Abstract
We have shown, the outcome of antifungal activity of phenazine derivatives which is produced by fluorescent pseudomonads (FPs) for the control of sheath blight of rice. A total of 50 fluorescent pseudomonads (FPs) were isolated from rice rhizosphere. Off which, 36 FPs exhibited antagonistic activity against Rhizoctonia solani, Macrophomina phaseolina, Fusarium oxysporum, Alternaria alternata and Sclerotium rolfsii up to 70–80% compared to control by dual culture method. BOX-PCR analyses of antagonistic isolates indicated that two phylogenetic group, where group I consisted of 28 isolates and eight isolates belongs to group II. Among 36 FPs, a total of 10 FPs revealed that the presence of phenazine derivatives on thin layer chromatography (TLC), which is coincided with that of authentic phenazine with Rf value 0.57. Similar to TLC analysis, antibiotic encoding gene phenazine-1-carboxamide (PCN) was detected in 10 FPs by PCR analysis with respective primer. Among, PCN detected isolates of FPs, a significant biocontrol potential possessing isolate designated as VSMKU1 and it was showed prominent antifungal activity against R. solani and other tested fungal pathogens. Hence, the isolate VSMKU1 was selected for further studies. The selected isolate VSMKU1 was identified as Pseudomonas aeruginosa by 16S rDNA sequence analysis. The antifungal metabolite phenazine like compound produced by VSMKU1 was confirmed by UV, FT-IR and HPLC analysis. The phenazine compound from VSMKU1 significantly arrest the growth of R. solani compared to carbendazim by well diffusion method. The detached leaf assay showed remarkable inhibition of lesion height 80 to 85% by the treatments of culture (VSMKU1), cell free culure filtrate and phenazine like compound compared to control and other treatments was observed in detached leaves of rice. These results emphasized that VSMKU1 isolate can be used as an alternative potential biocontrol agent against sheath blight of rice, instead of using commercial fungicide such as validamycin and carbendazim which cause environmental pollution and health hazards.
Keywords: Fluorescent pseudomonads, Rhizoctonia solani, Phenazine-1-carboxamide, Thin layer chromatography, Sheath blight of rice, Detached leaf assay
1. Introduction
Sheath blight of rice caused by Rhizoctonia solani Kühn is one of the economically important critical disease and provide quality issues and severe loss in rice production worldwide especially Asian countries like India and China (Slaton et al., 2003, Shanmugaiah et al., 2010, Xia et al., 2017). The soil-borne pathogen R. solani is an important basidiomycete fungus infecting with wide host range of agriculture crop (Zheng et al., 2013, Zhang et al., 2019). R. solani control is a very difficult because of high survival rate with fruiting bodies (sclerotia) of R. solani under various environmental conditions (Harikrishnan et al., 2014, Lu et al., 2016). Sheath blight of rice has gradually become one of the second most important disease next to blast disease of rice (Singh et al., 2010). So, far there is no sheath blight of rice resistant variety is available. Carbendazim and Validamycin are still widely used for the management of sheath blight of rice (Peng et al., 2014). Indiscriminate use of chemical fungicides is causing a rigorous intimidation to the environment and community health and they are dangerous to other beneficial rhizosphere micro flora existing in an agricultural environment (Shanmugaiah et al., 2010). Hence, in this background, here is a vital necessitate to discover, the alternative approach for crop protection from fungal and bacterial pathogens with the perception of environment friendly and sustainable agriculture (Shanmugaiah et al., 2010, Harikrishnan et al., 2016, Rahman et al., 2018, Ahirwar et al., 2020).
Rhizospheric beneficial bacteria are target-specific and they have been used as bioinoculants and biopesticides to enhance the plant enlargement and crop yield (Gnanamanickam et al., 1998, Shanmugaiah et al., 2006, Nascente et al., 2017). Among the beneficial antagonistic microbial inhabitants, fluorescent pseudomonads (FPs) are drawn much attention worldwide, because of their plant growth promotion effectiveness and bio- control prospective with broad spectral activity to control of various plant pathogens by the manufacture of metabolites, siderophore and hydrogen cyanide (Shanmugaiah et al., 2015, Li et al., 2018). FPs have been reported as the most predominant bacteria in the plant rhizosphere and this beneficial microbe is a part of its capacity to build a protective cover that enhances pathogen suppressive in the rhizosphere (Kumar and Dube, 1992, Singh et al., 2016).
Fluorescent pseudomonad’s (FPs) are major diverse, ecologically and cost-effectively important group of microorganisms. These microbes are well established in terms of the effective plant interaction (Haas and Défago, 2005). FPs directly influences the plant growth and indirectly inhibits the harmful effect of phytopathogenic microorganisms (Kloepper et al., 1980, Nam et al., 2018). The previous two decades, the use of plant rhizosphere associated bacteria having antagonistic potential has become one of the most important for managing plant diseases and maintaining ecological balance. Recently, more attention has given to FPs with reference to biocontrol and bio-fertilizing capabilities owing to their extensive colonization in the rhizosphere (Harman et al., 2004). FPs can be introduced by seed inoculation, soil application and foliar spray, where the effectiveness of various secondary metabolites can inhibit pathogenic microorganisms (Nandakumar et al., 2001). Hence, in the current scenario, to exploit genetic diversity and functional characters of FPs from rice rhizosphere is need of an hour to develop potential plant growth promoting and biocontrol agents against sheath blight of rice.
The biocontrol potential of FPs by the production of an array of antimicrobial compounds such as phenazine-1-carboxamide (Shanmugaiah et al., 2010), pyrrolnitrin (Howell and Stipanovic, 1979), pyoluteorin (Howell and Stipanovic, 1980), hydrogen cyanide (Voisard et al., 1989) and 2, 4 -diacetylphloroglucinol (Jousset et al., 2006) against soil borne pathogenic fungi were reported. Besides, Indole acetic acid (Kandel et al., 2017), 1-aminocyclo-propane-1-carboxylate deaminase and phosphate solubilizing capability has been observed in FPs as part of Plant growth promoting (PGP) traits (Sarma and Saikia, 2014). Phenazines derivatives are heterocyclic compounds that differ based on the substitution of assorted functional groups on the core phenazine ring structure (Mavrodi et al., 2001, Mavrodi et al., 2006, Biessy and Filion, 2018). The heterocyclic molecule phenazine derivatives are secreted by diverse bacterial genera such as Brevibacterium, Burkholderia, Streptomyces and Pseudomonas (Budzikiewicz, 1993, Shanmugaiah et al., 2010, Harikrishnan et al., 2016). The phenazine is predominantly synthesized by P. aeruginosa and all phenazine derivatives showed wide spectrum activity against numerous plant pathogenic bacteria and fungi (Smirnov and Kiprianova, 1990).
Phenazine producing bacteria are prevalent in the rhizosphere of wheat and rice (Mahmoudi et al., 2019, Harikrishnan et al., 2016, Shanmugaiah et al., 2010). FPs having phenazine biosynthetic genes were identified at higher number in wheat rhizosphere grown in dry land as compared to irrigated land (Mavrodi et al., 2012). The current study focus on bacterially mediate improvements in rice seedling for their growth and disease management.
Rhizobacterium mediated biological control have diverse mechanisms against plant pathogens of fungi and bacteria include antibiotics, iron chelating siderophore, salicylic acid and cell wall degrading enzymes (Santoyo et al., 2016, Carmona-Hernandez et al., 2019). Biocontrol and plant growth promoting rhizobacterium FPs are increasing plant growth through a biological nitrogen fixation (Bhattacharjee et al., 2008), phosphorus uptake (Yazdani et al., 2009), hydrogen cyanide (Voisard et al., 1989) and siderophores production (Rosenblueth and Martínez-Romero, 2006).
Our main focus of this research is to detect phenazine producing FPs from rice rhizosphere and to identify the most significant isolate inhibiting the sheath blight pathogen R. solani. Rice is a significant cultivated crop all over the world, especially tropical Asian countries like India and China. In India, rice cultivation is mainly concentrated in the southern parts of India, where Tamil Nadu is one of the leading states for rice production. Tamil Nadu is one of the rich microbial biodiversity with in India however, the biodiversity from the Vaigai river agriculture belt of Tamil Nadu is unexplored. Genetic diversity and functional study of fluorescent pseudomonads in the rice rhizosphere has not been explored in southern province of Tamil Nadu. Hence, we attempt to study sheath blight of rice control by new isolate of VSMKU1 which was isolated from the rice rhizosphere. This study mainly paying attention of existing Pseudomonas spp with the antibiotic encoding genes for the production of antimicrobial secondary metabolites associated with the control of sheath blight of rice, specifically phenazine like derivatives.
The objective of this study was carried out to (i) To study the genetic diversity and functional characters of antagonistic FPs associated with rice rhizosphere, (ii) Detection of phenazine-1-carboxamide encoding gene among antagonistic FPs (iii) To evaluate the FPs producing phenazine like compound against R. solani for the control of sheath blight of rice by detached leaf assay and (iv) The partially purified phenazine like compound was characterized by TLC, UV-VIS, and FT-IR spectrum.
2. Materials and methods
2.1. Sample collection and isolation of FPs
A total of 10 rice field rhizosphere samples were collected from various locations in the province of Dindigul, Tamil Nadu, India. The rhizosphere samples were stored at 4 °C until further process. Ten gram of rice rhizosphere sample were dissolved in 90 ml of sterile distilled water and vigorously shaken at 180 rpm at room temperature for 30 min. After that, the soil suspension was serially diluted up to 10 9. From all the dilution 100 µl of aliquot was spread on King’s B agar (King et al., 1954) in triplicates and plates were incubated at 28 °C for 24 h. Fluorescent colonies were visualized under UV trans-illuminator at 365 nm. All the isolates of FPs were kept in 30% (v/v) glycerol stocks at −80 °C for further study.
2.2. In vitro screening of FPs against fungal phytopathogens
FPs were tested towards fungal pathogens such as Rhizoctonia solani, Sclerotium rolfsii, Macrophomina phaseolina, Alternaria alternata and Fusarium oxysporum have been confirmed by a dual culture assay on potato dextrose agar (PDA), with three replicates of each FP (Harikrishnan et al., 2014).
2.3. 16S rDNA gene sequence analysis
Based on the best antagonistic performance, the strain VSMKU1 were selected for further studies. 16S rDNA gene was amplified using universal primers. 27F (5′AGAGTTTGATCCTGG -TCAGAACGCT) and 1492R (5′ TACGGCTACCTTGTACGACTTCACCCC) (Saikia et al., 2011).
2.4. Hydrolytic enzyme production by antagonistic fluorescent pseudomonads
The antagonistic FPs were tested for production of lytic enzymes like chitinase, cellulase, pectinase, protease and amylase. Chitinase production was evaluated in 0.1 % colloidal chitin agar medium (g/l) (Shanmugaiah et al., 2008), cellulase activity was determined in nutrient agar medium (NA) (g/l) with 0.1% (w/v) carboxymethylcellulose (CMC) and pectinase in nutrient agar medium (Aneja, 2001). Proteolytic activity was assessed using skim milk agar medium (g/l) and amylase activity was screened on nutrient agar (NA) added with 0.1% skim milk and starch (Benson, 1990).
2.5. Production of Indole acetic acid (IAA) and secondary metabolites by FPs
Indole acetic acid (IAA) production by FPs was assessed by the revised method of Gordon and Weber (1951). One ml of cell-free culture filtrate and 2 ml of Salkowski reagent (1 ml of 0.5 M FeCl3 in 50 ml of 35% HClO4) was added and incubated at 25 °C for 30 min in dark condition. The development of pink color indicates the production of IAA. Hydrogen cyanide production was assessed from FPs on nutrient sucrose agar medium (Lorck, 1948). Siderophore production by antagonistic FPs were tested (Schwyn and Neilands, 1987) and phosphate solubilization ability of the antagonistic FPs was assessed on Pikovaskaya’s agar medium (Pikovskaya, 1948).
2.6. Genetic diversity of fluorescent pseudomonads
2.6.1. Extraction of total genomic DNA
Total genomic DNA from fluorescent pseudomonads was isolated by the method of Keel et al. (1996).
2.6.2. BOX-PCR based genotypic analysis
BOX-PCR fingerprinting was performed for each antagonistic strain using BOXAIR primer (5′-CTACGGCAAGGCGACGCTGACG-3′) with slight modification (Jin et al., 2011).
2.6.3. Detection of Phenazine -1- carboxamide
Phenazine-1-carboxamide encoding gene detected from antagonistic FPs. The isolation of genomic DNA from FPs was performed by heat lysis method. After that DNA were amplified using specific primer of PhzHup and PhzHlow (5′-CGCACGGATCCTTTCAGAATGTTC-3′ and 5′-GCCACGCCAAGCTTCACGCTCA-3′). PCR reaction was conducted in a total volume of 25 μl, containing 50 ng DNA template,10X buffer (with 2.5 mM MgCl2), 25 mM dNTPs, 1 µl (10 pM) of primer sets, 1U Taq polymerase (GeNet Bio, Bangalore). The PCR reaction was carried out in an Agilent thermocycler through certain terms: initial denaturation at 95 °C for 3 min followed by 30 cycles of denaturation at 94 °C for 30 s, annealing at 54 °C for 30 s, extension at 72 °C for 1 min and final extension at 72 °C for 7 min (Mavrodi et al., 2001). The PCR product was separated by electrophoresis on 1% agarose gel in 1X TAE buffer at 70 V for 60 min. The gels were visualized using gel documentation system (Bio-Rad, Japan).
2.7. Thin layer chromatography (TLC)
The ethyl acetate extracts were air dried by rotary evaporator at 50 °C. The crude extracts of FPs were analyzed by TLC. On silica gel plates (Merck, Germany), 1 mg of commercial phenazine and crude extracts from the FPs were spotted with methanol solution (Sigma Aldrich, Mumbai). The TLC plates were developed with a solvent system of hexane: ethyl acetate at 4:2 ratio (Perneel et al., 2007). The spots were detected under UV at 254 nm and the Rf values of the crude metabolites were determined by compared with that of commercial phenazine.
2.7.1. Antifungal activity of phenazine like compound against R. solani
Antifungal activity was carried out by partially purified phenazine like compound, which is correlate with the authentic phenazine on TLC. The phenazine like compound was dissolved in ethyl acetate and the extract was introduced into each well with various concentrations such as 25, 50, 75 and 100 µg/ml on PDA plates. Three days old mycelial discs of R. solani (9 mm in diameter) was placed on the center of the PDA plates. Plates were hatched at 28 °C for three days.
2.8. Characterization of phenazine like compound
Phenazine like compound were dissolved at the concentration of 100 µg/ml with ethyl acetate and the sample were recorded between the wavelength of 200 and 400 nm after calibration with EA as blank using a UV–Vis spectrophotometer (Shimadzu, Kyoto, Japan). IR spectrum was recorded at 400–4000 cm−1 in dry chloroform solution using a Fourier transform infrared spectroscopy (FT- IR) (Shimadzu). Both ultraviolet (UV) and infrared (IR) spectrum was taken for pure phenazine as positive control. The crude metabolites were analyzed using an HPLC system which consisted of a photodiode array detector, analytical column of 250 × 4.60 mm (5 µm). Acetonitrile and 10% trifluoracetic acid (TFA) were used as mobile phase. The experiments were tested at 1.0 ml/min. The peaks were compared to pure phenazine.
2.9. Detached leaf assay
The extraction of secondary metabolites from the antagonistic FPs was done by adding 1 ml of pre-inoculation of FPs in KB broth and grown at 37 °C for 16 h. Liquid cultures of FPs were used at 108 Cfu/ml. The cell-free culture filtrates of FPs from 24 h old cultures grown in KB broth were diluted with sterile condensed water to achieve a final concentration of 10% (v/v). The crude phenazine like compound were prepared at the concentration of 5 µg/ml. The rice leaf segments were screened against sheath blight of rice (Guleria et al., 2007).
2.10. Data analysis
The data from the zone of inhibition for in vitro antagonism, efficacy of crude metabolites and their evaluation against pathogens were examined by Analysis of Variance (ANOVA) and the treatment means were compared through least significant difference (LSD) value of Duncan’s Multiple Range Test (DMRT) test at P < 0.05 using CoStat statistical software (Cohort Berkeley, California) (Cardinali and Nason, 2013). The significance analysis of variance was p < 0.001. For BOX PCR analyses, the dendrogram were constructed using the neighbor-joining method. Distances were corrected for multiple base changes (Jukes and Cantor, 1969).
3. Results
3.1. Antagonistic properties of FPs against phytopathogens
A total of 50 FPs was isolated from the rice rhizosphere of healthy rice plants in ten different places in the district of Dindigul, southern Tamil Nadu, India (Fig. 1). Of which, 36 isolates of FPs were showed potential antifungal activity against prevalent rice pathogen R. solani and other fungal pathogens such as Macrophomina phaseolina, Fusarium oxysporum, Alternaria alternata and Sclerotium rolfsii with different level of zone of inhibition ranging from 7.0 to 44.2 mm, 12.0 to 29.7 mm, 8.0 to 25.0 mm, 13.0 to 28.0 mm, and 13.0 to 27.0 mm respectively (Table 1). Among 36 FPs, VSMKU1 was selected as potent biocontrol agent based on the maximum zone of inhibition (Fig. 2) against R. solani when compared to rest of the isolates and control.
Fig. 1.
Rice rhizosphere sampling collection from dindigul district. (A) India map showing the place of Dindigul district, (B) displays the ten different sites of different paddy cultivating area from Dindigul district of Tamil Nadu.
Table 1.
In vitro antagonism of FPs against Phytopathogens.
| Antagonistic strain | Zone of inhibition (mm) (Mean ± SD) |
||||
|---|---|---|---|---|---|
| Rhizoctonia solani | Macrophomina phaseolina | Fusarium oxysporum | Alternaria alternata | Sclerotium rolfsii | |
| VSMKU1 | 44.2 ± 0.075 | 29.7 ± 0.082 | 25.0 ± 0.063 | 20.0 ± 0.063 | 27.0 ± 0.063 |
| VSMKU2 | 35.0 ± 0.084 | 26.3 ± 0.082 | 20.0 ± 0.063 | 21.80 ± 0.063 | 25.0 ± 0.063 |
| VSMKU3 | 18.8 ± 0.075 | 13.8 ± 0.075 | 15.0 ± 0.063 | 13.8 ± 0.075 | 15.0 ± 0.063 |
| VSMKU4 | 21.7 ± 0.103 | 14.3 ± 0.082 | 18.0 ± 0.063 | 15.0 ± 0.063 | 13.0 ± 0.063 |
| VSMKU5 | 17.0 ± 0.063 | 13.0 ± 0.063 | 16.0 ± 0.063 | 14.5 ± 0.084 | 18.0 ± 0.063 |
| VSMKU6 | 36.0 ± 0.087 | 12.0 ± 0.063 | 12.8 ± 0.075 | 14.5 ± 0.084 | 16.0 ± 0.063 |
| VSMKU7 | 16.0 ± 0.063 | 20.5 ± 0.084 | 16.8 ± 0.075 | 16.0 ± 0.063 | 17.0 ± 0.063 |
| VSMKU8 | 28.5 ± 0.084 | 20.0 ± 0.063 | 18.0 ± 0.063 | 14.0 ± 0.063 | 14.7 ± 0.082 |
| VSMKU17 | 8.50 ± 0.084 | 15.0 ± 0.063 | 15.0 ± 0.063 | 13.7 ± 0.082 | 18.0 ± 0.063 |
| VSMKU18 | 18.0 ± 0.063 | 13.0 ± 0.063 | 16.0 ± 0.063 | 11.7 ± 0.103 | 15.0 ± 0.063 |
| VSMKU19 | 19.0 ± 0.063 | 18.5 ± 0.084 | 18.0 ± 0.063 | 15.0 ± 0.063 | 18.0 ± 0.063 |
| VSMKU20 | 16.0 ± 0.063 | 17.0 ± 0.063 | 14.5 ± 0.084 | 14.0 ± 0.063 | 15.0 ± 0.063 |
| VSMKU23 | 17.0 ± 0.063 | 16.0 ± 0.063 | 13.8 ± 0.063 | 13.2 ± 0.075 | 16.0 ± 0.063 |
| VSMKU27 | 18.0 ± 0.063 | 15.0 ± 0.063 | 8.0 ± 0.063 | 13.0 ± 0.063 | 16.0 ± 0.063 |
| VSMKU28 | 18.5 ± 0.084 | 16.5 ± 0.084 | 16.0 ± 0.063 | 16.5 ± 0.084 | 19.0 ± 0.063 |
| VSMKU30 | 17.5 ± 0.084 | 14.5 ± 0.084 | 17.0 ± 0.063 | 14.0 ± 0.063 | 14.0 ± 0.063 |
| VSMKU31 | 17.0 ± 0.063 | 14.0 ± 0.063 | 15.0 ± 0.063 | 15.0 ± 0.063 | 16.0 ± 0.063 |
| VSMKU34 | 14.5 ± 0.084 | 18.0 ± 0.063 | 15.0 ± 0.063 | 15.0 ± 0.063 | 15.0 ± 0.063 |
| One way ANOVA | |||||
| LSD (P = 0.05) | 0.19 | 0.16 | 0.18 | 0.15 | 0.13 |
| Two way ANOVA | |||||
| LSD (P = 0.05) | |||||
| Isolates, | 0.072 (***) | ||||
| Pathogens, | 0.038 (***) | ||||
| Interaction effect (isolates × pathogens) | *** | ||||
Fig. 2.
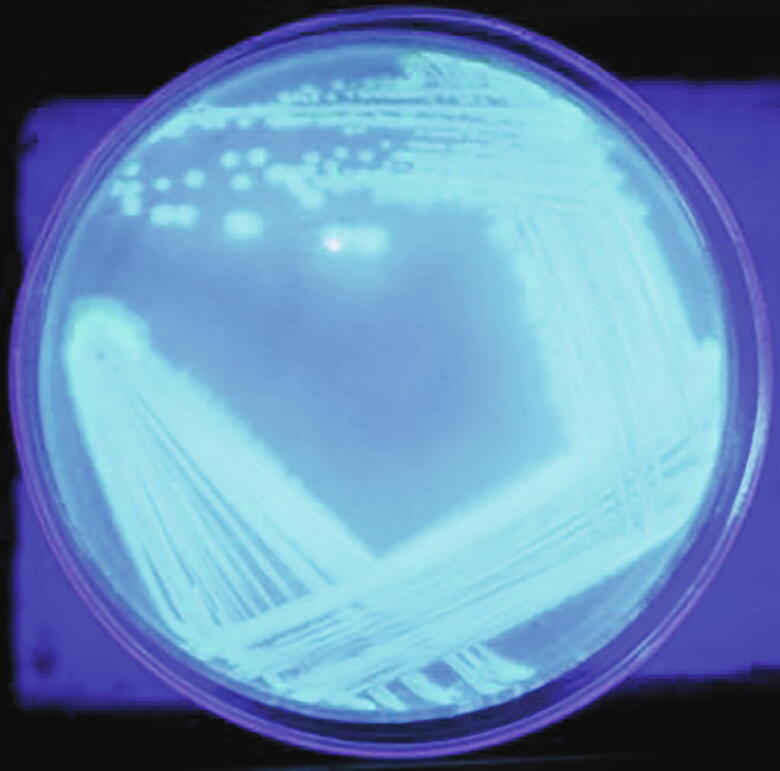
Pure culture of P. aeruginosa VSMKU1 exhibiting on King’s B agar medium when visualized under UV trans-illuminator at 365 nm.
3.2. Identification of the selected strain VSMKU1
The strain VSMKU1 16S rDNA partial sequence was matched to 11 reference species of Pseudomonas denoted in the Genbank. The highest score sequences were retrieved from Genbank, aligned, distance matrices were calculated and the phylogenetic tree was constructed (Data not shown). The strain VSMKU1 showed 99% similarity with Pseudomonas aeruginosa. The 16S rDNA sequence analysis was submitted to the Genbank database with the accession number KM583892.
3.3. Functional characterization of fluorescent pseudomonads
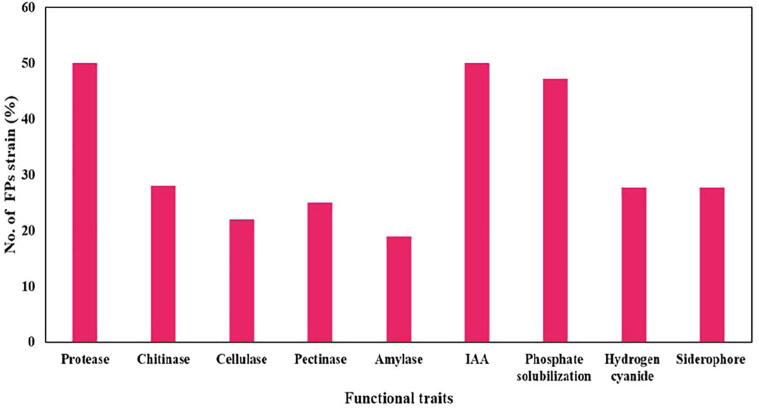
The hydrolytic enzymes, plant growth hormones and antimicrobial metabolites production by FPs were assessed. Out of 36 antagonistic FPs, 50% of the isolates produced protease and IAA. However, 28%, 22%, 25% and 19% produced chitinase, cellulase, pectinase and amylase. Whereas 47.2% and 27.7% showed positive for phosphate solubilization, hydrogen cyanide and siderophore (Fig. 3).
Fig. 3.
Functional characterization of 36 antagonistic FPs. Of which 18 strains produced protease, 10 strains produced chitinase, 8 strains produced cellulase, 9 strains produced pectinase, 7 strains produced amylase, 18 strains produced IAA, 17 strains showed positive for phosphate solubilization and 10 strains produced hydrogen cyanide and siderophore.
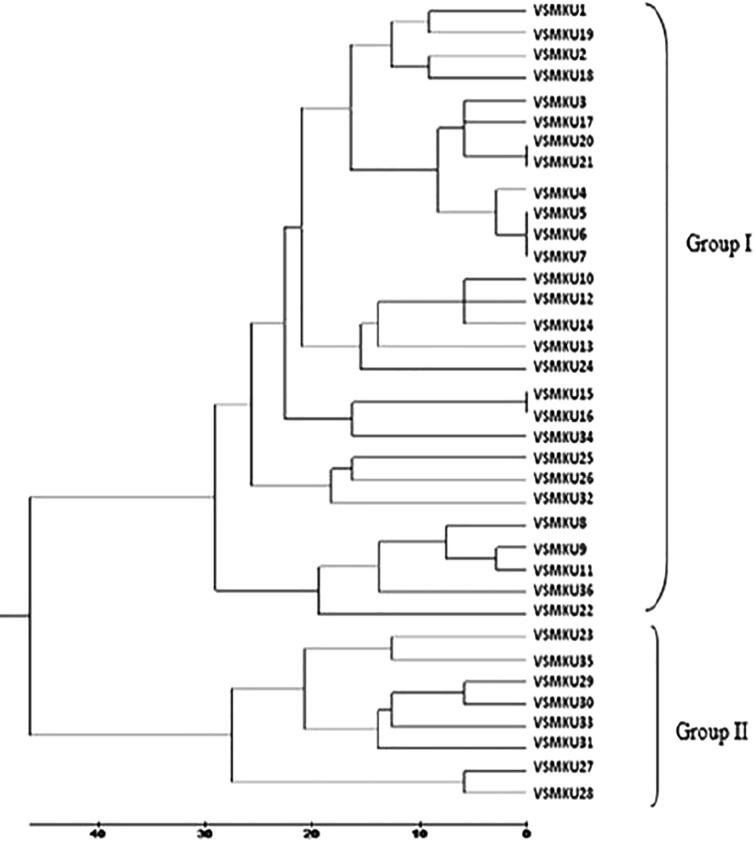
3.4. BOX-PCR based genetic diversity of fluorescent pseudomonads
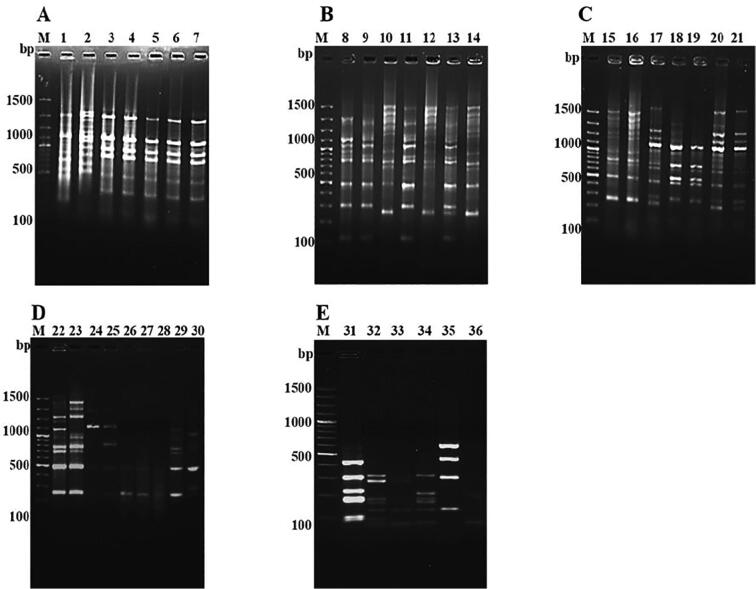
To study the diversity of 36 antagonistic FPs by BOX-PCR. BOX-PCR data resulted that 26 distinct polymorphic banding patterns ranging between 100 bp and 1500 bp (Fig. 4A-E). The polymorphic banding pattern showed 80% similarity coefficient with two distinct genomic clusters. The distances were correlated with multiple base changes by the method of Jukes and Cantor. While generating dendrogram, out of 36 FPs the tree forms two major clades Group I and Group II (Fig. 5). The group I was subdivided into 6 clusters consisting 28 isolates of FPs. Group II subdivided into 3 clusters which included 8 isolates of FPs, where VSMKU31 strain forming a separate cluster. Due to their high degree of genetic heterogeneity among the different species of antagonistic FPs, all strains displayed a large variance in fingerprinting patterns and thus resulted in their distribution in different clusters.
Fig. 4.
BOX PCR results of 36 antagonistic FPs. A-E showing distinct polymorphic banding patterns ranging between 100 bp and 1500 bp with the corresponding lanes are as follows: Lane M:100 bp DNA ladder, Lane 1: VSMKU1, Lane 2: VSMKU2, Lane 3: VSMKU3, Lane 4: VSMKU4, Lane 5: VSMKU5, Lane 6: VSMKU6, Lane 7: VSMKU7, Lane 8: VSMKU8, Lane 9: VSMKU9, Lane 10: VSMKU10, Lane 11: VSMKU11, Lane 12: VSMKU12, Lane 13: VSMKU13, Lane 14: VSMKU14, Lane 15: VSMKU15, Lane 16: VSMKU16, Lane 17: VSMKU17, Lane 18: VSMKU18, Lane 19: VSMKU19, Lane 20: VSMKU20, Lane 21: VSMKU21. D-E is Lane 22: VSMKU22, Lane 23: VSMKU23, Lane 24: VSMKU24, Lane 25: VSMKU25, Lane 26: VSMKU26, Lane 27: VSMKU27, Lane 28: VSMU28, Lane 29: VSMKU29, Lane 30: VSMKU30, Lane 31: VSMKU31, Lane 32: VSMKU32, Lane 33: VSMKU33, Lane 34: VSMKU34, Lane 35: VSMKU35, Lane 36: VSMKU36.
Fig. 5.
Phylogenetic analysis of 36 antagonistic FPs by BOX-PCR. The phylogenetic tree forms two major clades Group I and Group II. The group I was subdivided into 6 clusters consisting 28 isolates. Group II subdivided into 3 clusters which included 8 isolates where VSMKU31 strain forming a separate cluster.
3.5. Detection of phenazine-1-carboxamide encoding gene and phenazine like compound by PCR and TLC
A total 10 FPs (28%) were showed the presence of phenazine-1-carboxamide encoding gene with 500 bp and it was matches with PCN encoding gene P. aeruginosa MML2212 (Fig. 6). Similarly, out of 36 antagonistic FPs, 18 FPs were showed the production of phenazine like compound compared with that of authentic phenazine with Rf value 0.57 (Table 2; Fig. 7).
Fig. 6.
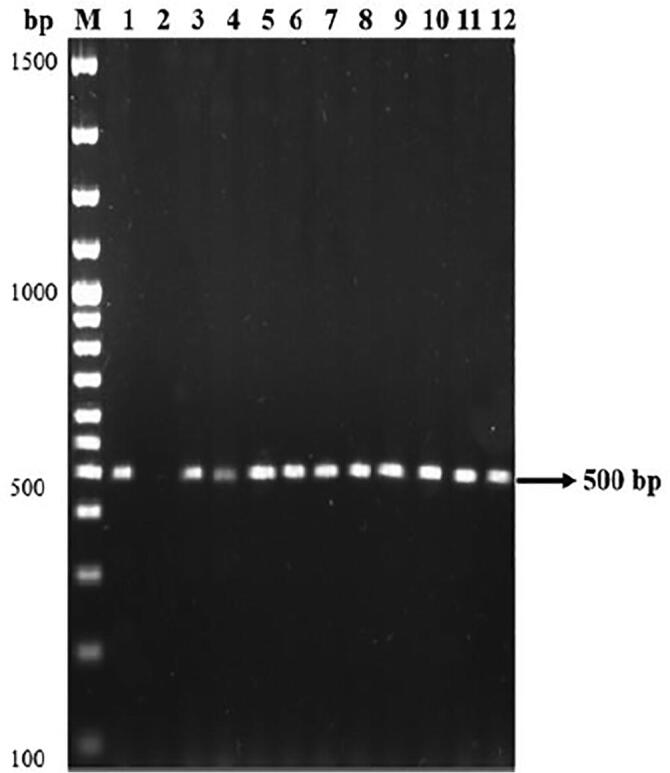
Detection of phenazine-1-carboxamide gene among the antagonistic FPs. The positive strains for PCN gene corresponds to the following lanes are as follows; Lane M: 100 bp DNA ladder, Lane 1: positive control (MML2212), Lane 2: Negative control, Lane 3: VSMKU1, Lane 4: VSMKU2, Lane 5: VSMKU3, Lane 6: VSMKU4, Lane 7: VSMKU6, Lane 8: VSMKU18, Lane 9: VSMKU20, Lane 10: VSMKU23, Lane 11: VSMKU28, Lane 12: VSMKU34.
Table 2.
Detection of Phenazine-1-carboxamide by TLC and PCR.
| FP isolate | TLC | PCR | FP isolate | TLC | PCR |
|---|---|---|---|---|---|
| VSMKU1a | + | + | VSMKU19b | + | – |
| VSMKU2a | + | + | VSMKU20a | + | + |
| VSMKU3a | + | + | VSMKU21c | – | – |
| VSMKU4a | + | + | VSMKU22c | – | – |
| VSMKU5b | + | – | VSMKU23a | + | + |
| VSMKU6a | + | + | VSMKU24c | – | – |
| VSMKU7b | + | – | VSMKU25c | – | – |
| VSMKU8b | + | – | VSMKU26c | – | – |
| VSMKU9c | – | – | VSMKU27b | + | – |
| VSMKU10c | – | – | VSMKU28a | + | + |
| VSMKU11c | – | – | VSMKU29c | – | – |
| VSMKU12c | – | – | VSMKU30b | + | – |
| VSMKU13c | – | – | VSMKU31b | + | – |
| VSMKU14c | – | – | VSMKU32c | – | – |
| VSMKU15c | – | – | VSMKU33c | – | – |
| VSMKU16c | – | – | VSMKU34a | + | + |
| VSMKU17b | + | – | VSMKU35c | – | – |
| VSMKU18a | + | + | VSMKU36 | – | – |
Indicates the presence phenazine derivative in TLC and PCN gene in the isolate.
Plus indicated the presence of phenazine derivative in TLC and absence of PCN gene in the isolate.
Indicates the absence phenazine derivative in TLC and PCN gene in the isolate.
Fig. 7.
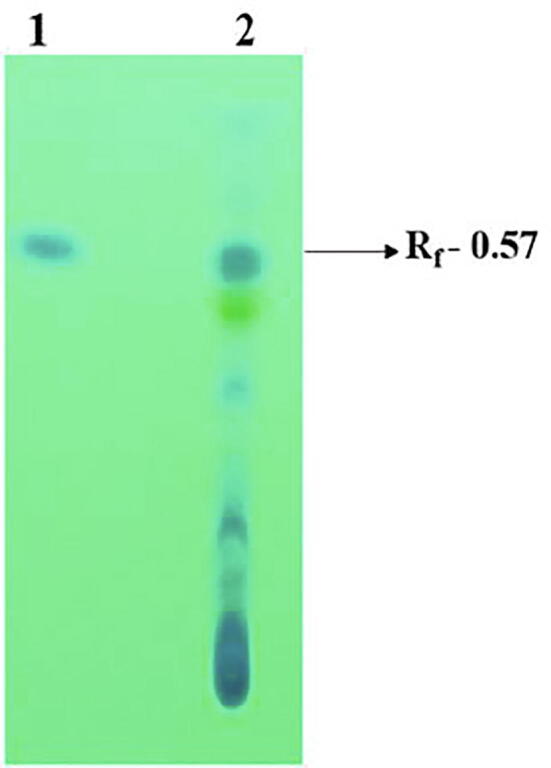
Thin-layer chromatography of Phenazine like compound from P. aeruginosa VSMKU1. Lane 1: Authentic phenazine exhibiting single pure band, Lane 2: Phenazine like compound of VSMKU1 showed many distinct bands and a single band of the crude metabolite from P. aeruginosa VSMKU1 coincides with the single band of authentic phenazine with the Rf value of 0. 57.
3.6. Antifungal activity of phenazine like compound against R. solani
The antifungal activity of phenazine like compound from antagonistic FPs wasshowed various level of zones of inhibition (ZOI) against R. solani using dissimilar concentrations of phenazine like compound (25, 50, 75 and 100 µg/ml) with diverse level of zone of inhibition ranged from 12.0 to 20.0 mm (25 µg/ml), 12.5 to 21.8 mm (50 µg/ml), 15.0 to 24.0 mm (75 µg/ml) and 17.0 to 25.6 mm (100 µg/ml) compared to authentic phenazine and carbendazim. However, phenazine like compound from VSMKU1 showed significant antifungal activity against R. solani in compared to rest of the other strains. Among all the concentration, the application of 100 µg/ml of phenazine derivatives from the isolate VSMKU1 was found to have a higher zone of inhibition against R. solani. The statistical analysis carried out using two-way ANOVA, indicate that irrespective of pathogens and concentration of cell-free culture, amongst all the strains VSMKU1 was found to have a significantly higher zone of inhibition for all the pathogens when compared to rest of the isolates (Table 3).
Table 3.
Zone of inhibition of different concentration of crude extract of metabolite from antagonistic FPs, Carbendazim and authentic phenazine against Rhizoctonia solani.
| Groups | Zone of inhibition in different concentration (mm) |
|||
|---|---|---|---|---|
| 25 µl | 50 µl | 75 µl | 100 µl | |
| Authentic Phenazine | 18.8 ± 0.07 | 19.5 ± 0.10 | 23.8 ± 0.07 | 24.8 ± 0.07 |
| Carbendazim | 18.3 ± 0.08 | 19.6 ± 0.10 | 23.1 ± 0.09 | 23.8 ± 0.07 |
| VSMKU1 | 20.0 ± 0.06 | 21.8 ± 0.07 | 24.0 ± 0.06 | 25.6 ± 0.05 |
| VSMKU3 | 16.0 ± 0.06 | 17.0 ± 0.06 | 18.3 ± 0.08 | 20.0 ± 0.08 |
| VSMKU4 | 15.5 ± 0.08 | 16.6 ± 0.05 | 20.8 ± 0.07 | 21.3 ± 0.08 |
| VSMKU5 | 14.1 ± 0.07 | 17.0 ± 0.06 | 17.6 ± 0.05 | 19.3 ± 0.08 |
| VSMKU6 | 13.0 ± 0.06 | 14.6 ± 0.05 | 16.3 ± 0.08 | 20.1 ± 0.07 |
| VSMKU7 | 16.8 ± 0.07 | 18.3 ± 0.08 | 20.1 ± 0.07 | 23.1 ± 0.07 |
| VSMKU8 | 16.3 ± 0.07 | 18.5 ± 0.08 | 21.5 ± 0.07 | 23.1 ± 0.07 |
| VSMKU17 | 15.3 ± 0.08 | 17.3 ± 0.08 | 19.6 ± 0.10 | 21.5 ± 0.08 |
| VSMKU18 | 16.5 ± 0.08 | 18.3 ± 0.08 | 20.5 ± 0.08 | 23.0 ± 0.06 |
| VSMKU19 | 16.1 ± 0.07 | 17.8 ± 0.07 | 19.5 ± 0.08 | 21.8 ± 0.07 |
| VSMKU20 | 13.3 ± 0.08 | 15.5 ± 0.08 | 18.8 ± 0.06 | 20.0 ± 0.06 |
| VSMKU23 | 14.5 ± 0.08 | 15.5 ± 0.08 | 17.0 ± 0.06 | 20.1 ± 0.07 |
| VSMKU27 | 16.3 ± 0.10 | 18.0 ± 0.06 | 19.0 ± 0.06 | 22.0 ± 0.06 |
| VSMKU28 | 17.0 ± 0.06 | 19.0 ± 0.06 | 20.6 ± 0.08 | 22.1 ± 0.07 |
| VSMKU30 | 16.0 ± 0.06 | 18.0 ± 0.06 | 19.0 ± 0.06 | 21.5 ± 0.08 |
| VSMKU31 | 14.0 ± 0.06 | 16.0 ± 0.06 | 18.0 ± 0.06 | 20.0 ± 0.06 |
| VSMKU34 | 12.0 ± 0.06 | 12.5 ± 0.08 | 15.0 ± 0.06 | 17.0 ± 0.06 |
| Two way ANOVA | ||||
| LSD (P = 0.05) | ||||
| Concentration | 0.032 (***) | |||
| Isolates/groups, | 0.073 (***) | |||
| Interaction effect (concentration of filtrate × isolates) | ** | |||
3.7. Characterization of the crude metabolite
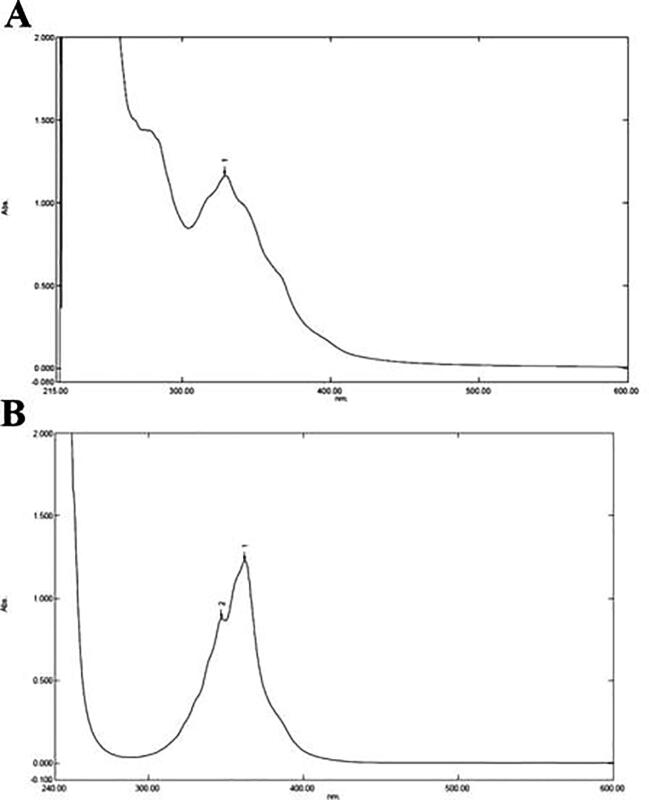
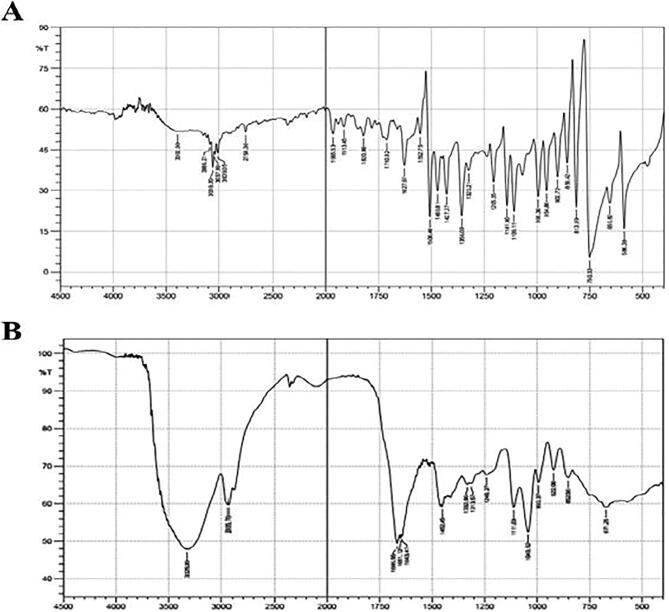
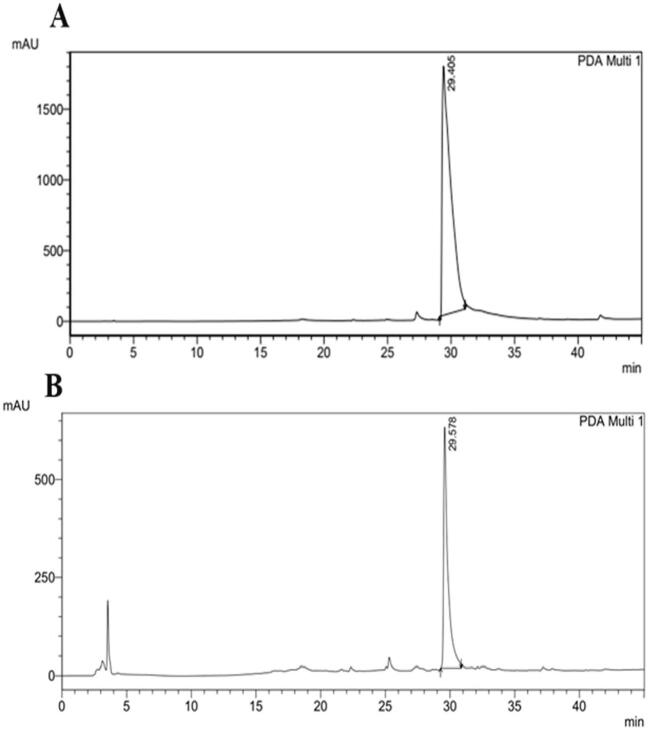
The UV spectrum of the phenazine like compound was showed kmax values at 300–400 nm, similar to a spectrum profile of authentic phenazine with kmax value range of 300–400 nm (Fig. 8A and 8B). The FT-IR spectrum ofphenazine like compound showed absorption at 3325.39 cm−1 and 2935.76 cm−1 with functional groups, particularly C—H aromatic stretch, NH amine stretch and CH alkene stretch. At 1666.55 cm−1, 1651.12 cm−1, 1643.41 cm−1 and 1452.45 cm−1 displayed the functional groups of C C alkene stretch, a C O carboxylic acid group, an amide stretch with a strong bond and an α, β unsaturated ketone with a strong bond. The spectra absorption values at 1332.86 cm−1, 1313.57 cm−1, 1111.03 cm−1 and 1043.52 cm−1 specified the existence of C—N amine stretch groups. The absorption value at 1240.27 cm−1 indicated a C—O carbonyl acid with strong intensity (Fig. 9A and B). The phenazine like compound was analyzed by HPLC. The crude phenazine like compound HPLC chromatogram (Fig. 10A) displays a similar peak value to that of authentic phenazine (Fig. 10B), with a retention time of 29.5 min at 254 nm.
Fig. 8.
UV spectrum analysis of Phenazine like compound from P. aeruginosa VSMKU1. A and B are UV spectrum profile in which A reveals the pure phenazine compound showing a high absorbance line was observed at k max at 362 nm while B Phenazine like compound produced by P. aeruginosa VSMKU1 exhibited a high absorbance line at 362 nm and lower absorbance line showing a peak at 347 nm. The peak observed at 362 nm of the crude metabolite from P. aeruginosa VSMKU1 coincides with that of pure phenazine.
Fig. 9.
FT-IR spectrum analysis of Phenazine like compound from P. aeruginosa VSMKU1.A - functional groups present in pure phenazine compound while B showing the FTIR spectrum of Phenazine like compound exhibiting absorption at 3325.39 cm−1 and 2935.76 cm−1 with functional groups, particularly C-H aromatic stretch, NH amine stretch, and CH alkene stretch. At 1666.55cm−1, 1651.12cm−1, 1643.41cm−1, and 1452.45cm−1 displayed the functional groups a C=C alkene stretch, a C=O carboxylic acid group, an amide stretch with a strong bond, and an α, β unsaturated ketone with a strong bond. The spectra absorption values at 1332.86cm−1, 1313.57cm−1, 1111.03cm−1 and 1043.52cm−1 indicated the presence of C-N amine stretch groups. The absorption value at 1240.27 cm−1 indicated a C-O carbonyl acid with strong intensity.
Fig. 10.
HPLC chromatogram of the Phenazine like compound from P. aeruginosa VSMKU1. A- HPLC chromatogram of authentic phenazine, B- Phenazine like compound from P. aeruginosa VSMKU1 respectively, and the first peak was observed at a high absorbance line at 254 nm at a retention time of 29.5 minutes that coincides with the peak of pure phenazine at 254 nm at a retention time of 29.4 minutes.
3.8. Effect of antagonistic culture, cell free culture filtrate and crude metabolite against sheath blight of rice by detached leaf assay
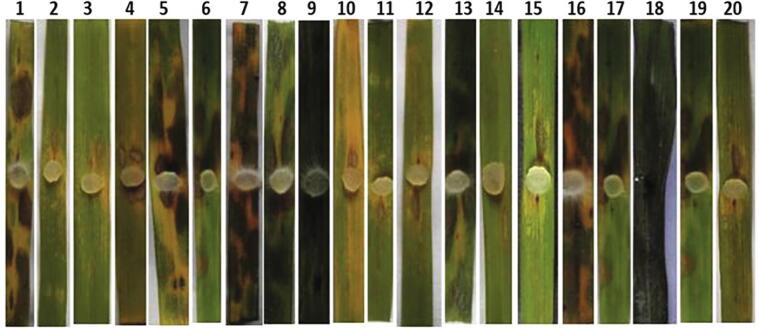
Treatment of detached rice leaves with culture, cell free culture filtrate and crude metabolites at 5 µg/ml significantly reduced the sheath blight lesions compared to carbendazim and control after five days of incubation (Table 4). In addition, the leaves treated with the culture of VSMKU1, crude metabolites showed significant reduction compared to cell-free culture filtrates, fungicide carbendazim and control. The crude metabolite of the 18 isolates showed significant reduction in lesion height (9.93% to 30.03%) followed by culture (12.27% to 33.00%) and least lesion height reduction was recorded in the cell-free culture filtrate (14.20% to 86.02%). Moreover, among all the treatment with different isolates, the strain VSMKU1 exhibited significant lesion height reduction in the culture (12.27%), cell free culture filtrate (14.20%) and crude metabolite (9.93%) compared to carbendazim (16.20%) and control (97.82%) (Table 4; Fig. 11). Overall, amongst all the FPs cell-free culture filtrate, irrespective of type of isolates the maximum disease reduction was observed while applying the crude metabolites (Table 4). However, VSMKU1 when applied as crude metabolite showed highest sheath blight control when compared to rest of isolates and found at par with commercial product carbendazim (Table 4).
Table 4.
Evaluation of sheath blight of rice by antagonistic FPs through detached leaf assay.
| Strain No | Distilled water | Culture | Culture Filtrate | Crude Metabolite | Carbendazim |
|---|---|---|---|---|---|
| VSMKU1 | 97.82 ± 0.04 | 19.27 ± 0.05 | 17.20 ± 0.08 | 16.93 ± 0.13 | 16.20 ± 0.06 |
| VSMKU2 | 97.82 ± 0.04 | 22.05 ± 0.16 | 24.20 ± 0.10 | 19.88 ± 0.11 | 16.20 ± 0.06 |
| VSMKU3 | 97.82 ± 0.04 | 32.27 ± 0.05 | 34.13 ± 0.18 | 30.95 ± 0.16 | 16.20 ± 0.06 |
| VSMKU4 | 97.82 ± 0.04 | 52.18 ± 0.19 | 54.08 ± 0.19 | 49.88 ± 0.11 | 16.20 ± 0.06 |
| VSMKU5 | 97.82 ± 0.04 | 83.00 ± 0.15 | 65.10 ± 0.20 | 59.95 ± 0.16 | 16.20 ± 0.06 |
| VSMKU6 | 97.82 ± 0.04 | 57.00 ± 0.18 | 58.95 ± 0.16 | 54.10 ± 0.10 | 16.20 ± 0.06 |
| VSMKU7 | 97.82 ± 0.04 | 62.12 ± 0.18 | 64.05 ± 0.16 | 60.03 ± 0.19 | 16.20 ± 0.06 |
| VSMKU8 | 97.82 ± 0.04 | 58.98 ± 0.65 | 62.15 ± 0.50 | 61.00 ± 0.23 | 16.20 ± 0.06 |
| VSMKU17 | 97.82 ± 0.04 | 63.08 ± 0.21 | 65.30 ± 0.46 | 59.97 ± 0.15 | 16.20 ± 0.06 |
| VSMKU18 | 97.82 ± 0.04 | 57.00 ± 0.18 | 58.50 ± 0.54 | 54.02 ± 0.20 | 16.20 ± 0.06 |
| VSMKU19 | 97.82 ± 0.04 | 57.00 ± 0.18 | 58.50 ± 0.54 | 54.02 ± 0.20 | 16.20 ± 0.06 |
| VSMKU20 | 97.82 ± 0.04 | 32.95 ± 0.16 | 34.98 ± 0.17 | 31.08 ± 0.14 | 16.20 ± 0.06 |
| VSMKU23 | 97.82 ± 0.04 | 32.95 ± 0.16 | 34.95 ± 0.16 | 31.05 ± 0.16 | 16.20 ± 0.06 |
| VSMKU27 | 97.82 ± 0.04 | 63.97 ± 0.15 | 67.05 ± 0.16 | 62.02 ± 0.21 | 16.20 ± 0.06 |
| VSMKU28 | 97.82 ± 0.04 | 32.95 ± 0.16 | 35.95 ± 0.16 | 30.05 ± 0.16 | 16.20 ± 0.06 |
| VSMKU30 | 97.82 ± 0.04 | 64.97 ± 0.15 | 67.22 ± 0.36 | 63.02 ± 0.21 | 16.20 ± 0.06 |
| VSMKU31 | 97.82 ± 0.04 | 65.97 ± 0.15 | 69.05 ± 0.16 | 64.02 ± 0.21 | 16.20 ± 0.06 |
| VSMKU34 | 97.82 ± 0.04 | 33.00 ± 0.18 | 86.02 ± 0.13 | 30.03 ± 0.15 | 16.20 ± 0.06 |
| Two way ANOVA | |||||
| LSD (P = 0.05) | |||||
| Culture filtrate, | 0.16 (***) | ||||
| isolates, | 0.31 (***) | ||||
| Interaction effect (culture filtrate × isolates) | *** |
Data represents average of 3 replicates in percentage; SD, Standard deviation; LSD, least significant difference carried out by DMRT through two way ANOVA.
Fig. 11.
Leaf detached assay by FPs. It shows the different percentage of sheath blight disease incidence by the treatment of Phenazine like compound produced from 18 antagonistic FPs as follows, 1: Distilled water, 2: Carbendazim, 3: VSMKU1, 4: VSMKU2, 5: VSMKU3, 6: VSMKU4, 7: VSMKU5, 8: VSMKU6, 9: VSMKU7, 10: VSMKU8, 11: VSMKU17, 12: VSMKU18, 13: VSMKU19, 14: VSMKU20, 15: VSMKU23, 16: VSMKU27, 17: VSMKU28, 18: VSMKU30, 19: VSMKU31, 20: VSMKU34..
4. Discussion
Fluorescent pseudomonads (FPs) is highly adaptable and cosmopolitan tailored bacterium in different habitats such as soil, plant rhizosphere, epiphytic and entophytic colonization in many plant systems. In recent days, FPs were considered as a prominent candidate of beneficial bacteria for controlling soil borne plant pathogens (Ellis et al., 2000, Duke et al., 2017) because of their highly competitive adaptation towards the exterior and interior tissues of leaf, roots and stems. (Capdevila et al., 2004). The present study focuses on functional and genetic characterization of rice rhizobacterium Pseudomonas spp. and validates its functional identity by using phenotypic and genotypic tools. We have recovered 52% of FPs isolates in this sample which have been found to have antagonistic activity against five fungal pathogens such as R. solani, M. phaseolina, F. oxysporum, A. alternata and S. rolfsii. Among the antagonistic FPs, the isolate VSMKU1 exhibited efficient capability to control fungal mycelium and could be used for the prevention of sheath blight disease during rice production. The broad spectrum antifungal activity of FPs is consistent with the results reported through various genetically and functionally diverse antagonistic FPs isolated from tea, banana and rice rhizosphere exhibiting different level of zone of inhibition towards fungal pathogen such as F. oxysporum f. sp.raphani (For), F. oxysporum. f.sp. ciceri (foc) and R. solani (Ayyadurai et al., 2007, Compant et al., 2010, Saikia et al., 2011, Schlemper et al., 2018, Hua et al., 2020). Our finding also reports a significant correlation between fungal phytopathogen growth suppression by the different level of production of secondary metabolites and hydrolytic enzymes by the antagonistic FPs, which implies that antibiosis is the dominant mode of action for disease control (Patel et al., 2019). Globally, Pseudomonas possesses many traits which make them efficient biocontrol and growth-promoting agents (Qessaoui et al., 2019) due to their production of wide spectrum bioactive metabolites (i.e., antibiotics, siderophore, volatiles, and growth-promoting substances) and more specifically found to very effective antibiotic compounds, such as phenazine-1-carboxamide (Shanmugaiah et al., 2010), phenazine-1-carboxylic acid (PCA) and 2, 4-diacetylphloroglucinol (DAPG) that result in antibiosis (Weller et al., 2007). In our study, 10 FPs isolates showed positive for phenazine-1- carboxamide (PCN) encoding gene. Thus the Pseudomonas spp., whose control mechanism was confirmed by the presence of biosynthetic genes encoding the antimicrobial antibiotics phenazine-1-carboxamide.
The phylogenetic tree constructed from the BOXAIR-PCR analysis has produced better option for determining the genetic variation among the fluorescent pseudomonads isolated from different niches (Picard and Bosco, 2003, Li et al., 2017). The antagonistic FPs recovered from this study were subdivided into the group I consisting of 6 clusters with 28 FPs and group II subdivide into 3 clusters with 8 FPs. All the strains showed wide variation of finger printing pattern due to the presence of a high degree of genetic variation and distribution of different clusters. The genetic variability between various FPs isolates may be due to mutation and other genetic modifications, such as recombination (Ochman et al., 2000). In addition, mutation rates in bacteria are commonly known to rise under stress due to SOS response and reduced capacity to manage metabolism generated DNA damaging free radicals (Friedberg et al., 2005). Furthermore, the high degree of variance among phosphate solubilizing bacteria isolated from banana rhizosphere employing BOX- PCR based genotypic analyses (Naik et al., 2008).
The phenazine like compound extracted from the strain VSMKU1 showed remarkable antifungal activity against R. solani compared to other strains and control. Currently well-known analytical and spectral standard methods are being employed for the characterization of the phenazine like compound with authentic phenazine through UV, IR and HPLC techniques. Our results on characterizing phenazine and its authenticity concurrence with the previously published results of other phenazine producing antagonistic bacteria such as P. aeruginosa MML2212 and Streptomyces aurantiogriseus VSMGT1014 were identified through TLC, UV, IR and HPLC and compared with that of commercial phenazine has been achieved (Shanmugaiah et al., 2010, Harikrishnan et al., 2016, Miguelez-Sierra et al., 2019.).
We also evaluated the reduction of lesion height percentage among the antagonistic FPs using the culture, cell-free culture filtrate and crude metabolite ranged from 12.27 to 83.00, 14.20 to 86.02 and 9.93 to 64.02% compared to carbendazim (16.20%) and control (97.82%). Among all the FPs examined culture, crude metabolites, cell-free culture filtrate of VSMKU1 exhibited remarkable control of sheath blight lesion height when compared to control or reduction was at par with crude phenazine used in the study. Similar results we also obtained, when rice seedlings treated with cell free culture filtrate and crude metabolite of S. aurantiogriseus VSMGT1014, which significantly controlled the sheath blight incidence compare to carbendazim (Harikrishnan et al., 2014). Moreover, result concurrent with a similar report (Prabavathy et al., 2006, Li et al., 2011, Mathivanan and Shanmugaiah, 2011) where, the culture filtrates of S. globisporous Jk-1, Streptomyces sp PM5 and P. aeruginosa MML2212 was used to control rice blast and sheath blight of rice caused by Magnaporthe oryzae and R. solani through the production antibiotic substances at specific concentrations showed low levels of sheath blight disease compared to commercial fungicides.
5. Conclusion
Owing to the harmful effects of chemical and environmental considerations, the use of modern chemical fungicides has therefore been prohibited from handling sheath blight of rice from a farmer's field by biological means. There is no “silver bullet” control method for Sheath blight disease of rice. In the current investigation, the VSMKU1 strain has been identified as higher phenazine producing and evaluated as most efficacious strain when applied as crude metabolite in controlling R. solani. Therefore, this strain P. aeruginosa VSMKU1 can be used to control rice sheath blight disease as a possible bio inoculant. This study also explored that P. aeruginosa strain VSMKU1 successfully inhibit the mycelium growth of R. solani due to the presence of broad-spectrum fungal antibiosis and innate potential of producing IAA, HCN, siderophore, phosphate solubilization and lytic enzyme production which stands as a viable environmental solution to control phytopathogens. Moreover, the presence of phenazine-1-carboxamide encoding gene in VSMKU1 is an additional fact for its antagonistic property. Hence, further research is needed to elucidate the efficient development for purification, characterization and production of a single metabolite from phenazine like compoundas a bio control agent for the control of different plant disease through in vitro and in vivo conditions.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors are grateful to the University Grants Commission, New Delhi, India for financial support through Major Research Project (39-214/2010 (SR) dated: 27.12.2010). The authors also would like to thank DBT- IPLS and DST- PURSE program for the financial support and The Chairperson, School of Biological Science, Madurai Kamaraj University, Madurai for providing laboratory facilities. The authors extend their appreciation to the Researchers supporting project number (RSP-2020/190) King Saud University, Riyadh, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ahirwar N.K., Singh R., Chaurasia S., Chandra R., Ramana S. Effective role of beneficial microbes in achieving the sustainable agriculture and eco-friendly environment development goals: a review. Front. Microbiol. 2020;5:111–123. [Google Scholar]
- Aneja K.R. fourth ed. New Age International (P) Ltd.; New Delhi: 2001. Experiments in Microbiology, Plant Pathology, Tissue Culture and Mushroom Cultivation; pp. 251–253. [Google Scholar]
- Ayyadurai N., Naik P.R., Sakthivel N. Functional characterization of antagonistic fluorescent pseudomonads associated with rhizospheric soil of rice (Oryza sativaL.) J. Microbiol. Biotechnol. 2007;17(6):919–927. [PubMed] [Google Scholar]
- Benson, H.J., 1990. Microbiological Applications: a laboratory manual in general microbiology. William C. Brown Pub: Dubuque, IA, USA.
- Biessy A., Filion M. Phenazines in plant-beneficial Pseudomonas spp.: biosynthesis, regulation, function and genomics. Environ. Microbiol. 2018;20(11):3905–3917. doi: 10.1111/1462-2920.14395. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee R.B., Singh A., Mukhopadhyay S.N. Use of nitrogen-fixing bacteria as biofertiliser for non-legumes: prospects and challenges. Appl. Microbiol. Biotech. 2008;80(2):199–209. doi: 10.1007/s00253-008-1567-2. [DOI] [PubMed] [Google Scholar]
- Budzikiewicz H. Secondary metabolites from fluorescent pseudomonads. FEMS Microbiol. Lett. 1993;104(3–4):209–228. doi: 10.1111/j.1574-6968.1993.tb05868.x. [DOI] [PubMed] [Google Scholar]
- Capdevila S., Martínez-Granero F.M., Sánchez-Contreras M., Rivilla R., Martín M. Analysis of Pseudomonas fluorescens F113 genes implicated in flagellar filament synthesis and their role in competitive root colonization. Microbiology. 2004;150(11):3889–3897. doi: 10.1099/mic.0.27362-0. [DOI] [PubMed] [Google Scholar]
- Cardinali A., Nason G.P. Costationarity of locally stationary time series using costat. J. Stat. Softw. 2013;55(1):1–22. [Google Scholar]
- Carmona-Hernandez S., Reyes-Pérez J.J., Chiquito-Contreras R.G., Rincon-Enriquez G., Cerdan-Cabrera C.R., Hernandez-Montiel L.G. Biocontrol of postharvest fruit fungal diseases by bacterial antagonists: a review. Agronomy. 2019;9(3):121–135. [Google Scholar]
- Compant S., Clément C., Sessitsch A. Plant growth-promoting bacteria in the rhizo-and endosphere of plants: their role, colonization, mechanisms involved and prospects for utilization. Soil Biol. Biochem. 2010;42(5):669–678. [Google Scholar]
- Duke K.A., Becker M.G., Girard I.J., Millar J.L., Fernando W.D., Belmonte M.F., de Kievit T.R. The biocontrol agent Pseudomonas chlororaphis PA23 primes Brassica napus defenses through distinct gene networks. BMC Genomics. 2017;18(1):467. doi: 10.1186/s12864-017-3848-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R.J., Timms-Wilson T.M., Bailey M.J. Identification of conserved traits in fluorescent pseudomonads with antifungal activity. Environ. Microbiol. 2000;2:274–284. doi: 10.1046/j.1462-2920.2000.00102.x. [DOI] [PubMed] [Google Scholar]
- Friedberg E.C., Walker G.C., Siede W., Wood R.D., Schultz R.A., Ellenberger T. second ed. American Society for Microbiology; Washington, D.C.: 2005. DNA repair and mutagenesis. [Google Scholar]
- Gnanamanickam, S.S., Valasubramanian, R., Thara, K.V., Chatterjee, A.K., 1998. Microbial antagonists for rice diseases control: Molecular Approaches. Microbes: for Health, Wealth and Sustainable Environment. Ed. Ajit Varma, pp. 371–388.
- Gordon S.A., Weber R.P. Colorimetric estimation of indole acetic acid. Plant Physiol. 1951;26(1):192–195. doi: 10.1104/pp.26.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guleria S., Aggarwal R., Thind T.S., Sharma T.R. Morphological and pathological variability in rice isolates of Rhizoctonia solani and molecular analysis of their genetic variability. J. Phytopathol. 2007;155:654–661. [Google Scholar]
- Haas D., Défago G. Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat. Rev. Microbiol. 2005;3(4):307–319. doi: 10.1038/nrmicro1129. [DOI] [PubMed] [Google Scholar]
- Harikrishnan H., Shanmugaiah V., Balasubramanian N., Sharma M.P., Kotchoni S.O. Antagonistic potential of native strain Streptomyces aurantiogriseus VSMGT1014 against sheath blight of rice disease. W. J. Microbiol Biotech. 2014;30(12):3149–3161. doi: 10.1007/s11274-014-1742-9. [DOI] [PubMed] [Google Scholar]
- Harikrishnan H., Shanmugaiah V., Nithya K., Balasubramanian N., Sharma M.P., Gachomo E.W., Kotchoni S.O. Enhanced production of phenazine-like metabolite produced by Streptomyces aurantiogriseus VSMGT1014 against rice pathogen, Rhizoctonia solani. J. Basic Microbiol. 2016;56(2):153–161. doi: 10.1002/jobm.201500362. [DOI] [PubMed] [Google Scholar]
- Harman G.E., Petzoldt R., Comis A., Chen J. Interactions between Trichoderma harzianum strain T22 and maize inbred line Mo17 and effects of these interactions on diseases caused by Pythium ultimum and Colletotrichum graminicola. Phytopathology. 2004;94(2):147–153. doi: 10.1094/PHYTO.2004.94.2.147. [DOI] [PubMed] [Google Scholar]
- Howell C.R., Stipanovic R.D. Control of Rhizoctonia solani on cotton seedlings with Pseudomonas fluorescens and with all antibiotic produced by the bacterium. Phytopathology. 1979;69:596–598. [Google Scholar]
- Howell C.R., Stipanovic R.D. Suppression of Phythium ultimum induced damping-off of cotton seedlings by Pseudomonas fluorescens and its antibiotic pyoluteorin. Phytopatholology. 1980;70:712–715. [Google Scholar]
- Hua G.K.H., Wang L., Chen J., Ji P. Biological control of Fusarium wilt on watermelon by fluorescent pseudomonads. Biocontrol Sci. Technol. 2020;30(3):212–227. [Google Scholar]
- Jin F., Ding Y., Ding W., Reddy M.S., Dilantha Fernando W.G., Du B. Genetic diversity and phylogeny of antagonistic bacteria against Phytophthora nicotianae isolated from tobacco rhizosphere. Int. J. Mol. Sci. 2011;12:3055–3071. doi: 10.3390/ijms12053055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jousset A., Lara E., Wall L.G., Valverde C. Secondary metabolites help biocontrol strain Pseudomonas fluorescens CHA0 to escape protozoan grazing. Appl. Environ. Microbiol. 2006;72(11):7083–7090. doi: 10.1128/AEM.00557-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jukes T.H., Cantor C.R. In: Mammalian Protein Metabolism. third ed. Munro H.N., editor. Academic Press; New York: 1969. Evolution of protein molecules; pp. 21–132. [Google Scholar]
- Kandel S.L., Firrincieli A., Joubert P.M., Okubara P.A., Leston N.D., McGeorge K.M., Mugnozza G.S., Harfouche A., Kim S.H., Doty S.L. An in vitro study of bio-control and plant growth promotion potential of Salicaceae endophytes. Front. Microbiol. 2017;8:386–401. doi: 10.3389/fmicb.2017.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keel C., Weller D.M., Natsch A., Défago G., Cook R.J., Thomashow L.S. Conservation of the 2, 4 -diacetylphloroglucinol biosynthesis locus among fluorescent Pseudomonas strains from diverse geographic locations. Appl. Environ. Microbiol. 1996;62(2):552–556. doi: 10.1128/aem.62.2.552-563.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King E.O., Ward M.K., Raney D.E. Two simple media for the demonstration of pyocyanin and fluorescein. J. Lab. Clin. Med. 1954;44:301–307. [PubMed] [Google Scholar]
- Kloepper J.W., Leong J., Teintze M., Schroth M.N. Pseudomonas-siderophores: a mechanism explaining disease -suppressive soil. Curr. Microbiol. 1980;4:317–320. [Google Scholar]
- Kumar B.D., Dube H.C. Seed bacterization with a fluorescent Pseudomonas for enhanced plant growth, yield and disease control. Soil Biol Biochem. 1992;24(6):539–542. [Google Scholar]
- Li F., Jiang P., Zheng H., Wang S., Zhao G., Qin S., Liu Z. Draft genome sequence of the marine bacterium Streptomyces griseoaurantiacus M045, which produces novel manumycin-type antibiotics with a pABA core component. J. Bacteriol. 2011;193(13):3417–3418. doi: 10.1128/JB.05053-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H.B., Singh R.K., Singh P., Song Q.Q., Xing Y.X., Yang L.T., Li Y.R. Genetic diversity of nitrogen-fixing and plant growth promoting Pseudomonas species isolated from sugarcane rhizosphere. Front Microbiol. 2017;8:1268. doi: 10.3389/fmicb.2017.01268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N., Alfiky A., Wang W., Islam M., Nourollahi K., Liu X., Kang S. Volatile compound-mediated recognition and inhibition between Trichoderma biocontrol agents and Fusarium oxysporum. Front. Microbiol. 2018;9:2614. doi: 10.3389/fmicb.2018.02614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorck H. Production of hydrocyanic acid by bacteria. Physiol. Plant. 1948;1(2):142–146. [Google Scholar]
- Lu L., Shu C., Liu C., Wang C., Zhou E. The impacts of natural antioxidants on sclerotial differentiation and development in Rhizoctonia solani AG-1 IA.Eur. J. Plant Pathol. 2016;146(4):729–740. [Google Scholar]
- Mahmoudi T.R., Yu J.M., Liu S., Pierson L.S., III, Pierson E.A. Drought-stress tolerance in wheat seedlings conferred by phenazine-producing rhizobacteria. Front. Microbiol. 2019;10:1590. doi: 10.3389/fmicb.2019.01590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathivanan, N., Shanmugaiah, V., 2011. Management of sheath blight disease in rice by Pseudomonas aeruginosa MML2212. Plant growth promoting rhizobacteria (PGPR) for sustainable agriculture. 507.
- Mavrodi D.V., Bonsall R.F., Delaney S.M., Soule M.J., Phillips G., Thomashow L.S. Functional analysis of genes for biosynthesis of pyocyanin and phenazine-1-carboxamide from Pseudomonas aeruginosa PAO1. J. Bacteriol. 2001;183(21):6454–6465. doi: 10.1128/JB.183.21.6454-6465.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavrodi D.V., Blankenfeldt W., Thomashow L.S. Phenazine compounds in fluorescent Pseudomonas spp. biosynthesis and regulation. Annu. Rev. Phytopathol. 2006;44:417–445. doi: 10.1146/annurev.phyto.44.013106.145710. [DOI] [PubMed] [Google Scholar]
- Mavrodi D.V., Mavrodi O.V., Parejko J.A., Bonsall R.F., Kwak Y.S., Paulitz T.C., Thomashow L.S., Weller D.M. Accumulation of the antibiotic phenazine-1-carboxylic acid in the rhizosphere of dryland cereals. Appl. Environ. Microbiol. 2012;78(3):804–812. doi: 10.1128/AEM.06784-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguelez-Sierra Y., Acebo-Guerrero Y., El Jaziri M., Bertin P., Hernández-Rodríguez A. Pseudomonas chlororaphis CP07 strain reduces disease severity caused by Phytophthora palmivora in genotypes of Theobroma cacao. Eur. J. Plant Pathol. 2019;155(4):1133–1143. [Google Scholar]
- Naik P.R., Sahoo N., Goswami D., Ayyadurai N., Sakthivel N. Genetic and functional diversity among fluorescent pseudomonads isolated from the rhizosphere of banana. Microbiol. Ecol. 2008;56:492–504. doi: 10.1007/s00248-008-9368-9. [DOI] [PubMed] [Google Scholar]
- Nam H.S., Anderson A.J., Kim Y.C. Biocontrol efficacy of formulated Pseudomonas chlororaphis O6 against plant diseases and root-knot nematodes. Plant Pathol. J. 2018;34(3):241. doi: 10.5423/PPJ.NT.12.2017.0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandakumar R., Babu S., Viswanathan R., Raguchander T., Samiyappan R. Induction of systemic resistance in rice against sheath blight disease by Pseudomonas fluorescens. Soil Biol. Biochem. 2001;33(4–5):603–612. [Google Scholar]
- Nascente A.S., de Filippi M.C.C., Lanna A.C., de Sousa T.P., de Souza A.C.A., da Silva Lobo V.L., da Silva G.B. Effects of beneficial microorganisms on lowland rice development. Environ. Sci. Pollut. Res. 2017;24(32):25233–25242. doi: 10.1007/s11356-017-0212-y. [DOI] [PubMed] [Google Scholar]
- Ochman H., Lawrence J.G., Groisman E.A. Lateral gene transfer and the nature of bacterial innovation. Nature. 2000;405:299–304. doi: 10.1038/35012500. [DOI] [PubMed] [Google Scholar]
- Patel P., Shah R., Joshi B., Ramar K., Natarajan A. Molecular identification and biocontrol activity of sugarcane rhizosphere bacteria against red rot pathogen Colletotrichum falcatum. Biotechnol. Rep. 2019;21:317–324. doi: 10.1016/j.btre.2019.e00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng D., Li S., Wang J., Chen C., Zhou M. Integrated biological and chemical control of rice sheath blight by Bacillus subtilis NJ-18 and jinggangmycin. Pest Manag. Sci. 2014;70(2):258–263. doi: 10.1002/ps.3551. [DOI] [PubMed] [Google Scholar]
- Perneel M., Heyrman J., Adiobo A., De Maeyer K., Raaijmakers J.M., De Vos P., Höfte M. Characterization of CMR5c and CMR12a, novel fluorescent Pseudomonas strains from the cocoyam rhizosphere with biocontrol activity. J. Appl. Microbiol. 2007;103(4):1007–1020. doi: 10.1111/j.1365-2672.2007.03345.x. [DOI] [PubMed] [Google Scholar]
- Picard C., Bosco M. Genetic diversity of phlD gene from 2, 4- diacetylphloroglucinol producing Pseudomonas spp. strains from the maize rhizosphere. FEMS Microbiol. Lett. 2003;219:167–172. doi: 10.1016/S0378-1097(03)00027-2. [DOI] [PubMed] [Google Scholar]
- Pikovskaya R.E. Mobilization of phosphorus in soil in concentration with vital activity of some microbial species. Microbiology. 1948;17:362–370. [Google Scholar]
- Prabavathy V.R., Mathivanan N., Murugesan K. Control of blast and sheath blight diseases of rice using antifungal metabolites produced by Streptomyces sp. PM5. Biol. Control. 2006;39(3):313–319. [Google Scholar]
- Qessaoui R., Bouharroud R., Furze J.N., El Aalaoui M., Akroud H., Amarraque A., Van Vaerenbergh J., Tahzima R., Mayad E.H., Chebli B. Applications of new rhizobacteria Pseudomonas isolates in agroecology via fundamental processes complementing plant growth. Sci. Rep. 2019;9(1):1–10. doi: 10.1038/s41598-019-49216-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman S.F.S., Singh E., Pieterse C.M., Schenk P.M. Emerging microbial biocontrol strategies for plant pathogens. Plant Sci. 2018;267:102–111. doi: 10.1016/j.plantsci.2017.11.012. [DOI] [PubMed] [Google Scholar]
- Rosenblueth M., Martínez-Romero E. Bacterial endophytes and their interactions with hosts. Mol. Plant-Microbe Interact. 2006;19(8):827–837. doi: 10.1094/MPMI-19-0827. [DOI] [PubMed] [Google Scholar]
- Saikia R., Sarma R.K., Yadav A., Bora T.C. Genetic and functional diversity among the antagonistic potential fluorescent pseudomonads isolated from tea rhizosphere. Curr. Microbiol. 2011;62(2):434–444. doi: 10.1007/s00284-010-9726-y. [DOI] [PubMed] [Google Scholar]
- Santoyo G., Moreno-Hagelsieb G., Del Carmen Orozco-Mosqueda M., Glick B.R. Plant growth-promoting bacterial endophytes. Microbiol. Res. 2016;183:92–99. doi: 10.1016/j.micres.2015.11.008. [DOI] [PubMed] [Google Scholar]
- Sarma R.K., Saikia R. Alleviation of drought stress in mung bean by strain Pseudomonas aeruginosa GGRJ21. Plant Soil. 2014;377(1–2):111–126. [Google Scholar]
- Schlemper T.R., Dimitrov M.R., Gutierrez F.A.S., Van Veen J.A., Silveira A.P., Kuramae E.E. Effect of Burkholderia tropica and Herbaspirillum frisingense strains on sorghum growth is plant genotype dependent. PeerJ. 2018;6:5346. doi: 10.7717/peerj.5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwyn B., Neilands J.B. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987;160(1):47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- Shanmugaiah, V., Ramesh, S., Jayaprakashvel, M., Mathivanan, N., 2006. Biocontrol and plant growth promoting potential of Pseudomonas sp. MML2212 from the rice rhizosphere. Mitteilungen-Biologischen Bundesanstalt fur Land und Forstwirtschaft, 408, 320.
- Shanmugaiah V., Mathivanan N., Balasubramanian N., Manoharan P.T. Optimization of cultural conditions for production of chitinase by Bacillus laterosporous MML2270 isolated from rice rhizosphere soil. Afr. J. Biotechnol. 2008;7(15):2562–2568. [Google Scholar]
- Shanmugaiah V., Mathivanan N., Varghese B. Purification, crystal structure and antimicrobial activity of phenazine-1-carboxamide produced by a growth-promoting biocontrol bacterium, Pseudomonas aeruginosa MML2212. J. Appl. Microbiol. 2010;108:703–711. doi: 10.1111/j.1365-2672.2009.04466.x. [DOI] [PubMed] [Google Scholar]
- Shanmugaiah, V., Nithya, K., Harikrishnan, H., Jayaprakashvel, M., Balasubramanian, N., 2015. Biocontrol mechanisms of siderophores against bacterial plant pathogens. Sustainable approaches to controlling plant pathogenic bacteria, pp. 167–190.
- Singh R., Singh L.S., Prasad D., Kureel R.S., Sengar R., Singh A. Relationship of susceptibility and growth stages of plant for development of epidemic of sheath blight in rice. J. Nat. Appl. Sci. 2010;2(2):230–233. [Google Scholar]
- Singh, S.K., Pathak, R., Choudhary, V., 2016. Plant growth-promoting rhizobacteria-mediated acquired systemic resistance in plants against pests and diseases. In Microbial-mediated Induced Systemic Resistance in Plants pp. Springer, Singapore, pp. 125–134.
- Slaton N.A., Cartwright R.D., Meng J., Gbur E.E., Norman R.J. Sheath blight severity and rice yield as affected by nitrogen fertilizer rate, application method, and fungicide. J. Agron. 2003;95(6):1489–1496. [Google Scholar]
- Smirnov, V.A., Kiprianova, E.A., 1990. Bacteria of Pseudomonas genus. Naukova Dumka, Kiev. Ukraine, pp. 100–111.
- Voisard C., Keel C., Haas D., Defago G. Cyanide production by Pseudomonas fluorescens helps suppress black root rot of tobacco under gnotobiotic conditions. Eur. Mol. Biol. Organ. J. 1989;8:351–358. doi: 10.1002/j.1460-2075.1989.tb03384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller D.M., Landa B.B., Mavrodi O.V., Schroeder K.L., De La Fuente L., Bankhead S.B., Molar R.A., Bonsall R.F., Mavrodi D.V., Thomashow L.S. Role of 2, 4-diacetylphloroglucinol producing fluorescent Pseudomonas spp. in the defense of plant roots. Plant Biol. 2007;9:4–20. doi: 10.1055/s-2006-924473. [DOI] [PubMed] [Google Scholar]
- Xia Y., Fei B., He J., Zhou M., Zhang D., Pan L., Li S., Liang Y., Wang L., Zhu J., Li P. Transcriptome analysis reveals the host selection fitness mechanisms of the Rhizoctonia solani AG1IA pathogen. Sci. Rep. 2017;7(1):1–16. doi: 10.1038/s41598-017-10804-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdani M., Bahmanyar M.A., Pirdashti H., Esmaili M.A. Effect of phosphate solubilization microorganisms (PSM) and plant growth promoting rhizobacteria (PGPR) on yield and yield components of corn (Zea mays L.) World Acad. Sci. Eng. Technol. 2009;49:90–92. [Google Scholar]
- Zhang F., Zeng D., Zhang C.S., Lu J.L., Chen T.J., Xie J.P., Zhou Y.L. Genome-wide association analysis of the genetic basis for sheath blight resistance in rice. Rice. 2019;12(1):1–13. doi: 10.1186/s12284-019-0351-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L., Liu H., Zhang M., Cao X., Zhou E. The complete genomic sequence of a novel mycovirus from Rhizoctonia solani AG-1 IA strain B275. Arch. Virol. 2013;158(7):1609–1612. doi: 10.1007/s00705-013-1637-3. [DOI] [PubMed] [Google Scholar]
Further Reading
- Charulatha, R., Harikrishnan, H., Manoharan, P.T., Shanmugaiah, V., 2013. Characterization of Groundnut Rhizosphere Pseudomonas sp. VSMKU 2013 for control of phytopathogens. In: Velu, R.K. (Ed) Microbiological Research in Agroecosystem Management. Springer India, New Delhi, pp. 121–127.
- Kim S.G., Zakaullah K., Jeon Y.H., Kim Y.H. Inhibitory effect of Paenibacillus polymyxa GBR-462 on Phytophthora capsici causing phytophthora blight in chili pepper. J. Phytopathol. 2009;157:329–337. [Google Scholar]