Abstract
Objective:
This study aimed to investigate if CT-detected extramural venous invasion (ctEMVI) was associated with the presence of lymph node metastasis (LNM) and survival outcomes in patients with gastric cancer.
Methods:
We retrospectively reviewed 105 patients with pathologically proved gastric cancer who underwent pre-operative CT examinations and received radical gastrectomy with extended lymphadenectomy. Differences in CT characteristics between the LNM-positive and -negative groups were assessed by two observers. Binary logistic regression analysis was performed to determine the risk factors of lymph node metastasis in gastric cancer. Progression-free survival analysis was performed by Kaplan–Meier method.
Results:
Two observers reached good inter-reader agreements in ctEMVI and ctN status with κ values of 0.711 and 0.751, respectively. The frequency of ctEMVI-positive status was 58.1% (61/105) in patients with gastric cancer. The LNM-positive group showed higher possibility of ctEMVI-positive status (81.7% vs 26.7%, p<0.001), larger tumor volume (mean volume, 40.77 vs 22.09 mL, p<0.001), poor tumor margin (45.0% vs 26.7% , p = 0.054) and high enhancement on arterial phase (43.3% vs 26.7%, p = 0.023) and venous phase (60.0% vs 44.4%, p = 0.048), than LNM-negative group. In multivariate analysis, ctEMVI status and tumor volume were identified as independent risk factors for lymph node metastasis with odds ratio (OR) of 9.804 (95% CI, 3.076-31.246; p<0.001) and 1.030 (95% CI, 1.001-1.060; p = 0.044). CT-detected EMVI presented better diagnostic efficiency for lymph node metastasis than CT-defined N status, with sensitivity (81.7% vs 70.0%), specificity (73.3% vs 71.1%), accuracy (78.1% vs 70.5), PPV (80.3% vs 76.4%), and NPV (75.0% vs 64.0%), respectively. Kaplan–Meier curves showed that patients with positive ctEMVI findings has lower PFS rate than patients with negative ctEMVI findings (Log-rank test, p = 0.007).
Conclusion:
CT-detected EMVI was significantly associated with lymph node metastasis and progression free survival in patients with gastric cancer. Compared to CT-defined N status, ctEMVI provided superior diagnostic performance to predict pathologic Nstatus.
Advances in knowledge:
Our study proved that CT-detected EMVI is a promising imaging marker to predict lymph node metastasis and poor prognosis, which may contribute to the precise evaluation of gastric cancer before surgery.
Introduction
Gastric cancer is the fourth most common malignant tumor and the second most death-related neoplastic disease worldwide.1 Curative surgical resection and combination chemotherapy are widely recommended for the treatment of gastric cancer. Several studies have revealed that one important factor affecting the overall survival (OS) in resectable gastric cancer is lymph node metastasis (LNM),2 which is related to the selection of treatment strategies and the prognosis of gastric cancer.
Extramural vessel invasion (EMVI), pathologically defined as tumor cells invading the vasculature beyond the muscularis propria,3 has been extensively studied in rectal cancer. Histopathological evidences showed that EMVI is presented in 28.0% of patients with gastric cancer.4 Although not a part of the AJCC/UICC staging system, EMVI is widely recognized as a predictor of poor prognosis,5 which is usually related to higher incidence rate of metastasis and disease recurrence.
MRI has exhibited excellent application for detecting EMVI status among patients with rectal cancer, due to its high resolution of soft tissue. A study conducted by Hunter et al6 demonstrated that EMVI defined by MRI can be used as an adverse imaging feature for synchronous distant metastasis in patients with rectal cancer. However, the application of MRI in gastric cancer is rarely seen due to the motion artifacts. With the development of multidetector CT techniques, the spatial resolution of CT images is continuously improving, along with the multiplanar reconstruction technique now been increasingly used in clinical practice, which makes the detection of EMVI on CT images a lot more accurate. As is reported in previous study,7 contrast-enhanced CT is valuable for detecting EMVI status in patients with gastric cancer before curative surgery. To our knowledge, only a few studies have reported about the importance of EMVI in the pre-operative radiological evaluation of gastric cancer. In a study by Cheng et al,8 EMVI-positive status detected on CT was identified as an adverse imaging feature of synchronous metastasis in patients with T4 gastric cancer. However, the patients in this study were involved with peritoneal metastases and distant metastases, rather than pathologically proved lymph nodes metastases.
Therefore, this study aimed to retrospectively analyze the relationship between ctEMVI status and pathologic N status and progression free survival, and to compare the predictive value of ctEMVI to that of ctN status as well as other qualitative tumor characteristics.
Methods and materials
Patients
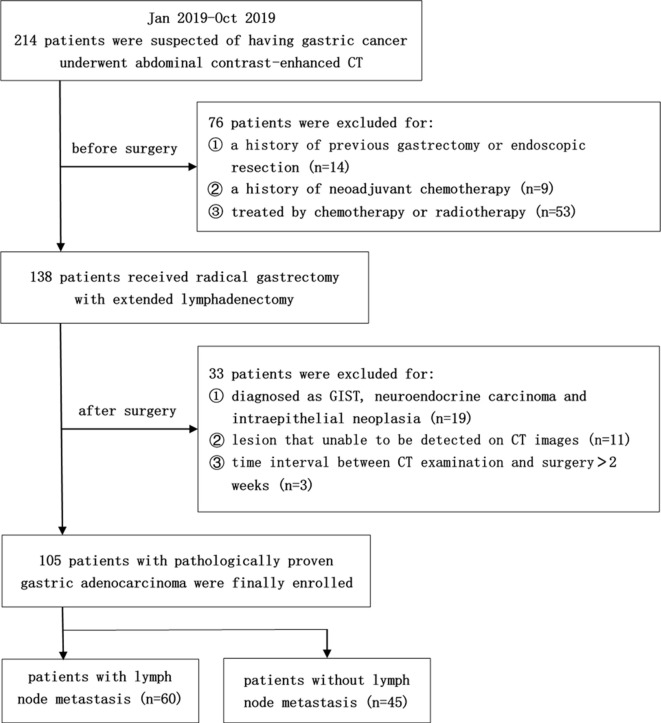
This retrospective study was approved by the institutional review board of Zhongshan Hospital of Fudan University, and a waiver of informed consent was obtained. Between January 2019 and October 2019, a total of 214 patients who had undergone abdominal contrast-enhanced CT examinations with pathologically proved gastric cancers were preliminary included. Among these cases, we further selected patients according to the following criteria: (1) patients who had gone through radical gastrectomy with extended lymphadenectomy for primary gastric cancer within 2 weeks upon CT examination; (2) no history of previous gastrectomy or endoscopic resection; (3) no history of neoadjuvant chemotherapy; (4) lesion that unable to be detected on CT images; (5) with a histological type of adenocarcinoma. Finally, 105 patients with pathologically proved gastric cancer were included in the study. The flow chart of patient inclusion and exclusion process is presented in Figure 1.
Figure 1.
Flow chart of patient inclusion and exclusion process. GIST, gastrointestinal stromal tumor.
Post-operative follow-up of all enrolled patients had been implemented until July 20, 2020. The presence of radiography/ultrasonography or laboratory testing-proved local recurrence, distant metastasis, or gastric cancer-related death was considered as disease progression. Progression-free survival (PFS) was defined as the period of time between surgery and the disease progression status, or the latest follow-up day for patient without any progression.
Surgical procedure
All patients underwent radical gastrectomy with extended lymphadenectomy in our general surgery department. According to the Japanese gastric cancer treatment guidelines 2014 (v. 4),9 the perigastric lymph nodes along with the lymph nodes that accompany the named vessels of the celiac trunk were dissected. D2 resection was implemented for all patients as a standard surgical procedure. At least more than 16 lymph nodes were dissected and sent separately for pathological examination to confirm whether there was a presence of metastasis.
Acquisition of contrast-enhanced CT images
All abdominal CT examinations were performed with the following CT scanner in Zhongshan Hospital of Fudan University: a 128-slice CT scanner (SOMATOM Definition AS, Siemens Healthineers, Germany). All patients were asked to fast for at least 8 h before CT scan, and to drink 800–1000 ml water 20 min before the examination in order to sufficiently distend their stomachs. CT scans covered the top of the diaphragm to hypogastric region with the patient supine. Contrast-enhanced CT images were acquired after intravenous injection of 100 ml non-ionic contrast agent (300 mg I/mL; Ultravist, Bayer Schering Pharma, Berlin, Germany), using an automated injector at a rate of 3 ml s−1. The arterial and portal venous CT phases were acquired by scanning the images 30–35 and 80 s respectively. CT scan parameters were as follows: tube voltage of 120 kVp; tube current of 160 mAs; rotation time of 0.5 s; pitch of 0.9, detector collimation of 32 × 1.2 mm; field of view of 387 × 387 mm; matrix size of 512 × 512; slice thickness of 1.5 mm and slice interval of 1.5 mm. Multiplane reconstructions including axial, sagittal and coronal planes with 1.5 mm thickness were performed on a separate workstation. All CT scanning data were transferred to the picture archiving and communications systemin our institution.
CT image analyses
All CT images were retrieved from the picture archiving and communications system for retrospective analysis, using the DICOM reader software Centricity DICOM Viewer 3.1 (GE Healthcare, Chicago, IL). Two radiologists (YT.Y and SY.D with 3 and 5 years of clinical experience in abdominal CT, respectively) who were blinded to the histopathology reports analyzed the CT images independently. A third radiologist (SX.R with 20 years of clinical experience in abdominal CT) was asked to review the images and make the final diagnosis if there was a controversy between the two younger observers. All CT image analyses were performed on multiplanar reconstruction images to determine the CT features in a more accurate manner.
In this study, ctEMVI and ctN status were considered two main factors under investigation, and the additional qualitative CT features included tumor location, tumor morphology, tumor margin, tumor volume, degree of enhancement on arterial and venous phases, and dynamic pattern of enhancement.
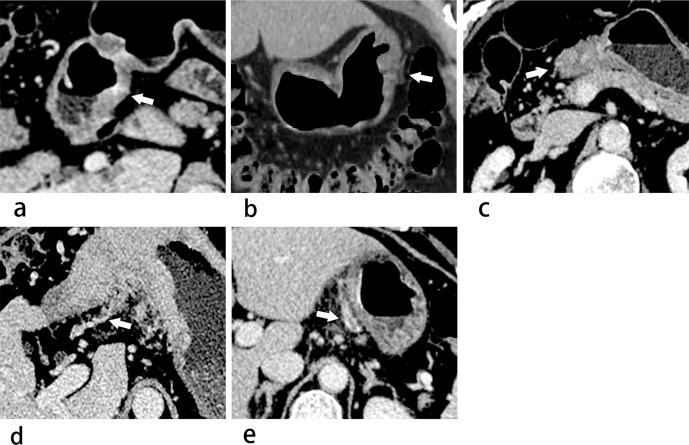
ctEMVI-positive status was defined as a contiguous tubular or nodular soft tissue which extended from the tumor lesion to the extramural blood vessels, leading to dilated vessels or exhibiting filling defects within the dilated veins along the vessels of the mesentery. With reference to the MR-defined EMVI scoring standard of the rectal cancer,10 along with the evaluation of ctEMVI in gastric cancer reported by Tan et al,7 we scored the ctEMVI according to the following criteria presented in Table 1. 0 to 2 points were defined as ctEMVI-negative status and 3 to 4 points were ctEMVI-positive stastus. Examples of ctEMVI evaluation were shown in Figure 2. ctN-positive status was defined as any celiac lymph node with a short-axis diameter larger than 10 mm11. Primary tumor location included three area: cardia, body and antrum. Tumor morphology was assessed as focal or diffuse type. Tumor region was characterized as well-defined margin or poorly defined margin. Tumor region was manually draftedon each axial slice on venous phase. Whole-tumor volume was calculated by multiplying each cross-sectional tumor area by section thickness.The tumor region avoided the gas and liquid inside the gastric cavity, as well as excluding the perigastric lymph nodes, curving vessels and adjacent organs outside the gastric wall. According to the enhancement difference between the tumor region and normal gastric tissue, the degree of enhancement was defined as low, moderate and high enhancement on arterial and portal venous phases.12 Subsequently, the dynamic enhancement pattern was defined as persistently low enhancement (low/low), progressively enhanced pattern (low/moderate, low/high, or moderate/high), gradually attenuated pattern, (high/moderate, high/low or moderate/low) and persistently high enhancement pattern (high/high).
Table 1.
CT criteria of EMVI status for gastric cancer
| CT-defined EMVI Score |
Typical Imaging Features |
|---|---|
| 0 | The lesion does not penetrate the gastric wall, and there are no extramural blood vessels adjacent to the tumor area |
| 1 | Slight extramural nodular extension, but no exhibition of any invasion into the vascular structure outside the tumor region |
| 2 | The mass penetrates the gastric wall,with extramural blood vessels in the vicinity of the tumor area, but there is no tumor density shadow within the vascular lumen, and these vessels are of normal caliber |
| 3 | The mass penetrates the gastric wall and extends in a strip shape into the extramural vascular lumen, whereas the caliber of these involved vessels is only slightly expanded |
| 4 | Obvious tubular or nodular soft-tissue extends irregularly into the extramural vascular luminal cavity with distinct distention of the vessel lumen |
EMVI, extramural venous invasion.
Figure 2.
(a–e) Images show examples of ctEMVI evaluation criteria using 5-point method in gastric cancer. (a) 0 points: a transverse image of venous phase shows there are no extramural blood vessels adjacent to the tumor lesion, which does not penetrate the gastric wall (white arrow). (b) 1 point: a coronal reconstruction image of venous phase shows minimal extramural stranding of the tumor lesion, but the adjacent blood vessel is not involved (white arrow). (c) 2 points: a transverse venous phase image shows the mass penetrates the gastric wall, with curved vessels around the tumor region (white arrow), but there is no exhibition of tumor density shadow inside the vascular lumen. (d) 3 points: a transverse venous phase image shows the mass extend in a tubular shape into the perigastric vascular lumen, with the involved vessel slightly curved and dilated (white arrow). (e) 4 points: a transverse venous phase image exhibits filling defects within the extramural vascular lumen, whose caliber is of distinct distention (white arrow). ctEMVI, CT-detected extramural venous invasion.
Histopathological evaluation
The radical gastrectomy specimens and extended lymphadenectomy specimens were fixed in formalin and stained with hematoxylin and eosin. According to the American Joint Committee on Cancer criteria (v. 8), the pathologic lymph node metastasis staging was precisely evaluated. Based on N stages of all lymphadenectomy specimens, the patients were divided into two groups: the LNM-positive and LNM-negative group. Moreover, the histologic type and differentiation of each tumor were also recorded.
Statistical analyses
Inter-reader agreements were assessed using Cohen’s κ coefficient (κ) to check the reproducibility of ctEMVI and ctN status since they were the major factors investigated in our study. The κ value degree was divided into four grades: poor agreement (κ<0.20); fair agreement (0.21-0.40); moderate agreement (0.41-0.60); good agreement (0.61-0.80); and perfect agreement (κ>0.8).
Clinical, histopathological and CT characteristics between LNM-positive and -negative groups were compared, using univariate statistical tests such as χ2 test, Fisher’s exact test, Mann–Whitney U test and the Student’s t test. Any factors with p-value lower than 0.1 in univariate analyses were chosen for further multivariate logistic regression analysis, with the enter selection procedure. Sensitivity, specificity, accuracy, positive predictive value (PPV), and negative predictive value (NPV) assessed with ctEMVI and ctN were calculated, using the pathology-proved N status as reference standard.
PFS analysis was performed using the Kaplan–Meier method, and Log-rank test was used to compare the difference between groups. All statistical analyses were performed using SPSS 25.0 software (SPSS Inc., Chicago, IL). All categoric variables were presented as number and percentage, and all quantitative variables were presented as mean ± SD. For all statistical analyses, a two-tailed p-value less than 0.05 was considered as statistically significant.
Results
Clinical and histopathological information
There were 74 males and 31 females included in this study, with a mean age of 63.90 ± 9.35 years (range, 28–84 years; median, 65 years). No distant metastases were found in those patients either on pre-operative CT images or during surgery. The clinical and histopathological information of 105 patients were presented in Table 2. There were no significant differences in age and gender between the LNM-positive and -negative groups. Histopathological examinations showed that the LNM-positive gastric cancer tended to have a poorer differentiation (78.3%, 47/60), while well or moderate differentiation type (48.9%, 22/45) was more common in LNM-negative cancers (p = 0.003). The Lauren classification showed no significant difference between the two groups (p = 0.098).
Table 2.
Clinical and histopathological characteristics of gastric cancer patients
| Characteristics | LNM-positive (n = 60) | LNM-negative (n = 45) | p-value |
|---|---|---|---|
| Age, years (mean ± SD) | 64.32 ± 9.44 | 63.33 ± 9.28 | 0.596 |
| Gender | 0.459 | ||
| Male | 44 (73.3) | 30 (66.7) | |
| Female | 16 (26.7) | 15 (33.3) | |
| Lauren classification | 0.098 | ||
| Intestinal | 21 (35.0) | 23 (51.1) | |
| Diffuse/Mixed | 39 (65.0) | 22 (48.9) | |
| Tumor differentiation | 0.003 | ||
| Well or moderate | 13 (21.7) | 22 (48.9) | |
| Poor | 47 (78.3) | 23 (51.1) |
SD, standard deviation.
Data in parentheses are percentages.
CT characteristics for lymph node metastasis
Two observers reached good inter-reader agreements in recognition of ctEMVI and ctN status with κ values of 0.711 and 0.751, respectively. CT characteristics of gastric cancer were presented in Table 3. The frequency of ctEMVI-positive status in this study was 58.1% (61/105). As it was shown in the table, the LNM-positive group had a much higher possibility of ctEMVI-positive status [81.7% (49/60) vs 26.7% (12/45), respectively], and the difference was statistically significant (p<0.001). In terms of the ctN status, LNM-positive group had a significantly higher frequency of ctN-positive status [70.0% (42/60) vs 28.9% (13/45), respectively; (p<0.001)]. The LNM-positive group more frequently showed a poorly-defined margin than the LNM-negative group [45.0% (27/60) vs 26.7% (12/45), respectively] and the difference was nearly significant (p = 0.054). Furthermore, the tumor volume of LNM-positive group was significantly larger than the LNM-negative group (mean volume, 40.77 ml vs 22.09 ml, p<0.001). As for the degree of enhancement of the tumor lesion, the LNM-positive group was more likely to have high enhancement on both arterial phase (43.3%, 26/60) and venous phase (60.0%, 36/60), compared with the LNM-negative group [(26.7%, 12/45) (p = 0.023); (44.4%, 20/45) (p = 0.048); respectively]. However, the dynamic enhancement pattern of tumor regions presented no significant difference (p = 0.455). In terms of the location and morphology of tumor, the difference between the two groups was not statistically significant (p = 0.507 and 0.213, respectively).
Table 3.
Prevalence of CT characteristics between LNM-positive and -negative gastric cancers
| Characteristics | LNM-positive (n = 60) | LNM-negative (n = 45) | p-value |
|---|---|---|---|
| ctEMVI status | <0.001 | ||
| Positive | 49 (81.7) | 12 (26.7) | |
| Negative | 11 (18.3) | 33 (73.3) | |
| ctN status | <0.001 | ||
| Positive | 42 (70.0) | 13 (28.9) | |
| Negative | 18 (30.0) | 32 (71.1) | |
| Tumor location | 0.507 | ||
| Cardia | 13 (21.7) | 11 (24.4) | |
| Body | 21 (35.0) | 11 (24.4) | |
| Antrum | 26 (43.3) | 23 (51.1) | |
| Morphology | 0.213 | ||
| Focal | 50 (83.3) | 33 (73.3) | |
| Diffuse | 10 (16.7) | 12 (26.7) | |
| Tumor margin | 0.054 | ||
| Well-defined margin | 33 (55.0) | 33 (73.3) | |
| Poorly defined margin | 27 (45.0) | 12 (26.7) | |
| Tumor volume, mL | 40.77 ± 31.86 | 22.09 ± 17.03 | <0.001 |
| Degree of enhancement | |||
| Arterial phase | 0.023 | ||
| Low | 13 (21.7) | 21 (46.7) | |
| Moderate | 21 (35.0) | 12 (26.7) | |
| High | 26 (43.3) | 12 (26.7) | |
| Venous phase | 0.048 | ||
| Low | 8 (13.3) | 15 (33.3) | |
| Moderate | 16 (26.7) | 10 (22.2) | |
| High | 36 (60.0) | 20 (44.4) | |
| Dynamic enhancement pattern | 0.455 | ||
| Persistently low enhancement | 18 (30.0) | 20 (44.4) | |
| Progressively enhanced pattern | 22 (36.7) | 13 (28.9) | |
| Gradually attenuated pattern | 2 (3.3) | 2 (4.4) | |
| Persistently high enhancement | 18 (30.0) | 10 (22.2) |
LNM, lymph node metastasis; ctEMVI, CT-detected extramural venous invasion.
Data in parentheses are percentages.
Risk factors for lymph node metastasis
Results of binary logistic regression analysis were presented in Table 4. Multivariate analysis showed that ctEMVI status and tumor volume were independent risk factors for pathological lymph node metastasis, with an OR of 9.804 (95% CI, 3.076-31.246; p<0.001) and 1.030 (95% CI, 1.001-1.060; p = 0.044), respectively.
Table 4.
Multivariate analysis of CT characteristics between LNM-positive and -negative gastric cancers
| Characteristics | Odds ratio | 95% Confidence Interval | p-value |
|---|---|---|---|
| ctEMVI status | |||
| Negative | |||
| Positive | 9.804 | 3.076–31.246 | <0.001 |
| ctN status | |||
| Negative | |||
| Positive | 1.931 | 0.609–6.122 | 0.264 |
| Tumor volume | 1.030 | 1.001–1.060 | 0.044 |
| Tumor margin | |||
| Well-defined margin | |||
| Poorly-defined margin | 0.616 | 0.187–2.023 | 0.424 |
| Degree of enhancement | |||
| Arterial phase | 0.329 | ||
| Low (reference) | |||
| Moderate | 1.008 | 0.157–6.476 | 0.993 |
| High | 2.834 | 0.345–23.268 | 0.332 |
| Venous phase | 0.513 | ||
| Low (reference) | |||
| Moderate | 1.270 | 0.202–7.993 | 0.799 |
| High | 0.521 | 0.053–5.158 | 0.577 |
LNM, lymph node metastasis; ctEMVI, CT-detected extramural venous invasion.
Diagnostic performance of ctEMVI for lymph node metastasis
The diagnostic performances of ctEMVI and ctN for lymph node metastasis were presented in Table 5. Compared with ctN, ctEMVI had an overall advantage in aspects of sensitivity (81.7% vs 70.0%), specificity (73.3% vs 71.1%), accuracy (78.1% vs 70.5), PPV (80.3% vs 76.4%), and NPV (75.0% vs 64.0%).
Table 5.
Diagnostic efficiency of ctEMVI vs ctN for lymph node metastasis
| Sensitivity (%) | Specificity (%) | Accuracy (%) | PPV (%) | NPV (%) | |
|---|---|---|---|---|---|
| ctN status | 70.0 | 71.1 | 70.5 | 76.4 | 64.0 |
| ctEMVI status | 81.7 | 73.3 | 78.1 | 80.3 | 75.0 |
ctEMVI, CT-detected extramural venous invasion.
Progression-free survival outcomes
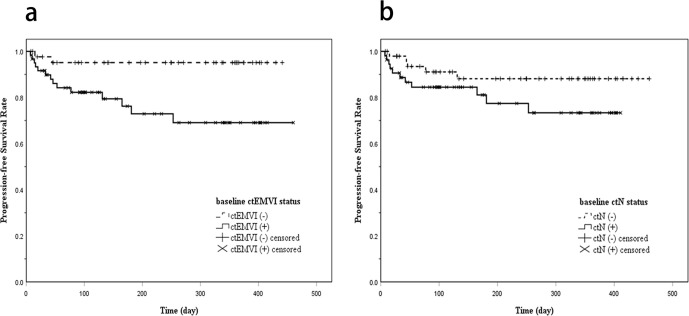
During the whole follow-up period (range, 7–460 days; median, 165 days), there were 16 (15.2%) cases reported disease progression after surgery. In addition, 7 (6.7%) cases were considered loss to follow-up due to a follow-up interval less than 30 days. Among ctEMVI-positive patients, 23.0% (14/61) of them developed disease progression. In contrast, 4.5% (2/44) developed disease progression among ctEMVI-negative patients. For patients with ctN-positive status, 20.0% (11/55) of them developed disease progression, while 10.0% (5/50) of patients with ctN-negative status developed disease progression. Kaplan–Meier curves showed the differences of PFS stratified by the ctEMVI and ctN status (Figure 3). The difference of PFS between patients with and without ctEMVI findings was of statistical significance (Log-rank test, χ2 = 7.203, p = 0.007). However, no significant difference was obtained in the PFS stratified by ctN status (Log-rank test, χ2 = 2.352, p = 0.125).
Figure 3.
(a) Kaplan–Meier curve showing disease progression-free survival according to baseline CT-detected EMVI status (Log-rank test, p = 0.007). (b) Kaplan–Meier curve showing disease progression-free survival according to baseline CT-defined N status (Log-rank test, p = 0.125). EMVI, CT-detected extramural venous invasion.
Discussion
This retrospective study investigated several CT characteristics of gastric cancer to differentiate patients with and without lymph node metastasis. The results of multivariate analysis revealed that CT-detected EMVI (OR, 9.804; 95% CI, 3.076–31.246; p<0.001) and tumor volume (OR, 1.030; 95% CI, 1.001–1.060; p = 0.044) were significantly correlated with lymph node metastasis in gastric cancers. Among LNM-positive patients, there was a higher incidence rate of ctEMVI-positive status (81.7%, 49/60; p<0.001), as well as a tendency of larger tumor size (40.77 ± 31.86 ml; p<0.001).
Tumor volume was one of the risk factors found in our study associated with lymph node metastasis. The possibility of lymph node metastasis increased as the tumor volume increased (OR, 1.030; 95% CI, 1.001–1.060). However, the OR value and its confidence interval obtained in our study were both equal to or very close to 1. This situation is probably caused by too much variation of the tumor volume, since the increasement of tumor volume by one unit has little effect on the outcome. Tumor volume has been proved to be associated with lymph node metastasis in many previous studies, and our finding was similar to those conclusions. Hallinan et al13 reported that tumor volume measured by CT could provide additional information for TNM staging of gastric cancer. Similarly, tumor volume of esophagogastric junction adenocarcinoma measured by CT was associated with regional lymph node metastasis and N stage, according to Li et al.14 In a study by Wang et al,15 post-chemotherapy measurement of tumor volume among patients with gastric cancer was of potential value for predicting N0 stage, with a sensitivity and specificity of 92.31 and 58.62%, respectively. However, in Cheng’s study,16 univariate and multivariate analyses showed that the tumor size was not a risk factor for reduced 3 year progression-free survival. This is probably because they only measured the longest diameter of the tumor region instead of whole-tumor volume. Considering that invasive gastric cancer always presents as a tumor peripherally involving the gastric wall, leading to a difficulty of measuring the longest diameter. Therefore, the maximum tumor diameter on axial images may be incapable of reflecting the growth situation of the whole tumor.
CT-detected EMVI was another independent risk factor for lymph node metastasis. Previous study demonstrated that EMVI usually coexisted with the invasion of perigastric nerves and lymphatic vessels, which was considered to be a pattern of tumor spreading along the neurovascular bundles.7 Pathologic findings demonstrated that EMVI was more likely to occur in patients with lymph node metastasis.17 Thus, detection of ctEMVI in gastric cancer may indicate that the occurrence of lymph node metastasis has already existed. Our study proved that ctEMVI has a close relationship with the metastases in lymph nodes. A ctEMVI-positive patient had a much higher possibility to develop lymph node metastasis than a ctEMVI-negative patient (OR, 9.804; 95% CI, 3.076–31.246). Similarly, Cheng et al8 reported a significant difference in the incidence of synchronous metastases in T4a gastric cancer between the ctEMVI-positive group and -negative group (40.3% vs 21.3%, respectively).
Despite of that ctN status presented a significant difference in the chi-square test, it was not recognized as a risk factor for lymph node metastasis in multivariate analysis. Conventional preoperative assessment of N status by CT usually obtained an unsatisfactory accuracy at about 60%.18 Due to the detection of ctN largely depends on node size, non-enlarged tumor-harboring lymph nodes and enlarged inflammatory nodes might impair the diagnostic performance.19,20 In our study, ctEMVI signs on pre-operative CT images performed comprehensively better diagnostic efficiency than that of ctN. These results indicated that ctEMVI has a great predictive capability for lymph node metastasis in gastric cancer.
With regard to progression-free survival rates, Kaplan–Meier curves showed that ctEMVI was also associate with the prognosis of gastric cancer. Patients with positive EMVI status on baseline CT images tended to develop distant metastasis or local recurrence in early period after surgery. In contrast, ctN status didn’t present a statistical significance in survival outcomes between subgroups. Other studies have also explored the prognostic value of ctEMVI for predicting 1 year-PFS and 2 year-PFS of gastric cancer patient, proving that ctEMVI was a relevant factor.21,22 According to some pathological studies, tumor cells could embolize via the portal circulation when perigastric vessels invasion was present, leading to distant metastasis through hematogenous spread.23 Therefore, ctEMVIpotentially has the ability to serve as a prognostic factor in gastric cancer, similar to the application of EMVI detected by MRI in rectal cancer.
A consensus that an accurate risk stratification before surgery and neoadjuvant chemotherapy, as well as the development of an individualized treatment plan, has been broadly reached, with the purpose of improving R0 resection rate and prolonging the overall survival. It is of great importance to pre-operatively identify lymph node metastasis among patients with gastric cancer, especially when the lymph nodes involve beyond the intended resection extension.19 Accurate diagnoses of lymph nodes metastases can lead to a focused extended lymphadenectomy.24 In addition, for patient with resectable gastric cancer, if lymph node metastasis is found by pathological examination, then a following post-operative chemotherapy should be performed. Therefore, a new marker like ctEMVI is of great value for improving the accuracy of identifying lymph node metastasis and distant metastasis in pre-operative examinations, as well as stratifying patient with high risk who may need a more aggressive treatment plan.
We should address some limitations of this study. Firstly, our study was performed retrospectively, and the number of patients involved in this study was relatively small. Besides, we included both early and advanced gastric cancer, which might introduce some confounding factors. Secondly, the record of tumor volume was measured by manually tracing the tumor boundaries, which might affect the accuracy of tumor volume calculation, especially for small tumors. Thirdly, the evaluation of enhancement in different phases might be subjective since we didn’t take a quantitative approach. Fourthly, due to the relatively short follow-up period, the survival information of some patients is incomplete.
In summary, pre-operative evaluation of EMVI on CT images showed great capability of indicating lymph node metastasis in patients with gastric cancer. Furthermore, ctEMVI was also correlated with the disease progression-free survival rates of gastric cancer patients. CT-detected EMVI is a potentially useful imaging marker for predicting lymph node metastasis and poor prognosis in gastric cancer.
Footnotes
The authors Yu-Tao Yang and San-Yuan Dong contributed equally to the work.
Contributor Information
Yu-Tao Yang, Email: yangyutao_0622@163.com.
San-Yuan Dong, Email: dsymri@163.com.
Jue Zhao, Email: zhaojue_coca@163.com.
Wen-Tao Wang, Email: 787356268@qq.com.
Meng-Su Zeng, Email: zeng.mengsu@zs-hospital.sh.cn.
Sheng-Xiang Rao, Email: raoxray@163.com.
REFERENCES
- 1., Fitzmaurice C, Dicker D, Pain A, Hamavid H, Moradi-Lakeh M, et al.Global Burden of Disease Cancer Collaboration . The global burden of cancer 2013. JAMA Oncol 2015; 1: 505–27. doi: 10.1001/jamaoncol.2015.0735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deng J-Y, Liang H. Clinical significance of lymph node metastasis in gastric cancer. WJG 2014; 20: 3967–75. doi: 10.3748/wjg.v20.i14.3967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor FGM, Swift RI, Blomqvist L, Brown G. A systematic approach to the interpretation of preoperative staging MRI for rectal cancer. American Journal of Roentgenology 2008; 191: 1827–35. doi: 10.2214/AJR.08.1004 [DOI] [PubMed] [Google Scholar]
- 4.CY D, Chen JG, Zhou Y, Zhao GF, Fu H, Zhou XK, et al. Impact of lymphatic and/or blood vessel invasion in stage II gastric cancer. World J Gastroenterol 2012; 18: 3610–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chand M, Siddiqui MR, Swift I, Brown G. Systematic review of prognostic importance of extramural venous invasion in rectal cancer. WJG 2016; 22: 1721–6. doi: 10.3748/wjg.v22.i4.1721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hunter CJ, Garant A, Vuong T, Artho G, Lisbona R, Tekkis P, et al. Adverse features on rectal MRI identify a high-risk group that may benefit from more intensive preoperative staging and treatment. Ann Surg Oncol 2012; 19: 1199–205. doi: 10.1245/s10434-011-2036-1 [DOI] [PubMed] [Google Scholar]
- 7.Tan CH, Vikram R, Boonsirikamchai P, Bhosale P, Marcal L, Faria S, et al. Extramural venous invasion by gastrointestinal malignancies: CT appearances. Abdom Imaging 2011; 36: 491–502. doi: 10.1007/s00261-010-9667-8 [DOI] [PubMed] [Google Scholar]
- 8.Cheng J, Wu J, Ye Y, Zhang C, Zhang Y, Wang Y. Extramural venous invasion detected by MDCT as an adverse imaging feature for predicting synchronous metastases in T4 gastric cancer. Acta radiol 2017; 58: 387–93. doi: 10.1177/0284185116658323 [DOI] [PubMed] [Google Scholar]
- 9.Japanese Gastric Cancer Association Japanese gastric cancer treatment guidelines 2014 (VER. 4. Gastric Cancer 2017; 20: 1–19. doi: 10.1007/s10120-016-0622-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang X-Y, Wang S, Li X-T, Wang Y-P, Shi Y-J, Wang L, et al. Mri of extramural venous invasion in locally advanced rectal cancer: relationship to tumor recurrence and overall survival. Radiology 2018; 289: 677–85. doi: 10.1148/radiol.2018172889 [DOI] [PubMed] [Google Scholar]
- 11.Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA: A Cancer Journal for Clinicians 2017; 67: 93–9. doi: 10.3322/caac.21388 [DOI] [PubMed] [Google Scholar]
- 12.Lee JS, Kim SH, Im S-A, Kim MA, Han JK. Human epidermal growth factor receptor 2 expression in unresectable gastric cancers: relationship with CT characteristics. Korean J Radiol 2017; 18: 809–20. doi: 10.3348/kjr.2017.18.5.809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hallinan JTPD, Venkatesh SK, Peter L, Makmur A, Yong WP, So JBY. Ct volumetry for gastric carcinoma: association with TNM stage. Eur Radiol 2014; 24: 3105–14. doi: 10.1007/s00330-014-3316-5 [DOI] [PubMed] [Google Scholar]
- 14.Li R, Chen T-wu, Hu J, Guo D-dan, Zhang X-ming, Deng D, et al. Tumor volume of resectable adenocarcinoma of the esophagogastric junction at multidetector CT: association with regional lymph node metastasis and N stage. Radiology 2013; 269: 130–8. doi: 10.1148/radiol.13122269 [DOI] [PubMed] [Google Scholar]
- 15.Wang Z-C, Wang C, Ding Y, Ji Y, Zeng MS, Rao SX. Ct volumetry can potentially predict the local stage for gastric cancer after chemotherapy. Diagn Interv Radiol 2017; 23: 257–62. doi: 10.5152/dir.2017.16517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng J, Feng C, Zhang Y, Hong N, Ye Y, Wang Y. CT-Detected extramural vessel invasion and regional lymph node involvement in stage T4a gastric cancer for predicting progression-free survival. AJR Am J Roentgenol 2019;: 1–7. [DOI] [PubMed] [Google Scholar]
- 17.Castonguay MC, Li-Chang HH, Driman DK. Venous invasion in oesophageal adenocarcinoma: enhanced detection using elastic stain and association with adverse histological features and clinical outcomes. Histopathology 2014; 64: 693–700. doi: 10.1111/his.12308 [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Liu W, Yu Y, Liu J-juan, Xue H-dan, Qi Y-fei, Liu JJ, YF Q, et al. Ct radiomics nomogram for the preoperative prediction of lymph node metastasis in gastric cancer. Eur Radiol 2020; 30: 976–86. doi: 10.1007/s00330-019-06398-z [DOI] [PubMed] [Google Scholar]
- 19.Borggreve AS, Goense L, Brenkman HJF, Mook S, Meijer GJ, Wessels FJ, et al. Imaging strategies in the management of gastric cancer: current role and future potential of MRI. Br J Radiol 2019; 92: 20181044. doi: 10.1259/bjr.20181044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wani AH, Parry AH, Feroz I, Choh NA. Preoperative staging of gastric cancer using computed tomography and its correlation with histopathology with emphasis on Multi-planar Reformations and virtual gastroscopy. J Gastrointest Cancer 2020; 14. doi: 10.1007/s12029-020-00436-6 [DOI] [PubMed] [Google Scholar]
- 21.Kim TU, Kim S, Lee NK, Kim HJ, Han GJ, Lee JW, et al. Prognostic value of computed Tomography–Detected extramural venous invasion to predict disease-free survival in patients with gastric cancer. J Comput Assist Tomogr 2017; 41: 430–6. doi: 10.1097/RCT.0000000000000543 [DOI] [PubMed] [Google Scholar]
- 22.Cheng J, Wu J, Ye Y, Zhang C, Zhang Y, Wang Y. The prognostic significance of extramural venous invasion detected by multiple-row detector computed tomography in stage III gastric cancer. Abdom Radiol 2016; 41: 1219–26. doi: 10.1007/s00261-015-0627-1 [DOI] [PubMed] [Google Scholar]
- 23.Bugg WG, Andreou AK, Biswas D, Toms AP, Williams SM. The prognostic significance of MRI-detected extramural venous invasion in rectal carcinoma. Clin Radiol 2014; 69: 619–23. doi: 10.1016/j.crad.2014.01.010 [DOI] [PubMed] [Google Scholar]
- 24.Vergadis C, Schizas D. Is Accurate N - Staging for Gastric Cancer Possible? Front Surg 2018; 5: 41. doi: 10.3389/fsurg.2018.00041 [DOI] [PMC free article] [PubMed] [Google Scholar]