Abstract
In the present study, four Lactobacillus strains from the cheese were analyzed for its probiotic potential against enteropathogenic bacteria. The probiotic properties of the selected strains were also analyzed and the selected bacterial strains showed high tolerance in bile salts and organic acid. The strain L. plantarum LP049 showed maximum survival rate (92 ± 4.2% and 93.3 ± 2%) after 3 h of treatment at 0.25% (w/v) bile salts and 0.25% (w/v) organic acid concentrations. The ability of the Lactobacillus strains to adhere to human epithelial cells (HT-29 cell lines) was evaluated and L. plantarum LP049 showed maximum adhesion property (19.2 ± 1.1%) than other tested strains. The Lactobacillus strains produced lactic acid at various concentrations. Compared with other strains, maximum level of lactic acid (3.1 g/L), hydrogen peroxide (4.31 mM) and bacteriocin (31 AU/mg) was detected in LB049. The inhibitory activity of culture supernatant against various bacterial pathogens was observed. The zone of inhibition ranged between 6 ± 2 mm and 23 ± 2 mm. The cell free extract showed activity against, Escherichia coli (ATCC 10536), Salmonella enteritidis (ATCC 13076), Shigella flexneri (ATCC 29903), and Enterococcus faecium (ATCC 8459). Consequently, L. plantarum LP049 may be considered as a potential candidate for the production of novel bioactive metabolites for therapeutic and bio-protective applications.
Keywords: Cheese, Lactobacillus, Bacterocin, Enteropathogens, Antibacterial
1. Introduction
Probiotics are live bacteria or other micro-organisms, that when ingested in suitable quantities confer numerous health benefits on the host organisms (Aarti et al., 2016, Al-Dhabi et al., 2020, Nagpal et al., 2012). Lactic acid bacteria (LAB) are Gram-positive, which are naturally available in the gastrointestinal tract of human and ruminants (Aarti et al., 2018). These LAB play significant role in maintaining the balance of microbial flora (Aarti et al., 2017, Kirtzalidou et al., 2011, Arasu et al., 2014a, Arasu et al., 2016, George Kerry et al., 2018). Many of probiotic bacteria that effectively colonize the gut of neonates have various origins and may be affected by many factors such as contact with hospital staff, parents, antibiotic treatment, feeding pattern, and type of delivery (Savino et al., 2011). In developing and under developing countries, diarrhea is one of the important diseases causing mortality and morbidity among infants (Lanata et al., 2013). The microorganisms such as, Vibrio cholera, Campylobacter, Escherichia coli, and Rotavirus cause diarrhea (Lanata et al., 2013). Probiotic bacteria effectively resist the growth and activities of various enteropathogenic microorganisms (Arasu et al., 2014b, Arasu et al., 2014c, Fijan et al., 2018). Probiotics from the genus Lactobacillus are widely reported for their beneficial properties as adjunctive/preventive therapy for highly infectious diarrhea in the pediatric group (Arasu et al., 2014b, Delcaru et al., 2016). Some of the LAB produce bacteriocins and these substances are mainly considered as GRAS (generally recognized as safe) substances. In a study, Miao et al. (2014) characterized a novel bacteriocin F1 producing Lactobacillus paracasei strain FX-6 isolated from Tibetan kefir with 18 amino acids and the molecular weight was 2112.842 Da (Miao et al., 2016a, Miao et al., 2016b). Probiotics are highly effective in regulating adipocytes in both animal and human subjects (Kadooka et al., 2010). Also, probiotic food showed functional properties related to anti-obesity (Cui et al., 2015). The anti-obesity characteristics of fermented food may be attributed to various bioactive compounds and the LAB in fermented food (Park et al., 2012). Lactobacillus also enhanced immune organ indexes, SIgA secretion level and spleen lymphocyte transformation rate in experimental animal (Ren et al., 2015). It has been reported that the probiotic bacterium, Lactobacillus acidophilus could gently regulate the intestinal microbial flora of experimental mice with intestinal dysfunction and provide a protective response of immune system, which could be effectively replace synthetic antibiotics for broilers (Sun et al., 2015). Hence, probiotic bacteria have lot of potential in animal husbandry as an effective alternative to commercial antibiotics. However, it could be noted that the bacteria should have the ability to withstand various adverse climatic conditions such as resist to digestive juices, various proteases, bile salt and should tolerant to gastric acid (Erkkilä and Petäjä, 2000). The probiotic organisms should have potent colonization ability, so that rapid adhere to the intestinal mucosa is possible (Valan Arasu et al., 2013). Also, the selected probiotic organisms should not have any side effects and must be safe for host organisms (Saxami et al., 2012). LAB produce various antimicrobial agents. The synthesized metabolites showed an acidic pH in the gastrointestinal tract to facilitate the antagonistic properties against various acid sensitive bacterial and viral pathogens by affecting with the cell membrane. Probiotic bacteria showed activity against various Gram-negative and Gram-positive bacteria, fungi and yeast (Arasu et al., 2014b, Valan Arasu et al., 2014). Lactobacilli also showed protective activity in food-borne illness and in food preservation (Valan Arasu et al., 2015, Valan Arasu et al., 2013). In recent years an attempt has been made to analyze the health benefits of host organisms by improving the balance of gut microbial flora, reducing cholesterol, vitamin production providing immunomodulatory effects and regulating lactase activity and various metabolic processes. The bacteria from the genera, Bifidobacterium and Lactobacillus are widely reported probiotic culture, however the genera such as, Saccharomyces, Enterococcus, Streptococcus and Lactococcus are commonly used as probiotics (García-Ruiz et al., 2014, Ilavenil et al., 2015a, Valan Arasu et al., 2013). The potential health benefits of probiotics on host health namely, production of anti-microbial metabolites and synthesis of B-group vitamins, anti-obesity effect (Kim et al., 2018), anti-diabetic effect, cholesterol-lowering effect and oxidative stress down-regulation (Yadav et al., 2018) have been reported previously. However, antibacterial potential and other characteristics vary from strains to strains. Hence, many studies are underway to evaluate probiotic characters of novel bacterial strain. The present study aimed to analyze the probiotic properties of Lactobacillus species isolated from the cheese to evaluate the antimicrobial property against various enteropathogenic bacteria. Fig. 1.
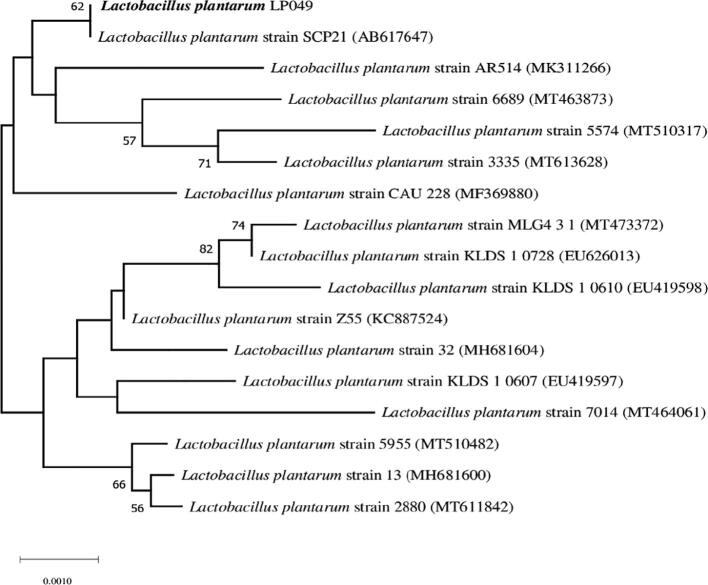
Fig. 1.
Phylogenetic relation of L. plantarum LP049 with other relevant strains.
2. Materials and methods
2.1. Characterization of Lactobacillus isolates from cheese
This experiment was performed between July 2019 and May 2020. A total of 130 samples were collected from cheese. The collected samples were transferred to the laboratory within 4 h and cultured using MRS broth for 72 h at 37 °C. Then, the samples were cultured in MRS agar and incubated for 48 h at 37 °C. The isolated bacterial strains were tested based on their glucose-fermentation property, catalytic property, morphological characters and Gram’s staining. The isolated organisms were incubated at various temperatures (30 °C, 35 °C and 40 °C) (Davoodabadi et al., 2015b, Kiliç and Karahan, 2010). The screened Lactobacillus species were stored in MRS agar slants and the broth was suspended in glycerol (20%, v/v) and stored at – 80 °C. The identification of the tested isolates was performed by biochemical tests and 16S rDNA sequence analysis. Chromosomal DNA of the selected bacteria was extracted and purified as suggested earlier (Heilig et al., 2002). A forward primer (5 -AGAGTTTGATCCTGGCTCAG-3) and a reverse primer (5 -CCGTCAATTCCTTTGAGTTT-3) were used for the amplification of Lactobacillus 16S rRNA gene. The PCR reaction was carried out in an ppendorf thermal cycler PCR system (Germany) and the master mix was performed as suggested by the manufacturer (Ampliqon, Denmark). Millipore water has been used until otherwise stated. The amplified fragments were sequenced using Applied Biosystems, according to the instructions of the manufacturers. The sequences were compared and the submitted in GenBank data base
2.2. Characteristic feature of probiotics
2.2.1. Acid and bile tolerance tests
The bile and acid tolerance of bacterial strains were analyzed as described earlier in literatures with little modifications (Donatien and Hagreacute tou, S.L., Mamoudou, H.D., Breacute hima, D., Mogens, J., , 2012, Klingberg et al., 2005). Briefly, the acid resistance of the organisms were examined in MRS broth (Himedia, Mumbai, India) adjusted the pH to 2.5 using 1 N HCl. About 0.2 mL of 18 h culture of Lactobacillus isolates (106 CFU/ml) was inoculated into 25 mL of MRS liquid medium and the pH was adjusted earlier. After 18 h incubation, the viable count of bacterial strain was performed using plating technique on MRS agar. The survival rate (%) of the bacterial strain was analyzed using plate count method on MRS agar, after incubation of 0 and 2 h. Lactobacillus isolates that grown in the acid medium were subjected to bile tolerance assay. Lactobacillus strains were inoculated in Erlenmeyer flask containing 50-mL (106 CFU/ml) of 18 h grown culture was inoculated on 50 mL of MRS liquid medium with 0.3% (w/v) oxgall bile salt (Sigma, USA). To the control, oxgall bile salt was not added.
(N/N0) × 100%.
2.2.2. Lactobacillus adhesion assay
The ability of the Lactobacillus strains to adhere to human epithelial cells was evaluated according to the method of Verdenelli et al. (2009). The percentage of adhesion of Lactobacillus strains was evaluated by comparison of adhered cells and total cells determined in bacterial suspension. Adhesion assay was performed in triplicates and an average value was considered for statistical analysis.
2.3. Antibacterial assay
The antibacterial activity of the selected Lactobacillus species against enteropathogenic bacterial strain was evaluated as described by Davoodabadi et al. (2015a) by well diffusion method. The pathogenic bacterial strains such as, Escherichia coli (ATCC 10536), Salmonella enteritidis (ATCC 13076), Shigella flexneri (ATCC 29903), and Enterococcus faecium (ATCC 8459) were inoculated in liquid medium (Nutrient broth) (Himedia, Mumbai, India) and cultured for overnight.
2.4. Antimicrobial agent characterization
In this study, the nature of the substances produced by Lactobacillus species responsible for antimicrobial activity was tested. The antimicrobial agents generally synthesized by Lactobacillus strains such as, bacteriocin, hydrogen peroxide and organic acid were determined as described previously by Shokryazdan et al. (2014). Lactobacillus strains were cultured in 50 mL MRS medium and centrifuged the sample for 10 min at 5000 × g at 4 °C. The cell free extract was used as the sample for all experiments. For the determination of bacteriocin, the culture supernatant (10 mL) was treated with 2 mg/mL trypsin (Sigma, USA). For the analysis of organic acids, the pH of the culture supernatant (10 mL) was adjusted to 6.5 using 1 N NaOH, and for the determination of hydrogen peroxide, the cell free sample (10 mL) was incubated with 1 mg/mL catalase. The cell free extract was sterilized using filtration (0.22 mm) and 50 µL sample was loaded into wells made in MRS agar plates, which was overlaid with soft nutrient agar (10 mL) (Himedia, Mumbai, India), and further inoculated with test pathogens.
2.5. Antimicrobial properties of L. plantarum strains
In this study, antibacterial potential of Lactobacillus species was evaluated by well diffusion method on MHA plates as described earlier by Valan Arasu et al. (2013a). The isolated Lactobacilli were cultured in MRS liquid medium at pH 6.5 and incubated for 24. After incubation period, antimicrobial properties were tested from the cell free supernatant. The cultures were centrifuged at 5000 × g for 10 min at 4 °C and the extract was sterilized using 0.22‐µm hydrophilic Durapore PVDF membrane. The filtered sample was used for the determination antibacterial activity. To analyze the chemical nature of the inhibitory compound synthesized by Lactobacillus species, the filtered sample was subjected to various treatments as suggested by Herreros et al. (2005) with little modifications. The culture supernatant was heated for 5 min at 100 °C in the presence of 1 N NaOH and the pH value was attained to 6.5. To the supernatant pepsin was added in order to evaluate the role of antimicrobial peptides on antibacterial activity. Proteinase K and pepsin were added at the concentrations of 1 mg/mL.
3. Results and discussion
3.1. Identification and characterization of Lactobacillus species isolated from cheese
A total of 18 bacteria were characterized as Lactobacillus strains by various phenotypic tests and Gram’s staining. The tested bacterial strains were catalytic negative and all are Gram’s positive bacterial strains. In this study, Lactobacillus species from five different species were identified. Among the species, five strains were characterized from Lactobacillus plantarum (Table 1). All ninteen bacterial strains were initially selected for acid tolerance, bile tolerance and adhesion assay. Later, potent strains were identified based on morphological characters and 16S rDNA gene sequencing. Lactobacillus species are probiotic organisms which help to balance the gastrointestinal microbial flora, destroy pathogenic microorganisms and produce various nutrients (Reid et al., 2003). Gastrointestinal diseases are mainly associated with maintaining microbial balance with the gastrointestinal tract (Delcenserie et al., 2008). Davoodabadi et al. (2015a) reported that Lactobacillus inhibit in the gastrointestinal of human and play very critical role in the balance of microbial consortium in the intestine. The selection of probiotic Lactobacillus was mainly based on antibiotic activity against bacterial pathogens, survival ability in the gastreointestinal tract, antibiotic resistant, ability to adhere to the human intestinal cells and tolerance to bile salts and acidic pHs (Arasu and Al-Dhabi, 2017, Ilavenil et al., 2015b, Plessas et al., 2017)
Table 1.
Lactobacillus species isolated from the cheese and their numbers.
| Bacterial strains | Strain number and number of strains |
|---|---|
| L. plantarum | Five strains, LP032, LP037, LP041, LP049 and LP058 |
| L. brevis | Four strains, LB062, LB067, LB068 and LB0112 |
| L. fermentum, | Two strains, LF06 and LF089 |
| L. rhamnosus | Three strains, LR013, LR019, LR087 and LR093 |
| L. furfuricola | Three strains, LF038, LF073 and LF0162 |
| L. paracasei | One strain, LP01 |
3.2. Acid and bile tolerance ability of Lactobacillus species
The viability of the selected eighteen isolates was tested against acid (pH 2.5) and oxgall bile (0.25%) and the results were tabulated in Table 2. Among the Lactobacillus strains, L. plantarum LP049 showed potent tolerance against acid and bile salts (0.25%) and the survival rate was 92 ± 4.2% and 93.3 ± 2, respectively. All isolated Lactobacillus strains were examined for bile and acid tolerance in the cell viabilities after 3 h of treatment showed variation in pH tolerance. At low pH, loss of bacterial population was registered because of loss of viable bacterial cells in the medium. It was previously reported that hydrochloric acid severely affect the biomolecules of cells, DNA, proteins and fatty acids (Sahadeva et al., 2011). At low pH, metabolism, viability of Lactobacilli and growth were significantly affected. Earlier studies also showed that exposure of Lactobacilli to gastric acid for 3 h caused marked reduction in the bacterial population (Mandal et al., 2006, Sahadeva et al., 2011). According to Sahadeva et al., 2011, Prasad et al., 1998, the threshold limit to describe acid tolerance for gastrointestinal bacterial isolate was set between pH 2.0 and 3.0 for about 3 h incubation, as it generally stimulates microbial residency in the stomach of animals or humans. In a study, Liong and Shah (2005) described pH 3.0 as a standard value for the determination of pH tolerance test for any probiotic strains. The present finding revealed moderate inhibition at this pH indicated potential probiotic properties. The ability of tolerance on bile salt was evaluated in this study. P. plantarum LP049 showed maximum (93.3 ± 2.8%) bile tolerant rate followed by L. brevis LB0112 (90 ± 4.3%). Bile salt affect cellular homeostasis that cause the dissocation of lipid bilayer, affect integral protein of their cell membranes, leading in the leakage of intracellular content and finally death of cell (Mandal et al., 2006). There were various Lactobacillus strains with probiotic properties, thus they have potential applications in the prevention and treatment of various diseases. Their inhibitory properties were mainly due to the production of deacetyl, H2O2 and bacteriocins (Dave and Shah, 1998).
Table 2.
Tolerance of isolated Lactobacillus strains at pH 3.0.
| Lactobacillus strains | Acid resistant survival rate (%) | Oxgall bile tolerance rate (%) |
|---|---|---|
| L. plantarum LP032 | 89 ± 2.5 | 75 ± 1.3 |
| L. plantarum LP037 | 62 ± 1.3 | 82 ± 1.1 |
| L. plantarum LP041 | 43 ± 0.7 | 59 ± 3.4 |
| L. plantarum LP058 | 87 ± 3.8 | 65 ± 1.4 |
| P. plantarum LP049 | 92 ± 4.2 | 93.3 ± 2.8 |
| L. brevis LB062 | 90 ± 1.38 | 85 ± 1.6 |
| L. brevis LB067 | 52 ± 2.3 | 49 ± 1.3 |
| L. brevis LB068 | 35 ± 1.8 | 53 ± 1.8 |
| L. brevis LB0112 | 86 ± 4.2 | 90 ± 4.3 |
| L. fermentum LF06 | 48 ± 3.3 | 59 ± 3.2 |
| L. fermentum LF089 | 59 ± 2.2 | 62.3 ± 2.7 |
| L. rhamnosus LR013 | 57 ± 1.1 | 59.5 ± 2.2 |
| L. rhamnosus LR019 | 40 ± 2.0 | 45.5 ± 1.9 |
| L. rhamnosus LR087 | 67 ± 3.2 | 67.5 ± 2.8 |
| L. rhamnosus LR093 | 84 ± 0.0 | 58 ± 2.7 |
| L. furfuricola LF038 | 29 ± 4.3 | 33 ± 0.08 |
| L. furfuricola LF073 | 19 ± 1.2 | 13.3 ± 0.0 |
| L. furfuricola LF0162 | 4 ± 2.5 | 5.1 ± 1.03 |
| L. paracasei LP01 | 59 ± 2.8 | 60.2 ± 1.05 |
3.3. Adhesion assay
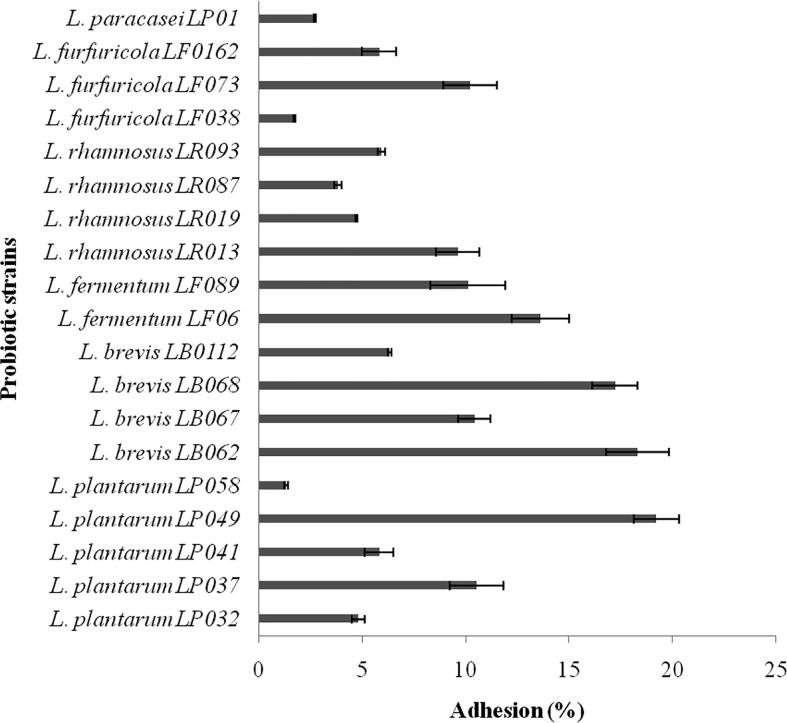
The selected Lactobacillus strains were subjected to HT-29 cell adhesion analysis. The strains showed varying adhesion properties and among all strains, L. plantarum LP049 showed maximum adhesion property (19.2 ± 1.1%). The other bacterial strains, L. brevis LB062 and L. brevis LB068 showed 18.3 ± 1.5 and 17.2 ± 1.1% adhesion power, respectively (Fig. 2). The other tested Lactobacillus strains showed low and moderate adhesive properties. Probiotic bacteria have the ability to adhere intestinal epithelial cells and the selection of any bacterial strain based on adhesion properties. Adhesion properties of Lactobacillus strains have been described previously by Ren et al., 2014, Davoodabadi et al., 2015b. The present finding is consistent with the finding of Halimi and Mirsalehian (2016). Also variation of adhesion level has also been reported previously by Shokryazdan et al. (2014). Bacteria from the genus Lactobacillus have adhesion abilities and this property is related to surface proteins. In bacteria various components from their cell surface involved in adherence to the epithelial cells, including, proteins, polysaccharides and (lipo) teichoic acids. Previously trypsin treated Lactobacillus strains showed least adhesive potentials indicated surface proteins have major role in bacterial adhesion (Glenting et al., 2013, Jensen et al., 2014). In some experiments revealed adhesive properties after trypsin treatment, indicated the role of non-proteinaceous compounds and non-surface proteins in the adhesion of Lactobacillus species (Polak-Berecka et al., 2014, Yadav et al., 2018). Also moonlighting proteins reported in Lactobacillus species have various functions due to multiple proteolytic fragments, splice variants and gene fusions. These proteins have been reported in various Lactobacillus species (Kinoshita et al., 2016). The surface proteins of Lactobacilli have the ability to adhere with the host cell. In a study Glenting et al. (2013) observed removal of various surface proteins from Lactobacilli after trypsin treatment and these confirmed the use of adhesive proteins on adhesion.
Fig. 2.
Lactobacilli strains and their adhesion property.
3.4. Characterization of antimicrobial agents
The Lactobacillus strains produced lactic acid at various concentrations. Compared with other strains, maximum level of lactic acid (3.1 g/L) was detected with strain LB049. The other strains, LB068, LB062 and LF06 showed 2.92 g/L, 2.65 g/L and 1.08 g/L, respectively (Fig. 3). Lactobacilli such as, L. plantarum produced organic acids reduce the pH of the environment (Ołdak et al., 2017). Li et al. (2016) reported that most of the L. plantarum produce various organic acids and these were useful to preserve fermented food. The antibacterial mechanism of organic acids varied widely. The previous findings showed that various kinds of lactobacilli produce various types of organic acids, and some of the lactobacilli produce more quantities of acidic acid than lactic acid (Rowland et al., 2010). This antimicrobial phenomenon clearly indicated that antimicrobial property of the selected bacterial strain is mainly due to the secretion of organic acids. Lactobacilli showed antibacterial activity mainly because of the production of lactic acid. However, other acids such as, citric acid and tartaric acid were also reported previously (Zalán et al., 2010). Hydrogen peroxide production improved along with the growth of the organisms. Hydrogen peroxide production was maximum and detected 4.31 mM in medium inoculated with the probiotic strain LB049. The other strains, LB062, LB068, and LF06 showed 3.87 mM, 2.14 mM and 0.98 mM, H2O2 respectively. Hydrogen peroxide production and antimicrobial activity were positively correlated. In Lactobacillus crispatus cell free extract total H2O2 level reached maximum (3.29 mM) under optimized agitation condition. This H2O2 producing property by Lactobacillus has been described previously by various researchers. Yap and Gilliland (2000) reported hydrogen peroxide producing ability by the strain, Lactobacillus delbrueckii subsp. lactis. Martín et al. (2005) characterized hydrogen peroxide-producing strain, Lactobacillus coryniformis which was isolated from milk cheese. All selected Lactobacterial strains showed bacteriocin in the cell free extract. The specific activity of bacteriocin was 31 AU/mg, 29.3 AU/mg, 11.8 AU/mg, and 3.9 AU/mg, respectively for the Lactobacillus strains, LB049, LB062, LB068, and LF06. Bacteriocins producing Lactobacilli has been identified by various researchers. In a study, Ghanbari et al. (2013) isolated bacteriocins producing lactobacilli strains from Sturgeon fish. In another study, Mohammadi et al. (2018) characterized bacteriocin from the cell free extract of Lactobacillus spp. isolated from mother's milk.
Fig. 3.
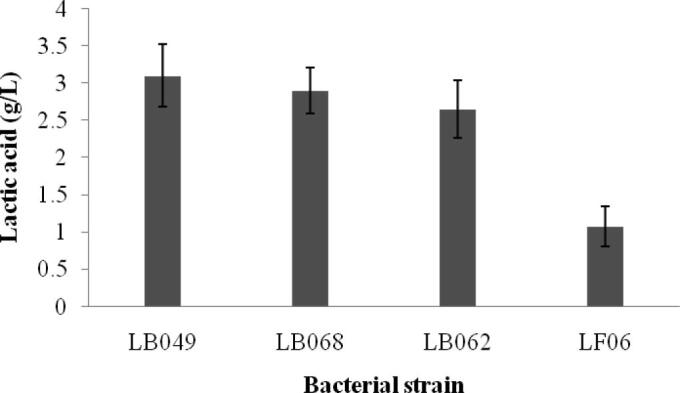
Lactic acid production by the bacterial strain islated from the cheese.
3.5. Antibacterial activity of Lactobacillus sp.
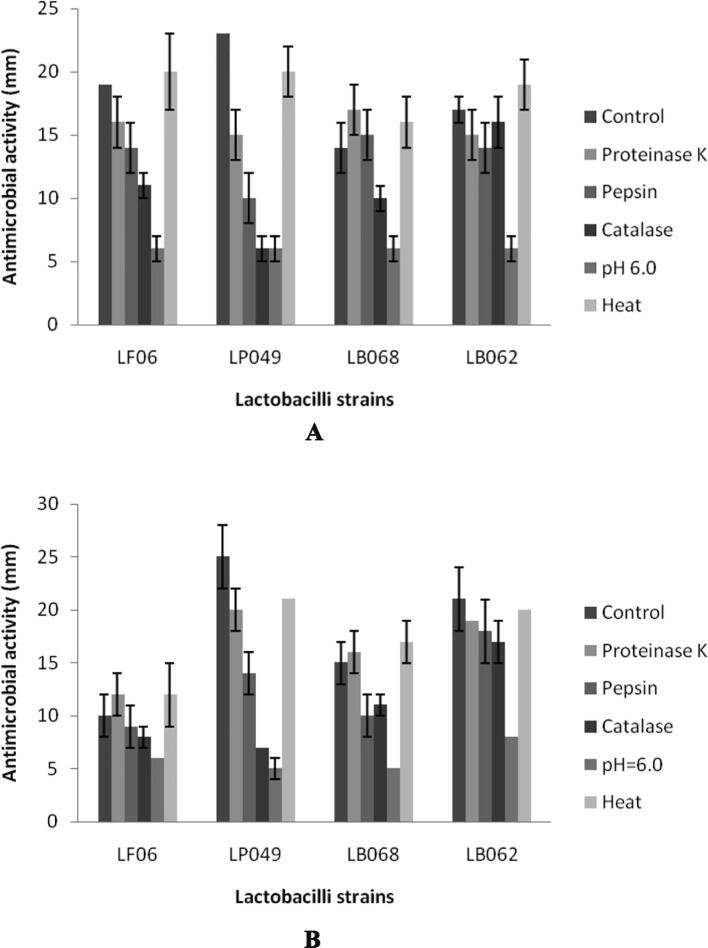
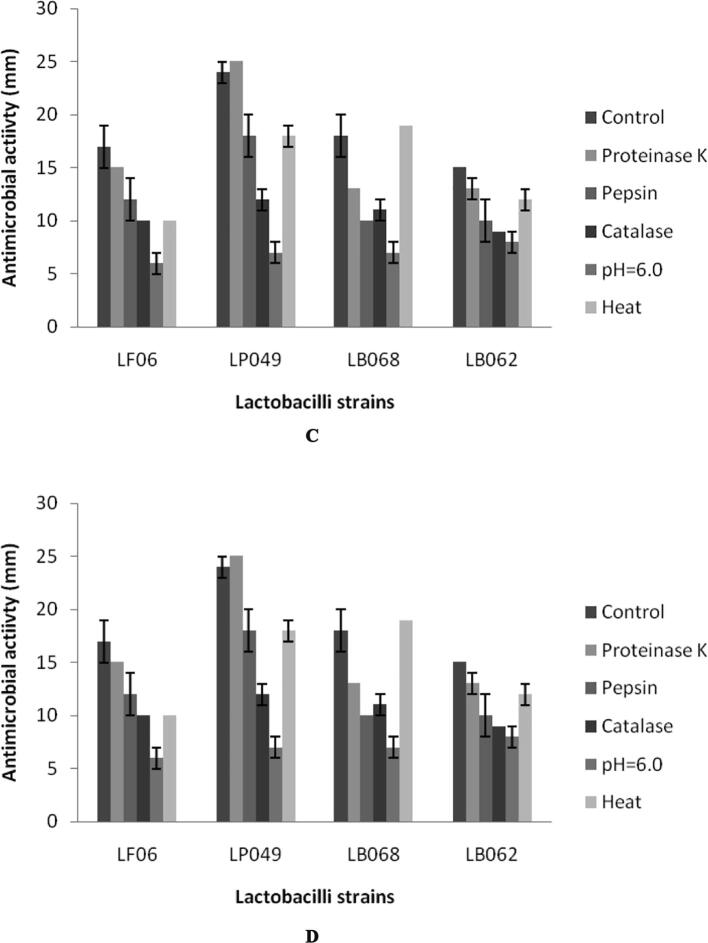
The inhibitory activity of culture supernatant was observed and the zone of diameter ranged between 6 ± 2 mm and 23 ± 2 mm. Four bacterial pathogens were selected to determine antimicrobial potential (Fig. 4a-d). The crude sample showed activity against, Escherichia coli (ATCC 10536), Salmonella enteritidis (ATCC 13076), Shigella flexneri (ATCC 29903), and Enterococcus faecium (ATCC 8459). Antibacterial activity varied among the selected bacterial species. As described in the Fig. 4, pepsin and catalase treated samples did not vary much than control experiments. At increased pH of the sample, (pH 6.0) antibacterial activity suddenly decreased than other treatments. Lactobacilli are Gram-positive, nonspore forming, fermenting various sugars into organic acids. This low pH value generally affected the growth of other group of bacteria. In some studies, it was reported the production of H2O2 during metabolic process critically inhibit other pathogenic bacterial growth (Charlier et al., 2009, Ilavenil et al., 2016). Some lactobacilli produce bacteriocin-like substances or bacteriocins to affect the growth of pathogens (Zhao et al., 2016). These kinds of lactobacilli have the ability to affect the growth of types of pathogenic bacteria and widely used for the preservation of food. Zhao et al. (2016) isolated antimicrobial compounds produced by L. plantarum JLA–9 which showed potential activity against Bacillus spp. have been used in various commercial applications. Some of the Lactobacillus species produce phenyllactic acid, a natural food preservative (Valerio et al., 2004, Vijayakumar et al., 2015).
Fig. 4.
Antibacterial activity of fermentation broth of four Lactobacillus strains after subjected to various treatments. Control sample: Filtered, unheated sample; Proteinase K: Samples treated with proteinase K at 1 mg/mL concentration; Pepsin: Samples treated with Pepsin at 1 mg/mL concentration; Catalase: Sample treated with catalase at 1 mg/mL concentration; pH: Samples adjusted the pH to 6.0; Heat: Sample treated at 100 °C for 5 min. A: Escherichia coli (ATCC 10536), B: Salmonella enteritidis (ATCC 13076), C: Shigella flexneri (ATCC 29903), and D: Enterococcus faecium (ATCC 8459).
4. Conclusions
The present results revealed that, the selected strains were highly tolerant to various in vitro gastrointestinal conditions and increased survival rate. The Lactobacillus strains possessed a good antibacterial activity against various gastrointestinal bacterial pathogens. These bacterial strains also showed high quantities of hydrogen peroxide, lactic acid and bacteriocins. Therefore, all Lactobacilli, especially, L. plantarum LP049 could be used as a novel probiotic strain for feed, food and various technological applications.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding of this research through the Research Group Project No. RG-1440-107.
Footnotes
Peer review under responsibility of King Saud University.
References
- Aarti C., Khusro A., Arasu M.V., Agastian P., Al-Dhabi N.A. Biological potency and characterization of antibacterial substances produced by Lactobacillus pentosus isolated from Hentak, a fermented fish product of North-East India. Springerplus. 2016;5:1743. doi: 10.1186/s40064-016-3452-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarti C., Khusro A., Varghese R., Arasu M.V., Agastian P., Al-Dhabi N.A., Ilavenil S., Choi K.C. In vitro investigation on probiotic, anti-Candida, and antibiofilm properties of Lactobacillus pentosus strain LAP1. Arch. Oral Biol. 2018;89:99–106. doi: 10.1016/j.archoralbio.2018.02.014. [DOI] [PubMed] [Google Scholar]
- Aarti C., Khusro A., Varghese R., Arasu M.V., Agastian P., Al-Dhabi N.A., Ilavenil S., Choi K.C. In vitro studies on probiotic and antioxidant properties of Lactobacillus brevis strain LAP2 isolated from Hentak, a fermented fish product of North-East India. LWT - Food Sci. Technol. 2017;86:438–446. doi: 10.1016/j.lwt.2017.07.055. [DOI] [Google Scholar]
- Al-Dhabi N.A., Arasu M.V., Vijayaraghavan P., Esmail G.A., Duraipandiyan V., Kim Y.O., Kim H., Kim H.J. Probiotic and antioxidant potential of lactobacillus reuterilr12 and lactobacillus lactisll 10 isolated from pineapple puree and quality analysis of pineapple-flavored goat milk yoghurt during storage. Microorganisms. 2020;8:1–15. doi: 10.3390/microorganisms8101461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arasu M.V., Al-Dhabi N.A. In vitro antifungal, probiotic, and antioxidant functional properties of a novel Lactobacillus paraplantarum isolated from fermented dates in Saudi Arabia. J. Sci. Food Agric. 2017;97:5287–5295. doi: 10.1002/jsfa.8413. [DOI] [PubMed] [Google Scholar]
- Arasu M.V., Al-Dhabi N.A., Ilavenil S., Choi K.C., Srigopalram S. In vitro importance of probiotic Lactobacillus plantarum related to medical field. Saudi J. Biol. Sci. 2016;23:S6–S10. doi: 10.1016/j.sjbs.2015.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arasu M.V., Al-Dhabi N.A., Rejiniemon T.S., Lee K.D., Huxley V.A.J., Kim D.H., Duraipandiyan V., Karuppiah P., Choi K.C. Identification and Characterization of Lactobacillus brevis P68 with Antifungal, Antioxidant and Probiotic Functional Properties. Indian J. Microbiol. 2014;55:19–28. doi: 10.1007/s12088-014-0495-3. [DOI] [Google Scholar]
- Arasu M.V., Jung M.W., Kim D.H., Ilavenil S., Jane M., Park H.S., Al-Dhabi N.A., Jeon B.T., Choi K.C. Enhancing Nutritional Quality of Silage by Fermentation with Lactobacillus plantarum. Indian J. Microbiol. 2014;54:396–402. doi: 10.1007/s12088-014-0473-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arasu M.V., Kim D.H., Kim P.I., Jung M.W., Ilavenil S., Jane M., Lee K.D., Al-Dhabi N.A., Choi K.C. In vitro antifungal, probiotic and antioxidant properties of novel Lactobacillus plantarum K46 isolated from fermented sesame leaf. Ann. Microbiol. 2014;64:1333–1346. doi: 10.1007/s13213-013-0777-8. [DOI] [Google Scholar]
- Charlier C., Cretenet M., Even S., Le Loir Y. Interactions between Staphylococcus aureus and lactic acid bacteria: An old story with new perspectives. Int. J. Food Microbiol. 2009;131:30–39. doi: 10.1016/j.ijfoodmicro.2008.06.032. [DOI] [PubMed] [Google Scholar]
- Cui M., Kim H.Y., Lee K.H., Jeong J.K., Hwang J.H., Yeo K.Y., Ryu B.H., Choi J.H., Park K.Y. Antiobesity effects of kimchi in diet-induced obese mice. J. Ethn. Foods. 2015;2:137–144. doi: 10.1016/j.jef.2015.08.001. [DOI] [Google Scholar]
- Dave R.I., Shah N.P. Ingredient Supplementation Effects on Viability of Probiotic Bacteria in Yogurt. J. Dairy Sci. 1998;81:2804–2816. doi: 10.3168/jds.S0022-0302(98)75839-4. [DOI] [PubMed] [Google Scholar]
- Davoodabadi A., Dallal M.M.S., Lashani E., Ebrahimi M.T. Antimicrobial activity of Lactobacillus spp. isolated from fecal flora of healthy breast-fed infants against diarrheagenic Escherichia coli. Jundishapur. J. Microbiol. 2015;8:27852. doi: 10.5812/jjm.27852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davoodabadi A., Soltan Dallal M.M., Rahimi Foroushani A., Douraghi M., Sharifi Yazdi M. kazem, Amin Harati F. Antibacterial activity of Lactobacillus spp. isolated from the feces of healthy infants against enteropathogenic bacteria. Anaerobe. 2015;34:53–58. doi: 10.1016/j.anaerobe.2015.04.014. [DOI] [PubMed] [Google Scholar]
- Delcaru C., Alexandru I., Podgoreanu P., Cristea V.C., Bleotu C., Chifiriuc M.C., Bezirtzoglou E., Lazar V. Antagonistic activities of some Bifidobacterium sp. strains isolated from resident infant gastrointestinal microbiota on Gram-negative enteric pathogens. Anaerobe. 2016;39:39–44. doi: 10.1016/j.anaerobe.2016.02.010. [DOI] [PubMed] [Google Scholar]
- Delcenserie, V., Martel, D., Lamoureux, M., Amiot, J., Boutin, Y., Roy, D., n.d. Immunomodulatory Effects of Probiotics in the Intestinal Tract 37 Immunomodulatory Effects of Probiotics in the Intestinal Tract, Curr. Issues Mol. Biol. [PubMed]
- Donatien K., Hagreacute tou S.L., Mamoudou H.D., Breacute Hima D., Mogens J. Acid resistance, bile tolerance and antimicrobial properties of dominant lactic acid bacteria isolated from traditional maari baobab seeds fermented condiment. African J. Biotechnol. 2012;11:1197–1205. doi: 10.5897/ajb11.2667. [DOI] [Google Scholar]
- Erkkilä S., Petäjä E. Screening of commercial meat starter cultures at low pH and in the presence of bile salts for potential probiotic use. Meat Sci. 2000;55:297–300. doi: 10.1016/S0309-1740(99)00156-4. [DOI] [PubMed] [Google Scholar]
- Fijan S., Šulc D., Steyer A. Study of the in vitro antagonistic activity of various single-strain and multi-strain probiotics against Escherichia coli. Int. J. Environ. Res. Public Health. 2018;15 doi: 10.3390/ijerph15071539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Ruiz A., González de Llano D., Esteban-Fernández A., Requena T., Bartolomé B., Moreno-Arribas M.V. Assessment of probiotic properties in lactic acid bacteria isolated from wine. Food Microbiol. 2014;44:220–225. doi: 10.1016/j.fm.2014.06.015. [DOI] [PubMed] [Google Scholar]
- George Kerry R., Patra J.K., Gouda S., Park Y., Shin H.S., Das G. Benefaction of probiotics for human health: A review. J. Food Drug Anal. 2018 doi: 10.1016/j.jfda.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanbari M., Jami M., Kneifel W., Domig K.J. Antimicrobial activity and partial characterization of bacteriocins produced by lactobacilli isolated from Sturgeon fish. Food Control. 2013;32:379–385. doi: 10.1016/j.foodcont.2012.12.024. [DOI] [Google Scholar]
- Glenting J., Beck H.C., Vrang A., Riemann H., Ravn P., Hansen A.M., Antonsson M., Ahrné S., Israelsen H., Madsen S. Anchorless surface associated glycolytic enzymes from Lactobacillus plantarum 299v bind to epithelial cells and extracellular matrix proteins. Microbiol. Res. 2013;168:245–253. doi: 10.1016/j.micres.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Halimi S., Mirsalehian A. Assessment and comparison of probiotic potential of four Lactobacillus species isolated from feces samples of Iranian infants. Microbiol. Immunol. 2016;60:73–81. doi: 10.1111/1348-0421.12352. [DOI] [PubMed] [Google Scholar]
- Heilig H.G.H.J., Zoetendal E.G., Vaughan E.E., Marteau P., Akkermans A.D.L., De Vos W.M. Molecular diversity of Lactobacillus spp. and other lactic acid bacteria in the human intestine as determined by specific amplification of 16S ribosomal DNA. Appl. Environ. Microbiol. 2002;68:114–123. doi: 10.1128/AEM.68.1.114-123.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herreros M.A., Sandoval H., González L., Castro J.M., Fresno J.M., Tornadijo M.E. Antimicrobial activity and antibiotic resistance of lactic acid bacteria isolated from Armada cheese (a Spanish goats’ milk cheese) Food Microbiol. 2005;22:455–459. doi: 10.1016/j.fm.2004.11.007. [DOI] [Google Scholar]
- Ilavenil S., Kim D.H., Arasu M.V., Srigopalram S., Sivanesan R., Choi K.C. Phenyllactic acid from Lactobacillus plantarum promotes adipogenic activity in 3T3-L1 adipocyte via up-regulation of PPAR-γ2. Molecules. 2015;20:15359–15373. doi: 10.3390/molecules200815359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilavenil S., Park H.S., Vijayakumar M., Valan Arasu M., Kim D.H., Ravikumar S., Choi K.C. Probiotic Potential of Lactobacillus Strains with Antifungal Activity Isolated from Animal Manure. Sci. World J. 2015;2015 doi: 10.1155/2015/802570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilavenil S., Vijayakumar M., Kim D.H., Valan Arasu M., Park H.S., Ravikumar S., Choi K.C. Assessment of probiotic, antifungal and cholesterol lowering properties of Pediococcus pentosaceus KCC-23 isolated from Italian ryegrass. J. Sci. Food Agric. 2016;96:593–601. doi: 10.1002/jsfa.7128. [DOI] [PubMed] [Google Scholar]
- Jensen H., Roos S., Jonsson H., Rud I., Grimmer S., van Pijkeren J.P., Britton R.A., Axelsson L. Role of Lactobacillus reuteri cell and mucusbinding protein A (CmbA) in adhesion to intestinal epithelial cells and mucus in vitro. Microbiol. (United Kingdom) 2014;160:671–681. doi: 10.1099/mic.0.073551-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadooka Y., Sato M., Imaizumi K., Ogawa A., Ikuyama K., Akai Y., Okano M., Kagoshima M., Tsuchida T. Regulation of abdominal adiposity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. Eur. J. Clin. Nutr. 2010;64:636–643. doi: 10.1038/ejcn.2010.19. [DOI] [PubMed] [Google Scholar]
- Kiliç G.B., Karahan A.G. Identification of lactic acid bacteria isolated from the fecal samples of healthy humans and patients with dyspepsia, and determination of their pH, bile, and antibiotic tolerance properties. J. Mol. Microbiol. Biotechnol. 2010;18:220–229. doi: 10.1159/000319597. [DOI] [PubMed] [Google Scholar]
- Kim S., Huang E., Park S., Holzapfel W., Lim S.D. Physiological characteristics and anti-obesity effect of lactobacillus plantarum K10. Korean J. Food Sci. Anim. Resour. 2018;38:554–569. doi: 10.5851/kosfa.2018.38.3.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita H., Ohuchi S., Arakawa K., Watanabe M., Kitazawa H., Saito T. Isolation of lactic acid bacteria bound to the porcine intestinal mucosa and an analysis of their moonlighting adhesins. Biosci. Microbiota, Food Heal. 2016;35:185–196. doi: 10.12938/bmfh.16-012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirtzalidou E., Pramateftaki P., Kotsou M., Kyriacou A. Screening for lactobacilli with probiotic properties in the infant gut microbiota. Anaerobe. 2011;17:440–443. doi: 10.1016/j.anaerobe.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Klingberg T.D., Axelsson L., Naterstad K., Elsser D., Budde B.B. Identification of potential probiotic starter cultures for Scandinavian-type fermented sausages. Int. J. Food Microbiol. 2005;105:419–431. doi: 10.1016/j.ijfoodmicro.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Lanata C.F., Fischer-Walker C.L., Olascoaga A.C., Torres C.X., Aryee M.J., Black R.E. Global Causes of Diarrheal Disease Mortality in Children <5 Years of Age: A Systematic Review. PLoS One. 2013;8:72788. doi: 10.1371/journal.pone.0072788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Xu W., Yang J., Zhao H., Pan C., Ding X., Zhang Y. Effects of applying lactic acid bacteria to the fermentation on a mixture of corn steep liquor and air-dried rice straw. Anim. Nutr. 2016;2:229–233. doi: 10.1016/j.aninu.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liong M.T., Shah N.P. Acid and bile tolerance and cholesterol removal ability of lactobacilli strains. J. Dairy Sci. 2005;88:55–66. doi: 10.3168/jds.S0022-0302(05)72662-X. [DOI] [PubMed] [Google Scholar]
- Mandal S., Puniya A.K., Singh K. Effect of alginate concentrations on survival of microencapsulated Lactobacillus casei NCDC-298. Int. Dairy J. 2006;16:1190–1195. doi: 10.1016/j.idairyj.2005.10.005. [DOI] [Google Scholar]
- Martín R., Olivares M., Marín M.L., Xaus J., Fernández L., Rodríguez J.M. Characterization of a reuterin-producing Lactobacillus coryniformis strain isolated from a goat’s milk cheese. Int. J. Food Microbiol. 2005;104:267–277. doi: 10.1016/j.ijfoodmicro.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Miao J., Guo H., Ou Y., Liu G., Fang X., Liao Z., Ke C., Chen Y., Zhao L., Cao Y. Purification and characterization of bacteriocin F1, a novel bacteriocin produced by Lactobacillus paracasei subsp. tolerans FX-6 from Tibetan kefir, a traditional fermented milk from Tibet. China. Food Control. 2014;42:48–53. doi: 10.1016/j.foodcont.2014.01.041. [DOI] [Google Scholar]
- Miao J., Liu G., Ke C., Fan W., Li C., Chen Y., Dixon W., Song M., Cao Y., Xiao H. Inhibitory effects of a novel antimicrobial peptide from kefir against Escherichia coli. Food Control. 2016;65:63–72. doi: 10.1016/j.foodcont.2016.01.023. [DOI] [Google Scholar]
- Miao J., Zhou J., Liu G., Chen F., Chen Y., Gao X., Dixon W., Song M., Xiao H., Cao Y. Membrane disruption and DNA binding of Staphylococcus aureus cell induced by a novel antimicrobial peptide produced by Lactobacillus paracasei subsp. tolerans FX-6. Food Control. 2016;59:609–613. doi: 10.1016/j.foodcont.2015.06.044. [DOI] [Google Scholar]
- Mohammadi F., Eshaghi M., Razavi S., Sarokhalil D.D., Talebi M., Pourshafie M.R. Characterization of bacteriocin production in Lactobacillus spp. isolated from mother’s milk. Microb. Pathog. 2018;118:242–246. doi: 10.1016/j.micpath.2018.03.020. [DOI] [PubMed] [Google Scholar]
- Nagpal R., Kumar A., Kumar M., Behare P.V., Jain S., Yadav H. Probiotics, their health benefits and applications for developing healthier foods: A review. FEMS Microbiol. Lett. 2012 doi: 10.1111/j.1574-6968.2012.02593.x. [DOI] [PubMed] [Google Scholar]
- Ołdak A., Zielińska D., Rzepkowska A., Kołozyn-Krajewska D. Comparison of Antibacterial Activity of Lactobacillus plantarum Strains Isolated from Two Different Kinds of Regional Cheeses from Poland: Oscypek and Korycinski Cheese. Biomed Res. Int. 2017;2017 doi: 10.1155/2017/6820369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.A., Tirupathi Pichiah P.B., Yu J.J., Oh S.H., Daily J.W., Cha Y.S. Anti-obesity effect of kimchi fermented with Weissella koreensis OK1-6 as starter in high-fat diet-induced obese C57BL/6J mice. J. Appl. Microbiol. 2012;113:1507–1516. doi: 10.1111/jam.12017. [DOI] [PubMed] [Google Scholar]
- Plessas S., Nouska C., Karapetsas A., Kazakos S., Alexopoulos A., Mantzourani I., Chondrou P., Fournomiti M., Galanis A., Bezirtzoglou E. Isolation, characterization and evaluation of the probiotic potential of a novel Lactobacillus strain isolated from Feta-type cheese. Food Chem. 2017;226:102–108. doi: 10.1016/j.foodchem.2017.01.052. [DOI] [PubMed] [Google Scholar]
- Polak-Berecka M., Waśko A., Paduch R., Skrzypek T., Sroka-Bartnicka A. The effect of cell surface components on adhesion ability of Lactobacillus rhamnosus. Antonie van Leeuwenhoek. Int. J. Gen. Mol. Microbiol. 2014;106:751–762. doi: 10.1007/s10482-014-0245-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad J., Gill H., Smart J., Gopal P.K. Selection and characterisation of Lactobacillus and Bifidobacterium strains for use as probiotics. Int. Dairy J. 1998;8:993–1002. doi: 10.1016/S0958-6946(99)00024-2. [DOI] [Google Scholar]
- Reid G., Jass J., Sebulsky M.T., McCormick J.K. Potential Uses of Probiotics in Clinical Practice. Clin. Microbiol. Rev. 2003 doi: 10.1128/CMR.16.4.658-672.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren D., Li C., Qin Y., Yin R., Du S., Liu H., Zhang Y., Wang C., Rong F., Jin N. Evaluation of immunomodulatory activity of two potential probiotic Lactobacillus strains by invivo tests. Anaerobe. 2015;35:22–27. doi: 10.1016/j.anaerobe.2015.06.008. [DOI] [PubMed] [Google Scholar]
- Ren D., Li C., Qin Y., Yin R., Du S., Ye F., Liu C., Liu H., Wang M., Li Y., Sun Y., Li X., Tian M., Jin N. Invitro evaluation of the probiotic and functional potential of Lactobacillus strains isolated from fermented food and human intestine. Anaerobe. 2014;30:1–10. doi: 10.1016/j.anaerobe.2014.07.004. [DOI] [PubMed] [Google Scholar]
- Rowland I., Capurso L., Collins K., Cummings J., Delzenne N., Goulet O., Guarner F., Marteau P., Meier R. Current level of consensus on probiotic science report of an expert meeting-London, 23 november 2009. Gut Microbes. 2010;1:436. doi: 10.4161/gmic.1.6.13610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahadeva R.P.K., Leong S.F., Chua K.H., Tan C.H., Chan H.Y., Tong E.V., Wong S.Y.W., Chan H.K. Survival of commercial probiotic strains to pH and bile. Int. Food Res. J. 2011;18:1515–1522. [Google Scholar]
- Savino F., Cordisco L., Tarasco V., Locatelli E., Di Gioia D., Oggero R., Matteuzzi D. Antagonistic effect of Lactobacillus strains against gas-producing coliforms isolated from colicky infants. BMC Microbiol. 2011;11:157. doi: 10.1186/1471-2180-11-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxami G., Ypsilantis P., Sidira M., Simopoulos C., Kourkoutas Y., Galanis A. Distinct adhesion of probiotic strain Lactobacillus casei ATCC 393 to rat intestinal mucosa. Anaerobe. 2012;18:417–420. doi: 10.1016/j.anaerobe.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Shokryazdan P., Sieo C.C., Kalavathy R., Liang J.B., Alitheen N.B., Faseleh Jahromi M., Ho Y.W. Probiotic potential of Lactobacillus strains with antimicrobial activity against some human pathogenic strains. Biomed Res. Int. 2014;2014 doi: 10.1155/2014/927268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Qingshen, Shi Y., Wang F., Han D., Lei H., Zhao Y., Sun Quan. Study on the effects of microencapsulated Lactobacillus delbrueckii on the mouse intestinal flora. J. Microencapsul. 2015;32:669–676. doi: 10.3109/02652048.2015.1057249. [DOI] [PubMed] [Google Scholar]
- Valan Arasu M., Jung M.-W., Ilavenil S., Jane M., Kim D.-H., Lee K.-D., Park H.-S., Hur T.-Y., Choi G.-J., Lim Y.-C., Al-Dhabi N.A., Choi K.-C. Isolation and characterization of antifungal compound from Lactobacillus plantarum KCC-10 from forage silage with potential beneficial properties. J. Appl. Microbiol. 2013;115:1172–1185. doi: 10.1111/jam.12319. [DOI] [PubMed] [Google Scholar]
- Valan Arasu M., Jung M.W., Ilavenil S., Kim D.H., Park H.S., Park J.W., Al-Dhabi N.A., Choi K.C. Characterization, phylogenetic affiliation and probiotic properties of high cell density Lactobacillus strains recovered from silage. J. Sci. Food Agric. 2014;94:2429–2440. doi: 10.1002/jsfa.6573. [DOI] [PubMed] [Google Scholar]
- Valan Arasu M., Jung M.W., Kim D.H., Park H.S., Ilavenil S., Al-Dhabi N.A., Choon Choi K. Identification and phylogenetic characterization of novel Lactobacillus plantarum species and their metabolite profiles in grass silage. Ann. Microbiol. 2015;65:15–25. doi: 10.1007/s13213-014-0830-2. [DOI] [Google Scholar]
- Valerio F., Lavermicocca P., Pascale M., Visconti A. Production of phenyllactic acid by lactic acid bacteria: An approach to the selection of strains contributing to food quality and preservation. FEMS Microbiol. Lett. 2004;233:289–295. doi: 10.1016/j.femsle.2004.02.020. [DOI] [PubMed] [Google Scholar]
- Verdenelli M.C., Ghelfi F., Silvi S., Orpianesi C., Cecchini C., Cresci A. Probiotic properties of Lactobacillus rhamnosus and Lactobacillus paracasei isolated from human faeces. Eur. J. Nutr. 2009;48:355–363. doi: 10.1007/s00394-009-0021-2. [DOI] [PubMed] [Google Scholar]
- Vijayakumar M., Ilavenil S., Kim D.H., Arasu M.V., Priya K., Choi K.C. In-vitro assessment of the probiotic potential of Lactobacillus plantarum KCC-24 isolated from Italian rye-grass (Lolium multiflorum) forage. Anaerobe. 2015;32:90–97. doi: 10.1016/j.anaerobe.2015.01.003. [DOI] [PubMed] [Google Scholar]
- Yadav R., Dey D.K., Vij R., Meena S., Kapila R., Kapila S. Evaluation of anti-diabetic attributes of Lactobacillus rhamnosus MTCC: 5957, Lactobacillus rhamnosus MTCC: 5897 and Lactobacillus fermentum MTCC: 5898 in streptozotocin induced diabetic rats. Microb. Pathog. 2018;125:454–462. doi: 10.1016/j.micpath.2018.10.015. [DOI] [PubMed] [Google Scholar]
- Yap P.S., Gilliland S.E. Comparison of newly isolated strains of Lactobacillus delbrueckii subsp. Lactis for hydrogen peroxide production at 5°C. J. Dairy Sci. 2000;83:628–632. doi: 10.3168/jds.S0022-0302(00)74922-8. [DOI] [PubMed] [Google Scholar]
- Zalán Z., Hudáček J., Štětina J., Chumchalová J., Halász A. Production of organic acids by Lactobacillus strains in three different media. Eur. Food Res. Technol. 2010;230:395–404. doi: 10.1007/s00217-009-1179-9. [DOI] [Google Scholar]
- Zhao S., Han J., Bie X., Lu Z., Zhang C., Lv F. Purification and Characterization of Plantaricin JLA-9: A Novel Bacteriocin against Bacillus spp. Produced by Lactobacillus plantarum JLA-9 from Suan-Tsai, a Traditional Chinese Fermented Cabbage. J. Agric. Food Chem. 2016;64:2754–2764. doi: 10.1021/acs.jafc.5b05717. [DOI] [PubMed] [Google Scholar]