Abstract
The complex tumor microenvironment is a most important factor in cancer development. The biological microenvironment is composed of a variety of barriers including the extracellular matrix and associated cells such as endothelia cells, pericytes, and cancer-associated fibroblasts. Different strategies can be utilized to enhance nanoparticle-based drug delivery and distribution into tumor tissues addressing the extracellular matrix or cellular components. In addition to the biological microenvironment, the immunological conditions around the tumor tissue can be very complicated and cancer cells have various ways of evading immune surveillance. Nanoparticle drug delivery systems can enhance cancer immunotherapy by tuning the immunological response and memory of various immune cells such as T cells, B cells, macrophages, and dendritic cells. In this review, the main components in the tumor biological and immunological environment are discussed. The focus is on recent advances in nanoparticle-based drug delivery systems towards targets within the tumor microenvironment to improve cancer chemotherapy and immunotherapy.
Keywords: Tumor microenvironment, Nanoparticles, Drug delivery, Tumor immunology, Tumor treatment, Tumor targeting, Immunotherapy, Combinational therapy
Graphical abstract
The main components in the tumor biological and immunological environment are discussed. The focus is on recent advances in nanoparticle-based drug delivery systems towards targets within the tumor microenvironment to improve cancer chemotherapy and immunotherapy.
1. Introduction
Cancer is a leading cause of death globally. Due to the complexity of tumors, the development of effective anti-cancer therapies remains challenging. Tumor tissue includes not only malignant cells but also other cells that are recruited to the tumor area. Together these cells create the tumor microenvironment1. In the tumor microenvironment, cancer cells are usually surrounded by cellular and non-cellular components, which consist of extracellular matrix, immune cells and stromal cells2. The tumor microenvironment supports tumor progression, drives therapeutic response or resistance, and creates immunological tolerance. It is heterogeneous and complex, in terms of composition, spatial arrangement, and other features such as receptor expression levels3, 4, 5. For example, in glioblastoma, epidermal growth factor receptor (EGFR) and platelet-derived growth factor receptor (PDGFR) are over expressed in cancer cells located in different proportions of the tumor. Cancer cells with EGFR are present in poorly vascularized areas, while cancer cells with PDGFR are present near the endothelial cells6. Another example of the heterogeneity of the microenvironment is the cancer-associated fibroblasts, which have different markers-associated with various functions in different tissues7.
Although the tumor microenvironment can be highly heterogeneous at the advanced stages, many common features are shared among various types of tumors. Therefore, targeting malignant and metastatic components in tumor microenvironment are potential strategies for tumor treatment2,8,9. Conventional drug delivery systems may fail to deliver adequate quantity of therapeutics to kill cancer cells effectively without undesirable side effects due to the complexity of the tumor microenvironment. Different drug targeting strategies can be used to increase the therapeutic efficiency, achieve tumor specificity, reduce the side effect and multidrug resistance, as well as improve the pharmacokinetic profile10,11. An example of targeted therapy that has been intensively studied is nanoparticle-based drug delivery systems. The theory of nanomedicine first came out in the late 1990s, achieved through utilization of the enhanced permeability and retention effect (EPR effect) of the tumors12. A variety of materials have been used to make nanocarriers, including: polymers10,13,14, lipids15, 16, 17, silica18,19, and metals20, 21, 22, 23. The nanometer size range of these delivery systems make them ideal for delivery of chemotherapeutic agents to the interstitial space of tumors with enhanced vascular leakiness. In addition to passive targeting, based on their size, nanoparticles can also be modified with targeting ligands that bind to specific receptors overexpressed on tumor cells to achieve active targeting. Currently, there are several anticancer nanomedicines approved by the U.S. Food and Drug Administration (FDA), such as Doxil (doxorubicin liposomes), DaunoXome (daunorubicin liposomes) and Abraxane (paclitaxel albumin-bound nanoparticles)24, 25, 26, 27. A list of nanomedicines currently under clinical trial can be found in a recent published review28. However, the heterogeneity of tumors can lead to low therapeutic efficacy, acquired resistance and side effects29. Nevertheless, targeting and modulating tumor microenvironment using nanomedicines are important strategies to improve cancer treatment outcomes.
The complex physiological features of the tumor microenvironment include hypoxia, acidosis and high tumor interstitial fluid pressure, etc.4. Tumor hypoxia is a result of overconsumption of oxygen due to rapid tumor development. About 50%–60% of solid tumors exhibit decreased oxygen levels30. Hypoxia is associated with tumor growth, metastasis and resistance to tumor therapies. Therefore, down-regulating hypoxia could potentially improve treatment outcomes31,32. Acidosis is another hallmark of the tumor microenvironment. Rapid glycolysis in tumor cells leads to acidic extracellular pH in the tumor microenvironment. The acidic pH may affect the conformation, solubility, binding affinity, as well as the delivery of many antitumor drugs33,34. pH-sensitive nanoparticle drug delivery systems can potentially enhance the antitumor outcome35,36. Another feature of the tumor microenvironment is the dysregulated enzyme expression34. Enzymes play a critical role in the metabolic processes in tumor progression. Nanomedicines have been designed to be responsive to enzymes that overexpress in the tumor microenvironment, such as matrix metalloproteinases and hyaluronidase37, 38, 39.
Nanomedicine-based drug delivery strategies also provide complementary opportunities for cancer immunotherapy. Cancer immunotherapy uses or involves components of the immune system to treat cancer. With the recent approval of chimeric antigen receptor (CAR) T cell therapy40,41 and immune checkpoint inhibitors42, 43, 44, cancer immunotherapy has become a most attractive anticancer strategy. Although this approach has strong potential, clinical researches have shown that many cancer patients do not show a therapeutic response to immunotherapy as a result of tumor heterogeneity. In this review, the current therapies targeting the tumor biological microenvironment, as well as the tumor microenvironment targeted therapies involved in cancer immunotherapy are discussed.
2. Targeting the tumor biological microenvironment
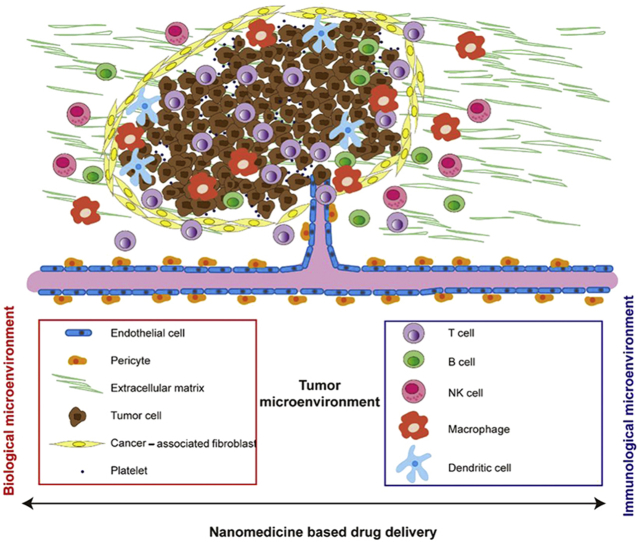
Apart from the malignant cells, the tumor microenvironment includes immune cells, stromal cells and extracellular matrix. As shown in Fig. 1, immune cells in the tumor microenvironment include: T lymphocytes, B lymphocytes, NK cells, dendritic cells, tumor-associated macrophages, and tumor-associated neutrophils. Stromal cells are connective tissue cells, such as fibroblasts, pericytes, vascular and endothelial cells.
Figure 1.
Tumor biological and immunological microenvironment.
2.1. Extracellular matrix
The extracellular matrix (ECM) is the non-cellular component of cells in all tissues. It is a complex network of extracellular macromolecules, including collagen, enzymes, and glycoproteins. ECM provides physical scaffolding and biochemical support to the surrounding cells45, which is necessary for cell growth and development, as well as other key biological processes, such as inflammation, differentiation and migration46.
In general, the extracellular matrix in the tumor tissue is often dense and stiff compared with the normal cell ECM, and this may contribute to tumor growth and metastasis47. ECM is part of all the biological barriers, the dense and stiff ECM in heterogeneous tumor tissues can prevent nanoparticle penetration and delivery, which leads to low accumulation and hinders the therapeutic efficacy46. Details on interactions between nanoparticles and the extracellular matrix have been reviewed by Engin et al.46 and Bandara et al.48. Multiple strategies have been investigated to overcome the dense and stiff biological barrier of the ECM to improve the anti-tumor efficiency of nanomedicines, such as using collagenase and hyaluronidase to “loosen” the ECM. Collagen is the major ECM structural component-associated with fibrosis49. Common methods to target collagen includes approaches involving the promotion of collagen degradation50, collagen synthesis inhibition51,52 and collagen cross-linking remediation53.
Collagenase54,55 is the enzyme that breaks down collagen and increases the penetration of the nanoparticles. It has been shown that collagenase can be used to improve drug delivery in cancer treatment. For example, Kato et al.56 injected collagenase intravenously in a lung tumor xenograft model followed with liposome/plasmid DNA complex (lipoplex) treatment to improve gene expression and lipoplex accumulation. Abdolahinia et al.57 synthesized collagenase and metformin-conjugated gold-nanoparticles and were able to show increased cytotoxicity in a breast cancer cell 3D spheroid culture model using these nanoparticles. Xu et al.58 developed a collagenase-modified polymeric micelle formulation, where the collagenase was utilized to digest the collagen fibers thus enhancing the permeation of the nanoparticles into tumor tissue. In addition, this drug delivery system was designed using a pH-sensitive ligand to allow nanoparticle expansion in size after reaching the tumor microenvironment and thus prolonging retention. Utilizing this strategy, cisplatin nanoparticles achieved excellent anticancer efficacy in a mouse model, releasing drug into the mitochondria of the cancer cells.
Hyaluronic acid is a glycolsaminoglycan that contributes to low elasticity and high gelation pressure in tumor tissues, and promotes tumor growth, metastasis and angiogenesis59. Hyaluronidase60,61 is an enzyme that has been used to degrade hyaluronic acid in the tumor ECM and has been shown to alleviate progression of many cancers, such as bladder cancer and brain cancer62,63. PEGPH20, a PEGylated recombinant human hyaluronidase, has been studied in multiple clinical trials in combination with chemotherapeutics, such as gemcitabine and nab-paclitaxel64, 65, 66, 67.
Hyaluronidase conjugated nanoparticles have also been investigated in efforts to improve their therapeutic efficiency68. Zhou et al.69 reported that PLGA-PEG nanoparticles conjugated with hyaluronidase demonstrated enhanced tumor penetration and anti-tumor efficacy in a 4T1 syngeneic mouse breast tumor model. These methods often require complicated chemical conjugation reactions. Lee et al.70 utilized pulsed high intensity focused ultrasound to break down the dense ECM structure of an A549 tumor mouse model, leading to enhanced tumor infiltration of drug-loaded nanoparticles. Using this strategy, the tumor targeting efficiency was shown to be increased by 2.5 times compared to the control group, which was not treated with high intensity focused ultrasound.
Many other ECM components, such as tenascin-C71, fibronectin/fibrin72,73, and galectin-174, have been targeted to improve nanomedicine delivery75. For example, tenascin-C is a glycoprotein overexpressed in the ECM of many tumors, such as breast cancer76, lung cancer77 and ovarian cancer78. It plays a critical role in supporting tumor proliferation and metastasis79. Tenascin-C is almost absent in the ECM of normal cells and can be a good ECM target79. Kang et al.80 synthesized Ft peptide to target both tenascin-C in the ECM and neuropilin-1 in neovasculature and glioma cells. Paclitaxel-loaded Ft nanoparticles (Ft-NP-PTX) enhanced the antitumor efficacy with prolonged survival rate in a glioblastoma mouse model. Lingasamy et al.81 developed a bi-specific peptide PL1 (PPRRGLIKLKTS) that can target both tenascin-C and fibronectin. PL1 nanoscale model payloads (iron oxide nanoworms and metallic silver nanoparticles) were shown to accumulate in glioblastoma (GBM) and prostate carcinoma models. Reduced tumor growth and increased survival were observed in a glioblastoma mouse model after PL-1 nanoworm treatment.
Targeting ECM for anti-cancer therapy is a promising strategy. Despite all the efforts and progress, there remain challenges and problems. For example, tumor collagen depletion can lead to cytokine release and potential inflammatory responses that promotes tumorigenesis82. Evidence has shown enhanced tumor progression after collagen depletion due to the elevated access of tumor cells to the blood stream83,84. Excessive breakdown of ECM components could cause ECM to lose its function and collapse its structure and worsen the therapeutic outcomes85,86. Further studies of the diverse properties and functions of the ECM are necessary to utilize ECM-targeted therapy.
2.2. Endothelial cells and angiogenesis
The vascular niche is a key component in tumor microenvironment87. The development of new blood vessels from pre-existing vessels is called angiogenesis. Angiogenesis is a major contributor to tumor growth and metastasis, and this makes it a promising target for cancer treatment88.
Clinically approved anti-angiogenic agents, such as thalidomide, bevacizumab, sunitinib, sorafenib and pazopanib, have led to significant therapeutic improvement in cancer treatment87. Angiogenesis-targeted delivery systems have been extensively studied to effectively deliver anti-angiogenic agents to tumor microenvironment with minimized systemic toxicity89. For example, receptors that overexpressed during angiogenesis, such as the epidermal growth factor receptor (EGFR), can be used as targets to prevent angiogenesis90,91.
The major mechanism of angiogenesis depends on interaction between the components in ECM including endothelial cells, pericytes and stoma cells87,92. Endothelial cells are frequently targeted in antiangiogenic therapies and a variety of ligands can be used for this purpose, such as peptides, antibodies, and cationic components93. The over expression of αv integrins on the endothelial cell surface is usually a marker of angiogenesis. The integrins facilitate the growth of newly formed vessels by attaching to tumor ECM proteins and/or neighboring cells94. Therefore, many drug delivery systems modified with ligands which can bind to integrins have been developed to target angiogenesis for cancer therapy. For example, the cyclic RGD (Arginine-Glycine-Aspartic acid) peptide is an essential motif that specifically binds to the integrin in tumor endothelial cells. Zhan et al.95 synthesized paclitaxel-loaded cyclic RGD modified PEG-PLA micelles and demonstrated that the micelles enhanced the anti-glioblastoma effect in U87MG glioblastoma xenografts with a significantly longer median survival time, when compared to the PEG-PLA paclitaxel micelles or paclitaxel controls. Sakurai et al.96 encapsulated an anti-angiogenic siRNA in an RGD peptide modified lipid nanoparticle (RGD-LNP). The continuous treatment of RGD-LNP encapsulating siRNA effectively improved the overall survival of metastasized lung model mice. A variety of other peptides can also be used to target endothelial cells, such as the NGR (asparagine-glycine-arginine) motif97, and the CGKRK peptide98.
Cancer nanomedicines must penetrate and accumulate in tumors through the tumor microenvironment vascular network in order to take effect. EPR effect-based delivery systems have yet to led to success in the clinical setting due to the heterogeneous properties of tumors. Therefore, it is essential to facilitate the delivery of the nanomedicine. Antiangiogenic therapy has drawn a lot of attention and has shown good results in many preclinical studies. However, this type of therapy could activate multiple signaling pathways, such as the vascular endothelial growth factor (VEGF) pathway, which may lead to drug resistance, tumor invasion and metastasis99,100. Gyanchandani et al.100 studied molecular changes in a bevacizumab resistant xenograft model using human-specific microarray analysis. Increased levels of fibroblast growth factor-2 (FGF2) were observed. FGF-2 is a pro-angiogenic factor regulated by the overexpression of upstream genes in extracellular signal-regulated kinase (ERK) pathways, including phospholipase C (PLCg2), frizzled receptor-4 (FZD4), chemokine [C-X3-C motif] (CX3CL1), and chemokine [C-C motif] ligand 5 (CCL5). Upregulation of FGF-2 induces tumor resistance against cancer therapy.
2.3. Pericytes
Pericytes are another promising target for anti-cancer treatment101. Pericytes are perivascular cells that surround the endothelium of capillaries and vessels. Pericytes interact with endothelial cells and are important cellular components of tumor microenvironment102. The function of pericytes in tumor progression is complicated and not fully understood as yet103,104. For example, pericytes are found to regulate angiogenesis and control endothelial cell proliferation through the vascular endothelial growth factor (VEGF) signaling pathway105,106. For drug delivery for the treatment of brain cancers, overcoming the blood‒brain barrier (BBB) is a major challenge. Pericytes are embedded in the basement membrane and ensure the integrity of the BBB. Increased brain permeability of both low and high molecular tracers was observed in pericyte-deficient mouse models107.
Several researches have shown improved anti-cancer efficiency using drug delivery systems modified with peptide moieties that bind to specific proteins on pericytes. For example, nanoparticles were developed to target the NG2 receptor, a proteoglycan highly expressed in tumor pericytes. The TH10 peptide (TAASGVRSMH) has been conjugated to docetaxel-loaded nanoparticles (TH10-DTX-NP)108. The TH10 peptide was screened and isolated through phage display and has a strong affinity for the NG2 receptors on pericytes. Antitumor effects have been observed using TH10-DTX-NP in a melanoma experimental lung metastasis mouse model with prolonged survival. The TH10 peptide enhanced the nanoparticle internalization through interaction between TH10 and NG2.
Another strategy to enhance nanoparticle permeation into tumor tissue is to reduce pericyte coverage. Chaudhuri et al.109 demonstrated that the administration of a smoothened inhibitor, erismodegib, could significantly enhance doxorubicin-loaded nanoparticle accumulation into the adenocarcinoma cell-enriched tumor region. Smoothened inhibitors may disrupt the hedgehog-signaling pathway (Hh) leading to the deactivation of tumor fibroblasts. In this study, the decrease in the pericyte coverage of the vascular endothelium structure was observed to be-associated with enhanced nanoparticle distribution, contributing to the improved therapeutic effect in a mouse model of pancreatic cancer.
There are still challenges to target pericytes for cancer therapy, including a limited knowledge of the identification, ontogeny, and progeny of pericytes110. Pericytes depletion may also fail to improve the anti-tumor effect and lead to unexpected tumor growth111. Moreover, low pericyte coverage has been reported to be-associated with tumor metastasis and poor prognosis110,112. A deeper understanding of the interaction between tumor pericytes and tumor progression is needed.
2.4. Cancer-associated fibroblasts
Cancer-associated fibroblasts (CAFs) are a type of mesenchymal stromal cells abundant in tumor microenvironment that play important roles in cancer progression113,114. CAFs provide physical support for tumor cells and are important in stroma remodeling. They are the major cell type involved in collagen production and crosslinking which contribute to increased ECM stiffness115. CAFs are also associated with tumorigenesis and are involved in immune evasion116.
CAFs could cause lower tumor penetration of nanomedicines and lead to poor therapeutic outcomes. Anti-cancer-associated fibroblast therapy has become a promising strategy to improve drug sensitivity. For example, Zhang et al.117 used the clinically-relevant cyclooxygenase-2 (COX-2) inhibitor, celecoxib, to improve the delivery of paclitaxel-loaded micelles. Celecoxib leads to reduced CAFs, disruption of the fibronectin bundle and improved tumor perfusion. As a result, the penetration and accumulation of paclitaxel-loaded micelles were enhanced and their therapeutic benefits improved in human lung A549 tumor xenografts. Chen et al.118 modified navitoclax-loaded nanoliposomes with peptide FH (FH-SSL-Nav). Small molecule navitoclax can induce the apoptosis in CAFs and peptide FH can specifically bind to tenascin-C. The peptide liposome FH-SSL-Nav exhibited increased cellular uptake and cytotoxicity in vitro, as well as improved antitumor efficacy in a Hep G2 tumor mouse model. A recent report119 has shown that the tumor cell-promoting behavior of CAFs can be attenuated by gold-core silver-shell hybrid nanomaterials. In the in vivo study, the nanomaterial showed promising results in inhibiting tumor metastasis.
A key challenge in CAF research is the lack of simple nomenclature of CAFs and the fibroblast subtypes for broader use in cancer and stromal biology. There is also a lack of robust biomarkers for CAFs detections in the clinical setting120. Deeper understanding of the CAFs origin, diverse function, heterogeneity and plasticity will be beneficial in modulating CAFs for anti-cancer therapy120. The design of sophisticated nanoparticulate drug delivery systems targeting CAFs relies on a more fundamental understanding of CAFs.
2.5. Platelets
Platelets are anucleate blood cells that are present in the tumor microenvironment. Besides their role in blood coagulation, platelets have been recognized for supporting tumor growth and metastasis121,122. Platelets interact with tumor cells through different ways. In brief, tumors relying on the vascular network for growth can induce aggregation, activation, and secretion of the platelets flowing through the tumor vessels122. Platelets not only protect tumor cells from blood sheer stress and immune cell-mediated elimination123, but also interact with other components in the tumor microenvironment, such as endothelial cells, pericytes, fibroblasts and immune cells thereby contributing to tumor progression and inflammation124.
Targeting platelets using nanoparticle-based drug delivery systems can potentially inhibit tumor metastasis. For example Zhang et al.125 designed nanoparticles modified with the tumor-homing pentapeptide CREKA (Cys-Arg-Glu-Lys-Ala) to deliver platelet inhibitor (ticagrelor). These nanoparticles were determined to efficiently inhibit platelet-tumor cell interaction and block tumor cell transition into mesenchymal-like invasive cells in a mammary tumor xenograft mouse model. Interestingly, platelet drug-loading and platelet membrane biomimetic systems are also very popular for tumor therapy126, 127, 128. Xu et al.129 conjugated doxorubicin-loaded platelets with anti-CD22 monoclonal antibodies for tumor targeting. The platelet drug carriers prolonged the circulation time of doxorubicin. Enhanced antitumor activity was observed both in vitro and in vivo. Wang et al.130 prepared platelet membrane (PLTM)-coated nanoparticles loaded with the anti-cancer drug bufalin (PLTM-CS-pPLGA/Bu NPs). Increased accumulation of the nanoparticles and more effective tumor growth inhibition were observed in H22 hepatocellular carcinoma tumor-bearing mice treated with platelet membrane-coated nanoparticles (PLTM-CS-pPLGA/Bu NPs) when compared to uncoated groups (CS-pPLGA/Bu NPs).
The platelet-like nanoparticles have demonstrated promising preclinical results. However, scale-up manufacturing and a thorough investigation of safety have yet to be established, which are necessary to translate the technology. Targeting platelets for cancer therapy may interfere with the hemostasis of cancer patients131. The risk-benefit of targeting platelets should be balanced as some of the platelet targeting approaches may impair their critical physiological function in coagulation. A better understanding of the interaction between platelets and other components in the tumor microenvironment is very important to improve therapeutic outcomes.
3. Targeting the immunological microenvironment
The interaction between the tumor microenvironment and the immune cells can influence the clinical outcomes of immunotherapy. Immune cell infiltration and cytokine production leads to heterogeneous inflammatory tumor microenvironment. The main immune cells in tumor microenvironment include: T lymphocytes, B lymphocytes, macrophages, NK and dendritic cells. The functions and components of the immune cells are diverse and complex1,132 and are listed below in Table 1133, 134, 135, 136, 137, 138, 139, 140, 141, 142, 143, 144, 145, 146, 147, 148, 149, 150, 151, 152, 153, 154, 155.
Table 1.
Quick guide for phenotype identification of the main immune cells within the tumor microenvironment.
| Lineage | Cell | Function | Positive | Ref. | |
|---|---|---|---|---|---|
| Lymphoid | T cells | Cytotoxic T cells | Immunostimulatory | CD3, CD8 | 133,134 |
| Helper T cells | Immunostimulatory | CD3, CD4 | 133, 134 | ||
| Gamma delta T cells | Immunostimulatory | CD3, γδTCR | 136,136 | ||
| NK T cells | Immunostimulatory | CD3, CD161, CD94, CD1d-α-GalCer | 137,138 | ||
| Memory T cells | Immunostimulatory | CD3, CD27, CD45RO | 139,140 | ||
| Regulatory T cells | Immunosuppressive | CD4, CD25, FoxP3 | 141,142,143 | ||
| B cells | Plasma cells | Immunostimulatory | CD38, CD138 | 141, 142, 143 | |
| Memory B cells | Immunostimulatory | CD19, CD20, CD27, CD38 | 141, 142, 143 | ||
| Regulatory B cells | Immunosuppressive | CD19, IL10, CD1d | 144,146 | ||
| NK cells | Immunostimulatory | CD16, CD56 | 144, 145, 146 | ||
| Myeloid | Tumor-associated macrophages (TAMs) | M1 phenotype | Immunostimulatory | CD68, iNOS, HLA-DR, CD80 | 148,149 |
| M2 phenotype | Immunosuppressive | CD68, CD163, VEGF, CD206 | 148,149,150 | ||
| Myeloid derived suppressor cells (MDSC) | Polymorphonuclear (PMN-MDSC) | Immunosuppressive | CD11b, CD15 (or CD66b), CD33 | 148, 149, 150 | |
| Monocytic (M-MDSC) | |||||
| Tumor-associated neutrophils (TANs) | Can be pro- or anti-tumor activity | CD66b | 148, 149, 150 | ||
| Dendritic cells (DC) | May be defective in TME as terminally differentiated myeloid DC | MHCII, CD103 or CD11b | 153,154,155 | ||
3.1. Tumor-associated macrophages
Tumor-associated macrophages (TAMs) are macrophages that are abundant in the tumor microenvironment. Monocytes generated in the bone marrow and spleen can infiltrate the tumor microenvironment and differentiate into macrophages156. Depending on the type of cytokines they are exposed to, the activated macrophages can be classified into two different phenotypes, M1 and M2 phenotype157. M1 and M2 macrophages exhibit pro-inflammatory or anti-inflammatory activities, respectively158. Likewise, TAMs include the classically activated (immunostimulatory) M1 phenotype as well as the alternatively activated (immunosuppressive) M2 phenotype159. The M1 phenotype can switch to the M2 phenotype and vice versa in response to microenvironmental signals, such as cytokines, chemokines, growth factors, as well as signals derived from other cells160. This process is called macrophage polarization. TAMs are usually M2 phenotype although they can exhibit either polarization phenotype161, 162, 163. The TAMs in the tumor microenvironment contribute to tumor progression, survival and metastasis and may result in a poor clinical outcome164.
Targeting TAMs to prevent tumor progression and metastasis has become a promising anticancer strategy. TAM-targeted therapy is mainly focused on inhibition of macrophage recruitment165,166, elimination of M2-TAMs167 or re-polarization of M2-TAMs to M1-TAMs168,169. For example, Das et al.170 reported a pancreatic cancer therapy involving activation of the innate immune receptor retinoic acid-inducible gene 1 (RIG-1) by a short interfering RNA agonist using surface-modified nanoparticles. This resulted in a higher M1:M2 macrophage ratio, increased proportion of cytotoxic T cells over regulatory T cells, as well as a reduction in regulatory B cells and plasma cells. Rong et al.171 introduced Fe3+ into PEGylated polydopamine to form iron chelated nanoparticles (Fe@PDA-PEG). As shown in Fig. 2, Fe@PDA-PEG nanoparticles induced M2-TAMs to M1 repolarization and enhanced anti-tumor efficacy in colon carcinoma and breast carcinoma mouse models. Pang et al.172 developed PLGA nanoparticles that were coated with M2-macrophages binding peptide (M2pep) to encapsulate PLX3397, a receptor tyrosine kinase inhibitor that was shown to deplete macrophages in tumors173. Results showed an increased uptake of M2pep-coated PLGA nanoparticles in M2-TAMs and reduced tumor growth in a mouse melanoma model.
Figure 2.
Iron chelated melanin like nanoparticles (Fe@PDA-PEG) induced M2-TAMs to M1 repolarization. Combining with photothermal therapy (PTT)-induced tumor-associated antigens (TAAs) release altered the tumor microenvironment to immune-induced cancer cell killing mode. (MΦ, macrophages; MHC II, major histocompatibility complex class II; TCR, T cell receptor). Reprinted with the permission from Ref. 171. Copyright © 2019 Elsevier.
3.2. Chronic inflammation in tumor development
Chronic inflammation is critically related to tumor progression174. On the one hand, cancers may arise from sites of infection and chronic inflammation, such as colorectal cancer associated with inflammatory bowel disease175 and esophageal adenocarcinoma associated with reflux esophagitis176. On the other hand, tumor progression can often lead to chronic inflammation due to the inflammatory cytokines or other inflammatory stimuli177. In the tumor microenvironment, cytokines such as tumor necrosis factor (TNF-α), Interleukins (IL-1, IL6 and IL-10) and transforming growth factor β (TGF-β), play a critical role in cancer-related chronic inflammation178,179.
For example, IL-1 binding to specific IL-1 receptors (IL-1R) initiates IL-1 signaling180. The IL-1R family includes 10 members, which assemble as heterodimers and signal through the MyD88/IRAK/NFκB pathway177,181. IL-1R-targeted delivery therapies have been studied to inhibit IL-1 induced inflammation. Shevtsov et al.182 conjugated recombinant IL-R antagonist (IL-1Ra) to superparamagnetic iron oxide (SPION) nanoparticles to image and target glioblastoma in an experimental rat model. SPION-IL-1Ra nanoparticles significantly improved the lifespan of C6 glioblastoma rats.
Increased levels of immunosuppressive cytokines, such as TGF-β and IL-10, are accumulated in the tumor microenvironment183. These cytokines are involved in a variety of activities in tumorigenesis and play important roles in the chronic inflammation processes184,185. Combinations of inflammation modulating agents can improve the efficacy of nanomedicines. Zuo et al.186 demonstrated that the TGF-β signaling pathway inhibition by the TGF-β type I receptor inhibitor LY364947 improves tumor penetration of the nanoparticles carrying siRNA targeting the breast cancer stem cells. This synergistic treatment delayed tumor growth and enhanced cancer stem cell clearance in breast cancer mouse xenografts. Panagi et al.187 showed that a combination of tranilast (TGF-β inhibitor) with Doxil (doxorubicin liposomes) increased tumor perfusion and oxygenation, reprogramed macrophage polarization towards the M1 phenotype, and caused reduction in tumor size in triple-negative breast cancer (TNBC) mouse models. Moreover, tranilast and Doxil co-treatment also improves the efficacy of immunotherapy of immune checkpoint blocking antibodies (anti-PD-1/anti-CTLA-4).
3.3. Driving “cold” tumors “hot”
TME plays essential roles in regulating the interactions between cancer cells and immune cells during tumor progression. Immunologically, the TME can be broadly classified as ‘hot’ or “cold” based on the tumor antigenicity188. “Hot” tumors are T cell-inflamed and highly immunogenic. The infiltrated tumor-specific T cells lose their tumor-killing capacity due to immune evasion. One of the immune evasion mechanisms is via the expression of the programmed death-ligand 1 (PD-L1), which can bind to the programmed death protein 1 (PD-1) on T cells and deactivate the T cells, which are the major players in the adaptive immune system. Therefore, the “hot” tumors are usually good responders to immune checkpoint inhibitors. In contrast, “cold” tumors are poorly immunogenic, there are less lymphocyte infiltrations and more immune suppressing cells189. Tumor immune evasion as a result of the immune responses likely happens at an early stage and affects the immune cell trafficking. Cold tumors, like pancreatic cancer, typically respond poorly to the checkpoint inhibitor therapy163.
To improve immunotherapy outcomes of “cold” tumors, activating the innate immune system has become an attractive strategy to increase tumor immunogenicity and turn “cold” tumors to “hot” tumors.
The innate immune system plays an integral role in the activation of adaptive immunity. Innate immune cells (i.e., macrophages and dendritic cells) activate inflammatory signaling through pattern recognition receptors (PRRs) in response to the binding of pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs)190.
3.3.1. Activate stimulator of interferon genes (STING) pathway
One strategy to activate the innate immune system to increase tumor immunogenicity is through activation of the STING pathway. STING is an endoplasmic reticulum (ER) transmembrane protein and mediates the innate immune responses induced by cytosolic DNA191. Upon sensing the DNA, enzyme cyclic GMP-AMP synthase (cGAS), a cytoplasmic PRR, cyclizes GTP and ATP and produces cyclic GMP-AMP (cGAMP). cGAMP binds to STING and activates the transcriptional gene signaling and host immune responses192. Activating STING is a promising way to stimulate the immune suppression reversion and induce tumor regression in experimental cancer models163,193, 194, 195, 196. Consistently, multiple STING agonists are in clinical trials for immunotherapy and combinational therapy with immune checkpoint inhibitors192. Targeted delivery of STING agonists could potentially activate immune suppressed tumors197, 198, 199. Cyclic dinucleotide (CDN) agonists of STING are widely studied immunotherapeutics to activate innate immunity.
The use of nanoparticulate drug delivery systems can facilitate the intracellular delivery of STING therefore inhibiting tumor growth. In a recent publication, Chattopadhyay et al.200 described the development of hollow polymeric nanoshells using PLGA to encapsulate STING for intracellular delivery. This resulted in synthetic immunogenic cell death (sICD) of cancer cells in three mouse tumor models. The sICD was in synergy with chemotherapeutic administration into the mouse models and resulted in enhanced therapeutic effect and restrained tumor progression. Shae et al.199 utilized STING-activating nanoparticles (STING-NPs) as a drug carrier with high bioavailability, to enhance the cytosolic delivery of the STING CDN agonist cGAMP. STING-NPs activate the STING signaling pathway in the tumor microenvironment, increase the immunostimulatory potency of immunotherapeutic cGAMP and induce the immunosuppressive “cold” tumors to immunogenic “hot” tumors. STING-NPs exhibited improved therapeutic efficacy in a melanoma mouse model, with decreased tumor growth rate and increased survival time in comparison to the immunotherapeutic cGAMP.
3.3.2. Activate Toll-like receptors (TLRs)
Another promising way to activate the innate system to increase tumor immunogenicity is through TLRs activation. Among the PRRs, TLRs have been most extensively investigated. TLRs are expressed across many immune cells, including T cells, B cells, dendritic cells, macrophages and NK cells. TLR-mediated signaling pathways play a critical role in the initiation of adaptive immune responses201. TLR signaling pathways include various signaling components, such as MyD88, Toll-interacting protein (TOLLIP), IL-1R-associated kinase (IRAK) and TNF receptor-associated factor 6 (TRAF6)201. Lately, TLR agonists have drawn extensive attention in cancer immunotherapy202. Several TLR agonists have been approved by FDA, such as Imiquimod (Aldara or R-837) and Bacillus Calmette-Guérin (BCG) vaccine. Moreover, various clinical trials in different cancer patients are ongoing to investigate the safety and efficacy of TLR agonists for monotherapy or combinational therapy203.
Targeted delivery of TLR agonists has also been extensively studied to improve the TLR-mediated immune-stimulatory effects for cancer immunotherapy204, 205, 206, 207, 208, 209.
For example, multiple studies have been conducted to stimulate TLR 7 and TLR 8, which are structurally conserved receptors. Therefore, in certain circumstances they recognize the same ligand210. One of the best-characterized TLR7/8 agonists is the imidazoquinoline, such as imiquimod. Imiquimod has been approved by the FDA for topical use in basal cell carcinoma and has also been investigated under clinical trials in metastatic melanoma and localized bladder cancer patients211.
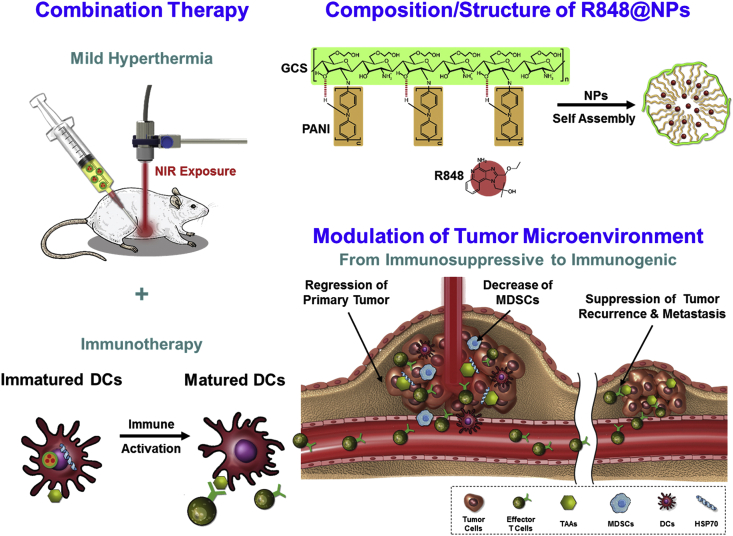
Rodell et al.207 studied the TLR agonist R848 loaded into β-cyclodextrin nanoparticles (CDNPs) in different mouse tumor models. The CDNP-R848 induced the repolarization of TAMs toward an M1 phenotype in tumor microenvironment. In combinational therapy with a PD-1 inhibitor, improved response rates were observed in a PD-1 resistant tumor model. In Fig. 3, Chen et al.212 designed a combined therapy involved low-temperature hyperthermia (44 °C) and immunotherapy using NIR (near-infrared) absorbing R848-loaded nanoparticles (R848NPs/+NIR). Results showed elevated immune activation, delayed tumor regression and extended survival in tumor-bearing mouse model.
Figure 3.
Composition and structure of TLR-7/8 agonist-loaded nanoparticles (R848@NPs). R848@NPs exert low-temperature hyperthermia and activate immune cells in the tumor microenvironment. Reprinted with the permission from Ref. 212. Copyright © 2020 Elsevier.
The activation of anti-tumor activity can also be utilized to develop cancer vaccines based on nanoparticles. Lynn et al.213 developed personalized cancer vaccines based on self-assembled nanoparticles composed of charge-modified peptide-TLR-7/8a conjugates and peptide neoantigens. The charge-modifying group and hydrophobic block in the platform is tunable for the modification of diverse peptide neoantigens for personalized use. The imidazoquinoline-based TLR-7/8a adjuvant used in the vaccine allows broad activation of human dendritic cell subsets to promote cytokine production, which can activate T-cell immunity. Moreover, the nanoparticles can form in a defined size through a simple self-assembly process.
Some examples of utilization of nano-carriers targeting the TLR for immune cancer therapy are listed in Table 2212, 213, 214, 215, 216, 217, 218, 219, 220 below.
Table 2.
Immuno-therapeutics targeting TLRs using nanocarriers.
| Nano carrier | Agonist and type of TLR | Application | Ref. |
|---|---|---|---|
| Polyaniline conjugated glycol-chitosan nanoparticles | Resiquimod (R848) (TLR7/8) | Synergy between hyperthermia and immune-suppression alleviation | 153, 154, 155 |
| Poly (lactic-co-glycolic acid) nanoparticle | Racemic mixture ‘522’, stereoisomer ‘528’ (TLR7/8) | Induced high levels of pro-inflammatory cytokines, effective in multiple tumor models | 153, 154, 155 |
| Antigen ovalbumin and phospholipid-loaded zinc doped iron oxide nanoparticles | PolyIC (TLR3), imiquimod (TLR7) | Checkpoint inhibition and protective antitumour responses for cancer immunotherapy | 153, 154, 155 |
| CpG DNA Nano-cocoon | Synthetic oligonucleotides contain unmethylated cytosine and guanosine (CpG ODN) (TLR9) | Co-delivery of anti-PD-1 with CpG induced immune response and prolonged survival time in mouse model | 153, 154, 155 |
| Poly (d,l-lactic-co-glycolic acid) (PLGA)/mPEG-PLA nanoparticles | Resiquimod (R848) (TLR7/8) | Nanoparticles detected in dendritic cells and macrophages in the draining lymph nodes with activated immune response | 153, 154, 155 |
| Qβ-VLPs as a nano vaccine | B-type CpGs with phosphorothioate backbone (TLR9) | Induced effective CD8+ T-cell responses | 153, 154, 155 |
| Magnetite nanoparticles-loaded polyethylene glycol phospholipid micelles | Monophosphoryl lipid A (MPLA) derived from lipooligosaccharide (TLR4) | Long-term protection against repeated tumor challenge in combination with checkpoint inhibition | 153, 154, 155 |
| Self-assembled nanoparticles of acetyl modifed glucomannan polysaccharide | Glucomannan polysaccharide (TLR2) | Intratumoral injection of acGM-1.8 suppresses the growth of two tumor models | 153, 154, 155 |
3.3.3. Activate unconventional T cells
In addition to the activation of conventional alpha beta T cells, an emerging group of T cells that have been frequently studied are gammadelta T (γδ T) cells. γδ T cells link the innate and adaptive immune responses221. Multiple studies have shown that γδ T cells are abundant components among the tumor-infiltrating lymphocytes222,223. γδ T cells are a less frequent type of T cells (1%–5% of circulating T cells) with a different type of T cell receptor that is composed of a γ chain and a δ chain224. Unlike conventional T cells which depend on MHC class I or class II molecules to recognize the antigens (i.e., peptides), γδ T cells respond to ligands that are fundamentally different. For example, the dominant subset of γδ T cells in peripheral blood is the Vδ2 T cell, which is sensitive to phosphorus-containing small molecules known as phosphoantigens225. Activated γδ T cells can either directly kill cancer cells or kill them through cytokine production226,227.
Bisphosphonate drugs, such as zoledronate, risedronate, alendronate and pamidronate, were approved by the FDA to treat osteoporosis. In clinical use, they were found to indirectly activate the gammadelta T cells224. Modulating the activation of γδ T cells using targeted delivery of these small molecules can be a potential way to stimulate anti-tumor immunotherapy. Man et al.228 utilized alendronate liposomes to activate the [89Zr]Zr (oxinate) labeled γδ T cells. Positron emission tomography (PET) tracking showed that liposome treatment increased the accumulation of γδ T cells in tumors in a human breast cancer xenograft mouse model.
The combination of immune-stimulating nanomedicine and immunotherapy has been extensively studied229. Engineering of nanoparticles by facilitating the targeted delivery to modulate the immune response in the tumor microenvironment may improve the outcome of anticancer immunotherapy. In addition to the innate immune system, the adaptive immune cells can also be directly targeted by nanoparticles. For example, Yu et al.230 prepared hyaluronidase-responsive nanoparticles (mCAuNCs@HA) carrying the photosensitizer (pheophorbide A) and the paclitaxel prodrug (PXTK). The immune checkpoint inhibitor anti-PD-L1 peptide (dPPA) is also loaded these nanoparticles and this combination therapy activates CD4+T cells, CD8+ T cells and NK cells in vivo. Elevated TNF-α and IL-12 levels were observed. This combination therapy leads to an improved anti-tumor efficacy with the tumor inhibition rate increased to 84.2% in mouse tumor models.
Although nanomedicine is capable of enhancing the cancer immunotherapy in various aspects, there are still challenges to targeting immune cells using nanoparticle-based immunomodulatory strategies. For instance, T cells are the main players in fighting cancer and are a therapeutically relevant target for ex vivo gene delivery. It is challenging to transfect T cells using most commercially-available reagents231. Engineering cationic polymers for gene delivery to T cells may be of assistance. Olden et al.232 designed and evaluated a panel of cationic polymers to deliver genes to T cells. Results show that comb- and sunflower-shaped pHEMA-g-pDMAEMA polymers can successfully deliver genes into human T cell line (Jurkat cells), human primary CD4+T cells, and CD8+ T cells with minimal cytotoxicity and the transfection efficiencies were up to 50%, 25% and 18%, respectively.
4. Summary, conclusions and further perspectives
Cancer remains one of the leading causes of death worldwide despite the huge effort and resources that continue to be applied in the area of cancer therapy. Tumors consist of not only the malignant cell masses but also the surrounding tumor microenvironment including cellular and non-cellular components. Dynamic and complex tumor microenvironment promotes tumor progression and is a promising target for anticancer treatment. In addition to targeting the tumor biological and immunological microenvironment as has been discussed here, tumor physical and chemical microenvironments, such as tumor interstitial fluid pressure, flow dynamics, pH, enzymes, and hypoxia, have also been widely studied as targeting strategies. With a growing knowledge of tumor microenvironment, various approaches have been applied to enhance the efficiency of nano-therapeutics compared to traditional passive targeting such as EPR effect.
The recent advancement in immunology offers hope for curing cancer. The approval of immune checkpoint inhibitors and CAR-T cell therapy has taken cancer immunotherapy to a new era. However, many cancer patients still do not obtain a therapeutic benefit from immunotherapy due to the heterogeneity of immune responses and various sorts of counter-measures by tumor cells. Unique tumor microenvironment creates immunological tolerance and causes therapeutic resistance. The tumor-infiltrating lymphocytes could become exhausted and express a variety of immune-inhibitory receptors, such as PD-1, LAG-3, CTLA-4 and TIM-3. In this article, several different strategies targeting the tumor microenvironment that can promote immune responses via increased drug release, tumor penetration, as well as prolonged immune activation have been reviewed. Nanotechnology-based drug delivery systems have shown promising results in delivering immune-therapeutics to enhance the outcome of cancer treatment in preclinical settings. The synergy between immunotherapy and nanomedicine has been gaining momentum.
Although progress has been made in the development of nanotechnology-based drug delivery systems to target tumor microenvironment, challenges remain, particularly in clinical translation and large-scale manufacturing. Researchers must be cautious about over-engineering the nanoparticulate drug delivery systems (which is a major obstacle hampering commercialization of nanocarriers in general). Stability, manufacturability and bioperformance are three aspects that must be carefully balanced when designing a nanocarrier system involving complex processes, such as chemical conjugation, targeting ligand modification, bioresponsive linker ligation, and particle size control. Academic scientists and pharmaceutical companies are making efforts to improve quality control and manufacturing reproducibility of these systems. For example, our team is leading an effort in continuous manufacturing of nanoparticles to improve quality and throughput while reducing cost. This manufacturing platform consists of a coaxial turbulent jet in co-flow system coupled with process analytical technology originally designed for the production of liposomes17 and has been extended to other nanoparticles such as polymeric micelles. The nanoparticles (for example, liposomes) can be produced with precise control over particle size in a monodisperse fashion, as well as precise control over other critical quality attributes such as drug loading and coating with targeting ligand. Such manufacturing control can assist in targeting anticancer therapeutics to specific sites, while ensuring quality and safety.
Acknowledgments
This work was supported by the University of Connecticut (USA).
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Author contributions
Jin Li and Diane Burgess conceived and designed this review. Jin Li wrote the manuscript. Jin Li and Diane Burgess revised the manuscript. All of the authors have read and approved the final manuscript.
Conflicts of interest
The authors have no conflicts of interest to declare.
References
- 1.Balkwill F.R., Capasso M., Hagemann T. The tumor microenvironment at a glance. J Cell Sci. 2012;23:5591–5596. doi: 10.1242/jcs.116392. [DOI] [PubMed] [Google Scholar]
- 2.Roma-Rodrigues C., Mendes R., Baptista P.V., Fernandes A.R. Targeting tumor microenvironment for cancer therapy. Int J Mol Sci. 2019;4:840–871. doi: 10.3390/ijms20040840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Runa F., Hamalian S., Meade K., Shisgal P., Gray P.C., Kelber J.A. Tumor microenvironment heterogeneity: challenges and opportunities. Curr Mol Biol Rep. 2017;4:218–229. doi: 10.1007/s40610-017-0073-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang S., Gao H. Nanoparticles for modulating tumor microenvironment to improve drug delivery and tumor therapy. Pharmacol Res. 2017;126:97–108. doi: 10.1016/j.phrs.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Yuan Y. Spatial heterogeneity in the tumor microenvironment. Cold Spring Harb Perspect Med. 2016;8:a026583–a026599. doi: 10.1101/cshperspect.a026583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Little S.E., Popov S., Jury A., Bax D.A., Doey L., Al-Sarraj S. Receptor tyrosine kinase genes amplified in glioblastoma exhibit a mutual exclusivity in variable proportions reflective of individual tumor heterogeneity. Cancer Res. 2012;7:1614–1620. doi: 10.1158/0008-5472.CAN-11-4069. [DOI] [PubMed] [Google Scholar]
- 7.Bu L., Baba H., Yoshida N., Miyake K., Yasuda T., Uchihara T. Biological heterogeneity and versatility of cancer-associated fibroblasts in the tumor microenvironment. Oncogene. 2019;25:4887–4901. doi: 10.1038/s41388-019-0765-y. [DOI] [PubMed] [Google Scholar]
- 8.Cheng Y.T., Yang C.C., Shyur L.F. Phytomedicine-modulating oxidative stress and the tumor microenvironment for cancer therapy. Pharmacol Res. 2016;114:128–143. doi: 10.1016/j.phrs.2016.10.022. [DOI] [PubMed] [Google Scholar]
- 9.Bahrami A., Hassanian S.M. The therapeutic potential of targeting tumor microenvironment in breast cancer: rational strategies and recent progress. J Cell Biochem. 2018;1:111–122. doi: 10.1002/jcb.26183. [DOI] [PubMed] [Google Scholar]
- 10.Li J., Gu B., Meng Q., Yan Z., Gao H., Chen X. The use of myristic acid as a ligand of polyethylenimine/DNA nanoparticles for targeted gene therapy of glioblastoma. Nanotechnology. 2011;43:435101–435109. doi: 10.1088/0957-4484/22/43/435101. [DOI] [PubMed] [Google Scholar]
- 11.Danhier F. To exploit the tumor microenvironment: since the EPR effect fails in the clinic, what is the future of nanomedicine?. J Control Release. 2016:108–121. doi: 10.1016/j.jconrel.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 12.Wagner V., Dullaart A., Bock A.K., Zweck A. The emerging nanomedicine landscape. Nat Biotechnol. 2006;10:1211–1217. doi: 10.1038/nbt1006-1211. [DOI] [PubMed] [Google Scholar]
- 13.Dahlman J.E., Barnes C., Khan O., Thiriot A., Jhunjunwala S., Shaw T.E. In vivo endothelial siRNA delivery using polymeric nanoparticles with low molecular weight. Nat Nanotechnol. 2014;8:648–655. doi: 10.1038/nnano.2014.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chowdhury P., Nagesh P.K.B., Khan S., Hafeez B.B., Chauhan S.C., Jaggi M. Development of polyvinylpyrrolidone/paclitaxel self-assemblies for breast cancer. Acta Pharm Sin B. 2018;4:602–614. doi: 10.1016/j.apsb.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu X., Khan M.A., Burgess D.J. A quality by design (QbD) case study on liposomes containing hydrophilic api: I. formulation, processing design and risk assessment. Int J Pharm. 2011;1‒2:52–59. doi: 10.1016/j.ijpharm.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 16.Costa A.P., Xu X., Burgess D.J. Freeze-anneal-thaw cycling of unilamellar liposomes: effect on encapsulation efficiency. Pharm Res (N Y) 2014;1:97–103. doi: 10.1007/s11095-013-1135-z. [DOI] [PubMed] [Google Scholar]
- 17.Costa A.P., Xu X., Khan M.A., Burgess D.J. Liposome formation using a coaxial turbulent jet in co-flow. Pharm Res (N Y) 2016;2:404–416. doi: 10.1007/s11095-015-1798-8. [DOI] [PubMed] [Google Scholar]
- 18.He Q., Shi J., Chen F., Zhu M., Zhang L. An anticancer drug delivery system based on surfactant-templated mesoporous silica nanoparticles. Biomaterials. 2010;12:3335–3346. doi: 10.1016/j.biomaterials.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 19.Chaudhary Z., Subramaniam S., Khan G.M., Abeer M.M., Qu Z., Janjua T. Encapsulation and controlled release of resveratrol within functionalized mesoporous silica nanoparticles for prostate cancer therapy. Front Bioeng Biotechnol. 2019;7:225–234. doi: 10.3389/fbioe.2019.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arvizo R., Bhattacharya R., Mukherjee P. Gold nanoparticles: opportunities and challenges in nanomedicine. Expet Opin Drug Deliv. 2010;6:753–763. doi: 10.1517/17425241003777010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thoidingjam S., Tiku A.B. New developments in breast cancer therapy: role of iron oxide nanoparticles. Adv Nat Sci-Nanosci. 2017;2:23002–23011. [Google Scholar]
- 22.Ruan S., He Q., Gao H. Matrix metalloproteinase triggered size-shrinkable gelatin-gold fabricated nanoparticles for tumor microenvironment sensitive penetration and diagnosis of glioma. Nanoscale. 2015;21:9487–9496. doi: 10.1039/c5nr01408e. [DOI] [PubMed] [Google Scholar]
- 23.Ruan S., Hu C., Tang X., Cun X., Xiao W., Shi K. Increased gold nanoparticle retention in brain tumors by in situ enzyme-induced aggregation. ACS Nano. 2016;11:10086–10098. doi: 10.1021/acsnano.6b05070. [DOI] [PubMed] [Google Scholar]
- 24.Yardley D.A. Nab-paclitaxel mechanisms of action and delivery. J Control Release. 2013;3:365–372. doi: 10.1016/j.jconrel.2013.05.041. [DOI] [PubMed] [Google Scholar]
- 25.Ventola C.L. Progress in nanomedicine: approved and investigational nanodrugs. P T. 2017;12:742–755. [PMC free article] [PubMed] [Google Scholar]
- 26.Tran S., DeGiovanni P.-J., Piel B., Rai P. Cancer nanomedicine: a review of recent success in drug delivery. Clin Transl Med. 2017;1:44–64. doi: 10.1186/s40169-017-0175-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Overchuk M., Zheng G. Overcoming obstacles in the tumor microenvironment: recent advancements in nanoparticle delivery for cancer theranostics. Biomaterials. 2018;156:217–237. doi: 10.1016/j.biomaterials.2017.10.024. [DOI] [PubMed] [Google Scholar]
- 28.McGoron A.J. Perspectives on the future of nanomedicine to impact patients: an analysis of us federal funding and interventional clinical trials. Bioconjugate Chem. 2020;3:436–447. doi: 10.1021/acs.bioconjchem.9b00818. [DOI] [PubMed] [Google Scholar]
- 29.Dagogo-Jack I., Shaw A.T. Tumour heterogeneity and resistance to cancer therapies. Nat Rev Clin Oncol. 2018;2:81–94. doi: 10.1038/nrclinonc.2017.166. [DOI] [PubMed] [Google Scholar]
- 30.Vaupel P., Mayer A. Hypoxia in cancer: significance and impact on clinical outcome. Canc Metastasis Rev. 2007;2:225–239. doi: 10.1007/s10555-007-9055-1. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y., Shang W., Niu M., Tian J., Xu K. Hypoxia-active nanoparticles used in tumor theranostic. Int J Nanomed. 2019;14:3705–3722. doi: 10.2147/IJN.S196959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumari R., Sunil D., Ningthoujam R.S. Hypoxia-responsive nanoparticle based drug delivery systems in cancer therapy: an up-to-date review. J Control Release. 2020;319:135–156. doi: 10.1016/j.jconrel.2019.12.041. [DOI] [PubMed] [Google Scholar]
- 33.Bae Y.M., Park Y.I., Nam S.H., Kim J.H., Lee K., Kim H.M. Endocytosis, intracellular transport, and exocytosis of lanthanide-doped upconverting nanoparticles in single living cells. Biomaterials. 2012;35:9080–9086. doi: 10.1016/j.biomaterials.2012.08.039. [DOI] [PubMed] [Google Scholar]
- 34.Kwon E.J., Lo J.H., Bhatia S.N. Smart nanosystems: bio-inspired technologies that interact with the host environment. Proc Natl Acad Sci U S A. 2015;47:14460–14466. doi: 10.1073/pnas.1508522112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Colby A.H., Oberlies N.H., Pearce C.J., Herrera V.L.M., Colson Y.L., Grinstaff M.W. Nanoparticle drug-delivery systems for peritoneal cancers: a case study of the design, characterization and development of the expansile nanoparticle. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2017;3:1451–1482. doi: 10.1002/wnan.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruan S., Cao X., Cun X., Hu G., Zhou Y., Zhang Y. Matrix metalloproteinase-sensitive size-shrinkable nanoparticles for deep tumor penetration and ph triggered doxorubicin release. Biomaterials. 2015;60:100–110. doi: 10.1016/j.biomaterials.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 37.Hu C., Cun X., Ruan S., Liu R., Xiao W., Yang X. Enzyme-triggered size shrink and laser-enhanced no release nanoparticles for deep tumor penetration and combination therapy. Biomaterials. 2018;168:64–75. doi: 10.1016/j.biomaterials.2018.03.046. [DOI] [PubMed] [Google Scholar]
- 38.Meng H., Xing G., Blanco E., Song Y., Zhao L., Sun B. Gadolinium metallofullerenol nanoparticles inhibit cancer metastasis through matrix metalloproteinase inhibition: imprisoning instead of poisoning cancer cells. Nanomed. 2012;2:136–146. doi: 10.1016/j.nano.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cathcart J., Pulkoski-Gross A., Cao J. Targeting matrix metalloproteinases in cancer: bringing new life to old ideas. Genes Dis. 2015;1:26–34. doi: 10.1016/j.gendis.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Y., Chen X., Han W., Zhang Y. Tisagenlecleucel, an approved anti-cd19 chimeric antigen receptor t-cell therapy for the treatment of leukemia. Drugs Today. 2017;11:597–608. doi: 10.1358/dot.2017.53.11.2725754. [DOI] [PubMed] [Google Scholar]
- 41.Boyiadzis M.M., Dhodapkar M.V., Brentjens R.J., Kochenderfer J.N., Neelapu S.S., Maus M.V. Chimeric antigen receptor (CAT) T therapies for the treatment of hematologic malignancies: clinical perspective and significance. J Immunother Cancer. 2018;1:137–148. doi: 10.1186/s40425-018-0460-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raedler L.A. Keytruda (pembrolizumab): first PD-1 inhibitor approved for previously treated unresectable or metastatic melanoma. Am Health Drug Benefits. 2015;8:96–100. [PMC free article] [PubMed] [Google Scholar]
- 43.Gong J., Chehrazi-Raffle A., Reddi S., Salgia R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J Immunother Cancer. 2018;1:8–25. doi: 10.1186/s40425-018-0316-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seidel J.A., Otsuka A., Kabashima K. Anti-PD-1 and anti-CTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations. Front Oncol. 2018;8:86–99. doi: 10.3389/fonc.2018.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frantz C., Stewart K.M., Weaver V.M. The extracellular matrix at a glance. J Cell Sci. 2010;24:4195–4200. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Engin A.B., Nikitovic D., Neagu M., Henrich-Noack P., Docea A.O., Shtilman M.I. Mechanistic understanding of nanoparticles' interactions with extracellular matrix: the cell and immune system. Part Fibre Toxicol. 2017;1:22–37. doi: 10.1186/s12989-017-0199-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reid S.E., Kay E.J., Neilson L.J., Henze A.T., Serneels J., McGhee E.J. Tumor matrix stiffness promotes metastatic cancer cell interaction with the endothelium. EMBO J. 2017;16:2373–2389. doi: 10.15252/embj.201694912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bandara S.R., Molley T.G., Kim H., Bharath P.A., Kilian K.A., Leal C. The structural fate of lipid nanoparticles in the extracellular matrix. Mater Horiz. 2020;1:125–134. doi: 10.1039/C9MH00835G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McKleroy W., Lee T.-H., Atabai K. Always cleave up your mess: targeting collagen degradation to treat tissue fibrosis. Am J Physiol Lung Cell Mol Physiol. 2013;11:L709–L721. doi: 10.1152/ajplung.00418.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pan A., Wang Z., Chen B., Dai W., Zhang H., He B. Localized co-delivery of collagenase and trastuzumab by thermosensitive hydrogels for enhanced antitumor efficacy in human breast xenograft. Drug Deliv. 2018;1:1495–1503. doi: 10.1080/10717544.2018.1474971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu J., Liao S., Diop-Frimpong B., Chen W., Goel S., Naxerova K. Tgf-β blockade improves the distribution and efficacy of therapeutics in breast carcinoma by normalizing the tumor stroma. Proc Natl Acad Sci U S A. 2012;41:16618–16623. doi: 10.1073/pnas.1117610109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li B., Clemons T.D., Agarwal V., Kretzmann J., Bradshaw M., Toshniwal P. Regulation of collagen expression using nanoparticle mediated inhibition of TGF-β activation. New J Chem. 2016;2:1091–1095. [Google Scholar]
- 53.Kanapathipillai M., Mammoto A., Mammoto T., Kang J.H., Jiang E., Ghosh K. Inhibition of mammary tumor growth using lysyl oxidase-targeting nanoparticles to modify extracellular matrix. Nano Lett. 2012;6:3213–3217. doi: 10.1021/nl301206p. [DOI] [PubMed] [Google Scholar]
- 54.Eikenes L., Bruland O.S., Brekken C., Davies Cde L. Collagenase increases the transcapillary pressure gradient and improves the uptake and distribution of monoclonal antibodies in human osteosarcoma xenografts. Cancer Res. 2004;14:4768–4773. doi: 10.1158/0008-5472.CAN-03-1472. [DOI] [PubMed] [Google Scholar]
- 55.Murty S., Gilliland T., Qiao P., Tabtieng T., Higbee E., Al Zaki A. Nanoparticles functionalized with collagenase exhibit improved tumor accumulation in a murine xenograft model. Part Part Syst Char. 2014;12:1307–1312. doi: 10.1002/ppsc.201400169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kato M., Hattori Y., Kubo M., Maitani Y. Collagenase-1 injection improved tumor distribution and gene expression of cationic lipoplex. Int J Pharm. 2012;2:428–434. doi: 10.1016/j.ijpharm.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 57.Abdolahinia E.D., Nadri S., Rahbarghazi R., Barar J., Aghanejad A., Omidi Y. Enhanced penetration and cytotoxicity of metformin and collagenase conjugated gold nanoparticles in breast cancer spheroids. Life Sci. 2019;231:116545–116554. doi: 10.1016/j.lfs.2019.116545. [DOI] [PubMed] [Google Scholar]
- 58.Xu F., Huang X., Wang Y., Zhou S. A size-changeable collagenase-modified nanoscavenger for increasing penetration and retention of nanomedicine in deep tumor tissue. Adv Mater. 2020;16:1906745–1906756. doi: 10.1002/adma.201906745. [DOI] [PubMed] [Google Scholar]
- 59.Lokeshwar V.B., Mirza S., Jordan A. Targeting hyaluronic acid family for cancer chemoprevention and therapy. Adv Canc Res. 2014:35–65. doi: 10.1016/B978-0-12-800092-2.00002-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim J.H., Moon M.J., Kim D.Y., Heo S.H., Jeong Y.Y. Hyaluronic acid-based nanomaterials for cancer therapy. Polymers. 2018;10:1133–1147. doi: 10.3390/polym10101133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim K., Choi H., Choi E.S., Park M.-H., Ryu J.-H. Hyaluronic acid-coated nanomedicine for targeted cancer therapy. Pharmaceutics. 2019;7:301–322. doi: 10.3390/pharmaceutics11070301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Whatcott C.J., Han H., Posner R.G., Hostetter G., Von Hoff D.D. Targeting the tumor microenvironment in cancer: why hyaluronidase deserves a second look. Canc Discov. 2011;4:291–296. doi: 10.1158/2159-8290.CD-11-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abyaneh H.S., Regenold M., McKee T.D., Allen C., Gauthier M.A. Towards extracellular matrix normalization for improved treatment of solid tumors. Theranostics. 2020;4:1960–1980. doi: 10.7150/thno.39995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Infante J.R., Korn R.L., Rosen L.S., LoRusso P., Dychter S.S., Zhu J. Phase 1 trials of pegylated recombinant human hyaluronidase ph20 in patients with advanced solid tumours. Br J Canc. 2018;2:153–161. doi: 10.1038/bjc.2017.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Doherty G.J., Tempero M., Corrie P.G. Halo-109-301: a phase iii trial of pegph20 (with gemcitabine and NAB-paclitaxel) in hyaluronic acid-high stage iv pancreatic cancer. Future Oncol. 2018;1:13–22. doi: 10.2217/fon-2017-0338. [DOI] [PubMed] [Google Scholar]
- 66.Hingorani S.R., Zheng L., Bullock A.J., Seery T.E., Harris W.P., Sigal D.S. Halo 202: randomized phase ii study of pegph20 plus BAN-paclitaxel/gemcitabine versus NAB-paclitaxel/gemcitabine in patients with untreated, metastatic pancreatic ductal adenocarcinoma. J Clin Oncol. 2018;4:359–366. doi: 10.1200/JCO.2017.74.9564. [DOI] [PubMed] [Google Scholar]
- 67.Ramanathan R.K., McDonough S.L., Philip P.A., Hingorani S.R., Lacy J., Kortmansky J.S. Phase Ib/II randomized study of folfirinox plus pegylated recombinant human hyaluronidase versus folfirinox alone in patients with metastatic pancreatic adenocarcinoma: SWOG S1313. J Clin Oncol. 2019;13:1062–1069. doi: 10.1200/JCO.18.01295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Luo Z., Dai Y., Gao H. Development and application of hyaluronic acid in tumor targeting drug delivery. Acta Pharm Sin B. 2019;6:1099–1112. doi: 10.1016/j.apsb.2019.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou H., Fan Z., Deng J., Lemons P.K., Arhontoulis D.C., Bowne W.B. Hyaluronidase embedded in nanocarrier peg shell for enhanced tumor penetration and highly efficient antitumor efficacy. Nano Lett. 2016;5:3268–3277. doi: 10.1021/acs.nanolett.6b00820. [DOI] [PubMed] [Google Scholar]
- 70.Lee S., Han H., Koo H., Na J.H., Yoon H.Y., Lee K.E. Extracellular matrix remodeling in vivo for enhancing tumor-targeting efficiency of nanoparticle drug carriers using the pulsed high intensity focused ultrasound. J Control Release. 2017;263:68–78. doi: 10.1016/j.jconrel.2017.02.035. [DOI] [PubMed] [Google Scholar]
- 71.Shao K., Hou Q., Go M.L., Duan W., Cheung N.S., Feng S.S. Sulfatide-tenascin interaction mediates binding to the extracellular matrix and endocytic uptake of liposomes in glioma cells. Cell Mol Life Sci. 2007;4:506–515. doi: 10.1007/s00018-007-6419-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kumra H., Reinhardt D.P. Fibronectin-targeted drug delivery in cancer. Adv Drug Deliv Rev. 2016;97:101–110. doi: 10.1016/j.addr.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 73.Zhang B., Shen S., Liao Z., Shi W., Wang Y., Zhao J. Targeting fibronectins of glioma extracellular matrix by CLT1 peptide-conjugated nanoparticles. Biomaterials. 2014;13:4088–4098. doi: 10.1016/j.biomaterials.2014.01.046. [DOI] [PubMed] [Google Scholar]
- 74.Upreti M., Jyoti A., Johnson S.E., Swindell E.P., Napier D., Sethi P. Radiation-enhanced therapeutic targeting of galectin-1 enriched malignant stroma in triple negative breast cancer. Oncotarget. 2016;27:41559–41574. doi: 10.18632/oncotarget.9490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Raavé R., van Kuppevelt T.H., Daamen W.F. Chemotherapeutic drug delivery by tumoral extracellular matrix targeting. J Control Release. 2018;274:1–8. doi: 10.1016/j.jconrel.2018.01.029. [DOI] [PubMed] [Google Scholar]
- 76.Lowy C.M., Oskarsson T. Tenascin c in metastasis: a view from the invasive front. Cell Adhes Migrat. 2015;1‒2:112–124. doi: 10.1080/19336918.2015.1008331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gocheva V., Naba A., Bhutkar A., Guardia T., Miller K.M., Li C.M. Quantitative proteomics identify tenascin-c as a promoter of lung cancer progression and contributor to a signature prognostic of patient survival. Proc Natl Acad Sci U S A. 2017;28:5625–5634. doi: 10.1073/pnas.1707054114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Didem T., Faruk T., Senem K., Derya D., Murat S., Murat G. Clinical significance of serum tenascin-C levels in epithelial ovarian cancer. Tumour Biol. 2014;7:6777–6782. doi: 10.1007/s13277-014-1923-z. [DOI] [PubMed] [Google Scholar]
- 79.Orend G., Chiquet-Ehrismann R. Tenascin-C induced signaling in cancer. Canc Lett. 2006;2:143–163. doi: 10.1016/j.canlet.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 80.Kang T., Zhu Q., Jiang D., Feng X., Feng J., Jiang T. Synergistic targeting tenascin C and neuropilin-1 for specific penetration of nanoparticles for anti-glioblastoma treatment. Biomaterials. 2016;101:60–75. doi: 10.1016/j.biomaterials.2016.05.037. [DOI] [PubMed] [Google Scholar]
- 81.Lingasamy P., Tobi A., Haugas M., Hunt H., Paiste P., Asser T. Bi-specific tenascin-C and fibronectin targeted peptide for solid tumor delivery. Biomaterials. 2019;219:119373–119383. doi: 10.1016/j.biomaterials.2019.119373. [DOI] [PubMed] [Google Scholar]
- 82.Parks W.C., Wilson C.L., Lopez-Boado Y.S. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004;8:617–629. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- 83.Fang M., Yuan J., Peng C., Li Y. Collagen as a double-edged sword in tumor progression. Tumour Biol. 2014;4:2871–2882. doi: 10.1007/s13277-013-1511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Page-McCaw A., Ewald A.J., Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;3:221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Walker C., Mojares E., Hernández Del Río. A role of extracellular matrix in development and cancer progression. Int J Mol Sci. 2018;10:3028–3058. doi: 10.3390/ijms19103028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bonnans C., Chou J., Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;12:786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fan F., Schimming A., Jaeger D., Podar K. Targeting the tumor microenvironment: focus on angiogenesis. J Oncol. 2012;2012:281261–281277. doi: 10.1155/2012/281261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Teichert M., Milde L., Holm A., Stanicek L., Gengenbacher N., Savant S. Pericyte-expressed tie 2 controls angiogenesis and vessel maturation. Nat Commun. 2017;1:16106–16117. doi: 10.1038/ncomms16106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wei X., Chen X., Ying M., Lu W. Brain tumor-targeted drug delivery strategies. Acta Pharm Sin B. 2014;3:193–201. doi: 10.1016/j.apsb.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.van Cruijsen H., Giaccone G., Hoekman K. Epidermal growth factor receptor and angiogenesis: opportunities for combined anticancer strategies. Int J Canc. 2005;6:883–888. doi: 10.1002/ijc.21479. [DOI] [PubMed] [Google Scholar]
- 91.Minder P., Zajac E., Quigley J.P., Deryugina E.I. EGFR regulates the development and microarchitecture of intratumoral angiogenic vasculature capable of sustaining cancer cell intravasation. Neoplasia. 2015;8:634–649. doi: 10.1016/j.neo.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chung A.S., Lee J., Ferrara N. Targeting the tumour vasculature: insights from physiological angiogenesis. Nat Rev Canc. 2010;7:505–514. doi: 10.1038/nrc2868. [DOI] [PubMed] [Google Scholar]
- 93.Sakurai Y., Akita H., Harashima H. Targeting tumor endothelial cells with nanoparticles. Int J Mol Sci. 2019;23:5819–5833. doi: 10.3390/ijms20235819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Weis S.M., Cheresh D.A. Αv integrins in angiogenesis and cancer. Cold Spring Harb Perspect Med. 2011;1:a006478. doi: 10.1101/cshperspect.a006478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhan C., Gu B., Xie C., Li J., Liu Y., Lu W. Cyclic RGD conjugated poly(ethylene glycol)-co-poly(lactic acid) micelle enhances paclitaxel anti-glioblastoma effect. J Control Release. 2010;1:136–142. doi: 10.1016/j.jconrel.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 96.Sakurai Y., Hada T., Kato A., Hagino Y., Mizumura W., Harashima H. Effective therapy using a liposomal siRNA that targets the tumor vasculature in a model murine breast cancer with lung metastasis. Mol Ther Oncolytics. 2018;11:102–108. doi: 10.1016/j.omto.2018.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Seidi K., Jahanban-Esfahlan R., Monhemi H., Zare P., Minofar B., Daei Farshchi Adli A. NGR (Asn-Gly-Arg)-targeted delivery of coagulase to tumor vasculature arrests cancer cell growth. Oncogene. 2018;29:3967–3980. doi: 10.1038/s41388-018-0213-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Agemy L., Kotamraju V.R., Friedmann-Morvinski D., Sharma S., Sugahara K.N., Ruoslahti E. Proapoptotic peptide-mediated cancer therapy targeted to cell surface p32. Mol Ther. 2013;12:2195–2204. doi: 10.1038/mt.2013.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ebos J.M., Lee C.R., Kerbel R.S. Tumor and host-mediated pathways of resistance and disease progression in response to antiangiogenic therapy. Clin Canc Res. 2009;16:5020–5025. doi: 10.1158/1078-0432.CCR-09-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Abdalla A.M.E., Xiao L., Ullah M.W., Yu M., Ouyang C., Yang G. Current challenges of cancer anti-angiogenic therapy and the promise of nanotherapeutics. Theranostics. 2018;2:533–548. doi: 10.7150/thno.21674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen M., Lei X., Shi C., Huang M., Li X., Wu B. Pericyte-targeting prodrug overcomes tumor resistance to vascular disrupting agents. J Clin Invest. 2017;10:3689–3701. doi: 10.1172/JCI94258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Martin J.D., Seano G., Jain R.K. Normalizing function of tumor vessels: progress, opportunities, and challenges. Annu Rev Physiol. 2019;81:505–534. doi: 10.1146/annurev-physiol-020518-114700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kelly-Goss M.R., Sweat R.S., Stapor P.C., Peirce S.M., Murfee W.L. Targeting pericytes for angiogenic therapies. Microcirculation (N Y) 2014;4:345–357. doi: 10.1111/micc.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ribeiro A.L., Okamoto O.K. Combined effects of pericytes in the tumor microenvironment. Stem Cell Int. 2015;n/a:868475. doi: 10.1155/2015/868475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Franco M., Roswall P., Cortez E., Hanahan D., Pietras K. Pericytes promote endothelial cell survival through induction of autocrine VEGF-a signaling and BCL-w expression. Blood. 2011;10:2906–2917. doi: 10.1182/blood-2011-01-331694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Eilken H.M., Diéguez-Hurtado R., Schmidt I., Nakayama M., Jeong H.-W., Arf H. Pericytes regulate vegf-induced endothelial sprouting through VEGFR1. Nat Commun. 2017;1:1574–1587. doi: 10.1038/s41467-017-01738-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Armulik A., Genové G., Mäe M., Nisancioglu M.H., Wallgard E., Niaudet C. Pericytes regulate the blood–brain barrier. Nature. 2010;7323:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 108.Guan Y.Y., Luan X., Xu J.R., Liu Y.R., Lu Q., Wang C. Selective eradication of tumor vascular pericytes by peptide-conjugated nanoparticles for antiangiogenic therapy of melanoma lung metastasis. Biomaterials. 2014;9:3060–3070. doi: 10.1016/j.biomaterials.2013.12.027. [DOI] [PubMed] [Google Scholar]
- 109.Roy Chaudhuri T., Straubinger N.L., Pitoniak R.F., Hylander B.L., Repasky E.A., Ma W.W. Tumor-priming smoothened inhibitor enhances deposition and efficacy of cytotoxic nanoparticles in a pancreatic cancer model. Mol Canc Therapeut. 2016;1:84–93. doi: 10.1158/1535-7163.MCT-15-0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Armulik A., Genove G., Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;2:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 111.Nisancioglu M.H., Betsholtz C., Genove G. The absence of pericytes does not increase the sensitivity of tumor vasculature to vascular endothelial growth factor-a blockade. Cancer Res. 2010;12:5109–5115. doi: 10.1158/0008-5472.CAN-09-4245. [DOI] [PubMed] [Google Scholar]
- 112.Ramaswamy S., Ross K.N., Lander E.S., Golub T.R. A molecular signature of metastasis in primary solid tumors. Nat Genet. 2003;1:49–54. doi: 10.1038/ng1060. [DOI] [PubMed] [Google Scholar]
- 113.Brennen W.N., Rosen D.M., Wang H., Isaacs J.T., Denmeade S.R. Targeting carcinoma-associated fibroblasts within the tumor stroma with a fibroblast activation protein-activated prodrug. J Natl Cancer Inst. 2012;17:1320–1334. doi: 10.1093/jnci/djs336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang B., Hu Y., Pang Z. Modulating the tumor microenvironment to enhance tumor nanomedicine delivery. Front Pharmacol. 2017;8:952. doi: 10.3389/fphar.2017.00952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liu T., Zhou L., Li D., Andl T., Zhang Y. Cancer-associated fibroblasts build and secure the tumor microenvironment. Front Cell Dev Biol. 2019;7:60–73. doi: 10.3389/fcell.2019.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu T., Han C., Wang S., Fang P., Ma Z., Xu L. Cancer-associated fibroblasts: an emerging target of anti-cancer immunotherapy. J Hematol Oncol. 2019;12:86–100. doi: 10.1186/s13045-019-0770-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang B., Jin K., Jiang T., Wang L., Shen S., Luo Z. Celecoxib normalizes the tumor microenvironment and enhances small nanotherapeutics delivery to a549 tumors in nude mice. Sci Rep. 2017;1:10071–10082. doi: 10.1038/s41598-017-09520-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chen B., Wang Z., Sun J., Song Q., He B., Zhang H. A tenascin C targeted nanoliposome with navitoclax for specifically eradicating of cancer-associated fibroblasts. Nanomed. 2016;1:131–141. doi: 10.1016/j.nano.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 119.Kovács D., Igaz N., Marton A., Rónavári A., Bélteky P., Bodai L. Core–shell nanoparticles suppress metastasis and modify the tumour-supportive activity of cancer-associated fibroblasts. J Nanobiotechnol. 2020;1:18–37. doi: 10.1186/s12951-020-0576-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sahai E., Astsaturov I., Cukierman E., DeNardo D.G., Egeblad M., Evans R.M. A framework for advancing our understanding of cancer-associated fibroblasts. Nat Rev Canc. 2020;3:174–186. doi: 10.1038/s41568-019-0238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schlesinger M. Role of platelets and platelet receptors in cancer metastasis. J Hematol Oncol. 2018;1:125–139. doi: 10.1186/s13045-018-0669-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yan M., Jurasz P. The role of platelets in the tumor microenvironment: from solid tumors to leukemia. Biochim Biophys Acta Mol Cell Res. 2016;3:392–400. doi: 10.1016/j.bbamcr.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 123.Palacios-Acedo A.L., Mège D., Crescence L., Dignat-George F., Dubois C., Panicot-Dubois L. Platelets, thrombo-inflammation, and cancer: collaborating with the enemy. Front Immunol. 2019;10:1805–1814. doi: 10.3389/fimmu.2019.01805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ho-Tin-Noé B., Goerge T., Wagner D.D. Platelets: guardians of tumor vasculature. Cancer Res. 2009;14:5623–5626. doi: 10.1158/0008-5472.CAN-09-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhang Y., Wei J., Liu S., Wang J., Han X., Qin H. Inhibition of platelet function using liposomal nanoparticles blocks tumor metastasis. Theranostics. 2017;5:1062–1071. doi: 10.7150/thno.17908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hu C.M., Fang R.H., Wang K.C., Luk B.T., Thamphiwatana S., Dehaini D. Nanoparticle biointerfacing by platelet membrane cloaking. Nature. 2015;7571:118–121. doi: 10.1038/nature15373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rao L., Bu L.L., Ma L., Wang W., Liu H., Wan D. Platelet-facilitated photothermal therapy of head and neck squamous cell carcinoma. Angew Chem Int Ed Engl. 2018;4:986–991. doi: 10.1002/anie.201709457. [DOI] [PubMed] [Google Scholar]
- 128.Hu Q., Sun W., Qian C., Wang C., Bomba H.N., Gu Z. Anticancer platelet-mimicking nanovehicles. Adv Mater. 2015;44:7043–7050. doi: 10.1002/adma.201503323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Xu P., Zuo H., Zhou R., Wang F., Liu X., Ouyang J. Doxorubicin-loaded platelets conjugated with anti-CD22 mabs: a novel targeted delivery system for lymphoma treatment with cardiopulmonary avoidance. Oncotarget. 2017;35:58322–58337. doi: 10.18632/oncotarget.16871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang H., Wu J., Williams G.R., Fan Q., Niu S., Wu J. Platelet-membrane-biomimetic nanoparticles for targeted antitumor drug delivery. J Nanobiotechnol. 2019;1:60–75. doi: 10.1186/s12951-019-0494-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Haemmerle M., Stone R.L., Menter D.G., Afshar-Kharghan V., Sood A.K. The platelet lifeline to cancer: challenges and opportunities. Canc Cell. 2018;6:965–983. doi: 10.1016/j.ccell.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Han S., Huang K., Gu Z., Wu J. Tumor immune microenvironment modulation-based drug delivery strategies for cancer immunotherapy. Nanoscale. 2020;2:413–436. doi: 10.1039/c9nr08086d. [DOI] [PubMed] [Google Scholar]
- 133.Zhang N., Bevan M.J. CD8+ T cells: foot soldiers of the immune system. Immunity. 2011;2:161–168. doi: 10.1016/j.immuni.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Andersen M.H., Schrama D., thor Straten P., Becker J.C. Cytotoxic T cells. J Invest Dermatol. 2006;1:32–41. doi: 10.1038/sj.jid.5700001. [DOI] [PubMed] [Google Scholar]
- 135.Bohner P., Chevalier M.F., Cesson V., Rodrigues-Dias S.-C., Dartiguenave F., Burruni R. Double positive CD4+CD8+ T cells are enriched in urological cancers and favor T helper-2 polarization. Front Immunol. 2019;10:622–631. doi: 10.3389/fimmu.2019.00622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nguyen K., Li J., Puthenveetil R., Lin X., Poe M.M., Hsiao C.-H.C. The butyrophilin 3a1 intracellular domain undergoes a conformational change involving the juxtamembrane region. Faseb J. 2017;11:4697–4706. doi: 10.1096/fj.201601370RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Balato A., Unutmaz D., Gaspari A.A. Natural killer t cells: an unconventional t-cell subset with diverse effector and regulatory functions. J Invest Dermatol. 2009;7:1628–1642. doi: 10.1038/jid.2009.30. [DOI] [PubMed] [Google Scholar]
- 138.Patel O., Cameron G., Pellicci D.G., Liu Z., Byun H.-S., Beddoe T. NKT TCR recognition of CD1d-α-C-galactosylceramide. J Immunol. 2011;9:4705–4713. doi: 10.4049/jimmunol.1100794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mahnke Y.D., Brodie T.M., Sallusto F., Roederer M., Lugli E. The who's who of T-cell differentiation: human memory T-cell subsets. Eur J Immunol. 2013;11:2797–2809. doi: 10.1002/eji.201343751. [DOI] [PubMed] [Google Scholar]
- 140.Kuss I., Donnenberg A.D., Gooding W., Whiteside T.L. Effector CD8+CD45RO−Cd27−T cells have signalling defects in patients with squamous cell carcinoma of the head and neck. Br J Canc. 2003;2:223–230. doi: 10.1038/sj.bjc.6600694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Curiel T.J., Coukos G., Zou L., Alvarez X., Cheng P., Mottram P. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;9:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 142.Tsuda S., Nakashima A., Shima T., Saito S. New paradigm in the role of regulatory T cells during pregnancy. Front Immunol. 2019;10:573–583. doi: 10.3389/fimmu.2019.00573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Corthay A. How do regulatory T cells work?. Scand J Immunol. 2009;4:326–336. doi: 10.1111/j.1365-3083.2009.02308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Guo F.F., Cui J.W. The role of tumor-infiltrating B cells in tumor immunity. J Oncol. 2019;n/a:2592419–2592427. doi: 10.1155/2019/2592419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sarvaria A., Madrigal J.A., Saudemont A. B cell regulation in cancer and anti-tumor immunity. Cell Mol Immunol. 2017;8:662–674. doi: 10.1038/cmi.2017.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Oleinika K., Rosser E.C., Matei D.E., Nistala K., Bosma A., Drozdov I. CD1d-dependent immune suppression mediated by regulatory B cells through modulations of iNKT cells. Nat Commun. 2018;1:684–700. doi: 10.1038/s41467-018-02911-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bassani B., Baci D., Gallazzi M., Poggi A., Bruno A., Mortara L. Natural killer cells as key players of tumor progression and angiogenesis: old and novel tools to divert their pro-tumor activities into potent anti-tumor effects. Cancers. 2019;4:461–489. doi: 10.3390/cancers11040461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhang M., He Y., Sun X., Li Q., Wang W., Zhao A. A high M1/M2 ratio of tumor-associated macrophages is associated with extended survival in ovarian cancer patients. J Ovarian Res. 2014;1:7–19. doi: 10.1186/1757-2215-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pinto M.L., Rios E., Durães C., Ribeiro R., Machado J.C., Mantovani A. The two faces of tumor-associated macrophages and their clinical significance in colorectal cancer. Front Immunol. 2019;10:1875–1886. doi: 10.3389/fimmu.2019.01875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Haque A.S.M.R., Moriyama M., Kubota K., Ishiguro N., Sakamoto M., Chinju A. CD206+ tumor-associated macrophages promote proliferation and invasion in oral squamous cell carcinoma via EGF production. Sci Rep. 2019;1:14611–14619. doi: 10.1038/s41598-019-51149-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Veglia F., Perego M., Gabrilovich D. Myeloid-derived suppressor cells coming of age. Nat Immunol. 2018;2:108–119. doi: 10.1038/s41590-017-0022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Carus A., Ladekarl M., Hager H., Nedergaard B.S., Donskov F. Tumour-associated CD66b+ neutrophil count is an independent prognostic factor for recurrence in localised cervical cancer. Br J Canc. 2013;10:2116–2122. doi: 10.1038/bjc.2013.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Rhodes J.W., Tong O., Harman A.N., Turville S.G. Human dendritic cell subsets, ontogeny, and impact on HIV infection. Front Immunol. 2019;10:1088–1111. doi: 10.3389/fimmu.2019.01088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Satpathy A.T., Wu X., Albring J.C., Murphy K.M. Re(de)fining the dendritic cell lineage. Nat Immunol. 2012;12:1145–1154. doi: 10.1038/ni.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jing W., McAllister D., Vonderhaar E.P., Palen K., Riese M.J., Gershan J. Sting agonist inflames the pancreatic cancer immune microenvironment and reduces tumor burden in mouse models. 2019;1:115. doi: 10.1186/s40425-019-0573-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Olingy C.E., Dinh H.Q., Hedrick C.C. Monocyte heterogeneity and functions in cancer. J Leukoc Biol. 2019;2:309–322. doi: 10.1002/JLB.4RI0818-311R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ley K. M1 means kill; M2 means heal. J Immunol. 2017;7:2191–2193. doi: 10.4049/jimmunol.1701135. [DOI] [PubMed] [Google Scholar]
- 158.Quail D.F., Joyce J.A. The microenvironmental landscape of brain tumors. Canc Cell. 2017;3:326–341. doi: 10.1016/j.ccell.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kim J., Bae J.S. Tumor-associated macrophages and neutrophils in tumor microenvironment. Mediat Inflamm. 2016;n/a:6058147. doi: 10.1155/2016/6058147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Liu Y., Cao X. The origin and function of tumor-associated macrophages. Cell Mol Immunol. 2015;1:1–4. doi: 10.1038/cmi.2014.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sica A., Larghi P., Mancino A., Rubino L., Porta C., Totaro M.G. Macrophage polarization in tumour progression. Semin Canc Biol. 2008;5:349–455. doi: 10.1016/j.semcancer.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 162.Laoui D., Movahedi K., Van Overmeire E., Van den Bossche J., Schouppe E., Mommer C. Tumor-associated macrophages in breast cancer: distinct subsets, distinct functions. Int J Dev Biol. 2011;7–9:861–867. doi: 10.1387/ijdb.113371dl. [DOI] [PubMed] [Google Scholar]
- 163.Jing W., McAllister D., Vonderhaar E.P., Palen K., Riese M.J., Gershan J. Sting agonist inflames the pancreatic cancer immune microenvironment and reduces tumor burden in mouse models. J Immunother Cancer. 2019;1:115–132. doi: 10.1186/s40425-019-0573-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhang M., He Y., Sun X., Li Q., Wang W., Zhao A. A high M1/M2 ratio of tumor-associated macrophages is associated with extended survival in ovarian cancer patients. J Ovarian Res. 2014;7:19. doi: 10.1186/1757-2215-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Conde J., Bao C., Tan Y., Cui D., Edelman E.R., Azevedo H.S. Dual targeted immunotherapy via in vivo delivery of biohybrid RNAi-peptide nanoparticles to tumour-associated macrophages and cancer cells. Adv Funct Mater. 2015;27:4183–4194. doi: 10.1002/adfm.201501283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Shen S., Zhang Y., Chen K.G., Luo Y.L., Wang J. Cationic polymeric nanoparticle delivering CCR2 siRNA to inflammatory monocytes for tumor microenvironment modification and cancer therapy. Mol Pharm. 2018;9:3642–3653. doi: 10.1021/acs.molpharmaceut.7b00997. [DOI] [PubMed] [Google Scholar]
- 167.Huang Z., Zhang Z., Jiang Y., Zhang D., Chen J., Dong L. Targeted delivery of oligonucleotides into tumor-associated macrophages for cancer immunotherapy. J Control Release. 2012;2:286–292. doi: 10.1016/j.jconrel.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 168.Li K., Lu L., Xue C., Liu J., He Y. Polarization of tumor-associated macrophage phenotype via porous hollow iron nanoparticles for tumor immunotherapy in vivo. Nanoscale. 2020;1:130–144. doi: 10.1039/c9nr06505a. [DOI] [PubMed] [Google Scholar]
- 169.Song M., Liu T., Shi C., Zhang X., Chen X. Bioconjugated manganese dioxide nanoparticles enhance chemotherapy response by priming tumor-associated macrophages toward M1-like phenotype and attenuating tumor hypoxia. ACS Nano. 2016;1:633–647. doi: 10.1021/acsnano.5b06779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Das M., Shen L., Liu Q., Goodwin T.J., Huang L. Nanoparticle delivery of RIG-I agonist enables effective and safe adjuvant therapy in pancreatic cancer. Mol Ther. 2019;3:507–517. doi: 10.1016/j.ymthe.2018.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Rong L., Zhang Y., Li W.-S., Su Z., Fadhil J.I., Zhang C. Iron chelated melanin-like nanoparticles for tumor-associated macrophage repolarization and cancer therapy. Biomaterials. 2019;225:119515–119523. doi: 10.1016/j.biomaterials.2019.119515. [DOI] [PubMed] [Google Scholar]
- 172.Pang L., Pei Y., Uzunalli G., Hyun H., Lyle L.T., Yeo Y. Surface modification of polymeric nanoparticles with M2pep peptide for drug delivery to tumor-associated macrophages. Pharm Res (N Y) 2019;4:65–87. doi: 10.1007/s11095-019-2596-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Patwardhan P.P., Surriga O., Beckman M.J., de Stanchina E., Dematteo R.P., Tap W.D. Sustained inhibition of receptor tyrosine kinases and macrophage depletion by PLX3397 and rapamycin as a potential new approach for the treatment of MPNSTs. Clin Canc Res. 2014;12:3146–3158. doi: 10.1158/1078-0432.CCR-13-2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Coussens L.M., Werb Z. Inflammation and cancer. Nature. 2002;6917:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kim E.R., Chang D.K. Colorectal cancer in inflammatory bowel disease: the risk, pathogenesis, prevention and diagnosis. World J Gastroenterol. 2014;29:9872–9881. doi: 10.3748/wjg.v20.i29.9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Souza R.F. From reflux esophagitis to esophageal adenocarcinoma. Dig Dis. 2016;5:483–490. doi: 10.1159/000445225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mantovani A., Barajon I., Garlanda C. IL-1 and IL-1 regulatory pathways in cancer progression and therapy. Immunol Rev. 2018;1:57–61. doi: 10.1111/imr.12614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Pelegrin P. Targeting interleukin-1 signaling in chronic inflammation: focus on P2X7 receptor and pannexin-1. Drug News Perspect. 2008;8:424–433. doi: 10.1358/dnp.2008.21.8.1265800. [DOI] [PubMed] [Google Scholar]
- 179.Landskron G., De la Fuente M., Thuwajit P., Thuwajit C., Hermoso M.A. Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res. 2014;n/a:149185–149203. doi: 10.1155/2014/149185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Boraschi D., Italiani P., Weil S., Martin M.U. The family of the interleukin-1 receptors. Immunol Rev. 2018;1:197–232. doi: 10.1111/imr.12606. [DOI] [PubMed] [Google Scholar]
- 181.Garlanda C., Dinarello C.A., Mantovani A. The interleukin-1 family: back to the future. Immunity. 2013;6:1003–1018. doi: 10.1016/j.immuni.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Shevtsov M.A., Nikolaev B.P., Yakovleva L.Y., Dobrodumov A.V., Zhakhov A.V., Mikhrina A.L. Recombinant interleukin-1 receptor antagonist conjugated to superparamagnetic iron oxide nanoparticles for theranostic targeting of experimental glioblastoma. Neoplasia. 2015;1:32–42. doi: 10.1016/j.neo.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ponomarev A.V., Shubina I.Z. Insights into mechanisms of tumor and immune system interaction: association with wound healing. Front Oncol. 2019;9:1115–1130. doi: 10.3389/fonc.2019.01115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Marek A., Brodzicki J., Liberek A., Korzon M. TGF-beta (transforming growth factor-beta) in chronic inflammatory conditions—a new diagnostic and prognostic marker? Med Sci Mon Int Med J Exp Clin Res. 2002;7:145–151. [PubMed] [Google Scholar]
- 185.Yoshimura A., Wakabayashi Y., Mori T. Cellular and molecular basis for the regulation of inflammation by TGF-β. J Biochem. 2010;6:781–792. doi: 10.1093/jb/mvq043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zuo Z.Q., Chen K.G., Yu X.Y., Zhao G., Shen S., Cao Z.T. Promoting tumor penetration of nanoparticles for cancer stem cell therapy by TGF-beta signaling pathway inhibition. Biomaterials. 2016;82:48–59. doi: 10.1016/j.biomaterials.2015.12.014. [DOI] [PubMed] [Google Scholar]
- 187.Panagi M., Voutouri C., Mpekris F., Papageorgis P., Martin M.R., Martin J.D. TGF-β inhibition combined with cytotoxic nanomedicine normalizes triple negative breast cancer microenvironment towards anti-tumor immunity. Theranostics. 2020;4:1910–1922. doi: 10.7150/thno.36936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Galon J., Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov. 2019;3:197–218. doi: 10.1038/s41573-018-0007-y. [DOI] [PubMed] [Google Scholar]
- 189.Sacchetti B., Botticelli A., Pierelli L., Nuti M., Alimandi M. CAR-T with license to kill solid tumors in search of a winning strategy. Int J Mol Sci. 2019;8:1903–1917. doi: 10.3390/ijms20081903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Mogensen T.H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev. 2009;2:240–273. doi: 10.1128/CMR.00046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Motwani M., Pesiridis S., Fitzgerald K.A. DNA sensing by the cGAS–STING pathway in health and disease. Nat Rev Genet. 2019;11:657–674. doi: 10.1038/s41576-019-0151-1. [DOI] [PubMed] [Google Scholar]
- 192.Zhang H., You Q.-D., Xu X.-L. Targeting stimulator of interferon genes (STING): a medicinal chemistry perspective. J Med Chem. 2019;63:3785–3816. doi: 10.1021/acs.jmedchem.9b01039. [DOI] [PubMed] [Google Scholar]
- 193.Zhu Q., Man S.M., Gurung P., Liu Z., Vogel P., Lamkanfi M. Cutting edge: sting mediates protection against colorectal tumorigenesis by governing the magnitude of intestinal inflammation. J Immunol. 2014;10:4779–4782. doi: 10.4049/jimmunol.1402051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Demaria O., De Gassart A., Coso S., Gestermann N., Di Domizio J., Flatz L. Sting activation of tumor endothelial cells initiates spontaneous and therapeutic antitumor immunity. Proc Natl Acad Sci U S A. 2015;50:15408–15413. doi: 10.1073/pnas.1512832112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wang-Bishop L., Wehbe M., Shae D., James J., Hacker B.C., Garland K. Potent STING activation stimulates immunogenic cell death to enhance antitumor immunity in neuroblastoma. J Immunother Cancer. 2020;1:e000282–e000298. doi: 10.1136/jitc-2019-000282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Miao L., Qi J., Zhao Q., Wu Q.N., Wei D.L., Wei X.L. Targeting the sting pathway in tumor-associated macrophages regulates innate immune sensing of gastric cancer cells. Theranostics. 2020;2:498–515. doi: 10.7150/thno.37745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Wilson D.R., Sen R., Sunshine J.C., Pardoll D.M., Green J.J., Kim Y.J. Biodegradable sting agonist nanoparticles for enhanced cancer immunotherapy. Nanomed. 2018;2:237–246. doi: 10.1016/j.nano.2017.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Cheng N., Watkins-Schulz R., Junkins R.D., David C.N., Johnson B.M., Montgomery S.A. A nanoparticle-incorporated sting activator enhances antitumor immunity in PD-L1-insensitive models of triple-negative breast cancer. JCI Insight. 2018;22:120638–120657. doi: 10.1172/jci.insight.120638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Shae D., Becker K.W., Christov P., Yun D.S., Lytton-Jean A.K.R., Sevimli S. Endosomolytic polymersomes increase the activity of cyclic dinucleotide sting agonists to enhance cancer immunotherapy. Nat Nanotechnol. 2019;3:269–278. doi: 10.1038/s41565-018-0342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Chattopadhyay S., Liu Y.H., Fang Z.S., Lin C.L., Lin J.C., Yao B.Y. Synthetic immunogenic cell death mediated by intracellular delivery of sting agonist nanoshells enhances anticancer chemo-immunotherapy. Nano Lett. 2020;4:2246–2256. doi: 10.1021/acs.nanolett.9b04094. [DOI] [PubMed] [Google Scholar]
- 201.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;2:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 202.Hennessy E.J., Parker A.E., O'Neill L.A. Targeting toll-like receptors: emerging therapeutics?. Nat Rev Drug Discov. 2010;4:293–307. doi: 10.1038/nrd3203. [DOI] [PubMed] [Google Scholar]
- 203.Smith M., García-Martínez E., Pitter M.R., Fucikova J., Spisek R., Zitvogel L. Trial watch: toll-like receptor agonists in cancer immunotherapy. OncoImmunology. 2018;12:e1526250–e1526265. doi: 10.1080/2162402X.2018.1526250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Lee Y.-R., Lee Y.-H., Im S.-A., Yang I.-H., Ahn G.W., Kim K. Biodegradable nanoparticles containing TLR3 or TLR9 agonists together with antigen enhance MHC-restricted presentation of the antigen. Arch Pharm Res (Seoul) 2010;11:1859–1866. doi: 10.1007/s12272-010-1119-z. [DOI] [PubMed] [Google Scholar]
- 205.Fox C.B., Sivananthan S.J., Duthie M.S., Vergara J., Guderian J.A., Moon E. A nanoliposome delivery system to synergistically trigger TLR4 and TLR7. J Nanobiotechnol. 2014;1:12–17. doi: 10.1186/1477-3155-12-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Wilkinson A., Lattmann E., Roces C.B., Pedersen G.K., Christensen D., Perrie Y. Lipid conjugation of TLR7 agonist resiquimod ensures co-delivery with the liposomal cationic adjuvant formulation 01 (CAF01) but does not enhance immunopotentiation compared to non-conjugated resiquimod+CAF01. J Control Release. 2018;291:1–10. doi: 10.1016/j.jconrel.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 207.Rodell C.B., Arlauckas S.P., Cuccarese M.F., Garris C.S., Li R., Ahmed M.S. TLR7/8-agonist-loaded nanoparticles promote the polarization of tumour-associated macrophages to enhance cancer immunotherapy. Nat Biomed Eng. 2018;2:578–588. doi: 10.1038/s41551-018-0236-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kim H., Sehgal D., Kucaba T.A., Ferguson D.M., Griffith T.S., Panyam J. Acidic ph-responsive polymer nanoparticles as a TLR7/8 agonist delivery platform for cancer immunotherapy. Nanoscale. 2018;44:20851–20862. doi: 10.1039/c8nr07201a. [DOI] [PubMed] [Google Scholar]
- 209.Schau I., Michen S., Hagstotz A., Janke A., Schackert G., Appelhans D. Targeted delivery of TLR3 agonist to tumor cells with single chain antibody fragment-conjugated nanoparticles induces type I-interferon response and apoptosis. Sci Rep. 2019;1:3299–3313. doi: 10.1038/s41598-019-40032-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Takeda K., Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;1:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 211.Kerr W.G., Chisholm J.D. The next generation of immunotherapy for cancer: small molecules could make big waves. J Immunol. 2019;1:11–19. doi: 10.4049/jimmunol.1800991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Chen P.-M., Pan W.-Y., Wu C.-Y., Yeh C.-Y., Korupalli C., Luo P.-K. Modulation of tumor microenvironment using a TLR-7/8 agonist-loaded nanoparticle system that exerts low-temperature hyperthermia and immunotherapy for in situ cancer vaccination. Biomaterials. 2020;230:119629–119639. doi: 10.1016/j.biomaterials.2019.119629. [DOI] [PubMed] [Google Scholar]
- 213.Lynn G.M., Sedlik C. Peptide-TLR-7/8a conjugate vaccines chemically programmed for nanoparticle self-assembly enhance CD8 T-cell immunity to tumor antigens. Nat Biotechnol. 2020;3:320–332. doi: 10.1038/s41587-019-0390-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Kim H., Niu L., Larson P., Kucaba T.A., Murphy K.A., James B.R. Polymeric nanoparticles encapsulating novel TLR7/8 agonists as immunostimulatory adjuvants for enhanced cancer immunotherapy. Biomaterials. 2018;164:38–53. doi: 10.1016/j.biomaterials.2018.02.034. [DOI] [PubMed] [Google Scholar]
- 215.Bocanegra Gondan A.I., Ruiz-de-Angulo A., Zabaleta A., Gómez Blanco N., Cobaleda-Siles B.M., García-Granda M.J. Effective cancer immunotherapy in mice by polyIC-imiquimod complexes and engineered magnetic nanoparticles. Biomaterials. 2018;170:95–115. doi: 10.1016/j.biomaterials.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 216.Wang C., Sun W., Wright G., Wang A.Z., Gu Z. Inflammation-triggered cancer immunotherapy by programmed delivery of CpG and anti-PD1 antibody. Adv Mater. 2016;40:8912–8920. doi: 10.1002/adma.201506312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Widmer J., Thauvin C., Mottas I., Nguyen V.N., Delie F., Allémann E. Polymer-based nanoparticles loaded with a TLR7 ligand to target the lymph node for immunostimulation. Int J Pharm. 2018;1:444–451. doi: 10.1016/j.ijpharm.2017.11.031. [DOI] [PubMed] [Google Scholar]
- 218.Mohsen M.O., Vogel M., Riether C., Muller J., Salatino S., Ternette N. Targeting mutated plus germline epitopes confers pre-clinical efficacy of an instantly formulated cancer nano-vaccine. Front Immunol. 2019;10:1015–1028. doi: 10.3389/fimmu.2019.01015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Traini G., Ruiz-de-Angulo A., Blanco-Canosa J.B., Zamacola Bascarán K., Molinaro A., Silipo A. Cancer immunotherapy of TLR4 agonist–antigen constructs enhanced with pathogen-mimicking magnetite nanoparticles and checkpoint blockade of PD-L1. Small. 2019;4:1803993–1804008. doi: 10.1002/smll.201803993. [DOI] [PubMed] [Google Scholar]
- 220.Feng Y., Mu R., Wang Z., Xing P., Zhang J., Dong L. A toll-like receptor agonist mimicking microbial signal to generate tumor-suppressive macrophages. Nat Commun. 2019;1:227285. doi: 10.1038/s41467-019-10354-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Holtmeier W., Kabelitz D. Gammadelta T cells link innate and adaptive immune responses. Chem Immunol Allergy. 2005;86:151–183. doi: 10.1159/000086659. [DOI] [PubMed] [Google Scholar]
- 222.Daley D., Zambirinis C.P., Seifert L., Akkad N., Mohan N., Werba G. Gammadelta T cells support pancreatic oncogenesis by restraining alpha beta T cell activation. Cell. 2016;6:1485–1499. doi: 10.1016/j.cell.2016.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Chen X., Shang W., Xu R., Wu M., Zhang X., Huang P. Distribution and functions of gammadelta T cells infiltrated in the ovarian cancer microenvironment. J Transl Med. 2019;1:144–156. doi: 10.1186/s12967-019-1897-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Li J., Lentini N.A., Wiemer D.F., Wiemer A.J. A luciferase lysis assay reveals in vivo malignant cell sensitization by phosphoantigen prodrugs. Biochem Pharmacol. 2019;170:113668–113676. doi: 10.1016/j.bcp.2019.113668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Hsiao C.H., Lin X., Barney R.J., Shippy R.R., Li J., Vinogradova O. Synthesis of a phosphoantigen prodrug that potently activates Vgamma9Vdelta2 T-lymphocytes. Chem Biol. 2014;8:945–954. doi: 10.1016/j.chembiol.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 226.Pistoia V., Tumino N., Vacca P., Veneziani I., Moretta A., Locatelli F. Human γδ T-cells: from surface receptors to the therapy of high-risk leukemias. Front Immunol. 2018;9:984–991. doi: 10.3389/fimmu.2018.00984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Zhao Y., Niu C., Cui J. Gamma-delta (γδ) T cells: friend or foe in cancer development? J Transl Med. 2018;1:3–15. doi: 10.1186/s12967-017-1378-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Man F., Lim L., Volpe A., Gabizon A., Shmeeda H., Draper B. In vivo pet tracking of (89)Zr-labeled Vgamma9Vdelta2 T cells to mouse xenograft breast tumors activated with liposomal alendronate. Mol Ther. 2019;1:219–229. doi: 10.1016/j.ymthe.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Ruan S., Xie R., Qin L., Yu M., Xiao W., Hu C. Aggregable nanoparticles-enabled chemotherapy and autophagy inhibition combined with anti-PD-L1 antibody for improved glioma treatment. Nano Lett. 2019;11:8318–8332. doi: 10.1021/acs.nanolett.9b03968. [DOI] [PubMed] [Google Scholar]
- 230.Yu W., He X., Yang Z., Yang X., Xiao W., Liu R. Sequentially responsive biomimetic nanoparticles with optimal size in combination with checkpoint blockade for cascade synergetic treatment of breast cancer and lung metastasis. Biomaterials. 2019;217:119309–119323. doi: 10.1016/j.biomaterials.2019.119309. [DOI] [PubMed] [Google Scholar]
- 231.Olden B.R., Cheng E., Cheng Y., Pun S.H. Identifying key barriers in cationic polymer gene delivery to human T cells. Biomater Sci. 2019;3:789–797. doi: 10.1039/c8bm01262h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Olden B.R., Cheng Y., Yu J.L., Pun S.H. Cationic polymers for non-viral gene delivery to human T cells. J Control Release. 2018;282:140–147. doi: 10.1016/j.jconrel.2018.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]