Abstract
The current study aimed to assess the antiulcerogenic impact of mesenchymal bone marrow stem cells (BMMSCs) against gastric ulcer induced by the use of piroxicam in rats and to compare this effect with the antiulcer drug “Pantoloc ®” proton pump inhibitors. The study included histological, histochemical, immunohistochemical and ultrastructural examination in stomach of rats in different study groups. In the ulcerated group, the glandular region of the stomach displayed clear mucosal lesions occurring as perforations along the stomach axis. In addition, stomach displayed degeneration of surface mucous cells accompanied by pyknosis, vacuolation among parietal cells in ishmus region, basal region with vacuolated chief cells and karyolitic nucleus of parietal cells. Moreover, Stomach sections of ulcer model rats showed intensive immunoreactivity to cytokeratin 20, Cox 2 and PCNA. Findings of the present study have shown that BMMSCs have an ameliorative effect against piroxicam-induced gastric ulcer in rats. Collectively, the proposed work has shown that BMMSCs have a curative capacity as an antiulcer due to their high antioxidant activity. Further studies are required in molecular levels to understand the mechanism of action during treatment.
Keywords: Stem cells, Piroxicam, Gastric ulcer, Rat, Histology, Ultrastructure
1. Introduction
Peptic ulcer disease (PUD) is among the most severe gastrointestinal ulcers. It is mucosal erosions caused by many factors such as drugs, stress and alcohol, due to the disturbance between the aggressive acid- pepsin secretion and defensive mucosal factors like mucin secretion and cell shedding. Long-term use of non-steroidal anti-inflammatory drugs (NSAIDs) may cause disturbances of the gastric mucosa, associated with toxicity, which may eventually cause ulceration at very high morbidity and mortality levels. (Al-Asmari et al., 2015).
Various medications such as prostaglandin analogs, histamine receptor antagonists, proton pump inhibitors, and cytoprotective agents are commonly used in the treatment of peptic ulcers. Recently, due to several adverse effects and toxicity of chemical drugs, natural medications are generally used in treatment (Al-Hussaini, 2014).
Pantoloc ® (pentaprazole, a proton pump inhibitor), works by irreversibly suppressing the enzyme mechanism of hydrogen H+/potassium K + adenosine tri-phosphatase (H+/K + ATPase), the proton pump of the parietal gastric cells. The proton pump is the final step in the secretion of gastric acid, which is primarily responsible for the secretion of hydrogen ions into the gastric lumen, thereby becoming an optimal target for decreasing acid secretion. Targeting the final stage in acid formation and its irresponsibility in inhibition makes this class of drugs more effective than H2 antagonists and decreases the secretion of gastric acid by almost 99%. Declining stomach acid can improve the healing of peptic ulcers and decrease indigestion pain (Ransburg and Cheer, 2012).
The proton pump inhibitors (PPIs) are in-active type, which is neutrally charged because it is lipophilic and can traverse the cell membranes as the parietal cell canaliculus in acidic conditions into the intracellular compartments. In this acid environment, the in-active form of the drug is then rearranged in its active form. Therefore, the active form also will bind to the gastric proton pump by covalent and irreversible bond, leading to its deactivation (Sachar et al., 2014). All PPIs give excellent healing of peptic ulcers and produce good results in reflux esophagitis. Gastric acids, however, are naturally required to digest proteins, absorb vitamin B12, calcium, and other nutrients and can induce hypochlorhydria when the acid decreases (Sakai et al., 2016).
Hoping to make significant medical breakthroughs, stem cell research is being pursued. Scientists are trying to develop therapies that regenerate or substitute tumor cells with tissues grown from stem cells and provide hope for people with cancer, diabetes, spinal cord injuries and several other ailments. The adult and embryonic stem cells could provide scientists with a route to developing useful new drug development and research methods (El-Azab et al., 2018).
Stem cell therapy is any procedure that makes use of stem cells or activates them. It is typically to help replace or restore damaged cells or tissues, but may also be used mainly to prevent damage. Stem cell therapy might either involve transplanting stem cells or giving drugs that target stem cells already in the body (Rashed et al., 2016). This research was planned to evaluate the beneficial regenerative effects of MSCs on gastric ulcers caused by the use of piroxicam.
2. Materials and methods
2.1. Animals
Fifty healthy adult female albino rats weighting about 160–165 gm, were purchased from the animal facility of the Egyptian Organization of Biological Products and Vaccines (Vacsera ®, Cairo, Egypt).Rats were kept under ideal hygienic conditions. They were fed at libitum and allowed free water supply and standard chow pellets. All experimental animals following the National Institutes of health guide for the care and use of laboratory animals (NIH publications No. 8023, revised 1985), and the experimental design was approved by Helwan University Research Ethics Committee.
2.2. Induction of gastric ulcer model
In this study rats were fasted overnight but had free water supply until 4 h before administration of brexin (Chiesi Company for pharmaceuticals and chemical industries, Cairo, Egypt). Gastric mucosal injury was caused by a single oral dose of brexin (piroxicam, 5 mg/kg body weight), then animals were killed after 3 h according to the procedure mentioned in Avila et al. (1996).
2.3. Experimental design
The animals were classified into four groups. Group I received saline solution as a negative control group. Group II received a single dose of brexin (piroxicam, 5 mg/kg) after fasting overnight which served as ulcer induced group (Avila et al., 1996). Group III received 20 mg/kg Pantoloc® (Medical union pharmaceuticals, Cairo, Egypt) as daily dosage for 2 weeks after induction of peptic ulcer (Talaat et al., 2014). Group IV received 106 cells of Bone marrow derived Mesenchymal Stem cells (BMMSCs) for each rat given by direct intravenous injection at the rat tail vein after induction of peptic ulcer (Rashed et al., 2016). BMMSCs extract was purchased from the stem cells research unit at Biochemistry Department, Faculty of Medicine, Cairo University. Animals of groups II and IV were killed after 2 weeks.
All animals were killed by decapitation twenty-four hours after the last dose and then, stomachs were dissected and opened around the greater curvature, then gently washed with saline solution to eliminate any debris or blood clots. As the gastric mucosal surface turned upward, tissues were bound to wax paper. Digital photographs have been taken for classification of gross pathology. Stomachs were cut and processed for histological, ultrastructural, and histochemical evaluations.
2.4. Histopathological assessment of gastric damage
Sections of the gastric glandular region were fixed in bouin solution for about 24 h, then dehydrated and impregnated in parablast for blocking, 5 µm thick sections were prepared, and then stained by hematoxylin and eosin (H&E) for microscopic examination of gastric injury and any regenerative process and stained with periodic acid Schiff's (PAS) to evaluate mucosal glycoprotein production (Bancroft et al., 2018). Sections were photographed using a LEICA® microscope (Wetzlar, Germany).
For immunohistochemical examination, sections of stomach were fixed in bouin fixative and then immune-stained using cytokeratine 20, Cox2 and PCNA antibody (Labvision, Neomarkers, USA) for 90 min. And then followed by the secondary antibody application using immune-peroxidase technique (Vectastain ABC kit; Vector Laboratories, Burlingame, CA) (Bancroft et al., 2018).
2.5. Electron microscopic studies
For transmission electron microscopy (TEM), small pieces of stomach (about 1 mm) were immediately fixed in 2.5% 0.1 M phosphate- buffered gluteraldehyde, and then specimens were embedded in epoxy resin mixture. Semi- thin sections (1micron thick) were cut and stained with toluidine blue (TB), then examined with the light microscope. After the proper areas were selected, ultrathin sections (60–90 nm) and finally stained sections were examined with a JEOL 1010 Transmission Electron Microscope (Tokio, Japan) (Bancroft et al., 2018).
According to the method mentioned in Bancroft et al. (2018), tissue are washed in phosphate buffer saline (PBS) and then fixed in 1 ml of 2.5% glutaraldehyde in PBS or cacodylate and processed for scanning electron microscopy (SEM). Examination was performed with a Jeol 840A or a Jeol JM6700 F scanning microscope (Tokio, Japan) (Bancroft et al., 2018).
2.6. Morphometrical analysis
Data were expressed as mean ± SD. Significant differences were determined by using ANOVA and post-hoc tests for multiple comparisons using GraphPad Prism (version 5.0) computer Software. Results were considered significant at P < 0.05 (Dawson and Trapp, 2001).
3. Results
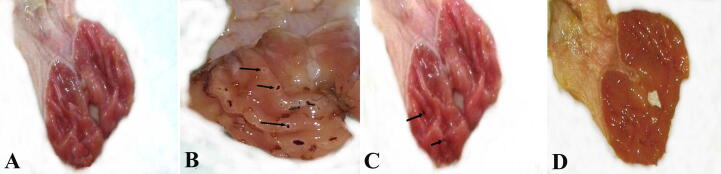
Gross examination of the open stomachs of negative control rats showed that the mucosa of the fore stomach were whitish in color, while the glandular part appeared pink to reddish in color (Fig. 1A). In the ulcerated group, the glandular region of the stomach displayed clear mucosal lesions occurring as perforations along the stomach axis (Fig. 1B), while in the group treated with Pantoloc ®, the glandular portion of the stomach showed small lesions relative to the group associated with ulcer (Fig. 1C). The stomachs of the treated stem cell group eventually displayed the same gross appearance as in the negative control group (Fig. 1D).
Fig. 1.
Photographs showing different gross morphology of different groups. (A) Negative control group showing no injury in gastric mucosa. (B) Ulcer model group showing a lot of mucosal lesions (arrows). (C) Pantoloc® treated group showing a slight improvement of the gastric mucosa with little gastric lesions (arrows). (D) Treated group with stem cells showing noticeable improvement with no mucosal lesions.
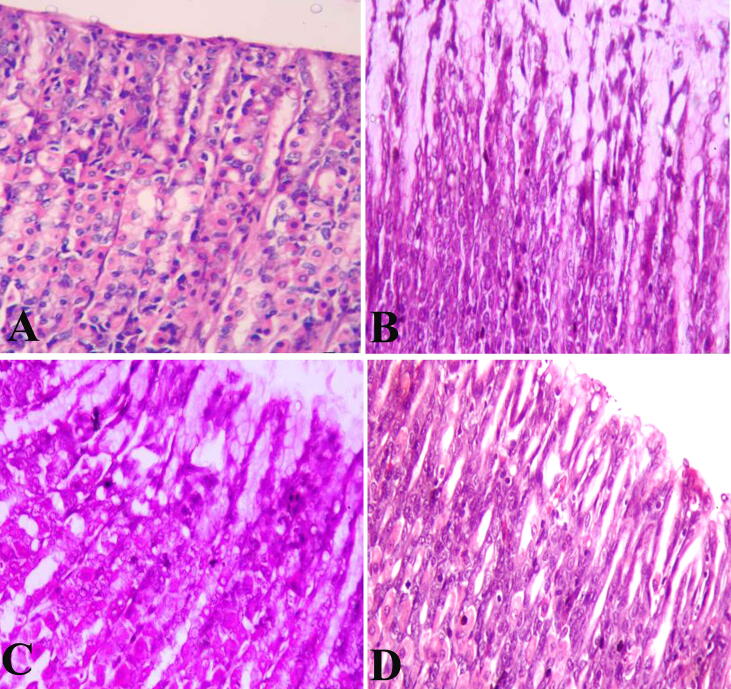
Examination of H&E-stained control rats revealed typical histological structures (Fig. 2A). Ulcer model rat group showed a degeneration of surface mucous cells accompanied by pyknosis, vacuolation among parietal cells in ishmus region, basal region with vacuolated chief cells and karyolitic nucleus of parietal cells (Fig. 2b). Pantoloc® treated rat appeared with degenerated surface mucous cells, pyknotic, karyolitic parietal cells and chief cells in the base region (Fig. 2C). Stem cells treated rats had almost regular stomach mucosal architecture and natural presence of the stomach glandular cells, main, parietal and surface mucous cells (Fig. 2D).
Fig. 2.
Photomicrograph showing the gastric mucosal layer in stomach of different groups (H&E stain). (A and D) stomachs of negative control group and stem cell treated group showing normal architecture in the gastric mucosa. (B) ulcer model group showing degeneration of the gastric mucosal layer. (C) Pantoloc® treated group showing less degree of degeneration among gastric mucosa. Magnification 400×.
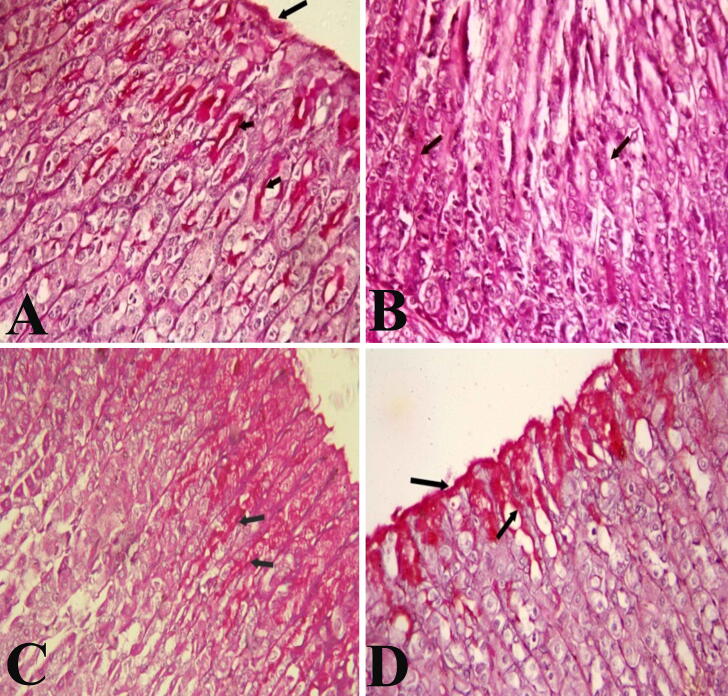
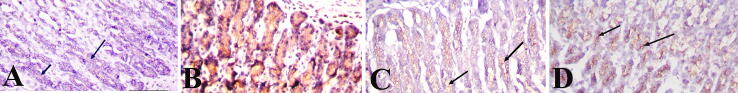
Fig. 3 showed sections of the stomach stained with PAS. In the negative control group, the surface epithelial cells and gastric glands showed a strong PAS reaction indicating the presence of a thick layer of mucosa (Fig. 3A). Stomach sections of ulcer induced group showed weak PAS reaction (Fig. 3B). The Pantoloc ® treated rats showed moderate PAS reactions (Fig. 3C), while the treated stem cell group showed a strong PAS response in the gastric mucosal cells similar to the control group (Fig. 3D).
Fig. 3.
Photomicrographs showing different reactions among mucosa layer in stomach sections in different groups (PAS stain). (A and D) control negative group and stem cell treated group showing strong reaction among surface mucous and neck cells (arrows). (B) Ulcer model group showing erosions of the surface epithelial cells and faint PAS reaction (arrows). (C) Pantoloc® treated group showing moderate PAS reaction (arrows). Magnification 400×.
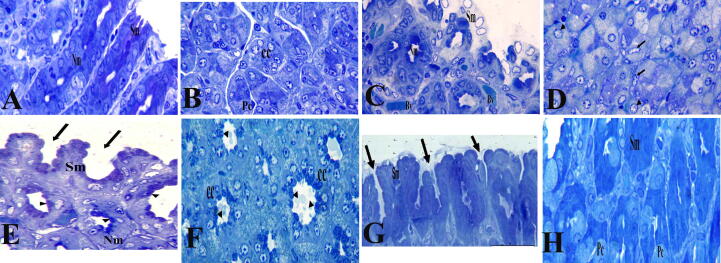
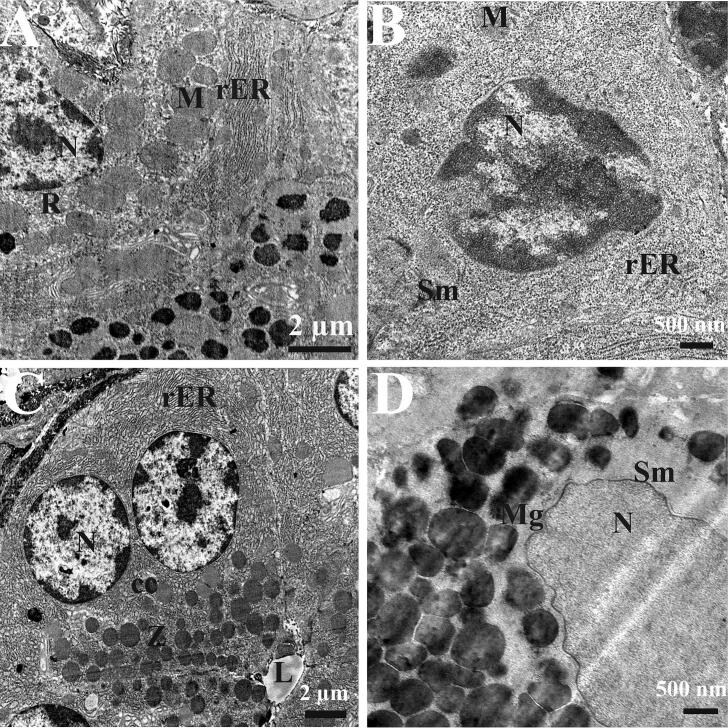
Through the examination of semi-thin sections (Fig. 4) and TEM (Fig. 5), we found that control rat 's stomach stained with toluidine blue showed normal cell appearance (Fig. 4A, B). The ultrastructure examinations of gastric gland revealed normal structure of different cells covering the gastric mucosa and lining the glands. The chief cells showing regular, intact nucleus, normal mitochondria, free ribosomes, intact rough endoplasmic reticulum and apical zymogenic secretory granules (Fig. 5A). Semi-thin section of an ulcer model rat's gastric mucosa showed mild degeneration in surface mucous cells, nearly normal appearance of neck mucous cells with mucous granules at their apex, and isthmus parietal cells are also seen with karyolitic nuclei and karyohexis among some other parietal cells (Fig. 4C, D). The ultrastructure examinations of gastric glands revealed slight degeneration in surface mucous cells with irregular picnotic nucleus, fragmented rough endoplasmic reticulum, and mitochondria (Fig. 5B). Semi-thin section of the gastric mucosa of a Pantoloc® drug treated rats showed more or less improvement in the surface mucous cells with gastric pits in between and approximately normal neck mucous cells with mucus granules at the apical region and chief cells appeared with zymogen granules at the apex (Fig. 4E, F). The ultrastructure examination of gastric gland revealed more or less normal chief cells with regular, rounded, intact nucleus, numerous apical zymogen granules and well developed rough endoplasmic reticulum is also shown (Fig. 5C). Semi-thin section of the stomach mucosa of rats treated with stem cells revealed normal appearance and intact surface mucosal cells with stomach pits in between and intact parietal cells in the isthmus area (Fig. 4G, H). The ultrastructure examinations of gastric glands revealed intact surface mucous cells with oval nucleus and intensive mucous granules at the apical part (Fig. 5D).
Fig. 4.
Semi-thin section of the gastric mucosa stained with Toluidine blue. Control negative group (A and B) and stem cell treated group (G and H) showing normal histological appearance of the surface mucous cells (Sm), neck mucous cells (Nm), parietal cells and chief cells in the glandular region. (C,D) ulcer model group showing degenerated surface mucous epithelial cells with abnormal histological appearance of parietal and chief cells (arrows and arrowhead). (E,F) Pantoloc® treated group showing slight improvement of the surface mucous cells (Sm) and normal histological appearance of chief cells (cc). Magnification 400×.
Fig. 5.
Electron micrograph of gastric mucosa. (A) A magnified part of the chief cell of the gastric mucosa of control negative rat group showing regular, intact nucleus (N), mitochondria (M), free ribosomes (R) and intact rough endoplasmic reticulum (rER). (B) An electron miocrograph of surface mucous cell (Sm) of the gastric mucosa of ulcer model rat showing irregular picknotic nucleus (N), fragmented rough endoplasmic reticulum (rER), and mitochondria (M). (C) An electron micrograph of chief cell (cc) of the gastric mucosa of a Pantoloc® treated rat showing regular, rounded, intact nucleus (N), numerous apical zymogen granules (z), well developed rough endoplasmic reticulum (rER) and lumen of the gastric gland are obviously shown (L). (D) An electron micrograph of the surface mucous cell of the gastric mucosa of a stem cell treated rat showing normal intact nucleus (N) and numerous mucous granules at its apical part (Mg).
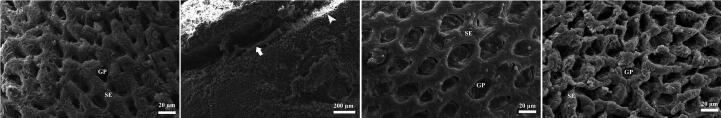
Scanning examination of control rats showed normal folds of gastric glandular region with normal gastric pits (Fig. 6A). The examination of ulcer model rats revealed degeneration of the surface of the “gastric ulcer” gastric mucosa (Fig. 6B). There was an improvement at the glandular surface in the Pantoloc® treated group (Fig. 6C). Also, a marked improvement among glandular surface was noticed in stem cell treated group similar to control negative group (Fig. 6D).
Fig. 6.
Scanning electron microscopic examination. (A and D) scanning electron micrograph of a control negative group and stem cell treated group stomach, showing normal folds, surface epithelial cell lining (SE) and gastric pits (GP). (B) Ulcer model rat showing the surface of the gastric mucosa (arrow head) and gastric ulcer (arrows). (C) Pantoloc® treated group showing improvement among surface epithelial cell lining (SE) and gastric pits (GP).
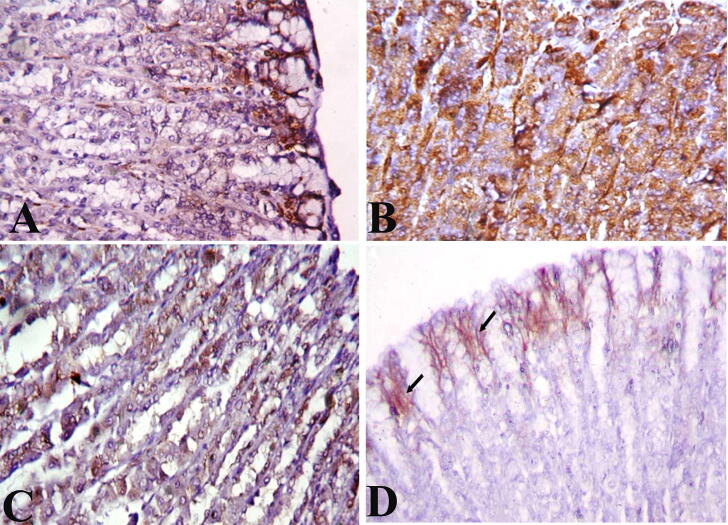
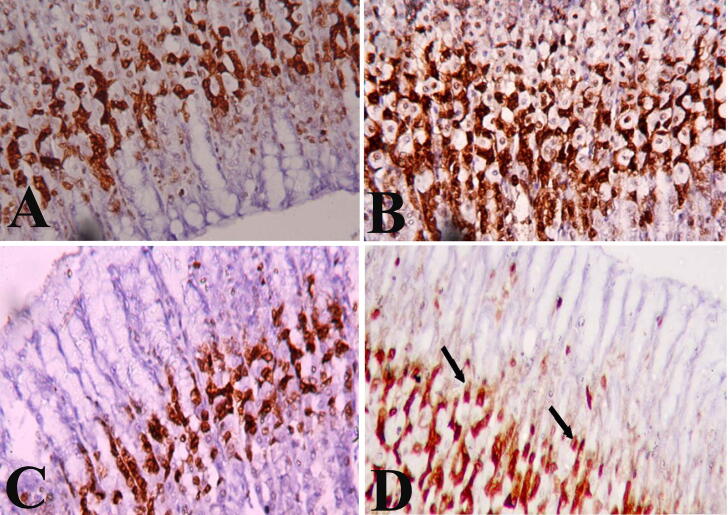
The cells of the gastric mucosa of negative control rats illustrate the normal immunoreactivity of cytokeratin 20 filaments as brown colour filaments stained with avidin–biotin immune-peroxidase technique and using the monoclonal antibody against cytokeratin 20. The cytokeratin 20 filaments are more evident at the luminal surface and lateral borders of the cells. The basal glandular cells showed weak immunoreactivity (Fig. 7A). Stomach sections of ulcer model rat showed intensive immunoreactivity to cytokeratin 20 (Fig. 7B). The Pantoloc ® -treated rat cells in the glandular area displayed mild immunoreactivity to cytokeratin 20 (Fig. 7C). However, the cells of the glandular region of the stem cell gastric mucosa treated rat showed a mild immunoreactivity to cytokeratin 20 approximately close to the control rat group (Fig. 7D).
Fig. 7.
Cross section of the gastric mucosa showing immunohistochemical reaction among different groups for anticytokeratin antibody in the glandular cells. (A) Control negative group showing weak reaction. (B) Ulcer model group showing intensive reaction. (C) Pantoloc® treated group showing moderate reaction. (D) Stem cell treated group showing weak to mild reaction. Magnification 400×.
With typical Cox 2 filaments immunoreactivity, the cells of the gastric mucosa of control rats appeared as brown colored filaments stained with avidin–biotin immunoperoxidase technique and the use of monoclonal antibodies against Cox 2. The Cox 2 filaments are more evident at the apical part and lateral borders of the cells. The surface glandular cells showed weak to mild immunoreactivity (Fig. 8A), stomach sections of the gastric mucosa of ulcer model rat showed intensive immunoreactivity to Cox 2 (Fig. 8B). While stomach sections of Pantoloc® treated rat group showed moderate immunoreactivity to Cox 2 (Fig. 8C). Stomach sections of the gastric mucosa of the rat treated with stem cells displayed low to moderate immunoreactivity to Cox2 relatively close to the control group of negative rats (Fig. 8D).
Fig. 8.
Cross section of the gastric mucosa showing immunohistochemical reaction among different groups for antiCox 2 antibody in the glandular cells. (A) Control negative group showing week to mild immunohistochemical reaction. (B) Ulcer model group showing intensive reaction. (C) Pantoloc® treated group showing moderate reaction. (D) Stem cell treated group showing weak to mild reaction. Magnification 400×.
The cells of the gastric mucosa of control rats illustrates the normal PCNA filaments immunoreactivity as brown colour filaments stained with avidin–biotin immunoperoxidase technique and using monoclonal antibody against PCNA. The PCNA are more evident at the nuclear level. The glandular cells showed moderate immunoreactivity in specific layer at the neck region of gastric mucosa (Fig. 9A). While the cells of the glandular region of the gastric mucosa of ulcer model rat showed intensiveimmunoreactivity to PCNA (Fig. 9B). The cells of the glandular region of the gastric mucosa of Pantoloc® drug treated rat showed moderate immunoreactivity to PCNA(Fig. 9C). On the other hand gastric mucosa of stem cell treated rat showed mild to moderate immunoreactivity to PCNA (Fig. 9D).
Fig. 9.
Cross section of the gastric mucosa showing immunohistochemical reaction among different groups for PCNA antibody in the glandular cells. (A) Control negative group showing moderate immunohistochemical reaction. (B) Ulcer model group showing intensive reaction. (C) Pantoloc® treated group showing mild reaction. (D) Stem cell treated group showing mild to moderate reaction. Magnification 400×.
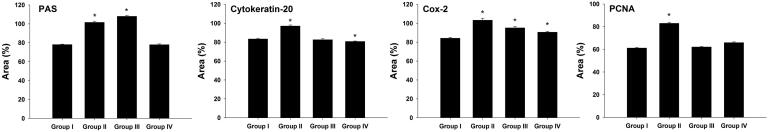
Fig. 10 represents a quantitative analysis of PAS satiability and the expression of cytokeratin 20, Cox-2 and PCNA in the gastric mucosa of rats. For the ulcer model group, we recorded the highest immune-reactivity to carbohydrates, cytokeratin 20, Cox2 and PCNA (Fig. 10) while after treatment with stem cells, stomachs showed less immunnoreactivity toward carbohydrates, cytokeratin 20, Cox2 and PCNA (Fig. 10).
Fig. 10.
Quantitative analysis of PAS satiability and the expression of cytokeratin 20, Cox-2 and PCNA in the gastric mucosa of rats. Values are mean ± SD. *, significance against control group at p ≤ 0.05.
4. Discussion
In the present study intragastric administration of piroxicam, an enolic acid- derived NSAID, revealed different changes in the gastric glandular cells, degeneration of surface mucous cells and pyknosis, vacuolation among parietal cells in ishmus region, basal region with vacuolated chief cells and karyolitic nucleus of parietal cells, this results is consisted with previous studies (Avila et al., 1996, Sabiua et al., 2015, Kotob et al., 2018). The mechanism underlying for these adverse effects are related to the ability of NSAIDs to inhibit gastric prostaglandin synthesis and this inhibition contributes to increased acid production and decreased cytoprotective mucus formation, which are essential events in mucosal ulceration etiology. In a study reported that indomethacin has caused alterations in the gastric secretions of rats (Bech et al., 2000, Biplab et al., 2011, Muhammed et al., 2012). Indomethacin has also been reported to decrease the function of antioxidant enzymes and induce lipid peroxidation which is a significant event in NSAID's toxicity process resulting in stomach oxidative injury (Halici et al., 2005).
Gastric damage exerted by NSAIDs may be initiated on mitochondria. Indeed, NSAIDs can dissipate mitochondrial transmembrane potential with subsequent increase in membrane permeability and liberation of cytochrome C from intermembranous space to cytosol. The cytochrome C then releases reactive oxygen species (ROS), activating caspase-9 and caspase-3, and cellular lipid peroxidation, leading to cellular apoptosis (Nagano et al., 2005).
Shoen and Vender, 1989, Avila et al., 1996 explained the instant effect of piroxicam on the surface glandular mucus secreting cells as it diffuse across the mucosa and damage the gastric mucosal barrier by altering cell membrane permeability and physicochemical characteristics of the gastric mucus allowing back diffusion of hydrogen ion, while chief cell was observed to enclose apical zymogen granules, intact nucleus, degenerated mitochondria with degenerated cristea and microvilli on its apical surface, while parietal cell showed rounded intact nucleus and numerous mitochondria in its cytoplasm, this revealed that not all the mucosal layer is affected by sudden erosion reason not a long term causing factor as stress.
Our scanning electron microscope results of the ulcer model rats showed degeneration of the gastric mucosa in concordance with the study of Halter et al. (2001) which reported that the acute mucosal damage caused by aspirin in humans happened within 60 min and is visualised as severe intramucosal petechial haemorrhage and erosions.
Our histochemical results of ulcer model rats group showed negative or weak distribution of polysaccharides at the surface, isthmus, neck and base regions and this also supported by the morphometric analysis to the gastric ulcer slides, this results is consisted with Mahmoud and Abd El-Ghffar (2019), who investigated the ulcerative effect of aspirin on gastric mucosa histochemically and evidenced that aspirin as NSAIDs drug cause noticeable decrease in the mucus polysaccharide secretion at the mucosal lining of mice model.
In our present study the immunohistochemical results revealed intensive immunoreactivity toward cytokeratin 20 in ulcer model rats group, in concordance to Vera et al. (2006). Masato et al. (2005) reported that the cytokeratin 20 positivity reflects epithelial genomic damage and repairing processes through increasing inflammatory factors and oxidative stress promoting cytoskeleton damage and epithelial differentiation.
The immunohistochemical result showed intense immunoreactivity to Cox 2 in the ulcer-induced model group, this finding is consistent with Mahmoud and Abd El-Ghffar (2019) and can be attributed to the fact that prostaglandin (PG) inhibition is caused by NSAIDs. PGs are a major player in the protection of gastric mucosal integrity by local promoting blood flow and increasing synthesis and secretion of mucus and bicarbonate (Byron and Kenneth 2014), while the bicarbonate helps protection by decreasing the acidity of the gastric lumen, the mucus layer acts as a barrier to protect against the effect of pepsin and HCL (Nurhidayah et al., 2014).
Chan and Leung, 2002, Talaat et al., 2014 reported that Cox 2 is rapidly induced at sites of inflammation, Cox 2 has been localized to the surface epithelium, lamina propria and endothelium.
We have shown that the glandular area of ulcer model rat gastric mucosa displayed intense immunoreactivity to PCNA distributed among all gastric glandular cells. Throughout the investigation into the impact of stress on gastric mucosa, Wael et al. (2018) reported similar findings, which can be explained as PCNA is an significant marker of tissue proliferation, increased PCNA reactivity accompanied by increased cell proliferation, which is evidence of ulcer re-epithelialization (Polo et al., 2012). Gastric mucosa can be repaired after mucosal injury by mucosal restitution and re-epithelisation by sloughing off the damaged cells. This is achieved by rapid migration of viable stem cells to the injured site to replace the dead cells during ulcer healing (Coskun et al., 2004).
Our histological examination revealed that Pantoloc® treated rats showed degenerated surface mucous cells, pyknotic, karyolitic parietal cells and karyohexis nuclei among chief cells in the basal region, this result is consisted with Abid et al. (2012) who reported that omeprazole treated ulcer group showed less severe mucosal necrosis and hemorrhage than that occur in induced group. Also, Mahmood et al. (2017) who investigated the effect of rabeprazole for treatment of ulcer and they concluded that parietal cells regenerated and have purple cytoplasm as a result of decreasing secretion of HCL.
Our results of semi-thin section of the gastric mucosa of a Pantoloc® treated rats agreed with our ultrastructural results including improvement in the surface mucous cells, approximately normal neck mucous cells and chief cells appeared with zymogen granules at the apex, the ultrastructure results of gastric glands supported the semi thin section results chief cells with regular, rounded, intact nucleus, numerous apical zymogen granules and well developed rough endoplasmic reticulum, these results can be explained as PPIs administration allow the damaged tissue to heal and regenerate.
A number of evidence suggests that the effectiveness of PPIs depends on their ability to inhibit the secretion of gastric acid (Goldstein et al., 2006). Reducing acid secretion is thus the main function of the defensive activities carried out by PPIs and that acid-independent mechanisms can make a significant contribution to their therapeutic effects (Suzuki and Hibi, 2005).
The present scanning results of a Pantoloc® treated rats showed noticed improvement as mucus covers the surface mucosal linning and this can be explained as PPIs cause decrease in the gastric HCL and increase mucus secretion to allow the gastric tissue to heal and this is in consistent with Kotob et al. (2018) who reported that histochemical examination of stomach of rat treated with omeprazole showed an increase in the polysaccharides at the surface epithelium and mucous neck cells of the gastric mucosa. Our histochemical results supported the scanning results which showed moderate distribution of polysaccharides among isthmus region and weak reaction at surface cells.
Our immunohistochemical results of Pantoloc® treated rats showed moderate immunoreactivity to cytokeratin 20 and Cox 2. This can be illustrated as the cytokeratin 20 is upregulated in the site of inflammation and explained by the fact that Cox 2 expression increased as an infilammatory mediator to let re-epithelialisation and proliferation to occur.
The cells of the glandular region of the gastric mucosa of Pantoloc® treated rats showed moderate immunoreactivity to PCNA as there are still proliferations occurring in attempting to restore normal proliferation level. This was agreed with Shen-Yung et al. (2015) who stated that 3-day pantoprazole administration (15 μmol/kg/day) induced a further increase in PCNA expression, either alone or in combination with indomethacin. Moreover, Barkun et al. (2012) reported that Pantoprazole® and famotidine®, when administered with equivalent acid-inhibiting doses, can promote the healing of chronic gastric ulcers with the same efficacy, suggesting that the inhibition of acid secretion have a major role in the repair of ulcerative lesions.
The PPIs (e.g. omeprazole, pantoprazole, esomeprazole, and lansoprazole) are replaced benzimidazole drugs targeting the final common pathway of acid production. The PPIs are found to be more significantly effective than H2RAs in elevating gastric pH and preventing and healing of the acid‐related tissue injury (Maity et al., 2008).
Stem cell treated rats in our study restored the normal architecture of the gastric mucosa and the glandular cells; chief, parietal and surface mucous cells and these results is in agreement with El-Azab et al. (2018) where their histological examination of stem cell treated ulcerated gastric tissue showed restoration of surface epithelium of gastric mucosa with almost normal mucous cells and gastric pits.
In the current study semi-thin section of the gastric mucosa of stem cell treated group showed normal appearance and intact surface mucous cells with gastric pits, in addition to the ultrastructure examinations in our study revealed intact surface mucous cells with oval nucleus and intensive mucus granules at the apical part and these results reflect the complete restoration of the normal gastric mucosal integrity and this is explained by Sayed and Rashed (2016), they reported that MSCs can migrate in to damaged tissues and organs, differentiate into their corresponding cells.
The scanning electron microscope results of a stem cell treated rats showed a marked improvement as undulating pattern of surface epithelial lining with mucus patches and regular gastric pits more or less similar to normal organization of gastric mucosal surface lining, these results support our results of ultrastructural examination of complete restoration of MSCs to mucosal integrity. Our histochemical results of the stem cell treated group showed intensive polysaccharides distribution among glandular cells and these is consisted with results documented by El-Azab et al. (2018). Furthermore, Lin et al. (2015) stated that MSCs had a special effectiveness in treating gastric ulcer as a result of increasing growth factor secretion, which caused angiogenesis and maintained the vascular permeability.
Our immunohistochemical results of stem cell treated rats showed mild immunoreactivity to cytokeratin 20 similar to normal result, these is illustrated as the cytokeratin expression back to its normal level production after complete restoration of mucosal integrity. Also, there was a mild immunoreactivity to Cox 2 approximately similar to the control negative group and this came in agreement with Rashed et al. (2016) stated that one of the functions of MSCs is to act as guardians against excessive inflammatory responses. As MSCs create a negative feedback loop through its activation to secrete PGE2 whereby lipopolysaccharide, TNF-α, nitric oxide and other damage-associated molecular patterns (DAMPs) from injured tissues and macrophages initiate this activation.
There were mild to moderate immunoreactivity in neck region to PCNA approximately similar to the control negative group and this is as a result of the ability of MSCs to regenerate damaged tissue and normalize the proliferation process after re-epithelialisation, differentionand success in gastric ulcer healing and this came in agreement with Salem et al. (2015).
MSCs labeled with PKH26 fluorescent dye have been observed in the gastric tissues confirming that these cells are homed into the gastric tissue and this finding is confirmed by Rashed and Fayez, 2015, Rashed et al., 2016.
5. Conclusion
From the present results, it can be concluded that the NSAID drugs causing gastric ulcer play a role in the induction of many histological, ultrastructural, histochemical and immunohistochemical alterations in the stomach. Ulcer has harmful effects on the stomach, and stem cell therapy has a clear recurrence of ulcers. Therefore, it is recommended that stem cell therapy can be used as a curative remedy. Further studies are required in molecular levels to understand the mechanism of action during treatment.
conflict of interest
No conflict of interest.
Acknowledgements
This study was supported by Research Supporting Project (RSP-2020/23), King Saud University, Riyadh, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Ahmed S. Alazzouni, Email: drahmedalazzouni@gmail.com.
Mohamed A. Dkhil, Email: mdkhil@ksu.edu.sa.
References
- Abid S.A., Mohamed N.J., Haider A.A. Effect of liqurice and chamomile extracts in the management of gastric ulcer in rats. Med. J. Babylon. 2012;9(3):583–597. [Google Scholar]
- Al-Asmari A., Al-Shahranib H., Al-Masric N., Al-Faraidid A., El-Fakia I., Arshaduddina M. Vanillinabrogates ethanol induced gastric injury in rats via modulation of gastric secretion, oxidative stress and inflammation. Toxicol. Rep. 2015;3:105–113. doi: 10.1016/j.toxrep.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hussaini J.A.S. Protective effect of Punicagranatumpeel extract against gastric mucosal erosions induced by ethanol in experimental rabbit models. AL-Qadisiya J. Vet. Med. Sci. 2014;13(1):52–58. [Google Scholar]
- Avila J.R., Alarcon D.C., Martin M.J., Motilva V., Luque L., Delgado D., Esteban J., Herrerias J. Role of endogenous sulphydryls and neutrophil infilteration in the pathogenesis of gastric mucosal injury induced by piroxicam in rats. Inflamm. Res. 1996;45(2):83–88. doi: 10.1007/BF02265120. [DOI] [PubMed] [Google Scholar]
- Bancroft, J., Suvarna, K., Layton, C., 2018. Bancroft,sTheory and Practice of Histological Techniques, eighth ed.
- Barkun A.N., Bardou M., Pham C.Q., Martel M. Proton pump inhibitors vs. histamine 2 receptor antagonists for stress-related mucosal bleeding prophylaxis in critically ill patients: a meta-analysis. Amer. J. Gastroenterol. 2012;107:507–520. doi: 10.1038/ajg.2011.474. [DOI] [PubMed] [Google Scholar]
- Bech P.L., Xavier R., Lu N., Nanda N.N., Dinauer M., Podolsky D.K. Mechanisms of NSAID- induced gastrointestinal injury defined using mutant mice. Gastroenterology. 2000;119(3):699–705. doi: 10.1053/gast.2000.16497. [DOI] [PubMed] [Google Scholar]
- Biplab A., Sudhir K.Y., Kshama R., Sandip K.B., Subrata C. Black tea and the aflavins assist healing of indomethacin-induced gastric ulceration in mice by antioxidative action. Evid. Based Complemnt. Alternat. Med. 2011;11:11–22. doi: 10.1155/2011/546560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byron C., Kenneth W.M. Gastrointestinal ulcers; role of aspirin andclinical outcomes in pathobiology, diagnosis and treatment. J. Multidisciplnary Health. 2014;7:137–146. doi: 10.2147/JMDH.S54324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan F.K., Leung W.K. peptic ulcer disease“. Lancet. 2002;21(360):933–941. doi: 10.1016/s0140-6736(02)11030-0. [DOI] [PubMed] [Google Scholar]
- Coskun O., Kanter M., Armautcu F., Çetin K., Kaybolmaz B., Yazgan O. Protective effects of quercetin, a flavonoid antioxidant in absolute ethanol- induced acute gastric ulcer. Europ. J. General Med. 2004;1:37–42. [Google Scholar]
- Dawson, B., Trapp, R.G., 2001. Basic & Clinical Biostatistics, Lange Medical Book. McGraw-Hill, Medical Publishing Division. 3rd ed., pp. 161–218 (Ch. 7-9).
- El-Azab N.E., Mansy A.E., El-Mahalaway A.M., Sabry D. Comparative study of the therapeutic effect of pomegranate alone or in combination with bone marrow mesenchymal stem cells on experimentally induced gastric ulcer in adult male rats: a histological and immunohistochemical study. Egypt. J. Histol. 2018;41(2):150–166. [Google Scholar]
- Goldstein J.L., Miner P.B., Schlesinger P.K., Liu S., Silberg D.G. Intragastric acid control in non-steroidal anti-inflammatory drug users: comparison of esomeprazole, lansoprazole and pantoprazole. Aliment. Pharmacol. Therap. 2006;26:1189–1196. doi: 10.1111/j.1365-2036.2006.02867.x. [DOI] [PubMed] [Google Scholar]
- Halter F., Tarnawski A., Schmassmann A., Peskar A. Cyclooxygenase 2 implications on maintenance of gastric mucosal integrity and ulcer healing: controversial issues and perspectives. Gut. 2001;49:443–453. doi: 10.1136/gut.49.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halici M., Odabasoglu F., Suleyman H., Cakir A., Aslam A., Bayir Y. Effect of water extract of Usnealongissima on antioxidant enzyme activity and mucosal damage caused by indomethacin in rats. Phytomedicine. 2005;12:656–662. doi: 10.1016/j.phymed.2004.06.021. [DOI] [PubMed] [Google Scholar]
- Kotob S.E., Alaa S., Safaa M., Hanaa A. Quercetin and ellagic acid in gastric ulcer prevention: an integrated scheme of the potential mechanisms of action from in vivo study. Asian J. Pharmaceut. Clin. Res. 2018;11(1):381–389. [Google Scholar]
- Lin Z.L., Zheng G.W., Zhang L., Zheng J.T., Chen H. Effect of transplantation of BMMSCs on pathological change of gastric precancerous lesions of rats. Asian Pacif. J. Trop. Med. 2015;10:1–4. doi: 10.1016/j.apjtm.2015.11.006. [DOI] [PubMed] [Google Scholar]
- Mahmood H.B., Alitaqe H.N., Dawood G.A. Histological changes of gastric ulcer in rabbits treated with rapebrazole. J. Univ. Karbala. 2017;15(1):31–39. [Google Scholar]
- Mahmoud Y.I., Abd El-Ghffar E.A. Spirulina ameliorates aspirin induced gastric ulcer in albino mice by alleviating oxidative stress and inflammation. Biomed. Pharmacother. 2019;109:314–321. doi: 10.1016/j.biopha.2018.10.118. [DOI] [PubMed] [Google Scholar]
- Maity P., Bindu S., Choubey V., Alam A., Mitra K., Goyal M., Dey S., Guha M., Pal C., Bandyopadhyay U. Lansoprazole protects and heals gastric mucosa from NSAID-induced gastropathy by inhibiting mitochondrial as well as Fas-mediated death pathways with concurrent induction of mucosal cell renewal. J. Biol. Chem. 2008;283:14391–14401. doi: 10.1074/jbc.M800414200. [DOI] [PubMed] [Google Scholar]
- Masato K., Shingo T., Masahiko T., Hiroaki M., Hideki I., Masakazu Y., Tsutomu N., Takanobu I., Sunao K., Masatsugu H. Efficiency of bone marrow derived cells in regeneration of the stomach after induction of ethanol- induced ulcers in rats. J. Gastroenterol. 2005;40:591–599. doi: 10.1007/s00535-005-1593-0. [DOI] [PubMed] [Google Scholar]
- Muhammed V.K., Thamotharan G., Sengottuvelu S., Haja-Sherief S., Sivakumar T. Evaluation of antiulcer activity of Ficuspumila L. leaf extract in albino rats, Global. J. Res. Med. Plants Indigenous Med. 2012;8:340–351. [Google Scholar]
- Nagano Y., Matsui H., Muramatsu M., Shimokawa O., Shibahara T., Yanaka A., Nakahara A., Matsuzaki Y., Tanaka N., Nakamura Y. Rebamipide significantly inhibits indomethacin-induced mitochondrial damage, lipid peroxidation, and apoptosis in gastric epithelial RGM-1 cells. Dig. Dis. Sci. 2005;50:76–83. doi: 10.1007/s10620-005-2810-7. [DOI] [PubMed] [Google Scholar]
- Nurhidayah B.R., Pouya H., Shahram G., Salmah I., Saad T., Mahmood A.A. Gastroprotective effect of ethanolic extract of Curcumaxanthorrhiza leaf against ethanol-induced gastric mucosal lesions in Sprague-Dawley rats. BioMed Res. Int. 2014:1–10. doi: 10.1155/2014/416409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polo C.M., Moraes T.M., Pellizzon C.H., Marques M.O., Rocha L.R., Hiruma-Lima C.A. Gastric ulcers in middle-aged rats: the healing effect of essential oil from Citrus aurantium L (Rutaceae) Evid. Based Complem. Altern. Med. 2012:509451. doi: 10.1155/2012/509451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransburg C.J., Cheer S. Pantoprazole for the treatment of peptic ulcer bleeding and prevention of rebleeding. Clin. Med. Insights Gastroenterol. 2012;5:51–60. doi: 10.4137/CGast.S9893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashed L., Fayez S. Therapeutic potential of mesenchymal stem cells in the treatment of gastric ulcer. Adv. Environ. Biol. 2015;9(18):1–7. [Google Scholar]
- Rashed L., Gharib D.M., Hussein R.E., Tork O., Abusree A. Combined effect of bone marrow derived mesenchymal stem cells and nitric oxide inducer on injured gastric mucosa in a rat model. Tissue Cell. 2016;48(6):644–652. doi: 10.1016/j.tice.2016.09.006. [DOI] [PubMed] [Google Scholar]
- Sabiua S., Garubab T., Sunmonuc T., Ajania E., Sulymana A., Nuraina I., Balogunc A. Indomethacin-induced gastric ulceration in rats, protective roles of Spondiasmombin and Ficus exasperate. Toxicol. Rep. 2015;2:261–267. doi: 10.1016/j.toxrep.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachar H., Vaidya K., Laine L. Intermittent vs continuous proton pump inhibitor therapy for high risk bleeding ulcers: a systematic review and meta-analysis. JAMA Int. Med. 2014;174(11):1755–1762. doi: 10.1001/jamainternmed.2014.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai H., Fujii T., Takeguchi N. Proton-Potassium (H(+)/K(+)) ATPases: Properties and Roles in Health and Diseases. Metal ions life sci. 2016;16:459–483. doi: 10.1007/978-3-319-21756-7_13. [DOI] [PubMed] [Google Scholar]
- Salem M.Y., El-Azab N.E., Helal O.K., Metwaly H.G., Bayoumi H.E. Does selenium improve the stem cell therapeutic effect on isoproterenol-induced myocardial infarction in rats? A histological and immunohistochemical study. Egypt. J. Histol. 2015;38:679–691. [Google Scholar]
- Sayed W.M., Rashed L.A. Therapeutic role of bone marrow derived mesenchymal stem cells in cyclo-phosphamide induced cardiotoxicity in adult male albino rat: a morphological and immunohistochemical study. Egypt. J. Histol. 2016;39:281–293. [Google Scholar]
- Shen-Yung W., Horng-Yuan W., Tsang-En W., Hsueh-Hsiao W., Wen-Hsiung C., Cheng-Hsin C., Shee-Chan L., Hung-I Y., Shou-Chuan S. Delayed healing of gastric ulcer is associated with down regulation of connexin 32 in the gastric mucosa. Adv. Dig. Med. 2015;2:67–73. [Google Scholar]
- Shoen R.T., Vender R.J. Mechanisms of non steroidal anti-infilammatory drugs- induced gastric damage. Amer. J. Med. 1989;86:449–457. doi: 10.1016/0002-9343(89)90344-6. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Hibi T. Novel effects other than antisecretory action and off-label use of proton pump inhibitors. Expert Opin. Pharmacoth. 2005;6:59–67. doi: 10.1517/14656566.6.1.59. [DOI] [PubMed] [Google Scholar]
- Talaat R., Samaka R., El-Toumy S., Mohamed A.Z. Anti-angiogenic and anti-infilammatory activity of Punicagranatum peel on experimently induced gastric ulcer in rats. Res. J. Pharmaceut. Biol. Chem. Sci. 2014;5(5):42–56. [Google Scholar]
- Vera T., Aleksandra S., Neda D., Marjan M., Olivera M., Ivan N., Thomas W., Tomica M., Peter M. Expression of cytokeratins in Helicobacter pylori associated chronic gastritis of adult patients infected with cagA+ strains: an immunohistochemicalstudy. World J. Gastroenterol. 2006;12(12):1865–1873. doi: 10.3748/wjg.v12.i12.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wael M.E., Abdulaziz M.A., Basil T.A., Jumana A.T., Raghad M.T. Gastroprotective and antioxidant effects of fluvoxamine on stress-induced peptic ulcer in rats. J. Taibah Uni. Med. Sci. 2018;13(5):422–431. doi: 10.1016/j.jtumed.2018.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]