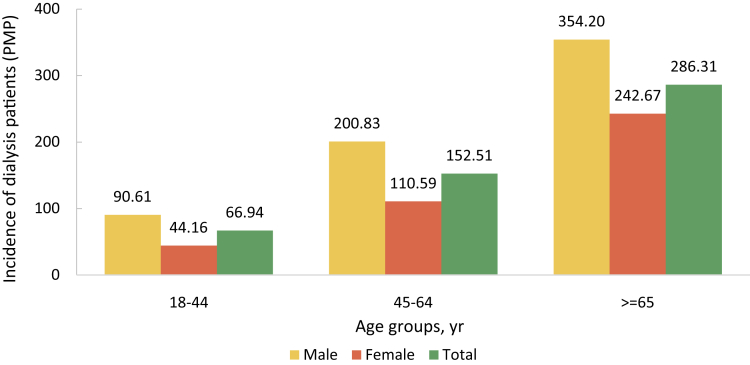CK-NET Executive Committee
Honorary chairman:
Qi-Min Zhan
Peking University Health Science Center, Beijing, China
Chairman:
Ming-Hui Zhao
Renal Division, Department of Medicine, Peking University First Hospital, Peking University Institute of Nephrology, Beijing, China; Research Units of Diagnosis and Treatment of Immune-Mediated Kidney Diseases, Chinese Academy of Medical Sciences, Beijing, China; Peking-Tsinghua Center for Life Sciences, Beijing, China
Executive chairman:
Luxia Zhang
Renal Division, Department of Medicine, Peking University First Hospital, Peking University Institute of Nephrology, Beijing, China; National Institute of Health Data Science at Peking University, Beijing, China; Advanced Institute of Information Technology, Peking University, Hangzhou, Zhejiang, China
Vice chairmen:
Li Zuo
Department of Nephrology, Peking University People's Hospital, Beijing, China
Yue Wang
Department of Nephrology, Peking University Third Hospital, Beijing, China
Feng Yu
Renal Division, Department of Medicine, Peking University First Hospital, Peking University Institute of Nephrology, Beijing, China; Research Units of Diagnosis and Treatment of Immune-Mediated Kidney Diseases, Chinese Academy of Medical Sciences, Beijing, China; Department of Nephrology, Peking University International Hospital, Beijing, China
Jie Ding
Department of Pediatrics, Peking University First Hospital, Beijing, China
Haibo Wang
National Institute of Health Data Science at Peking University, Beijing, China
CK-NET Work Group (alphabetically)
Rui Chen
Renal Division, Department of Medicine, Peking University First Hospital, Peking University Institute of Nephrology, Beijing, China; Research Units of Diagnosis and Treatment of Immune-Mediated Kidney Diseases, Chinese Academy of Medical Sciences, Beijing, China
Hong Chu
Renal Division, Department of Medicine, Peking University First Hospital, Peking University Institute of Nephrology, Beijing, China; Research Units of Diagnosis and Treatment of Immune-Mediated Kidney Diseases, Chinese Academy of Medical Sciences, Beijing, China
Xinwei Deng
Renal Division, Department of Medicine, Peking University First Hospital, Peking University Institute of Nephrology, Beijing, China; Research Units of Diagnosis and Treatment of Immune-Mediated Kidney Diseases, Chinese Academy of Medical Sciences, Beijing, China
Lanxia Gan
China Standard Medical Information Research Center, Shenzhen, Guangdong, China
Bixia Gao
Renal Division, Department of Medicine, Peking University First Hospital, Peking University Institute of Nephrology, Beijing, China; Research Units of Diagnosis and Treatment of Immune-Mediated Kidney Diseases, Chinese Academy of Medical Sciences, Beijing, China
Yifang Jiang
Renal Division, Department of Medicine, Peking University First Hospital, Peking University Institute of Nephrology, Beijing, China; Research Units of Diagnosis and Treatment of Immune-Mediated Kidney Diseases, Chinese Academy of Medical Sciences, Beijing, China
Lili Liu
Renal Division, Department of Medicine, Peking University First Hospital, Peking University Institute of Nephrology, Beijing, China; Research Units of Diagnosis and Treatment of Immune-Mediated Kidney Diseases, Chinese Academy of Medical Sciences, Beijing, China
Jianyan Long
Clinical Trial Unit, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, Guangdong, China
Ying Shi
China Standard Medical Information Research Center, Shenzhen, Guangdong, China
Zaiming Su
National Institute of Health Data Science at Peking University, Beijing, China
Xiaoyu Sun
National Institute of Health Data Science at Peking University, Beijing, China
Wen Tang
Department of Nephrology, Peking University Third Hospital, Beijing, China
Fang Wang
Renal Division, Department of Medicine, Peking University First Hospital, Peking University Institute of Nephrology, Beijing, China; Research Units of Diagnosis and Treatment of Immune-Mediated Kidney Diseases, Chinese Academy of Medical Sciences, Beijing, China
Huai-Yu Wang
National Institute of Health Data Science at Peking University, Beijing, China
Jinwei Wang
Renal Division, Department of Medicine, Peking University First Hospital, Peking University Institute of Nephrology, Beijing, China; Research Units of Diagnosis and Treatment of Immune-Mediated Kidney Diseases, Chinese Academy of Medical Sciences, Beijing, China
Song Wang
Department of Nephrology, Peking University Third Hospital, Beijing, China
Chao Yang
Renal Division, Department of Medicine, Peking University First Hospital, Peking University Institute of Nephrology, Beijing, China; Research Units of Diagnosis and Treatment of Immune-Mediated Kidney Diseases, Chinese Academy of Medical Sciences, Beijing, China
Dongliang Zhang
Department of Nephrology, Peking University International Hospital, Beijing, China
Xinju Zhao
Department of Nephrology, Peking University People's Hospital, Beijing, China
Liren Zheng
Department of Nephrology, Peking University International Hospital, Beijing, China
Zhiye Zhou
China Standard Medical Information Research Center, Shenzhen, Guangdong, China
| CK-NET International Advisory Committee (alphabetically) | |
| Joseph Coresh | Harold Feldman |
| David Jayne | Vivekanand Jha |
| Andrew Levey | Adeera Levin |
| Vlado Perkovic | Pierre Ronco |
| Rajiv Saran | Sydney Tang |
| CK-NET Domestic Advisory Committee (alphabetically) | |
| Chairman: Jianghua Chen | |
| Menghua Chen | Ping Fu |
| Detian Li | Guisen Li |
| Shaomei Li | Xinling Liang |
| Yunhua Liao | Hongli Lin |
| Jian Liu | Zhangsuo Liu |
| Yingchun Ma | Yonghui Mao |
| Luying Sun | Caili Wang |
| Rong Wang | Weiming Wang |
| Wenke Wang | Xiaoqin Wang |
| Changying Xing | Zuying Xiong |
| Xudong Xu | Dongmei Xu |
| Xiangdong Yang | Xiaoping Yang |
| Fan Yi | Yan Zha |
| Aihua Zhang | Chun Zhang |
| Jinghong Zhao | Qiaoling Zhou |
| CK-NET Technical Advisory Committee (alphabetically) | |
| Jennifer Bragg-Gresham | Zhihong Deng |
| Kevin He | Guilan Kong |
| Dawei Xie | Xiaohua Zhou |
Table of contents
| e102 | Dedication |
| e103 | Abbreviations |
| e104 | Preface |
| e106 | Analytical methods |
| e108 | Section I. Chronic kidney disease |
| e108 | Chapter 1. Identification and characteristics of hospitalized patients with CKD |
| e109 | 1.1 Prevalence of CKD among different types of underlying disease |
| e111 | 1.2 Demographics of CKD |
| e113 | 1.3 Cause of CKD |
| e116 | 1.4 Staging of CKD |
| e117 | 1.5 Travel pattern of hospitalized patients with CKD |
| e118 | Chapter 2. Cardiovascular disease in hospitalized patients with CKD |
| e119 | 2.1 Prevalence of CVD, stratified by patient group |
| e119 | 2.1.1 Prevalence of CHD |
| e121 | 2.1.2 Prevalence of stroke |
| e123 | 2.1.3 Prevalence of heart failure |
| e124 | 2.1.4 Prevalence of atrial fibrillation |
| e126 | 2.2 Prevalence of CVD among patients with CKD |
| e127 | 2.2.1 Prevalence of CHD among patients with CKD |
| e128 | 2.2.2 Prevalence of stroke among patients with CKD |
| e130 | 2.2.3 Prevalence of heart failure among patients with CKD |
| e132 | 2.2.4 Prevalence of atrial fibrillation among patients with CKD |
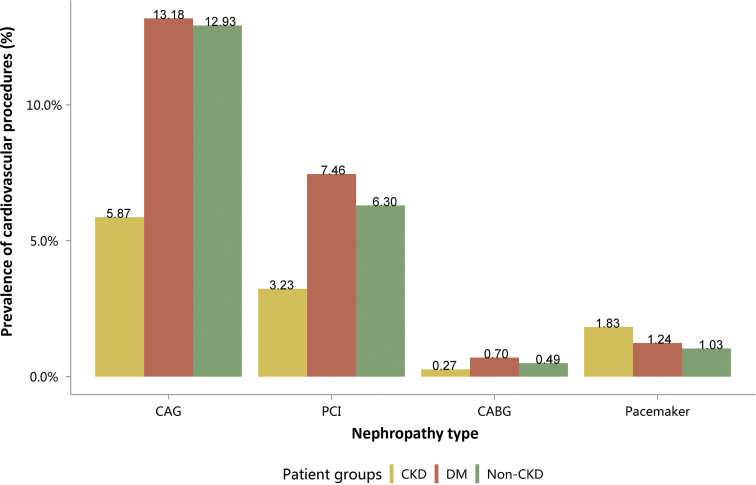
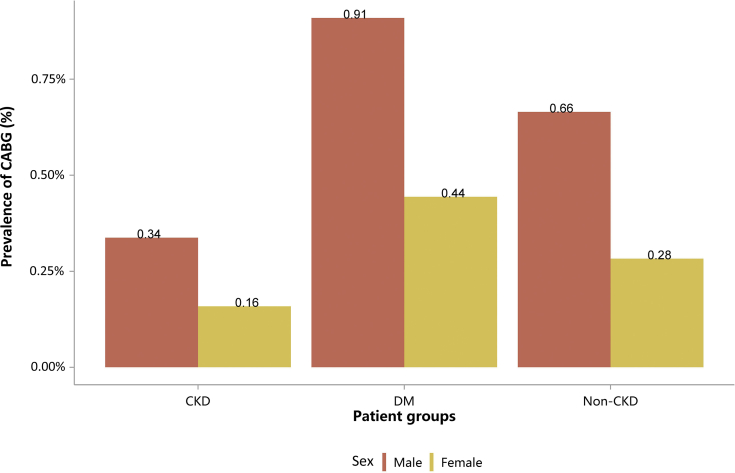
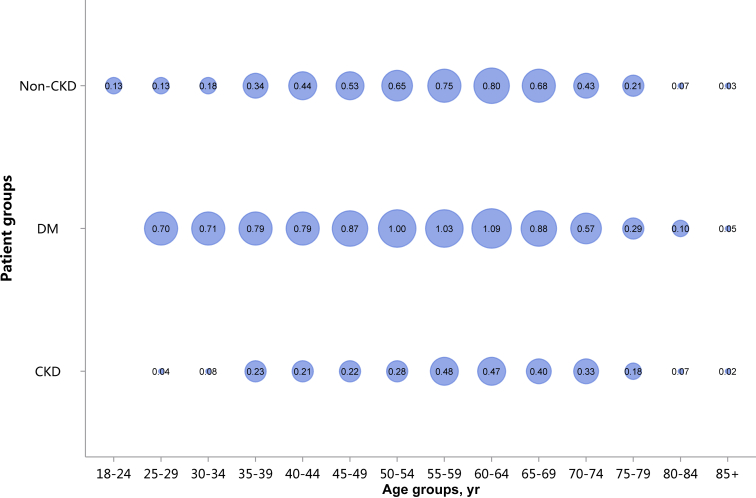
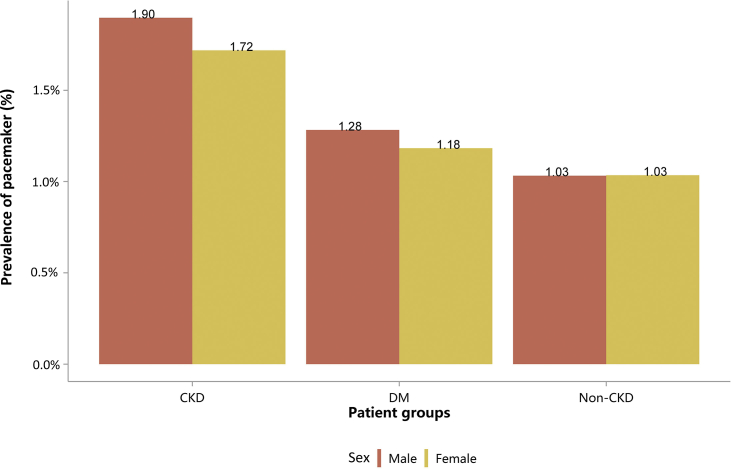
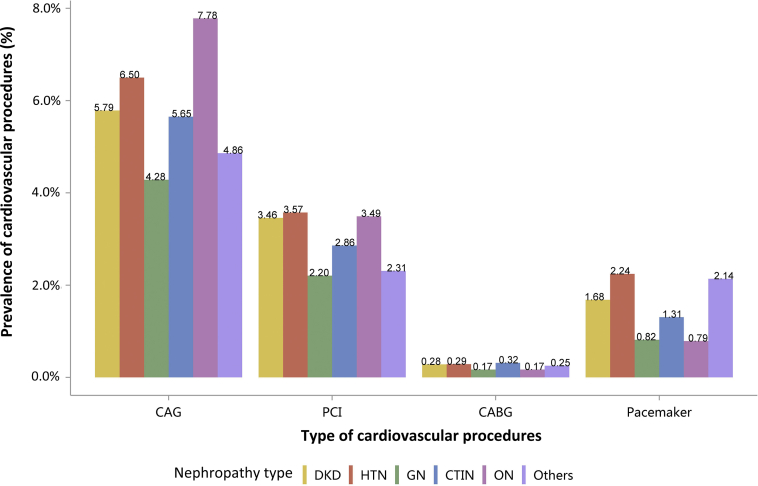
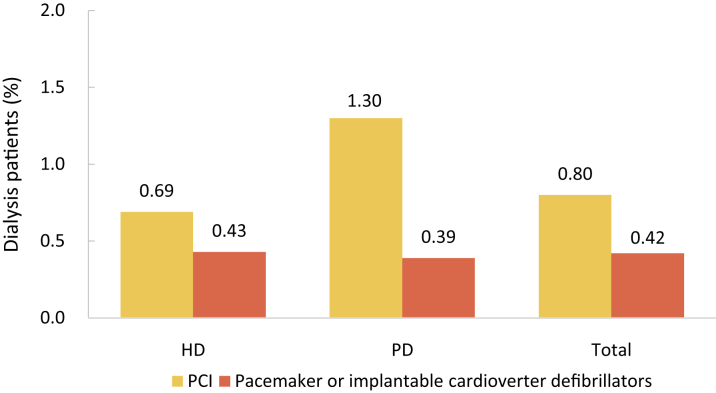
| e134 | 2.3 Cardiovascular procedures stratified by patient group |
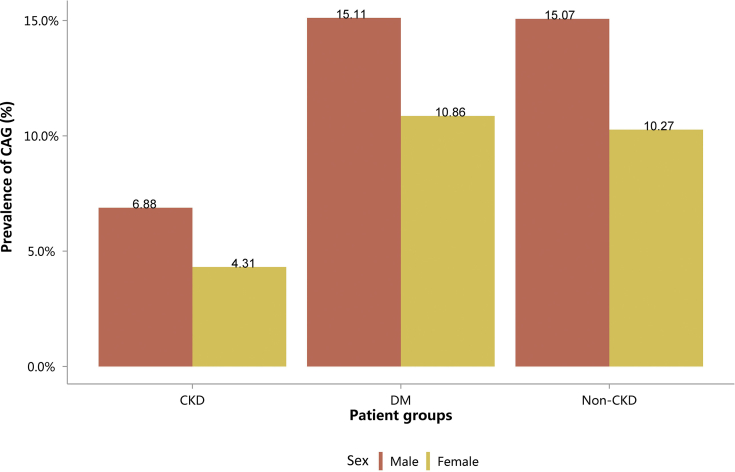
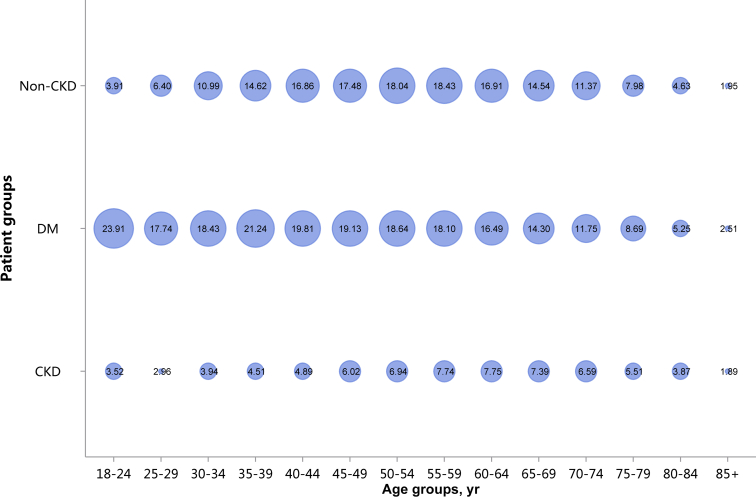
| e134 | 2.3.1 Cardiovascular procedure: coronarography |
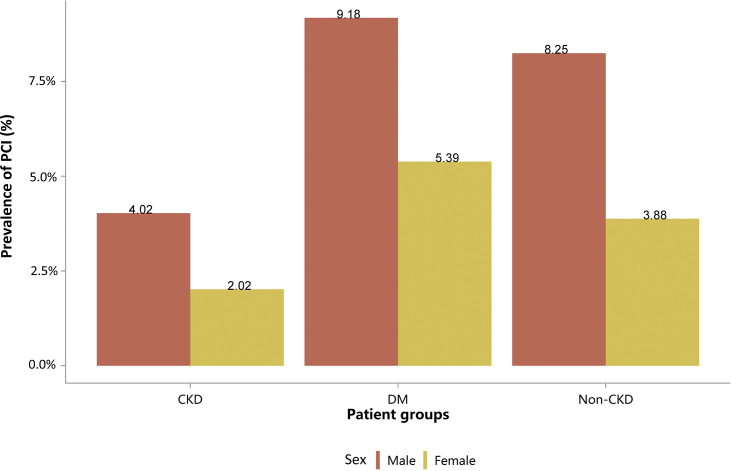
| e136 | 2.3.2 Cardiovascular procedure: percutaneous coronary intervention |
| e137 | 2.3.3 Cardiovascular procedure: coronary artery bypass grafting |
| e139 | 2.3.4 Cardiovascular procedure: pacemaker |
| e140 | 2.4 Cardiovascular procedures among patients with CKD |
| e141 | Chapter 3. Health care resource utilization of hospitalized patients with CKD |
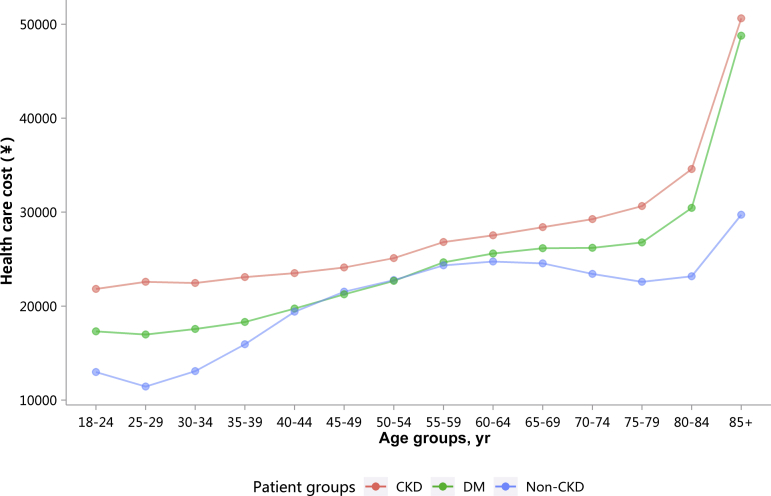
| e141 | 3.1 Costs |
| e141 | 3.1.1 Overall medical costs stratified by CKD, diabetes, and heart failure |
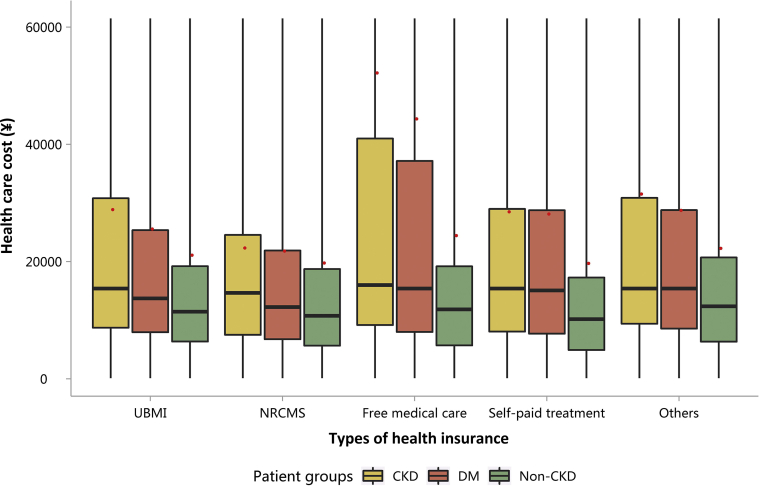
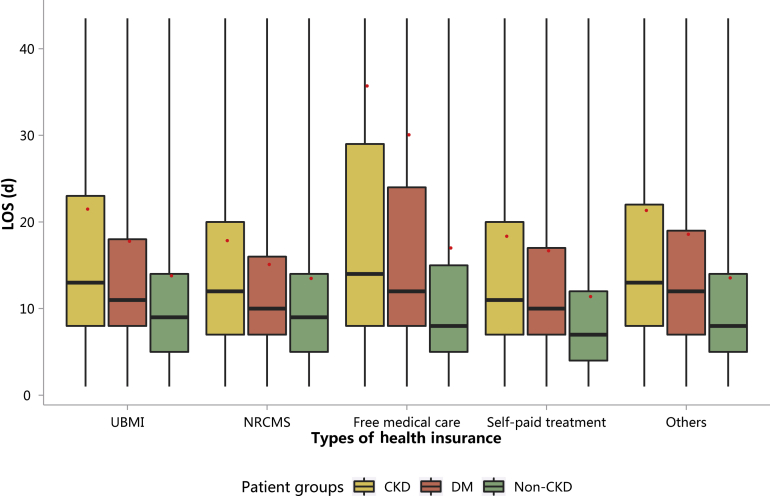
| e142 | 3.1.2 Costs stratified by types of health insurance |
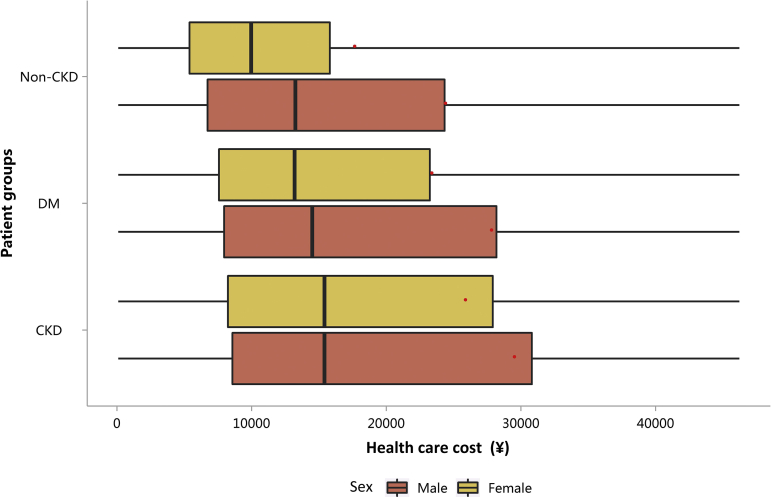
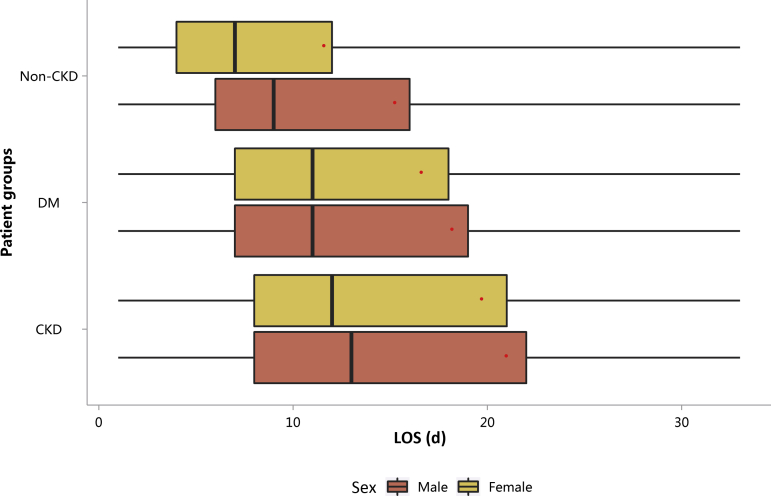
| e143 | 3.1.3 Costs stratified by sex |
| e144 | 3.1.4 Costs stratified by age |
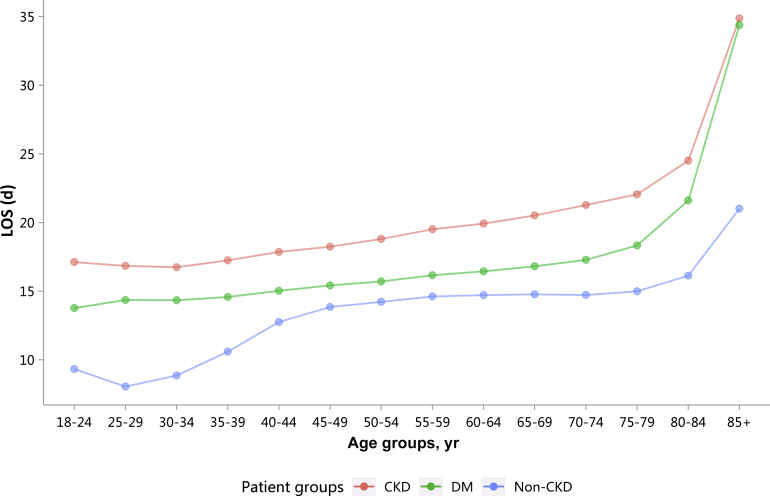
| e145 | 3.2 Length of hospital stay |
| e145 | 3.2.1 Overall length of hospital stay stratified by CKD, diabetes, and heart failure |
| e145 | 3.2.2 Length of hospital stay stratified by types of health insurance |
| e146 | 3.2.3 Length of hospital stay stratified by sex |
| e147 | 3.2.4 Length of hospital stay stratified by age |
| e148 | Chapter 4. In-hospital mortality of hospitalized patients with CKD |
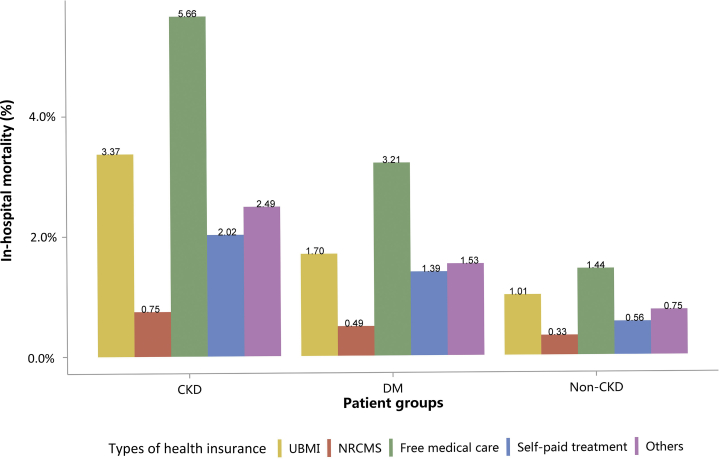
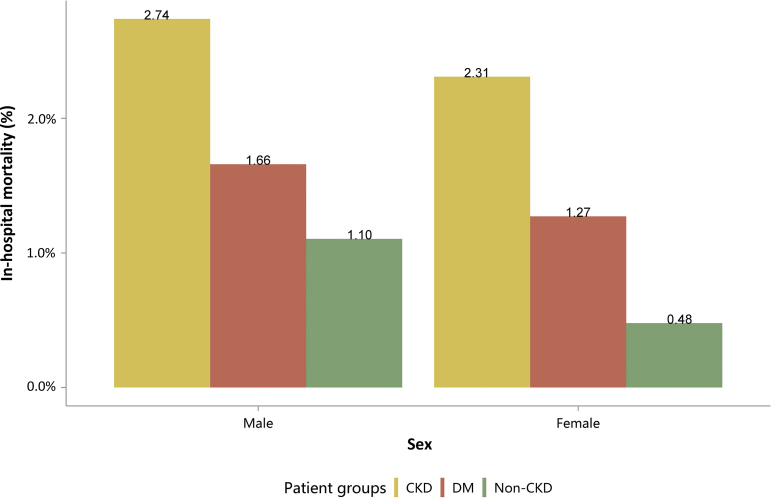
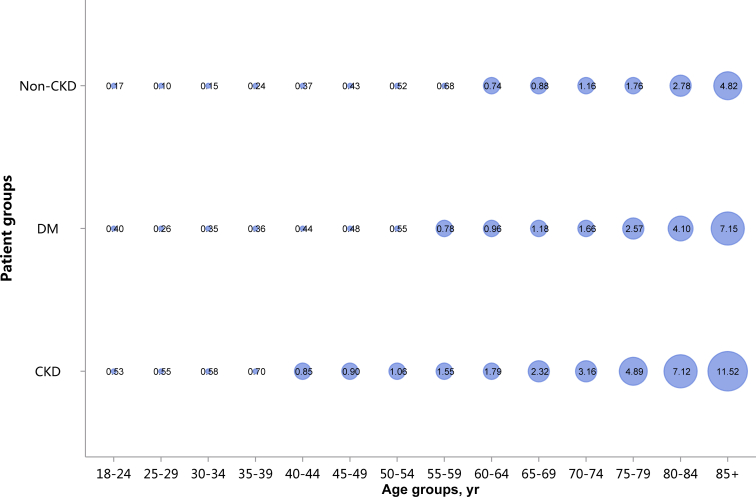
| e148 | 4.1 In-hospital mortality stratified by CKD, diabetes, and heart failure |
| e149 | 4.2 In-hospital mortality stratified by types of insurance |
| e150 | 4.3 In-hospital mortality stratified by sex |
| e151 | 4.4 In-hospital mortality stratified by age |
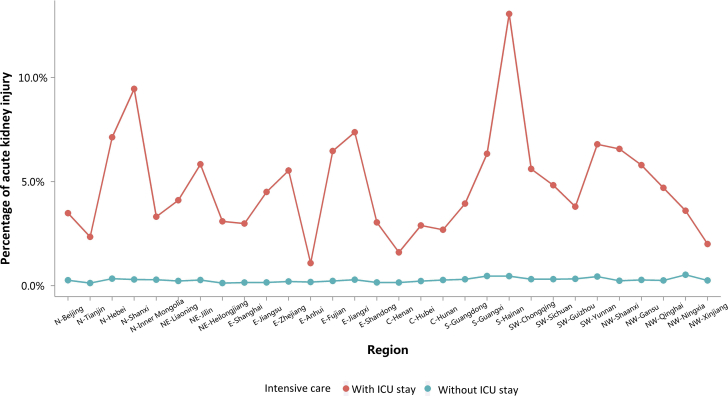
| e152 | Chapter 5. Acute kidney injury |
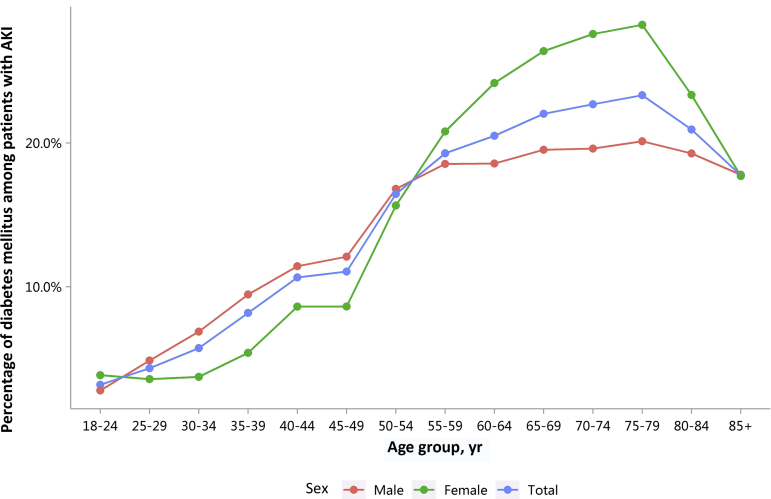
| e152 | 5.1 Percentage of AKI |
| e154 | 5.2 Characteristics of AKI |
| e154 | 5.2.1 Age distribution of AKI, stratified by sex |
| e155 | 5.2.2 Sex distribution of AKI, stratified by age |
| e156 | 5.3 Percentage of CKD and diabetes among patients with AKI |
| e158 | Section II. End-stage kidney disease |
| e158 | Chapter 6. Prevalence, incidence, and characteristics of dialysis patients |
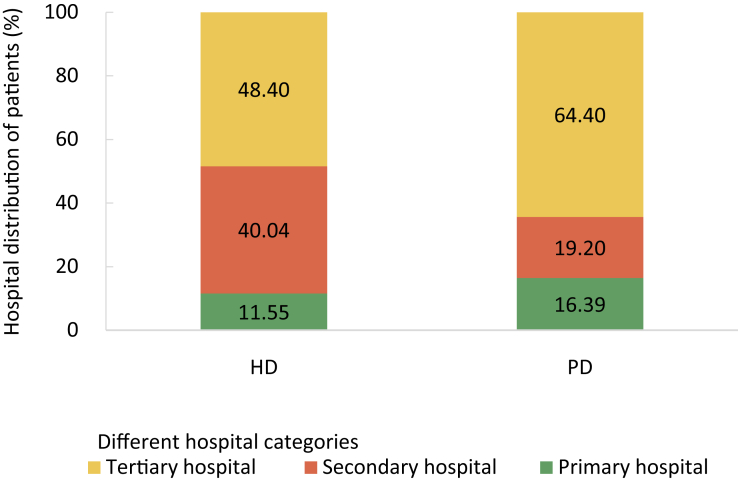
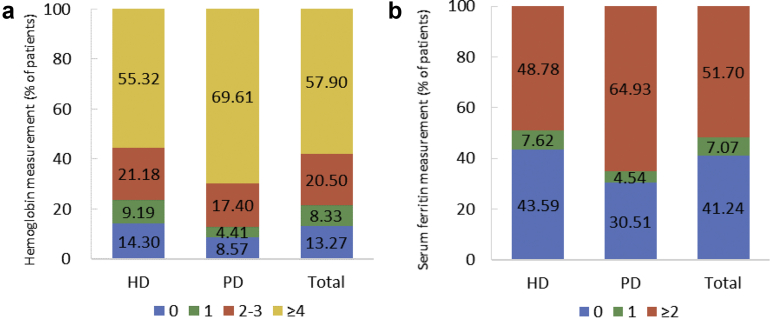
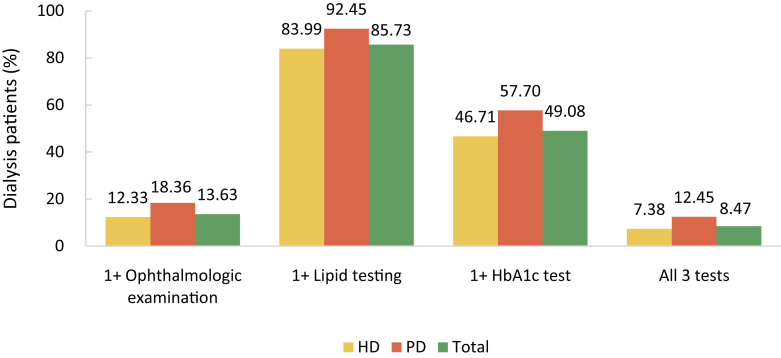
| e161 | Chapter 7. Clinical measurement and treatment among dialysis patients |
| e164 | Chapter 8. Vascular access |
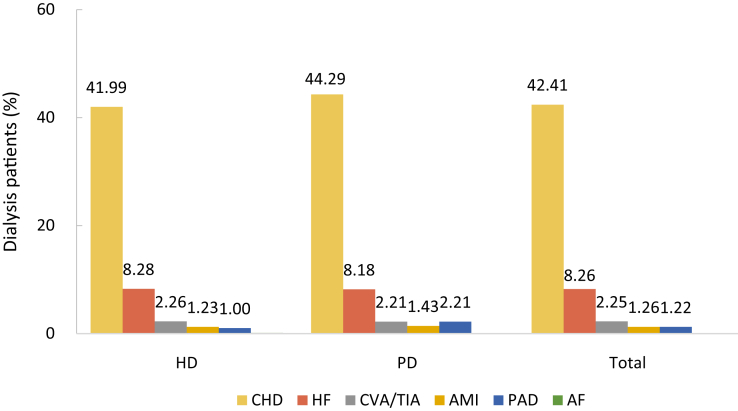
| e166 | Chapter 9. Cardiovascular disease and diabetes among dialysis patients |
| e169 | Chapter 10. Hospitalization among dialysis patients |
| e172 | Chapter 11. Medical expenditures for dialysis patients |
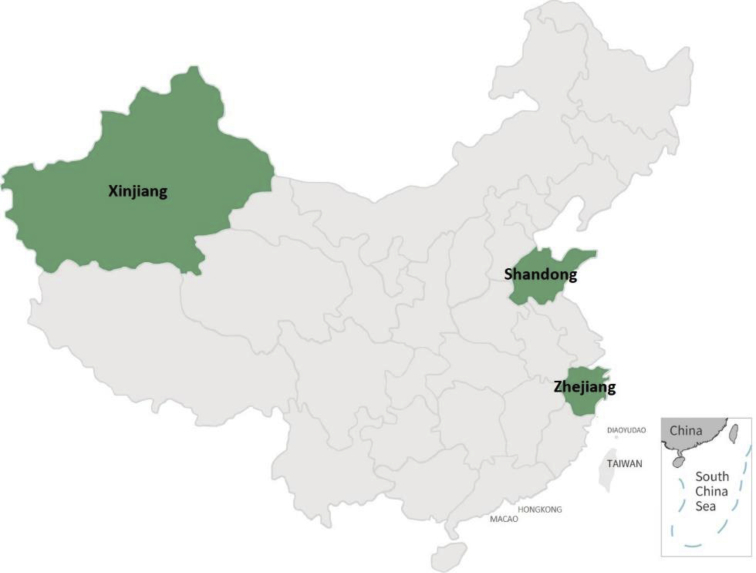
| e174 | Chapter 12. Regional data from dialysis registry system |
| e177 | Chapter 13. Kidney transplant waiting list |
| e178 | Chapter 14. Discussion |
| e179 | References |
| e180 | Appendices: Definitions of ICD coding |
| e180 | Appendix 1. Coding of various CKD etiologies |
| e181 | Appendix 2. Coding of CKD stages |
| e182 | Appendix 3. Coding of diabetes mellitus |
| e182 | Appendix 4. Coding of hypertension |
| e182 | Appendix 5. Coding of CVD |
| e184 | Appendix 6. Coding of CVD operations |
| e185 | Appendix 7. Coding of AKI |
Dedication
Establishing the prevention and control system of chronic kidney disease in china: exploration and practice of china kidney disease network
In recent years, the morbidity and mortality of chronic kidney disease (CKD) in China have increased significantly, accompanied with the rapidly rising incidence of metabolic diseases such as diabetes and hypertension, which poses a major threat to people’s health. Currently, the national prevention and control system of chronic diseases has a remarkable effect on reducing the burden of several major chronic diseases in China. However, the prevention and control system of CKD has not been established. Moreover, the standardized management of and intervention in patients have not been conducted in a timely and effective manner, which leads to a significant increase in the prevalence of end-stage kidney disease and huge consumption of health care resources in China. It would be helpful to promote the hierarchical medical system of CKD and ensure the medical needs of patients if CKD could be integrated into the national prevention strategy of chronic diseases.
At present, the prevention and control of CKD in China still faces many challenges, including low early awareness rate, low diagnosis rate, poor long-term prognosis, and high medical costs. Furthermore, the ability and capacity of primary care institutions still need to be improved. Thus, prevention and early screening are the key to reducing the incidence and development of CKD. Collecting, integrating, analyzing, and interpreting information on CKD using a comprehensive approach will be instrumental in prevention for this chronic disease and has the potential to inform policy makers.
Since the establishment of China Kidney Disease Network (CK-NET), it has continuously provided valuable “Chinese data” for the epidemiology of CKD in China, by integrating various sources of data involving kidney diseases. Without a doubt, the CK-NET has become an important part of the prevention and control system of CKD. Now this is the third annual data report published by the CK-NET team with more abundant contents and data compared with the previous reports. Under the joint efforts of experts from different disciplines, such as nephrology, public health, and data science, these reports have gradually become a treasure trove to understand the characteristics and trends of CKD and end-stage kidney disease in China. In addition, the exploration and practice of CK-NET through interdisciplinary cooperation created a replicable application model of big data, which not only accumulated unique experiences for the innovative research in nephrology, but also provided a large amount of population-level evidence for the management and decision of kidney diseases.
As the president of the Chinese Society of Nephrology, I am very pleased to see the contribution CK-NET has made to the prevention and control of kidney diseases in China, and also proud of the continuous endeavor of the CK-NET team in the past few decades. The establishment of the prevention and control system of CKD is a long-term systematic project. The road ahead will be long and our climb will be steep. I believe that with the integrated efforts of the government, medical community, and the public, the status of kidney diseases in China will usher in a new development.
Jianghua Chen, MD
Chairman, Chinese Society of Nephrology
Division of Nephrology
The First Affiliated Hospital of Zhejiang University
Hangzhou, Zhejiang, China
Abbreviations
| ADR | Annual Data Report |
| AF | atrial fibrillation |
| AKI | acute kidney injury |
| AMI | acute myocardial infarction |
| ATC | Anatomical Therapeutic Chemical |
| AVF | arteriovenous fistula |
| AVG | arteriovenous graft |
| CABG | coronary artery bypass grafting |
| CAD | coronary artery disease |
| CAG | coronarography |
| CHD | coronary heart disease |
| CHI | commercial health insurance |
| CHIRA | China Health Insurance Research Association |
| CKD | chronic kidney disease |
| CK-NET | China Kidney Disease Network |
| COTRS | China Organ Transplant Response System |
| CRRT | continuous renal replacement therapy |
| CTIN | chronic tubulointerstitial nephritis |
| CVA | cerebrovascular accident |
| CVC | central venous catheter |
| CVD | cardiovascular disease |
| DKD | diabetic kidney disease |
| DM | diabetes mellitus |
| EPO | erythropoietin |
| ESKD | end-stage kidney disease |
| GN | glomerulonephritis |
| HbA1c | hemoglobin A1c |
| HD | hemodialysis |
| HF | heart failure |
| HQMS | Hospital Quality Monitoring System |
| HT | hypertension |
| HTN | hypertensive nephropathy |
| IBNR | incurred but not reported |
| ICD | International Classification of Diseases |
| ICU | intensive care unit |
| iPTH | intact parathyroid hormone |
| IQR | interquartile range |
| ISN | International Society of Nephrology |
| IV | Intravenous |
| LOS | length of stay |
| MBD | mineral and bone disorder |
| NCC | noncuffed catheter |
| NRCMS | new rural co-operative medical care |
| ON | obstructive nephropathy |
| PAD | peripheral arterial disease |
| PCI | percutaneous coronary intervention |
| PD | peritoneal dialysis |
| PGN | primary glomerular nephropathy |
| PMP | per million population |
| PPPY | per person per year |
| PTH | parathyroid hormone |
| SD | standard deviation |
| SGN | secondary glomerular nephropathy |
| spKt/V | single-pool kt/V |
| TCC | tunneled cuffed catheter |
| TIA | transient ischemic attack |
| UBMI | urban basic medical insurance |
| UEBMI | Urban Employee Basic Medical Insurance |
| URBMI | Urban Residents Basic Medical Insurance |
| USRDS | United States Renal Data System |
| VA | vascular access |
Preface
In the last decade, chronic kidney disease (CKD) has been recognized as a major public health problem globally. CKD is a highly prevalent condition that contributes substantially to disease burden, both as a direct cause of global morbidity and mortality and as an important risk factor for cardiovascular disease.1 It is predicted that CKD will rise from 16th to 5th in the leading causes of early death between 2016 and 2040.2 The recent growth in the CKD population also implies an increasing burden of patients with end-stage kidney disease requiring kidney replacement therapy. China is a developing country with the largest population in the world, and CKD is prevalent in the country;3 however, there has been no well-established national surveillance system for kidney disease. Moreover, China still faces several challenges related to kidney care, including limited capacity and efficiency, suboptimal awareness, and huge heterogeneity in diagnosis and treatment.3,4
The unmet needs in nephrology have left ample space for leveraging big data and health information systems to improve the status of kidney health care.5,6 The China Kidney Disease Network (CK-NET), an initiative proposed by the late Professor Hai-Yan Wang in 2014,7 is now rapidly developing in accordance with the national strategy of prompting big data application in China. Currently, CK-NET is run by the Center for Data Science in Kidney Disease, Peking University Health Science Center, and supported by the Intelligent Medical Research Center, Advanced Institute of Information Technology, Peking University. Until now, more than 60 large renal centers and several regional medical data platforms in China have joined the collaborative network. Several large databases involving over 1 million patients with kidney disease including national administrative and claims databases, multicenter cohort studies, and regional electronic health records are available for use, under the authorization of relevant management departments. The website of CK-NET is http://www.chinakidney.net/.
One major output of CK-NET is to generate Annual Data Report (ADR) regarding kidney disease in China. The first CK-NET ADR consisted of 11 chapters, focusing on predialysis hospitalized patients, and was published as a supplement of American Journal of Kidney Diseases in June 2017.7 In that issue, Professor Rajiv Saran wrote an editorial entitled “The China Kidney Disease Network (CK-NET): Big Data-Big Dreams,”8 in which he said: “The CK-NET 2014 annual data report will undoubtedly serve as an important benchmark for kidney disease surveillance in China.” In March 2019, the executive summary and full text of CK-NET 2015 ADR were published in Kidney International and Kidney International Supplements, respectively.9,10 More information regarding adult dialysis patients in China has been included in this second CK-NET ADR and certain parts, especially the cardiovascular chapter, have been enriched, which provided detailed data for understanding the burden of CKD and end-stage kidney disease in China.
This year marks the third publication of CK-NET ADR. With the expansion of research group and data sources, the content of this report was further enriched. The travel patterns of patients with CKD were delineated from a national perspective in Chapter 1. An independent chapter regarding statistics from 3 provincial dialysis quality control centers (Shandong, Zhejiang, and Xinjiang) has been added to provide regional data. Furthermore, dialysis patients aged <18 years and covered by Urban Employee Basic Medical Insurance (UEBMI) and Urban Residents Basic Medical Insurance (URBMI) were also included so that the status of children and adolescents could be understood and corresponding prevention and control strategies could be formulated. This CK-NET 2016 ADR symbolizes a successful team effort in the era of big data, with support from the specialists and partners of our collaboration network.
However, the following limitations should be considered when interpreting the results in this report: first, selection bias cannot be ruled out because of limitations in data sampling. Second, International Classification of Diseases-10 codes are used to define CKD and other related diseases probably with low sensitivity and high specificity. Third, the percentage of CKD in our report comprehensively reflects the prevalence, hospitalization rate, and diagnostic rate, which was analyzed based on Hospital Quality Monitoring System. In 2016, the total number of hospitalizations in tertiary hospitals in China was reported to be 76.86 million,11 of which 48.0% were covered by Hospital Quality Monitoring System. Interpretations of results and epidemiologic definitions require careful consideration. Finally, the current ADR is only based on cross-sectional data, making causal inference difficult. A brief interpretation is included in each chapter to facilitate the understanding of the contents.
Disclosure
This article is published as a supplement supported by Peking University. Dr. Luxia Zhang received research funding from AstraZeneca. All the other authors declared no competing interests.
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (91846101, 81771938, 81301296, 81900665), Beijing Nova Programme Interdisciplinary Cooperation Project (Z191100001119008), the National Key R&D Program of the Ministry of Science and Technology of China (2016YFC1305405, 2019YFC2005000), the University of Michigan Health System-Peking University Health Science Center Joint Institute for Translational and Clinical Research (BMU20160466, BMU2018JI012, BMU2019JI005), CAMS Innovation Fund for Medical Sciences (2019-I2M-5-046), PKU-Baidu Fund (2019BD017), and Peking University (BMU2018MX020, PKU2017LCX05). We thank the National Health Commission of China, Ministry of Science and Technology of China, National Natural Science Foundation of China, Beijing Municipal Science and Technology Commission, China Health Insurance Research Association, China Organ Transplantation Development Foundation, Peking University, and China Standard Medical Information Research Center for the support of this study. We also thank CK-NET collaborating centers, members, and volunteers for their hard work and efforts, and every participant who has contributed important data to this work.
Analytical methods
Introduction
The analytical methods chapter describes the data sources, database definition, and analytical methods of the China Kidney Disease Network (CK-NET) 2016 Annual Data Report (ADR). For this ADR, we report on data from January 1, 2016, to December 31, 2016. The analyses are based on 4 national databases: the Hospital Quality Monitoring System (HQMS) database, China Health Insurance Research Association (CHIRA) database, Commercial Health Insurance (CHI) database, and China Organ Transplant Response System (COTRS) database.
The ethics committee of Peking University First Hospital approved this study. The contents of this report have been internally and externally reviewed and submitted to the National Health Commission of the People's Republic of China. The statistical analyses were performed using Microsoft Excel 2016 (Microsoft Corp., Redmond, WA) and SAS 9.4 (SAS Institute Inc., Cary, NC).
Data sources
HQMS database
The HQMS database is a mandatory national inpatient database system under the authority of the National Health Commission of the People’s Republic of China. All tertiary hospitals in China have been requested to submit standardized inpatient discharge records to HQMS on a daily basis and in an automated manner since 2013. Tertiary hospitals constitute the top tier of the medical system in China; at a minimum, they must have 500 beds and accreditation from health authorities. As opposed to tertiary hospitals in the Western medical system, tertiary hospitals in China provide primary, secondary, and tertiary care and specialist health services, which are exposed to a nationwide patient population. By contrast, primary hospitals are defined as community medical institutions that provided primary health services (<100 beds), and secondary hospitals are local medical institutions that provide comprehensive health services (100–499 beds).
Patient-level data were collected from the nationally uniform front page of the hospitalization medical record. Altogether, 353 variables including patient demographics, diagnoses in the form of International Classification of Diseases-10 (ICD-10) codes, procedures and operations, financial breakdowns, and information of affiliated hospitals or divisions were collected. As a part of stringent standard practice in China, the front page has legal validity and must be filled by the care-giving doctors who have the most accurate and comprehensive understanding of the patient’s medical condition. The diagnoses were then coded according to the ICD-10 coding system by certified professional medical coders at each hospital. The HQMS data reporting system performs automated data quality control on a daily basis at the time of data submission to ensure the completeness, consistency, and accuracy of data. If inconsistencies are detected, the entire daily data package of the hospital will be rejected and the hospital will be required to review and resubmit data.
As of December 2016, the HQMS database automated data exchange network covered 961 tertiary hospitals in 31 provinces (excluding Hong Kong, Macao, and Taiwan), accounting for more than 52% of the total number of tertiary hospitals in 2016, and had collected over 80 million hospitalization records. The number of tertiary hospitals covered by HQMS increased by 26 (from 935 in 2015 to 961 in 2016) and that of hospitalizations by 2.63 million (from 34.23 million in 2015 to 36.86 million in 2016).
CHIRA database
Urban basic medical insurance (UBMI) is the predominant medical insurance program in urban areas of China, covering 31 provinces and municipalities (excluding Hong Kong, Macao, and Taiwan). UBMI comprises the Urban Employee Basic Medical Insurance (UEBMI) and the Urban Resident Basic Medical Insurance (URBMI). By the end of 2016, the number of insured people reached 295 million and 448 million, respectively.
The CHIRA database is a national claims database initiated in 2007, which covers information on diagnosis, demographics, frequency of lab tests, prescription drugs, operation procedures, and medical expenditures of outpatients and inpatients at all levels of hospital (primary, secondary, and tertiary hospitals). A 2-stage sampling design was used to extract a national sample insured by UEBMI and URBMI in 22 provinces, 5 autonomous regions, and 4 municipalities directly under the central government in mainland China (excluding Hong Kong, Macao, and Taiwan). In the first stage, convenience sampling was conducted in 4 municipalities directly under the Central Government (Beijing, Shanghai, Tianjin, and Chongqing), 27 provincial capital cities, and a certain number of prefecture cities. In the second stage, a systematic random sampling sorted by age was used to extract approximately 2% of insured population from the municipalities/provincial capital cities and approximately 5% of that from prefecture-level cities. The number of sampling beneficiaries in the CHIRA database in 2016 was 8,516,679, an increase of 1.38 million from 2015, with their whole-year claims data recorded. All personal information including name, identity card number, medical insurance number, telephone number, and home address was anonymized and de-identified before analysis for the privacy protection reasons.
CHI database
The CHI database was extracted from 6 top commercial insurance companies with the largest market share in mainland China, covering 22 kinds of major diseases and over 60 million customers in 2016. The number of insurance policies exceeded 95 million from 1995 to 2016 in 31 provinces, autonomous regions, and municipalities directly under the central government (excluding Hong Kong, Macao, and Taiwan). Information of sex, age, insured amount, region, occupation, income, and disease diagnosis of policy holders was recorded in the database. Incidence of dialysis people was analyzed based on the CHI database.
COTRS database
Since September 1, 2013, it has become mandatory to allocate organs through COTRS in China, which is a national open transparent organ allocation computer system. The COTRS database is maintained by an impartial third party. The matching of donor organs to the recipients includes medical emergency, time spent on the waiting list, and histocompatibility. The chapter about the waiting list for kidney transplantation in China was based on the analysis of the COTRS database. The data regarding the waiting list for kidney transplantation were provided by the Report on Organ Transplantation Development in China (2015–2018), so this year's report did not present the detailed data.
Database definitions
Identifying patients with CKD
Three sets of ICD-10 disease codes were used to identify adult patients (≥18 years) with chronic kidney disease (CKD) in tertiary hospitals in China based on the HQMS database: Beijing version 4.0, National Standard version 1.0, and National clinical version 1.0. Codes for procedures and operations were based on the Beijing version and National clinical version. Patients with diabetes and CKD were defined as those diagnosed with both diabetes and CKD, but without the presence of nondiabetic kidney diseases evaluated by physicians.12,13 Results of renal biopsy were not available for most patients. Patients with acute kidney injury (AKI) were identified by ICD-10 coding in the HQMS database. Despite being aware of that AKI might be substantially underestimated by ICD-10 coding, we kept the chapter because it could reflect the reality of diagnoses. All relevant ICD codes are listed in Appendices 1–7.
Identifying dialysis patients
Dialysis patients were identified on the basis of the service items in medical billings and ICD-10 codes, which were defined as CKD requiring dialysis (hemodialysis [HD] and peritoneal dialysis [PD]), excluding acute renal failure. PD patients were identified by claim records of peritoneal dialysis fluid, and HD patients were identified by claim records of HD, including hemodialyzer and related operations.
Cardiovascular disease
Patients with cardiovascular disease (CVD) were identified by the diagnosis of CVD (ICD-10 coding), claim records of therapeutic drugs for CVD based on the Anatomical Therapeutic Chemical (ATC) codes (C01, cardiac therapy) and related operation procedures, such as coronary artery computed tomography and coronary arteriography. Coronary heart disease, acute myocardial infarction, heart failure, cerebrovascular accident/transient ischemic attack, peripheral arterial disease, atrial fibrillation, and cardiovascular procedures (percutaneous coronary intervention and pacemaker) were also identified by ICD-10 coding and related claim records.
Diabetes
Diabetic patients were identified by the diagnosis of diabetes (ICD-10 coding) and claim records of therapeutic drugs for diabetes (A10, drugs used in diabetes). The subgroup of “patients with diabetes” in the results did not necessarily have kidney disease.
Hypertension
Patients with hypertension were identified by the diagnosis of hypertension (ICD-10 coding) in the HQMS database.
Infectious disease
Infectious disease was identified by the top three ICD-10 coding of infection by various pathogens.
Clinical indicators
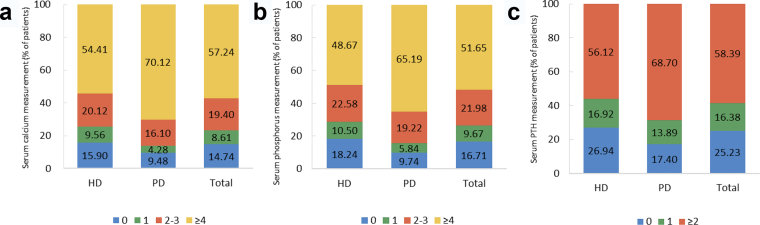
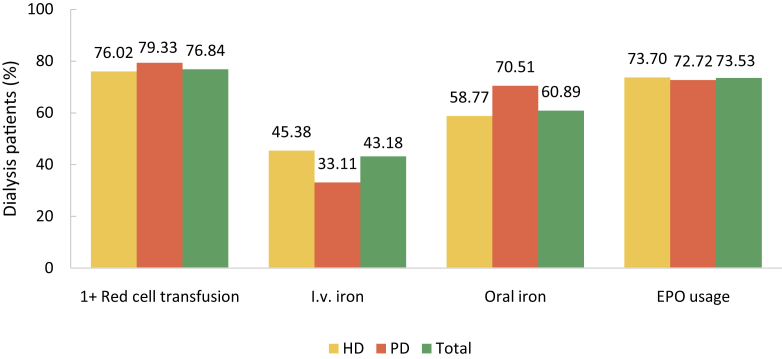
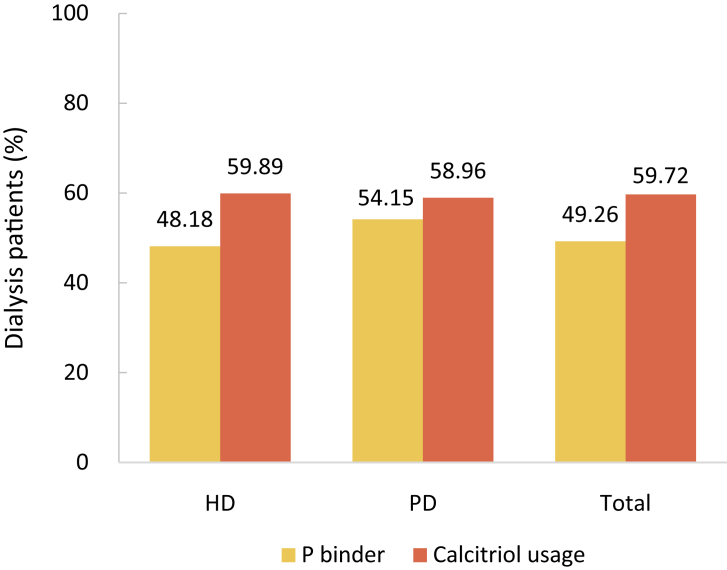
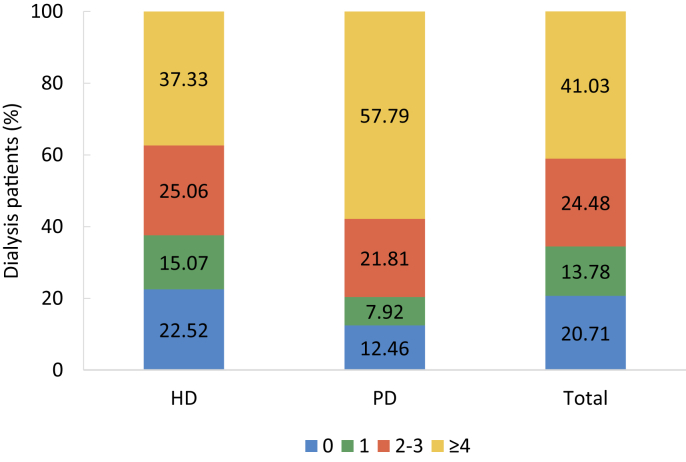
Lab tests and drug usage were identified by claim records. Lab tests included blood hemoglobin, serum levels of iron, total calcium, phosphorus, parathyroid hormone (PTH), albumin, lipids and HbA1c, and fundus examination for search of diabetic retinopathy. But the results of these tests were not recorded in the database. Drug usage included erythropoietin, iron (intravenous iron and oral iron), calcitriol, phosphate binder, and transfusion therapy.
Vascular access
The definitions of tunneled and cuffed catheter (TCC), noncuffed catheter (NCC), interventions for autogenous arteriovenous fistula (AVF)/autogenous arteriovenous graft (AVG), and stable AVF/AVG for HD patients were based on the claim records of surgical interventions, medical materials, and nursing treatments. For PD, newly inserted peritoneal catheters, transient central venous catheters (CVC), and stable patients were identified in the same way.
Statistical methods
Statistical methods included descriptive statistics, such as frequency, percentage, median with interquartile range, mean, and SD. The results were generally described by sex, age, geographic distribution, comorbidity, and dialysis modality. P values were not included because of large sample sizes.
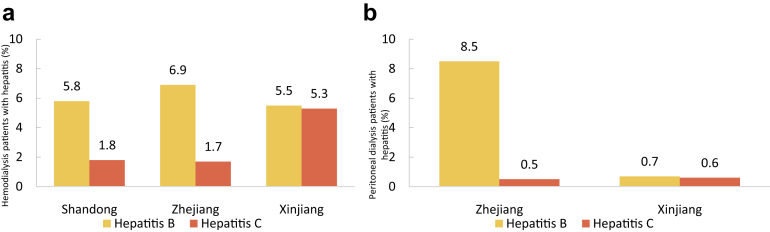
The comparisons between the 2 groups of patients with diabetes and those with CKD were based on the overall reference population, respectively, which meant we did not exclude patients with diabetes also having CKD or patients with CKD also having diabetes. Medical migration was defined as patients leaving their permanent residence to travel to other provinces for hospitalization. The prevalence of dialysis was estimated by multiplying percentage of dialysis patients in sampled data from the CHIRA database and the relevant UBMI utilization rate (data were from 2017 China Statistics Yearbook and Statistical Communique of the People’s Republic of China on the 2017 National Economic and Social Development). The incidence count in the CHI database has taken into account incurred but not reported (IBNR) events, which were often used to estimate the corresponding incidence rates in insurance industries. The age-adjusted prevalence and incidence of dialysis was standardized by the direct method using the 2010 national population census data. Dialysis data from the local renal registry systems in 3 provinces (Shandong, Zhejiang, and Xinjiang) were analyzed, and the results were collected through a standardized form via e-mail.
In the scenario that the interval between hospital discharge and following readmission was less than 3 days, we considered this as a continuous hospitalization. One hospitalization with a length of stay of ≥180 days was excluded. In the chapter on vascular access, HD patients would belong to only 1 group by a certain filter sequence from operational AVF/AVG, TCC, to NCC at last. If more than one kind of intervention were performed, the anterior filter situation would be selected. Patients without any intervention would be recognized as belonging to stable AVF/AVG. We could not distinguish AVF from AVG in the present database. Patients who had new PD catheter placement operations were considered as new-onset PD patients. Among these patients, patients who had CVC placement were considered to be transitional. Patients without new PD catheter placement operations were considered as maintenance PD patients. Stable PD patients were defined as maintenance PD patients without CVC placement operations. We did not separate TCC and NCC in the CVC group further because TCC was seldom used.
Section I. Chronic kidney disease
Chapter 1: Identification and characteristics of hospitalized patients with CKD
Bixia Gao1,2, Lanxia Gan3, Chao Yang1,2, Zaiming Su4, Jinwei Wang1,2, Ying Shi3 and Fang Wang1,2
1Renal Division, Department of Medicine, Peking University First Hospital, Peking University Institute of Nephrology, Beijing, China; 2Research Units of Diagnosis and Treatment of Immune-Mediated Kidney Diseases, Chinese Academy of Medical Sciences, Beijing, China; 3China Standard Medical Information Research Center, Shenzhen, Guangdong, China; and 4National Institute of Health Data Science at Peking University, Beijing, China
This chapter focuses on the prevalence, characteristics, and travel pattern of patients with CKD among the hospitalized population in tertiary hospitals in China.
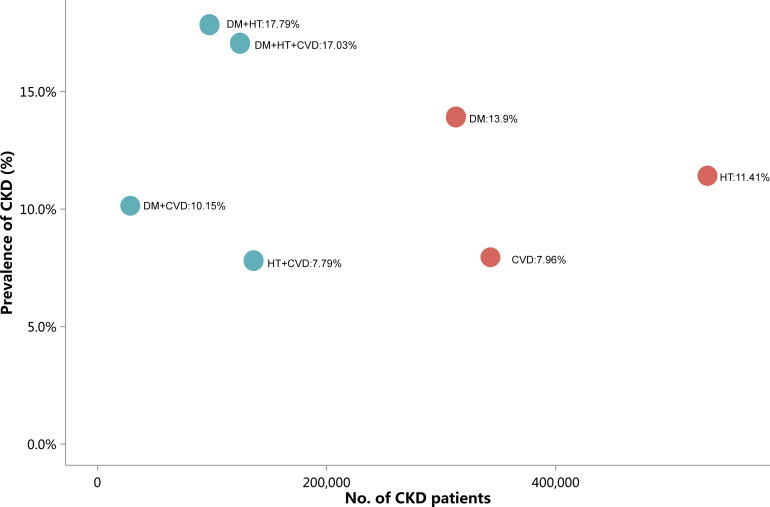
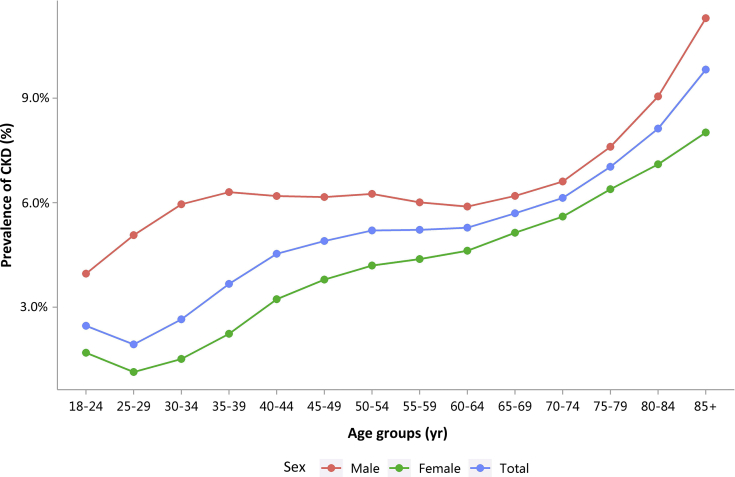
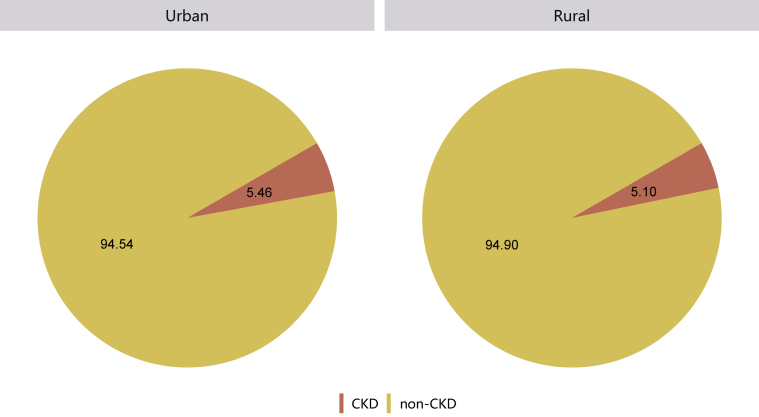
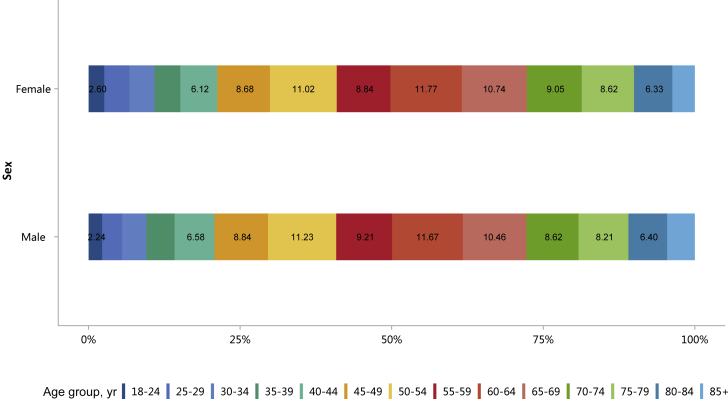
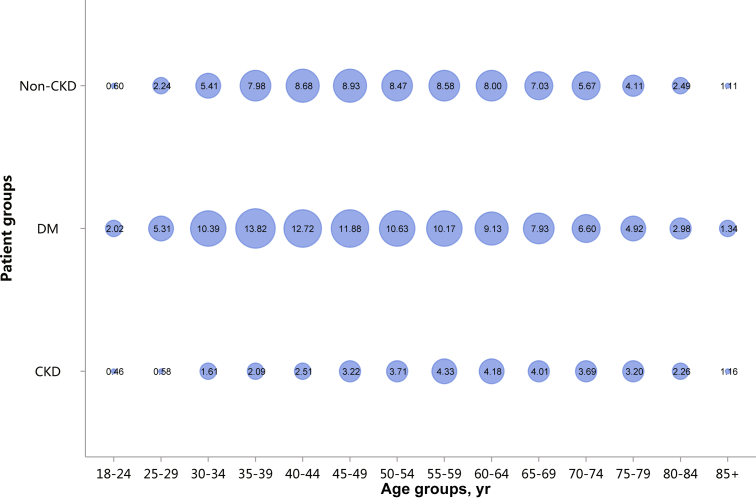
Patients with CKD constituted 4.86% of all inpatients, which was slightly higher than that in 2015 (4.80%).10 This percentage was particularly high among those with other major noncommunicable diseases, such as diabetes and hypertension (Figure 1 and Table 1). Moreover, the percentage of CKD increased with age (Figure 2 and Table 2), and the proportion of CKD in urban areas was higher than that in rural areas (5.46% vs. 5.10%; Figure 3 and Table 3). It should be noted that these percentages comprehensively reflected both the actual prevalence and the diagnosis rate because of the occurrence of missed diagnosis. Over one-half of the patients with CKD were 60 years or older (Figure 4 and Table 4), and a male predominance was observed in each age group (Figure 5 and Table 5).
Figure 1.
Prevalence of CKD among different types of underlying disease. CKD, chronic kidney disease; CVD, cardiovascular disease; DM, diabetes mellitus; HT, hypertension.
Table 1.
Prevalence of CKD among different types of underlying disease, N (%)
| Patient group | No. of patients with CKD | Prevalence of CKD (%) |
|---|---|---|
| HQMS | 992,727 | 4.86 |
| HT | 532,564 | 11.41 |
| CVD | 343,069 | 7.96 |
| DM | 312,854 | 13.90 |
| HT+CVD | 136,273 | 7.79 |
| DM+HT+CVD | 124,373 | 17.03 |
| DM+HT | 97,748 | 17.79 |
| DM+CVD | 28,549 | 10.15 |
CKD, chronic kidney disease; CVD, cardiovascular disease; DM, diabetes mellitus; HQMS, Hospital Quality Monitoring System; HT, hypertension.
Figure 2.
Patients with CKD, stratified by sex and age. CKD, chronic kidney disease.
Table 2.
Patients with CKD, stratified by sex and age, N (%)
| Age group, yr | Male | Female | Total |
|---|---|---|---|
| 18–24 | 12,995 (3.96) | 10,739 (1.69) | 23,734 (2.47) |
| 25–29 | 19,116 (5.07) | 17,054 (1.14) | 36,170 (1.93) |
| 30–34 | 23,053 (5.95) | 17,033 (1.52) | 40,086 (2.65) |
| 35–39 | 27,031 (6.30) | 17,710 (2.24) | 44,741 (3.67) |
| 40–44 | 38,140 (6.19) | 25,289 (3.23) | 63,429 (4.53) |
| 45–49 | 51,195 (6.16) | 35,878 (3.79) | 87,073 (4.90) |
| 50–54 | 65,079 (6.25) | 45,549 (4.19) | 110,628 (5.20) |
| 55–59 | 53,377 (6.01) | 36,555 (4.38) | 89,932 (5.22) |
| 60–64 | 67,617 (5.89) | 48,652 (4.62) | 116,269 (5.28) |
| 65–69 | 60,623 (6.19) | 44,383 (5.14) | 105,006 (5.70) |
| 70–74 | 49,914 (6.61) | 37,411 (5.60) | 87,325 (6.14) |
| 75–79 | 47,589 (7.60) | 35,641 (6.39) | 83,230 (7.03) |
| 80–84 | 37,090 (9.05) | 26,180 (7.10) | 63,270 (8.13) |
| 85+ | 26,476 (11.29) | 15,358 (8.02) | 41,834 (9.82) |
| Total | 579,295 (6.40) | 413,432 (3.63) | 992,727 (4.86) |
CKD, chronic kidney disease.
Figure 3.
Patients with CKD, stratified by urban versus rural area (percent values). CKD, chronic kidney disease.
Table 3.
Patients with CKD, stratified by urban versus rural area, N (% of general population)
| Residence | CKD |
|---|---|
| Urban | 509,412 (5.46) |
| Rural | 218,041 (5.10) |
| Total | 727,453 (5.35) |
CKD, chronic kidney disease; HQMS, Hospital Quality Monitoring System.
Patients with missing data for residence were not included in the analysis. HQMS: 6,844,236 (33.48%).
Figure 4.
Age distribution of patients with CKD, stratified by sex. CKD, chronic kidney disease.
Table 4.
Age distribution of patients with CKD, stratified by sex, N (%)
| Age group, yr | Male | Female | Total |
|---|---|---|---|
| 18–24 | 12,995 (2.24) | 10,739 (2.60) | 23,734 (2.39) |
| 25–29 | 19,116 (3.30) | 17,054 (4.12) | 36,170 (3.64) |
| 30–34 | 23,053 (3.98) | 17,033 (4.12) | 40,086 (4.04) |
| 35–39 | 27,031 (4.67) | 17,710 (4.28) | 44,741 (4.51) |
| 40–44 | 38,140 (6.58) | 25,289 (6.12) | 63,429 (6.39) |
| 45–49 | 51,195 (8.84) | 35,878 (8.68) | 87,073 (8.77) |
| 50–54 | 65,079 (11.23) | 45,549 (11.02) | 110,628 (11.14) |
| 55–59 | 53,377 (9.21) | 36,555 (8.84) | 89,932 (9.06) |
| 60–64 | 67,617 (11.67) | 48,652 (11.77) | 116,269 (11.71) |
| 65–69 | 60,623 (10.46) | 44,383 (10.74) | 105,006 (10.58) |
| 70–74 | 49,914 (8.62) | 37,411 (9.05) | 87,325 (8.80) |
| 75–79 | 47,589 (8.21) | 35,641 (8.62) | 83,230 (8.38) |
| 80–84 | 37,090 (6.40) | 26,180 (6.33) | 63,270 (6.37) |
| 85+ | 26,476 (4.57) | 15,358 (3.71) | 41,834 (4.21) |
| Total | 579,295 | 413,432 | 992,727 |
CKD, chronic kidney disease.
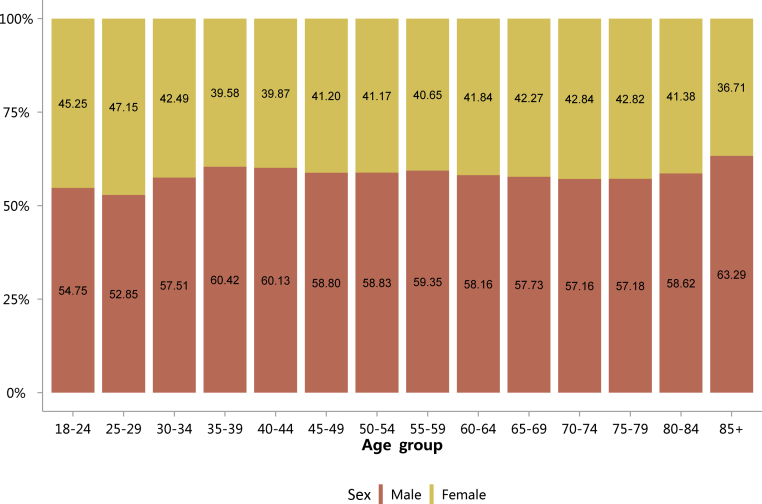
Figure 5.
Sex distribution of patients with CKD, stratified by age. Digits in columns represent percent values. CKD, chronic kidney disease.
Table 5.
Sex distribution of patients with CKD, stratified by age, N (%)
| Age group, yr | Male | Female | Total |
|---|---|---|---|
| 18–24 | 12,995 (54.75) | 10,739 (45.25) | 23,734 |
| 25–29 | 19,116 (52.85) | 17,054 (47.15) | 36,170 |
| 30–34 | 23,053 (57.51) | 17,033 (42.49) | 40,086 |
| 35–39 | 27,031 (60.42) | 17,710 (39.58) | 44,741 |
| 40–44 | 38,140 (60.13) | 25,289 (39.87) | 63,429 |
| 45–49 | 51,195 (58.80) | 35,878 (41.20) | 87,073 |
| 50–54 | 65,079 (58.83) | 45,549 (41.17) | 110,628 |
| 55–59 | 53,377 (59.35) | 36,555 (40.65) | 89,932 |
| 60–64 | 67,617 (58.16) | 48,652 (41.84) | 116,269 |
| 65–69 | 60,623 (57.73) | 44,383 (42.27) | 105,006 |
| 70–74 | 49,914 (57.16) | 37,411 (42.84) | 87,325 |
| 75–79 | 47,589 (57.18) | 35,641 (42.82) | 83,230 |
| 80–84 | 37,090 (58.62) | 26,180 (41.38) | 63,270 |
| 85+ | 26,476 (63.29) | 15,358 (36.71) | 41,834 |
| Total | 579,295 (58.35) | 413,432 (41.65) | 992,727 |
CKD, chronic kidney disease.
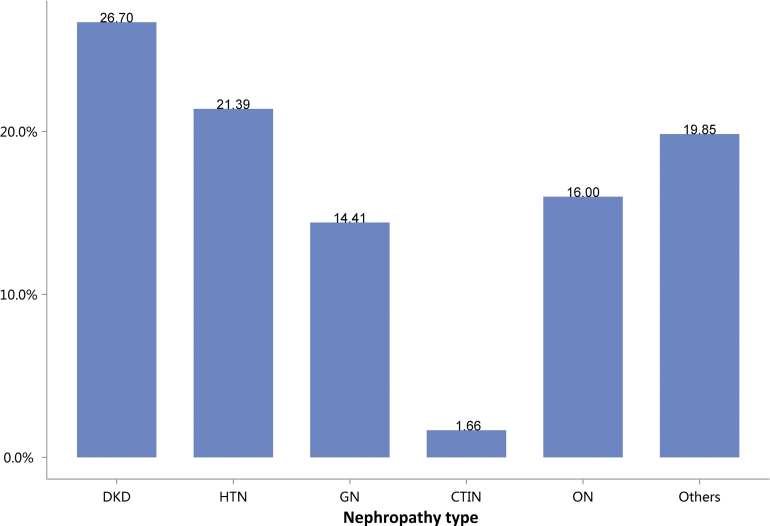
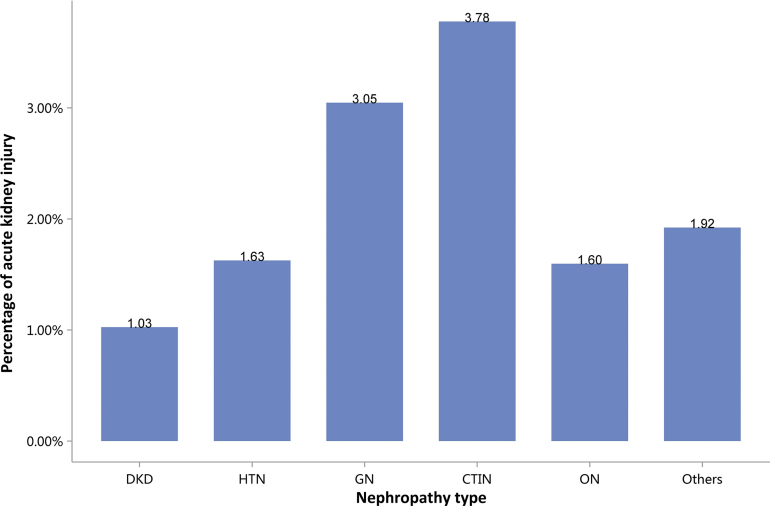
Regarding the etiology of CKD, the most commonly coded causes included diabetes (26.70%), hypertensive nephropathy (HTN, 21.39%), obstructive nephropathy (ON, 16.00%), and glomerulonephritis (GN, 14.41%; Figure 6 and Table 6). It must be noted that, in this chapter and subsequent chapters, we used the term diabetic kidney disease (DKD) in tables and figures to make the presentation of results more concise, but in fact these patients should be patients with both diabetes and CKD in the absence of a kidney biopsy.
Figure 6.
Cause distribution of patients with CKD. Digits above columns represent percent values. CKD, chronic kidney disease; CTIN, chronic tubulointerstitial nephropathy; DKD, diabetic kidney disease; GN, glomerulonephritis; HTN, hypertensive nephropathy; ON, obstructive nephropathy; Others, CKD due to other reasons.
Table 6.
Cause distribution of patients with CKD
| Cause | N (%) |
|---|---|
| DKD | 265,067 (26.70) |
| HTN | 212,309 (21.39) |
| GN | 143,024 (14.41) |
| CTIN | 16,494 (1.66) |
| ON | 158,824 (16.00) |
| Others | 197,009 (19.85) |
| Total | 992,727 |
CKD, chronic kidney disease; CTIN, chronic tubulointerstitial nephropathy; DKD, diabetic kidney disease; GN, glomerulonephritis; HTN, hypertensive nephropathy; ON, obstructive nephropathy; Others, CKD due to other reasons.
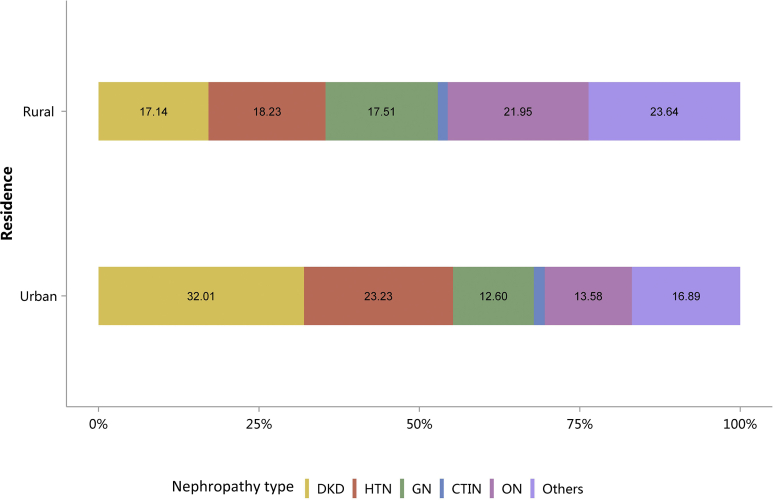
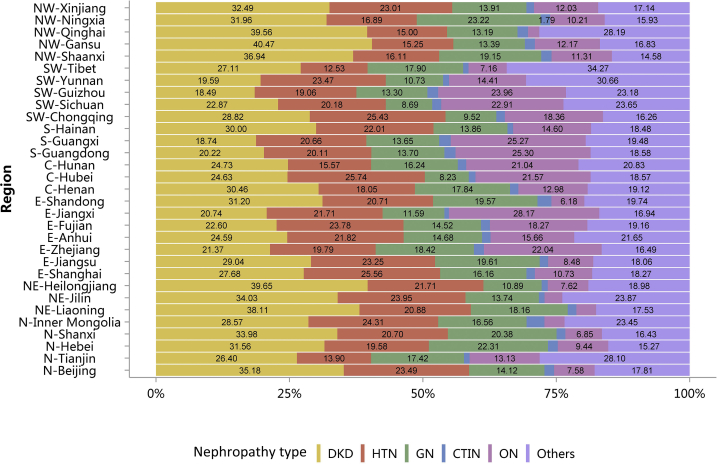
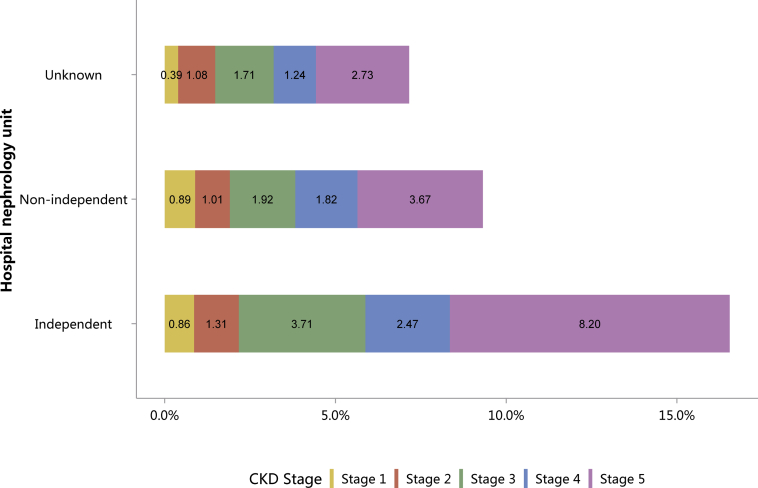
The percentages of HTN and ON have increased slightly compared with those in 2015.10 Furthermore, the spectrum of CKD varied between urban and rural areas. The top 3 causes in urban areas were diabetes (32.01%), HTN (23.23%), and ON (13.58%), whereas for rural residents, the leading causes were ON (21.95%), HTN (18.23%), and GN (17.51%), followed by diabetes (17.14%; Figure 7 and Table 7). There was an obvious geographic variation of incriminated etiologies, where a relatively high percentage of diabetes was found in the northeast and northwest of China, whereas a high percentage of ON was reported in the south and several provinces in the east of the country (Figure 8 and Table 8). Only 15.53% of inpatients with CKD had diagnostic codes for CKD staging, which reflected the pattern of diagnosis rather than patient characteristics (Figure 9 and Table 9).
Figure 7.
Cause of patients with CKD, stratified by urban versus rural area. Digits above columns represent percent values. CKD, chronic kidney disease; CTIN, chronic tubulointerstitial nephropathy; DKD, diabetic kidney disease; GN, glomerulonephritis; HTN, hypertensive nephropathy; ON, obstructive nephropathy; Others, CKD due to other reasons.
Table 7.
Cause of patients with CKD, stratified by urban versus rural area, N (%)
| Cause | Urban | Rural | Total |
|---|---|---|---|
| DKD | 163,076 (32.01) | 37,367 (17.14) | 200,443 (27.55) |
| HTN | 118,330 (23.23) | 39,741 (18.23) | 158,071 (21.73) |
| GN | 64,203 (12.60) | 38,177 (17.51) | 102,380 (14.07) |
| CTIN | 8620 (1.69) | 3354 (1.54) | 11,974 (1.65) |
| ON | 69,155 (13.58) | 47,858 (21.95) | 117,013 (16.09) |
| Others | 86,028 (16.89) | 51,544 (23.64) | 137,572 (18.91) |
| Total | 509,412 | 218,041 | 727,453 |
CKD, chronic kidney disease; CTIN, chronic tubulointerstitial nephropathy; DKD, diabetic kidney disease; GN, glomerulonephritis; HTN, hypertensive nephropathy; ON, obstructive nephropathy; Others, CKD due to other reasons.
Patients with missing data for residence were not included in the analysis. CKD: 265,274 (26.72%).
Figure 8.
Cause of patients with CKD, stratified by geographic region. C, Central China; CKD, chronic kidney disease; CTIN, chronic tubulointerstitial nephropathy; DKD, diabetic kidney disease; E, East China; GN, glomerulonephritis; HTN, hypertensive nephropathy; N, North China; NE, Northeast China; NW, Northwest China; ON, obstructive nephropathy; Others, CKD due to other reasons; S, South China; SW, Southwest China.
Table 8.
Cause of patients with CKD, stratified by geographic region, N (%)
| Region | DKD | HTN | GN | CTIN | ON | Others | Total |
|---|---|---|---|---|---|---|---|
| N-Beijing | 9129 (35.18) | 6094 (23.49) | 3665 (14.12) | 469 (1.81) | 1968 (7.58) | 4622 (17.81) | 25,947 |
| N-Tianjin | 1164 (26.40) | 613 (13.90) | 768 (17.42) | 46 (1.04) | 579 (13.13) | 1239 (28.10) | 4409 |
| N-Hebei | 9093 (31.56) | 5643 (19.58) | 6428 (22.31) | 533 (1.85) | 2720 (9.44) | 4399 (15.27) | 28,816 |
| N-Shanxi | 7814 (33.98) | 4760 (20.70) | 4686 (20.38) | 381 (1.66) | 1575 (6.85) | 3779 (16.43) | 22,995 |
| N-Inner Mongolia | 6645 (28.57) | 5654 (24.31) | 3852 (16.56) | 778 (3.34) | 877 (3.77) | 5456 (23.45) | 23,262 |
| NE-Liaoning | 11,310 (38.11) | 6197 (20.88) | 5389 (18.16) | 478 (1.61) | 1100 (3.71) | 5202 (17.53) | 29,676 |
| NE-Jilin | 6466 (34.03) | 4551 (23.95) | 2611 (13.74) | 206 (1.08) | 631 (3.32) | 4535 (23.87) | 19,000 |
| NE-Heilongjiang | 9585 (39.65) | 5248 (21.71) | 2633 (10.89) | 276 (1.14) | 1841 (7.62) | 4589 (18.98) | 24,172 |
| E-Shanghai | 6746 (27.68) | 6231 (25.56) | 3940 (16.16) | 388 (1.59) | 2616 (10.73) | 4454 (18.27) | 24,375 |
| E-Jiangsu | 16,229 (29.04) | 12,991 (23.25) | 10,955 (19.61) | 877 (1.57) | 4737 (8.48) | 10,089 (18.06) | 55,878 |
| E-Zhejiang | 8433 (21.37) | 7812 (19.79) | 7272 (18.42) | 743 (1.88) | 8701 (22.04) | 6510 (16.49) | 39,471 |
| E-Anhui | 7659 (24.59) | 6798 (21.82) | 4572 (14.68) | 500 (1.60) | 4880 (15.66) | 6744 (21.65) | 31,153 |
| E-Fujian | 5806 (22.60) | 6109 (23.78) | 3729 (14.52) | 431 (1.68) | 4692 (18.27) | 4921 (19.16) | 25,688 |
| E-Jiangxi | 9853 (20.74) | 10,315 (21.71) | 5504 (11.59) | 405 (0.85) | 13,384 (28.17) | 8048 (16.94) | 47,509 |
| E-Shandong | 9951 (31.20) | 6606 (20.71) | 6241 (19.57) | 832 (2.61) | 1970 (6.18) | 6296 (19.74) | 31,896 |
| C-Henan | 13,120 (30.46) | 7774 (18.05) | 7683 (17.84) | 667 (1.55) | 5593 (12.98) | 8237 (19.12) | 43,074 |
| C-Hubei | 22,774 (24.63) | 23,796 (25.74) | 7608 (8.23) | 1167 (1.26) | 19,941 (21.57) | 17,164 (18.57) | 92,450 |
| C-Hunan | 9553 (24.73) | 6017 (15.57) | 6276 (16.24) | 615 (1.59) | 8128 (21.04) | 8047 (20.83) | 38,636 |
| S-Guangdong | 16,923 (20.22) | 16,833 (20.11) | 11,462 (13.70) | 1748 (2.09) | 21,173 (25.30) | 15,546 (18.58) | 83,685 |
| S-Guangxi | 6353 (18.74) | 7004 (20.66) | 4626 (13.65) | 744 (2.19) | 8567 (25.27) | 6605 (19.48) | 33,899 |
| S-Hainan | 3125 (30.00) | 2293 (22.01) | 1444 (13.86) | 110 (1.06) | 1521 (14.60) | 1925 (18.48) | 10,418 |
| SW-Chongqing | 3740 (28.82) | 3301 (25.43) | 1235 (9.52) | 210 (1.62) | 2383 (18.36) | 2110 (16.26) | 12,979 |
| SW-Sichuan | 16,218 (22.87) | 14,306 (20.18) | 6163 (8.69) | 1206 (1.70) | 16,242 (22.91) | 16,768 (23.65) | 70,903 |
| SW-Guizhou | 3096 (18.49) | 3190 (19.06) | 2226 (13.30) | 338 (2.02) | 4011 (23.96) | 3880 (23.18) | 16,741 |
| SW-Yunnan | 10,576 (19.59) | 12,675 (23.47) | 5794 (10.73) | 612 (1.13) | 7781 (14.41) | 16,557 (30.66) | 53,995 |
| SW-Tibet | 106 (27.11) | 49 (12.53) | 70 (17.90) | 4 (1.02) | 28 (7.16) | 134 (34.27) | 391 |
| NW-Shaanxi | 9871 (36.94) | 4304 (16.11) | 5117 (19.15) | 514 (1.92) | 3021 (11.31) | 3895 (14.58) | 26,722 |
| NW-Gansu | 3494 (40.47) | 1317 (15.25) | 1156 (13.39) | 163 (1.89) | 1051 (12.17) | 1453 (16.83) | 8634 |
| NW-Qinghai | 1820 (39.56) | 690 (15.00) | 607 (13.19) | 90 (1.96) | 97 (2.11) | 1297 (28.19) | 4601 |
| NW-Ningxia | 1430 (31.96) | 756 (16.89) | 1039 (23.22) | 80 (1.79) | 457 (10.21) | 713 (15.93) | 4475 |
| NW-Xinjiang | 5220 (32.49) | 3697 (23.01) | 2234 (13.91) | 228 (1.42) | 1933 (12.03) | 2753 (17.14) | 16,065 |
| Total | 253,302 (26.61) | 203,624 (21.39) | 136,985 (14.39) | 15,839 (1.66) | 154,198 (16.20) | 187,967 (19.75) | 951,915 |
C, Central China; CKD, chronic kidney disease; CTIN, chronic tubulointerstitial nephropathy; DKD, diabetic kidney disease; E, East China; GN, glomerulonephritis; HTN, hypertensive nephropathy; N, North China; NE, Northeast China; NW, Northwest China; ON, obstructive nephropathy; Others, CKD due to other reasons; S, South China; SW, Southwest China.
Patients with missing data for geographic region were not included in the analysis. CKD: 40,775 (4.11%).
Figure 9.
Staging of CKD, stratified by hospital nephrology unit. CKD, chronic kidney disease.
Table 9.
Staging of CKD, stratified by hospital nephrology unit, N (%)
| CKD stage | Independent | Non-independent | Unknown | Total |
|---|---|---|---|---|
| Stage 1 | 7528 (0.86) | 422 (0.89) | 282 (0.39) | 8232 (0.83) |
| Stage 2 | 11,422 (1.31) | 479 (1.01) | 773 (1.08) | 12,674 (1.28) |
| Stage 3 | 32,403 (3.71) | 907 (1.92) | 1223 (1.71) | 34,533 (3.48) |
| Stage 4 | 21,621 (2.47) | 860 (1.82) | 884 (1.24) | 23,365 (2.35) |
| Stage 5 | 71,656 (8.20) | 1736 (3.67) | 1950 (2.73) | 75,342 (7.59) |
| Unknown | 729,345 (83.45) | 42,865 (90.68) | 66,298 (92.84) | 838,508 (84.47) |
| Total | 873,975 | 47,269 | 71,410 | 992,654 |
CKD, chronic kidney disease.
Patients with missing data and/or controversial data for stage were not included in the analysis. CKD: 73 (0.01%).
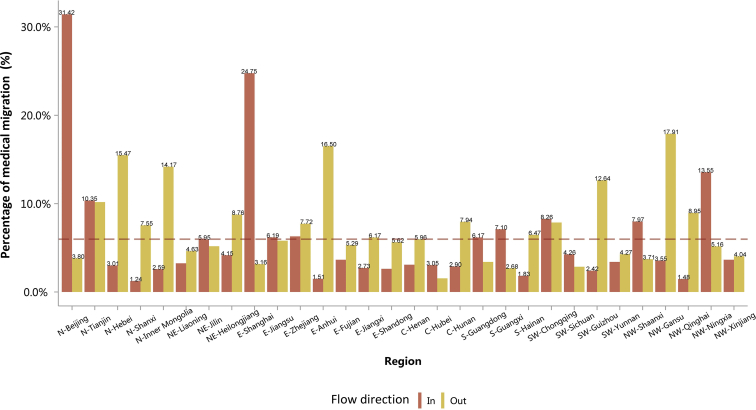
Overall, the percentage of medical migration (interprovince) among patients with CKD was 5.98% (Figure 10). In 2016, the top 3 provinces with the highest proportion of patient outflow were Gansu (17.91%), Anhui (16.50%), and Hebei (15.47%), whereas the top 3 provinces with the highest proportion of patient inflow were Beijing (31.42%), Shanghai (24.75%), and Ningxia (13.55%; Figure 10 and Table 10). The travel pattern of patients with CKD showed that the diagnosis and treatment level and resources of kidney care were unbalanced across regions.
Figure 10.
The travel pattern of patients with CKD. Reference line represents the overall percentage of flow of CKD (5.98%). C, Central China; CKD, chronic kidney disease; E, East China; N, North China; NE, Northeast China; NW, Northwest China; S, South China; SW, Southwest China.
Table 10.
The travel pattern of patients with CKD, N (%)
| Province | Hospital location |
Patient residence |
||
|---|---|---|---|---|
| Local | In | Local | Out | |
| Overall | 895,039 (94.02) | 56,913 (5.98) | 895,039 (94.02) | 56,913 (5.98) |
| N-Beijing | 24,961 (68.58) | 11,437 (31.42) | 24,961 (96.20) | 986 (3.80) |
| N-Tianjin | 3960 (89.65) | 457 (10.35) | 3960 (89.82) | 449 (10.18) |
| N-Hebei | 24,358 (96.99) | 755 (3.01) | 24,358 (84.53) | 4458 (15.47) |
| N-Shanxi | 21,260 (98.76) | 268 (1.24) | 21,260 (92.45) | 1735 (7.55) |
| N-Inner Mongolia | 19,965 (97.41) | 530 (2.59) | 19,965 (85.83) | 3297 (14.17) |
| NE-Liaoning | 28,302 (96.75) | 950 (3.25) | 28,302 (95.37) | 1374 (4.63) |
| NE-Jilin | 18,016 (94.05) | 1140 (5.95) | 18,016 (94.82) | 984 (5.18) |
| NE-Heilongjiang | 22,055 (95.85) | 956 (4.15) | 22,055 (91.24) | 2117 (8.76) |
| E-Shanghai | 23,605 (75.25) | 7763 (24.75) | 23,605 (96.84) | 770 (3.16) |
| E-Jiangsu | 52,634 (93.81) | 3471 (6.19) | 52,634 (94.19) | 3244 (5.81) |
| E-Zhejiang | 36,422 (93.71) | 2446 (6.29) | 36,422 (92.28) | 3049 (7.72) |
| E-Anhui | 26,012 (98.49) | 398 (1.51) | 26,012 (83.50) | 5141 (16.50) |
| E-Fujian | 24,330 (96.35) | 922 (3.65) | 24,330 (94.71) | 1358 (5.29) |
| E-Jiangxi | 44,579 (97.27) | 1252 (2.73) | 44,579 (93.83) | 2930 (6.17) |
| E-Shandong | 30,102 (97.38) | 811 (2.62) | 30,102 (94.38) | 1794 (5.62) |
| C-Henan | 40,505 (96.91) | 1293 (3.09) | 40,505 (94.04) | 2569 (5.96) |
| C-Hubei | 91,014 (96.95) | 2859 (3.05) | 91,014 (98.45) | 1436 (1.55) |
| C-Hunan | 35,567 (97.10) | 1062 (2.90) | 35,567 (92.06) | 3069 (7.94) |
| S-Guangdong | 80,842 (93.83) | 5318 (6.17) | 80,842 (96.60) | 2843 (3.40) |
| S-Guangxi | 32,989 (92.90) | 2520 (7.10) | 32,989 (97.32) | 910 (2.68) |
| S-Hainan | 9744 (98.17) | 182 (1.83) | 9744 (93.53) | 674 (6.47) |
| SW-Chongqing | 11,958 (91.74) | 1077 (8.26) | 11,958 (92.13) | 1021 (7.87) |
| SW-Sichuan | 68,877 (95.74) | 3065 (4.26) | 68,877 (97.14) | 2026 (2.86) |
| SW-Guizhou | 14,625 (97.58) | 363 (2.42) | 14,625 (87.36) | 2116 (12.64) |
| SW-Yunnan | 51,689 (96.60) | 1817 (3.40) | 51,689 (95.73) | 2306 (4.27) |
| NW-Shaanxi | 25,731 (92.03) | 2228 (7.97) | 25,731 (96.29) | 991 (3.71) |
| NW-Gansu | 7088 (96.45) | 261 (3.55) | 7088 (82.09) | 1546 (17.91) |
| NW-Qinghai | 4189 (98.52) | 63 (1.48) | 4189 (91.05) | 412 (8.95) |
| NW-Ningxia | 4244 (86.45) | 665 (13.55) | 4244 (94.84) | 231 (5.16) |
| NW-Xinjiang | 15,416 (96.35) | 584 (3.65) | 15,416 (95.96) | 649 (4.04) |
C, Central China; CKD, chronic kidney disease; E, East China; N, North China; NE, Northeast China; NW, Northwest China; S, South China; SW, Southwest China.
Patients with missing data for residence were not included in the analysis. CKD: 40,775 (4.11%).
1.1. Prevalence of CKD among different types of underlying disease
1.2. Demographics of CKD
1.3. Cause of CKD
1.4. Staging of CKD
1.5. Travel pattern of hospitalized patients with CKD
Chapter 2: Cardiovascular disease in hospitalized patients with CKD
Bixia Gao1,2, Chao Yang1,2, Huai-Yu Wang3, Xinwei Deng1,2, Zaiming Su3, Lanxia Gan4 and Ying Shi4
1Renal Division, Department of Medicine, Peking University First Hospital, Peking University Institute of Nephrology, Beijing, China; 2Research Units of Diagnosis and Treatment of Immune-Mediated Kidney Diseases, Chinese Academy of Medical Sciences, Beijing, China; 3National Institute of Health Data Science at Peking University, Beijing, China; and 4China Standard Medical Information Research Center, Shenzhen, Guangdong, China
Patients with chronic kidney disease (CKD) are at increased risk of cardiovascular disease (CVD), and this often manifests clinically like heart failure.14 This chapter focuses on the clinical pattern and treatment of CVD in hospitalized patients with CKD in China. The comparisons between the 2 groups of patients with diabetes and those with CKD were based on the overall reference population, respectively, which meant we did not exclude patients with diabetes also having CKD or patients with CKD also having diabetes.
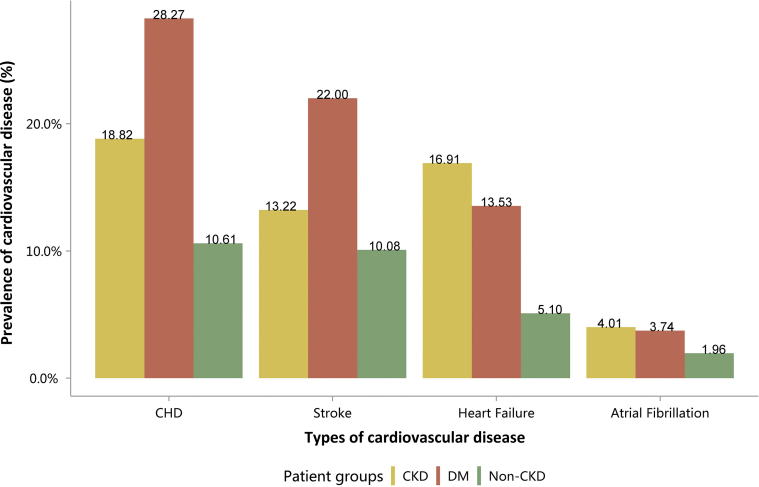
Coronary heart disease (CHD) was the most common CVD among inpatients with CKD (18.82%), followed by heart failure (16.91%), stroke (13.22%), and atrial fibrillation (4.01%; Figure 11 and Table 11). The pattern of CVD was consistent with that in 2015,10 but the percentage of each subtype had increased slightly. The overall percentage of CVD among patients with CKD was higher compared with those without CKD, but lower than that of patients with diabetes (Figure 11 and Table 11).
Figure 11.
Prevalence of CVD, stratified by patient group. CHD, coronary heart disease; CKD, chronic kidney disease; CVD, cardiovascular disease; DM, diabetes mellitus.
Table 11.
Prevalence of CVD, stratified by patient group, N (%)
| Patient group | CHD | Stroke | Heart failure | Atrial fibrillation |
|---|---|---|---|---|
| CKD | 186,871 (18.82) | 131,279 (13.22) | 167,839 (16.91) | 39,833 (4.01) |
| DM | 554,239 (28.27) | 431,304 (22.00) | 265,307 (13.53) | 73,289 (3.74) |
| Non-CKD | 2,063,083 (10.61) | 1961,573 (10.08) | 992,269 (5.10) | 381,399 (1.96) |
CHD, coronary heart disease; CKD, chronic kidney disease; CVD, cardiovascular disease; DM, diabetes mellitus.
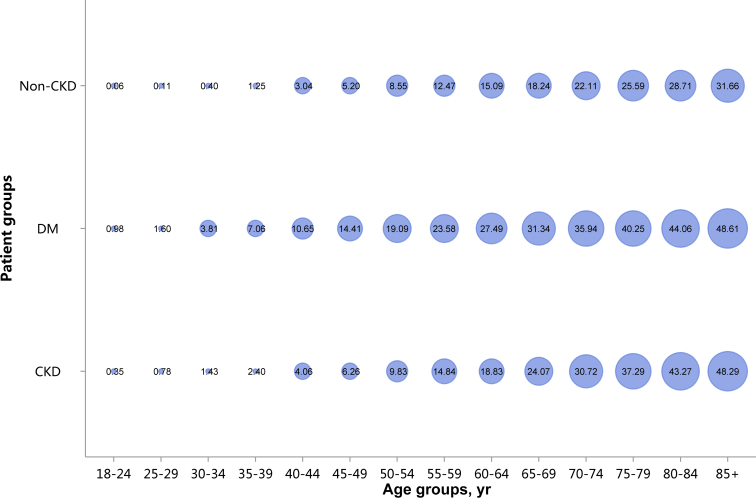
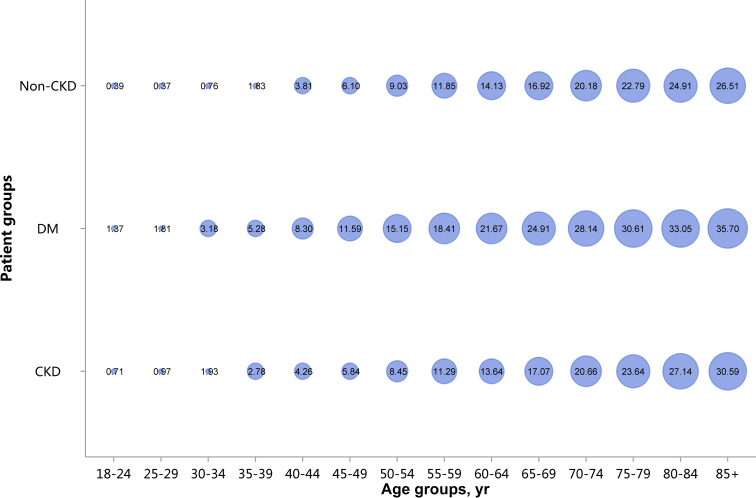
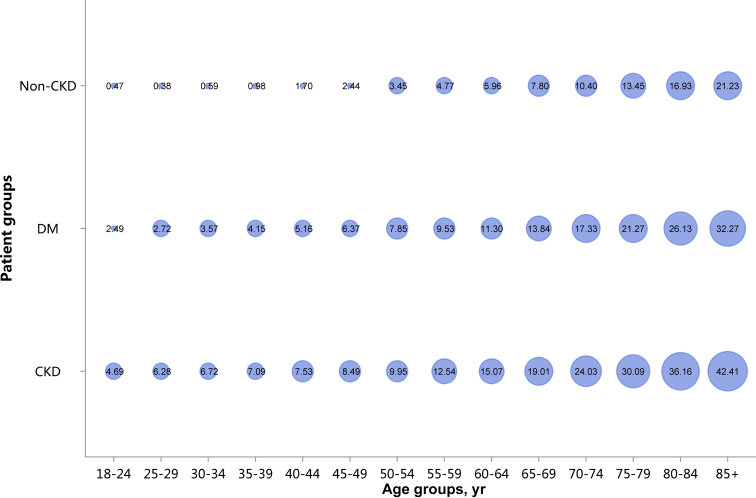
Patients with CKD had lower percentages of CHD and stroke (18.82% vs. 28.27%, 13.22% vs. 22.00%), and higher percentages of heart failure and atrial fibrillation (16.91% vs. 13.53%, 4.01% vs. 3.74%), compared with patients with diabetes (Figure 11 and Table 11). These trends were largely consistent across various age and sex subgroups (Figure 12, Figure 13, Figure 14, Figure 15, Figure 16, Figure 17, Figure 18, Figure 19 and Table 12, Table 13, Table 14, Table 15, Table 16, Table 17, Table 18, Table 19). With the increase of age, the percentage of various types of CVD was on the rise.
Figure 12.
Prevalence of CHD, stratified by sex. CHD, coronary heart disease; CKD, chronic kidney disease; DM, diabetes mellitus.
Figure 13.
Prevalence of CHD, stratified by age. Point size refers to the percentage of CHD. CHD, coronary heart disease; CKD, chronic kidney disease; DM, diabetes mellitus.
Figure 14.
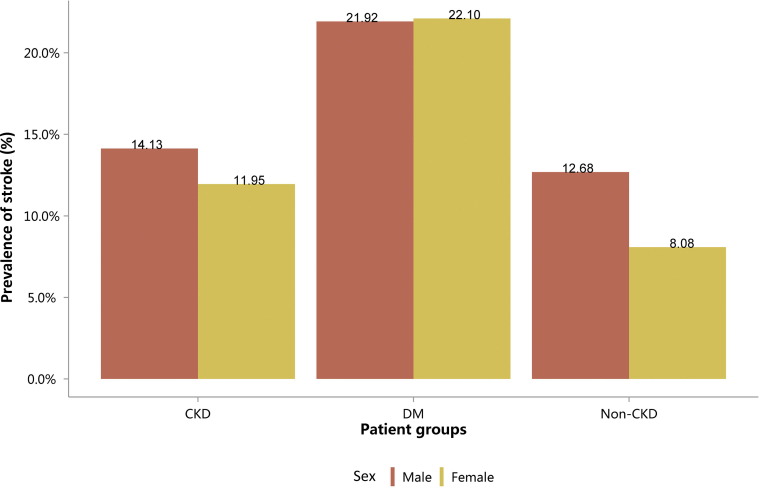
Prevalence of stroke, stratified by sex. CKD, chronic kidney disease; DM, diabetes mellitus.
Figure 15.
Prevalence of stroke, stratified by age. Point size refers to the percentage of stroke. CKD, chronic kidney disease; DM, diabetes mellitus.
Figure 16.
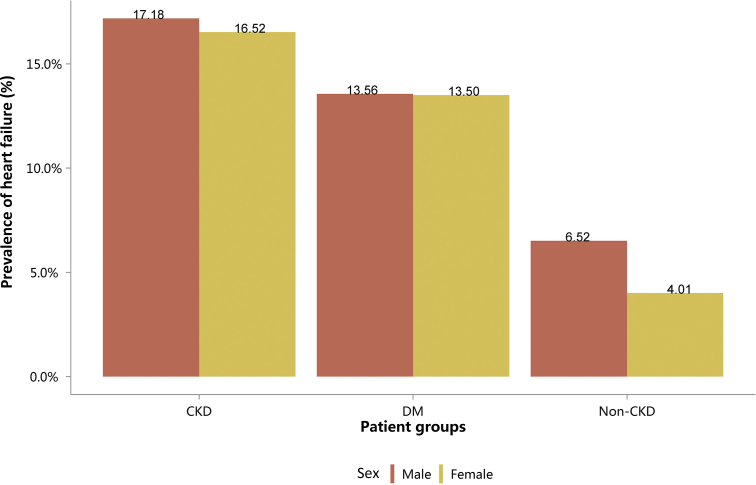
Prevalence of heart failure, stratified by sex. CKD, chronic kidney disease; DM, diabetes mellitus.
Figure 17.
Prevalence of heart failure, stratified by age. Point size refers to the percentage of heart failure. CKD, chronic kidney disease; DM, diabetes mellitus.
Figure 18.
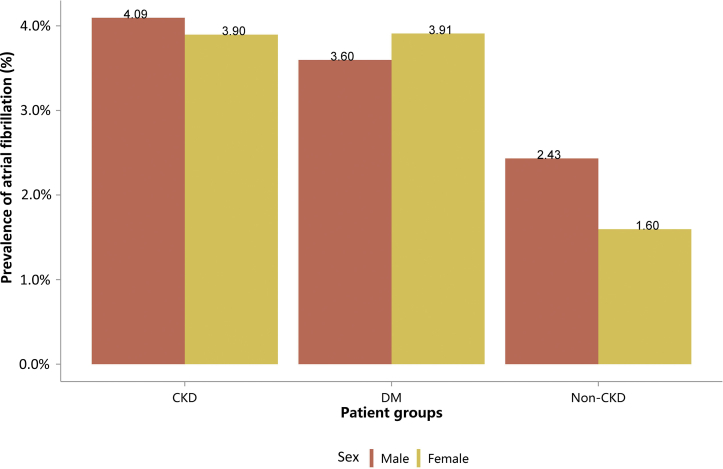
Prevalence of atrial fibrillation, stratified by sex. CKD, chronic kidney disease; DM, diabetes mellitus.
Figure 19.
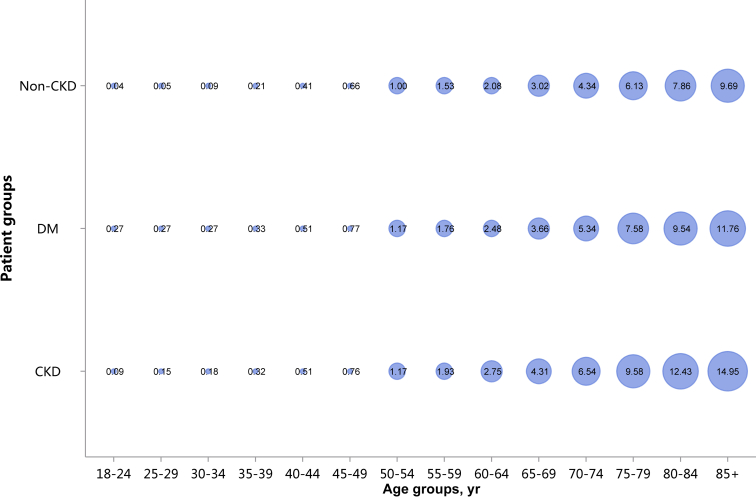
Prevalence of atrial fibrillation, stratified by age. Point size refers to the percentage of atrial fibrillation. CKD, chronic kidney disease; DM, diabetes mellitus.
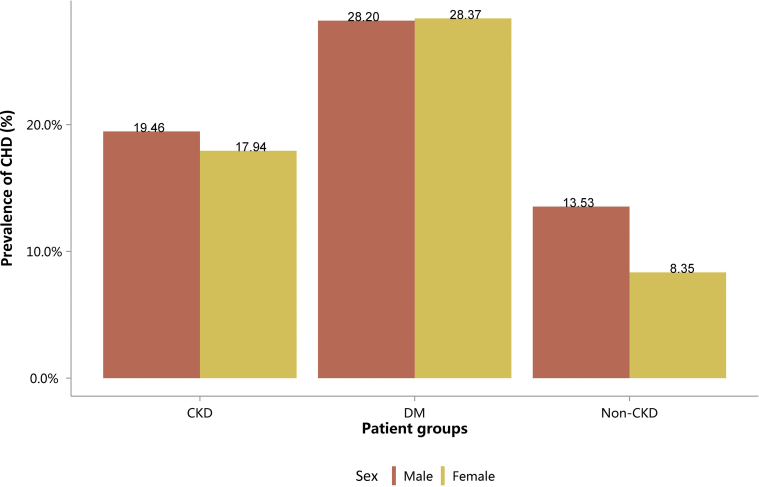
Table 12.
Prevalence of CHD, stratified by sex, N (%)
| Sex | CKD | DM | Non-CKD |
|---|---|---|---|
| Male | 112,708 (19.46) | 301,185 (28.20) | 1146,380 (13.53) |
| Female | 74,163 (17.94) | 253,054 (28.37) | 916,703 (8.35) |
| Total | 186,871 (18.82) | 554,239 (28.27) | 2,063,083 (10.61) |
CHD, coronary heart disease; CKD, chronic kidney disease; DM, diabetes mellitus.
Table 13.
Prevalence of CHD, stratified by age, N (%)
| Age group, yr | CKD | DM | Non-CKD |
|---|---|---|---|
| 18–24 | 84 (0.35) | 68 (0.98) | 596 (0.06) |
| 25–29 | 283 (0.78) | 211 (1.60) | 1979 (0.11) |
| 30–34 | 573 (1.43) | 822 (3.81) | 5860 (0.40) |
| 35–39 | 1073 (2.40) | 2469 (7.06) | 14,714 (1.25) |
| 40–44 | 2574 (4.06) | 7409 (10.65) | 40,616 (3.04) |
| 45–49 | 5452 (6.26) | 18,464 (14.41) | 87,828 (5.20) |
| 50–54 | 10,873 (9.83) | 43,431 (19.09) | 172,484 (8.55) |
| 55–59 | 13,342 (14.84) | 54,901 (23.58) | 203,671 (12.47) |
| 60–64 | 21,897 (18.83) | 86,381 (27.49) | 314,451 (15.09) |
| 65–69 | 25,276 (24.07) | 91,593 (31.34) | 316,912 (18.24) |
| 70–74 | 26,830 (30.72) | 86,086 (35.94) | 295,336 (22.11) |
| 75–79 | 31,038 (37.29) | 79,133 (40.25) | 281,692 (25.59) |
| 80–84 | 27,376 (43.27) | 54,206 (44.06) | 205,312 (28.71) |
| 85+ | 20,200 (48.29) | 29,065 (48.61) | 121,632 (31.66) |
| Total | 186,871 (18.82) | 554,239 (28.27) | 2,063,083 (10.61) |
CHD, coronary heart disease; CKD, chronic kidney disease; DM, diabetes mellitus.
Table 14.
Prevalence of stroke, stratified by sex, N (%)
| Sex | CKD | DM | Non-CKD |
|---|---|---|---|
| Male | 81,882 (14.13) | 234,132 (21.92) | 1074,340 (12.68) |
| Female | 49,397 (11.95) | 197,172 (22.10) | 887,233 (8.08) |
| Total | 131,279 (13.22) | 431,304 (22.00) | 1,961,573 (10.08) |
CKD, chronic kidney disease; DM, diabetes mellitus.
Table 15.
Prevalence of stroke, stratified by age, N (%)
| Age group, yr | CKD | DM | Non-CKD |
|---|---|---|---|
| 18–24 | 168 (0.71) | 95 (1.37) | 3642 (0.39) |
| 25–29 | 352 (0.97) | 239 (1.81) | 6827 (0.37) |
| 30–34 | 774 (1.93) | 686 (3.18) | 11,216 (0.76) |
| 35–39 | 1245 (2.78) | 1846 (5.28) | 21,548 (1.83) |
| 40–44 | 2704 (4.26) | 5776 (8.30) | 50,929 (3.81) |
| 45–49 | 5082 (5.84) | 14,852 (11.59) | 103,011 (6.10) |
| 50–54 | 9343 (8.45) | 34,465 (15.15) | 182,152 (9.03) |
| 55–59 | 10,153 (11.29) | 42,861 (18.41) | 193,501 (11.85) |
| 60–64 | 15,857 (13.64) | 68,098 (21.67) | 294,437 (14.13) |
| 65–69 | 17,920 (17.07) | 72,795 (24.91) | 293,957 (16.92) |
| 70–74 | 18,038 (20.66) | 67,407 (28.14) | 269,503 (20.18) |
| 75–79 | 19,677 (23.64) | 60,179 (30.61) | 250,829 (22.79) |
| 80–84 | 17,170 (27.14) | 40,664 (33.05) | 178,163 (24.91) |
| 85+ | 12,796 (30.59) | 21,341 (35.70) | 101,858 (26.51) |
| Total | 131,279 (13.22) | 431,304 (22.00) | 1,961,573 (10.08) |
CKD, chronic kidney disease; DM, diabetes mellitus.
Table 16.
Prevalence of heart failure, stratified by sex, N (%)
| Sex | CKD | DM | Non-CKD |
|---|---|---|---|
| Male | 99,529 (17.18) | 144,838 (13.56) | 552,061 (6.52) |
| Female | 68,310 (16.52) | 120,469 (13.50) | 440,208 (4.01) |
| Total | 167,839 (16.91) | 265,307 (13.53) | 992,269 (5.10) |
CKD, chronic kidney disease; DM, diabetes mellitus.
Table 17.
Prevalence of heart failure, stratified by age, N (%)
| Age group, yr | CKD | DM | Non-CKD |
|---|---|---|---|
| 18–24 | 1112 (4.69) | 172 (2.49) | 4390 (0.47) |
| 25–29 | 2271 (6.28) | 359 (2.72) | 6898 (0.38) |
| 30–34 | 2694 (6.72) | 772 (3.57) | 8613 (0.59) |
| 35–39 | 3173 (7.09) | 1451 (4.15) | 11,454 (0.98) |
| 40–44 | 4775 (7.53) | 3587 (5.16) | 22,639 (1.70) |
| 45–49 | 7390 (8.49) | 8157 (6.37) | 41,264 (2.44) |
| 50–54 | 11,011 (9.95) | 17,858 (7.85) | 69,645 (3.45) |
| 55–59 | 11,273 (12.54) | 22,184 (9.53) | 77,904 (4.77) |
| 60–64 | 17,526 (15.07) | 35,520 (11.30) | 124,248 (5.96) |
| 65–69 | 19,962 (19.01) | 40,462 (13.84) | 135,607 (7.80) |
| 70–74 | 20,986 (24.03) | 41,521 (17.33) | 138,931 (10.40) |
| 75–79 | 25,046 (30.09) | 41,822 (21.27) | 148,036 (13.45) |
| 80–84 | 22,878 (36.16) | 32,151 (26.13) | 121,080 (16.93) |
| 85+ | 17,742 (42.41) | 19,291 (32.27) | 81,560 (21.23) |
| Total | 167,839 (16.91) | 265,307 (13.53) | 992,269 (5.10) |
CKD, chronic kidney disease; DM, diabetes mellitus.
Table 18.
Prevalence of atrial fibrillation, stratified by sex, N (%)
| Sex | CKD | DM | Non-CKD |
|---|---|---|---|
| Male | 23,722 (4.09) | 38,416 (3.60) | 206,078 (2.43) |
| Female | 16,111 (3.90) | 34,873 (3.91) | 175,321 (1.60) |
| Total | 39,833 (4.01) | 73,289 (3.74) | 381,399 (1.96) |
CKD, chronic kidney disease; DM, diabetes mellitus.
Table 19.
Prevalence of atrial fibrillation, stratified by age, N (%)
| Age group, yr | CKD | DM | Non-CKD |
|---|---|---|---|
| 18–24 | 22 (0.09) | 19 (0.27) | 386 (0.04) |
| 25–29 | 55 (0.15) | 36 (0.27) | 863 (0.05) |
| 30–34 | 72 (0.18) | 59 (0.27) | 1361 (0.09) |
| 35–39 | 141 (0.32) | 114 (0.33) | 2422 (0.21) |
| 40–44 | 325 (0.51) | 353 (0.51) | 5523 (0.41) |
| 45–49 | 660 (0.76) | 987 (0.77) | 11,100 (0.66) |
| 50–54 | 1293 (1.17) | 2663 (1.17) | 20,109 (1.00) |
| 55–59 | 1740 (1.93) | 4099 (1.76) | 24,982 (1.53) |
| 60–64 | 3202 (2.75) | 7795 (2.48) | 43,338 (2.08) |
| 65–69 | 4523 (4.31) | 10,695 (3.66) | 52,409 (3.02) |
| 70–74 | 5708 (6.54) | 12,787 (5.34) | 57,925 (4.34) |
| 75–79 | 7970 (9.58) | 14,909 (7.58) | 67,502 (6.13) |
| 80–84 | 7866 (12.43) | 11,741 (9.54) | 56,233 (7.86) |
| 85+ | 6256 (14.95) | 7032 (11.76) | 37,246 (9.69) |
| Total | 39,833 (4.01) | 73,289 (3.74) | 381,399 (1.96) |
CKD, chronic kidney disease; DM, diabetes mellitus.
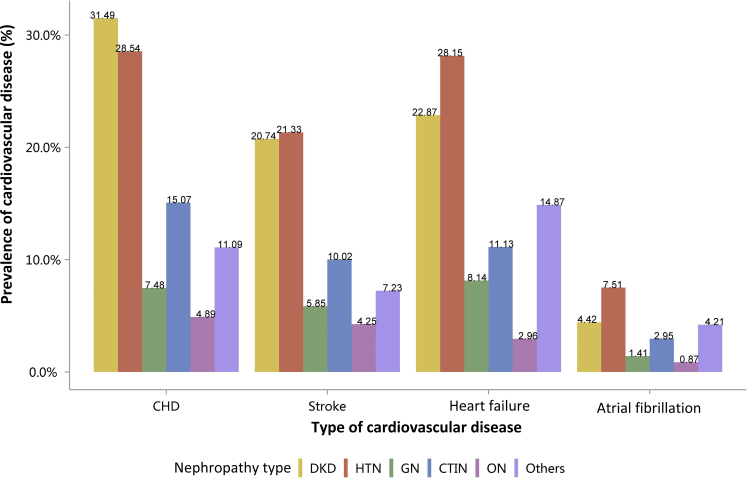
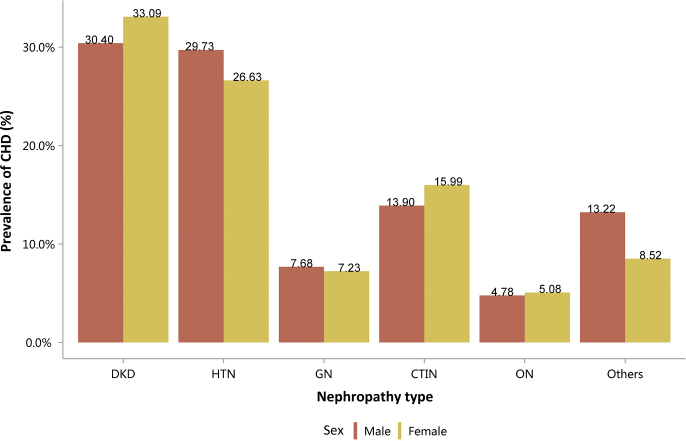
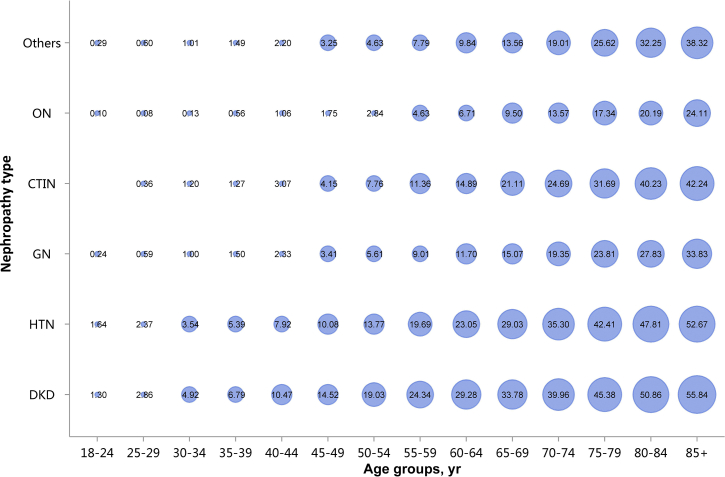
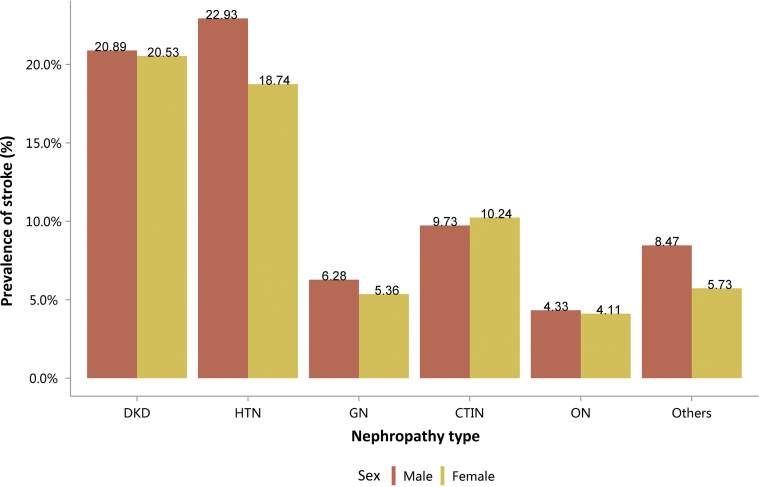
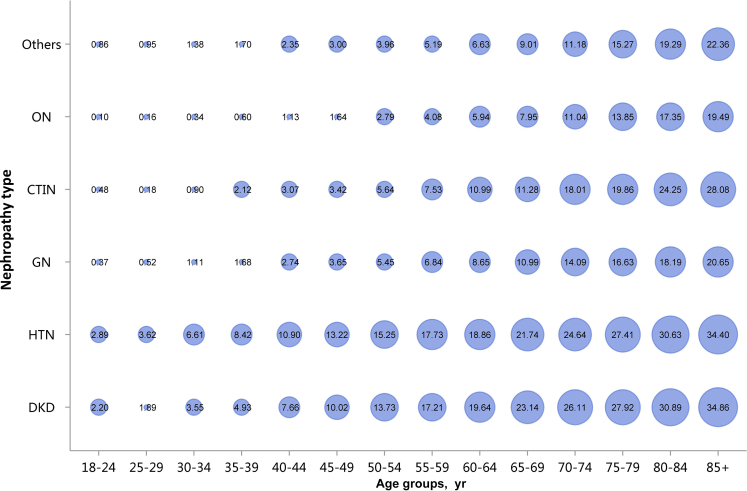
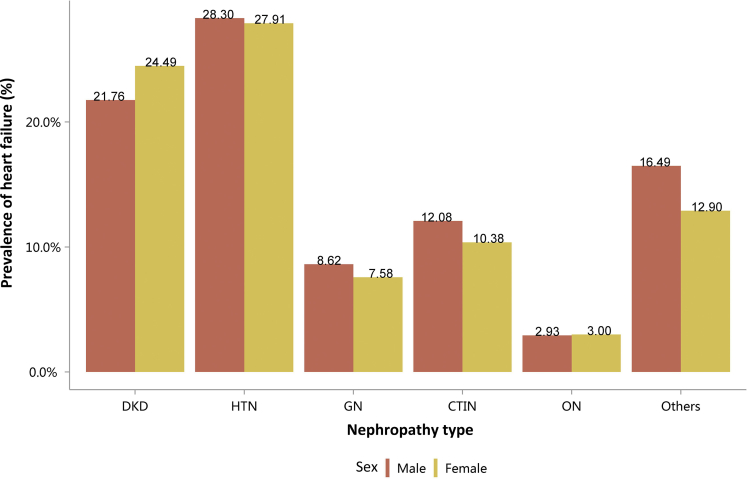
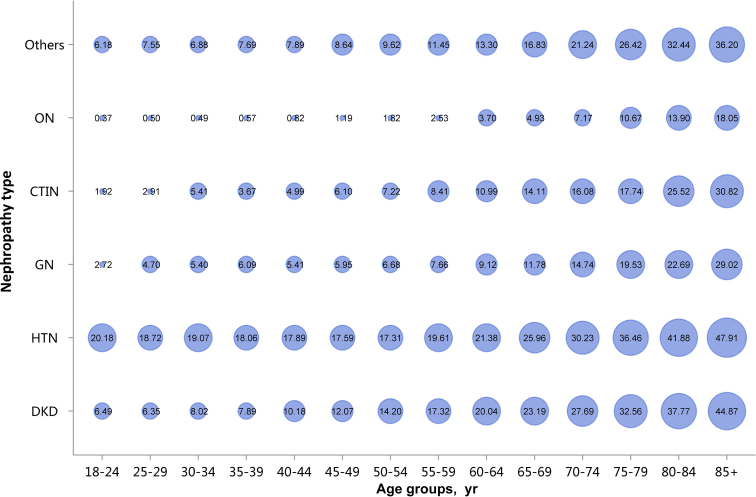
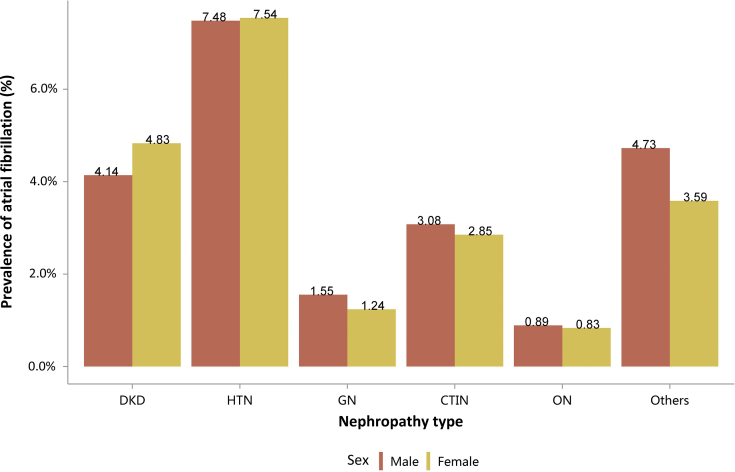
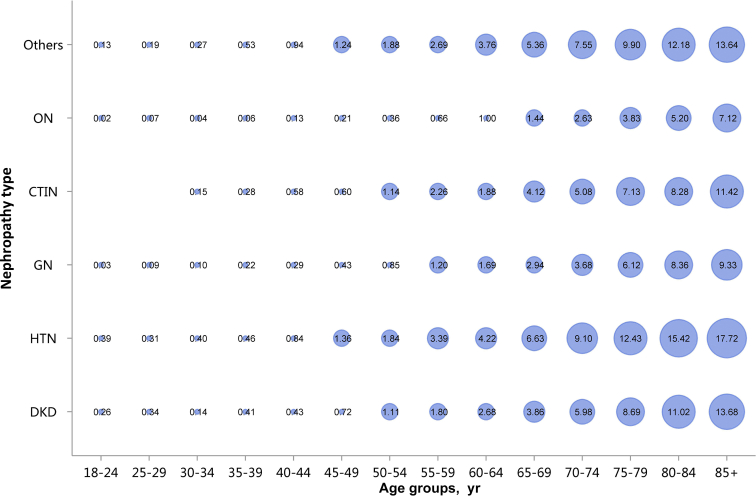
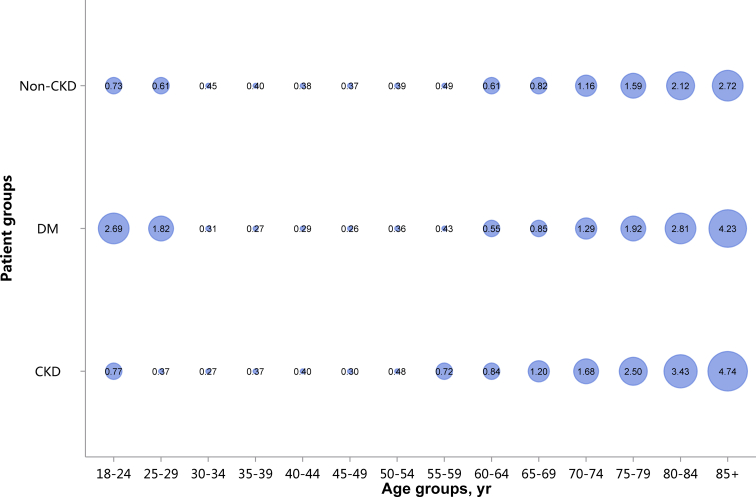
Among the causes of CKD, patients with diabetic kidney disease or hypertensive nephropathy had a higher percentage of CVD, followed by chronic tubulointerstitial nephritis (Figure 20 and Table 20). Stroke, heart failure, and atrial fibrillation were most common among hospitalized patients with hypertensive nephropathy (Figure 20 and Table 20). The trends were largely consistent across various age and sex subgroups (Figure 21, Figure 22, Figure 23, Figure 24, Figure 25, Figure 26, Figure 27, Figure 28 and Table 21, Table 22, Table 23, Table 24, Table 25, Table 26, Table 27, Table 28).
Figure 20.
Prevalence of CVD among patients with CKD. CHD, coronary heart disease; CKD, chronic kidney disease; CTIN, chronic tubulointerstitial nephropathy; CVD, cardiovascular disease; DKD, diabetic kidney disease; GN, glomerulonephritis; HTN, hypertensive nephropathy; ON, obstructive nephropathy; Others, CKD due to other reasons.
Table 20.
Prevalence of CVD among patients with CKD, N (%)
| Cause | CHD | Stroke | Heart failure | Atrial fibrillation |
|---|---|---|---|---|
| DKD | 83,482 (31.49) | 54,987 (20.74) | 60,611 (22.87) | 11,719 (4.42) |
| HTN | 60,595 (28.54) | 45,280 (21.33) | 59,769 (28.15) | 15,938 (7.51) |
| GN | 10,692 (7.48) | 8373 (5.85) | 11,642 (8.14) | 2015 (1.41) |
| CTIN | 2486 (15.07) | 1652 (10.02) | 1835 (11.13) | 487 (2.95) |
| ON | 7767 (4.89) | 6750 (4.25) | 4696 (2.96) | 1381 (0.87) |
| Others | 21,849 (11.09) | 14,237 (7.23) | 29,286 (14.87) | 8293 (4.21) |
| Total | 186,871 (18.82) | 131,279 (13.22) | 167,839 (16.91) | 39,833 (4.01) |
CHD, coronary heart disease; CKD, chronic kidney disease; CTIN, chronic tubulointerstitial nephropathy; CVD, cardiovascular disease; DKD, diabetic kidney disease; GN, glomerulonephritis; HTN, hypertensive nephropathy; ON, obstructive nephropathy; Others, CKD due to other reasons.
Figure 21.
Prevalence of CHD among patients with CKD, stratified by nephropathy type and sex. CHD, coronary heart disease; CKD, chronic kidney disease; CTIN, chronic tubulointerstitial nephropathy; DKD, diabetic kidney disease; GN, glomerulonephritis; HTN, hypertensive nephropathy; ON, obstructive nephropathy; Others, CKD due to other reasons.
Figure 22.
Prevalence of CHD among patients with CKD, stratified by nephropathy type and age. Point size refers to the percentage of CHD. CHD, coronary heart disease; CKD, chronic kidney disease; CTIN, chronic tubulointerstitial nephropathy; DKD, diabetic kidney disease; GN, glomerulonephritis; HTN, hypertensive nephropathy; ON, obstructive nephropathy; Others, CKD due to other reasons.
Figure 23.
Prevalence of stroke among patients with CKD, stratified by nephropathy type and sex. CKD, chronic kidney disease; CTIN, chronic tubulointerstitial nephropathy; DKD, diabetic kidney disease; GN, glomerulonephritis; HTN, hypertensive nephropathy; ON, obstructive nephropathy; Others, CKD due to other reasons.
Figure 24.
Prevalence of stroke among patients with CKD, stratified by nephropathy type and age. Point size refers to the percentage of stroke. CKD, chronic kidney disease; CTIN, chronic tubulointerstitial nephropathy; DKD, diabetic kidney disease; GN, glomerulonephritis; HTN, hypertensive nephropathy; ON, obstructive nephropathy; Others, CKD due to other reasons.
Figure 25.
Prevalence of heart failure among patients with CKD, stratified by nephropathy type and sex. CKD, chronic kidney disease; CTIN, chronic tubulointerstitial nephropathy; DKD, diabetic kidney disease; GN, glomerulonephritis; HTN, hypertensive nephropathy; ON, obstructive nephropathy; Others, CKD due to other reasons.
Figure 26.
Prevalence of heart failure among patients with CKD, stratified by nephropathy type and age. Point size refers to the percentage of heart failure. CKD, chronic kidney disease; CTIN, chronic tubulointerstitial nephropathy; DKD, diabetic kidney disease; GN, glomerulonephritis; HTN, hypertensive nephropathy; ON, obstructive nephropathy; Others, CKD due to other reasons.
Figure 27.
Prevalence of atrial fibrillation among patients with CKD, stratified by nephropathy type and sex. CKD, chronic kidney disease; CTIN, chronic tubulointerstitial nephropathy; DKD, diabetic kidney disease; GN, glomerulonephritis; HTN, hypertensive nephropathy; ON, obstructive nephropathy; Others, CKD due to other reasons.
Figure 28.
Prevalence of atrial fibrillation among patients with CKD, stratified by nephropathy type and age. Point size refers to the percentage of atrial fibrillation. CKD, chronic kidney disease; CTIN, chronic tubulointerstitial nephropathy; DKD, diabetic kidney disease; GN, glomerulonephritis; HTN, hypertensive nephropathy; ON, obstructive nephropathy; Others, CKD due to other reasons.
Table 21.
Prevalence of CHD among patients with CKD, stratified by cause and sex, N (%)
| Sex | DKD | HTN | GN | CTIN | ON | Others | Total |
|---|---|---|---|---|---|---|---|
| Male | 47,844 (30.40) | 38,963 (29.73) | 5932 (7.68) | 1007 (13.90) | 4711 (4.78) | 14,251 (13.22) | 112,708 (19.46) |
| Female | 35,638 (33.09) | 21,632 (26.63) | 4760 (7.23) | 1479 (15.99) | 3056 (5.08) | 7598 (8.52) | 74,163 (17.94) |
| Total | 83,482 (31.49) | 60,595 (28.54) | 10,692 (7.48) | 2486 (15.07) | 7767 (4.89) | 21,849 (11.09) | 186,871 (18.82) |
CHD, coronary heart disease; CKD, chronic kidney disease; CTIN, chronic tubulointerstitial nephropathy; DKD, diabetic kidney disease; GN, glomerulonephritis; HTN, hypertensive nephropathy; ON, obstructive nephropathy; Others, CKD due to other reasons.
Table 22.
Prevalence of CHD among patients with CKD, stratified by cause and age, N (%)
| Age group, yr | DKD | HTN | GN | CTIN | ON | Others | Total |
|---|---|---|---|---|---|---|---|
| 18–24 | 10 (1.30) | 25 (1.64) | 22 (0.24) | 0 (0.00) | 4 (0.10) | 23 (0.29) | 84 (0.35) |
| 25–29 | 50 (2.86) | 91 (2.37) | 65 (0.59) | 2 (0.36) | 6 (0.08) | 69 (0.60) | 283 (0.78) |
| 30–34 | 140 (4.92) | 195 (3.54) | 108 (1.00) | 8 (1.20) | 12 (0.13) | 110 (1.01) | 573 (1.43) |
| 35–39 | 314 (6.79) | 364 (5.39) | 161 (1.50) | 9 (1.27) | 61 (0.56) | 164 (1.49) | 1073 (2.40) |
| 40–44 | 954 (10.47) | 792 (7.92) | 316 (2.33) | 32 (3.07) | 160 (1.06) | 320 (2.20) | 2574 (4.06) |
| 45–49 | 2486 (14.52) | 1411 (10.08) | 545 (3.41) | 62 (4.15) | 354 (1.75) | 594 (3.25) | 5452 (6.26) |
| 50–54 | 5687 (19.03) | 2484 (13.77) | 946 (5.61) | 143 (7.76) | 672 (2.84) | 941 (4.63) | 10,873 (9.83) |
| 55–59 | 7125 (24.34) | 3013 (19.69) | 1068 (9.01) | 181 (11.36) | 775 (4.63) | 1180 (7.79) | 13,342 (14.84) |
| 60–64 | 11,610 (29.28) | 5151 (23.05) | 1673 (11.70) | 317 (14.89) | 1237 (6.71) | 1909 (9.84) | 21,897 (18.83) |
| 65–69 | 12,791 (33.78) | 6815 (29.03) | 1688 (15.07) | 395 (21.11) | 1312 (9.50) | 2275 (13.56) | 25,276 (24.07) |
| 70–74 | 12,932 (39.96) | 8213 (35.30) | 1441 (19.35) | 384 (24.69) | 1134 (13.57) | 2726 (19.01) | 26,830 (30.72) |
| 75–79 | 13,094 (45.38) | 11,405 (42.41) | 1330 (23.81) | 418 (31.69) | 1009 (17.34) | 3782 (25.62) | 31,038 (37.29) |
| 80–84 | 10,189 (50.86) | 11,361 (47.81) | 872 (27.83) | 350 (40.23) | 645 (20.19) | 3959 (32.25) | 27,376 (43.27) |
| 85+ | 6100 (55.84) | 9275 (52.67) | 457 (33.83) | 185 (42.24) | 386 (24.11) | 3797 (38.32) | 20,200 (48.29) |
| Total | 83,482 (31.49) | 60,595 (28.54) | 10,692 (7.48) | 2486 (15.07) | 7767 (4.89) | 21,849 (11.09) | 186,871 (18.82) |
CHD, coronary heart disease; CKD, chronic kidney disease; CTIN, chronic tubulointerstitial nephropathy; DKD, diabetic kidney disease; GN, glomerulonephritis; HTN, hypertensive nephropathy; ON, obstructive nephropathy; Others, CKD due to other reasons.
Table 23.
Prevalence of stroke among patients with CKD, stratified by cause and sex, N (%)
| Sex | DKD | HTN | GN | CTIN | ON | Others | Total |
|---|---|---|---|---|---|---|---|
| Male | 32,872 (20.89) | 30,056 (22.93) | 4848 (6.28) | 705 (9.73) | 4273 (4.33) | 9128 (8.47) | 81,882 (14.13) |
| Female | 22,115 (20.53) | 15,224 (18.74) | 3525 (5.36) | 947 (10.24) | 2477 (4.11) | 5109 (5.73) | 49,397 (11.95) |
| Total | 54,987 (20.74) | 45,280 (21.33) | 8373 (5.85) | 1652 (10.02) | 6750 (4.25) | 14,237 (7.23) | 131,279 (13.22) |
CKD, chronic kidney disease; CTIN, chronic tubulointerstitial nephropathy; DKD, diabetic kidney disease; GN, glomerulonephritis; HTN, hypertensive nephropathy; ON, obstructive nephropathy; Others, CKD due to other reasons.
Table 24.
Prevalence of stroke among patients with CKD, stratified by cause and age, N (%)
| Age group, yr | DKD | HTN | GN | CTIN | ON | Others | Total |
|---|---|---|---|---|---|---|---|
| 18–24 | 17 (2.20) | 44 (2.89) | 34 (0.37) | 2 (0.48) | 4 (0.10) | 67 (0.86) | 168 (0.71) |
| 25–29 | 33 (1.89) | 139 (3.62) | 58 (0.52) | 1 (0.18) | 12 (0.16) | 109 (0.95) | 352 (0.97) |
| 30–34 | 101 (3.55) | 364 (6.61) | 120 (1.11) | 6 (0.90) | 32 (0.34) | 151 (1.38) | 774 (1.93) |
| 35–39 | 228 (4.93) | 569 (8.42) | 180 (1.68) | 15 (2.12) | 66 (0.60) | 187 (1.70) | 1245 (2.78) |
| 40–44 | 698 (7.66) | 1090 (10.90) | 371 (2.74) | 32 (3.07) | 171 (1.13) | 342 (2.35) | 2704 (4.26) |
| 45–49 | 1716 (10.02) | 1850 (13.22) | 584 (3.65) | 51 (3.42) | 332 (1.64) | 549 (3.00) | 5082 (5.84) |
| 50–54 | 4104 (13.73) | 2750 (15.25) | 920 (5.45) | 104 (5.64) | 660 (2.79) | 805 (3.96) | 9343 (8.45) |
| 55–59 | 5038 (17.21) | 2713 (17.73) | 811 (6.84) | 120 (7.53) | 684 (4.08) | 787 (5.19) | 10,153 (11.29) |
| 60–64 | 7788 (19.64) | 4216 (18.86) | 1237 (8.65) | 234 (10.99) | 1096 (5.94) | 1286 (6.63) | 15,857 (13.64) |
| 65–69 | 8764 (23.14) | 5104 (21.74) | 1231 (10.99) | 211 (11.28) | 1098 (7.95) | 1512 (9.01) | 17,920 (17.07) |
| 70–74 | 8449 (26.11) | 5733 (24.64) | 1049 (14.09) | 280 (18.01) | 923 (11.04) | 1604 (11.18) | 18,038 (20.66) |
| 75–79 | 8055 (27.92) | 7371 (27.41) | 929 (16.63) | 262 (19.86) | 806 (13.85) | 2254 (15.27) | 19,677 (23.64) |
| 80–84 | 6188 (30.89) | 7279 (30.63) | 570 (18.19) | 211 (24.25) | 554 (17.35) | 2368 (19.29) | 17,170 (27.14) |
| 85+ | 3808 (34.86) | 6058 (34.40) | 279 (20.65) | 123 (28.08) | 312 (19.49) | 2216 (22.36) | 12,796 (30.59) |
| Total | 54,987 (20.74) | 45,280 (21.33) | 8373 (5.85) | 1652 (10.02) | 6750 (4.25) | 14,237 (7.23) | 131,279 (13.22) |
CKD, chronic kidney disease; CTIN, chronic tubulointerstitial nephropathy; DKD, diabetic kidney disease; GN, glomerulonephritis; HTN, hypertensive nephropathy; ON, obstructive nephropathy; Others, CKD due to other reasons.
Table 25.
Prevalence of heart failure among patients with CKD, stratified by cause and sex, N (%)
| Sex | DKD | HTN | GN | CTIN | ON | Others | Total |
|---|---|---|---|---|---|---|---|
| Male | 34,237 (21.76) | 37,101 (28.30) | 6653 (8.62) | 875 (12.08) | 2891 (2.93) | 17,772 (16.49) | 99,529 (17.18) |
| Female | 26,374 (24.49) | 22,668 (27.91) | 4989 (7.58) | 960 (10.38) | 1805 (3.00) | 11,514 (12.90) | 68,310 (16.52) |
| Total | 60,611 (22.87) | 59,769 (28.15) | 11,642 (8.14) | 1835 (11.13) | 4696 (2.96) | 29,286 (14.87) | 167,839 (16.91) |
CKD, chronic kidney disease; CTIN, chronic tubulointerstitial nephropathy; DKD, diabetic kidney disease; GN, glomerulonephritis; HTN, hypertensive nephropathy; ON, obstructive nephropathy; Others, CKD due to other reasons.
Table 26.
Prevalence of heart failure among patients with CKD, stratified by cause and age, N (%)
| Age group, yr | DKD | HTN | GN | CTIN | ON | Others | Total |
|---|---|---|---|---|---|---|---|
| 18–24 | 50 (6.49) | 307 (20.18) | 248 (2.72) | 8 (1.92) | 15 (0.37) | 484 (6.18) | 1112 (4.69) |
| 25–29 | 111 (6.35) | 719 (18.72) | 521 (4.70) | 16 (2.91) | 37 (0.50) | 867 (7.55) | 2271 (6.28) |
| 30–34 | 228 (8.02) | 1050 (19.07) | 582 (5.40) | 36 (5.41) | 46 (0.49) | 752 (6.88) | 2694 (6.72) |
| 35–39 | 365 (7.89) | 1220 (18.06) | 654 (6.09) | 26 (3.67) | 62 (0.57) | 846 (7.69) | 3173 (7.09) |
| 40–44 | 928 (10.18) | 1789 (17.89) | 734 (5.41) | 52 (4.99) | 124 (0.82) | 1148 (7.89) | 4775 (7.53) |
| 45–49 | 2067 (12.07) | 2462 (17.59) | 952 (5.95) | 91 (6.10) | 240 (1.19) | 1578 (8.64) | 7390 (8.49) |
| 50–54 | 4243 (14.20) | 3122 (17.31) | 1127 (6.68) | 133 (7.22) | 431 (1.82) | 1955 (9.62) | 11,011 (9.95) |
| 55–59 | 5070 (17.32) | 3001 (19.61) | 908 (7.66) | 134 (8.41) | 424 (2.53) | 1736 (11.45) | 11,273 (12.54) |
| 60–64 | 7946 (20.04) | 4778 (21.38) | 1304 (9.12) | 234 (10.99) | 683 (3.70) | 2581 (13.30) | 17,526 (15.07) |
| 65–69 | 8780 (23.19) | 6094 (25.96) | 1320 (11.78) | 264 (14.11) | 681 (4.93) | 2823 (16.83) | 19,962 (19.01) |
| 70–74 | 8960 (27.69) | 7032 (30.23) | 1098 (14.74) | 250 (16.08) | 599 (7.17) | 3047 (21.24) | 20,986 (24.03) |
| 75–79 | 9395 (32.56) | 9805 (36.46) | 1091 (19.53) | 234 (17.74) | 621 (10.67) | 3900 (26.42) | 25,046 (30.09) |
| 80–84 | 7566 (37.77) | 9953 (41.88) | 711 (22.69) | 222 (25.52) | 444 (13.90) | 3982 (32.44) | 22,878 (36.16) |
| 85+ | 4902 (44.87) | 8437 (47.91) | 392 (29.02) | 135 (30.82) | 289 (18.05) | 3587 (36.20) | 17,742 (42.41) |
| Total | 60,611 (22.87) | 59,769 (28.15) | 11,642 (8.14) | 1835 (11.13) | 4696 (2.96) | 29,286 (14.87) | 167,839 (16.91) |
CKD, chronic kidney disease; CTIN, chronic tubulointerstitial nephropathy; DKD, diabetic kidney disease; GN, glomerulonephritis; HTN, hypertensive nephropathy; ON, obstructive nephropathy; Others, CKD due to other reasons.
Table 27.
Prevalence of atrial fibrillation among patients with CKD, stratified by cause and sex, N (%)
| Sex | DKD | HTN | GN | CTIN | ON | Others | Total |
|---|---|---|---|---|---|---|---|
| Male | 6516 (4.14) | 9810 (7.48) | 1200 (1.55) | 223 (3.08) | 879 (0.89) | 5094 (4.73) | 23,722 (4.09) |
| Female | 5203 (4.83) | 6128 (7.54) | 815 (1.24) | 264 (2.85) | 502 (0.83) | 3199 (3.59) | 16,111 (3.90) |
| Total | 11,719 (4.42) | 15,938 (7.51) | 2015 (1.41) | 487 (2.95) | 1381 (0.87) | 8293 (4.21) | 39,833 (4.01) |
CKD, chronic kidney disease; CTIN, chronic tubulointerstitial nephropathy; DKD, diabetic kidney disease; GN, glomerulonephritis; HTN, hypertensive nephropathy; ON, obstructive nephropathy; Others, CKD due to other reasons.
Table 28.
Prevalence of atrial fibrillation among patients with CKD, stratified by cause and age, N (%)
| Age group, yr | DKD | HTN | GN | CTIN | ON | Others | Total |
|---|---|---|---|---|---|---|---|
| 18–24 | 2 (0.26) | 6 (0.39) | 3 (0.03) | 0 (0.00) | 1 (0.02) | 10 (0.13) | 22 (0.09) |
| 25–29 | 6 (0.34) | 12 (0.31) | 10 (0.09) | 0 (0.00) | 5 (0.07) | 22 (0.19) | 55 (0.15) |
| 30–34 | 4 (0.14) | 22 (0.40) | 11 (0.10) | 1 (0.15) | 4 (0.04) | 30 (0.27) | 72 (0.18) |
| 35–39 | 19 (0.41) | 31 (0.46) | 24 (0.22) | 2 (0.28) | 7 (0.06) | 58 (0.53) | 141 (0.32) |
| 40–44 | 39 (0.43) | 84 (0.84) | 40 (0.29) | 6 (0.58) | 19 (0.13) | 137 (0.94) | 325 (0.51) |
| 45–49 | 123 (0.72) | 190 (1.36) | 68 (0.43) | 9 (0.60) | 43 (0.21) | 227 (1.24) | 660 (0.76) |
| 50–54 | 331 (1.11) | 331 (1.84) | 143 (0.85) | 21 (1.14) | 85 (0.36) | 382 (1.88) | 1293 (1.17) |
| 55–59 | 526 (1.80) | 519 (3.39) | 142 (1.20) | 36 (2.26) | 110 (0.66) | 407 (2.69) | 1740 (1.93) |
| 60–64 | 1064 (2.68) | 943 (4.22) | 241 (1.69) | 40 (1.88) | 185 (1.00) | 729 (3.76) | 3202 (2.75) |
| 65–69 | 1462 (3.86) | 1557 (6.63) | 329 (2.94) | 77 (4.12) | 199 (1.44) | 899 (5.36) | 4523 (4.31) |
| 70–74 | 1935 (5.98) | 2117 (9.10) | 274 (3.68) | 79 (5.08) | 220 (2.63) | 1083 (7.55) | 5708 (6.54) |
| 75–79 | 2507 (8.69) | 3342 (12.43) | 342 (6.12) | 94 (7.13) | 223 (3.83) | 1462 (9.90) | 7970 (9.58) |
| 80–84 | 2207 (11.02) | 3664 (15.42) | 262 (8.36) | 72 (8.28) | 166 (5.20) | 1495 (12.18) | 7866 (12.43) |
| 85+ | 1494 (13.68) | 3120 (17.72) | 126 (9.33) | 50 (11.42) | 114 (7.12) | 1352 (13.64) | 6256 (14.95) |
| Total | 11,719 (4.42) | 15,938 (7.51) | 2015 (1.41) | 487 (2.95) | 1381 (0.87) | 8293 (4.21) | 39,833 (4.01) |
CKD, chronic kidney disease; CTIN, chronic tubulointerstitial nephropathy; DKD, diabetic kidney disease; GN, glomerulonephritis; HTN, hypertensive nephropathy; ON, obstructive nephropathy; Others, CKD due to other reasons.
Despite the relatively high burden of CHD among patients with CKD, the percentages of related cardiovascular procedures including conventional coronarography, percutaneous coronary intervention, and coronary artery bypass graft were much lower than in patients without CKD (Figure 29, Figure 30, Figure 31, Figure 32, Figure 33, Figure 34, Figure 35 and Table 29, Table 30, Table 31, Table 32, Table 33, Table 34, Table 35). The percentage of pacemaker implantation was higher among patients with CKD than in patients with diabetes or without CKD (Figures 36 and 37; Tables 36 and 37). The trends did not vary substantially across causes of CKD, except for patients with obstructive nephropathy, who had the lowest percentage of CHD but the highest percentage of conventional coronarography (Figure 38 and Table 38).
Figure 29.
Cardiovascular procedures stratified by patient group. CABG, coronary artery bypass grafting; CAG, coronarography; CKD, chronic kidney disease; DM, diabetes mellitus; PCI, percutaneous coronary intervention.
Figure 30.
Cardiovascular procedure: CAG, stratified by sex. CAG, coronarography; CKD, chronic kidney disease; DM, diabetes mellitus.
Figure 31.
Cardiovascular procedure: CAG, stratified by age. CAG, coronarography; CKD, chronic kidney disease; DM, diabetes mellitus. Point size refers to the percentage of CAG.
Figure 32.
Cardiovascular procedure: PCI, stratified by sex. CKD, chronic kidney disease; DM, diabetes mellitus; PCI, percutaneous coronary intervention.
Figure 33.
Cardiovascular procedure: PCI, stratified by age. CKD, chronic kidney disease; DM, diabetes mellitus; PCI, percutaneous coronary intervention. Point size refers to the percentage of PCI.
Figure 34.
Cardiovascular procedure: CABG, stratified by sex. CABG, coronary artery bypass grafting; CKD, chronic kidney disease; DM, diabetes mellitus.
Figure 35.
Cardiovascular procedure: CABG, stratified by age. CABG, coronary artery bypass grafting; CKD, chronic kidney disease; DM, diabetes mellitus. Point size refers to the percentage of CABG.
Table 29.
Cardiovascular procedures stratified by patient group, N (%)
| Patient group | CAG | PCI | CABG | Pacemaker |
|---|---|---|---|---|
| CKD | 20,132 (5.87) | 11,090 (3.23) | 916 (0.27) | 6265 (1.83) |
| DM | 120,445 (13.18) | 68,114 (7.46) | 6377 (0.70) | 11,304 (1.24) |
| Non-CKD | 513,092 (12.93) | 249,898 (6.30) | 19,617 (0.49) | 41,016 (1.03) |
CABG, coronary artery bypass grafting; CAG, coronarography; CKD, chronic kidney disease; DM, diabetes mellitus; PCI, percutaneous coronary intervention.
Table 30.
Cardiovascular procedure: CAG, stratified by sex, N (%)
| Sex | CKD | DM | Non-CKD |
|---|---|---|---|
| Male | 14,296 (6.88) | 75,400 (15.11) | 331,353 (15.07) |
| Female | 5836 (4.31) | 45,045 (10.86) | 181,739 (10.27) |
| Total | 20,132 (5.87) | 120,445 (13.18) | 513,092 (12.93) |
CAG, coronarography; CKD, chronic kidney disease; DM, diabetes mellitus.
Table 31.
Cardiovascular procedure: CAG, stratified by age, N (%)
| Age group, yr | CKD | DM | Non-CKD |
|---|---|---|---|
| 18–24 | 46 (3.52) | 71 (23.91) | 334 (3.91) |
| 25–29 | 81 (2.96) | 127 (17.74) | 988 (6.40) |
| 30–34 | 145 (3.94) | 362 (18.43) | 2689 (10.99) |
| 35–39 | 220 (4.51) | 1025 (21.24) | 6449 (14.62) |
| 40–44 | 417 (4.89) | 2750 (19.81) | 17,290 (16.86) |
| 45–49 | 892 (6.02) | 6474 (19.13) | 35,815 (17.48) |
| 50–54 | 1731 (6.94) | 14,309 (18.64) | 65,913 (18.04) |
| 55–59 | 2059 (7.74) | 17,027 (18.10) | 73,420 (18.43) |
| 60–64 | 3183 (7.75) | 23,944 (16.49) | 101,662 (16.91) |
| 65–69 | 3362 (7.39) | 21,758 (14.30) | 86,406 (14.54) |
| 70–74 | 3007 (6.59) | 16,364 (11.75) | 61,301 (11.37) |
| 75–79 | 2756 (5.51) | 10,775 (8.69) | 40,114 (7.98) |
| 80–84 | 1654 (3.87) | 4375 (5.25) | 16,638 (4.63) |
| 85+ | 579 (1.89) | 1084 (2.51) | 4073 (1.95) |
| Total | 20,132 (5.87) | 120,445 (13.18) | 513,092 (12.93) |
CAG, coronarography; CKD, chronic kidney disease; DM, diabetes mellitus.
Table 32.
Cardiovascular procedure: PCI, stratified by sex, N (%)
| Sex | CKD | DM | Non-CKD |
|---|---|---|---|
| Male | 8361 (4.02) | 45,785 (9.18) | 181,270 (8.25) |
| Female | 2729 (2.02) | 22,329 (5.39) | 68,628 (3.88) |
| Total | 11,090 (3.23) | 68,114 (7.46) | 249,898 (6.30) |
CKD, chronic kidney disease; DM, diabetes mellitus; PCI, percutaneous coronary intervention.
Table 33.
Cardiovascular procedure: PCI, stratified by age, N (%)
| Age group, yr | CKD | DM | Non-CKD |
|---|---|---|---|
| 18–24 | 6 (0.46) | 6 (2.02) | 51 (0.60) |
| 25–29 | 16 (0.58) | 38 (5.31) | 346 (2.24) |
| 30–34 | 59 (1.61) | 204 (10.39) | 1323 (5.41) |
| 35–39 | 102 (2.09) | 667 (13.82) | 3522 (7.98) |
| 40–44 | 214 (2.51) | 1766 (12.72) | 8901 (8.68) |
| 45–49 | 477 (3.22) | 4021 (11.88) | 18,283 (8.93) |
| 50–54 | 924 (3.71) | 8158 (10.63) | 30,943 (8.47) |
| 55–59 | 1151 (4.33) | 9567 (10.17) | 34,181 (8.58) |
| 60–64 | 1714 (4.18) | 13,267 (9.13) | 48,071 (8.00) |
| 65–69 | 1822 (4.01) | 12,062 (7.93) | 41,791 (7.03) |
| 70–74 | 1682 (3.69) | 9193 (6.60) | 30,578 (5.67) |
| 75–79 | 1601 (3.20) | 6106 (4.92) | 20,646 (4.11) |
| 80–84 | 966 (2.26) | 2480 (2.98) | 8943 (2.49) |
| 85+ | 356 (1.16) | 579 (1.34) | 2319 (1.11) |
| Total | 11,090 (3.23) | 68,114 (7.46) | 249,898 (6.30) |
CKD, chronic kidney disease; DM, diabetes mellitus; PCI, percutaneous coronary intervention.
Table 34.
Cardiovascular procedure: CABG, stratified by sex, N (%)
| Sex | CKD | DM | Non-CKD |
|---|---|---|---|
| Male | 701 (0.34) | 4537 (0.91) | 14,611 (0.66) |
| Female | 215 (0.16) | 1840 (0.44) | 5006 (0.28) |
| Total | 916 (0.27) | 6377 (0.70) | 19,617 (0.49) |
CABG, coronary artery bypass grafting; CKD, chronic kidney disease; DM, diabetes mellitus.
Table 35.
Cardiovascular procedure: CABG, stratified by age, N (%)
| Age group, yr | CKD | DM | Non-CKD |
|---|---|---|---|
| 18–24 | 0 (0.00) | 0 (0.00) | 11 (0.13) |
| 25–29 | 1 (0.04) | 5 (0.70) | 20 (0.13) |
| 30–34 | 3 (0.08) | 14 (0.71) | 44 (0.18) |
| 35–39 | 11 (0.23) | 38 (0.79) | 152 (0.34) |
| 40–44 | 18 (0.21) | 110 (0.79) | 453 (0.44) |
| 45–49 | 32 (0.22) | 295 (0.87) | 1092 (0.53) |
| 50–54 | 71 (0.28) | 767 (1.00) | 2371 (0.65) |
| 55–59 | 128 (0.48) | 968 (1.03) | 2987 (0.75) |
| 60–64 | 193 (0.47) | 1585 (1.09) | 4791 (0.80) |
| 65–69 | 183 (0.40) | 1346 (0.88) | 4027 (0.68) |
| 70–74 | 149 (0.33) | 788 (0.57) | 2308 (0.43) |
| 75–79 | 89 (0.18) | 358 (0.29) | 1036 (0.21) |
| 80–84 | 31 (0.07) | 82 (0.10) | 253 (0.07) |
| 85+ | 7 (0.02) | 21 (0.05) | 72 (0.03) |
| Total | 916 (0.27) | 6377 (0.70) | 19,617 (0.49) |
CABG, coronary artery bypass grafting; CKD, chronic kidney disease; DM, diabetes mellitus.
Figure 36.
Cardiovascular procedure: pacemaker, stratified by sex. CKD, chronic kidney disease; DM, diabetes mellitus.
Figure 37.
Cardiovascular procedure: pacemaker, stratified by age. Point size refers to the percentage of pacemaker. CKD, chronic kidney disease; DM, diabetes mellitus.
Table 36.
Cardiovascular procedure: pacemaker, stratified by sex, N (%)
| Sex | CKD | DM | Non-CKD |
|---|---|---|---|
| Male | 3940 (1.90) | 6400 (1.28) | 22,696 (1.03) |
| Female | 2325 (1.72) | 4904 (1.18) | 18,320 (1.03) |
| Total | 6265 (1.83) | 11,304 (1.24) | 41,016 (1.03) |
CKD, chronic kidney disease; DM, diabetes mellitus.
Table 37.
Cardiovascular procedure: pacemaker, stratified by age, N (%)
| Age group, yr | CKD | DM | Non-CKD |
|---|---|---|---|
| 18–24 | 10 (0.77) | 8 (2.69) | 62 (0.73) |
| 25–29 | 10 (0.37) | 13 (1.82) | 94 (0.61) |
| 30–34 | 10 (0.27) | 6 (0.31) | 111 (0.45) |
| 35–39 | 18 (0.37) | 13 (0.27) | 178 (0.40) |
| 40–44 | 34 (0.40) | 40 (0.29) | 392 (0.38) |
| 45–49 | 45 (0.30) | 88 (0.26) | 750 (0.37) |
| 50–54 | 119 (0.48) | 279 (0.36) | 1436 (0.39) |
| 55–59 | 191 (0.72) | 407 (0.43) | 1965 (0.49) |
| 60–64 | 346 (0.84) | 804 (0.55) | 3651 (0.61) |
| 65–69 | 546 (1.20) | 1295 (0.85) | 4849 (0.82) |
| 70–74 | 765 (1.68) | 1792 (1.29) | 6236 (1.16) |
| 75–79 | 1252 (2.50) | 2387 (1.92) | 8012 (1.59) |
| 80–84 | 1466 (3.43) | 2343 (2.81) | 7596 (2.12) |
| 85+ | 1453 (4.74) | 1829 (4.23) | 5684 (2.72) |
| Total | 6265 (1.83) | 11,304 (1.24) | 41,016 (1.03) |
CKD, chronic kidney disease; DM, diabetes mellitus.
Figure 38.
Cardiovascular procedures among patients with CKD. CABG, coronary artery bypass grafting; CAG, coronarography; CKD, chronic kidney disease; CTIN, chronic tubulointerstitial nephropathy; DKD, diabetic kidney disease; GN, glomerulonephritis; HTN, hypertensive nephropathy; ON, obstructive nephropathy; Others, CKD due to other reasons; PCI, percutaneous coronary intervention.
Table 38.
Cardiovascular procedures among patients with CKD, N (%)
| Cause | CAG | PCI | CABG | Pacemaker | Total |
|---|---|---|---|---|---|
| DKD | 7805 (5.79) | 4665 (3.46) | 381 (0.28) | 2270 (1.68) | 15,121 (2.80) |
| HTN | 7372 (6.50) | 4054 (3.57) | 326 (0.29) | 2546 (2.24) | 14,298 (3.15) |
| GN | 1061 (4.28) | 546 (2.20) | 42 (0.17) | 202 (0.82) | 1851 (1.87) |
| CTIN | 251 (5.65) | 127 (2.86) | 14 (0.32) | 58 (1.31) | 450 (2.53) |
| ON | 1223 (7.78) | 549 (3.49) | 27 (0.17) | 124 (0.79) | 1923 (3.06) |
| Others | 2420 (4.86) | 1149 (2.31) | 126 (0.25) | 1065 (2.14) | 4760 (2.39) |
| Total | 20,132 (5.87) | 11,090 (3.23) | 916 (0.27) | 6265 (1.83) | 38,403 (2.80) |
CABG, coronary artery bypass grafting; CAG, coronarography; CKD, chronic kidney disease; CTIN, chronic tubulointerstitial nephropathy; DKD, diabetic kidney disease; GN, glomerulonephritis; HTN, hypertensive nephropathy; ON, obstructive nephropathy; Others, CKD due to other reasons; PCI, percutaneous coronary intervention.
2.1. Prevalence of CVD, stratified by patient group
2.1.1. Prevalence of CHD
2.1.2. Prevalence of stroke
2.1.3. Prevalence of heart failure
2.1.4. Prevalence of atrial fibrillation
2.2. Prevalence of CVD among patients with CKD
2.2.1. Prevalence of CHD among patients with CKD
2.2.2. Prevalence of stroke among patients with CKD
2.2.3. Prevalence of heart failure among patients with CKD
2.2.4. Prevalence of atrial fibrillation among patients with CKD
2.3. Cardiovascular procedures stratified by patient group
2.3.1. Cardiovascular procedure: coronarography
2.3.2. Cardiovascular procedure: percutaneous coronary intervention
2.3.3. Cardiovascular procedure: coronary artery bypass grafting
2.3.4. Cardiovascular procedure: pacemaker
2.4. Cardiovascular procedures among patients with CKD
Chapter 3: Health care resource utilization of hospitalized patients with CKD
Bixia Gao1,2, Chao Yang1,2, Xinwei Deng1,2, Zaiming Su3, Lanxia Gan4, Ying Shi4 and Fang Wang1,2
1Renal Division, Department of Medicine, Peking University First Hospital, Peking University Institute of Nephrology, Beijing, China; 2Research Units of Diagnosis and Treatment of Immune-Mediated Kidney Diseases, Chinese Academy of Medical Sciences, Beijing, China; 3National Institute of Health Data Science at Peking University, Beijing, China; and 4China Standard Medical Information Research Center, Shenzhen, Guangdong, China
Medical expenditure and length of stay (LOS) are both important indicators for health care resource utilization, which are critical to resource allocation and government decisions. This chapter describes the medical expenditure and LOS of inpatients with chronic kidney disease. Because of the high skewness of cost and LOS, the results are displayed as the median and interquartile range (IQR), and mean and SD are also provided. The comparisons between the 2 groups of patients with diabetes and those with CKD were based on the overall reference population, respectively, which meant we did not exclude patients with diabetes also having CKD or patients with CKD also having diabetes.
The total medical expenditure of all patients with CKD included in the analysis of 2016 was 27,646 million RMB (approximately 3916 million USD as of May 2020), accounting for 6.50% of the overall expenditure in the database; however, the percentage of inpatients with CKD was only 4.86% (Table 39). Compared with other comorbidities, patients with CKD and heart failure incurred higher medical costs (44,419 RMB per person per year [PPPY]) (Table 39).
The median cost per patient with CKD was 15,405 (IQR: 8435–29,542) RMB, which was higher than that in 2015 (14,965 [IQR: 8302–28,282] RMB).10 Moreover, the median cost per patient with CKD was higher than that in patients with diabetes (13,868 [IQR: 7779–25,688] RMB) and those without CKD (11,182 [IQR: 5916–18,922] RMB) (Figure 39 and Table 40). The trends were consistent across age-sex subgroups and across different types of health insurance (Figure 39, Figure 40, Figure 41 and Table 40, Table 41, Table 42, Table 43, Table 44, Table 45). There was no significant difference in median cost of patients with CKD between different subgroups of sex and health insurance (Figures 39 and 40; Table 40, Table 41, Table 42, Table 43), but the cost increased with age.
Figure 39.
Costs stratified by types of health insurance. The box plot shows the distribution of hospitalization costs for different types of health insurance. Limited to 1.5 * third quartile. Red points refer to cost per person per year. CKD, chronic kidney disease; DM, diabetes mellitus; NRCMS, new rural co-operative medical care; UBMI, urban basic medical insurance.
Table 40.
Costs stratified by types of health insurance (median [IQR])
| Types of health insurance | CKD | DM | Non-CKD |
|---|---|---|---|
| UBMI | 15,405 (8701–30,810) | 13,720 (7941–25,355) | 11,449 (6355–19,210) |
| NRCMS | 14,654 (7503–24,548) | 12,242 (6764–21,890) | 10,764 (5653–18,730) |
| Free medical care | 15,992 (9179–40,978) | 15,405 (7986–37,172) | 11,848 (5713–19,188) |
| Self-paid treatment | 15,405 (8057–28,976) | 15,088 (7697–28,761) | 10,189 (4905–17,277) |
| Others | 15,405 (9401–30,859) | 15,405 (8560–28,774) | 12,368 (6330–20,703) |
| Total | 15,405 (8435–29,542) | 13,868 (7779–25,688) | 11,182 (5916–18,922) |
CKD, chronic kidney disease; DM, diabetes mellitus; IQR, interquartile range; NRCMS, new rural co-operative medical care; UBMI, urban basic medical insurance.
Figure 40.
Costs stratified by sex. The box plot shows the distribution of hospitalization costs by sex. Limited to 1.5 * third quartile. Red points refer to cost per person per year. CKD, chronic kidney disease; DM, diabetes mellitus.
Figure 41.
Costs stratified by age group (mean). CKD, chronic kidney disease; DM, diabetes mellitus.
Table 41.
Costs stratified by types of health insurance (mean [SD])
| Types of health insurance | CKD | DM | Non-CKD |
|---|---|---|---|
| UBMI | 28,571 (45,728) | 25,252 (39,809) | 20,802 (33,334) |
| NRCMS | 22,014 (31,083) | 21,502 (30,955) | 19,451 (28,227) |
| Free medical care | 51,889 (120,882) | 44,053 (105,654) | 24,138 (58,256) |
| Self-paid treatment | 28,185 (52,990) | 27,822 (49,905) | 19,377 (33,696) |
| Others | 31,213 (54,743) | 28,422 (48,907) | 21,965 (35,731) |
| Total | 27,849 (48,004) | 25,642 (43,183) | 20,437 (33,295) |
CKD, chronic kidney disease; DM, diabetes mellitus; NRCMS, new rural co-operative medical care; UBMI, urban basic medical insurance.
Table 42.
Costs stratified by sex (median [IQR])
| Sex | CKD | DM | Non-CKD |
|---|---|---|---|
| Male | 15,405 (8576–30,810) | 14,499 (7958–28,180) | 13,245 (6727–24,330) |
| Female | 15,405 (8243–27,911) | 13,185 (7576–23,237) | 9,967 (5388–15,813) |
| Total | 15,405 (8435–29,542) | 13,868 (7779–25,688) | 11,182 (5916–18,922) |
CKD, chronic kidney disease; DM, diabetes mellitus; IQR, interquartile range.
Table 43.
Costs stratified by sex (mean [SD])
| Sex | CKD | DM | Non-CKD |
|---|---|---|---|
| Male | 29,364 (52,231) | 27,659 (47,974) | 24,242 (39,111) |
| Female | 25,726 (41,267) | 23,227 (36,485) | 17,502 (27,638) |
| Total | 27,849 (48,004) | 25,642 (43,183) | 20,437 (33,295) |
CKD, chronic kidney disease; DM, diabetes mellitus.
Table 44.
Costs stratified by age group (median [IQR])
| Age group, yr | CKD | DM | Non-CKD |
|---|---|---|---|
| 18–24 | 12,498 (6418–21,184) | 8438 (5171–15,405) | 7331 (3779–15,405) |
| 25–29 | 12,932 (6533–22,383) | 9019 (5566–15,405) | 7197 (4013–14,584) |
| 30–34 | 13,411 (6822–22,739) | 9390 (5828–15,405) | 8165 (4393–15,405) |
| 35–39 | 13,952 (7067–23,331) | 9989 (6149–16,084) | 9385 (4991–15,405) |
| 40–44 | 14,540 (7325–24,503) | 10,784 (6522–17,754) | 11,066 (5712–17,906) |
| 45–49 | 15,202 (7715–25,585) | 11,635 (6830–19,825) | 12,145 (6158–20,662) |
| 50–54 | 15,405 (8093–27,107) | 12,254 (7172–21,548) | 12,471 (6433–22,401) |
| 55–59 | 15,405 (8617–29,298) | 13,243 (7605–24,582) | 13,213 (6873–24,986) |
| 60–64 | 15,405 (8805–30,429) | 13,834 (7868–26,143) | 13,549 (7049–26,016) |
| 65–69 | 15,405 (9088–30,810) | 14,537 (8141–27,466) | 13,796 (7217–25,943) |
| 70–74 | 15,405 (9237–31,476) | 15,119 (8391–28,071) | 13,655 (7258–24,442) |
| 75–79 | 15,718 (9524–33,215) | 15,405 (8659–29,178) | 13,682 (7332–23,440) |
| 80–84 | 16,951 (9940–36,348) | 15,405 (9080–30,810) | 13,884 (7376–23,805) |
| 85+ | 19,897 (10,866–46,114) | 17,940 (10,375–42,786) | 15,397 (7643–27,911) |
| Total | 15,405 (8435–29,542) | 13,868 (7779–25,688) | 11,182 (5916–18,922) |
CKD, chronic kidney disease; DM, diabetes mellitus; IQR, interquartile range.
Table 45.
Costs stratified by age group (mean [SD])
| Age group, yr | CKD | DM | Non-CKD |
|---|---|---|---|
| 18–24 | 21,832 (43,991) | 17,310 (45,222) | 12,989 (25,672) |
| 25–29 | 22,591 (50,116) | 16,984 (59,430) | 11,439 (20,395) |
| 30–34 | 22,468 (39,758) | 17,565 (38,731) | 13,083 (23,231) |
| 35–39 | 23,088 (38,987) | 18,314 (33,121) | 15,942 (27,348) |
| 40–44 | 23,515 (39,688) | 19,748 (34,015) | 19,401 (31,552) |
| 45–49 | 24,118 (38,872) | 21,251 (34,244) | 21,535 (33,278) |
| 50–54 | 25,107 (38,859) | 22,681 (35,411) | 22,773 (34,241) |
| 55–59 | 26,817 (40,873) | 24,658 (36,627) | 24,346 (35,324) |
| 60–64 | 27,532 (40,635) | 25,609 (37,611) | 24,750 (35,151) |
| 65–69 | 28,409 (41,269) | 26,161 (37,637) | 24,551 (34,574) |
| 70–74 | 29,263 (42,277) | 26,212 (37,190) | 23,430 (33,269) |
| 75–79 | 30,651 (45,385) | 26,787 (39,491) | 22,588 (32,921) |
| 80–84 | 34,585 (61,972) | 30,458 (56,928) | 23,177 (40,641) |
| 85+ | 50,623 (108,954) | 48,777 (112,238) | 29,728 (70,956) |
| Total | 27,849 (48,004) | 25,642 (43,183) | 20,437 (33,295) |
CKD, chronic kidney disease; DM, diabetes mellitus.
The average LOS of inpatients with CKD was 20.33 (SD: 31.65) days PPPY and the median LOS was 13 (IQR: 8–22) days, which was higher than that of patients with diabetes (11 [IQR: 7–18] days) and patients without CKD (8 [IQR: 5–14] days) (Figure 42 and Table 46, Table 47, Table 48). The trends were consistent across age-sex subgroups and across types of health insurance as well (Figure 42, Figure 43, Figure 44 and Table 47, Table 48, Table 49, Table 50, Table 51, Table 52). Patients aged ≥85 years had the longest hospitalization days (Figure 44 and Tables 51 and 52).
Figure 42.
Length of hospital stay stratified by types of health insurance. Limited to 1.5 * third quartile. Red points refer to LOS per person per year. CKD, chronic kidney disease; DM, diabetes mellitus; LOS, length of hospital stay; NRCMS, new rural co-operative medical care; UBMI, urban basic medical insurance.
Table 47.
Length of hospital stay stratified by types of health insurance (median [IQR])
| Types of health insurance | CKD | DM | Non-CKD |
|---|---|---|---|
| UBMI | 13 (8–23) | 11 (8–18) | 9 (5–14) |
| NRCMS | 12 (7–20) | 10 (7–16) | 9 (5–14) |
| Free medical care | 14 (8–29) | 12 (8–24) | 8 (5–15) |
| Self-paid treatment | 11 (7–20) | 10 (7–17) | 7 (4–12) |
| Others | 13 (8–22) | 12 (7–19) | 8 (5–14) |
| Total | 13 (8–22) | 11 (7–18) | 8 (5–14) |
CKD, chronic kidney disease; DM, diabetes mellitus; IQR, interquartile range; NRCMS, new rural co-operative medical care; UBMI, urban basic medical insurance.
Table 48.
Length of hospital stay stratified by types of health insurance (mean [SD])
| Types of health insurance | CKD | DM | Non-CKD |
|---|---|---|---|
| UBMI | 21.28 (31.44) | 17.56 (25.28) | 13.58 (21.05) |
| NRCMS | 17.64 (25.89) | 14.89 (17.80) | 13.28 (17.61) |
| Free medical care | 35.49 (66.81) | 29.86 (58.90) | 16.79 (36.14) |
| Self-paid treatment | 18.15 (31.31) | 16.47 (27.31) | 11.18 (18.31) |
| Others | 21.12 (32.80) | 18.37 (28.97) | 13.33 (20.87) |
| Total | 20.33 (31.65) | 17.34 (26.07) | 13.06 (20.20) |
CKD, chronic kidney disease; DM, diabetes mellitus; NRCMS, new rural co-operative medical care; UBMI, urban basic medical insurance.
Figure 43.
Length of hospital stay stratified by sex. Limited to 1.5 * third quartile. Red points refer to LOS per person per year. CKD, chronic kidney disease; DM, diabetes mellitus; LOS, length of hospital stay.
Figure 44.
Length of hospital stay stratified by age group (mean). CKD, chronic kidney disease; DM, diabetes mellitus; LOS, length of hospital stay.
Table 49.
Length of hospital stay stratified by sex (median [IQR])
| Sex | CKD | DM | Non-CKD |
|---|---|---|---|
| Male | 13 (8–22) | 11 (7–19) | 9 (6–16) |
| Female | 12 (8–21) | 11 (7–18) | 7 (4–12) |
| Total | 13 (8–22) | 11 (7–18) | 8 (5–14) |
CKD, chronic kidney disease; DM, diabetes mellitus; IQR, interquartile range.
Table 50.
Length of hospital stay stratified by sex (mean [SD])
| Sex | CKD | DM | Non-CKD |
|---|---|---|---|
| Male | 20.86 (33.18) | 18.07 (27.94) | 15.12 (23.23) |
| Female | 19.59 (29.35) | 16.48 (23.60) | 11.47 (17.34) |
| Total | 20.33 (31.65) | 17.34 (26.07) | 13.06 (20.20) |
CKD, chronic kidney disease; DM, diabetes mellitus.
Table 51.
Length of hospital stay stratified by age group (median [IQR])
| Age group, yr | CKD | DM | Non-CKD |
|---|---|---|---|
| 18–24 | 11 (7–19) | 10 (7–14) | 6 (4–10) |
| 25–29 | 10 (6–18) | 10 (7–15) | 5 (4–8) |
| 30–34 | 10 (6–18) | 10 (7–15) | 6 (4–9) |
| 35–39 | 11 (7–18) | 10 (7–15) | 7 (4–11) |
| 40–44 | 11 (7–19) | 10 (7–16) | 8 (5–13) |
| 45–49 | 12 (7–19) | 10 (7–16) | 9 (5–15) |
| 50–54 | 12 (7–20) | 11 (7–16) | 9 (6–15) |
| 55–59 | 12 (8–21) | 11 (7–17) | 9 (6–16) |
| 60–64 | 13 (8–22) | 11 (7–18) | 10 (6–16) |
| 65–69 | 13 (8–23) | 11 (7–18) | 10 (6–16) |
| 70–74 | 14 (8–24) | 12 (8–19) | 10 (6–16) |
| 75–79 | 14 (8–25) | 12 (8–20) | 10 (6–17) |
| 80–84 | 14 (8–26) | 13 (8–22) | 10 (6–17) |
| 85+ | 16 (9–32) | 14 (8–29) | 11 (6–19) |
| Total | 13 (8–22) | 11 (7–18) | 8 (5–14) |
CKD, chronic kidney disease; DM, diabetes mellitus; IQR, interquartile range.
Table 52.
Length of hospital stay stratified by age group (mean [SD])
| Age group, yr | CKD | DM | Non-CKD |
|---|---|---|---|
| 18–24 | 17.12 (28.10) | 13.77 (17.73) | 9.32 (16.66) |
| 25–29 | 16.84 (33.03) | 14.36 (39.98) | 8.05 (13.28) |
| 30–34 | 16.75 (27.13) | 14.34 (20.73) | 8.86 (14.49) |
| 35–39 | 17.25 (28.80) | 14.57 (20.73) | 10.60 (17.71) |
| 40–44 | 17.85 (29.30) | 15.03 (21.19) | 12.75 (20.24) |
| 45–49 | 18.24 (28.14) | 15.42 (21.25) | 13.84 (21.27) |
| 50–54 | 18.80 (28.06) | 15.71 (21.67) | 14.22 (20.72) |
| 55–59 | 19.51 (28.25) | 16.16 (22.02) | 14.62 (20.85) |
| 60–64 | 19.92 (27.76) | 16.44 (22.35) | 14.71 (19.89) |
| 65–69 | 20.51 (27.62) | 16.81 (21.81) | 14.77 (19.24) |
| 70–74 | 21.28 (29.05) | 17.28 (22.49) | 14.72 (19.00) |
| 75–79 | 22.05 (30.19) | 18.33 (25.14) | 14.99 (20.85) |
| 80–84 | 24.51 (38.29) | 21.61 (36.37) | 16.13 (26.40) |
| 85+ | 34.87 (62.26) | 34.38 (64.50) | 21.01 (43.03) |
| Total | 20.33 (31.65) | 17.34 (26.07) | 13.06 (20.20) |
CKD, chronic kidney disease; DM, diabetes mellitus.
3.1. Costs
3.1.1. Overall medical costs stratified by CKD, diabetes, and heart failure
Table 39.
Overall medical costs, stratified by CKD, diabetes, and heart failure
| Patient group | HQMS population | Total costs (millions,¥) | PPPY (¥) | Population (%) | Costs (%) |
|---|---|---|---|---|---|
| All | 20,444,645 | 425,184 | 20,797 | 100.00 | 100.00 |
| With HF or CKD or DM | 3,450,824 | 94,034 | 27,250 | 16.88 | 22.12 |
| CKD only | 595,833 | 14,603 | 24,508 | 2.91 | 3.43 |
| DM only | 1,465,828 | 34,252 | 23,367 | 7.17 | 8.06 |
| HF only | 792,903 | 24,836 | 31,323 | 3.88 | 5.84 |
| CKD and DM only | 229,055 | 5588 | 24,397 | 1.12 | 1.31 |
| CKD and HF only | 101,898 | 4332 | 42,514 | 0.50 | 1.02 |
| DM and HF only | 199,366 | 7300 | 36,618 | 0.98 | 1.72 |
| CKD and HF and DM | 65,941 | 3123 | 47,363 | 0.32 | 0.73 |
| No CKD or HF or DM | 16,993,821 | 331,150 | 19,486 | 83.12 | 77.88 |
| All CKD | 992,727 | 27,646 | 27,849 | 4.86 | 6.50 |
| All DM | 1,960,190 | 50,263 | 25,642 | 9.59 | 11.82 |
| All HF | 1,160,108 | 39,591 | 34,127 | 5.67 | 9.31 |
| CKD and DM | 294,996 | 8711 | 29,531 | 1.44 | 2.05 |
| CKD and HF | 167,839 | 7455 | 44,419 | 0.82 | 1.75 |
| DM and HF | 265,307 | 10,424 | 39,289 | 1.30 | 2.45 |
CKD, chronic kidney disease; DM, diabetes mellitus; HF, heart failure; HQMS, Hospital Quality Monitoring System; PPPY, per person per year.
3.1.2. Costs stratified by types of health insurance
3.1.3. Costs stratified by sex
3.1.4. Costs stratified by age
3.2. Length of hospital stay
3.2.1. Overall length of hospital stay stratified by CKD, diabetes, and heart failure
Table 46.
Overall length of hospital stay stratified by CKD, diabetes, and heart failure
| Patient group | HQMS population | Total LOS (thousands, d) | PPPY (d) | Population (%) | LOS (%) |
|---|---|---|---|---|---|
| All | 20,444,645 | 274,232 | 13.41 | 100.00 | 100.00 |
| With HF or CKD or DM | 3,450,824 | 60,829 | 17.63 | 16.88 | 22.18 |
| CKD only | 595,833 | 10,659 | 17.89 | 2.91 | 3.89 |
| DM only | 1,465,828 | 23,414 | 15.97 | 7.17 | 8.54 |
| HF only | 792,903 | 13,254 | 16.72 | 3.88 | 4.83 |
| CKD and DM only | 229,055 | 4488 | 19.59 | 1.12 | 1.64 |
| CKD and HF only | 101,898 | 2917 | 28.63 | 0.50 | 1.06 |
| DM and HF only | 199,366 | 3976 | 19.94 | 0.98 | 1.45 |
| CKD and HF and DM | 65,941 | 2121 | 32.16 | 0.32 | 0.77 |
| No CKD or HF or DM | 16,993,821 | 213,402 | 12.56 | 83.12 | 77.82 |
| All CKD | 992,727 | 20,185 | 20.33 | 4.86 | 7.36 |
| All DM | 1,960,190 | 33,999 | 17.34 | 9.59 | 12.40 |
| All HF | 1,160,108 | 22,268 | 19.19 | 5.67 | 8.12 |
| CKD and DM | 294,996 | 6609 | 22.40 | 1.44 | 2.41 |
| CKD and HF | 167,839 | 5038 | 30.02 | 0.82 | 1.84 |
| DM and HF | 265,307 | 6097 | 22.98 | 1.30 | 2.22 |
CKD, chronic kidney disease; DM, diabetes mellitus; HF, heart failure; HQMS, Hospital Quality Monitoring System; LOS, length of hospital stay; PPPY, per person per year.
3.2.2. Length of hospital stay stratified by types of health insurance
3.2.3. Length of hospital stay stratified by sex
3.2.4. Length of hospital stay stratified by age
Chapter 4: In-hospital mortality of hospitalized patients with CKD
Bixia Gao1,2, Chao Yang1,2, Zaiming Su3, Lanxia Gan4, Ying Shi4 and Fang Wang1,2
1Renal Division, Department of Medicine, Peking University First Hospital, Peking University Institute of Nephrology, Beijing, China; 2Research Units of Diagnosis and Treatment of Immune-Mediated Kidney Diseases, Chinese Academy of Medical Sciences, Beijing, China; 3National Institute of Health Data Science at Peking University, Beijing, China; and 4China Standard Medical Information Research Center, Shenzhen, Guangdong, China
This chapter focuses on the in-hospital mortality of inpatients with chronic kidney disease (CKD). We conducted stratified analyses based on comorbidity, types of insurance, age, and sex. The comparisons between the 2 groups of patients with diabetes and those with CKD were based on the overall reference population, respectively, which meant we did not exclude patients with diabetes also having CKD or patients with CKD also having diabetes.
In 2016, the in-hospital mortality rate of inpatients with CKD was 2.56% (Table 53), slightly lower than that in 2015 (2.63%).10 Moreover, the mortality rate was higher than that of all inpatients (0.84%) and patients with diabetes (1.48%), but lower than that of patients with heart failure (4.52%; Table 53). This trend was consistent across different types of medical insurance and subgroups of age and sex (Figure 45, Figure 46, Figure 47 and Table 54, Table 55, Table 56).
Figure 45.
In-hospital mortality stratified by different types of insurance. CKD, chronic kidney disease; DM, diabetes mellitus; NRCMS, new rural co-operative medical care; UBMI, urban basic medical insurance.
Figure 46.
In-hospital mortality stratified by sex. CKD, chronic kidney disease; DM, diabetes mellitus.
Figure 47.
In-hospital mortality stratified by age group. Point size refers to mortality rate. CKD, chronic kidney disease; DM, diabetes mellitus.
Table 54.
In-hospital mortality stratified by different types of insurance, N (%)
| Types of Health Insurance | CKD | DM | Non-CKD |
|---|---|---|---|
| UBMI | 17,170 (3.37) | 20,039 (1.70) | 88,726 (1.01) |
| NRCMS | 1627 (0.75) | 1545 (0.49) | 13,305 (0.33) |
| Free medical care | 1074 (5.66) | 1195 (3.21) | 4478 (1.44) |
| Self-paid treatment | 2543 (2.02) | 2883 (1.39) | 21,399 (0.56) |
| Others | 3001 (2.49) | 3415 (1.53) | 18,187 (0.75) |
| Total | 25,415 (2.56) | 29,077 (1.48) | 146,095 (0.75) |
CKD, chronic kidney disease; DM, diabetes mellitus; NRCMS, new rural co-operative medical care; UBMI, urban basic medical insurance.
Table 55.
In-hospital mortality stratified by sex, N (%)
| Patient group | Male | Female | Total |
|---|---|---|---|
| CKD | 15,866 (2.74) | 9549 (2.31) | 25,415 (2.56) |
| DM | 17,726 (1.66) | 11,351 (1.27) | 29,077 (1.48) |
| Non-CKD | 93,583 (1.10) | 52,512 (0.48) | 146,095 (0.75) |
CKD, chronic kidney disease; DM, diabetes mellitus
Table 56.
In-hospital mortality stratified by age group, N (%)
| Age group, yr | CKD | DM | Non-CKD |
|---|---|---|---|
| 18–24 | 125 (0.53) | 28 (0.40) | 1594 (0.17) |
| 25–29 | 200 (0.55) | 35 (0.26) | 1877 (0.10) |
| 30–34 | 231 (0.58) | 76 (0.35) | 2138 (0.15) |
| 35–39 | 312 (0.70) | 125 (0.36) | 2809 (0.24) |
| 40–44 | 541 (0.85) | 307 (0.44) | 4944 (0.37) |
| 45–49 | 783 (0.90) | 614 (0.48) | 7249 (0.43) |
| 50–54 | 1170 (1.06) | 1255 (0.55) | 10,386 (0.52) |
| 55–59 | 1393 (1.55) | 1811 (0.78) | 11,055 (0.68) |
| 60–64 | 2076 (1.79) | 3016 (0.96) | 15,477 (0.74) |
| 65–69 | 2433 (2.32) | 3452 (1.18) | 15,249 (0.88) |
| 70–74 | 2756 (3.16) | 3974 (1.66) | 15,538 (1.16) |
| 75–79 | 4070 (4.89) | 5062 (2.57) | 19,380 (1.76) |
| 80–84 | 4505 (7.12) | 5050 (4.10) | 19,896 (2.78) |
| 85+ | 4820 (11.52) | 4272 (7.15) | 18,503 (4.82) |
| Total | 25,415 (2.56) | 29,077 (1.48) | 146,095 (0.75) |
CKD, chronic kidney disease; DM, diabetes mellitus.
Patients covered by free medical care had the highest in-hospital mortality rate (5.66%), followed by those with urban basic medical insurance (3.37%) (Figure 45 and Table 54). This might be related to the characteristics of health care resource utilization of various insurance types and higher percentages of diabetic kidney disease and hypertensive nephropathy among urban residents. The in-hospital mortality rate of male patients with CKD (2.74%) was higher than that of female patients (2.31%; Figure 46 and Table 55). Moreover, the mortality rate increased with age (Figure 47 and Table 56). Hospitalized patients with CKD who were aged ≥85 years had the highest mortality rate (11.52%; Figure 47 and Table 56).
4.1. In-hospital mortality stratified by CKD, diabetes, and heart failure
Table 53.
In-hospital mortality stratified by CKD, diabetes, and heart failure
| Patient group | Hospital mortality | HQMS population | Mortality rate (%) | Proportion (%) |
|---|---|---|---|---|
| All | 171,510 | 20,444,645 | 0.84 | 100.00 |
| With HF or CKD or DM | 78,482 | 3,450,824 | 2.27 | 45.76 |
| CKD only | 9055 | 595,833 | 1.52 | 5.28 |
| DM only | 13,505 | 1,465,828 | 0.92 | 7.87 |
| HF only | 32,121 | 792,903 | 4.05 | 18.73 |
| CKD and DM only | 3522 | 229,055 | 1.54 | 2.05 |
| CKD and HF only | 8229 | 101,898 | 8.08 | 4.80 |
| DM and HF only | 7441 | 199,366 | 3.73 | 4.34 |
| CKD and HF and DM | 4609 | 65,941 | 6.99 | 2.69 |
| No CKD or HF or DM | 93,028 | 16,993,821 | 0.55 | 54.24 |
| All CKD | 25,415 | 992,727 | 2.56 | 14.82 |
| All DM | 29,077 | 1,960,190 | 1.48 | 16.95 |
| All HF | 52,400 | 1,160,108 | 4.52 | 30.55 |
| CKD and DM | 8131 | 294,996 | 2.76 | 4.74 |
| CKD and HF | 12,838 | 167,839 | 7.65 | 7.49 |
| DM and HF | 12,050 | 265,307 | 4.54 | 7.03 |
CKD, chronic kidney disease; DM, diabetes mellitus; HF, heart failure; HQMS, Hospital Quality Monitoring System.
4.2. In-hospital mortality stratified by types of insurance
4.3. In-hospital mortality stratified by sex
4.4. In-hospital mortality stratified by age
Chapter 5: Acute kidney injury
Bixia Gao1,2, Chao Yang1,2, Xinwei Deng1,2, Zaiming Su3, Lanxia Gan4, Ying Shi4 and Li Yang1,2
1Renal Division, Department of Medicine, Peking University First Hospital, Peking University Institute of Nephrology, Beijing, China; 2Research Units of Diagnosis and Treatment of Immune-Mediated Kidney Diseases, Chinese Academy of Medical Sciences, Beijing, China; 3National Institute of Health Data Science at Peking University, Beijing, China; and 4China Standard Medical Information Research Center, Shenzhen, Guangdong, China
Acute kidney injury (AKI) is associated with significant morbidity and subsequent chronic kidney disease (CKD) development.15 This chapter focuses on the characteristics of inpatients diagnosed with AKI. It should be noted that because AKI is often underdiagnosed, our results reflect both the actual diagnostic rate and potential burdens.
There were substantial geographic variations regarding the percentage of AKI among patients who stayed in an intensive care unit (ICU), compared with those without ICU stay (Figure 48 and Table 57). Patients with ICU stay in Hainan Province, China, had the highest percentage of AKI (13.06%, Table 57). The percentage of patients with the diagnostic coding of AKI was 0.30% (Table 57), which was the same as that in 2015.10
Figure 48.
Percentage of AKI with and without ICU stay, stratified by geographic region. AKI, acute kidney injury; C, Central of China; E, East of China; ICU, intensive care unit; N, North of China; NE, Northeast of China; NW, Northwest of China; S, South of China; SW, Southwest of China.
Table 57.
Percentage of AKI with and without ICU stay, stratified by geographic region, N (%)
| Region | With ICU stay | Without ICU stay | Total |
|---|---|---|---|
| N-Beijing | 1273 (3.48) | 2030 (0.26) | 3303 (0.41) |
| N-Tianjin | 29 (2.34) | 255 (0.12) | 284 (0.14) |
| N-Hebei | 341 (7.13) | 1945 (0.34) | 2286 (0.39) |
| N-Shanxi | 350 (9.46) | 1561 (0.30) | 1911 (0.36) |
| N-Inner Mongolia | 64 (3.31) | 1464 (0.29) | 1528 (0.30) |
| NE-Liaoning | 328 (4.11) | 1284 (0.23) | 1612 (0.28) |
| NE-Jilin | 133 (5.84) | 1099 (0.27) | 1232 (0.30) |
| NE-Heilongjiang | 203 (3.09) | 707 (0.12) | 910 (0.16) |
| E-Shanghai | 69 (2.99) | 1440 (0.15) | 1509 (0.16) |
| E-Jiangsu | 852 (4.51) | 2563 (0.16) | 3415 (0.21) |
| E-Zhejiang | 647 (5.53) | 1944 (0.20) | 2591 (0.27) |
| E-Anhui | 140 (1.08) | 1250 (0.18) | 1390 (0.19) |
| E-Fujian | 521 (6.47) | 1418 (0.23) | 1939 (0.31) |
| E-Jiangxi | 413 (7.37) | 2035 (0.29) | 2448 (0.34) |
| E-Shandong | 347 (3.04) | 1365 (0.16) | 1712 (0.20) |
| C-Henan | 310 (1.60) | 1576 (0.15) | 1886 (0.18) |
| C-Hubei | 748 (2.90) | 3698 (0.22) | 4446 (0.26) |
| C-Hunan | 232 (2.69) | 1403 (0.27) | 1635 (0.31) |
| S-Guangdong | 1089 (3.95) | 4865 (0.31) | 5954 (0.37) |
| S-Guangxi | 580 (6.34) | 2231 (0.46) | 2811 (0.57) |
| S-Hainan | 200 (13.06) | 834 (0.46) | 1034 (0.56) |
| SW-Chongqing | 121 (5.61) | 853 (0.31) | 974 (0.36) |
| SW-Sichuan | 1042 (4.83) | 4321 (0.31) | 5363 (0.38) |
| SW-Guizhou | 64 (3.80) | 1017 (0.33) | 1081 (0.35) |
| SW-Yunnan | 559 (6.79) | 3632 (0.44) | 4191 (0.50) |
| SW-Tibet | — | — | — |
| NW-Shaanxi | 201 (6.57) | 1361 (0.24) | 1562 (0.27) |
| NW-Gansu | 116 (5.79) | 630 (0.28) | 746 (0.32) |
| NW-Qinghai | 85 (4.70) | 185 (0.25) | 270 (0.36) |
| NW-Ningxia | 89 (3.60) | 607 (0.52) | 696 (0.59) |
| NW-Xinjiang | 230 (2.00) | 866 (0.25) | 1096 (0.31) |
| Total | 11,376 (4.03) | 50,439 (0.25) | 61,815 (0.30) |
AKI, acute kidney injury; C, Central of China; E, East of China; ICU, intensive care unit; N, North of China; NE, Northeast of China; NW, Northwest of China; S, South of China; SW, Southwest of China.
Altogether, 1.76% of patients with CKD were diagnosed with AKI. Regarding the causes of CKD, patients with chronic tubulointerstitial nephropathy and glomerulonephritis had higher percentages of AKI (3.78% and 3.05%, respectively), whereas patients with diabetic kidney disease had the lowest AKI percentage (1.03%; Figure 49 and Table 58). The analysis of characteristics showed that patients aged 50 to 54 and 60 to 79 years accounted for a high proportion, for both male and female (Figure 50 and Table 59). For all age groups, the proportion of male was higher than that of female (Figure 51 and Table 60).
Figure 49.
Percentage of AKI among patients with CKD. AKI, acute kidney injury; CKD, chronic kidney disease; CTIN, chronic tubulointerstitial nephropathy; DKD, diabetic kidney disease; GN, glomerulonephritis; HTN, hypertensive nephropathy; ON, obstructive nephropathy; Others, CKD due to other reasons.
Table 58.
Percentage of AKI among patients with CKD, N (%)
| Cause | AKI |
|---|---|
| DKD | 2718 (1.03) |
| HTN | 3453 (1.63) |
| GN | 4357 (3.05) |
| CTIN | 623 (3.78) |
| ON | 2536 (1.60) |
| Others | 3788 (1.92) |
| Total | 17,475 (1.76) |
AKI, acute kidney injury; CKD, chronic kidney disease; CTIN, chronic tubulointerstitial nephropathy; DKD, diabetic kidney disease; GN, glomerulonephritis; HTN, hypertensive nephropathy; ON, obstructive nephropathy; Others, CKD due to other reasons.
Figure 50.
Age distribution of AKI patients, stratified by sex. AKI, acute kidney injury.
Table 59.
Age distribution of AKI patients, stratified by sex, N (%)
| Age group, yr | Male | Female | Total |
|---|---|---|---|
| 18–24 | 1116 (2.79) | 677 (3.10) | 1793 (2.90) |
| 25–29 | 1254 (3.14) | 895 (4.09) | 2149 (3.48) |
| 30–34 | 1308 (3.27) | 751 (3.43) | 2059 (3.33) |
| 35–39 | 1511 (3.78) | 703 (3.21) | 2214 (3.58) |
| 40–44 | 2380 (5.96) | 917 (4.19) | 3297 (5.33) |
| 45–49 | 3292 (8.24) | 1392 (6.37) | 4684 (7.58) |
| 50–54 | 4153 (10.40) | 1834 (8.39) | 5987 (9.69) |
| 55–59 | 3310 (8.29) | 1591 (7.28) | 4901 (7.93) |
| 60–64 | 4549 (11.39) | 2391 (10.93) | 6940 (11.23) |
| 65–69 | 4321 (10.82) | 2474 (11.31) | 6795 (10.99) |
| 70–74 | 3646 (9.13) | 2305 (10.54) | 5951 (9.63) |
| 75–79 | 3752 (9.39) | 2452 (11.21) | 6204 (10.04) |
| 80–84 | 2993 (7.49) | 2069 (9.46) | 5062 (8.19) |
| 85+ | 2361 (5.91) | 1418 (6.48) | 3779 (6.11) |
| Total | 39,946 | 21,869 | 61,815 |
AKI, acute kidney injury.
Figure 51.
Sex distribution of AKI patients, stratified by age. AKI, acute kidney injury.
Table 60.
Sex distribution of AKI patients, stratified by age, N (%)
| Age group, yr | Male | Female | Total |
|---|---|---|---|
| 18–24 | 1116 (62.24) | 677 (37.76) | 1793 |
| 25–29 | 1254 (58.35) | 895 (41.65) | 2149 |
| 30–34 | 1308 (63.53) | 751 (36.47) | 2059 |
| 35–39 | 1511 (68.25) | 703 (31.75) | 2214 |
| 40–44 | 2380 (72.19) | 917 (27.81) | 3297 |
| 45–49 | 3292 (70.28) | 1392 (29.72) | 4684 |
| 50–54 | 4153 (69.37) | 1834 (30.63) | 5987 |
| 55–59 | 3310 (67.54) | 1591 (32.46) | 4901 |
| 60–64 | 4549 (65.55) | 2391 (34.45) | 6940 |
| 65–69 | 4321 (63.59) | 2474 (36.41) | 6795 |
| 70–74 | 3646 (61.27) | 2305 (38.73) | 5951 |
| 75–79 | 3752 (60.48) | 2452 (39.52) | 6204 |
| 80–84 | 2993 (59.13) | 2069 (40.87) | 5062 |
| 85+ | 2361 (62.48) | 1418 (37.52) | 3779 |
| Total | 39,946 (64.62) | 21,869 (35.38) | 61,815 |
AKI, acute kidney injury.
The overall trends of CKD and diabetes among patients with AKI were similar to those in 2015.10 Altogether, 28.27% had a diagnosis of CKD, and the percentage of CKD decreased with age and was significantly higher among female patients aged <70 years (Figure 52 and Table 61), which might partly reflect survivorship bias. The percentage of diabetes among patients with AKI was 17.30% (Figure 53 and Table 62). Patients aged 75 to 79 years had the highest percentage of diabetes (Figure 53 and Table 62).
Figure 52.
Percentage of CKD among patients with AKI, stratified by sex and age. AKI, acute kidney injury; CKD, chronic kidney disease.
Table 61.
Percentage of CKD among patients with AKI, stratified by sex and age, N (%)
| Age group, yr | Male | Female | Total |
|---|---|---|---|
| 18–24 | 481 (43.10) | 318 (46.97) | 799 (44.56) |
| 25–29 | 420 (33.49) | 373 (41.68) | 793 (36.90) |
| 30–34 | 386 (29.51) | 325 (43.28) | 711 (34.53) |
| 35–39 | 409 (27.07) | 281 (39.97) | 690 (31.17) |
| 40–44 | 628 (26.39) | 357 (38.93) | 985 (29.88) |
| 45–49 | 817 (24.82) | 522 (37.50) | 1339 (28.59) |
| 50–54 | 1080 (26.01) | 630 (34.35) | 1710 (28.56) |
| 55–59 | 892 (26.95) | 507 (31.87) | 1399 (28.55) |
| 60–64 | 1246 (27.39) | 765 (31.99) | 2011 (28.98) |
| 65–69 | 1259 (29.14) | 770 (31.12) | 2029 (29.86) |
| 70–74 | 992 (27.21) | 623 (27.03) | 1615 (27.14) |
| 75–79 | 884 (23.56) | 597 (24.35) | 1481 (23.87) |
| 80–84 | 704 (23.52) | 476 (23.01) | 1180 (23.31) |
| 85+ | 484 (20.50) | 249 (17.56) | 733 (19.40) |
| Total | 10,682 (26.74) | 6793 (31.06) | 17,475 (28.27) |
AKI, acute kidney injury; CKD, chronic kidney disease.
Figure 53.
Percentage of diabetes mellitus among patients with AKI, stratified by sex and age. AKI, acute kidney injury.
Table 62.
Percentage of diabetes mellitus among patients with AKI, stratified by sex and age, N (%)
| Age group, yr | Male | Female | Total |
|---|---|---|---|
| 18–24 | 31 (2.78) | 26 (3.84) | 57 (3.18) |
| 25–29 | 61 (4.86) | 32 (3.58) | 93 (4.33) |
| 30–34 | 90 (6.88) | 28 (3.73) | 118 (5.73) |
| 35–39 | 143 (9.46) | 38 (5.41) | 181 (8.18) |
| 40–44 | 272 (11.43) | 79 (8.62) | 351 (10.65) |
| 45–49 | 398 (12.09) | 120 (8.62) | 518 (11.06) |
| 50–54 | 698 (16.81) | 287 (15.65) | 985 (16.45) |
| 55–59 | 614 (18.55) | 331 (20.80) | 945 (19.28) |
| 60–64 | 845 (18.58) | 578 (24.17) | 1423 (20.50) |
| 65–69 | 844 (19.53) | 653 (26.39) | 1497 (22.03) |
| 70–74 | 715 (19.61) | 636 (27.59) | 1351 (22.70) |
| 75–79 | 755 (20.12) | 692 (28.22) | 1447 (23.32) |
| 80–84 | 577 (19.28) | 483 (23.34) | 1060 (20.94) |
| 85+ | 420 (17.79) | 251 (17.70) | 671 (17.76) |
| Total | 6463 (16.18) | 4234 (19.36) | 10,697 (17.30) |
AKI, acute kidney injury.
5.1. Percentage of AKI
5.2. Characteristics of AKI
5.2.1. Age distribution of AKI, stratified by sex
5.2.2. Sex distribution of AKI, stratified by age
5.3. Percentage of CKD and diabetes among patients with AKI
Section II. End-stage kidney disease
Chapter 6: Prevalence, incidence, and characteristics of dialysis patients
Chao Yang1,2, Xiaoyu Sun3, Rui Chen1,2, Huai-Yu Wang3, Zaiming Su3 and Fang Wang1,2
1Renal Division, Department of Medicine, Peking University First Hospital, Peking University Institute of Nephrology, Beijing, China; 2Research Units of Diagnosis and Treatment of Immune-Mediated Kidney Diseases, Chinese Academy of Medical Sciences, Beijing, China; and 3National Institute of Health Data Science at Peking University, Beijing, China
This chapter focuses on the prevalence and demographic characteristics of patients receiving hemodialysis (HD) and peritoneal dialysis (PD) in China based on the China Health Insurance Research Association (CHIRA) database. Furthermore, the age-adjusted incidence was also provided based on the Commercial Health Insurance (CHI) database.
In the CHIRA database, the number of dialysis patients we identified from the 8,516,679 insured population in 2016 was 18,083 (0.21%), with a male predominance (57.73%; Table 63). The mean age of patients was 55.6 years, much lower than that from Japan (67.2 years),16 with 1.29% aged <18 years old (Table 64). For all prevalent dialysis patients, HD was the major treatment modality (91.94%) (Tables 63 and 64). HD and PD patients were both concentrated in tertiary hospitals (48.40% and 64.40%, respectively, Figure 54). The geographical distribution of dialysis patients included in this study is shown in Table 65.
Table 63.
Number of dialysis patients, stratified by sex and modality
| Sex | HD |
PD |
Total |
|||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Male | 9669 | 58.16 | 771 | 52.88 | 10,440 | 57.73 |
| Female | 6956 | 41.84 | 687 | 47.12 | 7643 | 42.27 |
| Total | 16,625 | 100 | 1458 | 100 | 18,083 | 100 |
HD, hemodialysis; PD, peritoneal dialysis.
Table 64.
Number of dialysis patients, stratified by age and modality
| Age (yr) | HD |
PD |
Total |
|||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Mean ± SD | 55.8 ± 16.3 | 54.2 ± 16.7 | 55.6 ± 16.3 | |||
| <18 | 213 | 1.28 | 21 | 1.44 | 234 | 1.29 |
| 18–44 | 3820 | 22.98 | 400 | 27.43 | 4220 | 23.34 |
| 45–64 | 7223 | 43.45 | 606 | 41.56 | 7829 | 43.29 |
| ≥65 | 5351 | 32.19 | 429 | 29.42 | 5780 | 31.96 |
| Unknown | 18 | 0.11 | 2 | 0.14 | 20 | 0.11 |
| Total | 16,625 | 100 | 1458 | 100 | 18,083 | 100 |
HD, hemodialysis; PD, peritoneal dialysis.
Figure 54.
Distribution of HD and PD patients among different hospital levels. HD, hemodialysis; PD, peritoneal dialysis.
Table 65.
Number of dialysis patients, stratified by geographic region and modality
| Geographic distribution | HD |
PD |
Total |
|||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| East China | 3297 | 19.83 | 468 | 32.10 | 3765 | 20.82 |
| North China | 602 | 3.62 | 130 | 8.92 | 732 | 4.05 |
| Central China | 2848 | 17.13 | 314 | 21.54 | 3162 | 17.49 |
| South China | 663 | 3.99 | 158 | 10.84 | 821 | 4.54 |
| Northwest China | 638 | 3.84 | 49 | 3.36 | 687 | 3.80 |
| Southwest China | 7603 | 45.73 | 224 | 15.36 | 7827 | 43.28 |
| Northeast China | 974 | 5.86 | 115 | 7.89 | 1089 | 6.02 |
| Total | 16,625 | 100 | 1458 | 100 | 18,083 | 100 |
HD, hemodialysis; PD, peritoneal dialysis.
In 2016, the age-adjusted prevalence of patients receiving dialysis was estimated to be 419.12 per million population (PMP), with a significant increase compared with that in 2015 (311.29 PMP; Table 66). The age-adjusted prevalence of HD and PD was 384.13 PMP and 34.99 PMP, respectively (Table 66). The prevalence of male patients (468.99 PMP) was higher than that of female patients (367.26 PMP; Table 66). Based on estimations from the CHIRA database, the corresponding number of Chinese prevalent dialysis patients in 2016 was approximately 578,000.
Table 66.
Age-adjusted prevalence of dialysis patients (PMP) in 2015 and 2016, stratified by sex and modalitya
| Sex | HD |
PD |
Total |
|||
|---|---|---|---|---|---|---|
| 2015 | 2016 | 2015 | 2016 | 2015 | 2016 | |
| Male | 315.00 | 433.16 | 25.70 | 35.84 | 340.70 | 468.99 |
| Female | 250.23 | 333.21 | 31.73 | 34.05 | 281.97 | 367.26 |
| Total | 282.60 | 384.13 | 28.69 | 34.99 | 311.29 | 419.12 |
HD, hemodialysis; PD, peritoneal dialysis; PMP, per million population.
Age-adjusted prevalence was standardized by the direct method using the 2010 national census population.
The age-adjusted incidence rate for dialysis was 116.10 PMP in 2016 based on the analysis of the CHI database, which was slightly lower than that in 2015 (122.19 PMP; Table 67).10 The incidence rates increased with age in both men and women (Table 68 and Figure 55). The northeast of China had the highest incidence of dialysis (152.81 PMP; Table 69). It should be noted that people covered by commercial insurance may have a higher socioeconomic status and better health literacy compared with the general population, so the interpretation of incidence rates should be cautious.
Table 67.
Incidence of dialysis patients, stratified by sex
| Sex | Incidence counta | Exposure count (person-years) | Crude incidence rate (PMP) | Adjusted incidence rate (PMP)b |
|---|---|---|---|---|
| Male | 4300.56 | 29,657,383.95 | 145.01 | 151.03 |
| Female | 2618.17 | 32,592,996.61 | 80.33 | 86.61 |
| Total | 6918.73 | 62,250,380.56 | 111.14 | 116.10 |
PMP, per million population.
Incidence count had taken into account of incurred but not reported (IBNR).
Age-adjusted incidence rate was standardized by the direct method using the 2010 national census population.
Table 68.
Incidence of dialysis patients, stratified by age and sex
| Age group, yr | Male |
Female |
Total |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Incidence counta | Exposure count (person-years) | Incidence rate (PMP) | Incidence counta | Exposure count (person-years) | Incidence rate (PMP) | Incidence counta | Exposure count (person-years) | Incidence rate (PMP) | |
| 18–44 | 1419.31 | 15,663,855.24 | 90.61 | 718.88 | 16,277,863.40 | 44.16 | 2138.19 | 31,941,718.63 | 66.94 |
| 45–64 | 2717.49 | 13,531,211.10 | 200.83 | 1724.76 | 15,595,901.19 | 110.59 | 4442.25 | 29,127,112.30 | 152.51 |
| ≥65 | 163.75 | 462,317.61 | 354.20 | 174.54 | 719,232.02 | 242.67 | 338.29 | 1,181,549.63 | 286.31 |
PMP, per million population.
Incidence count had taken into account of incurred but not reported (IBNR).
Figure 55.
Incidence of dialysis patients, stratified by age and sex. PMP, per million population.
Table 69.
Incidence of dialysis patients, stratified by geographic distribution and sex
| Geographic distribution | Male |
Female |
Total |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Incidence counta | Exposure count (person-years) | Crude Incidence rate (PMP) | Adjusted incidence rate (PMP)b | Incidence counta | Exposure count (person-years) | Crude Incidence rate (PMP) | Adjusted incidence rate (PMP)b | Incidence counta | Exposure count (person-years) | Crude Incidence rate (PMP) | Adjusted incidence rate (PMP)b | |
| East China | 1190.83 | 9,256,990.44 | 128.64 | 129.32 | 735.39 | 9,959,195.70 | 73.84 | 80.31 | 1926.22 | 19,216,186.14 | 100.24 | 103.58 |
| North China | 593.16 | 4,648,068.27 | 127.61 | 122.76 | 379.77 | 4,923,737.90 | 77.13 | 117.21 | 972.93 | 9,571,806.17 | 101.65 | 124.43 |
| Central China | 783.63 | 4,915,134.99 | 159.43 | 173.90 | 468.11 | 5,018,025.24 | 93.29 | 89.20 | 1251.74 | 9,933,160.24 | 126.02 | 127.78 |
| South China | 355.88 | 2,440,165.28 | 145.84 | 156.00 | 209.49 | 2,978,643.65 | 70.33 | 82.76 | 565.37 | 5,418,808.93 | 104.34 | 115.40 |
| Northwest China | 339.29 | 2,236,328.74 | 151.72 | 163.01 | 179.07 | 2,544,359.44 | 70.38 | 68.09 | 518.37 | 4,780,688.18 | 108.43 | 109.69 |
| Southwest China | 335.50 | 2,432,554.34 | 137.92 | 135.49 | 234.90 | 2,862,938.72 | 82.05 | 88.46 | 570.40 | 5,295,493.06 | 107.71 | 109.82 |
| Northeast China | 663.89 | 3,345,510.45 | 198.44 | 204.53 | 394.97 | 3,906,879.51 | 101.10 | 112.35 | 1058.86 | 7,252,389.96 | 146.00 | 152.81 |
| Missing | 38.36 | 382,631.43 | 100.25 | 79.76 | 16.48 | 399,216.45 | 41.28 | 30.45 | 54.84 | 781,847.88 | 70.14 | 54.69 |
PMP, per million population.
Incidence count had taken into account of incurred but not reported (IBNR).
Age-adjusted incidence rate was standardized by the direct method using the 2010 national census population.
Because mortality rates of dialysis patients are not provided in this report, we have quoted data from the National Medical Service and Quality Safety Report 2017, which showed a mortality rate of approximately 4.1% for HD patients and approximately 2.7% for PD patients.17
Chapter 7: Clinical measurement and treatment among dialysis patients
Chao Yang1,2, Wen Tang3, Song Wang3, Huai-Yu Wang4, Rui Chen1,2, Hong Chu1,2 and Yue Wang3
1Renal Division, Department of Medicine, Peking University First Hospital, Peking University Institute of Nephrology, Beijing, China; 2Research Units of Diagnosis and Treatment of Immune-Mediated Kidney Diseases, Chinese Academy of Medical Sciences, Beijing, China; 3Department of Nephrology, Peking University Third Hospital, Beijing, China; and 4National Institute of Health Data Science at Peking University, Beijing, China
The quality of dialysis delivered to patients varies from country to country.18 This chapter focuses on the clinical measurement and treatment of several major complications of dialysis patients, including anemia, mineral bone disorder, and malnutrition.
The percentages of dialysis patients who achieved the monitoring frequency of hemoglobin, ferritin, phosphorus, and parathyroid hormone, recommended by “Kidney Disease: Improving Global Outcomes” guidelines,19,20 were 55.32%, 48.78%, 48.67%, and 56.12% for hemodialysis (HD), and 69.61%, 64.93%, 65.19%, and 68.70% for peritoneal dialysis (PD), respectively (Figures 56 and 57). The percentage of patients using erythropoietin, phosphorus binder, and calcitriol was 73.70%, 48.18%, and 59.89% for HD, and 72.72%, 54.15%, and 58.96% for PD, respectively (Figures 58 and 59). In general, these percentages have increased compared with those in 2015.10
Figure 56.
Percentage of dialysis patients who underwent 1 or more measurements of (a) hemoglobin and (b) serum ferritin in 2016. HD, hemodialysis; PD, peritoneal dialysis.
Figure 57.
Percentage of dialysis patients who underwent 1 or more measurements of (a) serum calcium, (b) serum phosphorus, and (c) serum parathyroid hormone in 2016. HD, hemodialysis; PD, peritoneal dialysis; PTH, parathyroid hormone.
Figure 58.
Percentage of dialysis patients receiving anemia-related treatment. EPO, erythropoietin; HD, hemodialysis; PD, peritoneal dialysis.
Figure 59.
Percentage of dialysis patients receiving MBD-related treatment. HD, hemodialysis; MBD, mineral and bone disorder; PD, peritoneal dialysis.
Regarding the monitoring frequency of blood albumin, 37.33% of HD patients and 57.79% of PD patients achieved the recommended goal (Figure 60). For patients with diabetes, the percentage of those had an ophthalmologic examination, lipid testing, and hemoglobin A1c test at least once a year was only 7.38% and 12.45% for HD and PD patients, respectively (Figure 61), which was higher than in 2015 (5.70% and 6.49% for HD and PD patients, respectively).19
Figure 60.
Percentage of dialysis patients who underwent blood albumin testing. HD, hemodialysis; PD, peritoneal dialysis.
Figure 61.
Diabetes-related examinations among dialysis patients with diabetes. HD, hemodialysis; PD, peritoneal dialysis.
Chapter 8: Vascular access
Xinju Zhao1, Chao Yang2,3, Dongliang Zhang4, Liren Zheng4, Zaiming Su5 and Feng Yu2,3,4
1Department of Nephrology, Peking University People’s Hospital, Beijing, China; 2Renal Division, Department of Medicine, Peking University First Hospital, Peking University Institute of Nephrology, Beijing, China; 3Research Units of Diagnosis and Treatment of Immune-Mediated Kidney Diseases, Chinese Academy of Medical Sciences, Beijing, China; 4Department of Nephrology, Peking University International Hospital, Beijing, China; and 5National Institute of Health Data Science at Peking University, Beijing, China
This chapter focuses on vascular access (VA) operations of prevalent dialysis patients. Arteriovenous fistula (AVF) or arteriovenous graft (AVG) was the predominant type of VA in prevalent hemodialysis (HD) patients, accounting for 77.12% (Table 70). The highest proportion was seen in the age group of 18–44 years (82.98%), and the lowest proportion was found in the age group of ≥65 years (69.69%; Table 70). Moreover, patients with diabetes tended to have a lower proportion of AVF/AVG usage than those without diabetes (47.70% vs. 86.16%; Table 70).
Table 70.
Type of vascular access operations among HD patients
| Operations for AVF/AVG |
Tunneled cuffed catheter |
Noncuffed catheter |
Stable AVF/AVG |
|||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | |
| Sex | ||||||||
| Male | 578 | 5.98 | 150 | 1.55 | 1596 | 16.51 | 7371 | 76.23 |
| Female | 354 | 5.09 | 112 | 1.61 | 1050 | 15.09 | 5450 | 78.35 |
| Age group, yr | ||||||||
| <18 | 3 | 1.41 | 1 | 0.47 | 45 | 21.13 | 164 | 77.00 |
| 18–44 | 204 | 5.34 | 32 | 0.84 | 418 | 10.94 | 3170 | 82.98 |
| 45–64 | 417 | 5.77 | 98 | 1.36 | 978 | 13.54 | 5740 | 79.47 |
| ≥65 | 308 | 5.76 | 131 | 2.45 | 1205 | 22.52 | 3729 | 69.69 |
| Insurance type | ||||||||
| UEBMI | 593 | 5.51 | 182 | 1.69 | 1916 | 17.80 | 8099 | 75.23 |
| URBMI | 339 | 5.79 | 80 | 1.37 | 730 | 12.46 | 4722 | 80.59 |
| Diabetes | ||||||||
| No | 489 | 3.85 | 106 | 0.83 | 1179 | 9.27 | 10957 | 86.16 |
| Yes | 443 | 11.34 | 156 | 3.99 | 1467 | 37.54 | 1864 | 47.70 |
| Total | 932 | 5.61 | 262 | 1.58 | 2646 | 15.92 | 12821 | 77.12 |
AVF, arteriovenous fistula; AVG, arteriovenous graft; HD, hemodialysis; UEBMI, Urban Employee Basic Medical Insurance; URBMI, Urban Resident Basic Medical Insurance.
Among the sampled HD patients, 5.61% of them had new operations for AVF/AVG, with a male predominance (62.02%; Table 70). Only 1.58% patients had a tunneled cuffed catheter (TCC) (Table 70). Most of the operations for AVF or AVG had been performed in tertiary hospitals and secondary hospitals compared with primary hospitals (83.98% vs. 28.17% vs. 6.34%). Most central venous catheter (CVC) operations had also been performed more likely in tertiary and secondary hospitals. However, the interpretation should be cautious because of the sampling scheme.
Among 1458 peritoneal dialysis (PD) patients, 18.45% of them had new PD catheter placement operations, which indicated that these people were new-onset PD patients (Table 71). Altogether, 7.41% of the new PD patients had central venous catheter (CVC) placement operations (Table 71), which suggested that they had transitional HD treatments. The highest percentage of patients who needed transitional HD treatments were those who were 65 years or older (10.02%; Table 71). There were differences between 2 different insurance types. Patients covered by Urban Resident Basic Medical Insurance (URBMI) had a higher percentage of transitional HD treatments compared with those covered by Urban Employee Basic Medical Insurance (UEBMI) (10.61% vs. 6.29%; Table 71). Patients with diabetes had a higher percentage of transitional HD treatments than those without diabetes (12.95% vs. 4.76%; Table 71). Patients were less likely to initiate their dialysis in primary hospitals (4.09%).
Table 71.
Type of dialysis access operations among new PD patients
| New PD catheter placement |
Transitional CVC for new PD patients |
|||
|---|---|---|---|---|
| N | % | N | % | |
| Sex | ||||
| Male | 147 | 19.07 | 65 | 8.43 |
| Female | 122 | 17.76 | 43 | 6.26 |
| Age group, yr | ||||
| <18 | 3 | 14.29 | 2 | 9.52 |
| 18–44 | 58 | 14.50 | 17 | 4.25 |
| 45–64 | 108 | 17.82 | 46 | 7.59 |
| ≥65 | 100 | 23.31 | 43 | 10.02 |
| Insurance type | ||||
| UEBMI | 183 | 16.93 | 68 | 6.29 |
| URBMI | 86 | 22.81 | 40 | 10.61 |
| Diabetes | ||||
| No | 138 | 13.98 | 47 | 4.76 |
| Yes | 131 | 27.81 | 61 | 12.95 |
| Total | 269 | 18.45 | 108 | 7.41 |
CVC, central venous catheter; PD, peritoneal dialysis; UEBMI, Urban Employee Basic Medical Insurance; URBMI, Urban Resident Basic Medical Insurance.
Patients without new PD catheter placement operations were considered as maintenance PD patients. Among these patients, 17.41% had transitional CVC inserts (Table 72). We speculated that these patients might have some complications or comorbidities needing transitional HD treatments or continuous renal replacement therapy (CRRT). But we could not identify these reasons. Stable PD patients were defined as maintenance PD patients without CVC placement operations (82.59%; Table 72). The percentage of PD transfer set exchange was only 29.86% in maintenance PD patients (Table 72). Patients covered by UEBMI had a higher proportion of PD transfer set exchange compared with those covered by URBMI (34.30% vs. 16.15%; Table 72). Patients with diabetes had a higher percentage of exchange than those without diabetes (39.12% vs. 26.15%; Table 72).
Table 72.
Transitional CVC treatments and PD transfer set exchange rates for maintenance PD patients
| Maintenance PD patients |
Transitional CVC for maintenance PD patients |
Stable PD patients |
PD transfer set exchange |
|||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | |
| Sex | ||||||||
| Male | 624 | 80.93 | 124 | 19.87 | 500 | 80.13 | 202 | 32.37 |
| Female | 565 | 82.24 | 83 | 14.69 | 482 | 85.31 | 153 | 27.08 |
| Age group, yr | ||||||||
| <18 | 18 | 85.71 | 8 | 44.44 | 10 | 55.56 | 1 | 5.56 |
| 18–44 | 342 | 85.50 | 42 | 12.28 | 300 | 87.72 | 95 | 27.78 |
| 45–64 | 498 | 82.18 | 74 | 14.86 | 424 | 85.14 | 145 | 29.12 |
| ≥65 | 329 | 76.69 | 83 | 25.23 | 246 | 74.77 | 114 | 34.65 |
| Insurance type | ||||||||
| UEBMI | 898 | 83.07 | 165 | 18.37 | 733 | 81.63 | 308 | 34.30 |
| URBMI | 291 | 77.19 | 42 | 14.43 | 249 | 85.57 | 47 | 16.15 |
| Diabetes | ||||||||
| No | 849 | 86.02 | 95 | 11.19 | 754 | 88.81 | 222 | 26.15 |
| Yes | 340 | 72.19 | 112 | 32.94 | 228 | 67.06 | 133 | 39.12 |
| Total | 1189 | 81.55 | 207 | 17.41 | 982 | 82.59 | 355 | 29.86 |
CVC, central venous catheter; PD, peritoneal dialysis; UEBMI, Urban Employee Basic Medical Insurance; URBMI, Urban Resident Basic Medical Insurance.
Chapter 9: Cardiovascular disease and diabetes among dialysis patients
Chao Yang1,2, Xinju Zhao3, Hong Chu1,2, Zaiming Su4, Fang Wang1,2 and Li Zuo3
1Renal Division, Department of Medicine, Peking University First Hospital, Peking University Institute of Nephrology, Beijing, China; 2Research Units of Diagnosis and Treatment of Immune-Mediated Kidney Diseases, Chinese Academy of Medical Sciences, Beijing, China; 3Department of Nephrology, Peking University People’s Hospital, Beijing, China; and 4National Institute of Health Data Science at Peking University, Beijing, China
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality in patients with chronic kidney disease.21 Patients receiving dialysis are at increased risk of CVD and diabetes. In this chapter, we provide a description of CVD and diabetes among dialysis patients, stratified by age, sex, geographical region, and treatment modalities.
CVD was common in patients receiving dialysis, with a prevalence rate of 45.92% in 2016 (Table 73), close to that in 2015 (45.49%).10 The prevalence of CVD among peritoneal dialysis (PD) patients (47.14%) was slightly higher than in hemodialysis (HD) patients (45.65%), and it increased with age (Table 73). The highest prevalence of CVD was found in North China (70.39%; Table 73).
Table 73.
Prevalence of CVD among dialysis patients, by modality, age, sex, and geographical region (%)
| CVD | HD | PD | Total |
|---|---|---|---|
| Sex | |||
| Male | 44.41 | 46.33 | 44.74 |
| Female | 47.54 | 48.29 | 47.68 |
| Age group, yr | |||
| <18 | 0 | 0 | 0 |
| 18–44 | 30.36 | 38.37 | 31.85 |
| 45–64 | 45.70 | 43.84 | 45.38 |
| ≥65 | 56.41 | 58.85 | 56.85 |
| Unknown | 33.33 | 50.00 | 35.29 |
| Geographic region | |||
| East China | 38.93 | 39.36 | 39.01 |
| North China | 71.03 | 68.42 | 70.39 |
| Central China | 56.34 | 54.86 | 56.16 |
| South China | 34.12 | 43.86 | 37.13 |
| Northwest China | 40.25 | 20.00 | 37.50 |
| Southwest China | 22.28 | 31.03 | 24.23 |
| Northeast China | 39.06 | 56.47 | 41.84 |
| Total | 45.65 | 47.14 | 45.92 |
CVD, cardiovascular disease; HD, hemodialysis; PD, peritoneal dialysis.
Patients receiving dialysis had a high burden of CVD across a wide range of conditions, including coronary heart disease (CHD), heart failure (HF), cerebrovascular accident/transient ischemic attack (CVA/TIA), acute myocardial infarction (AMI), peripheral arterial disease (PAD), and atrial fibrillation (AF). CHD and HF were the 2 leading CVDs for dialysis patients (42.41% and 8.26%, respectively), whereas CVA/TIA, AMI, PAD, and AF were less common (2.25%, 1.26%, 1.22%, and 0.09%, respectively; Figure 62). Notably, only 0.80% and 0.42% of patients underwent percutaneous coronary intervention (PCI) and received pacemaker or implantable cardioverter defibrillators, respectively (Figure 63).
Figure 62.
Percentages of different types of CVD among dialysis patients, by modality. AF, atrial fibrillation; AMI, acute myocardial infarction; CHD, coronary heart disease; CVA/TIA, cerebrovascular accident/transient ischemic attack; CVD, cardiovascular disease; HD, hemodialysis; HF, heart failure; PAD, peripheral arterial disease; PD, peritoneal dialysis.
Figure 63.
Percentages of dialysis patients receiving cardiovascular procedures, by modality. HD, hemodialysis; PCI, percutaneous coronary intervention; PD, peritoneal dialysis.
The prevalence of diabetes among dialysis patients was 33.14% in 2016 (Table 74), higher than that in 2015 (27.12%).10 Diabetes was most common in PD patients, men, and patients older than 65 years (Table 74). The prevalence of diabetes among dialysis patients varied between different regions, among which East China had the highest prevalence (33.33%; Table 74). The prevalence of CVD was higher in patients with diabetes than in patients without diabetes regardless of dialysis modality (Table 75).
Table 74.
Prevalence of diabetes among dialysis patients, by modality, age, sex, and geographical region (%)
| Diabetes | HD | PD | Total |
|---|---|---|---|
| Sex | |||
| Male | 59.68 | 62.30 | 60.24 |
| Female | 40.32 | 37.70 | 39.76 |
| Age group, yr | |||
| <18 | 0 | 0 | 0 |
| 18–44 | 8.55 | 9.51 | 8.76 |
| 45–64 | 50.68 | 44.92 | 49.44 |
| ≥65 | 40.50 | 45.57 | 41.60 |
| Unknown | 0.27 | 0 | 0.21 |
| Geographic region | |||
| East China | 33.57 | 32.46 | 33.33 |
| North China | 10.62 | 18.36 | 12.29 |
| Central China | 24.75 | 16.39 | 22.95 |
| South China | 6.57 | 11.48 | 7.63 |
| Northwest China | 5.58 | 1.97 | 4.80 |
| Southwest China | 3.60 | 4.92 | 3.88 |
| Northeast China | 15.30 | 14.43 | 15.11 |
| Total | 31.72 | 39.61 | 33.14 |
HD, hemodialysis; PD, peritoneal dialysis.
Table 75.
Prevalence of CVD among dialysis patients with and without diabetes (%)
| CVD | HD | PD | Total |
|---|---|---|---|
| Diabetes | |||
| Yes | 60.31 | 63.28 | 60.95 |
| No | 38.84 | 36.56 | 38.47 |
| Total | 45.65 | 47.14 | 45.92 |
CVD, cardiovascular disease; HD, hemodialysis; PD, peritoneal dialysis.
Chapter 10: Hospitalization among dialysis patients
Chao Yang1,2, Huai-Yu Wang3, Xinju Zhao4, Hong Chu1,2, Zaiming Su3 and Fang Wang1,2
1Renal Division, Department of Medicine, Peking University First Hospital, Peking University Institute of Nephrology, Beijing, China; 2Research Units of Diagnosis and Treatment of Immune-Mediated Kidney Diseases, Chinese Academy of Medical Sciences, Beijing, China; 3National Institute of Health Data Science at Peking University, Beijing, China; and 4Department of Nephrology, Peking University People’s Hospital, Beijing, China
Hospital admissions and readmissions among dialysis patients represent important indicators regarding the quality of care as well as health care resource utilizations. It has been reported that hospitalization was associated with more severe comorbidities, poorer outcomes, and higher medical expenditures. In this chapter, we focus on admission rates, length of stay (LOS), and rehospitalization within 30 days among dialysis patients.
The all-cause hospitalization rate for dialysis patients was 2.67 per person per year (PPPY) (Table 76), which was higher than that in 2015 (1.78 PPPY).10 Patients with diabetes tended to have a higher all-cause hospitalization rate (2.94 PPPY; Table 76). The hospitalization rate in tertiary hospitals was lower than that in secondary and primary hospitals, for both hemodialysis (HD) and peritoneal dialysis (PD) patients (Table 76). The average LOS in dialysis patients in 2016 was 35.90 days PPPY. PD patients, female, and patients with diabetes had longer hospital stays (Table 77). Among patients aged <18 years, the LOS in HD patients was notably lower than in PD patients (17.00 days vs. 37.00 days PPPY; Table 77).
Table 76.
All-cause hospitalization rate for dialysis patients, stratified by modality (PPPY)
| HD |
PD |
Total |
||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Sex | ||||||
| Male | 2.60 | 2.48 | 2.72 | 1.76 | 2.63 | 2.34 |
| Female | 2.75 | 2.40 | 2.69 | 1.89 | 2.73 | 2.30 |
| Age group, yr | ||||||
| <18 | 2.00 | 1.73 | 2.67 | 1.53 | 2.33 | 1.51 |
| 18–44 | 2.50 | 2.47 | 2.49 | 1.50 | 2.50 | 2.27 |
| 45–64 | 2.66 | 2.36 | 2.78 | 1.91 | 2.69 | 2.27 |
| ≥65 | 2.75 | 2.57 | 2.74 | 1.87 | 2.75 | 2.43 |
| Unknown | 1.00 | — | — | — | 1.00 | — |
| Diabetes | ||||||
| No | 2.49 | 2.37 | 2.53 | 1.74 | 2.50 | 2.25 |
| Yes | 2.94 | 2.57 | 2.93 | 1.89 | 2.94 | 2.42 |
| Hospital level | ||||||
| Primary hospital | 3.14 | 2.62 | 2.93 | 1.77 | 3.07 | 2.36 |
| Secondary hospital | 2.91 | 2.54 | 2.90 | 1.59 | 2.91 | 2.43 |
| Tertiary hospital | 2.48 | 2.37 | 2.63 | 1.87 | 2.52 | 2.26 |
| Admits/PPPY | 2.66 | 2.45 | 2.71 | 1.81 | 2.67 | 2.32 |
HD, hemodialysis; PD, peritoneal dialysis; PPPY, per person per year.
Table 77.
Length of stay for dialysis patients, stratified by modality (days)
| HD |
PD |
Total |
||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Sex | ||||||
| Male | 34.67 | 40.20 | 35.80 | 32.71 | 34.91 | 38.68 |
| Female | 36.92 | 42.88 | 38.80 | 33.65 | 37.36 | 40.94 |
| Age group, yr | ||||||
| <18 | 17.00 | 11.53 | 37.00 | 41.61 | 27.00 | 29.42 |
| 18–44 | 30.69 | 36.92 | 34.88 | 32.20 | 31.71 | 35.85 |
| 45–64 | 34.73 | 38.45 | 36.76 | 31.99 | 35.18 | 37.12 |
| ≥65 | 39.55 | 46.75 | 38.92 | 35.29 | 39.42 | 44.55 |
| Unknown | 3.00 | — | — | — | 3.00 | — |
| Diabetes | ||||||
| No | 31.32 | 38.69 | 33.68 | 31.97 | 31.81 | 37.40 |
| Yes | 42.59 | 44.44 | 41.41 | 34.11 | 42.30 | 42.09 |
| Hospital level | ||||||
| Primary hospital | 42.10 | 50.50 | 35.75 | 30.27 | 39.93 | 44.64 |
| Secondary hospital | 36.16 | 41.13 | 40.08 | 26.63 | 36.71 | 39.45 |
| Tertiary hospital | 34.62 | 40.33 | 36.50 | 34.99 | 35.08 | 39.08 |
| Days/PPPY | 35.57 | 41.30 | 37.05 | 33.12 | 35.90 | 39.62 |
HD, hemodialysis; PD, peritoneal dialysis; PPPY, per person per year.
In terms of cause-specific hospitalization among HD patients, cardiovascular disease (CVD) was more common than infectious diseases or vascular access events, accounting for 18.83% of all admissions (Table 78). For PD patients, as we slightly modified the identification strategy of access events, CVD has replaced access events as the leading cause of hospitalization compared with the results in 2015 (Table 79).10 For patients under 18 years, more attention should also be paid to CVD prevention.
Table 78.
Cause-specific hospitalization in HD patients
| CVD |
Infectious diseases |
Access events |
||||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Sex | ||||||
| Male | 864 | 19.46 | 271 | 6.10 | 508 | 11.44 |
| Female | 578 | 17.96 | 168 | 5.22 | 311 | 9.66 |
| Age group, yr | ||||||
| <18 | 15 | 12.10 | 5 | 4.03 | 2 | 1.61 |
| 18–44 | 113 | 8.36 | 80 | 5.92 | 182 | 13.46 |
| 45–64 | 597 | 18.71 | 184 | 5.77 | 366 | 11.47 |
| ≥65 | 717 | 23.96 | 170 | 5.68 | 269 | 8.99 |
| Diabetes | ||||||
| No | 696 | 14.73 | 244 | 5.17 | 436 | 9.23 |
| Yes | 746 | 25.42 | 195 | 6.64 | 383 | 13.05 |
| Hospital level | ||||||
| Primary hospital | 272 | 22.19 | 80 | 6.53 | 95 | 7.75 |
| Secondary hospital | 354 | 16.71 | 112 | 5.29 | 238 | 11.23 |
| Tertiary hospital | 816 | 18.92 | 247 | 5.73 | 486 | 11.27 |
| Total | 1442 | 18.83 | 439 | 5.73 | 819 | 10.69 |
CVD, cardiovascular disease; HD, hemodialysis.
Table 79.
Cause-specific hospitalization in PD patients
| CVD |
Infectious diseases |
Access events |
||||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Sex | ||||||
| Male | 75 | 13.02 | 40 | 6.94 | 47 | 8.16 |
| Female | 51 | 9.43 | 39 | 7.21 | 20 | 3.70 |
| Age group, yr | ||||||
| <18 | 6 | 35.29 | 0 | 0 | 0 | 0 |
| 18–44 | 18 | 6.19 | 16 | 5.50 | 10 | 3.44 |
| 45–64 | 36 | 7.84 | 28 | 6.10 | 38 | 8.28 |
| ≥65 | 66 | 18.86 | 35 | 10.00 | 19 | 5.43 |
| Diabetes | ||||||
| No | 57 | 7.95 | 44 | 6.14 | 39 | 5.44 |
| Yes | 69 | 17.25 | 35 | 8.75 | 28 | 7.00 |
| Hospital level | ||||||
| Primary hospital | 21 | 12.14 | 16 | 9.25 | 11 | 6.36 |
| Secondary hospital | 26 | 12.04 | 14 | 6.48 | 16 | 7.41 |
| Tertiary hospital | 79 | 10.85 | 49 | 6.73 | 40 | 5.49 |
| Total | 126 | 11.28 | 79 | 7.07 | 67 | 6.00 |
CVD, cardiovascular disease; PD, peritoneal dialysis.
Rehospitalization rate within 30 days for dialysis patients was 24.18% in 2016, which was higher than that in 2015 (23.18%).10 The rehospitalization rates increased with age and were substantially higher in the diabetic population (Table 80).
Table 80.
Rehospitalization rate within 30 days for dialysis patients, stratified by modality
| HD |
PD |
Total |
||||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Sex | ||||||
| Male | 1060 | 23.87 | 177 | 30.73 | 1237 | 24.66 |
| Female | 762 | 23.67 | 123 | 22.74 | 885 | 23.54 |
| Age group, yr | ||||||
| <18 | 20 | 16.13 | 1 | 5.88 | 21 | 14.89 |
| 18–44 | 249 | 18.42 | 55 | 18.90 | 304 | 18.50 |
| 45–64 | 757 | 23.73 | 128 | 27.89 | 885 | 24.25 |
| ≥65 | 796 | 26.60 | 116 | 33.14 | 912 | 27.29 |
| Diabetes | ||||||
| No | 908 | 19.22 | 162 | 22.59 | 1070 | 19.67 |
| Yes | 914 | 31.14 | 138 | 34.50 | 1052 | 31.54 |
| Hospital level | ||||||
| Primary hospital | 299 | 24.39 | 42 | 24.28 | 341 | 24.37 |
| Secondary hospital | 497 | 23.45 | 61 | 28.24 | 558 | 23.90 |
| Tertiary hospital | 1026 | 23.78 | 197 | 27.06 | 1223 | 24.26 |
| Total | 1822 | 23.79 | 300 | 26.86 | 2122 | 24.18 |
CVD, cardiovascular disease; HD, hemodialysis; PD, peritoneal dialysis.
Chapter 11: Medical expenditures for dialysis patients
Chao Yang1,2, Zaiming Su3, Huai-Yu Wang3, Bixia Gao1,2 and Fang Wang1,2
1Renal Division, Department of Medicine, Peking University First Hospital, Peking University Institute of Nephrology, Beijing, China; 2Research Units of Diagnosis and Treatment of Immune-Mediated Kidney Diseases, Chinese Academy of Medical Sciences, Beijing, China; and 3National Institute of Health Data Science at Peking University, Beijing, China
Patients receiving dialysis usually experience catastrophic medical expenditures. In this chapter, we focus on the pattern of medical expenditures for dialysis patients, as well as its impacts on the health care system.
The total medical expenditure for 18,083 dialysis patients in 2016 was 911 million RMB, of which 75.6% was covered by urban basic health insurance. Patients aged ≥45 years spent more than 80% of the total medical expenditure (Table 81). The direct cost of dialysis procedure was the leading expenditure in HD patients (30.79%), followed by drugs (30.27%), whereas the order was reversed for PD patients (28.74% and 34.48% for dialysis procedure and drugs, respectively; Table 81).
Table 81.
Overall costs of dialysis patients, stratified by modality
| Variables | HD | PD | Total |
|---|---|---|---|
| Sex | |||
| Male | 61.74 | 59.00 | 61.42 |
| Female | 38.26 | 41.00 | 38.58 |
| Age group, yr | |||
| <18 | 0.75 | 1.76 | 0.87 |
| 18–44 | 18.32 | 21.52 | 18.68 |
| 45–64 | 42.12 | 38.35 | 41.69 |
| ≥65 | 38.64 | 38.28 | 38.60 |
| Unknown | 0.17 | 0.09 | 0.16 |
| Breakdown of costs | |||
| Laboratory examinations | 6.67 | 8.52 | 6.88 |
| Other examinations | 4.72 | 4.41 | 4.69 |
| Drugs | 30.27 | 34.48 | 30.76 |
| Direct costs of dialysis | 30.79 | 28.74 | 30.56 |
| Others | 27.55 | 23.84 | 27.12 |
| Pattern of payment | |||
| UBMI paid | 75.81 | 74.29 | 75.64 |
| Out-of-pocket | 24.19 | 25.71 | 24.36 |
| Hospital level | |||
| Primary hospital | 5.83 | 6.88 | 5.95 |
| Secondary hospital | 22.51 | 13.44 | 21.47 |
| Tertiary hospital | 71.65 | 79.68 | 72.57 |
| Total absolute cost (RMB PPPY) | 806,886,272 | 104,572,915 | 911,459,186 |
HD, hemodialysis; PD, peritoneal dialysis; UBMI, urban basic medical insurance.
Data are % unless otherwise noted.
The median annual cost per patient in 2016 increased compared with that in 2015 (HD: 89,257 RMB vs. 87,125 RMB; PD: 79,563 RMB vs. 73,266 RMB; Table 82).10 In outpatients, the median cost was higher in HD patients than in PD patients (60,896 RMB vs. 50,669 RMB); in contrast, among inpatients, the costs of PD exceeded the costs of HD (36,363 RMB vs. 27,805 RMB; Table 82).
Table 82.
Costs of dialysis patients per patient, stratified by modality
| RMB PPPY | HD |
PD |
Total |
|---|---|---|---|
| Median (IQR) | Median (IQR) | Median (IQR) | |
| Outpatient | 60,896 (46,056–93,358) | 50,669 (25,276–69,828) | 60,062 (41,596–88,995) |
| Inpatient | 27,805 (12,952–58,870) | 36,363 (18,196–71,890) | 29,195 (14,059–61,223) |
| Overall | 89,257 (66,390–123,050) | 79,563 (58,485–113,313) | 87,776 (64,531–121,764) |
HD, hemodialysis; IQR, interquartile range; PD, peritoneal dialysis; PPPY, per patient per year.
Different from the pattern of inpatient cost, the leading cost for outpatients on dialysis was direct expense related to dialysis treatment, accounting for 57.33% of the overall expenditures (58.17% for HD patients and 50.68% for PD patients, respectively; Tables 83 and 84). The proportion of out-of-pocket cost among inpatients was much higher than that among outpatients (29.05% vs. 17.49%; Tables 83 and 84).
Table 83.
Inpatient costs of dialysis patients, stratified by modality
| Variables | HD | PD | Total |
|---|---|---|---|
| Inpatient costs: RMB | 479,059,071 | 62,878,177 | 541,937,248 |
| Inpatient/Overall | 59.37 | 60.13 | 59.46 |
| Sex | |||
| Male | 61.59 | 57.36 | 61.10 |
| Female | 38.41 | 42.64 | 38.90 |
| Age group, yr | |||
| <18 | 1.05 | 2.64 | 1.24 |
| 18–44 | 16.24 | 21.80 | 16.89 |
| 45–64 | 39.03 | 33.84 | 38.43 |
| ≥65 | 43.68 | 41.72 | 43.45 |
| Unknown | 0.00 | — | 0.00 |
| Breakdown of costs | |||
| Laboratory examinations | 9.97 | 12.25 | 10.23 |
| Other examinations | 7.20 | 6.84 | 7.16 |
| Drugs | 33.57 | 34.72 | 33.70 |
| Direct costs of dialysis | 12.05 | 14.20 | 12.30 |
| Others | 37.21 | 31.99 | 36.61 |
| Pattern of payment | |||
| UBMI paid | 71.15 | 69.43 | 70.95 |
| Out-of-pocket | 28.85 | 30.57 | 29.05 |
| Hospital level | |||
| Primary hospital | 4.05 | 3.53 | 3.99 |
| Secondary hospital | 18.69 | 11.44 | 17.85 |
| Tertiary hospital | 77.25 | 85.03 | 78.16 |
HD, hemodialysis; PD, peritoneal dialysis; UBMI, urban basic medical insurance.
Data are % unless otherwise noted.
Table 84.
Outpatient costs of dialysis patients, stratified by treatment modality
| Variables | HD | PD | Total |
|---|---|---|---|
| Outpatient costs: RMB | 327,827,201 | 41,694,738 | 369,521,938 |
| Outpatient/Overall | 40.63 | 39.87 | 40.54 |
| Sex | |||
| Male | 61.96 | 61.47 | 61.91 |
| Female | 38.04 | 38.53 | 38.09 |
| Age group, yr | |||
| <18 | 0.32 | 0.45 | 0.33 |
| 18–44 | 21.35 | 21.08 | 21.32 |
| 45–64 | 46.63 | 45.14 | 46.47 |
| ≥65 | 31.28 | 33.09 | 31.49 |
| Unknown | 0.41 | 0.24 | 0.39 |
| Breakdown of costs | |||
| Laboratory examinations | 1.84 | 2.89 | 1.96 |
| Other examinations | 1.10 | 0.74 | 1.06 |
| Drugs | 25.46 | 34.13 | 26.43 |
| Direct costs of dialysis | 58.17 | 50.68 | 57.33 |
| Others | 13.42 | 11.56 | 13.21 |
| Pattern of payment | |||
| UBMI paid | 82.63 | 81.62 | 82.51 |
| Out-of-pocket | 17.37 | 18.38 | 17.49 |
| Hospital level | |||
| Primary hospital | 8.43 | 11.93 | 8.83 |
| Secondary hospital | 28.10 | 16.46 | 26.79 |
| Tertiary hospital | 63.47 | 71.61 | 64.39 |
HD, hemodialysis; PD, peritoneal dialysis; UBMI, urban basic medical insurance.
Data are % unless otherwise noted.
Chapter 12: Regional data from dialysis registry system
Jianghua Chen1, Bixia Gao2,3, Jian Liu4, Zaiming Su5, Jing Sun6, Yingping Sun4, Huai-Yu Wang5, Rong Wang6, Chao Yang2,3, Xi Yao1 and Ping Zhang1
1Kidney Disease Center, the First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, Zhejiang, China; 2Renal Division, Department of Medicine, Peking University First Hospital, Peking University Institute of Nephrology, Beijing, China; 3Research Units of Diagnosis and Treatment of Immune-Mediated Kidney Diseases, Chinese Academy of Medical Sciences, Beijing, China; 4Division of Nephrology, the First Hospital of Xinjiang University of Medicine, Uramuqi, Xinjiang, China; 5National Institute of Health Data Science at Peking University, Beijing, China; and 6Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, Shandong, China
In this chapter, regional data from 3 provincial dialysis quality control centers (Shandong, Zhejiang, Xinjiang) were provided to better understand the epidemiology and treatment of dialysis patients in different regions of China.
In terms of geographic distribution, Shandong and Zhejiang are in the east of China, and Xinjiang is in the northwest of China (Figure 64). The general information of the 3 provinces in 2016 is shown in Table 85.22,23 The prevalence and incidence of dialysis in Zhejiang were the highest (Table 86), and higher than the national data in the previous chapter. Overall, the prevalence and incidence rates of dialysis therapy increased with improved local economic status reflected mainly in the gross domestic product per capita (Tables 85 and 86). Zhejiang also had the highest mortality rate of hemodialysis (HD) and peritoneal dialysis (PD), whereas data on PD in Shandong were not provided (Table 86). Compared with the other 2 provinces, patients receiving dialysis in Xinjiang were younger (Table 87).
Figure 64.
Geographical location of Shandong, Zhejiang, and Xinjiang provinces in China. Shandong and Zhejiang are in the east of China, and Xinjiang is in the northwest of China.
Table 85.
General information of Shandong, Zhejiang, and Xinjiang in China in 2016a
| Province | Area (million square kilometers) | Population (million) | Health expenditure (billion RMB) | Proportion of health expenditure in GDP (%) | GDP per capita (RMB) | Health expenditure per capita (RMB) |
|---|---|---|---|---|---|---|
| Shandong | 0.15 | 99.47 | 335.47 | 4.93 | 68,733 | 3372.70 |
| Zhejiang | 0.10 | 55.90 | 257.36 | 5.45 | 84,916 | 4603.84 |
| Xinjiang | 1.66 | 23.98 | 96.23 | 9.97 | 40,564 | 4012.89 |
GDP, gross domestic product.
Data from China Statistics Yearbook 2017 and China Health Statistics Yearbook 2018.
Table 86.
Prevalence, incidence and mortality of dialysis patients in Shandong, Zhejiang, and Xinjiang in China
| Province | HD |
PD |
||||||
|---|---|---|---|---|---|---|---|---|
| Number of patients | Prevalence (PMP) | Incidence (PMP) | Mortality (%) | Number of patients | Prevalence (PMP) | Incidence (PMP) | Mortality (%) | |
| Shandong | 25,678 | 260.8 | 84.7 | 8.6 | — | — | — | — |
| Zhejiang | 21,716 | 390.3 | 91.8 | 12.5 | 6065 | 109.0 | 27.2 | 6.1 |
| Xinjiang | 4698 | 195.0 | 51.0 | 9.6 | 663 | 31.0 | 4.0 | 4.8 |
HD, hemodialysis; PD, peritoneal dialysis; PMP, per million population.
Data on PD in Shandong Province were not provided.
Table 87.
Demographic characteristics of dialysis patients in Shandong, Zhejiang, and Xinjiang in China
| Province | HD |
PD |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Male | Mean age (yr) | 18–44 years | 45–64 years | ≥65 years | Male | Mean age (yr) | 18–44 years | 45–64 years | ≥65 years | |
| Shandong | 55.9 | 55.3 | 16.4 | 48.5 | 32.2 | — | — | — | — | — |
| Zhejiang | 59.0 | 60.8 | 14.8 | 42.6 | 38.4 | 52.4 | 60.6 | 19.7 | 46.8 | 27.4 |
| Xinjiang | 64.7 | 51.8 | 29.8 | 45.2 | 20.4 | 56.0 | 49.9 | 18.6 | 26.2 | 6.2 |
HD, hemodialysis; PD, peritoneal dialysis.
Data are %.
Data on PD in Shandong Province were not provided.
Glomerulonephritis was still the leading cause in both incident and prevalent dialysis patients in 3 provinces (Table 88), which is different from reports indicating diabetic kidney disease as the major cause of incident dialysis patients in Japan in 2013.16 The top 3 causes of death of HD and PD patients in the 3 provinces were cardiovascular events, cerebrovascular events, and infection in the 3 provinces (Table 89).
Table 88.
Top 3 primary causes of incident and prevalent dialysis patients in Shandong, Zhejiang, and Xinjiang in China
| Province | Incident dialysis |
Prevalent dialysis |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HD |
PD |
HD |
PD |
|||||||||
| 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | |
| Shandong | GN (—) | DKD (—) | HTN (—) | — | — | — | GN (—) | DKD (—) | HTN (—) | — | — | |
| Zhejiang | GN (45.1) | DKD (22.9) | HTN (7.2) | GN (51.1) | DKD (14.6) | HTN (8.0) | GN (44.7) | DKD (22.6) | HTN (7.9) | GN (50.3) | DKD (14.3) | HTN (8.9) |
| Xinjiang | PGN (30.5) | DKD (30.5) | HTN (12.9) | PGN (21.0) | SGN (5.0) | Urinary tumor (1.0) | PGN (37.4) | DKD (26.5) | HTN (15.0) | PGN (9.3) | SGN (2.5) | Urinary infection and stone (0.2) |
DKD, diabetic kidney disease; GN, glomerulonephritis; HTN, hypertensive nephropathy; PGN, primary glomerular nephropathy; SGN, secondary glomerular nephropathy.
Data are %.
Data on PD and percentages in Shandong Province were not provided.
Table 89.
Top 3 causes of death of dialysis patients in Shandong, Zhejiang, and Xinjiang in China
| Province | HD |
PD |
||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 1 | 2 | 3 | |
| Shandong | Cardiovascular events (—) | Cerebrovascular events (—) | Infection (—) | — | — | — |
| Zhejiang | Cardiovascular events (27.7) | Cerebrovascular events (18.4) | Infection (17.9) | Cardiovascular events (25.7) | Cerebrovascular events (19.3) | Infection (17.7) |
| Xinjiang | Cardiovascular events (40.0) | Cerebrovascular events (35.9) | Infection (9.9) | Cardiovascular events (58.2) | Cerebrovascular events (8.2) | Infection (2.7) |
HD, hemodialysis; PD, peritoneal dialysis.
Data are %.
Data on PD and percentages in Shandong Province were not provided.
The hepatitis B virus infection rate of HD patients in the 3 provinces was close, fluctuating around 6%, whereas the hepatitis C virus infection rate in Xinjiang was the highest (5.3%; Figure 65). PD patients in Zhejiang had the highest infection rate of hepatitis B virus (8.5%; Figure 65). The percentage of dialysis patients who achieved the recommended goals for different laboratory tests, including hemoglobin, transferrin saturation, ferritin, serum calcium, serum phosphorus, intact parathyroid hormone (iPTH), serum albumin, and single-pool kt/V (spKt/V), varied greatly among the 3 provinces, suggesting that management of dialysis patients in China should be further improved (Table 90).
Figure 65.
Hepatitis B/C virus infection in (a) HD and (b) PD patients in Shandong, Zhejiang, and Xinjiang in China (%). Data on PD in Shandong Province were not provided. HD, hemodialysis; PD, peritoneal dialysis.
Table 90.
The percentage of dialysis patients who achieved the recommended goals for laboratory tests in Shandong, Zhejiang, and Xinjiang in Chinaa
| Modality | Province | Hemoglobin | Transferrin saturation | Ferritin | Serum calcium | Serum phosphorus | iPTH | Serum albumin | SpKt/V |
|---|---|---|---|---|---|---|---|---|---|
| HD | Shandong | 45.9 | — | 57.3 | 52.5 | 21.3 | 24.9 | 56.2 | 46.5 |
| Zhejiang | 18.5 | 79.2 | 52.7 | 68.6 | 26.3 | 27.7 | 38.4 | 87.6 | |
| Xinjiang | 63.5 | 27.1 | 51.2 | 51.5 | 39.4 | 54.7 | 76.7 | 40.9 | |
| PD | Shandong | — | — | — | — | — | — | — | — |
| Zhejiang | 19.8 | 79.5 | 47.6 | 73.8 | 36.8 | 31.2 | 22.9 | 90.3 | |
| Xinjiang | 10.7 | 2.4 | 10.0 | 18.0 | 10.3 | 6.0 | 2.0 | — |
HD, hemodialysis; iPTH, intact parathyroid hormone; PD, peritoneal dialysis; spKt/V, single-pool kt/V.
Data are %.
Data on PD in Shandong Province were not provided.
The analysis was performed based on the patient’s last values of laboratory tests in that year. The recommended goal of each laboratory test was as follows: (i) hemoglobin ≥110 g/l; (ii) percentage of transferrin saturation >20%; (iii) ferritin >200 μg/l; (iv) serum calcium 2.10–2.50 mmol/l; (v) serum phosphorus 0.87–1.45 mmol/l; (vi) iPTH 150–300 pg/ml; (vii) serum albumin >40 g/l; (viii) spKt/V ≥1.2.
Chapter 13: Kidney transplant waiting list
Kidney transplantation is an alternative kidney replacement therapy for patients with end-stage kidney disease (ESKD). China has made significant progress in reforming its organ donation and transplantation processes during the last decade. The new “China model” of organ transplantation is based on the China Organ Transplant Response System (COTRS), which is a national open and transparent organ allocation computer system. As of September 1, 2013, it became mandatory to allocate organs through the COTRS.
The data regarding the waiting list for kidney transplantation were provided by the Report on Organ Transplantation Development in China (2015–2018),24 which showed that there were 26,039 candidates on the kidney transplant waiting list at the end of 2016 (excluding Hong Kong, Macao, and Taiwan), so this year's report did not present the detailed data.
According to the data from Chinese Scientific Registry of Kidney Transplantation (CSRKT), there were 7224 kidney transplantations from deceased donations (DD) and 1795 from living-related donations in 2016.24 Since 2015, DD kidney transplantation has become the main type in mainland China.24 Pediatric kidney transplantation (<18 years old) has also received extensive attention in recent years, accounting for 2.3% of all kidney transplantations in mainland China in 2016.24
Chapter 14: Discussion
By integrating and mining national administrative or claims databases from various sources, the China Kidney Disease Network (CK-NET) 2016 Annual Data Report (ADR) has provided a comprehensive description of the burden of chronic kidney disease (CKD) and end-stage kidney disease (ESKD) in China, serving as a regularly updated surveillance system for kidney diseases. This work has appreciable policy implications. It is of substantial value for understanding the burden of kidney diseases in China and developing prevention and control strategies.
Globally in 2017, 697.5 million cases suffered from CKD, with the largest number in China (132.3 million).1 Moreover, China had more than 170,000 of the 1.2 million deaths caused by CKD, ranking second in the world after India.1 We found that patients with CKD constituted 4.86% of all admissions in tertiary hospitals in China, slightly higher than those previously reported (4.47% in 2014 and 4.80% in 2015).7,10 The United States Renal Data System (USRDS) reported that the prevalence of recognized CKD has steadily risen year after year, and the proportion of Medicare patients with CKD increased from 13.8% to 14.5% from 2016 to 2017.25 However, we should emphasize that the percentage of CKD in our analysis comprehensively reflects the prevalence, hospitalization rate, and diagnostic rate. Therefore, it is necessary to be careful when making an international comparison. Furthermore, the percentage of CKD was higher among those with other major noncommunicable diseases (NCDs), such as diabetes and hypertension, indicating the importance of management of these high-risk populations. Diabetic kidney disease (DKD) was supposed to be the most common cause of CKD, and the spectrum of CKD varied among different groups of geographic region and socioeconomic status. Consequently, effective prevention and management of DKD is essential to attenuate the future burden of ESKD.
With the rapid development of China’s economy and the increase of aging population, the demands for high-quality health care have been growing.26 As medical resources are generally concentrated in relatively developed areas, more and more patients choose to seek health care outside their own residential areas. Compared with other NCDs, huge geographic disparities in capacity exist in nephrology.5 Overall, the percentage of medical migration (interprovince) among patients with CKD was 5.98%. The travel pattern of patients with CKD showed that the diagnosis and treatment level and resources of kidney care were unbalanced across regions, with a “siphon effect” in areas with relatively developed economic and medical conditions. Despite the efforts of the government, the phenomenon of patient travel is still common, which deviates from the policy of “serious disease treating in county-level hospital” advocated by the State Council.27 Therefore, optimizing the allocation of resources and enhancing the capacity and accessibility of kidney care in vulnerable areas is an emerging policy priority.
Findings from the International Society of Nephrology (ISN) survey showed global variations in the prevalence of kidney replacement therapy, varying from 4 per million population (PMP) in Rwanda to 3392 PMP in Taiwan, China.28 In 2017, the prevalence of ESKD in the United States reached 2203.5 PMP, and a third of incident ESKD patients had received little or no pre-ESKD nephrology care.25 In this ADR, we calculated the age-adjusted prevalence and incidence of patients receiving dialysis based on the government-funded and commercial claims database. The prevalence rate (419.12 PMP in 2016) is lower than those reported in the United States and Taiwan,25,28 and slightly lower than data reported from Zhejiang Province (499.3 PMP in 2016), whose economic levels are relatively good. Meanwhile, according to data from 3 provincial dialysis quality control centers, the prevalence and incidence rates of dialysis increased with the local economic level, especially the gross domestic product per capita.
Some other findings, including the effects of metabolic diseases on the spectrum of CKD, disproportionate high cost of CKD, underdiagnoses of acute kidney injury among hospitalized patients, less-optimal laboratory tests and treatment among dialysis patients, and status of children and adolescents on dialysis, would inspire the future research as well as policy making. Overall, competing priorities and limited resources will coexist in China for a long time. China still faces several challenges of managing the growing number of patients with ESKD, such as the lack of financial and clinical resources, inequalities in access to health care across regions, and relatively low awareness rate of CKD. Addressing these challenges needs joint efforts from the government, multiple organizations, health care professionals, and the public. Incorporating CKD into existing national prevention strategies of NCDs may help raise the awareness of CKD and reduce the burden of ESKD.
Under the support of National Health Commission of China and Chinese Society of Nephrology, the series of CK-NET ADR could serve as an example of leveraging the power of big data to monitor kidney diseases in developing countries. We hope that the novel model and practical experiences of CK-NET could be shared with other countries or regions facing the similar threats of CKD, which would provide insights into the development of surveillance and prevention strategies of kidney diseases. We sincerely welcome cooperation from all walks of life to improve kidney health worldwide.
Contributor Information
CK-NET Work Group:
Rui Chen, Hong Chu, Xinwei Deng, Lanxia Gan, Bixia Gao, Yifang Jiang, Lili Liu, Jianyan Long, Ying Shi, Zaiming Su, Xiaoyu Sun, Wen Tang, Fang Wang, Huai-Yu Wang, Jinwei Wang, Song Wang, Chao Yang, Dongliang Zhang, Xinju Zhao, Liren Zheng, and Zhiye Zhou
Appendices: Definitions of ICD coding
Appendix 1.
Coding of various CKD etiologies
| Etiology of CKD | All editions | China edition | Beijing edition | Clinic edition | |||
|---|---|---|---|---|---|---|---|
| 1. Diabetes mellitus | |||||||
| Type 1 diabetes mellitus with renal complications | E10.2+ N08.3 | ||||||
| Type 2 diabetes mellitus with renal complications | E11.2+ N08.3 | ||||||
| Unspecified diabetes mellitus with renal complications | E14.2 | ||||||
| Malnutrition-related diabetes mellitus with renal complications | E12.200+N08.3 | E12.200 | |||||
| Other specified diabetes mellitus with renal complications | E13.2 | E13.200 | |||||
| 2. Hypertensive diseases | |||||||
| Hypertensive renal disease with renal failure | I12 | ||||||
| Hypertensive heart and renal disease with (congestive) heart failure | I13 | ||||||
| Pregnancy with hypertensive heart and renal disease | O10.301 | ||||||
| Pregnancy with essential hypertension and proteinuria | O11.x01 | ||||||
| Pre-existing hypertensive renal disease during pregnancy, childbirth, and puerperium | O10.200 | O10.200 | |||||
| Pregnancy with hypertensive renal disease | O10.201 | O10.201 | |||||
| Pre-existing hypertensive heart and renal disease during pregnancy, childbirth, and puerperium | O10.300 | O10.300 | |||||
| Pre-existing hypertension with proteinuria | O11.x00 | O11.x00 | |||||
| 3. Glomerular diseases | |||||||
| Recurrent and persistent hematuria | N02 | ||||||
| Chronic nephritic syndrome | N03 | ||||||
| Nephrotic syndrome | N04 | ||||||
| Unspecified nephritic syndrome | N05 | ||||||
| Isolated proteinuria with specified morphologic lesion | N06 | ||||||
| Persistent proteinuria, unspecified | N39.1 | ||||||
| 4. Renal tubulointerstitial diseases | |||||||
| Chronic tubulointerstitial nephritis | N11 | ||||||
| Tubulointerstitial nephritis, not specified as acute or chronic | N12 | ||||||
| Drug- and heavy metal–induced tubulointerstitial and tubular conditions | N14 | ||||||
| Renal tubulointerstitial disorders in diseases classified elsewhere | N16 | ||||||
| Other specified disorders of carbohydrate metabolism | E74.8 | ||||||
| Disorders of amino acid transport | E72.0 | ||||||
| Nephrogenic diabetes insipidus | N25.1 | N25.1 | |||||
| Renal tubule acidosis | N25.8 | ||||||
| Balkan nephropathy | N15.000 | N15.001 | N15.000 | ||||
| Renal tubulointerstitial disease, specified | N15.800 | N15.800 | |||||
| Renal granuloma | N15.801 | N15.801 | |||||
| Renal tubulointerstitial disease | N15.900 | N15.900 | |||||
| Impaired renal tubular function–related disease | N25.9 | N25.9 | |||||
| Liddle syndrome | I15.101 | I15.101 | |||||
| Urate nephropathy | M10.001+N16.8 | N28.905 | M10.001+N16.8 | ||||
| Systemic lupus erythematosus + renal tubulointerstitial diseases | M32.102+N16.4 | M32.113+N16.4 | M32.102+N16.4 | ||||
| Sicca syndrome + renal tubulointerstitial diseases | M35.006+N16.4 | M35.005+N16.4 | M35.006+N16.4 | ||||
| 5. Obstructive nephropathy | |||||||
| Hydronephrosis with ureteropelvic junction obstruction | N13.0 | ||||||
| Hydronephrosis with ureteral stricture, not elsewhere classified | N13.1 | ||||||
| Hydronephrosis with renal and ureteral calculous obstruction | N13.2 | N13.2 | N13.200 | ||||
| Other obstructive nephropathy | N13.8 | N13.8 | N13.801 | ||||
| 6. Other related diagnosis | |||||||
| Hereditary nephropathy, not elsewhere classified | N07 | N07.901 | N07 | ||||
| Glomerular disorders in diseases classified elsewhere | N08, exclude N08.5 | ||||||
| Renal agenesis and other reduction defects of kidney | Q60 | ||||||
| Polycystic kidney, autosomal recessive | Q61.1 | ||||||
| Polycystic kidney, autosomal dominant | Q61.2 | ||||||
| Polycystic kidney, unspecified | Q61.3 | ||||||
| Medullary cystic kidney, sponge kidney NOS | Q61.5 | ||||||
| Lobulated, fused and horseshoe kidney | Q63.1 | ||||||
| Congenital malformation of kidney, unspecified | Q63.9 | ||||||
| Gout due to impairment of renal function | M10.300 | M10.393 | M10.300 | ||||
| Unspecified contracted kidney | N26 | ||||||
| Ischemia and infarction of kidney | N28.0 | ||||||
| Other specified disorders of kidney and ureter | N28.8 | ||||||
| Disorders of kidney and ureter, unspecified | N28.9 | ||||||
| Congenital renal failure | P96.0 | P96.0 | P96.000 | ||||
| Extrarenal uremia | R39.2 | ||||||
| Aortic arch syndrome + renovascular hypertension | M31.4 + I15.0 | I77.604 + I15.0 | I77.600x004 + I15.0 | ||||
| Goodpasture syndrome | M31.001 | ||||||
| Renal osteodystrophy | N25.0 | ||||||
| Failure and rejection of renal transplantation | T86.1 | ||||||
| Hemolytic uremic syndrome | D59.3 | ||||||
| Dialysis | Z49 | ||||||
| Renal allergic purpura | D69.005+N08.2 | ||||||
| Lupus nephritis | M32.101+N08.5 | M32.105+N08.5 | M32.101+N08.5 | ||||
| Goodpasture syndrome–related glomerulonephritis | M31.003+N08.5 | M31.003+N08.5 | |||||
| Antiglomerular basement membrane antibody–related disease | M31.002+N08.5 | M31.005+N08.5 | M31.002+N08.5 | ||||
| Microscopic polyangitis | M31.700 | M31.701 | M31.700 | ||||
| ANCA-related nephritis | M31.701+N08.5 | M31.802 | M31.701+N08.5 | ||||
| Thrombotic thrombocytopenic purpura–related glomerulonephritis | M31.102+N08.5 | M31.102+N08.5 | |||||
| Wegener’s granulomatosis-related glomerulonephritis | M31.303+N08.5 | M31.303+N08.5 | |||||
| Pregnancy with nephrotic syndrome | O26.801 | O26.811 | O26.801 | ||||
| Pregnancy with glomerulonephritis | O26.804 | O26.812 | O26.804 | ||||
| Pregnancy with renal failure | O26.802 | O26.813 | O26.802 | ||||
| HBV-related nephritis | B18.103+N08.0 | B18.102 | B18.103+N08.0 | ||||
| HCV-related nephritis | B18.205+N08.0 | B18.208 | B18.205+N08.0 | ||||
| Cryoglobulinemia-related glomerulonephritis | D89.101+N08.2 | D89.101+N08.2 | |||||
| Hereditary amyloidosis nephropathy | E85.002 | E85.003 | E85.002 | ||||
| Amyloidosis-related nephropathy | E85.411+N29.8 | E85.410+N08.4 | E85.411+N29.8 | ||||
| Psoriatic nephritis | L40.803+ | L40.802+N05.9 | L40.800x002+N05.9 | ||||
| Kidney injury–related gout | M10.300 | M10.393 | M10.300 | ||||
| Syphilitic nephritis | A52.712+N08.0 | A52.700x012+N08.0 | |||||
| Lupus kidney injury | M32.112+N08.5 | ||||||
| Lupus nephritis | M32.101+N08.5 | M32.105+N08.5 | M32.101+N08.5 | ||||
| Lupus tubulointerstitial kidney | M32.102+N16.4 | M32.113+N16.4 | M32.102+N16.4 | ||||
| Gouty nephropathy | M10.391 | M10.300x091 | |||||
| Gouty nephrolithiasis | M10.005+N22.8 | M10.392 | M10.005+N22.8 | ||||
ANCA, antineutrophil cytoplasmic antibodies; CKD, chronic kidney disease; HBV, hepatitis B virus; HCV, hepatitis C virus.
Appendix 2.
Coding of CKD stages
| Stage of CKD | China edition | Beijing edition | Clinic edition |
|---|---|---|---|
| CKD stage 1 | N18.801 | N18.914 | N18.801 |
| CKD stage 2 | N18.802 | N18.915 | N18.802 |
| CKD stage 3 | N18.803 | N18.916 | N18.803 |
| CKD stage 4 | N18.804 | N18.917 | N18.804 |
| CKD stage 5 | N18.001 | N18.918 | N18.001 |
CKD, chronic kidney disease.
Appendix 3.
Coding of diabetes mellitus
| Etiology | ICD coding |
|---|---|
| Type 1 diabetes mellitus | E10 |
| Type 2 diabetes mellitus | E11 |
| Malnutrition-related diabetes mellitus | E12 |
| Other specified diabetes mellitus | E13 |
| Unspecified diabetes mellitus | E14 |
ICD, International Classification of Diseases.
The definition of the control group in chapters 2, 3, and 4 was based on the ICD coding of E10, E11, and E13.
Appendix 4.
Coding of hypertension
| Etiology | ICD coding |
|---|---|
| Essential (primary) hypertension | I10 |
| Hypertensive heart disease | I11 |
| Hypertensive renal disease | I12 |
| Hypertensive heart and renal disease | I13 |
| Secondary hypertension | I15 |
ICD, International Classification of Diseases.
Appendix 5.
Coding of CVD
| Etiology | All editions | China edition | Beijing edition | Clinic edition |
|---|---|---|---|---|
| 1. Cerebral stroke | ||||
| Subarachnoid hemorrhage | I60 | |||
| Intracerebral hemorrhage | I61 | |||
| Acute ischemic cerebral stroke | I63 | |||
| I64 | ||||
| H34.1 | ||||
| Transient ischemic attack | G45 | |||
| 2. Coronary heart disease | ||||
| Angina pectoris | I20 | |||
| Acute myocardial infarction | I21 | |||
| Subacute myocardial infarction | I22 | |||
| Complications after myocardial infarction | I23 | |||
| Other acute ischemic heart disease | I24 | |||
| Chronic ischemic heart disease | I25 | |||
| 3. Heart failure | ||||
| Whole-heart failure | I50.003 | I50.002 | ||
| Right heart failure | I50.001 | I50.004 | I50.001 | |
| Right ventricular failure | I50.005 | I50.000x005 | ||
| Acute right heart failure | I50.000x006 | |||
| Left heart failure | I50.100 | I50.106 | I50.100x006 | |
| Left ventricular failure | I50.100 | |||
| Left atrial failure | I50.102 | |||
| Chronic left heart insufficiency | I50.103 | I50.105 | ||
| Left heart failure with acute pulmonary edema | I50.107 | I50.103 | ||
| Congestive heart failure | I50.000 | I50.001 | I50.000 | |
| Acute heart failure | I50.904 | I50.907 | ||
| Chronic heart failure | I50.905 | I50.908 | ||
| Other heart failure | I50.900 | I50.911 | I50.900 | |
| Postoperative heart failure and pulmonary edema | I97.104 | I97.100x004 | ||
| Heart failure of newborns | P29.000 | P29.001 | P29.000 | |
| Hypertensive heart failure | I11.001 | |||
| Hypertensive heart disease with (congestive) heart failure | I11.000 | I11.000 | ||
| Hypertensive heart disease without (congestive) heart failure | I11.900 | |||
| Hypertensive heart disease and kidney disease with congestive heart failure | I13.000 | I13.000 | ||
| Hypertensive heart disease and kidney disease with congestive heart failure and renal failure | I13.200 | I13.200 | ||
| Intractable heart failure | I50.900x017 | |||
| Heart failure after cardiac surgery | I97.102 | I97.106 | I97.102 | |
| Postoperative heart failure | I97.803 | I97.803 | ||
| Chronic left heart insufficiency | I50.103 | I50.105 | ||
| Cardiac insufficiency | I50.901 | I50.902 | I50.900x002 | |
| Cardiac insufficiency of newborns | P29.001 | |||
| Acute exacerbation of chronic cardiac insufficiency | I50.900x018 | |||
| Acute left heart failure | I50.102 | I50.101 | ||
| Acute pulmonary edema | J81xx02 | J81.x00x002 | ||
| Pregnancy with heart failure | O99.417 | O99.408 | O99.400x008 | |
| Pregnancy with cardiac insufficiency | O99.429 | O99.429 | O99.414 | |
| Childbirth with heart failure | O75.403 | O75.403 | ||
| Pregnancy with left heart failure | O99.423 | O99.424 | ||
| Puerperal cardiac insufficiency | O99.402 | O99.434 | O99.402 | |
| Acute pulmonary edema after postpartum | O99.507 | O99.508 | O99.508 | |
| Heart failure due to anesthesia during pregnancy | O29.102 | O99.500x008 | ||
| Heart failure due to anesthesia during childbirth | O74.202 | O74.200x002 | ||
| Heart failure after obstetric surgery or operation | O75.402 | |||
| Heart failure due to anesthesia during puerperium | O89.102 | O89.100x002 | ||
| Low cardiac output syndrome | I50.901 | I50.901 | ||
| Cardiac function, class I | I50.902 | I50.902 | ||
| Cardiac function, class II | I50.903 | I50.907 | I50.903 | |
| Cardiac function, class III | I50.904 | I50.908 | I50.904 | |
| Cardiac function, class IV | I50.905 | I50.910 | I50.905 | |
| Cardiac function, class II (NYHA) | I50.900x007 | |||
| Cardiac function, class III (NYHA) | I50.900x008 | |||
| Cardiac function, classes II and III (NYHA) | I50.900x009 | |||
| Cardiac function, class IV (NYHA) | I50.900x010 | |||
| Circulatory failure | R57.901 | I50.913 | R57.901 | |
| Pulmonary edema | J81.x00 | J81xx03 | J81.x00 | |
| Cardiogenic shock | R57.000 | R57.001 | R57.000 | |
| Respiratory and circulatory failure | J96.102 | J96.900 | ||
| Cardiogenic asthma | I50.104 | I50.104 | ||
| 4. Atrial fibrillation | ||||
| Atrial fibrillation | I48.x01 | I48xx04 | I48.x01 | |
| Idiopathic atrial fibrillation | I48.x02 | I48xx02 | I48.x05 | |
| Persistent atrial fibrillation | I48xx07 | I48.x00x007 | ||
| Chronic atrial fibrillation | I48xx08 | I48.x00x008 | ||
| Pregnancy with atrial fibrillation | O99.427 | O99.427 | O99.400x027 | |
| Atrial fibrillation with flutter | I48.x00 | I48xx01 | I48.x00 | |
| Primary atrial fibrillation | I48.x00x009 | |||
| Long-term persistent atrial fibrillation | I48.x00x011 | |||
| Acute atrial fibrillation | I48.x00x012 | |||
| Permanent atrial fibrillation | I48.x00x013 | |||
| Long-range persistent atrial fibrillation | I48.x00x014 | |||
| New diagnosis of atrial fibrillation | I48.x00x015 | |||
| Paroxysmal atrial fibrillation | I48.x02 | I48xx06 | I48.x02 |
CVD, cardiovascular disease; NYHA, New York Heart Association.
Appendix 6.
Coding of CVD operations
| Operation | China edition | Beijing edition | Clinic edition |
|---|---|---|---|
| Coronary angiography (CAG) | 88.55001 | 88.5500 | |
| 88.5,500x002 | |||
| 88.56001 | 88.5600 | ||
| 88.5,600x002 | |||
| 88.57002 | 88.5701 | ||
| 88.5700 | |||
| 88.5,700x003 | |||
| 88.5900 | |||
| Percutaneous coronary intervention (PCI) | 36.06003 | 36.0602 | |
| 36.0601 | |||
| 36.06004 | 36.0600 | ||
| 36.07003 | 36.0700 | ||
| 36.0,700x004 | |||
| 36.0701 | |||
| Coronary artery bypass grafting (CABG) | 36.11001 | ||
| 36.12001 | |||
| 36.13001 | |||
| 36.14001 | |||
| 36.15001 | |||
| 36.16001 | |||
| 36.17001 | |||
| 36.2 001 | |||
| Pacemaker | Z95.000 | Z95.000 | |
| T82.700 | T82.700 | ||
| T82.703 | T82.702 | T82.703 | |
| T82.100 | |||
| T82.101 | |||
| T82.102 | |||
| T82.103 | |||
| T82.800 | T82.800 | ||
| T82.903 | T82.801 | T82.903 | |
| T82.904 | T82.904 | ||
| T85.707 | |||
| Z45.007 | |||
| Z45.001 | Z45.001 | ||
| Z45.002 | |||
| Z95.001 | |||
| Z45.004 | Z45.003 | Z45.004 | |
| T82.100x002 | |||
| T82.100x003 | |||
| T82.702 | T82.700x002 | ||
| Z45.000 | |||
| Z45.003 | Z45.003 | ||
| Z45.005 | Z45.005 | ||
| Z45.006 | Z45.006 | ||
| 37.89001 | 37.8901 | ||
| 89.4500 | |||
| 37.7501 | |||
| 37.7800 | |||
| 37.80001 | 37.8,000x001 | ||
| 37.80002 | 37.8,000x002 | ||
| 37.8001 | |||
| 37.7701 | |||
| 37.7600 | |||
| 37.78001 | |||
| Implantable defibrillator/cardiac resynchronization therapy defibrillator | Z95.800x007 | ||
| Z45.800x006 | |||
| T82.100x011 | |||
| T82.100x010 | |||
| 00.5100 | |||
| 00.51001 | 00.5,100x001 | ||
| 00.5101 | |||
| 00.5102 | |||
| 00.53001 | 00.5301 | ||
| 00.53002 | 00.5302 | ||
| 00.5400 | |||
| 00.54001 | 00.5401 | ||
| 00.54002 | 00.5402 | ||
| 37.9400 | |||
| 37.94001 | 37.9401 | ||
| 37.9403 | |||
| 37.9404 | |||
| 37.9500 | |||
| 37.9,500x001 | |||
| 37.9600 | |||
| 37.9700 | |||
| 37.9,700x001 | |||
| 37.9,700x002 | |||
| 37.9800 | |||
| 37.9,800x002 | |||
| 99.6202 |
CVD, cardiovascular disease.
Appendix 7.
Coding of AKI
| AKI | All editions | China edition | Beijing edition | Clinic edition |
|---|---|---|---|---|
| Acute renal failure | N17 | |||
| Rapidly progressive nephritic syndrome | N01 | |||
| Traumatic anuria | T79.5 | |||
| Hemolytic-uremic syndrome | D59.3 | |||
| Hepatorenal syndrome | K76.7 | |||
| Postpartum acute renal failure | O90.4 | |||
| Renal failure after abortion | O08.4 | |||
| Postprocedural disorders of genitourinary system, not elsewhere classified | N99.0 | |||
| Acute tubulointerstitial nephritis | N10.x00 | N10.x00 | ||
| Acute interstitial nephritis | N10.x01 | N10.x01 | ||
| Chronic glomerulonephritis with rapidly progressive glomerulonephritis | N00.908 | N00.900x009 | ||
| Acute Infectious interstitial nephritis | N10xx03 | N10.x00x003 | ||
| TINU syndrome | N10xx04+H20.9 | N12.x00x005 |
AKI, acute kidney injury; TINU, tubulointerstitial nephritis and uveitis.
References
- 1.GBD Chronic Kidney Disease Collaboration Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet (London, England) 2020;395:709–733. doi: 10.1016/S0140-6736(20)30045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foreman K.J., Marquez N., Dolgert A. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016–40 for 195 countries and territories. Lancet (London, England) 2018;392:2052–2090. doi: 10.1016/S0140-6736(18)31694-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang L., Wang F., Wang L. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet (London, England) 2012;379:815–822. doi: 10.1016/S0140-6736(12)60033-6. [DOI] [PubMed] [Google Scholar]
- 4.Liu Z.H. Nephrology in china. Nat Rev Nephrol. 2013;9:523–528. doi: 10.1038/nrneph.2013.146. [DOI] [PubMed] [Google Scholar]
- 5.Yang C., Kong G., Wang L. Big data in nephrology: Are we ready for the change? Nephrology (Carlton) 2019;24:1097–1102. doi: 10.1111/nep.13636. [DOI] [PubMed] [Google Scholar]
- 6.Nadkarni G.N., Coca S.G., Wyatt C.M. Big data in nephrology: promises and pitfalls. Kidney Int. 2016;90:240–241. doi: 10.1016/j.kint.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Zhang L., Wang H., Long J. China Kidney Disease Network (CK-NET) 2014 Annual Data Report. Am J Kidney Dis. 2017;69:A4. doi: 10.1053/j.ajkd.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saran R., Steffick D., Bragg-Gresham J. The China Kidney Disease Network (CK-NET): “Big Data-Big Dreams”. Am J Kidney Dis. 2017;69:713–716. doi: 10.1053/j.ajkd.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Wang F., Yang C., Long J. Executive summary for the 2015 Annual Data Report of the China Kidney Disease Network (CK-NET) Kidney Int. 2019;95:501–505. doi: 10.1016/j.kint.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 10.Zhang L., Zhao M.-H., Zuo L. China Kidney Disease Network (CK-NET) 2015 Annual Data Report. Kidney Int Suppl. 2019;9:e1–e81. doi: 10.1016/j.kisu.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Health Commission Statistical Communique on Healthcare and Family Planning Development in China in 2016. http://www.nhc.gov.cn/guihuaxxs/s10748/201708/d82fa7141696407abb4ef764f3edf095.shtml Available at:
- 12.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet (London, England) 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Afkarian M., Zelnick L.R., Hall Y.N. Clinical manifestations of kidney disease among US adults with diabetes, 1988–2014. JAMA. 2016;316:602–610. doi: 10.1001/jama.2016.10924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haynes R., Zhu D., Judge P.K. Chronic kidney disease, heart failure and neprilysin inhibition. Nephrol Dial Transplant. 2020;35:558–564. doi: 10.1093/ndt/gfz058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sato Y., Takahashi M., Yanagita M. Pathophysiology of AKI to CKD progression. Semin Nephrol. 2020;40:206–215. doi: 10.1016/j.semnephrol.2020.01.011. [DOI] [PubMed] [Google Scholar]
- 16.Masakane I., Nakai S., Ogata S. An overview of regular dialysis treatment in Japan (as of 31 December 2013) Ther Apher Dial. 2015;19:540–574. doi: 10.1111/1744-9987.12378. [DOI] [PubMed] [Google Scholar]
- 17.National Health Commission . Scientific and Technical Documentation Press; Beijing, China: 2018. National Medical Service and Quality Safety Report, 2017. [Google Scholar]
- 18.Sola L., Levin N.W., Johnson D.W. Development of a framework for minimum and optimal safety and quality standards for hemodialysis and peritoneal dialysis. Kidney Int Suppl. 2020;10:e55–e62. doi: 10.1016/j.kisu.2019.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Kidney Int Suppl. 2017;7:1–59. doi: 10.1016/j.kisu.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group KDIGO Clinical Practice Guideline for Anemia in Chronic Kidney Disease. Kidney Int Suppl. 2012;2:279–335. [Google Scholar]
- 21.Sarnak M.J., Amann K., Bangalore S. Chronic kidney disease and coronary artery disease: JACC state-of-the-art review. J Am Coll Cardiol. 2019;74:1823–1838. doi: 10.1016/j.jacc.2019.08.1017. [DOI] [PubMed] [Google Scholar]
- 22.National Bureau of Statistics of China. China Statistics Yearbook 2017. Available at: http://www.stats.gov.cn/tjsj/ndsj/2017/indexeh.htm. Published 2017. Accessed March 19, 2020.
- 23.National Health Commission. China Health Statistics Yearbook 2018. Beijing, China: Peking Union Medical College Press; 2018.
- 24.China Organ Transplantation Development Foundation. Report on Organ Transplantation Development in China (2015–2018); 2019. Available at: http://www.cotdf.org.cn/. Accessed March 13, 2020.
- 25.Saran R., Robinson B., Abbott K.C. US Renal Data System 2019 Annual Data Report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2020;75:A6–A7. doi: 10.1053/j.ajkd.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 26.Yang G., Wang Y., Zeng Y. Rapid health transition in China, 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet (London, England) 2013;381:1987–2015. doi: 10.1016/S0140-6736(13)61097-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.The State Council China sets up new system to optimize medical resources. http://english.www.gov.cn/policies/latest_releases/2015/09/11/content_281475187605730.htm Available at:
- 28.Bello A.K., Levin A., Lunney M. Status of care for end stage kidney disease in countries and regions worldwide: international cross sectional survey. BMJ. 2019;367:l5873. doi: 10.1136/bmj.l5873. [DOI] [PubMed] [Google Scholar]