Abstract
Objective:
To compare the efficacy and tolerance of 7-days-a-week accelerated postoperative radiotherapy (p-CAIR) vs postoperative radio-chemotherapy (p-RTCT)
Methods:
Between September 2007 and October 2013, 111 patients were enrolled and randomly assigned to receive 63 Gy in 1.8 Gy fractions 7-days-a-week (n = 57, p-CAIR) or 63 Gy in 1.8 Gy fractions 5-days-a-week with concurrent cisplatin 80–100 mg per square meter of body-surface area on days 1, 22 and 43 of the radiotherapy course (p-RTCT). It represents approximately 40% of the intended trial size, that was closed prematurely due to slowing accrual. Only high-risk patients with squamous cell cancer of the oropharynx/oral cavity, considered fit for concurrent treatment were enrolled.
Results:
The rate of locoregional control (LRC) did not differ significantly between treatment arms (p = 0.18, HR = 0.56), 5 year LRC tended, however, to favour p-RTCT (81%) vs p-CAIR (62%). There was no difference in overall survival between treatment arms (p = 0.90, HR = 1.03).
The incidence and severity of acute mucosal reactions and late reactions did not differ significantly between treatment arms. Haematological toxicity of p-RTCT was, however, considerably increased compared to p-CAIR
Conclusion:
Concurrent postoperative RTCT tended to improve locoregional control rate as compared to p-CAIR. This, however, did not transferred into improved overall survival. Postoperative RTCT was associated with a substantial increase in haematological toxicity that negatively affected treatment compliance in this arm.
Advances in knowledge:
To our knowledge, this is the first trial that compares accelerated radiotherapy and radio-chemotherapy in postoperative treatment for oralcavity/oropharyngeal cancer
Introduction
Despite improvements in surgical and radiation techniques, locoregional recurrences remain among the major causes of failure in combined treatment for locally advanced cancer of the oropharynx and oral cavity. Two large randomised clinical trials demonstrated that postoperative concurrent administration of high-dose cisplatin with radiotherapy provide favourable locoregional control compared to postoperative radiotherapy alone.1,2 This resulted in a widespread acceptance of postoperative radio-chemotherapy as a new standard in adjuvant treatment after surgery for high risk head and neck cancer. The combined treatment that incorporated concurrent radiotherapy and chemotherapy was, however, associated with a substantial increase in adverse effects and the benefit in locoregional control did not result in improved overall survival in RTOG 9501/Intergroup trial.2
An alternative approach in attempts to enhance the effectiveness of combined treatment for locally advanced cancer of the head and neck is represented by the trials in which the overall radiation treatment time of postoperative radiotherapy was shortened, compared to standard fractionation.3 This may improve locoregional tumour control by hampering tumour repopulation that can be triggered by cell depletion from surgery and successive fractions of radiotherapy. The outcome of these trials is largely conflicting, with some of them demonstrating a significant improvement in locoregional control that favours accelerated postoperative radiotherapy,4 some demonstrating a non-significant trend towards such improvement,5–7 while others show no beneficial effect of accelerated postoperative radiotherapy.8 Such disparity can be explained by relatively small sample size of these trials, heterogeneity in patient selection criteria and, thus risk of recurrence, diversity in dose-fractionation schedules and heterogeneity in individual time intervals surgery-radiotherapy and average values reported in the trials.
The largest trial that compared accelerated vs conventional postoperative radiotherapy for high-risk head and neck cancer was performed in Maria Sklodowska-Curie National Research Institute of Oncology and recruited 279 patients with cancer of the larynx, oral cavity and oropharynx.7 The results of this trial have shown a non-significant trend towards improvement in locoregional control in a whole group of 279 patients. A significant improvement in LRC attributable to acceleration of postoperative radiotherapy was, however, demonstrated in a subgroup of 121 patients with cancer of the oropharynx/oral cavity. Also, we were able to identify, based on molecular marker profiles, subgroup of the patients with even more significant improvement in locoregional control from accelerated postoperative radiotherapy.9,10 Patients with cancer of the larynx, and those with a molecular profile unfavourable for accelerated radiotherapy, did not have clinical benefit from shortening overall radiation treatment time. Our supposed ability to select the patients who may benefit from accelerated postoperative radiotherapy (i.e. mainly those with cancer of the oral cavity/oropharynx) created the basis for the present trial.
The hypothesis to be tested in this trial is that the patients with cancer of the oral cavity/oropharynx treated with 7-days-a-week conventional accelerated postoperative irradiation (p-CAIR) may have a similar locoregional tumour control and survival as those treated with postoperative radio-chemotherapy (p-RTCT), but may have a favourable tolerance of treatment. Also, we aim to seek subsets of patients that may benefit from a given treatment schedule (p-CAIR vs p-RTCT). We previously published an interim report that focused on acute mucosal reactions.11 Here, we present the mature final results of the study.
Methods and materials
The trial design
Eligible patients had to be at least 18 years old; provide a written informed consent for participation in a trial, had squamous-cell cancer of the oral cavity or oropharynx; had undergone macroscopically complete major surgery; could tolerate chemotherapy as defined by good performance status (ZUBROD 0–1), white cell count (WBC) of at least 3500 per cubic millimetre, a platelet (PLT) count of at least 100,000 per cubic millimetre and creatinine clearance of more than 50 ml per minute. Aminotransferase and bilirubin values could not exceed twice the normal upper limit. Patients after reconstructive surgery with free-style flaps and those with stage pT1N0 disease were excluded. The other exclusion criteria were history of invasive cancer (except for non-melanoma skin cancer), or prior treatment. The protocol of the study was approved by the local bioethical committee in accordance to the national regulations
Statistical considerations and flow diagram
Randomisation was performed at the time of appointment for radiotherapy by telephone call to the trial office. The patients were assigned to receive continuous 7-days-a-week postoperative radiotherapy (p-CAIR) or conventionally fractionated postoperative concurrent radio-chemotherapy (p-RTCT). Random number generator was used to assign treatment arm.
The intended number of patients enrolled (280) was designed to be large enough to detect at least 15% difference in locoregional tumour control in trial arms with a power of 80% and an error of 5%. We assumed that the difference in locoregional tumour control larger than 15% would be not consistent with equal effectiveness of both arms in terms of locoregional tumour control. On the other hand, such sample size would allow to detect approximately 5–10% difference in complication rate. Considering relatively small intended trial size (280 patients) and large number of factors that could, potentially, influence the outcome (surgical margin, extracapsular extension, pT3-4, pN+, performance status, age, oropharyngeal/oral cavity site etc.) we did not stratify assignment of the patients to a given trial arm.
Kaplan–Meier method was used to plot survival curves. Median follow-up, as estimated by reverse Kaplan–Meier method, was 5.4 years. Log-rank test was used to assess significance of differences in survival distributions: p-value of less than 0.05 was considered as significant. To further explore the data, Cox proportional hazard regression model was used to quantify hazard ratios (HRs). We note that the number of uncensored events was not sufficient to calculate HRs in some subsets of patients (e.g. lack of uncensored events in low-risk patients treated with p-RTCT).Considering this, p-values, as shown on the graphs, refer to log-rank test estimates.
The analysis of survival (if not otherwise stated in the text) was based on intention to treat basis. For the assessment of acute/late toxicity, the off-protocol radiotherapy treatments were, however, excluded.
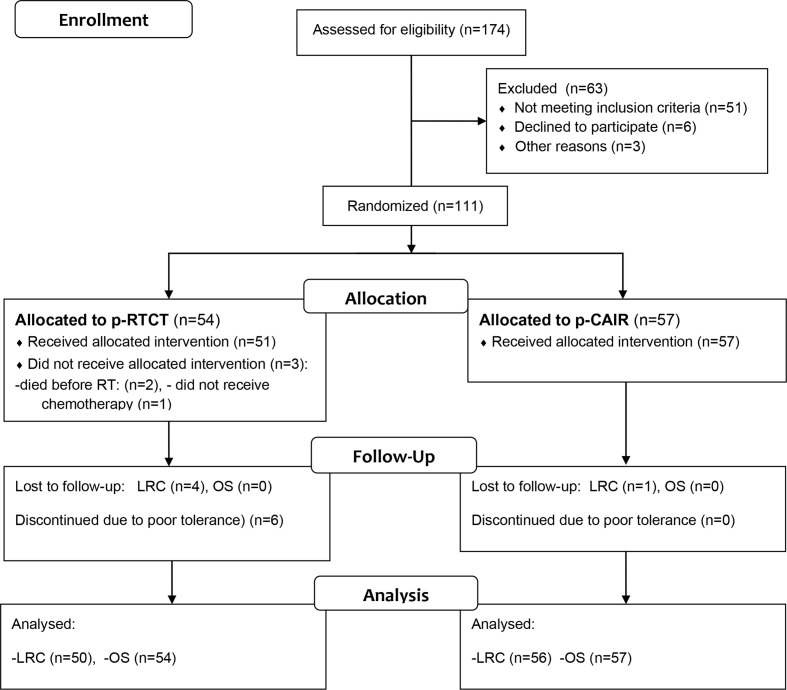
Unfortunately, the trial was closed prematurely due to slowing accrual. This was mainly due to increase in use of reconstructive surgery in our institution; patients with free-style flaps were recruited to the other studies that used different dose-fractionation than described in the present protocol. Overall, between September 2007 and October 2013, 111 patients were recruited, including 57 (51.4%) in p-CAIR and 54 (48.6%) in p-RTCT. It represents approximately 40% of the intended trial size. Figure 1 shows flow diagram of the patients assessed for eligibility and recruited to the trial.
Figure 1.
Consort flow diagram of the trial. Note that some patients lost to follow-up for LRC were available for the analysis of OS. LRC, locoregional tumour control; OS, overall survival.
Patient characteristics
Table 1 summarises characteristics of 111 patients enrolled. There were 54 patients with cancer of the oral cavity (48.6%) and 57 patients with cancer of the oropharynx (51.4%). Clinical and pathological staging was performed according to the seventh edition of the AJCC Manual.12 Most of the patients were in advanced clinical and pathological stages of disease, including 81 individuals (73.0%) in pathological stage III or IV. In general, the patients were well balanced in the trial arms with respect to the probable prognostic factors such as age, stage, site, clinical and pathological stage, pathological margins, performance status and interval surgery-radiotherapy (Table 1). Unfortunately, the data on extracapsular extension were incomplete in this series. In p-RTCT, 6/27 (22%) of patients assessed had exctracapsular extension, compared to 6/31 (19%) in p-CAIR.
Table 1.
Characteristics of the patients by treatment group
| Variable | Subgroups | p-RTCT | p-CAIR |
| (n = 54) | (n = 57) | ||
| Age | <57 years | 28 (52%) | 24 (42%) |
| ≥57 years | 25 (48%) | 33 (57%) | |
| Sex | M | 37 (69%) | 39 (68%) |
| F | 17 (31%) | 18 (32%) | |
| Tumor site | Oral cavity | 25 (46%) | 29 (51%) |
| Oropharynx | 29 (54%) | 28 (49%) | |
| cT stage | T1, T2 | 36 (67%) | 39 (68%) |
| T3, T4 | 18 (33%) | 18 (32%) | |
| cN stage | N0 | 17 (31%) | 19 (33%) |
| N1, N2, N3 | 37 (69%) | 38 (67%) | |
| ZUBROD | 0 | 23 (43%) | 25 (44%) |
| 1 | 31 (57%) | 32 (56%) | |
| Pathological | Negative | 36 (67%) | 36 (63%) |
| margin | Positive | 12 (22%) | 18 (32%) |
| Uncertain | 6 (11%) | 3 (5%) | |
| Number of invaded nodes | 0 | 17 (31%) | 12 (21%) |
| 1 | 11 (20%) | 13 (23%) | |
| ≥2 | 25 (46%) | 30 (53%) | |
| Uncertain | 1 (2%) | 2 (3%) | |
| Interval surgery-RT | ≤9 weeks | 10 (19%) | 11 (19%) |
| >9 weeks | 52 (78%) | 46 (81%) | |
| N/A | 2 (3%) | 0 (0%) | |
| Pathological | Stage II | 19 (35%) | 11 (19%) |
| Stage | Stage III | 18 (33%) | 22 (39%) |
| (AJCC 7-th Ed) | Stage IV A | 14 (26%) | 22 (39%) |
| Stage IVB | 3 (6%) | 2 (3%) | |
| LRC risk score | <7 | 11 (21%) | 29 (51%) |
| ≥7 | 43 (79%) | 28 (49%) |
To better assess the pre-treatment risk of locoregional recurrence, we used a simple rank scale that has been established based on results of the study performed by Peters et al13 and described in our earlier study.7 According to this scale, all patients recruited to the present study may be considered as “high risk”. One may, however, arbitrarily divide this category into subsets of “very high risk” (score ≥7) or “intermediately high risk” (score 3–6). When such partition was employed, we noticed some trend in favour of p-CAIR arm with higher proportion of “intermediately high risk” patients recruited to this arm, compared to p-RTCT. The difference, however, did not appear significant when formally tested using Wald–Wolfowitz test (p = 0.23).
Surgery and radiotherapy
Patients were appointed for radiotherapy shortly after the surgery, i.e. as soon as the pathological specimens had been evaluated. The protocol required postoperative radiotherapy to begin as soon as possible, i.e. as adequate healing had occurred and radiation treatment plan had been approved. This may optimally occur 4–6 weeks after the surgery, but with increasing complexity of radiation treatment techniques and waiting lists for radiotherapy the interval surgery-radiotherapy was frequently longer than originally intended (Table 1). All patients underwent major surgery, as intended, lymphadenectomy was performed in 101/111 patients (91.0%).
Postoperative radiotherapy
The prescribed total dose, dose per fraction and radiation treatment technique was the same in both arms of the trial; the assigned treatments differed, however, with respect to the overall radiation treatment time: it was 5 weeks in p-CAIR and 7 weeks in p-RTCT. The total dose at volumes considered to be at high risk of recurrence was 63 Gy in 1.8 Gy per fraction, i.e. the same as used in our earlier trial that compared continuous 7-days-a-week postoperative radiotherapy (p-CAIR) and conventional postoperative radiotherapy (p-CF).7 A support for such dose selection provided earlier study that had been performed in MD Anderson, Houston.13
Treatment technique was previously described.11 The dose delivered to electively treated volumes was 45 Gy, supraclavicular nodes were electively treated whenever pathological specimen revealed involvement of the neck nodes. In patients assigned to p-CAIR 'large' portals covering clinical target volume were irradiated to the total dose of 45 Gy and were treated 5 days -a-week. By contrast, ’small' portals, limited to the areas considered to be at intermediate/high risk of recurrence excluded the spinal cord and were treated 7 days-a-week. In patients assigned to p-RTCT 'large' portals were irradiated 5-days-a-week to the total dose of 45 Gy over the first 5 weeks of treatment, while ’small fields' were irradiated at weeks 6–7.
Orphit masks were used for immobilisation. The patients were treated using linear accelerators with 6 MV photons. The quality assurance procedures included repeated in vivo dosimetry, image-guided procedures (kV or cone beam CT), double check of treatment plans and portals and pre-treatment and weekly audits during therapy. The protocol allowed use of intensity modulated radiotherapy (IMRT) or 3D conformal radiotherapy, eventually, IMRT was used in 75% of the patients.
Chemotherapy
Chemotherapy consisted of cisplatin 80–100 mg per square meter of body-surface area on days 1, 22 and 43 of the course of radiotherapy. Prophylactic hydration and antiemetic agents were given to patients that received chemotherapy. The original protocol of the trial that allowed cisplatin dose of 100 mg per square meter was amended after completion of the pilot study allowing use of cisplatin doses in the range of 80–100 mg, with lower doses prescribed to the patients with ZUBROD one performance status and/or comorbidities.
Scoring and analysing of acute mucosal and haematological reactions and late toxicity
We have previously published an interim report on acute mucosal and haematological reactions in the present trial based on assessment in initial 84 patients, including the description of the methodology used.11 We also described the supportive treatment that was used in the trial. In general, the acute reactions were scored using modified Dische system.14 This report demonstrated that both schedules were tolerable with respect to acute toxicity, and that early mucosal reactions were comparable in both trial arms but haematological toxicity was more pronounced during radio-chemotherapy. The late reactions were scored according to RTOG/EORTC scoring system.15
Study end points
The primary end point of the trial is assessment of cumulative incidence of locoregional recurrences. The secondary end points include overall survival, metastases-free survival, local control, nodal control, disease-free survival, second cancer free survival, and morbidity of combined treatment.
Results
Treatment compliance
Overall, 90/111 patients (81.1%) complied with the assigned radiation treatment schedule (Table 2). Two patients in p-RTCT died before radiotherapy was started, both due to rapid progression of disease after surgery. No other treatment-related death were recorded. Three patients in p-RTCT and six in p-CAIR received radiation doses higher than in the protocol, due to local/nodal progression during treatment or treatment planning. Higher total doses were delivered in those individuals in 1.8 Gy per fraction. Likewise, six patients in p-RTCT and one in p-CAIR received doses lower than planned due to deteriorating general performance of the patient and/or detection of distant metastases before or during radiotherapy. The other reasons for non-compliance are specified in Table 2. In summary, 13/54 patients in p-RTCT (24.1%) and 8/57 in p-CAIR (14.0%) did not fully comply to the assigned radiation treatment.
Table 2.
Radiotherapy compliance by trial arm
| Variable | p-RTCT (n = 54) | p-CAIR (n = 57) |
|---|---|---|
| Death before RT | 2 (5.0%) | 0 (0%) |
| RT doses higher than in the protocol (70–72 Gy) | 3 (6%) | 6 (10%) |
| RT doses lower than in the protocol | 6 (11.0%) | 1 (2%) |
| Surgery for nodal recurrence before RT | 1 (2%) | 0% (0%) |
| Consent withdrawal | 0% (0%) | 1 (2%) |
| Distant metastases before RT | 1 (25%) | 0% (0%) |
| Total | 13 (24.0%) | 8 (14%) |
RT, radiation therapy.
Radiation treatment interruptions were recorded in 11 patients (19%) treated in p-RTCT arm, compared to 7 patients (13%) in p-CAIR. The median overall radiation treatment time, was 50 days in p-RTCT, compared to 35 days in p-CAIR, including all patients who did not comply to treatment and those who had unplanned radiation treatment interruptions.
Out of 54 patients assigned to p-RTCT, 25 patients (46.3%) received 3 courses of chemotherapy, 21 (38.9%) received 2 courses of chemotherapy, 1 received 1 course of chemotherapy and 6 (11.1%) did not receive chemotherapy. The reason for not receiving chemotherapy was rapid deterioration of general performance of the patient before the onset of radiotherapy and/or local/distant progression of disease. The reason for receiving less than three chemotherapy courses was poor tolerance of treatment. We note that all patients who were withdrawn from chemotherapy received lower or higher radiation doses than specified in the protocol and were, thus, accounted for as non-compliant (Table 2).
Locoregional tumour control in p-RTCT vs p-CAIR
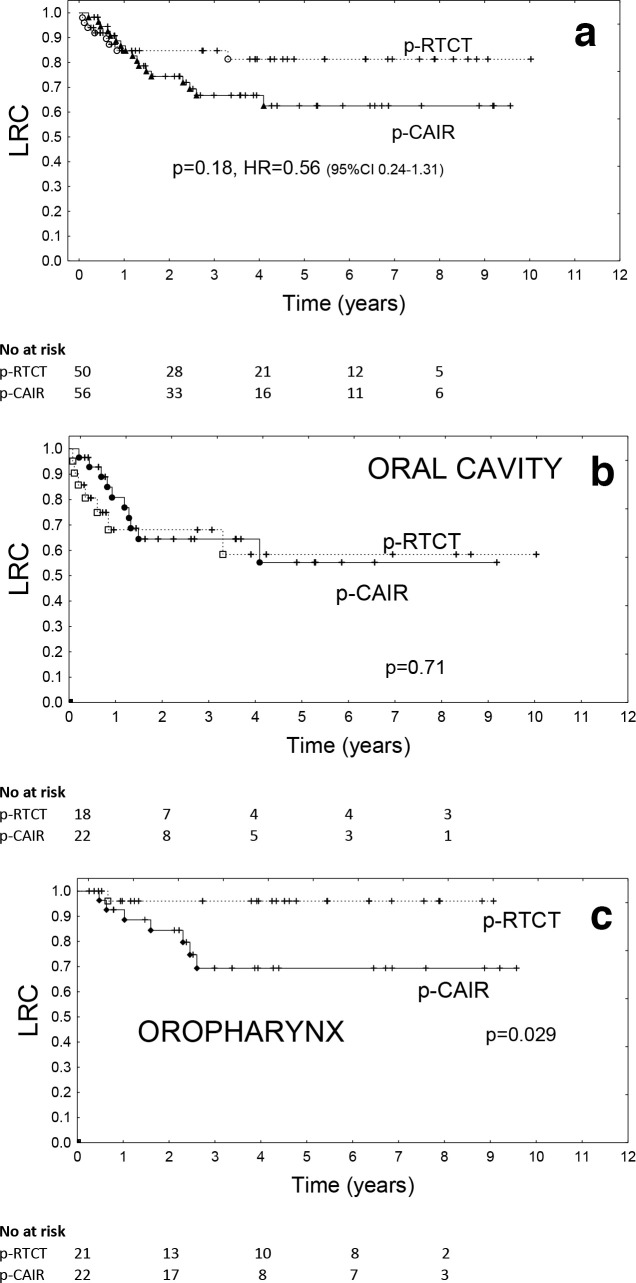
Because of deaths before radiotherapy and loses to follow-up (Figure 1, Table 2), locoregional tumour control was assessed in 50 patients in p-RTCT and 56 patients in p-CAIR. The crude rate of locoregional recurrences was 16.0% (8/50) in p-RTCT and 30.3% (17/56) in p-CAIR, the difference, while tending to favour radio-chemotherapy,did not appear significant (p = 0.18). The actuarial 3 year locoregional control rates were 84.7% (p-RTCT) vs 66.7% (p-CAIR), the difference (log-rank-test) was not significant (p = 0.18, HR = 0.56, 95% CI 0.24–1.31) Figure 2a. The respective actuarial 5 year locoregional control rates were 62.4% vs 81.3%.
Figure 2.
Locoregional tumour control in all patients recruited to the trial (a), and in subset of the patients with cancer of the oral cavity (b) vs oropharynx (c).
Restriction of the data to only those individuals who received intended total radiation dose of 63 Gy did not change the qualitative outcome of the study: the difference in locoregional tumour control in favour of p-RTCT appeared, however, larger with 3 year locoregional control rates of 91.9% (p-RTCT) vs 67.9% (p-CAIR), p = 0.05.
Locoregional control differed significantly depending on primary tumour site: the actuarial 3 year locoregional control rate was (irrespectively of treatment arm) 84.7% for cancer of the oropharynx and 65.1% for cancer of the oral cavity. Interestingly, primary tumour site appeared to be predictive for the gain in locoregional tumour control from RT-CT: Among patients with cancer of the oral cavity, the actuarial 3 year locoregional control rates were 68.1% vs 64.5% for p-RTCT and p-CAIR, respectively, the difference was not significant (p = 0.71, Figure 2b). By contrast, among patients with cancer of the oropharynx the actuarial 3 year locoregional control rates were 96.0% vs 69.3% for p-RTCT and p-CAIR, respectively, the difference was statistically significant (p = 0.029, Figure 2c).
Figure 3 provides graphical display of hazard ratio estimates for comparison of locoregional tumour control rates (p-RTCT vs p-CAIR) in selected subgroups of patients. It can be assessed from the graph that, apart from oropharyngeal tumour location, patients with long interval surgery-radiotherapy (≥9 weeks) tended to benefit from p-RTCT, unlike those with short interval. Risk score for recurrence did not appear to be predictive for the gain in locoregional tumour control from RT-CT: Patients with “intermediately high” risk (score <7, N = 40) tended to benefit from p-RTCT in similar extent, as patients with “extreme risk” (score ≥7,N = 70), but the difference in LRC between p-RTCT and p-CAIR in both groups did not appear significant (p = 0.14 and p = 0.19, respectively).
Figure 3.
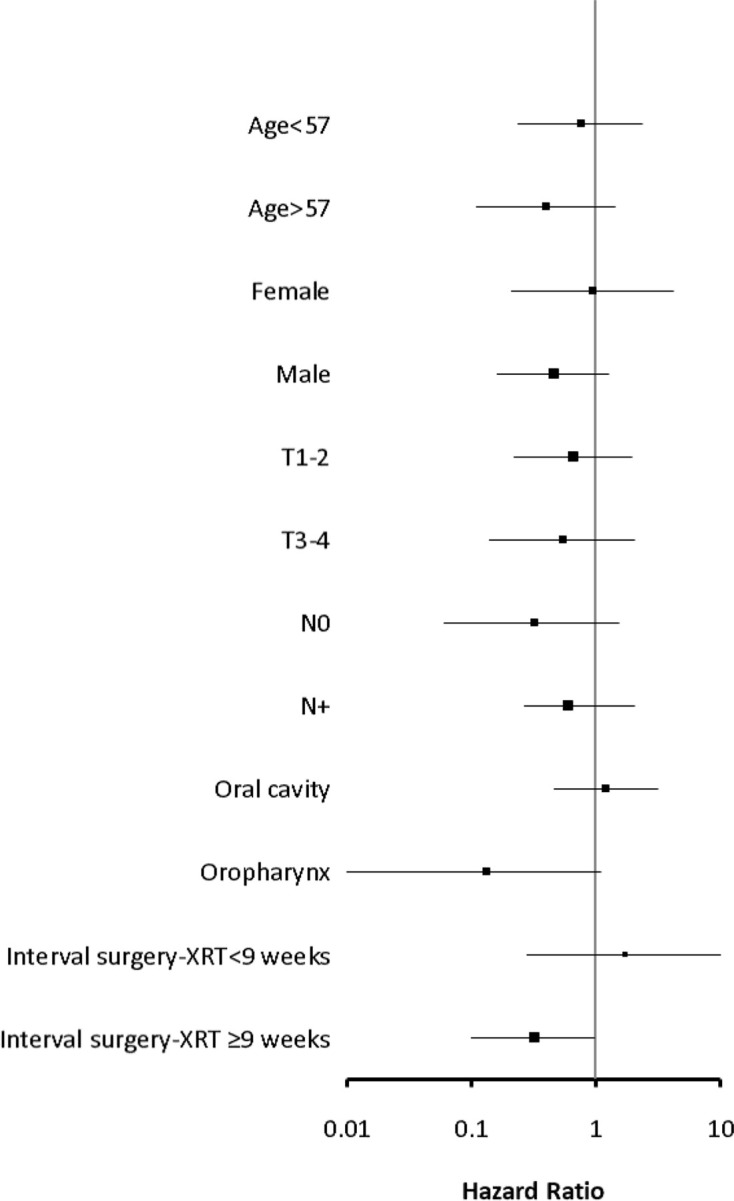
Graphical display of hazard ratio estimates for comparison of locoregional tumour control (p-RTCT vs p-CAIR) in selected subgroups of patients
Other outcomes
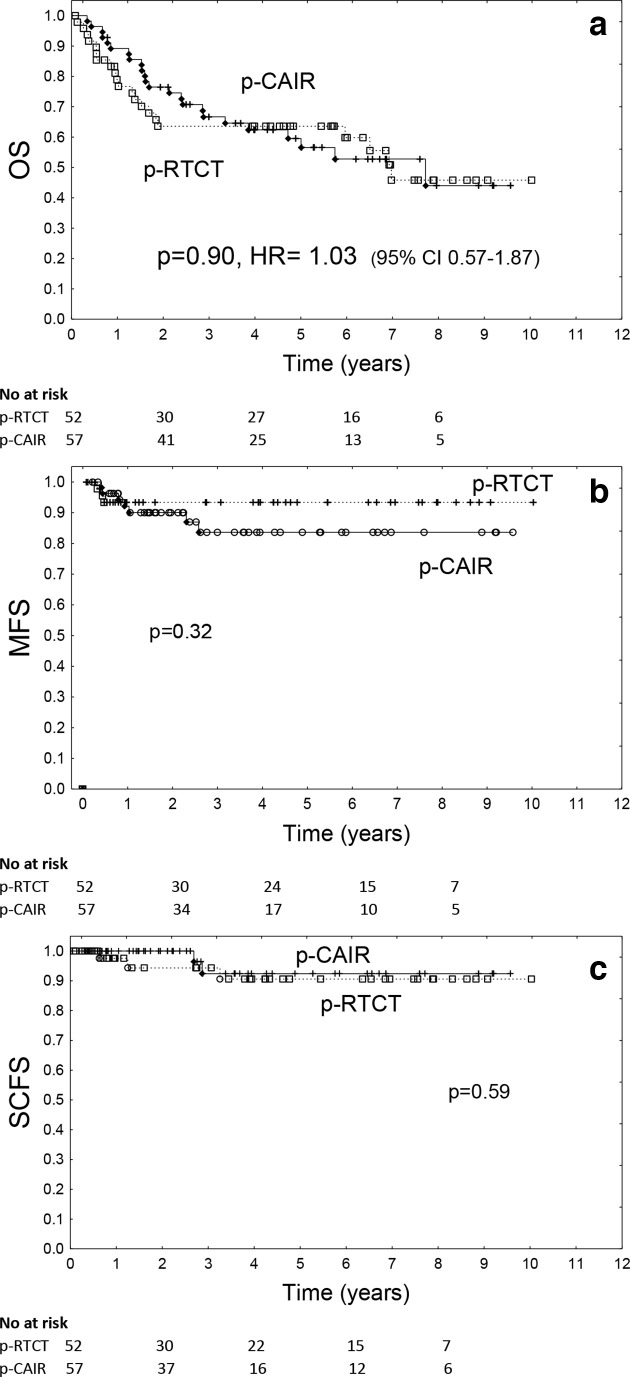
Overall survival did not differ significantly between trial arms (p = 0.90, HR = 1.03, 95% CI 0.57–1.87, Figure 4a). 3 year actuarial overall survival was 66.7% in p-CAIR vs 63.6% in p-RTCT, 5 year overall survival was 59.5% vs 63.6%, respectively. Restriction of the data to the patients who received intended total radiation dose of 63 Gy did not change the qualitative outcome of OS analysis: the difference between p-RTCT and p-CAIR remained not significant (p = 0.66). Metastases-free survival also did not differ significantly between treatment arms (p = 0.32), 3 year accumulated rate of metastases was 16.4% in p-CAIR vs 6.6% in p-RTCT, and 5 year rate was 83.6% vs 93.4%, respectively (Figure 4b). Freedom from second cancer also did not differ between treatment arm (p = 0.59): after 3 years, it was approximately 10%, irrespectively of trial arm and 92.4% vs 90.6% after 5 years for p-CAIR vs p-RTCT (Figure 4c).
Figure 4.
Overall survival (a), metastases-free survival (b) and second cancer-free survival (c) according to treatment arm (p-CHRT vs p-CAIR).
Unlike LRC, overall survival according to treatment arm did not differ significantly neither in subset of patients with oropharyngeal cancer (p = 0.96), nor in subset of patients with cancer of the oral cavity (p = 0.68).
Tolerance of treatment
The acute and late toxicity analysis, as shown, did not include 21 patients with off-protocol radiation treatments that were described in Table 2. This is because the therapeutic interventions used in these patients differed, to major extend, from those intended and diverse symptoms related to deteriorating performance, early recurrences or dissemination disrupted proper assessment of acute/late normal tissue reactions.
The average maximum mucosal severity score was 14.8 (sdt ±3.6) in p-CAIR compared to 12.9 (std ±4.1) in p-RTCT; the difference, according to U Mann–Whitney test, appeared statistically significant (p = 0.024). The duration of confluent mucositis (Dische score ≥10) was 2.9 weeks in p-CAIR compared to 2.4 weeks in p-RTCT, the difference did not appear significant (p = 0.13). Percent of the weight loss did not differ significantly between treatment arms (p = 0.43); it was 8.3%, std ±3.5 vs 7.4%, std ±5.1 for p-CAIR and p-RTCT, respectively.
Haematological reactions were considerably more pronounced in p-RTCT. The average haemoglobin concentration at the end of radiotherapy was 13.7 g dl−1 in p-CAIR vs 12.3 g dl−1 in p-RTCT, the difference was highly significant (p < 0.00001). Likewise, the average white blood cell count at the end of radiotherapy was significantly lower in p-RTCT, compared to p-CAIR (3.8 × 109/l vs 6.9 × 109/l), the difference was highly significant (p < 0.00001). Platelet count at the end of radiotherapy was 236 × 103(p-CAIR) vs 208 × 103(p-RTCT) per microliter of blood, the difference was not significant (p = 0.1).
Table 3 describes acute and late toxicity of treatment, including peak incidence of Grade 2–3 late reactions that were recorded during the follow-up. Grade 4–5 reactions were not observed. Median time to development of late reaction was 2.1 year (range 0.6–9.0 years). Grade 2 xerostomia was the most frequently observed late reaction (9 individuals in p-CAIR including 2 with Grade 3 reaction vs 6 individuals in p-RTCT, none with Grade 3 reaction). Two patients (one in p-CAIR and one in p-RTCT) developed Grade 3 bone reactions. Overall, Grade 2–3 late reactions were more frequent in p-CAIR vs p-RTCT (38 vs 24 individuals, including 5 vs 3 with Grade 3 reaction, respectively), the difference according to U Mann–Whitney test was, however, not significant (p = 0.3).
Table 3.
Acute and late toxicity by treatment arm
| p-CAIR | p-RTCT | |
|---|---|---|
| Acute reactions | ||
| Maximum mucosal severity score (Dische system) | 14.8 | 12.9 |
| Average percent of the weight loss | 8.3% | 7.4% |
| Average Hb concentration (end of RT) | 13.7 g dl−1 | 12.3 g dl−1 |
| Average WBC count (end of RT) | 3.8 × 109/l | 6.9 × 109/l |
| Late reactions | ||
| Mucosa | 5 | 4 |
| Skin | 4 | 1 |
| Subcutaneous | 6 (1) | 3 |
| Xerostomia | 9 (2) | 6 |
| Bone | 1 (1) | 1 (1) |
| Spinal | 0 | 0 |
| Taste | 5 | 3 (1) |
| Carries | 8 (1) | 5 (1) |
| Ear | 0 | 1 |
| Total for late reactions | 38 (5) | 24 (3) |
RT, radiation therapy; WBC, white blood cell.
For late reactions, the number of patients with Grade 2–3 late reactions according to treatment arm is shown (number of Grade 3 reactions is in the brackets)
Discussion
The main outcome of the present trial is that in a group of patient with cancer of the oral cavity/oropharynx locoregional tumour control in accelerated postoperative 7-days-a-week radiotherapy (p-CAIR) did not differ significantly, compared to postoperative radio-chemotherapy (p-RTCT). Considering limitations of the present research (premature termination of the trial, and resulting small sample size) it would be inadequate, however, to postulate non-inferiority of p-CAIR, compared to p-RTCT. While difference in locoregional control did not appear significant an apparent trend in favour of RTCT was observed, with 18% improvement in LRC after 3 years (Figure 2a). The benefit in terms of LRC from p-RTCT was particularly apparent in subgroup of the patients with cancer of the oropharynx (Figure 2c), the difference in this subgroup appeared statistically significant. By contrast, no difference in LRC was observed in patients with cancer of the oral cavity. Also, p-RTCT tended to improve LRC among the patients with long interval surgery-radiotherapy (Figure 3). Subset analysis in randomised trials have its apparent limitations, particularly when the sample size is small. It seems, however, justified to postulate that more research is required to better assess the benefit from a specified treatment schedule, particularly at given primary tumour site.
An important finding of the present research is that the trend for improvement in LRC from p-RTCT did not translate into any apparent improvement in overall survival, irrespectively of primary site and risk group. A possible explanation is that p-RTCT was associated with a substantial increase in haematological toxicity that negatively affected treatment compliance in this arm (Table 2). Acute mucosal reaction were, on the other hand, somewhat more severe in p-CAIR, both in terms of peak incidence and duration of reaction. It appears that it translated into higher rate of Grade 2–3 late reactions that tended to be more frequently observed in p-CAIR arm (Table 3). Likely, the mechanism of consequential late effect may explain the observed outcome.16 Considering that the difference in incidence of late reactions did not appear statistically significant, it seems appropriate to conclude that no improvement in late toxicity was associated with use of accelerated postoperative radiotherapy, as compared to radio-chemotherapy.
One of important limitations of this trial is lack of complete data on HPV infection among the patients included. We note, however, that among 32 individuals from this trial with known PCR-assessed, HPV status 26/32 (81%) patients were HPV negative and 6/32 (19%) were HPV positive. This included 17 patients with oropharyngeal cancer out of whom 5/17 (29%) were HPV positive. Relatively low incidence of HPV infection in this series is consistent with previously published data from our institution.17
Conclusion
While the conclusions from this study are limited due to its premature termination and underpowered size, concurrent postoperative RTCT tended to improve locoregional control rate as compared to p-CAIR. This, however, did not transferred into improved overall survival. Postoperative RTCT was associated with a substantial increase in haematological toxicity that negatively affected treatment compliance in this arm.
Footnotes
Acknowledgment: The following number was assigned upon registration of the protocol of the trial: ISRCTN65457367.
Funding: This study was sponsored by the Polish Ministry of Science and Higher Education, grant number: N402 1801 34
Previous presentation of data: The data were presented at the 2019 ASTRO Annual Meeting, Chicago USA
Contributor Information
Grzegorz Wozniak, Email: grzeswo@wp.pl.
Maciej Misiołek, Email: msmisiolek@gmail.com.
Adam Idasiak, Email: adam.idasiak@io.gliwice.pl.
Iwona Dębosz-Suwińska, Email: iwonawzk@o2.pl.
Magdalena Jaworska, Email: magdalena.jaworska@io.gliwice.pl.
Wieslaw Bal, Email: wieslaw.bal@io.gliwice.pl.
Boguslaw Maciejewski, Email: boguslaw.maciejewski@io.gliwice.pl.
Leszek Miszczyk, Email: leszek.miszczyk@io.gliwice.pl.
Krzysztof Składowski, Email: krzysztof.skladowski@io.gliwice.pl.
Rafal Suwinski, Email: rafal.suwinski@io.gliwice.pl.
REFERENCES
- 1.Bernier J, Domenge C, Ozsahin M, Matuszewska K, Lefèbvre J-L, Greiner RH, et al. . Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med 2004; 350: 1945–52. doi: 10.1056/NEJMoa032641 [DOI] [PubMed] [Google Scholar]
- 2.Cooper JS, Pajak TF, Forastiere AA, Jacobs J, Campbell BH, Saxman SB, et al. . Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med 2004; 350: 1937–44. doi: 10.1056/NEJMoa032646 [DOI] [PubMed] [Google Scholar]
- 3.Matuschek C, Haussmann J, Bölke E, Gripp S, Schuler PJ, Tamaskovics B, et al. . Accelerated vs. conventionally fractionated adjuvant radiotherapy in high-risk head and neck cancer: a meta-analysis. Radiat Oncol 2018; 13: 195. doi: 10.1186/s13014-018-1133-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Awwad HK, Lotayef M, Shouman T, Begg AC, Wilson G, Bentzen SM, et al. . Accelerated hyperfractionation (AHF) compared to conventional fractionation (CF) in the postoperative radiotherapy of locally advanced head and neck cancer: influence of proliferation. Br J Cancer 2002; 86: 517–23. doi: 10.1038/sj.bjc.6600119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ang KK, Trotti A, Brown BW, Garden AS, Foote RL, Morrison WH, et al. . Randomized trial addressing risk features and time factors of surgery plus radiotherapy in advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys 2001; 51: 571–8. doi: 10.1016/S0360-3016(01)01690-X [DOI] [PubMed] [Google Scholar]
- 6.Langendijk H, Kaanders JH, Doornaert P, Burlage FR, Van den Ende PLA, Oei SB, et al. . OC-008: POPART vs CPORT in squamous cell head and neck cancer: results of a multicenter randomised study of the Dutch head and neck Study Group. Radiotherapy and Oncology 2015; 114: 9–10. doi: 10.1016/S0167-8140(15)34768-X [DOI] [Google Scholar]
- 7.Suwiński R, Bańkowska-Woźniak M, Majewski W, Idasiak A, Maciejewski A, Ziółkowska E, et al. . Randomized clinical trial on 7-days-a-week postoperative radiotherapy for high-risk squamous cell head and neck cancer. Radiother Oncol 2008; 87: 155–63. doi: 10.1016/j.radonc.2008.02.009 [DOI] [PubMed] [Google Scholar]
- 8.Sanguineti G, Richetti A, Bignardi M, Corvo' R, Gabriele P, Sormani MP, et al. . Accelerated versus conventional fractionated postoperative radiotherapy for advanced head and neck cancer: results of a multicenter phase III study. Int J Radiat Oncol Biol Phys 2005; 61: 762–71. doi: 10.1016/j.ijrobp.2004.07.682 [DOI] [PubMed] [Google Scholar]
- 9.Suwinski R, Jaworska M, Nikiel B, Grzegorz W, Bankowska-Wozniak M, Wojciech M, et al. . Predicting the effect of accelerated fractionation in postoperative radiotherapy for head and neck cancer based on molecular marker profiles: data from a randomized clinical trial. Int J Radiat Oncol Biol Phys 2010; 77: 438–46. doi: 10.1016/j.ijrobp.2009.05.021 [DOI] [PubMed] [Google Scholar]
- 10.Snietura M, Jaworska M, Mlynarczyk-Liszka J, Goraj-Zajac A, Piglowski W, Lange D, et al. . Pten as a prognostic and predictive marker in postoperative radiotherapy for squamous cell cancer of the head and neck. PLoS One 2012; 7: e33396. doi: 10.1371/journal.pone.0033396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suwinski R, Wozniak G, Misiolek M, Jaworska M, Kozaczka M, Bal W, et al. . Randomized clinical trial on 7-days-a-week post-operative radiotherapy vs concurrent post-operative radiochemotherapy in locally advanced cancer of the oral cavity/oropharynx: a report on acute normal tissue reactions. Br J Radiol 2016; 89: 20150805. doi: 10.1259/bjr.20150805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edge S. B, Byrd D. R, Compton C. C, Fritz A. G, Greene F. L et al.. AJCC cancer staging manual, 7th ed . New York, NY: Springer; 2010. . [Google Scholar]
- 13.Peters LJ, Goepfert H, Ang KK, Byers RM, Maor MH, Guillamondegui O, et al. . Evaluation of the dose for postoperative radiation therapy of head and neck cancer: first report of a prospective randomized trial. Int J Radiat Oncol Biol Phys 1993; 26: 3–11. doi: 10.1016/0360-3016(93)90167-T [DOI] [PubMed] [Google Scholar]
- 14.Dische S, Warburton MF, Jones D, Lartigau E. The recording of morbidity related to radiotherapy. Radiother Oncol 1989; 16: 103–8. doi: 10.1016/0167-8140(89)90026-1 [DOI] [PubMed] [Google Scholar]
- 15.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the radiation therapy Oncology Group (RTOG) and the European organization for research and treatment of cancer (EORTC. Int J Radiat Oncol Biol Phys 1995; 31: 1341–6. doi: 10.1016/0360-3016(95)00060-C [DOI] [PubMed] [Google Scholar]
- 16.Dörr W, Hendry JH. Consequential late effects in normal tissues. Radiother Oncol 2001; 61: 223–31. doi: 10.1016/S0167-8140(01)00429-7 [DOI] [PubMed] [Google Scholar]
- 17.Snietura M, Piglowski W, Jaworska M, Mucha-Malecka A, Wozniak G, Lange D, et al. . Impact of HPV infection on the clinical outcome of p-CAIR trial in head and neck cancer. Eur Arch Otorhinolaryngol 2011; 268: 721–6. doi: 10.1007/s00405-010-1396-7 [DOI] [PMC free article] [PubMed] [Google Scholar]