Abstract
Despite numerous efforts to overcome neuropathic pain, various pharmacological drugs often fail to meet the needs and have many side effects. Muscovite is an aluminosilicate mineral that has been reported to have an anti-inflammatory effect, but the efficacy of muscovite for neuropathic pain has not been investigated. Here, we assessed whether muscovite nanoparticles can reduce the symptoms of pain by controlling the inflammatory process observed in neuropathic pain. The analgesic effects of muscovite nanoparticles were explored using partial sciatic nerve ligation model of neuropathic pain, in which one-third to one-half of the nerve trifurcation of the sciatic nerve was tightly tied to the dorsal side. Muscovite nanoparticles (4 mg/100 μL) was given intramuscularly to evaluate its effects on neuropathic pain (3 days per week for 4 weeks). The results showed that the muscovite nanoparticle injections significantly alleviated partial sciatic nerve ligation-induced mechanical and cold allodynia. In the spinal cord, the muscovite nanoparticle injections exhibited inhibitory effects on astrocyte and microglia activation and reduced the expression of pro-inflammatory cytokines, such as interleukin-1β, tumor necrosis factor-α, interleiukin-6 and monocyte chemoattractant protein-1, which were upregulated in the partial sciatic nerve ligation model. Moreover, the muscovite nanoparticle injections resulted in a decrease in activating transcription factor 3, a neuronal injury marker, in the sciatic nerve. These results suggest that the analgesic effects of muscovite nanoparticle on partial sciatic nerve ligation-induced neuropathic pain may result from inhibiting activation of astrocytes and microglia as well as pro-inflammatory cytokines. We propose that muscovite nanoparticle is a potential anti-nociceptive candidate for neuropathic pain. All experimental protocols in this study were approved by the Institutional Animal Ethics Committee (IACUC) at Dongguk University, South Korea (approval No. 2017-022-1) on September 28, 2017.
Keywords: astrocyte, microglia, muscovite, nanoparticle, neuropathic pain, partial sciatic nerve ligation, pharmacopuncture, pro-inflammatory cytokine, spinal cord
Chinese Library Classification No. R453; R364; R741
Introduction
Neuropathic pain is a major health problem that represents a considerable social and economic burden worldwide (Breivik et al., 2013; Schaefer et al., 2014a; Global Burden of Disease Study, 2015; Witt et al., 2016). Patients with neuropathic pain report more severe pain and experience greater functional and quality of life disorders than patients diagnosed with a non-neuropathic mechanisms (Gureje et al., 1998; Siniscalco et al., 2007; Haanpaa, 2013; Schaefer et al., 2014b). However, various pharmacological treatments often fail to meet patients’ needs due to unsatisfactory efficacy and adverse side effects (Andreev et al., 1994; Fisher et al., 2000; Benyamin et al., 2008).
Effective treatment for neuropathic pain has focused on the primary sensory neurons and their effect on activity of spinal dorsal horn neurons (Woolf and Salter, 2000). In general, inflammation is a pathophysiological condition associated with neuropathic pain (Sommer et al., 2018). Neuroinflammation involves complex bidirectional signaling between neurons and glial cells (Clark et al., 2013; Ji et al., 2013; Sommer et al., 2018). Activation of glial cells, including spinal cord astrocytes and microglia, initiates and maintains long-lasting thermal hyperalgesia and mechanical allodynia after a nerve injury (Latremoliere and Woolf, 2009; Gao and Ji, 2010; Gwak et al., 2017). Peripheral changes result in the release of pro-inflammatory cytokines, which include the activation of sensory neurons, including immune-like glial cells in the injured nerve, dorsal root ganglia, and spinal cord (Gao and Ji, 2010; Clark et al., 2013). Therefore, targeting glial activation and spinal cord pro-inflammatory cytokine action can be a potential therapeutic strategy for neuropathic pain. Pro-inflammatory cytokines [interleukin (IL)-6, IL-1β, and tumor necrosis factor (TNF)-α] in the spinal cord cause pain hypersensitivity through central sensitization, thus participating in the pathogenesis of neuropathic pain (Nantel et al., 1999).
We tested muscovite nanoparticles to explore a new approach to inhibit glial cells and inflammatory cytokines. Muscovite has been used to treat bleeding, dysentery, and inflammation in traditional medicine, including East Asian medicine and Ayurveda. Previous studies have shown that muscovite has anti-tumor activity through chemopreventive effects and blocking the proliferation of cancer cells (Cho et al., 2013; Kang et al., 2015). On the other hand, several studies have shown that muscovite nanoparticles have immune enhancing effects via the lysosome and phagosome pathway (Jung et al., 2010, 2015). In addition, muscovite has been reported to be effective in modulating the inflammatory response and apoptosis in the chronic atrophic gastritis rat model (Zhu et al., 2006, 2008). Although muscovite has been shown to modulate the inflammatory response, no study has investigated the analgesic effect of muscovite on neuropathic pain.
In the present study, we examined the effect of muscovite on spinal glial cell activation (astrocyte and microglia) in a partial sciatic nerve ligation (PSNL) mouse model of neuropathic pain. We also analyzed the changes in pro-inflammatory cytokines as well as astrocytes and microglia in the model.
Materials and Methods
Animals
Eight-week-old adult male C57BL/6 mice (Samtako Animal Co., Seoul, Korea), weighing 24–26 g, were used in all experiments. All mice were acclimated for at least 1 week before the experiments and maintained with a 12-hour light/dark cycle (lights on at 8:00 a.m. and off at 8:00 p.m.) under a controlled temperature of 22 ± 2°C and relative humidity of 55 ± 15%. A total of 30 mice were randomly assigned to each group (n = 6/group): sham-operated (Sham), PSNL (PSNL), PSNL + GB30 (GB30), PSNL + GB34 (HL), PSNL + BL25 (LB). All experimental protocols in this study followed the “Guide for Animal Experiments” edited by the Korean Academy of Medical Sciences and were approved by the Institutional Animal Ethics Committee (IACUC) at Dongguk University, South Korea (approval No. 2017-022-1) on September 28, 2017.
Induction of neuropathic pain induced by PSNL
The animal’s left sciatic nerve was exposed at the mid-thigh level, and the dorsal one-third to one-half of the sciatic nerve was tightly ligated with an 8-0 silk (AILEE, Busan, Korea) suture (Figure 1). The sham group underwent the same surgery as the PSNL animals except that sutures were not inserted nor ligated into the nerve (Jang et al., 2018). Animals were randomly assigned to each group and a baseline testing was performed 7 days after surgery.
Figure 1.
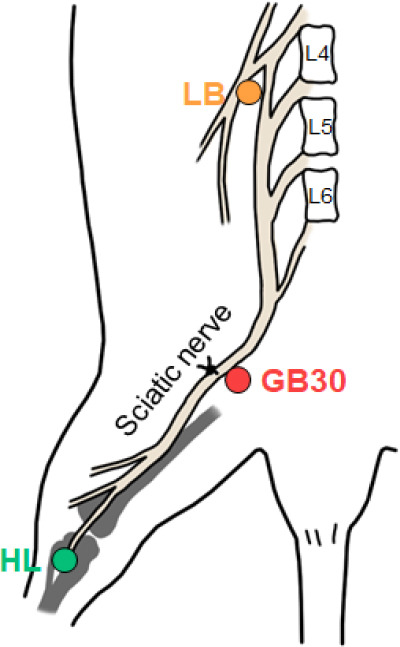
Schematic diagram of surgical ligation and muscovite injection points.
Ligature was tied around the sciatic nerve in the PSNL mice. Muscovite was injected at GB30, HL, and LB. L4: Lumbar 4; L5: lumbar 5; L6: lumbar 6; LB: lumbar (GB34); HL: hindlimb (BL25); PSNL: partial sciatic nerve ligation.
Drug preparation
The muscovite was provided by SoltoBio (Seoul, South Korea) for the research use. It is a traditionally used mineral compounds, which mainly contains KAl2(AlSi3O10)(OH)2. The nanoparticle was generated by high speed collision methods. After boiling the muscovite powder in sterile distilled water, the supernatant was centrifuged (Centrifuge 5415R, Eppendorf, Westbury, NY, USA) at 4000 × g for 10 minutes, then filtered at 0.2 μm membrane (Whatman; Micron Separation, Inc, Westborough, MA). The size of muscovite in diameter was 419 (359–489) nm in median (10–90%) value when measured by Laser Diffraction Grain Size Analyzer in Korea Quality & Testing Institute (Suwon, South Korea).
Muscovite nanoparticle injection
GB30, LB, and HL groups received intramuscularly (i.m.) injection of muscovite at GB30, BL25, and GB34 of acupoint, respectively (Figure 1). These points follow the World Health Organization standard. GB30 is in the buttock region, at the junction of the lateral one third and medial two thirds of the line connecting the prominence of the greater trochanter with the sacral hiatus. This point is close to the sciatic nerve. GB34 is on the fibular aspect of the leg, in the depression anterior and distal to the head of the fibula, which is close to the lumbar spinal cord. BL25 is in the lumbar region, at the same level as the inferior border of the spinous process of the fourth lumbar vertebra (L4). We intramuscularly injected the 4 mg/100 μL saline muscovite nanoparticles into the three points with a depth of 1–2 mm.
Behavioral tests
Mechanical allodynia frequency was assessed in the hind paw using electronic von Frey filaments (IITC, Woodland Hills, CA, USA). Mechanical frequency was measured by applying 10 filament to each mouse with a force of 0.6 g to the surface of the hind paw with a 5-second interval between each application, as described previously (Figure 2A) (Jang et al., 2018).
Figure 2.
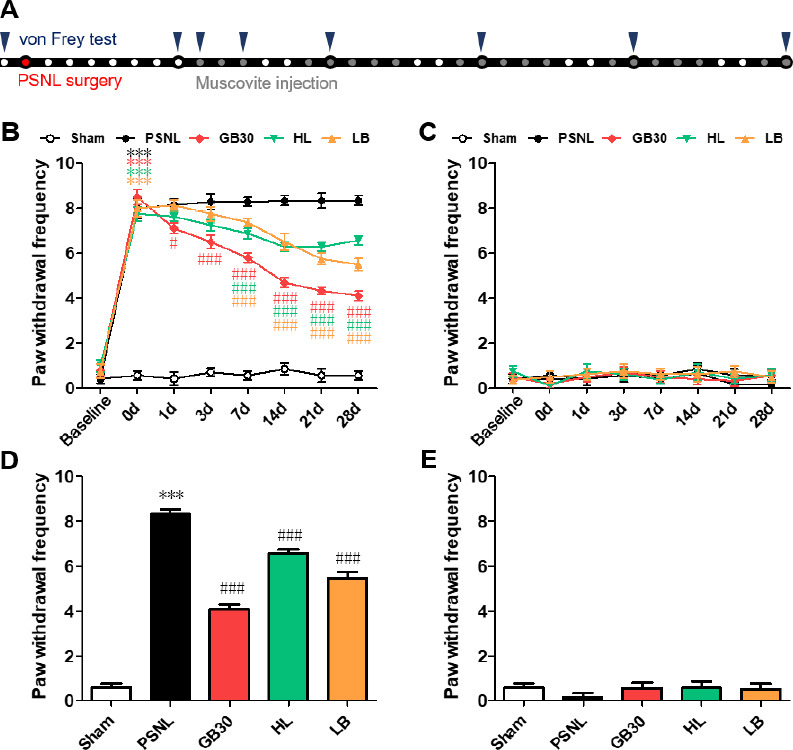
Mechanical allodynia changes for 4 weeks after muscovite injections following PSNL.
(A) Experimental schedule of the muscovite injections and von Frey test. (B–E) Anti-allodynic effects of muscovite was measured on the ipsilateral (B, D) and contralateral (C, E) plantar surfaces 2 hours after the muscovite injection for 0, 1, 3, 7, 14, 21, and 28 days. Data are expressed as the mean ± SEM (n = 6/group). ***P < 0.001 vs. sham group; #P < 0.05, ###P < 0.001, vs. PSNL group (two-way repeated measures analysis of variance followed by Bonferroni post hoc tests). HL: Hindlimb (BL25); LB: lumbar (GB34); PSNL: partial sciatic nerve ligation.
To test cold allodynia, mice were confined in acrylic glass cylinders on an elevated wire mesh. The surface of the hind paw was exposed to 20 μL of acetone, without touching the skin (Figure 3A).
Figure 3.
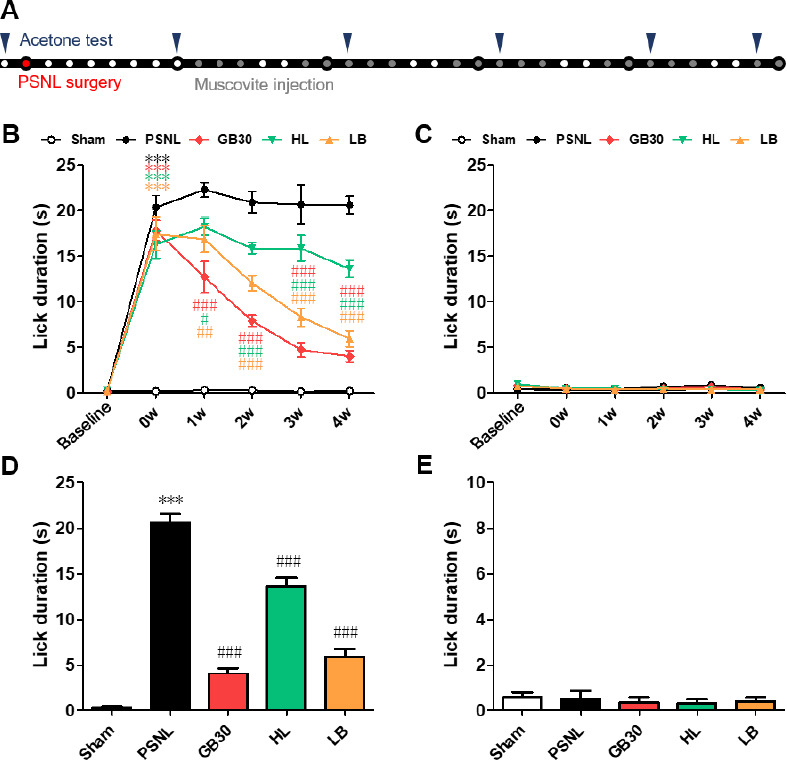
Cold allodynia changes for 4 weeks after muscovite injections following PSNL.
(A) Experimental schedule of the muscovite injections and acetone test. (B–E) Cold allodynia in the acetone test in the ipsilateral (B, D) and contralateral paw (C, E) surfaces 2 hours after the muscovite injection for 0, 1, 2, 3, and 4 weeks. Data are expressed as the mean ± SEM (n = 6/group). ***P < 0.001 vs. sham group; #P < 0.05, ##P < 0.01, ###P < 0.001, vs. PSNL group (two-way repeated measures analysis of variance (ANOVA) followed by Bonferroni post-hoc tests). HL: Hindlimb (BL25); LB: lumbar (GB34); PSNL: partial sciatic nerve ligation.
Western blot assay
The lumbar portion of the spinal cord (L1–6) and sciatic nerve samples were homogenized in lysis buffer (CyQUANT; Invitrogen, Carlsbad, CA, USA). Protein sample (10μg) was subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis, then transferred to polyvinylidene fluoride membranes, and finally incubated with primary antibodies: interleukin-6 (IL-6; 1:500; Cell Signaling Technology, Danvers, MA, USA; #12912), IL-1β (1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA; sc-7884), TNF-α (1:500; Novus Biologicals, Milano, Italy; NBP1-19532), activating transcription factor 3 (ATF-3; 1:500; Cell Signaling Technology; #33593), glial fibrillary acidic protein (GFAP; 1:500; Invitrogen; 14-9892-82), Iba-1 (1:500; Millipore, Billerica, MA, USA; MABN92) and β-actin (1:1000; Santa Cruz Biotechnology; sc-47778) for 72 hours at 4°C. The membranes were incubated with the secondary rabbit (1:5000; Thermo Scientific, Madison, WI, USA; PA1-30359) or mouse (1:10,000; Thermo Scientific; PA1-30355) antibodies for 1 hour at room temperature. The bands were visualized using an enhanced chemiluminescence kit (Super Signal West Pico; Pierce, Rockford, IL, USA), and the band intensity was analyzed with the Image J software (National Institutes of Health, Bethesda, MD, USA).
Immunofluorescence
Mice were perfused with PBS followed by 10% formalin. The separated spinal cords were post-fixed overnight, cryoprotected, and incubated with 10%, 20%, and 30% sucrose, respectively, for 12 hours. Spinal cords were cut into 40 μm-thick coronal sections. Three sections per mice were selected for later immunofluorescence. Nonspecific binding sites were blocked using 0.3% bovine serum albumin (BSA) with 0.3% Triton X-100 in PBS for 1 hour at room temperature. After blocking, the sections were incubated with mouse anti-GFAP (marker of astrocyte; 1:500; Invitrogen; 14-9892-82) or mouse anti-Iba-1 (marker of microglia; 1:500; Millipore; MABN92) for 72 hours at 4°C. The sections were then incubated with secondary antibody of Alexa Fluor 488 donkey anti-rabbit IgG (A21206, 1:500 dilution) diluted in PBS with 0.3% Triton X-100 for 1 hour at room temperature. Then fluorescence mounting medium with 4′,6-diamidino-2-phenylindole (DAPI) was finally added. The sections were observed under an Olympus FV-1000 laser confocal scanning microscope (Olympus Corporation, Shinjuku, Tokyo, Japan) equipped with a plan apochromatic × 60 oil immersion objective.
Statistical analysis
GraphPad Prism 5 software (GraphPad Software Inc., San Diego, CA, USA) was used for all statistical analyses. All data are expressed as the mean ± standard error. Analyses of the von Frey and acetone test were performed using two-way repeated measures analysis of variance (ANOVA) and the Bonferroni post-hoc tests for pairwise multiple comparisons. Group comparisons of inflammatory cytokine activation in the spinal cord were analyzed by one-way analysis of variance (ANOVA) followed by the Newman–Keuls post-hoc test. In all of the analyses, the differences were considered statistically significant at P < 0.05.
Results
Muscovite injections induce mechanical and cold allodynia
To investigate the behavior of muscovite for regulating neuropathic pain, we examined the effect of muscovite on neuropathic pain development by assessing mechanical and cold allodynia. After the muscovite injections, two-way ANOVA revealed a significant main effect of group (F(4, 272) = 710, P < 0.001) with a significant group × time interaction (F(7, 272) = 255, P < 0.001) in the von Frey test, and revealed a significant main effect of group (F(4, 186) = 264, P < 0.001) with a significant group × time interaction (F(5, 186) = 150, P < 0.001) in the acetone test. One-way ANOVA revealed a significant effect of the muscovite injections after 1 month in the von Frey test (F(4, 36) = 157.9, P < 0.001) and acetone test (F(4, 35) = 104.9, P < 0.001).
The mechanical withdrawal frequency of the muscovite-injected groups exhibited remarkable decreases on postoperative days 3–28 compared with the PSNL group. Notably, when injected at GB30, the mechanical withdrawal frequency was significantly improved compared to the other muscovite-injected groups (all P < 0.001 vs. PSNL; Figure 2B). However, there was no difference in the contralateral paw (Figure 2C). As a result, mechanical allodynia decreased in all groups after muscovite injections for 1 month, particularly when injected at GB30 (Figure 2D), while no significant differences were detected in the contralateral paw (Figure 2E). These results indicate that muscovite could exhibit an ameliorative effect in PSNL mice.
The PSNL group exhibited pronounced cold allodynia compared to the sham group, as indicated by the significant improvement in the frequency of the paw withdrawal response postoperatively (Figure 3B). The muscovite-injected groups showed a significant attenuation of cold allodynia, with a remarkable decrease in the paw withdrawal response frequency compared with that of the PSNL group during 1–4 weeks (all P < 0.001, vs. PSNL). However, no difference was observed in the contralateral paw (Figure 3C). As a result, cold allodynia decreased in all groups after 1 month of muscovite injections, particularly when injected at GB30 (Figure 3D). No significant differences were detected in the contralateral paw (Figure 3E). These data suggest that muscovite injections could alter cold allodynia in PSNL mice.
Muscovite reverses the PSNL-induced expression of neuroinflammatory cytokines
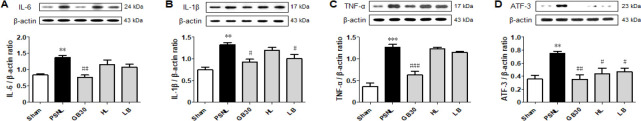
To investigate the effects of muscovite on PSNL-induced neuroinflammation, the protein levels of pro-inflammatory cytokines were measured. One-way ANOVA revealed significant differences in relative protein expression of pro-inflammatory cytokine IL-6 (F(4, 20) = 8.870, P = 0.0027), IL-1β (F(4, 20) = 9.248, P = 0.0022), and TNF-α (F(4, 20) = 39.26, P < 0.0001). Determination of pro-inflammatory cytokines revealed higher levels in the PSNL than in the sham group (P < 0.01, Figure 4A; P < 0.01, Figure 4B; P < 0.001, Figure 4C). Following muscovite injections at GB30, the pro-inflammatory cytokine levels decreased significantly (P < 0.01, Figure 4A; P < 0.05, Figure 4B; P < 0.001, Figure 4C).
Figure 4.
Expression levels of pro-inflammatory cytokines after muscovite injection.
(A) IL-6, (B) IL-1β, and (C) TNF-α were detected by western blot assay in the spinal cord tissue. (D) ATF-3 was detected in the sciatic nerve. Data are expressed as the mean ± SEM (n = 3/group). **P < 0.01, ***P < 0.001, vs. sham group; #P < 0.05, ##P < 0.01, ###P < 0.001, vs. PSNL group. n = 3/group (one-way analysis of variance followed by the Student-Newman-Keuls test). ATF-3: Activating transcription factor 3; HL: hindlimb (BL25); IL-6 interleukin-6; IL-1β: interleukin-1beta; LB: lumbar (GB34); PSNL: partial sciatic nerve ligation; TNF-α: tumor necrosis factor-alpha.
We next assessed expression of ATF-3 on the sciatic nerve following neuronal damage or stress (Tsujino et al., 2000). One-way ANOVA revealed a significant difference in the relative protein expression level of ATF-3 (F(4, 20) = 6.375, P = 0.0081). ATF-3 increased after PSNL compared to the sham group, but decreased significantly in the GB30 group compared to the PSNL group (P < 0.01; Figure 4D).
Muscovite inhibits astrocyte and microglia activation in the spinal cord
The activation of astrocytes releases a series of substances that specifically regulate the transmission of pain. To determine whether muscovite inactivated astrocytes, we observed changes in the immunoactivity and expression of GFAP, a marker of astrocyte activity. One-way ANOVA revealed significant differences in astrocyte-positive cells (F(4, 20) = 11.19, P = 0.0010) and protein levels (F(4, 20) = 4.770, P = 0.0206). Post-hoc Neuman-Keuls analysis showed that the GFAP-labeled astrocytes had thin processes and smaller cell bodies in the lamina I to II of the Sham group, suggesting no activation, but exhibited an obvious hypertrophic body with thicker processes in the PSNL group (Figure 5A). However, GFAP staining decreased significantly in the GB30 group (P < 0.01), while HL and LB groups exhibited no significant differences compared with the PSNL group. The same conclusion was reached from optical density of GFAP (Figure 5A and B). Furthermore, muscovite treatment significantly decreased GFAP protein expression in the GB30 group (P < 0.05; Figure 5C). These indicate that the GB30 group had significantly reduced astrocyte activity, thereby reducing the transmission of pain perception in the central nervous system, which might be another mechanism of the analgesic effect of muscovite in PSNL mice.
Figure 5.
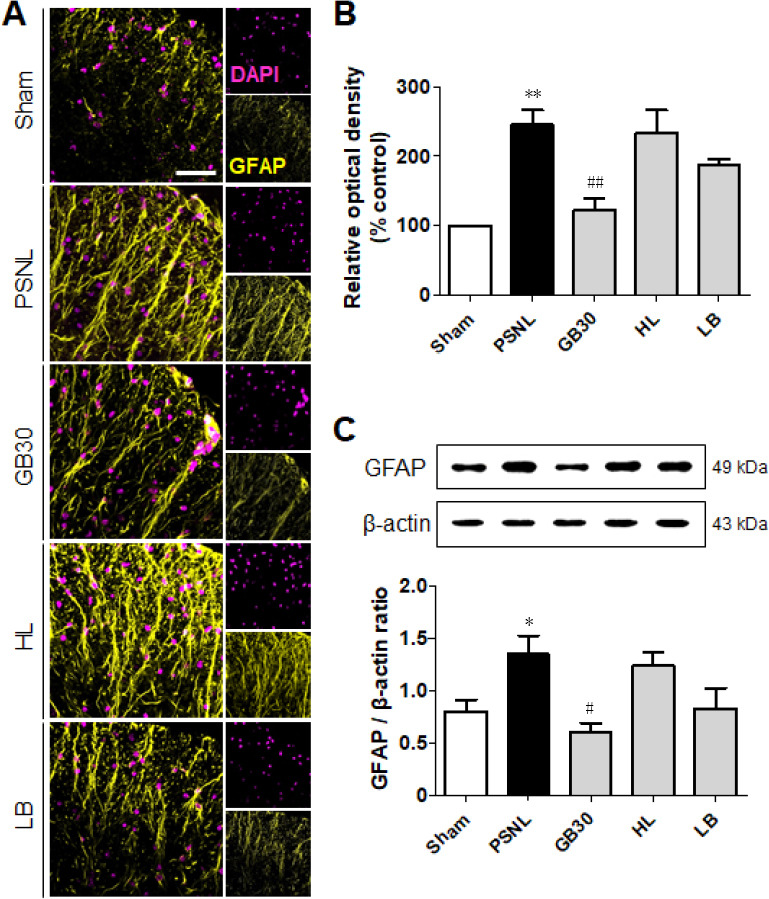
Regulation of muscovite in activated astrocytes.
(A, B) Immunofluorescence staining demonstrates that GFAP is regulated for muscovite injection. (C) Western blot assay confirming GFAP expression after muscovite injection. β-Actin was used as the loading control. Scale bar: 100 µm. Data are expressed as the mean ± SEM (n = 3/group). *P < 0.05, **P < 0.01, vs. sham group; #P < 0.05, ##P < 0.01, vs. PSNL group (one-way analysis of variance followed by the Student-Newman-Keuls test). DAPI: 4′,6-Diamidino-2-phenylindole; GFAP: glial fibrillary acidic protein; HL: hindlimb (BL25); LB: lumbar (GB34); PSNL: partial sciatic nerve ligation.
To determine whether muscovite inactivates microglia, we observed changes in the immunoactivity and protein level of Iba-1, a marker of microglia activity. One-way ANOVA revealed significantly different immunoactivity (F(4, 20) = 79.74, P < 0.0001) and protein level (F(4, 20) = 5.752, P = 0.0114) in microglia-positive cells. Post-hoc Neuman-Keuls analysis showed that Iba-1-positive cells had thinner processes and smaller cell bodies in the lamina I to II of the sham group, suggesting no activation. Comparably, an obvious hypertrophic body with thicker processes were exhibited in the PSNL group (Figure 6A). However, Iba-1 staining was decreased significantly in the GB30, HL, and LB groups (P < 0.001), and the same conclusion was reached from the optical density of Iba-1 (Figure 6A and B). Furthermore, muscovite treatment significantly decreased Iba-1 protein expression in the GB30 group (P < 0.01; Figure 6C). These results show that muscovite administration at GB30 inhibited PSNL-induced activation of microglia.
Figure 6.
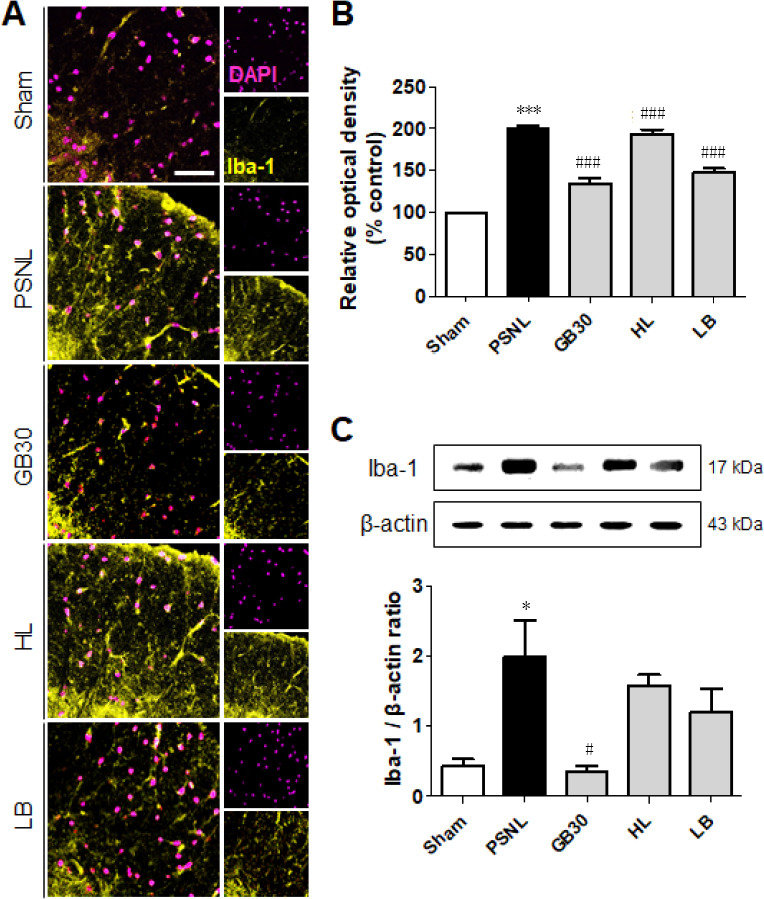
Regulation of muscovite in activated microglia.
(A, B) Immunofluorescence staining demonstrates that Iba-1 is regulated for muscovite injection. (C) Western blot assay confirming Iba-1 expression after muscovite injection. β-Actin was used as the loading control. Scale bar: 100 µm. Data are expressed as the mean ± SEM (n = 3/group). *P < 0.05, ***P < 0.001, vs. sham group; #P < 0.05, ###P < 0.001, vs. PSNL group (one-way analysis of variance followed by the Student-Newman-Keuls test). DAPI: 4′,6-Diamidino-2-phenylindole; HL: hindlimb (BL25); LB: lumbar (GB34); PSNL: partial sciatic nerve ligation.
Discussion
Neuropathic pain affects millions of people around the world, often accompanied by changes in quality of life. Since the needs of patients with neuropathic pain are not largely met due to the poor efficacy and side effects of current treatments, new treatments are needed. Accumulating evidence demonstrates that neuroinflammatory responses are often characterized by the construction of pro-inflammatory cytokines. This plays an important role in the activation of glial cells and the pathophysiology of neuropathic pain induced by PSNL (Malmberg and Basbaum, 1998). By targeting neuroinflammation, the treatment of neuropathic pain has becoming mainstream and has been found to be an effective therapeutic strategy. This study showed that muscovite injection inhibited mechanical and cold allodynia induced in PSNL mice. Moreover, it decreased the activation of astrocytes and microglia in the spinal cord, and attenuated the expression of pro-inflammatory cytokines in the spinal cord.
We first investigated whether muscovite reduced mechanical and cold allodynia in the neuropathic pain model using the von Frey and acetone tests. PSNL is a well-characterized animal model of neuropathic pain (Colleoni and Sacerdote, 2010). By tightly tying one-third to one-half of the sciatic nerve, the mice exhibit reliable signs, such as mechanical and cold allodynia with persistent spontaneous pain that lasts for several months (Colleoni and Sacerdote, 2010). Our data were consistent with the literature: the animals developed significant mechanical and cold allodynia as the frequency for paw withdrawal increased after PSNL. Also, they showed associated aversive behavior, such as licking and limping of the affected paw, and enlarged movements. These were reduced after muscovite injection, with significant decrease in the mechanical and cold allodynia, suggesting that muscovite possesses pain-relieving effects and reduces the transmission of pain in the central nervous system. Notably, mechanical and cold allodynia were further reduced when muscovite was injected at GB30 compared with at the other injection points. Moreover, the ameliorative effect of GB30 group even preceded that of amitriptyline, the positive control group (Additional Figure 1 (382.9KB, tif) ). These findings suggest that the effect of muscovite is injection site-dependent.
In addition, we measured levels of the pro-inflammatory cytokines IL-6, IL-1β, and TNF-α. In neuropathic pain, increased pro-inflammatory cytokine expression is considered the main cause of hyperalgesia (Denkers et al., 2004). The present study revealed that the muscovite injections significantly decreased the expression of these pro-inflammatory elements in the PSNL mice. Based on these observations, analgesia of muscovite can be attributed to its alleviating effect on pro-inflammatory mediator attenuation such as TNF-α, IL-1β and IL-6 in PSNL-induced neuropathic pain. Muscovite injections at GB30 may decrease the production of pro-inflammatory cytokines in the spinal cord, thus attenuating hyperalgesia. Observations of functional recovery were also mapped to changes in neurochemical expression including ATF-3. ATF-3 is upregulated after nerve injury (Tsujino et al., 2000; Averill et al., 2004) and downregulated after re-innervation of peripheral targets (Seijffers et al., 2006). ATF-3 was significantly decreased in sciatic nerves harvested from muscovite-injected mice compared with PSNL mice. Since ATF-3 is reduced after neuroprotection or re-innervation of targets, our result shows that muscovite partially contributes to nerve regeneration. Furthermore, decrease of ATF-3 was remarkable in GB30 group, which was injected closest to the ligation site of sciatic nerve.
An increasing amount of evidence suggests that neuroinflammation in the spinal cord plays an important role in the development and maintenance of neuropathic pain by activating glial cells (Kiguchi et al., 2012; Ellis and Bennett, 2013). Activated glial cells produce numerous mediators, such as pro-inflammatory cytokines, which result in a cascading activation of surrounding glia that enhance neuronal activity (Guo et al., 2007; Calvo et al., 2012; Ji et al., 2014). Furthermore, these activated pro-inflammatory cytokines play an important role in the development of neuropathic pain following peripheral nerve injury (Frank et al., 2007; Mika et al., 2013). For example, peripheral nerve injury upregulates the level of cytokines, such as IL-6, IL-1β, and TNF-α, in the central nervous system (Fregnan et al., 2012). We assessed, by fluorescence and western blot analysis, whether muscovite injection could produce changes in glial cells at the spinal cord in PSNL mice. We discovered that the expression of GFAP and Iba-1 in the spinal cord decreased significantly following injection of muscovite at GB30, indicating suppression of astrocytes and microglial activation. These results suggest that the analgesic effects of the muscovite injections at GB30 may be associated with suppressing glial cell activation.
Taken together, muscovite injection moderates nociceptive behavior of neuropathic pain by suppressing inflammatory response and neuroglial activation. Subsequently, increased levels of IL-6, IL-1β and TNF-α were detected in mice with PSNL along with activation of astrocytes and microglia. Inversely, muscovite injections decreased the levels of pro-inflammatory cytokines as well as the activation of astrocytes and microglia, thus exerting an analgesic effect. Therefore, muscovite may be a potential analgesic agent to treat neuropathic pain. However, further experimental studies on the effective safe dosage for humans and specific activation mechanisms should be warranted to determine the effective use of muscovite for treating and managing neuropathic pain.
The present study suggests that muscovite injections at GB30 attenuated nociceptive behaviors in PSNL mice by inhibiting pro-inflammatory cytokines. Furthermore, the suppression of astrocytes and microglia was involved in the analgesic effects of muscovite. These results suggest that muscovite may be a potential candidate with which to regulate and attenuate the development of neuropathic pain.
Additional files:
Additional Figure 1 (382.9KB, tif) : Behavioral changes for 4 weeks after muscovite or amitriptyline injections following PSNL using von Frey test.
Behavioral changes for 4 weeks after muscovite or amitriptyline injections following PSNL using von Frey test.
Amitriptyline, tricyclic antidepressant was intraperitoneally injected at 10 mg/kg using positive control. The mechanical withdrawal threshold in both the amitriptyline and muscovite injection on the GB30 groups exhibited remarkable decreases on postoperative days 14-28 compared with the PSNL group. In addition, the ameliorative effect of muscovite injected at GB30 took effect faster than that of amitriptyline. Data are expressed as the mean ± SEM (n = 5/group). ***P < 0.001, vs. sham group; ##P < 0.01, ###P < 0.001, vs. PSNL group (data were analyzed with two-way repeated measures analysis of variance followed by Bonferroni post hoc tests). HL: Hindlimb (BL25); LB: lumbar (GB34); PSNL: partial sciatic nerve ligation.
Additional file 1: Open peer review report 1 (77.2KB, pdf) .
Footnotes
Conflicts of interest: None declared.
Financial support: This study was supported by grants from the National Research Foundation of Korea funded by the Korean government (NRF-2017R1A2B4009963; to JYO, TYH, JHJ and HJP) and from the Korea Institute of Oriental Medicine (grant K18182; to JYP, YR and HJP).
Institutional review board statement: All experimental protocols in this study were approved by the Institutional Animal Ethics Committee (IACUC) at Dongguk University, South Korea (approval No. 2017-022-1) on September 28, 2017.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: Ana Maria Blanco Martinez, Universidade Federal do Rio de Janeiro, Brazil; Askin Esen Hasturk, Oncology Training and Research Hospital, Turkey.
Funding: This study was supported by grants from the National Research Foundation of Korea funded by the Korean government (NRF-2017R1A2B4009963; to JYO, TYH, JHJ and HJP) and from the Korea Institute of Oriental Medicine (grant K18182; to JYP, YR and HJP).
P-Reviewers: Martinez AMB, Hasturk AE; C-Editor: Zhao M; S-Editor: Li CH; L-Editor: Song LP; T-Editor: Jia Y
References
- 1.Andreev N, Urban L, Dray A. Opioids suppress spontaneous activity of polymodal nociceptors in rat paw skin induced by ultraviolet irradiation. Neuroscience. 1994;58:793–798. doi: 10.1016/0306-4522(94)90456-1. [DOI] [PubMed] [Google Scholar]
- 2.Averill S, Michael GJ, Shortland PJ, Leavesley RC, King VR, Bradbury EJ, McMahon SB, Priestley JV. NGF and GDNF ameliorate the increase in ATF3 expression which occurs in dorsal root ganglion cells in response to peripheral nerve injury. Eur J Neurosci. 2004;19:1437–1445. doi: 10.1111/j.1460-9568.2004.03241.x. [DOI] [PubMed] [Google Scholar]
- 3.Benyamin R, Trescot AM, Datta S, Buenaventura R, Adlaka R, Sehgal N, Glaser SE, Vallejo R. Opioid complications and side effects. Pain Physician. 2008;11(2 Suppl):S105–120. [PubMed] [Google Scholar]
- 4.Breivik H, Eisenberg E, O’Brien T OPENMinds. The individual and societal burden of chronic pain in Europe: the case for strategic prioritisation and action to improve knowledge and availability of appropriate care. BMC Public Health. 2013;13:1229. doi: 10.1186/1471-2458-13-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calvo M, Dawes JM, Bennett DL. The role of the immune system in the generation of neuropathic pain. Lancet Neurol. 2012;11:629–642. doi: 10.1016/S1474-4422(12)70134-5. [DOI] [PubMed] [Google Scholar]
- 6.Cho SY, Lee HJ, Cho SM, Kim B, Jung YK, Kim SH. Particled Mica, STB-HO has chemopreventive potential via G1 arrest, and inhibition of proliferation and vascular endothelial growth factor receptor 2 in HCT colorectal cancer cells. BMC Complement Altern Med. 2013;13:189. doi: 10.1186/1472-6882-13-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark AK, Old EA, Malcangio M. Neuropathic pain and cytokines: current perspectives. J Pain Res. 2013;6:803–814. doi: 10.2147/JPR.S53660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colleoni M, Sacerdote P. Murine models of human neuropathic pain. Biochim Biophys Acta. 2010;1802:924–933. doi: 10.1016/j.bbadis.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 9.Denkers EY, Butcher BA, Del Rio L, Kim L. Manipulation of mitogen-activated protein kinase/nuclear factor-kappaB-signaling cascades during intracellular Toxoplasma gondii infection. Immunol Rev. 2004;201:191–205. doi: 10.1111/j.0105-2896.2004.00180.x. [DOI] [PubMed] [Google Scholar]
- 10.Ellis A, Bennett DL. Neuroinflammation and the generation of neuropathic pain. Br J Anaesth. 2013;111:26–37. doi: 10.1093/bja/aet128. [DOI] [PubMed] [Google Scholar]
- 11.Fisher K, Coderre TJ, Hagen NA. Targeting the N-methyl-D-aspartate receptor for chronic pain management. Preclinical animal studies, recent clinical experience and future research directions. J Pain Symptom Manage. 2000;20:358–373. doi: 10.1016/s0885-3924(00)00213-x. [DOI] [PubMed] [Google Scholar]
- 12.Frank MG, Baratta MV, Sprunger DB, Watkins LR, Maier SF. Microglia serve as a neuroimmune substrate for stress-induced potentiation of CNS pro-inflammatory cytokine responses. Brain Behav Immun. 2007;21:47–59. doi: 10.1016/j.bbi.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 13.Fregnan F, Muratori L, Simoes AR, Giacobini-Robecchi MG, Raimondo S. Role of inflammatory cytokines in peripheral nerve injury. Neural Regen Res. 2012;7:2259–2266. doi: 10.3969/j.issn.1673-5374.2012.29.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao YJ, Ji RR. Chemokines, neuronal-glial interactions, and central processing of neuropathic pain. Pharmacol Ther. 2010;126:56–68. doi: 10.1016/j.pharmthera.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries: 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo W, Wang H, Watanabe M, Shimizu K, Zou S, LaGraize SC, Wei F, Dubner R, Ren K. Glial-cytokine-neuronal interactions underlying the mechanisms of persistent pain. J Neurosci. 2007;27:6006–6018. doi: 10.1523/JNEUROSCI.0176-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gureje O, Von Korff M, Simon GE, Gater R. Persistent pain and well-being: a World Health Organization Study in Primary Care. JAMA. 1998;280:147–151. doi: 10.1001/jama.280.2.147. [DOI] [PubMed] [Google Scholar]
- 18.Gwak YS, Hulsebosch CE, Leem JW. Neuronal-glial interactions maintain chronic neuropathic pain after spinal cord injury. Neural Plast. 2017;2017:2480689. doi: 10.1155/2017/2480689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haanpaa M. The assessment of neuropathic pain patients. Pain Manag. 2013;3:59–65. doi: 10.2217/pmt.12.71. [DOI] [PubMed] [Google Scholar]
- 20.Jang JH, Park JY, Oh JY, Bae SJ, Jang H, Jeon S, Kim J, Park HJ. Novel analgesic effects of melanin-concentrating hormone on persistent neuropathic and inflammatory pain in mice. Sci Rep. 2018;8:707. doi: 10.1038/s41598-018-19145-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ji RR, Berta T, Nedergaard M. Glia and pain: is chronic pain a gliopathy? Pain. 2013;154(Suppl 1):S10–28. doi: 10.1016/j.pain.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ji RR, Xu ZZ, Gao YJ. Emerging targets in neuroinflammation-driven chronic pain. Nat Rev Drug Discov. 2014;13:533–548. doi: 10.1038/nrd4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jung BG, Toan NT, Cho SJ, Ko JH, Jung YK, Lee BJ. Dietary aluminosilicate supplement enhances immune activity in mice and reinforces clearance of porcine circovirus type 2 in experimentally infected pigs. Vet Microbiol. 2010;143:117–125. doi: 10.1016/j.vetmic.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 24.Jung M, Shin MK, Jung YK, Yoo HS. Modulation of macrophage activities in proliferation, lysosome, and phagosome by the nonspecific immunostimulator, mica. PLoS One. 2015;10:e0117838. doi: 10.1371/journal.pone.0117838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang TW, Kim HS, Lee BC, Shin TH, Choi SW, Kim YJ, Lee HY, Jung YK, Seo KW, Kang KS. Mica nanoparticle, STB-HO eliminates the human breast carcinoma cells by regulating the interaction of tumor with its immune microenvironment. Sci Rep. 2015;5:17515. doi: 10.1038/srep17515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kiguchi N, Kobayashi Y, Kishioka S. Chemokines and cytokines in neuroinflammation leading to neuropathic pain. Curr Opin Pharmacol. 2012;12:55–61. doi: 10.1016/j.coph.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 27.Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10:895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malmberg AB, Basbaum AI. Partial sciatic nerve injury in the mouse as a model of neuropathic pain: behavioral and neuroanatomical correlates. Pain. 1998;76:215–222. doi: 10.1016/s0304-3959(98)00045-1. [DOI] [PubMed] [Google Scholar]
- 29.Mika J, Zychowska M, Popiolek-Barczyk K, Rojewska E, Przewlocka B. Importance of glial activation in neuropathic pain. Eur J Pharmacol. 2013;716:106–119. doi: 10.1016/j.ejphar.2013.01.072. [DOI] [PubMed] [Google Scholar]
- 30.Nantel F, Denis D, Gordon R, Northey A, Cirino M, Metters KM, Chan CC. Distribution and regulation of cyclooxygenase-2 in carrageenan-induced inflammation. Br J Pharmacol. 1999;128:853–859. doi: 10.1038/sj.bjp.0702866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schaefer C, Mann R, Sadosky A, Daniel S, Parsons B, Nieshoff E, Tuchman M, Nalamachu S, Anschel A, Stacey BR. Burden of illness associated with peripheral and central neuropathic pain among adults seeking treatment in the United States: a patient-centered evaluation. Pain Med. 2014a;15:2105–2119. doi: 10.1111/pme.12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schaefer C, Sadosky A, Mann R, Daniel S, Parsons B, Tuchman M, Anschel A, Stacey BR, Nalamachu S, Nieshoff E. Pain severity and the economic burden of neuropathic pain in the United States: BEAT Neuropathic Pain Observational Study. Clinicoecon Outcomes Res. 2014b;6:483–496. doi: 10.2147/CEOR.S63323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seijffers R, Allchorne AJ, Woolf CJ. The transcription factor ATF-3 promotes neurite outgrowth. Mol Cell Neurosci. 2006;32:143–154. doi: 10.1016/j.mcn.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 34.Siniscalco D, Rossi F, Maione S. Molecular approaches for neuropathic pain treatment. Curr Med Chem. 2007;14:1783–1787. doi: 10.2174/092986707781058913. [DOI] [PubMed] [Google Scholar]
- 35.Sommer C, Leinders M, Uceyler N. Inflammation in the pathophysiology of neuropathic pain. Pain. 2018;159:595–602. doi: 10.1097/j.pain.0000000000001122. [DOI] [PubMed] [Google Scholar]
- 36.Tsujino H, Kondo E, Fukuoka T, Dai Y, Tokunaga A, Miki K, Yonenobu K, Ochi T, Noguchi K. Activating transcription factor 3 (ATF3) induction by axotomy in sensory and motoneurons: A novel neuronal marker of nerve injury. Mol Cell Neurosci. 2000;15:170–182. doi: 10.1006/mcne.1999.0814. [DOI] [PubMed] [Google Scholar]
- 37.Witt EA, Kenworthy J, Isherwood G, Dunlop WC. Examining the association between pain severity and quality-of-life, work-productivity loss, and healthcare resource use among European adults diagnosed with pain. J Med Econ. 2016;19:858–865. doi: 10.1080/13696998.2016.1178127. [DOI] [PubMed] [Google Scholar]
- 38.Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- 39.Zhu FS, Si JM, Wang LJ, Wang DF, Chen P. Effect of mica granule on the expression of gene-protein associated with cancer in gastric mucosa tissue of chronic atrophic gastritis rats. Zhongguo Zhong Yao Za Zhi. 2006;31:312–316. [PubMed] [Google Scholar]
- 40.Zhu FS, Si JM, Wang LJ, Wang DF, Chen P. Effect of mica monomer powder on chief and parietal cells as well as G and D cells in gastric mucosa of chronic atrophic gastritis in rats. Chin J Integr Med. 2008;14:111–116. doi: 10.1007/s11655-007-9003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Behavioral changes for 4 weeks after muscovite or amitriptyline injections following PSNL using von Frey test.
Amitriptyline, tricyclic antidepressant was intraperitoneally injected at 10 mg/kg using positive control. The mechanical withdrawal threshold in both the amitriptyline and muscovite injection on the GB30 groups exhibited remarkable decreases on postoperative days 14-28 compared with the PSNL group. In addition, the ameliorative effect of muscovite injected at GB30 took effect faster than that of amitriptyline. Data are expressed as the mean ± SEM (n = 5/group). ***P < 0.001, vs. sham group; ##P < 0.01, ###P < 0.001, vs. PSNL group (data were analyzed with two-way repeated measures analysis of variance followed by Bonferroni post hoc tests). HL: Hindlimb (BL25); LB: lumbar (GB34); PSNL: partial sciatic nerve ligation.