Keywords: developmental neurobiology, glycogen synthase kinase 3, intrauterine, learning, lithium, memory, offspring, placenta diseases, signal pathways, ß-catenin
Abstract
Mild intrauterine hypoperfusion (MIUH) is a serious pathological event that affects the growth and development of fetuses and offspring. MIUH can lead to growth restriction, low birth weight, neurodevelopmental disorders, and other adverse clinical outcomes. To study the effects of MIUH on learning and memory function in offspring, a model of MIUH was established by placing a coil (length 2.5 mm, diameter 0.24 mm) on the uterine artery and ovarian uterine artery of Sprague-Dawley rats in the second trimester of pregnancy (day 17). Next, 120 mg/kg lithium chloride (the MIUH + Li group) or normal saline (the MIUH group) was injected intraperitoneally into these rats. In addition, 120 mg/kg lithium chloride (the Li group) or normal saline (the SHAM group) was injected intraperitoneally into pregnant rats without coil placement. The Morris water maze was used to detect changes in learning and memory ability in the offspring at 4 weeks after birth. In the MIUH group, the escape latency and journey length before reaching the platform were both increased, and the number of times that the platform was crossed and the activity time in the target quadrant within 90 seconds were both decreased compared with the SHAM group. Immunofluorescence double staining and western blot assays demonstrated that hippocampal nestin and Ki67 (both cell-proliferation-related proteins) expression was significantly downregulated in the MIUH group compared with the SHAM group. Furthermore, western blot assays were conducted to investigate changes in related signaling pathway proteins in the brains of offspring rats, and revealed that glycogen synthase kinase 3β (GSK3β) expression was upregulated and β-catenin expression was downregulated in the MIUH group compared with the SHAM group. In addition, compared with the MIUH group, the expression levels of p-GSK3β and β-catenin were upregulated in the MIUH + Li group. These results suggest that MIUH may affect learning and memory function in rat offspring by regulating the GSK3β signaling pathway. The experimental procedures were approved by Animal Ethics Committee of Shengjing Hospital of China Medical University (approval No. 2018PS07K) in June 2018.
Chinese Library Classification No. R453; R363; R364
Introduction
Placental hypoperfusion is a common pathological feature of many complex twin and fetal abnormalities, such as twin transfusion syndrome, selective growth restriction, twin stillbirth, and placental hemangioma (Ginosar et al., 2018). With the rapid development of fetal intrauterine therapy and fetal surgery, the number of cases of iatrogenic placental hypoperfusion has increased. Fetal placental vascular coagulation, radiofrequency ablation, and fetal diaphragmatic hernia repair can all cause hypoperfusion of the placenta to some extent (Pierce et al., 2000; Bendon and Cantor, 2007, Noroozzadeh et al., 2019). Studies have reported that placental hypoperfusion may have both short- and long-term adverse effects on the nervous system of offspring, which may lead to perinatal brain damage and abnormal nerve development (Pierce et al., 2000; Bendon and Cantor, 2007; Ginosar et al., 2018). It has been demonstrated that placental hypoperfusion can reduce brain weight and hippocampal volume of offspring in rats, and that it also has an effect on long-term myelination and cellular protrusions in offspring rats (Piazza et al., 2019). Placental hypoperfusion may also have a negative effect on short-term neurogenesis and the nervous system microenvironment in offspring. Learning and memory function is one of the most important nervous system functions, and it is also an important index to evaluate nervous system development as a whole. Therefore, it is clinically significant to study the effects of exposure to placental hypoperfusion in the second trimester on learning and memory in offspring.
In mammals, the hippocampus is the core region of learning and memory, and neurogenesis and the number of neural stem cells (NSCs) in the hippocampus are strongly associated with both learning and memory. Studies have demonstrated that when newborn rats are repeatedly exposed to adverse stimulations, cell death in the dentate gyrus does not increase, but NSC proliferation is inhibited, and the spatial memory ability of newborn rats is affected (Pierce et al., 2000; Piazza et al., 2019). Another study reported that proliferation of NSCs is associated with hippocampal-dependent cognitive impairment; for example, ethanol and ionizing radiation noticeably reduces hippocampal NSC proliferation, leading to hippocampal-dependent cognitive impairment (Pierce et al., 2000; Bendon and Cantor, 2007; Bolton et al., 2014; Ginosar et al., 2018; Piazza et al., 2019). Nestin and Ki67 are markers of NSC proliferation and directly reflect this process (Jantzie et al., 2015). The long-term prognosis of the neonatal nervous system that has experienced a pathological state and survived is the focus of the previous study (Driscoll et al., 2018). At present, the mechanism by which placental hypoperfusion affects the development of the nervous system in offspring remains controversial. The glycogen synthase kinase 3β (GSK3β) pathway plays a key role in the maintenance of neuronal networks, cell survival, and longevity (Sun et al., 2020). However, no reports have yet been published regarding the regulatory effects of mild intrauterine hypoperfusion (MIUH) on the GSK3β signaling pathway.
Therefore, this study aimed to investigate the effects of MIUH in the second trimester of pregnancy on learning and memory function in offspring, and explore the possible underlying mechanisms of these effects.
Materials and Methods
Animals
In the current study, 160 clean and healthy female six-week-old Sprague-Dawley rats with a gestational period of 17.5 days, weighing 140–160 g, were provided by the Animal Center, Shengjing Hospital of China Medical University, China (license No. SCXK (Jing) 2014-0004). Eight male rats weighing 100–150 g each were randomly selected from the offspring of each pregnant rat. The experiments were carried out 4 weeks after the birth of the offspring. All rats were given free access to water and food, and housed at 20–25°C with a relative humidity of 40–60% under natural light. The experimental procedures were approved by the Animal Ethics Committee of Shengjing Hospital of China Medical University (approval No. 2018PS07K) in June 2018. In addition, the experimental procedures followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996).
Placental hypoperfusion model in the second trimester
The rats were randomly divided into four groups (n = 40 per group): the SHAM group, MIUH group, Li group, and MIUH + Li group. Rats at 17.5 days of gestation were selected. After inhalation anesthesia with 3% isoflurane (Shanghai Hansi Chemical Co., Ltd., Shanghai, China), the rats were fixed, disinfected, and covered with towels. A median abdominal incision was made to expose the uterus, fetal rats, bilateral uterine arteries, and ovarian uterine arteries. In the SHAM group, 120 mg/kg saline was injected into the abdominal cavity of each pregnant rat. No operation was performed after the abdominal incision was made, and the abdomen was then closed. In the Li group, 120 mg/kg lithium chloride (Shanghai Yuanye Biotechnology Co., Ltd., Shanghai, China) was injected into the abdominal cavity of each pregnant rat. Again, no operation was performed after the abdominal incision was made, and the abdomen was then closed. In the MIUH and MIUH + Li groups, a coil with a length of 2.5 mm and an inner diameter of 0.24 mm (SAMINI Co., Ltd., Shizuoka, Japan) was placed around the uterine artery and ovarian uterine artery, and each pregnant rat was then intraperitoneally injected with 200 μL normal saline (MIUH group) or 120 mg/kg lithium chloride (MIUH + Li group) before the abdomen was closed. In the MIUH group and MIUH + Li group, the peak blood flow velocities of the uterine and umbilical arteries were examined by ultrasound 2 hours after surgery. The blood flow velocity decreased by more than 20%, but not more than 30%, which is considered to be a successful establishment of the MIUH model (Ohshima et al., 2016).
Morris water maze test
The Morris water maze consists of a round bucket made of stainless steel with a diameter of 120 cm and a height of 60 cm, an underwater platform with a movable position, and an image acquisition and processing system (including computer, camera, and analysis software; Dongguan Bozhiyuan Biotechnology Co., Ltd., Guangzhou, China). The water temperature was kept at 24.0 ± 0.5°C. For the Morris water maze test, four male rats were randomly selected from the offspring of each pregnant rat 4 weeks after birth (Jantzie et al., 2015). During the test, the laboratory was dimly lit, quiet, and there was no food or drink present to avoid the interference of smell. The experiments started at 8:00 a.m. After each experiment, the rats were dried and returned to the cage, and were kept warm. The place navigation test and the spatial probe test were used. Outcome measures included the number of times that the platform was crossed and the activity time spent in the target quadrant within 90 seconds. Eight rats were used in each experiment.
The place navigation test was carried out over 5 days. Each rat was tested in four rounds each day. The test time of each round was set at 90 seconds, and the test interval of each round was not less than 30 minutes to ensure that the rats had sufficient rest. Each rat was randomly placed into the bucket from the midpoint wall of each quadrant, and the time from placing the rat in the water to the rat finding the platform (escape latency) and staying on the platform was automatically recorded by the image acquisition system and analysis software (Beijing Ji’ander Technology Co., Ltd., Beijing, China). The computer also automatically recorded the swimming speed and distance during this period, (i.e., the journey before reaching the platform). If the rat did not find the platform within the specified 90 seconds, the computer automatically stopped recording, and the tester gently guided the rat to the platform and ensured that the rat stayed on the platform for 30 seconds. On day 5, the underwater platform was removed after the place navigation test. The quadrant midpoint corresponding to the original platform area was selected as the water entry point. The rat was placed in the bucket, facing the wall. The number of times the rat crossed the platform and the activity time spent in the target quadrant within 90 seconds were automatically recorded by the image acquisition and analysis system.
Immunofluorescence double staining
At 12, 24, 48, and 72 hours after anesthesia, hippocampi from the offspring of each group (n = 8 litters, with 3 offspring rats per litter as replicates) were harvested for immunofluorescence double staining. The brain tissue was fixed in 4% paraformaldehyde, and after treatment, coronal slices of 8 μm thickness were cut. Microwave antigen retrieval was conducted in 0.01 M citrate buffer. Primary antibodies (anti-nestin, a cell-proliferation-related protein, sc-23927, rat, monoclonal, 1:100 dilution; anti-Ki67, a cell-proliferation-related protein, sc-23900, rat, monoclonal, 1:100 dilution) were added at 4°C overnight. The next day, mouse anti-rat IgG secondary antibody was added at 1:500 and incubated at 37°C for 2 hours, and the sections were then observed under a fluorescence microscope (Leica Microsystems, Wetzlar, Germany; red for Nestin, green for Ki67). Slides were imaged using NIS Elements BR imaging software (Nikon, Tokyo, Japan). The fluorescence intensity has been quantified. DAPI staining intensity was used to normalize the intensities between different sections.
Western blot assays
At 12, 24, and 48 hours and on postnatal day 0 (P0), P14, and P28 after anesthesia, fetal brains from each group (n = 8 litters, with 3 offspring rats per litter as replicates) were harvested to analyze protein expression. In addition, on P0, P14, and P28, offspring hippocampi from each group (n = 8) were also harvested to analyze protein expression. The tissue was homogenized using protein lysates (20 mM Tris pH 7.4, 250 mM sodium chloride, 0.1% Triton-X-100, 2 mM EDTA, 10 μg/mL leupeptin, 10 μg/mL aprotinin, 0.5 mM phenylmethylsulfonyl fluoride, 4 mM sodium orthovanadate, and 1 mM DTT) and the supernatant was reserved. Next, 30 μg of protein from each sample was subjected to 12% sodium dodecyl sulfate polyacrylamide gels and transferred onto a nitrocellulose membrane. The target proteins were then probed using specific antibodies (mouse anti-nestin, a cell-proliferation-related protein, sc-23927, monoclonal, 1:1000 dilution; mouse anti-Ki67, a cell-proliferation-related protein, sc-23900, monoclonal, 1:1000 dilution; mouse anti-GSK-3β, a signaling-pathway-related protein, sc-81462, monoclonal, 1:1000 dilution; mouse anti-p-GSK-3β, a signaling-pathway-related protein, sc-81496, monoclonal, 1:1000 dilution; mouse anti-β-catenin, a signaling-pathway-related protein, sc-53483, monoclonal, 1:1000 dilution) at 4°C overnight. Next, the membranes were incubated at room temperature for 1 hour in secondary antibody (rat anti-rat IgG, 1:5000). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH; sc-47724, housekeeping protein, mouse, monoclonal, 1:1000) was detected simultaneously as an internal reference. The Molecular Imager VersaDoc MP 5000 system was used to image the blots. Signal intensities were determined using an imaging system (Quantity One, Bio-Rad, Beijing, China) and imaging software (National Institutes of Health Image Version 1.37, National Institutes of Health, Bethesda, MD, USA). For the western blot assays, we obtained a relative ratio of the gray value of each protein to the corresponding internal reference (GAPDH).
Statistical analysis
The data are expressed as the mean ± SD. All data were analyzed using GraphPad Prism version 6.0 software (GraphPad, San Diego, CA, USA). Differences between the experimental groups were determined using independent-sample t-test and one-way analysis of variance followed by a Bonferroni post hoc test. P < 0.05 was considered statistically significant.
Results
Effects of MIUH on peak velocity of the umbilical artery in fetal rats
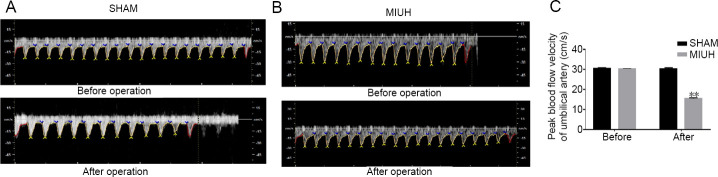
Abnormal umbilical artery blood flow is related to adverse pregnancy outcomes; in the presence of high-risk factors, fetal umbilical artery blood flow monitoring has a high practical value in predicting adverse pregnancy outcomes. Heightened monitoring during pregnancy and timely termination of pregnancy can improve the prognosis of both mothers and infants. After establishing the animal model in the present study, we monitored peak blood flow velocity of the fetal rat umbilical artery. In the SHAM group, there was no significant change in blood flow of the umbilical artery when comparing before and 2 hours after the operation. In the MIUH group, the umbilical artery blood flow was significantly decreased by approximately 30% at 2 hours after the operation (Figure 1A–C). Furthermore, there was a significant difference in blood flow velocity between the SHAM and MIUH groups (P < 0.01).
Figure 1.
Effects of MIUH on peak blood flow velocity of the umbilical artery in fetal rats.
(A, B) Images of peak blood flow velocity of umbilical artery in fetal rats before and 2 hours after the operation in the SHAM and MIUH groups. (C) Graph showing peak blood flow velocity of the umbilical artery before and 2 hours after the operation in the SHAM and MIUH groups. At 2 hours, peak blood flow velocity was significantly lower in the MIUH group than in the SHAM group. Data are shown as the mean ± SD (n = 40; independent-sample t-test). All experiments were conducted in triplicate. **P < 0.01, vs. SHAM group. MIUH: Mild intrauterine hypoperfusion.
Effects of MIUH on learning and memory function in offspring rats
The learning and memory function of the offspring rats was detected using the Morris water maze test. In the spatial probe test, the activity track of the offspring was clearly focused in the target quadrant in the SHAM group, while the activity track in the MIUH group lacked a clear purpose and was distributed over the entire area (Figure 2A). In addition, the results from the place navigation test revealed that the escape latency in the MIUH group was significantly longer, by 50%, on day 5 compared with the SHAM group (Figure 2B). Furthermore, the path length before reaching the platform was significantly longer, by 63%, in the MIUH group compared with the SHAM group (Figure 2C). In the spatial probe test, the number of times crossing the platform and the activity time in the target quadrant were lower in the MIUH group compared with the SHAM group, by approximately 47% and 54%, respectively (Figure 2D and E).
Figure 2.
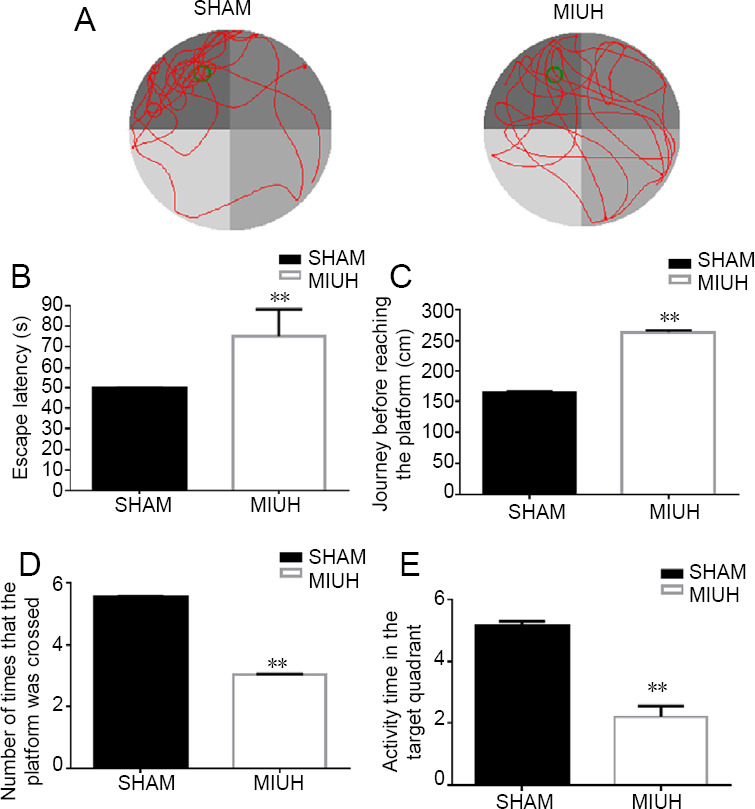
Effects of MIUH on learning and memory function in offspring rats.
(A) The Morris water maze test of learning and memory function was performed in the offspring rats. Four male rats were randomly selected from the offspring of each pregnant rat, 4 weeks after birth. The activity track of the offspring was clearly focused in the target quadrant in the SHAM group, while the activity track in the MIUH group lacked a clear purpose and was distributed over the entire area. (B) The place navigation test revealed that escape latency on day 5 was longer in the MIUH group than in the SHAM group. (C) In the place navigation test, the journey before reaching the platform was longer in the MIUH group than in the SHAM group on day 5. (D) In the spatial probe test, the number of times that the platform was crossed was lower in the MIUH group than in the SHAM group. (E) In the spatial probe test, the activity time in the target quadrant was shorter in the MIUH group than in the SHAM group. Data are expressed as the mean ± SD (n = 40; independent-sample t-test). All experiments were conducted in triplicate. **P < 0.01, vs. SHAM group. MIUH: Mild intrauterine hypoperfusion.
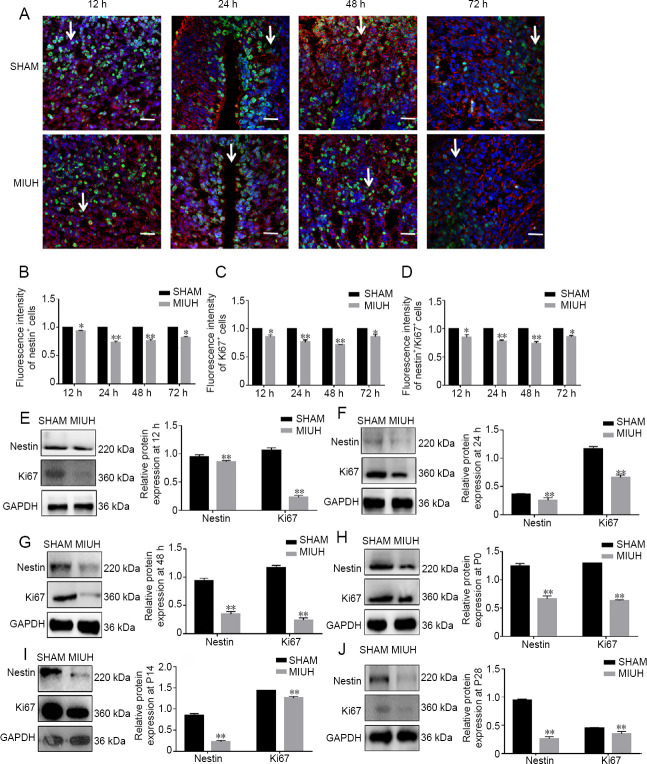
Effects of MIUH on NSC proliferation in the fetal rat brain
Immunofluorescence results revealed that the expression levels of nestin and Ki67, which are markers of NSCs, were significantly lower in the hippocampus of rats in the MIUH group compared with the SHAM group at 12, 24, and 72 hours (Figure 3A–D). Western blot assay results revealed that MIUH significantly downregulated the expression of nestin and Ki67 in fetal rats at 12 hours (11% and 72% lower than SHAM, respectively), 24 hours (25% and 47% lower than SHAM), 48 hours (51% and 67% lower than SHAM), P0 (52% and 53% lower than SHAM), P14 (64% and 10% lower than SHAM), and P28 (61% and 11% lower than SHAM) (Figure 3E–J).
Figure 3.
Effects of MIUH on NSC proliferation in the rat brain at different time points.
(A–D) Immunofluorescence staining results of NSCs in the fetal rat brain at different time points. Original magnification, 400×; red for nestin; green for Ki67. Arrows point to nestin+/Ki67+ cells. The fluorescence intensities of nestin, Ki67, and Nestin and Ki67 merged were lower in the MIUH group than in the SHAM group. The arrows show the proliferation of NSCs. (E–J) Western blot assays. MIUH significantly downregulated the expression of nestin and Ki67 in fetal rats at 12, 24, and 48 hours, and at P0, P14, and P28. Data are shown as the mean ± SD (B–D: n = 40; E–J: n = 3; independent t-test). All experiments were conducted in triplicate. **P < 0.01, vs. SHAM group. MIUH: Mild intrauterine hypoperfusion; NSC: neural stem cell; P: postnatal day.
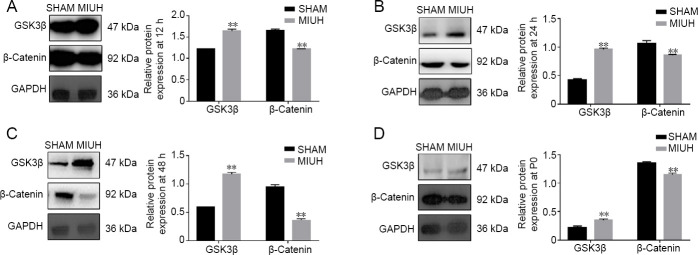
Regulatory effects of MIUH on the Wnt/β-catenin signaling pathway
The Wnt/β-catenin signaling pathway is one of the most important signaling pathways in cells, and plays an important role in neurodevelopment. The effects of MIUH on the expression of GSK3β and β-catenin in tissues at 12 hours, 24 hours, 48 hours, and P0 were studied using western blot assays. The results revealed that MIUH upregulated GSK3β expression levels and downregulated β-catenin expression levels (P < 0.01; Figure 4A–D).
Figure 4.
Regulatory effects of MIUH on the Wnt/β-catenin signaling pathway.
(A–D) Western blot assays showing the effects of MIUH on the expression of GSK3β and β-catenin in tissues at 12, 24, and 48 hours, and at P0. GSK3β expression was higher in the MIUH group than in the SHAM group, while β-catenin expression was lower in the MIUH group than in the SHAM group. Data are expressed as the mean ± SD (n = 3; independent t-test). All experiments were conducted in triplicate. **P < 0.01, vs. SHAM group. MIUH: Mild intrauterine hypoperfusion; P: postnatal day.
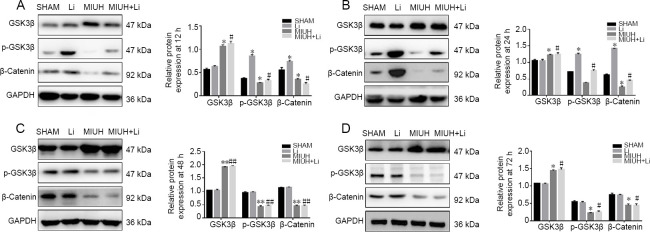
LiCl affects β-catenin levels by upregulating p-GSK3β
Western blot assay results revealed that LiCl treatment upregulated the expression of p-GSK3β and β-catenin. At 12 and 24 hours, LiCl treatment led to a partial recovery from the downregulation of p-GSK3β and β-catenin caused by MIUH (P < 0.01); however, the effect was weak at 48 hours (P < 0.05). In contrast, the regulatory effect of LiCl on GSK3β was weak, and there were no significant differences between the groups (P > 0.05; Figure 5A–D).
Figure 5.
LiCl affects β-catenin levels by upregulating p-GSK3β.
(A–D) Western blot assays of the expression of GSK3β, p-GSK3β, and β-catenin at 12, 24, and 48 hours, and at P0. GSK3β expression was higher in the MIUH group than in the SHAM group. The expression levels of β-catenin and p-GSK3β were lower in the MIUH group than in the SHAM group. The expression levels of GSK3β and p-GSK3β were higher in the Li group than in the SHAM group at 12 and 24 hours. The expression levels of β-catenin and GSK3β were higher in the MIUH + Li group than in the MIUH group, while the expression of p-GSK3β was lower in the MIUH + Li group than in the MIUH group. Data are expressed as the mean ± SD (n = 3; one-way analysis of variance followed by the Bonferroni post hoc test). All experiments were conducted in triplicate. *P < 0.05, **P < 0.01, vs. SHAM group; #P < 0.05, ##P < 0.01, vs. the MIUH group. MIUH: Mild intrauterine hypoperfusion; P: postnatal day.
Discussion
Placental hypoperfusion is a serious pathological event that affects the growth and development of fetuses and offspring, and can cause many adverse clinical outcomes, such as growth restriction, low birth weight, and premature delivery (Gidday and Park, 2010). One of the most noteworthy effects is on nervous system development in offspring. However, the relationship between hypoperfusion and fetal nervous system development is still unclear, as are the mechanisms that may be involved. The purpose of the present study was to explore the mechanisms of placental hypoperfusion on central nervous system development in offspring. An MIUH model was established in the second trimester of pregnancy. The peak blood flow velocity of the fetal rat umbilical artery was decreased in the MIUH group 2 hours after the operation.
Learning and memory function is one of the most important nervous system functions in young rats, and it is also an important index for the evaluation of nervous system development. Therefore, to study the effects of placental hypoperfusion on nervous system development in offspring, learning and memory function is an inevitable choice (Koltz et al., 2011; Jantzie et al., 2015). The effects of MIUH on learning and memory ability in offspring rats was tested using the Morris water maze test. In the spatial probe test, the activity track of rats in the SHAM group was focused to the target quadrant, while the activity of rats in the MIUH group lacked a clear focus and was distributed throughout the enclosure. On day 5, the escape latency of the MIUH group was significantly prolonged. In addition, the number of times in which the platform was crossed before reaching the platform was significantly higher in the MIUH group than in the SHAM group, and the activity time in the target quadrant was significantly reduced. Thus, our experimental results demonstrate that MIUH has a significant inhibitory effect on learning and memory function in offspring rats.
In mammals, the hippocampus is the core area of learning and memory, and neurogenesis and the number of NSCs in the hippocampus are strongly associated with both learning and memory. It has been reported that mutant mice with relatively high NSC proliferation have better learning and memory ability than normal mice (Choi and Choi, 2016; Klein et al., 2016; Sun et al., 2016; Lin et al., 2017; Saha et al., 2017; Tuoc et al., 2017; Peters et al., 2018; Wang et al., 2018; Abreu et al., 2019; Gu et al., 2019; Karimipour et al., 2019). Placental hypoperfusion can cause severe demyelination of the nervous system and decrease hippocampal volume in offspring. This suggests that hippocampal NSCs may be involved in the effects of placental hypoperfusion on learning and memory in offspring (Ghasemi et al., 2020). In the present study, hippocampal nestin and Ki67 expression was markedly downregulated in the MIUH group, as revealed by immunofluorescence staining and western blot assay. Therefore, MIUH significantly inhibits the proliferation of hippocampal cells, thus inhibiting the learning and memory function of offspring rats.
GSK3β is one of the key signaling molecules downstream of the PI3K/Akt signaling pathway, which participates in various biochemical reactions in vivo (Dey et al., 2017; Solar and Paul, 2017). Many studies have reported that GSK3β is involved in the control of various neuronal functions as well as in the regulation of NSC proliferation (Nguyen et al., 2017; Lei et al., 2019). In the current study, MIUH upregulated GSK3β and downregulated β-catenin, which suggests that MIUH might affect the learning and memory function of offspring rats by regulating the GSK3β signaling pathway. Lithium has been demonstrated to affect many neurotransmitter systems, including those of serotonin, dopamine, and glutamate. Lithium can directly affect GSK3β by activating Akt both in vitro and in vivo (Gao et al., 2019). To investigate whether MIUH plays a role through GSK3β, we investigated the Li group and the MIUH + Li group. By adding LiCl to the MIUH group, we revealed that LiCl upregulates p-GSK3β and β-catenin, and to some extent reduces the injury caused by MIUH. Li is believed to affect the Wnt pathway, which further suggests that MIUH may affect learning and memory function in offspring by regulating the GSK3β signaling pathway. Our study has some limitations. The time points used in this study were not perfect, and the detection of pathway proteins was not very comprehensive. We only investigated cell proliferation markers and β-catenin in Wnt/β-catenin pathway. In future studies we should look at other neurogenic markers and other markers in the Wnt/β-catenin pathway. We will test more time points in future experiments, and we will further supplement the detection of pathway-related proteins.
In conclusion, MIUH can inhibit cell proliferation by inhibiting the GSK3β signaling pathway, which then affects the learning and memory function of offspring rats. Our study suggests new possibilities for the treatment of MIUH.
Footnotes
Conflicts of interest: The authors declare that there are no conflicts of interest associated with this manuscript.
Financial support: This work was supported by the National Key Research & Department Program of China, No. 2018YFC1002902 (to CXL). The funding source had no role in study conception and design, data analysis or interpretation, paper writing or deciding to submit this paper for publication.
Institutional review board statement: The experimental procedures were approved by Animal Ethics Committee of Shengjing Hospital of China Medical University (approval No. 2018PS07K) in June 2018. The experimental procedure followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This work was supported by the National Key Research & Department Program of China, No. 2018YFC1002902 (to CXL).
C-Editor: Zhao M; S-Editors: Wang J, Li CH; L-Editors: Gardner B, Stow A, Qiu Y, Song LP; T-Editor: Jia Y
References
- 1.Abreu CC, Fernandes TN, Henrique EP, Pereira PDC, Marques SB, Herdeiro SLS, Oliveira FRR, Magalhaes NGM, Anthony DC, Melo MAD, Guerreiro-Diniz C, Diniz DG, Picanco-Diniz CW. Small-scale environmental enrichment and exercise enhance learning and spatial memory of Carassius auratus, and increase cell proliferation in the telencephalon: an exploratory study. Braz J Med Biol Res. 2019;52:e8026. doi: 10.1590/1414-431X20198026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bendon RW, Cantor DB. Stillbirth due to placental hypoperfusion after salpingo-oophorectomy for an ovarian cyst. Obstet Gynecol. 2007;110:482–484. doi: 10.1097/01.AOG.0000259334.31330.91. [DOI] [PubMed] [Google Scholar]
- 3.Bolton JL, Auten RL, Bilbo SD. Prenatal air pollution exposure induces sexually dimorphic fetal programming of metabolic and neuroinflammatory outcomes in adult offspring. Brain Behav Immun. 2014;37:30–44. doi: 10.1016/j.bbi.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 4.Choi YJ, Choi YS. Effects of electromagnetic radiation from smartphones on learning ability and hippocampal progenitor cell proliferation in rat. Osong Public Health Res Perspect. 2016;7:12–17. doi: 10.1016/j.phrp.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dey G, Bharti R, Ojha PK, Pal I, Rajesh Y, Banerjee I, Banik P, Parida S, Parekh A, Sen R, Mandal M. Therapeutic implication of ‘Iturin A’ for targeting MD-2/TLR4 complex to overcome angiogenesis and invasion. Cell Signal. 2017;35:24–36. doi: 10.1016/j.cellsig.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 6.Driscoll DJO, Felice VD, Kenny LC, Boylan GB, O’Keeffe GW. Mild prenatal hypoxia-ischemia leads to social deficits and central and peripheral inflammation in exposed offspring. Brain Behav Immun. 2018;69:418–427. doi: 10.1016/j.bbi.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Ghasemi E, Afkhami Aghda F, Rezvani ME, Shahrokhi Raeini A, Hafizibarjin Z, Zare Mehrjerdi F. Effect of endogenous sulfur dioxide on spatial learning and memory and hippocampal damages in the experimental model of chronic cerebral hypoperfusion. J Basic Clin Physiol Pharmacol. 2020 doi: 10.1515/jbcpp-2019-0227. doi: 101515/jbcpp-2019-0227. [DOI] [PubMed] [Google Scholar]
- 8.Gidday JM, Park TS. Erythropoietin and prenatal hypoxia-ischemia. J Neurosurg Pediatr. 2010;6:203–205. doi: 10.3171/2010.4.PEDS10103. [DOI] [PubMed] [Google Scholar]
- 9.Ginosar Y, Gielchinsky Y, Nachmansson N, Hagai L, Shapiro J, Elchalal U, Abramovitch R. BOLD-MRI demonstrates acute placental and fetal organ hypoperfusion with fetal brain sparing during hypercapnia. Placenta. 2018;63:53–60. doi: 10.1016/j.placenta.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Gu Q, Feng Z, Liang Q, Li M, Deng J, Ma M, Wang W, Liu J, Liu P, Rong P. Machine learning-based radiomics strategy for prediction of cell proliferation in non-small cell lung cancer. Eur J Radiol. 2019;118:32–37. doi: 10.1016/j.ejrad.2019.06.025. [DOI] [PubMed] [Google Scholar]
- 11.Jantzie LL, Getsy PM, Denson JL, Firl DJ, Maxwell JR, Rogers DA, Wilson CG, Robinson S. Prenatal hypoxia-ischemia induces abnormalities in CA3 microstructure, potassium chloride co-transporter 2 expression and inhibitory tone. Front Cell Neurosci. 2015;9:347. doi: 10.3389/fncel.2015.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karimipour M, Rahbarghazi R, Tayefi H, Shimia M, Ghanadian M, Mahmoudi J, Bagheri HS. Quercetin promotes learning and memory performance concomitantly with neural stem/progenitor cell proliferation and neurogenesis in the adult rat dentate gyrus. Int J Dev Neurosci. 2019;74:18–26. doi: 10.1016/j.ijdevneu.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 13.Klein C, Rasinska J, Empl L, Sparenberg M, Poshtiban A, Hain EG, Iggena D, Rivalan M, Winter Y, Steiner B. Physical exercise counteracts MPTP-induced changes in neural precursor cell proliferation in the hippocampus and restores spatial learning but not memory performance in the water maze. Behav Brain Res. 2016;307:227–238. doi: 10.1016/j.bbr.2016.02.040. [DOI] [PubMed] [Google Scholar]
- 14.Koltz MT, Tosun C, Kurland DB, Coksaygan T, Castellani RJ, Ivanova S, Gerzanich V, Simard JM. Tandem insults of prenatal ischemia plus postnatal raised intrathoracic pressure in a novel rat model of encephalopathy of prematurity. J Neurosurg Pediatr. 2011;8:628–639. doi: 10.3171/2011.9.PEDS11174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lei F, He W, Tian X, Zhou Q, Zheng L, Kang J, Song Y, Feng D. GSK-3 inhibitor promotes neuronal cell regeneration and functional recovery in a rat model of spinal cord injury. Biomed Res Int. 2019;2019:9628065. doi: 10.1155/2019/9628065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin YL, Persaud SD, Nhieu J, Wei LN. Cellular retinoic acid-binding protein 1 modulates stem cell proliferation to affect learning and memory in male rat. Endocrinology. 2017;158:3004–3014. doi: 10.1210/en.2017-00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nguyen T, Fan T, George SR, Perreault ML. Disparate effects of lithium and a GSK-3 inhibitor on neuronal oscillatory activity in prefrontal cortex and hippocampus. Front Aging Neurosci. 2017;9:434. doi: 10.3389/fnagi.2017.00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noroozzadeh M, Raoufy MR, Bidhendi Yarandi R, Faraji Shahrivar F, Ramezani Tehrani F. The effects of prenatal androgen exposure on cardiac function and tolerance to ischemia/reperfusion injury in male and female rats during adulthood. Life Sci. 2019;229:251–260. doi: 10.1016/j.lfs.2019.05.044. [DOI] [PubMed] [Google Scholar]
- 19.Ohshima M, Coq JO, Otani K, Hattori Y, Ogawa Y, Sato Y, Harada-Shiba M, Ihara M, Tsuji M. Mild intrauterine hypoperfusion reproduces neurodevelopmental disorders observed in prematurity. Sci Rep. 2016;6:39377. doi: 10.1038/srep39377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peters AJ, Villasana LE, Schnell E. Ketamine alters hippocampal cell proliferation and improves learning in rat after traumatic brain injury. Anesthesiology. 2018;129:278–295. doi: 10.1097/ALN.0000000000002197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piazza FV, Segabinazi E, de Meireles ALF, Mega F, Spindler CF, Augustin OA, Salvalaggio GDS, Achaval M, Kruse MS, Coirini H, Marcuzzo S. Severe uncontrolled maternal hyperglycemia induces microsomia and neurodevelopment delay accompanied by apoptosis, cellular survival, and neuroinflammatory deregulation in rat offspring hippocampus. Cell Mol Neurobiol. 2019;39:401–414. doi: 10.1007/s10571-019-00658-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pierce BT, Pierce LM, Wagner RK, Apodaca CC, Hume RF, Jr, Nielsen PE, Calhoun BC. Hypoperfusion causes increased production of interleukin 6 and tumor necrosis factor alpha in the isolated, dually perfused placental cotyledon. Am J Obstet Gynecol. 2000;183:863–867. doi: 10.1067/mob.2000.108887. [DOI] [PubMed] [Google Scholar]
- 23.Saha M, Chakraborty C, Arun I, Ahmed R, Chatterjee S. An advanced deep learning approach for ki-67 stained hotspot detection and proliferation rate scoring for prognostic evaluation of breast cancer. Sci Rep. 2017;7:3213. doi: 10.1038/s41598-017-03405-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Solar M, Paul W. Chain relaxation in thin polymer films: turning a dielectric type-B polymer into a type-A’ one. Soft Matter. 2017;13:1646–1653. doi: 10.1039/c6sm02557a. [DOI] [PubMed] [Google Scholar]
- 25.Sun Y, Hong F, Zhang L, Feng L. The sphingosine-1-phosphate analogue, FTY-720, promotes the proliferation of embryonic neural stem cells, enhances hippocampal neurogenesis and learning and memory abilities in adult rat. Br J Pharmacol. 2016;173:2793–2807. doi: 10.1111/bph.13557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun Y, Wu A, Li X, Qin D, Jin B, Liu J, Tang Y, Wu J, Yu C. The seed of Litchi chinensis fraction ameliorates hippocampal neuronal injury in an Abeta25-35-induced Alzheimer’s disease rat model via the AKT/GSK-3beta pathway. Pharm Biol. 2020;58:35–43. doi: 10.1080/13880209.2019.1697298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tuoc T, Dere E, Radyushkin K, Pham L, Nguyen H, Tonchev AB, Sun G, Ronnenberg A, Shi Y, Staiger JF, Ehrenreich H, Stoykova A. Ablation of BAF170 in developing and postnatal dentate gyrus affects neural stem cell proliferation, differentiation, and learning. Mol Neurobiol. 2017;54:4618–4635. doi: 10.1007/s12035-016-9948-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y, Yin S, Xue H, Yang Y, Zhang N, Zhao P. Mid-gestational sevoflurane exposure inhibits fetal neural stem cell proliferation and impairs postnatal learning and memory function in a dose-dependent manner. Dev Biol. 2018;435:185–197. doi: 10.1016/j.ydbio.2018.01.022. [DOI] [PubMed] [Google Scholar]