Abstract
Amylases take part with vital role in industries such as food, fermentation; starch processing, textile and paper etc. Increasing amylases demand, high nutrient expenditure and environmental pollution have forced to utilize agro-industrial residues as a low-cost feedstock for enzyme production. In present study, three soil samples were collected from agro-industrial waste dumping areas in District Faisalabad. Ten thermophilic bacterial isolates were separated at 55 °C on the basis of colonial morphology, three isolates (F6, F11, F17) showed prominent zone of clearance applying iodine test on starch agar plates. Bacterial isolate F-11 showed highest amylase activity with DNS method and molecularly identified through 16S RNA sequencing as Bacillus sp. with Accession number MH917294. Four unconventional food wastes (banana, lemon, mango and potato) pretreated with 0.8% sulphuric acid concentrations taking 1000 g/L weight released the highest sugars contents and phenolic components. Maximum amylase activity i.e. 29.23 mg/ml was achieved in mango waste at, 40 °C, with pH 6.0 and 0.17% nitrogenous source adding 8% inoculum size (2 days old) using Response Surface Methodology (RSM) for optimization. Crude amylase confirmed its efficiency in starch hydrolysis that suggested it as potential candidate for application in starch industries.
Keywords: Food wastes, Thermophilic, Bacillus sp., Thermostable amylases
1. Introduction
Enzymes are biocatalysts that lead a large number of biochemical processes and hence play a very vital role in life processes (Awan et al., 2018). Enzymes are obtained preferably from microbes rather than plants and animals because this method is advantageous economically as well as gives higher yields in short fermentation time (Chowdary and Prakash, 2018). Microbes from different sources e.g. fungi, bacteria and yeast have been used in order to obtain the best enzyme yield. Production cost of enzyme depends on fermentation cost of media. In order to produce economically safe bioactives, different food wastes are being used to get low cost enzymes (Rigo et al., 2010).
A huge amount of residues is generated yearly by different agricultural industries. These residues are source of environmental pollution and adversely affect the animal and human health if these wastes are directly thrown in the surroundings without proper disposal (Sadh et al., 2018). It is reported that most agro industrial wastes are directly disposed through dumping, burning and land filling without planning because these wastes are untreated and underutilized, so different harmful effects arise in the atmosphere such as addition of greenhouse gases in the environment (Rodríguez Couto, 2008). In actual the compositions of these wastes have high nutritional value but these residues are considered as agro-industrial by-products (Graminha et al., 2008). Sugar, protein, fibers and minerals are primary component of these wastes (Benabda et al., 2019). The integral components of agro-industrial wastes contain cellulose, hemicellulose and lignin on the whole being called “lignocellulosic materials” (Mussatto et al., 2012).
Enzyme market of world contains 25% of this industrially important class (Rajagopalan and Krishnan, 2008). Among these amylases are important in starch liquefaction to reduce viscosity, maltose production, high fructose syrup and maltotetrose syrup (John, 2017). Different sources such as animals, plant and microorganisms are used for amylases production but microbes are preferable source for industries (Aiyer, 2005, Awan et al., 2018, John, 2017, Mushtaq et al., 2017, Sodhi et al., 2005). Amylases are used in a number of important industries such as food, brewing, textile, paper, detergent and pharmaceutical (Chakraborty et al., 2009, Mojumdar and Deka, 2019, Sahnoun et al., 2015). Today thermophilic microbes are used in industrial processes due to the reason of low chances of contamination and decreased cost of cooling. Isolation of new effective strains of amylase producing microbes is necessary for the production of large quantity of α-amylases that have a large industrially importance (Singh et al., 2016).
The purpose of this study is to isolate efficient thermophilic amylolytic bacterium from local industrial habitat, increase its amylolytic activity by RSM and evaluate its potential by employing food wastes as low cost medium. Application of crude amylases showed great potential as an additive in detergent industry as well as saccharification of starch in other industries.
2. Materials and methods
2.1. Collection of sample and isolation of thermophilic amylolytic bacterium
Three soil samples were collected from agro-industrial wastes dumping areas in vicinity of Faisalabad, Pakistan in sterilized capped bottles. After collection, the samples were processed for isolation of thermophilic amylolytic bacteria. Different colonies were isolated at 55 °C and sub-cultured in order to get pure culture of thermophilic bacteria. Starch agar medium was used to separate amylolytic bacteria.
2.2. Screening of amylase producing microbes
Isolated colonies were taken from pure culture plate and were spread on starch agar plates containing 0.2 g starch, 2.0 g agar and 1.3 g nutrient broth. This method was followed according to Singh with little modifications. Incubation was done at 55 °C for 24–48 h. Every plate was bathed with Gram’s iodine to confirm starch hydrolysis. All the isolates were preserved in agar slants and glycerol stocks were also prepared.
2.3. Molecular identification and phylogenetic analysis of bacterial isolate
The select bacterial isolate was cultured in nutrient broth and processed for DNA extraction. PCR amplification using primer 907R 5′ (CCG TCA ATT CMT TTR AGT TT) 3′ and 1492R 5′ (TAC GGY TAC CTT GTT ACG ACT T) 3′ were carried out following gel electrophoresis and get sequenced from Macrogen Company Korea. All the procedure was done by following method (Jabeen and Qazi, 2014). The sequence was compared with available database bank on NCBI using BLAST tool. The sequence was carried to ClustalW program and aligned with closest relative. ClustalW program package was used to plot phylogenetic tree.
2.4. α-Amylase assay
By estimating total released reducing sugars, alpha amylase activity was estimated by following method of Bernfeld (1955). One unit of enzyme activity is defined as the amount of enzyme, which produces 1 μmole of reducing sugar per minute (U/ml/min).
2.5. Optimization for amylase yield of selected bacterium through RSM
Five independent variables (inoculum size. inoculum age, media concentration, yeast extract, pH) with five different levels (+2, +1, 0, −1, −2) were planned by using Central Composite Design of RSM leading to a total of 32 sets per experiment was formulated to optimize the process parameters. Different coded values showing minimum and maximum ranges of independent variable were determined.
2.6. Cultivation of selected amylolytic bacteria in agro-industrial wastes
2.6.1. Sample collection and processing
Four different types of food wastes banana, lemon, mango and potato peels were used as substrate. All food wastes were obtained from different areas of Faisalabad. After collection food wastes weret washed with simple tap water and then with distilled water until soil were removed from wastes peels. After washing, all these peels were dried in sunlight and then in oven at 70 °C until constant weight were achieved.
2.6.2. Pretreatment of wastes
All four wastes after processing were pretreated with different concentrations of H2SO4. For acid pretreatment different concentrations of biomass 500, 1000 and 1500 (g/L) were taken in 0.6%, 0.8% and 1% sulphuric acid.
2.6.3. Determination of total sugar and phenol content
For the determination of total sugar content of all biomass, phenol sulphuric acid method was used Dubois et al. (1956). For the determination of total phenolic components of pretreated biomass, the method was used according to Sanz et al. (2005).
2.7. Cultivation of bacterial strain on pretreated agro-industrial wastes through submerge fermentation
For cultivation of bacterial strain on agro-industrial wastes, 2.5 g of every pretreated waste powder was mixed in 250 ml dH2O, mixed well and left for 2 h at room temperature. After that filtered these wastes and the collected filtrate was divided into two flasks each containing 100 ml filtrate. In one flask added 0.6% NaCl and 0.17% yeast extract and in second flask no nutrients were added and again autoclaved. Now optimized inoculum size and age of selected bacterial strain was added in all flasks, optimized conditions were followed and optical density was taken at 540 nm by using spectrophotometer.
2.8. Application of crude amylase
In order to check the effect of crude amylase, medium containing starch 0.2%, NaCl 0.6% and yeast extract 0.17% were mixed in 100 ml distilled water. Optimized pH was set i.e. 6.0 and autoclaved at 121 °C. After that optimized inoculum of identified bacterial strain F-11 was added in this reaction mixture, incubation was done at optimized temperature i.e. 60 °C for 24 h and divided this mixture into two beakers. Starch solution was prepared by adding 0.05 g starch in 10 ml dH2O. Cotton cloth was used, spread 0.5 ml starch solution on it, dried in air, dipped this cloth having crude amylase enzyme of Bacillus sp. F11 in one beaker and left for 30 mins at 50 °C. After incubation DNS test was applied on the mixture containing starch stained cotton piece and also on mixture containing no starch stained cloth that acted as control. Glucose release was compared in both situations by taking optical density at 540 nm through spectrophotometer (Simair et al., 2017).
Starch was calculated by multiplying 0.9 with total glucose as described by (Saxena and Singh, 2011). Where glucose amount (g/ml) represented by A and B is total starch amount (g/ml).
3. Results
3.1. Isolation of thermophilic amylolytic bacteria
For isolation of thermophilic amylolytic bacteria three different soil samples were used from Faisalabad district near Government College University Faisalabad. Ten different isolates of thermophilic bacteria were isolated from soil samples in nutrient agar medium at 55 °C showed zone of clearance on starch agar plates but out of these ten bacterial isolates only three showed prominent starch hydrolysis zone.
3.2. Screening of the bacterial isolates
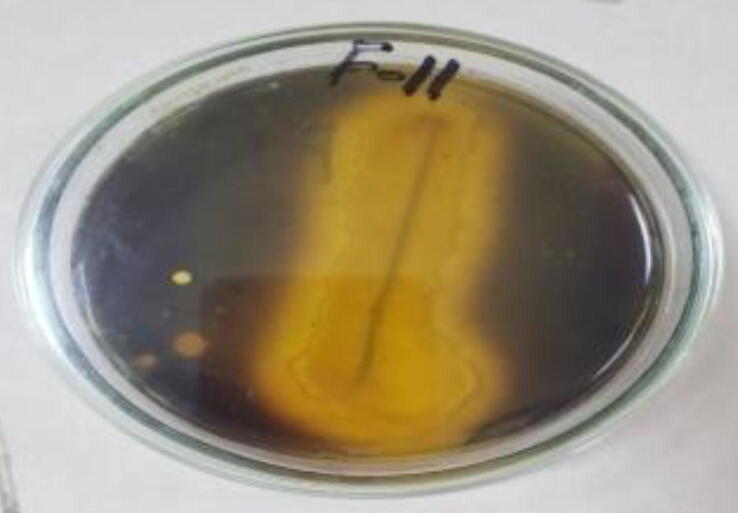
Main purpose of this study was to isolate, characterize and identify extra- cellular amylolytic bacteria from nearest localities. Further screening of bacteria was done on the base of amylolytic activity. Out of ten, three strains F-6, F-11 and F-17 were showed large zone in starch-iodine test and were selected for further studies. Out of these three, F-11 was showed large zone in Fig. 3.1.
Fig. 3.1.
Zone of starch clearance by Bacillus sp. F11.
3.3. Morphology of ten different colonies of bacterial isolates:
Morphology of bacterial isolates was observed and most isolates were off-white in color, but others were reddish brown. Most isolates showed opaque characteristic but few of them were translucent. Bacterial isolates were also different in other characters such as shape, edge, elevation etc.
3.4. Identification of isolate by 16 S RNA sequencing
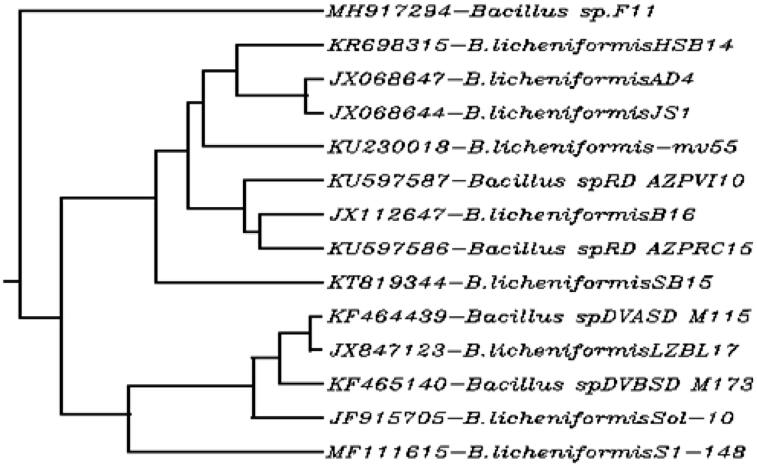
The most active producer of amylase was get sequenced and found to be the closest homolog of Bacillus sp. with accession no. MH917294. The total GC content was 52.77% with 99% similarity index. Sequencing based phylogenetic tree was constructed represented in Fig. 3.2.
Fig. 3.2.
Phylogenetic tree of Bacillus sp. F11.
3.5. Optimization of amylase production by Response Surface Methodology
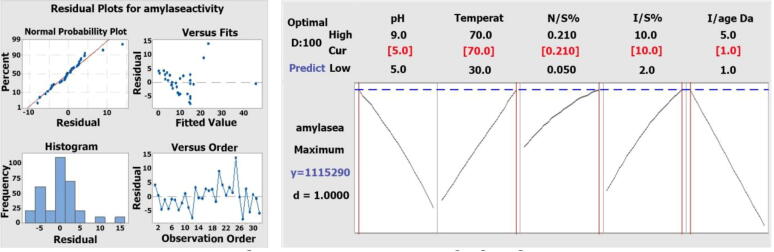
Central composite design runs with their experimental and predicted values for the production of high amylase activity. The relationship between five independent variables (pH, temperature, nitrogenous source, inocula size and inocula age) and total amylase activity is represented through polynomial equation, generated by applying Response Surface Methodology on application data. In this equation some terms are linear while others are quadratic. In this equation pH, temperature, N/S%, I/S%, I/age days, pH* I/age days, temperature*I/S% have positive values which showed that these terms either linear or quadratic were highly significant for amylase production. While pH*pH, N/S%*N/S%, I/S%*IS%, pH* temperature, pH*N/S%, pH*I/S%, temperature*I/age days, N/S%* I/age days and I/S%*I/age days have negative values that represented less significance for amylase activity. Bacillus licheniformis F-11 showed amylase activity ranged from minimum 3.59 mg/ml in run 13 (pH 6, temperature 40 °C, nitrogenous source 0.17%, inocula size 8% and inocula age 4 days) to maximum 45.31 in run 15 (pH 6, 40 °C, nitrogenous source 0.17%, inoculum size 8% and inoculum age 2 day) in all experimentally observed responses. Response optimization is shown in Fig. 3.3.
Fig. 3.3.
Optimization of amylase activity Bacillus sp. F11 by RSM.
3.6. Pretreatment of food wastes with H2SO4 acid
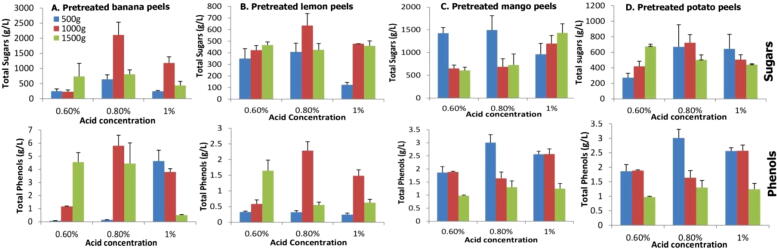
Pretreatment of wastes i.e. banana, potato, lemon and mango was performed with 0.6% 0.8% and 1% H2SO4 acid, by using 500, 1000 and 1500 (g/L) quantity of wastes. Results declared that 0.8% acid with 1000 g waste gave the highest sugars contents and phenolic components while these contents decreases either when raises the acid quantity or amount of wastes shown in Fig. 3.4.
Fig. 3.4.
Comparison of sugar and phenolic contents of pretreated wastes.
3.7. Cultivation of Bacillus sp. F11 on pretreated agro-industrial wastes through submerge fermentation
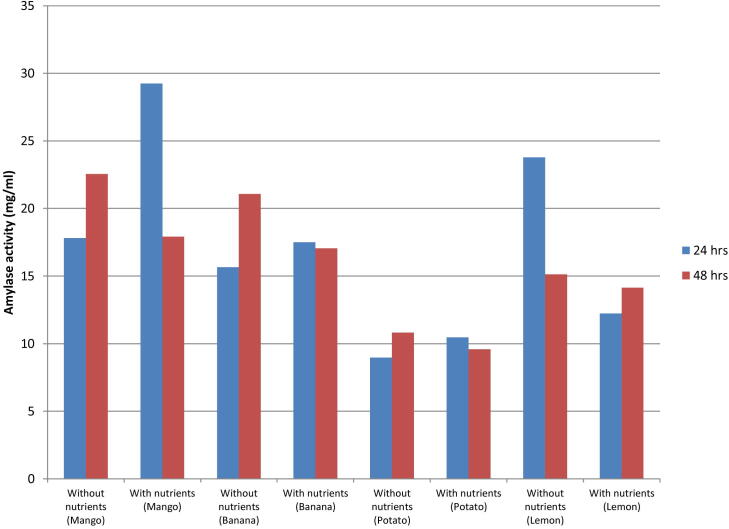
Bacillus sp. F11 was cultivated in pretreated banana, lemon, mango and potato peels waste under optimized conditions. After 24 h amylase production increases in medium having nutrients but trend was turned to opposite side while passing 48 h shown in Fig. 3.5. Among all wastes maximum amylase production was estimated in mango peels i.e. 29.23 mg/ml.
Fig. 3.5.
Cultivation of bacterial strain F-11 on pretreated potato waste.
3.8. Application of crude amylase
Bacillus licheniformis was cultivated in optimized conditions and growth was taken after 24 h at 60 °C. Starch stained cotton cloth soaked in amylase solution of Bacillus sp. F11 showed high amylase activity and represented 104.94% hydrolysis that showed these amylases can be used as an additive in detergent for starch hydrolysis.
4. Discussion
The use of food wastes as low cost substrate for production of enzymes is valuable for the agriculture countries like Pakistan producing abundant wastes. Keeping in this view the purpose of the present study was to isolate thermophilic amylolytic bacterium from industrial area and to utilize kitchen wastes for production of amylases. Employment of such wastes to produce valuable product resolve the environmental problems. Thermostable amylases alongwith the benefit of growth in optimized conditions through the method of RSM enhanced the quality of product.
In the present study a total of 10 bacterial isolates were isolated from soil samples. Three soil samples were collected from nearby areas of Government College University Faisalabad for the isolation of thermophilic amylolytic bacteria. Starch iodine test was considered best for selection of amylolytic bacteria. Many workers isolated amylolytic bacteria for production of amylases. Selected bacterial isolate through analysis of 16S rRNA showed that specific bacteria belonged to genus Bacillus. Most species of genus Bacillus are found in soil. Bacillus sp. is found everywhere because by forming endospore they are able to bear extreme conditions. Four species of thermophilic Bacillus licheniformis through 16S rRNA sequencing as active amylase producer were identified (Awan et al., 2018). In order to fulfill all industrial needs Bacillus species. are considered good producers of amylase (Krishnan et al., 2017, Tiwari et al., 2015).
Response surface methodology is statistical technique and efficient tool used for analysis of large number of variables. In order to optimize one factor only one variable is changed while all others remain constant. During fermentation process different parameters effect on one another and their combined effect show response i.e. formation of amylase (Almanaa et al., 2020). So, one factor optimization process cannot show precise amount of amylase (Mushtaq et al., 2017). Five different independent factors pH, temperature, yeast extract, inoculum size and inoculum age were used with different ranges for optimization of the selected bacterial isolate F-11 through RSM. In all 32 runs, Bacillus sp. F-11 showed maximum amylase activity 45.304 mg/ml at pH 6.0. For optimum amylase production, 6 pH was found suitable (Saxena and Singh, 2011). In one research, amylase yield was best in pH 5.5 in rice bran and 13U/mg was its specific activity (Suganthi et al., 2011). One experiment showed 5–9 working pH range for amylase activity with Bacillus licheniformis (Awan et al., 2018). By using Pseudomonas mendocina for amylase production, optimum amount (1.303 U) was produced at pH 7.0 (Padhiar and Kommu, 2016).
Temperature is significant parameter influenced amylase activity. In this study 60 °C was found to be highly optimized for high yield of amylase production (Finore et al., 2014). The same results were found by working on thermophilic Bacillus licheniformis (KA-2, KA-6 and KA-9) and showed that 55 °C was optimized temperature for high yield of amylase production i.e. 0.046 U/ml, 0.052 U/ml and 0.07 U/ml respectively (Awan et al., 2010). Maximum yield of α-amylase was 6.37 ± 0.67 at 50 °C by using Bacillus sp. (Singh et al., 2016). By using potato peels, groundnut husk, maize straw and corn chaff, it was found that maximum production was detected at 40 °C (Obi et al., 2019). For enzyme production another important factor for high yield includes inoculum size. In our study 8% was the most suitable for amylase production. A research was completed on Bacillus sp. for amylase production and found that inoculum size of 20% was the best for high yield (Saxena and Singh, 2011). Amylase produce by using wheat bran and found that inoculum size of 20% was paramount for high yield (Saxena and Singh, 2011) but in an experiment enzyme formation was badly affected by increasing inoculum size (Anto et al., 2006) Inoculum age was also important for high enzyme yield and in the present study inoculum of 48 h was appropriate for amylase production. Bacillus licheniformis strains KA-2, KA-5, KA-6 and KA-9 produced 0.043 mg/ml, 0.0428 mg/ml, 0.0483 mg/ml 0.025 U/ml amylase activities at 24 h. Nitrogen source play chief role in production of proteins so its quantity also affects the quantity of enzyme production. Yeast extract was the nitrogen source utilize for amylases production. In this study 0.17% yeast extract was the best for high amylase yield from F-11. Yeast extract was the most suitable nitrogen source for enzyme yield by using Bacillus licheniformis (Awan et al., 2018).
Several researchers worked on different agro-industrial wastes employing several microbes who exhibited their abilities to produce amylase (Muhammad et al., 2010). Four agro-industrial wastes banana, lemon, mango and potato were used for amylase production by using Bacillus sp. (F-11). Mushtaq et al. (2017) also worked on potato peels, washed and dried at 70 °C for amylase production by using Bacillus sp. Jadhav et al. (2013) also found potato peels the potential producer of amylases. Siddiqui et al. (2014) also reported amylase from Bacillus sp. AY3 growing on fruit peels. Kumar et al. (2013) investigated that amylase was produced by using mango kernel by Fusarium solani through submerge fermentation. In an research wheat bran and potato peels were found to be best substrate for high yield of amylase with a specific enzyme activity 1.2 U/ug and 1.1 U/ug recpectively (Mojumdar and Deka, 2019). Amylase was also produced from Bacillus subtilis D19 and shows highest amylase ativity 640 U/g in wheat bran (Almanaa et al., 2020).
Pre-treatment is a significant mean for breakdown of the structure of these agroindustrial wastes mainly composed of cellulose, hemicellulose and lignin. Cellulose is major component present in high quantity in agro-industrial residues. It is well known that lignified hemicellulose and cellulose are present in the cell wall that result in need to have an effective method to separate them from cell wall. Chemical pretreatment methods are the most effective way to breakdown the material in the agroindustrial wastes and expose the components for utilization. Sulphuric acid (H2SO4) was used for pretreatment of all four wastes by adding different concentration of acid such as 0.6%, 0.8% and 1% and determined total sugar and total phenol. In our study maximum concentration of total sugar and total phenol was found in 0.8% acid in all four wastes. In an research wheat straw was pretreated with dilute sulphuric acid and found that 4% dilute sulphuric acid gave rise to high production of product (Mirza et al., 2013).
In washing process, the use of α-amylase has been proved efficient in removing starch stain by estimating glucose released. Bacillus sp. BCC 01-50 produced crude α- amylase by the utilization of raw starch sources such as soluble starch, potato, wheat and corn starches whose effectiveness was examined by the amount of breakdown of various starch materials during a short period (Simair et al., 2017). These thermostable amylases can be used in various industries as in this study starch stained fabric released sugars showed hydrolysis of starch, produced by selected bacterial strain (Prakash and Jaiswal, 2011, Shukla and Singh, 2015).
5. Conclusion
From this study it was concluded that agro-industrial wastes cause serious pollution in our environment and by utilization of these wastes we can produce different enzymes like α-amylase which has large number of applications in starch industry. So, in this way we not only removed pollution from our surroundings but at the same time produce industrially important enzyme through low cost methods. In addition thermostable amylases showed its additional potential in working harsh industrial applications.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Faiza Jabeen, Email: acancerian@hotmail.com.
Samina Qamer, Email: saminabee@gmail.com.
References
- Aiyer P.V. Amylases and their applications. Afr. J. Biotechnol. 2005;4 [Google Scholar]
- Almanaa T.N., Vijayaraghavan P., Alharbi N.S., Kadaikunnan S., Khaled J.M., Alyahya S.A. Solid state fermentation of amylase production from Bacillus subtilis D19 using agro-residues. J. King Saud Univ.-Sci. 2020;32:1555–1561. [Google Scholar]
- Anto H., Trivedi U., Patel K. α-Amylase production by Bacillus cereus MTCC 1305 using solid-state fermentation. Food Technol. Biotechnol. 2006;44:241–245. [Google Scholar]
- Awan K., Jabeen F., Manzoor M., Qazi J.I. Potential of thermophilic amylolytic bacteria for growth in unconventional media: Potato peels. J. Food Process Eng. 2018;41:e12635. [Google Scholar]
- Awan M.S., Khan S.A., Rehman Z., Saleem A., Rana S., Rajoka M. Influence of nitrogen sources on production of βb-galactosidase by Aspergillus niger. Afr. J. Biotechnol. 2010;9 [Google Scholar]
- Benabda O., Mhir S., Kasmi M., Mnif W., Hamdi M. Optimization of protease and amylase production by Rhizopus oryzae cultivated on bread waste using solid-state fermentation. J. Chem. 2019;2019:1–9. doi: 10.1155/2019/3738181. [DOI] [Google Scholar]
- Bernfeld P. α-and β-amylases. Methods Enzymol. 1955;1:149–158. [Google Scholar]
- Chakraborty S., Khopade A., Kokare C., Mahadik K., Chopade B. Isolation and characterization of novel α-amylase from marine Streptomyces sp. D1. J. Mol. Catal. B Enzym. 2009;58:17–23. [Google Scholar]
- Chowdary A.R., Prakash P.O. Optimization of amylase production from bacillus cereus using solid state fermentation. J. Biotechnol. Res. 2018;4:58–65. [Google Scholar]
- Dubois M., Gilles K.A., Hamilton J.K., Rebers P.T., Smith F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956;28:350–356. [Google Scholar]
- Finore I., Di Donato P., Poli A., Kirdar B., Kasavi C., Toksoy E.O., Nicolaus B., Lama L. Use of agro waste biomass for α-amylase production by Anoxybacillus amylolyticus: purification and properties. J. Microb. Biochem. Technol. 2014;6:320–326. [Google Scholar]
- Graminha E., Gonçalves A., Pirota R., Balsalobre M., Da Silva R., Gomes E. Enzyme production by solid-state fermentation: Application to animal nutrition. Anim. Feed Sci. Technol. 2008;144:1–22. [Google Scholar]
- Jabeen, F., Qazi, J., 2014. Isolation of chitinase yielding Bacillus cereus JF68 from soil employing an edible crab shell chitin.
- Jadhav, S.A., Kataria, P.K., Bhise, K.K., Chougule, S.A., 2013. Amylase production from potato and banana peel waste 5.
- John J. Amylases-bioprocess and potential applications: A review. Int. J. Bioinforma. Biol. Sci. 2017;5:41. doi: 10.5958/2321-7111.2017.00006.3. [DOI] [Google Scholar]
- Krishnan M., Nagendran A., Moorthi V. Isolation and characterization of amylase producers and optimization of enzyme production. Int. J. Dev. Res. 2017;7:18128–18134. [Google Scholar]
- Kumar D., Yadav K.K., Muthukumar M., Garg N. Production and characterization of alpha-amylase from mango kernel by Fusarium solani NAIMCC-F-02956 using submerged fermentation. J. Environ. Biol. 2013;34:1053–1058. [PubMed] [Google Scholar]
- Mirza S.S., Qazi J.I., Zhao Q., Chen S. Photo-biohydrogen production potential of Rhodobacter capsulatus-PK from wheat straw. Biotechnol. Biofuels. 2013;6:144. doi: 10.1186/1754-6834-6-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mojumdar A., Deka J. Recycling agro-industrial waste to produce amylase and characterizing amylase–gold nanoparticle composite. Int. J. Recycl. Org. Waste Agric. 2019;8:263–269. doi: 10.1007/s40093-019-00298-4. [DOI] [Google Scholar]
- Muhammad I., Sajjad A., Shahjhan B., Muhammad G., Muhammad N., Quratualain S. Pretreatment: a potential technique to enhance the enzymatic hydrolysis. World J. Agric. Sci. 2010;6:440–445. [Google Scholar]
- Mushtaq Q., Irfan M., Tabssum F., Iqbal Qazi J. Potato peels: A potential food waste for amylase production. J. Food Process Eng. 2017;40:e12512. [Google Scholar]
- Mussatto S.I., Ballesteros L.F., Martins S.L.F., Teixeira J.A. InTech; 2012. Use of Agro-industrial Wastes in Solid-state Fermentation Processes. [Google Scholar]
- Obi C.N., Okezie O., Ezugwu A.N. Amylase production by solid state fermentation of agro-industrial wastes using bacillus species. Eur. J. Nutr. Food Saf. 2019;408–414 doi: 10.9734/ejnfs/2019/v9i430087. [DOI] [Google Scholar]
- Padhiar A., Kommu S. Isolation, characterization and optimization of bacteria producing amylase. Int. J. Adv. Res. Biol. Sci. 2016;3:1–7. [Google Scholar]
- Prakash O., Jaiswal N. Immobilization of a thermostable“-amylase on agarose and agar matrices and its application in starch stain removal. World Appl. Sci. J. 2011;13:572–577. [Google Scholar]
- Rajagopalan G., Krishnan C. α-Amylase production from catabolite derepressed Bacillus subtilis KCC103 utilizing sugarcane bagasse hydrolysate. Bioresour. Technol. 2008;99:3044–3050. doi: 10.1016/j.biortech.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Rigo E., Ninow J.L., Di Luccio M., Oliveira J.V., Polloni A.E., Remonatto D., Arbter F., Vardanega R., de Oliveira D., Treichel H. Lipase production by solid fermentation of soybean meal with different supplements. LWT-Food Sci. Technol. 2010;43:1132–1137. [Google Scholar]
- Rodríguez Couto S. Exploitation of biological wastes for the production of value-added products under solid-state fermentation conditions. Biotechnol. J. Healthc. Nutr. Technol. 2008;3:859–870. doi: 10.1002/biot.200800031. [DOI] [PubMed] [Google Scholar]
- Sadh P.K., Duhan S., Duhan J.S. Agro-industrial wastes and their utilization using solid state fermentation: a review. Bioresour. Bioprocess. 2018;5:1. doi: 10.1186/s40643-017-0187-z. [DOI] [Google Scholar]
- Sahnoun M., Kriaa M., Elgharbi F., Ayadi D.-Z., Bejar S., Kammoun R. Aspergillus oryzae S2 alpha-amylase production under solid state fermentation: optimization of culture conditions. Int. J. Biol. Macromol. 2015;75:73–80. doi: 10.1016/j.ijbiomac.2015.01.026. [DOI] [PubMed] [Google Scholar]
- Sanz V.C., Mena M.L., González-Cortés A., Yanez-Sedeno P., Pingarrón J. Development of a tyrosinase biosensor based on gold nanoparticles-modified glassy carbon electrodes: Application to the measurement of a bioelectrochemical polyphenols index in wines. Anal. Chim. Acta. 2005;528:1–8. [Google Scholar]
- Saxena R., Singh R. Amylase production by solid-state fermentation of agro-industrial wastes using Bacillus sp. Braz. J. Microbiol. 2011;42:1334–1342. doi: 10.1590/S1517-838220110004000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla R.J., Singh S.P. Characteristics and thermodynamics of α-amylase from thermophilic actinobacterium, Laceyella sacchari TSI-2. Process Biochem. 2015;50:2128–2136. [Google Scholar]
- Siddiqui A., Salahuddin T., Riaz A., Zohra R.R., Naheed S. Production of amylase from Bacillus sp. AY3 using fruit peels as substrate. FUUAST. J. Biol. 2014;4:213. [Google Scholar]
- Simair A.A., Qureshi A.S., Khushk I., Ali C.H., Lashari S., Bhutto M.A., Mangrio G.S., Lu C. Production and partial characterization of α-amylase enzyme from Bacillus sp. BCC 01–50 and potential applications. BioMed Res. Int. 2017 doi: 10.1155/2017/9173040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R.N., Bahuguna A., Chauhan P., Sharma V.K., Kaur S., Singh S.K., Khan A. Production, purification and characterization of thermostable α-amylase from soil isolate Bacillus sp. strain B-10. J. Biosci. Biotechnol. 2016;5 [Google Scholar]
- Sodhi H.K., Sharma K., Gupta J.K., Soni S.K. Production of a thermostable α-amylase from Bacillus sp. PS-7 by solid state fermentation and its synergistic use in the hydrolysis of malt starch for alcohol production. Process Biochem. 2005;40:525–534. [Google Scholar]
- Suganthi R., Benazir J.F., Santhi R., Ramesh Kumar V., Hari A., Meenakshi N., Nidhiya K.A., Kavitha G., Lakshmi R. Amylase production by Aspergillus niger under solid state fermentation using agroindustrial wastes. Int. J. Eng. Sci. Technol. 2011;3:1756–1763. [Google Scholar]
- Tiwari, S., Srivastava, R., Singh, C., Shukla, K., Singh, RK, Singh, P., Singh, R, Singh, N., Sharma, R., 2015. Amylases: An overview with special reference to alpha amylase 4, 16.