Abstract
Purpose
Ongoing symptoms and impairments in quality of life (QOL) among breast cancer survivors remain a significant problem. We tested the feasibility and acceptability of a manualized Ayurvedic nutrition and lifestyle intervention for breast cancer survivors.
Methods
Eligible participants had Stage I–III breast cancer, underwent treatment within the past year that included chemotherapy, and were without active disease. The 4-month individualized Ayurvedic intervention included counseling on nutrition, lifestyle, yoga, and marma (like acupressure) during 8 one-on-one visits with an Ayurvedic practitioner. Feasibility and acceptability were the primary outcomes. QOL (European Organization for Research and Treatment of Cancer Quality of Life Questionnaire [EORTC QLQ C30]) and symptoms—sleep disturbance (General Sleep Disturbance Scale [GSDS]), fatigue (Lee Fatigue Scale [LFS]), depressive symptoms (Center for Epidemiological Studies—Depression Scale [CES-D]), anxiety (Spielberger State-Trait Anxiety Inventory [STAI-S, STAI-T]), and stress (Perceived Stress Scale [PSS])—were measured prior to, at midpoint, and at the end of the 4-month intervention. Effect sizes (Cohen’s d) were calculated along with paired t tests comparing baseline to end of month 4 time points. Mixed effects models were used for repeated measures analyses.
Results
Participants (n = 32) had a mean age of 48 years (SD = 10). Retention at the end of the intervention was 84%. Among those who completed the intervention (n = 27), adherence was high (99.5% of visits with practitioners attended). Large improvements were seen in QLQ-C30 emotional functioning (d = 0.84, P < 0.001), QLQ-C30 cognitive functioning (d = 0.86, P < 0.001), GSDS (d = –1.23, P < 0.001), and CES-D (d = –1.21, P < 0.001). Moderate improvements were seen in QLQ-C30 global health (d = 0.65, p = 0.003), LFS (d = –0.68, P = 0.002), and PSS (d = –0.75, P < 0.001). No adverse events were observed due to the intervention.
Conclusion
This 4-month Ayurvedic whole-systems multimodal nutrition and lifestyle intervention was feasible and acceptable for breast cancer survivors. Promise of clinical benefit was seen in terms of improvements in symptoms and QOL that warrants further investigation.
Keywords: Ayurvedic Medicine, nutritional therapy, cancer survivorship, breast cancer, yoga, integrative medicine, complementary therapies
Introduction
Breast cancer is the leading cancer diagnosis among women.1 In 2018, 30,000 cases of breast cancer were reported in the United States alone, and nearly 2.1 million women were diagnosed worldwide.2 Breast cancer survival rates have improved over the years, likely due to advancements in screening, awareness, and treatment.3 Currently, an estimated 3.8 million women in the United States are breast cancer survivors.4
Addressing the needs of an increasing breast cancer survivor population is important and has proven challenging.5 Numerous symptoms often persist years after the completion of treatment.6–8 These symptoms include fatigue,9–11 sleep disturbance,12,13 anxiety, and depression,14 along with impaired cognitive functioning,15 that can be disabling and adversely impact quality of life (QOL).16,17 Despite growing evidence that a patient’s psychosocial health outcome can be improved through effective interventions,14 and encouragement by the National Academy of Medicine (NAM) for health care providers to address these growing health needs, survivors continue to describe the paucity of care after the completion of primary cancer treatment.18,19
While Survivorship Care Plans (SCPs) have been encouraged by the NAM and professional societies to address the paucity of care,20,21 evidence-based studies on SCPs show varying results,22,23 with either no benefits or benefits that last for a short duration.24,25 While patients may respond positively to elements of SCPs, such as satisfaction with the information received,24 SCPs do not address symptoms or QOL outcomes.23 Therefore, the investigation of other approaches that address symptoms and QOL may prove complementary to SCPs.
Due in part to the persistence of symptoms and impairments in QOL, up to 86% of breast cancer survivors utilize complementary and integrative medicine (CIM) approaches,26,27 which include acupuncture,28,29 yoga,30–32 mindfulness-based stress reduction,33 diet,34 and exercise.35 While CIM approaches have shown promise in improving symptoms and QOL, there is currently no standardized approach. Moreover, studies of individualized, multimodality CIM interventions are lacking.
With the long-term goal of addressing cancer survivor symptoms and QOL impairments, we developed, using a modified Delphi method,36,37 a whole-systems multimodality nutrition and lifestyle approach based on Ayurvedic Medicine.38 Originating from South Asia, Ayurveda (meaning “the science of life”) is a comprehensive whole system of medicine that aims to promote health and healing.39 Ayurveda maintains a guiding principle in attaining health through maintaining or restoring physiologic homeostasis.39 Ayurvedic theory states that each individual has an in-born mind–body constitution (called prakruti) that is described by 3 physiologic principles (called doshas).40 These 3 doshas can become imbalanced, which is an illness state called vikruti.39 In the case of vikruti, the goal is to restore the balance of the doshas to the prakruti state (ie, physiologic homeostasis) through nutrition, lifestyle, and other treatment approaches.39 Digestion, absorption, and metabolism (called agni) are key concepts in Ayurvedic nutrition. Posttreatment cancer survivors who are in a vikruti (imbalanced) state and have problems with agni (digestion and metabolism) may benefit from an Ayurvedic nutrition and lifestyle approach.38 However, this has not previously been studied.
Given the inconsistencies and gaps in care for survivors, as well as a lack of consensus on best approaches, we tested the feasibility and acceptability of a 4-month whole systems, individualized, multimodality nutrition and lifestyle approach, based on Ayurvedic Medicine, for breast cancer survivors. We hypothesized that this traditional Ayurvedic approach would be feasible and acceptable and show promise of clinical benefit.
Methods
Study Design
We conducted a single-arm pilot clinical trial of a manualized38,41 4-month nutrition and lifestyle intervention based on Ayurvedic Medicine. The study was designed to assess the feasibility and acceptability of the manualized Ayurvedic intervention. The participants were enrolled in 3 cohorts. The feasibility and acceptability data from each cohort were used to evaluate the manualized intervention and study procedures in order to make modifications, if indicated. The study was approved by the University of California, San Francisco (UCSF) Institutional Review Board (IRB), and written informed consent was obtained from all the participants.
Study Population
Women with Stage I–III breast cancer, who were without active disease, and within 1 to 12 months of completing primary treatment (chemotherapy, radiation therapy, or surgery, whichever was the last received) were eligible. Chemotherapy in the adjuvant or neoadjuvant setting was required. The participants had to be 18 years or older, had a Karnofsky Performance Status42 score of greater than 60, be able to read and write English, and be able to give informed consent. Exclusion criteria included receiving chemotherapy or radiation at the time of enrollment (trastuzumab was allowed), less than 1 month from surgery for breast cancer (including reconstructive surgery), receipt of adjuvant hormone therapy for less than 1 month prior to enrollment, and having received Ayurvedic treatment within 2 months of study enrollment.
Participants were recruited from the breast oncology practice at the UCSF Helen Diller Family Comprehensive Cancer Center, the breast oncology practice at Zuckerberg San Francisco General Hospital, other oncology practices in the greater San Francisco Bay Area, the UCSF Osher Center for Integrative Medicine, IRB approved mailings to patients, fliers, and clinicaltrials.gov.
Study Intervention
The Ayurvedic intervention in this study was based on the traditional Ayurvedic practice of swastha vritta (attaining and maintaining well-being).39,40 Swastha vritta includes a focus on ahara and vihara (nutrition, lifestyle, yoga, and marma).39,40 In Ayurvedic clinical practice, the focus of swastha vritta matches with the needs of cancer survivors, posttreatment, to regain homeostasis and well-being.
The study followed the protocol described in the treatment manual that was developed from a prior study by our group utilizing a Delphi approach.43 The Ayurvedic intervention was delivered by 2 Ayurvedic practitioners. One had a Bachelor’s in Ayurvedic Medicine and Surgery, which is a 6-year graduate degree earned at an Ayurvedic university in India, and more than 10 years of clinical experience as an Ayurvedic practitioner. The other practitioner was trained in Ayurveda in the United States in a 1500-h training program and had over 20 years of clinical experience as an Ayurvedic practitioner. Both had experience working with cancer patients, including having worked with more than 50 cancer patients in their career.
During the 4-month intervention, the participants attended a total of 8 one-on-one clinic visits with an Ayurvedic practitioner that focused on a whole systems, individualized, multimodality intervention comprised of nutrition, lifestyle, yoga, and marma44 (similar to acupressure) treatment. The manual-directed intervention consisted of a total of 9 intervention hours that included an initial 120-min consultation, during which the Ayurvedic diagnoses were made and the corresponding individualized treatment plan was created and implemented (Supplementary Figure 1). The remaining 7 sessions were each 60-min follow-ups in which counseling (education and prescriptions) was provided for Ayurvedic nutrition, lifestyle, yoga, and/or marma. The Ayurvedic diagnoses were reevaluated at each of the follow-up visits, resulting in modifications to the treatment plan, if indicated. The Ayurvedic diagnoses included prakruti (constitution) and vikruti (current imbalance). Both are defined by the relative proportions of the 3 doshas, which are vata (principle of movement), pitta (principle of transformation), and kapha (principle of structure). Additionally, the diagnoses of the state of agni and ama (digestion and metabolism) and dhatus and srotas (body systems) were made.
The Ayurvedic intervention components—nutrition, lifestyle, yoga, and marma—were individualized based on the Ayurvedic diagnoses (Supplementary Figure 1). Based on these diagnoses, specific nutrition guidance was provided that included particular foods to emphasize or limit. Lifestyle recommendations were provided for daily and seasonal routines; stage of life; for planning sleep-times, wake-times, and mealtimes; and relaxation. Components of yoga, which were prescribed based on the Ayurvedic diagnoses, included postures, breathing, and meditation and were taught in a gentle and simple manner. Marma, known as healing touch, utilizes 108 points along srotas (channels), analogous to Traditional Chinese Medicine acupuncture points and meridians but without the use of needles. Marma, which was based on the Ayurvedic diagnoses, was administered by delivering gentle pressure to specific points on the body by the Ayurvedic practitioner during study visits.
In addition to attending clinic visits with Ayurvedic practitioners, the participants engaged in home practice. They were asked to incorporate nutrition and lifestyle recommendations into their daily routines at home. They were taught and given yoga and marma regimens to follow on their own, on a daily basis. Adherence to these home practices was measured using approaches described below in the study measures section.
Study Measures
Feasibility and acceptability were assessed through recruitment (ie, number screened), enrollment (ie, reaching enrollment benchmarks, time to enroll), retention (ie, number of participants completing the study; benchmark >80%), and adherence. Adherence to the study intervention was evaluated based on the percentage of Ayurvedic clinical visit attendance (benchmark >80%) and according to home practice of the 4 main intervention components–nutrition, lifestyle, yoga, and marma. Adherence to each of these components was assessed from the participants using a 0–10 rating scale (with 0 representing no adherence and 10 representing full adherence to all recommendations). The participants were sent weekly text messages and were asked to rate their adherence considering their performance during the previous 24-h window. Over the 4-month intervention period, all participants were asked about adherence, time, and effort devoted to each component of the intervention. The intervention was evaluated for fidelity by direct observation and recordings of a subset of sessions with participants and compared to the study manual.
The patient-reported outcomes (PROs) that were collected included symptom and QOL outcomes. Among the QOL outcomes were global health status, functional scales, symptom scales, and a measure of spiritual well-being. Symptoms of interest included sleep disturbance, fatigue, depressive symptoms, anxiety, and perceived stress. These PROs were collected to determine the promise of clinical benefit of the intervention. These are described in detail below.
The European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ C30)45 was used to assess QOL, in conjunction with the breast cancer specific module (BR23). The EORTC QLQ C30 is a 30-item cancer-specific measure with 3 multi-item scales: global health status/QOL; functional scales (physical, role, cognitive, emotional, and social); and symptom scales (fatigue, nausea and vomiting, pain, dyspnea, insomnia, appetite loss, constipation, diarrhea, and financial difficulties).46 The BR23 includes 2 scales: functional scales (body image, sexual functioning, sexual enjoyment, and future perspective) and symptom scales (systemic therapy side effects, breast symptoms, arm symptoms, and upset over loss of hair).46 Higher scores for functional scales indicate healthier levels of functioning, while higher scores for symptoms scale represent more severe symptoms. These validated, cancer-specific instruments were chosen because they are brief and evaluate dimensions that may be impacted by a holistic intervention, such as the one in this study. The EORTC QLQ C30 B23 was administered at baseline (BL), and at the end of weeks 4, 8, 12, and 16.
Sleep disturbance was measured by the 21-item General Sleep Disturbance Scale (GSDS) that has been utilized in studies with cancer patients and has well-established reliability and validity.47,48 Each item is rated on a 0–7 (0 = never to 7 = daily) numeric rating scale (NRS). The GSDS total score represents a global measure of sleep, while the 7 subscales capture specific dimensions of sleep (quality of sleep, quantity of sleep, sleep onset latency, midsleep awakenings, early awakenings, medications for sleep, and excessive daytime sleepiness). The GSDS total score ranges from 0 to 147 (0 = no sleep disturbance, 147 = extreme sleep disturbance), with a cutoff score between good and poor sleepers at 43.49 Subscale items have narrower score ranges, but the same directionality (higher numbers represent poorer sleep). The GSDS was administered at BL, midpoint (at the end of week 8), and at the end of the study (at the end of week 16).
The 18-item Lee Fatigue Scale (LFS) was used to measure fatigue and energy.50–52 Each item is rated on a 0–10 NRS. The mean of the 13 items for fatigue and 5 items for energy was calculated. For fatigue, a higher score represents greater fatigue (cutoff ≥4.4). For energy, higher scores represent better energy levels (cutoff ≤ 4.8). The LFS was administered at BL, midpoint (at the end of week 8), and at the end of the study (at the end of week 16).
Depressive symptoms were measured using the 20-item Center for Epidemiological Studies—Depression Scale (CES-D)53,54 that assesses real-time levels of depressive symptoms. A total summary score ranges from 0 to 60, with a score of ≥16 indicating the need for diagnostic evaluation for major depression. The CES-D was administered at BL, midpoint (at the end of week 8), and at the end of study (at the end of week 16).
Symptoms of anxiety were measured using the Spielberger State-Trait Anxiety Inventory (STAI-S, STAI-T).55 Each measure consisted of 20 items that were summed and total scores range from 20 to 80, with a higher score indicating greater anxiety. The cutoff scores for State and Trait anxiety are ≥31.8 and ≥32.2, respectively. State anxiety56 (STAI-S) questions measured a participant’s transitory emotional responses to current stressful situations. The STAI-S was administered at BL, midpoint (at the end of week 8), and the end of study (at week 16). The STAI-T assesses an individual’s inherent predisposition to anxiety according to their personality and is considered consistent over time. It was administered at BL.
The Perceived Stress Scale (PSS)57 is a 10-item scale rating that evaluates the frequency of feelings and thoughts related to general stress (0 = never to 4 = very often). A higher summed score, which ranges from 27 to 40, is considered higher perceived stress. The PSS was administered at BL, midpoint (at the end of week 8), and at the end of study (at week 16).
Spiritual well-being was measured using the 12-item Functional Assessment of Chronic Illness Therapy—Spiritual Well-Being (FACIT-SP-12)58,59 that evaluates a sense of meaning, peace, and the role of faith in illness. Higher scores reflect higher well-being. This measure was administered at BL and at the end of study (at week 16).58
Participants were additionally asked questions pertaining to their expectations for the intervention. This measure was adapted from Kalauokalani et al.60 Each of the 3 items on the questionnaire was rated on a 0 (not at all helpful) to 10 (extremely helpful) scale. The following expectation questions were asked at BL: BL expectancy question 1 asked how helpful the participant thought the intervention would be for them; BL expectancy question 2 asked how helpful the participants believed the intervention would be for their QOL; and BL expectancy question 3 asked how helpful the participants believed the intervention would be for their cancer treatment-associated symptoms.
Analysis
An intent-to-treat approach was utilized. The data were used to assess the strengths and weaknesses of the study protocol and Ayurvedic treatment manual and to inform subsequent studies. The key benchmarks for feasibility and acceptability were prespecified as reaching an 80% retention rate and at least 80% adherence with Ayurvedic clinic visits among those retained.
Descriptive statistics were used to summarize demographic, health history, and adherence parameters. Paired t tests were used to evaluate for differences between BL and end of week 16 time points, along with effect sizes (Cohen’s d) for all PROs. Additionally, mixed effects models were used for repeated measures analyses to incorporate all measurement time points for all PROs and to stay as true as possible to an intent-to-treat approach. Maximum likelihood estimation of linear mixed effects models allows all available data to be used and effectively manages missingness under missing at random assumptions.61 In these models, time was used as the predictor variable, and the individual scale scores for each of the PRO measures was the outcome variable (one model for each PRO). Models included a random person effect to account for the correlation of repeated measures. Estimated means for the measures of interest at each study time point, along with their 95% confidence intervals, were derived from the mixed effects models.
Two additional sets of mixed effects models were run: first to see if there were differences between the 3 cohorts, which were enrolled in 3 separate waves with minor protocol modifications between the waves. A cohort indicator was used as a covariate to assess whether observed effects were moderated by cohort. A second set of adjusted models included BL expectations (one set of models for each of the 3 expectation questions) as covariates, to assess whether expectations at BL moderated the impact of the intervention on PROs.
Our choice of sample size was determined by several factors. The key consideration was the ability to assess feasibility and acceptability. Enrolling 32 participants who each underwent 8 visits yielded a total of 256 visits across which we could assess adherence. Other considerations included cost relative to available funding, time available for recruitment, and that this pilot study was the first implementation of an intervention of this kind in this patient population. Determining acceptability also influenced the choice of sample size. We needed sufficient participants to reach saturation for qualitative data (reported elsewhere) and to determine the promise of clinical benefits of the intervention, based on the PROs.
Results
Participant Flow
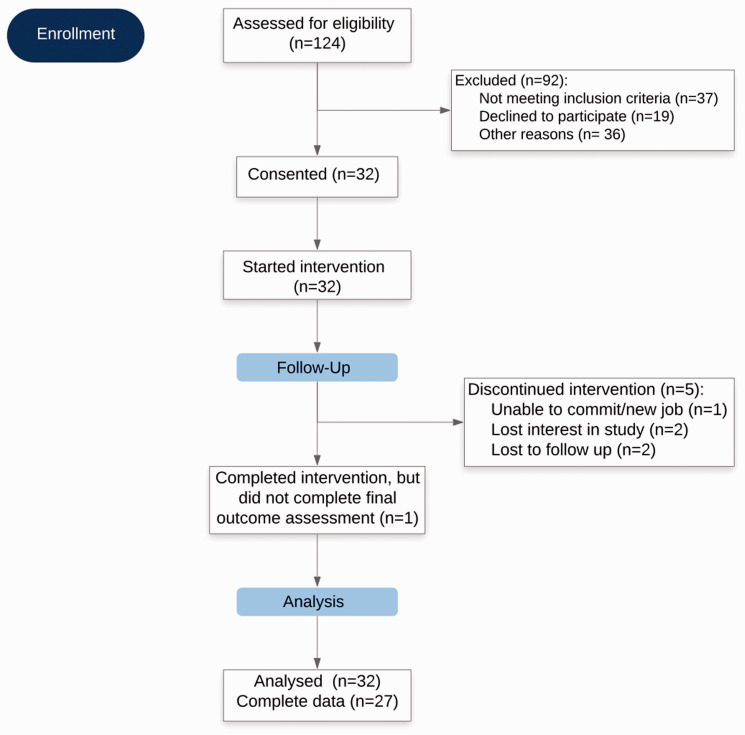
Of the 124 women who initially expressed interest and were screened, 92 were excluded (Figure 1). Of the 92 who were excluded, 37 women did not meet eligibility criteria for the following reasons: more than 1 year since completing treatment (n = 15), had not received chemotherapy (n = 10), receiving ongoing cancer treatment at the time of screening (n = 9), receiving hormone therapy for <2 months at the time of screening (n = 2), or recent exposure to Ayurveda (n = 1). Nineteen declined to participate due to logistical challenges including distance (n = 9), time commitment (n = 6), and/or lack of motivation to change diet and lifestyle practices (n = 4). Those individuals who initially expressed interest but who could not be reached thereafter were categorized as “unknown” (n = 36). Of the 32 participants who consented and began the intervention, 5 dropped out or were lost to follow-up. The remaining 27 (85%) participants completed the full intervention.
Figure 1.
The CONSORT Flow Diagram.
Study Sample
The mean age of the participants was 48 years (Table 1). Participants were well educated, and most were employed during the time of the intervention (91%; n = 29). The majority of the participants had Stage II or III breast cancer (75%, n = 29). Seventy-eight percent had received radiation (n = 25) and 53% (n = 17) had received adjuvant chemotherapy (the rest received neoadjuvant chemotherapy).
Table 1.
Demographic and Clinical Characteristics.
| Characteristic | Level | % | a |
|---|---|---|---|
| Age at enrollment (years) | 18–40 | 21.9 | 7 |
| 41–50 | 43.8 | 14 | |
| 51–60 | 25.0 | 8 | |
| >60 | 9.4 | 3 | |
| Race | White | 75.0 | 24 |
| Black or African-American | 6.3 | 2 | |
| Asian | 9.4 | 3 | |
| Native Hawaiian or Pacific Islander | 3.1 | 1 | |
| >1 Race | 6.3 | 2 | |
| Hispanic | 12.5 | 4 | |
| Non-Hispanic | 87.5 | 28 | |
| Marital status | Single | 37.5 | 12 |
| Married | 53.1 | 17 | |
| Divorced | 3.1 | 1 | |
| Widowed | 3.1 | 1 | |
| Unmarried partners | 3.1 | 1 | |
| Education | Some college | 12.5 | 4 |
| BA degree | 34.4 | 11 | |
| Advanced degree | 50 | 16 | |
| Did not respond | 3.1 | 1 | |
| Employment status | Currently employed | 90.6 | 29 |
| Unknown | 9.4 | 3 | |
| BMI at enrollment | <18—underweight | 0 | 0 |
| 18.5 to 24.9—normal weight | 57.1 | 16 | |
| 25 to 29.9—overweight | 25.0 | 7 | |
| 30—obese | 17.9 | 5 | |
| Frequency missing BMI | 4 | ||
| Cancer stage | IA | 6.25 | 2 |
| IB | 3.1 | 1 | |
| IIA | 40.6 | 13 | |
| IIB | 25 | 8 | |
| IIIA | 18.8 | 6 | |
| IIIB | 3.1 | 1 | |
| IIIC | 3.1 | 1 | |
| Time since diagnosisb | 12 months | 62.5 | 20 |
| 13–24 months | 31.3 | 10 | |
| 25–48 months | 6.3 | 2 | |
| Treatment history: radiation | Yes | 78.1 | 25 |
| No | 21.9 | 7 | |
| Treatment history: surgical procedure | Mastectomy | 56.3 | 18 |
| Lumpectomy | 56.3 | 18 | |
| Lymph node dissection | 90.6 | 29 | |
| Full axillary | 21.9 | 7 | |
| Sentinel node | 68.8 | 22 | |
| Treatment history: chemotherapy | |||
| Type | Neoadjuvant | 37.5 | 12 |
| Adjuvant | 56.3 | 18 | |
| Dose-dense | 46.9 | 15 | |
| Regular | 31.3 | 10 | |
| Drug | Adriamycin | 65.6 | 21 |
| Cyclophosphamide | 65.6 | 21 | |
| Paclitaxel | 65.6 | 21 | |
| Docetaxel | 15.6 | 5 | |
| Other | 28.1 | 9 |
Abbreviations: BMI, body mass index.
32 study participants were enrolled; 27 study participants completed intervention.
Time since diagnosis and signed consent.
Study Outcomes
Feasibility and acceptability
Screening and recruitment occurred over 32 months - divided into 3 distinct periods (one for each cohort) between March 2012 and December 2015. Twenty-seven participants (84%) completed the study intervention, which exceeded our retention benchmark (>80%). Among those retained, nearly 100% attendance in clinic visits with the Ayurvedic practitioner was achieved (1 participant missed 1 clinic visit with the practitioner; 215/216 visits attended [99.5%]), which exceeded our other adherence benchmark (>80%).
The participant’s mean self-reported adherence on a 0–10 scale to nutrition was 6.9 (SD = 1.8) and lifestyle was 6.6 (SD = 2.0). Adherence was rated somewhat lower for yoga (4.6, SD = 3.0) and marma (5.8, SD = 2.8).
Patient-reported outcomes
The summary of QOL outcomes (EORTC QLQ-C30 and BR23) across all time points are shown in Table 2. Large effect sizes reflecting improvements over time were seen in emotional functioning (d = 0.84, P < 0.001) and cognitive functioning (d = 0.86, P < 0.001). Medium effect sizes reflecting improvements over time were seen in global health (d = 0.65, P = 0.003), social functioning (d = 0.47, P = 0.024), fatigue (d = –0.57, P = 0.007), insomnia (d = –0.52, P = 0.014), sexual enjoyment (d = 0.73, P = 0.18), and arm symptoms (d = –0.57, P = 0.008). Small effect sizes, most of which were not statistically significant, were seen in physical functioning (d = 0.32, P = 0.11), role functioning (d = 0.23, P = 0.25), nausea and vomiting (d = –0.06, P = 0.76), dyspnea (d = –0.26, P = 0.19), appetite loss (d = –0.05, P = 0.80), constipation (d = –0.18, P = 0.38), diarrhea (d = –0.27, P = 0.18), financial difficulties (d = –0.25, P = 0.22), body image (d = 0.48, P = 0.022), sexual functioning (d = 0.10, P = 0.62), future perspective (d = 0.48, P = 0.022), systematic therapy side effects (d = –0.40, P = 0.055), and breast symptoms (d = –0.17, P = 0.40).
Table 2.
Summary of Outcome Measures for QOL.
| Outcome Measurea | Baseline Mean (SD) | Month 1 Mean (SD) | Month 2 Mean (SD) | Month 3 Mean (SD) | Month 4 Mean (SD) | Effect Sizeb | 95% CI for Effect Size | P Valuec |
|---|---|---|---|---|---|---|---|---|
| EORTC QLQ-30 | ||||||||
| Global health | ||||||||
| Global health status | 64.3 (16.0) | 65.5 (21.5) | 67.7 (17.8) | 69.1 (13.2) | 78.2 (12.9) | 0.65 | (0.3, 1.1) | 0.003 |
| Functional scales | ||||||||
| Physical function | 87.1 (12.1) | 87.6 (15.7) | 91.1 (7.5) | 94.1 (7.5) | 91.5 (13.4) | 0.32 | (–0.1, 0.7) | 0.11 |
| Role functioning | 79.9 (19.8) | 77.0 (25.0) | 79.6 (26.5) | 85.9 (14.8) | 87.2 (20.7) | 0.23 | (–0.2, 0.6) | 0.25 |
| Emotional functioning | 65.5 (18.5) | 71.3 (15.4) | 73.2 (16.0) | 76.1 (15.7) | 78.5 (12.3) | 0.84 | (0.4, 1.3) | <0.001 |
| Cognitive functioning | 69.1 (18.9) | 74.7 (23.8) | 79.9 (23.0) | 79.6 (18.5) | 81.7 (18.0) | 0.86 | (0.5, 1.3) | <0.001 |
| Social functioning | 75.0 (22.4) | 75.3 (27.3) | 79.9 (22.5) | 86.8 (14.5) | 89.1 (15.6) | 0.47 | (0.1, 0.9) | 0.024 |
| Symptom scales | ||||||||
| Fatigue | 37.6 (21.4) | 34.1 (24.5) | 26.9 (14.3) | 26.1 (17.0) | 25.0 (15.7) | –0.57 | (–1.0, –0.2) | 0.007 |
| Nausea and vomiting | 7.5 (12.6) | 4.0 (10.6) | 6.0 (10.6) | 4.8 (9.1) | 7.1 (15.8) | –0.06 | (–0.5, 0.3) | 0.76 |
| Pain | 21.5 (21.3) | 23.0 (23.7) | 22.2 (23.4) | 16.9 (15.5) | 14.7 (16.6) | –0.44 | (–0.8, –0.03) | 0.035 |
| Dyspnea | 14.1 (16.5) | 12.6 (18.7) | 6.9 (13.8) | 6.1 (13.2) | 8.3 (17.2) | –0.26 | (–0.7, 0.1) | 0.19 |
| Insomnia | 43.8 (24.6) | 34.5 (24.4) | 35.6 (21.2) | 36.4 (22.8) | 30.1 (25.8) | –0.52 | (–0.9, –0.1) | 0.014 |
| Appetite loss | 9.6 (17.0) | 6.9 (16.4) | 6.9 (17.0) | 1.5 (7.1) | 7.7 (27.2) | –0.05 | (–0.5, 0.4) | 0.80 |
| Constipation | 15.6 (19.0) | 18.4 (22.9) | 9.7 (18.3) | 9.6 (15.1) | 11.5 (18.7) | –0.18 | (–0.6, 0.2) | 0.38 |
| Diarrhea | 7.6 (14.0) | 4.6 (11.7) | 8.3 (14.7) | 6.1 (16.7) | 3.9 (10.9) | –0.27 | (–0.7, 0.1) | 0.18 |
| Financial difficulties | 31.8 (33.4) | 32.2 (33.9) | 25.0 (31.5) | 36.9 (35.0) | 28.2 (32.2) | –0.25 | (–0.7, 0.2) | 0.22 |
| BR23 | ||||||||
| Body image | 67.6 (28.0) | 72.7 (25.7) | 74.7 (24.0) | 75.9 (21.0) | 79.3 (22.6) | 0.48 | (0.1, 0.9) | 0.022 |
| Sexual functioning | 25.8 (23.3) | 24.7 (19.2) | 24.3 (15.5) | 24.2 (20.4) | 24 (19.5) | 0.10 | (–0.3, 0.5) | 0.62 |
| Sexual enjoyment | 56.9 (36.8) | 50.0 (29.8) | 59.5 (23.3) | 51.5 (27.3) | 63.3 (33.1) | 0.73 | (–0.5, 2.0) | 0.18 |
| Future perspective | 49.0 (22.4) | 59.8 (22.5) | 54.2 (23.7) | 51.5 (28.6) | 61.5 (15.5) | 0.48 | (0.07, 0.9) | 0.022 |
| Systemic therapy side effects | 15.4 (9.6) | 12.6 (11.6) | 12.2 (9.7) | 14.2 (11.8) | 11.6 (12.0) | –0.40 | (–0.8, 0.01) | 0.055 |
| Breast symptoms | 20.7 (15.3) | 21.6 (23.4) | 23.3 (20.1) | 15.7 (13.8) | 19.9 (20.3) | –0.17 | (–0.6, 0.2) | 0.40 |
| Arm symptoms | 20.5 (17.4) | 17.2 (16.7) | 17.1 (18.8) | 12.6 (14.3) | 12.0 (13.3) | –0.57 | (–1.0, –0.2) | 0.008 |
| Upset by hair loss | 33.3 (n/a)d | 0.0 (0.0)e | 50.0 (57.7) | 33.3 (0.0) | 16.7 (23.6) | (n/a)d | (n/a)d | (n/a)d |
Abbreviations: CI, confidence interval; EORTC QLQ-30, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire.
Increasing scores on functional scales represent improvements in function, and decreasing symptom scores represent improvements in symptoms.
Effect size: baseline to month 4.
P value: baseline to month 4; P value for t test, level of significance < 0.05.
n/a could not be calculated due to insufficient data; this was a conditional question asked only of those who suffered hair loss during time of intervention.
Skip pattern survey item with only limited responses.
The summary of symptom outcomes across all time points is shown in Table 3. Large effect sizes reflecting improvements over time were seen in total sleep disturbance (GSDS) (d = –1.23, P < 0.001), quality of sleep (GSDS) (d = –1.16, P < 0.001), sleep onset latency (GSDS) (d = –1.17, P < 0.001), midsleep wakes (GSDS) (d = –0.93, P < 0.001), early awakenings (GSDS) (d = –0.84, P < 0.001), excessive daytime sleepiness (GSDS) (d = –0.93, P < 0.001), depressive symptoms (CES-D) (d = –1.21, P < 0.001), and spiritual well-being (FACIT)—peace (d = 0.86, P < 0.001). Medium effect sizes reflecting improvements over time were seen in quantity of sleep (GSDS) (d = –0.73, P = 0.005), medications for sleep (GSDS) (d = –0.50, P = 0.043), energy (LFS) (d = 0.54, P = 0.011), fatigue (LFS) (d = –0.68, P = 0.002), state anxiety (STAI) (d = –0.75, P < 0.001), stress (PSS) (d = –0.75, P < 0.001), and spiritual well-being—meaning (FACTIT-SP-12) (d = 0.50, P = 0.020). A small effect size was seen in spiritual well-being—faith (FACTIT-SP-12) (d = 0.33, P = 0.11)
Table 3.
Summary of Symptom Outcome Measures.
| Outcome Measuresa | Baseline Mean (SD) | Month 2 Mean (SD) | Month 4 Mean (SD) | Effect Sizeb | 95% CI for Effect Size | P Valuec |
|---|---|---|---|---|---|---|
| Total sleep disturbance (GSDS) | 61.4 (16.4) | 46.9 (21.5) | 40.8 (17.1) | –1.23 | (–26.5, –11.6) | <0.001 |
| Quality of sleep | 13.2 (4.9) | 10.7 (5.6) | 8.1 (4.3) | –1.16 | (–7.6, –3.7) | <0.001 |
| Quantity of sleep | 4.8 (2.2) | 4.0 (2.4) | 3.0 (2.0) | –0.73 | (–2.8, –0.6) | 0.005 |
| Sleep onset latency | 3.4 (2.1) | 2.7 (2.0) | 1.2 (1.3) | –1.17 | (–1.6, –0.8) | <0.001 |
| Midsleep wakes | 5.8 (1.8) | 4.8 (2.2) | 3.7 (2.1) | –0.93 | (–1.3, –0.5) | <0.001 |
| Early awakenings | 4.9 (2.0) | 3.0 (2.6) | 2.6 (2.4) | –0.84 | (–1.3, –0.4) | <0.001 |
| Medications for sleep | 13.4 (9.2) | 10.8 (11.7) | 9.3 (9.9) | –0.50 | (–5.4, –0.1) | 0.043 |
| Excessive daytime sleepiness | 19.2 (8.8) | 12.8 (7.6) | 12.0 (7.0) | –0.93 | (–10.4, –3.3) | <0.001 |
| Energy (LFS) | 4.4 (2.1) | 4.0 (1.8) | 5.7 (1.9) | 0.54 | (0.1, 1.0) | 0.011 |
| Fatigue (LFS) | 4.0 (2.6) | 3.4 (2.5) | 2.1 (2.1) | –0.68 | (–1.1, –0.3) | 0.002 |
| Depressive symptoms (CES-D) | 15.8 (8.8) | 8.7 (8.1) | 6.6 (6.4) | –1.21 | (–1.6, –0.9) | <0.001 |
| State anxiety (STAI) | 36.5 (12.6) | 33.5 (9.7) | 29.1 (9.5) | –0.75 | (–1.2, –0.4) | <0.001 |
| Stress (PSS) | 16.5 (6.6) | 11.1 (9.3) | 10.4 (7.4) | –0.75 | (–1.1, –0.4) | <0.001 |
| Spiritual well-being (FACIT) | ||||||
| Meaning | 12.9 (3.0) | 14.6 (2.0) | 0.50 | (0.1, 0.9) | 0.020 | |
| Peace | 9.8 (3.9) | 12.1 (3.2) | 0.86 | (0.5, 1.3) | <0.001 | |
| Faith | 8.9 (5.1) | 10.6 (5.0) | 0.33 | (–0.1, 0.7) | 0.11 |
Abbreviations: CES-D, Center for Epidemiological Studies—Depression Scale; CI, confidence interval; FACIT, Functional Assessment of Chronic Illness Therapy; GSDS, General Sleep Disturbance Scale; LFS, Lee Fatigue Scale; PSS, Perceived Stress Scale; STAI, Spielberger State-Trait Inventory.
Decreasing symptom scores represent improvements in symptoms. Increases in energy and spiritual well-being represent improvements in spiritual well-being.
Baseline to month 4.
Baseline to month 4; P value for t test, level of significance ≤ 0.05.
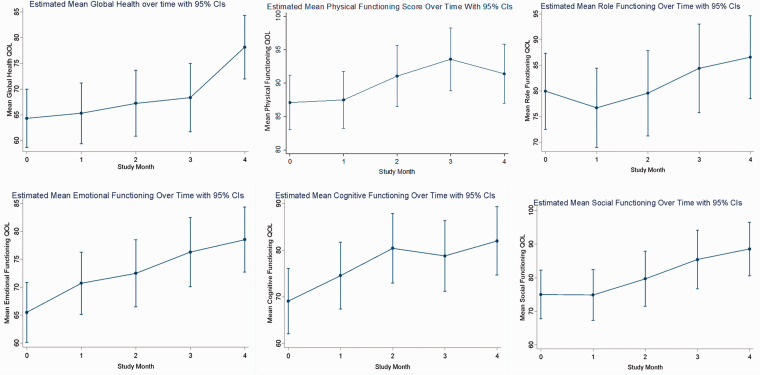
Graphs of marginal predicted means from the mixed effects models for QOL and symptoms are plotted graphically with marginal predicted means shown in Figure 2. The corresponding repeated measures analyses can be found in Supplementary Tables 1 to 3. Improvements from BL to final assessment were seen with positive slopes in QOL global health, emotional functioning, and cognitive functioning, as well as energy (LFS) and spiritual well-being—peace (FACIT). Improvements in sleep disturbance (GSDS total), fatigue (LFS), depressive symptoms (CES-D), state anxiety (STAI), and perceived stress (PSS) were seen, indicated by the favorable direction of the negative slope. Most outcomes showed gradual improvements over the course of the 4-month intervention and had not plateaued by month 4 (global health, role functioning, emotional functioning, social functioning, sleep, fatigue, and anxiety). Some outcomes appear to have plateaued by the end of month 4—physical functioning and cognitive functioning.
Figure 2.
Graphs of Marginal Predicted Means Compared at Baseline to Month 2 and Month 4.
Abbreviations: CIs, confidence intervals; QOL, quality of life.
Based on participant input, minor modifications were made to study procedures and manualized intervention between cohorts. For example, after cohort 1, self-reported adherence was measured through text messaging instead of from paper study logbooks. Similarly, after cohort 1, detailed yoga and marma prescriptions were provided to participants to help with implementation of these practices at home. QOL and symptom models including cohort as a covariate found no evidence of cohort effects (data not shown).
Models including participant expectations as covariates also did not find statistically significant effects of expectations on QOL and symptom outcomes, except for BR23 systematic therapy side effects (P = 0.012, P = 0.005, P = 0.002 for expectation questions 1, 2, and 3, respectively). The means of self-rated expectations of benefit from the intervention at BL were 8.2 (SD = 1.6) for overall benefit, 8.2 (SD = 1.7) for expectation of improvement in QOL, and 8.0 (SD = 1.8) for expectation of improvement in cancer treatment-associated symptoms, on the 0–10 expectation scales.
Discussion
This study is the first to test the feasibility, acceptability, and promise of clinical benefit of a multimodality, whole-systems Ayurvedic nutrition and lifestyle intervention for breast cancer survivors. The results from this pilot clinical trial support the feasibility and acceptability of this 4-month intervention in these women.
Our feasibility and acceptability benchmarks were met or exceeded. We screened and recruited participants in a timely manner and met enrollment benchmarks. Of the 124 who were screened, 92 (74%) were excluded, but most (40%) were due to eligibility requirements. Receipt of chemotherapy was an inclusion criterion because these patients are more likely to have residual symptoms and impairments in QOL.62,63 Of the participants who enrolled, there was high retention (84%, n = 27 of 32). Study visit attendance among those retained was nearly 100%, which is an indication of strong adherence and participant engagement throughout the intervention despite the fact that 91% were employed and working at the time of study entry. Our results for retention matched or exceeded those seen in other studies of similar interventions in this patient population, and our results for adherence exceeded that reported in other studies of similar interventions in this patient population.64–66
The improvements seen in QOL and symptoms suggest the promise of clinical benefit of the Ayurvedic intervention. Our hypothesis is that the holistic, individualized, and multimodality aspects of the Ayurvedic intervention contributed to these improvements. Meaningful improvements, as evidenced by medium to large effect sizes, were seen in different dimensions of QOL including global health, emotional functioning, cognitive functioning, and spiritual well-being—peace, as well as a number of symptom measures including energy, fatigue, depressive symptoms, anxiety, stress, and sleep disturbance. These findings, if supported by future randomized studies, suggest that some of the largest improvements cluster around psychological and emotional well-being, which potentially address an area often neglected in cancer survivorship.67–69
For some of the EORTC QLQ QOL outcomes, minimally important differences (MID) have been determined that indicate the smallest change in the outcome that would be considered important by a patient or clinician.70 We found that improvements in average scores in our study population demonstrated MIDs for EORTC QLQ C30 global health, cognitive functioning, and social functioning indicating clinically meaningful improvements in these parameters. Similarly, for a number of symptom measures that have validated thresholds above or below which symptoms are considered clinically important, we found that average scores in our study population crossed these thresholds by 4 months, implying clinically meaningful improvement. These included sleep disturbance (mean GSDS total score was 61.4 at BL and 40.8 at 4 months, with values above 43 representing substantial sleep disturbance), energy (mean BL: 4.42 to mean at 4 months 5.73, with clinical threshold of 4.8), depressive symptoms (BL mean 15.8 to mean at 4 months of 6.64; clinical threshold for major depression 16 or higher), and state anxiety (BL mean 36.5 to mean at 4 months 29.1, with clinical threshold of 31.8). These findings suggest the possibility that the Ayurvedic intervention may contribute to clinically meaningful improvements in this survivorship population, which merits future study in a randomized controlled trial.
Several outcomes continued to improve over the course of the 4-month intervention and did not reach a plateau by the end of the intervention. We saw improvements in QOL global heath from month 3 to month 4, energy and fatigue from month 2 to month 4, and state anxiety from month 2 to month 4. These findings suggest the possibility of a dose-effect for the intervention, where increasing time and/or exposure to the intervention is associated with continued improvements in outcomes. These continued improvements over the course of the 4-month intervention may reflect gradually better integration of nutrition, lifestyle, yoga, and marma recommendations over an extended period of time and greater time required for mastery of the intervention. These findings offer a number of hypotheses that merit further study.
The participants’ expectations of the benefit of the Ayurvedic intervention were not associated with changes in PROs, with a single exception of BR23 systematic therapy side effects. The relationship of expectation to placebo is debated.71 However, if they are related, our findings suggest that the expectation component of the placebo effect did not play a major role in QOL and symptom improvements for this sample, which lends some support to the idea that the improvements in PROs seen in this study could be due to the efficacy of the Ayurvedic intervention itself. However, this study was not intended or designed to study efficacy, and these findings need to be further tested in a randomized controlled study.
Several study limitations are worth noting. This nonrandomized pilot feasibility study cannot, by design, prove efficacy, which was not a study aim. Due to the nonrandomized design, we cannot say with certainty that the improvements seen in QOL and symptoms were due to the study intervention, rather than improvements that may occur with the passage of time. However, the extent of these improvements appears to be more than that typically seen in our clinical experience over a 4-month time period for similar patient populations. For example, we found very large effect sizes for improvements in some QOL parameters as well as sleep disturbance. Additionally, research suggests that some survivors have persistent symptoms many years after the completion of treatment.8,72 Other limitations of this study include the fact that the majority of the participants were white, married, and highly educated and that the study includes a single cancer diagnosis. The study includes patients who are inclined to consent to an integrative medicine intervention such as this, limiting the generalizability of this study.
If the improvements in QOL and symptoms observed in this study are shown to be due to the Ayurvedic intervention, it would represent a meaningful advancement in care of cancer survivors. QOL improvements have been associated with an increase in adherence to cancer treatment, such as adjuvant hormone therapy,73 that could impact survival. The Ayurvedic intervention redirects focus toward more personal, patient-centered care, which is much needed for patients seeking well-structured guidance during this significant time.74,75 Additionally, the Ayurvedic intervention, with the potential to improve symptoms and QOL, might integrate well with SCPs, which have not yet shown benefit in improving symptoms and QOL. These hypotheses warrant further investigation in a future randomized control study.
Conclusion
The results from this study support the feasibility and acceptability of this Ayurvedic nutrition and lifestyle intervention for breast cancer survivors, warranting further research including intervention optimization in a future study. Breast cancer survivors have reported uncertainty about how to proceed after the completion of their cancer treatment, due in part to a lack of clinical guidance and structure during this important phase of survivorship.19 Improvements in symptoms and QOL outcomes for survivors may be well accomplished with a comprehensive CIM approach to survivorship care.76,77
Supplemental Material
Supplemental material, sj-pdf-1-gam-10.1177_2164956120964712 for A 4-Month Whole-Systems Ayurvedic Medicine Nutrition and Lifestyle Intervention Is Feasible and Acceptable for Breast Cancer Survivors: Results of a Single-Arm Pilot Clinical Trial by Anand Dhruva MD Cairn Wu BA Christine Miaskowski RN, PhD Wendy Hartogensis Phd, MPH Hope S Rugo MD Shelley R Adler PhD Ted J Kaptchuk, Rucha Kelkar BAMS, PT, DPT Sangeeta Agarawal RN, MS, CAS Amisha Vadodaria BA Ellen Garris CAS Frederick M Hecht MD in Global Advances in Health and Medicine
Supplemental material, sj-pdf-2-gam-10.1177_2164956120964712 for A 4-Month Whole-Systems Ayurvedic Medicine Nutrition and Lifestyle Intervention Is Feasible and Acceptable for Breast Cancer Survivors: Results of a Single-Arm Pilot Clinical Trial by Anand Dhruva MD Cairn Wu BA Christine Miaskowski RN, PhD Wendy Hartogensis Phd, MPH Hope S Rugo MD Shelley R Adler PhD Ted J Kaptchuk, Rucha Kelkar BAMS, PT, DPT Sangeeta Agarawal RN, MS, CAS Amisha Vadodaria BA Ellen Garris CAS Frederick M Hecht MD in Global Advances in Health and Medicine
Supplemental material, sj-pdf-3-gam-10.1177_2164956120964712 for A 4-Month Whole-Systems Ayurvedic Medicine Nutrition and Lifestyle Intervention Is Feasible and Acceptable for Breast Cancer Survivors: Results of a Single-Arm Pilot Clinical Trial by Anand Dhruva MD Cairn Wu BA Christine Miaskowski RN, PhD Wendy Hartogensis Phd, MPH Hope S Rugo MD Shelley R Adler PhD Ted J Kaptchuk, Rucha Kelkar BAMS, PT, DPT Sangeeta Agarawal RN, MS, CAS Amisha Vadodaria BA Ellen Garris CAS Frederick M Hecht MD in Global Advances in Health and Medicine
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Center for Complementary and Integrative Health (1K23AT005340-01).
Supplemental Material: Supplemental material for this article is available online.
ORCID iDs
Anand Dhruva https://orcid.org/0000-0001-5552-7131
References
- 1.Torre LA, Islami F, Siegel RL, Ward EM, Jemal A. Global cancer in women: burden and trends. Cancer Epidemiol Biomarkers Prev. 2017; 26(4):444–457. [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 3.Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005; 353(17):1784–1792. [DOI] [PubMed] [Google Scholar]
- 4.Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019; 69(5):363–385. [DOI] [PubMed] [Google Scholar]
- 5.O’Malley DM, Hudson SV, Ohman-Strickland PA, et al. Follow-up care education and information: identifying cancer survivors in need of more guidance. J Cancer Educ. 2016; 31(1):63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bower JE, Ganz PA, Desmond KA, et al. Fatigue in long-term breast carcinoma survivors: a longitudinal investigation. Cancer. 2006; 106(4):751–758. [DOI] [PubMed] [Google Scholar]
- 7.Joly F, Lange M, Dos Santos M, Vaz-Luis I, Di Meglio A. Long-term fatigue and cognitive disorders in breast cancer survivors. Cancers. 2019; 11(12):1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maass SWMC, Roorda C, Berendsen AJ, Verhaak PFM, De Bock GH. The prevalence of long-term symptoms of depression and anxiety after breast cancer treatment: a systematic review. Maturitas. 2015; 82(1):100–108. [DOI] [PubMed] [Google Scholar]
- 9.Bower JE, Lamkin DM. Inflammation and cancer-related fatigue: mechanisms, contributing factors, and treatment implications. Brain Behav Immun. 2013; 30(Suppl):S48–S57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donovan KA, Jacobsen PB, Andrykowski MA, et al. Course of fatigue in women receiving chemotherapy and/or radiotherapy for early stage breast cancer. J Pain Symptom Manage. 2004; 28(4):373–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bower JE, Ganz PA, Desmond KA, Rowland JH, Meyerowitz BE, Belin TR. Fatigue in breast cancer survivors: occurrence, correlates, and impact on quality of life. J Clin Oncol. 2000; 18(4):743–743. [DOI] [PubMed] [Google Scholar]
- 12.Dhruva A, Paul SM, Cooper BA, et al. A longitudinal study of measures of objective and subjective sleep disturbance in patients with breast cancer before, during, and after radiation therapy. J Pain Symptom Manag. 2012; 44(2):215–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lowery-Allison AE, Passik SD, Cribbet MR, et al. Sleep problems in breast cancer survivors 1–10 years posttreatment. Palliat Support Care. 2018; 16(3):325–334. [DOI] [PubMed] [Google Scholar]
- 14.Fallowfield L, Jenkins V. Psychosocial/survivorship issues in breast cancer: are we doing better? J Natl Cancer Inst. 2015; 107(1):335. [DOI] [PubMed] [Google Scholar]
- 15.Ganz PA, Kwan L, Castellon SA, et al. Cognitive complaints after breast cancer treatments: examining the relationship with neuropsychological test performance. J Natl Cancer Inst. 2013; 105(11):791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Penttinen HM, Saarto T, Kellokumpu-Lehtinen P, et al. Quality of life and physical performance and activity of breast cancer patients after adjuvant treatments. Psychooncology. 2011; 20(11):1211–1220. [DOI] [PubMed] [Google Scholar]
- 17.Adler NE, Page AEK. Cancer Care for the Whole Patient: Meeting Psychosocial Health Needs. Washington, DC: National Academy of Sciences; 2008. [PubMed] [Google Scholar]
- 18.Forsythe LP, Alfano CM, Leach CR, Ganz PA, Stefanek ME, Rowland JH. Who provides psychosocial follow-up care for post-treatment cancer survivors? A survey of medical oncologists and primary care physicians. J Clin Oncol. 2012; 30(23):2897–2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weaver KE, Aziz NM, Arora NK, et al. Follow-up care experiences and perceived quality of care among long-term survivors of breast, prostate, colorectal, and gynecologic cancers. J Oncol Pract. 2014; 10(4):e231–e239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ganz PA, Levit LA. Charting a new course for the delivery of high-quality cancer care. J Clin Oncol. 2013; 31(36):4485–4487. [DOI] [PubMed] [Google Scholar]
- 21.Mayer DK, Nekhlyudov L, Snyder CF, Merrill JK, Wollins DS, Shulman LN. American Society of Clinical Oncology clinical expert statement on cancer survivorship care planning. J Oncol Pract. 2014; 10(6):345–351. [DOI] [PubMed] [Google Scholar]
- 22.Grunfeld E, Julian JA, Pond G, et al. Evaluating survivorship care plans: results of a randomized, clinical trial of patients with breast cancer. J Clin Oncol. 2011; 29(36):4755–4762. [DOI] [PubMed] [Google Scholar]
- 23.Jacobsen PB, Derosa AP, Henderson TO, et al. Systematic review of the impact of cancer survivorship care plans on health outcomes and health care delivery. J Clin Oncol. 2018; 36(20):2088–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brennan ME, Gormally JF, Butow P, Boyle FM, Spillane AJ. Survivorship care plans in cancer: a systematic review of care plan outcomes. Br J Cancer. 2014; 111(10):1899–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greenlee H, Molmenti CL, Crew KD, et al. Survivorship care plans and adherence to lifestyle recommendations among breast cancer survivors. J Cancer Surviv. 2016; 10(6):956–963. [DOI] [PubMed] [Google Scholar]
- 26.Greenlee H, Kwan ML, Ergas IJ, et al. Complementary and alternative therapy use before and after breast cancer diagnosis: the Pathways Study. Breast Cancer Res Treat. 2009; 117(3):653–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hammersen F, Pursche T, Fischer D, Katalinic A, Waldmann A. Use of complementary and alternative medicine among young patients with breast cancer. Breast Care (Basel). 2020; 15(2):163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, Lin L, Li H, Hu Y, Tian L. Effects of acupuncture on cancer-related fatigue: a meta-analysis. Support Care Cancer. 2018; 26(2):415–425. [DOI] [PubMed] [Google Scholar]
- 29.Zia FZ., Olaku O., Bao T., et al The National Cancer Institute’s Conference on Acupuncture for Symptom Management in Oncology: state of the science, evidence, and research gaps. JNCI Monogr. 2017. 2017 (52) 68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bower JE, Garet D, Sternlieb B, et al. Yoga for persistent fatigue in breast cancer survivors: a randomized controlled trial. Cancer. 2012; 118(15):3766–3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cramer H, Lauche R, Klose P, Lange S, Langhorst J, Dobos GJ. Yoga for improving health-related quality of life, mental health and cancer-related symptoms in women diagnosed with breast cancer. Cochrane Database Syst Rev. 2017; 1(1):CD010802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dong B, Xie C, Jing X, Lin L, Tian L. Yoga has a solid effect on cancer-related fatigue in patients with breast cancer: a meta-analysis. Breast Cancer Res Treat. 2019; 177(1):5–16. [DOI] [PubMed] [Google Scholar]
- 33.Schell LK, Monsef I, Wöckel A, Skoetz N. Mindfulness-based stress reduction for women diagnosed with breast cancer. Cochrane Database Syst Rev. 2019; 3(3):CD011518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwedhelm C, Boeing H, Hoffmann G, Aleksandrova K, Schwingshackl L. Effect of diet on mortality and cancer recurrence among cancer survivors: a systematic review and meta-analysis of cohort studies. Nutr Rev. 2016; 74(12):737–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spei ME, Samoli E, Bravi F, La Vecchia C, Bamia C, Benetou V. Physical activity in breast cancer survivors: a systematic review and meta-analysis on overall and breast cancer survival. Breast. 2019; 44:144–152. [DOI] [PubMed] [Google Scholar]
- 36.Hasson F, Keeney S, McKenna H. Research guidelines for the Delphi survey technique. J Adv Nurs. 2000; 32(4):1008–1015. [PubMed] [Google Scholar]
- 37.Hsu C-C, Sandford BA. The Delphi technique: making sense of consensus. Pract Assess Res Eval. 2007; 12(1):10. [Google Scholar]
- 38.Dhruva A, Hecht FM, Miaskowski C, et al. Correlating traditional Ayurvedic and modern medical perspectives on cancer: results of a qualitative study. J Altern Complement Med (New York, NY). 2014; 20(5):364–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lad V. Textbook of Ayurveda. Vol 1 1st ed. Albuquerque, NM: The Ayurvedic Press; 2002. [Google Scholar]
- 40.Svoboda RE. Prakriti Your Ayurvedic Constitution. Twin Lakes, WI: Lotus Press; 1998. [Google Scholar]
- 41.Schnyer RN, Allen JJ. Bridging the gap in complementary and alternative medicine research: manualization as a means of promoting standardization and flexibility of treatment in clinical trials of acupuncture. J Altern Complement Med. 2002; 8(5):623–634. [DOI] [PubMed] [Google Scholar]
- 42.Karnofsky D. Performance Scale. New York, NY: Plenum Press; 1977. [Google Scholar]
- 43.Dhruva A, Hecht FM, Miaskowski C, et al. Correlating traditional ayurvedic and modern medical perspectives on cancer: results of a qualitative study. J Altern Complement Med. 2014; 20(5):364–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vasant Lad AD. Marma Points of Ayurveda: The Energy Pathways for Healing Body, Mind, and Consciousness with a Comparison to Traditional Chinese Medicine. Albuquerque, NM: The Ayurvedic Press; 2015. [Google Scholar]
- 45.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993; 85(5):365–376. [DOI] [PubMed] [Google Scholar]
- 46.Fayers PM, Aaronson NK, Bjordal K, Groenvold M, Curran D, Bottomley A. EORTC QLQ-C30 Scoring Manual. 3rd ed. Brussels, Belgium: European Organisation for Research and Treatment of Cancer; 2001. [Google Scholar]
- 47.Yo YT, Lee KA. Pain, fatigue, and sleep disturbances in oncology outpatients receiving radiation therapy for bone metastasis: a pilot study. J Pain Symptom Manage. 1999; 17(5):320–332. [DOI] [PubMed] [Google Scholar]
- 48.Lee KA. Self-reported sleep disturbances in employed women. Sleep. 1992; 15(6):493–498. [DOI] [PubMed] [Google Scholar]
- 49.Fletcher BS, Paul SM, Dodd MJ, et al. Prevalence, severity, and impact of symptoms on female family caregivers of patients at the initiation of radiation therapy for prostate cancer. J Clin Oncol. 2008; 26(4):599–605. [DOI] [PubMed] [Google Scholar]
- 50.Dhruva A, Dodd M, Paul SM, et al. Trajectories of fatigue in patients with breast cancer before, during, and after radiation therapy. Cancer Nurs. 2010; 33(3):201–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee KA, Hicks G, Nino-Murcia G. Validity and reliability of a scale to assess fatigue. Psychiatry Res. 1991; 36(3):291–298. [DOI] [PubMed] [Google Scholar]
- 52.Lee KA, Lentz MJ, Taylor DL, Mitchell ES, Woods NF. Fatigue as a response to environmental demands in women's lives. Image J Nurs Sch. 1994; 26(2):149–154. [DOI] [PubMed] [Google Scholar]
- 53.Orme JG, Reis J, Herz EJ. Factorial and discriminant validity of the Center for Epidemiological Studies Depression (CES-D) scale. J Clin Psychol. 1986; 42(1):28–33. [DOI] [PubMed] [Google Scholar]
- 54.Radloff LS. The CES-D scale. Appl Psychol Meas. 1977; 1(3):385–401. [Google Scholar]
- 55.Spielberger CG, Gorsuch RL, Suchene R, Vagg PR, Jacobs GA. Manual for the State-Anxiety Inventory (Form Y): Self-Evaluation Questionnaire. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 56.Bieling PJ, Antony MM, Swinson RP. The State-Trait Anxiety Inventory, Trait version: structure and content re-examined. Behav Res Ther. 1998; 36(7–8):777–788. [DOI] [PubMed] [Google Scholar]
- 57.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983; 24(4):385–396. [PubMed] [Google Scholar]
- 58.Munoz AR, Salsman JM, Stein KD, Cella D. Reference values of the functional assessment of chronic illness therapy-spiritual well-being: a report from the American Cancer Society’s studies of cancer survivors. Cancer. 2015; 121(11):1838–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peterman AH, Fitchett G, Brady MJ, Hernandez L, Cella D. Measuring spiritual well-being in people with cancer: the functional assessment of chronic illness therapy–Spiritual Well-being Scale (FACIT-SP). Ann Behav Med. 2002; 24(1):49–58. [DOI] [PubMed] [Google Scholar]
- 60.Kalauokalani D, Cherkin DC, Sherman KJ, Koepsell TD, Deyo RA. Lessons from a trial of acupuncture and massage for low back pain: patient expectations and treatment effects. Spine (Phila Pa 1976). 2001; 26(13):1418–1424. [DOI] [PubMed] [Google Scholar]
- 61.Allison P. Missing Data. Thousand Oaks, CA: SAGE Publications; 2001. [Google Scholar]
- 62.Bower JE, Ganz PA, Desmond KA, Rowland JH, Meyerowitz BE, Belin TR. Fatigue in breast cancer survivors: occurrence, correlates, and impact on quality of life. J Clin Oncol. 2000; 18(4):743–753. [DOI] [PubMed] [Google Scholar]
- 63.Mandelblatt JS, Zhai W, Ahn J, et al. Symptom burden among older breast cancer survivors: the Thinking and Living With Cancer (TLC) study. Cancer. 2020; 126(6):1183–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Campbell KL, Van Patten CL, Neil SE, et al. Feasibility of a lifestyle intervention on body weight and serum biomarkers in breast cancer survivors with overweight and obesity. J Acad Nutr Diet. 2012; 112(4):559–567. [DOI] [PubMed] [Google Scholar]
- 65.Derry HM, Jaremka LM, Bennett JM, et al. Yoga and self-reported cognitive problems in breast cancer survivors: a randomized controlled trial. Psychooncology. 2015; 24(8):958–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lapen K, Benusis L, Pearson S, et al. A feasibility study of restorative yoga versus vigorous yoga intervention for sedentary breast and ovarian cancer survivors. Int J Yoga Therap. 2018; 28(1):79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bower JE. Behavioral symptoms in patients with breast cancer and survivors. J Clin Oncol. 2008; 26(5):768–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maass SWMC, Boerman LM, Verhaak PFM, Du J, de Bock GH, Berendsen AJ. Long-term psychological distress in breast cancer survivors and their matched controls: a cross-sectional study. Maturitas. 2019; 130:6–12. [DOI] [PubMed] [Google Scholar]
- 69.Martínez Arroyo O, Andreu Vaíllo Y, Martínez López P, Galdón Garrido MJ. Emotional distress and unmet supportive care needs in survivors of breast cancer beyond the end of primary treatment. Support Care Cancer. 2019; 27(3):1049–1057. [DOI] [PubMed] [Google Scholar]
- 70.Musoro JZ, Coens C, Fiteni F, et al. Minimally important differences for interpreting EORTC QLQ-C30 scores in patients with advanced breast cancer. JNCI Cancer Spectr. 2019; 3(3):pkz037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kaptchuk TJ, Hemond CC, Miller FG. Placebos in chronic pain: evidence, theory, ethics, and use in clinical practice. BMJ (Clinical Research ed). 2020; 370:m1668. [DOI] [PubMed] [Google Scholar]
- 72.Joly F, Lange M, Dos Santos M, Vaz-Luis I, Di Meglio A. Long-term fatigue and cognitive disorders in breast cancer survivors. Cancers (Basel ). 2019; 11(12):1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Van Liew JR, Christensen AJ, De Moor JS. Psychosocial factors in adjuvant hormone therapy for breast cancer: an emerging context for adherence research. J Cancer Surviv. 2014; 8(3):521–531. [DOI] [PubMed] [Google Scholar]
- 74.Grunfeld E. Survivorship 2.0. J Clin Oncol. 2019; 37(34):3179–3182. [DOI] [PubMed] [Google Scholar]
- 75.Shapiro CL. Cancer survivorship. N Engl J Med. 2018; 379(25):2438–2450. [DOI] [PubMed] [Google Scholar]
- 76.Jacobsen PB, Wagner LI. A new quality standard: the integration of psychosocial care into routine cancer care. J Clin Oncol. 2012; 30(11):1154–1159. [DOI] [PubMed] [Google Scholar]
- 77.Pettiford J, Felts S, Wischkaemper E, Miller D, Crawford S, Layeequr Rahman R. A bio-psychosocial intervention program for improving quality of life in breast cancer survivors – final outcome of a prospective randomized trial. Breast J. 2017; 23(5):537–544. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-gam-10.1177_2164956120964712 for A 4-Month Whole-Systems Ayurvedic Medicine Nutrition and Lifestyle Intervention Is Feasible and Acceptable for Breast Cancer Survivors: Results of a Single-Arm Pilot Clinical Trial by Anand Dhruva MD Cairn Wu BA Christine Miaskowski RN, PhD Wendy Hartogensis Phd, MPH Hope S Rugo MD Shelley R Adler PhD Ted J Kaptchuk, Rucha Kelkar BAMS, PT, DPT Sangeeta Agarawal RN, MS, CAS Amisha Vadodaria BA Ellen Garris CAS Frederick M Hecht MD in Global Advances in Health and Medicine
Supplemental material, sj-pdf-2-gam-10.1177_2164956120964712 for A 4-Month Whole-Systems Ayurvedic Medicine Nutrition and Lifestyle Intervention Is Feasible and Acceptable for Breast Cancer Survivors: Results of a Single-Arm Pilot Clinical Trial by Anand Dhruva MD Cairn Wu BA Christine Miaskowski RN, PhD Wendy Hartogensis Phd, MPH Hope S Rugo MD Shelley R Adler PhD Ted J Kaptchuk, Rucha Kelkar BAMS, PT, DPT Sangeeta Agarawal RN, MS, CAS Amisha Vadodaria BA Ellen Garris CAS Frederick M Hecht MD in Global Advances in Health and Medicine
Supplemental material, sj-pdf-3-gam-10.1177_2164956120964712 for A 4-Month Whole-Systems Ayurvedic Medicine Nutrition and Lifestyle Intervention Is Feasible and Acceptable for Breast Cancer Survivors: Results of a Single-Arm Pilot Clinical Trial by Anand Dhruva MD Cairn Wu BA Christine Miaskowski RN, PhD Wendy Hartogensis Phd, MPH Hope S Rugo MD Shelley R Adler PhD Ted J Kaptchuk, Rucha Kelkar BAMS, PT, DPT Sangeeta Agarawal RN, MS, CAS Amisha Vadodaria BA Ellen Garris CAS Frederick M Hecht MD in Global Advances in Health and Medicine