Abstract
Background: Gastric cancer (GC) is one of the most common causes of cancer death. GSE83521 microarray analysis suggested that circular RNA circ_ASAP2 (hsa_circ_0008768) expression was increased in GC tissues. However, the molecular mechanism of circ_ASAP2 remains unknown. Methods: Expression levels of circ_ASAP2, microRNA-770-5p (miR-770-5p), and the cyclin-dependent kinase 6 (CDK6) were detected by using real time PCR (RT-PCR). 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) and transwell assays were applied to explore cell viability, migration, and invasion, respectively. The interactions between miR-770-5p and circ_ASAP2 or CDK6 was predicted by using Starbase software, and then confirmed by luciferase reporter assay. Xenograft tumor model was also used to estimate the effect of circ_ASAP2 on tumor growth in vivo. Results: The expression levels of circ_ASAP2 and CDK6 were increased, and miR-770-5p level was decreased in GC tissues and cells. Furthermore, circ_ASAP2 knockdown inhibited cell viability, migration, and invasion of GC cells. Mechanically, circ_ASAP2 functioned as a sponge of miR-770-5p to regulate CDK6 expression, thereby boosting the progression of GC cells. Circ_ASAP2 silencing hindered the tumor growth of GC in vivo. Conclusion: Circ_ASAP2 knockdown can repress the development of GC cells partly through regulating the miR-770-5p/CDK6 axis, suggesting an underlying circRNA-targeted therapy for GC treatment.
Keywords: Gastric cancer, circ_ASAP2, miR-770-5p, CDK6
Introduction
Gastric cancer (GC) is one of the most common causes of cancer death in the world [9], the incidence and mortality of which were on the second rank in China [4]. GC is caused by complicated interactions among host, bacterial factors and environmental, which induces genetic and epigenetic dysregulation of oncogenic and tumor suppressive genes [2]. During past years, despite the discovery of some potential biomarkers and target to enhance the diagnosis and treatment of GC patients, the prognosis of GC is still unsatisfactory [1]. Hence, seeking novel effective biomarkers and exploration of the potential molecular mechanism would be of substantially clinical value for diagnosis and treatment.
Circular RNA (circRNA), a newly discovered untranslated RNA, forms a covalently closed loop through specific splicing. Widely expressed in the cytoplasm, circRNA exhibits a higher degree of stability than linear mRNA [16,30]. Increasing evidence suggests that the dysregulation of circRNA was involved with the formation and development of diverse tumors, acting as a tumor suppressor gene or oncogene [24,40]. Several articles have conveyed that a large number of circRNAs take part in the regulation of tumor growth and metastasis, including GC. For example, Pan et al. showed that the overexpression of circUBA1 acted as a competitive endogenous RNA (ceRNA) of miR-375 to increase TEAD4, thereby promoting GC cell proliferation, migration, and invasion [11]. Consistently, Jiang et al. reported that circ_oo32821 served as an oncogene through boosting proliferation and metastasis in GC development [38]. Interestingly, we found that circ_ASAP2 displayed a high expression in GC tissues in the GEO dataset (GSE83521). However, the exact role and molecular mechanism of circ_ASAP2 in GC is uncertain.
MicroRNAs (miRNAs) are a class of small non-coding RNAs with a length of ~22 nt, and negatively regulate the gene expression by binding to the 3’-untranslated region (3’UTR) of target mRNAs [3]. In recent years, miRNAs were found to be related to the regulation of GC including miR-19b-3p, miR-375, miR-218, and miR-449 [7,8,21,42]. Moreover, previous literature revealed that the abnormal expression of miR-770-5p could predict progression in various cancers, containing glioma [19], ovarian cancer [12], and gastric cardia adenocarcinoma [33]. Yet, the role of miR-770-5p in GC is still not fully understood.
Cyclin-dependent kinase 6 (CDK6), a regulator of the cell cycle, has a specific oncogenic role in a variety of tumors [28]. Relevant literature indicated that CDK6 was upregulated in GC tissues and cells [5]. Moreover, some research confirmed that CDK6 participated in the regulation of GC cell proliferation, invasion, and cell cycle through interacting with miRNAs [18,23], implying that CDK6 has a role in GC progression.
Here, our results show that circ_ASAP2 was increased in GC tissues and cells, and circ_ASAP2 knockdown suppressed proliferation, migration, and invasion of GC cells. Moreover, the biological information tool predicted that there might be a binding site in circ_ASAP2 or CDK6 3’UTR complementary to miR-770-5p. Hence, we aimed to identify whether circ_ASAP2 could regulate GC development through the miR-770-5p/CDK6 axis.
Methods
Samples and cell culture
Approved by the First Affiliated Hospital of Xinjiang Medical University Ethics Committee, 45 patients with GC gave informed consent to be in the study. They had not received any other treatment before the surgery. Pair-matched tumorous tissues and adjacent nontumorous gastric tissues from the 45 patients were obtained. The tissue samples were immediately frozen in liquid nitrogen, and then stored at -80°C until RNA isolation.
The nontumorous gastric cell line GES-1 and GC cell lines (AGS and MKN45) were purchased from Procell (Wuhan, China). These cells were cultured in 25 ml flasks in 5% CO2 environment at a temperature of 37°C. RPMI-1640 was used for AGS and MKN45 cells, and DMEM was for GES-1. The media were also supplemented with 10% fetal bovine serum (FBS), streptomycin and penicillin (Gibco, Carlsbad, CA, US).
Transfection
Circ_ASAP2 small interfering RNA (si-circ_ASAP2) and the corresponding negative control (si-NC), miR-770-5p mimics (miR-770-5p), inhibitor (anti-miR-770-5), negative control (miR-NC, anti-miR-NC), pcDNA-circ_ASAP2 (circ_ASAP2) and pcDNA-CDK6 (CDK6), and their negative pcDNA3.1 (Vector and pcDNA) were obtained from Syngentech (Beijing, China), and were transfected using Lipofectamine 3000 reagent (Invitrogen, Carlsbad, CA, USA) into GC cells at a final concentration of 10-100 nM.
RNA extraction and RT-PCR
Total RNA from tissue samples and cell lines was extracted using the Animal miRNA Isolation Kit (ForeGene, Chengdu, China) according to the manufacturer’s protocol. RNA concentrations and quality were detected with a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Inc., Waltham, MA, US). The PrimeScript RT Reagent (Takara, Tokyo, Japan) was used to reverse transcribe 500 ng total RNA to cDNA. The circ_ASAP2, miR-770-5p, and CDK6 expression was determined with the SYBR Green Master (Vazyme, Shanghai, China). Normalized to the internal controls glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and snRNA U6, these genes’ expression was calculated by the 2-ΔΔCt method. Primer sequences were: circ_ASAP2: 5’-CTGCCAGGTGAAGACCAACC-3’ (sense), 5’-TGCATCCTGGAATAACACAGACA-3’ (antisense); miR-770-5p: 5’-TGCAGCACGCCAACATGGCACATGTATACATATG-3’ (sense), 5’-ATTAGGTATATCTCCAAATGCTATCCTTCCCC-3’ (antisense); CDK6: 5’-ACGTGGTCAGGTTGTTT-3’ (sense), 5’-TTTATGGTTTCAGTGGG-3’ (antisense); U6: 5’-CTCGCTTCGGCAGCACA-3’ (sense), 5’-AACGCTTCACGAATTTGCGT-3’ (antisense); GAPDH: 5’-ACCACAGTCCATGCCATCAC-3’ (sense), 5’-TCCACCACCCTGTTGCTGTA-3’ (antisense).
RNase R digestion
Total RNA (5 μL) was incubated at 37°C 15 min with 3 U/μg of RNase R (Epicentre Biotechnologies, Shanghai, China), followed by RNase R digestion reaction twice as previously published.
Cell viability assay
Transfected AGS and MKN45 cells were seeded in 96-well plates (Corning Costar, Corning, NY, US) for 24 h, at the concentration of 2.0 × 103 cells per well. Then 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT, Beyotime, Shanghai, China) solution was added into each well at 24 h, 48 h, 72 h and 96 h after transfection, respectively. Cell viability was measured by determining the absorbance at 490 nm with a microplate reader.
Transwell migration and invasion assay
Cell migratory and invasive rates were detected by the 24-well culture plates and chamber with or without Matrigel. The upper chamber got 100 μl of 1 × 106 cells/ml DMEM medium containing 0.1% FBS and 500 μl DMEM medium containing 15% FBS was put into the lower chamber. After 12 h, we removed the cells on the matrigel gently with several cotton swabs, and the migratory or invasive cells were stained with Crystal Violet, the result was counted and imaged under a microscope (Bx41, Olympus, Japan).
Western blot assay
Total protein from cells and tumor tissues were extracted by RIPA lysis buffer (Merck Millipore, Darmstadt, Germany). After being heated for 10 min at 95°C with the loading buffer containing 0.003% bromophenol blue, the 30 μg protein samples were separated by 12% SDS-PAGE and transferred onto the PVDF membranes (Millipore Corporation, Billerica, MA, USA).The membranes were blocked with 5% nonfat milk with Tris-buffered saline and 0.1% Tween 20 (TBST) at least for 1 h at 37°C, then were incubated with the primary antibodies (CDK6, 1:2000, Abcam, Cambridge, UK; β-actin, 1:5000, Easybio, Beijing, China) overnight at 4°C. After washing in TBST three times, the membranes were incubated with horseradish peroxidase-conjugated secondary antibody at room temperature for 2 h. Results were visualized using chemiluminescence after washing by TBST solution.
Luciferase reporter assay
The sequences of circ_ASAP2 or CDK6 3’UTR involving miR-16-5p putative binding sites or its mutation were constructed into downstream part of firefly luciferase gene in pGL3 vectors (Promega Corporation, Fitchburg, WI, US), named as circ_ASAP2-wild type (circ_ASAP2-wt), circ_ASAP2-mutant (circ_ASAP2-mut), CDK6-wt, and CDK6-mut. GC cells of AGS and MKN45 were seeded into a 24-well plate for 24 h before transfection. After that, GC cells were co-transfected with 100 ng constructed luciferase reporter plasmid, 50 ng of Renilla luciferase reporter vector (pRL-TK), and 50 nM of miR-770-5p, or miR-NC using the Lipofectaine3000 (Invitrogen, Waltham, MA, US). Cells were collected at 48 h after transfection, and the luciferase activity was examined with the Dual Luciferase Reporter Assay Kit (Promega).
Xenograft tumor model
AGS cells (5 × 106) stably transfected with the circ_ASAP2 knockdown lentiviral vector (sh-circ_ASAP2, GeneChem, Shanghai, China) or the negative control vector (sh-NC, GeneChem) were subcutaneously injected into two groups of 4-week-old nude mice (n=6 mice/group, Shanghai Experimental Animal Center, Shanghai, China), respectively. The tumor volumes were measured once a week by a caliper according to the formula: volume = length × width2/2. Five weeks later, the mice were sacrificed and the resected tumor masses were collected for subsequent weight, followed by analysis with RT-PCR and western blot. All the animal experiments were approved by the First Affiliated Hospital of Xinjiang Medical University and were performed in accordance with the guide for the care and use of laboratory animals.
Statistical analysis
All data are shown as the mean ± standard deviation (SD) of at least 3 individual experiments. The differences between two or more groups were estimated with the Student t-test or one-way analysis of variance with Tukey’s tests. The statistical analyses were analyzed using the GraphPad 7.0 software. P < 0.05 was considered significant.
Results
Circ_ASAP2 was increased in GC tissues and cells
GSE83521 microarray analysis verified that circ_ASAP2 presented high expression in GC tissues compared with normal tissues (Figure 1A). Hence, to further explore the role of circ_ASAP2 in GC, an RT-qPCR assay was conducted to measure the expression level of circ_ASAP2 in GC tissues (n=45) compared with tumor-adjacent normal tissues (n=45), and the outcome indicated that the level of circ_ASAP2 was significantly increased in GC tissues (Figure 1B). Moreover, our data also suggested that circ_ASAP2 was upregulated in two GC cell lines (AGS and MKN45) relative to the gastric mucosal epithelial cell line (GES-1) (Figure 1C). Since RNase R enzyme (a highly processive 3’ to 5’ exoribonuclease) does not act on circular RNAs but only linear RNAs [32], RNase R was used for verification the features of circ_ASAP2 in AGS and MKN45 cells. As displayed in Figure 1D and 1E, circular RNA was resistant to RNase R treatment when compared with the linear RNAs. Thus, circ_ASAP2 might be involved with GC progression.
Figure 1.
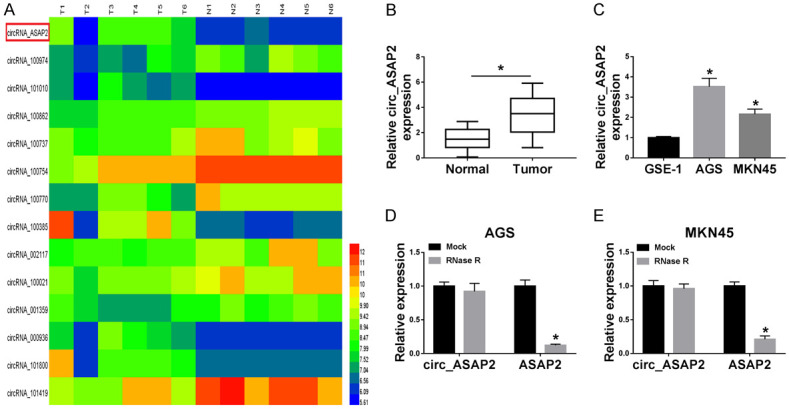
The relative expression of circ_ASAP2 was upregulated in GC tissues and cells. A. GSE83521 microarray analyzed the circRNA expression profiling between GC tissues and adjacent normal tissues. B. Relative expression of circ_ASAP2 in GC tissues (n=45) matched to the adjacent normal tissues (n=45) by RT-qPCR. C. Expression levels of circ_ASAP2 in gastric cancer cell lines (AGS and MKN45) relative to the gastric mucosal epithelial cell line (GES-1). D, E. Expression levels of circ_ASAP2 and ASAP2 were detected in AGS and MKN45 cells treated with RNase R or Mock. *P < 0.05.
Circ_ASAP2 knockdown suppressed cell viability, migration, and invasion in GC cells
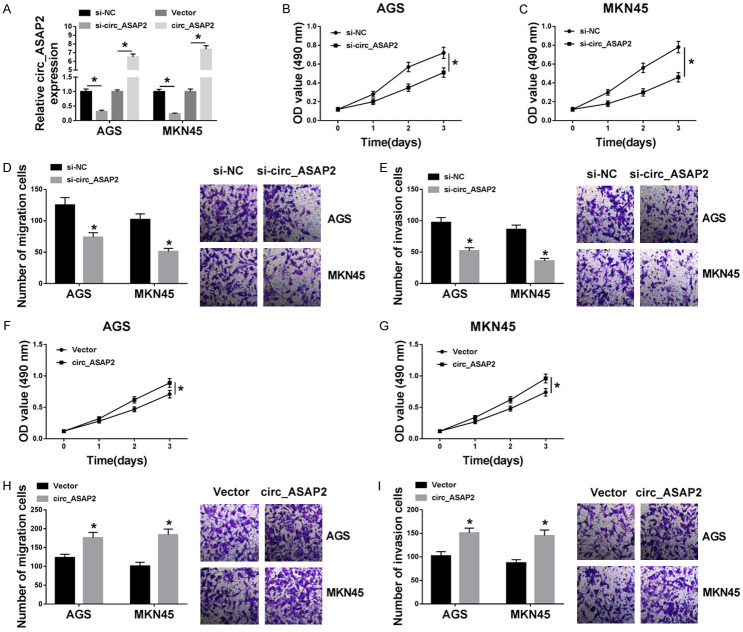
Next, to identify the biological function of circ_ASAP2 in GC cells, the knockdown or overexpression efficiency of circ_ASAP2 in AGS and MKN45 cells was examined and exhibited in Figure 2A. Functionally, MTT assay revealed that circ_ASAP2 reduction hindered the proliferation (Figure 2B and 2C) of EC cells in contrast to controls. According to the results of Transwell assay, the knockdown of circ_ASAP2 repressed the abilities of migration (Figure 2D), and invasion (Figure 2E) of EC cells relative to cells with si-NC. On the contrary, enhanced proliferative ability (Figure 2F and 2G), and the capacities for migration and invasion (Figure 2H and 2I) resulted from upregulation of circ_ASAP2 in AGS and MKN45 cells. All these data suggested that circ_ASAP2 deficiency could inhibit cell viability, migration, and invasion of GC cells.
Figure 2.
The effect of overexpressing or silencing circ_ASAP2 on cell viability, migration, and invasion in gastric cancer cells. A. The circ_ASAP2 expression level in AGS and MKN45 transfected with si-NC, si-circ_ASAP2, Vector, and circ_ASAP2. B, C. MTT assay was used to detect cell viability in si-NC or si-circ_ASAP2-transfected AGS and MKN45. D, E. Transwell assay was carried out to measure migration and invasion in si-NC or si-circ_ASAP2-transfected AGS and MKN45. F, G. Cell viability was examined in Vector or circ_ASAP2-transfected AGS and MKN45. H, I. Migration and invasion were tested in Vector-transfected or circ_ASAP2-transfected AGS and MKN45. *P < 0.05.
MiR-770-5p acted as the target of circ_ASAP2 in GC cells
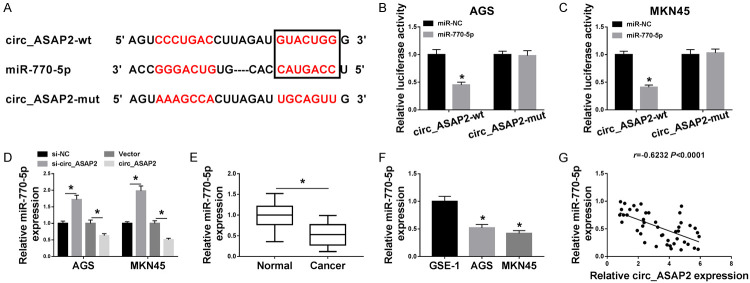
It has widely accepted that circRNAs serve as competing endogenous RNAs (ceRNAs) to sponge corresponding miRNAs, thereby regulating their biologic roles [29,39]. Hence, starbase software was employed to forecast the underlying binding targets of circ_ASAP2. As shown in Figure 3A, there were some binding sites between circ_ASAP2 and miR-770-5p, followed by demonstration with dual-luciferase reporter assay. Results unfolded decreased luciferase activity in AGS and MKN45 cells co-transfected with circ_ASAP2-wt and miR-770-5p. However, after co-transfection with circ_ASAP2-mut and miR-NC, there was no significant change in luciferase activity (Figure 3B and 3C). Moreover, RT-qPCR results presented that miR-770-5p was increased in si-circ_ASAP2-transfected GC cells, while miR-770-5p was reduced due to upregulation of circ_ASAP2 (Figure 3D). Besides, miR-770-5p displayed a low expression in EC tissues and cells relative to their respective controls (Figure 3E and 3F), and its expression was negatively correlated with circ_ASAP2 in EC tissues (Figure 3G). Overall, these data implied that miR-770-5p directly bound to circ_ASAP2 in GC cells.
Figure 3.
Circ_ASAP2 bound with miR-770-5p in gastric cancer cells. A. The predicted binding sites between circ_ASAP2 and miR-770-5p were analyzed by using starbase. B, C. Dual-luciferase reporter assay was performed to confirm the direct target sites. D. MiR-770-5p level was detected in AGS and MKN45 cells transfected with si-NC, si-circ_ASAP2, Vector, and circ_ASAP2. E. MiR-770-5p level was assessed in GC tissues and normal tissues. F. MiR-770-5p was detected in GES-1, AGS and MKN45 cells. G. The correlation between miR-770-5p and circ_ASAP2 expression in gastric cancer tissues was analyzed by Pearson’s correlation analysis. *P < 0.05.
Knockdown of miR-770-5p alleviated the suppressive action of circ_ASAP2 silencing on cell viability, migration and invasion of GC cells
Considering that miR-770-5p might act as the target of circ_ASAP2, we further explored whether circ_ASAP2 could regulate the biologic processes of GC cells through targeting miR-770-5p. As exhibited in Figure 4A, circ_ASAP2 knockdown boosted the expression level of miR-770-5p, while the introduction of anti-miR-770-5p relieved the effect in AGS and MKN45 cells. Functional analysis suggested that co-transfection of miR-770-5p inhibitor could reverse the negative effect of single deficiency of circ_ASAP2 on cell viability in GC cells (Figure 4B and 4C). Meanwhile, the results from Transwell assays indicated that the improvedmigration and invasion caused by the knockdown of circ_ASAP2 was mitigated through miR-770-5p downregulation in AGS and MKN45 cells (Figure 4D and 4E). The above data hinted that circ_ASAP2 knockdown could inhibit the development of GC cells by regulating miR-770-5p.
Figure 4.
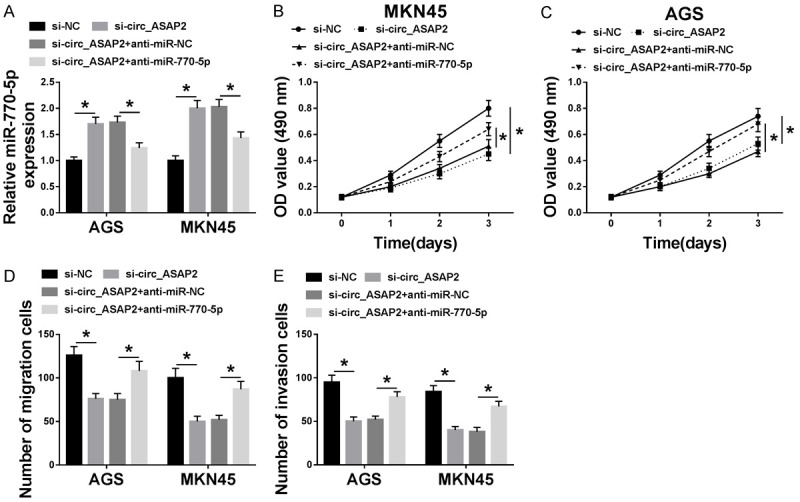
Knockdown of miR-770-5p alleviated circ_ASAP2 downregulation-mediated cell viability, migration, and invasion in GC cells. A. The expression level of miR-770-5p was assessed by RT-qPCR assay in AGS and MKN45 cells transfected with si-NC, si-circ_ASAP2, si-circ_ASAP2+anti-miR-NC, and si-circ_ASAP2+anti-miR-770-5p. B, C. Cell viability in transfected AGS and MKN45 cells was examined by MTT assay. D, E. Migration and invasion in transfected AGS and MKN45 cells were detected by transwell assay. *P < 0.05.
CDK6 served as the target of miR-770-5p
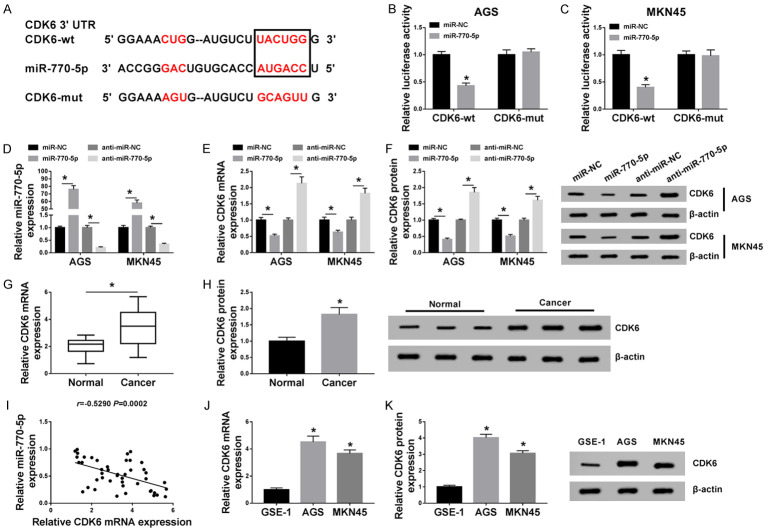
miRNAs can modulate tumor progression by interacting with mRNAs. Therefore, the latent possible target genes of miR-770-5p were predicted by using starbase software. As a result, CDK6 3’UTR was found to possess some binding sites with miR-770-5p (Figure 5A). To verify the prediction result, dual-luciferase reporter assay in AGS and MKN45 cells was carried out. Data suggested that the upregulation of miR-770-5p decreased the luciferase activity of CDK6-wt reporter vector in AGS and MKN45 cells, but not that of CDK6-mut reporter vector (Figure 5B and 5C). Furthermore, the transfection rate of miR-770-5p mimics or inhibitor was determined by RT-qPCR assay (Figure 5D). Whereafter, both mRNA level and protein levels of CDK6 were determined in transfected AGS and MKN45 cells. As displayed in Figure 5E and 5F, CDK6 was distinctly reduced by the overexpression of miR-770-5p, while CDK6 expression was enhanced by miR-770-5p knockdown. RT-qPCR and western blot assays presented that CDK6 expression levels were increased in GC tissues (Figure 5G and 5H) and cells (Figure 5J and 5K) when compared with their respective controls. In addition, an inverse correlation between CDK6 and miR-770-5p in GC tissues was observed (Figure 5I). Collectively, these findings implied that miR-770-5p could interact with CDK6 to suppress its expression.
Figure 5.
CDK6 is a direct target of miR-770-5p. A. Starbase software was applied to analyze the relationship between miR-770-5p and CDK6. B, C. The binding sites of CDK6 in miR-770-5p were verified by dual-luciferase reporter assay in AGS and MKN45 cells. D, E. Levels of miR-770-5p or CDK6 were assessed in AGS and MKN4 transfected with miR-NC, miR-770-5p, anti-miR-NC or anti-miR-770-5p. F. CDK6 protein level in transfected AGS and MKN4 cells was detected by western blot. G, H. Both mRNA level and protein level of CDK6 were examined in GC tissues and adjacent normal tissues. I. Pearson’s correlation analysis was conducted to analyze the association between miR-770-5p and CDK6 in GC tissues. J, K. CDK6 level in GES-1, AGS, and MKN45 cells was tested by RT-qPCR assay and western blot. *P < 0.05.
MiR-770-5p regulated the development of GC cells by targeting CDK6 in GC cells
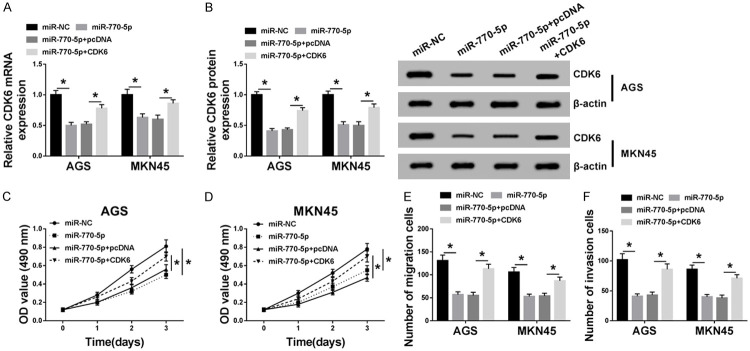
Then, to further investigate whether the miR-770-5p-mediated GC progression was regulated by CDK6, recovery experiments were performed in AGS and MKN45 cells. As illustrated in Figure 6A and 6B, co-transfection of CDK6 could alleviate the inhibiting effect of miR-770-5p overexpression on CDK6 mRNA level and protein level in GC cells. Notably, the influence of miR-770-5p upregulation on cell viability, migration, and invasion was similar to si-circ_ASAP2. MTT and Transwell results indicated that miR-770-5p mimics repressed cell viability (Figure 6C and 6D), migration (Figure 6E), and invasion (Figure 6F) of GC cells, which was lightened by the overexpression of CDK6. Taken together, miR-770-5p suppressed cell viability, migration, and invasion of GC cells through regulating CDK6 expression.
Figure 6.
Overexpression of CDK6 relieved the inhibitory action of miR-770-5p upregulation on cell viability, migration, and invasion in GC cells. A, B. The levels of CDK6 in AGS and MKN45 cells transfected with miR-NC, miR-770-5p, miR-770-5p+pcDNA, and miR-770-5p+CDK6 were measured by RT-qPCR and western blot. C, D. MTT assay was used to examine the cell viability of transfected AGS and MKN45 cells. E, F. The cell migration and invasion of transfected AGS and MKN45 cells were detected by transwell assay. *P < 0.05.
Verification of circ_ASAP2/miR-770-5p/CDK6 axis in GC cells
To determine whether circ_ASAP2 could exert a function through the miR-770-5p/CDK6 axis in GC cells, the effect of circ_ASAP2 on CDK6 expression was detected in AGS and MKN45 cells. Recovery experiments suggested that circ_ASAP2 knockdown reduced the mRNA level and protein level of CDK6, while the re-introduction of anti-miR-770-5p alleviated the negative action of si-circ_ASAP2 on CDK6 levels in GC cells (Figure 7A and 7B). In general, circ_ASAP2 could regulate CDK6 expression by sponging miR-770-5p in GC cells.
Figure 7.
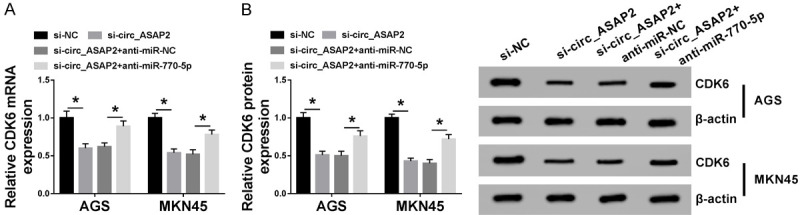
Circ_ASAP2 regulated CDK6 expression level by sponging miR-770-5p in GC cells. A. The levels of CDK6 in AGS and MKN45 cells transfected with si-NC, si-circ_ASAP2, si-circ_ASAP2+anti-miR-NC and si-circ_ASAP2+anti-miR-770-5p was examined by RT-qPCR. B. CDK6 protein level was detected in transfected AGS and MKN45 cells.
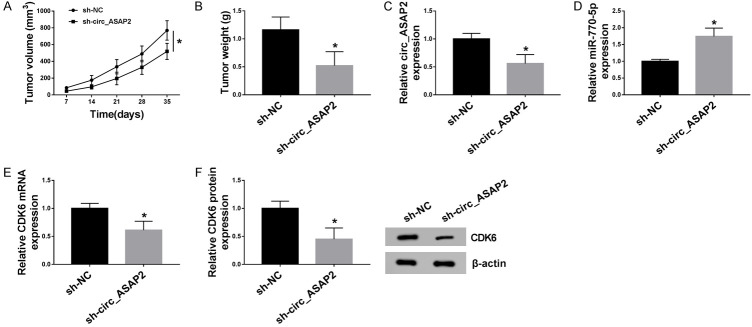
Circ_ASAP2 knockdown inhibited tumor growth in vivo
In order to explore the clinical application of circ_ASAP2 on GC in vivo, the xenograft tumor model was applied. AGS cells with sh-circ_ASAP2 or negative control (sh-NC) were subcutaneously injected into athymic nude mice. Subsequently, we viewed that intratumoral injection of sh-circ_ASAP2 suppressed tumor growth, as presented by reduced tumor volume and weight (Figure 8A and 8B). Apart from that, the tumor tissues acquired from the nude mice had a decreased expression level of circ_ASAP2 in sh-circ_ASAP2 group relative to sh-NC group (Figure 8C), accompanied by higher miR-770-5p and lower CDK6 expression levels (Figure 8D and 8E). CDK6 protein level also declined, further supporting the down-regulation of sh-circ_ASAP2 on CDK6 expression (Figure 8F). The above data hinted that circ_ASAP2 knockdown restrained xenograft tumor growth by regulating the miR-770-5p/CDK6 axis.
Figure 8.
Circ_ASAP2 knockdown hindered tumor growth of EC in vivo. A, B. Tumor growth was inhibited in the sh-circ_ASAP2 mouse group compared with the sh-NC. C-E. Relative expression levels of circ_ASAP2, miR-770-5p and CDK6 in xenografts were detected by RT-PCR. F. CDK6 protein level was examined in xenografts by western blot. *P < 0.05.
Discussion
With the development of sequencing technology and bioinformatics analysis, the involvement of circRNAs in cancer progression has attracted extensive attention [13,22]. Studies in the past few years have shown that circRNAs, a novel highly stable and abundant endogenous non-coding RNA, have been confirmed to serve functionally as oncogenes or tumor suppressor genes in the regulation of multiple human cancers [25,26]. Nevertheless, few circRNAs have been well functionally and mechanistically characterized in GC, and the biologic function of most circRNAs remains to be explored. Here, a new circRNA circ_ASAP2 was first identified to be increased in GC tissues and cells. Functionally, circ_ASAP2 reduction blocked proliferation, migration, and invasion of GC cells. Furthermore, the inhibitory action of circ_ASAP2 knockdown on tumor growth was also validated in GC xenografts in nude mice. These data implied a pivotal role of circ_ASAP2 in the progression of GC.
Recent research displayed that circRNAs could exert a biologic function by working as a molecular sponge for miRNAs, thereby resulting in the de-repression of miRNA targets [6,10]. In the present study, we first verified that miR-770-5p was a direct target of circ_ASAP2 in GC cells. Moreover, some reports illuminated that miR-770-5p, a form of mature miR-770, was a tumor suppressor in diverse cancers, such as lung cancer [41], breast cancer [27], and glioma [15]. In our study, miR-770-5p was down-regulated, and negatively associated with circ_ASAP2 level in GC tissues. Furthermore, functional analysis verified that inhibition of miR-770-5p reversed the circ_ASAP2-deficiency-mediated decrease in proliferation, migration, and invasion of GC cells, further supporting that circ_ASAP2 could regulate GC development through targeting miR-770-5p in GC cells.
It is widely accepted that miRNAs modulate tumor progression by interacting with mRNAs [17]. Moreover, miRNAs target the downstream genes and negatively regulate gene expression. By base pairing to the sequence regions in mRNAs’ 3’UTR with perfect or near perfect complementarity, miRNAs play a role in regulating target expression [20,36]. In this study, CDK6 served as a target of miR-770-5p in GC cells. CDK6, with a functional role in tissue homeostasis and differentiation, was directly involved in transcription in tumor cells and in hematopoietic stem cells [31]. Apart from that, CDK6 was closely associated with proliferation, metastasis, and cell cycle in cancers [14,35]. In this paper, a high expression of CDK6 was viewed in GC tissues and cells. Importantly, CDK6 overexpression weakened the suppression effect of miR-770-5p upregulation on proliferation, migration, and invasion in GC cells. In agreement with our data, CDK6 promoted migration and proliferation in GC cells [34,37]. Western blot analysis proved that anti-miR-770-5p alleviated the inhibitory effect of circ_ASAP2 knockdown on CDK6 level in GC cells, further supporting the regulatory role of the circ_ASAP2/miR-770-5p/CDK6 in GC cells.
Conclusion
In summary, we showed that circ_ASAP2 functioned as a sponge of miR-770-5p to positively regulate CDK6, thus boosting GC progression. These findings provided an underlying therapeutic strategy for GC treatment.
Disclosure of conflict of interest
None.
References
- 1.Wagner AD, Syn NL, Moehler M, Grothe W, Yong WP, Tai BC, Ho J, Unverzagt S. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2017;8:CD004064. doi: 10.1002/14651858.CD004064.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ando T, Goto Y, Ishiguro K, Maeda O, Watanabe O, Ohmiya N, Niwa Y, Hamajima N, El-Omar E, Goto H. The interaction of host genetic factors and helicobacter pylori infection. Inflammopharmacology. 2007;15:10–14. doi: 10.1007/s10787-006-1556-y. [DOI] [PubMed] [Google Scholar]
- 3.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 4.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 5.Wang D, Sun Y, Li W, Ye F, Zhang Y, Guo Y, Zhang DY, Suo J. Antiproliferative effects of the CDK6 inhibitor PD0332991 and its effect on signaling networks in gastric cancer cells. Int J Mol Med. 2018;41:2473–2484. doi: 10.3892/ijmm.2018.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiong DD, Dang YW, Lin P, Wen DY, He RQ, Luo DZ, Feng ZB, Chen G. A circRNA-miRNA-mRNA network identification for exploring underlying pathogenesis and therapy strategy of hepatocellular carcinoma. J Transl Med. 2018;16:220. doi: 10.1186/s12967-018-1593-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deng M, Zeng C, Lu X, He X, Zhang R, Qiu Q, Zheng G, Jia X, Liu H, He Z. miR-218 suppresses gastric cancer cell cycle progression through the CDK6/Cyclin D1/E2F1 axis in a feedback loop. Cancer Lett. 2017;403:175–185. doi: 10.1016/j.canlet.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Ding L, Xu Y, Zhang W, Deng Y, Si M, Du Y, Yao H, Liu X, Ke Y, Si J, Zhou T. MiR-375 frequently downregulated in gastric cancer inhibits cell proliferation by targeting JAK2. Cell Res. 2010;20:784–793. doi: 10.1038/cr.2010.79. [DOI] [PubMed] [Google Scholar]
- 9.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 10.Kulcheski FR, Christoff AP, Margis R. Circular RNAs are miRNA sponges and can be used as a new class of biomarker. J Biotechnol. 2016;238:42–51. doi: 10.1016/j.jbiotec.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 11.Pan H, Pan J, Chen P, Gao J, Guo D, Yang Z, Ji L, Lv H, Guo Y, Xu D. Circular RNA circUBA1 promotes gastric cancer proliferation and metastasis by acting as a competitive endogenous RNA through sponging miR-375 and regulating TEAD4. Cancer Lett. 2020 doi: 10.1016/j.canlet.2020.02.022. S0304-3835(20)30085-9. [DOI] [PubMed] [Google Scholar]
- 12.Zhao H, Yu X, Ding Y, Zhao J, Wang G, Wu X, Jiang J, Peng C, Guo GZ, Cui S. MiR-770-5p inhibits cisplatin chemoresistance in human ovarian cancer by targeting ERCC2. Oncotarget. 2016;7:53254–53268. doi: 10.18632/oncotarget.10736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang HD, Jiang LH, Sun DW, Hou JC, Ji ZL. CircRNA: a novel type of biomarker for cancer. Breast Cancer. 2018;25:1–7. doi: 10.1007/s12282-017-0793-9. [DOI] [PubMed] [Google Scholar]
- 14.Jin J, Chen X, Chen J, Geng X. Long noncoding RNA MACC1-AS1 is a potential sponge of microRNA-34a in cervical squamous cell carcinoma and upregulates cyclin-dependent kinase 6. Oncol Lett. 2020;19:2339–2345. doi: 10.3892/ol.2020.11346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang JF, Zhang JS, Zhao ZH, Yang PB, Ji SF, Li N, Shi QD, Tan J, Xu X, Xu CB, Zhao LY. MicroRNA-770 affects proliferation and cell cycle transition by directly targeting CDK8 in glioma. Cancer Cell Int. 2018;18:195. doi: 10.1186/s12935-018-0694-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo JU, Agarwal V, Guo H, Bartel DP. Expanded identification and characterization of mammalian circular RNAs. Genome Biol. 2014;15:409. doi: 10.1186/s13059-014-0409-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reddy KB. MicroRNA (miRNA) in cancer. Cancer Cell Int. 2015;15:38. doi: 10.1186/s12935-015-0185-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng L, Xie Y, Zhang H, Wu Y. miR-107 targets cyclin-dependent kinase 6 expression, induces cell cycle G1 arrest and inhibits invasion in gastric cancer cells. Med Oncol. 2012;29:856–863. doi: 10.1007/s12032-011-9823-1. [DOI] [PubMed] [Google Scholar]
- 19.Jia L, Song Y, Mu L, Li Q, Tang J, Yang Z, Meng W. Long noncoding RNA TPT1-AS1 downregulates the microRNA-770-5p expression to inhibit glioma cell autophagy and promote proliferation through STMN1 upregulation. J Cell Physiol. 2020;235:3679–3689. doi: 10.1002/jcp.29262. [DOI] [PubMed] [Google Scholar]
- 20.Lai EC. Micro RNAs are complementary to 3’UTR sequence motifs that mediate negative post-transcriptional regulation. Nat Genet. 2002;30:363–364. doi: 10.1038/ng865. [DOI] [PubMed] [Google Scholar]
- 21.Li LP, Wu WJ, Sun DY, Xie ZY, Ma YC, Zhao YG. miR-449a and CDK6 in gastric carcinoma. Oncol Lett. 2014;8:1533–1538. doi: 10.3892/ol.2014.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kristensen LS, Hansen TB, Venø MT, Kjems J. Circular RNAs in cancer: opportunities and challenges in the field. Oncogene. 2018;37:555–565. doi: 10.1038/onc.2017.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Zhou X, Shan B, Han J, Wang F, Fan X, Lv Y, Chang L, Liu W. Downregulation of microRNA‑33a promotes cyclin‑dependent kinase 6, cyclin D1 and PIM1 expression and gastric cancer cell proliferation. Mol Med Rep. 2015;12:6491–6500. doi: 10.3892/mmr.2015.4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang M, Chen B, Ru Z, Cong L. CircRNA circ-ITCH suppresses papillary thyroid cancer progression through miR-22-3p/CBL/β-catenin pathway. Biochem Biophys Res Commun. 2018;504:283–288. doi: 10.1016/j.bbrc.2018.08.175. [DOI] [PubMed] [Google Scholar]
- 25.Li P, Yang X, Yuan W, Yang C, Zhang X, Han J, Wang J, Deng X, Yang H, Li P, Tao J, Lu Q, Gu M. CircRNA-Cdr1as exerts anti-oncogenic functions in bladder cancer by sponging microRNA-135a. Cell Physiol Biochem. 2018;46:1606–1616. doi: 10.1159/000489208. [DOI] [PubMed] [Google Scholar]
- 26.Yang R, Xing L, Zheng X, Sun Y, Wang X, Chen J. The circRNA circAGFG1 acts as a sponge of miR-195-5p to promote triple-negative breast cancer progression through regulating CCNE1 expression. Mol Cancer. 2019;18:4. doi: 10.1186/s12943-018-0933-7. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Noyan S, Gurdal H, Gur Dedeoglu B. Involvement of miR-770-5p in trastuzumab response in HER2 positive breast cancer cells. PLoS One. 2019;14:e0215894. doi: 10.1371/journal.pone.0215894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tadesse S, Yu M, Kumarasiri M, Le BT, Wang S. Targeting CDK6 in cancer: state of the art and new insights. Cell Cycle. 2015;14:3220–3230. doi: 10.1080/15384101.2015.1084445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang S, Zhu D, Li H, Li H, Feng C, Zhang W. Characterization of circRNA-associated-ceRNA networks in a senescence-accelerated mouse prone 8 brain. Mol Ther. 2017;25:2053–2061. doi: 10.1016/j.ymthe.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conn SJ, Pillman KA, Toubia J, Conn VM, Salmanidis M, Phillips CA, Roslan S, Schreiber AW, Gregory PA, Goodall GJ. The RNA binding protein quaking regulates formation of circRNAs. Cell. 2015;160:1125–1134. doi: 10.1016/j.cell.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 31.Tigan AS, Bellutti F, Kollmann K, Tebb G, Sexl V. CDK6-a review of the past and a glimpse into the future: from cell-cycle control to transcriptional regulation. Oncogene. 2016;35:3083–3091. doi: 10.1038/onc.2015.407. [DOI] [PubMed] [Google Scholar]
- 32.Vincent HA, Deutscher MP. Insights into how RNase R degrades structured RNA: analysis of the nuclease domain. J Mol Biol. 2009;387:570–583. doi: 10.1016/j.jmb.2009.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo W, Dong Z, Liu S, Qiao Y, Kuang G, Guo Y, Shen S, Liang J. Promoter hypermethylation-mediated downregulation of miR-770 and its host gene MEG3, a long non-coding RNA, in the development of gastric cardia adenocarcinoma. Mol Carcinog. 2017;56:1924–1934. doi: 10.1002/mc.22650. [DOI] [PubMed] [Google Scholar]
- 34.Wu W, Wei N, Shao G, Jiang C, Zhang S, Wang L. circZNF609 promotes the proliferation and migration of gastric cancer by sponging miR-483-3p and regulating CDK6. Onco Targets Ther. 2019;12:8197–8205. doi: 10.2147/OTT.S193031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yuan W, Wang D, Liu Y, Tian D, Wang Y, Zhang R, Yin L, Deng Z. miR‑494 inhibits cell proliferation and metastasis via targeting of CDK6 in osteosarcoma. Mol Med Rep. 2017;16:8627–8634. doi: 10.3892/mmr.2017.7709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xie X, Lu J, Kulbokas EJ, Golub TR, Mootha V, Lindblad-Toh K, Lander ES, Kellis M. Systematic discovery of regulatory motifs in human promoters and 3’UTRs by comparison of several mammals. Nature. 2005;434:338–345. doi: 10.1038/nature03441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jia Y, Zhao LM, Bai HY, Zhang C, Dai SL, Lv HL, Shan BE. The tumor-suppressive function of miR-1296-5p by targeting EGFR and CDK6 in gastric cancer. Biosci Rep. 2019;39:BSR20181556. doi: 10.1042/BSR20181556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang Y, Zhang Y, Chu F, Xu L, Wu H. Circ_0032821 acts as an oncogene in cell proliferation, metastasis and autophagy in human gastric cancer cells in vitro and in vivo through activating MEK1/ERK1/2 signaling pathway. Cancer Cell Int. 2020;20:74. doi: 10.1186/s12935-020-1151-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhong Y, Du Y, Yang X, Mo Y, Fan C, Xiong F, Ren D, Ye X, Li C, Wang Y, Wei F, Guo C, Wu X, Li X, Li Y, Li G, Zeng Z, Xiong W. Circular RNAs function as ceRNAs to regulate and control human cancer progression. Mol Cancer. 2018;17:79. doi: 10.1186/s12943-018-0827-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen Z, Xiao K, Chen S, Huang Z, Ye Y, Chen T. Circular RNA hsa_circ_001895 serves as a sponge of microRNA-296-5p to promote clear cell renal cell carcinoma progression by regulating SOX12. Cancer Sci. 2020;111:713–726. doi: 10.1111/cas.14261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Z, Yang Y, Zhang X. MiR-770 inhibits tumorigenesis and EMT by targeting JMJD6 and regulating WNT/β-catenin pathway in non-small cell lung cancer. Life Sci. 2017;188:163–171. doi: 10.1016/j.lfs.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 42.Zhang J, Song Y, Zhang C, Zhi X, Fu H, Ma Y, Chen Y, Pan F, Wang K, Ni J, Jin W, He X, Su H, Cui D. Circulating mir-16-5p and mir-19b-3p as two novel potential biomarkers to indicate progression of gastric cancer. Theranostics. 2015;5:733–745. doi: 10.7150/thno.10305. [DOI] [PMC free article] [PubMed] [Google Scholar]