Abstract
The development of antibiotic resistant in K. pneumoniae is an emerging thread worldwide due to the poor antimicrobial drugs. To overcome this issue, researchers are focused on plant material and their essential oils to fight against multi drug resistant bacteria. In this context, the current study was concentrated in medicinal plant of guva leaves and their essential oils to combat multi drug resistant bacterial infections. The essential oils were successfully screened and confirmed by HRLC-MS analysis. The anti-bacterial ability of the compounds were loaded into the chitosan nanoparticles and proved by FT-IR analysis. In addition, the chitosan loaded essential oils morphology was compared with chitosan alone in SEM analysis and suggested that the material was loaded successfully. Further, the anti-bacterial ability of the chitosan loaded essential oils were primarily confirmed by agar well diffusion method. At the 100 µg/mL of lowest concentration of chitosan loaded essential oils, the multi-drug resistant K. pneumoniae was inhibited with 96% and confirmed by minimum inhibition concentration experiment. Hence, all the experiments were proved that the essential oils were successfully loaded into the chitosan nanoparticles, and it has more anti-bacterial activity against multi-drug resistant K. pneumoniae.
Keywords: Medicinal plant, Essential oils, Chitosan, Nanomaterials, Anti-bacterial activity, Multi-drug resistant bacteria, Minimum inhibition concentration
1. Introduction
Last few decades, the nanoencapsulation of bioactive materials, drugs, and antibiotics using natural biopolymer is attracted significant interest (Cui et al., 2018). Because, natural biopolymers increased all the compound materials after encapsulation including biodegradability, biocompatibility, enhanced stability, gradual drug release capacity, increased stability, highest antimicrobial efficacy and low toxicity. Among the different biopolymers, chitosan is an excellent biopolymer which is used as an encapsulation process, it owing to its biodegradability, biocompatibility and toxicity level Hadidi et al., 2020. It also delivered as a variety forms like beads, fibers, films and membranes. In addition, the most rich polysaccharide content of highest biodegradable ability containing biopolymer is chitosan (Hasheminej et al., 2019). It is a very well-known biopolymer with increased properties of biodegradable, biocompatible and biomedical. It is an excellent natural polymer that synthesized from chitin through cationic process. It is an important biopolymer driven by alkaline deacetylation process, which is used in the entire field including agriculture, food, environmental, pharmaceutical, biomedical and clinical (Badawy et al., 2020). Chitosan is a second most biopolymer available in naturally with high content. The marine crustaceans are the important source to synthesize the natural chitosan (Zhang et al., 2020). Naturally, biopolymers of chitosan have high biodegradability, biocompatibility, antimicrobial activity, anti-biofilm activity, film forming ability with decreased toxicity level (Maruthupandy et al., 2019). Previously, more researchers are reported chitosan as carrier a molecule, which is used and increase the drug nature. The increased natures of the drugs are working 80% against various infections after successful delivery of chitosan. Also, chitosan is a functionalized biopolymer, highly used in all the applications including food for packaging, biomedical for anti-bacterial and anti-biofilm activity. In clinical, it is acted as successful carrier molecules for various drugs (Jiang et al., 2020). Particularly, chitosan is used as a important biological material in food packaging and biomedical applications due to the decreased toxicity nature. Sometimes, chitosan is used to improve the drug nature for improving the anti-microbial and anti-biofilm and anti-oxidant nature that it could expand its applications in food active packaging.
Chemically, when the process of deacetylation process, the weaker hydrogen bonds of chitin are linked with N-acetyl glucosamine chins and formed as a chitosan, which possesses positively charged amino groups when it diluted in acidic solvent (Mishra et al., 2018, Rajivgandhi et al., 2019b). Chitosan is formed with the help of negatively charged crosslinking agents in the natural polymers moieties of itself through intra- and intermolecular hydrogen bonds (Karimirad et al., 2019). When the synthesis process, chitosan beads is acted as a stable loading system, but it depends on the size and types of the crosslinking agents (Topuz and Uyar, 2020). The universal crosslinking agent is sodium tripolyphosphate (TPP), which is used in nanoencapsulation, even formed capsules are labile in the aqueous system based on the pH and ionic strength (Rajkumar et al., 2020). Basically, it shows on rod-like, crystal structures with larger surface area and hold the negatively charged sulphate groups on the cellulose backbone due to the process of hydrolysis. It can be formed as polymeric matrix with the mechanical behavior. Previously, this process is increased the rigid film matrix without stable condition for improved antimicrobial and mechanical properties (Tatiana et al., 2018).
Recent years, the plant derived essential oil has got increased attention to use as active biomedical agents that can be incorporated into biodegradable active films (López-Meneses et al., 2018a, López-Meneses et al., 2018b). The well-known effects of potential biological activities against various infections are received from various aromatic plant and spices in liquid form. Plant essential oils have more phenolic and flavonoid content, which have the excellent anti-oxidant properties. Also, terpenoid content of essential oils has increased anti-oxidant activity (Su et al., 2020). Essential oils have excellent anti-oxidant activity with increased biological activity such as antimicrobial, anti-cancer, antiviral and larvicidal. Previously more bioactive compounds with different biological activity such as anti-inflammatory, immunomodulatory and wound healing properties were screened from essential oil contents (Jahed et al., 2017a, Jahed et al., 2017b). Some of the essential oil compounds have anti-diabetics activity. The purification of essential oils from degradation affected by extreme processing conditions is their encapsulation (Jahed et al., 2017a, Jahed et al., 2017b). To increase the essential oil properties, recent studies are concentrated encapsulation methods. Among the various encapsulations, the biopolymers mediated encapsulation was applied more in biomedical research. Among the biopolymer mediated encapsulation, chitosan is a naturally derived biopolymer, which has the ability to decrease the water vapor due to the increased permeability in essential oils (Mohammadi et al., 2015). Chitosan mediated encapsulation of essential oils not only protects the environment, thereby extending the shelf life of the product but also enables a controlled release of active compounds. Nanoencapsulation of essential oil into the chitosan is an emerging route of encapsulation. In this route of encapsulation, the nanomaterial is loaded to envelop or reservoirs where nanomaterial are trapped and released from large surface area. Based on the above facts, the present study was focused on chitosan loaded Guava leaves essential oil against multi drug resistant K. pneumoniae.
2. Materials and methods
2.1. Needed materials
The natural polymer of low molecular Chitosan (50,000 Da) with 75% degree of deacetylation was purchased from Hi-media laboratories, Mumbai, India. For purify the essential oils, the Guava fruits were obtained from Pottanam village, Namakkal District. The plant was authenticated by Dr.S.R.Sivakumar, Assistant Professor, Department of plant Science, Bharathidasan University, Tiruchirappalli, Tamil nadu, India. The chemical and media was purchased from Suresh Scientific & Co. All the glass wares of this study were procured from Merck, Mumbai, India. The bacterial strain of multi drug resistant bacteria K. pneumonia was obtained from Department of Marine Science, Bharathidasan University, Tiruchirappalli, Tamil nadu, India.
2.2. Purification and identification of available essential oil from Guava leaves
Hydrodistillation method is the best method to purify and quantify the essential oil from plant and it also one of the universally approved method (Rajivgandhi et al., 2020a, Rajivgandhi et al., 2020b). Briefly, the collected leaves were dried after surface sterilization with D·H2O and maintained 15 days in dark condition. The dried plant leaves were ground by using master and pestle for nice powder. The nice thin content of the plant powder was performed to hydrodistillation 12 h in Clevenger’s apparatus. After 12 h, the essential oil was collected and separated the organic and aqueous phase. Finally, the organic phase of was dried over using sodium anhydrous sulphate and stored at 4 °C for further use. After, the crude essential oil powder was mixed into methanol (99%) to dissolve the powder. Consequently, the sample was analyzed by using HRLC-MS (Model 6745, Agilent Technology, CA, America) for complete analysis of the powder content including phytochemical compounds, proteins, amino acids, nutrients and bioactive compounds. The HRLC-MS was connected with DB-5 colomn 925 × 0.22 mm), the film thickness was used as o. 30 µm. The software was fitted by turbo mass gold mass spectrometer. The injector temperature was maintained at 240 °C. The temperatures of 40 °C and 240 °C were set initially and finally respectively. The injected volume and helium carrier gas was designed as 1 mL and 1 mL /m respectively. The solution of methanol was used for analysis. In addition, the 100 K Pa of pressure, 70 eV of energy, 1000 rate of decomposition and 0.50 range of decomposition were set. After successful completion of program, the instrument was run and allowed 4 h for complete analysis. After completion of the program, the available chemical constituents of the compounds were noted. The resulted chemical constituents were compared with Wiley library database of the Bharathidasan University NIST and confirm the available compounds.
2.3. Preparation of essential oils loaded chitosan nanoparticles
The identified essential oil was loaded with chitosan molecules by using two step nanoencapsulaiton process including oil-in water emulsification and ionic gelation method. The method was followed by previously reported evidence of Zhang et al. (2020) with some alterations. Equal volume of chitosan solution, 1% of oil and acetic acid 1: 1 ratio (1% w/v of chitosan + 1% v/v acetic acid) were taken and dissolved completely by using ultra sonicator (Suresh Scientific@Co, Tiruchirappalli, India) at 1 h. The diluted mixture was filtered by using whatman NO.1 filter paper and removes the undissolved materials. After, tween 80 solutions were added in to 50 mL of filtered chitosan solution. The pH was maintained at 4.2 with the help of 1 N NaOH solution. The mixture was further sonicated and received the homogeneous solution after two hours’ time interval. Required quantity of purified essential oil was gradually added on the wall of the tubes constantly at the time of homogenization with 1000 rpm for 10 min. After, the sample was constantly maintained in ice-both conditions for make the oil-in-water emulsion. Next, the cross linker of sodium tripolyphosphate (TPP) was added gradually to stimulate the ionic gelation of chitosan. The agitation was maintained constantly with 30 min and formed particles were collected. Then, the formed particles were centrifuged at 10000 rpm for 30 min at 4 °C. After centrifugation, the purified suspensions were washed three times using deionized water. Finally, the collected suspensions were dried immediately −30 °C for 1 h using freez drier (Cryodos 50/230 V, Telstar, Madrid, Spain).
2.4. Characterization of chitosan loaded essential oil
The physiochemical characterization of chitosan loaded essential oil containing material was analyzed by UV–vis spectroscopy and XRD. Further, the morphological observation and size and shape of the nanomaterial were observed by SEM and TEM.
2.4.1. FT-IR analysis of chitosan loaded essential oil
The structural interaction of prepared chitosan film incorporated plant essential oil was analyzed by FT-IR with the following reference of Jiang et al. (2020). The tested powder samples of chitosan, Chitosan loaded essential oil were recorded from the peak range at 4000–400 cm-1 by F-IR (Nexus, Shimadzhu, Japan) with 64 scans recorded at 4 cm-1 resolution.
2.4.2. Scanning electron microscopy analysis of chitosan loaded essential oil
The prepared chitosan loaded essential oil morphological structure compared with chitosan alone was observed by SEM analysis using JSM-3456, JEOL, Japan at 15 kv (Mishra et al., 2018). After complete preparation of essential oil within the chitosan, the samples were fixed in aluminum support with aid of carbon rib, being coated in liquid nitrogen contains gold metal surface to internal structure visualization. After, 1 h, the coated samples were directly observed under SEM with magnification of ~40.0 x.
2.4.3. Transmission electron microscopy analysis of chitosan loaded essential oil
The surface morphology size and shape of the chitosan, chitosan loaded essential oils structure was viewed by transmission electron microscopy (LEO EVO 40 Model, Japan). Approximately, 10 mg samples were coated on the gold coated aluminum surface plate and analyzed by transmission electron microscope.
2.5. Anti-bacterial activity
The agar well diffusion experiment method was used to detect the anti-bacterial activity of MDRs K. pneumoniae in the presence of chitosan loaded plant essential and followed by previous reports of Rajivgandhi et al., 2018. The muller hinton agar plates were used to check the anti-bacterial activity in our study. After complete solidification, 5 mm gap between the wells were made aseptically using sterile gel borer, which had been previously swabbed with 24 h old K. pneumoniae culture. Then, various concentration of chitosan loaded essential oil was added into the cutted wells and maintained at room temperature 1 day. After incubation, the plates were taken and observed the diameter zones of the wells. The inhibition zone of the wells were measured in diameter and noted.
2.6. Minimum inhibition concentration experiment
The minimum inhibition concentration effect of synthesized chitosan loaded essential oil sample was detected by 96-well microtiter plate followed by the earliest report of Yilmaz et al. (2019). Briefly, 24 h staled K. pneumonia culture was diluted into the 96-well plate containing fresh tryptic soy broth. After dilution, different concentration of chitosan loaded essential oil solution was added into respective wells. Whereas, without addition of the chitosan loaded essential oil containing well acted as a control. The plate was maintained at room temperature for one day. After incubation, the turbidity of the wells was observed on the naked eye and lowest concentration of the well that shown with higher turbidity of the well was confirmed as minimum inhibition concentration. This minimum inhibition effect was converted to percentages using universal formula,
3. Result
3.1. Purification and identification of available essential oil from Guava leaves
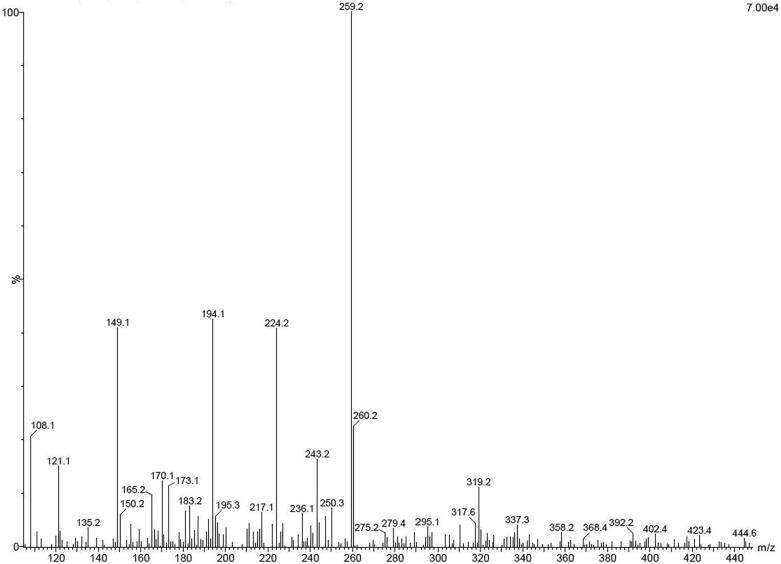
After careful purification through hydro-distillation method using Clevenger apparatus, the highest yield of the Guva leaves were obtained approximately 15.5 mL/l. The obtained essential oil was exhibited with whitish color and available phytochemicals, proteins, amino acid, and other compounds were observed by HRLC-MS peaks of Fig. 1. Among the peaks, the anti-microbial activity compounds were screened. All the screened compounds were identified based on the retention time, occupied area and occupied percentages. Among the entire identified compounds, there are 13 antimicrobial compounds were identified from previous reports (Paula et al., 2011, Zhang et al., 2020, and our identified compounds may also have anti-bacterial activity against tested K. pneumoniae. Some of the major compounds are phytochemical derivatives; they are also listed including thujene, ɑ-pinene, β-pinene, sabinene, cubenol, P-aucubin and L-scopoletin. Therefore our result was indicated that the anti-microbial ability of the bioactive compounds and phytochemicals compounds were highly present in the Guva essential oil. The result was agreed by previous report of Chaturvedi et al. (2019) and Guva essential oil was one of the important sources for biological activities. Recently, Joseph and Priya (2010), was reported that the purified guva essential oils have more anti-bacterial and anti-cancer activity against multi drug resistant bacteria and cancer cells. Usually, the traditional medicinal plants have increased bioactive compounds and phytochemical derivatives. In addition, the more alkaloid and flavonoid derivatives are also present in the essential oil derived compounds (Soliman Magda et al., 2016, Mahfuzul Hoque et al., 2007). Supportively, Garode and Waghode (2014) documented that the traditional medicinal plant derived essential oils have rich phenolic and flavonoid content; it may be leads to high anti-oxidant activity. Sometimes, the phyto-chemical derivatives and bioactive compounds were presented in different ratio due to the various environmental factors including pH, temperature, salt content, and genetic factors, nutritional status, harvesting time as well as the organs used for EO extraction (Joseph and Priya, 2011, Farhana et al., 2017). Finally, our result was confirmed that the Guva leaves derived essential oils have enormous bioactive compounds and phytochemical derivatives with increased biological activity.
Fig. 1.
Detection of phytochemical constituents of Guava leaf extract by HRLC-MS analysis.
3.2. Preparation of EOs-loaded chitosan nanoparticles
Recent years, the chitosan loaded essential oils formed nanogel is participated as a promising drug delivery system against antimicrobial activity of different bacterial infections (Kavaz and MaryamIdris, 2019). In addition, the chitosan loaded essential oils formed nanogel is a one of the excellent materials in biomedical applications due to the more entrapment efficiency, stability and control release of drugs (Joseph and Priya, 2010, Salehi et al., 2020), it is a two way process, the formation of chitosan loaded essential oils was emulsified using ionic gelation between the chitosan and cross linker of TPP by electrostatic interaction. In our study, we have used TPP as a cross linker in the ionic gelation of chitosan to essential oils. Previously, more researchers Jahed et al., 2017a, Jahed et al., 2017b, López-Meneses et al., 2018a, were reported that the chitosan loaded essential oil method is one of the best method for drug delivery due to the amide linkage between carboxylic group of plant essential oil and primary amino groups of chitosan without leaving a spacer molecules. In the present study, we have formulated the Guva leaves mediated essential oils with chitosan and form a cage like structure due to the self-association of hydrophobic segment of Guva essential oil inside and outside of chitosan with hydrophilic segment (López-Meneses et al., 2018a, López-Meneses et al., 2018b). In our study, we have used sonicator for chitosan plus essential oil mixer. The maximum encapsulation efficiency and loading capacity have been detected for 1: 1 ratio of chitosan and guva essential oils. Therefore, our result was initially confirmed that the essential oil was successfully loaded into the chitosan polymer. The confirmation of this nanogel formation was identified by FT-IR analysis.
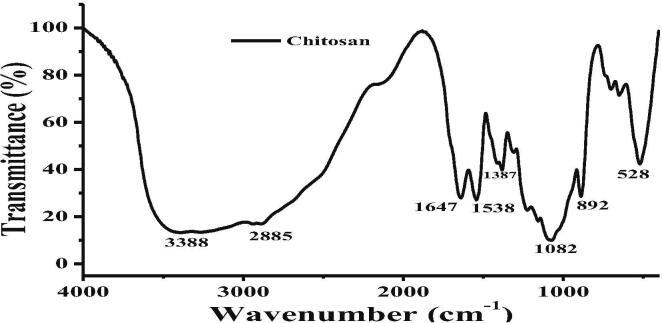
3.3. FT-IR analysis of chitosan and chitosan loaded guva leaf essential oil
FT-IR is one of the important techniques that used for detection of possible chemical groups present in the tested materials (Rajkumar et al., 2020). In our study we have confirmed the available functional groups of chitosan and presenting converted chemicals groups of chitosan loaded plant essential oil. The result of our tested material was present in Fig. 2a, At the peaks of 3388, 2885, 1647, 1538, 1387, 1082, 892, 528 was corresponding to respective chemical groups of —OH and NH stretching, —CH stretching, amide linkages I and II with N—H bending, carboxylic stretching of C—O—C with glucose like ring were exhibited. The exhibited peaks and their respective chemical groups were confirmed that the sample was chitosan (Fig. 2a). After addition of TPP, the new chemical groups of P—O and P O was observed within the place of amide II (—NH bending). It was confirmed that the new chemical group’s peaks of 2211, 1641, 1290 and 506 cm-1 were shifted from chitosan peaks of 2885–1082. The new P—O and P O peaks were observed from this process. It has shifted by the process of electrostatic linkage between ammonium ions of chitosan and phosphoric ions of TPP in nanoparticles. Further, after addition of purified guva essential oils into the chitosan, the peaks were exhibited with 3457, 2211, 1641 and 506 cm-1 for the respective chemical groups of O—H stretching, C—H stretching, C O stretching, C—C aromatic ring stretch, CH2 bend and C—H aromatic bend. The result was clearly indicated that the essential oil was successfully loaded into the chitosan-TPP mixture (Fig. 2b). The resulted absorption peaks of FT-IR result was indicated as same wave number. In addition, among the combination of chitosan-TPP-essential oils, the C—H stretching peak intensity was significantly increased due to the loading efficiency of essential oils. The exhibited peaks of 2866–2925 and 1238 cm-1 clearly indicated that the essential oils were successfully loaded in to the chitosan nanoparticles. Our result was agreed by recent report of Su et al. (2020), and the chemical groups of the shifted places were exhibited the loading efficiency of essential oils into chitosan. Supported evidence was published by K. Myszka, et al. (2020), and the shifted peaks without change of wave number containing chitosan loaded essential oil combination were successful. Therefore, our result was confirmed that the guva essential oils were encapsulated into the chitosan nanoparticles without inclusion of any chemical reaction. The unchanged structure and role of the chitosan loaded essential oils were indicated that the nanomaterial increased their biological properties (Yadav et al., 2020).
Fig. 2.
Detection of functional group changes between the chitosan.
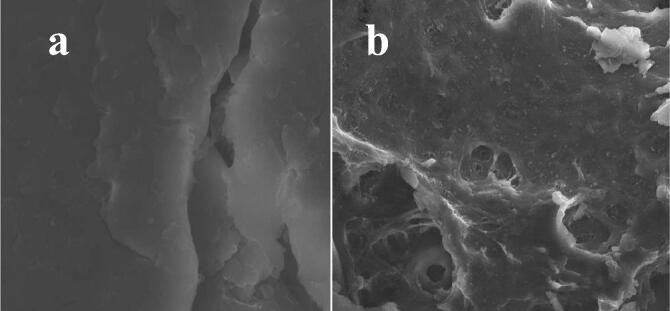
3.4. Scanning electron microscopy analysis
Scanning electron microscopic analysis is an important tool for analysis of morphology and particle size of the chitosan; chitosan loaded essential oils (Rajivgandhi et al., 2019b, Rajivgandhi et al., 2019a). In result, the chitosan nanoparticle was exhibited with spherical shape with smooth morphology and various sizes. In addition, the resulted morphological mediated chitosan was exhibited with uniform particles, and their average size was 40–96 nm. After loading with essential oil, when compared with chitosan alone (Fig. 3a), the chitosan loaded essential oil morphology (Fig. 3b) of was shown with swelling in inside the chitosan layer, it surrounded by individual particles (Rajivgandhi et al., 2020a, Rajivgandhi et al., 2020b). However, when we find, the particle was clearly aggregated. In addition, the result was suggested that the essential oil was combined and melted with chitosan with each other, which might have the presence of some essential oil content on the chitosan surface. It exhibited that the chitosan may decreased slightly and increased the essential oil content (Hasheminej et al., 2019). Therefore, our result was confirmed that the essential oil was loaded into the chitosan surface. Our result was agreed by Hadidi et al. (2020) and the swelling and aggregation variation between the chitosan alone and chitosan loaded essential oils.
Fig. 3.
Confirmation of plant essential oil loaded chitosan nanoparticles images by scanning electron microscope. Original morphology of chitosan (a) after loading of essential oil in the chitosan nanoparticle morphology (b).
3.5. Anti-bacterial activity
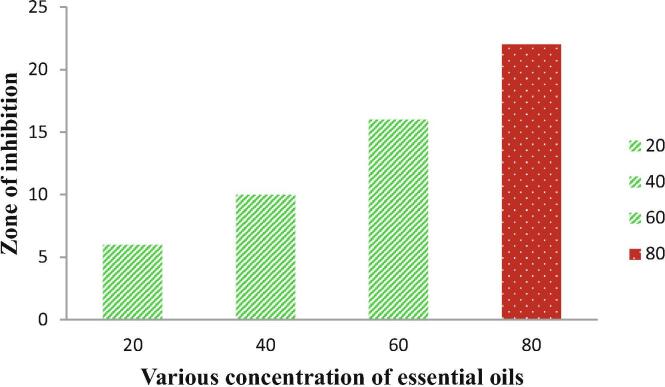
The DMSO diluted chitosan loaded essential oil was showed increased anti-bacterial activity against tested K. pneuminiae after overnight incubation when compared with previous reports of Dima et al., 2014, Hafsa et al., 2016, Rieger and Schiffman, 2014. Previously the guva leaf extract was showed with 10, 12 mm zone of inhibition against P. aeruginosa and K. pneumoniae respectively. Interestingly, our resulted chitosan loaded essential oils material was exhibited with 22 mm zone of inhibition against tested multi drug resistant K. pneumoniae at 70 µg/mL concentration (Fig. 4). This concentration was very low compared with previous reported essential oil loaded chitosan (Tatiana et al., 2018). Our result was suggested that the increased zone of inhibition may stimulate by chitosan. The available essential oil compounds of Karimirad et al., 2019, Topuz and Uyar, 2020, Mishra et al., 2018 may play a anti-bacterial inhibition role against K. pneumoniae in this study. These compounds are major compounds in guva essential oils. Our result was also agreed by previous documented reports of Hasheminej et al. (2020), and clove essential oils β-Pinene, Thujene and β-Farnesene have excellent anti-microbial properties. In mechanistic role, the chitosan loaded essential oils may enter in inside of the cytoplasm through ion channels and leads to membrane damage and protein damage. After the cytoplasmic leakages were done continuously and the production of pathogenicity molecules were lost.
Fig. 4.
Anti-bacterial activity result of chitosan loaded essential oil against multi drug resistant K. pneumoniae.
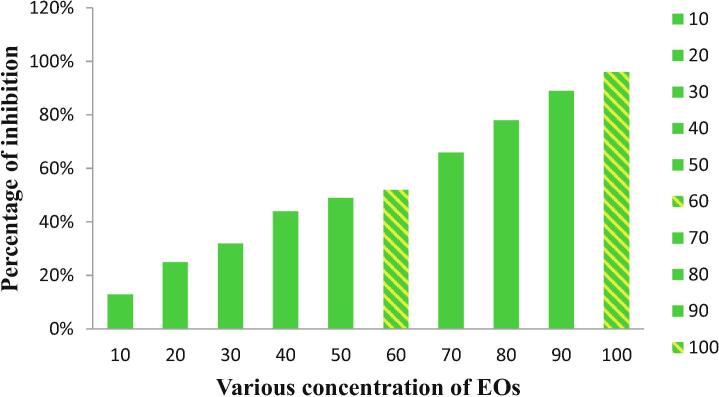
3.6. Minimum inhibition concentration of chitosan loaded essential oil
The anti-bacterial activity of the chitosan loaded essential oil was further confirmed by using minimum inhibition concentration assay. In this assay, we have found the minimum concentration of the chitosan loaded essential oil against tested K. pneumoniae by 96-well plate assay. After 24 h incubation, the 96-well plates were showed with more turbidity at increasing concentration. When the concentration was increased that the inhibition activity also increased in 96-well plates of treated wells (Deepika et al., 2019). The result was indicated that the chitosan loaded essential oil has antibacterial activity at lowest concentration. When, the diluted sample at 10 µg/mL, the chitosan loaded essential oil found with 13% inhibition rate against tested K. pneumoniae. The half minimum inhibition concentration of 52% was exhibited at 60 µg/mL concentration and the complete inhibition of 96% was inhibited at 100 µg/mL concentration. This concentration was very lowest concentration compare with previous study. Therefore, the lowest concentration of 100 µg/mL was fixed as a minimum inhibition concentration for this study (Fig. 5). Similar result was published by Alharbi and Alarfaj (2020), and the chitosan loaded essential oil improved the anti-bacterial activity and exhibited the result with very lowest concentration. Previous reported documents were agreed this study result, and stated that the minimum concentration of chitosan loaded essential oil has excellent inhibition activity against various bacteria. Recently, Williams and Stavrinides, 2020, TchindaIgor et al., 2017, reported that the minimum inhibition concentration method was one of the best method for identification of lowest concentration.
Fig. 5.
Minimum inhibition concentration analysis of chitosan loaded essential oil against multi drug resistant K. pneumoniae.
4. Conclusion
Based on our study result, we have confirmed that the essential oils were successfully loaded into the chitosan surface and it confirmed by comparison of chitosan alone result. The confirmation of chitosan loaded essential oils was showed by transferred chemical groups of FT-IR. In addition, the rough surface morphology of chitosan loaded essential oil was confirmed that the oils were present inside of the chitosan. Further, the anti-bacterial activity of the chitosan loaded essential oil was found at excellent activity against selected multi drug resistant K. pneumoniae. Furthermore, the minimum inhibition concentration study result was confirmed that the chitosan loaded essential oils was inhibited the K. pneumoniae at very lowest concentration (100 µg/mL). Finally, we have concluded that the present findings were confirmed that the chitosan loaded essential oil has increased anti-bacterial activity against K. pneumoniae.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The work was supported by University Research Fellowship (2013-2016) funded by Ref. No-05441/URF/K7/2013) Bharathidasan University, Tiruchirappalli-24 for entire of this study. The authors extend their appreciation to the Researchers Supporting Project number (RSP-2020/119), King Saud University, Riyadh, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
References
- Alharbi F.A., Alarfaj A.A. Green synthesis of silver nanoparticles from Neurada procumbens and its antibacterial activity against multi-drug resistant microbial pathogens. J. King Saud Univers. Sci. 2020;32:1346–1352. [Google Scholar]
- Badawy M.E.I., Lotfy T.M.R., Shawir S.M.S. Facile synthesis and characterizations of antibacterial and antioxidant of chitosan monoterpene nanoparticles and their applications in preserving minced meat. Int. J. Biolog. Macromol. 2020;156:127–136. doi: 10.1016/j.ijbiomac.2020.04.044. [DOI] [PubMed] [Google Scholar]
- Chaturvedi, T., Singh, S., Nishad, I., Kumar, A., Tiwari, N., Tandon, S., Saikia, D., Swaroop Verma, R., 2019. Chemical composition and antimicrobial activity of the essential oil of senescent leaves of guava (Psidiumguajava L.), Nat. Product Res. doi.org/10.1080/14786419.2019.1648462. [DOI] [PubMed]
- Cui H., Bai M., Marwan M.A., Lin Rashed L. The antibacterial activity of clove oil/chitosan nanoparticles embedded gelatin nanofibers against Escherichia coli O157:H7 biofilms on cucumber. Int. J. Food Microb. 2018;266:69–78. doi: 10.1016/j.ijfoodmicro.2017.11.019. [DOI] [PubMed] [Google Scholar]
- Deepika M.S., Thangam R., Vijayakumar T.S., Sasirekha R., Vimala R., Sivasubramanian S., Arun S., Babu M.D., Thirumurugan R. Antibacterial synergy between rutin and florfenicol enhances therapeutic spectrum against drug resistant Aeromonas hydrophila. Microbial Pathogenesis. 2019 doi: 10.1016/j.micpath.2019.103612. 103612. [DOI] [PubMed] [Google Scholar]
- Dima C., Cotârlet M., Alexe P., Dima S. Reprint of “Microencapsulation of essential oil of pimento [Pimenta dioica (L) Merr.] by chitosan/k-carrageenan complex coacervation method. Innovat. Food Sci. Emerg. Technol. 2014;25:97–105. [Google Scholar]
- Farhana J.A., Hossain Md.F., Mowlah A. Antibacterial Effects of Guava (Psidium guajava L.) Extracts Against Food Borne Pathogens. Int. J. Nutrit. Food Sci. 2017;6:1–5. [Google Scholar]
- Garode A.M., Waghode S.M. Antibacterial activity of psidium guajava linn (guava) leaves extracts on bacterial pathogens. Int. J. Bioassays. 2014;3(02):1794–1796. [Google Scholar]
- Hadidi M., Pouramin S., Adinepour F., Haghani S., MahdiJafari S. Chitosan nanoparticles loaded with clove essential oil: Characterization, antioxidant and antibacterial activities. Carbohyd. Polym. 2020;236 doi: 10.1016/j.carbpol.2020.116075. [DOI] [PubMed] [Google Scholar]
- Hafsa J., Smach M., Khedher M.R.B., Charfeddine B., Limem K., Majdoub H., Rouatbi S. Physical, antioxidant and antimicrobial properties of chitosan films containing Eucalyptus globulus essential oil. LWT - Food Sci. Technol. 2016;68:356–364. [Google Scholar]
- Hasheminej N., Khodaiyan F., Safari M. Improving the antifungal activity of clove essential oil encapsulated by chitosan nanoparticles. Food Chem. 2019;275:113–122. doi: 10.1016/j.foodchem.2018.09.085. [DOI] [PubMed] [Google Scholar]
- Jahed E., Khaledabad M.A., Bari M.R., Almasi H. Effect of cellulose and lignocellulose nanofibers on the properties ofOriganum vulgare ssp. gracile essential oil-loaded chitosan films. React. Funct. Polym. 2017;117:70–80. [Google Scholar]
- Jahed E., Khaledabad M.A., Almasia H., Hasanzadeh R. Physicochemical properties of Carum copticum essential oil loadedchitosan films containing organic nanoreinforcement. Carbohyd. Poly. 2017;164:325–338. doi: 10.1016/j.carbpol.2017.02.022. [DOI] [PubMed] [Google Scholar]
- Jiang Y., Lan W., Sameen D.E., Ahmed S., Qin W., Zhang Q., Chen H., Dai J., He L., Liu Y. Preparation and characterization of grass carp collagen-chitosan-lemon essential oil composite films for application as food packaging. Int. J. Biolog. Macromol. 2020;160:340–351. doi: 10.1016/j.ijbiomac.2020.05.202. [DOI] [PubMed] [Google Scholar]
- Joseph B., Priya M. Invitro antimicrobial activity of psidium guajava l. leaf essential oil and extracts using agar well diffusion method. Int. J. Current. Pharm. Res. 2010;2:1–6. [Google Scholar]
- Joseph B., Priya M. Phytochemical and biopharmaceutical aspects of Psidium guajava(L) essential oil: A review. Res. J. medi. Plant. 2011;5:432–442. [Google Scholar]
- Karimirad R., Behnamian M., Dezhsetan S. Application of chitosan nanoparticles containing Cuminum cyminum oil as a delivery system for shelf life extension of Agaricus bisporus. LWT. 2019;106:218–228. [Google Scholar]
- Kavaz D., MaryamIdris C. Onyebuchi. Physiochemical characterization, antioxidative, anticancer cells proliferation and food pathogens antibacterial activity of chitosan nanoparticles loaded with Cyperus articulatus rhizome essential oils. Int. J. Biolog. Macromol. 2019;123:837–845. doi: 10.1016/j.ijbiomac.2018.11.177. [DOI] [PubMed] [Google Scholar]
- López-Meneses A.K., Plascencia-Jatomea M., Lizardi-Mendoz J., Fernández-Quiroz D., Rodríguez-Félix F., Mouriño-Pérez R.R., Cortez-Rocha M.O. Schinus molle L. essential oil-loaded chitosan nanoparticles: Preparation, characterization, antifungal and anti-aflatoxigenic properties. LWT - Food Sci. Technol. 2018;96:597–603. [Google Scholar]
- López-Meneses A.K., Plascencia-Jatomea M., Lizardi-Mendoza J., Fernández-Quiroz D., Rodríguez-Félix F., Mouriño-Pérez R.R., Cortez-Rocha M.O. Schinus molle L. essential oil-loaded chitosan nanoparticles: Preparation, characterization, antifungal and anti-aflatoxigenic properties. LWT. 2018;96:597–603. [Google Scholar]
- Mahfuzul Hoque M.D., Bari M.L., Inatsu Y., Juneja Vijay K., Kawamoto S. Antibacterial Activity of Guava (Psidium guajava L.) and Neem (Azadirachta indica A. Juss.) Extracts Against Foodborne Pathogens and Spoilage Bacteria. Foodborn Pathog. DIS. 2007;4:1–8. doi: 10.1089/fpd.2007.0040. [DOI] [PubMed] [Google Scholar]
- Maruthupandy M., Rajivgandhi G., Muneeswaran T., Vennila T., Quero F., Song J.M. Chitosan/silver nanocomposites for colorimetric detection of glucose molecules. Int. J. Biolog. Macromol. 2019;121:822–828. doi: 10.1016/j.ijbiomac.2018.10.063. [DOI] [PubMed] [Google Scholar]
- Mishra D., Khare P., Singh D.K., Luqman S., Ajaya Kumar P.V., Yadav A., Das T., Saikia B.K. Retention of antibacterial and antioxidant properties of lemongrass oil loaded on cellulose nanofibre-poly ethylene glycol composite. Indust. Crops Prod. 2018;114:68–80. [Google Scholar]
- Mohammadi A., Hashemi M., Hosseini S.M. Chitosan nanoparticles loaded with Cinnamomum zeylanicum essential oil enhance the shelf life of cucumber during cold storage. Postharv. Biol. Technol. 2015;110:203–213. [Google Scholar]
- Myszka K., Sobieszczańska N., Olejnik A., Majcher M., Szwengiel A., Wolko Ł., Juzwa W. Studies on the anti-proliferative and anti-quorum sensing potentials of Myrtus communis L. essential oil for the improved microbial stability of salmon-based products. LWT. 2020;127 109380. [Google Scholar]
- Paula H.C.B., Sombra F.M., de Freitas Cavalcante R., Abreu O.M.S.F., de Paula R.C.M. Preparation and characterization of chitosan/cashew gum beads loaded with Lippia sidoides essential oil. Mat. Sci. Engin. C. 2011;31:173–178. [Google Scholar]
- Rajivgandhi G., Muneeswaran T., Maruthupandy M., Ramakritinan C.M., Saravanan K., Ravikumar V., Manoharan N. Antibacterial and anticancer potential of marine endophytic actinomycetes Streptomyces coeruleorubidus GRG 4 (KY457708) compound against colistin resistant uropathogens and A549 lung cancer cells. Microb. Pathog. 2018;125:325–335. doi: 10.1016/j.micpath.2018.09.025. [DOI] [PubMed] [Google Scholar]
- Rajivgandhi G., Maruthupandy M., Quero F., Li W.J. Graphene/nickel oxide nanocomposites against isolated ESBL producing bacteria and A549 cancer cells. Mat. Sci. Engin. C. 2019;102:829–843. doi: 10.1016/j.msec.2019.05.008. [DOI] [PubMed] [Google Scholar]
- Rajivgandhi G., Maruthupandy M., Veeramani T., Quero F., Li W.J. Anti-ESBL investigation of chitosan/silver nanocomposites against carbapenem resistant Pseudomonas aeruginosa. Int. J. Biolog. Macromol. 2019;132:1221–1234. doi: 10.1016/j.ijbiomac.2019.03.238. [DOI] [PubMed] [Google Scholar]
- Rajivgandhi G.N., Maruthupandy M., Li J.L., Dong L., Alharbi N.S., Kadaikunnan S., Khaled J.M., Alanzi K.F., Li W.J. Photocatalytic reduction and anti-bacterial activity of biosynthesized silver nanoparticles against multi drug resistant Staphylococcus saprophyticus BDUMS 5 (MN310601) Mat. Sci. Engin. C. 2020;114 doi: 10.1016/j.msec.2020.111024. [DOI] [PubMed] [Google Scholar]
- Rajivgandhi G.N., Ramachandran G., Maruthupandy M., Manoharan N., Alharbi N.S., Kadaikunnan S., Khaled J.M., Almanaa T.N., Li W.J. Anti-oxidant, anti-bacterial and anti-biofilm activity of biosynthesized silver nanoparticles using Gracilaria corticata against biofilm producing K. pneumonia. Colloid. Surf. A: Physicochem. Eng. Asp. 2020;600 124830. [Google Scholar]
- Rajkumar V., Gunasekaran C., Dharmaraj J., Chinnaraj P., Paul C.A., Kanithachristy I. Structural characterization of chitosan nanoparticle loaded with Piper nigrum essential oil for biological efficacy against the stored grain pest control. Pest. Biochem. Physiol. 2020;166 doi: 10.1016/j.pestbp.2020.104566. [DOI] [PubMed] [Google Scholar]
- Rieger K.A., Schiffman J.D. Electrospinning an essential oil: Cinnamaldehyde enhances the antimicrobial efficacy of chitosan/poly(ethylene oxide) nanofibers. Carbohyd. Polym. 2014;113:561–568. doi: 10.1016/j.carbpol.2014.06.075. [DOI] [PubMed] [Google Scholar]
- Salehi F., Behboudi H., Kavoosi G., Ardestani S.K. Incorporation of Zataria multiflora essential oil into chitosan biopolymer nanoparticles: A nanoemulsion based delivery system to improve the in-vitro efficacy, stability and anticancer activity of ZEO against breast cancer cells. Int. J. Biolog. Macromol. 2020;143:382–392. doi: 10.1016/j.ijbiomac.2019.12.058. [DOI] [PubMed] [Google Scholar]
- Soliman Magda F.M., Maha M.F., Salama Fatema M., Saber R. Comparative study of the volatile oil content and antimicrobial activity of Psidium guajava L. and Psidium cattleianum Sabine leaves. Bullet. Faculty of Pharm. 2016;54:219–225. [Google Scholar]
- Su H., Huang C., Liu Y., Kong S., Wang J., Huang H., Zhang B. Preparation and Characterization of Cinnamomum Essential Oil-Chitosan Nanocomposites: Phys. Struct. Antiox. Activit. Proces. 2020;8:834. doi: 10.3390/pr8070834. [DOI] [Google Scholar]
- Tatiana E., Damascenoa S., Almeid R.R., de Carvalho S.Y.B., de Carvalho G.S.G., Mano V., de Lima Guimarães A.C.P.L.G. Lippia origanoides Kunth. essential oil loaded in nanogel based on the chitosan and ρ-coumaric acid: Encapsulation efficiency and antioxidant activity. Indust. Crop. Prod. 2018;125:85–94. [Google Scholar]
- TchindaIgor C.F., Voukeng K., Beng V.P., Kuete V. Antibacterial activities of the methanol extracts of Albizia adianthifolia, Alchornea laxiflora, Laportea ovalifolia and three other Cameroonian plants against multi-drug resistant Gram-negative bacteria. Saudi J. Biolog. Sci. 2017;24:950–955. doi: 10.1016/j.sjbs.2016.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topuz F., Uyar T. Antioxidant, antibacterial and antifungal electrospun nanofibers for food packaging applications. Food Res. Int. 2020;130 doi: 10.1016/j.foodres.2019.108927. [DOI] [PubMed] [Google Scholar]
- Williams A.N., Stavrinides J. Pantoea Natural Product 3 is encoded by an eight-gene biosynthetic gene cluster and exhibits antimicrobial activity against multi-drug resistant Acinetobacter baumannii and Pseudomonas aeruginosa. Microbiol. Res. 2020;234 doi: 10.1016/j.micres.2020.126412. 126412. [DOI] [PubMed] [Google Scholar]
- Yadav A., Kujur A.K., Prem P., Singh V., Gupta B. Prakash, Encapsulation of Bunium persicum essential oil using chitosan nanopolymer: Preparation, characterization, antifungal assessment, and thermal stability. Int. J. Biolog. Macromol. 2020;142:172–180. doi: 10.1016/j.ijbiomac.2019.09.089. [DOI] [PubMed] [Google Scholar]
- Yilmaz M.T., Yilmaz A., Akman P.K., Bozkurt F., Dertli E., Basahel A., Al-Sasi B., Taylan O., Sagdic O. Electrospraying method for fabrication of essential oil loaded-chitosan nanoparticle delivery systems characterized by molecular, thermal, morphological and antifungal properties. Innovat. Food Sci. Emerg. Technol. 2019;52:166–178. [Google Scholar]
- Zhang H., Liang Y., Li X., Kang H. Effect of chitosan-gelatin coating containing nano-encapsulated tarragon essential oil on the preservation of pork slices. Meat Sci. 2020;166 doi: 10.1016/j.meatsci.2020.108137. [DOI] [PubMed] [Google Scholar]