Abstract
Antioxidants are one of the effective treatment lines in managing type 2 diabetes (typ2diab) and its complications. Nanoformulations could help in ameliorating the oral bioavailability and biocompatibility properties. Ellagic acid (Ella) is a natural antioxidant compound commonly present in fruits. This study examined the effect Ella nanoparticles (Ella NPs) alone and combined with metformin, the standard antidiabetic drug, on controlling blood glucose in typ2diab. Forty-eight adult Sprague-Dawley rats were used in this study. Except for the control group that was fed a regular pellet diet, all animals were fed a high-fat diet (HFD) for 9 weeks. For the last 4 weeks, rats were injected with streptozotocin (35 mg/kg). Then the rats were randomized into 8 groups: control, HFD, diabetic, Ella, Ella + metformin, Ella NPs, and Ella NPs + metformin. Data showed that Ella NPs improved blood glucose levels and the body weights of diabetic rats throughout all the weeks of the experiment whereas effects of the regular Ella were limited to the last two weeks of the treatment. Additionally, data demonstrated that the antidiabetic action of Ella NPs and its effective duration were similar to metformin. Ella NPs led to a lowering effect on lipid profile markers (total cholesterol (TC), triglyceride (TG), low-density lipoprotein (LDL), and very-low-density lipoprotein (VLDL)), superior to the regular Ella, which reduced only TG and VLDL. Results of the pathological examination showed improved number and activity of beta islets in all treatment groups. The most enhanced islets were in the Ella NPs and metformin group. The different treatments decreased caspase 3 and increased insulin gene expression, and the effect was superior in the Ella NPs and metformin group. The results of this study confirmed that Ella could manage typ2diab by lowering glucose and lipid levels and improving body weight with the superiority of Ella NPs. The mechanisms behind these effects are inhibition of beta-cell apoptosis and stimulation of insulin production.
Keywords: Ellagic acid, Nanoformulations, Metformin, Beta islet, Glucose, Lipids, Caspase 3, Insulin, Bodyweight
1. Introduction
Type 2 diabetes (typ2diab) is the most common metabolic disease whose primary cause is obesity-linked insulin resistance. Obesity, mental stress, infection, and/or genetic predisposition might contribute to the pathogenesis of typ2diab by increasing insulin resistance or impairing insulin secretion (Tilg et al., 2009). Diabetes is rapidly rising all over the world at a high rate (Huizinga and Rothman, 2006). The global spread of diabetes mellitus has been estimated to be 171 million people and is projected to more than double to 366 million people by 2030 (Wild et al., 2004). In the Arab world, the countries with the highest prevalence of typ2diab are: Saudi Arabia, Oman, Kuwait, Bahrain, and UAE; this prevalence was significantly associated with high Gross Domestic Product (GDP) and energy consumption (Meo et al., 2017). There are many oral antidiabetic drugs used to control typ2diab. However, most of them have little efficacy and many unwanted side effects (Karagiannis et al., 2012). Therefore, the development of antidiabetic drugs with low toxicity and high efficacy along with cost effectiveness is still needed.
Naturally occurring biologically active compounds found in plants are non-toxic and inexpensive. Therefore, they are widely used to prevent morbidity and mortality from chronic diseases (Gaziano et al., 2007). In recent years, natural antioxidants have acquired increasing attention over synthetic ones in the protection against typ2diab because of their proven benefits, low toxicity, and ease of access through natural diets and dietary supplements (Montonen et al., 2004). Ellagic acid (2,3,7,8-tetrahydroxy-chromeno [5,4,3-cde] chromene-5, 10-dione) (Ella) is a natural compound classified as polyphenolic and is mainly present in fruits like grapes, pomegranates, blackcurrants, raspberries, and in nuts (Rozentsvit et al., 2017). Owing to its antiproliferative activity in certain tumors and its antioxidant and anti-inflammatory effects, Ella has been extensively investigated. An increasing body of evidence indicates that the intake of Ella is effective in alleviating obesity and improving metabolic complications caused by obesity, such as insulin resistance and typ2diab (Rozentsvit et al., 2017). Bala et al. (2006) developed a nanoparticulate formulation for sustained-release with antioxidant Ella as a possible prophylaxis when administered orally (Bala et al., 2006). Such Ella-loaded nanoparticles demonstrated a more efficient free radical scavenging efficacy in a yeast cell culture model and a cell-free system.
The present study examined the effect Ella nanoparticles (Ella NPs) alone and combined with metformin, the standard antidiabetic drug, on controlling blood glucose in a typ2diab model induced in rats.
2. Materials and methods
2.1. Chemicals
Streptozotocin (STZ) was obtained from Sigma Chemicals Co. (St. Louis, MO, USA). Glucophage tablets and 500 mg metformin (Merck, Santa, France) were used. Ella NPs were formulated and characterized as recently published (El-Shitany et al., 2019).
2.2. Animals
Thirty-six adult Sprague-Dawley rats weighing 180–200 g were used. The animals were kept in cages at ambient temperature at 25 ± 5 °C under a typical half-light and half-dark cycle. They were allowed ad libitum access to food and water with a one week period for acclimatization. Ethical approval from the Ethical Review Committee of King Abdulaziz University was received and all animals were treated according to the Declaration of Helsinki with the care guidelines of using experimental animals in line with the NIH protocol (Touitou et al., 2004).
2.3. Induction of typ2diab
The control group was fed the regular pellet diet, and all other rats were put on a high-fat diet (HFD) for 9 weeks. The composition of the HFD was 58% fat, 25% protein, and 17% carbohydrate, as a percentage of total kcal. At 4 weeks after the start of the experiment, intraperitoneal injection (i.p.) of one dose of STZ (35 mg/kg) in 0.01 M cold citrate buffer (pH 4.5) was administered. Animals that served as control were injected with only the vehicle (0.01 M citrate buffer, pH 4.5). At 24 h, the glucose levels were measured, and diabetes was confirmed when the glucose level was more than 220 mg/dl. The weight of diabetic rats was recorded and then they were randomly divided into 6 groups, 6 rats per group (Guo et al., 2018).
2.4. Experimental design
All diabetic rats were treated with the indicated doses every day for 9 weeks. Group 1 (Control): The non-fatty non-diabetic group was i.p. injected with 0.9% saline solutions. Group 2 (HFD): Fatty non-diabetic group was i.p. injected with 0.9% saline solution. Group 3 (Diabetic): Fatty and diabetic group. Group 4 (Metformin): Diabetic rats were i.p. injected with metformin 300 mg/kg (Katakam et al., 2000). Group 5 (Ella): Diabetic rats were i.p. injected with Ella 10 mg/kg (Amin and Arbid, 2017). Group 6 (Ella NPs): Diabetic rats were i.p. injected with Ella NPs 10 mg/kg. Group 7 (Ella + Metformin): Diabetic rats were i.p. injected with a combination of Ella 10 mg/kg and metformin 300 mg/kg. Group 8 (Ella NPs + Metformin): Diabetic rats were i.p. injected with a combination of Ella NPs 10 mg/kg and metformin 300 mg/kg.
2.5. Assessment of body weight gain and blood glucose level
The rats' body weight and blood sugar levels were assessed weekly for 9 weeks. Body weight gain was calculated for each group. Blood glucose was tested using Ames Ames glucometer GX (Miles Inc., Indiana, USA). Briefly, the tail was inserted into a water bath (45 °C) and around 1 ml of its end was cut to produce blood, and one drop of the blood was used to determine the blood glucose level.
2.6. Samples collection
At the end of the experimental period, rats were euthanized using deep ether anesthesia and blood was collected from retro-orbital venous plexus into EDTA-tubes. The blood was centrifuged at 3,000 xg for 20 min using a benchtop centrifuge (Anke TGL-16B) to recover the plasma. The pancreas samples were extracted, washed with 0.9% saline solution, and preserved in a 10% neutral solution of formalin for the histopathological and immunohistochemical examinations.
2.7. Assessment of plasma lipid profile
Triglycerides (TG), low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, total cholesterol (TC), and very-low-density lipoprotein (VLDL) cholesterol levels were measured in the plasma using Triglyceride Quantification Colorimetric Kit (Biovision, Inc., Milpitas, CA) and HDL and LDL/VLDL Cholesterol Assay Kit (Cat # STA-391, Cell Biolabs, Inc., USA).
2.8. Assessment of pancreatic histopathological alteration
The formalin-preserved pancreases were stained with hematoxylin and eosin (H & E) and looked over microscopically for any histopathological variations.
2.9. Immunohistochemical assessment of pancreatic insulin and caspase-3
The immunoperoxidase (PAP, peroxidase/anti peroxidase) reaction was performed using the insulin and caspase-3 antibodies of Lab Vision (Thermo Scientific, Fremont, CA, USA, catalog no. RB1197R7). The slides were then examined under the light microscope and photographed in a blinded manner by a pathologist.
2.10. Statistical analysis
All data are presented as average ± standard deviation (SD). Statistical software SPSS 22.0 (IBM, Yorktown Heights, NY, USA) was utilized for data entry. Statistical analyses were performed using a one-way analysis of variance (ANOVA) test. Statistically significant differences were determined at the 95% level.
3. Results
3.1. Effect of Ella, Ella NPs, and their combination with metformin on body weight measured weekly over 9 weeks and the body weight gain after 9 weeks
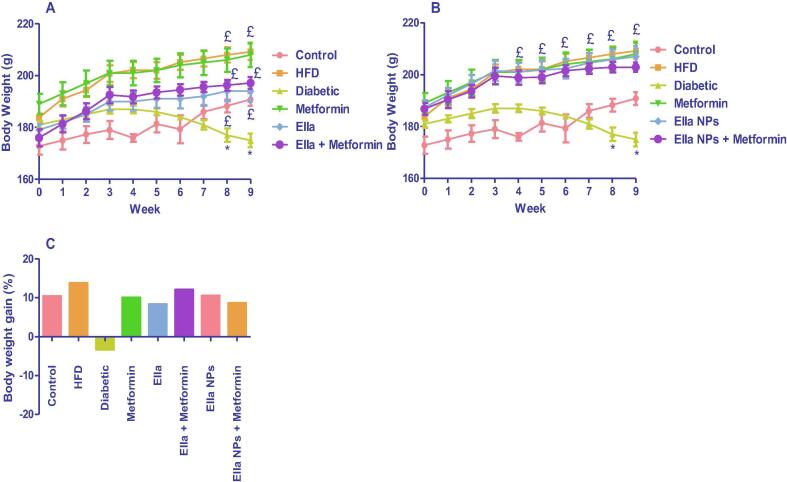
HFD/STZ-induced typ2diab (diabetic group) resulted in a significant decrease in rats' body weight compared with control rats at week 8 and week 9. The diabetic rats treated with metformin (300 mg/kg) or Ella (10 mg/kg) as monotherapy and combined therapy with metformin showed a significant increase in rats' body weight at week 8 and week 9 compared to the diabetic group (Fig. 1A). The diabetic rats treated with metformin (300 mg/kg) or Ella NPs (10 mg/kg) as monotherapy and combined therapy with metformin showed significantly increased rats’ body weight from week 4 to week 9 compared to the diabetic group (Fig. 1B). Unfortunately, although the monotherapy with either metformin, Ella, or Ella NPs effectively increased rats' body weight, there was no significant difference between the weight of rats post-metformin monotherapy, and metformin combined therapy with either Ella or Ella NPs (Fig. 1A and B). All treatment groups had increased body weight gain (%) compared with the diabetic group (Fig. 1C).
Fig. 1.
Effects of A: ellagic acid (Ella), metformin, and their combination, B: ellagic acid nanoparticles (Ella NPs), metformin, and their combination on rats' body weight measured weekly over 9 weeks. C: Effects of Ella, Ella NPs, metformin, and their combination on rats' body weight gain % calculated after 9 weeks of treatment. Results are presented as mean ± SD. * significant relative to control; £ significant relative to diabetic. (P < 0.05).
3.2. Effect of Ella, Ella NPs, and their combination with metformin on blood glucose levels measured weekly over 9 weeks
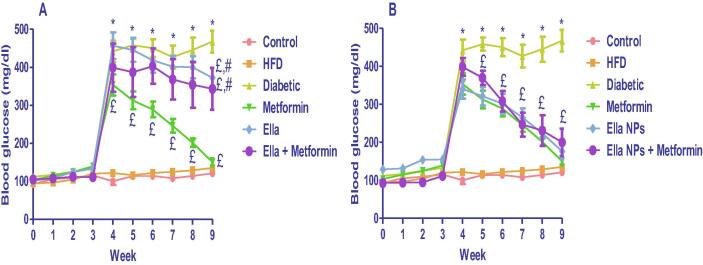
HFD/STZ-induced typ2diab (diabetic group) resulted in a significant increase in blood glucose levels compared with control rats from week 4 to week 9. The diabetic rats treated with metformin (300 mg/kg) monotherapy showed significant decreases in blood glucose levels from week 4 to week 9 compared to the diabetic group. The diabetic rats treated with Ella (10 mg/kg) monotherapy and Ella combined therapy with metformin showed a significant decrease in blood glucose levels at week 9 only compared to diabetic rats (Fig. 2A). The diabetic rats treated with Ella NPs (10 mg/kg) monotherapy and Ella NPs combined therapy with metformin showed significantly decreased blood glucose levels from week 5 to week 9 compared to the diabetic group. Metformin monotherapy induced a significant hypoglycaemic action compared to either Ella monotherapy or Ella combined with metformin at week 9. Unfortunately, while the monotherapy with either metformin or Ella NPs effectively reduced the blood glucose level, there was no significant difference between the hypoglycaemic effect of metformin monotherapy and metformin combined therapy with Ella NPs (Fig. 2B).
Fig. 2.
Effects of A: ellagic acid (Ella), metformin, and their combination, B: ellagic acid nanoparticles (Ella NPs), metformin, and their combination on blood glucose levels measured weekly over 9 weeks. Results are presented as mean ± SD. * significant relative to control; £ significant relative to diabetic. # significant relative to metformin. (P < 0.05).
3.3. Effect of Ella, Ella NPs, and their combination with metformin on plasma lipid profile measured after 9 weeks
HFD/STZ-induced typ2diab (diabetic group) resulted in a significant increase in plasma TG and VLDL levels compared with control rats at week 9. On the other hand, the diabetic rats showed a significant decrease in plasma HDL levels compared with the control group at week 9. The diabetic rats treated with metformin (300 mg/kg), Ella (10 mg/kg), Ella NPs as monotherapy, and combined therapy with metformin showed a significant decrease in plasma TG and VLDL at week 9 compared to diabetic group. The diabetic rats treated with Ella NPs as monotherapy and combined therapy with metformin showed a significant decrease in plasma TC and LDL at week 9 compared to the diabetic group. The hypolipidemic effect of the combined treatment of metformin with either Ella or Ella NPs was superior to the effect of metformin monotherapy (Table 1).
Table 1.
Effect of ellagic acid (Ella), ellagic acid nanoparticles (Ella NPs), metformin, and their combination plasma lipid profile measured after 9 weeks of treatment.
| Group | TC (mg/dl) | TG (mg/dl) | HDL (mg/dl) | LDL (mg/dl) | VLDL (mg/dl) |
|---|---|---|---|---|---|
| Control | 90 ± 9.3 | 58 ± 6.3 | 37 ± 1.1 | 36 ± 6.8 | 11 ± 1.2 |
| HFD | 94 ± 6.1 | 93 ± 14.7 a | 23 ± 2.1 a | 53 ± 6.8 | 19 ± 2.9 a |
| Diabetic | 90 ± 8.8 | 126 ± 17.5 a | 28 ± 3.6 a | 42 ± 9.5 | 25 ± 3.4 a |
| Metformin | 83 ± 13.0 | 90 ± 10.0 b | 27 ± 2.6 | 49 ± 13.5 | 18 ± 1.9 b |
| Ella | 86 ± 2.7 | 51 ± 2.9 b, c | 25 ± 1.7 | 51 ± 0.8 | 10 ± 0.4 b, c |
| Ella + Metformin | 73 ± 12.4 | 60 ± 2.3 b, c | 27 ± 4.3 | 34 ± 8.3 | 12 ± 0.4 b, c |
| Ella NPs | 60 ± 4.7 b, c | 62 ± 3.3 b, c | 25 ± 1.8 | 26 ± 2.9 b, c | 12 ± 0.6 b, c |
| Ella NPs + Metformin | 68 ± 10.0 b, c | 43 ± 2.7 b, c | 26 ± 2.9 | 33 ± 6.6 b, c | 9 ± 0.9 b, c |
Results are presented as mean ± SD.
TC: total cholesterol; TG: triglyceride; HDL: high-density lipoprotein; LDL: low-density lipoprotein; VLDL: very-low-density lipoprotein.
Significant relative to control.
Significant relative to diabetic.
Significant relative to metformin. (P < 0.05).
3.4. Effect of Ella, Ella NPs, and their combination with metformin on histopathology of pancreatic islets of Langerhans after 9 weeks
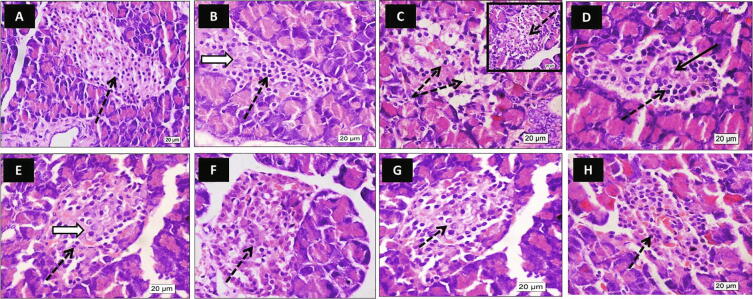
The control and HFD groups showed Langerhans islets with an average population of centrally located beta cells. However, some cells in the HFD group looked hypertrophied. The HFD/STZ-induced typ2diab (diabetic group) group showed islets with a decreased population of centrally located beta cells; most of them looked swollen with cytoplasmic vacuolation and small pyknotic nuclei. The metformin group showed preservation of islets cells compared to the diabetic group with few centrally located beta cells showing degenerated nuclei. The beta cells appeared with their average density and active vesicular nuclei in all the other treatment groups (Ella, Ella + metformin, Ella NPs, and Ella NPs + metformin). The group of Ella NPs + metformin showed more preservation of centrally located beta cells compared to all groups (Fig. 3).
Fig. 3.
Effect of ellagic acid (Ella), ellagic acid nanoparticles (Ella NPs), and their combination with metformin on histopathology of pancreatic islets of Langerhans (H & E) after 9 weeks. Photos represents pancreas of control [A], high fat diet (HFD) [B], diabetic [C], metformin [D], Ella [E], Ella + metformin [F], Ella NPs [G], Ella NPs + metformin [H]. The control and HFD photos showed Langerhans islets with a normal population of centrally located beta cells (dotted arrows). Some cells in the HFD photo looked hypertrophied (white arrow). The diabetic photo showed islets with a decreased population of centrally located beta cells; most of them looked swollen with cytoplasmic vacuolation and small pyknotic nuclei (dotted arrow). The metformin photo showed preservation of islets cells compared to the diabetic photo (dotted arrow) with few centrally located beta cells showing degenerated nuclei (black arrow). The Ella photo showed Langerhans islets with normal cell population (dotted arrows) similar to the control photo. Central beta cells have active nuclei (white arrow). Ella + metformin showed Langerhans islets with normal beta cell density; most of them have active vesicular nuclei (dotted arrow). Ella NPs photo showed preservation of normal islets cell population with apparent active nuclei of centrally located beta cells (dotted arrow). Ella NPs + metformin showed more preservation of centrally located beta cells compared to other groups (dotted arrows). (H & E X 600).
3.5. Effect of Ella, Ella NPs, and their combination with metformin on insulin immunostaining in pancreatic islets of Langerhans after 9 weeks
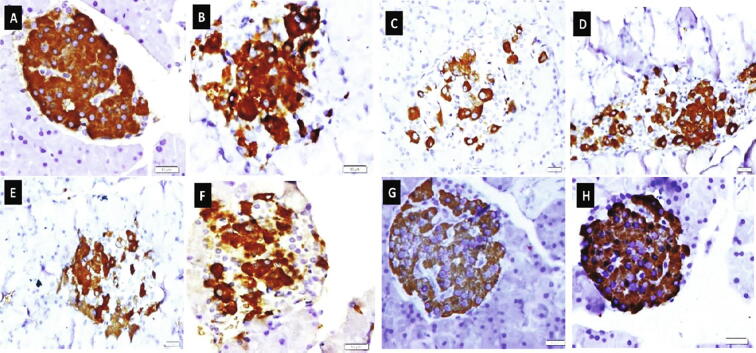
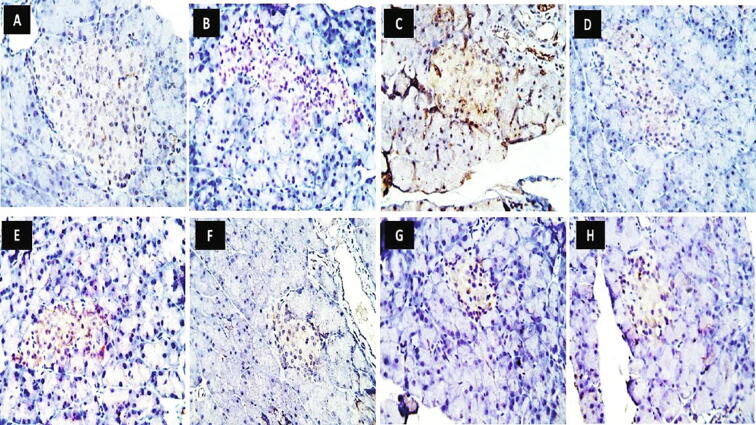
The control group showed marked insulin immuno-expression, which occupies most of the islet's cells. The islet's cells of the HFD group showed nearly similar insulin immuno-expression compared to the control group. A marked reduction in the insulin immuno-expression was observed within scattered islet's cells of the diabetic group. Moderate positive insulin immuno-expressions were found within islet's cells of diabetic rats treated with metformin, Ella, and Ella + metformin groups, whereas marked positive insulin immuno-expressions were observed within most islet's cells of diabetic rats treated with Ella NPs monotherapy and Ella NPs combined therapy with metformin (Fig. 4).
Fig. 4.
Effect of ellagic acid (Ella), ellagic acid nanoparticles (Ella NPs), and their combination with metformin on insulin immunostaining in pancreatic islets of Langerhans after 9 weeks. Photos represents pancreas of control [A], high fat diet (HFD) [B], diabetic [C], metformin [D], Ella [E], Ella + metformin [F], Ella NPs [G], Ella NPs + metformin [H]. (Insulin immunoreactivity X 600).
3.6. Effect of Ella, Ella NPs, and their combination with metformin on caspase-3 immunostaining in pancreatic islets of Langerhans after 9 weeks
The control group showed negative caspase-3 immuno-expression within the islet's cells. A strong positive caspase-3 immuno-expression was observed within the islet's cells of the diabetic group. Moderate positive caspase-3 immuno-expressions were observed within islet's cells of diabetic rats treated with Ella, Ella NPs, and HFD groups. In contrast, mild positive caspase-3 immuno-expressions were found within islet's cells of diabetic rats treated with metformin monotherapy and metformin combined therapy with either Ella or Ella NPs (Fig. 5).
Fig. 5.
Effect of ellagic acid (Ella), ellagic acid nanoparticles (Ella NPs), and their combination with metformin on caspase-3 immunostaining in pancreatic islets of Langerhans after 9 weeks. Photos represents pancreas of control [A], high fat diet (HFD) [B], diabetic [C], metformin [D], Ella [E], Ella + metformin [F], Ella NPs [G], Ella NPs + metformin [H]. (Caspase-3 immunoreactivity X 400).
4. Discussion
The results of this study showed a therapeutic effect of Ella NPs against typ2diab induced by STZ in rats fed on the HFD diet. It was observed that Ella NPs treated diabetic rats had weight reduction associated with diabetes throughout all weeks of the experiment and until its end, while the effect of the regular Ella was limited to the last 2 weeks of the experiment only. It was also noted that the treatment of diabetic rats with Ella NPs caused a significant decrease in the level of blood glucose over the time of the experiment, while the effect of the regular Ella was limited to the last 2 weeks only. Both the time course and the antidiabetic action of Ella NPs were similar to metformin, and their combination did not show an additional benefit concerning either body weight or serum glucose.
Antioxidants are one of the effective treatment lines in managing diabetes and its complications. However, their poor biopharmaceutical properties, such as poor solubility and poor bioavailability, interfere with their use (Sule et al., 2008). From the present study, it appears that Ella NPs alone or combined with metformin could be more efficient than Ella itself, and this may be attributed to the protection of Ella NPs from GI degradation and sustained release of encapsulated drug in the circulation, resulting in improved bioavailability (Bhardwaj et al., 2006, Patra et al., 1007). In addition, nanoformulations could help in ameliorating bioavailability and biocompatibility issues, while preserving the therapeutic effectiveness of the medicine (Mansoor et al., 2019). In an earlier study, a blood-hypoglycemic effect of Ella in the STZ-induced diabetes model in rats was found, although the Ella doses used were approximately 5 and 10 times those used in the current study (Fatima et al., 2017, Malini et al., 2011). Ratnam et al. (Ratnam et al., 2008) reported improved efficacy of Ella NPs when used at a dose much lower than the regular Ella. These findings further confirm the enhanced bioavailability and enhanced potency of Ella NPs.
Typ2diab is commonly connected with an alteration of lipid picture with elevated TC, TG, and LDL, besides a marked decrease in serum HDL content (De MagalhÃes et al., 2019). The results of this HFD/STZ typ2diab model showed that the synthesis of NPs of Ella led to a lowering effect of all components of the lipid profile in the blood (TC, TG, LDL, and VLDL), superior to the regular Ella that reduced only TG and VLDL. In line with the results of the current study, treatment of STZ diabetic rats with a combination of Ella NPs and coenzyme Q10 resulted in a significant reduction of blood lipids compared to regular Ella (Ratnam et al., 2008). Moreover, a previous study showed that treating hamsters fed with HFD with pomegranate peel extract rich in Ella in a dose exceeding our dose by around 3 times lowered blood TC, TG, LDL, and VLDL while increasing HDL (Liu et al., 2015). Contrary to results of Liu et al. (Liu et al., 2015), neither Ella nor Ella NPs increased the level of HDL in the HFD/STZ typ2diab model. The difference in the effect of Ella between our study and their results may be due to the different diabetic models adopted. In our study, two factors affected the level of HDL–the HFD and STZ–both of them could increase the level of HDL statistically compared to the control, and in addition the HFD alone could increase HDL.
Rsesults of the pathological examination of the pancreas confirmed all the biochemical results because the number and activity of beta cells improved in all treatment groups. It was observed that the least improved islets were the metformin islets, and the most enhanced islets were as a result of the combination of the Ella NPs and metformin. Chronic hyperglycemia associated with typ2diab leads to loss of mass and function of pancreatic beta cells. Oxidative stress associated with increased glucose and lipids levels stimulates programmed beta cell death (Ding et al., 2019). As shown by the immunopathological examination of the apoptotic factor caspase 3 in the pancreatic islets, it increased in the diabetic group. At the same time, the different treatments decreased its gene expression, and the effect was superior to the Ella NPs and metformin group. Caspase 3 is one of the main factors causing apoptosis in mammals (Feng et al., 2016). It is well known that inhibition of its action is essential for apoptosis suppression (Grippa et al., 2015). Ella was found to inhibit the activity of caspase 3 in the liver and brain of rats with liver and brain damage resulting from D-galactose-induced aging (Chen et al., 2018).
Treatment of diabetic rats with Ella NPs alone or in combination with metformin increased gene expression of insulin in the beta islets compared to both the diabetic group and metformin alone. The improvement in beta cell count and inhibition of programmed cell death are the main reasons behind the increase in cell function and improved insulin production in these groups. In agreement with the results of the present study, the treatment of STZ diabetic rats with a combination of Ella NPs and coenzyme Q10 resulted in significant increase in plasma insulin concentration compared to the regular Ella (Ratnam et al., 2008).
5. Conclusion
The results of this study confirmed that Ella could manage typ2diab by lowering glucose and lipid levels and improving body weight by using Ella NPs. The mechanisms behind these effects are inhibition of beta-cell apoptosis and stimulation of insulin production.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, under grant no. G: 687-140-1439. The authors, therefore, acknowledge with thanks The DSR for their technical and financial support.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Steve Harakeh, Email: sharakeh@gmail.com.
Shaker A. Mousa, Email: shaker.mousa@acphs.edu.
References
- Amin M.M., Arbid M.S. Estimation of ellagic acid and/or repaglinide effects on insulin signaling, oxidative stress, and inflammatory mediators of liver, pancreas, adipose tissue, and brain in insulin resistant/type 2 diabetic rats. Appl. Physiol. Nutr. Metab. 2017;42:181–192. doi: 10.1139/apnm-2016-0429. [DOI] [PubMed] [Google Scholar]
- Bala I., Bhardwaj V., Hariharan S., Kharade S.V., Roy N., Kumar M.N.V.R. Sustained release nanoparticulate formulation containing antioxidant-ellagic acid as potential prophylaxis system for oral administration. J. Drug Target. 2006;14:27–34. doi: 10.1080/10611860600565987. [DOI] [PubMed] [Google Scholar]
- Bhardwaj V., Hariharan S., Bala I., Lamprecht A., Kumar N., Panchagnula R. Pharmaceutical aspects of polymeric nanoparticles for oral drug delivery. J. Biomed. Nanotechnol. 2006;1:235–258. doi: 10.1166/jbn.2005.033. [DOI] [Google Scholar]
- Chen P., Chen F., Zhou B. Antioxidative, anti-inflammatory and anti-apoptotic effects of ellagic acid in liver and brain of rats treated by D-galactose. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-19732-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding M., Fang Q., Cui Y., Shen Q., Liu Q., Wang P. Liraglutide prevents β-cell apoptosis via inactivation of NOX2 and its related signaling pathway. J. Diab. Complicat. 2019;33:267–277. doi: 10.1016/j.jdiacomp.2018.12.013. [DOI] [PubMed] [Google Scholar]
- El-Shitany N.-A.-E.-A., Abbas A.T., Ali S.S., Eid B., Harakeh S., Neamatalla T. Nanoparticles ellagic acid protects against cisplatin-induced hepatotoxicity in rats without inhibiting its cytotoxic activity. Int. J. Pharmacol. 2019;15:465–477. doi: 10.3923/ijp.2019.465.477. [DOI] [Google Scholar]
- Fatima N., Hafizur R.M., Hameed A., Ahmed S., Nisar M., Kabir N. Ellagic acid in Emblica officinalis exerts antidiabetic activity through the action on β-cells of pancreas. Eur. J. Nutr. 2017;56:591–601. doi: 10.1007/s00394-015-1103-y. [DOI] [PubMed] [Google Scholar]
- Feng Y., Yu Y., Wang S., Ren J., Camer D., Hua Y. Chlorogenic acid protects d -galactose-induced liver and kidney injury via antioxidation and anti-inflammation effects in mice. Pharm. Biol. 2016;54:1027–1034. doi: 10.3109/13880209.2015.1093510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaziano T.A., Galea G., Reddy K.S. Scaling up interventions for chronic disease prevention: the evidence. Lancet. 2007;370:1939–1946. doi: 10.1016/S0140-6736(07)61697-3. [DOI] [PubMed] [Google Scholar]
- Grippa A., Buxó L., Mora G., Funaya C., Idrissi F., Mancuso F. The seipin complex Fld1/Ldb16 stabilizes ER-lipid droplet contact sites. J. Cell Biol. 2015;211:829–844. doi: 10.1083/jcb.201502070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X.X., Wang Y., Wang K., Ji B.P., Zhou F. Stability of a type 2 diabetes rat model induced by high-fat diet feeding with low-dose streptozotocin injection. J. Zhejiang Univ. Sci. B. 2018;19:559–569. doi: 10.1631/jzus.B1700254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizinga M.M., Rothman R.L. Addressing the diabetes pandemic: a comprehensive approach. Indian J. Med. Res. 2006;124:481–484. [PubMed] [Google Scholar]
- Karagiannis T., Paschos P., Paletas K., Matthews D.R., Tsapas A. Dipeptidyl peptidase-4 inhibitors for treatment of type 2 diabetes mellitus in the clinical setting: systematic review and meta-analysis. BMJ. 2012;344:17. doi: 10.1136/bmj.e1369. [DOI] [PubMed] [Google Scholar]
- Katakam P.V.G., Ujhelyi M.R., Hoenig M., Miller A.W. Metformin improves vascular function in insulin-resistant rats. Hypertension. 2000;35:108. doi: 10.1161/01.HYP.35.1.108. [DOI] [PubMed] [Google Scholar]
- Liu R., Li J., Cheng Y., Huo T., Xue J., Liu Y. Effects of ellagic acid-rich extract of pomegranates peel on regulation of cholesterol metabolism and its molecular mechanism in hamsters. Food Funct. 2015;6:780–787. doi: 10.1039/c4fo00759j. [DOI] [PubMed] [Google Scholar]
- De MagalhÃes D., Kume W., Correia F., Queiroz T., Allebrandt Neto E., Dos Santos M. High-fat diet and streptozotocin in the induction of type 2 diabetes mellitus: a new proposal. An Acad. Bras. Cienc. 2019;91:20180314. doi: 10.1590/0001-3765201920180314. [DOI] [PubMed] [Google Scholar]
- Malini P., Kanchana G., Rajadurai M. Antibiabetic efficacy of ellagic acid in streptozotoc-ininduced diabetes mellitus in albino wistar rats. Asian J. Pharm. Clin. Res. 2011;4:124–128. [Google Scholar]
- Mansoor S., Kondiah P., Choonara Y., Pillay V. Polymer-based nanoparticle strategies for insulin delivery. Polymers (Basel) 2019;11:1380. doi: 10.3390/polym11091380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meo S.A., Usmani A.M., Qalbani E. Prevalence of type 2 diabetes in the Arab world: impact of GDP and energy consumption. Eur. Rev. Med. Pharmacol. Sci. 2017;21:1303–1312. [PubMed] [Google Scholar]
- Montonen J., Knekt P., Järvinen R., Reunanen A. Dietary antioxidant intake and risk of type 2 diabetes. Diab. Care. 2004;27:362–366. doi: 10.2337/diacare.27.2.362. [DOI] [PubMed] [Google Scholar]
- Patra J., Das G., Fraceto L., Campos E., Rodriguez-Torres M., Acosta-Torres L. Nano based drug delivery systems: recent developments and future prospects 10 Technology 1007 Nanotechnology 03 Chemical Sciences 0306 Physical Chemistry (incl. Structural) 03 Chemical Sciences 0303 Macromolecular and Materials Chemistry 11 Medical and He. J. Nanobiotechnol. 2018;16 doi: 10.1186/s12951-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnam D., Chandraiah G., Sonaje K., Viswanad B., Bhardwaj V., Ramarao P. A potential therapeutic strategy for diabetes and its complications in the form of co-encapsulated antioxidant nanoparticles (NanoCAPs) of ellagic acid and coenzyme Q10: preparation and evaluation in streptozotocin induced diabetic rats. J. Biomed. Nanotechnol. 2008;4:33–43. doi: 10.1166/jbn.2008.011. [DOI] [Google Scholar]
- Rozentsvit A., Vinokur K., Samuel S., Li Y., Gerdes A.M., Carrillo-Sepulveda M.A. Ellagic acid reduces high glucose-induced vascular oxidative stress through ERK1/2/NOX4 signaling pathway. Cell. Physiol. Biochem. 2017;44:1174–1187. doi: 10.1159/000485448. [DOI] [PubMed] [Google Scholar]
- Sule N., Singh R., Srivastava D. Alternative modes of binding of recombinant human histone deacetylase 8 to colloidal gold nanoparticles. J. Biomed. Nanotechnol. 2008;4:463–468. doi: 10.1166/jbn.2008.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilg H., Moschen A.R., Kaser A. Obesity and the Microbiota. Gastroenterology. 2009;136:1476–1483. doi: 10.1053/j.gastro.2009.03.030. [DOI] [PubMed] [Google Scholar]
- Touitou Y., Portaluppi F., Smolensky M.H., Rensing L. Ethical principles and standards for the conduct of human and animal biological rhythm research. Chronobiol. Int. 2004;21:161–170. doi: 10.1081/CBI-120030045. [DOI] [PubMed] [Google Scholar]
- Wild S., Roglic G., Green A., Sicree R., King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diab. Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]