble V.L. Peptide-TLR-7/8a conjugate vaccines chemically programmed for nanoparticle self-assembly enhance CD8 T-cell immunity to tumor antigens. Nat Biotechnol. 2020;38:320–332. doi: 10.1038/s41587-019-0390-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Qin L., Cao J., Shao K., Tong F., Yang Z., Lei T. A tumor-to-lymph procedure navigated versatile gel system for combinatorial therapy against tumor recurrence and metastasis. Sci Adv. 2020;6:eabb3116. doi: 10.1126/sciadv.abb3116. [DOI] [PMC free article] [PubMed] [Google Scholar]