Abstract
This context was investigated to assess the in vitro antioxidant, anti-diabetic, anti-obesity, and angiotensin-converting enzyme (ACE) inhibition traits of Punica granatum fruits peel extract. Initially, among various extracts tested, aqueous and ethanolic peel extracts depicted the presence of diverse phytoconstituents. In vitro antioxidative properties of peel extracts were determined using standard methodologies. Results showed that aqueous and ethanolic extracts had IC50 values of 471.7 and 509.16 μg/mL, respectively in terms of 1,1,diphenyl 2,2,picrylhydrazyl scavenging. Likewise, IC50 values of aqueous and ethanol extract were obtained as 488.76 and 478.47 μg/mL towards the degradation of hydrogen peroxide. The ethanolic extract exhibited the highest inhibition of α-glucosidase by showing activity of 53.34 ± 2.0 to 15.18 ± 1.4 U/L in a dose dependent manner (100–1000 µg/mL). Ethanolic extract was reported as the most active inhibitor of lipase with an IC50 value of 603.50 µg/mL. Ethanolic extract showed increased inhibition of ACE in a concentration dependent manner (100–1000 µg/mL) with IC50 value of 519.45 µg/mL. Fourier transform-infrared spectrum revealed the availability of various functional groups in the ethanolic extract of peel. Gas chromatography-mass spectrometry chromatogram of peel extract illustrated 23 diversified chemical constituents including 1,2,3,4-butanetetrol, Dimethyl sulfone, 9-octadecenamide, and Pentadecanoic acid as predominant compounds. In summary, P. granatum fruits peel extract revealed promising antioxidant, anti-diabetic, anti-obesity, and anti-hypertensive properties.
Keywords: Antioxidant, Anti-obesity, Angiotensin-converting enzyme, FT-IR, GC-MS, P. granatum peel
1. Introduction
Diabetes mellitus (DM) is a major chronic disease with persistent hyperglycaemia which is affecting global population at an alarming rate. In 2019, the global DM prevalence is estimated to be 9.3% (463 million people) and the total count is predicted to rise by 2045 (Saeedi et al., 2019). Diabetes mellitus can deteriorate various organ systems due to the metabolic aberrations and immune dysfunction which can lead to various complications such as retinopathy, nephropathy, neuropathy, and increased risk of cardiovascular diseases (Goyal and Jialal, 2020). The prevalence of co-morbidities like hypertension and obesity are seen commonly among people with DM (Chaudhary et al., 2019). In DM, immune dysfunction occurring in a hyperglycaemic environment leads to the greater frequency of bacterial infections associated with acute and emphysematous pyelonephritis, abscess, gangrene, foot infections, and external otitis as well as fungal infections associated with cystitis and candidiasis (Casqueiro et al., 2012). Without integrated pharmaceutical and medical nutrition therapy, DM could be disabling, life-threatening, and expensive.
Hypertension is another common progressive disorder worldwide which leads to varied chronic diseases. Angiotensin converting enzyme (ACE) produced from the lungs converts angiotensin I into angiotensin II which causes vasoconstriction followed by hypertension. At present, antioxidants are widely used for their preventive role against cardiovascular diseases as well as potentiality for scavenging free radicals (Nwaji et al., 2016, Jäkälä and Vapaatalo, 2010). A huge population of the world is affected by hypertension, and this metabolic disorder is expected to increase globally in future (Mittal and Singh, 2010). In spite of adopting life style changes (exercise and healthy diets) as common preventive approaches, the administration of drugs is imperative at critical stages. Hence, there is urgency to prevent and cure hypertension associated disorders by identifying natural therapeutics.
Phytochemicals offer wide varieties of therapeutically active compounds, which are considered less toxic, safer, and cheaper with better drug delivery and therapeutic properties compared to synthetic compounds (Khusro et al., 2013, Al-Dhabi et al., 2015, Al-Dhabi and Arasu, 2016, Barathikannan et al., 2016, Park et al., 2016a, Park et al., 2016b, Cuong et al., 2017, Ilavenil et al., 2017, Parveen et al., 2018, Esther Lydia et al., 2019). Punica granatum or pomegranate belongs to Lythraceae family. Constituents of P. granatum fruits and its peel are known to depict varied biological properties (Barbosa-Filho et al., 2006, Janani and Esther Lydia, 2013, Barathikannan et al., 2016, Esther Lydia et al., 2020). P. granatum peel has several phytochemicals like polyphenolic compounds, phenolic acids, anthocyanins, and flavonoids as potent antioxidants (Middha et al., 2013, Madugula et al., 2017, Di Sotto et al., 2019).
Considering the pivotal therapeutic attributes of P. granatum, this investigation was aimed to demonstrate the in vitro antioxidant, anti-diabetic, anti-obesity, and ACE inhibitory traits of P. granatum fruits peel extract. Further, different types of bioactive components in P. granatum extract were identified using analytical assays.
2. Materials and methods
2.1. Collection of fruits
P. granatum fruits were procured from Pazhamudhir Nilayam, Chennai and authenticated. Peels of the fruits were manually removed and was spread on a muslin cloth for 5–6 days for drying. Fruits peels were then subjected to grinding for further processing.
2.2. Sequential extraction
Sequential extraction of fruits peel was performed as per the method of Esther Lydia et al. (2019) using acetone, ethanol, distilled water, petroleum ether, and chloroform as solvents. Solvents extracts were used for further experimental analyses.
2.3. Phytochemical analysis
The presence of varied phytocomponents (carbohydrate, flavonoid, tannins, saponins, alkaloids, quinines, glycosides, cardio glycoside, terpenoids, phenols, coumarins, steroids, phytosteroids, and anthraquinone) in various solvent extracts of peel was determined as per the method of Harborne (1993).
2.4. In vitro antioxidative activities-
2.4.1. 1, 1, diphenyl 2, 2, picrylhydrazyl (DPPH) scavenging assay
Among various extracts tested, the aqueous and ethanolic extracts were reported to be promising in terms of the presence of essential phytochemicals, and thus, only these two extracts were used for in vitro antioxidant activities and other analyses. The DPPH degrading properties of selected extracts of P. granatum peel at varied concentrations (100–1000 μg/ml) were determined as per the method of Shimada et al. (1992). Percentage (%) DPPH scavenging traits of peel extracts were calculated according to the equations as given below:
2.4.2. Hydrogen peroxide (H2O2) degradation assay
Hydrogen peroxide scavenging traits of aqueous and ethanolic extracts of P. granatum peel at varied doses (100–1000 μg/mL) were estimated according to the methodology of Ruch et al. (1989) using the equation mentioned below:
where, A0 is the absorbance of the control and A1 is the absorbance of the sample.
2.5. In vitro α-glucosidase activities
α-glucosidase activities of aqueous and ethanolic extracts were estimated as per the method of Esther Lydia et al. (2019).
2.6. In vitro anti-obesity test
Porcine pancreatic lipase (PPL type II) inhibitory properties of aqueous and ethanolic extracts of P. granatum peel were estimated following the methodology of Zheng et al. (2010). The inhibitory characteristic was estimated as per the equation mentioned below:
where, A - activity without the inhibitor, a - negative control without the inhibitor, B - activity with inhibitor, and b - negative control with inhibitor.
2.7. In vitro anti-hypertensive activities
Anti-hypertensive properties of extracts were determined in terms of ACE inhibition using UV–Vis spectrophotometer based on hippuric acid formation ability from hippuryl-L-histidyl-L-leusine (HHL) catalyzed by ACE (Chaudhary et al., 2014). Angiotensin converting enzyme (50 µL; 25 mU/mL) was mixed with 50 μL of the test solution at 37 °C for 10 min. On the other hand, substrate (150 μL; 8.3 mM of HHL in 50 mM sodium borate buffer constituting 0.5 M sodium chloride, pH 8.2) was mixed with solution and kept for 30 min at room temperature. Further, 250 μL of 1 M HCl was added into the mixture to stop the reaction, followed by the addition of 0.5 mL of ethyl acetate and centrifugation of mixture at 800 g for 10 min. Upper component (0.2 mL) was collected and evaporated under vacuum. Hippuric acid was mixed with distilled water and read spectrophotometrically at 228 nm. Ramipril (3.6 ng/mL) was used as standard and ACE inhibition was estimated according to the equation given below:
where, A is the optical density at 228 nm with ACE but without inhibitor, B is the optical density of both ACE and inhibitor, C is the optical density without ACE and inhibitor.
2.8. Analytical assays-
2.8.1. Fourier transform infra-red (FT-IR) spectroscopy
The FT-IR spectrum of ethanolic extract was carried out according to the methodology of Khusro et al. (2014).
2.8.2. Gas chromatography-mass spectrometry (GC-MS)
The GC-MS spectrum of ethanolic extract was performed as per the procedure of Esther Lydia et al. (2019) and relative peak areas were calculated.
2.9. Statistical analyses
Experiments were performed in triplicate and values were represented as mean ± standard deviation.
3. Results
3.1. Phytocomponent analysis
Table 1 illustrates the presence of fundamental preliminary phytochemical components in different solvent extracts of P. granatum peel. The investigation uncovered the availability of disparate phytochemicals in aqueous peel extract. Ethanolic extract was also reported rich in carbohydrates, tannins, flavonoids, alkaloids, quinones, glycosides, cardiac glycosides, terpenoids, phenols, coumarins, and steroids. In contrary, acetone extract demonstrated the availability of limited phytoconstituents while chloroform extract contained tannins, alkaloids, quinones, terpenoids, and steroids. Quinones and phenols were observed only phytochemicals present in petroleum ether extract.
Table 1.
Phytocomponents of P. granatum fruits peel extracts.
| Phytochemicals | Aqueous extract | Ethanol extract | Acetone extract | Chloroform extract | Petroleum ether extract |
|---|---|---|---|---|---|
| Carbohydrates | + | + | + | − | − |
| Tannins | + | + | − | + | − |
| Saponins | + | + | − | − | − |
| Flavonoids | + | + | + | − | − |
| Alkaloids | + | + | + | + | − |
| Quinones | + | + | − | + | + |
| Glycosides | − | + | + | − | − |
| Cardiac glycosides | + | + | + | − | − |
| Terpenoids | + | + | − | + | − |
| Phenols | + | + | − | − | + |
| Coumarins | + | + | + | − | − |
| Steroids | + | + | − | + | − |
| Phytosteroids | − | − | − | − | |
| Anthraquinone | − | − | − | − | − |
‘+’: present; ‘−’: absent.
3.2. Antioxidant activities
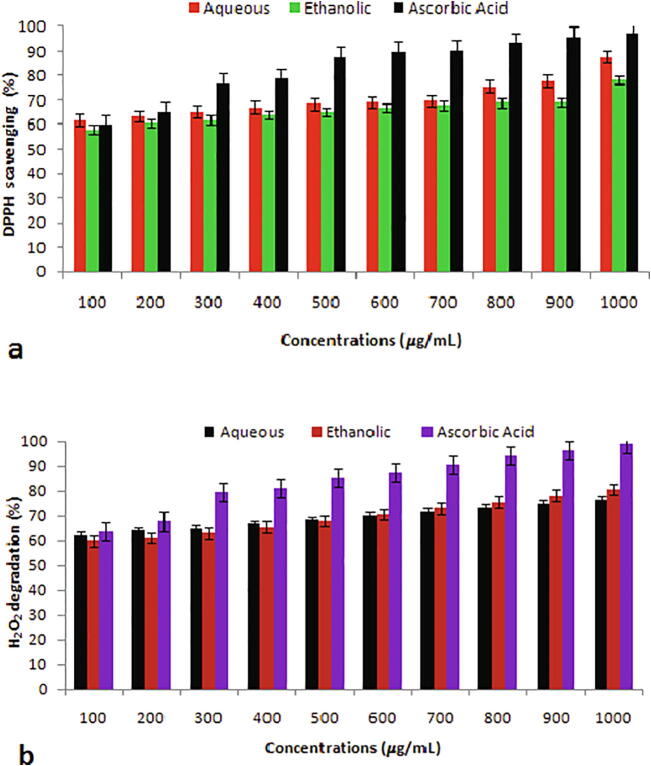
Fig. 1a shows the DPPH scavenging activities of peel extracts at different concentrations. At the highest concentration (1000 μg/mL), extracts showed DPPH radical degrading activities of 87.6 ± 1.8 and 78.32 ± 1.9%, respectively with respect to ascorbic acid (97.35 ± 1.6%). Result disclosed that aqueous extract, ethanolic extract, and ascorbic acid had an IC50 value of 471.70, 509.16, and 390.93 μg/mL, respectively.
Fig. 1.
(a) DPPH scavenging and (b) H2O2 degrading properties of peel extracts at various concentrations (100–1000 µg/mL).
The potential of P. granatum peel extracts to scavenge H2O2 is expressed in Fig. 1b. The extracts exhibited H2O2 degradation at distinct concentrations, however, the aqueous extract of fruit peel exhibited a high H2O2 restraint impact at various concentrations (100–400 μg/mL), while at 700 to 1000 μg/mL, ethanolic extract possessed a higher potential in degrading H2O2 with scavenging rate of 73.12 ± 1.7, 75.64 ± 1.6, 78.16 ± 1.7, and 80.68 ± 1.6%. IC50 values of aqueous and ethanolic extract, and standard were obtained as 488.76, 478.47, and 388.20 μg/mL, respectively.
3.3. α-glucosidase activities
The ethanolic extract depicted α-glucosidase inhibition by showing activity of 53.34 ± 2.0 to 15.18 ± 1.4 U/L in a concentration-dependent manner. In contrary, aqueous extract depicted activity of 65.48 ± 1.8 to 20.23 ± 1.3 U/L at varied concentrations tested (Fig. 2).
Fig. 2.
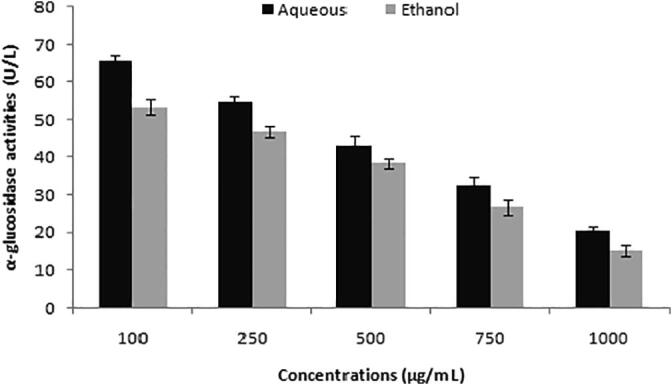
α-glucosidase activities of peel extracts at various concentrations (100–1000 µg/mL).
3.4. Anti-obesity properties
Fig. 3 illustrates lipase inhibitory potentials of aqueous and ethanolic extracts. With increase in concentrations, lipase inhibition also increased but the ethanolic extract was reported as the most active inhibitor of lipase with an IC50 value of 603.50 µg/mL.
Fig. 3.
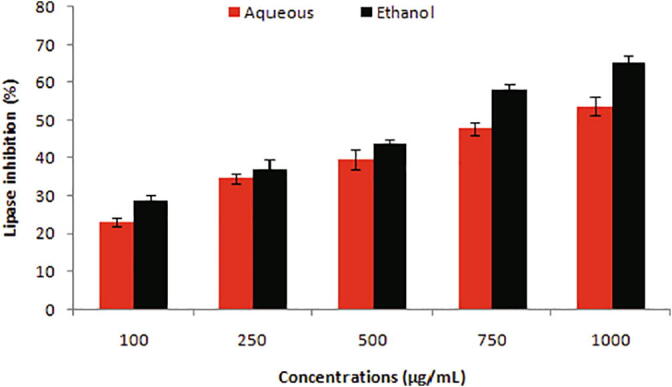
Lipase inhibition traits of peel extracts at various concentrations (100–1000 µg/mL).
3.5. Anti-hypertensive activities
The inhibition of ACE activity using different concentrations of the extracts is shown in Fig. 4. Ethanolic extract revealed increased inhibition of ACE at varied ranges (100–1000 µg/mL) with an IC50 value of 519.45 µg/mL with respect to the aqueous extract.
Fig. 4.
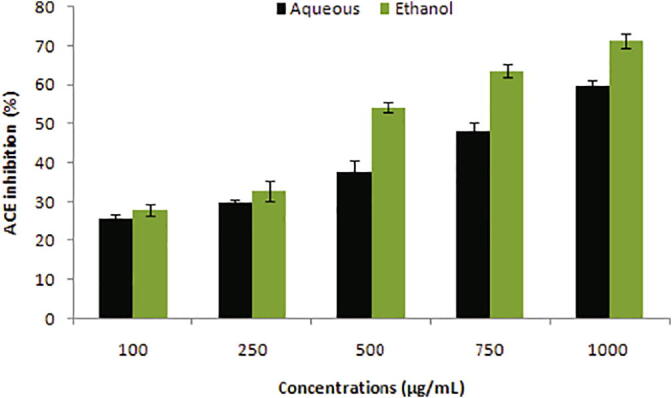
ACE inhibition traits of peel extracts at various concentrations (100–1000 µg/mL).
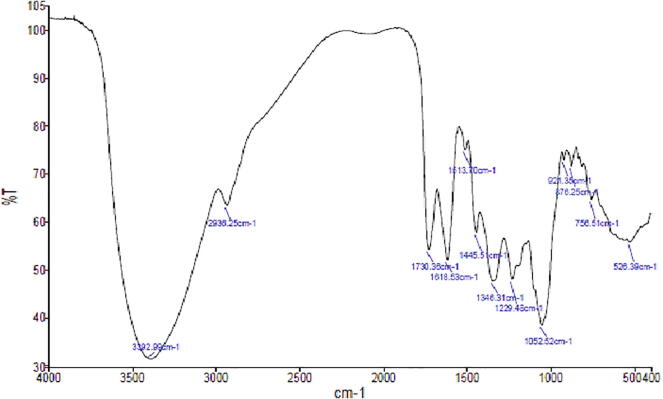
3.6. FT-IR spectrum
The FT-IR spectrum of ethanolic extract of P. granatum peel is shown in Fig. 5. The characteristic absorption band exhibited C—I stretching/halo compound, C—H bending/alkane, P—O—C stretching/aromatic phosphates, S O stretching/sulfoxide/sulfone, C—O stretching/alkyl aryl ether, N—O stretching/Nitro compound, C C stretching/ketone, C O stretching/aldehyde, and N—H stretching/amine.
Fig. 5.
FT-IR spectrum of ethanolic extract of P. granatum fruits peel.
3.7. GC-MS chromatogram
GC-MS chromatogram of the peel extract is illustrated in Table 2, incorporating the retention time and area of bioactive components present. Among those bioactive components, 1,2,3,4-butanetetrol (33.22%), Dimethyl sulfone (20.47%), 9-octadecenamide (13.14%), and Pentadecanoic acid (6.62%) were reported as the major compounds.
Table 2.
List of various compounds present in ethanolic extract of P. granatum fruits peel.
| Peak | Retention time | Area (%) | Compound name |
|---|---|---|---|
| 1 | 7.341 | 20.47 | Dimethyl sulfone |
| 2 | 7.452 | 33.22 | 1,2,3,4-butanetetrol, Erythritol |
| 4 | 7.645 | 1.53 | Nickel (II) bis(N,N-dioctyldithiocarbamate) |
| 5 | 7.727 | 2.26 | S-methyl methanethiosulphonate, Methyl 2-hydroxyethyl sulfoxide |
| 6 | 8.091 | 1.46 | Ethanol, 2,2′-[1,2-phenylenebis[(2-chloro-2,1-ethadediyl)oxy-2,1-ethanediyloxy]] bis-Thiohypophosphoric acid |
| 7 | 8.239 | 1.86 | Methane, (Methylsulfinyl) (Methylthio)-3,5-dithiahexanol 5,5-dioxide p-dioxane-2,3-diol |
| 8 | 8.388 | 1.56 | Dimethyl sulfoxide, 5,6-dihydro-4H-1-benzazonine-2,7-dione |
| 9 | 8.470 | 1.49 | 2,2′-sulfinyldiethanol |
| 10 | 8.507 | 0.81 | 2,2′-sulfinyldiethanol propanamide, 2-hydroxy-1-ethanol |
| 11 | 8.603 | 1.63 | 2-chloroethyl thiocyanate butane, 2,3-dichloro-dimethyl sulfoxide |
| 12 | 8.700 | 2.25 | 3,7-octadiene-1,1,8-tricarboxylic acid, 3,7-dimethyl, trimethyl ester, 1,4-Dimethyl-pyridinium chloride, Formaldehyde oxime trimer |
| 13 | 9.643 | 1.37 | Carbonic acid, 2-chloroethyl 4-nitrophenyl ester, 6-Methoxybenzofuroxan |
| 14 | 10.572 | 2.58 | 1,2,3-benzenetriol |
| 15 | 10.884 | 1.78 | Ethanol, 2,2′-sulfonylbis-1-propene, 1-methylthio-2-trifluromethyl-1,3,3,3-tetrafluoro-propylene glycol |
| 16 | 11.909 | 1.34 | 4-Hepten-3-one, 4-methyl-benzenamine, 2-methoxy-5-[5-(1H-pyrazol-1-yl)-1H-1,2,3,4-tetrazol-1-yl]-2H-1,4-oxazino quinolone |
| 19 | 17.956 | 0.56 | [4,8-bis (decyloxy)-5-(4-flurobenzenzoyl) naphthalene, 1-(4-flurobenzoyl)-Vitexin |
| 20 | 19.063 | 1.31 | Tetradecanamide, Decanamide, 2,2-dimethyl-tetrahydro-[1,4] dioxo 6,7-diol |
| 21 | 20.638 | 13.14 | 9-octadecenamide, (2-butanamide, 3,3-dimethyl-pentanamide) |
| 22 | 20.823 | 1.38 | Hexadecanamide, Tetradecanamide |
| 23 | 23.349 | 6.62 | Pentadecanoic acid, 2-hydroxyl-1-(hydroxymethyl) ethyl ester, Hexadecanoic acid. Octadecanoic acid |
4. Discussion
In recent times, there is profound admiration in the estimation and utilization of medicinal plants as the well being of option/traditional medicine and some fundamental dietary supplements for maintaining several ailments relating to human health (Latha et al., 2019). The massive therapeutic significance of plants exclusively relies upon the bioactive composites, particularly phytochemicals that produce physiological effects on human health. In this regard, an alternative approach to diversiform chemical constituents of the medicinal plant becomes imperative to the scientific community to validate and document its medicinal applications just as precursors for organizing complex synthetic substances. Therefore, this investigation established a distinctive role of P. granatum fruits peel as adaptogenic and bio-therapeutics. The phytochemical analysis of the fruits peel extracts exhibited the availability of diversified components in different solvent extracts. Findings supported the reports of Bhandary et al., 2012, Sangeetha, 2015 who observed the availability of identical phytoconstituents in different extracts of P. granatum fruits peel. These phytoconstituents are known to offer imperative biological activities on physiological systems (Karthikeyan and Vidya, 2019).
At present, the emergence of synthetic oral anti-diabetic inhibitors has improved the performance in the management of DM, and its multifaceted complications as well as susceptibility to infections. But the regular use of these inhibitors comes with diluted effects. The present study revealed a protective efficacy of P. granatum fruits peel extracts against chronic hyperglycemia-induced oxidative stress linked to DM, thereby suggesting an approach to ameliorate its potential burden through the antioxidant application. In the antioxidant traits, aqueous and ethanolic extracts showed varied ranges of DPPH scavenging activities. The present findings agreed with the reports of Venkatadri et al. (2017) who illustrated pronounced degradation of DPPH at disparate concentrations of the extracts. However, this investigation uncovered that aqueous extract elicited the potent antioxidant activity, linking the fact that active phytochemicals of P. granatum fruits peel are readily dissolved in an aqueous medium. Plants rich in phytoconstituents, such as alkaloids, saponins, flavonoids, and phenols have been marked to protect the human body from free radicals, improves the normal metabolism of aerobic cells, and confer strong antioxidant defence mechanisms (Oboh et al., 2014). In this investigation, the DPPH degrading potential of aqueous extract suggests the availability of many hydroxyl groups in the structure of saponins as contained in the aqueous extract than ethanolic, acetone, chloroform, and petroleum ether extracts responsible for the enhancement of antioxidant activity in DM (Elekofehinti, 2015). Hydrogen peroxide deactivates enzymes by the oxidation of essential thiol (-SH) groups and reacts with Fe2+ similar to Cu2+ ions for forming hydroxyl radicals (Venkatadri et al., 2017). In this study, P. granatum peel extracts showed moderate scavenging of H2O2 which might be due to glycosides found in the extracts.
Diabetes mellitus is a multi-factorial disorder which causes several other complications such as diminished response of T-cells, neutrophil function, and humoral immunity disorders (Muller et al., 2005). Therefore, it demands multiple therapeutic approaches. α-glucosidase aids the digestion of dietary carbohydrates and starches to produce glucose for intestinal absorption, which thus prompts upsurge in blood glucose concentrations. In diabetics, this enzyme inhibition leads to delayed glucose absorption and lowering of postprandial hyperglycemia. In fact, Ngozi and Olatunbosun (2016) reported that α-glucosidase inhibition partially diminished the degree of HbA1c. In a similar manner, α-glucosidase inhibitors suppressed postprandial hyperglycemia in DM (Lordan et al., 2013). In the present study, ethanolic extract was observed as the most active agent to inhibit α-glucosidase with respect to the aqueous extract. The high α-glucosidase inhibitory trait might be because of saponins present in the extract. Saponins have been found to play a significant impact in the cause of diabetic complications, and its hypoglycemic effect is mediated by distinct mechanisms, particularly the restoration of insulin response, α-glucosidase activity inhibition, and gluconeogenesis inhibition (Elekofehinti, 2015). Furthermore, the prominent α-glucosidase inhibition characteristic of extracts might also be because of phenols and flavonoids. Wang et al. (2004) established that hydroxyl group corresponds to the inhibition mechanism. In fact, it forms hydrogen bonds with the polar side chains of the amino acids, thereby modifying the molecular conformation of enzyme along with its hydrophilic and hydrophobic characteristics, prompting a decline in enzyme activity.
In furtherance of the efforts to explore the anti-obesity potency of P. granatum fruits peel, we investigated pancreatic lipase activity, a key enzyme for lipid digestion and dietary fats absorption (Yang et al., 2014). Ethanolic extract of the peel was observed as the most potent inhibitor of pancreatic lipase. This in vitro anti-obesity activity of P. granatum fruits peel extract might be adduced to its phytoconstituents especially phenols and flavonoids. Previous studies have observed the presence of pancreatic lipase inhibitors in some natural sources (Inthongkaew et al., 2017, Hengpratom et al., 2018).
Angiotensin-converting enzyme is a key enzyme which is known to catalyze the conversion of angiotensin I into the active vasoconstrictor, angiotensin II. ACE inhibitors are one of the mechanistic therapeutic strategies targeted for stabilizing blood pressure in hypertensive patients. The anti-hypertensive activities of medicinal plants act through the inhibition of ACE. A wide range of medicinal plants with ACE inhibitory activities have been reported in the past (Barbosa-Filho et al., 2006), and this activity was attributed to the synergistic action of secondary metabolites viz. alkaloids, flavonoids, tannins, proanthocyanidins, fatty acids, and terpenoids (Loizzo et al., 2007, Park et al., 2017). The ACE inhibitory activities of extracts might be because of flavonoid, alkaloid, and tannin contents, possibly through sequestration of enzyme metal co-factor, protein precipitation or through other mechanisms.
FT-IR and GC-MS chromatograms of ethanolic extract of fruits peel revealed the occurrence of a diverse bioactive components, particularly 1,2,3,4-butanetetrol, Dimethyl sulfone, 9-octadecenamide, and Pentadecanoic acid. Findings hypothesized that these bioactive metabolites may prevent metabolic diseases by inhibiting carbohydrate digestion and stimulating the secretion of insulin from pancreatic beta-cells (Hanhineva et al., 2010, Mohamed, 2014). In addition, these metabolites present in the extract could also be promising agents for exhibiting ACE inhibitory traits.
5. Conclusions
The present study demonstrated that P. granatum fruits peel extract attenuated enzyme inhibition in DM, obesity, and hypertension. In addition, FT-IR and GC-MS analyses of ethanolic extract of fruits peel revealed the occurrence of diverse bioactive compounds. Further extensive studies are required to get a deeper insight of not only on in silico molecular docking mechanisms of the bioactive substances in the metabolic pathway of obesity and hypertension in DM but also pivotal therapeutic role of P. granatum fruits peel through in vivo studies.
Declaration of Competing Interest
The authors declared that they do not have any conflict in publishing this research article.
Acknowledgement
We duly acknowledge the funding body University Grants Commission (Minor Project F. MRP-5503/15 SERO/UGC) for providing financial support to Dr. D. Esther Lydia. The authors also acknowledge sincerely the management of Loyola College, Chennai, India and Entomology Research Institute, Loyola College, Chennai, India for providing the facility for carrying out the research. The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for funding this Research Group No. (RG -1437-024).
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
D. Esther Lydia, Email: esilydi@yahoo.co.in.
Ameer Khusro, Email: armankhan0301@gmail.com.
Hak-Jae Kim, Email: hak3962@sch.ac.kr.
References
- Al-Dhabi N.A., Arasu M.V., Rejiniemon T.S. In vitro antibacterial, antifungal, antibiofilm, antioxidant, and anticancer properties of isosteviol isolated from endangered medicinal plant Pittosporum tetraspermum. Evidence-Based Complement. Alter. Med. 2015:2015. doi: 10.1155/2015/164261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Dhabi N.A., Arasu M.V. Quantification of phytochemicals from commercial Spirulina products and their antioxidant activities. Evidence-Based Complement. Alter. Med. 2016;2016 doi: 10.1155/2016/7631864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barathikannan K., Venkatadri B., Khusro A., Al-Dhabi N.A., Agastian P., Arasu M.V., et al. Chemical analysis of Punica granatum fruit peel and its in vitro and in vivo biological properties. BMC Complement. Alter. Med. 2016;16:264. doi: 10.1186/s12906-016-1237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa-Filho J.M., Martins V.K.M., Rabelo L.A., Moura M.D., Silva M.S., Cunha E.V.L., et al. Natural products inhibitors of the angiotensin-converting enzyme (ACE): a review between 1980–2000. Rev. Bras. Farmacogn. 2006;16:421–446. [Google Scholar]
- Bhandary S., Kumari S., Bhat V., Sharmila K., Beka M. Preliminary phytochemical screening of various extracts of Punica granatum peel, whole fruit, and seeds. J. Health Sci. 2012;2:35–38. [Google Scholar]
- Casqueiro J., Casqueiro J., Alves C. Infections in patients with diabetes mellitus: A review of pathogenesis. Indian J. Endocrinol. Metab. 2012;16(Suppl1):S27–S36. doi: 10.4103/2230-8210.94253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary G., TameezUd Din A., Chaudhary F., Tanveer A., Siddiqui K.H., TameezUd Din A. Association of obesity indicators with hypertension in type 2 diabetes mellitus patients. Cureus. 2019;11:e5050. doi: 10.7759/cureus.5050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary S.K., Mukherjee P.K., Maity N., Nema N.K., Bhadra S., Saha B.P. Ocimum sanctum L. A potential angiotensin converting enzyme (ACE) inhibitor useful in hypertension. Indian J. Nat. Prod. Res. 2014;5:83–87. [Google Scholar]
- Cuong D.M., Arasu M.V., Jeon J., Park Y.J., Kwon S.-J., Al-Dhabi N.A., Park S.U. Medically important carotenoids from Momordica charantia and their gene expressions in different organs. Saudi J. Biol. Sci. 2017;24:1913–1919. doi: 10.1016/j.sjbs.2016.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Sotto A., Locatelli M., Macone A., Toniolo C., Cesa S., Carradori S. Hypoglycemic, antiglycation, and cytoprotective properties of a phenol-rich extract from waste peel of Punica granatum L. var. Dente di Cavallo DC2. Molecules. 2019;24:3103. doi: 10.3390/molecules24173103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elekofehinti O.O. Saponins : Anti-diabetic principles from medicinal plants – A review. Pathophysiology. 2015;22:95–103. doi: 10.1016/j.pathophys.2015.02.001. [DOI] [PubMed] [Google Scholar]
- Esther Lydia D., Gupta C., Khusro A., Salem A.Z.M. Susceptibility of poultry associated bacterial pathogens to Momordica charantia fruits and evaluation of in vitro biological properties. Microb. Pathog. 2019;132:222–229. doi: 10.1016/j.micpath.2019.05.002. [DOI] [PubMed] [Google Scholar]
- Esther Lydia D., Khusro A., Immanuel P., Esmail G.A., Al-Dhabi N.A., Arasu M.V. Photo-activated synthesis and characterization of gold nanoparticles from Punica granatum L. seed oil: An assessment on antioxidant and anticancer properties for functional yoghurt nutraceuticals. J. Photochem. Photobiol. B: Biol. 2020;206:111868. doi: 10.1016/j.jphotobiol.2020.111868. [DOI] [PubMed] [Google Scholar]
- Goyal, R., Jialal, I., 2020. Diabetes Mellitus Type 2. [Updated 2020 Feb 28]. In: Stat Pearls [Internet]. Treasure Island (FL): Stat Pearls Publishing; 2020 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK513253.
- Hanhineva K., Törrönen R., Bondia-Pons I., Pekkinen J., Kolehmainen M., Mykkä-nen H., et al. Impact of dietary polyphenols on carbohydrate metabolism. Int. J. Mol. Sci. 2010;11:1365–1402. doi: 10.3390/ijms11041365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harborne J.B. Academic Press; London: 1993. Phytochemistry; pp. 89–131. [Google Scholar]
- Hengpratom T., Lowe G.M., Thumanu K., Suknasang S., Tiamyom K., Eumkeb G. Oroxylum indicum (L.) Kurz extract inhibits adipogenesis and lipase activity in vitro. BMC Complement. Altern. Med. 2018;18:1–14. doi: 10.1186/s12906-018-2244-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilavenil S., Kim D.H., Srigopalram S., Kuppusamy P., Arasu M.V., Lee K.D., Lee J.C., Song Y.H., Jeong Y.I., Choi K.C. Ferulic acid in Lolium multiflorum inhibits adipogenesis in 3T3-L1 cells and reduced high-fat-diet-induced obesity in Swiss albino mice via regulating p38MAPK and p44/42 signal pathways. J. Funct. Foods. 2017;37:293–302. [Google Scholar]
- Inthongkaew P., Chatsumpun N., Supasuteekul C., Kitisripanya T., Putalun W., Likhitwitayawuid K., et al. α-glucosidase and pancreatic lipase inhibitory activities and glucose uptake stimulatory effect of phenolic compounds from Dendrobium formosum. Braz. J. Pharmacogn. 2017;27:480–487. [Google Scholar]
- Jäkälä P., Vapaatalo H. Antihypertensive peptides from milk proteins. Pharmaceuticals. 2010;3:251–272. doi: 10.3390/ph3010251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janani J., Esther Lydia D. Total polyphenol content and minimum inhibitory concentration of pomegranate (Punica granatum Linn) extracts against oral microorganisms. Serbian Dental J. 2013;60:183–190. [Google Scholar]
- Karthikeyan G., Vidya A.K. Phytochemical analysis, antioxidant and antibacterial activity of pomegranate peel. Life Sci. Informat. Publ. 2019;5:218–231. [Google Scholar]
- Khusro A., Aarti C., Preetamraj J.P., Panicker S.G. In vitro studies on antibacterial activity of aqueous extracts of spices and vegetables against Bacillus licheniformis strain 018 and Bacillus tequilensis strain ARMATI. Int. J. Curr. Microbiol. Appl. Sci. 2013;2:79–88. [Google Scholar]
- Khusro A., Aarti C., Preetamraj J.P., Panicker S.G. Comparative study on the effect of different solvent extracts of Calotropis gigantea and Carica papaya latex against new bacterial isolates – An in vitro study. Int. J. Pharm. Pharm. Sci. 2014;6:874–879. [Google Scholar]
- Latha R., Rajanathan T.M.C., Khusro A., Chidambaranathan N., Agastian P., Nagarajan S. Anticancer activity of Mahonia leschenaultii methanolic root extract and berberine on Dalton’s ascitic lymphoma in mice. Asian Pac. J. Trop. Med. 2019;12:264–271. [Google Scholar]
- Loizzo M.R., Said A., Tundis R., Rashed K., Statti G.A., Hufner A., et al. Inhibition of angiotensin-converting enzyme (ACE) by flavonoids isolated from Ailanthus excelsa (Roxb) (Simaroubaceae) Phytother. Res. 2007;21:32–36. doi: 10.1002/ptr.2008. [DOI] [PubMed] [Google Scholar]
- Lordan S., Smyth T.J., Soler-Vila A., Stanton C., Ross R.P. The α-amylase and α-glucosidase inhibitory effects of Irish seaweed extracts. Food Chem. 2013;141:2170–2176. doi: 10.1016/j.foodchem.2013.04.123. [DOI] [PubMed] [Google Scholar]
- Madugula P., Reddy S., Koneru J., Rao A.S., Sruthi R., Dalli D.T. Rhetoric to Reality- Efficacy of Punica granatum peel extract on oral Candidiasis: An in vitro study. J. Clin. Diagnos. Res. 2017;11:ZC114–ZC117. doi: 10.7860/JCDR/2017/22810.9304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middha S.K., Usha T., Pande V. HPLC Evaluation of phenolic profile, nutritive content, and antioxidant capacity of extracts obtained from Punica granatum fruit peel. Adv. Pharmacol. Sci. 2013:296236. doi: 10.1155/2013/296236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal B.V., Singh A.K. Hypertension in the developing world: challenges and opportunities. Am. J. Kidney Dis. 2010;55:590–598. doi: 10.1053/j.ajkd.2009.06.044. [DOI] [PubMed] [Google Scholar]
- Mohamed S. Functional foods against metabolic syndrome (obesity, diabetes, hypertension, and dyslipidemia) and cardiovascular disease. Trends Food Sci. Technol. 2014;35:114–128. [Google Scholar]
- Muller L.M., Gorter K.J., Hak E., Goudzwaard W.L., Schellevis F.G., Hoepelman A.I. Increased risk of common infections in patients with type 1 and type 2 diabetes mellitus. Clin. Infect. Dis. 2005;41:281–288. doi: 10.1086/431587. [DOI] [PubMed] [Google Scholar]
- Ngozi R., Olatunbosun S. FT-IR analysis of Sapium ellipticum (Hochst) pax ethanol leaf extract and its inhibitory effects on pancreatic α -amylase and intestinal α -glucosidase activities in vitro. Egyptian J. Basic Appl. Sci. 2016;3:343–349. [Google Scholar]
- Nwaji N.N., Ojo O.O., Ayinla Z.A., Ajayi-Smith A.F., Mgbenka U.R., Nkwor A.N., et al. Antioxidant and angiotensin converting enzyme inhibition activity of Landophia owariensis. Int. J. Pharmacog. Phytochem. Res. 2016;8:871–880. [Google Scholar]
- Oboh G., Adelusi T., Akinyemi A.J., Ajani R.A. Inhibition of key enzymes linked to type 2 diabetes and sodium nitroprusside induced lipid peroxidation in rats ’ pancreas by phenolic extracts of avocado pear leaves and fruit. Int. J. Biomed. Sci. 2014;10:208–216. [PMC free article] [PubMed] [Google Scholar]
- Park C.H., Baskar T.B., Park S.-Y., Kim S.-J., Arasu M.V., Al-Dhabi N.A., Kim J.K., Park S.U. Metabolic profiling and antioxidant assay of metabolites from three radish cultivars (Raphanus sativus) Molecules. 2016;21:157. doi: 10.3390/molecules21020157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y.J., Baskar T.B., Yeo S.K., Arasu M.V., Al-Dhabi N.A., Lim S.S., Park S.U. Composition of volatile compounds and in vitro antimicrobial activity of nine Mentha spp. SpringerPlus. 2016;5:1628. doi: 10.1186/s40064-016-3283-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y.J., Park S.-Y., Arasu M.V., Al-Dhabi N.A., Ahn H.-G., Kim J.K., Park S.U. Accumulation of carotenoids and metabolic profiling in different cultivars of Tagetes flowers. Molecules. 2017;22:313. doi: 10.3390/molecules22020313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parveen A., Kim J.H., Oh B.G., Subedi L., Khan Z., Kim S.Y. Phytochemicals: Target-based therapeutic strategies for diabetic retinopathy. Molecules. 2018;23:1519. doi: 10.3390/molecules23071519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruch R.J., Cheng S.J., Klaunig J.E. Prevention of cytotoxicity and inhibition of intracellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis. 1989;10:1003. doi: 10.1093/carcin/10.6.1003. [DOI] [PubMed] [Google Scholar]
- Saeedi P., Petersohn I., Salpea P., Malanda B., Karuranga S., Unwin N., et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res. Clin. Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- Sangeetha, Jayaprakash., 2015. Phytochemical screening of Punica granatum Linn. peel extracts. J. Acad. Ind. Res. 4, 160–162.
- Shimada K., Fujikawa K., Yahara K., Nakamura T. Antioxidative properties of xanthan on the autioxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem. 1992;40:945–948. [Google Scholar]
- Venkatadri B., Khusro A., Aarti C., Rameshkumar M.R., Agastian P. In vitro assessment on medicinal properties and chemical composition of Michelia nilagirica bark. Asian Pac. J. Trop. Biomed. 2017;7:782–790. [Google Scholar]
- Wang Y., Ma L., Li Z., Du Z., Liu Z., Qin J. Synergetic inhibition of metal ions and genistein on alpha-glucosidase. FEBS Lett. 2004;576:46–50. doi: 10.1016/j.febslet.2004.08.059. [DOI] [PubMed] [Google Scholar]
- Yang M.H., Chin Y.W., Yoon K.D., Kim J. Phenolic compounds with pancreatic lipase inhibitory activity from Korean yam (Dioscorea opposita) J. Enzyme Inhib. Med. Chem. 2014;29:1–6. doi: 10.3109/14756366.2012.742517. [DOI] [PubMed] [Google Scholar]
- Zheng C.D., Duan Y.Q., Gao J.M., Ruan Z.G. Screening of anti-lipase properties of 37 traditional Chinese medicinal herbs. J. Chin. Med. Assoc. 2010;73:319–324. doi: 10.1016/S1726-4901(10)70068-X. [DOI] [PubMed] [Google Scholar]