Abstract
Macrophages have a leading position in the tumor microenvironment (TME) which paves the way to carcinogenesis. Initially, monocytes and macrophages are recruited to the sites where the tumor develops. Under the guidance of different microenvironmental signals, macrophages would polarize into two functional phenotypes, named as classically activated macrophages (M1) and alternatively activated macrophages (M2). Contrary to the anti-tumor effect of M1, M2 exerts anti-inflammatory and tumorigenic characters. In progressive tumor, M2 tumor-associated macrophages (TAMs) are in the majority, being vital regulators reacting upon TME. This review elaborates on the role of TAMs in tumor progression. Furthermore, prospective macrophage-focused therapeutic strategies, including drugs not only in clinical trials but also at primary research stages, are summarized followed by a discussion about their clinical application values. Nanoparticulate systems with efficient drug delivery and improved antitumor effect are also summed up in this article.
Key words: Tumor-associated macrophages, Tumor microenvironment, Targeted drug delivery, Tumor treatment, Combined therapy, Immunotherapy, Extracellular vesicles, Nano-drug
Graphical abstract
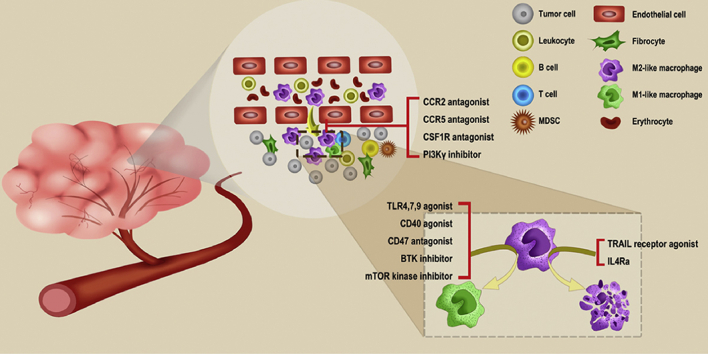
Tumor-associated macrophages (TAMs) as a major part in the tumor environment (TME) are associated with the progression of tumor. This review summarizes the roles TAMs play and some TAM targeting strategies. Also, this review provides some perspectives about drug targets for TAMs and some potential combination therapies.
1. Introduction
Cancer is the major disease threating human health which accounts for estimated 9.6 million deaths in 2018. Therefore, the growing burden of cancer pushes forward the development of various therapies against cancer cells. However, it is generally believed that cancer cells do not work alone. Cooperating closely with stromal cells, immune cells, and extracellular matrices, tumor cells promote chronic inflammation and immunosuppression. Besides, an angiogenic intratumoral environment that feeds the tumor comes into being. Human body has its immune operation to protect us from the internal threats (such as malignant cells) which was called the tumor immune cycle1. In this cycle, effector T lymphocytes will infiltrate into tumors and kill them. However, some essential steps for T cell immunosurveillance are often impaired in tumorigenesis, resulting in tumor escape2.
For nearly a century, much endeavor has centered around “immune-enhancing” strategies to combat with cancer, such as IL-23, IFNs4, CAR-T cells5, and anti-CTLA-4 mAbs6. Scientists hope that enhanced immunity can eliminate tumors just like kill invasive viruses or bacteria. But the results are not quite satisfactory. The weapon for malignant tumors to fight the immune response is not some powerful reproductive activity like those pathogenic microorganisms have, but their tricks to cheat our immune system. Thus, enhancing immunity not only fail to get objective remission of cancer but also trigger severe immune-related side effects (irAE)7. With the guard of these tricks, so called "immune evasion mechanisms", tumor cells will disrupt the routine immune processes, such as altering the antigen presentation process, secreting immunosuppressive factors that induce lymphocyte apoptosis, or activating adverse regulatory pathways8,9. These immune evasion mechanisms continue to develop and become more diverse and complex during cancer progression. How to block them and reactivate our immune system is the problem required to be solved in tumor immunotherapy.
Tumor escape involves pretty complicated elements in which the tumor microenvironment (TME) dominates the major part. As one of the chief members in TME, the role of tumor-associated macrophage (TAM) cannot be overlooked. As a brand-new target, more and more researches are focusing on TAMs and trying to find some breakthroughs about antitumor therapy. Before getting it to the introduction of novel therapeutic strategies targeting TAMs, some basic characters of TAMs should be noticed.
We summarize the critical functions of TAMs in tumor development as follows. Firstly, TAMs secrete manifold growth factors to help oncogenesis10,11; secondly, they are beneficial to produce several proteolytic enzymes and motor-related proteins to support the invasion and metastasis of tumors12,13; thirdly, TAMs encode multiple gene products to promote angiogenesis14,15; finally, TAM is a powerful manufacturer of numerous immunosuppressive molecules. Besides, the increased TAMs population is associated with low survival rates in many human malignant neoplasms. Therefore, TAMs cannot be neglected in tumor development and become a promising new target for tumor therapy. In recent years, researches on TAM-targeting therapy have focused on the following aspects: suppressing the recruitment of macrophages, activating the repolarization of the original tumorigenic M2-like into an anti-tumor M1-like phenotype, and depleting TAMs16.
At present, nanotechnology is the focus of concern in the field of drug delivery system, especially standing out in targeting therapy. These drug-loaded systems have the characteristics of large specific surface area, low immunogenicity, and long circulation time in the blood. The same goes for TAM-targeting therapy, nano-drugs including nanocapsules17,18, nanospheres19, nanomicelles20, nanoliposomes21 with biological or physicochemical modification have the advantage of more precise locational drug release and less toxic effects on healthy tissue, like myelosuppression, gastrointestinal reaction, organ toxicity and some other common side effects caused by chemotherapy drugs.
The nanocarrier system relies on the EPR effect (enhanced permeability and retention effect) to passively target tumor sites22, which is applicable to most of the solid tumors. Due to vascular damage and lymphatic system loss in tumor tissues, nanoparticles pass through the interstitial vascular barrier and remain in the tumors with the help of pressure difference induced by poor lymphatic drainage, which requires the particle size to be generally between 10 and 200 nm23, and its circulation time to be longer than 6 h. With the help of a nanocarrier system, TAM-targeting treatment is realizable and feasible. Therefore, we will sum up some advanced TAM-targeting immune-nanomedicine applied to clinical practice, as well as researches in preclinical trials in this review.
2. The conversion of TAMs from progenitors
Macrophage plays an influential role in the host defense system24, as one of the determinants in the immune response to pathogens. Meanwhile, they are the crucial mediators of the inflammatory process in the human organism25. After released from the bone marrow or yolk sac, immature mononuclear macrophages enter the tissues via blood circulation and eventually differentiate into tissue-resident macrophages26. As is well known, macrophages often gathers at the injury or infected sites27 to perform several functions: 1) producing cytokines and chemokines as the mediators to modulate other immune cells; 2) producing growth factors, angiogenic factors, and proteases to promote tissue repair; 3) killing pathogens by producing reactive oxygen and nitrogen free radicals. Also, they can present extrinsic antigens to cytotoxic T cells14.
The TME is a complex cellular ecosystem, jointly formed by leukocytes, fibroblasts, and vascular endothelial cells28, accompanying the evolution of cancer cells and backing their conversion into malignant tumors29. As one of the hallmarks of TME in tumorigenesis30, inflammation is mainly contributed by the immune cells together with tumor cells31. Among all immune cells being recruited to tumor sites, macrophages are in the majority and present as a special coordinator of multiple factors in the whole journey of tumor progression32.
Mononuclear phagocytes generally originate from bone marrow. After drifting in the circulating bloodstream for hours or days, peripheral blood mononuclear cells, with the assistance of the adhesion molecules from selectin family, will adhere to the vessel wall via specific receptors on endothelial cells. With the assistance of tumor-secreting chemokines, monocytes migrate across the vessels through the gap between vascular endothelial cells, and intrude surrounding tumor tissues where they differentiate into TAMs33. Relevant chemokines include the CXC chemokine family (interleukin IL-8), CXC-chemokine ligand (CXCL1, CXCL6, and CXCL8), CC-chemokine ligand (CCL2, CCL5, and CCL18). Also, there are other recruitment factors like vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), CX3C and C chemokines, hypoxia-inducible factor (HIF) and transforming growth factor β (TGF-β) which have also been verified to be promoters of monocyte efflux34,35. It is reported that serum level of CCL2 is higher in human gastric cancer, breast cancer and lung cancer36, which could bind to a specific receptor (CCR2 mainly) highly expressed in macrophages, promoting their infiltration to tumors.
There are two polarized pathways for macrophages in tissues: the classically activated M1 pathway and the alternatively activated M2 pathway. Induced by lipopolysaccharide (LPS) or interferon C in vitro, M1 macrophages secrete nitric oxide, inflammatory factors, and chemokines, like IL-12, IL-23, MHC-II and B7 family members like B7-1 (CD80) and B7-2 (CD86), whose primary function is activating Th1 immune response to exert anti-tumor effect37,38. The M2 pathway requires two types of signal molecules: the Th2 cytokine and the inducer of endogenous or exogenous tumor necrosis factor39,40. Cytokines (IL-4, IL-13), vitamin D3, TGF-β, PGE2, and glucocorticoids are the main factors to induce M2 type activation41. Then M2 macrophages will secrete IL-1 chemokine receptor antagonists, matrix metalloproteinase (MMP), and upregulate the expression of MHC, restraining immune response and stimulating tumor invasion, growth and metastasis by secreting IL-10, TGF-β, etc., preventing T cells from effectively exerting anti-tumor effects42.
Likewise, TAM can be classified as M1 and M2 type co-existing in the TME. On account of its plasticity, TAMs can switch from one type to another depending on the environment they reside43. There is no single unique marker for TAMs, though a combination of markers has been listed. And scientists have identified selective cell surface markers for M1 type (CD86, CD64, CD16, CD120b, TLR2, and SLAMF7) and M2 type (CD23, CD163, CD206, CD1a, CD1b, CD93, and CD226) macrophages43,44. Surprisingly, recent research indicated that TAMs frequently co-express M1 and M2 type genes in individual cells which enhanced the difficulty to take them apart45, which may provide evidence to the existence of the intermediate state of TAMs.
3. Promoting tumor progression—the crime committed by TAMs
What trick dose the tumor play in the human body? The tumor can effectively incite defection of immune cells into immunosuppressive cells. For example, regulatory cells (Tregs) can produce IL-10, TGF-β and express the negative costimulatory molecules CTLA-4, PD-1 to inhibit the function of T cells46,47. Myeloid-derived suppressor cells (MDSCs) promote tumor immune escape through impairing the function of NK cells, DCs, T cells and inhibiting their proliferation via l-arginine depletion. Beyond that, MDSCs also interfere with the efficiency of checkpoint blockade48, 49, 50. TAMs inhibit the expression of IL-12 in intratumoral DCs and CD8+ T cell-mediated anti-tumor immune responses by producing high levels of IL-1046,51. To sum up, the immune system is a double-edged sword for cancer patients. On the one hand, it kills tumor cells and inhibits outgrowth. On the other hand, it can promote tumor progression and metastasis by establishing the microenvironment which is conducive to tumor growth.
The heterogeneity of TAMs depends on the variation of TME and the type of tumor. M1 macrophage factors, such as interferon-inducing factors (CCL5, CXCL9, CXCL10, and CXCL16), also exist in tumors52. Even in the same neoplasm model, different phenotypes of TAMs are distributed in different regions53. In the protumoral TME, with the production of M2-favoring cytokines and the lack of proinflammatory factors, M2 TAMs are the dominator which are comprised by various subtypes like M2a, M2b, M2c, and others54. IL-4 and IL-13 as the Th2 cytokines can prejudicially induce macrophage to M2a type. Differentiating to M2b mostly credits to immune complexes, lipopolysaccharide and IL-1, while IL-10 and glucocorticoids can elicit the M2c form of macrophage activation. Different induction factors induce varying M2 type macrophages with various functions: M2a TAMs inhibit Th1 but activate Th2 immune response, and then consequently initiate immune response to resist parasites, yet M2b and M2c both exhibit immunosuppressive effects55. TAMs play a role in producing arginase, chitinase family proteins, macrophage galectin, MMP and promoting vascular endothelial cells secreting cytokines. IL-10 is highly expressed, but the expression of immunosuppressive factors like TGF, IL-12, MHC and reactive nitrogen intermediates is downregulated in TAMs56. M2 TAMs promote tumor progression through a variety of pathways, which will be expounded below in various aspects.
3.1. Favoring tumor growth
By detecting the biochemical indicators of carcinoma, like MIB-1 in breast cancer, Ki67 in endometrial cancer, and the mitotic index of renal cell carcinoma, it is verified that TAMs infiltration is positively correlated with tumor malignancy57, 58, 59, which is also proved by co-culture cell model in vitro60. Although normal macrophages generate cytotoxicity by synthesizing NO through the iNOS pathway with the substrate l-arginine, this pathway is blocked in M2 macrophages, substituted by the synthesis of ornithine and polyamines to promote tumor cell proliferation. And further studies have shown that TAMs express a variety of cytokines including epidermal growth factor (EGF), TGF-β, PDGF, and fibroblast growth factor (bFGF)61 to expand the affected area of cancer.
3.2. Promoting cancer invasion and metastasis
Data display that metastasis cause 90% of cancer deaths worldwide62. Supportive evidences have illustrated that tumor invasion and metastasis is contributed by the interactions among different types of interstitial protumoral cells including TAMs63. Subcutaneous administration of chlorophosphonate-coated liposomes has been exploited to deplete monocytes/macrophages in the hamster model. Scavenging macrophages in peripheral blood and tumor stroma by sodium clodronate effectively inhibited the metastasis of HARA-B cells to the bone and muscle64. In the polyomavirus intermediate T gene (PyMT)-induced mouse breast cancer model, chloropropionate-mediated TAMs exhaustion typically reduced lung metastasis65. Moreover, macrophages always locate in the rupture sites of the basement membrane and the infiltration front of malignant tumors.
ECM degrading enzymes (serine proteases, metalloproteinases, cathepsins, and many other types of proteases) released by TAMs would relax the fibrous connective tissue around the neoplasm. As a result, tumor cells will detach from the solid tumor tissue and get a free ride on the systemic circulation, therefore generating distant metastasis. Besides, TAMs release ECM degrading enzymes to break and deform the collagen fibers tightly surrounding the cancer cells66. As collagen fibers expand outward, anchors of the tumor cells wrapped around blood vessels are more likely to metastasize distantly.
Though being insufficient to simulate the TME in vivo considering the multifarious factors, an in vitro model established on the co-culture of macrophages and breast cancer MCF-7 cells or IGROV-1 ovarian cancer cells is qualified to mimic the mutual effects between macrophages and cancer cells. As a direct result, c-Jun N-terminal kinase and nuclear factor kappa B (NF-κB) signaling pathways are activated in both tumor cell lines67. As soon as the activation of the transcription factors, extracellular MMP inducer and macrophage migration inhibitory factor are accordingly increased, enhancing the production and activity of MMP. The knockdown of macrophage cathepsin or urokinase-type plasminogen activator (uPA) reduced the neoplasm invasion in a murine model of breast cancer68. The osteopontin (OPN, a secreted glycoprotein which promotes the activity and invasiveness of cancer cells) knockout tumor cells cultured in the condition with macrophages or its medium can regain the metastatic potential69, clearly demonstrating that pro-invasive factors like OPN can be derived from macrophages to help with metastasis. Moreover, the co-culture of pancreatic cancer cells with M2 TAMs speeds up the epithelial–mesenchymal transition process70,71. It has been certificated that TAMs produce the inflammatory factor TNF-α and up-regulate the zinc finger protein transcription factor Snail72 which inhibits the expression of E-cadherin so as to promote tumor invasion, recurrence, and metastasis.
3.3. Participating in neovascularization
As an essentials process in solid tumor evolution, angiogenesis is affected by a variety of regulatory factors. As one of the pivotal modulators, TAMs population is positively correlated with microvessel density and the level of VEGF in the carcinoma73. TAMs are also regarded as independent prognostic factors affecting the survival rate. Compared with untreated mice, after ablation of macrophages by clodronate liposome (Clod-Lip), angiogenesis can be effectively prevented60, which illustrated that TAMs are a critical promoter for tumor angiogenesis.
TAMs can secret factors like IL-8, CCL8, bFGF, VEGF, and so forth, which are involved in angiogenesis. Co-incubation of macrophages with MDA-MB-231 breast cancer cells increased expression of angiogenic factors (CXC chemokines and CC chemokines)74. Subsequently, a significant increase of medium-induced angiogenesis was observed in the chicken chorioallantoic membrane. In the in vitro model where HUVECs co-cultured with macrophages and basal cell carcinoma, the levels of VEGF-A and bFGF in the supernatant increased with enhancement of culture medium75. The angiogenic response caused by the implantation of human T47D breast tumorspheres into the back of nude mice was aggravated. Compared to tumorspheres consisting only of tumor cells60, the presence of macrophages in mixed tumorspheres resulted in the up-regulation of VEGF. Another crucial angiogenesis regulator is angiopoietin (Ang) whose mRNA expression can be enhanced by TAMs-derived IL-1 and TNF-α in colon cancer cells, on this account, promoting angiogenesis76.
Macrophage chemoattractants, such as endothelial-monocyte activated polypeptide II (EMAP II), endothelin-2, and VEGF, are concentrated at the sites of neoplastic hypoxia, ischemia, and necrosis. It is exactly the reason why a subpopulation of TAMs accumulate in those areas77. Hypoxia stimulates TAMs to express a large number of pro-angiogenic molecules, e.g., VEGF and MMP-7. Microarray experiments revealed up-regulation of more than thirty angiogenic protein-coding genes in macrophages, like CXCL8, ANG, cyclooxygenase-2, IL-6, IL-7, MMP-1, MMP-9, VEGF-A, and VEGF-C78.
3.4. Immunosuppression
Existence of M2 TAMs in tumor tissues is an important contributing factor for the formation of the immunosuppressive TME. Classically activated macrophages in the early stage of the tumor progression could produce NO, which leads to the extensive death of tumor cells. Nevertheless, as tumors develop, PGE2 and IL-10 secreted by cancer cells will impair M1-mediated immune responses79. M2 TAMs do not express tumor-associated antigens in vitro, nor enhance the anti-cancer effect of T cells and NK cells80. The deletion of these biological functions will directly limit the host's anti-tumor immunity.
The close relationship between the phenotype of macrophages and tumor-derived cytokines has been found. Tumor cells secreting IL-4 and TGF-β can reduce the IL-12 secreting by macrophages and limit the proliferation of NK cells and cytotoxic T cells81. Necrotic tumor cells releasing intracellular components, mainly immunosuppressive factors (IL-10, S1P, etc.), are responsible for the polarization of macrophages to M2 TAMs. As soon as TAMs turned into the M2-like phenotype, the production of NO and iNOS would be reduced82,83. Also, MMP expression induced by TAMs cleaves the Fas ligand between cancer cells so that the cancer is not only less sensitive to chemotherapeutic drugs but also hardly lysed by NK cells and T cells. Alterations of these immune regulatory elements caused by TAMs play a vital role in immunosuppression.
3.5. The communication between TAMs and other cells—extracellular vesicles (EVs)
Extracellular vesicle (EV) as an information transmitter among individual cells is released by all cell types. The studies have turned their points to TAM-derived EVs from their compatriots derived from cancer cells. For antitumor therapy, various EVs produced by TAMs are turned out to be a double-edged sword. MicroRNA (miR) is a significant regulator of gene expression capsuled in EVs. TAM-derived EVs containing miR-146a84 are involved in the repolarization of M2 to M1 type while miR-19a-3p85 and miR-15586 are associated with the polarization of M1 type. Also, TAM-derived EVs are related with the immunosuppressive microenvironment. For example, miR-29a-3p and miR-21–5P suppress STAT3 in CD4+ T cells inducing a Treg/Th17 cell imbalance in epithelial ovarian cancer (EOC) which facilitates the cancer progression87, as well as miR-223 that is delivered to EOC cells by TAM-derived EVs88. And transferring miR-21–5p and miR-155–5p in colorectal cancer cells promotes the metastasis of cancer cells89. In a study, Zheng et al.90 have found that apolipoprotein E (ApoE) is a specific protein in M2 TAM-derived exosomes which promotes the migration of gastric cancer cells by activating PI3K-Akt signaling pathway. However, opposing views were pointed out by Cianciaruso et al.91 that the molecular profiles of M2 TAM-derived EVs are similar to M1 TAMs that present a potency to stimulate the immune response for they detected several positive regulators of the immune response including STING and some proteins related to TLR signaling. Therefore, the effects of TAM-derived EVs need to be further explored.
4. Treatment strategies targeting TAMs via nanovehicle
There are preclinical and clinical data demonstrating that immune checkpoint inhibitors (ICIs) targeting CTLA-4, PD-1, and PD-L1 can relieve the tumor restraint of anti-tumor T cell immunity92. In a recent research, a new drug target, SHP2, showed up to synergize with PD-1 blockade in tumor immunotherapy93. As one of the hottest options in immunotherapy94, ICIs administration tremendously improves the prognosis of a patient with advanced cancer. However, the curative effect of ICIs depends on the T cells activation. Immunosuppressive cells contributing to the immune evasion will lead to the failure of ICIs. Therefore, how to inhibit the activity of these immunosuppressive cells is currently a matter of concern, among which TAM is one of the typical immunosuppressive cells that occupies a significant part of tumor mass95. As described above, macrophages differentiate into TAMs after recruited to tumor sites, promoting tumor progression in different cancer models, which makes TAMs-targeting treatment an alternative option in cancer therapy. In recent years, researches in TAMs-targeting therapy have concentrated on the following aspects: suppression of macrophage recruitment, the repolarization of the M2-like into the M1-like phenotype, and inhibition of TAM survival. The strategies related to TAM targeting are summarized in Table 165,96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, and we list some clinical trials including agents that target TAMs in Supporting Information Table S1.
Table 1.
Strategies related to TAM-targeting.
| Strategy | Passive sensitive factor | Active target | Nanomaterial | Drug | Drug target | Cancer | Ref. |
|---|---|---|---|---|---|---|---|
| Inhibiting the recruitment of macrophages | – | M2pep receptor | Gold nanoparticle | siRNA | VEGF mRNA | Lung cancer | 96 |
| – | – | Nanoparticle | siRNA | CX3CL1 mRNA | Colorectal cancer | 97 | |
| – | – | Cationic nanoparticle | siCCR2 | CCR2 | Orthotopic Murine breast cancer | 98 | |
| – | – | – | Maraviroc | CCR5 | Malignant phyllodes tumor | 99 | |
| Eliminating TAMs | – | – | Liposome | Sodium clodronate | – | Lung cancer | 65 |
| pH | – | Polymer | Anti-IL-10 and anti-IL-10R oligonucleotides | Galactose | Allograft hepatoma murine model | 100 | |
| pH | CD206 | Nanoparticle | Doxorubicin | – | Triple-negative breast cancer | 101 | |
| – | SR-B1 AND M2pep receptor | Nanoparticle | siCSR1R | CSF1R | Melanoma tumors | 102 | |
| Matrix metalloproteinases | Peptide receptor | Nanoparticle | PTX | – | Lung cancer | 103 | |
| Repolarizing TAMs | – | Legumain | Liposome | Hydrozinocurcumin | STAT3 | Breast cancer | 104 |
| Hypoxia | CD206 | Metal oxide nanoparticle | MnO2 and hyaluronic acid | TLR | Breast cancer | 105 | |
| – | RIG-I-like receptor | Lipid calcium phosphate nanoparticle | ppp dsRNA | BCL2 | Pancreatic cancer | 106 | |
| – | – | Cyclodextrin nanoparticle | R848-Ad | TLR7/8 | Murine colon cancer | 107 | |
| pH | CD206 | Lipid-coated calcium phosphonate nanoparticle | miRNA 155 | – | S180 heterotropic tumor | 108 | |
| High redox properties (disulfide bond) | CD206 | Porous hollow iron oxide nanoparticle | 3-methyladenine | P13Kγ | Breast cancer | 109 |
‒Not applicable.
4.1. Inhibition of recruitment of mononuclear macrophages
Blocking up the infiltration of macrophages into tumor sites by modulating chemotactic agents is an underlying strategy. Tumors and chemoattractants act important parts in the recruitment of macrophages. Given that CCL2-CCR2 takes an essential role, many strategies to disrupt the combination of CCL2 and its receptors have been born. Pharmacological inhibition of CCL2 using Bindarit reduced the recruitment of macrophages and inhibited the progression of cancer. In murine models, CCL2 blockers (carlumab, CNTO88) have been proved to successfully suppress the growth of several cancers110,111. Sanford et al.112 applied CCR2 antagonist to block the recruitment of CCR2+ monocytes from the bone marrow to the tumor in a mouse pancreatic cancer model, resulting in suppression of tumor growth and metastasis. In addition to CCL2, CCL5 and CXCL12 also serve as macrophage chemoattractants which may become promising targets to select111. Besides, blocking colony-stimulating factor one receptor (CSF-1R) is another way to control the number of macrophages in tumors113, 114, 115, 116. For example, PLX3397, a potent tyrosine kinase inhibitor of CSF-1R, has been confirmed to enhance the immunotherapy by reducing macrophage recruitment and activating tumor-infiltrating lymphocytes113,117, 118, 119. The clinical activity of an inhibitor of CSF-1R dimerization, RG7155, has been affirmed in patients to significantly eliminate CSF-1R+CD163+ macrophages in diffuse giant cell tumors120. Thus, blocking the mononuclear macrophages infiltrating into tumors is proven to be effective in some oncology researches and a portion of clinical trials.
To sum up, as tumor-derived chemoattractants, such as CCL2, MIP1α, MIP1β, CSF1, CXCL12, SDF-1α, VEGFα, and IL1β, etc. being major factors to recruit macrophages, blocking these pathways would be promising to assist antitumor therapy. However, it is too naïve to completely cut off the recruitment of macrophages to the tumor by controlling one of the numerous chemoattractants. Researchers should be told that there are many clinical trials for inhibitors of chemoattractant receptors whose results are not so satisfactory. In spite of it, once the TAMs invade the tumor, there are other strategies against them.
4.2. Eliminating activated TAMs precisely
The TAMs can be cleared by various methods, e.g., directly inducing their apoptosis by chemical synthesis drugs. Trabectedin (ET-743) is an anti-tumor drug that depletes TAMs through activating the exogenous apoptotic pathway via TRAIL receptors in the soft tissue sarcoma and platinum-sensitive ovarian cancer recurrence121. But for the off-target effects on monocyte/macrophage-mediated host defense, the drugs that specifically targeting TAMs are in urgent need122. Cieslewicz et al.123 developed a specific peptide, M2pep which in priority drawn to M2 macrophages as a target, carrying pro-apoptotic peptides to kill TAMs selectively.
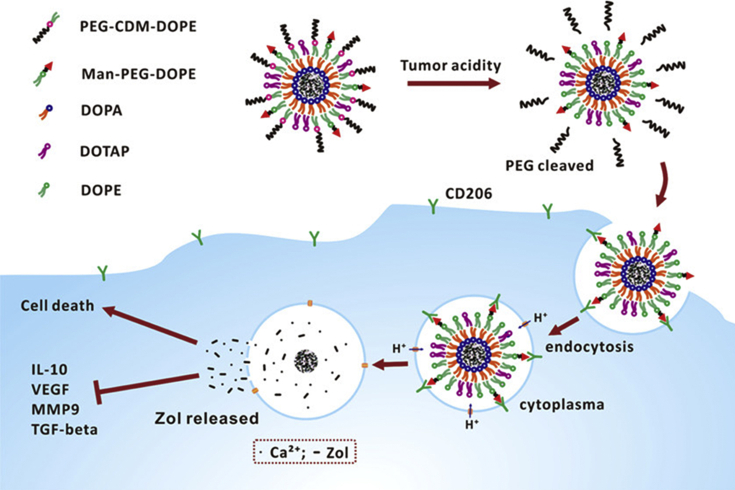
Markers existed on macrophage's surface can serve as effective therapeutic target sites as well. For example, the mannose receptor CD206 is a much representative one124,125. Nanocarriers modified with single-stranded peptides with the high affinity of CD206 are capable of selectively TAMs targeting. As is shown in Fig. 1, mannose conjugated lipid-coated nanoparticles wrapped with extracellular pH-sensitive overcoat specifically induced the apoptosis of TAMs126. Legumin overexpressed in TAMs, a stress protein that belongs to the asparagine endopeptidase family, is also employed as an effective therapeutic target127,128. Another wildly focused surface receptor is the folate receptor (FR), a glycoprotein that has a high affinity with folic acid. Initially, it was found that FR is overexpressed on epithelial-derived cancer cells and was therefore used to deliver anti-tumor drugs specifically. Then in an ovarian cancer model, it was found that the internalization of folate-conjugated liposomes in TAMs was ten times as much as that in tumor cells, and tumor cells accounted for only 50% of total absorption by FR endocytosis129, which makes FR a promising target for TAMs. In exploring new methods for specific oligonucleotides delivery to TAMs, in particular, CpG, anti-IL-10, and anti-IL-10R, Huang et al.130 developed a PEG-histidine modified alginate nanoparticle, using galactosylated cationic dextran to prepare stable nanochains which relied on the high level of macrophage galactose-type lectin. These nanoparticles are capable of binding and protecting oligonucleotides.
Figure 1.
Schematic Illustration of lipid-coated calcium zoledronate nanoparticles (CaZol@) containing conjugated mannose targeting TAMs and inducing their apoptosis. Reprinted with permission from Ref. 127. Copyright © 2019 American Chemical Society.
Although eliminating TAMs is an attractive way to adopt, it will bring a big safety issue—how to avoid the damage of cytotoxic drugs to normal tissues. In addition to TAMs, many tissue-resident macrophages in different organs may also have the same certain receptors. For example, the mannose receptor CD206 is also expressed on the surface of Kupffer cells in the liver. There is no doubt that the cytotoxic drugs circulating in blood will deplete tissue-resident macrophages and greatly affect the microenvironment of normal tissue inducing severe adverse events.
4.3. Repolarizing TAMs
TAMs are highly malleable, of which classical M1 macrophages exert anti-tumor activity. Therefore, repolarization of TAMs appears to be a better means of treating tumors. The key point of immunotherapy is to inhibit local immunosuppression in the TME and re-activate the killing-tumor immune defense. Zhen et al.130 designed a kind of nanocarrier delivering oligonucleotide to TAMs. A CpG oligonucleotide, known as a kind of immunostimulatory agent, was encapsulated in a nanocarrier to facilitate TAMs uptake. The combination of CpG oligonucleotide, anti-IL-10, and anti-IL-10 receptor antisense oligonucleotides showed effective TAMs reprogramming ability in a murine model of liver cancer. Several studies have demonstrated that TLR activation induces the repolarization of macrophages from secreting tumor-promoting cytokines to secreting tumor-killing factors. In tumor-bearing mice, Seya et al.131 activated TLR3/Toll via Polyl (I:C)-IL-1 receptor, which rapidly increased the pro-inflammatory cytokines, thereby accelerating the polarization macrophages. Rolny et al.132 inhibited tumor growth and metastasis by downregulating placental growth factor (PlGF), inducing macrophage repolarization and vascular normalization. Thymosin-α, as an immunomodulator133, can make TAMs differentiate to dendritic cells that produce large numbers of cytokines, such as IL-1 and TNF-α, thereby exerting an anti-tumor immune response. The delivery of Thymosin-α through nanocarriers is considered to be an effective approach to boost the immune reaction in cancer patients. Gene therapy to regulate the polarization of TAMs is also a promising strategy. It has been reported that miR-155, miR-146, and miR-511 play important roles in regulating TAM activation and function134.
Some small molecule drugs have been found to function as repolarization factor of TAMs135. Copper N-(2-hydroxyacetophenone) glycine chelate induces the formation of ROS and triggers p38MARK and ERK1/2 activation pathways, resulting in the up-regulation of intracellular glutathione and down-regulation of TGF-β, which then transfer macrophages to the M1 anti-tumor type135. Similarly, when inhibit the NK-κB signaling pathway, a pivotal part in the progression of tumor inflammation, TAMs would develop into pro-inflammatory cells with tumor cytotoxicity and high expression of IL-12136. 5,6-Dimethylflavone-4-acetic acid (DMXAA) is a small molecule flavonoid initially developed as a vascular blocker137. With further research, it reveals a strong influence on the TME of murine model80, 81, 82, 83 to induce TAMs to secrete inflammatory cytokines and chemokines, and then stimulate anti-tumor CD8 responses138, 139, 140, 141. Fridlender et al.142 illustrated that both individual and combination administration with immunotherapy of DMXAA could improve the anti-tumor effect. The research provides a new idea for the combined application of anti-tumor immunotherapy and macrophage activation. Hydrozinocurcumin (HC), synthetic curcumin analog with better water solubility, stability, cell permeability, and bioavailability than curcumin143 is a transcriptional signal transducer and a potent inhibitor of activator 3 (STAT3) phosphorylation144, which down-regulates a range of STAT3 downstream targets, inhibits cell proliferation, reduces colony formation, and inhibits cell migration and invasion, also induces apoptosis. Zhang et al.104 synthesized a legumain-targeted nanoliposomes carrying HC for the repolarization of TAMs. The results revealed that HC nanoliposomes effectively transformed the macrophage phenotype into M1 type, which effectively inhibited tumor growth, angiogenesis, and metastasis. Mukherjee et al.145 developed a curcumin mixture (TriCurin), including curcumin, resveratrol, epicatechin gallate, for improving the in vivo degradation and low bioavailability of curcumin to effectively repolarize TAMs into M1 phenotype in tumor-bearing mice.
The polarization of TAMs mainly depends on specific cytokines in the TME. One way to polarize TAMs towards M1 type is replenishing Th1 cytokines, including LPS, IFN-γ and GM-CSF, or disrupting the M2 pathway. In most of the researches, directly or indirectly increase of Th1 cytokines has obtained impressive anti-tumor effects. Repolarizing TAMs is the most reliable strategy among the three above introduced strategies. But considering that TAMs polarization is reversible and adjustable, it nearly cannot entirely prevent M1 macrophages repolarize to M2 type with the progression of the tumor. How to maintain the M1 state of TAMs is a hard problem to solve. Therefore, it may be worth a try to combine repolarizing TAMs with other TAM targeting strategies.
4.4. Dual-targeting strategies
Dual targeting treatment is an attractive method with enhanced therapeutic effect. We summarized some research facts about dual targeting as follows.
Considering respective defects of every strategy, combined treatment among those strategies can yet be regarded as an excellent choice, proved by several researches. For example, CD40 agonist treatment can stimulate the repolarization of TAMs towards a profitable situation for treatment146, and so as CSF-1R inhibitor147. Compared with monotherapy, a combination with CSF-1/CSF-1R inhibition which suppresses the myeloid population and CD40 agonist which activate antigen-presenting cells was confirmed to be more effective on anti-tumor ability148. One article pointed out that eliminating TAMs by CSF-1R inhibitor will trigger the deposition of MDSCs in the tumor by increasing CXCL1 which consequently weakens the immune attack. Thus, scientists combined CSF1R inhibitor with a CXCR2 antagonist to block granulocyte infiltration of tumors to show strong anti-tumor effects149. Another research has shown that PRC1 promotes metastatic outgrowth for its capacity of recruiting M2 TAMs and Tregs. Combined PRC1 inhibitor with DCIT (anti-CTLA-4 and anti-PD-1) which is complementary to each other consequently significantly reduces the TAMs and Tregs, suppresses the neoangiogenesis and considerably increases the CD4+ and CD8+ cells150.
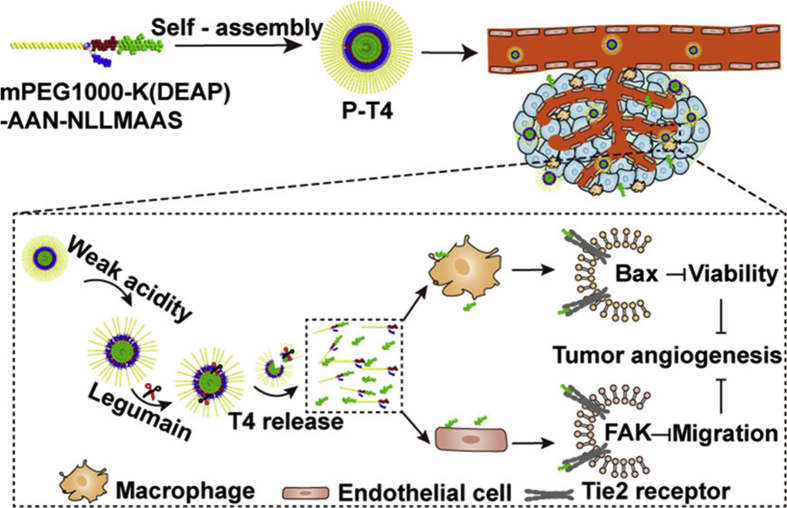
Das et al.106 designed a system involving the lipid calcium phosphate nanoparticles and a short interfering double-stranded RNA (dsRNA) with 5′ triphosphate end specifically silencing Bcl2 and then inducing tumor apoptosis. After the combination of the retinoic acid-inducible gene I (RIG-I)-like receptors and 5′ triphosphate dsRNA (ppp dsRNA), type I interferon is generated, transforming T helper cells response from anti-inflammatory to pro-inflammatory subtype which assists TAM repolarizing. Sun et al.103 integrated a kind of drug-loaded carrier and a versatile polypeptide to develop a novel nanoparticle dual-targeting TME and cancer cells. The polypeptide is composed of a cell-penetrating peptide (TAT) and a new tumor-homing peptide (LinTT1) which can not only target cancer cells and vascular cells but also has a specific affinity to TAMs. Another team applied a Ly6C antibody to modify the surface of porous silicon nanocarriers to realize dual-targeting by means of Ly6C expressed both in tumor-associated endothelial cells, as well as in macrophages within the stroma151. Taking advantage of the angiopoietin Tie2 receptor's co-expression on both macrophages and endothelial cells, Zhang et al.152 designed a dual-responsive amphiphilic peptide to decorate the small peptide T4 which inhibited Tie2 and angiogenesis (Fig. 2). A fusion protein, AS16-Fc consisting of peptide AS16 and IgG Fc fragment with prolonged circulating half-life, presented as a magic bullet that hits both VEGFR and Ang-2 to inhibit the microvessels generation and tumor growth effectively. Also, AS16-Fc stimulates the repolarization of macrophages and reduces the M2-like macrophages in the TME153.
Figure 2.
Schematic illustration to demonstrate the progress of releasing the small peptide T4 from a dual-responsive amphiphilic peptide and targeting to both macrophage and endothelial cell, which consequently inhibit tumor angiogenesis. Reprinted with permission from Ref. 153. Copyright © 2019 American Chemical Society.
A review154 suggests that there is a piece of strong evidence that TAMs are related to hypoxia, cancer progression and enhanced extravasation of macrophages. Researchers have found that hypoxia can polarize TAMs towards M2 type by upregulating TGF-β and M-CSFR which is also the promoter for M2 type polarization. This condition can be partially reversed by HIF inhibitor acriflavine (ACF) which confirmed that overturning the tumor hypoxia microenvironment is helpful to TAMs repolarization155. Thus, a hypoxia-sensitive drug delivery system simultaneously targeting TAMs is worth to develop, which is anticipated to strengthen the efficacy of chemotherapy and overcome drug resistance in solid and metastatic tumors.
We have referred before that every single strategy has its deficiencies. Using one means is hardly to get an inspiring anti-tumor result in clinical treatment. The most key point is to realize the maximum efficiency of the dual-targeting strategy, which requires scientists in various fields to deeply explore the mechanisms of tumor and TME.
4.5. The obstructions of TAM-targeting treatment
The obstructions of TAM-targeting treatment mainly displayed at the quick clearance of nano-drugs, the limitation of the EPR effect, the drug-reservation in TME and the low specificity of active delivery.
The vast majority of administered nanomedicines will be eliminated in blood circulation as a result of metabolism. The macrophages and neutrophils will recognize the protein combined to the nanoparticles and activate phagocytosis of relative immune cells to eliminate nanoparticles from the blood. Besides, the reticuloendothelial system (RES) in the lung, spleen and bone marrow has a potent capacity to clear exogenous nanomedications, as well as the renal elimination of small-size nanoparticle (<5 nm)156. The acknowledged solution at present is modifying polyethylene glycol (PEG) on the surface of nanoparticles which constrains the combination of serum albumin and nanoparticles to prolong the circulation time.
For a long time, the EPR effect has been taken for a theoretical basis of nanomedicines’ passive delivery. But clinical and preclinical facts verified that the often overstated EPR effect is highly heterogeneous. Because the tumor itself is a heterogeneous tissue, vascular permeability does not exist in every part of a tumor. Thus, the extravasation of nanomedicines in blood vessels depending on the EPR effect is not so reliable156,157. Moreover, the EPR effect is more noticeable in some animal models than human beings. Most rodent tumors grow much faster, therefore, the new vessels cannot develop completely and they become more permeable158. However, the EPR effect is still valuable in human drug delivery research. Though human tumors express high levels of VEGF and they are well-vascularized, some of the tumors are still leaky159. And to resolve the limitation, scientists found some pharmacologically active agents like tumor necrosis factor-α (TNF-α) and histamine can improve the extravasation and the penetration of nanomedicines160.
TME as the nest of a tumor, its physicochemical property is much different from the normal internal environment. Thanks to its physical characteristics (hypoxia and low-pH), TME is exactly suitable for tumor proliferation, invasion, and angiogenesis. Moreover, TME can obstacle the nano-drug penetration process banking on its high interstitial fluid pressure, poor blood perfusion and high matrix density161. In practical application, some methods should be intervened to help nanomedicines to get away with the reservation of TME. Anti-angiogenic drugs normalizing the tumor vasculature inhibit the extravasation of blood components and lower tumor interstitial fluid pressure. Additionally, modulating tumor hypoxia and reducing the density of tumor matrix are helpful, e.g., a low dose of a transforming growth factor-β (TGF-β) inhibitor can reduce fibrosis in the TME162,163.
Active targeting strategy utilizes some specific ligands-modifying nanoparticle to combine with the overexpressed receptor on the surface of TAMs with effectively increased cellular uptake of nanomedicine. Nevertheless, the presence of ligand or antibody on the nanoparticle cannot change their biodistribution, targeting ligands increase the possibility of contact of nano-medicines and target cells to passively increase the cellular uptake. An overexpressed certain receptor in target cells would also be presented by other normal cells164. Honestly speaking, it's not accurately clear that nanoparticles are more endocytosed by normal cells or target cells. Also, active delivery is under the influence of tumor heterogeneity and individual difference. Even in the same patient, cancer will present different properties on different stages165. Although the active targeting strategy is still imperfect, its creditable achievements in oncotherapy cannot be ignored.
5. Combination of TAM-targeted therapy and other treatments
Inhibiting the recruitment of TAMs and eliminating the TAMs both show the perfect capacity for fighting the tumor. Also, repolarizing TAMs into M1-like macrophages is an excellent way to choose. The application of the nanomedicines in targeting-TAM strategy dramatically improves the therapeutic effect. We have referred that TAMs in TME present an essential function in improving tumor development and going against the treatment effect of other therapeutic strategies. Combined treatment has been one of the megatrends of neoplastic disease therapy. Therefore, taking advantage of combined therapy will be likely to overcome the resistance caused by the TAMs and maximize the clinical outcome in cancer patients. According to the researches exhibited in this review, TAM-targeted therapy with checkpoint blockers is attractive to coordinate with radiotherapy or chemotherapy, which may obtain unexpected results.
Coordinating targeting therapy with immune modulation will trigger intensive anti-tumor responses. A research showed that erdafitinib and anti-PD-1 combination treatment induced unprecedented changes, including a depletion of immunosuppressive TAMs in the TME166. However, the mechanisms of the specific immune subsets change in the TME remain unclear. Sorafenib, a multi-kinase receptor inhibitor, assisting with an anti-CTLA-4 antibody in a murine cancer model enhanced the anti-tumor ability depending on its direct cytotoxicity to tumor cells and inhibition of MDSCs from expressing immunosuppressive factors167.
Radiation therapy with the advantage of less systemic side effects compared to chemotherapy, has been applied as one of the major anti-cancer therapies. However, influenced by acquired tumor resistance which leads to the relapse and metastasis of tumor, the efficacy of radiotherapy is limited168. It has been shown that in response to tumor irradiation, CSF-1 will be increasingly secreted by tumor cells. Subsequent recruitment of immunosuppressive macrophages resulted in the TME resistant to immune-mediated tumor cell killing. The addition of an anti-PD-L1 antibody to the anti-CSF antibody is necessarily required for tumor inhibition since the expression of PD-L1 on tumor cells increase following radiation, which impedes the robust and lasting anti-tumor immunity169. Although the combination of radiotherapy and ICI leads to an enhanced antineoplastic effect, challenges still exist. Radiotherapy associated pro-immune factors and the radiation-mediated immune suppressors will compete with each other170,171. A series of factors induced by radiation which have the potential to be immune regulators to synergize tumor control with radiotherapy is summarized by Ozpiskin et al168. How to break the balance to incline to the anti-tumor side is a challenging problem remained to be solved in the future.
Recent studies have revealed that macrophages would limit the efficacy of chemotherapy in cancer treatment. To solve this problem, Salvagno et al.172 used CSF-1R blockade to target TAMs in breast cancer which stimulated intratumoral type I interferon signaling, and consequently enhanced the anti-tumor effect of platinum-based chemotherapeutics. Also, Wanderley and colleagues173 provided new and exciting evidence that the TLR4 agonist taxol can restore anti-tumor activities in vivo by reprogramming TAM to an immunocompetent M1-profile, thus contributing to the anti-tumor effect of taxol in combination with ICIs.
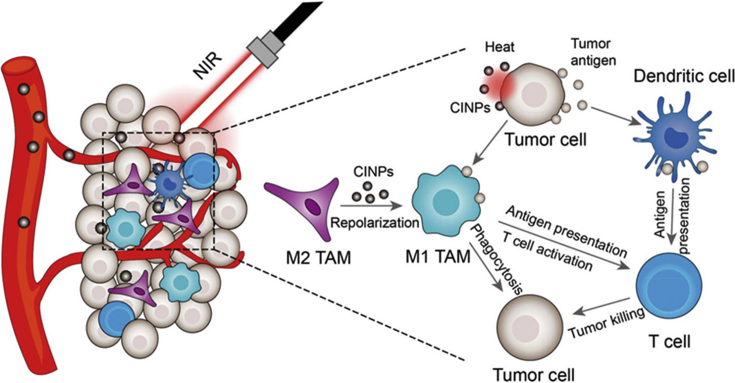
Many other combined treatments are also in studying and show attractive results. For example, in Fig. 3, CINPs can efficiently reprogram M2 TAMs to M1-like phenotype. Besides, with high photothermal effect and cancer cell killing ability, CINPs can be an underlying candidate in photothermal therapy (PTT) for tumor174 to realize the combination of TAM-targeting and PTT treatment.
Figure 3.
Schematic illustration of CINPs for tumorigenesis inhibition by inducting macrophage repolarization and synergizing photothermal therapy. Reprinted with permission from Ref. 175. Copyright © 2019 American Chemical Society.
We believe that there will be more researches supplying reference value to the birth of more effective combination treatments in the future. TAM targeting therapy, which acts as a treatment to disrupt the tumor microenvironment, combining with the treatment directly killing tumor cells can theoretically and practically obtain a better anti-tumor effect.
6. Further perspectives
Cancer has been one of the diseases that seriously affect humor health currently, but the treatments of it still develop slowly. Target therapy is a quite pop choice for cancer treatment considering its low toxicity to normal tissue. However, the results of most target therapies are not satisfactory. Recently, EV as a potential drug carrier has been under exploitation. It possesses multiple advantages over other traditional drug delivery systems, such as biocompatibility for its biological origin, direct fusion with targeted cell membrane175, hydrophilic shell with anti-phagocytosis markers176 and intrinsic cell targeting property. These prominent characters help EVs grab scientists’ attention in the drug delivery system. There have been numerous researches about the application of EVs in tumor drug deliver177, 178, 179, 180. However, several obstacles need to be resolved before clinical application, for example the absent simple approaches of EVs isolation181, the unsatisfactory drug encapsulation rate, and the inefficiency of specific targeting of engineered EVs182. To solve the problem, Piffoux et al.183 have designed a drug delivery system by fusing EVs with liposomes which increased the loading rate as well as retained the targeting efficiency. Sensibility to the regulation of TME and other cell types by the signal transmitted by EVs makes EVs a potential drug-carrier in TAM-targeting drug delivery184.
Although thousands of cancer cells targeting drugs have already been explored, researchers have undervalued TME in tumor progression, which plays an important role as a “fuel pool” supporting the metastasis of tumors. TAM is a major participator in TME which associates with tumor immune escape. According to the above reasons, blocking TAMs function in tumor development has also become one of the therapeutic methods and has achieved some success in clinical trials.
Increasing the specificity of targeting-treatment is always an ongoing issue in the field of drug delivery. In the TAM targeting therapy, the off-target effect is common due to the complex phenotype of macrophages widely distributed in humor body. An in-depth study of the differences among these multifarious macrophages is the key to solve the targeted bottleneck problem of TAM treatment. And exploring the specific characteristics and effects of macrophages in tumors is necessary to maximize the efficacy of targeted TAM treatment. Nowadays many studies have used nanotechnology to improve TAM targeting, but both passive and active targeting have their limitations. If the biological differences between normal macrophages and TAMs can be distinguished, it will be a breakthrough in the TAMs targeting therapy. Multi-sites targeting can reduce the off-target effect to a certain extent in which internal responsiveness and external responsiveness of tumor sites are two major strategies. On the one hand, making use of a variety of different physiological characteristics between the TME and normal tissues, like hypoxia, low pH and high expression of certain enzymes in TME, a series of TME-sensitive targeted preparations can be designed. On the other hand, the use of external stimuli, such as the effects of light, heat, magnetic fields, ultrasound, etc., allows the drugs to be accurately released at the tumor sites and exert its efficacy. Taking advantage of the synergy of multiple targeting strategies, once the delivery system locates at the tumor site, another target strategy will accurately send them to TAMs. "Logistical” delivery will increase the specificity of the drug delivery system. Also, the combination of targeted TAM treatment and other treatments can help to improve the effectiveness of targeting therapy to a certain extent. TAM targeting therapy combined with chemotherapy, radiotherapy, surgery, and immunotherapy have already been explored. But in combination therapy, we believe that different timing of TAM-targeting drug administration will result in different therapeutic effects based on the important and special role of TAM in tumor progression. And in different clinical conditions, the three strategies referred above may exhibit entirely different effects. How to get the greatest utility of targeting TAM therapy needs more exploration further. We consider that TAM targeting therapy still needs to be studied in terms of mechanism, drug delivery, and other aspects. But once the key research results are obtained, it will be a powerful weapon in cancer treatment.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (Nos. 81673022, 81572952 and 81373346), and National Key R&D Program of China (No.2017YFE0102200).
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences
Supporting data to this article can be found online at https://doi.org/10.1016/j.apsb.2020.04.004.
Contributor Information
Qichun Wei, Email: qichun_wei@zju.edu.cn.
Min Han, Email: hanmin@zju.edu.cn.
Author contributions
Qiyao Yang conceived and designed this review. Ningning Guo analyzed the literatures and summarized the results. Yizhou and Jiejian Chen reviewed and edited this review. Qichun Wei and Min Han revised this review. All of the authors have read and approved the final manuscript.
Conflicts of interest
The authors have no conflicts of interest to declare.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Chen D.S., Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 2.Dunn G.P., Bruce A.T., Ikeda H., Old L.J., Schreiber R.D. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg S.A., Mule J.J., Spiess P.J., Reichert C.M., Schwarz S.L. Regression of established pulmonary metastases and subcutaneous tumor mediated by the systemic administration of high-dose recombinant interleukin 2. J Exp Med. 1985;161:1169–1188. doi: 10.1084/jem.161.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quesada J.R.R.J., Manning J.T., Hersh E.M., Gutterman J.U. Alpha interferon for induction of remission in hairy-cell leukemia. N Engl J Med. 1984;310:15–18. doi: 10.1056/NEJM198401053100104. [DOI] [PubMed] [Google Scholar]
- 5.June C., Rosenberg S.A., Sadelain M., Weber J.S. T-cell therapy at the threshold. Nat Biotechnol. 2012;30:611–614. doi: 10.1038/nbt.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leach D.R., Krummel M.F., Allison J.P. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 7.Sanmamed M.F., Chen L. A paradigm shift in cancer immunotherapy: from enhancement to normalization. Cell. 2018;175:313–326. doi: 10.1016/j.cell.2018.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finke J., Ferrone S., Frey A., Mufson A., Ochoa A. Where have all the T cells gone? Mechanisms of immune evasion by tumors. Immunol Today. 1999;20:158–160. doi: 10.1016/s0167-5699(98)01435-2. [DOI] [PubMed] [Google Scholar]
- 9.Drake C.G., Jaffee E., Pardoll D.M. Mechanisms of immune evasion by tumors. Adv Immunol. 2006;90:51–81. doi: 10.1016/S0065-2776(06)90002-9. [DOI] [PubMed] [Google Scholar]
- 10.Grivennikov S.I., Wang K., Mucida D., Stewart C.A., Schnabl B., Jauch D. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 2012;491:254–258. doi: 10.1038/nature11465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kong L., Zhou Y., Bu H., Lv T., Shi Y., Yang J. Deletion of interleukin-6 in monocytes/macrophages suppresses the initiation of hepatocellular carcinoma in mice. J Exp Clin Canc Res. 2016;35 doi: 10.1186/s13046-016-0412-1. 131-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schulz M., Salamero Boix A., Niesel K., Alekseeva T., Sevenich L. Microenvironmental regulation of tumor progression and therapeutic response in brain metastasis. Front Immunol. 2019;10:1713. doi: 10.3389/fimmu.2019.01713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kai K., Vicki P., Zena W. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prenen H., Mazzone M. Tumor-associated macrophages: a short compendium. Cell Mol Life Sci. 2019;76:1447–1458. doi: 10.1007/s00018-018-2997-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu C., Kros J.M., Cheng C., Mustafa D. The contribution of tumor-associated macrophages in glioma neo-angiogenesis and implications for anti-angiogenic strategies. Neuro Oncol. 2017;19:1435–1446. doi: 10.1093/neuonc/nox081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andon F.T., Digifico E., Maeda A., Erreni M., Mantovani A., Alonso M.J. Targeting tumor associated macrophages: the new challenge for nanomedicine. Semin Immunol. 2017;34:103–113. doi: 10.1016/j.smim.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Kwon O.S., Song H.S., Conde J., Kim H.I., Artzi N., Kim J.H. Dual-color emissive upconversion nanocapsules for differential cancer bioimaging in vivo. ACS Nano. 2016;10:1512–1521. doi: 10.1021/acsnano.5b07075. [DOI] [PubMed] [Google Scholar]
- 18.Zhu G., Mei L., Vishwasrao H.D., Jacobson O., Wang Z., Liu Y. Intertwining DNA-RNA nanocapsules loaded with tumor neoantigens as synergistic nanovaccines for cancer immunotherapy. Nat Commun. 2017;8 doi: 10.1038/s41467-017-01386-7. 1482-1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dineshkumar S., Raj A., Srivastava A., Mukherjee S., Pasha S.S., Kachwal V. Facile incorporation of "aggregation-induced emission"-active conjugated polymer into mesoporous silica hollow nanospheres: synthesis, characterization, photophysical studies, and application in bioimaging. ACS Appl Mater Interfaces. 2019;11:31270–31282. doi: 10.1021/acsami.9b07664. [DOI] [PubMed] [Google Scholar]
- 20.Qian Y., Wang Y., Jia F., Wang Z., Yue C., Zhang W. Tumor-microenvironment controlled nanomicelles with AIE property for boosting cancer therapy and apoptosis monitoring. Biomaterials. 2019;188:96–106. doi: 10.1016/j.biomaterials.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Zamani P., Momtazi-Borojeni A.A., Nik M.E., Oskuee R.K., Sahebkar A. Nanoliposomes as the adjuvant delivery systems in cancer immunotherapy. J Cell Physiol. 2018;233:5189–5199. doi: 10.1002/jcp.26361. [DOI] [PubMed] [Google Scholar]
- 22.Wilhelm S., Tavares A.J., Qin D., Ohta S., Audet J., Dvorak H.F. Analysis of nanoparticle delivery to tumours. Nat Rev Mater. 2016;1:16014. [Google Scholar]
- 23.Adair J.H., Parette M.P., Altınoğlu E.İ., Kester M. Nanoparticulate alternatives for drug delivery. ACS Nano. 2010;4:4967–4970. doi: 10.1021/nn102324e. [DOI] [PubMed] [Google Scholar]
- 24.Gordon S., Martinez F.O. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 25.Liu G., Yang H. Modulation of macrophage activation and programming in immunity. J Cell Physiol. 2013;228:502–512. doi: 10.1002/jcp.24157. [DOI] [PubMed] [Google Scholar]
- 26.Siamon G., Taylor P.R. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 27.Dalit S.A., Conrad S.M., Mosser D.M. Monocyte subpopulations and their differentiation patterns during infection. J Leukoc Biol. 2007;82:244–252. doi: 10.1189/jlb.0307191. [DOI] [PubMed] [Google Scholar]
- 28.Denardo D.G., Barreto J.B., Andreu P., Vasquez L., Tawfik D., Kolhatkar N. CD4 T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Canc Cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Allinen M., Beroukhim R., Cai L., Brennan C., Lahti-Domenici J., Huang H. Molecular characterization of the tumor microenvironment in breast cancer. Canc Cell. 2004;6:17–32. doi: 10.1016/j.ccr.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 30.Grivennikov S.I., Greten F.R., Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colvin E.K. Tumor-associated macrophages contribute to tumor progression in ovarian cancer. Front Oncol. 2014;4:137. doi: 10.3389/fonc.2014.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quail D.F., Joyce J.A. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Condeelis J., Pollard J.W. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 34.Yue Z.Q., Liu Y.P., Ruan J.S., Zhou L., Lu Y. Tumor-associated macrophages: a novel potential target for cancer treatment. Chin Med J. 2012;125:3305–3311. [PubMed] [Google Scholar]
- 35.Cramer T., Yamanishi Y., Clausen B.E., Förster I., Pawlinski R., Mackman N. HIF-1α is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhong C., Qiuyan C., Yi L., Guozhi X., Zhihao W., Qinghua Z. Monocyte chemotactic protein 1 promotes lung cancer-induced bone resorptive lesions in vivo. Neoplasia. 2009;11:228–236. doi: 10.1593/neo.81282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Derlindati E., Dei C.A., Montanini B., Spigoni V., Curella V., Aldigeri R. Transcriptomic analysis of human polarized macrophages: more than one role of alternative activation?. PLoS One. 2015;10 doi: 10.1371/journal.pone.0119751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fagone P., Rosa M.D., Palumbo M., Gregorio C.D., Nicoletti F., Malaguarnera L. Modulation of heat shock proteins during macrophage differentiation. Inflamm Res. 2012;61:1131–1139. doi: 10.1007/s00011-012-0506-y. [DOI] [PubMed] [Google Scholar]
- 39.Sica A., Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haixia Z., Xinhua Z., Xuewei C., Ying L., Zunqiong K., Tian T. Isoliquiritigenin, a flavonoid from licorice, blocks M2 macrophage polarization in colitis-associated tumorigenesis through downregulating PGE2 and IL-6. Toxicol Appl Pharmacol. 2014;279:311–321. doi: 10.1016/j.taap.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 41.Josephs D.H., Bax H.J., Karagiannis S.N. Tumour-associated macrophage polarisation and re-education with immunotherapy. Front Biosci. 2015;7:334–351. doi: 10.2741/E735. [DOI] [PubMed] [Google Scholar]
- 42.Mira E., Carmona-Rodriguez L., Tardaguila M., Azcoitia I., Gonzalez-Martin A., Almonacid L. A lovastatin-elicited genetic program inhibits M2 macrophage polarization and enhances T cell infiltration into spontaneous mouse mammary tumors. Oncotarget. 2013;4:2288–2301. doi: 10.18632/oncotarget.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vitale I., Manic G., Coussens L., Kroemer G., Galluzzi L. Macrophages and metabolism in the tumor microenvironment. Cell Metabol. 2019;30:36–50. doi: 10.1016/j.cmet.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 44.Beyer M., Mallmann M.R., Xue J., Staratschek-Jox A., Vorholt D., Krebs W. High-resolution transcriptome of human macrophages. PLoS One. 2012;7 doi: 10.1371/journal.pone.0045466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Müller S.R., Kohanbash G., Liu S.J., Alvarado B., Carrera D., Bhaduri A. Single-cell profiling of human gliomas reveals macrophage ontogeny as a basis for regional differences in macrophage activation in the tumor microenvironment. Genome Biol. 2017;18:234. doi: 10.1186/s13059-017-1362-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kitamura T., Qian B.Z., Pollard J.W. Immune cell promotion of metastasis. Nat Rev Immunol. 2015;15:73–86. doi: 10.1038/nri3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saleh R., Elkord E. Treg-mediated acquired resistance to immune checkpoint inhibitors. Canc Lett. 2019;457:168–179. doi: 10.1016/j.canlet.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 48.Umansky V., Blattner C., Fleming V., Hu X., Gebhardt C., Altevogt P. Myeloid-derived suppressor cells and tumor escape from immune surveillance. Semin Immunopathol. 2017;39:295–305. doi: 10.1007/s00281-016-0597-6. [DOI] [PubMed] [Google Scholar]
- 49.Marzagalli M., Ebelt N.D., Manuel E.R. Unraveling the crosstalk between melanoma and immune cells in the tumor microenvironment. Semin Canc Biol. 2019;59:236–250. doi: 10.1016/j.semcancer.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 50.Rodriguez P.C., Ochoa A.C. Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: mechanisms and therapeutic perspectives. Immunol Rev. 2008;222:180–191. doi: 10.1111/j.1600-065X.2008.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Akalu Y.T., Rothlin C.V., Ghosh S. TAM receptor tyrosine kinases as emerging targets of innate immune checkpoint blockade for cancer therapy. Immunol Rev. 2017;276:165–177. doi: 10.1111/imr.12522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Allavena P., Chieppa M., Bianchi G., Solinas G., Fabbri M., Laskarin G. Engagement of the mannose receptor by tumoral mucins activates an immune suppressive phenotype in human tumor-associated macrophages. Clin Dev Immunol. 2010;2010:547179. doi: 10.1155/2010/547179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hu W., Li X., Zhang C., Yang Y., Jiang J., Wu C. Tumor-associated macrophages in cancers. Clin Transl Oncol. 2015;18:251–258. doi: 10.1007/s12094-015-1373-0. [DOI] [PubMed] [Google Scholar]
- 54.Gensel J.C., Zhang B. Macrophage activation and its role in repair and pathology after spinal cord injury. Brain Res. 2015;1619:1–11. doi: 10.1016/j.brainres.2014.12.045. [DOI] [PubMed] [Google Scholar]
- 55.Solinas G., Germano G., Mantovani A., P.A. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol. 2009;85:1065–1073. doi: 10.1189/jlb.0609385. [DOI] [PubMed] [Google Scholar]
- 56.Thorsten H., Julia W., Frances B., Hagen K., Ninfeng Fiona L., Annette P. Ovarian cancer cells polarize macrophages toward a tumor-associated phenotype. J Immunol. 2006;176:5023–5032. doi: 10.4049/jimmunol.176.8.5023. [DOI] [PubMed] [Google Scholar]
- 57.Yoshihiro K., Hasita H., Koji O., Yukio F., Bing B., Takenobu N. Importance of direct macrophage-tumor cell interaction on progression of human glioma. Canc Sci. 2012;103:2165–2172. doi: 10.1111/cas.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Komohara Y., Niino D., Saito Y., Ohnishi K., Horlad H., Ohshima K. Clinical significance of CD163+ tumor-associated macrophages in patients with adult T-cell leukemia/lymphoma. Canc Sci. 2013;104:945–951. doi: 10.1111/cas.12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hamada I., Kato M., Yamasaki T., Iwabuchi K., Watanabe T., Yamada T. Clinical effects of tumor-associated macrophages and dendritic cells on renal cell carcinoma. Anticancer Res. 2002;22:4281–4284. [PubMed] [Google Scholar]
- 60.Zeisberger S.M., Odermatt B., Marty C., Zehnder-Fjallman A.H., Ballmer-Hofer K., Schwendener R.A. Clodronate-liposome-mediated depletion of tumour-associated macrophages: a new and highly effective antiangiogenic therapy approach. Br J Canc. 2006;95:272–281. doi: 10.1038/sj.bjc.6603240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rigo A., Gottardi M., Zamo A., Mauri P., Bonifacio M., Krampera M. Macrophages may promote cancer growth via a GM-CSF/HB-EGF paracrine loop that is enhanced by CXCL12. Mol Canc. 2010;9:273. doi: 10.1186/1476-4598-9-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gupta G.P., Massague J. Cancer metastasis: building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 63.Guan X. Cancer metastases: challenges and opportunities. Acta Pharm Sin B. 2015;5:402–418. doi: 10.1016/j.apsb.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Polverini P.J., Leibovich S.J. Effect of macrophage depletion on growth and neovascularization of hamster buccal pouch carcinomas. J Oral Pathol. 2010;16:436–441. doi: 10.1111/j.1600-0714.1987.tb00714.x. [DOI] [PubMed] [Google Scholar]
- 65.Koji H., Michihisa Z., Kousuke W., Haruo I., Abbas F., Kimura Y.N. Inhibition of bone and muscle metastases of lung cancer cells by a decrease in the number of monocytes/macrophages. Canc Sci. 2010;99:1595–1602. doi: 10.1111/j.1349-7006.2008.00880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Belli C., Trapani D., Viale G., D'Amico P., Duso B.A., Della Vigna P. Targeting the microenvironment in solid tumors. Canc Treat Rev. 2018;65:22–32. doi: 10.1016/j.ctrv.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 67.Thorsten H., Julia W., Hagen K., Ningfeng Fiona L., Leinster D.A., Kellie C. Macrophages induce invasiveness of epithelial cancer cells via NF-kappa B and JNK. J Immunol. 2005;175:1197–1205. doi: 10.4049/jimmunol.175.2.1197. [DOI] [PubMed] [Google Scholar]
- 68.Vasilena G., Haowei W., Gadea B.B., Tanaya S., Hunter K.E., Garfall A.L. IL-4 induces cathepsin protease activity in tumor-associated macrophages to promote cancer growth and invasion. Genes Dev. 2010;24:241–255. doi: 10.1101/gad.1874010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jiasen C., De-Hua H., Dong-Ming K., Jine Y., Limin Z., Shi-Mei Z. Human macrophages promote the motility and invasiveness of osteopontin-knockdown tumor cells. Canc Res. 2007;67:5141–5147. doi: 10.1158/0008-5472.CAN-06-4763. [DOI] [PubMed] [Google Scholar]
- 70.Liu C.Y., Xu J.Y., Shi X.Y., Huang W., Ruan T.Y., Xie P. M2-polarized tumor-associated macrophages promoted epithelial-mesenchymal transition in pancreatic cancer cells, partially through TLR4/IL-10 signaling pathway. Lab Invest. 2013;93:844–854. doi: 10.1038/labinvest.2013.69. [DOI] [PubMed] [Google Scholar]
- 71.Partecke L.I., Günther C., Hagemann S., Jacobi C., Merkel M., Sendler M. Induction of M2-macrophages by tumour cells and tumour growth promotion by M2-macrophages: a quid pro quo in pancreatic cancer. Pancreatology. 2013;13:508–516. doi: 10.1016/j.pan.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 72.Olmeda D., Jorda M., Peinado H., Fabra A., Cano A. Snail silencing effectively suppresses tumour growth and invasiveness. Oncogene. 2007;26:1862–1874. doi: 10.1038/sj.onc.1209997. [DOI] [PubMed] [Google Scholar]
- 73.Yuri P., Hendri A.Z., Danarto R. Association between tumor-associated macrophages and microvessel density on prostate cancer progression. Prostate Int. 2015;3:93–98. doi: 10.1016/j.prnil.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Joimel U., Gest C., Soria J., Pritchard L.L., Alexandre J., Laurent M. Stimulation of angiogenesis resulting from cooperation between macrophages and MDA-MB-231 breast cancer cells: proposed molecular mechanism and effect of tetrathiomolybdate. BMC Canc. 2010;10:375. doi: 10.1186/1471-2407-10-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tjiu J.W., Chen J.S., Shun C.T., Lin S.J., Liao Y.H., Chu C.Y. Tumor-associated macrophage-induced invasion and angiogenesis of human basal cell carcinoma cells by cyclooxygenase-2 induction. J Invest Dermatol. 2009;129:1016–1025. doi: 10.1038/jid.2008.310. [DOI] [PubMed] [Google Scholar]
- 76.Shchors K., Evan G. Tumor angiogenesis: cause or consequence of cancer? Canc Res. 2007;67:7059–7061. doi: 10.1158/0008-5472.CAN-07-2053. [DOI] [PubMed] [Google Scholar]
- 77.Lewis C.E., Hughes R. Inflammation and breast cancer. Microenvironmental factors regulating macrophage function in breast tumours: hypoxia and angiopoietin-2. Breast Cancer Res. 2007;9:209. doi: 10.1186/bcr1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jonathan Richard W., Harris R.A., Lee S.R., Craigon M.H., Katie B., Toby P. Genetic amplification of the transcriptional response to hypoxia as a novel means of identifying regulators of angiogenesis. Genomics. 2004;83:1–8. doi: 10.1016/s0888-7543(03)00215-5. [DOI] [PubMed] [Google Scholar]
- 79.Ruhrberg C., De P.M. A double agent in cancer: deciphering macrophage roles in human tumors. Nat Med. 2010;16:861–862. doi: 10.1038/nm0810-861. [DOI] [PubMed] [Google Scholar]
- 80.Vasievich E.A., Leaf H. The suppressive tumor microenvironment: a challenge in cancer immunotherapy. Mol Pharm. 2011;8:635–641. doi: 10.1021/mp1004228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shabo I., Svanvik J. Expression of macrophage antigens by tumor cells. Adv Exp Med Biol. 2011;714:141–150. doi: 10.1007/978-94-007-0782-5_7. [DOI] [PubMed] [Google Scholar]
- 82.Weigert A., Brüne B. Nitric oxide, apoptosis and macrophage polarization during tumor progression. Nitric Oxide. 2008;19:95–102. doi: 10.1016/j.niox.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 83.Yasuda H. Solid tumor physiology and hypoxia-induced chemo/radio-resistance: novel strategy for cancer therapy: nitric oxide donor as a therapeutic enhancer. Nitric Oxide. 2008;19:205–216. doi: 10.1016/j.niox.2008.04.026. [DOI] [PubMed] [Google Scholar]
- 84.Li Y., Zhao L., Shi B., Ma S., Shi J. Functions of miR-146a and miR-222 in tumor-associated macrophages in breast cancer. Sci Rep. 2015;5:18648. doi: 10.1038/srep18648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yang J., Zhang Z., Chen C., Liu Y., Si Q., Chuang T.-H. MicroRNA-19a-3p inhibits breast cancer progression and metastasis by inducing macrophage polarization through downregulated expression of Fra-1 proto-oncogene. Oncogene. 2014;33:3014–3023. doi: 10.1038/onc.2013.258. [DOI] [PubMed] [Google Scholar]
- 86.Cai X., Yin Y., Li N., Zhu D., Zhang J., Zhang C.-Y. Re-polarization of tumor-associated macrophages to pro-inflammatory M1 macrophages by microRNA-155. J Mol Cell Biol. 2012;4:341–343. doi: 10.1093/jmcb/mjs044. [DOI] [PubMed] [Google Scholar]
- 87.Zhou J., Li X., Wu X., Zhang T., Zhu Q., Wang X. Exosomes released from tumor-associated macrophages transfer miRNAs that induce a Treg/Th17 cell imbalance in epithelial ovarian cancer. Cancer Immunol Res. 2018;6:1578–1592. doi: 10.1158/2326-6066.CIR-17-0479. [DOI] [PubMed] [Google Scholar]
- 88.Zhu X., Shen H., Yin X., Yang M., Wei H., Chen Q. Macrophages derived exosomes deliver miR-223 to epithelial ovarian cancer cells to elicit a chemoresistant phenotype. J Exp Clin Canc Res. 2019;38:81. doi: 10.1186/s13046-019-1095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lan J., Sun L., Xu F., Liu L., Hu F., Song D. M2 macrophage-derived exosomes promote cell migration and invasion in colon cancer. Canc Res. 2019;79:146–158. doi: 10.1158/0008-5472.CAN-18-0014. [DOI] [PubMed] [Google Scholar]
- 90.Zheng P., Luo Q., Wang W., Li J., Wang T., Wang P. Tumor-associated macrophages-derived exosomes promote the migration of gastric cancer cells by transfer of functional Apolipoprotein E. Cell Death Dis. 2018;9:434. doi: 10.1038/s41419-018-0465-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cianciaruso C., Beltraminelli T., Duval F., Nassiri S., Hamelin R., Mozes A. Molecular profiling and functional analysis of macrophage-derived tumor extracellular vesicles. Cell Rep. 2019;27:3062–3080. doi: 10.1016/j.celrep.2019.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sharma P., Allison J.P. The future of immune checkpoint therapy. Science. 2015;348:56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 93.Zhao M., Guo W., Wu Y., Yang C., Zhong L., Deng G. SHP2 inhibition triggers anti-tumor immunity and synergizes with PD-1 blockade. Acta Pharm Sin B. 2019;9:304–315. doi: 10.1016/j.apsb.2018.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yong S.-B., Chung J.Y., Song Y., Kim J., Ra S., Kim Y.-H. Non-viral nano-immunotherapeutics targeting tumor microenvironmental immune cells. Biomaterials. 2019;219:119401. doi: 10.1016/j.biomaterials.2019.119401. [DOI] [PubMed] [Google Scholar]
- 95.Pollard J.W. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Canc. 2004;4:71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 96.Conde J., Bao C., Tan Y., Cui D., Edelman E.R., Azevedo H.S. Dual targeted immunotherapy via in vivo delivery of biohybrid RNAi-peptide nanoparticles to tumour-associated macrophages and cancer cells. Adv Funct Mater. 2015;25:4183–4194. doi: 10.1002/adfm.201501283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jung K., Heishi T., Khan O.F., Kowalski P.S., Dai F. Ly6Clo monocytes drive immunosuppression and confer resistance to anti-VEGFR2 cancer therapy. J Clin Invest. 2017;127:3039–3051. doi: 10.1172/JCI93182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shen S., Zhang Y., Chen K.-G., Luo Y.-L., Wang J. Cationic polymeric nanoparticle delivering CCR2 siRNA to inflammatory monocytes for tumor microenvironment modification and cancer therapy. Mol Pharm. 2018;15:3642–3653. doi: 10.1021/acs.molpharmaceut.7b00997. [DOI] [PubMed] [Google Scholar]
- 99.Nie Y., Huang H., Guo M., Chen J., Wu W., Li W. Breast phyllodes tumors recruit and repolarize tumor-associated macrophages via secreting CCL5 to promote malignant progression, which can be inhibited by CCR5 inhibition therapy. Clin Canc Res. 2019;25:3873–3886. doi: 10.1158/1078-0432.CCR-18-3421. [DOI] [PubMed] [Google Scholar]
- 100.Huang Z., Zhang Z., Jiang Y., Zhang D., Zhang J. Targeted delivery of oligonucleotides into tumor-associated macrophages for cancer immunotherapy. J Control Release. 2011;158:286–292. doi: 10.1016/j.jconrel.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 101.Niu M., Valdes S., Naguib Y.W., Hursting S.D., Cui Z. Tumor-associated macrophage-mediated targeted therapy of triple-negative breast cancer. Mol Pharm. 2016;13:1833–1842. doi: 10.1021/acs.molpharmaceut.5b00987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Qian Y., Qiao S., Dai Y., Xu G., Dai B., Lu L. Molecular-targeted immunotherapeutic strategy for melanoma via dual-targeting nanoparticles delivering small interfering RNA to tumor-associated macrophages. ACS Nano. 2017;11:9536–9549. doi: 10.1021/acsnano.7b05465. [DOI] [PubMed] [Google Scholar]
- 103.Sun Z., Li R., Ji S., You P., Man W. Matrix metalloproteinase cleavable nanoparticles for tumor microenvironment and tumor cell dual-targeting drug delivery. ACS Appl Mater Interfaces. 2017;9:40614–40627. doi: 10.1021/acsami.7b11614. [DOI] [PubMed] [Google Scholar]
- 104.Zhang X., Tian W., Cai X., Wang X., Dang W., Tang H. Hydrazinocurcumin encapsuled nanoparticles “re-educate” tumor-associated macrophages and exhibit anti-tumor effects on breast cancer following STAT3 suppression. PLoS One. 2013;8 doi: 10.1371/journal.pone.0065896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Song M., Liu T., Shi C., Zhang X., Chen X. Bioconjugated manganese dioxide nanoparticles enhance chemotherapy response by priming tumor-associated macrophages toward M1-like phenotype and attenuating tumor hypoxia. ACS Nano. 2016;10:633–647. doi: 10.1021/acsnano.5b06779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Das M., Shen L., Liu Q., Goodwin T.J., Huang L. Nanoparticle delivery of RIG-I agonist enables effective and safe adjuvant therapy in pancreatic cancer. Mol Ther. 2019;27:507–517. doi: 10.1016/j.ymthe.2018.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rodell C.B., Ahmed M.S., Garris C.S., Pittet M.J., Weissleder R. Development of adamantane-conjugated TLR7/8 agonists for supramolecular delivery and cancer immunotherapy. Theranostics. 2019;9:8426–8436. doi: 10.7150/thno.35434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zang X., Zhang X., Zhao X., Hu H., Qiao M., Deng Y. Targeted delivery of miRNA 155 to tumor associated macrophages for tumor immunotherapy. Mol Pharm. 2019;16:1714–1722. doi: 10.1021/acs.molpharmaceut.9b00065. [DOI] [PubMed] [Google Scholar]
- 109.Li K., Lu L., Xue C., Liu J., He Y., Zhou J. Polarization of tumor-associated macrophage phenotype via porous hollow iron nanoparticles for tumor immunotherapy in vivo. Nanoscale. 2020;12:130–144. doi: 10.1039/c9nr06505a. [DOI] [PubMed] [Google Scholar]
- 110.Pienta Kenneth J., Machiels Jean-Pascal, Schrijvers Dirk. Phase 2 study of carlumab (CNTO 888), a human monoclonal antibody;against CC-chemokine ligand 2 (CCL2), in metastatic castration-resistant prostate cancer. Invest N Drugs. 2013;31:760–768. doi: 10.1007/s10637-012-9869-8. [DOI] [PubMed] [Google Scholar]
- 111.Sandhu Shahneen K., Fong Peter C., Messiou Christina. A first-in-human, first-in-class, phase I study of carlumab (CNTO 888), a human monoclonal antibody against CC-chemokine ligand 2 in patients with solid tumors. Canc Chemother Pharmacol. 2013;71:1041–1050. doi: 10.1007/s00280-013-2099-8. [DOI] [PubMed] [Google Scholar]
- 112.Sanford D.E., Belt B.A., Panni R.Z., Mayer A., Deshpande A.D., Carpenter D. Inflammatory monocyte mobilization decreases patient survival in pancreatic cancer: a role for targeting the CCL2/CCR2 axis. Clin Canc Res. 2013;19:3404–3415. doi: 10.1158/1078-0432.CCR-13-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Prada C.E., Jousma E., Rizvi T.A., Wu J., Dunn R.S., Mayes D.A. Neurofibroma-associated macrophages play roles in tumor growth and response to pharmacological inhibition. Acta Neuropathol. 2013;125:159–168. doi: 10.1007/s00401-012-1056-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Strachan D.C., Ruffell B., Oei Y., Bissell M.J., Coussens L.M., Pryer N. CSF1R inhibition delays cervical and mammary tumor growth in murine models by attenuating the turnover of tumor-associated macrophages and enhancing infiltration by CD8+ T cells. OncoImmunology. 2013;2 doi: 10.4161/onci.26968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Weizman N., Krelin Y., Shabtay-Orbach A., Amit M., Binenbaum Y., Wong R.J. Macrophages mediate gemcitabine resistance of pancreatic adenocarcinoma by upregulating cytidine deaminase. Oncogene. 2014;33:3812–3819. doi: 10.1038/onc.2013.357. [DOI] [PubMed] [Google Scholar]
- 116.Pyonteck S.M., Akkari L., Schuhmacher A.J., Bowman R.L., Sevenich L., Quail D.F. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013;19:1264–1272. doi: 10.1038/nm.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sluijter M., van der Sluis T.C., van der Velden P.A., Versluis M., West B.L., van der Burg S.H. Inhibition of CSF-1R supports T-cell mediated melanoma therapy. PLoS One. 2014;9 doi: 10.1371/journal.pone.0104230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kim T.S., Cavnar M.J., Cohen N.A., Sorenson E.C., Greer J.B., Seifert A.M. Increased KIT inhibition enhances therapeutic efficacy in gastrointestinal stromal tumor. Clin Canc Res. 2014;20:2350–2362. doi: 10.1158/1078-0432.CCR-13-3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Patwardhan P.P., Surriga O., Beckman M.J., de Stanchina E., Dematteo R.P., Tap W.D. Sustained inhibition of receptor tyrosine kinases and macrophage depletion by PLX3397 and rapamycin as a potential new approach for the treatment of MPNSTs. Clin Canc Res. 2014;20:3146–3158. doi: 10.1158/1078-0432.CCR-13-2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ries C.H., Cannarile M.A., Hoves S., Benz J., Wartha K., Runza V. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Canc Cell. 2014;25:846–859. doi: 10.1016/j.ccr.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 121.Guan Y., Sakai R., Rinehart K.L., Wang A.H. Molecular and crystal structures of ecteinascidins: potent antitumor compounds from the Caribbean tunicate Ecteinascidia turbinata. J Biomol Struct Dyn. 1993;10:793–818. [PubMed] [Google Scholar]
- 122.Giovanni G., Roberta F., Cristina B., Achille A., Samantha P., Manuela L. Role of macrophage targeting in the antitumor activity of trabectedin. Canc Cell. 2013;23:249–262. doi: 10.1016/j.ccr.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 123.Cieslewicz M., Tang J., Yu J.L., Cao H., Zavaljevski M., Motoyama K. Targeted delivery of proapoptotic peptides to tumor-associated macrophages improves survival. Proc Natl Acad Sci U S A. 2013;110:15919–15924. doi: 10.1073/pnas.1312197110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Perrie Y., Frederik P.M., Gregoriadis G. Liposome-mediated DNA vaccination: the effect of vesicle composition. Vaccine. 2001;19:3301–3310. doi: 10.1016/s0264-410x(00)00432-1. [DOI] [PubMed] [Google Scholar]
- 125.Yoshiyuki H., Shigeru K., Sachiko S., Fumiyoshi Y., Mitsuru H. Enhancement of immune responses by DNA vaccination through targeted gene delivery using mannosylated cationic liposome formulations following intravenous administration in mice. Biochem Biophys Res Commun. 2004;317:992–999. doi: 10.1016/j.bbrc.2004.03.141. [DOI] [PubMed] [Google Scholar]
- 126.Zang X., Zhang X., Hu H., Qiao M., Zhao X., Deng Y. Targeted delivery of zoledronate to tumor-associated macrophages for cancer immunotherapy. Mol Pharm. 2019;16:2249–2258. doi: 10.1021/acs.molpharmaceut.9b00261. [DOI] [PubMed] [Google Scholar]
- 127.Cheng L., Chengzao S., Haining H., Kim J., Thomas E. Overexpression of legumain in tumors is significant for invasion/metastasis and a candidate enzymatic target for prodrug therapy. Canc Res. 2003;63:2957–2964. [PubMed] [Google Scholar]
- 128.Murthy R.V., Arbman G., Gao J., Roodman G.D., Sun X.F. Legumain expression in relation to clinicopathologic and biological variables in colorectal cancer. Clin Canc Res. 2005;11:2293–2299. doi: 10.1158/1078-0432.CCR-04-1642. [DOI] [PubMed] [Google Scholar]
- 129.Mary Jo T., Waters D.J., Low P.S. Folate-conjugated liposomes preferentially target macrophages associated with ovarian carcinoma. Canc Lett. 2004;213:165–172. doi: 10.1016/j.canlet.2003.12.028. [DOI] [PubMed] [Google Scholar]
- 130.Zhen H., Zhengping Z., Yucui J., Dachuan Z., Jiangning C., Lei D. Targeted delivery of oligonucleotides into tumor-associated macrophages for cancer immunotherapy. J Control Release. 2012;77:286–292. doi: 10.1016/j.jconrel.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 131.Seya T., Shime H., Matsumoto M. TAMable tumor-associated macrophages in response to innate RNA sensing. OncoImmunology. 2012;1:1000–1001. doi: 10.4161/onci.19894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rolny C., Mazzone M., Tugues S., Laoui D., Johansson I., Coulon C. HRG inhibits tumor growth and metastasis by inducing macrophage polarization and vessel normalization through downregulation of PLGF. Canc Cell. 2011;19:31–44. doi: 10.1016/j.ccr.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 133.Shrivastava P., Singh S.M., Singh N. Effect of thymosin alpha 1 on the antitumor activity of tumor-associated macrophage-derived dendritic cells. J Biomed Sci. 2004;11:623–630. doi: 10.1007/BF02256128. [DOI] [PubMed] [Google Scholar]
- 134.Mario Leonardo S., Martin E., Michele D.P., Pittet M.J. MicroRNA-mediated control of macrophages and its implications for cancer. Trends Immunol. 2013;34:350–359. doi: 10.1016/j.it.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chakraborty P., Chatterjee S., Ganguly A., Saha P., Adhikary A., Das T. Reprogramming of TAM toward proimmunogenic type through regulation of MAP kinases using a redox-active copper chelate. J Leukoc Biol. 2012;91:609–619. doi: 10.1189/jlb.0611287. [DOI] [PubMed] [Google Scholar]
- 136.Thorsten H., Toby L., Iain M.N., Charles K.A., Hagen K., Thompson R.G. Re-educating" tumor-associated macrophages by targeting NF-kappaB. J Exp Med. 2008;205:1261–1268. doi: 10.1084/jem.20080108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zwi L.J., Baguley B.C., Gavin J.B., Wilson W.R. Necrosis in non-tumour tissues caused by flavone acetic acid and 5,6-dimethyl xanthenone acetic acid. Br J Canc. 1990;62:932–934. doi: 10.1038/bjc.1990.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ching L.M., Young H.A., Eberly K., Yu C.R. Induction of STAT and NFκB activation by the antitumor agents 5,6-dimethylxanthenone-4-acetic acid and flavone acetic acid in a murine macrophage cell line. Biochem Pharmacol. 1999;58:1173–1181. doi: 10.1016/s0006-2952(99)00194-x. [DOI] [PubMed] [Google Scholar]
- 139.Jassar A.S., Eiji S., Veena K., Jing S., Silverberg M.B., Lumei C. Activation of tumor-associated macrophages by the vascular disrupting agent 5,6-dimethylxanthenone-4-acetic acid induces an effective CD8+ T-cell-mediated antitumor immune response in murine models of lung cancer and mesothelioma. Canc Res. 2005;65:11752–11761. doi: 10.1158/0008-5472.CAN-05-1658. [DOI] [PubMed] [Google Scholar]
- 140.Roberts Z.J., Goutagny N., Perera P.Y., Kato H., Kumar H., Kawai T. The chemotherapeutic agent DMXAA potently and specifically activates the TBK1-IRF-3 signaling axis. J Exp Med. 2007;204:1559–1569. doi: 10.1084/jem.20061845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Larosa D.F., Veena K., Jing S., Guanjun C., Arminder J., Aaron B. The vascular disrupting agent, DMXAA, directly activates dendritic cells through a MyD88-independent mechanism and generates antitumor cytotoxic T lymphocytes. Canc Res. 2007;67:7011–7019. doi: 10.1158/0008-5472.CAN-06-3757. [DOI] [PubMed] [Google Scholar]
- 142.Fridlender Z.G., Jassar A., Mishalian I., Wang L.C., Kapoor V., Cheng G. Using macrophage activation to augment immunotherapy of established tumours. Br J Canc. 2013;108:1288–1297. doi: 10.1038/bjc.2013.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rathore R., Jain J.P., Srivastava A., Jachak S.M., Kumar N. Simultaneous determination of hydrazinocurcumin and phenol red in samples from rat intestinal permeability studies: HPLC method development and validation. J Pharmaceut Biomed Anal. 2008;46:374–380. doi: 10.1016/j.jpba.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 144.Wang X., Zhang Y., Zhang X., Tian W., Feng W., Chen T. The curcumin analogue hydrazinocurcumin exhibits potent suppressive activity on carcinogenicity of breast cancer cells via STAT3 inhibition. Int J Oncol. 2012;40:1189–1195. doi: 10.3892/ijo.2011.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mukherjee S., Hussaini R., White R., Atwi D., Fried A., Sampat S. TriCurin, a synergistic formulation of curcumin, resveratrol, and epicatechin gallate, repolarizes tumor-associated macrophages and triggers an immune response to cause suppression of HPV+ tumors. Cancer Immunol Immunother. 2018;67:761–774. doi: 10.1007/s00262-018-2130-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Quaranta V., Schmid M.C. Macrophage-mediated subversion of anti-tumour immunity. Cells. 2019;8:747. doi: 10.3390/cells8070747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Schmid M.C., Quaranta V., Rainer C., Nielsen S.R., Raymant M.L., Ahmed M.S. Macrophage-derived granulin drives resistance to immune checkpoint inhibition in metastatic pancreatic cancer. Canc Res. 2018;78:4253–4269. doi: 10.1158/0008-5472.CAN-17-3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wiehagen K.R., Girgis N.M., Yamada D.H., Smith A.A., Chan S.R., Grewal I.S. Combination of CD40 agonism and CSF-1R blockade reconditions tumor-associated macrophages and drives potent antitumor immunity. Cancer Immunol Res. 2017;5:1109–1121. doi: 10.1158/2326-6066.CIR-17-0258. [DOI] [PubMed] [Google Scholar]
- 149.Kumar V., Donthireddy L., Marvel D., Condamine T., Wang F., Lavilla-Alonso S. Cancer-associated fibroblasts neutralize the anti-tumor effect of CSF1 receptor blockade by inducing PMN-MDSC infiltration of tumors. Canc Cell. 2017;32:654–668. doi: 10.1016/j.ccell.2017.10.005. e655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Su W., Han H.H., Wang Y., Zhang B., Zhou B., Cheng Y. The polycomb repressor complex 1 drives double-negative prostate cancer metastasis by coordinating stemness and immune suppression. Canc Cell. 2019;36:139–155. doi: 10.1016/j.ccell.2019.06.009. e110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yokoi K., Godin B., Oborn C.J., Alexander J.F., Liu X., Fidler I.J. Porous silicon nanocarriers for dual targeting tumor associated endothelial cells and macrophages in stroma of orthotopic human pancreatic cancers. Canc Lett. 2013;334:319–327. doi: 10.1016/j.canlet.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhang L., Qi Y., Min H., Ni C., Wang F., Wang B. Cooperatively responsive peptide nanotherapeutic that regulates angiopoietin receptor Tie2 activity in tumor microenvironment to prevent breast tumor relapse after chemotherapy. ACS Nano. 2019;13:5091–5102. doi: 10.1021/acsnano.8b08142. [DOI] [PubMed] [Google Scholar]
- 153.Zhu X., Yang J., Gao Y., Wu C., Yi L., Li G. The dual effects of a novel peptibody on angiogenesis inhibition and M2 macrophage polarization on sarcoma. Canc Lett. 2018;416:1–10. doi: 10.1016/j.canlet.2017.10.043. [DOI] [PubMed] [Google Scholar]
- 154.Silva V.L., Al-Jamal W.T. Exploiting the cancer niche: tumor-associated macrophages and hypoxia as promising synergistic targets for nano-based therapy. J Control Release. 2017;253:82–96. doi: 10.1016/j.jconrel.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 155.Guo X., Xue H., Shao Q., Wang J., Guo X., Chen X. Hypoxia promotes glioma-associated macrophage infiltration via periostin and subsequent M2 polarization by upregulating TGF-beta and M-CSFR. Oncotarget. 2016;7:80521–80542. doi: 10.18632/oncotarget.11825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jain R.K., Stylianopoulos T. Delivering nanomedicine to solid tumors. Nat Rev Clin Oncol. 2010;7:653–664. doi: 10.1038/nrclinonc.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bae Y.H., Park K. Targeted drug delivery to tumors: myths, reality and possibility. J Control Release. 2011;153:198–205. doi: 10.1016/j.jconrel.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lammers T., Kiessling F., Hennink W.E., Storm G. Drug targeting to tumors: principles, pitfalls and (pre-) clinical progress. J Control Release. 2012;161:175–187. doi: 10.1016/j.jconrel.2011.09.063. [DOI] [PubMed] [Google Scholar]
- 159.Stiver S., Dvorak H. Vascular permeability factor/vascular endothelial growth factor (VPF/VEGF) J Clin Ligand Assay. 2000;23:193–205. [Google Scholar]
- 160.Seki T., Carroll F., Illingworth S., Green N., Cawood R., Bachtarzi H. Tumour necrosis factor-alpha increases extravasation of virus particles into tumour tissue by activating the Rho A/Rho kinase pathway. J Control Release. 2011;156:381–389. doi: 10.1016/j.jconrel.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 161.Gao H. Shaping tumor microenvironment for improving nanoparticle delivery. Curr Drug Metabol. 2016;17:731–736. doi: 10.2174/1389200217666160630203600. [DOI] [PubMed] [Google Scholar]
- 162.Yang S., Gao H. Nanoparticles for modulating tumor microenvironment to improve drug delivery and tumor therapy. Pharmacol Res. 2017;126:97–108. doi: 10.1016/j.phrs.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 163.Kano M.R., Bae Y., Iwata C., Morishita Y., Yashiro M., Oka M. Improvement of cancer-targeting therapy, using nanocarriers for intractable solid tumors by inhibition of TGF-beta signaling. Proc Natl Acad Sci U S A. 2007;104:3460–3465. doi: 10.1073/pnas.0611660104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kwon I.K., Lee S.C., Han B., Park K. Analysis on the current status of targeted drug delivery to tumors. J Control Release. 2012;164:108–114. doi: 10.1016/j.jconrel.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Paulos M.C. Ligand binding and kinetics offolate receptor recycling in vivo: impact on receptor-mediated drug delivery. Mol Pharmacol. 2004;66:1406–1414. doi: 10.1124/mol.104.003723. [DOI] [PubMed] [Google Scholar]
- 166.Palakurthi S., Kuraguchi M., Zacharek S.J., Zudaire E., Huang W., Bonal D.M. The combined effect of FGFR inhibition and PD-1 blockade promotes tumor-intrinsic induction of antitumor immunity. Cancer Immunol Res. 2019;7:1457–1471. doi: 10.1158/2326-6066.CIR-18-0595. [DOI] [PubMed] [Google Scholar]
- 167.Motoshima T., Komohara Y., Horlad H., Takeuchi A., Maeda Y., Tanoue K. Sorafenib enhances the antitumor effects of anti-CTLA-4 antibody in a murine cancer model by inhibiting myeloid-derived suppressor cells. Oncol Rep. 2015;33:2947–2953. doi: 10.3892/or.2015.3893. [DOI] [PubMed] [Google Scholar]
- 168.Ozpiskin O.M., Zhang L., Li J.J. Immune targets in the tumor microenvironment treated by radiotherapy. Theranostics. 2019;9:1215–1231. doi: 10.7150/thno.32648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Jones K.I. Radiation combined with macrophage depletion promotes adaptive immunity and potentiates checkpoint blockade. EMBO Mol Med. 2018;10 doi: 10.15252/emmm.201809342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Pitti R.M., Marsters S.A., Lawrence D.A., Roy M., Kischkel F.C., Dowd P. Genomic amplification of a decoy receptor for Fas ligand in lung and colon cancer. Nature. 1998;396:699–703. doi: 10.1038/25387. [DOI] [PubMed] [Google Scholar]
- 171.Chiang C.S., Fu S.Y., Wang S.C., Yu C.F., Chen F.H., Lin C.M. Irradiation promotes an M2 macrophage phenotype in tumor hypoxia. Front Oncol. 2012;2 doi: 10.3389/fonc.2012.00089. 89-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Salvagno C., Ciampricotti M., Tuit S., Hau C.S., Visser K.E.D. Therapeutic targeting of macrophages enhances chemotherapy efficacy by unleashing type I interferon response. Nat Cell Biol. 2019;21:1–11. doi: 10.1038/s41556-019-0298-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Garassino M.C., Torri V., Colombo M.P., Sica A. Choosing the best chemotherapy agent to boost immune checkpoint inhibition activity. Canc Res. 2018;78:5729–5730. doi: 10.1158/0008-5472.CAN-18-2245. [DOI] [PubMed] [Google Scholar]
- 174.Deng R.H., Zou M.Z., Zheng D., Peng S.Y., Liu W., Bai X.F. Nanoparticles from cuttlefish Ink inhibit tumor growth by synergizing immunotherapy and photothermal therapy. ACS Nano. 2019;13:8618–8629. doi: 10.1021/acsnano.9b02993. [DOI] [PubMed] [Google Scholar]
- 175.Tatischeff I., Alfsen A. A new biological strategy for drug delivery: eucaryotic cell-derived nanovesicles. J Biomaterials Nanobiotechnol. 2011;2:494–499. [Google Scholar]
- 176.Chauhan S., Danielson S., Clements V., Edwards N., Ostrand-Rosenberg S., Fenselau C. Surface glycoproteins of exosomes shed by myeloid-derived suppressor cells contribute to function. J Proteome Res. 2017;16:238–246. doi: 10.1021/acs.jproteome.6b00811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Alvarez-Erviti L., Seow Y., Yin H., Betts C., Lakhal S., Wood M.J.A. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29:341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 178.Kooijmans S.A.A., Aleza C.G., Roffler S.R., van Solinge W.W., Vader P., Schiffelers R.M. Display of GPI-anchored anti-EGFR nanobodies on extracellular vesicles promotes tumour cell targeting. J Extracell Vesicles. 2016;5:31053. doi: 10.3402/jev.v5.31053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Guo M., Wu F., Hu G., Chen L., Xu J., Xu P. Autologous tumor cell-derived microparticle-based targeted chemotherapy in lung cancer patients with malignant pleural effusion. Sci Transl Med. 2019;11 doi: 10.1126/scitranslmed.aat5690. [DOI] [PubMed] [Google Scholar]
- 180.Kooijmans S.A.A., Fliervoet L.A.L., van der Meel R., Fens M., Heijnen H.F.G., van Bergen En, Henegouwen P.M.P. PEGylated and targeted extracellular vesicles display enhanced cell specificity and circulation time. J Control Release. 2016;224:77–85. doi: 10.1016/j.jconrel.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 181.Jong OGd, Kooijmans S.A.A., Murphy D.E., Jiang L., Schiffelers R.M. Drug delivery with extracellular vesicles: from imagination to innovation. Acc Chem Res. 2019;52:1761–1770. doi: 10.1021/acs.accounts.9b00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Vader P., Mol E.A., Pasterkamp G., Schiffelers R.M. Extracellular vesicles for drug delivery. Adv Drug Deliv Rev. 2016;106:148–156. doi: 10.1016/j.addr.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 183.Piffoux M., Silva A.K.A., Wilhelm C., Gazeau F., Tareste D. Modification of extracellular vesicles by fusion with liposomes for the design of personalized biogenic drug delivery systems. ACS Nano. 2018;12:6830–6842. doi: 10.1021/acsnano.8b02053. [DOI] [PubMed] [Google Scholar]
- 184.Syed N.S., Frank A.-C., Raue R., Brüne B. MicroRNA—a tumor trojan horse for tumor-associated macrophages. Cells. 2019;8:1482. doi: 10.3390/cells8121482. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.