Abstract
Objective:
In contrast to traditional views of incurability, patients with oligometastatic disease present with an opportunity for disease eradication with aggressive treatment. There is mounting evidence in support of the role of stereotactic body radiotherapy (SBRT) in oligometastatic prostate cancer (OMPC).
Methods:
MEDLINE and EMBASE were queried for prospective cohort studies reporting the outcomes of metachronous OMPC treated with SBRT. The primary outcome was overall local control. Secondary outcomes included androgen deprivation therapy-free survival (ADTFS), biochemical recurrence free survival (BCFS), and progression-free survival (PFS). When appropriate, these endpoints were combined in a meta-analysis.
Results:
We screened 356 abstracts and identified 10 studies to include in our analysis, with a total of 653 patients and 1,111 lesions. The maximum number of lesions included in any single study ranged from 3 to 5. PET-CT staging occurred in 92.4% of all patients. SBRT dose varied, with BED1.5 ranging from 152 to 408. Only one Grade 3 bone toxicity was observed. Meta-analysis reported an overall local control rate of 97% (95% CI, 94–100). Median ADTFS was 24.7 months (95% CI, 20.1–29.2 months). Two-year BCFS, PFS, and ADTFS were 33% (95% CI, 11–55), 39% (95% CI, 24–54), and 52% (95%CI, 41–62), respectively. Patients treated with SBRT were half as likely to experience PSA progression than those on observation when looking at randomized control trial data alone.
Conclusion:
SBRT appears to be effective in controlling overall disease burden in metachronous OMPC patients and is associated with minimal significant toxicity. The current prospective literature is scarce, and further prospective data are needed to guide treatment recommendations.
Advances in knowledge:
This study provides a comprehensive summary of the prospective evidence reporting the outcomes of SBRT in the management of OMPC patients. We quantify the rates of local control, biochemical-free recurrence, progression-free survival, and ADT-free survival through meta-analysis.
Introduction
The oligometastatic state is a clinical state between localized disease and widespread metastases. In contrast to traditional views of incurability, patients with oligometastatic disease present with an opportunity for disease eradication with aggressive treatment. Oligometastases can manifest clinically as synchronous or metachronous disease. Patients with synchronous oligometastases present de novo with limited metastatic disease and an untreated primary. Conversely, patients who have had definitive therapy for their primary tumors may have limited recurrence after a disease-free period with metachronous oligometastases.1
Several trials have shown a benefit of additional local treatment of the primary tumor when compared to systemic therapy alone in synchronous oligometastatic prostate cancer (OMPC) patients with low disease burden.2 Similarly, there is mounting interest in investigating the role of radical management of the metastatic lesions themselves in metachronous disease. Recent phase 2 studies have reported promising outcomes utilizing stereotactic body radiotherapy (SBRT) in the treatment of the metastatic lesions in this patient population.3,4
We performed a systematic review and meta-analysis with the aim to synthesize the existing prospective evidence in order to provide oncologists guidance in the management of OMPC patients.
Methods
Search Strategy and Criteria: This study was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.5 MEDLINE and EMBASE databases were queried for studies that prospectively collected and reported the outcomes of SBRT in the management of metachronous OMPC from inception to April 1, 2020. Metachronous OMPC was defined as clinical or radiological recurrent disease, with five or less lesions, in patients who have previously had primary management of prostate cancer using local treatment modalities such as surgery or radiotherapy. SBRT was defined as conformal, external beam radiotherapy that accurately delivers high-dose irradiation within 1–10 fractions.6 The full search strategy is available in Supplementary Material 1. Search results were imported into Covidence (Veritas Health Innovation, Melbourne, Australia) for eligibility determination through abstract and subsequent full-text screening by two independent reviewers (MY and NM). Studies with less than 20 patients, synchronous OMPC, abstracts, and non-English language were excluded. The most recent publication was selected in the event of multiple publications of the same patient cohort. Conflicts were resolved by a third reviewer (FYM). Risk of bias assessment was performed by MY and NM independently according to the MINORS criteria for included single arm observational studies and the Cochrane RoB tool for randomized trials.7,8 This analysis was not registered with a prospective registry for systematic reviews.
Data Extraction: Data were abstracted by two reviewers (MY and NM) on a standard data abstraction form. Baseline data elements extracted included: number of patients/lesions, positron emission tomography (PET) utilization rate, dose fractionation, concomitant androgen deprivation therapy (ADT) utilization rate, toxicity, among others. Dose fractionation schedules were converted to biologically effective dose (BED) using the formula: BED = , where n is the number of fractions, d is the dose per fraction, and the α/β ratio is assumed to be 1.5 for prostate cancer.9
Outcomes Extraction and Statistical Analysis: Our primary outcome of interest is local control (LC). Secondary endpoints are local recurrence-free survival (LRFS), androgen deprivation-free survival (ADTFS), biochemical recurrence-free survival (BCFS), and progression-free survival (PFS), and toxicity. PFS and BCFS are differentiated in that the former describes clinically or radiographically detectable disease, while the latter is defined by PSA recurrence.
Studies reporting these endpoints, where appropriate, were weighted by inverse variance and combined in a meta-analysis using a random effects model. Where outcome measures and their variances were not stated, survival curves were reconstructed using Web Plot Digitizer and the ‘ifdpc’ function in Stata (StataCorp, College Station, TX) as previously described.10,11 Where variance data was unavailable, studies were weighted by sample size and combined. The individual treatment arms of randomized trials were analyzed separately unless otherwise stated. Heterogeneity among studies was quantified by the I2 statistic, with I2 values exceeding 25%, 50%, and 75% representing low, moderate, and high heterogeneity, respectively.12 Quantitative analyses for survival endpoints and proportional endpoints were performed using the ‘metan’ and ‘metaprop’ functions, respectively, in Stata v.15.
Results
Literature Search: We screened 355 abstracts and identified 10 studies to include in our analysis, with a total of 653 patients and 1,111 lesions. Six studies were observational cohort series:13–18 one phase 1 single arm prospective trial (POPSTAR),19 one phase 2 single arm prospective trial (TRANSFORM),20 and two phase 2 randomized control trials (RCT) (STOMP and ORIOLE)3,4 (Supplementary Material 1). Both RCTs enrolled patients with up to three asymptomatic metastatic lesions and did not have ADT for a pre-specified period before enrollment. The treatment arm in ORIOLE consisted entirely of SBRT, whereas STOMP allowed SBRT or surgical resection in their metastasis directed therapy (MDT) arm. Since subgroup analysis was not performed in STOMP, all participants in the MDT arm are analyzed in the current study as having received SBRT, since only a minority underwent resection (19%). Note that only the MDT arms of these RCTs are included in quantitative analyses with the other studies.
All single arm studies were of good or moderate quality as assessed by the MINORS criteria, with a median score of 11/16 (range 9–14) (Supplementary Material 1). The STOMP and ORIOLE trials were determined to have some concerns in the domain of outcome measurement in that the assessors were not blinded to treatment allocation; however the overall risk of bias was low (Supplementary Material 1).
Patients Characteristics: Study characteristics are described in Table 1. The maximum number of lesions included in any single study ranged from 2 to 5, with the majority (64%) limited to a maximum of three lesions. PET utilization ranged from 36% to 100% of included patients within a single study, with most studies (73%) having a 100% utilization rate. Overall, 601/653 (92%) patients were staged by PET. Choline PET was the most common modality. SBRT dose varied, but the most frequently utilized dose fractionation schedules included 50 Gy/10 (n = 3), 30 Gy/3 (n = 4), and 20 Gy/1 (n = 3); the BED1.5 ranged from 152 to 408. Five studies treated a proportion of patients who were concurrently on ADT, ranging from 14% to 78% of the total cohort of patients.
Table 1.
Characteristics of included prospective studies
| First Author | Muacevic (14) | Decaestecker (13) | Kneebone (16) | Jereczek-Fossa (15) | Osta (3) | Sivab (19) | Gomez-Iturriaga (17) | Bowdenb (20) | Pasqualetti (18) | Philipsa (4) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | 2013 | 2014 | 2016 | 2017 | 2018 | 2018 | 2019 | 2020 | 2020 | 2020 | |
| Country | Germany | Belgium | Australia | Italy | Belgium | Australia | Spain | Australia | Italy | United States | |
| Number of Patients | 40 | 50 | 57 | 94 | 31 | 33 | 49 | 199 | 46 | 54 | |
| Number of Lesions | 64 | 70 | 73 | 124 | 51 | 50 | 93 | 429 | 67 | 90 | |
| Age (mean) | 66 | 59 | 64 | 70.7 | 62 | 70 | 71 | 67.4 | 70 | 68 | |
| Maximum Lesions per Patient | 2 | 3 | 3 | 5 | 3 | 3 | 5 | 5 | 3 | 3 | |
| PET Utilization (proportion, type) | 1 (choline) | 1 (FDG, choline) | 1 (PSMA) | 0.96 (choline) | 1 (choline) | 1 (NaF) | 1 (choline) | 0.76 (choline) | 1 (choline) | 1 (PSMA) | |
| Median Time to SBRT from Diagnosis (years) | - | 4.8 | 5.6 | 5.2 | 5.3 | - | 3.2 | 3.8 | 5.8 | - | |
| Median Follow up (months) | 14 | 24 | 16 | 19 | 36 | 24 | 24 | 35 | 29 | 19 | |
| Primary Tumor Treatment Modality | EBRT (%) | 18 (16) | 7 (13) | 19 (20) | 7 (23) | 15 (45) | 7 (14) | 185 (93) | 2 (4) | 6 (17) | |
| RP (%) | 6 (12) | 20 (35) | 39 (42) | 2 (7) | 18 (54) | 14 (29) | 9 (4.5) | 17 (37) | 30 (83) | ||
| RP+EBRT (%) | 36 (72) | 30 (52) | 34 (36) | 22 (70) | 25 (51) | 27 (59) | |||||
| Other (%)c | 2 (2) | 1 (2) | 3 (1.5) | ||||||||
| Median BED1.5 (Gy) | 287 | 217 (70% patients) 230 (30% patients) | Bone: 216 (5% patients) and 287 (26% patients) Node: 383 (26% patients) and 230 (42% patients) | 152 | 230 | 287 | Bone: 152 Node: 147 | 217 | 189 | 211 | |
| Concomitant ADT utilization (proportion) | 0.48 | 0 | 0 | 0.36 | 0 | 0 | 0.78 | 0.14 | 0.53 | 0 | |
SBRT – stereotactic body radiotherapy, PET – positron emission tomography, FDG – fluorodeoxyglucose, PSMA - prostate specific membrane antigen, NaF – sodium fluoride, BED – biologically effective dose, ADT – androgen deprivation therapy, EBRT – external beam radiotherapy, RP – radical prostatectomy
Phase 2 randomized control trial.
Single arm prospective trial.
Other primary treatment modalities include brachytherapy and cryotherapy.
Outcomes and Quantitative Synthesis: Outcome measures are summarized in Table 2. One study uniquely reported treatment escalation-free survival (TEFS). This is a composite endpoint defined by ADT initiation for patients who were not on ADT, second-line ADT or chemotherapy for patients on concurrent ADT at enrollment, or palliative radiotherapy. Treatment escalation occurred at the discretion of the clinical team based on PSA progression, infield recurrence, development of >5 new metastases, or clinical concern at rate of disease progression.20 Notably, only one Grade 3 toxicity was reported among all studies; a vertebral compression fracture that required instrumentation.19
Table 2.
Study Outcomes
| First Author | Local Control | Local Recurrence-Free Survival | Androgen Deprivation-Free Survival | Biochemical Recurrence-Free Survival | Progression-Free Survival | Treatment Escalation-Free Survival | Grade ≥ 3 Toxicity (%) | |||||
| Overall (%) | 2 year (%) | Median (mo) | 2 year (%) | Median (mo) | 2 year (%) | Median (mo) | 2 year (%) | Median (mo) | 2 year (%) | Median (mo) | ||
| Muacevic (14) | 97 | 96 | NR | 0 | ||||||||
| Decaestecker (13) | 100 | 60 | 25 | 35 | 19 | 0 | ||||||
| Kneebone (16) | 16 | 11 | 0 | |||||||||
| Jereczek-Fossa (15) | 90 | 84 | NR | 30 | 17 | 0 | ||||||
| Ost* (3) | 100 | 44 | 21 | 28 | 10 | 0 | ||||||
| Siva†(19) | 93 | NR | 48 | 3 -VCF | ||||||||
| Gomez-Iturriaga (17) | 89 | 0 | ||||||||||
| Bowden†(20) | 52 | 27 | 0 | |||||||||
| Pasqualetti (18) | 96 | 29 | 0 | |||||||||
| Philips* (4) | 99 | 57 | NR | 58 | NR | 0 | ||||||
| Quantitative Synthesis (95% CI) | 97 (94–100) | 88.7 (5.4) ‡ | 52 (41–62) | 24.7 (20.1–29.2) | 33 (11–55) | 39 (24–54) | ||||||
mo – months, VCF – vertebral compression fracture, NR – not reached, CI – confidence interval
Phase 2 randomized control trial
Phase 1 single arm trial
Standard deviation
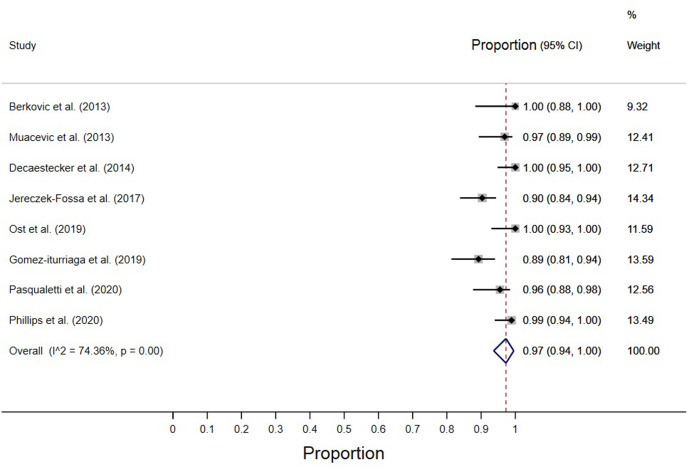
The overall local control rate was 97% (95% CI, 94–100) as reported by seven studies (Figure 1).3,4,13–15,17,18 The overall local control rates ranged from 89% to 100% of all treated lesions. The 2-year LRFS rate was reported by three studies; their weighted mean was 88.7% (SD = 5.4).14,15,19
Figure 1.
Overall local control.
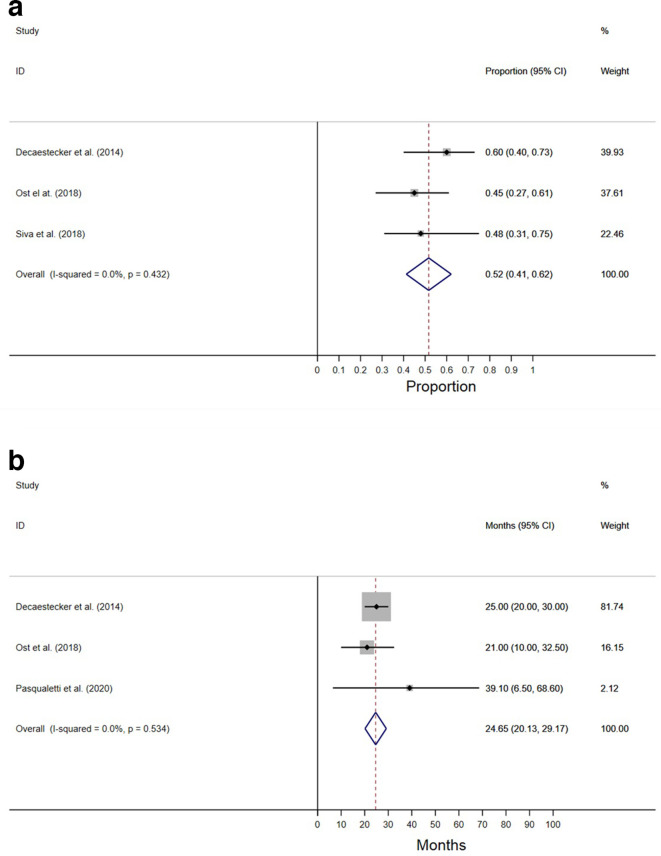
ADTFS is graphically summarized in Figure 2. Median and 2-year survivals were reported as an outcome by four studies.3,13,18,19 Meta-analysis of these outcomes reported an overall median ADTFS of 24.7 months (95% CI, 20.1–29.2), and a 2-year ADTFS of 52% (95% CI, 41–62).
Figure 2.
Androgen deprivation-free survival. (A) 2 year (B) median
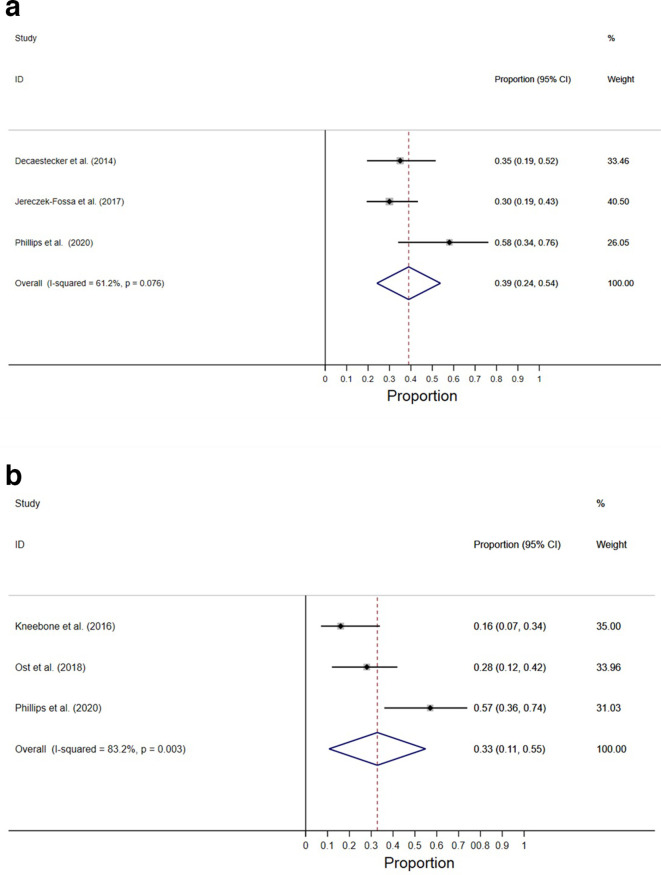
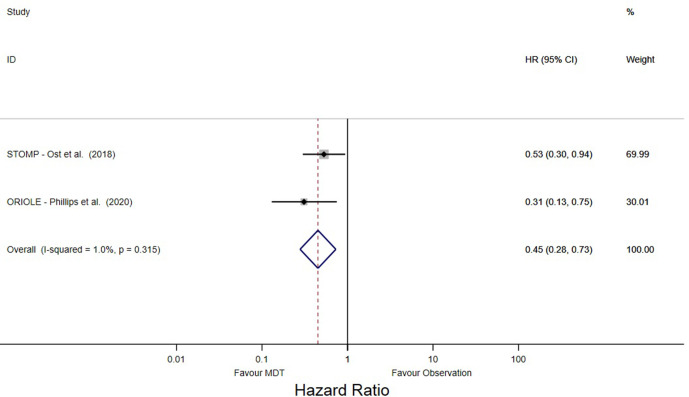
The 2-year PFS was 39% (95% CI, 24–54), as reported by three studies (Figure 3A).4,13,15 The 2-year BCFS was 33% (95% CI, 11–55) as synthesized from three studies (Figure 3B).3,4,16 The hazard ratios of the difference in risk of PSA progression between MDT and observation arms were also reported by the two included RCTs.3,4 Meta-analysis of these trials showed that MDT was associated with significantly lower PSA progression risk than compared to observation, with nearly half of the risk of progression (HR 0.45; 95% CI, 0.28–0.73) (Figure 4).
Figure 3.
2 year (A) progression-free survival (B) biochemical-free survival
Figure 4.
Hazard ratio from randomized control trials. Abbreviations: HR – hazard ratio, MDT – metastasis directed therapy
Discussion
OMPC represents a unique subset of metastatic prostate cancer patients in which the control of the limited metastatic disease burden is thought to decrease the seeding of other clinical sites of progression. As such, the paradigm of OMPC management is evolving, and there is a trend towards aggressive escalation in the management of these patients to sustain prolonged disease control.
The evidence supporting MDT in OMPC patients is developing. Both surgical resection and SBRT are recognized modalities in MDT; the former limited mainly to nodal metastases, while the latter can be implemented to treat boney or visceral disease.21 Through our systematic search, we found that a significant proportion of the OMPC SBRT literature consists of retrospective studies. By nature of their design, these studies are subject to selection and informational biases, and as such excluded from our systematic review.
To our knowledge, the current study is the first to synthesize only the existing high-quality, prospective literature. We identified several prospective cohort studies meeting our stringent eligibility criteria, including two single arm trials, as well as two phase 2 RCTs. Despite the smaller size of these studies, together they represent the best current evidence supporting SBRT in the MDT of metachronous OMPC patients.
Our results suggest that OMPC patients treated with SBRT may experience excellent local control rates greater than 95%. This is in keeping with the reported outcomes from several prospective trials investigating the role of SBRT in oligometastatic disease. The seminal SABR-COMET trial reported an overall local control rate of 70%. However, enrolled patients had mixed metastatic histologies, with only 21% of patients having OMPC and 17% of patients having lung primaries, which tend to be less indolent.22 Similarly, Gomez et al reported an 88% local control rate in the MDT arm of a phase 2 trial investigating local consolidative treatment in oligometastatic lung cancer patients.23
Because of the effective local control, most disease progression occurs distantly. As such, we observed sustained biochemical control rates, with roughly one-third of patients in our pooled analysis remaining free of biochemical failure at 2 years. Analysis of just the STOMP and ORIOLE trials showed that patients in the observation arm were twice as likely to experience PSA progression than if they received MDT. A recent update of the STOMP trial was presented at the 2020 American Society of Clinical Oncology (ASCO) meeting. At 5 years, ADTFS remained significantly superior in the MDT arm (34%) compared to the control (8%), with a HR of 0.57 (80%CI, 0.38–0.84).24
Furthermore, we found that clinical progression and the need for ADT initiation are prolonged for up to 18 months and over 2 years, respectively, for approximately half of these patients receiving SBRT. This is significant as ADT use, in addition to short-term vasomotor and erectile dysfunction adverse effects, is associated with significant long-term toxicities such as osteoporosis and coronary heart disease; it can decrease overall quality of life.25,26 Financial toxicity is also another consideration. The average 3-month injection costs a little over $1000 Canadian in a single payer system like Ontario, Canada.27 Additionally, there is evidence that complications from ADT utilization, such as insufficiency fractures, can nearly double healthcare costs for patients.28
Importantly, excess toxicity appears to be limited from SBRT. There was only one Grade 3 bone toxicity observed among over 600 patients and 1000 treated lesions. This is supported by a previous review of the retrospective literature surrounding SBRT utilization in this patient population, where Grade 2–3 toxicities were observed in only 13% of treated patients. Only one Grade 3 urinary toxicity occurred.29 The promising safety profile of SBRT certainly presents it as a promising treatment option in the management of OMPC patients.
Several comparative phase 2 and phase 3 trials are currently accruing to provide further comparative data on the efficacy of SBRT in OMPC. The GETUG 36, PLATON, ARTO, and PCS-IX trials are all phase 2 or 3 RCTs enrolling only OMPC patients that directly compare the addition of SBRT to standard of care systemic therapy modalities.30–33 The SABR-COMET-10 and CORE phase 3 trials enroll oligometastatic patients of multiple histologies including prostate, and similarly will compare the addition of SBRT with standard management alone.34 The mature results of these trials are highly anticipated.
There is evolving evidence that oligometastatic lesions represent a biologically distinct entity from widely disseminated disease. Favorable primary tumor biology, inhospitable target organ microenvironment, and decreased circulatory system viability are a few contributory factors posited to explain this distinction.35 Preclinical studies have proven the existence of genetic and phenotypic differences between metastatic tumor cells when compared to the primary.36 Early in vitro studies have shown that the primary tumor itself may possess a heterogeneous population of cells of varying metastatic potential.37–39 Clinically, specific genetic markers have been identified to be associated with worse outcomes.40,41
As a parallel component of the ORIOLE study, circulating tumor DNA (ctDNA) and peripheral T-cell receptor DNA from a subset of participants were analyzed and stratified based on the presence of high-risk genetic markers. The investigators reported a significant improvement in PFS in the non-high-risk subgroup with SBRT compared to observation, whereas no difference was observed in the high-risk subgroup. Furthermore, they found patients treated with SBRT had more pronounced T-cell clonal expansion. Interestingly, greater peripheral baseline clonality was associated with composite endpoint progression at 180 days in patients in the treatment arm, but not the observation arm, suggesting that this biological signal may portend a better prognosis in patients who receive SBRT.4 Translational correlative studies such as these are certainly encouraged in future prospective studies to better define the patient prototype who may benefit from aggressive MDT.
Clinical patient selection remains an essential component in determining the optimal management of OMPC patients. Four key prognostic factors associated with better prognosis in oligometastic patients include young age, good performance status, indolent disease (protracted disease-free period between primary and recurrence), and low disease burden.42
Nevertheless, there remains variation in aspects of the definition and management of OMPC patients among global experts. At the 2017 advanced prostate cancer consensus conference, opinion was divided on the definition of OMPC as well as the management of these patients. There appeared to be an even split of proponents advocating for the use of local ablative treatment with short course ADT, and those who advocated for ADT or other systemic therapy alone.43 Subsequently, in the 2019 Dutch Multidisciplinary Consensus Meeting, there remained divide on the optimal management of OMPC patients, including the role for SBRT. Panel consensus was limited by lack of evidence, further underscoring the need for ongoing prospective evaluation in this matter.44
Limitations of the current analysis include the small number of studies eligible for inclusion in our study, with some heterogeneity in the reported endpoints. For each endpoint, only 3–4 studies were eligible for appropriate quantitative synthesis. Furthermore, only two studies were randomized control trials that allowed for direct within study comparison of intervention and control arms.3,4 The remainder were single arm studies which precluded the pooling of treatment effects in the manner of a traditional meta-analysis. Some survival endpoints required recapitulation through digital reconstruction of published survival curves; although this methodology boasts a high degree of precision, it may not represent the exact study values.11 Nevertheless, we adhered to widely accepted methodology to ensure that only high-quality literature evidence was synthesized in an appropriate statistical manner in respect to the endpoints of interest. Other considerations include the heterogeneous utilization rate of ADT among included cohorts, which may ultimately influence the outcomes of interest in this current analysis. These studies, however, represent the minority of patients. The formal trials, STOMP, ORIOLE, or POPSTAR had strict eligibility criteria that did not allow for ADT utilization for a pre-specified period preceding trial enrollment. Future prospective trials are expected to have the same degree of stringency in order to improve the detection of the true treatment effect of SBRT.
Conclusion
SBRT appears to be effective in controlling local disease burden in metachronous OMPC patients and delaying clinical progression and the initiation of ADT. It is associated with minimal significant toxicities. Although extremely promising, there is limited, high-quality evidence to support its current use as standard of care. We await the results of ongoing RCTs to provide further guidance of the role of SBRT in the management of OMPC patients.
Footnotes
Acknowledgment: The authors would like to thank Dr. Alejandro Berlin for his critical review and suggestions to the present work, and Dr. Jetan Badhiwala for his review of the statistical methodology.
Contributor Information
Michael Yan, Email: michael.yan@kingstonhsc.ca.
Nikitha Moideen, Email: nikitha.moideen@kingstonhsc.ca.
Vanessa Freitas Bratti, Email: vanessabratti@hotmail.com.
Fabio Ynoe de Moraes, Email: fabio.ynoedemoraes@kingstonhsc.ca.
REFERENCES
- 1.Weichselbaum RR, Hellman S. Oligometastases revisited. Nat Rev Clin Oncol 2011; 8: 378–82. doi: 10.1038/nrclinonc.2011.44 [DOI] [PubMed] [Google Scholar]
- 2.Burdett S, Boevé LM, Ingleby FC, Fisher DJ, Rydzewska LH, Vale CL, et al. Prostate radiotherapy for metastatic hormone-sensitive prostate cancer: a STOPCAP systematic review and meta-analysis. Eur Urol 2019; 76: 115–24. doi: 10.1016/j.eururo.2019.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ost P, Reynders D, Decaestecker K, Fonteyne V, Lumen N, De Bruycker A, et al. Surveillance or Metastasis-Directed therapy for oligometastatic prostate cancer recurrence: a prospective, randomized, multicenter phase II trial. J Clin Oncol 2018; 36: 446–53. doi: 10.1200/JCO.2017.75.4853 [DOI] [PubMed] [Google Scholar]
- 4.Phillips R, Shi WY, Deek M, Radwan N, Lim SJ, Antonarakis ES, et al. Outcomes of observation vs stereotactic ablative radiation for oligometastatic prostate cancer. JAMA Oncol 2020; 6: 650. doi: 10.1001/jamaoncol.2020.0147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P, .PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009; 62: 1006–12. doi: 10.1016/j.jclinepi.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 6.Guckenberger M, Andratschke N, Alheit H, Holy R, Moustakis C, Nestle U, et al. Definition of stereotactic body radiotherapy: principles and practice for the treatment of stage I non-small cell lung cancer. Strahlenther Onkol 2014; 190: 26–33. doi: 10.1007/s00066-013-0450-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg 2003; 73: 712–6. doi: 10.1046/j.1445-2197.2003.02748.x [DOI] [PubMed] [Google Scholar]
- 8.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. Rob 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019; 366: l4898. doi: 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 9.Brenner DJ, Hall EJ. Fractionation and protraction for radiotherapy of prostate carcinoma. Int J Radiat Oncol Biol Phys 1999; 43: 1095–101. doi: 10.1016/S0360-3016(98)00438-6 [DOI] [PubMed] [Google Scholar]
- 10.Rohatgi A. WebPlotDigitizer. 4.2 ed San Francisco, California, USA; 2019. [Google Scholar]
- 11.Wei Y, Royston P. Reconstructing time-to-event data from published Kaplan-Meier curves. Stata J 2017; 17: 786–802. doi: 10.1177/1536867X1801700402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–60. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Decaestecker K, De Meerleer G, Lambert B, Delrue L, Fonteyne V, Claeys T, et al. Repeated stereotactic body radiotherapy for oligometastatic prostate cancer recurrence. Radiat Oncol 2014; 9: 135. doi: 10.1186/1748-717X-9-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muacevic A, Kufeld M, Rist C, Wowra B, Stief C, Staehler M. Safety and feasibility of image-guided robotic radiosurgery for patients with limited bone metastases of prostate cancer. Urol Oncol 2013; 31: 455–60. doi: 10.1016/j.urolonc.2011.02.023 [DOI] [PubMed] [Google Scholar]
- 15.Jereczek-Fossa BA, Fanetti G, Fodor C, Ciardo D, Santoro L, Francia CM, et al. Salvage stereotactic body radiotherapy for isolated lymph node recurrent prostate cancer: single institution series of 94 consecutive patients and 124 lymph nodes. Clin Genitourin Cancer 2017; 15: e623–32. doi: 10.1016/j.clgc.2017.01.004 [DOI] [PubMed] [Google Scholar]
- 16.Kneebone A, Hruby G, Ainsworth H, Byrne K, Brown C, Guo L, et al. Stereotactic body radiotherapy for oligometastatic prostate cancer detected via prostate-specific membrane antigen positron emission tomography. Eur Urol Oncol 2018; 1: 531–7. doi: 10.1016/j.euo.2018.04.017 [DOI] [PubMed] [Google Scholar]
- 17.Gomez-Iturriaga A, Casquero Ocio F, Ost P, Fernandez I, Rodeño E, Llarena R, et al. Outcomes after a first and/or second salvage treatment in patients with oligometastatic prostate cancer recurrence detected by (18-F) choline PET-CT. Eur J Cancer Care 2019; 28: e13093. doi: 10.1111/ecc.13093 [DOI] [PubMed] [Google Scholar]
- 18.Pasqualetti F, Panichi M, Sollini M, Sainato A, Galli L, Morganti R, et al. 18F]Fluorocholine PET/CT-guided stereotactic body radiotherapy in patients with recurrent oligometastatic prostate cancer. Eur J Nucl Med Mol Imaging 2020; 47: 185–91. doi: 10.1007/s00259-019-04482-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siva S, Bressel M, Murphy DG, Shaw M, Chander S, Violet J, et al. Stereotactic Abative body radiotherapy (SABR) for oligometastatic prostate cancer: a prospective clinical trial. Eur Urol 2018; 74: 455–62. doi: 10.1016/j.eururo.2018.06.004 [DOI] [PubMed] [Google Scholar]
- 20.Bowden P, See AW, Frydenberg M, Haxhimolla H, Costello AJ, Moon D, et al. Fractionated stereotactic body radiotherapy for up to five prostate cancer oligometastases: interim outcomes of a prospective clinical trial. Int J Cancer 2020; 146: 161–8. doi: 10.1002/ijc.32509 [DOI] [PubMed] [Google Scholar]
- 21.Bernard B, Gershman B, Karnes RJ, Sweeney CJ, Vapiwala N. Approach to oligometastatic prostate cancer. Am Soc Clin Oncol Educ Book 2016; 35: 119–29. doi: 10.1200/EDBK_159241 [DOI] [PubMed] [Google Scholar]
- 22.Palma DA, Olson R, Harrow S, Gaede S, Louie AV, Haasbeek C, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet 2019; 393: 2051–8. doi: 10.1016/S0140-6736(18)32487-5 [DOI] [PubMed] [Google Scholar]
- 23.Gomez DR, Blumenschein GR, Lee JJ, Hernandez M, Ye R, Camidge DR, et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol 2016; 17: 1672–82. doi: 10.1016/S1470-2045(16)30532-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ost P, Reynders D, Decaestecker K, Fonteyne V, Lumen N, De Bruycker A, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence (STOMP): five-year results of a randomized phase II trial. Journal of Clinical Oncology 2020; 38: 10. doi: 10.1200/JCO.2020.38.6_suppl.10 [DOI] [Google Scholar]
- 25.Alibhai SMH, Gogov S, Allibhai Z. Long-Term side effects of androgen deprivation therapy in men with non-metastatic prostate cancer: a systematic literature review. Crit Rev Oncol Hematol 2006; 60: 201–15. doi: 10.1016/j.critrevonc.2006.06.006 [DOI] [PubMed] [Google Scholar]
- 26.Alibhai SMH, Breunis H, Timilshina N, Naglie G, Tannock I, Krahn M, et al. Long-Term impact of androgen-deprivation therapy on physical function and quality of life. Cancer 2015; 121: 2350–7. doi: 10.1002/cncr.29355 [DOI] [PubMed] [Google Scholar]
- 27.Krahn MD, Bremner KE, Luo J, Alibhai SMH. Health care costs for prostate cancer patients receiving androgen deprivation therapy: treatment and adverse events. Curr Oncol 2014; 21: 457–65. doi: 10.3747/co.21.1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krupski TL, Foley KA, Baser O, Long S, Macarios D, Litwin MS. Health care cost associated with prostate cancer, androgen deprivation therapy and bone complications. J Urol 2007; 178(4 Pt 1): 1423–8. doi: 10.1016/j.juro.2007.05.135 [DOI] [PubMed] [Google Scholar]
- 29.Ost P, Bossi A, Decaestecker K, De Meerleer G, Giannarini G, Karnes RJ, et al. Metastasis-directed therapy of regional and distant recurrences after curative treatment of prostate cancer: a systematic review of the literature. Eur Urol 2015; 67: 852–63. doi: 10.1016/j.eururo.2014.09.004 [DOI] [PubMed] [Google Scholar]
- 30.Management of Castration-Resistant Prostate Cancer With Oligometastases (PCS IX) [Internet].. Available from: https://clinicaltrials.gov/ct2/show/NCT02685397 [cited March 14, 2020].
- 31.Local Ablative Therapy For Hormone Sensitive Oligometastatic Prostate Cancer (PLATON) [Internet].. Available from: https://clinicaltrials.gov/ct2/show/NCT03784755 [cited March 14, 2020].
- 32.Blanchard P, Foulon S, Louvel G, Habibian M, Fizazi K. A randomized controlled trial of metastases-directed treatment in patients with metastatic prostate cancer using stereotactic body irradiation: a GETUG-AFU trial. Cancer Radiother 2017; 21(6-7): 491–4. doi: 10.1016/j.canrad.2017.06.007 [DOI] [PubMed] [Google Scholar]
- 33.Phase II Randomized Trial of Radiation Therapy in Oligometastatic mCRPC Prostate Cancer (ARTO) [Internet].. Available from: https://clinicaltrials.gov/ct2/show/NCT03449719 [cited March 14, 2020].
- 34.Khoo V, Ahmed M, McDonald F, Kirby A, Van As N, Hawkins M, et al. 122: core: a randomised trial of conventional care versus Radioablation (stereotactic body radiotherapy) for extracranial oligometastases. Lung Cancer 2017; 103: S55–6. doi: 10.1016/S0169-5002(17)30172-1 [DOI] [Google Scholar]
- 35.Reyes DK, Pienta KJ. The biology and treatment of oligometastatic cancer. Oncotarget 2015; 6: 8491–524. doi: 10.18632/oncotarget.3455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pantel K, Brakenhoff RH. Dissecting the metastatic cascade. Nat Rev Cancer 2004; 4: 448–56. doi: 10.1038/nrc1370 [DOI] [PubMed] [Google Scholar]
- 37.Fidler IJ, Kripke ML. Metastasis results from preexisting variant cells within a malignant tumor. Science 1977; 197: 893–5. doi: 10.1126/science.887927 [DOI] [PubMed] [Google Scholar]
- 38.Harris JF, Chambers AF, Hill RP, Ling V. Metastatic variants are generated spontaneously at a high rate in mouse KHT tumor. Proc Natl Acad Sci U S A 1982; 79: 5547–51. doi: 10.1073/pnas.79.18.5547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gerlinger M, Rowan AJ, Horswell S, Math M, Larkin J, Endesfelder D, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med 2012; 366: 883–92. doi: 10.1056/NEJMoa1113205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Annala M, Vandekerkhove G, Khalaf D, Taavitsainen S, Beja K, Warner EW, et al. Circulating tumor DNA genomics correlate with resistance to abiraterone and enzalutamide in prostate cancer. Cancer Discov 2018; 8: 444–57. doi: 10.1158/2159-8290.CD-17-0937 [DOI] [PubMed] [Google Scholar]
- 41.Abida W, Cyrta J, Heller G, Prandi D, Armenia J, Coleman I, et al. Genomic correlates of clinical outcome in advanced prostate cancer. Proc Natl Acad Sci U S A 2019; 116: 11428–36. doi: 10.1073/pnas.1902651116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palma DA, Louie AV, Rodrigues GB. New strategies in stereotactic radiotherapy for Oligometastases. Clin Cancer Res 2015; 21: 5198–204. doi: 10.1158/1078-0432.CCR-15-0822 [DOI] [PubMed] [Google Scholar]
- 43.Gillessen S, Attard G, Beer TM, Beltran H, Bossi A, Bristow R, et al. Management of patients with advanced prostate cancer: the report of the advanced prostate cancer consensus conference APCCC 2017. Eur Urol 2018; 73: 178–211. doi: 10.1016/j.eururo.2017.06.002 [DOI] [PubMed] [Google Scholar]
- 44.Aluwini SS, Mehra N, Lolkema MP, Oprea-Lager DE, Yakar D, Stoevelaar H, et al. Oligometastatic prostate cancer: results of a Dutch multidisciplinary consensus meeting. Eur Urol Oncol 2020; 3: 231-238. doi: 10.1016/j.euo.2019.07.010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.