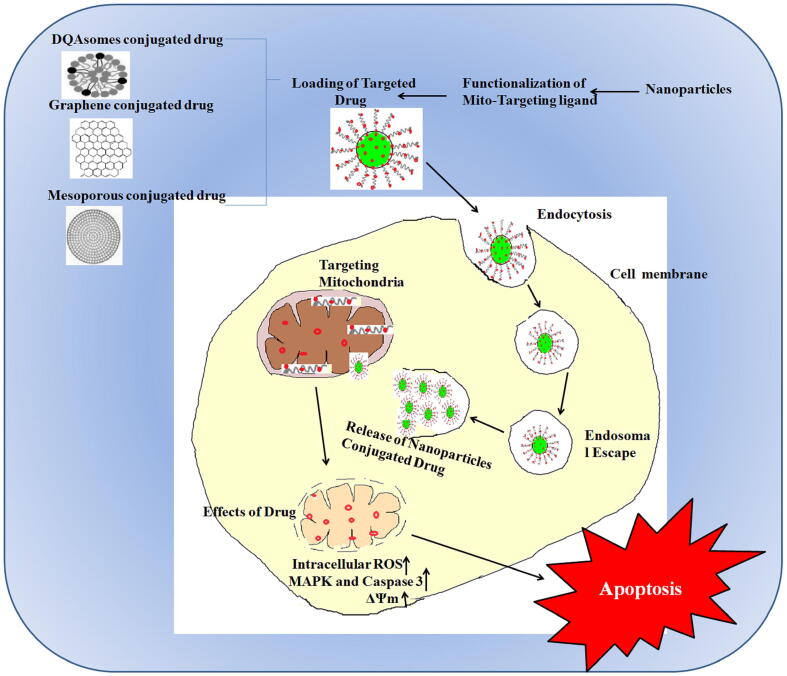
Abstract
Mitochondria are a major intracellular organelle for drug targeting due to its functional roles in cellular metabolism and cell signaling for proliferation and cell death. Mitochondria-targeted treatment strategy could be promising to improve the therapeutic efficacy of cancer while minimizing the adverse side effects. Over the last decades, several studies have explored and focused on mitochondrial functions, which has led to the emergence of mitochondria-specific therapies. Molecules in the mitochondria are considered to be prime targets, and a wide range of molecular strategies have been designed for targeting mitochondria compared with that of the cytosol. In this review, we focused on the molecular mechanisms of mitochondria-specific ligand targeting and selective drug action strategies for targeting mitochondria, including those premised on mitochondrial targeting of signal peptides (MTS), cell-penetrating peptides (CPPs), and use of lipophilic cations. Furthermore, most research has concentrated on specific conjugation of ligands to therapeutic molecules to enhance their effectiveness. There are several variations for the ideal design and development for mitochondrial-targeted drugs, such as selecting a suitable ligand and linker targets. However, some challenges related to drug solubility and selectivity could be resolved using the nanocarrier system. Nanoparticles yield excellent advantages for targeting and transmitting therapeutic drugs, and they offer elegant platforms for mitochondria-specific drug delivery. We explain many of the advanced and proven strategies for multifunctional mitochondria-specific targets, which should contribute to achieving better anticancer therapies in a promising future.
Keywords: Antitumoral therapy, Drug delivery system, Mitochondria-targeted drug delivery, Nanocarrier system, Nanomedicine, Targeted nanoparticles
1. Introduction
A therapeutic strategy at the most fundamental level depends on the interaction between subcellular components and drugs. Subcellular components of the cell interact with target-specific drugs, which results in functional alteration of the cells. These targeted drugs alter the metabolic and molecular function of subcellular organelles to enhance the treatment efficacy in patients and improve the health condition. Therapeutic drugs could have the ability to bind and interact with specifically targeted subcellular components (Nag and Delehanty, 2019). Drug targeting affects only selected cellular organelles of the malignant cells, with non-significant side effects to normal cells. Conventional treatments with most drugs are dose-dependent, and the drugs can be toxic at excessive doses. Nanoparticles are a powerful approach that circumvents the indiscriminate adverse effects of the drugs and can deliver at the target site to ensure the efficacy of the treatment. The architecture of the nanoparticles offers improvement in drug efficacy in the following ways by carrying concentrated cargo, its small size ensuring deep tissue delivery and accessibility, a facility for docking with different targeting moieties such as peptides and proteins and ability to modulate the unloading of the cargo at the target site (controlled release by photostimulation, enzyme or pH triggered release or application of magnetic field). All these attributes help overcome the challenges of poor bioavailability or biodistribution, limited or no water-solubility, immunogenic issues, pharmacokinetic hurdles, and higher toxicity.
Currently, therapeutic strategies for drug treatment of diseases are associated with the dysregulation of normal metabolic function in certain tissues and cells. Effective drug treatment without any adverse side effects to the normal biochemical and metabolic pathways is a major challenge (Ward et al., 2019). Therefore, drug molecules that can selectively accumulate at a targeted site are needed. This feature is especially important in designing and developing targeted drug delivery and therapies for cancer. Drug therapy has two distinct targeting strategies: selective and specific action on a target, and selective drug accumulation at a target (Singh et al., 2019). These strategies can be combined to target a particular site, tissue, or cells. Enhancing and improving the degree of selective drug accumulation can reduce the required drug dose, and increase the therapeutic efficacy and decrease toxicity to the normal cells. The properties of selective drug accumulation are associated with the concepts of biodistribution and bioavailability, which are connected with the physico-chemical properties of the drug molecules. The physico-chemical characteristics of drug molecules influence their pharmaceutical activity and help overcome their limitations. Large-scale screening analysis of chemical libraries has been used to detect the biological activity of desired compounds. This method can help determine high bioavailability and selective target action of drug molecules. Unfortunately, this type of analysis leads to many potent, active molecules being excluded from further analysis and development. Even if a drug has potent pharmacological activity toward a desired molecular target, it could also have poor bioavailability, and it may not be possible to deliver it exclusively to targeted tissues or cells.
There is a growing list of potent drug molecules that currently lack delivery strategies. Establishing a way to deliver them to their target molecules in the human body would make them more useful in clinical practice. After drug administration, the bioactive drugs have to reach the targeted tumor mass, which consists of tumor cells and supporting stroma. The stroma is the supporting blood vessels, connective tissues, normal cells, and extracellular matrix. Selective drug accumulation in a solid tumor is an initial step in selective metabolic function and molecular action on a tumor. Once the drug reaches the tumor cells and enters the cells, it then has to reach the sub-cellular organelles to target. The drug must be able to enter into the organelle from the cytosol and find its target subcellular organelles. Currently, cellular targeting strategies are well accepted, but strategies for a cell-specific accumulation of the targeted drug needs to be addressed. Moreover, sub-cellular targeting strategies are limited by technological issues, especially for mitochondria. Mitochondria play a key role in cellular function, cell survival, energy generation, stabilization of Reactive Oxygen Species (ROS), and regulation of cell death. The health status of the cells is mainly regulated by mitochondria, so it naturally implies that they may be involved in cancer development and progression (Giampazolias and Tait, 2016). The seminal work defines a phenomenon called Warburg effect shown by tumor cells, has increased glycolytic Adenosine Triphosphate (ATP) production, and reduced Oxidative Phosphorylation (OXPHOS). The important indicators of the cells becoming cancerous are large metabolic imbalances and increased resistance to cell death, both of these processes regulated by mitochondria (Pathania et al., 2009). Therefore, targeting this organelle may have a powerful impact on cancer. This review summarizes the selective drug accumulation, selective drug action, and selective drug combination to target mitochondrial function that is a potential target for cancer therapy.
2. Why target mitochondria?
Mitochondria are vital organelles in eukaryotic cells and mediate various cellular and metabolic processes, especially energy metabolism and the cell cycle. They are the mediators of life through energy generation and death via apoptosis and necrosis. The mitochondrial permeability transition pore complex (mPTPC) is the key regulator of cell death. Mitochondrial permeability transition (MPT) is a phenomenon that makes the highly impermeable inner mitochondrial membrane permeable that is modulated through the mPTPC and induced by high concentrations of mitochondrial Ca2+ and oxidative stress (Ilmarinen-Salo et al., 2012). Opening of the mPTPC results in collapse of the inner membrane potential, breakdown of the respiratory chain, blocking of the mitochondrial ATP synthesis and finally leading to swelling of the membranes, rupture and cell death. In recent years, various approaches are targeting to cure mitochondrial diseases by restoring cell function or stimulating programmed cell death.
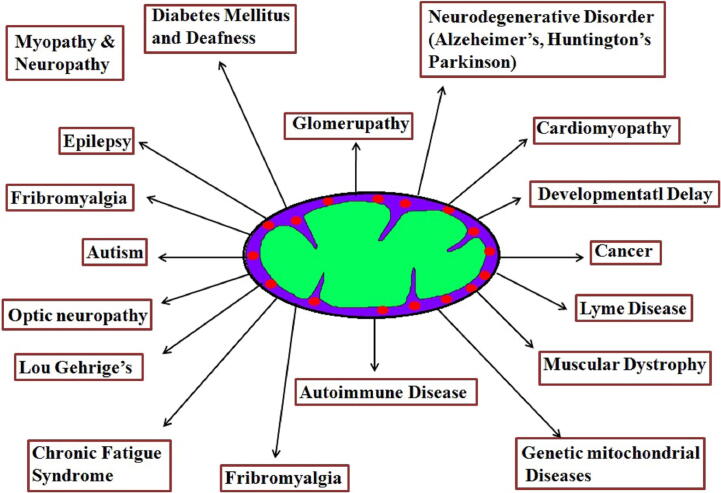
Apoptosis plays a major role in tissue cell homeostasis, and it is commonly recognized that alteration of apoptosis mechanism contributes to the transformation of normal cells into malignant cells. Dysfunction of apoptotic pathways has been interlinked with different types of tumors. Mitochondrial dysfunction has been recognized for various diseases (see Fig. 1a) for more than a decade. It leads to altered energy metabolism (biogenesis) and the electron transport chain (ETC) system of the malignant cells (Pathania et al., 2009), resulting in mutations in the mitochondrial DNA (mtDNA) that modulates the normal mitochondrial function. The four important strategies related to mitochondrial metabolism that enhance the cancer cell for survival are; i) Mitochondria responsible for cancer survival: Mitochondrial glycolytic flux enhances the production of ATP by mitochondrial glutaminolysis pathway to support the survival of cancer cells and is also involved in the development of BRAF mutation (a gene that encodes a protein called B-Raf) in lung cancer. Moreover, autophagy is a major source of glutamine which modulates the mitochondrial metabolism in cancer cells (Strohecker et al., 2013). Fatty acids also act as an alternative substrate for oxidation involved in mitochondrial ATP production and maintain the cancer cell survival (Carracedo et al., 2013). ii) Mitochondria promote cancer cell invasion and metastasis: Tumor invasion and metastasis mainly depends on mitochondrial oxidative phosphorylation. Peroxisome-proliferator regulator plays a major role in mitochondrial function and biogenesis that activates the mitochondrial oxidative phosphorylation and enhances the invasion and metastasis of the tumor cells (lung tumor) (LeBleu et al., 2014). The energy metabolism permits for the generation of ROS (Reactive Oxygen Species) resulting in activation of src and pyk2 protein tyrosine kinases which in turn activates and enhances the invasion and tumor development. In cancer, the intra-and extracellular pools of Ca2+ that accumulate in mitochondria affects the opening of mitochondrial permeability transition pore complex (mPTPC) that influences cell death (Halestrap, 2010). iii) Mitochondria associated with drug resistance of cancer cell: Genotoxic drugs stimulate a shift in mitochondria by modulating energy metabolism (mitochondria biogenesis) and mitochondrial oxidative phosphorylation (upregulation) inducing mitochondrial dependency, resulting in resistance to chemotherapy (colorectal tumors). Studies have shown that mitochondrial oxidative metabolism is slowed down with MAPK activation by repressing the MITF/PGC1α pathway (Vellinga et al., 2015). On the contrary, mitochondrial oxidative phosphorylation is stimulated by BRAF inhibitors in turn producing ROS in melanoma cells. Oxidative metabolism can be considered as an adaptive mechanism that limits the efficacy of BRAF inhibitors (Haq et al., 2013). A study found several BRAF inhibitor-resistant melanoma cell lines were highly sensitive to several mitochondria-targeted compounds including the mitochondria pro-oxidative drug, elesclomol. These findings indicate the development of mitochondria specific targeting drugs for BRAF inhibitor-resistant cancers (Haq et al., 2013). iv) Mitochondria responsible for energy metabolism and enhances the cell proliferation: Recent study in tumor metabolomics illustrates that cancer cells can oxidize glucose-derived pyruvate through pyruvate dehydrogenase (PDH) – dependent pathway in mitochondria and enhance the production of glutamine which is essential for tumor growth metabolism in tumor cells. These reports suggest that mitochondria play a crucial and major role in tumor development and an effective approach would be to develop mitochondria specific targeted drugs as a line of defense against cancer.
Fig. 1a.
Mitochondrial associated disorders.
2.1. Mitochondria-targeted drug delivery
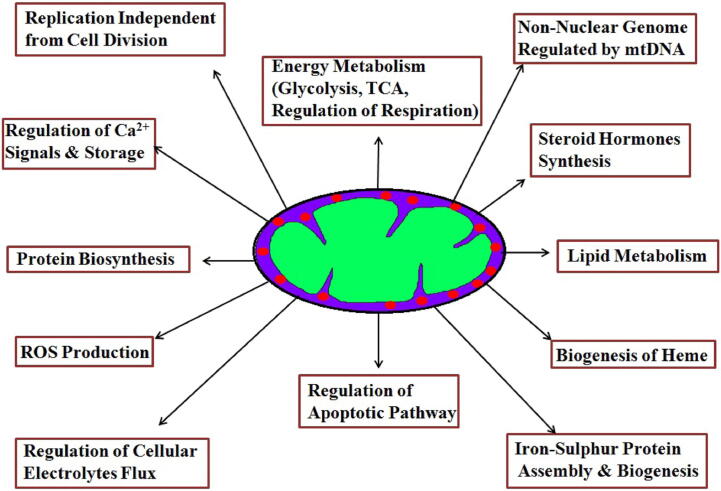
Fig. 1b summarizes the various targets on mitochondria for developing drug delivery strategies. Mitochondria are a prime target for new drugs and there is an increasing interest in molecular mechanisms of xenobiotics with cellular machinery located on or inside mitochondria (Rin Jean et al., 2014). Since mitochondria can switch from glycolysis to oxidative phosphorylation (OXPHOS) for their survival, the metabolism can be targeted. Among metabolism, drugs that target ETC (metformin, tamoxifen), glycolysis and OXPHOS (HKII inhibitors), and TCA cycle (inhibitors of isocitrate dehydrogenases) possess chemotherapeutic potential. Also, drugs that target the apoptotic machinery and ROS homeostasis is a part of the multi-pronged approach for mitochondrial-targeted drugs. These include the Bcl 2 family of proteins and targeting redox regulating enzymes and ROS production. Molecules that generate mitochondrial ROS production to induce apoptosis will enhance the chemotherapeutic potential. For drug therapy, targeting mitochondria in cancer cells involves two major approaches: i) a selective action on the target i.e. the action in itself is sophisticated enough to make the distinction between cancer and non-cancer cell and ii) selective accumulation of the drugs at the molecular target that is directly correlated to the physico-chemical properties of the molecule which would have an impact on the bioavailability and biodistribution of the drug.
Fig. 1b.
Mitochondrial targets for developing drug delivery strategies.
2.1.1. Selective drug action strategies for targeting mitochondria
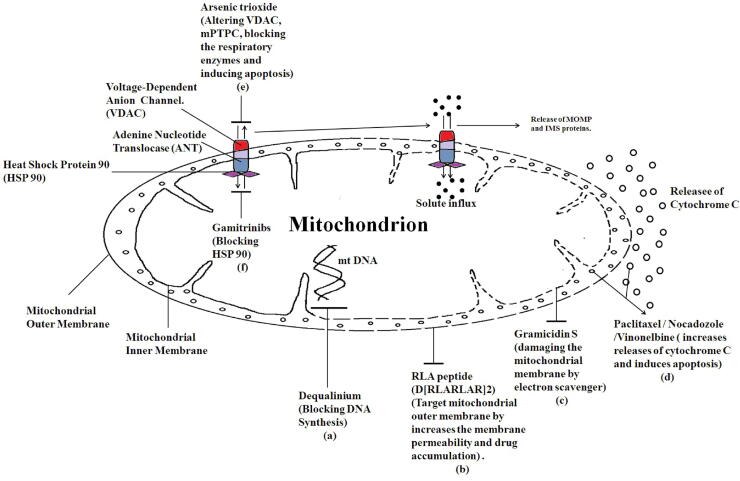
Many bioactive molecules are currently being used in clinical trials that target mitochondria. Targeted drug delivery to mitochondria results in improved therapeutic efficacy while minimizing the toxic effects on non-malignant cells. Various drugs have been used to target the function, structure and metabolic activity of mitochondria to induce cell death of cancer cells (see Fig. 2). Some of the clinically approved drugs are VP-16 (Custódio et al., 2001), paclitaxel (Yilmaz et al., 2017) and vinorelbine (Shi et al., 2015) with targets in mitochondria affect mitochondrial function (Table 1) along with the increasing bank of experimental drugs such as CD437, ceramide, MKT077 and Lonidamine (Han et al., 2016, Ma et al., 2016, Starenki and Park, 2015, Cervantes-Madrid et al., 2015).
Fig. 2.
Schematic representation of mitochondrial function explains the reason for target mitochondria. (a) Dequalinium binds to the mitochondrial DNA (mtDNA) and blocks the DNA synthesis. (b) RLA peptide (D[RLARLAR]2) targets the mitochondrial outer membrane by increasing the membrane permeability and drug accumulation. (c) Gramicidin S acts as an electron scavenger and damages the mitochondrial membrane. (d) Paclitaxel/Nocadozole/Vinonelbine increases the release of cytochrome C and induces apoptosis. (e) Arsenic trioxide which binds to the voltage dependent anionic channel (VDAC) that alters the function of VDAC and mitochondria permeability transition pore complex (mPTPC). It also inhibits blocks the respiratory enzymes resulting in induction of apoptosis. (f) Gamitrinibs inhibit and target the activity of heat shock protein 90 (HSP90) in the mitochondria of human cancer cells by acting as ATPase antagonists and modulating tumor necrosis factor receptor-associated protein 1 (TRAP1) and, exerts apoptotic functions. Thus, drugs specifically targeted to mitochondria display tumor-selective cytotoxic properties results in induction of tumor cell death.
Table 1.
Mitochondria specific drug delivery and targeting strategies of nanoparticles used in cancer treatment.
| Targeting Moieties | Nano-carriers and conjugates | Targeting strategies and molecular action | Outcome | Ref |
|---|---|---|---|---|
| CD437 | – | Inhibits POLA1; affects Mptpc; releases cytochrome C | Apoptosis | (Han et al., 2016) |
| Arsenic trioxide | – | Affects mPTPC; inhibits BCL2 and BCLXL | Apoptosis | (Cervantes-Madrid et al., 2015) |
| Cyclosporine A (CsA) | PLGA (Poly lactic co-glycolic acid) | Affects mPTPC | Apoptosis | (Zhang et al., 2019) |
| Lonidamine (LND) | – | Inhibits hexokinase 2 (HK2) | Apoptosis | (Wang et al., 2014) |
| Etoposide (VP-16) | – | Topoisomerase II inhibitor | Apoptosis | (Custódio et al., 2001) |
| MKT-007 (Rhodacyanin) | – | Prevents ATP synthesis in tumour cells; | Tumor cell death; nephrotoxicity | (Starenki and Park, 2015) |
| Elesclomol | – | Inhibits ETC; blocks ATP synthesis, induces ROS | Apotosis | (Nagai et al., 2012) |
| Metformin/Phenormin | – | AMPK activator; blocks ATP synthesis | Apoptosis | (Scotland et al., 2013) |
| Oligomycin A | – | Inhibits ATPase; affects mPTPC; induces ROS | Apoptosis | (Mandujano-Tinoco et al., 2013) |
| CB 839 | – | Inhibits glutaminase | Apoptosis | (Meric-Bernstam et al., 2019) |
| Tamoxifen | – | Inhibits topoisomerase; | Apoptosis | (Jafari and Fatemi, 2020) |
| Nucleic acid, peptides, CsA | Triphenylphosphonium (TPP) - mitochondriotropic | Drug accumulation | Cytotoxicity | (Trnka et al., 2015, Larosche et al., 2007) |
| Porphyrins | TPP | Drug accumulation | Photo-cytotoxicity | (Yang et al., 2019) |
| TEMPOL | TPP | Drug accumulation | Apoptosis | (Trnka et al., 2009) |
| Topotecan, DOX, Cisplatin | TPP- antibiotic peptide (KLAKLAK)2- Mesoporous silica nanoparticles (MSN); MSN-amino silane and EDC (1-ethyl-3-(3-dimethyl aminopropyl) carbodiimide and N-hydroxysuccinimide)-TPP | Lowered ATP production; decreased mitochondrial membrane potential , Drug accumulation, facilitates endocytosis | Cytotoxicity | (Zhang et al., 2012, Shi et al., 2018, Choi et al., 2015) |
| Bombesin receptor (BnR) antagonists | BnR peptides | Targeted delivery | Cytoxicity | (Moreno et al., 2016) |
| Mastoparan | Liposomes were altered with fusogenic and transferring peptide (Chol-GALA) | Affects MPT | Cell death | (Yamada et al., 2015) |
| DOX | Hypericin-Graphene oxide | Increased phototherapy and chemotherapy | Cell death | (Han et al., 2018) |
| Methyl coumarin | TPP | Inhibited proliferation, induced ROS generation, reduced mitochondrial membrane potential, promotes Ca2+ accumulation, | Apoptosis | (Wang and Xu, 2017) |
| Celastrol | PEGylated polyaminoacid-capped CST-loaded MSN | Controlled in vitro drug release; | cytotoxicity Apoptosis | (Choi et al., 2018) |
| Paclitaxel | Liposomes-rhodamine- 123-conjugated polymer; Dequalinium (DQA) DQA-PEG(5000)-DSPE –mitochondriotropic molecule. Steoryltriphenylphosphonium (STPP)-based liposomes | Affects mPTPC; Increases drug accumulation; DQAsomes; inhibiting the mtDNA synthesis in cancer cells; Increases intracellular Ca2+ homeostasis and cytochrome c release | Tumor cell death. | (Biswas et al., 2012, Solomon et al., 2013) |
| Gamitrinibs | TPP | Specifically antagonize the ATPase pocket on Hsp90 | Tumor cell death. | (Fiesel et al., 2017) |
| Green fluorescent protein (GFP) as a model | MITO-Porter | Facilitates macropinocytosis and delivers cargo (drugs) to mitochondrial matrices. | Targeted drug delivery to cancer cell. | (Kawamura et al., 2020) |
| Mesochlorin e6 | N-(2-hydroxylpropyl) methacrylamide (HPMA)-TPP | Enhanced effect of specific combined targeting of tumor cells and mitochondria | Apoptosis | (Cuchelkar et al., 2008) |
| Hypocrellin A | water-soluble amorphous silica nanocage (HANC) | Small size ensures specific targeting and delivery | Apoptosis | (Zangabad et al., 2017) |
| Proteins | Intracellular Delivery of Proteins with Cell-Penetrating Peptides for Therapeutic Uses in Human Disease | Uptake of exogenously delivered proteins | Apotosis | (Dinca et al., 2006) |
| Dye-labeled to track uptake | Mitochondria penetrating peptides (MPP) | Imparts positive charge and lipophilic potential | Maximizes drug delivery | (Alta et al., 2017) |
Drugs such as paclitaxel increase intracellular Ca2+ homeostasis that leads to the opening of mPTPC and stimulating the release of cytochrome c resulting in mitochondria-mediated apoptosis in tumor cells (Yilmaz et al., 2017). Fig. 3 explains that drugs induce cytochrome c release through direct interaction with the mPTPC of mitochondria, which subsequently results in apoptosis. While lonidamine (LND), an indazole carboxylate derivative is an inhibitor of hexokinase 2 (HK2) and induces p53-dependent apoptosis in tumor cells (Wang et al., 2014). VP-16 (Etoposide), a topoisomerase II inhibitor, incubated with rat liver mitochondria induces mitochondria permeability transition, increases the intracellular Ca2+ homeostasis resulting in mitochondrial swelling and depolarization of mitochondria (Custódio et al., 2001). Elesclomol preferentially binds to copper ions (extracellular ion) and transports the copper ion into mitochondria of the tumor cells thus, inhibiting the toxic effect to normal tissues (Nagai et al., 2012).
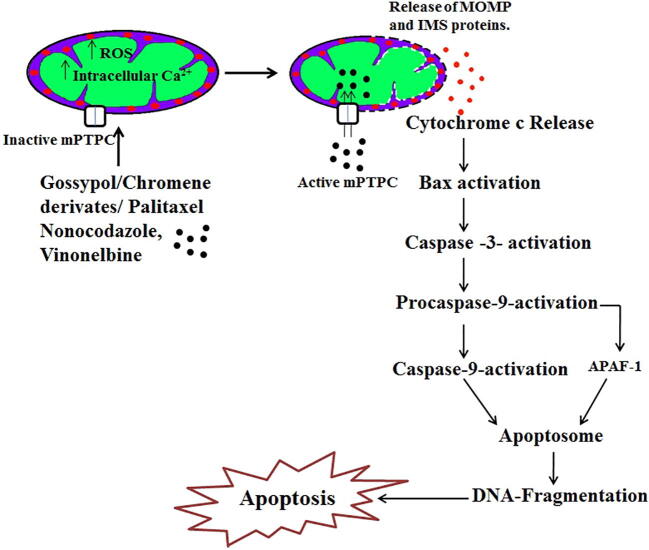
Fig. 3.
Schematic representation of mitochondria-specific targeted drug action. Drug (Paclitaxel/Gossypol/Chromene derivate/Nocodazole/Vinonelbine) induces the increase of reactive oxygen species (ROS) and intracellular calcium level in mitochondria that changes mitochondria permeability transition (MPT) and mitochondria permeability transition pore complex (mPTPC) resulting in solute influx and release of mitochondrial outer membrane permeabilization (MOMP) and mitochondrial intermembrane space (IMS) proteins present in the mitochondrial membrane. The release of cytochrome c activates Bax that in turn activates caspase-3. This induces procaspase-9 followed by activation of apoptotic protease activating factor 1 (APAF-1) which form the apoptosome that leads to DNA fragmentation resulting in apoptosis. Most of the mitochondrial specific anticancer drugs results in induction of apoptosis.
Even though, phenformin possesses a similar action as metformin but is a more potent mitochondrial inhibitor (García Rubiño et al., 2019). Tamoxifen is a well-known antitumor drug that accumulates inside mitochondria (40-fold) and suppresses both mitochondrial respiration and fatty acid oxidation (Larosche et al., 2007). Cisplatin is an anticancer drug that rapidly accumulates in the inner mitochondria and alters the structural stability and metabolic function of the mitochondria (Table 1).
Effective drug molecules do not sufficiently target mitochondria in tumors. There are challenges in developing a mitochondria-targeted drug. An important one is to evaluate non-invasively the involvement of mitochondrial function and damage in the development of the disease, implying that it is difficult to know when to propose a mitochondria treatment or assess if the treatment is selective. There is a need to identify biomarkers that are specific, sensitive for a short period and clinically useful (Steele et al., 2017). Another key point is maintaining the selectivity of the drug so that the adverse effects such as cardio- or hepatotoxicity can be minimized.
2.1.2. New paradigm to overcome metabolic challenges by targeting mitochondria
Mitochondria targeting strategies have greatly attracted opportunities for the development of potential anticancer agents. However, blockage of a mitochondrial metabolic pathway may activate alternative mechanisms that could maintain cancer cell survival and growth. A new targeting paradigm to enhance the cancer treatment regimen is to provide a combination of mitochondria targeting drugs with inhibitors of alternative metabolic pathways thereby developing a “combination of antimetabolic therapy”. This combination enhances therapeutic efficacy on tumor cells compared to the use of single mitochondrial inhibitors.
Antimetabolic combination therapy can be observed as the effects of pharmacological inhibition of various metabolic pathways to kill or destroy the tumor cells. Antioxidants such as mito-CP and mito-Q targeting mitochondria possess synergistic activity with an antiglycolytic drug, 2-deoxyglucose (2-DG) is phosphorylated by hexokinase and subsequently inhibits ATP generated via the glycolytic pathway to destroy breast cancer cells in vivo and in vitro (Dwarakanath and Jain, 2009) and are less toxic to normal cells. Cancer cells can metabolize lactate (preferred substrate) for mitochondrial oxidative phosphorylation. Thus, the inhibition of lactate importer (monocarboxylate transporter 1 (MCT-1)) in cancer cells, inhibits lactate dependent respiration and cancer cell growth, therefore, exhibiting its potential as a major anticancer drug (Park et al., 2018). Furthermore, the combination of 2-DG, metformin, and mitochondrial complex I inhibitor enhances the induction of complete (100%) cytotoxicity to prostate cancer without disturbing the normal epithelial cells. In addition to this study, there was enhanced suppression and killing of myeloma cells when treated with a combination of GLUT-4 inhibitor, metformin, and ritonavir that targets the mitochondrial metabolism as opposed to when treated with an individual drug (Dalva-Aydemir et al., 2015). Furthermore, preclinical analysis on tumor growth showed that the inhibitory effect was less when treated alone with an inhibitor of glycolysis than when followed by glutamine addition indicating that tumor cells utilize an alternative metabolic pathway for its survival. It would be interesting to identify the specific metabolic targets and develop an effective robust, screening approach to the relevant antimetabolic combination for the mitochondria target.
Molecular-targeted drugs illustrate an interesting response rate as in advanced stages of the disease in relapsed patients. The molecular targeted drug (like BRAF-inhibitor) unlike genotoxic drugs, does not inhibit massive cancer cell death, instead, it leads to the persistence of few subpopulations of cells developing drug-resistance. To overcome this problem, the combination of molecular targeted drug therapy (oncogene kinase inhibitors) with mitochondrial activity inhibitor was developed to better control cancer growth that suppresses the development of a tolerant subpopulation. The strategies include i) initial target: suppression of oncogenic molecules by molecular – targeted drugs thereby modulating mitochondrial metabolism leading to damage and/or disruption of cancer cells (suppression of mitochondria biogenesis and glycolysis) and cancer cells promoting mitochondrial metabolism in drug-resistant subpopulation cells. ii) final-target: The drug-tolerant subpopulation of cancer cells is infinitely sensitive to the lethal effects of mitochondrial activity inhibitors. This effective targeting strategy, notably the combination of oncogenic kinase inhibitor and mitochondrial activity inhibitors, seems to be the most efficient therapy in several preclinical models.
The mitochondrial protein of dihydrolipoamide S-acetyltransferase (DLAT), a component of the pyruvate dehydrogenase (PDH) enzyme is necessary for cell survival in the presence of BCR-ABL inhibitors in Ph+ leukemia cells (Alvarez-Calderon et al., 2015). Therefore treatment with a combination of BCR-ABL inhibitors and mitochondrial activity inhibitors results in enhanced inhibition of cell proliferation and promotes cell death. These new therapeutic combinations of pre-clinical models completely destroy the drug-resistant subpopulation of cancer cells and minimizethe risk of relapse in certain cancers. This therapeutic combination strategy would be less challenging to implement safely in clinical practice than mitochondrial inhibitors alone. Furthermore, the therapeutic combination of mitochondrial inhibitors and molecular targeted drugs needs further evaluation in preclinical and clinical trials.
2.1.3. Selective drug accumulation strategy for targeting mitochondria
There are two categories of selective drug accumulation strategies for targeting tumor mitochondria: i) targeted drug delivery and drug accumulation in or around the tumor, and ii) drug delivery and accumulation in the mitochondria of the cancer cells. The drug accumulation strategy may be modified based on the chemical structure to change physio-chemical properties that regulate the drug aggregation in mitochondria. The modification must enhance the accumulation of the targeted drug without changing the integrity of its molecular action. Mitochondrial drugs encapsulated in nano-carriers need to overcome the extracellular and intracellular barriers of the targeted areas, tissues, or cells. Suitable alteration of nano-carriers helps the drug to escape from the endocytic pathway and enhances the intracellular drug delivery which allows for selective localization, molecular action, and selective drug accumulation at a target site.
Some drug accumulation and targeting strategies use nano-carrier conjugated ligands that are bigger than a functional group of the bioactive drug molecules to modify the biodistribution. This strategy works as long as the nano-carrier conjugation does not modify or negatively alter the effects of the drug molecules at their molecular target. These strategies have been used effectively to enhance the drug distribution in the body and accomplish higher concentrations of the drug molecules in the targeted tissue. Some nano-carrier conjugated ligands show moderate drug aggregation in targeted tumor tissue. The few ligands that enhance the mitochondria-mediated drug aggregation are known as mitochondriotropics. Research on tumor-targeted delivery has shown interest in enhancing the concentration of anticancer molecules. The research aimed at targeted drug delivery in sub-cellular compartments has only recently received more attention for improved cancer therapy (Cervantes-Madrid et al., 2015).
2.1.3.1. Strategies of molecular modification
In normal cells, the cytoskeletal network of protein, distinct internal organelles, and a multitude of intracellular macromolecules and other diffused biomolecules form an environment that is very dissimilar from that of tumor cells. Classical Fickian diffusion is possibly the predominant method of non-specific transportation in the cells. The viscosity of the cell cytoplasm and the binding with intracellular components (mitochondria, lysosomes, nucleus, Golgi, endoplasmic reticulum) are also believed to be involved in the diffusion of bioactive drug molecules inside a cell (Rajendran et al., 2010). The physico-chemical properties generally determine the target of sub-cellular components (such as mitochondria), and they play a major role in targeted drug delivery. Intracellular drug distribution and cellular uptake of low-molecular-weight molecules are linked with a quantitative structure–activity relationship (QSAR) model. QSAR strategies have recently been used to recognize a potential common chemical structure of bioactive drug molecules that specifically accumulates at or inside the mitochondria of living cells. This strategy has also been useful for remodeling cationic transfection lipids (Jafari and Fatemi, 2020), predicting the intracellular deposition of drug molecules, and assisting targeted drug delivery to mitochondria. Several bioactive cargos are conjugated with a TPP cation, a mitochodriotropic molecule that enhances the targeted drug delivery to the mitochondria (Trnka et al., 2015) (Table 1).
Gamitrinib-triphenylphosphonium (G-TPP) targets mitochondrial-localized members of the HSP90 family including TNF receptor-associated protein-1 (TRAP1) (Fiesel et al., 2017).TRAP1 is responsible for the suppression of oxidative stress and prevents the opening of mPTPC. At the used sublethal dose, G-TPP is known to induce mitochondrial unfolded protein response (mitoUPR) and autophagy (Fiesel et al., 2017). The active geldanamycin molecules of gamitrinibs are linked with an amide containing a linker to cyclic guanidinium moieties, which serve as mitochondria targeting signals (MTS). A major advantage of gamitrinibs is that it is non-toxic to normal cells and has no adverse side effects on HSP90 in non-mitochondrial intracellular compartments (Fiesel et al., 2017). Similarly, a short leader sequence of matrix protein can facilitate mitochondria-specific targeted drug delivery of pro-apoptotic anticancer peptide sequences (KLAKLAK)2 to tumor cells (Luo et al., 2014). For the development of nano carrier-based drug targeting, an efficacious strategy is to design conjugating ligands that have a dual-specificity for targeting mitochondria as well as tumor cells.
2.1.3.2. Conjugated ligand strategies for targeting mitochondria
Solid nano-carrier-like micelles and liposomes are viewed as strategies for non-chemical targeted delivery to alter the accumulation of targeted drug molecules. These nano-carrier molecules can be altered for selectively targeting a specific cell type. Prolonged-circulating liposome nanoparticles are able to deliver a targeted drug through passive targeting of the leaky vasculature via enhanced retention potential and permeability of the targeted membrane. These liposomes can be conjugated with targeting antibodies or other targeting ligands for cell-specific recognition and drug delivery (Marzbali and Khosroushahi, 2017). Chemical strategies of drug delivery through nanoparticle conjugated bioactive molecules enable delivery to a targeted site. Nano-carriers enhance the tumor-targeting and mediate the mitochondria-targeted drug accumulation inside the cancer cell, which could be used for clinical therapy (Table 1).
Alteration with transferrin peptide augments endocytosis-mediated liposomal uptake into the targeted cells. These peptides then facilitate release from the endosomes into the cytosol. The strategies of targeted delivery attain a higher concentration of the drug molecules inside the cell to interact and facilitate the targeting of mitochondria. Micelle nanoparticles labeled with fluorescent dye were used to deliver targeted hydrophobic drugs distributed to several sub-cellular components. Fluorescent-labeled micelles enhance the distribution of drugs in cytoplasmic organelles, accumulate in mitochondria, and are not restricted to a single cell type (Shaw and Pease, 2019). Furthermore, the cargo of incorporated micelles shows enhanced cell internalization than the free cargo by itself.
DQAsomes loaded with folic-acid-conjugated paclitaxel show increased cytotoxicity on HeLa cells (expressing folate receptors) compared to folic-acid-conjugated paclitaxel encapsulated liposomes, paclitaxel loaded DQAsomes and folic acid conjugated paclitaxel alone (Vaidya et al., 2009). DQA80s that are functionalized DQAsomes having cationic lipids (Table 1), were found to be dually effective as drug carriers targeting mitochondria as well as the ability to target tumor cells (see Fig. 4). Polymer and liposomal based nanoparticles carry cargo conjugated with mitochondriotropic ligands such as TPP. Liposomes have the advantage of being biocompatible and can carry both hydrophilic and hydrophobic drugs, and malleable for surface modifications to enhance the specificity of targeted drug delivery. Liposomes with surface modification by a mitochondriotropic residue are known as proteoliposomes enhances the liposome penetration into sub-cellular components (Table 1) (Kang and Ko, 2019).
Fig. 4.
DQAsomes, mesoporous and graphene oxide nanoparticles conjugated drug for mitochondrion targeted therapeutics.
Another strategy involves MITO Porter, a surface-modified liposome (altered octaarginine residue) that facilitates drug delivery into cells as intact vesicles through micropinocytosis (Kawamura et al., 2020). Table 1 lists the different strategies for mitochondria-specific nanoparticle-mediated drug delivery and targeting strategies of nanoparticles used in cancer treatment (Cervantes-Madrid et al., 2015, Zhang et al., 2019, Zhang et al., 2012, Scotland et al., 2013, Mandujano-Tinoco et al., 2013, Meric-Bernstam et al., 2019, Yang et al., 2019, Trnka et al., 2009, Shi et al., 2018, Choi et al., 2015, Choi et al., 2018, Moreno et al., 2016, Yamada et al., 2015, Han et al., 2018, Wang and Xu, 2017, Biswas et al., 2012, Solomon et al., 2013, Cuchelkar et al., 2008, Zangabad et al., 2017, Dinca et al., 2006, Alta et al., 2017). Therefore, these nanosystems are applied to mitochondria-specific targeted drug delivery of proapoptotic substances for cancer treatment.
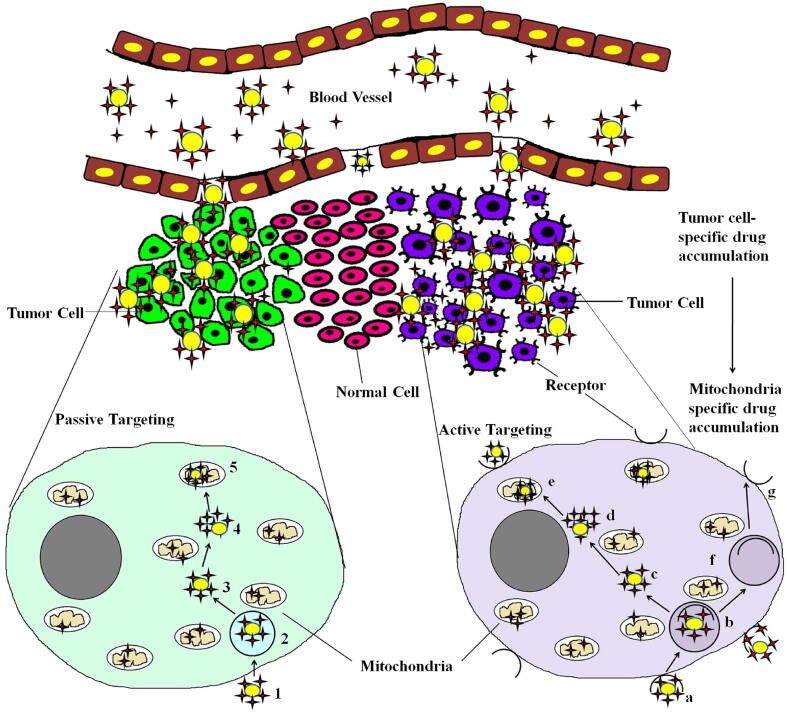
Cell-penetrating peptides (CPPs) are a group of small peptides that carry molecular cargo across the cell membrane enabling the uptake of exogenously delivered proteins. Peptides comprising of both natural and synthetic residues that specifically target the mitochondria are called mitochondria-penetrating peptides (MPPs). Another strategy that facilitates cellular entry and targeting to the mitochondria combines existing CPP motifs with mitochondria-targeting sequences (MTS) (Dinca et al., 2006, Alta et al., 2017). Fig. 5 illustrates nanoparticles that mediate drug accumulation and drug delivery at tumor sites specifically to the mitochondria by passive or active targeting. Drugs that reach the cytoplasm need to be addressed for specific targeting if their targets reside in the cytoplasm (and are membrane-bound), which are not reachable from the surface endocytic routes. Mitochondria act as major hotspots for specific targeted therapy in cancer cells. Mesoporous silica nanoparticles (MSNPs) functionalized with different targeting ligands have been used to deliver hydrophobic and hydrophilic drugs to subcellular organelles of cancer cells (Luo et al., 2014). TPP functionalized in mesoporous silica containing doxorubicin has been reported to be an effective transporter escaping the mitochondrial membrane (see Fig. 4). The dual loading of hypericin (natural anthraquinone derivative with photosensitizer property) and doxorubicin in graphene oxide has been reported to be beneficial for targeting mitochondria (see Fig. 5). After intraperitoneal administration, the drug targets mitochondria and concentrates in tumor cells due to enhanced permeability and retention effect (EPR) and releases hypericin due to cleavage of disulfide bonds by glutathione (Han et al., 2018). Wang and Xu (Wang and Xu, 2017) have reported a methyl coumarin -triphenylphosphonium for mitochondrial-targeted therapy. The design includes the reaction of methyl group substituted coumarin (6-methyl coumarin) with N-bromosuccinimide followed by coupling with triphenylphosphonium to form 6-bromomethyl coumarin (Table 1).
Fig. 5.
Graphic illustration of selective drug accumulation in drugs that are actively or passively targeted specifically to mitochondria of tumor cells. Nanoparticle encapsulated drug molecules reaches the tumor site selectively through the leaky vasculature in tumor. These nanoparticle conjugated drugs infiltrate the tumor cells by active and passive targeting strategies. The nanocarrier linked drug molecules can bind or enter the cell through endocytosis or receptor- mediated endocytic process. In active targeting strategy the drug molecule enters through: a) Nanoparticle conjugated drug is internalized via receptor mediated endocytic route. b) The released vesicles undergo fusion with existing early endosomes to produce receptor mediated early endosomes. c) The nanoparticle conjugated drug is released from receptor mediated endosomes. d) The drug is released from the nanoparticle. e) Drug enters into the mitochondria by its selective accumulation. f) After the drug is released from the receptor endosomes, the receptors are recycled via endosomes to the plasma membrane. g) The recycled receptor is present in the plasma membrane. In the passive targeting strategy the drug molecule enters through: 1) Nanoparticle conjugated drug is internalized via endocytic route. 2) The released vesicles undergo fusion with existing early endosomes to produce early endosomes. The drug is released from the endosomes and the recycling outer via endosomes. 3) The nanoparticle conjugated drug is released from endosomes. 4) The drug is released from the nanoparticle. 5) The drug enters the mitochondria by its selective accumulation. Drug, and nanoparticles.
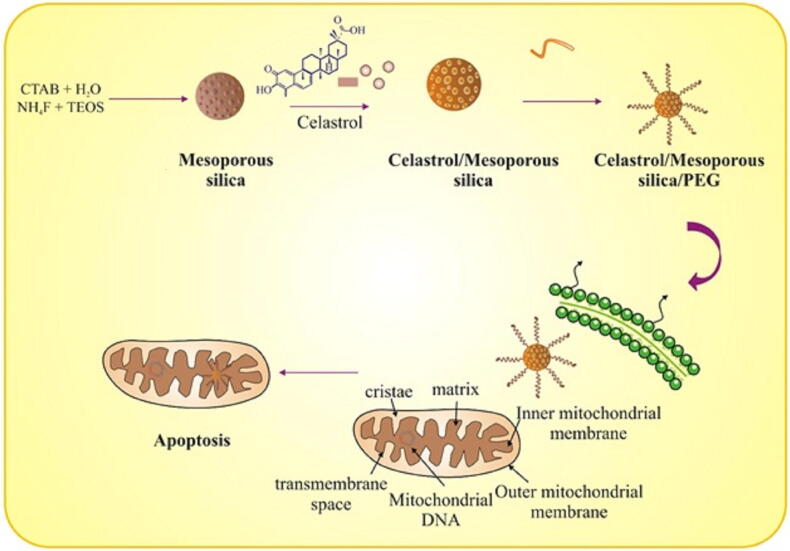
Celastrol (pentacyclic triterpenoid), extracted from Thunder god vine (Tripterygium wilfordii), exhibited anticancer effects after encapsulation into mesopores of silica nanoparticles (Choi et al., 2018). Compared to free celastrol, the functionalized nanosized compound increased expression of HIF-1α and mitochondrial apoptotic pathway (see Fig. 6). Taken together, the numerous studies presented strongly suggest that nanoparticles can positively regulate the targeted drug delivery and drug accumulation of bioactive components into sub-cellular organelles. Thus, they represent a functional tool for designing and developing mitochondria-specific targeted drug delivery for effective therapeutic interventions in cancer.
Fig. 6.
Celastrol encapsulated PEGylated polyaminoacid capped silica nanoparticles.
3. Summary and future perspectives
Due to its critical standpoint in cellular processes, mitochondria, play an important role in human health and disorders, that has led to the emergence of new field in nanotechnology for designing mitochondria-targeted drug delivery. Mitochondria targeting strategies in the design and development of anticancer agents rely on two basic drug targeting strategies: specific targeting action and specific drug accumulation at a targeted site. Bioactive compounds with selective drug targeting and selective drug accumulation strategies could provide economic value, and targeted delivery offers enhanced drug action and safety. Therefore, drug design and development studies must include specific strategies aimed at conferring selective drug accumulation with specific drug targeting strategies.
Direct bioconjugation of targeted drugs with ligands that facilitate sub-cellular accumulation in desired tumor cells has significant promise and effectiveness in cancer therapy. Nanoparticle-encapsulated drugs offer much more promise for targeting and delivering drugs into sub-cellular organelles in tumors, thereby avoiding the collateral damage to healthy and normal cells. Therefore, ideal targeting and delivery strategies for mitochondria-specific targeting approaches in cancer treatment might involve the identification of bioactive components with specific targeting action on mitochondria. The bioactive compounds may then be linked with mitochondria-specific targeting ligands and then encapsulated in nanoparticles for targeting cancer cells.
The ideal mitochondria-specific targeted drug delivery system should reach the targeted site and selectively target the sub-cellular organelles. A mitochondria-specific protein targeting system would have superior transportation characteristics compared to lipophilic cations. The ideal drug should also be designed to protect the drug-loaded nanoparticles from the energy metabolism of the body. Potential strategies could involve conjugating mitochondria-targeting ligands with nanoparticles, mitochondria-specific targeted peptides, proteins, or monoclonal antibodies. These therapeutic drugs have been encapsulated with nanoparticles and help to protect them from biodegradation in vivo, as well as enhance and facilitate the strategies of mitochondria-specific targeted drug delivery. The successful design and development of mitochondria-specific targeted drug delivery not only reduces adverse side effects but also enhances the efficacy of the therapeutic agents.
In the field of drug discovery, there is growing attention on intracellular drug targeting and delivery. Recent research has aided in the recognition of the molecular mechanisms and complex pathways in the transport of drug molecules into cells and specific subcellular organelles. However, better knowledge and understanding is needed regarding the cell sorting pathways and cell transport trafficking inside the cells. The clinical practice of nanoparticle-based drug delivery systems for sub-cellular targeting is difficult because of the complexity of the transport mechanism, and it is a great challenge to provide such therapies efficaciously on a commercial scale.
The ultimate target of sub-cellular delivery is to ensure that drug molecules are selectively delivered to their targeted site, tissue, or cells within the infected area while maximizing the therapeutic effects and minimizing harm. Selective drug delivery allows for much safer therapeutic drugs, especially for cancer, where numerous drugs are limited by the toxicity of effective doses, which leads to discontinuation of drug treatment. Targeted drug therapy allows for a much lower drug concentration to have a greater effect than the drug in its free form. The effective therapeutic concentration of the drug can be reduced by delivering the drug to its target site, and the therapeutic efficacy can be improved substantially.
Nanoparticles afford numerous benefits for drug targeting and delivery into sub-cellular compartments. Compared to the free form of therapeutic drugs, nano formulated drugs have enhanced specificity towards a drug target. Numerous nano-carriers and targeting ligands have paved the way for developing and designing nano formulated systems that could achieve both drug targeting and drug delivery into sub-cellular organelles. Targeting nanoparticles to specific cells and intracellular drug delivery significantly improves their performance as imaging probes, bioactive drug carriers, and molecular contrast agents. The armamentarium of drug-targeting ligands, nano-conjugates, and encapsulation of nanoparticles to allow intracellular drug delivery is expanding with the understanding of cell-signaling pathways, intracellular targets, molecular targets, physico-chemical properties of the nano-carriers, the molecular basis of diseases, and the cell sorting mechanism that regulates the drug environment.
Declaration of Competing Interest
The author declares no conflict of interest.
Acknowledgments
Acknowledgments
The author is very thankful to the Deanship of Scientific Research, Prince Sattam bin Abdulaziz University for supporting this work. The author SD personally grateful to Mr. Muthamil Selvan for his understanding, full-hearted support and encouragement. Further, this research holds no conflict of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Peer review under responsibility of King Saud University.
References
- Alta R.Y., Vitorino H.A., Goswami D., Liria C.W., Wisnovsky S.P., Kelley S.O., Machini M.T., Esposito B.P. Mitochondria-penetrating peptides conjugated to desferrioxamine as chelators for mitochondrial labile iron. PloS one. 2017;12(2) doi: 10.1371/journal.pone.0171729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Calderon F., Gregory M.A., Pham-Danis C., DeRyckere D., Stevens B.M., Zaberezhnyy V., Hill A.A., Gemta L., Kumar A., Kumar V., Wempe M.F. Tyrosine kinase inhibition in leukemia induces an altered metabolic state sensitive to mitochondrial perturbations. Clin. Cancer Res. 2015;21:1360–1372. doi: 10.1158/1078-0432.ccr-14-2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas, S., Dodwadkar, N.S., Deshpande, P.P., Torchilin, V.P., 2012. Liposomes loaded with paclitaxel and modified with novel triphenylphosphonium–PEG–PE conjugate possess low toxicity, target mitochondria and demonstrate enhanced antitumor effects in vitro and in vivo. J. Control Release 159, 393–402. http://doi.org/10.1016/ j.jconrel.2012.01.009. [DOI] [PMC free article] [PubMed]
- Carracedo A., Cantley L.C., Pandolfi P.P. Cancer metabolism: fatty acid oxidation in the limelight. Nat Rev Cancer. 2013;13:227–1132. doi: 10.1111/j.1742-4658.2007.05686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervantes-Madrid D., Romero Y., Dueñas-González A. Reviving lonidamine and 6-diazo-5-oxo-L-norleucine to be used in combination for metabolic cancer therapy. BioMed Res. Int. 2015 doi: 10.1155/2015/690492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J.Y., Gupta B., Ramasamy T., Jeong J.H., Jin S.G., Choi H.G., Yong C.S., Kim J.O. PEGylated polyaminoacid-capped mesoporous silica nanoparticles for mitochondria-targeted delivery of celastrol in solid tumors. Colloids Surf. B Biointerfaces. 2018;165:56–66. doi: 10.1016/j.colsurfb.2018.02.015. [DOI] [PubMed] [Google Scholar]
- Choi Y.M., Kim H.K., Shim W., Anwar M.A., Kwon J.W., Kwon H.K., Kim H.J., Jeong H., Kim H.M., Hwang D.M., Kim H.S. Mechanism of Cisplatin -Induced Cytotoxicity Is Correlated to Impaired Metabolism Due to Mitochondrial ROS Generation. PLoS One. 2015;10 doi: 10.1371/journal.pone.0135083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuchelkar V., Kopečková P., Kopeček J. Novel HPMA copolymer-bound constructs for combined tumor and mitochondrial targeting. Mol. Pharmaceutics. 2008;5(5):776–786. doi: 10.1021/mp800019g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Custódio J.B., Cardoso C.M., Madeira V.M., Almeida L.M. Mitochondrial permeability transition induced by the anticancer drug etoposide. Toxicol. in vitro. 2001;15(4–5):265–270. doi: 10.1016/S0887-2333(01)00019-4. [DOI] [PubMed] [Google Scholar]
- Dalva-Aydemir S., Bajpai R., Martinez M., Adekola K.U., Kandela I., Wei C., Singhal S., Koblinski J.E., Raje N.S., Rosen S.T., Shanmugam M. Targeting the metabolic plasticity of multiple myeloma with FDA-approved ritonavir and metformin. Clin. Cancer Res. 2015;21(5):1161–1171. doi: 10.1158/1078-0432.CCR-14-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinca A., Chien W.M., Chin M.T. Intracellular Delivery of Proteins with Cell Penetrating Peptides for Therapeutic uses in Human Disease. Int. J. Mol. Sci. 2006;17:263. doi: 10.3390/ijms17020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwarakanath B., Jain V. Targeting glucose metabolism with 2-deoxyD-glucose for improving cancer therapy. Future Oncol. 2009;5:581–585. doi: 10.2217/fon.09.44. [DOI] [PubMed] [Google Scholar]
- Fiesel, F.C., James, E.D., Hudec, R., Springer, W., 2017. Mitochondrial targeted HSP90 inhibitor Gamitrinib-TPP (G-TPP) induces PINK1/Parkin-dependent mitophagy. Oncotarget, 8(63), 106233. http://doi.org/10.18632/oncotarget.22287. [DOI] [PMC free article] [PubMed]
- García Rubiño M.E., Carrillo E., Ruiz Alcalá G., Domínguez-Martín A., Marchal J., Boulaiz H. Phenformin as an Anticancer Agent: Challenges and Prospects. Int. J. Mol. Sci. 2019;20(13):3316. doi: 10.3390/ijms20133316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giampazolias E., Tait S.W. Mitochondria and the hallmarks of cancer. FEBS J. 2016;283(5):803–814. doi: 10.1111/febs.13603. [DOI] [PubMed] [Google Scholar]
- Halestrap A.P. A pore way to die: the role of mitochondria in reperfusion injury and cardioprotection. Biochem. Soc. Trans. 2010;38(4):841–860. doi: 10.1042/BST0380841. [DOI] [PubMed] [Google Scholar]
- Han T., Goralski M., Capota E., Padrick S.B., Kim J., Xie Y., Nijhawan D. The antitumor toxin CD437 is a direct inhibitor of DNA polymerase α. Nat. Chem. Biol. 2016;12:511–515. doi: 10.1038/nchembio.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C., Zhang C., Ma T., Zhang C., Luo J., Xu X., Zhao H., Chen Y., Kong L. Hypericin-functionalized graphene oxide for enhanced mitochondria-targeting and synergistic anticancer effect. Acta Biomater. 2018;77:268–281. doi: 10.1016/j.actbio.2018.07.018. [DOI] [PubMed] [Google Scholar]
- Haq R., Shoag J., Andreu-Perez P., Yokoyama S., Edelman H., Rowe G.C., Frederick D.T., Hurley A.D., Nellore A., Kung A.L., Wargo J.A. Oncogenic BRAF regulates oxidative metabolism via PGC1α and MITF. Cancer Cell. 2013;23(3):302–315. doi: 10.1016/j.ccr.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilmarinen-Salo P., Moilanen E., Kinnula V.L., Kankaanranta H. Nitric oxide-induced eosinophil apoptosis is dependent on mitochondrial permeability transition (mPT), JNK and oxidative stress: apoptosis is preceded but not mediated by early mPT-dependent JNK activation. Respir. Res. 2012;13(1):73. doi: 10.1186/1465-9921-13-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafari K., Fatemi M.H. Application of nano-quantitative structure–property relationship paradigm to develop predictive models for thermal conductivity of metal oxide-based ethylene glycol nanofluids. J. Therm. Anal. Calorimetry. 2020;1–10 doi: 10.1007/s10973-019-09215-3. [DOI] [Google Scholar]
- Kang J.H., Ko Y.T. Enhanced subcellular trafficking of resveratrol using mitochondriotropic liposomes in cancer cells. Pharmaceutics. 2019;11(8):423. doi: 10.3390/pharmaceutics11080423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura E., Maruyama M., Abe J., Sudo A., Takeda A., Takada S., Yokota T., Kinugawa S., Harashima H., Yamada Y. Validation of Gene Therapy for Mutant Mitochondria by Delivering Mitochondrial RNA Using a MITO-Porter. Mol. Ther. Nucl. Acids. 2020;20:687–698. doi: 10.1016/j.omtn.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larosche I., Letteron P., Fromenty B., Vadrot N., Abbey-Toby A., Feldmann G., Pessayre D., Mansouri A. Tamoxifen inhibits topoisomerases, depletes mitochondrial DNA, and triggers steatosis in mouse liver. J. Pharmacol. Exp. Therapeutics. 2007;321(2):526–1435. doi: 10.1124/jpet.106.114546. [DOI] [PubMed] [Google Scholar]
- LeBleu V.S., O’Connell J.T., Herrera K.N.G., Wikman H., Pantel K., Haigis M.C., De Carvalho F.M., Damascena A., Chinen L.T.D., Rocha R.M., Asara J.M. PGC-1α mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat. Cell. Biol. 2014;16:992–1003. doi: 10.1038/ncb3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo G.F., Chen W.H., Liu Y., Lei Q., Zhuo R.X., Zhang X.Z. Multifunctional enveloped mesoporous silica nanoparticles for subcellular co-delivery of drug and therapeutic peptide. Sci. Rep. 2014;4:6064. doi: 10.1021/ja312004m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y.Y., Mou X.Z., Ding Y.H., Zou H., Huang D.S. Delivery systems of ceramide in targeted cancer therapy: ceramide alone or in combination with other anti-tumor agents. Expert Opin. Drug Deliv. 2016;213:1397–1406. doi: 10.1080/17425247.2016.1188803. [DOI] [PubMed] [Google Scholar]
- Mandujano-Tinoco E.A., Gallardo-Pérez J.C., Marín-Hernández A., Moreno-Sánchez R., Rodríguez-Enríquez S. Anti-mitochondrial therapy in human breast cancer multicellular spheroids. Biochim. Biophys. Acta. 2013;1833:541–551. doi: 10.1016/j.bbamcr.2012.11.013. [DOI] [PubMed] [Google Scholar]
- Marzbali M.Y., Khosroushahi A.Y. Polymeric micelles as mighty nanocarriers for cancer gene therapy: a review. Cancer Chemother. Pharmacol. 2017;79:637–649. doi: 10.1007/s00280-017-3273-1. [DOI] [PubMed] [Google Scholar]
- Meric-Bernstam, F., Lee, R.J., Carthon, B.C., Iliopoulos, O., Mier, J.W., Patel, M.R., Tannir, N.M., Owonikoko, T.K., Haas, N.B., Voss, M.H., Harding, J.J., 2019. CB-839, a glutaminase inhibitor, in combination with cabozantinib in patients with clear cell and papillary metastatic renal cell cancer (mRCC): Results of a phase I study.37,547. http://doi.org/10.1200/JCO.2019.37.7_suppl.549.
- Moreno, P., Ramos-Álvarez, I., Moody, T.W., Jensen, R.T., 2016. Bombesin related peptides/receptors and their promising therapeutic roles in cancer imaging, targeting and treatment. Expert Opin. Ther. Targets. 20, 1055–1073. http://doi.org/10.1517/ 14728222.2016.1164694. [DOI] [PMC free article] [PubMed]
- Nag A.O.K., Delehanty J.B. Active Cellular and Subcellular Targeting of Nanoparticles for Drug Delivery. Pharmaceutics. 2019;11(10):543. doi: 10.3390/pharmaceutics11100543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai M., Vo N.H., Ogawa L.S., Chimmanamada D., Inoue T., Chu J., Beaudette-Zlatanova B.C., Lu R., Blackman R.K., Barsoum J., Koya K. The oncology drug elesclomol selectively transports copper to the mitochondria to induce oxidative stress in cancer cells. Free Radi. Biol. Med. 2012;52(10):2142–2150. doi: 10.1016/j.freeradbiomed.2012.03.017. [DOI] [PubMed] [Google Scholar]
- Park, S.J., Smith, C.P., Wilbur, R.R., Cain, C.P., Kallu, S.R., Valasapalli, S., Sahoo, A., Guda, M.R., Tsung, A.J., Velpula, K.K., 2018. An overview of MCT1 and MCT4 in GBM: small molecule transporters with large implications. Am. J. Cancer Res. 8(10), 1967–1976. PMID: 30416849; PMCID: PMC6220151. [PMC free article] [PubMed]
- Pathania D., Millard M., Neamati N. Opportunities in discovery and delivery of anticancer drugs targeting mitochondria and cancer cell metabolism. Adv Drug Deliv Rev. 2009;61:1250–1275. doi: 10.1016/j.addr.2009.05.010. [DOI] [PubMed] [Google Scholar]
- Rajendran L., Knölker H.J., Simons K. Subcellular targeting strategies for drug design and delivery. Nat Rev Drug Discov. 2010;9:29–42. doi: 10.1038/nrd2897. [DOI] [PubMed] [Google Scholar]
- Rin Jean S., Tulumello D.V., Wisnovsky S.P., Lei E.K., Pereira M.P., Kelley S.O. Molecular vehicles for mitochondrial chemical biology and drug delivery. ACS Chem. Biol. 2014;9(2):323–333. doi: 10.1021/cb400821p. [DOI] [PubMed] [Google Scholar]
- Scotland S., Saland E., Skuli N., De Toni F., Boutzen H., Micklow E., Senegas I., Peyraud R., Peyriga L., Theodoro F., Dumon E. Mitochondrial energetic and AKT status mediate metabolic effects and apoptosis of metformin in human leukemic cells. Leukemia. 2013;27(11):2129–2138. doi: 10.1038/leu.2013.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw D.D., Pease L.F., III Release of pharmaceutical cocktails from small polymeric micelles. Chem. Engg. Sci. 2019;207:799–804. doi: 10.1016/j.ces.2019.05.052. [DOI] [Google Scholar]
- Shi J.F., Sun M.G., Li X.Y., Zhao Y., Ju R.J., Mu L.M., Yan Y., Li X.T., Zeng F., Lu W.L. A combination of targeted sunitinib liposomes and targeted vinorelbine liposomes for treating invasive breast cancer. J. Biomed. Nanotechnol. 2015;11(9):1568–1582. doi: 10.1166/jbn.2015.2075. [DOI] [PubMed] [Google Scholar]
- Shi M., Zhang J., Li X., Pan S., Li J., Yang C., Hu H., Qiao M., Chen D., Zhao X. Mitochondria-targeted delivery of doxorubicin to enhance antitumor activity with HER-2 peptide-mediated multifunctional pH-sensitive DQAsomes. Int. J. Nanomed. 2018;13:4209–4226. doi: 10.2147/IJN.S163858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A.P., Biswas A., Shukla A., Maiti P. Targeted therapy in chronic diseases using nanomaterial-based drug delivery vehicles. Sig Transduct Target Ther. 2019;4:33. doi: 10.1038/s41392-019-0068-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon M.A., Shah A.A., D'Souza G.G. In vitro assessment of the utility of stearyl triphenyl phosphonium modified liposomes in overcoming the resistance of ovarian carcinoma Ovcar-3 cells to paclitaxel. Mitochondrion. 2013;13:464–472. doi: 10.1016/j.mito.2012.10.013. [DOI] [PubMed] [Google Scholar]
- Starenki D., Park J.I. Selective mitochondrial uptake of MKT-077 can suppress medullary thyroid carcinoma cell survival in vitro and in vivo. Endocrinol. Metabolism. 2015;30(4):593–603. doi: 10.3803/EnM.2015.30.4.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele H.E., Horvath R., Lyon J.J., Chinnery P.F. Monitoring clinical progression with mitochondrial disease biomarkers. Brain. 2017;140:2530–2540. doi: 10.1093/brain/awx168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohecker A.M., Guo J.Y., Karsli-Uzunbas G., Price S.M., Chen G.J., Mathew R., McMahon M., White E. Autophagy sustains mitochondrial glutamine metabolism and growth of braf V600E-driven lung tumors. Cancer Discov. 2013;3:1272–1285. doi: 10.1158/2159-8290.CD-13-0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trnka J., Blaikie F.H., Logan A., Smith R.A., Murphy M.P. Antioxidant properties of Mito-TEMPOL and its hydroxylamine. Free Radic Res. 2009;43:4–12. doi: 10.1080/10715760802582183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trnka J., Elkalaf M., Anděl M. Lipophilic triphenylphosphonium cations inhibit mitochondrial electron transport chain and induce mitochondrial proton leak. PLoS One. 2015;30 doi: 10.1371/journal.pone.0121837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya B., Paliwal R., Rai S., Khatri K., Goyal A.K., Mishra N., Vyas S.P. Cell-selective mitochondrial targeting: A new approach for cancer therapy. Cancer Therapy. 2009;7(1):141–148. [Google Scholar]
- Vellinga T.T., Borovski T., de Boer V.C., Fatrai S., van Schelven S., Trumpi K., Verheem A., Snoeren N., Emmink B.L., Koster J., Rinkes I.H.B. SIRT1/PGC1α- dependent increase in oxidative phosphorylation supports chemotherapy resistance of colon cancer. Clin Cancer Res. 2015;2:2870–2879. doi: 10.1158/1078-0432.CCR-14-2290. [DOI] [PubMed] [Google Scholar]
- Wang L., Xiong H., Wu F., Zhang Y., Wang J., Zhao L., Guo X., Chang L.J., Zhang Y., You M.J., Koochekpour S., Saleem M., Huang H., Lu J., Deng Y. Hexokinase 2-mediated Warburg effect is required for PTEN- and p53-deficiency-driven prostate cancer growth. Cell Reports. 2014;8:1461–1474. doi: 10.1016/j.celrep.2014.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Xu W. Mito-methyl coumarin, a novel mitochondria-targeted drug with great antitumor potential was synthesized. Biochem. Biophys. Res. Commun. 2017;489(1):1–7. doi: 10.1016/j.bbrc.2017.05.116. [DOI] [PubMed] [Google Scholar]
- Ward N.C., Watts G.F., Eckel R.H. Statin toxicity: mechanistic insights and clinical implications. Cir Res. 2019;124(2):328–350. doi: 10.1161/CIRCRESAHA.119.315233. [DOI] [PubMed] [Google Scholar]
- Yamada Y., Nakamura K., Abe J., Hyodo M., Haga S., Ozaki M., Harashima H. Mitochondrial delivery of Coenzyme Q10 via systemic administration using a MITO-Porter prevents ischemia/reperfusion injury in the mouse liver. J. Control Release. 2015;213:86–95. doi: 10.1016/j.jconrel.2015.06.037. [DOI] [PubMed] [Google Scholar]
- Yang M., Deng J., Guo D., Sun Q., Wang Z., Wang K., Wu F. Mitochondria-targeting Pt/Mn porphyrins as efficient photosensitizers for magnetic resonance imaging and photodynamic therapy. Dyes Pigments. 2019;166:189–195. doi: 10.1016/j.dyepig.2019.03.048. [DOI] [Google Scholar]
- Yilmaz E., Watkins S.C., Gold M.S. Paclitaxel-induced increase in mitochondrial volume mediates dysregulation of intracellular Ca2+ in putative nociceptive glabrous skin neurons from the rat. Cell Calcium. 2017;62:16–28. doi: 10.1016/j.ceca.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zangabad P.S., Karimi M., Mehdizadeh F., Malekzad H., Ghasemi A., Bahrami S., Zare H., Moghoofei M., Hekmatmanesh A., Hamblin M.R. Nanocaged platforms: modification, drug delivery and nanotoxicity. Opening synthetic cages to release the tiger. Nanoscale. 2017;9(4):1356–1392. doi: 10.1039/C6NR07315H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C.X., Cheng Y., Liu D.Z., Liu M., Cui H., Mei Q.B., Zhou S.Y. Mitochondria-targeted cyclosporin A delivery system to treat myocardial ischemia reperfusion injury of rats. J. Nanobiotechnol. 2019;17(1):18. doi: 10.1186/s12951-019-0451-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Yao H.J., Yu Y., Zhang Y., Li R.J., Ju R.J., Wang X.X., Sun M.G., Shi J.F., Lu W.L. Mitochondrial targeting topotecan-loaded liposomes for treating drug-resistant breast cancer and inhibiting invasive metastases of melanoma. Biomaterials. 2012;33:1808–1820. doi: 10.1016/j.biomaterials.2011.10.085. [DOI] [PubMed] [Google Scholar]