Abstract
Screening for lung cancer with low radiation dose CT has been shown to be effective in reducing lung cancer mortality by two major randomised controlled trials. Lung cancer screening is set to become the largest targeted cancer screening programme globally, but the effectiveness of the programme is dependent on many different factors. This article describes the key evidence for lung cancer screening, the key factors important for optimisation and the progress towards implementation.
Lung cancer is responsible for more deaths than breast and bowel cancer combined. In the UK, the 5 year survival for lung cancer is only 16% compared with 58% for colorectal and 90% for breast cancer.1 The median survival of people with lung cancer is around 6 months in marked contrast with colorectal cancer where it is not reached at 10 years. Lung cancer is an aggressive cancer and two-thirds of people present with advanced disease, when treatment has only a limited effect on longer term survival. If found at an early stage, lung cancer has a much better prognosis, with 5 year survival of 80–90%.2 Screening programmes exist for breast and bowel cancer in many developed nations, but few have introduced lung cancer screening. The reason for this has been, until recently, a lack of evidence for a reduction in disease-specific mortality. In this review, the key evidence for efficacy of low radiation dose CT (LDCT) screening is described and the factors that are important in ensuring that LDCT screening programmes clinically and cost-effectively are discussed.
Efficacy of lung cancer screening
In October 2010, the national lung screening trial (NLST), was stopped 1 year earlier than planned, by the Trial Monitoring Committee as it had achieved the pre-specified stop criteria of a 20% reduction in lung cancer mortality rate.3 The trial also reported a 6.7% reduction in all-cause mortality despite being considerably underpowered for this outcome. It is important to understand the implications of this study for any future screening programmes, which go well beyond these headline results. The NLST design employed three annual LDCT screens vs three annual chest X-rays, so the screening period when cancer could be detected was just 2 years. Participants were followed up for over 4 further years when screening could have no impact on new lung cancers occurring over that period, in contrast to a screening programme, where the effect of screening continues for those who continue to be eligible. Whilst being adequately powered to detect a lung cancer specific mortality benefit, the NLST design also allowed estimation of overdiagnosis. An analysis using data with a further year of follow-up showed the difference in lung cancer mortality rate had fallen to 16% and also noted that it was markedly different for males (8%) and females (27%), with the all-cause mortality difference unchanged at 6.9%.4 As well as the relatively short screening period, there may also have been an underestimate of the mortality benefit because of the inclusion of chest X-ray in the control arm. When the control arm in NLST was compared with the NLST-eligible control participants in the Prostate Lung Colorectal and Ovarian (PLCO) trial after 6 years follow-up, there was a non-significant 6% reduction in mortality [RR 0.94, (95% CI 0.81–1.10)] with chest X-ray which may have had some impact on the observed difference in NLST.5 After 13 years of follow-up the RR was 0.99 for chest X-ray vs no screening.
10 years later, the results of the Dutch-Belgian NELSON trial confirmed the reduction in lung cancer mortality rate. This time the LDCT screening was for a duration of 5.5 years over four rounds, baseline and 1, 3 and 5.5 years from baseline and compared with no screening. A greater lung cancer specific mortality reduction was reported in NELSON of 24% in males, 33% in females.6 The latter was not statistically significant but was at 7, 8 and 9 years of follow-up when the differences were 54%, 59% and 41% respectively. The NELSON trial was less than one-third the size of NLST (53,452 randomised in NLST, 15,789 NELSON) and again was not powered to detect an all-cause mortality difference (in fact there were 860 male deaths in the control group and 868 in the screened group). The original aim of the NELSON trial was to screen only males. The study was later amended to include females, but females made up only 16% of total recruitment. Given that the benefit of screening in females might exceed that in males, and that age-standardised all-cause mortality is lower for females, this uneven sex distribution in NELSON might underestimate the benefit of screening when applied to the whole population.
Both NELSON and NLST had annual lung cancer detection rates of below 1%. The greater the detection rate, the greater is the mortality benefit (true, unless competing causes of death become dominant). Thus, better selection criteria would result in higher mortality reductions than seen even in NELSON. This, added to a continuous programme, will substantially increase the benefit. The Multicentric Italian Lung Disease (MILD) trial suggested the importance of continued screening. Despite being underpowered, a landmark analysis showed that between 5 and 10 years after the start of screening, there was a 39% reduction in lung cancer mortality, with greater differences after 5 years.7
Concerns about screening
Not everyone agrees that CT screening for lung cancer should be implemented. Concerns include the cost-effectiveness of the intervention, the applicability of the evidence from trials in the general at-risk population and the lack of an all-cause mortality benefit in NELSON. Cost-effectiveness is addressed later in this article. The concern about applicability of the evidence is that the two main trials may have recruited participants that might have benefitted more from screening than the general population at risk. This is almost certainly the case, and a feature of most screening trials, where participants are often healthier than the total eligible population. In both NLST and NELSON, this is reflected by the participants being better educated and more likely to be ex-smokers. Only two randomised trials have used a population approach to recruitment, NELSON and the UK Lung Screen (UKLS) trials.8 Both showed that the proportion of eligible people willing to be recruited was low, 19% for NELSON and 11% for UKLS. The lower figure for UKLS probably reflects the requirement for a higher risk of lung cancer for eligibility.
The key is the extent to which this selection bias could influence the predicted benefit in a fully implemented programme. The NELSON group have shown that their recruited population, although different from the general eligible population at risk, are not that dissimilar, and the difference would be unlikely to make a significant difference.9 In NLST, 42% of recruits were under the age of 60 and make a major contribution to the overall health of the study participants. It has been shown that at least 25% of the participants in NLST had little chance of benefitting from the 2 year screening duration (baseline and screen at 1 and 2 years from baseline).10 However, it is of concern that as the risk of lung cancer increases, so do the smoking-related competing causes of death. The relationship between saving more lives from lung cancer, because the population has a higher prevalence and incidence and the excess mortality from competing causes has not been studied except in modelling research.
Meta-analyses have shown that neither the breast or bowel screening trials show an all-cause mortality benefit, having ORs of 0.99 and 1.0 respectively.11,12 This is argued by some as a reason to discontinue the programmes, and there is ongoing debate.13 All-cause mortality is influenced by the frequency of the cancer in the population, the cancer-specific mortality rate reduction, the length of the screening programme, the frequency of competing causes of death and the additional benefits and harms from screening. Thus, it is important to understand that trials can only give an indication of the likely effect of a programme as trials usually include a follow-up period where there is no screening. During this time, there is no continued influence on disease-specific or all-cause mortality. Despite this, NLST showed a significant reduction in all-cause mortality, although was underpowered for this outcome.14
Optimising CT screening
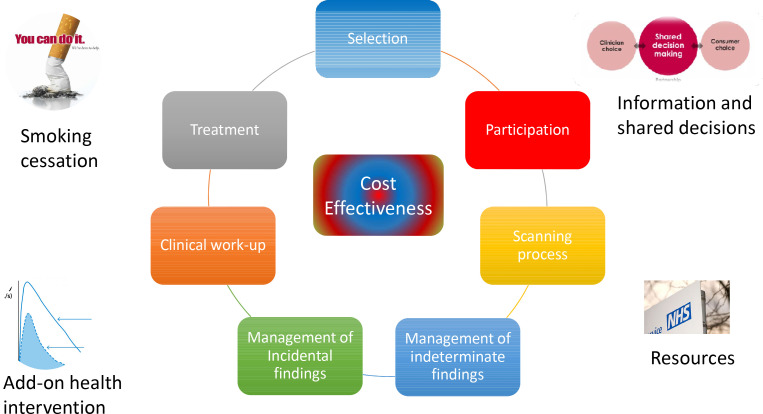
The key to implementing a successful programme is how the components, including those already mentioned (selection criteria and ongoing screening) are managed.15 In the USA, there are detailed guidance documents available.16,17 In the UK, most of these details have recently been addressed in the form of a national protocol and quality assurance standard.18,19 In many other countries, similar approaches are being taken. To understand why optimisation is so important, the various issues are discussed below; many are critically dependent on the skills and commitments of radiologists. Figure 1 summarises the key issues.
Figure 1.
Key issues that are important for optimisation of a CT screening programme.
Selection criteria
For screening to be effective, there needs to be a target population that is at risk of the disease. Current cancer screening programmes identify the target population on the basis of age and/or sex. Lung cancer screening is likely to be the first large-scale cancer screening programme that relies on additional risk factors to select the population at risk, mostly tobacco consumption. Both NELSON and NLST selected participants on the basis of age and smoking but other trials and pilot programmes have selected on the basis of multivariable risk prediction models.8,20 Currently, a number of these models have been externally validated and show improved sensitivity and specificity. Concerns about these models selecting participants that have more competing causes of death, have been raised and one expert panel recommended against using them.21 Although the recommendation was challenged, by showing that competing risks of death are similar for NLST and participants selected by the Prostate Lung Colorectal and Ovarian modified 2012 (PLCOm2012) model,22 when the lowest risk and least likely to benefit are excluded10 it remains a significant concern. In a programme, participants may be considerably less healthy, and many recommend screening until the age of 80, where randomised controlled evidence is lacking. In the UK, a major national first phase programme is underway, the Targeted Lung Health Check, where participants aged 55–75 are selected by either the Liverpool Lung project version two model23 or the PLCOm2012 the last screen is at age 77, corresponding to the last screen in NLST. Several ongoing trials will examine how the models work in practice, and newer models are being developed.
Participation
For screening programmes to have a significant impact on population health, there must be adequate numbers of at-risk people participating both at baseline and on subsequent screening. So far, participation rates are mostly unknown as screening programmes are only just beginning to start but in the USA, rates of participation are low, estimated at 3.3% of the eligible population in 2015 but possibly rising to as much as 14% by 2018.24,25 Approximately, two-fifths of people with lung cancer come from the most disadvantaged socioeconomic sectors of society where participation in health interventions is poor.26 Much work has been done on understanding the issues with engaging with this sector of society, particularly relevant is work in CT screening in deprived areas and in smoking cessation services.27 The most important factors, some of which have been shown to be effective in other cancer screening programmes and which may be important in encouraging participation in lung cancer screening, are show in Table 1.
Table 1.
Strategies associated with improved participation in cancer screening
| Strategy | Influence on participation |
|---|---|
| Pre-invitation letters | These have been shown to increase uptake of schedule appointments28 |
| Direct invitation from primary care doctor | Participants respond well to their GP invitation. In the Lung Screen Uptake trial participation rate was 53% in a very deprived population in London29 |
| Inviting to “Lung Health Check” | Not mentioning cancer screening at the outset may reduce fear and the concept of measuring health may help participants to see a potential benefit |
| Scheduled appointments | These increase attendance compared with no fixed date or time.30 |
| Reminder letters | This has been shown to increase participation in almost all screening programmes and has been confirmed in several of the UK pilots31 |
| No smoking cessation at invite | Initial mention of smoking cessation may put current smokers off attending, so the advice is to only offer this during the face-to-face visit29,32 |
| Positioning of CT scanner in convenient location | Distance to travel and inconvenience is one of the main practical barriers to participation.33 |
The lung health check
Lung health checks (LHC), or similar consultations are where the participant is confirmed to be eligible and informed consent is taken. There may also be other activities such as cardiovascular risk assessment and measuring lung function. Smoking cessation advice and assistance is essential for all current smokers. The LHC is also an opportunity to consent for research. The CT can be scheduled the same day or later, depending on the local set up. Some mobile units have the LHC room, smoking cessation room and CT scanner all in the same unit (Figure 2).
Figure 2.
A mobile lung health check and LDCT scanner used for the Yorkshire Lung Screening Trial. LDCT, low radiation dose CT.
CT scanning and reporting
Low-dose non-contrast CTs are the accepted standard with well-established scanning protocols. It is central to the success of any programme to have accurate and appropriate reporting. Reporting proformas are widely available in countries where CT screening has started. Radiologists vary in their sensitivity for detection of lung nodules but this can be improved by the use of modern computer-aided detection and volumetry software. Quality assurance should include initial second reads and ongoing feedback, as in mammography screening. Scans that are false negatives for cancer are unusual and training should include the pitfalls that lead to tumours being overlooked. What is probably more important, and currently somewhat controversial, is the reporting of incidental findings (see below).
Management of indeterminate findings
Most indeterminate findings are nodules. There are well-established guidelines that accurately manage them. The British Thoracic Society Guidelines34 are used in the UK and several European Countries and are due for a limited update, notably to include further recommendations on new (incident) nodules and management of subsolid nodules. Other European position statements have been published.35,36 In North America, Lung RADS is used but even the latest iteration does not use volumetry thresholds or volume doubling time, other than giving a conversion from diameter to volume.37 Even so, nodule management is generally done well if guidelines are followed. The very low benign resection rates achieved by the UK pilots31,38 and the IELCAP group39 are a testament to this. Nodule guidance is also crucial in the avoidance of overdiagnosis and over treatment.
Management of incidental findings
Incidental findings can lead to benefits to patients, e.g. other early stage tumours, treatable abdominal aneurysm, but more commonly just lead to unnecessary tests, costs and most importantly, distress for the patient. They are also subject to the pitfalls of overdiagnosis. In lung RADS, there is an “S” modifier for such findings and this can be used for more than 40% of studies.40 In the UK pilots and some trials, the rate of reported incidental findings ranged from over 40% down to under 4%, where a strict protocol was applied (M Callister and S Janes, personal communication). For this reason, an agreed incidental findings protocol is important, with an emphasis on only raising incidental findings when they can be addressed according to accepted treatment pathways. The English National Quality Assurance protocol that is used in the TLCH programme includes an incidental findings section that attempts to meet these criteria (Supplementary Material 1) and the target actionable incidental findings rate is 8% or less.19
Clinical work-up
Once participants are referred for suspected cancer, there is a greater potential for harm from higher radiation dose imaging and minimally invasive and invasive procedures. The process is also a cause of greater psychological distress. It is thus important to ensure that protocols are in place to avoid referral unless the probability of malignancy is high enough to justify. Most successful pilots and trials show that approximately half of the participants who are referred for clinical work-up are diagnosed with cancer. This concurs with the findings of NELSON and UKLS, where there were pre-specified management protocols for the management of indeterminate findings by interval imaging.8,41 In the UK pilots, where, British Thoracic Society nodule guidelines were employed, although the cancer detection rate was higher, the same proportion of referrals were confirmed cancer.31,38 Harms in clinical work-up need to be minimised, especially for participants who do not have cancer. This can be done by further clarifying the chance of nodules or masses being malignant by positron emission tomography-CT, and where the chance is intermediate or high, considering confirming with a biopsy technique, commonly transthoracic needle biopsy. This can be a particular challenge for radiologists in very early stage lung cancer. The latest TNM staging system emphasises the difference in prognosis even between T1a and T1b, so it is important to treat as early as possible.2 Many of the smaller nodules have an intermediate probability of cancer, so highly skilled radiologists can make a pivotal contribution to both early treatment and avoidance of benign resections. Recent data from the UK show that benign resection rates can be as low as 2% where this principle is applied.38,42 Improving localisation and sampling techniques is an important area for research and development, both improving safety and accuracy of transthoracic needle biopsy and evaluating emerging techniques such as electromagnetic navigation. It is important that evidence-based guidelines are followed for clinical work-up in the same way as for nodule management.34,43
Treatment
Between 70 and 85% of lung cancers detected by CT screening are Stage I and II. For larger tumours, treatment should proceed according to the standard of care but treatment of very early stage tumours is more of a challenge. It is important, before discussing treatment with the patients, to consider whether the tumour is likely to cause harm. This is best evaluated using a robust nodule management strategy that uses volume doubling time to establish whether a nodule, even if confirmed cancer, will limit life expectancy, taking into account age and comorbidities. Where the preferred strategy is further observation, this should be discussed with the patient. This will be an essential component of CT screening programmes to minimise overdiagnosis. Once it has been confirmed that a suspected early stage tumour should be treated, the usual considerations about patient fitness apply. Although entry criteria for clinical trials have mostly selected patients suitable for surgery, it can be expected that programmes may select some that are less fit and more suitable for radiotherapy. This was suggested by both the UK pilots and the London-based Lung Screen Uptake Trial where there was evidence of the inclusion of such participants.29,31,38
Surgery on smaller lesions requires that there is accurate localisation of the lesion which can be achieved by a variety of methods including marker injection (methylene blue, lipiodal) or by inserting a wire or coils. It is not clear which of these or other procedures are the most effective and safe.44–48 The majority of comparative trials show them to be equivalent with possibly more side-effects from wire insertion but a shorter surgical time.45,49 Most of the comparative trials have been in the context of localising small subsolid lesions where resection is often not indicated unless the lesion is part-solid and growing. Surgery for very early stage is still debated although the smaller the lesion, the harder it is to show differences between techniques (wedge resection, segmentectomy and lobectomy).50,51 The other area for debate is whether ablative techniques might have similar outcomes to surgery in smaller lesions. We still await the results of randomised trials on the subject, as it is very difficult to control for comorbidities and fitness in comparative cohorts.52
Smoking cessation
Combining smoking cessation with CT screening is one of the most important elements of a programme. Stopping smoking for 7 years achieves the same lung cancer mortality reduction as that shown in NLST.53 Smoking quit rates in CT screening trials have been higher than baseline population quit rates but this will be heavily influenced by bias in the trial population, where people with health-seeking behaviour are more likely to participate.54,55 Participants with indeterminate results may have a higher quit rate than others54 and this has led to further research testing whether detailed information about abnormalities on the CT such as emphysema and coronary artery calcification can help incentivise participants to quit.56 Smoking cessation rates, as expected strongly influence any cost-effectiveness analysis.57
Harms
Both NLST and NELSON report relatively high rates of invasive procedures and benign resections. Neither of these trials included a protocol for clinical work-up once participants were referred from the CT screening trial. In NLST, over three rounds of screening, the benign resection rate was 24 and 29% of people who had a surgical procedure to diagnose cancer had at least one complication. The rate of harm in people without cancer was much lower.3 In Round 1 of NELSON, 27% of invasive procedures showed benign disease.41 Reducing harms in screening is essential. Many of the ways in which this can be achieved have been discussed above as optimisation is about maximising the benefit to harm ratio. Minimising non-invasive, and invasive investigations and treatment is helped by following evidence-based guidelines for the management of nodules and for clinical work-up.34 The latter is likely to have contributed to the low rates of minimally invasive investigations and benign resection rates seen in the UK pilots.
Overdiagnosis is often cited as a concern in screening programmes. A recent long-term follow-up of NLST showed the overdiagnosis rate to be almost negligible,58 except where the diagnosis was bronchoalveolar cell carcinoma. The latter term was replaced in the new classification of pulmonary adenocarcinoma59 but many of these were probably lepidic predominant adenocarcinoma, which correlate with subsolid lesions on CT. There is still the potential, in implemented programmes, to overdiagnose and overtreat lesions that would be unlikely to cause harm. Careful clinical management, particularly of subsolid nodules is required taking into account growth rate, age and comorbidity of the participant.34 Harms can also be accrued from investigation of incidental findings, hence the need to follow an agreed evidence-based protocol as indicated above.
Cost-effectiveness
As depicted in Figure 1, cost-effectiveness depends on all of the elements of the programme discussed above. In analyses, a wide range of incremental cost-effectiveness ratios have been reported but to the authors’ knowledge, none include a comprehensive consideration of the various factors that optimise programmes. Publications have largely looked at cost-effectiveness in isolation by using results of randomised trials or have modelled the effect of varying elements of the intervention such as screening interval or risk of lung cancer. Common themes are that the cost of the CT is a major driver, that it is more cost-effective to screen the higher risk groups and that annual screening, although more costly overall, is more cost-effective than biennial screening, at least in higher risk groups.60,61
Practical implementation and pilot programmes
Globally, implementation of CT screening programmes is now underway, but many countries have had programmes in place for some years – notably Japan, Korea and China. Of the western countries only the USA and Canada have had national programmes approved for more than a year. Since the publication of the NELSON mortality outcomes, many European countries are developing national implementation plans. In the UK, a number of pilots have shown promising results in terms of participation rates of 40 to 50%, good early stage detection rates, and low rates of harm, especially in those eventually shown not to have cancer.29,38,42,62 Screening programmes require robust local and national quality assurance systems underpinned by agreed protocols and standards, such as those available in the USA16,17 and UK.18,19 These cover all aspects of screening including administration, the screening process and evaluation as well as defining the responsibilities of individuals running the programme. The resultant infrastructure is essential to support an efficient programme.
CT screening and COVID-19
An immediate challenge to implementation of CT screening is the COVID-19 pandemic. This has already led to the suspension of all cancer screening programmes. Resuming these programmes and initiating new ones requires that protocols include specifications for effective measures of infection control. Without this, there will be two consequences: firstly, patients and staff may be harmed through contracting the disease and secondly, participation rates may fall owing to concern about the risk of infection.
The future of CT screening
CT screening for lung cancer has the potential to be one of the most effective cancer screening programmes. Although estimates can be made of total number of deaths prevented and the impact on all-cause mortality,63 there are many unknowns that are relevant here. These include the proportion of people with cancer who are eligible for screening, the participation rate, the selection of people with competing causes of mortality, the adherence in the screening programme and the disease-specific mortality reduction achieved. Nevertheless, the fact that lung cancer screening is the only modality to have shown a reduction in all-cause mortality in a randomised trial, coupled with the significant contribution that lung cancer makes to all-cause mortality (7% of all deaths in the UK), means that lung screening has the potential to deliver major health gains at a population level. A major imperative is to ensure that high quality screening programmes help to realise these potential health gains. This will require the development of screening centres to deliver, co-ordinate and quality assure screening activity.
Contributor Information
David R Baldwin, Email: david.baldwin@nuh.nhs.uk.
Matthew E J Callister, Email: matthew.callister@nhs.net.
REFERENCES
- 1.CRUK. Cancer Survival Statistics. 2020. Available from: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type.
- 2.Rami-Porta R, Bolejack V, Crowley J, Ball D, Kim J, Lyons G, et al. The IASLC lung cancer staging project: proposals for the revisions of the T descriptors in the forthcoming eighth edition of the TNM classification for lung cancer. J Thorac Oncol 2015; 10: 990–1003. doi: 10.1097/JTO.0000000000000559 [DOI] [PubMed] [Google Scholar]
- 3., Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, et al.National Lung Screening Trial Research Team . Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011; 365: 395–409. doi: 10.1056/NEJMoa1102873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pinsky PF, Church TR, Izmirlian G, Kramer BS. The National lung screening trial: results stratified by demographics, smoking history, and lung cancer histology. Cancer 2013; 119: 3976–83. doi: 10.1002/cncr.28326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oken MM, Hocking WG, Kvale PA, Andriole GL, Buys SS, Church TR, et al. Screening by chest radiograph and lung cancer mortality: the prostate, lung, colorectal, and ovarian (PLCO) randomized trial. JAMA 2011; 306: 1865–73. doi: 10.1001/jama.2011.1591 [DOI] [PubMed] [Google Scholar]
- 6.de Koning HJ, van der Aalst CM, de Jong PA, Scholten ET, Nackaerts K, Heuvelmans MA, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med 2020; 382: 503–13. doi: 10.1056/NEJMoa1911793 [DOI] [PubMed] [Google Scholar]
- 7.Pastorino U, Silva M, Sestini S, Sabia F, Boeri M, Cantarutti A, et al. Prolonged lung cancer screening reduced 10-year mortality in the mild trial: new confirmation of lung cancer screening efficacy. Ann Oncol 2019; 30: 1162–9. doi: 10.1093/annonc/mdz117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Field JK, Duffy SW, Baldwin DR, Whynes DK, Devaraj A, Brain KE, et al. Uk lung cancer RCT pilot screening trial: baseline findings from the screening arm provide evidence for the potential implementation of lung cancer screening. Thorax 2016; 71: 161–70. doi: 10.1136/thoraxjnl-2015-207140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Aalst CM, van Iersel CA, van Klaveren RJ, Frenken FJM, Fracheboud J, Otto SJ, et al. Generalisability of the results of the Dutch-Belgian randomised controlled lung cancer CT screening trial (NELSON): does self-selection play a role? Lung Cancer 2012; 77: 51–7. doi: 10.1016/j.lungcan.2012.02.021 [DOI] [PubMed] [Google Scholar]
- 10.Tammemägi MC. Selecting lung cancer screenees using risk prediction models-where do we go from here. Transl Lung Cancer Res 2018; 7: 243–53. doi: 10.21037/tlcr.2018.06.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gotzsche PC, Jorgensen KJ. Screening for breast cancer with mammography. Cochrane Database Syst Rev 2013; 6: CD001877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fitzpatrick-Lewis D, Ali MU, Warren R, Kenny M, Sherifali D, Raina P. Screening for colorectal cancer: a systematic review and meta-analysis. Clin Colorectal Cancer 2016; 15: 298–313. doi: 10.1016/j.clcc.2016.03.003 [DOI] [PubMed] [Google Scholar]
- 13.Dobbin KK, Ebell M. Should we expect all-cause mortality reductions in large screening studies? Br J Gen Pract 2018; 68: 290–1. doi: 10.3399/bjgp18X696545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heijnsdijk EAM, Csanádi M, Gini A, Ten Haaf K, Bendes R, Anttila A, et al. All-Cause mortality versus cancer-specific mortality as outcome in cancer screening trials: a review and modeling study. Cancer Med 2019; 8: 6127–38. doi: 10.1002/cam4.2476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oudkerk M, Devaraj A, Vliegenthart R, Henzler T, Prosch H, Heussel CP, et al. European position statement on lung cancer screening. Lancet Oncol 2017; 18: e754–66. doi: 10.1016/S1470-2045(17)30861-6 [DOI] [PubMed] [Google Scholar]
- 16.Association ATSaAL. Implementation Guide for Lung Cancer Screening. 2019. Available from: https://www.lungcancerscreeningguide.org/wp-content/uploads/2018/10/implementation-guide-for-lung.pdf.
- 17.Mazzone P, Powell CA, Arenberg D, Bach P, Detterbeck F, Gould MK, et al. Components necessary for high-quality lung cancer screening: American College of chest physicians and American thoracic Society policy statement. Chest 2015; 147: 295–303. doi: 10.1378/chest.14-2500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Health Service England -N, Cancer, Programme,. Targeted screening for lung cancer with low radiation dose computed tomography. standard protocol prepared for the targeted lung health checks programme. 2019. Available from: https://www.england.nhs.uk/wp-content/uploads/2019/02/targeted-lung-health-checks-standard-protocol-v1.pdf.
- 19.National Health Service England -N, Cancer, Programme,. Targeted Screening for Lung Cancer with Low Radiation Dose Computed Tomography. Quality Assurance Standards prepared for the Targeted Lung Health Checks Programme. 2020. Available from: https://www.england.nhs.uk/wp-content/uploads/2019/02/targeted-screening-for-lung-cancer-quality-assurance-standard.pdf..
- 20.Tammemagi MC, Schmidt H, Martel S, McWilliams A, Goffin JR, Johnston MR, et al. Participant selection for lung cancer screening by risk modelling (the Pan-Canadian Early Detection of Lung Cancer [PanCan] study): a single-arm, prospective study. Lancet Oncol 2017; 18: 1523–31. doi: 10.1016/S1470-2045(17)30597-1 [DOI] [PubMed] [Google Scholar]
- 21.Mazzone PJ, Silvestri GA, Patel S, Kanne JP, Kinsinger LS, Wiener RS, et al. Screening for lung cancer: chest guideline and expert panel report. Chest 2018; 153: 954–85. doi: 10.1016/j.chest.2018.01.016 [DOI] [PubMed] [Google Scholar]
- 22.Tammemägi MC, Katki HA, Hocking WG, Church TR, Caporaso N, Kvale PA, et al. Selection criteria for lung-cancer screening. N Engl J Med 2013; 368: 728–36. doi: 10.1056/NEJMoa1211776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raji OY, Duffy SW, Agbaje OF, Baker SG, Christiani DC, Cassidy A, et al. Predictive accuracy of the Liverpool lung project risk model for stratifying patients for computed tomography screening for lung cancer: a case-control and cohort validation study. Ann Intern Med 2012; 157: 242–50. doi: 10.7326/0003-4819-157-4-201208210-00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jemal A, Fedewa SA. Lung cancer screening with low-dose computed tomography in the United States-2010 to 2015. JAMA Oncol 2017; 3: 1278–81. doi: 10.1001/jamaoncol.2016.6416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zahnd WE, Eberth JM. Lung cancer screening utilization: a behavioral risk factor surveillance system analysis. Am J Prev Med 2019; 57: 250–5. doi: 10.1016/j.amepre.2019.03.015 [DOI] [PubMed] [Google Scholar]
- 26.Maheswaran R, Pearson T, Jordan H, Black D, deprivation S, distance travel. Location of service, and uptake of breast cancer screening in North Derbyshire, UK. J Epidemiol Community Health 2006; 60: 208–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murray RL, Bauld L, Hackshaw LE, McNeill A. Improving access to smoking cessation services for disadvantaged groups: a systematic review. J Public Health 2009; 31: 258–77. doi: 10.1093/pubmed/fdp008 [DOI] [PubMed] [Google Scholar]
- 28.Libby G, Bray J, Champion J, Brownlee LA, Birrell J, Gorman DR, et al. Pre-notification increases uptake of colorectal cancer screening in all demographic groups: a randomized controlled trial. J Med Screen 2011; 18: 24–9. doi: 10.1258/jms.2011.011002 [DOI] [PubMed] [Google Scholar]
- 29.Quaife SL, Ruparel M, Dickson JL, Beeken RJ, McEwen A, Baldwin DR, et al. Lung screen uptake trial (LSUT): randomized controlled clinical trial testing targeted invitation materials. Am J Respir Crit Care Med 2020; 201: 965–75. doi: 10.1164/rccm.201905-0946OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duffy SW, Myles JP, Maroni R, Mohammad A. Rapid review of evaluation of interventions to improve participation in cancer screening services. J Med Screen 2017; 24: 127–45. doi: 10.1177/0969141316664757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghimire B, Maroni R, Vulkan D, Shah Z, Gaynor E, Timoney M, et al. Evaluation of a health service adopting proactive approach to reduce high risk of lung cancer: the Liverpool healthy lung programme. Lung Cancer 2019; 134: 66–71. doi: 10.1016/j.lungcan.2019.05.026 [DOI] [PubMed] [Google Scholar]
- 32.Quaife SL, Marlow LAV, McEwen A, Janes SM, Wardle J. Attitudes towards lung cancer screening in socioeconomically deprived and heavy smoking communities: informing screening communication. Health Expect 2017; 20: 563–73. doi: 10.1111/hex.12481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ali N, Lifford KJ, Carter B, McRonald F, Yadegarfar G, Baldwin DR, et al. Barriers to uptake among high-risk individuals declining participation in lung cancer screening: a mixed methods analysis of the UK lung cancer screening (UKLS) trial. BMJ Open 2015; 5: e008254. doi: 10.1136/bmjopen-2015-008254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Callister MEJ, Baldwin DR, Akram AR, Barnard S, Cane P, Draffan J, et al. British thoracic Society guidelines for the investigation and management of pulmonary nodules. Thorax 2015; 70 Suppl 2(Suppl 2): ii1–54. doi: 10.1136/thoraxjnl-2015-207168 [DOI] [PubMed] [Google Scholar]
- 35.Matthijs Oudkerk AD, Vliegenthart R, Henzler T, Prosch H, Heussel CP, Bastarrika G, et al. Maurizio V Infante up, Jesper H Pedersen, Eugenio Paci, Stephen W Duffy, Harry de Koning, John K field. European position statement on lung cancer screening. Lancet Oncology 2017; 18: e754–66. [DOI] [PubMed] [Google Scholar]
- 36.Kauczor H-U, Baird A-M, Blum TG, Bonomo L, Bostantzoglou C, Burghuber O, et al. ESR/ERS statement paper on lung cancer screening. Eur Respir J 2020; 55: 1900506. doi: 10.1183/13993003.00506-2019 [DOI] [PubMed] [Google Scholar]
- 37.Radiology ACo. Lung‐RADS® Version 1.1. 2019. Available from: https://www.acr.org/-/media/ACR/Files/RADS/Lung-RADS/LungRADSAssessmentCategoriesv1-1.pdf?la=en.
- 38.Crosbie PA, Balata H, Evison M, Atack M, Bayliss-Brideaux V, Colligan D, et al. Implementing lung cancer screening: baseline results from a community-based 'Lung Health Check' pilot in deprived areas of Manchester. Thorax 2019; 74: 405–9. doi: 10.1136/thoraxjnl-2017-211377 [DOI] [PubMed] [Google Scholar]
- 39.Flores R, Bauer T, Aye R, Andaz S, Kohman L, Sheppard B, et al. Balancing curability and unnecessary surgery in the context of computed tomography screening for lung cancer. J Thorac Cardiovasc Surg 2014; 147: 1619–26. doi: 10.1016/j.jtcvs.2013.11.001 [DOI] [PubMed] [Google Scholar]
- 40.Reiter MJ, Nemesure A, Madu E, Reagan L, Plank A. Frequency and distribution of incidental findings deemed appropriate for S modifier designation on low-dose CT in a lung cancer screening program. Lung Cancer 2018; 120: 1–6. doi: 10.1016/j.lungcan.2018.03.017 [DOI] [PubMed] [Google Scholar]
- 41.van Klaveren RJ, Oudkerk M, Prokop M, Scholten ET, Nackaerts K, Vernhout R, et al. Management of lung nodules detected by volume CT scanning. N Engl J Med 2009; 361: 2221–9. doi: 10.1056/NEJMoa0906085 [DOI] [PubMed] [Google Scholar]
- 42.Crosbie PA, Balata H, Evison M, Atack M, Bayliss-Brideaux V, Colligan D, et al. Second round results from the Manchester 'Lung Health Check' community-based targeted lung cancer screening pilot. Thorax 2019; 74: 700–4. doi: 10.1136/thoraxjnl-2018-212547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gould MK, Donington J, Lynch WR, Mazzone PJ, Midthun DE, Naidich DP, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? diagnosis and management of lung cancer, 3rd ED: American College of chest physicians evidence-based clinical practice guidelines. Chest 2013; 143(5 Suppl): e93S–120. doi: 10.1378/chest.12-2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun S-H, Gao J, Zeng X-M, Zhang Y-F. Computed tomography-guided localization for lung nodules: methylene-blue versus coil localization. Minim Invasive Ther Allied Technol 2020;: 1–6. doi: 10.1080/13645706.2020.1725579 [DOI] [PubMed] [Google Scholar]
- 45.Park CH, Lee SM, Lee JW, Hwang SH, Kwon W, Han K, et al. Hook-Wire localization versus lipiodol localization for patients with pulmonary lesions having ground-glass opacity. J Thorac Cardiovasc Surg 2020; 159: 1571–9. doi: 10.1016/j.jtcvs.2019.08.100 [DOI] [PubMed] [Google Scholar]
- 46.Liu J, Wang X, Wang Y, Sun M, Liang C, Kang L. Comparison of CT-guided localization using hook wire or coil before thoracoscopic surgery for ground glass nodules. Br J Radiol 2020; 93: 20190956. doi: 10.1259/bjr.20190956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li A, Chan S, Thung KH. Pre-Operative CT localization for patients with subsolid opacities expecting video-assisted thoracoscopic surgery-single center experience of fluorescent iodized emulsion and hook-wire localization technique. Br J Radiol 2020; 93: 20190938. doi: 10.1259/bjr.20190938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao G, Yu X, Chen W, Geng G, Li N, Liu H, et al. Computed tomography-guided preoperative semi-rigid hook-wire localization of small pulmonary nodules: 74 cases report. J Cardiothorac Surg 2019; 14: 149. doi: 10.1186/s13019-019-0958-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang M-Y, Liu Y-S, An X-B, Li K, Liu Y-J, Wang F. Cerebral arterial air embolism after computed tomography-guided hook-wire localization of a pulmonary nodule: a case report. Medicine 2019; 98: e15437. doi: 10.1097/MD.0000000000015437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cao J, Yuan P, Wang Y, Xu J, Yuan X, Wang Z, et al. Survival rates after lobectomy, Segmentectomy, and wedge resection for non-small cell lung cancer. Ann Thorac Surg 2018; 105: 1483–91. doi: 10.1016/j.athoracsur.2018.01.032 [DOI] [PubMed] [Google Scholar]
- 51.Dai C, Shen J, Ren Y, Zhong S, Zheng H, He J, et al. Choice of Surgical Procedure for Patients With Non-Small-Cell Lung Cancer ≤ 1 cm or > 1 to 2 cm Among Lobectomy, Segmentectomy, and Wedge Resection: A Population-Based Study. J Clin Oncol 2016; 34: 3175–82. doi: 10.1200/JCO.2015.64.6729 [DOI] [PubMed] [Google Scholar]
- 52.Khakwani A, Harden S, Beckett P, Baldwin D, Navani N, West D, et al. Post-Treatment survival difference between lobectomy and stereotactic ablative radiotherapy in stage I non-small cell lung cancer in England. Thorax 2020; 75: 237–43. doi: 10.1136/thoraxjnl-2018-212493 [DOI] [PubMed] [Google Scholar]
- 53.Tanner NT, Kanodra NM, Gebregziabher M, Payne E, Halbert CH, Warren GW, et al. The association between smoking abstinence and mortality in the National lung screening trial. Am J Respir Crit Care Med 2016; 193: 534–41. doi: 10.1164/rccm.201507-1420OC [DOI] [PubMed] [Google Scholar]
- 54.Brain K, Carter B, Lifford KJ, Burke O, Devaraj A, Baldwin DR, et al. Impact of low-dose CT screening on smoking cessation among high-risk participants in the UK lung cancer screening trial. Thorax 2017; 72: 912–8. doi: 10.1136/thoraxjnl-2016-209690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van der Aalst CM, van den Bergh KAM, Willemsen MC, de Koning HJ, van Klaveren RJ. Lung cancer screening and smoking abstinence: 2 year follow-up data from the Dutch-Belgian randomised controlled lung cancer screening trial. Thorax 2010; 65: 600–5. doi: 10.1136/thx.2009.133751 [DOI] [PubMed] [Google Scholar]
- 56.Murray RL, Britton J, Brain K, McCutchan G, Quaife SL, Lewis S, et al. AlexandraCurriculum vitae: honorary Professor David R BaldwinPage 20 updated 1.6.19Ashurst, Matthew Callister. The Yorkshire enhanced stop smoking study (YesS): a protocol for a randomisedcontrolled trial to evaluate the effect of adding a personalised smoking cessation intervention to a lung cancerscreening programme. BMJ Open in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Villanti AC, Jiang Y, Abrams DB, Pyenson BS. A cost-utility analysis of lung cancer screening and the additional benefits of incorporating smoking cessation interventions. PLoS One 2013; 8: e71379. doi: 10.1371/journal.pone.0071379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. National lung screening trial research T. lung cancer incidence and mortality with extended follow-up in the National lung screening trial. J Thorac Oncol 2019; 14: 1732–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger K, Yatabe Y, et al. International association for the study of lung Cancer/American thoracic Society/European respiratory Society: international multidisciplinary classification of lung adenocarcinoma: Executive summary. Proc Am Thorac Soc 2011; 8: 381–5. doi: 10.1513/pats.201107-042ST [DOI] [PubMed] [Google Scholar]
- 60.Ten Haaf K, Tammemägi MC, Bondy SJ, van der Aalst CM, Gu S, McGregor SE, et al. Performance and cost-effectiveness of computed tomography lung cancer screening scenarios in a population-based setting: a Microsimulation modeling analysis in Ontario, Canada. PLoS Med 2017; 14: e1002225. doi: 10.1371/journal.pmed.1002225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cressman S, Peacock SJ, Tammemägi MC, Evans WK, Leighl NB, Goffin JR, et al. The cost-effectiveness of high-risk lung cancer screening and drivers of program efficiency. J Thorac Oncol 2017; 12: 1210–22. doi: 10.1016/j.jtho.2017.04.021 [DOI] [PubMed] [Google Scholar]
- 62.Ghimire B, Maroni R, Vulkan D, Shah Z, Gaynor E, Timoney M, et al. Erratum to "Evaluation of a health service adopting proactive approach to reduce high risk of lung cancer: The Liverpool Healthy Lung Programme" [Lung Cancer 134 (August) (2019) 66-71. Lung Cancer 2020; 139: 224. doi: 10.1016/j.lungcan.2019.10.007 [DOI] [PubMed] [Google Scholar]
- 63.Stang A, Jöckel K-H. The impact of cancer screening on all-cause mortality. Dtsch Arztebl Int 2018; 115(29-30): 481–6. doi: 10.3238/arztebl.2018.0481 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.