Abstract
Pectinases are enzymes which are widely distributed in microbes that are present in pectin enriched sites. The agro-industrial residues can be utilized in the industrial scale for low-cost and efficient pectinase production in an eco-friendly approach. This study employs low-cost substrates (i.e. culinary fruit peels) for maximum pectinase production from novel Streptomyces fumigatiscleroticus VIT-SP4. The extraction and characterization of pectin from different fruit peels were investigated and pectinase activity was analyzed. The orange pectin gave maximum pectinase activity of about 45.93 (U/mL). Further, statistical optimization of process parameters was studied by using Taguchi method showed optimum values of pH-6, temperature −35 °C, orange pectin% − 2.5, incubation time- 48 h and RPM- 200 rpm and pectinase activity was found to be 98.65 (U/mL). The response surface methodology (RSM) was used for the optimization of media components which revealed that starch −1.17%, yeast extract-2%, and orange pectin% − 0.75% produces maximum pectinase of about 170.05 (U/mL). The drug-delivery study showed drug release was not observed at initial pH 3 after 4 h. The immediate drug release was noted at pH 6 caused due to disintegration of pectin by the pectinase activity. The self-healing of cracks by spray culture technique was investigated. The crack healing was observed up to 0.50 mm wide after 12 days. This confirms the ability of actinomycete spores to survive and they react to form calcite complex directly helps in crack healing process. This low-cost microbial pectinase can be used in drug delivery and concrete crack-healing applications sectors in future.
Keywords: Fruit pectin, Pectinase, Statistical optimization, Drug delivery, Crack-healing
1. Introduction
In recent times the market value of pectinases was increased and it acquires the highest position among commercially used enzymes in the industries and it is estimated to be 41.4$ billion in future (Garg et al., 2016, El Enshasy et al., 2018). As, enzymes are applied enormously in different fields and decreasing its production cost becomes the main problem and it has to be focused. Most of the fruit dump yard wastes lead to hazardous pollution and their disposal is alarming in less developed countries that require an eco-friendly approach. Citrus waste residues accumulated as unfavorable waste which are perceived as valueless but after degradation by pectinolytic actinomycetes results in production of by-products like cellulose, fibers, pectin that can be utilized for various industrial processes (Kaur, 2017). Many industries discard agro-industrial residues as municipal solid wastes which can be utilized for enzyme production (Marzo et al., 2019) and they are dumped by industries contain high amounts of carbon, nitrogen and other minerals and these wastes may be used as a substrate for pectinase production (Mehmood et al., 2019). The citrus peels and seeds of about 34 million tons were dumped in food processing industries and accumulation of them causes serious environmental problems. According to the industrial point of view, the production cost of enzymes is mainly caused due to the cost of the medium and it has to be minimized. Hence, citrus peels which are discarded as wastes that can be valorized as an alternate substrate for pectinase production (Viayaraghavan et al., 2019). The Taguchi method has been employed for the prediction of the important contribution of variables that are designed and optimum conditions of each variable by running experiments on flask experiments (John et al., 2019). Valorization of industrial wastes and their utilization by statistical analysis may be the positive approach to minimize the production cost of enzyme. Response surface methodology (secondary mathematical model) which is an integration of statistical and mathematical methodologies that are significant for their modelling and problem scrutiny. This model is predominantly used for optimizing the components of media required for microbial derived pectinase and it has been immensely used for the optimization of biochemical and chemical reactions like enzyme yield (Barbosa et al., 2010), optimization of media (Kunamneni and Singh, 2005), constraints for enzyme hydrolysis (Shieh and Lai, 2000), setting up of criteria for food processing (Ozer et al., 2004). Pectinase enzyme has been reported for the drug-release studies for colon treatment by the degradation of pectin-coated tablets and it also used for pulsatile drug delivery studies (Zhu et al., 2019, Liu et al., 2012). In recent scenario, there is an interest in the microbial self-healing process in case of crack repair of concrete due to its advantage like long-lasting, eco-friendly and efficiency (Wang et al., 2016). Microbial-induced calcium carbonate precipitation (MICP) plays an important role in bacterial healing of concrete cracks (Zhang et al., 2016). The calcium precipitation depends on the spore concentration of the bacteria (Achal and Mukherjee, 2015). On the other hand, some spores grown as vegetative cells because they cannot form germinated spores. There is a research lacuna on the statistical optimization of pectinase production from Streptomyces sp. by valorizing fruit pectin.
Thus, our current study focuses on extraction, morphological and chemical characterization of pectins from different fruit peels. The extracted pectin was utilized for the statistical optimization of pectinase from novel strain Streptomyces fumigatiscleroticus VIT-SP4 by Taguchi orthogonal array method and Response Surface Methodology (RSM). The yielded pectinase was utilized for controlled drug delivery study and potential isolate VIT-SP4 were investigated for concrete- crack healing process.
2. Materials and methods
2.1. Extraction and selection of economically viable pectin
The fruit peels like mosambi (Citrus limetta) – [MO], pomegranate (Punica granatum) [PO], Orange (Citrus aurantium) - [OR], mango (Mangifera indica) - [MA] and commercial pectin (control) were selected for the study. The pectin was extracted followed by the protocol. (Liew et al., 2014).
The percentage production of fruit peel pectin was determined by below formulae:
2.2. Characterization of pectin
2.2.1. Scanning Electron Microscopy (SEM) characterization of fruit pectin
The morphological features of pectin were visualized by Scanning Electron Microscope (SEM) (ZEISS EVO 18 RESEARCH) provided with retro-disperse electrons operated inside an Oxford X-ray probe under low vacuum conditions at the pressure at different magnifications at 1, 3 and 5 kV respectively.
2.2.2. Fourier Transform Infrared Spectroscopy (FTIR) characterization of fruit pectin
The samples were blended with potassium bromide (FT-IR grade) and characterized by FT-IR (Perkin Elmer-Spectrum RX1 spectrometer). The spectra were scanned wavenumber range between 4000 and 500 cm−1.
2.2.3. In vitro analysis of fruit peel pectin after pectinase activity
The pectin samples were dried in the oven at 105 °C to evaluate the moisture content present in it. Ash, proteins, lipids and crude fibers were analyzed according to AOAC methods (Feldsine et al., 2002).
2.3. Inoculum preparation for pectinase production
The potential pectinolytic isolate Streptomyces fumigatiscleroticus VIT-SP4 (Genbank accession no. KM875468) was selected for the study (Kumar and Suneetha, 2017). The isolate VIT-SP4 was inoculated in production (CSPY-ME) media contains (K2HPO4 0.5, casein 3.0, starch 10.0, peptone 1.0, yeast extract 1.0, pectin 1.0, malt extract 10.0 g L-1) where commercial pectin was replaced with extracted fruit pectins. The media were incubated at 28 ± 2 °C in a shaker at 100g for 72 h. incubation, then the suspension of potential isolate (1 mL) were pooled, centrifuged at 10,000 rpm for 10 min at 4 °C and analyzed for pectinase assay (Panda et al., 1999). The isolate Streptomyces lydicus MTCC 7505 was taken as control for all experiments.
2.4. Taguchi methodology: Optimization of process variables for maximum pectinase yield
The statistical optimization of pectinase was carried out by the Taguchi orthogonal array methodology by Design expert software version 7.0. Five main factors namely temperature, pH, Incubation time, pectin (%), RPM were considered. The experiment's size was designed by arrays with 25 experimental trails. The experiments were investigated by flask experiments for different trails which involve the 72 h-old culture of Streptomyces fumigatiscleroticus VIT-SP4 in production media (100 mL) with different parameters based on experimental design and it was pectinase activity was assayed for each trial. The results of the experiments were analyzed by design expert software for individual and interaction of the factors, optimal conditions for maximum pectinase production by Streptomyces strain. The optimized conditions were validated by the software. The signal to noise (S/N) ratio was calculated.
2.5. Central Composite design (CCD): Optimization of media components for pectinase yield by RSM
The effect of media components (critical variables) was optimized for maximal pectinase production from Streptomyces fumigatiscleroticus VIT-SP4 was carried out by Central Composite Design (CCD). The full factorial design was investigated with independent factors (3) namely starch, yeast extract, pectin% substrate levels (5) and replicates (3) to obtain second-order three-dimensional response surface over the factors and levels using Design Expert Version 11.0.0 software shown in supplementary information Table 3. Each variable was analyzed with 5 various levels and it results in 20 experimental runs with 6-axial and 6-centre points (Yu and Xu, 2018).
Table 3.
Experimental design for the optimization of variables for pectinase production.
| Std. order | Run | Starch (g/L) | Yeast extract (g/L) | Pectin % (g/L) | Actual Value | PredictedValue |
|---|---|---|---|---|---|---|
| 1 | 1 | 0.5 | 1 | 0.5 | 96.00 | 99.76 |
| 10 | 2 | 1.17045 | 2 | 0.75 | 170.05 | 168.84 |
| 5 | 3 | 0.5 | 1 | 1 | 100.15 | 98.33 |
| 2 | 4 | 1 | 1 | 0.5 | 115.33 | 113.38 |
| 12 | 5 | 0.75 | 3.68179 | 0.75 | 110.25 | 110.63 |
| 7 | 6 | 0.5 | 3 | 1 | 110.00 | 113.16 |
| 11 | 7 | 0.75 | 0.318207 | 0.75 | 96.21 | 94.12 |
| 9 | 8 | 0.329552 | 2 | 0.75 | 130.36 | 129.87 |
| 16 | 9 | 0.75 | 2 | 0.75 | 160.25 | 158.95 |
| 19 | 10 | 0.75 | 2 | 0.75 | 160.25 | 158.95 |
| 14 | 11 | 0.75 | 2 | 1.17045 | 123.54 | 122.18 |
| 13 | 12 | 0.75 | 2 | 0.329552 | 100.42 | 100.08 |
| 8 | 13 | 1 | 3 | 1 | 148.45 | 145.89 |
| 20 | 14 | 0.75 | 2 | 0.75 | 152.13 | 158.95 |
| 3 | 15 | 0.5 | 3 | 0.5 | 120.30 | 117.01 |
| 4 | 16 | 1 | 3 | 0.5 | 115.15 | 118.18 |
| 6 | 17 | 1 | 1 | 1 | 139.00 | 143.50 |
| 17 | 18 | 0.75 | 2 | 0.75 | 160.25 | 158.95 |
| 18 | 19 | 0.75 | 2 | 0.75 | 160.25 | 158.95 |
| 15 | 20 | 0.75 | 2 | 0.75 | 160.25 | 158.95 |
The analysis of variance (ANOVA) was assessed by determining the statistical analysis of the model and the significance of the model was determined. The F-value, variance’s measure was determined by RSM and it was represented as R2 value. The quadratic model was performed and the 2-Dcontour plot and 3D- response surface curves were generated to determine variable’s effect for maximum pectinase production by using Design Expert 11.0. Software (Wahab et al., 2018).
The shake flask experiments was performed to evaluate the predictive values of media components namely starch, yeast extract, and pectin%. The media components were taken in different concentrations based on the design. For this experiment, inoculums of the potential isolate were inoculated into 100 mL of media in 250 mL conical flask and it was incubated at 30 °C in a shaker with 100 rpm for 120 h. The physical parameters were optimized by full factorial design before the experiment.
2.6. Pectinase effect on a pectin-coated tablet in controlled drug-delivery system
The pectin-based drug delivery system was carried out in this study. Indomethacin, an anti-inflammatory drug (NSAID) was selected as a model drug. The Ca-pectinate tablets were prepared with extracted orange pectin and the purified enzyme from Streptomyces fumigatiscleroticus VIT-SP4 was chosen. The enzyme is purified and molecular weight was found to be 45 kDa. The purity of the enzyme was confirmed by HPLC prior to the experiment. The experiment was carried out for the observation of drug release at different pH in CSPYME media and time intervals (Maestrelli et al., 2008).
2.7. Crack healing of concrete specimen by Streptomyces fumigatiscleroticus VIT-SP4
2.7.1. Culture preparation and sporulation kinetics
The prepared inoculum of isolate S.fumigatiscleroticus VIT-SP4 were added to the sterilized CSPY-ME media and then it was incubated for 72 h under agitation 100 rpm at 28 ± 2 °C for maximum pectinase production. The endospores of S.fumigatiscleroticus VIT-SP4 (72 h-old culture) was inoculated in CSPY-ME media were applied to cement concrete varying in pH to determine the survivability of organisms in adverse conditions.
2.7.2. Cement specimen preparation and survivability of actinomycetes spores
The actinomycete spores were introduced into cement specimen in addition to water for determination of spore viability. The survivability of spores was studied for a period of 1 to 90 days. Each specimen was pulverized by sterilized mortars to escalate the extraction of spores. Then, it was serial-diluted in sterilized Tris-HCl buffer. The concrete cylinders were prepared for a particular size of about (75 × 75 mm). The cracks were made on the concrete cylinders by the compression machine. Then, culture was sprayed after the spore formation and observed for the crack healing process shown in Supplementary information (Fig. 1).
Fig. 1.
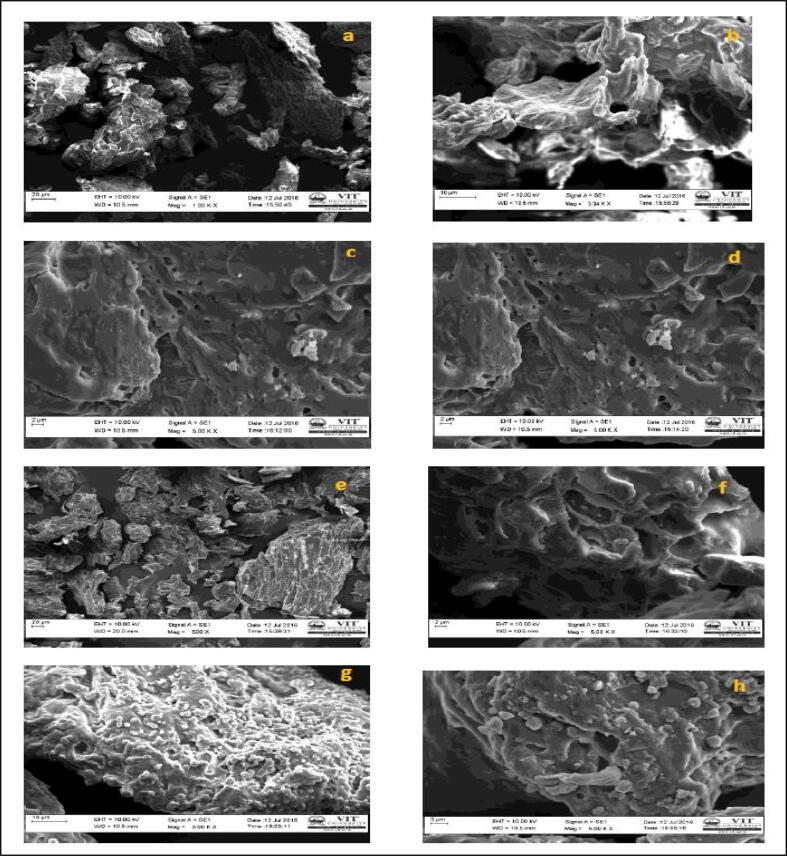
Morphological study of culinary fruit pectins by SEM (Zeiss EVO 18) analysis at different magnifications (a) and (b) Mosambi (Citrus limetta) (c) and (d) Pomegranate (Punica granatum); (e) and (f) Orange (Citrus aurantium); (g) and (h) Mango (Mangifera indica).
2.7.3. Ultrasonic treatment on concrete cracks
The ultrasonic waves (Steinkamp ultrasonic tester type BP III) of frequency range of 400–600 kHz were passed through hardened concrete cylinders of 75 × 75 mm with a crack of 0.50 mm with 10 mm depth on an open fissure in the concrete and observed for maximum transmission time. The instrument is connected to the machine which measures the time of a wave to passes from one sensor to another sensor. The sensors were placed at the upper end of a concrete specimen on the cracks and it was measured (Van Tittelboom et al., 2010).
3. Results
3.1. Characterization and utilization of pectin for pectinase production
This study deals with the valorization of fruit pectin for the production and statistical optimization of pectinase from potential isolate Streptomyces fumigatiscleroticus VIT-SP4 (Genbank accession no. KM875468). The pectin was extracted from different fruit peels and pectin yield was calculated by using the above formulae (Liew et al., 2014). The pectin yield was more in orange peel and it was estimated to be 42.3% respectively shown in Supplementary information (Table 1). The morphological characterization of pectin was analyzed by SEM (Scanning Electron Microscopy). The results revealed that mosambi pectin shows porous structure with hard and cervices; pomegranate pectin shows rough and uneven structures with pores; orange pectin shows heterogeneous structure with pores, mango pectin shows cuticles on cuticular wax ridges shown in (Fig. 1). The surface property of pectin was examined to understand pectin structures, its types namely protopectin or pectinic acid present in fruit peels and also structural changes in pectin caused by acid extraction method. Pectinase acts on different pectin by various mechanisms based on its pectin structures and this study helps for detailed study about the mechanism of pectinase on different pectins.
Table 1.
Experimental design for optimization of process parameters by Taguchi method.
| Run | Factor 1 A:pH |
Factor 2 B: Temp |
Factor 3 C:Pectin% |
Factor 4 D: Incubation time |
Factor 5 E: RPM |
Pectinase activity (Actual value) |
Pectinase activity (Predicted value) |
|
|---|---|---|---|---|---|---|---|---|
| 3 | 1 | 5 | 30 | 2 | 48 | 150 | 92.35 | 92.02 |
| 16 | 2 | 8 | 20 | 2.5 | 36 | 250 | 89.23 | 88.90 |
| 11 | 3 | 7 | 20 | 2 | 96 | 100 | 78.89 | 79.47 |
| 24 | 4 | 9 | 35 | 2 | 36 | 50 | 95.23 | 95.07 |
| 6 | 5 | 6 | 20 | 1.5 | 48 | 200 | 96.75 | 96.59 |
| 14 | 6 | 7 | 35 | 1 | 48 | 250 | 87.54 | 87.54 |
| 21 | 7 | 9 | 20 | 3 | 72 | 150 | 94.52 | 94.52 |
| 1 | 8 | 5 | 20 | 1 | 24 | 50 | 94.06 | 93.96 |
| 23 | 9 | 9 | 30 | 1.5 | 24 | 250 | 94.31 | 94.89 |
| 7 | 10 | 6 | 25 | 2 | 72 | 250 | 93.57 | 93.47 |
| 9 | 11 | 6 | 35 | 3 | 24 | 100 | 97.54 | 97.21 |
| 12 | 12 | 7 | 25 | 2.5 | 24 | 150 | 80.23 | 80.07 |
| 18 | 13 | 8 | 30 | 1 | 72 | 100 | 88.01 | 87.85 |
| 4 | 14 | 5 | 35 | 2.5 | 72 | 200 | 97.36 | 97.94 |
| 17 | 15 | 8 | 25 | 3 | 48 | 50 | 87.59 | 88.17 |
| 2 | 16 | 5 | 25 | 1.5 | 36 | 100 | 90.25 | 90.25 |
| 25 | 17 | 9 | 40 | 2.5 | 48 | 100 | 97.23 | 97.13 |
| 13 | 18 | 7 | 30 | 3 | 36 | 200 | 85.56 | 85.46 |
| 10 | 19 | 6 | 40 | 1 | 36 | 150 | 96.23 | 96.81 |
| 20 | 20 | 8 | 40 | 2 | 24 | 200 | 86.34 | 86.34 |
| 22 | 21 | 9 | 25 | 1 | 96 | 200 | 95.25 | 94.92 |
| 8 | 22 | 6 | 35 | 2.5 | 48 | 200 | 98.65 | 98.65 |
| 19 | 23 | 8 | 35 | 1.5 | 96 | 150 | 87.38 | 87.28 |
| 5 | 24 | 5 | 40 | 3 | 96 | 250 | 97.89 | 97.73 |
| 15 | 25 | 7 | 40 | 1.5 | 72 | 50 | 84.56 | 84.23 |
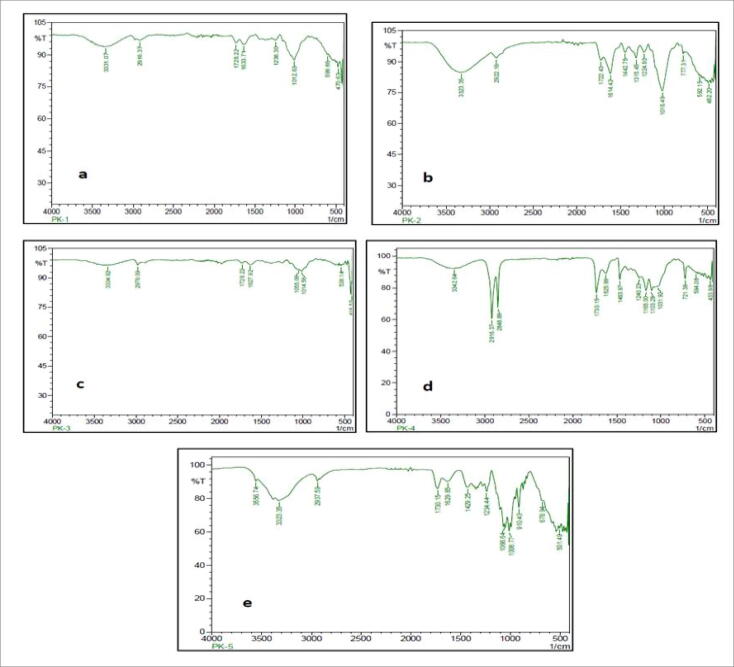
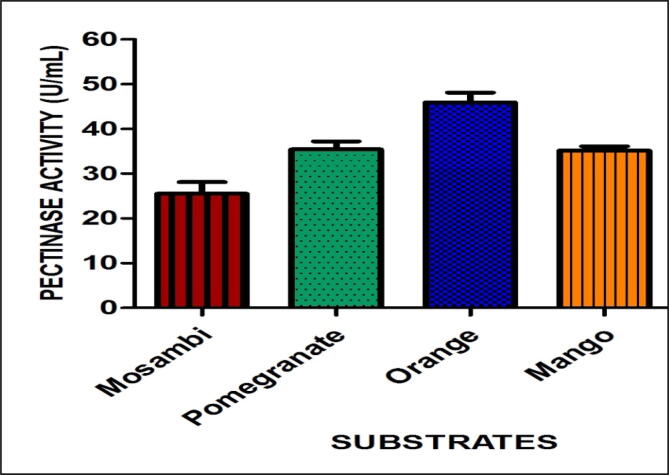
The interpretation of the FT-IR result was based on the corresponding peaks respective to the functional groups present in the fruit pectins. The spectra showed that peaks at 3331.07 cm−1 of Mosambi [MO], 3323.35 cm−1 of pomegranate [PO], 3334.92 cm−1 of orange [OR], 3342.64 cm−1 of mango [MA] and 3556.74 cm−1 of commercial pectin. The peaks at 2916.37 cm−1 of [MO], 2922.16 cm−1 of [PO], 2978.09 cm−1 of [OR], 2916.37 cm−1 of [MA] and 2937.59 cm−1 of commercial pectin. The peaks 1728.22 cm−1 of [MO], 1722.43 cm−1 of [PO], 1728.22 cm−1 of [OR], 2848.86 cm−1 of [MA] and 2937.59 cm−1 of pectin (commercial). The characteristic peak at 1633.71 cm−1 of [MO], 1614.42 cm-1of [PO], 1627.92 cm-1of [OR], 1625.99 cm−1 of [MA] 1629.85 cm−1 of commercial pectin. The peaks 1238.30 cm−1 of [MO], 1016.49 cm−1 of [PO], 1055.06 cm-1of [OR], 1165.00 cm-1of [MA],1012.63 cm−1 , 1066.64 cm−1,1103.28 cm−1, 1008.77 cm−1, 1031.92 cm−1. The FTIR spectral peaks of fruit pectins were compared with commercial pectin shown in (Fig. 2). The peaks correspond to the functional groups confirm the presence of pectic acid and other functional groups similar to that of pectin. The in-vitro analysis of fruit peels was characterized by using the AOAC method for the evaluation of proximate composition present in fruit peels (AOAC, 1995). The results showed the amount of lipid, ash, carbohydrate, and fiber present in the fruit peels shown in Supplementary information (Table 2). The potential pectinolytic isolate Streptomyces fumigatiscleroticus VIT-SP4 was inoculated in the production media (CSPYME) media in which commercial pectin was replaced with fruit pectin. After incubation, the supernatant was taken for pectinase assay. The Orange pectin gave maximum pectinase activity of about 45.93 (U/mL) shown in (Fig. 3). So, orange pectin will be valorized for further optimization studies for pectinase production by Streptomyces fumigatiscleroticus VIT-SP4.
Fig. 2.
Fourier Transform Infrared Spectroscopy analysis of (a) Mosambi (Citrus limetta) (b) Pomegranate (Punica granatum) (c) Orange (Citrus aurantium) (d) Mango (Mangifera indica) (e) Commercial pectin.
Table 2.
Analysis of variance of factors and summary of statistics for taguchi model.
| Source | Sum of squares | Df | Mean square | F-Value | p-value | |
|---|---|---|---|---|---|---|
| Model | 762.58 | 20 | 38.13 | 63.08 | 0.0005 | Significant |
| A-pH | 644.85 | 4 | 161.21 | 266.70 | <0.0001 | |
| B-Temp | 42.05 | 4 | 10.5 | 17.39 | 0.0085 | |
| C-Pectin% | 42.56 | 4 | 10.64 | 17.60 | 0.0084 | |
| D- Incubation time | 38.45 | 4 | 9.61 | 15.88 | 0.0078 | |
| E- RPM | 24.66 | 4 | 6.16 | 10.20 | 0.0225 | |
| Residual | 2.42 | 4 | 0.6045 | |||
| Cor total | 765.00 | 24 | ||||
| Std. dev | 0.7775 | R2 | 0.9968 | |||
| Mean | 91.46 | Adjusted R2 | 0.9810 | |||
| C.V.% | 0.8501 | Predicted R2 | 0.8765 | |||
| Adeq precision | 26.9112 |
Fig. 3.
Selection and conventional optimization of cheap substrates (fruit pectin) for maximum pectinase production.
3.2. Optimization of process parameters for maximum production of pectinase by Taguchi method
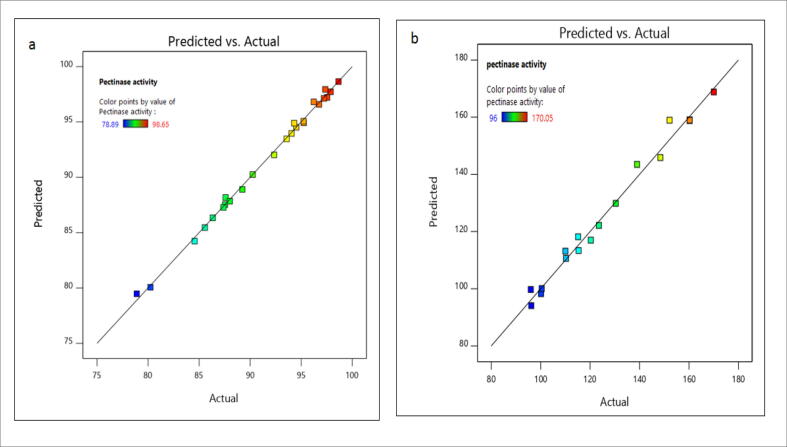
The results for the optimization of process parameters by Taguchi method was shown in (Table 1). The results revealed that the statistics for the Taguchi model were analyzed by F-value for ANOVA and results were shown in (Table 2). The F-value is 63.08 for the ANOVA model recommends it is the signature model for pectinase production. The chance is only 0.05% that F-value is large occur due to noise. The p-value of the model is<0.0500 indicates variables in the model are significant. In Taguchi model, all the model terms including A, B, C, D, and E are significant. The R2 value should be more than 0.75 suggests the model's fitness. In this Taguchi model, the R2 value is 0.9968. So, the model can analyze the response is correct. The (S/N) ratio for this model was determined by ‘Adeq precision'. The ratio should be greater than 4 to confirm the model is the desired model. In this, the ratio is 26.9112 implies an adequate signal. This model can be utilized to direct the design space. The point in the parity plot has two co-ordinates namely (x and y) where × indicates actual value and y indicates predicted value. The line equation (y = x) was considered to be a reference. When both experimental and predicted values are similar, the points lie on the same line (Fig. 6 (a). In our model, experimental and predicted values lay on the same straight line. This confirms the model's fitness.
Fig. 6.
Parity plot: (a) taguchi model (b) response surface methodology (The point in the parity plot has two co-ordinates namely (x and y) where x indicates actual value and y indicates predicted value. The line equation (y = x) were considered to be reference. The model is significant all points lie on same straight line).
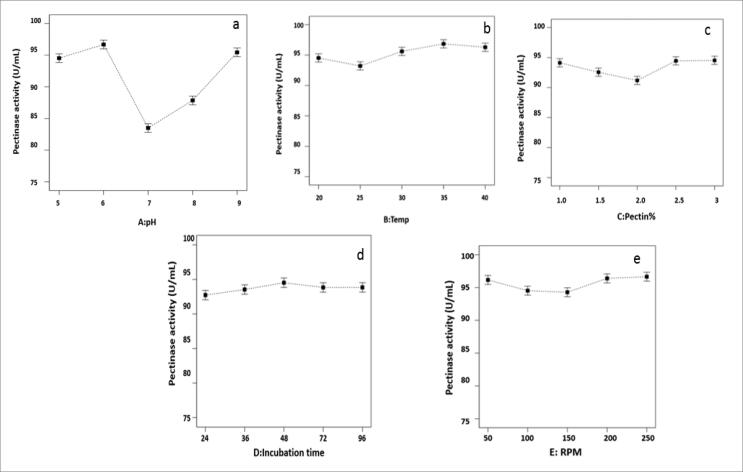
The results revealed that optimum conditions for maximum pectinase activity from isolate VIT-SP4 were found to be pH-6, temperature- 35 °C, orange pectin%- 2.5, incubation time- 48 h and RPM- 200 shown in (Fig. 4). The maximum pectinase activity produced by Streptomyces fumigatiscleroticus VIT-SP4 under these optimum conditions was found to be 98.65 (U/mL).
Fig. 4.
Taguchi model: Main effect plots of process parameters (a) pH (b) Temperature (c) Orange Pectin% (d) Incubation time (e) RPM.
3.3. Media components optimization: Response surface methodology (RSM)
The experiments were carried out based on the optimized values of media components and results were shown in (Table 3). The statistical results of the model was determined by F-Value for the analysis of variance (ANOVA) and results are shown in (Table 4). The F-value for the ANOVA model of 97.03 suggests that the model is significant. There is only a 0.01% chance that Fisher's value (F-value) this large may occur due to noise. The p-value of the model terms is less than 0.0500 implies terms are significant. In this study, A, B, C, AB, AC, A2, B2, C2 are the most significant model terms. In case, there are more insignificant model terms, the model reduction can be helpful to improve the model.
Table 4.
Analysis of Variance (ANOVA) for all variables in RSM model.
| Source | Sum of Squares | Df | Mean square | F-value | p-value | |
|---|---|---|---|---|---|---|
| Model | 12333.86 | 9 | 1370.43 | 97.03 | <0.0001 | Significant |
| A-starch | 1833.28 | 1 | 1833.28 | 129.81 | <0.0001 | Significant |
| B- Yeast extract | 392.02 | 1 | 329.02 | 23.30 | 0.0007 | Significant |
| C- Pectin% | 589.20 | 1 | 589.20 | 41.72 | <0.0001 | Significant |
| AB | 77.38 | 1 | 77.38 | 5.48 | 0.0413 | Significant |
| AC | 498.02 | 1 | 498.02 | 35.26 | 0.0001 | Significant |
| BC | 2.90 | 1 | 2.90 | 0.2056 | 0.6599 | Not significant |
| A2 | 165.72 | 1 | 165.72 | 1.73 | 0.0065 | |
| B2 | 5764.09 | 1 | 5764.09 | 408.13 | <0.0001 | |
| C2 | 4118.77 | 1 | 4118.77 | 291.63 | <0.0001 | |
| Residual | 141.23 | 10 | 14.12 | |||
| Lack of fit | 86.29 | 5 | 17.26 | 1.57 | 0.3162 | Not significant |
| Pure Error | 54.95 | 5 | 10.99 | |||
| Cor Total | 12475.09 | 19 |
The R2 value should be greater than 0.75 implies the fitness of the model. In this model, the Correlation coefficient (R2 value) is 0.9887. The ‘Predicted R2 value' is 0.9320 is a reasonable agreement with ‘Adjusted R2 Value' is 0.9785 (i.e.) difference is less than 0.2. So, the model can predict the response is correct. The signal to noise ratio for the model was calculated by ‘Adeq Precision'. This value should be greater than 4 to confirm the model is the desired model. In this model, the ratio is 28.116 indicates an adequate signal. This model can be utilized to route the design space shown in (Table 5).
Table 5.
Statistical Summary of model terms to determine suggested model for pectinase production.
| Source | Sequential p-value | Lack of fit p-value | Adjusted R2 | Predicted R2 | |
|---|---|---|---|---|---|
| Linear | 0.2503 | <0.0001 | 0.0744 | −0.1643 | |
| 2FI | 0.8431 | <0.0001 | −0.0714 | −0.8081 | |
| Quadratic | <0.0001 | 0.3162 | 0.9785 | 0.9320 | Suggested |
| Cubic | 0.2289 | 0.4533 | 0.9842 | 0.8654 | Aliased |
The Coefficient of variation (CV) implies a degree of precision which is utilized for the comparison of experimental values. The model's final equation in terms of coded factors was
The ‘Lack of Fit F-value’ shows 1.57 denotes lack of Fit is insignificant following pure error is shown in (Table 6). There is a 31.62% chance that ‘Lack of Fit F-value' was larger which obtains due to noise (i.e. Non-significant lack of fit is considered as good). When both experimental and predicted values are similar, the points lie on the same line (Fig. 6(b). In our model, experimental and predicted values lay on the same straight line. This confirms the model's fitness. The Response Surface Methodology (RSM) and 3-D graphical representation of the regression equation were generated by the model. These graphs were utilized to determine the factors which were significant for maximal pectinase production. Based on the combinations between the factors three response graphs were generated by the software.
Table 6.
Statistical summary of lack of fit.
| Source | Sum of Squares | Df | Mean Square | F-value | P-value | |
|---|---|---|---|---|---|---|
| Linear | 9668.65 | 11 | 878.97 | 79.99 | <0.0001 | |
| 2FI | 9090.35 | 8 | 1136.29 | 103.40 | <0.0001 | |
| Quadratic | 86.29 | 5 | 17.26 | 1.57 | 0.3162 | Suggested |
| Cubic | 7.26 | 1 | 7.26 | 0.6605 | 0.4533 | Aliased |
| Pure Error | 54.95 | 5 | 10.99 |
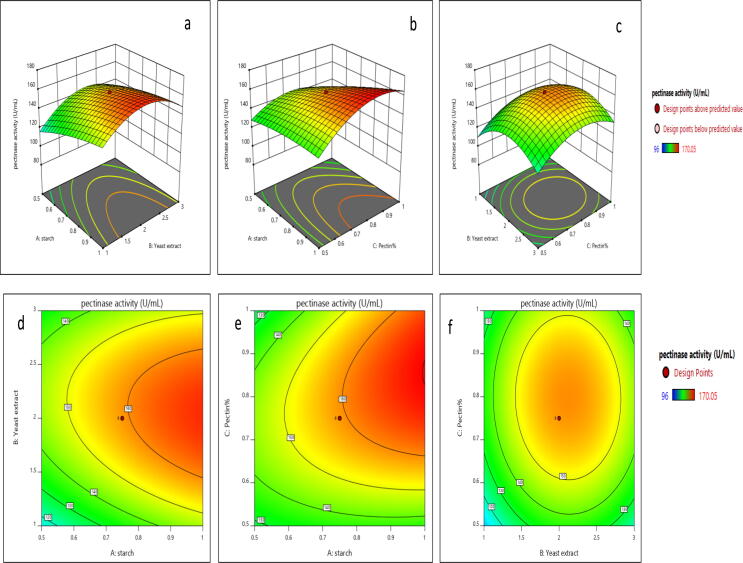
The response surface graph (Fig. 5 (a)) implies the concentration and interaction between starch (A) and Yeast extract (B) for maximum pectinase production. The results showed that when starch and yeast extract concentrations increase, there is a gradual increase in pectinase production. At high concentration of yeast extract leads to a decrease in the pectinase production. The P-value is 0.0413 which is less than 0.05 confirms the interaction between starch and yeast extract showed an increase in the pectinase production. Found to be significant. The response surface graph (Fig. 5 (b)) shows the particular concentration and interaction between starch (A) and orange pectin% (C). The yeast extract (B) was kept as constant for higher pectinase production. When starch and orange pectin % concentrations increase, the pectinase activity was also increased. At, the very higher concentration of orange pectin% mild decrease in the pectinase activity was observed. The p-value is 0.0001 which is less than 0.05 confirms the interaction between starch and orange pectin% was found to be significant. The response surface graph (Fig. 5 (c)) shows the interaction between yeast extract (B) and orange pectin% (C). The starch (A) was kept as constant for maximum production of pectinase. The results showed that a higher concentration of yeast extract decreases the pectinase production. When, orange pectin% concentration increases, there is a gradual increase in pectinase production. The p-value is 0.6599 which is greater than 0.05 confirms the interaction between yeast extract and orange pectin% influences less for the maximum pectinase production. Found to be insignificant. The contour plot (Fig. 5 (d)) represents a 2D-plot showed the contour curves are oval which confirms interaction between starch (A) and yeast extract (B) found to be is significant for pectinase production. The contour plot (Fig. 5 (e)) represents the 2D-plot of interaction between Starch (A) and Orange pectin% (C) for pectinase production. The oval shape of the contour curve indicates more interaction between factors starch (A) and orange pectin% (C) which confirms it is significant. The contour plot (Fig. 5 (f)) shows a 2D-plot of interaction between yeast extract (B) and orange pectin% (C) for pectinase production. The contour curve is a circular shape confirms the interaction between factors (B) and (C) are less interactive for pectinase production. The optimum concentrations were predicted for the media components by Design-Expert software (Ver.11.0) were Starch −1.17%, Yeast extract-2%, orange pectin%-0.75%. Based on the predicted concentrations of the media components, the predicted value for maximum pectinase activity was found to be 170.05 U/mL. To investigate the optimum concentrations of media components generated by the model experiments were carried out in shake flask experiments. The experimental actual value for maximal pectinase activity was revealed to be 168.84 U/mL which is in good agreement with the predicted value.
Fig. 5.
Response surface method: 3D-response graph relationship between (a) starch and yeast extract (b) starch and orange pectin% (c) yeast extract and orange pectin %, Counter plot for interaction between (d) starch and yeast extract (e) starch and orange pectin% (f) yeast extract and orange pectin%
The positive interaction between starch and yeast extract for pectinase production from isolate VIT-SP4 was observed. But, less interaction was observed between yeast extract and orange pectin% for the production of pectinase from isolate VIT-SP4. The optimal orange pectin concentration for the potential pectinolytic isolate was found to be 0.75%. The pectin (0.25%) utilized for maximum production of pectinase from Streptomyces sp. (Kuhad et al., 2004). The orange pectin% utilized by isolate VIT-SP4 is comparable to previous reports. The interaction between orange pectin% and starch showed positive interaction for maximum pectinase production. The interaction between orange pectin% and yeast extract showed less interaction for pectinase production by Streptomyces fumigatiscleroticus VIT-SP4.
3.4. Action of pectinase from potential isolate VIT-SP4 to pectin-based pellet for controlled drug delivery system
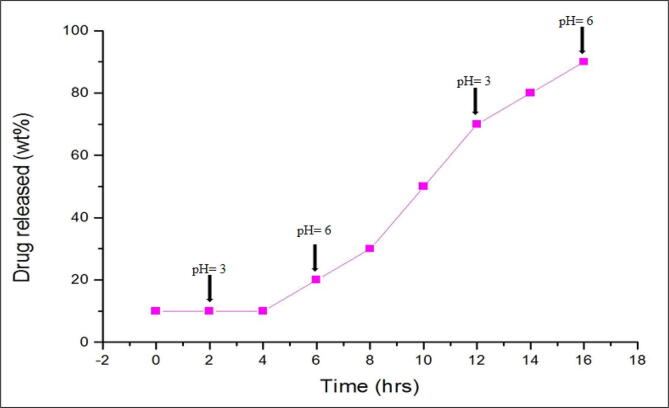
The controlled drug release parameter studied for continued hours in CSPY-ME media with variation in different pH at regular intervals. The result revealed that at initial pH (3.0) after 2 h there is an absence of drug release. When pH was attained to pH 6 after the period of 6 h rapid drug release was observed. At pH 3, drug release was absent and at pH 6, immediate drug release was observed after 17 h. The maximum pectinase activity from Streptomyces fumigatiscleroticus VIT-SP4 was observed at pH 6 which cause controlled drug release and it was shown in (Fig. 7).
Fig. 7.
In-vitro controlled drug release by pectinase enzyme.
3.5. Crack healing of concrete cement specimen by potential isolate
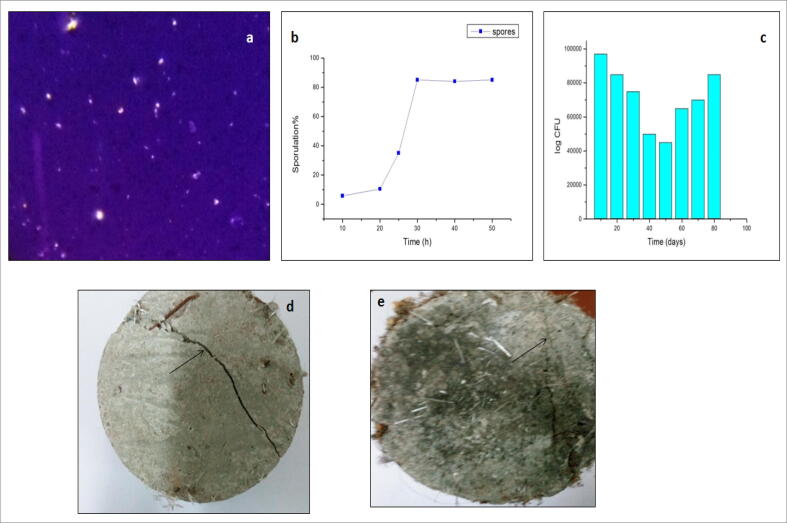
The self-healing of crack concrete blocks was investigated with potential isolate VIT-SP4 by spray culture technique. The stained spores were observed (Fig. 8 (a)). Sporulation % was shown in (Fig. 8(b)). Detection of viable spores was resistant after 90 days was shown in (Fig. 8(c)) confirms the survivability of spores. The cracked concrete block was taken as control (Fig. 8 (d)). After sporulation, the inoculum was sprayed on the cracks. The spores react with chemicals present in the concrete to form calcite and it helps in self-healing of cracks. The crack healing was observed up to 0.55 mm wide was recorded after the specimens were submerged in water for 12 days (Fig. 8(e)). The ultrasonic measurement test was carried out for both cracked and healed specimens. The results showed that the transmission time of the cracked specimen and healed concrete specimen were found to be 31.05 µs and 30.07 µs respectively. After, crack repair waves cannot pass through sealed crack leading to a decrease in the transmission time. Bio-mineralized calcite showed maximum resistance to dissolution compared to inorganic calcite formation.
Fig. 8.
Spore formation and sporulation kinetics in Streptomyces fumigatiscleroticus VIT-SP4 (a) spore-staining of isolate (b) % sporulation determined with stained spores (c) Survivability of Streptomyces fumigatiscleroticus VIT-SP4 spores in cement paste up to 90 days (d) shows cracks on the cement paste (control) (e) crack-healing of concrete block by spores of S.fumigatiscleroticus VIT-SP4 (test).
4. Discussion
The study deals with characterization of pectin and optimization of pectinase from novel Streptomyces fumigatiscleroticus VIT-SP4 and its industrial applications. The pectin was extracted from different fruit peels. The maximum pectin yield was obtained by citric acid extraction method was of about 42.3% respectively and it was higher comparable to the pectin yield of 19.24% (Prakash Maran et al., 2013), 20.44 ± 0.64 (Guo et al., 2012), 5.27% (Yeoh et al., 2008) seen in earlier reports. The media which has pectin materials can ultimately increase the production of enzymes that were reported earlier (Aguilar and Huitron, 1990). The characterization of pectin was carried out by scanning electron microscope. The results revealed the morphological features of fruit pectins were similar to earlier reports (Arias and Ramon-Laca, 2005, Mafra et al., 2001, Pathak et al., 2017, Wang et al., 2016). The FTIR spectra revealed that peaks at 3300.00 cm−1 correspond to the OH groups due to the presence of alcohol and pectic components of pectin molecule. The peaks at 2900.00 cm−1 is C—H asymmetrical stretching vibration due to aliphatic structures caused by the result of different vibration modes in carbohydrates and lignin. The peaks at 1700.00 cm−1 correspond to carbonyl group (C O) stretching of methyl esterified COCH3 in pectin which has been regarded as low methoxy pectin. The peaks at 1600.00 cm−1 represents stretching vibration due to ionic carboxyl groups. The peaks 1200.00 cm−1, 1100.00 cm−1, 1000.00 cm−1 are caused by C O and C C vibration of glycosidic bonds and pyrenoid ring. The FTIR spectra range around 800–1300 cm−1 represents the ‘fingerprint' region due to OH bending C—O—C stretching and CH3 plane deformation. Finally, IR spectra at 3300 cm−1 confirm the presence of pectic acid in the fruit peels (Chatjigakis et al., 1998, Silverstein and Bassler, 1962, Pappas et al., 2004, Kumar and Chauhan, 2010, Cerna et al., 2003, Gan et al., 2010). The in-vitro analysis of fruit peels after pectinase treatment was characterized by using the AOAC method and proximate composition present in fruit peels were evaluated (AOAC, 1995). The potential isolate VIT-SP4 was inoculated in the production (CSPYME) media with different fruit pectins. The result revealed that orange pectin gave maximal pectinase activity compared to other pectins which is higher compared to previous studies (Kuo et al., 2019).
The optimization of pectinase was carried out by Taguchi orthogonal array method. The maximal pectinase production from Streptomyces fumigatiscleroticus VIT-SP4 was observed at pH 6 which is similar to earlier study (Zhu et al., 2019) and it was comparable to pectinase production from Streptomyces erumpens MTCC 7317 at pH 7 (Kar and Ray, 2011). The optimal temperature for the maximum pectinase production by the potential isolate VIT-SP4 was found to be 35 °C which is the same compared to the soil temperature of dumpsites which was about 35 to 40 °C as reported. The optimal orange pectin% for maximum pectinase production from Streptomyces fumigatiscleroticus VIT-SP4 was found to be 2.5% which is nearly comparable to the pectin% used in the earlier studies. The concentration extracted pectin (2%) in media was utilized for pectinase production by bacteria. The result concludes actinomycetes utilize maximum pectin concentration for pectinase production (Sharma et al., 2019). The incubation time for maximal pectinase produced by Streptomyces fumigatiscleroticus VIT-SP4 was observed at 48 h which is similar compared to previous studies on Streptomyces lydicus (Jacob and Prema, 2006). The optimized agitation speed for pectinase production was found to be 200 RPM which is higher compared to the previous reports on bacteria (180 RPM) (Pant et al., 2015) and fungi (150 RPM) (Dinarvand et al., 2017).
The media optimization was carried out by Response surface methodology (CCD). The optimal starch concentration was observed at 1.17% for maximum pectinase production by Streptomyces fumigatiscleroticus VIT-SP4. The statistical optimization of pectinase production from Bacillus sp.Y1 by RSM and optimal starch concentration was found to be 3.8% (Guo et al., 2019). The interaction between starch and yeast extract, starch and orange pectin% showed positive interaction towards maximum pectinase production by isolate VIT-SP4. Maximum pectinase production was observed at 2% yeast extract concentration by potential isolate VIT-SP4. The yeast extract (0.3%) for maximal pectinase production by B.licheniformis KIBGE IB-21 (Rehman et al., 2012). The high concentration of nitrogen source has been utilized by isolate VIT-SP4 for maximum pectinase production compared to bacterial isolates.
The Controlled drug release study was carried out with pectinase from isolate VIT-SP4 to pectin based pellet. The main cause for drug release is the disintegration of pectin by pectinase enzyme at pH 6 whereas, at pH 3, there was no disintegration and it confirms the disintegration of pectin by pectinase which facilitate the controlled drug release as seen in earlier reports (Butte et al., 2014).
The self-healing of crack concrete blocks was investigated with potential isolate VIT-SP4 by spray culture technique. The crack healing was observed up to 0.55 mm wide was recorded and the specimens were submerged in water for 12 days. Whereas, crack healing was recorded up to 0.46 mm wide of 100 days period and which is comparable to previous studies (Wiktor and Jonkers, 2011). The results confirm that self-healing of cracks as there was a decrease in the transmission time of the healed concrete specimen compared to the cracked specimen (Van Tittelboom et al., 2010).
Streptomyces are spore-forming actinomycetes that are resistant to high pH and remained viable after casting the concrete for several days and helps directly in self-healing system. In the crack healing process, actinomycetes germinate spores and they get colonized at the time of harsh conditions in presence of H2O and causes CaCO3 precipitation to provide compressive strength and compatibility to the concrete. The self-healing of concrete by actinomycetes could decrease material permeability. As, it acts as catalyst it can be incorporated as mineral precursor to form CaCO3 precipitation which offer self-sufficient repair process. The self-healing of concrete with other inorganic materials like epoxy resin, silica fume and fly ash lead to various disadvantages like thermal expansion coefficient of the concrete that causes environmental and health problems. Since, microbes were involved in the process it seemed to be very cost-effective and recyclable in production compared to other inorganic materials.
5. Conclusion
The study highlights the cost-free production and statistical optimization of pectinase from novel pectinolytic isolate Streptomyces fumigatiscleroticus VIT-SP4 (Genbank accession no: KM875468) by utilizing fruit pectin. The Orange pectin gave maximum pectinase activity of about 45.93 (U/mL) compared to other peels. The statistical optimization of pectinase from isolate VIT-SP4 by Taguchi method produces pectinase activity of 98.65 U/mL. Further, optimization of media concentrations by response surface methodology (CCD) showed maximum pectinase activity of 170.05 U/mL. The results conclude a 1.7-fold increase in the pectinase activity after optimization. The yielded pectinase was used for controlled drug delivery study and drug release was observed at pH 6. Further, downstream processing of the pectinase can be carried out. The production and characterization of pectin-coated drug before and after pectinase treatment can be studied. The in-vivo and clinical studies can be done prior to commercialize pectin-coated tablets in the market as anti-inflammatory or colon-specific drugs that require specific drug delivery system. The potential isolate VIT-SP4 was utilized for concrete crack-healing process and self-healing of cracks was observed up to 0.50 mm wide after 12 days. Hence, large-scale production of this cost-effective pectinase in industrial level can be implemented as it makes less production cost.
Acknowledgments
Acknowledgements
The authors also acknowledge MTCC for the standard culture. The authors thank Dr. G.Viswanathan, Chancellor, VIT for his constant support to carry out this work, Department of Science and Technology (DST), India for financial support, Authors acknowledge Scanning Electron microscope facility of DST-FIST of VIT for the analysis.
Declaration of Competing Interest
The authors declared that they have no conflict of interest.
Data availability statement
All data generated or analyzed during this study are included in this published article.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.sjbs.2020.07.024.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Achal V., Mukherjee A. A review of microbial precipitation for sustainable construction. Constr. Build. Mater. 2015;93:1224–1235. [Google Scholar]
- Aguilar G., Huitron C. Constitutive exo-pectinase produced byAspergillus sp. CH-Y-1043 on different carbon source. Biotechnol. Lett. 1990;12:655–660. [Google Scholar]
- Arias B.A., Ramon-Laca L. Pharmacological properties of citrus and their ancient and medieval uses in the Mediterranean region. J. Ethnopharmacol. 2005;97:89–95. doi: 10.1016/j.jep.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Barbosa A.M., Giese E.C., Dekker R.F.H., Borsato D., Perez A.I.B., Iranzo J.F.U. Extracellular β-glucosidase production by the yeast Debaryomyces pseudopolymorphus UCLM-NS7A: optimization using response surface methodology. N. Biotechnol. 2010;27:374–381. doi: 10.1016/j.nbt.2010.05.013. [DOI] [PubMed] [Google Scholar]
- Butte K., Momin M., Deshmukh H. Optimisation and in vivo evaluation of pectin based drug delivery system containing curcumin for colon. Int. J. Biomater. 2014;2014(1):1–7. doi: 10.1155/2014/924278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerna M., Barros A.S., Nunes A., Rocha S.M., Delgadillo I., Copíkova J., Coimbra M.A. Use of FT-IR spectroscopy as a tool for the analysis of polysaccharide food additives. Carbohydr. Polym. 2003;51:383–389. doi: 10.1016/S0144-8617(02)00259-X. [DOI] [Google Scholar]
- Chatjigakis A.K., Pappas C., Proxenia N., Kalantzi O., Rodis P., Polissiou M. FT-IR spectroscopic determination of the degree of esterification of cell wall pectins from stored peaches and correlation to textural changes. Carbohydr. Polym. 1998;37:395–408. doi: 10.1016/S0144-8617(98)00057-5. [DOI] [Google Scholar]
- Dinarvand M., Rezaee M., Foroughi M. Optimizing culture conditions for production of intra and extracellular inulinase and invertase from Aspergillus niger ATCC 20611 by response surface methodology (RSM) Brazilian J. Microbiol. 2017;48:427–441. doi: 10.1016/j.bjm.2016.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Enshasy H.A., Elsayed E.A., Suhaimi N., Malek R.A., Esawy M. Bioprocess optimization for pectinase production using Aspergillus niger in a submerged cultivation system. BMC Biotechnol. 2018;18:1–13. doi: 10.1186/s12896-018-0481-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldsine P., Abeyta C., Andrews W.H. AOAC International methods committee guidelines for validation of qualitative and quantitative food microbiological official methods of analysis. J. AOAC Int. 2002;85:1187–1200. [PubMed] [Google Scholar]
- Gan C.Y., Abdul Manaf N.H., Latiff A.A. Physico-chemical properties of alcohol precipitate pectin-like polysaccharides from Parkia speciosa pod. Food Hydrocoll. 2010;24:471–478. doi: 10.1016/j.foodhyd.2009.11.014. [DOI] [Google Scholar]
- Garg G., Singh A., Kaur A., Singh R., Kaur J., Mahajan R. Microbial pectinases: an ecofriendly tool of nature for industries. 3. Biotech. 2016;6(1):1–13. doi: 10.1007/s13205-016-0371-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F., Li X., Zhao J., Li G., Gao P., Han X. Optimizing Culture Conditions by Statistical Approach to Enhance Production of Pectinase from Bacillus sp. Y1. Biomed. Res. Int. 2019;1–10 doi: 10.1155/2019/8146948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X., Han D., Xi H., Rao L., Liao X., Hu X., Wu J. Extraction of pectin from navel orange peel assisted by ultra-high pressure, microwave or traditional heating: A comparison. Carbohydr. Polym. 2012;88:441–448. doi: 10.1016/j.carbpol.2011.12.026. [DOI] [Google Scholar]
- Jacob N., Prema P. Influence of Mode of Fermentation on Production of Polygalacturonase by a Novel Strain of Streptomyces lydicus. Food Technol. Biotechnol. 2006;44:263–267. [Google Scholar]
- John I., Pola J., Thanabalan M., Appusamy A. Bioethanol Production from Musambi Peel by Acid Catalyzed Steam Pretreatment and Enzymatic Saccharification: Optimization of Delignification Using Taguchi Design. Waste Biomass Valorization. 2019:1–13. [Google Scholar]
- Kar S., Ray R.C. Purification, characterization and application of thermostable exo-polygalacturonase from Streptomyces erumpens MTCC 7317. J. Food Biochem. 2011;35:133–147. doi: 10.1111/j.1745-4514.2010.00372.x. [DOI] [Google Scholar]
- Kaur A. Approaches to Agro-industrial Solid Waste Disposal and Bioenergy Generation. Adv. Environ. Biotechnol. Springer. 2017:189–196. [Google Scholar]
- Kuhad R.C., Kapoor M., Rustagi R. Enhanced production of an alkaline pectinase from Streptomyces sp. RCK-SC by whole-cell immobilization and solid-state cultivation. World J. Microbiol. Biotechnol. 2004;20:257–263. doi: 10.1023/B:WIBI.0000023833.15866.45. [DOI] [Google Scholar]
- Kumar A., Chauhan G.S. Extraction and characterization of pectin from apple pomace and its evaluation as lipase (steapsin) inhibitor. Carbohydr. Polym. 2010;82:454–459. doi: 10.1016/j.carbpol.2010.05.001. [DOI] [Google Scholar]
- Kumar P., Suneetha V. Screening, Biochemical and Molecular identification of novel Streptomyces fumigatiscleroticus VIT-SP4 derived cocktail pectinase from border industrial pectin enriched places of Andhra Pradesh and Tamil Nadu. Res. J. Biotechnol. 2017;12(4):22–29. [Google Scholar]
- Kunamneni A., Singh S. Response surface optimization of enzymatic hydrolysis of maize starch for higher glucose production. Biochem. Eng. J. 2005;27:179–190. [Google Scholar]
- Kuo C.H., Huang C.Y., Shieh C.J., Wang H.M.D., Tseng C.Y. Hydrolysis of orange peel with cellulase and pectinase to produce bacterial cellulose using Gluconacetobacter xylinus. Waste Biomass Valorization. 2019;10:85–93. [Google Scholar]
- Liew S.Q., Chin N.L., Yusof Y.A. Extraction and Characterization of Pectin from Passion Fruit Peels. Agric. Agric. Sci. Procedia. 2014;2:231–236. doi: 10.1016/j.aaspro.2014.11.033. [DOI] [Google Scholar]
- Liu J., Zhang L., Jia Y., Hu W., Zhang J., Jiang H. Preparation and evaluation of pectin-based colon-specific pulsatile capsule in vitro and in vivo. Arch. Pharm. Res. 2012;35:1927–1934. doi: 10.1007/s12272-012-1109-4. [DOI] [PubMed] [Google Scholar]
- Maestrelli F., Cirri M., Corti G., Mennini N., Mura P. Development of enteric-coated calcium pectinate microspheres intended for colonic drug delivery. Eur. J. Pharm. Biopharm. 2008;69:508–518. doi: 10.1016/j.ejpb.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Mafra I., Lanza B., Reis A., Marsilio V., Campestre C., De Angelis M., Coimbra M.A. Effect of ripening on texture, microstructure and cell wall polysaccharide composition of olive fruit (Olea europaea) Physiol. Plant. 2001;111:439–447. doi: 10.1034/j.1399-3054.2001.1110403.x. [DOI] [PubMed] [Google Scholar]
- Marzo C., Díaz A.B., Caro I., Blandino A. Valorization of agro-industrial wastes to produce hydrolytic enzymes by fungal solid-state fermentation. Waste Manag. Res. 2019;37:149–156. doi: 10.1177/0734242X18798699. [DOI] [PubMed] [Google Scholar]
- Mehmood T., Saman T., Irfan M., Anwar F., Ikram M.S., Tabassam Q. Pectinase Production from Schizophyllum commune Through Central Composite Design Using Citrus Waste and Its Immobilization for Industrial Exploitation. Waste Biomass Valorization. 2019;10:2527–2536. [Google Scholar]
- Ozer E.A., Ibanoglu S., Ainsworth P., Yagmur C. Expansion characteristics of a nutritious extruded snack food using response surface methodology. Eur. Food Res. Technol. 2004;218:474–479. [Google Scholar]
- Panda T., Naidu G.S.N., Sinha J. Multiresponse analysis of microbiological parameters affecting the production of pectolytic enzymes by Aspergillus niger: a statistical view. Process Biochem. 1999;35:187–195. [Google Scholar]
- Pant G., Prakash A., Pavani J.V.P., Bera S., Deviram G.V.N.S., Kumar A., Panchpuri M., Prasuna R.G. Production, optimization and partial purification of protease from Bacillus subtilis. J. Taibah Univ. Sci. 2015;9:50–55. doi: 10.1016/j.jtusci.2014.04.010. [DOI] [Google Scholar]
- Pappas C.S., Malovikova A., Hromadkova Z., Tarantilis P.A., Ebringerova A., Polissiou M.G. Determination of the degree of esterification of pectinates with decyl and benzyl ester groups by diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) and curve-fitting deconvolution method. Carbohydr. Polym. 2004;56:465–469. doi: 10.1016/j.carbpol.2004.03.014. [DOI] [Google Scholar]
- Pathak, P.D., Mandavgane, S.A., Kulkarni, B.D., 2017. Fruit peel waste: Characterization and its potential uses. Curr. Sci. 113, 444–454. http://doi.org/10.18520/cs/v113/i03/444-454.
- Prakash Maran J., Sivakumar V., Thirugnanasambandham K., Sridhar R. Optimization of microwave assisted extraction of pectin from orange peel. Carbohydr. Polym. 2013;97:703–709. doi: 10.1016/j.carbpol.2013.05.052. [DOI] [PubMed] [Google Scholar]
- Rehman H.U., Qader S.A.U., Aman A. Polygalacturonase: Production of pectin depolymerising enzyme from Bacillus licheniformis KIBGE IB-21. Carbohydr. Polym. 2012;90:387–391. doi: 10.1016/j.carbpol.2012.05.055. [DOI] [PubMed] [Google Scholar]
- Sharma D., Sharma G., Mahajan R. Development of strategy for simultaneous enhanced production of alkaline xylanase-pectinase enzymes by a bacterial isolate in short submerged fermentation cycle. Enzyme Microb. Technol. 2019;122:90–100. doi: 10.1016/j.enzmictec.2018.12.008. [DOI] [PubMed] [Google Scholar]
- Shieh C.J., Lai Y.F. Application of response surface methodology to the study of methyl glucoside polyester synthesis parameters in a solvent-free system. J. Agric. Food Chem. 2000;48:1124–1128. doi: 10.1021/jf990460f. [DOI] [PubMed] [Google Scholar]
- Silverstein R.M., Bassler G.C. Spectrometric identification of organic compounds. J. Chem. Educ. 1962;39:546. [Google Scholar]
- Van Tittelboom K., De Belie N., De Muynck W., Verstraete W. Use of bacteria to repair cracks in concrete. Cem. Concr. Res. 2010;40:157–166. doi: 10.1016/j.cemconres.2009.08.025. [DOI] [Google Scholar]
- Viayaraghavan P., Jeba Kumar S., Valan Arasu M., Al-Dhabi N.A. Simultaneous production of commercial enzymes using agro industrial residues by statistical approach. J. Sci. Food Agric. 2019;99:2685–2696. doi: 10.1002/jsfa.9436. [DOI] [PubMed] [Google Scholar]
- Wahab W.A.A., Karam E.A., Hassan M.E., Kansoh A.L., Esawy M.A., Awad G.E.A. Optimization of pectinase immobilization on grafted alginate-agar gel beads by 24 full factorial CCD and thermodynamic profiling for evaluating of operational covalent immobilization. Int. J. Biol. Macromol. 2018;113:159–170. doi: 10.1016/j.ijbiomac.2018.02.086. [DOI] [PubMed] [Google Scholar]
- Wang M., Huang B., Fan C., Zhao K., Hu H., Xu X., Pan S., Liu F. Characterization and functional properties of mango peel pectin extracted by ultrasound assisted citric acid. Int. J. Biol. Macromol. 2016;91:974–1803. doi: 10.1016/j.ijbiomac.2016.06.011. [DOI] [PubMed] [Google Scholar]
- Wiktor V., Jonkers H.M. Quantification of crack-healing in novel bacteria-based self-healing concrete. Cem. Concr. Compos. 2011;33:763–770. doi: 10.1016/j.cemconcomp.2011.03.012. [DOI] [Google Scholar]
- Yeoh S., Shi J., Langrish T.A.G. Comparisons between different techniques for water-based extraction of pectin from orange peels. Desalination. 2008;218:229–237. doi: 10.1016/j.desal.2007.02.018. [DOI] [Google Scholar]
- Yu P., Xu C. Production optimization, purification and characterization of a heat-tolerant acidic pectinase from Bacillus sp. ZJ1407. Int. J. Biol. Macromol. 2018;108:972–980. doi: 10.1016/j.ijbiomac.2017.11.012. [DOI] [PubMed] [Google Scholar]
- Zhang J.L., Wu R.S., Li Y.M., Zhong J.Y., Deng X., Liu B., Han N.X., Xing F. Screening of bacteria for self-healing of concrete cracks and optimization of the microbial calcium precipitation process. Appl. Microbiol. Biotechnol. 2016;100:6661–6670. doi: 10.1007/s00253-016-7382-2. [DOI] [PubMed] [Google Scholar]
- Zhu W., Han C., Dong Y., Jian B. Enzyme-responsive mechanism based on multi-walled carbon nanotubes and pectin complex tablets for oral colon-specific drug delivery system. J. Radioanal. Nucl. Chem. 2019;320:503–512. doi: 10.1007/s10967-019-06501-0. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article.