Abstract
Objectives:
Among cancer prevention studies, little is known about knowledge, attitudes, and beliefs toward triage adherence in the context of the human papillomavirus self-collection test. This formative research aims to identify knowledge, attitudes, and beliefs related to human papillomavirus and cervical cancer prevention specifically about adherence to Pap triage among women residing in a low-income province in Argentina.
Methods:
We conducted six focus groups, stratified by residence and age. All participants were aged 30 or older and had performed human papillomavirus self-collection. Data collection and thematic analysis were carried out using constructs from the Health Belief Model.
Results:
Misinformation regarding human papillomavirus and cervical cancer was common and was a source of distress. Women could not distinguish Pap screening from triage; human papillomavirus risk perception was limited but cervical cancer was perceived as a threatening disease. Women were willing to follow-up after receiving an abnormal screening result. Negative views about clinician-collected screening/triage were common, defined as painful and shameful, and comes with an economic cost (transport/time). Lack of help from family/friends was an obstacle to adhering to triage. Health issues in the family’s records and a physician’s recommendation were a cue to adhere to triage.
Conclusion:
Lack of knowledge or misinformation of the causes of cervical cancer, human papillomavirus, and the multi-step screening and triage process are barriers to follow-up adherence. Interventions to improve communication between women and health providers about screening results and follow-up are needed. Also, health services should be organized to respond to women’s needs and reduce access barriers to follow-up.
Keywords: Argentina, cervical cancer, Health Belief Model, human papillomavirus self-collection test, Pap smear triage
Introduction
In Latin America, the high mortality rate due to cervical cancer (CC) is related to problems in the continuity of the screening process, including the low coverage of screening and high follow-up abandonment.1,2 Human papillomavirus (HPV) DNA tests are more effective than cytology for the detection of CC precursors and have high negative predictive value, therefore reducing screening frequency.3,4 HPV testing allows self-collection (SC), which is especially beneficial for women facing obstacles in accessing the health care system.5–7 SC is highly accepted by women in different countries and increases screening coverage.6,8,9 In an HPV-test-based program, triage tests are needed to identify which HPV-positive (HPV+) women need further diagnostic and treatment procedures. When cytology is used as triage, HPV+ women have to attend health care centers for a Pap test. However, adhering to triage and treatment can be challenging, especially among women with SC tests, who in general may not be regular health care system users, and therefore are at greater risk of not being able to continue with triage procedures.10,11 In Argentina, clinician-collected screening involves taking two samples for HPV screening and Pap triage. Evidence has shown that women using SC have lower triage adherence.11
Evidence from cytology-based screening studies has shown that in addition to socio-economic conditions, factors related to the health care organization and individual aspects, such as fears and beliefs, are key determinants of women’s capacity to adhere to follow-up procedures.12–15 In particular, studies found that poor knowledge or misconceptions regarding the need for follow-up, wrong beliefs regarding what screening results mean, fear of cancer, and low-risk perception were common among non-adherent women.16–20 Instead, most studies have focused on women’s knowledge, attitudes, and beliefs (KAB) related to traditional Pap screening, not the HPV SC screening process.13
An HPV+ result means the sexually transmitted infection (STI) is present and might cause cancer, but additional tests are needed to detect whether the virus infection has developed into cervical disease. This means screening results require women to process complex concepts regarding the different stages of the infection/disease progression and the role of screening, follow-up, diagnosis, and treatment.21 In a quantitative study carried out in Argentina, subjective reasons, such as fear of cancer or thinking that triage was not necessary, were among the main reasons reported by HPV+ women with self-collected samples for not continuing Pap triage.12 A systematic review conducted to analyze the impact on inequalities of the introduction of HPV-based screening showed that feelings related to an HPV+ result depended on women’s previous knowledge and their ability to understand the physicians’ indications.22 Patient anxiety and negative feelings (such as insecurity or fear) have also been shown to have a major role in the behavior to adhere to follow-up.23–26
The objective of this analysis was to understand women’s KAB regarding CC prevention and HPV among women who have performed HPV SC, and how they affect HPV+ women’s ability to complete Pap triage. In this article, we report results from formative research conducted as part of the ATICA study (Application of Information and Communication Technologies to Self-collection, for its initials in Spanish), a trial evaluating an mHealth intervention to increase compliance with triage testing among HPV+ women who performed SC.27 Data provided by this formative research will be key to inform researchers and health authorities implementing HPV SC strategies, as well as for implementation interventions aimed at increasing adherence to follow-up.
Methods
Theoretical framework
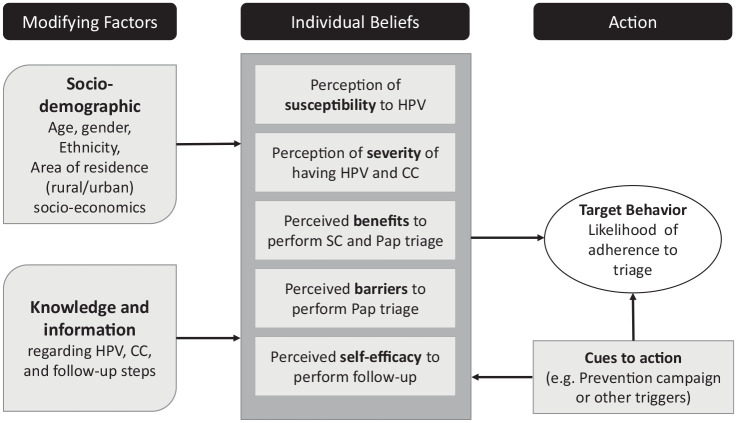
For data collection and analysis, we relied on constructs from the Health Belief Model (HBM),28,29 which has been extensively used to understand psychosocial factors regarding cervical screening behaviors (Figure 1).13,30–33
Figure 1.
Health Belief Model components and linkages.
Source: ATICA protocol,27 adapted from Skinner et al. (2015).28
The ATICA study includes a cluster-randomized trial aimed at evaluating the effectiveness of a multicomponent intervention consisting of SMS messages for women testing positive after HPV SC and automated reminders to community health workers (CHWs) to visit and encourage HPV+ women who have not completed triage to attend health centers.27 Following the HBM, we hypothesized that HPV+ women would complete Pap-based triage founded on their perceived susceptibility of getting CC; their feelings about the seriousness of contracting it (perceived severity), their perceived barriers to performing Pap-based triage, their evaluation about the benefits of doing it; as well as their confidence in their capability to attend triage (perceived self-efficacy). In this context, CHW visits and SMS were considered cues to action, they may also have an impact on individual’s beliefs.28 Perceptions and feelings would differ by women’s socio-demographic profile, previous knowledge, and health information (modifying factors).
Participant selection
Eligible women were aged 30 or older, residing in Jujuy province, who had performed an SC in the last 12 months, were literate, and were mobile phone users. Focus group (FG) participants were stratified by age (decile) and residential zone (rural/urban). We used a convenience sample of women recruited face-to-face by 23 CHWs from 6 different health care centers of the province (Table 1).
Table 1.
Focus group sample quotes.
| Age | Urban areas | Rural areas | Total |
|---|---|---|---|
| 30–50 years | FG1: n = 8 | FG2: n = 9 | 17 women |
| 51 years or older | FG3: n = 9 | FG4: n = 4 | 13 women |
| 40 years or oldera | FG6: n = 6 | FG5: n = 12 | 18 women |
| Total | 23 women | 25 women | 48 women |
Originally, four FGs were proposed. Two additional FGs were carried out with women aged 40 years and above. In those cases, age segmentation was redefined to obtain more data related to technology use.
Setting
The study took place in Jujuy province located in Northwest Argentina. Jujuy has around 673,000 inhabitants; 85% live in urban areas and 32% are poor. Three percent of those aged 10 or above is illiterate, 69% of whom are women.34 Primary HPV screening is offered free of cost in all public health care facilities since 2012.5 In 2014, SC was offered by CHWs during home visits to women aged 30 or older and who have not registered screening in the last 5 years.35 Currently, women receive instructions to retrieve their results 30 days after home SC.27 Pap triage among HPV+ women is performed by a health professional at health centers, also free of charge.5 The provincial public health system includes a tertiary referral hospital, 300 primary health care centers, 18 diagnostic centers, and 5 treatment services. Forty-five percent use free health care services.
Data collection
We used a semi-structured guide for FGs, organized in five modules: (1) cellphone and SMS use; (2) knowledge and information regarding HPV and CC; (3) dimensions of the HBM regarding HPV/CC, SC, and Pap; (4) relationship with the health system and barriers for Pap (triage); and (5) construction of SMS content (reported elsewhere).36 In this article, we report results from modules 2, 3, and 4. Each participant completed an anonymous profile survey about age, educational level, current economic activity, family status, and type of health insurance. At the end of each FG, we provided them with accurate information and answered their questions to reduce confusion.
The information was collected in Spanish by two female researchers with a background in social sciences: one acted as a moderator and the other as an observer; neither of them lived in Jujuy, nor did they have any relation with the health care institutions or their authorities. This was also stated during the FGs.
Fieldwork took place in January 2018 in locations that were easily accessible for participants; no health care professionals or CHWs were present. Each FG lasted 2 h on average and was digitally recorded to transcribe verbatim.
Analytic approach
FG data were analyzed thematically using the HBM constructs. From women’s preferences and opinions, we coded emergent categories as subthemes,37 allowing a thematic analysis of the discussions regarding women’s beliefs, feelings, and opinions about CC-prevention (SC and Pap) and issues regarding adherence. Atlas.Ti (version 7.5.4; ATLAS.ti Scientific Software Development GmbH, Berlin) software was used for data processing. Transcripts were analyzed independently by two researchers to later compare, debate, and resolve the inconsistencies with the other members of the ATICA team. Participant profile variables were used to compare differences in women’s statements and report differences relevant to the analysis. We used COREQ (Consolidated Criteria for Reporting Qualitative Research) for reporting our methodological approach.38
Ethical aspects
The ATICA study’s protocol, including the formative research phase, was approved by the CEMIC Institutional Review Board, the Ethics Research Committee of the Jujuy Ministry of Health, The Institutional Review Board of the Harvard T.H. Chan School of Public Health, and the Deakin University Human Research Ethics Committee. The trial was registered in ClinicalTrials.gov (no. NCT03478397) and the Deakin University Human Ethics Research Committee (no. APP 2018–039). Women had to sign an informed consent before the study began. The anonymity of participants was guaranteed.27
Results
Characteristics of the women
A total of 48 women participated in six FGs. Most of them had less than secondary-level education, were employed, lived with a partner and children, and had public health insurance (Table 2).
Table 2.
Characteristics of the participants.
| Total cases | Women | f |
% |
|---|---|---|---|
| 48 | 100 | ||
| Age group (years) | 30–39 | 14 | 29.2 |
| 40–49 | 19 | 39.6 | |
| 50–70 | 14 | 29.2 | |
| Non-data | 1 | 2.1 | |
| Mean: 45 years; range: 30–68 years | |||
| Educational level | Primary complete/secondary incomplete | 22 | 45.8 |
| Secondary complete | 11 | 22.9 | |
| Tertiary incomplete/complete | 14 | 29.2 | |
| Non-data | 1 | 2.1 | |
| Economic activity status | Economically inactive (out of labor force) | 16 | 33.3 |
| Economically active (labor force) | 32 | 66.7 | |
| Family status | In a partnership with children | 26 | 54.2 |
| In a partnership without children | 4 | 8.3 | |
| Single with children | 12 | 25.0 | |
| Single without children | 6 | 12.5 | |
| Health insurance | Public | 35 | 72.9 |
| Private/social security | 13 | 27.1 | |
Knowledge and information regarding HPV and CC
In most groups, some women were unaware: “I didn’t know what it [HPV] is. You are telling me now” (FG4), and several had misinformation regarding HPV, its relationship with CC, and the role of SC as prevention (FG1, FG3, FG4, and FG5). In the other groups, some participants demonstrated being well-informed (few from FG3, and most from FG2 and FG6) and were regular health care system users reporting periodic medical checkups.
When we asked about the purpose of SC, women knew it was to prevent CC. Younger participants (in FG1, FG2, and FG6) highlighted that CHWs did not explain that HPV is an STI. For that reason, after the CHW visit, some women sought information on the Internet (FG2 and FG6) or they obtained information about HPV transmission from other women (FG1 and FG6).
Regardless of previous knowledge, participants pointed out that faced with an HPV+ result they would visit a gynecologist. However, most of them could not specify why or what for (FG1, FG3, FG4, and FG5). Misconceptions included considering that an HPV+ result implied starting cancer-related treatments:
(What to do next on an HPV+ result) It is to do a deeper examination to see what state you are in if you are very advanced or in time to stop it, or if they have to remove the womb.
FG3
Only a few women had information regarding HPV, CC, transmission, and the need for follow-up. Women who had already received HPV+ results and attended a gynecologic consultation for triage were knowledgable about the follow-up process (FG1, FG2, and FG6):
You need to consult with the gynecologist to take the methods you have to follow. To start, you have to do a biopsy. If they performed the Pap. If it was positive, you have to have a biopsy, because the doctor will ask you for the biopsy. After the biopsy, they will send you to a specialist in case you have warts. They will send you to the gynecologist or the oncologist.
FG6
After SC, some women worried about whether they had done it correctly and if the SC was HPV+, then they wanted confirmation with Pap (FG2 and FG6). It was not clear if most women were able to distinguish between screening and triage Pap:
Is the positive (SC result) because we did it wrong? I prefer to go for sure . . . (To perform the Pap)
FG2
Perceived susceptibility to HPV and CC
The perceived susceptibility to HPV was linked to being a woman, which would result in the need for frequent gynecology care: “We as women are prone to get infected with anything” (FG2). HPV susceptibility was also related to being sexually active, having “different partners,” and using birth control methods (FG1, FG2, FG3, FG5, and FG6).
Among young women who knew about the sexual transmission of HPV, some mentioned doubts regarding the partner’s faithfulness and they felt at risk of HPV (FG1).
One woman did not consider herself at risk as she had not been sexually active for many years; therefore, she felt that doing the test was a formality. When the woman received her HPV+ result and was able to link it to cancer, she reacted with fatalistic beliefs and perceived herself as a possible victim of social stigmatization:
After being offered the self-collection I said, “Well, okay.” For me, it was just like a formality. It has been seven years since I’ve separated and I haven’t had another partner . . . and that’s why it felt like a formality, just in case.
(When I was told I was positive) I was ashamed to have a (positive) result because I said, “What are they going to say? That I’m an easy woman who has sex with everyone.” I started to realize, “Positive is cancer,” it is the first thing I thought. “I have a daughter, she is a girl, and cancer is to die. I cannot die . . .” Life felt overwhelming . . . Until that moment I considered that HPV meant promiscuity . . . later you understand it is not.
FG2
Gynecology-related health issues, such as endometriosis, breast cancer, ovarian cysts, or pregnancy loss, suffered by participants themselves or relatives were mentioned conditions that increased women’s susceptibility to CC:
I did the test mostly because . . . I had lost a baby, I said . . . “maybe they left something inside of me . . .”
FG1
Perceived severity of having HPV and CC
Most participants did not perceive that having HPV was a serious condition. Although the motive to perform SC was to prevent cancer (considered a serious disease), they could not link HPV and CC. Only those who received an HPV+ result and a physician’s explanation understood that HPV was associated with CC (FG1, FG2, and FG6). Women knew that HPV is an STI and they compared its severity to HIV:
WOMAN 1: To me, HPV is a very aggressive word because after AIDS, the HPV issue . . . Why is that virus coming? What does the virus come from? Then you say, “It is like AIDS.”
MODERATOR: Do you associate it with that?
WOMAN 2: It happens . . . it is not you. You can take it well, but it is society. Imagine they come to you: “You caught it (HPV) because you are dirty.” Look if they find out that you have HPV?
FG6
Perceived benefits and barriers to Pap smear
The women made no mention of the benefits of Pap smears. In all groups, the Pap smear was mentioned as a part of women’s routine checkups. Regarding perceived barriers, participants’ narratives focused on negative experiences with previous Paps, irrespective of whether they were for screening or triage. Most women expressed to feel embarrassed when they performed it. However, they thought that SC “is better than undressing in front of a stranger” (FG6). Also, most women complained about the rude behavior or mistreatment by some professionals: “They yell at you: ‘Open your legs’ (imperative tone)” (FG5). For some of them, this behavior was a motive for not accepting Paps (FG3, FG5, and FG6). Pap was also described as a painful experience: “My gynecologist tortures me every year (performing Pap).” (FG2) This kind of experiences was described as a barrier to repeat the Pap:
I did a Pap and they made me hurt a lot because it seemed that I was bitten, and then I said: “I never do anything again, never again.”
FG3
Others mentioned difficulties to take time off work or ask for permission to leave work and the potential economic loss. Women with children (FG1 and FG5) commonly mentioned the lack of family and social support to help look after children so they could go to the health center and undergo Pap, both for screening or triage. Among those who did get support, it was mainly from female close relatives. Few were supported by their partner or friends.
Perceived self-efficacy to perform SC and Pap triage
When we asked about SC, some women mentioned feeling insecure both before and after SC. Women expressed that when the CHW offered them the SC kit, they felt afraid of not being able to perform it correctly and hurting themselves (FG5):
WOMAN 1: I was afraid to do it alone. I didn’t know if I would be doing it correctly.
WOMAN 2: That fear seems to me that we have it all because you say “Have I put it well? Has it been contaminated? Could it be that . . .?”
FG2
Regarding Pap smear, both for screening or triage, most women said they would be able to attend the consultation, despite feeling embarrassed and preferring to avoid Pap:
WOMEN 1: For me, for example, it is difficult to go to the gynecologist. But well, I go because I’m a woman, I have to do my checkups, but . . . sometimes I’m ashamed, being there, opening my legs . . .
WOMEN 2: Its also is hard for me.
WOMEN 1: And yes, it’s hard, hard . . . but . . . well, as I say, to me every year I perform it . . . I told her (her doctor), “If I could skip a year,” but she told me “No, it is important for you, there is no other way.”
FG2
Cues to perform SC and Pap triage
A motive to perform HPV testing was knowing an acquaintance who tested HPV+ . In these situations, some women perceived themselves as part of an at-risk group (perception of susceptibility and severity of HPV):
My friends, when I told them (about her HPV + result) all went to do checkups, because they said, “Oh, we are not exempt, if (woman name) has it, then we too . . .”
FG2
According to women, a cue to adopt preventive behaviors was the activities implemented by CHWs. They usually assist women to get children’s immunizations, perform SC tests, and remind them medical appointments (e.g. Pap triage):
WOMAN 1: There are women who for example forget (to do medical checkups)
WOMAN 2: The CHW is for that . . . they are sometimes for that, they are mostly . . .
WOMAN 1: If you forget to do it . . . if you forgot the vaccine they knock on your door . . .
WOMAN 2: CHWs keep an eye on us.
WOMAN 3: Yes, they are more aware . . .
WOMAN 1: . . . if you have to go to the doctor . . . if the doctor is calling you to do the Pap . . .
WOMAN 2: It is very good. They will knock on the door and remind you . . .
FG1
Almost all women recognized the need for a Pap test to meet routine checkups (for screening). Although CHWs can remind them of the appointment, it is the doctor recommendation that is the main factor that leads them to do the Pap (FG2):
(After the SC) I came here (to the health center), the doctor attended me, explained the new results and she says “You have to do the Pap.”
FG1
Table 3 summarized the findings coded by themes conceptually defined according to the HBM, the subthemes, and the categories that emerged in the analysis.
Table 3.
Knowledge and beliefs about HPV and cervical cancer (themes and verbatim).
| Themes | Subthemes | Verbatim examples |
|---|---|---|
| Knowledge and information regarding HPV and CC | No information | “I came (to FG) to learn about it.” |
| Misinformation | “We consume some foods and vegetables which brings us the disease.” | |
| Some information on: | “Those who are over 30 years old.” “To people who do not have health care insurance.” |
|
| HPV: “It’s because of sexual transmission.” | ||
| “HPV is a virus.” | ||
| “SC is to prevent CC.” | ||
| Follow-up steps in case of HPV+: “You need to consult with the gynecologist to take the methods you have to follow.” | ||
| Perceived susceptibility of having HPV and CC | All women are at risk | “As a woman, we are prone to get infected.” |
| Sexually active women | “Who has sexual relations.” | |
| Who has doubts about partner faithfulness: “He infected me.” | ||
| “I have had more than one partner, two . . . some partners. Then I had the intrigue about . . .” | ||
| Personal/family history of gynecological issues | “My mother had uterine cancer at 47 years old and I thought it can happen to me too . . . I can have some issues. I always did the Pap, for that very reason.” | |
| Perceived severity of HPV and CC | No perceived severity | “It was one more checkup.” |
| Perceived severity | HPV: “What does the virus come from? Then you say, ‘It is like AIDS’.” | |
| “For me, it was if HPV is cancer, HPV is death . . .” | ||
| “Cancer is death.” | ||
| Evaluation of benefits and barriers | Benefits of Pap | None mentioned. |
| Barriers to undergoing Pap | Pap requires time: “You miss a day at work.” | |
| “You must do it yearly.” | ||
| “I felt shame.” “I felt embarrassed.” | ||
| “Pap is painful, torture.” | ||
| “Some professionals had rude behaviors.” | ||
| “I don’t have time, I have small children and I’m taking care of them. I have no one to leave them with.” | ||
| Self-efficacy | To not be able to do SC | “If SC was a positive result, maybe I did wrongly.” |
| To be capable to do Pap | “I felt embarrassed, but there is no other way.” | |
| Cues to perform Pap | “My sister had already tested positive, so I wanted to do it.” | |
| “My gynecologist, in particular, says if you have a good PAP result, other doctors recommend you every three years, but she makes me go every year [to perform Pap].” | ||
| “There are women who for example forget to do medical checkups . . . The CHW is for that . . . they are sometimes for that, they are mostly . . . If you forget to do it, they knock on your door . . .” |
HPV: human papillomavirus; CC: cervical cancer; FG: focus groups; SC: self-collection test.
Discussion
Our study showed that most women had misinformation regarding what HPV was, its sexual transmission, and its relation with CC. We also found that most women would visit a gynecologist if given a positive HPV result, although they could not specify why or what for. Pap smear was recognized as a part of women’s routine checkups, but women were not able to distinguish between a Pap for screening from a Pap for triage. The main barriers to adhering to triage were previous negative experiences with Paps and being able to take time off work or family responsibilities to go to the clinic.
Our findings showed that most women knew that SC was to prevent CC, but they had misinformation about HPV and its causal link with CC, as well as its sexual transmission. Similar findings were published by Waller et al.39 in Australia who found that the links between HPV, CC, and sexual activity were commonly absent. In our study, participants’ knowledge of the causes of CC was poor despite having performed SC. A study that analyzed barriers to information among women undergoing HPV tests found that after consultation some women felt overwhelmed with HPV information, preventing the absorption of the new knowledge. The study conducted in a public health facility in Ireland suggested that the complexities surrounding HPV infection require to evaluate what and how much information women need and how to convey this information.40 Screening behaviors are facilitated by trusted health providers41–43 such as Jujuy CHWs. Although CHWs provide national education materials to their patients and women accept SC, additional education may be needed to emphasize the link between HPV as an STI and the link with CC.
In our study, most women stated that they would visit a gynecologist if they tested HPV+, although they do not understand the meaning of the result or what additional tests are needed. In addition, women did not know the difference between screening and triage. They acknowledged Pap was routine care, but none was able to specify that after HPV SC, the Pap test was used to identify HPV+ women with precancerous lesions. Several studies have shown that misinformation regarding the purpose of the follow-up was a reason for non-adherence.16,44,45 A prior study conducted in Jujuy showed that after the gynecological consultation, most women still had doubts and unanswered questions regarding diagnosis and follow-up. However, other women said they understood what the provider explained, but demonstrated gaining only some of the information. In both groups, information needs were obstacles to adherence follow-up.21 In the current study, misconceptions about the causes of CC made the HPV+ result synonymous with cancer itself. And that may explain why some women in Jujuy believe that HPV+ requires cancer-related treatments.
Following the HBM, perceived risk influences women’s decisions to adopt a CC preventive behavior.46,47 In our study, many women did not perceive themselves as being at risk of HPV. For them, all women are prone to any STI based on gender-related susceptibility, not specific to HPV. The risk perception was linked to having multiple sexual partners and partner infidelity. Beliefs related to being sexually active were also reported in a systematic review conducted to examine socio-cultural factors influencing CC prevention of studies among immigrant and ethnic minorities in the United States.45
In our study, CC was considered a serious disease, but HPV was not considered a severe condition and many women did not perceive themselves at risk of HPV. An HPV+ result would increase their perceived susceptibility to CC. Perceived severity of cancer more generally prompted women with a family history of cancer to adhere to doctor recommendations.30,48,49 Although previous research has signaled fear of cancer as a barrier to follow-up and treatment after an abnormal Pap-based screening,17,19,50 in our study women did not mention the fear of cancer as a barrier. Instead, the perceived severity of cancer modified the women perceived susceptibility and influenced their willingness to continue the follow-up. This subject should be further analyzed, especially to develop educational materials and communication strategies that provide accurate information about the link of HPV and CC, without generating an increased and over-estimated perception of cancer risk among HPV-positive women. This is particularly important considering that evidence has shown that among HPV-positive women the negative psychosocial impact of HPV testing is mainly related to the fear of having CC.51
Women from Jujuy did not mention any benefits of Pap smear, but they reported negative views about clinician-collected screening/triage: the gynecological consultation was described as a painful and shameful experience. Our previous work showed that many women preferred SC due to previous bad experiences with health providers at health centers; SC also allowed them to elude the embarrassment of being examined by a health professional.24 In our study, women’s previous painful experiences during Pap-based screening, shame of the pelvic examination, and mistreatment by professionals were mentioned as a reason for not accepting Pap smears. This perception of Pap-based screening as a painful, shameful, threatening, and invasive procedure that could act as a barrier has also been reported by other studies.45,52–54 In Ecuador, researchers found that the Pap smear procedure provoked a sensation of great vulnerability and brought back memories of experiences of mistreatment and discrimination.55 Similar barriers to Pap were found in Peru56 and Chile.57 In addition, women in our study perceived Pap as producing economic and time loss due to the time taken off from work or family responsibilities, as other studies have also shown.53,57,58
We found that the lack of family and friends’ support made family and work responsibilities in an additional barrier to screening/triage uptake. Consistently, women in Jujuy reported that an HPV+ result requires family and social support to get over subjective (fear) and institutional (get an appointment) barriers, as well as to deal with home and children responsibilities. Our results suggest that these negative feelings related to Pap-based screening as well as the perception of the procedure as time-consuming might affect women’s adherence to triage in the same way that they have affected screening uptake and follow-up.17,57,59 This highlights the need of improving organizational aspects of gynecology services (i.e. organizing appointment times that respond to women needs), the importance of assuring a consultation environment respectful of women’s privacy, as well as training health providers in communicational strategies that take into account and are respectful of women’s cultural and socio-economic background.
This study has some limitations. We used a stratified sample to obtain different profiles, but in all nonprobabilistic samples, results may be subjected to bias. Results may disproportionately reflect opinions from participants who had especially negative or positive experiences. However, women who are not regular health system users may be less represented. Due to the small sample size and the specific study setting, the generalizability of our findings may be limited; however, it is considered sufficient for qualitative research and clear themes emerged from the data.
Conclusion
Lack of knowledge or misinformation of the causes of CC, HPV, and the triage process may be barriers to understanding the result of the SC test and subsequent need for follow-up and treatment.
Our findings suggest innovations to improve health providers’ communication and increase women’s knowledge about HPV testing and the role of triage are needed. Also, health services should be organized to respond to women’s needs and reduce access barriers to follow-up.
Acknowledgments
The authors thank the Ministry of Health of Jujuy province, their wonderful team health workers, and their coordinators for their support; Dr Alicia Campanera and Mariana Curotto for their support during the fieldwork. Finally, they thank all the women who generously donated their time to participate.
Footnotes
Author contributions: All authors made a substantial contribution to the design of the project; acquisition, analysis, and interpretation of data; they drafted the article and/or revised it critically; and they approved the final version to be published.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work is part of a Formative Research subcomponent of the main project “Mixed-methods approach to evaluate an mHealth intervention to increase Pap triage of HPV+ women who have performed self-collection” led by Dr Silvina Arrossi at the Center of Studies of State and Society (CEDES), in association with the Dana-Farber Cancer Institute/Harvard T.H. Chan School of Public Health in the United States, Deakin University from Australia and in collaboration with the Argentinean National Cancer Institute and Jujuy’s Ministry of Health. It is funded by the National Cancer Institute of the National Institutes of Health (Award No. R01CA218306). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
ORCID iD: Sánchez Antelo Victoria  https://orcid.org/0000-0003-4892-0394
https://orcid.org/0000-0003-4892-0394
References
- 1. Murillo R, Almonte M, Pereira A, et al. Cervical cancer screening programs in Latin America and the Caribbean. Vaccine 2008; 26: L37–L48. [DOI] [PubMed] [Google Scholar]
- 2. Arrossi S, Paolino M, Sankaranarayanan R. Challenges faced by cervical cancer prevention programs in developing countries: a situational analysis of program organization in Argentina. Rev Panam Salud Publica 2010; 28(4): 249–257. [DOI] [PubMed] [Google Scholar]
- 3. Cuzick J, Arbyn M, Sankaranarayanan R, et al. Overview of human papillomavirus-based and other novel options for cervical cancer screening in developed and developing countries. Vaccine 2008; 26: K29–K41. [DOI] [PubMed] [Google Scholar]
- 4. Sankaranarayanan R, Nene BM, Shastri SS, et al. HPV screening for cervical cancer in rural India. N Engl J Med 2009; 360: 1385–1394. [DOI] [PubMed] [Google Scholar]
- 5. Arrossi S, Thouyaret L, Laudi R, et al. Implementation of HPV-testing for cervical cancer screening in programmatic contexts: the Jujuy demonstration project in Argentina. Int J Cancer 2015; 137: 1709–1718. [DOI] [PubMed] [Google Scholar]
- 6. Arrossi S, Thouyaret L, Herrero R, et al. Effect of self-collection of HPV DNA offered by community health workers at home visits on uptake of screening for cervical cancer (the EMA study): A population-based cluster-randomised trial. Lancet Glob Heal 2015; 3: e85–e94. [DOI] [PubMed] [Google Scholar]
- 7. Madzima TR, Vahabi M, Lofters A. Emerging role of HPV self-sampling in cervical cancer screening for hard-To-reach women. Can Fam Physician 2017; 63(8): 597–601. [PMC free article] [PubMed] [Google Scholar]
- 8. Léniz J, Barriga MI, Lagos M, et al. HPV vaginal self-sampling among women non-adherent to Papanicolaou screening in Chile. Salud Publica Mex 2013; 55(2): 162–169. [DOI] [PubMed] [Google Scholar]
- 9. Zehbe I, Moeller H, Severini A, et al. Feasibility of self-sampling and human papillomavirus testing for cervical cancer screening in First Nation women from Northwest Ontario, Canada: a pilot study. BMJ Open 2011; 1: e000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Holme F, Maldonado F, Martinez-Granera OB, et al. HPV-based cervical cancer screening in Nicaragua: from testing to treatment. BMC Public Health 2020; 20: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arrossi S, Paolino M, Laudi R, et al. Programmatic human papillomavirus testing in cervical cancer prevention in the Jujuy demonstration project in Argentina: a population-based, before-and-after retrospective cohort study. Lancet Glob Health 2019; 7(6): e772–e783. [DOI] [PubMed] [Google Scholar]
- 12. Paolino M, Campanera A, Martiarena SN, et al. Triage of women with Human Papillomavirus self-collection in Jujuy province. Rev Argent Salud Publica 2019; 10: 7–14. [Google Scholar]
- 13. Garcés-Palacio IC, Ramos-Jaraba SM, Rubio-León DC. Health beliefs associated with the follow-up of pap smear abnormalities among low-income women in Medellín, Colombia. J Cancer Educ 2018; 33: 417–423. [DOI] [PubMed] [Google Scholar]
- 14. Ramos S, Tamburrino MC, Aguilera A, et al. Significaciones culturales, conocimientos y prácticas relativas al cáncer colorrectal, de mama y de cuello de útero: un estudio sociocultural para orientar la política comunicacional de los programas de prevención. CABA, 2013, https://www.semanticscholar.org/paper/Significaciones-culturales%2C-conocimientos-y-al-de-y-Ramos-Tamburrino/4971552044e90e9a0380a43009a55bcbf0a680f3
- 15. Gago J, Paolino M, Arrossi S. Factors associated with low adherence to cervical cancer follow-up retest among HPV+/ cytology negative women: A study in programmatic context in a low-income population in Argentina. BMC Cancer 2019; 19: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eggleston KS, Coker AL, Das IP, et al. Understanding barriers for adherence to follow-up care for abnormal pap tests. J Womens Health (Larchmt) 2007; 16(3): 311–330. [DOI] [PubMed] [Google Scholar]
- 17. Percac-Lima S, Aldrich LS, Gamba GB, et al. Barriers to follow-up of an abnormal Pap smear in Latina women referred for colposcopy. J Gen Intern Med 2010; 25: 1198–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Paolino M, Sankaranarayanan R, Arrossi S. Social determinants of dropout from diagnosis and treatment by women with abnormal Pap smears in Buenos Aires, Argentina. Rev Panam Salud Publica 2013; 34: 437–445. [PubMed] [Google Scholar]
- 19. Tejeda S, Darnell JS, Cho YI, et al. Patient barriers to follow-up care for breast and cervical cancer abnormalities. J Womens Health 2013; 22(6): 507–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Restivo V, Costantino C, Marras A, et al. Pap testing in a high-income country with suboptimal compliance levels: A survey on acceptance factors among Sicilian women. Int J Environ Res Public Health 2018; 15: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Szwarc L. [“I felt sick”: meanings and perceptions of women about a positive diagnosis of HPV in Jujuy]. XIV Jornadas Nacionales de Historia de las Mujeres y IX Congreso Iberoamericano de Estudios Género (in Spanish), https://fh.mdp.edu.ar/encuentros/index.php/historiadelasmujeres/jnhm2019
- 22. Giorgi Rossi P, Baldacchini F, Ronco G. The possible effects on socio-economic inequalities of introducing HPV testing as primary test in cervical cancer screening programs. Front Oncol 2014; 4: 20–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sancho-Garnier H, Tamalet C, Halfon P, et al. HPV self-sampling or the Pap-smear: a randomized study among cervical screening nonattenders from lower socioeconomic groups in France. Int J Cancer 2013; 133: 2681–2687. [DOI] [PubMed] [Google Scholar]
- 24. Arrossi S, Ramos S, Straw C, et al. HPV testing: a mixed-method approach to understand why women prefer self-collection in a middle-income country. BMC Public Health 2016; 16: 832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gupta S, Palmer C, Bik EM, et al. Self-sampling for human papillomavirus testing: increased cervical cancer screening participation and incorporation in international screening programs. Front Public Health 2018; 6: 77–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Paolino M, Arrossi S. Analysis of the reasons for abandoning the follow-up and treatment process in women with pre-cancerous cervical lesions in the province of Jujuy: implications for health management. Salud Colect 2012; 8(3): 247–261. [DOI] [PubMed] [Google Scholar]
- 27. Arrossi S, Paolino M, Orellana L, et al. Mixed-methods approach to evaluate an mHealth intervention to increase adherence to triage of human papillomavirus-positive women who have performed self-collection (the ATICA study): study protocol for a hybrid type i cluster randomized effectiveness-imp. Trials 2019; 20: 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Skinner CS, Tiro J, Champion VL. The health belief model. In: Glanz K, Rimer BK, Viswanath VK. (eds) Health behavior and health education. San Francisco, CA: Jossey-Bass, 2015, pp. 75–93. [Google Scholar]
- 29. Janz NK, Becker MH. The health belief model: a decade later. Health Educ Q 1984; 11: 1–47. [DOI] [PubMed] [Google Scholar]
- 30. Moore de, Peralta A, Holaday B, Hadoto IM. Cues to cervical cancer screening among U.S. Hispanic women. Hisp Heal Care Int 2017; 15: 5–12. [DOI] [PubMed] [Google Scholar]
- 31. Fleming K, Simmons VN, Christy SM, et al. Educating Hispanic women about cervical cancer prevention: feasibility of a promotora-led charla intervention in a farmworker community. Ethn Dis 2018; 28: 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yunus NA, Mohamed Yusoff H, Draman N. Non-adherence to recommended Pap smear screening guidelines and its associated factors among women attending health clinic in Malaysia. Malays Fam Physician 2018; 13(1): 10–17. [PMC free article] [PubMed] [Google Scholar]
- 33. León-Maldonado L, Wentzell E, Brown B, et al. Perceptions and experiences of human papillomavirus (HPV) infection and testing among low-income Mexican women. PLoS ONE 2016; 11: e0153367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Estructura de población. Dirección Provincial de Estadística y Censos [Internet]. Jujuy: DiPEC, 2020, [2020 August 8]. http://dipec.jujuy.gob.ar/poblacion/estructura-de-la-poblacion/ [Google Scholar]
- 35. Arrossi S, Paolino M, Thouyaret L, et al. Evaluation of scaling-up of HPV self-collection offered by community health workers at home visits to increase screening among socially vulnerable under-screened women in Jujuy Province, Argentina. Implement Sci 2017; 12: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sánchez Antelo V, Kohler RE, Curotto M, et al. Developing SMS content to promote Papanicolaou triage among women who performed HPV self-collection test: qualitative study. JMIR Form Res 2020; 4: e14652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. QualRIS team. Qualitative Methods in Implementation Science. White Paper. 1st ed, 2018, National Cancer Institute, https://cancercontrol.cancer.gov/IS/docs/NCI-DCCPS-ImplementationScience-WhitePaper.pdf [Google Scholar]
- 38. Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32- item checklist for interviews and focus group. Int J Qual Heal Care 2007; 19: 349–357. [DOI] [PubMed] [Google Scholar]
- 39. Waller J, McCaffery K, Nazroo J, et al. Making sense of information about HPV in cervical screening: a qualitative study. Br J Cancer 2005; 92: 265–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. O’Connor M, Costello L, Murphy J, et al. Influences on human papillomavirus (HPV)-related information needs among women having HPV tests for follow-up of abnormal cervical cytology. J Fam Plann Reprod Health Care 2015; 41(2): 134–141. [DOI] [PubMed] [Google Scholar]
- 41. Musa J, Achenbach CJ, O’Dwyer LC, et al. Effect of cervical cancer education and provider recommendation for screening on screening rates: A systematic review and meta-analysis. PLoS ONE 2017; 12: e0183924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nuno T, Castle PE, Harris R, et al. Breast and cervical cancer screening utilization among Hispanic women living near the United States-Mexico border. J Womens Health (Larchmt) 2011; 20(5): 685–693. [DOI] [PubMed] [Google Scholar]
- 43. Kolthoff SK, Hestbech MS, Jørgensen n KJ, et al. Do invitations for cervical screening provide sufficient information to enable informed choice? A cross-sectional study of invitations for publicly funded cervical screening. J R Soc Med 2016; 109(7): 274–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ell K, Vourlekis B, Muderspach L, et al. Abnormal cervical screen follow-up among low-income Latinas: project SAFe. J Womens Health Gend Based Med 2002; 11(7): 639–651. [DOI] [PubMed] [Google Scholar]
- 45. Johnson CE, Mues KE, Mayne SL, et al. Cervical cancer screening among immigrants and ethnic minorities. J Low Genit Tract Dis 2008; 12(3): 232–241. [DOI] [PubMed] [Google Scholar]
- 46. Morema EN, Atieli HE, Onyango RO, et al. Determinants of cervical screening services uptake among 18–49 year old women seeking services at the Jaramogi Oginga Odinga Teaching and Referral Hospital, Kisumu, Kenya. BMC Health Serv Res 2014; 14: 335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dhendup T, Tshering P. Cervical cancer knowledge and screening behaviors among female university graduates of year 2012 attending national graduate orientation program, Bhutan. BMC Womens Health 2014; 14: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wilson KL, Cowart CJ, Rosen BL, et al. Characteristics associated with HPV diagnosis and perceived risk for cervical cancer among unmarried, sexually active college women. J Cancer Educ 2018; 33(2): 404–416. [DOI] [PubMed] [Google Scholar]
- 49. Evangelista Rodrigues D, Alves Moreira KF, Souza de Oliveira T, et al. Barriers to prevention of cervical cancer in the city of Porto Velho, Rondônia, Brazil. Investig Y Educ En Enferm 2016; 34: 59–67. [DOI] [PubMed] [Google Scholar]
- 50. Nelson K, Geiger AM, Mangione CM. Effect of health beliefs on delays in care for abnormal. J Gen Intern Med 2002; 17(9): 709–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Arrossi S, Almonte M, Herrero R, et al. Psycho-social impact of positive human papillomavirus testing in Jujuy, Argentina results from the Psycho-Estampa study. Prev Med Reports 2020; 18: 101070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chorley AJ, Marlow LAV, Forster AS, et al. Experiences of cervical screening and barriers to participation in the context of an organised programme: a systematic review and thematic synthesis. Psychooncology 2017; 26(2): 161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Urrutia Soto MT, Araya A, Jaque MF. Why do Chilean women choose to have or not have pap tests? J Obstet Gynecol Neonatal Nurs 2017; 46: e3–e12. [DOI] [PubMed] [Google Scholar]
- 54. Liebermann EJ, VanDevanter N, Hammer MJ, et al. Social and cultural barriers to women’s participation in pap smear screening programs in low- and middle-income Latin American and Caribbean countries: an integrative review. J Transcult Nurs 2018; 29(6): 591–602. [DOI] [PubMed] [Google Scholar]
- 55. Godoy Y, Godoy C, Reyes J. Social representations of gynecologic cancer screening assessment a qualitative research on ecuadorian women. Rev Esc Enferm USP 2016; 50(Spec): 68–73. [DOI] [PubMed] [Google Scholar]
- 56. Olaza-Maguiña AF, De la Cruz-Ramirez YM. Barriers to the non-acceptance of cervical cancer screenings (pap smear test) in women of childbearing age in a rural area of Peru. Ecancermedicalscience 2019; 13: 901–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Urrutia Soto MT, Poupin L. Women with cervical cancer: perceptions about the Papanicolau test. Aquichan 2015; 15: 499–507. [Google Scholar]
- 58. Byrd TL, Chavez R, Wilson KM. Barriers and facilitators of cervical cancer screening among Hispanic women. Ethn Dis 2007; 17(1): 129–134. [PubMed] [Google Scholar]
- 59. Moore de, Peralta A, Holaday B, McDonell JR. Factors affecting Hispanic women’s participation in screening for cervical cancer. J Immigr Minor Health 2015; 17(3): 684–695. [DOI] [PubMed] [Google Scholar]