Abstract
Objectives:
To identify risk factors of geriatrics index of comorbidity (GIC) and multidetector CT (MDCT) findings for predicting mortality in patients with acute mesenteric ischemia (AMI) due to superior mesenteric artery (SMA) thromboembolism.
Methods:
33 patients with AMI due to SMA thromboembolism underwent abdominal MDCT and angiography. Patients’ comorbidities and MDCT findings of ischemic bowel/mesenteric injuries, regions of SMA involved by thromboembolism, and degrees of SMA stenosis were retrospectively reviewed. The comorbidities were classified into 1–4 levels according to GIC. The association of MDCT signs and GIC classification with mortality were analyzed. Diagnostic performances of risk factors associated with mortality were evaluated by receiver operating characteristic (ROC) curve analyses.
Results:
Eighteen patients (54.5%) died during hospitalization or follow-up, including one patient with class 1, two patients with class 2, eight patients with class 3, and seven patients with class 4 according to GIC. Three risk factors significantly associated with mortality were identified, including pneumatosis and/or portomesenteric venous gas (PPMVG) (p = 0.017), four regions of SMA involved by thromboembolism (region I + II + III + IV) (p = 0.036), and class 3 + 4 of comorbidities (p = 0.001). The sensitivity and specificity of PPMVG, region I + II + III + IV, class 3 + 4 of comorbidities, and the three risk factors combined for diagnosing mortality were 33.3 and 100%, 27.8 and 100%, 83.3 and 73.3%, and 88.9 and 73.3%, respectively. The areas under the ROC curve (AUC) of the three risk factors combined (0.88) and class 3 + 4 of comorbidities (0.78) were larger than that of PPMVG (0.67) and region I + II + III + IV (0.64). The mortality rate rose from 15.4% in patients without risk factor to 66.7%, 100%, and 100% in patients with one, two, and three factors, respectively.
Conclusion:
Three risk factors for mortality were identified in patients with AMI due to SMA thromboembolism, including PPMVG and four regions of SMA involved by thromboembolism on MDCT images, and class 3 + 4 of comorbidities. Close monitoring of these risk factors could possibly lower the mortality.
Advances in knowledge:
Risk factors based on GIC and MDCT findings may be used to predict mortality in patients with AMI. Close monitoring of these risk factors could possibly lower the mortality.
Introduction
Acute arterial thromboembolism is the most common cause of acute mesenteric ischemia (AMI), the superior mesenteric artery (SMA) is the visceral arterial branch that is most involved owing to its small branching angle from the aorta.1,2 AMI due to SMA thromboembolism is a life-threatening and frequently lethal vascular emergency with a very high mortality rate, early accurate diagnosis and timely treatment are crucial to improve the prognosis.3–5 Multidetector computed tomography (MDCT) is recommended as the first-step imaging approach for AMI because of a fast and accurate diagnostic tool for evaluating both the bowel and its vasculature.6,7 The sensitivity and specificity of MDCT angiography in the diagnosis of AMI are as high as 93–100%, which has the potential to improve the survival rate of patients.8,9 A number of studies have proved that some MDCT findings combining with clinical and laboratory parameters have the abilities to predict transmural bowel infarction, severity, and outcome of AMI.10–13 Thus, MDCT has played an important role in making diagnosis, treatment option and prognosis assessment for AMI.
AMI due to SMA thromboembolism remains a high mortality rate despite advances in diagnostic methods and treatment options.3,4,14 Higher morbidity rate in the aged and atypical clinical manifestation resulted in delayed diagnosis contribute to the high mortality rate, also elderly AMI patients with health status may have a strong influence on their overall risk of death.1,3,15 There is a wide range of data on risk stratification in patients with AMI based on the MDCT findings, laboratory tests, length of ischemic bowel necrosis, intensive care unit stay, etc.10,12,13,16 However, the data on risk stratification are few for all-cause mortality in patients with AMI on the basis of the presence of comorbidities. These data are very useful for both radiologists and surgeons who want to objectively assess the risk of death imposed by comorbidities in individual patients before making treatment plan.
Several indicators have been suggested to quantify comorbidity in adults, including Charlson comorbidity index, cumulative illness rating scale, index of coexisting disease, Kaplan index, geriatrics index of comorbidity (GIC) and chronic disease score.15,17,18 To compare the abilities of six validated comorbidity indicators to predict adverse hospitalization outcomes, GIC was the best predictor of prognosis in old patients with acute disease.15 The GIC classifies patients into levels 1–4 according to the number and severity of comorbidities.15,18 The purpose of the study was to identify the ability of MDCT findings and GIC to predict the mortality of patients with AMI due to SMA thromboembolism.
Methods and materials
Study population
This research was designed as a retrospective, observational, non-interventional, monocentric study, and was approved by our institutional ethics committee. The requirement for individual consent was waived due to the retrospective nature of the study. From February 2013 to December 2018, consecutive patients admitted with verified or clinically suspected AMI were referred to the radiology department for MDCT examinations. Prior to MDCT scanning, all patients underwent clinical examinations by either a gastroenterologist or a vascular surgeon. Of these, patients with venous mesenteric ischemia, non-occlusive mesenteric ischemia, and mechanically induced mesenteric ischemia were excluded. Only patients with acute SMA thromboembolism and abnormal findings of bowel/mesentery verified by MDCT were retrospectively analyzed. Finally, a total of 33 patients with AMI due to SMA thromboembolism were enrolled in the study.
MDCT scanning
All patients underwent unenhanced and dual-phase enhanced (hepatic arterial and portal venous phases) MDCT scans on either a 64-row MDCT scanner (GE Healthcare, United States) or a 256-slice MDCT scanner (Philips Healthcare, Cleveland) with a coverage extending from the dome of the diaphragm to the lower edge of the right kidney or the symphysis pubis. The scan parameters were as follows: collimation of 64 × 0.625 mm or 256 × 0.5 mm and table speed of 64 or 256 mm per rotation. The following were applied to all scans: pitch 0.984, matrix 512 × 512, field of view 240–400 mm, tube voltage 120 kV, and tube current 250–300 mA.
A dual-head power injector was used to inject the contrast agent (Ultravist 370 mg iodine/mL; Bayer Schering Pharma, Berlin, Germany) at 2 mL/kg body weight followed by 30 ml of saline, with an injection rate of 4 ml s−1 through an antecubital vein. The upper dose limit of contrast agent was set to 120 ml for each patient. Images of the hepatic arterial and portal venous phases were acquired at 20 and 50 s after completion of the contrast agent administration, respectively.
Image analyses
All data obtained were transferred to an off-line workstation (AW 4.3 or 4.5; General Electric Healthcare, Milwaukee, USA) for image post-processing and analysis. Multiplanar reformation (MPR), curved planar reformation (CPR), volume rendering (VR) and maximum intensity projection (MIP) were used to reformat images and analyze the MDCT findings of the SMA thromboembolism and secondary bowel/mesenteric ischemia by three abdominal radiologists with 12, 15 and 23 years of experience, respectively. The radiologists independently reviewed the images, and final agreements were achieved by consensus.
Images from each examination were evaluated for the MDCT signs of bowel/mesenteric ischemia,19,20 including (1) bowel wall thickening (>3 mm small bowel and >5 mm colonic wall thickness); (2) bowel wall edema was determined by the presence of submucosal edema with a visible target or halo sign; (3) bowel-loop dilatation was indicated as small bowel >2.5 cm and large bowel >6 cm in inner-diameter; (4) subjective presence of bowel wall thinning, defined as thin as paper because of severe bowel-loop dilatation; (5) increased bowel wall attenuation, compared with adjacent normal bowel segments on unenhanced MDCT images; (6) decreased bowel wall enhancement, compared with adjacent normal bowel segments on dual-phase enhanced MDCT images; (7) mesenteric haziness or fluid was defined as an area of hazy increased attenuation in the mesentery; (8) pneumatosis and/or portomesenteric venous gas (PPMVG); (9) mesenteric vascular engorgement, defined as relative dilatation of mesenteric vessels; (10) free intraperitoneal gas; (11) free intraperitoneal fluid; (12) solid organ infarction.
The radiologists also assessed the location and extension of SMA thromboembolism, and the degrees of SMA stenosis on MDCT images. The SMA can be divided into four regions21 : region I is the origin; region II, the main trunk including the origin of the middle colic artery (MCA); region III, the main trunk beyond the origin of the MCA; and region IV, the more peripheral portion of the SMA and its branches (Figure 1). As the thromboembolism may locate in more than one region of SMA, thromboembolism locating in more than one region of SMA was described as one region plus another. The degrees of SMA stenosis on MDCT images were graded as mild (<50%), moderate (50% to <75%), severe (75% to <100%), and complete occlusion (100%).4
Figure 1.

Illustration of four anatomical regions for the SMA. I, region I is the origin of the SMA; II, region II is the main trunk including the origin of the MCA; III, region III is the main trunk beyond the origin of the MCA; IV, region IV is the peripheral portion of the SMA and its branches. MCA, middle colic artery; ICA, ileocolic artery.
Comorbidity classification
Electronic medical records were reviewed by one gastroenterologist with over 10 years of experience for grading comorbidities according to GIC. The GIC was defined based on number of comorbidities, and severity of comorbidities which was graded from 0 to 4 scale according to the following criteria: 0 = absence of comorbidities, 1 = asymptomatic comorbidities, 2 = symptomatic comorbidities needing medication but under satisfactory control, 3 = symptomatic comorbidities uncontrolled by treatment, and 4 = life threatening or greatest severity of comorbidities. The GIC divided patients into 4 classes of increasing somatic comorbidity. Class one includes patients without comorbidities or patients who have no less than one comorbidities with grade of disease severity equal to 1, class two includes patients who have no less than one comorbidities with grade of disease severity equal to 2, class three includes patients who have one comorbidity with grade of disease severity equal to three and other comorbidities with grade of disease severity equal to or lower than 2, class four includes patients who have no less than two comorbidities with grade of disease severity equal to three or no less than one comorbidities with grade of disease severity equal to 4.15,18,22
Prognosis during hospitalization and Follow-up
Electronic medical records were reviewed for the prognosis of patients during hospitalization. A follow up of the surviving patients was performed through telephone calls and outpatient clinic visits for 0.3 to 24 months.
Statistical analysis
Data are presented as mean ± standard deviation (SD) or percentage. Statistical analyses were performed using Pearson χ2 test or Fisher exact test for categorical variables, and independent sample t test for continuous variables with normal distribution. Continuous variables without normal distribution were analyzed by non-parametric two-independent sample test. Diagnostic performances of risk factors associated with mortality significantly were evaluated by ROC curve analyses. Since patients with classes 1 and 2 have no coexisting diseases or coexisting diseases under satisfactory control which have only little effects on mortality, and patients with classes 3 and 4 have more severe coexisting diseases which have considerable effects on mortality, allowing us to combine classes 1 and 2, and classes 3 and 4 for analyzing the association with mortality, expressed as class 1 + 2 and class 3 + 4. The ROC curve analyses were performed using the MedCalc software V.17.2 (MedCalc Software, Mariakerke, Belgium) with the method of DeLong et al23 for the calculation of the area under the ROC curve (AUC), other statistical analyses were performed using the SPSS V.22.0 statistical software package (SPSS Inc., Chicago, USA). p < 0.05 was considered significantly different.
Results
Outcomes of hospitalization and Follow-up
Thirty-three patients with AMI included thirteen female and twenty male patients, with a mean age of 68.7 ± 11.7 years (range 45–87 years) on the day of study enrollment. Endovascular treatment was performed in sixteen patients, ischemic bowel necrosis occurred in five patients after the endovascular treatment and further underwent a laparotomy for resection of necrotic bowel. Open surgery was performed in nine patients, and the remaining eight patients only received conservative anticoagulant therapy because of older age, severe conditions, or contraindications for endovascular and surgery treatment. The treatment decision was made by attending physicians based on clinical, laboratory tests, and image findings.
Finally, among sixteen patients underwent endovascular treatment or necrotic bowel resection, six patients died during hospitalization and three patients died within 30 days follow up. Among nine patients underwent open surgery, three patients died during hospitalization and two patients died within 10 days follow up. Among eight patients received conservative anticoagulant therapy, four patients died during hospitalization. The causes of death included septic shock, multiple organ failure, gastrointestinal bleeding, or/and cardiac arrest. The mortality was not significant difference between endovascular or/and surgery (56%, 14/25) and conservative treatment (50%, 4/8) for the 33 patients (p = 0.541).
Eighteen died patients with mean age of 70.2 ± 11.2 years (49–87 years), comprised of ten males and eight females with duration of symptoms before admission of 2.8 ± 2.0 days (1–7 days). Fifteen survived patients with mean age of 66.9 ± 12.4 years (45–83 years), comprised of ten males and five females with duration of symptoms before admission of 3.4 ± 2.6 days (1–10 days). There was no significant difference between died and survived patients for the age (p = 0.437), sex (p = 0.515), and duration of symptoms before admission (p = 0.532) (Table 1).
Table 1.
Clinical characteristics and comorbidities in the 33 patients with AMI
| Clinical findings | All patients with AMI (n = 33) | Survived patients with AMI (n = 15) | Died patients with AMI (n = 18) | P valuea |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age (years; mean ± SD) | 68.7 ± 11.7 | 66.9 ± 12.4 | 70.2 ± 11.2 | 0.437 |
| Sex (male/female) | 20/13 | 10/5 | 10/8 | 0.515 |
| Duration of symptoms before admission (days ± SD) | 3.1 ± 2.2 | 3.4 ± 2.6 | 2.8 ± 2.0 | 0.532 |
| Endovascular or surgery/ conservative treatment (n) | 25/8 | 11/4 | 14/4 | 0.541 |
| Comorbidities [n (%)] | ||||
| Hypertension | 24 (72.7%) | 11 (73.3%) | 13 (72.2%) | 0.627 |
| Diabetes mellitus | 7 (21.2%) | 3 (20%) | 4 (22.2%) | 0.609 |
| Atrial fibrillation | 12 (36.4%) | 7 (46.7%) | 5 (27.8%) | 0.261 |
| Coronary heart disease | 14 (42.4%) | 5 (33.3%) | 9 (50%) | 0.335 |
| Cerebrovascular disease | 6 (18.2%) | 2 (13.3%) | 4 (22.2%) | 0.423 |
| Chronic pulmonary disease | 4 (12.1%) | 1 (6.7%) | 3 (16.7%) | 0.374 |
| Rheumatic heart disease | 3 (9.1%) | 1 (6.7%) | 2 (11.1%) | 0.570 |
| Peripheral vascular disease | 3 (9.1%) | 1 (6.7%) | 2 (11.1%) | 0.570 |
| Renal function insufficiency | 4 (12.1%) | 2 (13.3%) | 2 (11.1%) | 0.626 |
| Colon cancer | 1 (3%) | 0 | 1 (5.6%) | 0.545 |
| Urinary bladder cancer | 1 (3%) | 0 | 1 (5.6%) | 0.545 |
AMI, acute mesenteric ischemia; SD, standard deviation.
Comparison between survived and died patients.
MDCT findings and prediction of mortality
The number of patients having MDCT signs of bowel/mesenteric ischemia in 18 died and 15 survived patients are described in Table 2. Decreased bowel wall enhancement (63.6%), mesenteric vascular engorgement (54.5%), bowel wall thickening (42.4%) and bowel-loop dilatation (42.4%) (Figures 2–5) were found frequently on MDCT images in the 33 patients with AMI, only PPMVG (p = 0.017) (Figures 3–6) was significantly associated with mortality. In addition, solid organ infarction was observed in 3 (16.7%) of 18 died patients and 5 (33.3%) of 15 survived patients, and was located in the spleen, kidney, and liver (Figure 6). There was no significant difference for solid organ infarction association with mortality (p = 0.240).
Table 2.
MDCT findings of AMI in survived and died patients
| MDCT findings | All patients with AMI (n = 33) | Survived patients with AMI (n = 15) | Died patients with AMI (n = 18) | P valuea |
|---|---|---|---|---|
| MDCT signs | ||||
| Bowel wall thickening | 14 (42.4%) | 8 (53.3%) | 6 (33.3%) | 0.211 |
| Bowel wall edema | 3 (9.1%) | 2 (13.3%) | 1 (5.6%) | 0.430 |
| Bowel-loop dilatation | 14 (42.4%) | 6 (40%) | 8 (44.4%) | 0.797 |
| Bowel wall thinning | 5 (15.2%) | 1 (6.7%) | 4 (22.2%) | 0.229 |
| Increased bowel wall attenuation | 1 (3%) | 0 | 1 (5.6%) | 0.545 |
| Decreased bowel wall enhancement | 21 (63.6%) | 7 (46.7%) | 14 (77.8%) | 0.068 |
| PPMVG | 6 (18.2%) | 0 | 6 (33.3%) | 0.017 |
| Mesenteric haziness or fluid | 4 (12.1%) | 2 (13.3%) | 2 (11.1%) | 0.626 |
| Mesenteric vascular engorgement | 18 (54.5%) | 10 (66.7%) | 8 (44.4%) | 0.202 |
| Free intraperitoneal gas | 2 (6.1%) | 1 (6.7%) | 1 (5.6%) | 0.710 |
| Free intraperitoneal fluid | 5 (15.2%) | 3 (20%) | 2 (11.1%) | 0.409 |
| Solid organ infarction | 8 (24.2%) | 5 (33.3%) | 3 (16.7%) | 0.240 |
| MDCT angiographic findings | ||||
| Degree of SMA stenosis | ||||
| Mild (<50%) | 3 (9.1%) | 2 (13.3%) | 1 (5.6%) | 0.430 |
| Moderate (50% to <75%) | 5 (15.2%) | 2 (13.3%) | 3 (16.7%) | 0.591 |
| Severe (75% to <100%) | 9 (27.3%) | 6 (40%) | 3 (16.7%) | 0.135 |
| Complete occlusion (100%) | 16 (48.5%) | 5 (33.3%) | 11 (61.1%) | 0.112 |
| Region of SMA thromboembolism | ||||
| Region I | 2 (6.1%) | 1 (6.7%) | 1 (5.6%) | 0.710 |
| Region II | 4 (12.1%) | 1 (6.7%) | 3 (16.7%) | 0.374 |
| Region III | 5 (15.2%) | 3 (20%) | 2 (11.1%) | 0.409 |
| Region IV | 2 (6.1%) | 1 (6.7%) | 1 (5.6%) | 0.710 |
| Region II + III | 4 (12.1%) | 3 (20%) | 1 (5.6%) | 0.234 |
| Region III + IV | 8 (24.2%) | 4 (26.7%) | 4 (22.2%) | 0.541 |
| Region II + III + IV | 3 (9.1%) | 2 (13.3%) | 1 (5.6%) | 0.430 |
| Region I + II + III + IV | 5 (15.2%) | 0 | 5 (27.8%) | 0.036 |
AMI, acute mesenteric ischemia; MDCT, multidetector computed tomography; PPMVG, pneumatosis and/or portomesenteric venous gas; SMA, superior mesenteric artery.
Comparison between survived and died patients.
Figure 2.
An 81-year-old female patient with AMI due to SMA thromboembolism. (A) and B): Pretreatment MDCT images show decreased small bowel wall enhancement (thin arrows) and SMA thromboembolism (thick arrows) in its regions II and III, the degree of SMA stenosis is complete occlusion (100%). (C) and D): MDCT images after endovascular treatment show bowel wall edema (thin arrows), mesenteric haziness or fluid (☆), free intraperitoneal fluid (*), and a catheter (short arrow) in the SMA for thrombolysis.
Figure 3.
A 51-year-old male patient with AMI due to SMA thromboembolism. (A) and B): MDCT multiplanar reformation images show small bowel wall thickening (thin arrows), small bowel-loop dilatation (*), pneumatosis (arrowheads), and SMA thromboembolism (thick arrow). (C): MDCT maximum intensity projection image show that thromboembolism (arrows) is located in the region III of the SMA, and the degree of SMA stenosis is severe (>75%).
Figure 4.
A 60-year-old male patient with AMI due to SMA thromboembolism. (A) and B): MDCT multiplanar reformation images show bowel-loop dilatation (*) and pneumatosis (thin arrows), thromboembolism (thick arrows) is located in all 4 regions of the SMA (region I + II + III + IV) and the degree of SMA stenosis is complete occlusion (100%).
Figure 5.
A 59-year-old female patient with AMI due to SMA stenosis. (A) and B): MDCT multiplanar reformation images show pneumatosis (thin arrows), bowel-loop dilatation (*) and bowel wall thinning, infarction of the liver, spleen and kidney (☆), free intraperitoneal fluid (arrowheads), thromboembolism (thick arrows) is located in all 4 regions of the SMA (region I + II + III + IV) with a stenosis degree of 100% (complete occlusion).
Figure 6.
A 69-year-old female patient with AMI due to SMA thromboembolism. (A) and B): MDCT multiplanar reformation images show pneumatosis and portomesenteric venous gas (thin arrows), mesenteric haziness or fluid (☆), free intraperitoneal fluid (*), and SMA thromboembolism (thick arrows). (C): MDCT maximum intensity projection image show that thromboembolism (arrows) is located in the regions II–IV of the SMA with a stenosis degree of 100% (complete occlusion).
On MDCT angiographic images, the regions of SMA thromboembolism and degrees of SMA stenosis in 18 died and 15 survived patients are described in Table 2. Four regions of SMA involved by thromboembolism (region I + II + III + IV) was found only in five died patients (Figures 4 and 5), and was significantly associated with mortality (p = 0.036). No significant difference was found between died and survived patients for degrees of SMA stenosis.
GIC and prediction of mortality
The number of patients with AMI having different comorbidities at the time of admission is listed in Table 1. In the 33 patients with AMI, comorbidities mainly included diabetes mellitus, hypertension, atrial fibrillation, cardiovascular disease, cerebrovascular disease, and peripheral vascular disease. According to the GIC, Fifteen survived patients had two patients with class 1, nine patients with class 2, two patients with class 3, and two patients with class 4; while Eighteen died patients had one patient with class 1, two patients with class 2, eight patients with class 3, and seven patients with class 4. There were 14 patients (42.4%) with class 1 + 2 of comorbidities and 19 patients (57.6%) with class 3 + 4 of comorbidities. The mean age in class 3 + 4 of patients (71.4 ± 10.8 years [50–87 years]) was larger than that of class 1 + 2 of patients (65.0 ± 12.2 years [45–81 years]), but no significant difference was observed (p = 0.120). Various kinds of comorbidities were not significantly associated with mortality (p > 0.05). However, class 3 + 4 of comorbidities by GIC was significantly associated with mortality and class 1 + 2 was inversely correlated with mortality (p = 0.001) (Table 3).
Table 3.
Classification of comorbidities by GIC in survived and died patients with AMI
| GIC | All patients with AMI (n = 33) | Survived patients with AMI (n = 15) | Died patients with AMI (n = 18) | P valuea |
|---|---|---|---|---|
| Class 1 + 2 of comorbidities | 14 (42.4%) | 11 (78.6%) | 3 (21.4%) | 0.001 |
| Class 3 + 4 of comorbidities | 19 (57.6%) | 4 (21.1%) | 15 (78.9%) |
AMI, acute mesenteric ischemia; GIC, geriatrics index of comorbidity.
Comparison between AMI patients with class 1 + 2 and 3 + 4 of comorbidities for the percentage of survived and died patients.
ROC curve analyses of risk factors associated with mortality
For diagnosing mortality, the sensitivity and specificity of PPMVG, region I + II + III + IV, class 3 + 4 of comorbidities, and the three risk factors combined were 33.3 and 100%, 27.8 and 100%, 83.3 and 73.3%, and 88.9 and 73.3%, respectively. The AUCs of PPMVG, region I + II + III + IV, class 3 + 4 of comorbidities, and the three risk factors combined were 0.67 ± 0.09 (95% CI 0.48–0.82), 0.64 ± 0.10 (95% CI 0.45–0.80), 0.78 ± 0.07 (95% CI 0.61–0.91), and 0.88 ± 0.06 (95% CI 0.72–0.97), respectively. The AUCs of the three risk factors combined and class 3 + 4 of comorbidities were larger than that of PPMVG and region I + II + III + IV (Table 4, Figure 7). On the basis of these findings, the mortality rate in the 33 patients with AMI rose from 15.4% in patients without risk factor to 66.7%, 100%, and 100% in patients with one, two, and three factors, respectively.
Table 4.
ROC curve analyses of the variables associated with mortality
| Variables | AUC ± SD (95% CI) | Sensitivity | Specificity | P value |
|---|---|---|---|---|
| PPMVG | 0.67 ± 0.09(0.48–0.82) | 33.3% | 100% | 0.004 |
| Region I + II + III + IV | 0.64 ± 0.10(0.45–0.80) | 27.8% | 100% | 0.011 |
| Class 3 + 4 of comorbidities | 0.78 ± 0.07(0.61–0.91) | 83.3% | 73.3% | <0.001 |
| Three risk factors combineda | 0.88 ± 0.06(0.72–0.97) | 88.9% | 73.3% | <0.001 |
AUC, area under ROC curve; CI, confidence intervals; PPMVG, pneumatosis and/or portomesenteric venous gas; ROC, receiver operating characteristic; SD, Standard deviation.
Combined PPMVG, region I + II + III + IV and class 3 + 4 of comorbidities.
Figure 7.
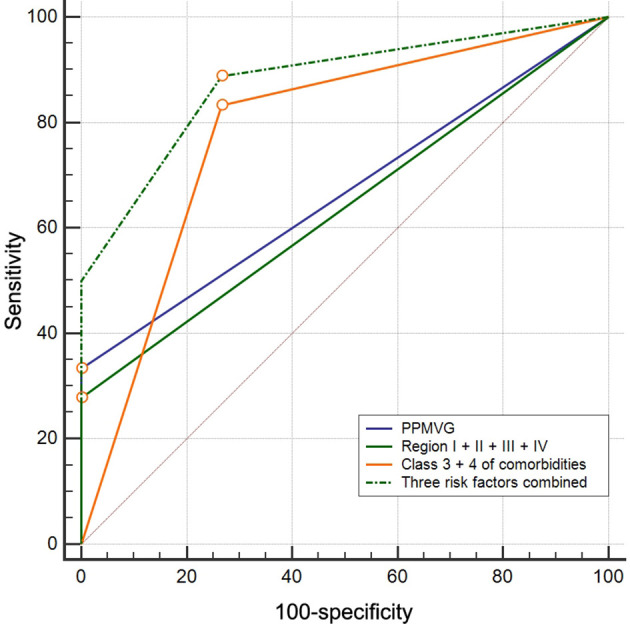
ROC curve analyses of PPMVG, region I + II + III + IV, class 3 + 4 of comorbidities, and the three risk factors combined for diagnosing mortality in patients with AMI due to SMA thromboembolism. The AUCs of the three risk factors combined (0.88) and class 3 + 4 of comorbidities (0.78) were larger than that of PPMVG (0.67) and region I + II + III + IV (0.64), which suggested that class 3 + 4 of comorbidities may be more discriminative than two MDCT signs in diagnosing mortality of patients with AMI, and each of the three risk factors had an additive effect. ROC, receiver operating characteristic; PPMVG, pneumatosis and/or portomesenteric venous gas; AUC, area under the ROC curve; AMI, acute mesenteric ischemia; SMA, superior mesenteric artery.
Discussion
In this retrospective study, including 33 AMI patients due to SMA thromboembolism treated in our hospital, we identified three risk factors that were significantly associated with mortality, including PPMVG and four regions of SMA involved by thromboembolism (region I + II + III + IV) on MDCT images, and class 3 + 4 of comorbidities by GIC. Furthermore, each of the three risk factors had an additive effect, AMI patients with two or three risk factors had mortality rate with 100%, and should be cared intensively by physician. This suggests that close monitoring of these risk factors could possibly lower the mortality.
On ROC curve analyses, the AUC of class 3 + 4 of comorbidities was larger than that of two MDCT signs, and the class 3 + 4 of comorbidities had 83.3% of sensitivity and 73.3% of specificity. This suggests that comorbidities (class 3 + 4) are more discriminative than MDCT findings for diagnosing mortality in patients with AMI due to SMA thromboembolism. Comorbidity is an inevitable prognostic factor for AMI due to SMA thromboembolism. First, the prevalence of AMI caused SMA thromboembolism is higher in the elderly people, and elderly people often have preexisting or coexisting diseases that may have a profound impact on their overall mortality risk.1,3,15,24 Second, some of the comorbidities are likely to affect the decision to make treatment, especially comorbidities in elderly people with AMI may affect the surgeons’ decision to operate or whether to remove necrotic bowel.3,25
To the best of our knowledge, there are very few reports about evaluation of comorbidities in patients with AMI. Grotelüschen et al16 reported that atrial fibrillation, diabetes, or atherosclerosis were not significantly associated with mortality of AMI patients. The study of Grotelüschen et al16 had some limitations, the severity of coexisting diseases was not considered in patients with AMI, and only three types of comorbidities were included. Nuzzo et al10 reported that higher incidence of atherosclerotic risk factors and cardiovascular comorbidities were found in AMI patients with bowel necrosis, which suggests that comorbidities are likely to association with prognosis of AMI patients. In our study, the number of co-occurrence diseases in one patient and severity of comorbidities were considered. As a patient with AMI may have more than one kind of co-occurrence disease, this explains why various kinds of comorbidities were not significantly associated with mortality, but the severity of comorbidities classified by GIC, class 3 + 4 of comorbidities can be as one of risk factors in predicting the mortality of AMI patients.
MDCT Imaging-based early diagnosis and management are recommended for AMI patients.6,7,26 A study demonstrated that the rate of in-hospital mortality was 42% for patients with acute SMA occlusion who underwent contrast-enhanced MDCT, versus 71% for patients without MDCT examination.26,27 Hence, contrast-enhanced MDCT plays an important role in prognosis improvement for AMI, because of fast acquisition of images, and imaging both bowel and vessels in real time.
Which MDCT findings associating with prognosis, and being as an independent risk factor exist in controversy.10–12,19,20 A prospective study from an intestinal stroke center reported that only bowel loop dilation on MDCT was identified as an independent risk factor for irreversible ischemic bowel necrosis requiring resection in the setting of AMI, and close monitoring of which could possibly lower the mortality.10 Another retrospective study confirmed that MDCT findings of both ischemic bowel injury and vascular occlusion were not significantly associated with mortality of AMI patients.16 Our results demonstrated that PPMVG and four regions of SMA involved by thromboembolism (region I + II + III + IV) were associated with mortality of AMI patients. Our study samples included only patients with arterial AMI, other studies’ samples were consisted of patients with arterial, venous, and non-occlusive AMI.10,12,16 The difference of study samples may be the cause of different results for the association between MDCT signs and mortality. In addition, the degree of SMA stenosis was not associated with mortality in our study, the reason may be that the mesenteric circulation has extensive collateral circulation.28
Compared with comorbidities, PPMVG and region I + II + III + IV have lower sensitivity and the highest specificity, their specificity for diagnosing mortality reach to 100% in our study. PPMVG is considered as a specific finding for diagnosis of ischemic bowel necrosis on MDCT images.10,29,30 The bowel necrosis often causes serious complications in AMI patients, such as septic shock, multiple organ function failure, short bowel syndrome.10 These serious complications are likely to cause death especially for elderly AMI patients with comorbidities. Similarly, the whole regions of SMA involved by thromboembolism (region I + II + III + IV) is likely to cause more extent of ischemic bowel injury or bowel necrosis, because the extent of ischemic bowel injury correlate with the site of SMA occlusion.21 And this situation is difficult to manage and often leads to death. This can explain that PPMVG and region I + II + III + IV have the highest specificity for diagnosing mortality in our study.
The time factor of duration of symptoms before admission affects mortality less in our study. It is likely that non-specific complaints and patient referral preclude our ability to record accurate time from acute onset to admission, especially for elder patients or patients with severe comorbidities. In addition, Edwards et al31 reported no beneficial effect on survival with shorter intervals to treatment because of higher percentage of established intestinal necrosis in AMI patients due to SMA thromboembolism, which is similar to our results.
The age factor deserves special attention in AMI patients with comorbidities. Both AMI and comorbidities are the high prevalence in the elderly people.3,15 As the mean age in patients with AMI increases due to the longer life expectancy, the proportion of AMI patients with serious comorbidities also increases. In our study, patients with class 3 + 4 of comorbidities were more than that with class 1 + 2 of comorbidities because most of these patients were old age. Although a difference in age existed between patients with class 3 + 4 of comorbidities and class 1 + 2 of comorbidities, this difference was not statistically significant. Similarly, the age was also not significantly related to the mortality of patients with AMI in our study. With the development of medical technology, the age factor can be overcome in the treatment. The patient’s function status and the presence of comorbid conditions that outweigh the age factor must be evaluated during the diagnosis and treatment for patients with AMI.
It was worth mentioning that our study sample consisted of 4 AMI patients with renal function insufficiency, the adverse effects of contrast agent should be remained a concern for these patients who underwent intravenous contrast-enhanced MDCT scan, because iodinated contrast can pose a risk of post-contrast acute kidney injury for patients with impaired renal function.32 However, contrast-enhanced MDCT is recommended to perform for AMI patients with renal function insufficiency in the emergent setting, as the consequences of delayed diagnosis, missed diagnosis, or mismanagement are far more detrimental to the post-contrast acute kidney injury.33 After MDCT examinations, serum creatinine and electrolytes were monitored, and intravenous fluid resuscitation with crystalloids were started promptly to correct the volume deficit and metabolic derangement for these AMI patients with renal function insufficiency. These management strategies could decrease the incidence and the severity of post-contrast acute kidney injury.
Our study has several limitations. First, this is a retrospective study, which might lead to patient selection bias and incompleteness of some information of patients. Second, the etiology of SMA thromboembolism did not be classified into embolic and thrombotic occlusion. An autopsy for 213 patients with acute thromboembolic occlusion of SMA found the etiology of SMA occlusion were thrombotic rather than embolic in 10 patients with cardiac thrombi and 14 patients with atrial fibrillation, this indicates that identification of SMA embolic occlusion based on atrial fibrillation or cardiac thrombi in clinical series will lead to an overestimation of patients with embolic occlusions.34 Thus, part of patients underwent endovascular or conservative treatment were difficult to define as embolic or thrombotic occlusion in our study.
In conclusion, it is well known that AMI due to SMA thromboembolism often occurs in elderly patients with preexisting or coexisting diseases. Our study’s findings indicated that PPMVG and four regions of SMA involved by thromboembolism on MDCT images, and class 3 + 4 of comorbidities were significantly associated with poor prognosis, it is important to emphasize that classes 3 and 4 of comorbidities by GIC are likely to affect the decision to make treatment plan in AMI patients. In this setting that requires emergent management, these risk factors can contribute to appropriate management of patients. Keeping a close watch on these factors should become a portion of the management strategy and possibly lower mortality. The management strategy with the help of three risk factors will be confirmed by further prospective validation studies.
Footnotes
Funding: This work was supported by the National Natural Science Foundation of China (81671943). The funder had no role in study design, data collection, data analysis, data interpretation, or the writing of the manuscript. There are no conflicts of interest to report.
Contributor Information
Wei Tang, Email: dpjinhua@live.com.
Bo Jin, Email: 13508342087@139.com.
Lian-Qin Kuang, Email: 909801791@qq.com.
Jing Zhang, Email: dpwanglu@live.com.
Chun-Xue Li, Email: dpliran@live.com.
Yi Wang, Email: ywhxl@qq.com.
REFERENCES
- 1.Clair DG, Beach JM. Mesenteric ischemia. N Engl J Med 2016; 374: 959–68. doi: 10.1056/NEJMra1503884 [DOI] [PubMed] [Google Scholar]
- 2.Kanasaki S, Furukawa A, Fumoto K, Hamanaka Y, Ota S, Hirose T, et al. Acute mesenteric ischemia: multidetector CT findings and endovascular management. Radiographics 2018; 38: 945–61. doi: 10.1148/rg.2018170163 [DOI] [PubMed] [Google Scholar]
- 3.Acosta S, Björck M. Acute thrombo-embolic occlusion of the superior mesenteric artery: a prospective study in a well defined population. Eur J Vasc Endovasc Surg 2003; 26: 179–83. doi: 10.1053/ejvs.2002.1893 [DOI] [PubMed] [Google Scholar]
- 4.Zhang Z, Wang D, Li G, Wang X, Wang Y, Li G, et al. Endovascular treatment for acute thromboembolic occlusion of the superior mesenteric artery and the outcome comparison between endovascular and open surgical treatments: a retrospective study. Biomed Res Int 2017; 2017: 1–10. doi: 10.1155/2017/1964765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Acosta S. Surgical management of peritonitis secondary to acute superior mesenteric artery occlusion. World J Gastroenterol 2014; 20: 9936–41. doi: 10.3748/wjg.v20.i29.9936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirkpatrick IDC, Kroeker MA, Greenberg HM. Biphasic CT with mesenteric CT angiography in the evaluation of acute mesenteric ischemia: initial experience. Radiology 2003; 229: 91–8. doi: 10.1148/radiol.2291020991 [DOI] [PubMed] [Google Scholar]
- 7.Aschoff AJ, Stuber G, Becker BW, Hoffmann MHK, Schmitz BL, Schelzig H, et al. Evaluation of acute mesenteric ischemia: accuracy of biphasic mesenteric multi-detector CT angiography. Abdom Imaging 2009; 34: 345–57. doi: 10.1007/s00261-008-9392-8 [DOI] [PubMed] [Google Scholar]
- 8.Lim S, Halandras PM, Bechara C, Aulivola B, Crisostomo P. Contemporary management of acute mesenteric ischemia in the endovascular era. Vasc Endovascular Surg 2019; 53: 42–50. doi: 10.1177/1538574418805228 [DOI] [PubMed] [Google Scholar]
- 9., Ginsburg M, Obara P, Lambert DL, Hanley M, Steigner ML, et al.Expert Panels on Vascular Imaging and Gastrointestinal Imaging: . ACR Appropriateness Criteria®Imaging of Mesenteric Ischemia. J Am Coll Radiol 2018; 15(11S): S332–40. doi: 10.1016/j.jacr.2018.09.018 [DOI] [PubMed] [Google Scholar]
- 10.Nuzzo A, Maggiori L, Ronot M, Becq A, Plessier A, Gault N, et al. Predictive factors of intestinal necrosis in acute mesenteric ischemia: prospective study from an intestinal stroke center. Am J Gastroenterol 2017; 112: 597–605. doi: 10.1038/ajg.2017.38 [DOI] [PubMed] [Google Scholar]
- 11.Zielinski MD, Eiken PW, Bannon MP, Heller SF, Lohse CM, Huebner M, et al. Small bowel obstruction-who needs an operation? A multivariate prediction model. World J Surg 2010; 34: 910–9. doi: 10.1007/s00268-010-0479-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yıldırım D, Hut A, Tatar C, Dönmez T, Akıncı M, Toptaş M. Prognostic factors in patients with acute mesenteric ischemia. Turk J Surg 2017; 33: 104–9. doi: 10.5152/UCD.2016.3534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zogheib E, Cosse C, Sabbagh C, Marx S, Caus T, Henry M, et al. Biological scoring system for early prediction of acute bowel ischemia after cardiac surgery: the palm score. Ann Intensive Care 2018; 8: 46. doi: 10.1186/s13613-018-0395-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kassahun WT, Schulz T, Richter O, Hauss J. Unchanged high mortality rates from acute occlusive intestinal ischemia: six year review. Langenbecks Arch Surg 2008; 393: 163–71. doi: 10.1007/s00423-007-0263-5 [DOI] [PubMed] [Google Scholar]
- 15.Zekry D, Loures Valle BH, Lardi C, Graf C, Michel J-P, Gold G, et al. Geriatrics index of comorbidity was the most accurate predictor of death in geriatric hospital among six comorbidity scores. J Clin Epidemiol 2010; 63: 1036–44. doi: 10.1016/j.jclinepi.2009.11.013 [DOI] [PubMed] [Google Scholar]
- 16.Grotelüschen R, Bergmann W, Welte MN, Reeh M, Izbicki JR, Bachmann K. What predicts the outcome in patients with intestinal ischemia? a single center experience. J Visc Surg 2019; 156: 405–11Epub ahead of print. doi: 10.1016/j.jviscsurg.2019.01.007 [DOI] [PubMed] [Google Scholar]
- 17.Chirinos JA, Veerani A, Zambrano JP, Schob A, Perez G, Mendez AJ, et al. Evaluation of comorbidity scores to predict all-cause mortality in patients with established coronary artery disease. Int J Cardiol 2007; 117: 97–102. doi: 10.1016/j.ijcard.2006.06.005 [DOI] [PubMed] [Google Scholar]
- 18.Zekry D, Loures Valle BH, Graf C, Michel J-P, Gold G, Krause K-H, et al. Prospective comparison of 6 comorbidity indices as predictors of 1-year post-hospital discharge institutionalization, readmission, and mortality in elderly individuals. J Am Med Dir Assoc 2012; 13: 272–8. doi: 10.1016/j.jamda.2010.11.011 [DOI] [PubMed] [Google Scholar]
- 19.Sheedy SP, Earnest F, Fletcher JG, Fidler JL, Hoskin TL. Ct of small-bowel ischemia associated with obstruction in emergency department patients: diagnostic performance evaluation. Radiology 2006; 241: 729–36. doi: 10.1148/radiol.2413050965 [DOI] [PubMed] [Google Scholar]
- 20.Millet I, Boutot D, Faget C, Pages-Bouic E, Molinari N, Zins M, et al. Assessment of strangulation in adhesive small bowel obstruction on the basis of combined CT findings: implications for clinical care. Radiology 2017; 285: 798–808. doi: 10.1148/radiol.2017162352 [DOI] [PubMed] [Google Scholar]
- 21.Ottinger LW. The surgical management of acute occlusion of the superior mesenteric artery. Ann Surg 1978; 188: 721–31. doi: 10.1097/00000658-197812000-00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rozzini R, Frisoni GB, Ferrucci L, Barbisoni P, Sabatini T, Ranieri P, et al. Geriatric index of comorbidity: validation and comparison with other measures of comorbidity. Age Ageing 2002; 31: 277–85. doi: 10.1093/ageing/31.4.277 [DOI] [PubMed] [Google Scholar]
- 23.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988; 44: 837–45. doi: 10.2307/2531595 [DOI] [PubMed] [Google Scholar]
- 24.Acosta S, Wadman M, Syk I, Elmståhl S, Ekberg O. Epidemiology and prognostic factors in acute superior mesenteric artery occlusion. J Gastrointest Surg 2010; 14: 628–35. doi: 10.1007/s11605-009-1130-1 [DOI] [PubMed] [Google Scholar]
- 25.Mamode N, Pickford I, Leiberman P. Failure to improve outcome in acute mesenteric ischaemia: seven-year review. Eur J Surg 1999; 165: 203–8. doi: 10.1080/110241599750007054 [DOI] [PubMed] [Google Scholar]
- 26.Copin P, Zins M, Nuzzo A, Purcell Y, Beranger-Gibert S, Maggiori L, et al. Acute mesenteric ischemia: a critical role for the radiologist. Diagn Interv Imaging 2018; 99: 123–34. doi: 10.1016/j.diii.2018.01.004 [DOI] [PubMed] [Google Scholar]
- 27.Wadman M, Block T, Ekberg O, Syk I, Elmståhl S, Acosta S. Impact of MDCT with intravenous contrast on the survival in patients with acute superior mesenteric artery occlusion. Emerg Radiol 2010; 17: 171–8. doi: 10.1007/s10140-009-0828-4 [DOI] [PubMed] [Google Scholar]
- 28.Barrett T, Upponi S, Benaglia T, Tasker AD. Multidetector CT findings in patients with mesenteric ischaemia following cardiopulmonary bypass surgery. Br J Radiol 2013; 86: 20130277. doi: 10.1259/bjr.20130277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferrada P, Callcut R, Bauza G, O'Bosky KR, Luo-Owen X, Mansfield NJ, et al. Pneumatosis intestinalis predictive evaluation study: a multicenter epidemiologic study of the American association for the surgery of trauma. J Trauma Acute Care Surg 2017; 82: 451–60. doi: 10.1097/TA.0000000000001360 [DOI] [PubMed] [Google Scholar]
- 30.van Petersen AS, Kolkman JJ, Meerwaldt R, Huisman AB, van der Palen J, Zeebregts CJ, et al. Mesenteric stenosis, collaterals, and compensatory blood flow. J Vasc Surg 2014; 60: 111–9. doi: 10.1016/j.jvs.2014.01.063 [DOI] [PubMed] [Google Scholar]
- 31.Edwards MS, Cherr GS, Craven TE, Olsen AW, Plonk GW, Geary RL, et al. Acute occlusive mesenteric ischemia: surgical management and outcomes. Ann Vasc Surg 2003; 17: 72–9. doi: 10.1007/s10016-001-0329-8 [DOI] [PubMed] [Google Scholar]
- 32.van der Molen AJ, Reimer P, Dekkers IA, Bongartz G, Bellin M-F, Bertolotto M, et al. Post-contrast acute kidney injury. Part 2: risk stratification, role of hydration and other prophylactic measures, patients taking metformin and chronic dialysis patients : Recommendations for updated ESUR Contrast Medium Safety Committee guidelines. Eur Radiol 2018; 28: 2856–69. doi: 10.1007/s00330-017-5247-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bala M, Kashuk J, Moore EE, Kluger Y, Biffl W, Gomes CA, et al. Acute mesenteric ischemia: guidelines of the world Society of emergency surgery. World J Emerg Surg 2017; 12: 38. doi: 10.1186/s13017-017-0150-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Acosta S, Ogren M, Sternby N-H, Bergqvist D, Björck M. Clinical implications for the management of acute thromboembolic occlusion of the superior mesenteric artery: autopsy findings in 213 patients. Ann Surg 2005; 241: 516–22. doi: 10.1097/01.sla.0000154269.52294.57 [DOI] [PMC free article] [PubMed] [Google Scholar]







