Abstract
Boophone disticha (B. disticha) is a bulbous tropical and subtropical flowering plant widespread in Africa, which is frequently used to treat several human ailments. Until the present, there is no scientific validation on the biological activity of this plant from the Eastern Cape Province of South Africa and as a result, this study aimed to assess the bioactive compounds, free radicals scavenging and anticancer potentials of crude bulb extracts (chloroform, acetone, and ethanol) of Boophone disticha obtained from this geographical location. Standard biochemical techniques and Gas-chromatography mass spectrometry (GCMS) analysis were used to pinpoint the bioactive compounds in the crude extracts sequel to their antioxidant potentials against radicals such as 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), hydrogen peroxide and nitric oxide as well as their ferric ion reducing power. In addition, their cytotoxicity effects against Human cervix adenocarcinoma (HeLa) cells were assessed as an in vitro model for anticancer. The phytochemical evaluation of the crude extracts showed the presence of phenolics, flavonoids, and alkaloids. GCMS profiles confirmed the presence of some bioactive compounds in the crude extracts of B. disticha that could be responsible for their biological activities. The plant extracts possessed considerable antioxidant activity and exhibited dose-dependent radicals’ inhibition from all assays carried out. Furthermore, the cytotoxicity effects against HeLa cells recorded inhibition concentration (IC50) of 1.5, 1.6, and 1.9 µg/mL for acetone, chloroform, and ethanolic extracts of B. disticha, respectively. Findings from the present study suggest that B. disticha could be a good prospective source of antioxidant and anticancer agents. Therefore, further research on the isolation and purification of compounds from these extracts are indispensable.
Keywords: Boophone disticha, Crude extract, Bioactive compounds, Antioxidant, Cytotoxicity
1. Introduction
The African continent is remarkably endowed with a diverse plant biodiversity members of which studies have shown that they possess bioactive compounds that possessed several biological activities such as antioxidant, anti-inflammatory, antiviral, antibacterial and anticancer (Vladimir-Knežević et al., 2014). These noteworthy attributes have enhanced their prospects as precursor substrates for new drug development (Anand et al., 2019, Yuan et al., 2016). Besides, African folkloric herbs have played a significant socio-economic role by satisfying health-care demands and providing job opportunities for the rural dwellers and traditional healers in the developing world, especially in Asian and African countries (Zofou et al., 2013). For many decades, folkloric medicine has been a common practice among the local population in the aforementioned regions for the treatment of all kinds of human infections and diseases (Ulac et al., 2019). In addition, natural products are less toxic as compared to synthetics, and this attribute makes them more superior and promising (Ertaş et al., 2014).
In Hogsback, a village in the vicinity of the Amathole Mountain in the Eastern Cape Province, South Africa, B. disticha (specifically the bulb part) is extensively utilized by rural dwellers as herbal mixtures. In this geographical location, B. disticha is commonly used to treat a wide range of health conditions such as headaches, chest pains, abdominal pain, and cancer. It is also used to sedate and calm psychotic patients as well as to treat mental illnesses (Neergaard et al., 2009). B. disticha is a bulbous plant that belongs to the family of Amaryllidaceae known to possess an exclusive group of alkaloids termed Amaryllidaceae alkaloids that are isolated from plants of all genera of this family (Nair and Van Staden, 2014). The bulb part of the plant is covered in thick dry scales and is found underground, whereas the flower protrudes above the ground. The Amaryllidaceae is a monocotyledonous family that is widely distributed across the globe and consisted of 59 genera with over 850 species (Cedrón et al., 2010). Countries such as South America and South Africa are reported to be regions with major diversity that contain 28 genera and 19 genera of this family, respectively (Cedrón et al., 2010). There are over 500 alkaloids that have been discovered in Amaryllid species across the globe with 193 of which are found in South Africa and thus, representing 39% of the global complement of structures that have been isolated from 88 species comprising 14 genera and three tribes (Bastida et al., 2006, Nair et al., 2013, Viladomat et al., 1997). As a result, the alkaloid compounds from B. disticha have attracted a huge interest in phytomedicine research due to the biological properties (Nair and Van Staden, 2014).
Phytochemicals of plant origin are natural compounds that are non-nutritious but are essential for normal human physiological processes (Rezaeian et al., 2015). These compounds are found in plants, food, or food their products and they serve as natural antioxidants (Koleva et al., 2002). Natural antioxidants are safe and they can protect the cells’ macromolecules such as proteins, nucleic acids, and lipids from the oxidative damage caused by free radicals from various biochemical reactions that occur in the body (Kampa and Castana 2008). A reaction of reactive oxygen species (ROS) with macromolecules induces apoptosis that could consequently lead to several physiological, neurological, and cardiovascular disorders (Floyd and Hensley 2002). For normal cellular processes, there must be a redox balance between the human’s antioxidant defense mechanism and ROS that are produced during cellular processes in the body. For example, continual stress conditions increase energy consumption from high metabolism processes, which consequently favours ROS generation in the body. This process consequently produces an imbalance or a shift in equilibrium between intracellular antioxidant and ROS, which results in oxidative stress, and perhaps causes oxidative damage to cells, tissues, and organs (Kannan and Jain 2000). Different kinds of phytochemicals such as flavonoids, steroids, phenolic acids, tannins, ascorbic acid, tocopherols, glycosides, and saponins have been reported to exhibit antioxidant activities thus increasing prospects for their usage in the treatment of human chronic diseases such as diabetes, atherosclerosis, neurodegenerative diseases, cancer, hepatitis, etc. (Granda and De Pascual-Teresa, 2018, Innocenti et al., 2005).
Considering the folkloric significance of B. disticha around the Hogback community in the Eastern Cape Province of South Africa, it is essential to scientifically validate the biological activities of B. disticha with in vitro studies. As a result, this present study aimed to investigate the bioactive compounds, antioxidant and antiproliferative activities of bulb solvent extracts (chloroform, acetone, and ethanol) of B. disticha.
2. Materials and methods
2.1. Plant collection and identification
Ethical clearance certificate (reference number MAB021STON01) was obtained from the University of Fort Hare ethic community prior the plant collection which was carried out around Hogsback community in Raymond Mhlaba Local Municipality in the Eastern Cape Province, South Africa at a geographical coordinate (S-32°38′78′′, E-26°55′38′′). The plant was transported to the laboratory at the Department of Biochemistry and Microbiology, University of Fort Hare. The expertise of Dr. Mayekiso, a botanist, was implored for plant identification and authentication, and the plants were deposited in the University of Fort Hare Botany Department Herbarium with a voucher specimen number ST2018060.
2.2. Preparation of plant extracts
The bulb of B. disticha was separated, washed severally with tap water followed by rinsing with double-distilled water and air-dried for two weeks. Thereafter, the dried samples were pulverized into powder with an electric blender. One hundred grams (100 g) of plant parts were each extracted in separate flasks containing 500 mL of organic solvents (acetone, chloroform, and ethanol); the mixtures were incubated in a shaker for 24 h at 25 °C, 200 rpm. The plant extracts were filtered using Whatman No. 1 filter paper and thereafter concentrated using IKA rotary evaporator and subsequently dried at room temperature to get the crude extracts, which were stored at 4 °C for use.
2.3. Screening of bioactive compounds in the crude extracts
2.3.1. Total phenolics content
The total phenolic content was estimated using the Folin–Ciocalteu protocol as highlighted by Otang et al. (2012) with some minor modifications where gallic acid (0–30 mg/mL) was used as a standard. Subsequently, 100 μL of the sample was mixed with 100 μL of 10% (v/v) Folin–Ciocalteu reagent and 100 μL of 2% (w/v) sodium carbonate. The mixture was stirred vigorously using a vortex and incubated for 60 min at room temperature, after which absorbance was read at 750 nm using an Ultrospec Visible Plate Reader II 96. The concentration of each sample was calculated from the standard curve as gallic acid equivalent per milligram (GAE) of dried plant material.
2.3.2. Total flavonoids content
Total flavonoid screening was evaluated by using the method of aluminium chloride colorimetric described by Adedapo et al. (2008). An aliquot amount of 0.5 mL of each sample was added to 0.5 mL of 2% (w/v) AlCl3 ethanolic solution and mixed gently. The mixtures were left at room temperature for 90 min and the absorbance was read at 440 nm using a microplate reader against a blank. The presence of flavonoids was confirmed by the appearance of a yellow colour. Quercetin was used as a standard. The flavonoid content was established in milligrams equivalents of quercetin per milligram of each fraction using the standard curve.
2.3.3. Qualitative determination of alkaloids
The presence of alkaloids was assessed using the method adapted from Yadav and Agarwal (2011). The crude extract was mixed with 2 mL of 1% (v/v) HCl and heated gently in a water bath. Mayer’s reagent was prepared by adding 1.36 g mercuric chloride and 5 g potassium iodide in 100 mL distilled water while Wagner’s reagent was prepared by dissolving 2 g of iodine and 6 g of potassium iodide in 100 mL of distilled water. The appropriate volumes of Mayer’s and Wagner’s reagents were then added to the mixture simultaneously. The formation of turbidity precipitate confirmed the existence of alkaloids.
2.4. GCMS analysis
The bioactive compounds in the crudes extracts were identified with GCMS analysis as described by Tonisi et al. (2020) and MassHunter software for use for the data analysis.
2.5. Evaluation of free radical scavenging potentials
The antiradical scavenging potentials of the crude extracts were evaluated against the radicals of DPPH (Brand-Williams et al., 1995), ABTS (Adedapo et al., 2008); H2O2 (Oyedemi et al., 2010), nitric oxide (Garrat 1964) as well as ferric ion reducing ability by Oyaizu (1986) following standard procedures established in the literature.
2.6. Cytotoxicity effect against HeLa cells
The cytotoxicity potential of the crude extracts against HeLa cells was carried out following the biological standard procedures highlighted by Okaiyeto et al. (2019).
2.7. Statistical analysis
Data generated from phytochemical and antioxidant studies were expressed as mean values ± standard deviation of three replicates and significant levels were tested at P < .05. For the cytotoxicity assays, GraphPad Prism was used for non-linear regression analysis to estimate the IC50 values as described by Okaiyeto et al. (2020).
3. Results and discussion
3.1. Screening of bioactive compounds in the crude extracts
3.1.1. Phenolics and flavonoid contents
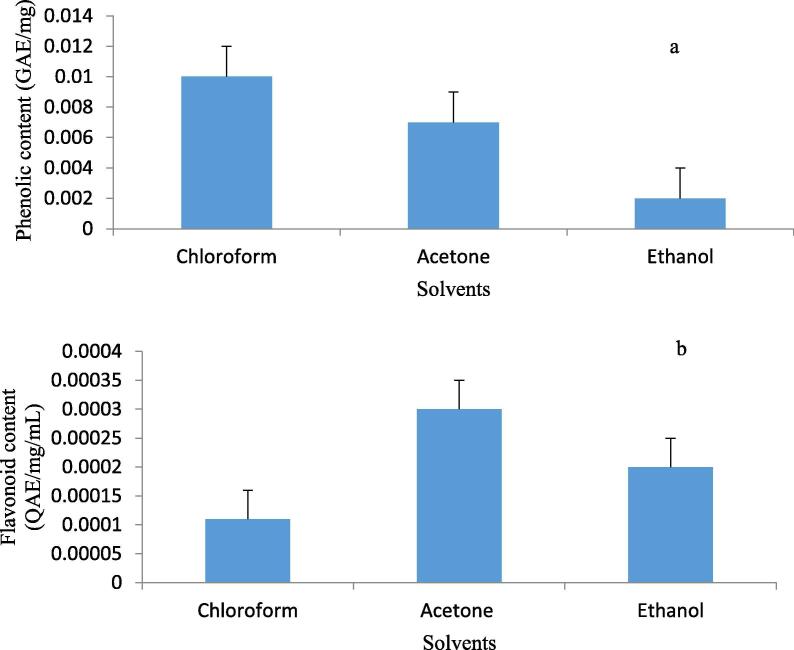
Bioactive ingredients such as tannins, phenolics, glycosides, flavonoids, and alkaloids present in plants have received a great interest and consideration in recent times due to their several health benefits in the treatment of human ailments (Złotek et al., 2016). In this study, solvent extracts of B. disticha have proven to be a source of bioactive compounds of interest. The highest phenolic content was recorded for chloroform leaf extracts with an estimated value of 0.01 mg/g gallic acid equivalent (GAE), followed by acetone and the least being ethanol extracts with 0.007 and 0.002 mg/g GAE, respectively (Fig. 1(a)). On the other hand, acetone proved to be a better solvent in extracting flavonoids compared to ethanol and chloroform with the following respective values 0.0003, 0.0002, and 0.00011 mg/g QE, respectively (Fig. 1(b)). The findings of Moyo et al. (2010) highlighted that the antioxidant activity of plants could be attributed to the redox function of phenolic compounds that enhance their reducing power radical scavenging capacities. Saxena et al. (2012) reported that phenolic acids and flavonoids are the most significant classes of bioactive compounds in plants and they serve as natural antioxidants in human diets. Thus, the presence of such compounds in B. disticha enhances the value and urgency of natural phytochemicals in modern medicine. Most studies on the phytochemistry of B. disticha have focused on analyzing alkaloids; therefore, this finding can provide new insight on the phytochemical information of B. disticha. As phenolics and flavonoids are known for a variety of biological functions and health benefits in humans, these results seem to support the traditional use of these plants for the benefit of humans.
Fig. 1.
Phenolic (a) and flavonoid (b) content of B. disticha bulb in the crude extracts. The content was calculated using the formula.
3.1.2. Qualitative determination of alkaloids
In this study, the appearance of yellow and brown turbidity in all the crude extracts qualitatively confirmed the presence of alkaloids, and our results agree with the report of Tonisi et al. (2020). In the same vein, the findings of Sasikala and Sundaraganapathy (2017) also corroborate with our study findings where alkaloids were observed to be part of the constituents of hydroalcoholic extract of Ipomoea aquatic by the appearance of a yellow and reddish precipitate. Thus, the findings of the present study as well as those gleaned from the literature support the view that B. disticha is comprised mostly of alkaloids.
3.2. GCMS analysis
GCMS was used to identify phytochemical compounds present in the extracts of B. disticha bulb. Based on the literature search, some compounds found in the studied extracts have been reported to display antioxidant, anti-inflammatory, antibacterial and antimicrobial activities (De Freitas et al., 2017). For example, apocynin, n-hexadecanoic acid, trifluoroacetoxy hexadecane, hexadecane, 9-nonadecene, 2.4-di-tert-butylphenol, cyclohexadecane and ethyl-cyclodocosane were some of the compounds identified by GCMS (Table 1). These compounds might be responsible for the antioxidant potential of B. disticha bulb extracts observed in this study. Anti-inflammatory as well as anti-cancerous compounds identified included, 2-methoxyl-4-vinylphenol, phytol, 9,12-octadecadienoic acid (z,z), octadecanoic acid, octadecanoic acid, heptadecyl triflouroacetate and alloaromadendrene. Major compounds were revealed to be 3,6-Dipropyl-1,2-dihydro-1,2,4,5-tetrazine (40.32%), from acetone extract, apocynin (21.8% and 15.53%), from acetone and ethanol extracts, respectively.
Table 1.
Compounds found in B. disticha bulb solvent extracts. UI = Unidentified, Un = Unknown, a = Component/compound, b = Chemical formula, c = Retention time, d = Percentage composition, e = Quality assurance of GC/MS library.
|
|
||||||
|---|---|---|---|---|---|---|
| B. disticha Acetone | B. disticha Ethanol | B. disticha Chloroform | ||||
| UI | 4.561 | 0.35 | – | – | 35 | |
| UI | 4.921 | 19 | – | – | 11 | |
| UI | 5.185 | 0.57 | – | – | 35 | |
| 2-Dodecene | C12H24 | 21.147 | – | – | 0.05 | 96 |
| 2-Methoxyl-4-vinylphenol | C9H10O2 | 26.176 | – | 0.45 | – | 95 |
| 1-Tetradecene | C14H28 | 29.212 | – | 0.16 | 2.15 | 98 |
| Ethanone, 1-(4-hydroxy-3,5-dimethoxyphenyl)- | C10H12O4 | 31.705 | 0.21 | 0.36 | – | 49 |
| Apocynin (Powerful antioxidant) | C9H10O3 | 33.054 | 21.80 | 15.53 | 1.08 | 97 |
| 2,4-Di-tert-butylphenol | C14H22O | 34.066 | – | 1.62 | 7.58 | 97 |
| 2-Tetradecene | C14H28 | 37.126 | – | 0.58 | 3.35 | 99 |
| Cyclohexadecane | C16H32 | 37.126 | – | 0.58 | 3.35 | 94 |
| 4-octyldodecyl cyclopentane | C25H50 | 37.461 | – | – | 0.2 | 60 |
| UI | 37.756 | 0.21 | – | – | 46 | |
| 6-methyl-5-(1-methylethyl) 5-hepten-3-yn-2-ol | C11H18O | 41.144 | – | – | 0.01 | 55 |
| Pyrimidine, 4,5-diamino-6-methoxy-meta-methoxybenzenethiol | Un | 42.859 | 0.43 | – | – | 64 |
| 7-Methyl-4-azafluorene | C13H11N | 43.126 | – | – | 0.03 | 50 |
| UI | 43.202 | – | – | 0.01 | 47 | |
| 1-Octadecene | CH3(CH2)15CH = CH2 | 44.337 | – | 0.79 | 3.97 | 99 |
| Heptadecyl heptafluorobutyrate | C21H35F7O2 | 44.604 | – | – | 0.18 | 51 |
| Isophthalic acid, dodecyl methyl ester | C21H32O4 | 44.740 | – | – | 0.01 | 60 |
| UI | 44.796 | – | – | 0.01 | 47 | |
| 6,10,14-Trimethyl-pentadecan-2-ol | C18H38O | 46.116 | 0.10 | – | – | 89 |
| 4-(Methylthio)phenyl isothiocyanat | C8H7NS2 | 46.224 | – | – | 0.17 | 50 |
| 1,2-Dimethoxy-4-(1,2,3-trimethoxyp ropyl)benzene | Un | 46.260 | – | – | 0.15 | 53 |
| UI | 46.589 | – | – | 0.17 | 49 | |
| 7-Bromo-3a,6,6-trimethyl-hexahydro -benzofuran-2(3H)-one | Un | 46.658 | – | – | 0.1 | 55 |
| UI | 46.701 | – | – | 0.08 | 47 | |
| UI | 46.761 | – | – | 0.15 | 47 | |
| Furane-3-carboxylic acid, 5-tert-b utyl-2-(4-tert-butylphenoxymethyl) | Un | 46.923 | – | – | 0.09 | 50 |
| 7-Bromo-3a,6,6-trimethyl-hexahydro -benzofuran-2(3H)-one | Un | 46.957 | – | – | 0.08 | 55 |
| Furane-3-carboxylic acid, 5-tert-b utyl-2-(4-tert-butylphenoxymethyl) | Un | 47.244 | – | – | 0.25 | 50 |
| Formaldehyde, O-[(pentafluoropheny l)methyl]oxime | C8H4F5NO | 47.329 | – | – | 0.09 | 50 |
| UI | 47.833 | – | 1.69 | – | 47 | |
| 3-Fluoro-p-anisidine | FC6H3(OCH3)NH2 | 47.836 | 1.16 | – | – | 50 |
| Furane-3-carboxylic acid, 5-tert-b utyl-2-(4-tert-butylphenoxymethyl) | Un | 48.063 | – | – | 0.29 | 50 |
| UI | 48.100 | – | – | 0.57 | 49 | |
| UI | 48.481 | – | – | 1.04 | 50 | |
| UI | 48.624 | – | – | 0.13 | 49 | |
| Desmedipham | C16H16N2O4 | 48.650 | – | – | 0.17 | 50 |
| UI | 49.001 | 0.15 | – | – | 30 | |
| UI | 49.753 | – | 12.21 | – | 46 | |
| 3,6-Dipropyl-1,2-dihydro-1,2,4,5-t etrazine | Un | 49.784 | – | – | 10.66 | 50 |
| 3,6-Dipropyl-1,2-dihydro-1,2,4,5-tetrazine | Un | 49.884 | 40.32 | – | – | 53 |
| n-Hexadecanoic acid | C16H32O2 | 50.079 | 3.13 | 0.56 | – | 99 |
| UI | 50.259 | – | – | 0.18 | 43 | |
| Trifluoroacetoxy hexadecane | C18H33F3O2 | 50.892 | – | 1.03 | – | 94 |
| 1-Nonadecene | C19H3 | 50.917 | – | – | 4.30 | 94 |
| UI | 51.937 | – | – | 0.03 | 35 | |
| UI | 52.257 | – | – | 0.06 | 38 | |
| UI | 52.332 | – | – | 0.03 | 42 | |
| UI | 52.836 | 0.63 | – | – | 45 | |
| UI | 53.691 | 0.84 | – | – | 45 | |
| Hexadecane | C16H34 | 54.176 | – | – | 0.10 | 70 |
| Phytol | C20H40O | 54.533 | 0.15 | – | – | 99 |
| 9,12-Octadecadienoic acid (z,z) | C18H32O2 | 55.227 | 2.32 | – | 0.27 | 99 |
| Z,E-2,13-octadecadien-1-ol | C18H34O | 55.238 | – | 0.28 | – | 90 |
| (z)-18-Octadec-9-enolide | C18H32O2 | 55.246 | 0.46 | – | – | 99 |
| UI | 55.396 | – | 0.51 | – | 46 | |
| (1aR,4s,4aR,7R,7aS,7bS)-1,1,4,7-Tetramethyldecahydro-1H-cyclopropa[e]azulen-4-ol | C15H26O | 55.681 | – | – | 0.07 | 90 |
| Z,z-10,12-Hexadecadien-1-ol acetate | C18H32O2 | 55.711 | – | – | 0.08 | 80 |
| 2-(3-Hydroxylbutyl) cyclooctanone | C12H22O2 | 55.742 | – | – | 0.05 | 64 |
| Octadecanoic acid | C17H35CO2H | 56.142 | 0.61 | – | – | 96 |
| 2-hydroxyl-cyclopentadecanone | C15H28O2 | 56.769 | – | – | 0.24 | 90 |
| 2-Dodecen-1-yl(-)succinic anhydrid | C16H26O3 | 56.782 | – | – | 0.11 | 58 |
| 9-Nonadecene | C19H38 | 56.903 | – | 0.67 | – | 91 |
| Heptadecyl triflouroacetate | C19H35F3O2 | 56.937 | – | – | 3.35 | 94 |
| 4-Octyldodecyl cyclopentane | C25H50 | 57.083 | – | – | 0.68 | 93 |
| Benzaldehyde, 4-[1-[4-(acetyloxy)- 3,5-dimethoxyphenyl]ethoxy]-3-methoxy | Un | 57.870 | – | – | 1.56 | 62 |
| Dodecyl methyl ester Isophthalic acid | C21H32O4 | 57.902 | – | – | 2.09 | 68 |
| 4-(4-Methylaminobenzylideneamino)benzonitrile | Un | 58.050 | 0.34 | – | – | 53 |
| UI | 58.977 | – | – | 1.61 | 43 | |
| UI | 59.542 | – | – | 12.08 | 49 | |
| UI | 59.897 | – | 1.18 | – | 44 | |
| Dodecyl phthalate | C20H30O4 | 60.209 | – | 2.21 | – | 95 |
| Undulatine | C18H21NO5 | 61.021 | – | 2.38 | – | 99 |
| UI | 61.059 | – | – | 0.08 | 35 | |
| UI | 61.086 | – | – | 0.03 | 35 | |
| UI | 61.119 | – | – | 0.03 | 45 | |
| 1,2-Benzenedicarboxylic acid, bis(8-methylnonyl) ester | Un | 61.253 | – | 1.8 | – | 98 |
| UI | 61.261 | 2.26 | – | – | 38 | |
| 2,2 dimethyl-6-methylene cyclohexanepropanol | C12H22O | 61.387 | – | – | 1.10 | 59 |
| UI | 61.631 | – | – | 0.33 | 35 | |
| UI | 61.786 | – | – | 1.64 | 41 | |
| 1,2-Benzenedicarboxylic acid Bis (8-methylnonyl) ester | Un | 61.776 | – | 2.33 | – | 98 |
| UI | 61.810 | 0.35 | – | – | 42 | |
| UI | 62.138 | – | – | 6.16 | 53 | |
| Ethyl-cyclodocosane | C24H48 | 62.426 | – | 0.82 | – | 95 |
| Cyclotetracosane | C24H48 | 62.444 | – | – | 2.25 | 96 |
| Epibuphanisine | C17H19NO3 | 62.571 | 2.31 | 6.16 | 5.92 | 99 |
| 2,3,5,6-Tetramethyl-para-phenylenediamine | (CH3)4C6(NH2)2 | 62.896 | 0.87 | – | – | 58 |
| UI | 63.269 | 0.67 | – | – | 47 | |
| Silane, diethylisohexyloxy(1-naphthydoxy)- | Un | 63.298 | – | – | 0.65 | 64 |
| 1,2-Benzenedicarboxylic acid, bis(8-methylnonyl) ester | Un | 63.583 | – | 1.14 | – | 98 |
| UI | 63.913 | – | – | 2.61 | 25 | |
| UI | 63.966 | – | – | 0.63 | 43 | |
| UI | 64.006 | – | – | 2.29 | 15 | |
| UI | 64.314 | 0.37 | – | – | 46 | |
| Bis (8-methylnonyl) ester 1,2-Benzenedicarboxylic acid | Un | 65.046 | 0.64 | 1.06 | – | 98 |
| Eicosane | C20H42 | 65.189 | – | – | 0.27 | 89 |
| Benzenemethanol, 4-(1,1-dimethylethyl)- | C11H16O | 65.287 | 0.19 | – | – | 58 |
| UI | 65.350 | – | – | 0.22 | 47 | |
| Buphandrin | C18H21NO4 | 65.554 | −3.27 | 1.06 | −2.05 | 99 |
| Guaia-9–11-diene | C15H24 | 65.606 | – | – | 0.22 | 55 |
| 1,3-bis([(2Z)-3,7-Dimethylocta-2,6-dien-1-yl]oxy)-1,1,3,3-tetramethyldisiloxane | C24H46O3Si2 | 65.689 | – | – | 0.18 | 90 |
| Alloaromadendrene | C15H24 | 65.730 | – | – | 0.09 | 59 |
| Alpha-Cadinol | C15H26O | 65.845 | – | – | 0.47 | 53 |
| UI | 65.930 | 0.4 | – | – | 43 | |
| 1,2-Benzenedicarboxylic acid, bis(8-methylnonyl) ester | Un | 66.615 | 0.17 | 1.06 | – | 98 |
| Buphandrin | C18H21NO4 | 67.561 | −3.27 | 1.06 | −2.05 | 99 |
| %Total content | 94.3 | 59.28 | 84.02 | |||
(–) denote no detection.
Apocynin has been reported as a powerful antioxidant that can inhibit the formation of hydrogen peroxide, hydroxyl ion, and peroxynitrite (Heumüller et al., 2008, Muijsers et al., 2000). Since the use of B. disticha bulb is associated with the treatment of neurodegenerative related diseases, acetylcholinesterase (AChE) inhibitor compounds were identified to be undulatine and epibuphanisine. The inhibition of AChE is the major trait targeted by researchers since the enzyme largely contributes to the progression of Alzheimer’s disease (AD). However, studies carried out by van Rijn et al. (2010), showed undulatine to have AChE inhibitory activity 10-folds lower than that of galanthamine. It was further discovered that all B. disticha bulb extracts contained epibuphanisine that is an alkaloid suspected to contain AChE inhibitory activity. A large number of compounds have unknown biological activity, as such, they need further investigation. Nonetheless, the compounds revealed in this study support the notion that B. disticha contains neuroprotective properties. Hence, these findings necessitate further studies on the AChE inhibitory potential of B. disticha.
3.3. Evaluation of antioxidant potentials of crude extracts of B. Disticha
3.3.1. DPPH radical scavenging activity
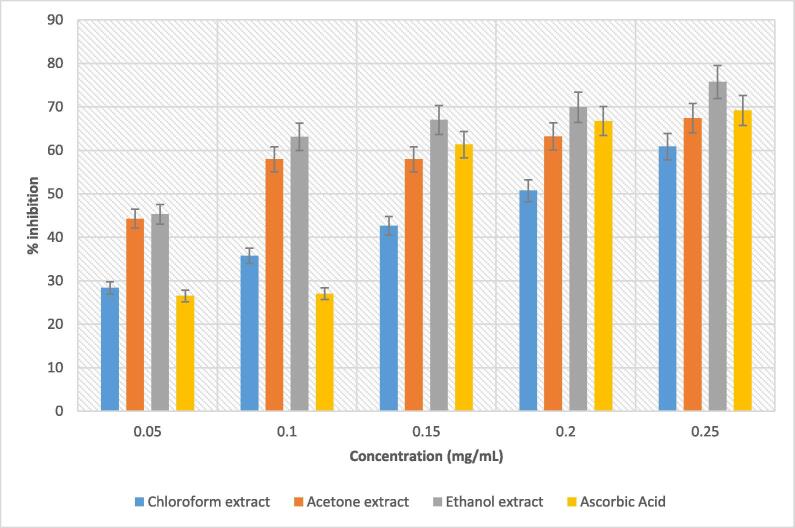
The DPPH scavenging potential of B. disticha extracts was evaluated and the results are represented in Fig. 2. the ethanolic extract exhibited the highest DPPH radical scavenging of 75.47% at 0.25 mg/mL followed by acetone and chloroform extracts with inhibition of 67.412% and 60.872%, respectively. The inhibitory effect of ethanolic extract surpassed even that of the standard (ascorbic acid), which is a clear indication of its high scavenging activity. This finding also implies that the ethanol was effective in extracting bioactive compounds with antioxidant properties against the DPPH radical. In a study conducted by Adewusi et al. (2012), they found that out the ethyl acetate and methanolic extracts of B. disticha bulb and root studied, the methanolic extract of the bulb did not show any scavenging activity whereas methanolic extract of the root demonstrated good scavenging potential against DPPH radical. However, results from this present study demonstrate a different perception that the B. disticha bulb plant part does possess a scavenging activity against DPPH radical. The dose-dependent increase in DPPH radical scavenging activity is directly proportional to the antioxidant capacity of a plant extract. A slightly similar findings were documented by Aremu et al. (2011) on the DPPH scavenging activity of leaf and stem petroleum ether (PE), dichloromethane (DCM) and 50% aqueous methanol (MeOH) extracts of Leucosidea sericea (L. sericea), a plant that is used for the treatment of neurological disorders in South Africa. When a plant possesses an efficient radical scavenging activity of DPPH, there is a high possibility that it may also contain antiradical activity against highly reactive molecules implicated in the pathology of various diseases.
Fig. 2.
DPPH radical scavenging capability of solvent bulb extracts of B. disticha.
3.3.2. ABTS radical scavenging activity
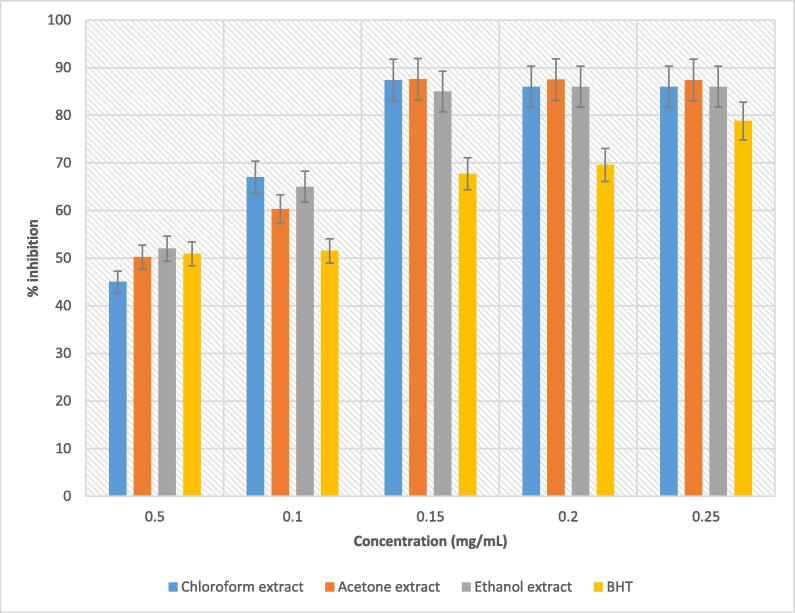
Similar to DPPH, ABTS assay is one of the methods commercially used to assess the antioxidant potential of an extract but the difference is that ABTS assay can be conducted under varying experimental conditions. ABTS assay is specifically used for establishing the presence of hydrogen donating antioxidants (Lee et al., 2011). In the present study, all B. disticha bulb extracts demonstrated effective scavenging activity against ABTS radical with values ranging between 86 and 87%, and acetone extract showing marginally higher inhibition within the concentration range from 0.15, 0.2, and 0.25 mg/mL (Fig. 3). Based on this observation, it can be deduced that 0.25 mg/mL is the inhibitory concentration limit for acetone extract. On the other hand, ethanol had a maximum inhibition of 86% at 0.25 mg/mL, with a similar trend being observed for chloroform extract. However, values among the three extracts varied from 0.21, 0.59, and 0.57 mg/mL for ethanol, chloroform and acetone extracts, respectively. Moreover, common among all extracts were significantly lower values compared to that of standard (BHT) which was 1.15 mg/mL. Nevertheless, these findings indicate the potential of B. disticha bulb as an effective antioxidant source. Similarly, ethanol extract of rosemary tea was reported to exhibit high antioxidant potential against ABTS radical (Oh et al., 2012).
Fig. 3.
ABTS radical scavenging activity of solvent extracts of B. disticha bulb compared with a standard (BHT).
3.3.3. Hydrogen peroxide scavenging activity
ROS have been recognized for their dual functions that include destructive and constructive species. ROS are involved in several redox-related activities in the cells for the maintenance of cellular homeostasis (Bardaweel et al., 2018). The mitochondrial hydrogen peroxide, for example, is reported to be released during physiological hypoxia (Sena and Chandel 2012). For the cells to survive during this process, hydrogen peroxide will activate a transcription factor called hypoxia-inducible factor 1 (HIF-1) that will initiate metabolic adaptation of cells under low oxygen (Chandel et al., 1998, Sena and Chandel, 2012). However, as much as hydrogen peroxide has physiological functions, it is also regarded as one of the reactive radicals that can cause serious damage to the cells when its amounts are beyond the normal physiological range. Hydrogen peroxide is also thought to be a source of hydroxyl ion when it encounters metals such as iron through the Fenton reaction (Bolisetty and Jaines, 2013). Therefore, hydrogen peroxide abundance leads to oxidative stress and an effective antioxidant activity against hydrogen peroxide from plant extracts can simultaneously lead to less production of hydroxyl ions. In the present study, all plant extracts of B. disticha bulb demonstrated a varying degree of scavenging potential against hydrogen peroxide radical.
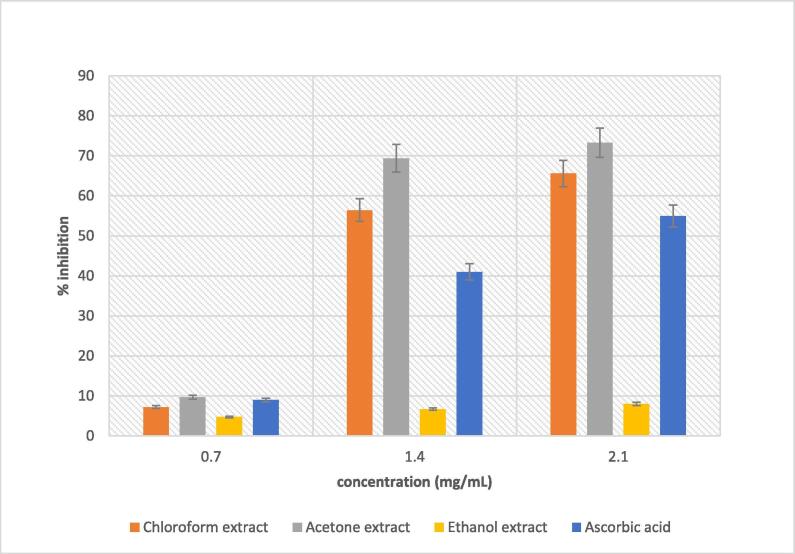
Results illustrated in Fig. 4 show that acetone extract had the highest scavenging activity compared to other extracts, throughout the concentration range tested. The maximum scavenging activity of 73.2% was recorded at 2.1 mg/mL, followed by chloroform extract with scavenging activity of 65.6%. With the lowest inhibiting rate of less than 10%, ethanol extract did not display any substantial scavenging activity against hydrogen peroxide radical (Fig. 4). All the extracts, as well as standard (ascorbic acid), demonstrated dose-dependent inhibition of hydrogen peroxide. A similar trend was observed in a study conducted by Kumaran and Karunakaran (2007) when assessing the antioxidant activity of methanol extracts from Phyllanthus species. However, hydrogen peroxide is not always considered as a highly reactive molecule, but due to its high potential of giving rise to hydroxyl ion, it can be very toxic to cells. Therefore, the noteworthy scavenging activity displayed by all extracts understudy against hydrogen peroxide indicates their potential effectiveness in limiting the chances of hydroxyl ion formation.
Fig. 4.
Hydrogen peroxide scavenging activity of solvent extracts of B. disticha.
3.3.4. Nitric oxide radical scavenging activity
Besides reactive oxygen species, there are also nitrogen species that can induce oxidative stress in implicated in various human diseases (Förstermann, 2010). Nitric oxide reacts with oxygen or superoxide to form reactive nitrogen species (RNS) such as nitrogen dioxide, dinitrogen tetroxide, dinitroazanide, nitrate, nitrite, and peroxynitrite, which are very unstable and highly reactive molecules. The presence of these molecules in the mitochondria can significantly alter normal cellular processes such as mitochondrial biogenesis, respiration and oxidative stress (Bolisetty and Jaines, 2013).
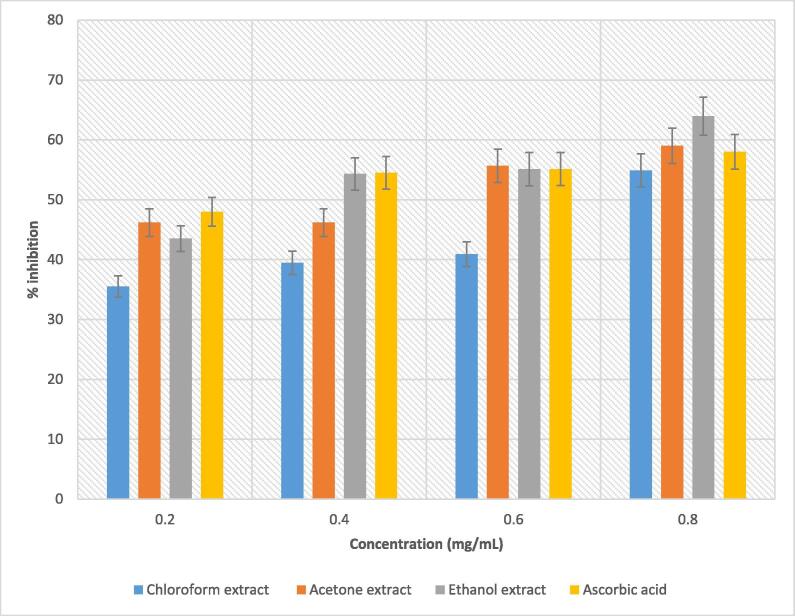
In the present study, all the studied extracts of B. disticha bulb revealed notable radical scavenging activity with a dose-dependent pattern (Fig. 5). Ethanolic bulb extract had the highest inhibiting activity at the concentration of 0.8 mg/mL, followed by acetone extract at 59% and chloroform extract at 54.09%. The effective nitric oxide inhibition displayed by plant extracts used in the present study indicates B. disticha may be of considerable interest in preventing health problems caused by excessive production of nitric oxide. Wang et al. (2016) stated that acute inflammation is one of the pathological features found in the brain of Alzheimer's disease (AD) and during this process, large amounts of nitric oxide are thought to be the causative agents of such neuropathology. Nitric oxide has been reported to be implicated in inflammation, cancer, and other pathological conditions (Choudhari et al., 2013). From these findings, B. disticha emerges as a source of bioactive compounds with potential as an effective nitric oxide radical scavenging agent.
Fig. 5.
Scavenging activity of nitric oxide radical by extracts of B. disticha bulb.
3.3.5. Ferric ion reducing antioxidant power
Although iron is considered vital for cellular health since it is actively involved in the transportation of oxygen, however, its levels above the normal range are considered dangerous since they can lead to the generation of radicals, which can largely contribute to cellular injury (Emerit et al., 2004). The ferric reducing assay was aimed to assess the ability of bioactive compounds to reduce ferric ion (III) to ferrous ion (II) by simply donating electrons rather than hydrogens (Gülçin, 2015).
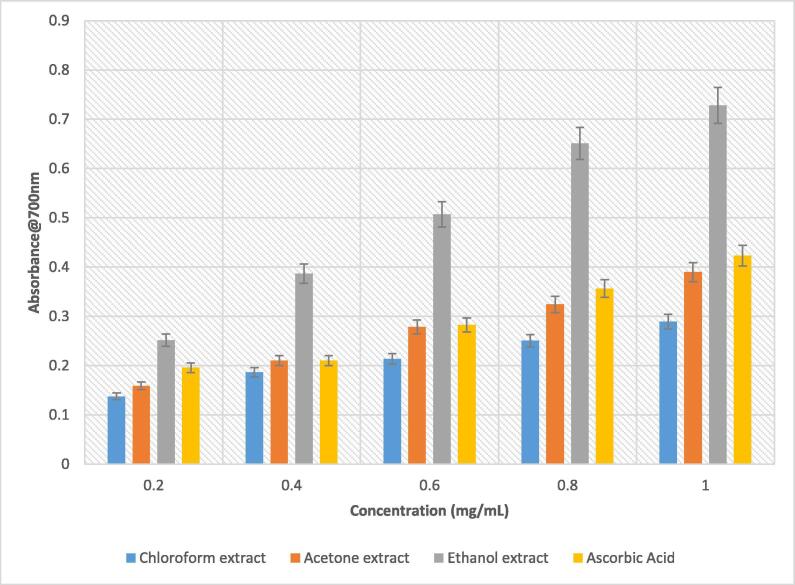
In this present study, B. disticha bulb extracts have been shown to possess excellent reducing power. As depicted in Fig. 6, the ethanolic extract exhibited high reducing power compared to the other extracts and ascorbic acid across the concentration range with lower reducing power attributed to acetone and chloroform extracts, respectively. These results can be attributed to the ability of the tested extracts to donate electrons, which then suggests that they can act as free radical scavengers (Lee et al., 2011). This is yet another observation in support of the ability of ethanolic solvent in extracting compounds with antioxidant properties. Furthermore, the reducing ability of these plant extracts demonstrated in this study is an indication of the potential medicinal value this plant holds, in terms of antioxidant power, and this makes it a good reference source for future research in drug development. The report of Masoko and Masiphephethu (2019) buttressed our findings in which the organic solvents of Schkuhria pinnata showed high ferric reducing power.
Fig. 6.
Reducing power of solvent extracts B. disticha bulb.
3.4. Cytotoxicity assessment
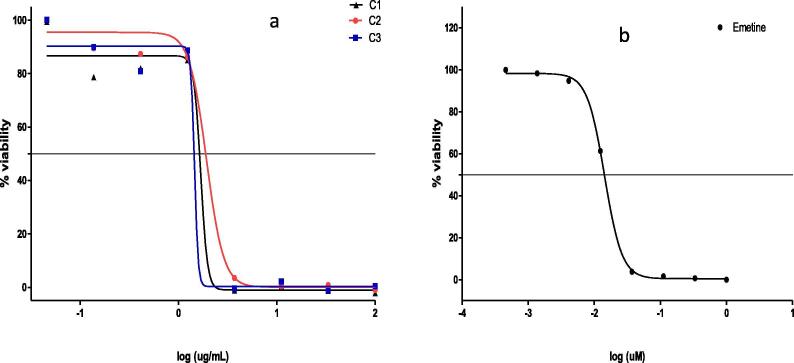
The Alamar blue assay is one of the most reliable techniques to determine the cytotoxicity effect of an extract on cells. This method uses resazurin, which is a non-toxic cell-permeable compound, to measure the number of viable cells (Rampersad 2012). Viable cells will reduce resazurin to resofurin; a compound that appears red-pink colour and emits strong fluorescence when exposed to ultraviolet (UV) light. In the current study, the cytotoxicity effects of B. disticha extracts were assessed against HeLa cancer cells. Although there have been published literature reports supporting the traditional usage of this plant (Nair and Van Staden, 2014), however, results depicted in Fig. 7a shows that the plant extracts was not extremely toxic effect on HeLa cells.
Fig. 7.
Cytotoxic effects of solvent extracts of B. disticha against HeLa cell measured in percentages (%). The experiment was.
As shown in Fig. 7a, the extract concentration is inversely proportional to the cell viability. At a very low concentration of 0.04 µg/mL, all extracts showed negligible or no effect at all on the viability of the cell population. Between the concentration ranges of 0.13 µg/mL to 1.23 µg/mL, it was observed that cell viability remained within the range of 79% and 90%. After log 0.091 µg/mL, a sharp decline in cell viability was recorded at 3.7 µg/mL, where after, there is almost no survival of cells. The calculated IC50 values were 1.6 µg/mL, 1.9 µg/mL and 1.5 µg/mL for C1 (B. disticha chloroform extract), C2 (B. disticha acetone extract) and (C3: B. disticha ethanolic extract), respectively. When comparing the cytotoxic effect of the studied extracts with the standard (emetine), it was found that B. disticha extracts demonstrated a less cytotoxic effect against HeLa cells than emetine (Fig. 7b). The IC50 values displayed by the crude extracts show that the plant could be used as a lead substrate in the development of antiproliferative agent against HeLa cancerous cells.
The use of HeLa cell lines has an added advantage of discovering an anticancer property of a plant, as such; it was observed in this present study that B. disticha extracts exerted effective cytotoxicity effect at concentration low as 1.23 µg/mL, little or no HeLa cells viability. As aforementioned, B. disticha belongs to the Amaryllidaceae family that is known to comprise a variety of alkaloid compounds that are toxic (Van Wyk and Gericke 2000). Besides, natural products are well recognized as sources of cytotoxic molecules (Gullett et al., 2010). Therefore, cytotoxicity results observed in this study could be attributed to the presence of alkaloid compounds. Our findings followed a similar trend to the report of Adewusi et al. (2012) on the cytotoxicity effect of a crinine alkaloid from B. disticha methanol extract on human neuroblastoma (SH-SY5Y). The GCMS analysis of the crude extracts showed the presence of alkaloid compounds such as buphanine, buphandrin and crinamidine that are known to have a cytotoxic effect against cancerous cells such as human leukaemic MoLT-4 cells (Zvetkova et al., 2001). Not only from B. disticha, but also from amaryllidaceae alkaloids isolated from Crinum augustum and Crinum bulbispermum have been reported to exhibit cytotoxic activity on cancerous cells (Zvetkova et al., 2001). Based on the present study and literature review undertaken, B. disticha has been shown to contain alkaloid compounds that possess and exert toxic effects on cancerous cells. As a result, further studies on cytotoxicity effects of studied extracts of B. disticha against other cancerous cells would be necessary to further validate their anticancer property.
4. Conclusion
The present study has shown that solvent extracts of B. disticha bulb could be prospective sources of antioxidants for the treatment of human diseases induced by oxidative stress as well as an anticancer agent against cervical cancer. The antioxidant and antiproliferative activities demonstrated by these crude extracts could be due to the presence of phenolic, flavonoid, and alkaloid. Thus, it is, therefore, necessary to carry out further study on the isolation of pure compounds from the most potent extract. In addition, further biological activities such as neuroprotective and cytotoxicity effects of the pure compounds from the plant extracts is currently a subject of active research in our laboratory.
Declaration of Competing Interest
The authors declare that they have no competing interests.
Acknowledgments
We are grateful to the National Research Foundation (NRF) of South Africa (student bursary grant number: 106821), the South African Medical Research Council (grant number: UFH/P790); and University of Fort Hare for their financial support. All authors would like to appreciate the effort of Dr Mayekiso for the plant collection and identification.
Footnotes
Peer review under responsibility of King Saud University.
References
- Adedapo A.A., Jimoh F.O., Afolayan A.J., Masika P.J. Antioxidant activities and phenolic contents of the methanol extracts of the stems of Acokanthera oppositifolia and Adenia gummifera. BMC Complement. Altern. Med. 2008;8:54. doi: 10.1186/1472-6882-8-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adewusi E.A., Fouche G., Steenkamp V. Cytotoxicity and acetylcholinesterase inhibitory activity of an isolated crinine alkaloid from Boophane disticha (Amaryllidaceae) J. Ethnopharmacol. 2012;143:572–578. doi: 10.1016/j.jep.2012.07.011. [DOI] [PubMed] [Google Scholar]
- Anand U., Jacobo-Herrera N., Altemimi A., Lakhssassi N. A Comprehensive review on medicinal plants as antimicrobial therapeutics: Potential avenues of biocompatible drug discovery. Metabolites. 2019;9:258. doi: 10.3390/metabo9110258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aremu A.O., Amoo S.O., Ndhlala A.R., Finnie J.F., Van Staden J. Antioxidant activity, acetylcholinesterase inhibition, iridoid content and mutagenic evaluation of Leucosidea sericea. Food Chem. Toxicol. 2011;49:1122–1128. doi: 10.1016/j.fct.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Bardaweel S.K., Gul M., Alzweiri M., Ishaqat A., AlSalamat H.A., Bashatwah R.M. Reactive oxygen species: The dual role in physiological and pathological conditions of the human body. Eurasian J. Med. 2018;50(3):193–201. doi: 10.5152/eurasianjmed.2018.17397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastida J., Lavilla R., Viladomat F. Chemical and biological aspects of Narcissus alkaloids. The Alkaloids. Chem. Biol. 2006;63:87–179. doi: 10.1016/S1099-4831(06)63003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolisetty S., Jaimes E.A. Mitochondria and reactive oxygen species: physiology and pathophysiology. Int. J. Mol. Sci. 2013;14:6306–6344. doi: 10.3390/ijms14036306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand-Williams W., Cuvelier M.E., Berset C.L.W.T. Use of a free radical method to evaluate antioxidant activity. LWT - Food Sci. Technol. 1995;28:25–30. [Google Scholar]
- Cedrón J.C., Del Arco-Aguilar M., Estévez-Braun A., Ravelo Á.G. Chemistry and biology of Pancratium alkaloids. Alkaloids Chem. Biol. 2010;68:1–37. doi: 10.1016/s1099-4831(10)06801-x. [DOI] [PubMed] [Google Scholar]
- Chandel N.S., Maltepe E., Goldwasser E., Mathieu C.E., Simon M.C., Schumacker P.T. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. PNAS. 1998;95:11715–11720. doi: 10.1073/pnas.95.20.11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhari S.K., Chaudhary M., Bagde S., Gadbail A.R., Joshi V. Nitric oxide and cancer: a review. World J. Surg. Oncol. 2013;11:118. doi: 10.1186/1477-7819-11-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Freitas M.C.D., de Miranda M.B., de Oliveira D.T., Vieira-Filho S.A., Caligiorne R.B., de Figueiredo S.M. Biological activities of red propolis: A review. Recent Pat. Endocr. Metab. Immun. Drug Discov. 2017;11:3–12. doi: 10.2174/1872214812666180223120316. [DOI] [PubMed] [Google Scholar]
- Emerit J., Edeas M., Bricaire F. Neurodegenerative diseases and oxidative stress. Biomed. Pharmacother. 2004;58:39–46. doi: 10.1016/j.biopha.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Ertaş A., Boğa M., Haşimi N., Yeşil Y., Gören A.C., Topçu G., Kolak U. Antioxidant, anticholinesterase, and antimicrobial activities and fatty acid constituents of Achillea cappadocica Hausskn. et Bornm. Turk. J. Chem. 2014;38:592–599. [Google Scholar]
- Floyd R.A., Hensley K. Oxidative stress in brain aging: implications for therapeutics of neurodegenerative diseases. Neurobiol Aging. 2002;23(5):795–807. doi: 10.1016/s0197-4580(02)00019-2. [DOI] [PubMed] [Google Scholar]
- Förstermann U. Nitric oxide and oxidative stress in vascular disease. Pflug Arch. Eur. J. Phy. 2010;459:923–939. doi: 10.1007/s00424-010-0808-2. [DOI] [PubMed] [Google Scholar]
- Garrat, D.C., 1964. The Quantitative Analysis of Drugs Japan. 3rd ed., 456-458.
- Granda H., De Pascual-Teresa S. Interaction of polyphenols with other food components as a means for their neurological health benefits. J. Agric. Food Chem. 2018;66(31):8224–8230. doi: 10.1021/acs.jafc.8b02839. [DOI] [PubMed] [Google Scholar]
- Gülçin İ. Fe3+–Fe2+ transformation method: an important antioxidant assay. Adv. Protocols Oxidat. Stress III. 2015;1208:233–246. doi: 10.1007/978-1-4939-1441-8_17. [DOI] [PubMed] [Google Scholar]
- Gullett N.P., Ruhul M., Amin A.R., Bayraktar S., Pezzuto J.M., Shin D.M., Khuri F.R., Aggarwal B.B., Surh Y.J., Kucuk O. Cancer prevention with natural compounds”. Semin Oncol. 2010;37(3):258–281. doi: 10.1053/j.seminoncol.2010.06.014. [DOI] [PubMed] [Google Scholar]
- Heumüller S., Wind S., Barbosa-Sicard E., Schmidt H.H., Busse R., Schröder K., Brandes R.P. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension. 2008;51:211–217. doi: 10.1161/HYPERTENSIONAHA.107.100214. [DOI] [PubMed] [Google Scholar]
- Innocenti M., Gallori S., Giaccherini C., Ieri F., Vincieri F.F., Mulinacci N. Evaluation of the phenolic content in the aerial parts of different varieties of Cichorium intybus L. J. Agric. Food Chem. 2005;53(16):6497–6502. doi: 10.1021/jf050541d. [DOI] [PubMed] [Google Scholar]
- Kampa M., Castanas E. Human health effects of air pollution. Environ. Pollut. 2008;151(2):362–367. doi: 10.1016/j.envpol.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Kannan K., Jain S.K. Oxidative stress and apoptosis. Pathophysiol. 2000;7(3):153–163. doi: 10.1016/s0928-4680(00)00053-5. [DOI] [PubMed] [Google Scholar]
- Koleva I.I., vanBeek T.A., Linssen J.P.H., deGroot A., Evstatieva L.N. Screening of plant extracts for antioxidant activity: A comparative study on three testing methods. Phytochem. Anal. 2002;13(1):8–17. doi: 10.1002/pca.611. [DOI] [PubMed] [Google Scholar]
- Kumaran A., Karunakaran R.J. In vitro antioxidant activities of methanol extracts of Phyllanthus species from India. LWT- Food Sci. Technol. 2007;40:344–352. [Google Scholar]
- Lee S.H., Bafna M.R., Sancheti S.S., Seo S.Y. Acetylcholineterase inhibitory and antioxidant properties of Rhododendron yedoense var Poukhanense bark. J. Med. Plant Res. 2011;5:248–254. [Google Scholar]
- Masoko P., Masiphephethu M.V. Phytochemical investigation, antioxidant and antimycobacterial activities of Schkuhria pinnata (Lam) Thell Extracts against Mycobacterium smegmatis. J. Evid. Based Integr. Med. 2019;24:1–8. doi: 10.1177/2515690X19866104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyo M., Ndhlala A.R., Finnie J.F., Van Staden J. Phenolic composition, antioxidant and acetylcholinesterase inhibitory activities of Sclerocarya birrea and Harpephyllum caffrum (Anacardiaceae) extracts. Food Chem. 2010;123:69–76. [Google Scholar]
- Muijsers R.B.R., van Den W.E., Folkerts G., Beukelman C.J., Koster A.S., Postma D.S., Nijkamp F.P. Apocynin inhibits peroxynitrite formation by murine macrophages. British J. Pharmacol. 2000;130:932–936. doi: 10.1038/sj.bjp.0703401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair J.J., Bastida J., Codina C., Viladomat F. Alkaloids of the South African Amaryllidaceae: A review. Nat. Prod. Commun. 2013;8:1335–1350. [PubMed] [Google Scholar]
- Nair J.J., Van Staden J. Traditional usage, phytochemistry and pharmacology of the South African medicinal plant Boophone disticha (Lf) Herb. (Amaryllidaceae) J. Ethnopharmacol. 2014;151:12–26. doi: 10.1016/j.jep.2013.10.053. [DOI] [PubMed] [Google Scholar]
- Neergaard J.S., Andersen J., Pedersen M.E., Stafford G.I., Van Staden J., Jäger A.K. Alkaloids from Boophone disticha with affinity to the serotonin transporter. South Afr. J. Bot. 2009;75:371–774. [Google Scholar]
- Oh J., Jo H., Cho A.R., Kim S.J., Han J. Antioxidant and antimicrobial activities of various leafy herbal teas. Food Control. 2012;31:403–409. [Google Scholar]
- Okaiyeto K., Hoppe H., Okoh A.I. Plant-based synthesis of silver nanoparticles using aqueous leaf extract of Salvia officinalis: Characterization and its antiplasmodial activity. J. Cluster Sci. 2020 doi: 10.1007/s10876-020-01766-y. [DOI] [Google Scholar]
- Okaiyeto K., Ojemaye M.O., Hoppe H., Mabinya L.V., Okoh A.I. Phytofabrication of silver/silver chloride nanoparticles using aqueous leaf extract of Oedera genistifolia: Characterization and antibacterial potential. Molecules. 2019;4:4382. doi: 10.3390/molecules24234382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otang W.M., Grierson D.S., Ndip R.N. Phytochemical studies and antioxidant activity of two South African medicinal plants traditionally used for the management of opportunistic fungal infections in HIV/AIDS patients. BMC Complement. Altern. Med. 2012;12:43. doi: 10.1186/1472-6882-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyaizu M. Studies on products of browning reaction. The Japanese Journal of Nutrition and Dietetics. Jpn. Acad. Nutr. Diet. 1986;44:307–315. [Google Scholar]
- Oyedemi S.O., Yakubu M.T., Afolayan A.J. Effect of aqueous extract of Leonotis leonurus (L.) R. Br. leaves in male Wistar rats. Hum. Exp. Toxicol. 2010;29:377–384. doi: 10.1177/0960327110363864. [DOI] [PubMed] [Google Scholar]
- Rampersad S.N. Multiple applications of Alamar Blue as an indicator of metabolic function and cellular health in cell viability bioassays. Sensors. 2012;12:12347–12360. doi: 10.3390/s120912347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaeian S., Pourianfar H.R., Janpoor J. Antioxidant properties of several medicinal plants growing wild in northeastern Iran. Asian J. Plant Sci. Res. 2015;5(2):63–68. [Google Scholar]
- Sasikala M., Sundaraganapathy R. Analytical Detection of Triterpenoids Present in the Hydroalcoholic Extract of Ipomoea Aquatica Forssk. in South India. Inter. J. ChemTech. Res. 2017;11:274–282. [Google Scholar]
- Saxena M., Saxena J., Pradhan A. Flavonoids and phenolic acids as antioxidants in plants and human health. Int. J. Pharm. Sci. Rev. Res. 2012;16:130–134. [Google Scholar]
- Sena L.A., Chandel N.S. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell. 2012;48:158–167. doi: 10.1016/j.molcel.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonisi S., Okaiyeto K., Hoppe H., Mabinya L.V., Nwodo U., Okoh A.I. Chemical constituents, antioxidant and cytotoxicity properties of Leonotis leonurus used in the folklore management of neurological disorders in the Eastern Cape, South Africa. 3. Biotech. 2020;10:141. doi: 10.1007/s13205-020-2126-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulaç E., Köseoğlu, Yılmaz P., Kolak U. Evaluation of antioxidant and cholinesterase inhibitory activities of some medicinal plants. Food Health. 2019;5(1):39–47. [Google Scholar]
- van Rijn R.M., Rhee I.K., Verpoorte R. Isolation of acetylcholinesterase inhibitory alkaloids from Nerine bowdenii. Nat. Prod. Res. 2010;24:222–225. doi: 10.1080/14786410802263758. [DOI] [PubMed] [Google Scholar]
- Van Wyk B.E., Gericke N. Briza Publications; 2000. People's plants: A guide to useful plants of Southern Africa. [Google Scholar]
- Viladomat F., Bastida J., Codina C., Nair J.J., Campbell W.E. Alkaloids of the South African Amaryllidaceae. Recent Res. Dev. Phytochem. 1997;1:131–171. [Google Scholar]
- Vladimir-Knežević S., Blažeković B., Kindl M., Vladić J., Lower-Nedza A.D., Brantner A.H. Acetylcholinesterase inhibitory, antioxidant and phytochemical properties of selected medicinal plants of the Lamiaceae family. Molecules. 2014;19:767–782. doi: 10.3390/molecules19010767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Song Y., Gao M., Bai X., Chen Z. Neuroprotective Effect of several phytochemicals and its potential application in the prevention of neurodegenerative diseases. Geriatrics. 2016;1:29. doi: 10.3390/geriatrics1040029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav R.N.S., Agarwal M. Phytochemical analysis of some medicinal plants. J. Phytol. 2011;3:10–14. [Google Scholar]
- Yuan H., Ma Q., Ye L., Piao G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules. 2016;21:559. doi: 10.3390/molecules21050559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Złotek U., Mikulska S., Nagajek M., Swieca M. The effect of different solvents and number of extraction steps on the polyphenol content and antioxidant capacity of basil leaves (Ocimum basilicum L.) extracts. Saudi J. Biol. Sci. 2016;23:628–633. doi: 10.1016/j.sjbs.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zofou D., Ntie-Kang F., Sippl W., Efange S.M.N. Bioactive natural products derived from the Central African flora against neglected tropical diseases and HIV. Nat. Prod. Rep. 2013;30:1098. doi: 10.1039/c3np70030e. [DOI] [PubMed] [Google Scholar]
- Zvetkova E., Wirleitner B., Tram N.T., Schennach H., Fuchs D. Aqueous extracts of Crinum latifolium (L.) and Camellia sinensis show immunomodulatory properties in human peripheral blood mononuclear cells. Int. Immunopharmacol. 2001;1:2143–2150. doi: 10.1016/s1567-5769(01)00140-0. [DOI] [PubMed] [Google Scholar]