Abstract
Background: Ischemic tolerance is an endogenous protective mechanism in organs or tissues undergoing one or more short-term sublethal ischemias. Intermittent hypobaric hypoxia preconditioning (IHHP) can induce tolerance and thus protect brain tissues from cerebral ischemic injury (CIR). The current study evaluated the neuroprotective effect of IHHP. Methods: The established xenograft model was divided into the ischemia/reperfusion (I/R), IHHP, IHHP+I/R, and sham groups. Transmission electron microscopy was used to observe alterations in neuron ultrastructure. Neuron damage was detected using Nissl staining. Western blot and qRT-PCR were used to evaluate the relative expression of genes and proteins related to apoptosis. Immunohistochemistry was used to determine the expression of proteins involved in the processes of neuroprotection and repair. Results: Our results indicated that the damage to the neurons, organelles, and axons was significantly less following ischemia/reperfusion and intermittent hypobaric hypoxia reconditioning treatment than that in the ischemia/reperfusion group. Compared to the ischemia/reperfusion group, significant downregulation of pro-apoptotic gene/protein expressions along with upregulation of anti-apoptotic and nerve regeneration gene/protein expressions in the IHHP+I/R group were observed. Conclusion: IHHP can significantly reduce ischemia/reperfusion injury in rat brain nerves and promote nerve repair.
Keywords: Intermittent hypobaric hypoxia, neuroprotective, cerebral ischemia/reperfusion, rats
Introduction
Stroke is a complex disease with symptoms of cerebral ischemia and hemorrhagic injury induced by transient or permanent cerebrovascular occlusion. It leads to neuronal death or behavioral deficits. Ischemic stroke is a common serious life-threatening medical problem among adults [1]. The high morbidity, disability, and mortality rates induced by ischemic stroke seriously affect the quality of the patient’s life and impose a heavy burden on society and the patients’ families. Unfortunately, current therapies have only a very limited chance of providing a good clinical outcome. Therefore, it is necessary to explore better treatment strategies.
Ischemic tolerance is a strategy developed to provide short-term mild ischemic stimulation or drug treatment to brain tissues in advance to attenuate neuronal damage induced by ischemic injury. In the past several years, research on preconditioning (also known as induction of tolerance) has led to promising clinical outcomes in patients with acute brain injury [2]. Previous studies report that cerebral ischemic preconditioning induces cerebral ischemic tolerance. For example, hydrogen inhalation can decrease neurological damage following cerebral ischemia/reperfusion in mice [3]. The Gualou Guizhi decoction could promote nerve function and neurogenesis after focal cerebral ischemia-reperfusion [4], while ceftriaxone pretreatment can protect neurons against cerebral ischemia-reperfusion injury [5], and 12/15-lipoxygenase metabolites of arachidonic acid play a neuroprotective role in cerebral ischemic injury (CIR) through the activation of peroxisome proliferator-activated receptor subtype γ [6]. Previous studies also found that distal limb ischemic regulation can reduce cerebral ischemic infarct size by enhancing collateral circulation [7]. Intermittent hypobaric hypoxia preconditioning (IHHP) is often used to treat or prevent a variety of cardiovascular and cerebrovascular diseases. Intermittent low-pressure hypoxia preconditioning has a protective effect in CIR by inhibiting the overexpression of nitric oxide synthase (NOS) and nitric oxide (NO) [8]. Intermittent hypoxia induces accumulation of Na+ in myocardial cells, subsequently suppressing Na+/H+ exchange-mediated acid extrusion [9], and activating reactive oxygen species and the gasotransmitter in the carotid artery [10]. It also improves renal vascular hypertension by upregulating NOS expression in the nucleus tractus solitarii [11]. Based on our previous research [12], this study evaluated the neuroprotective effect of IHHP in our cerebral ischemia/reperfusion rat model.
Neuroglobin (Ngb) is a kind of oxygen-carrying globulin, which is mainly expressed in nerve tissues. Ngb works as an oxygen carrier and remover of NO and oxygen-free radicals in cells. Some previous studies have found that Ngb can protect neurons from hypoxic damage [13-16]. Globin is mainly expressed in the brain following hypoxia or cerebral ischemia injury, and it plays a defense role in neuronal system insults [17,18]. Currently, neuroprotection therapy in brain injury patients remains a major challenge due to either ineffective drug treatment or unexpected side effects. Therapies based on the brain’s endogenous mechanisms that allow it to protect itself from harmful stimuli and damage are therefore widely studied. c-Fos is an immediate early gene, which can reflect the activation of neurons and is thus widely used to evaluate neuronal morphologic and functional alterations. The expression of c-Fos can be increased by a variety of physiological and pathological stimulations in the central nervous system [19]. Growth associated protein-43 (GAP-43) is a membrane phosphoprotein, which is involved in nerve development, axon regeneration, and synaptic reconstruction. It can mediate axon extension, change cell morphology, and play an important role in nerve function [20]. Our current study evaluated the neuroprotective effect of IHHP on the CIR model by observing nerve ultrastructure, and determining the expressions of Ngb, c-Fos, GAP-43, and apoptosis-related genes.
Materials and methods
Ethical statement
The study protocol was reviewed and approved by the Institutional Animal Care and Use Committee of Qinghai University, China. This study was conducted strictly in accordance with the recommendations and guidelines for laboratory animal care and use in the National Institutes of Health, China.
Experimental animals
Adult male Sprague-Dawley (SD) rats (weight: 280-300 g; Charles River Animal Center, China) were raised in cages at 23 ± 1.0°C and randomly divided into four groups: Sham, IHHP, I/R, IHHP+I/R.
Sham group
Only the bilateral vertebral arteries of the animals were clip-occluded for 2 days followed by bilateral common carotid artery exposure without blockage.
IHHP group
The rats were housed in a hypoxic environment for 4 days. For the next 4 days, the bilateral vertebral arteries of the rats were occluded once a day. On day 5, permanent bilateral vertebral artery occlusion was performed. After 2 days, the bilateral common carotid arteries were isolated without blockage.
I/R group
The bilateral vertebral arteries were clip-occluded for 2 days. The bilateral common carotid arteries were then blocked for 8 min and resumed blood reperfusion.
IHHP+I/R group
The rats were exposed to a hypoxic environment once a day for 4 consecutive days. The bilateral vertebral arteries were occluded on day 5 for 2 days. The I/R procedure was then performed.
Intermittent low-pressure hypoxia preconditioning
Based on the weight, the rats were housed in a sealed 2500 mL glass bottle mixture with hypoxic fresh air and anhydrous sodium (to absorb water and carbon dioxide). A decompressor was connected to adjust the pressure to 70.66-70.93 kp. IHHP was conducted once daily for 4 consecutive days and stopped immediately when dyspnea occurred in the rat.
Cerebral ischemia/reperfusion model
The rats were anesthetized with 10% chloral hydrate at an intraperitoneal dosage of 0.3 mL/kg. The common carotid, external carotid, and pterygopalatine arteries were identified. The 0.5 mm head of a 0.26 mm nylon monofilament suture was coated with paraffin and marked at a length of 20 mm. All insertions were processed through the incision of the left common carotid artery. The pterygopalatine artery was temporarily ligated to prevent accidental insertion. Depending on the weight, the length of the suture was about 18-20 mm from the bifurcation of the common carotid artery. The skin was sutured, and the nylon suture was fixed to it. The left middle cerebral artery was occluded for 2 h, and then, the suture was carefully withdrawn to allow reperfusion. The sham operation control experienced the same procedure as the experimental group, but the insertion of a nylon suture was excluded. The body temperature of each rat was maintained at 37 ± 0.5°C throughout the procedure. The successful model showed the paralyzed right limb, loss of standing ability, and one side turning when lifting the tail.
Experimental reagents
RIPA lysate (Beyotime, Jiangsu, China); BCA protein concentration determination kit (Beyotime, Jiangsu, China); Enhanced chemiluminescence reagent (Beyotime, Jiangsu, China); PrimeScriptTM RT reagent Kit with gDNA Eraser (Takara, Japan); PVDF membrane (Merck Millipore, United States); SDS-PAGE gel reagent box (Beyotime, Jiangsu, China); rabbit anti- mouse GAP-43 polyclonal antibody (Abcam, China); rabbit anti- mouse C-fos polyclonal antibody (Abcam, China); rabbit anti-mouse Ngb polyclonal antibody (Academy of Military Medical Sciences, China); rabbit anti-mouse of Bcl-2, Caspase-3, BAX, and Caspase-9 polyclonal antibody (Wuhan Boster biotechnology Co., Ltd., China); HRP-labeled goat anti-rabbit secondary antibodies (Abcam, China); Trizol (Takara, Japan); SYBR Premix Ex TaqTM II (Takara, Japan).
Transmission electron microscopy
The experimental animal models in each group were anesthetized by intraperitoneal injection of 10% chloral hydrate based on the weight, and then given transcardial perfusion fixation. Cortical tissue of 1 mm size was removed and immediately fixed in 4% glutaraldehyde for 2 h followed by fixing in 1% perosmic acid for 1 h. The slides were dehydrated with acetone and embedded within Epon812. After localization analysis in semi-thin sections, the slides were prepared to ultrathin sections, stained with uranyl acetate-lead citrate, and observed with a transmission electron microscope.
Nissl staining
The frozen slices were fixed with 4% paraformaldehyde for 20 min and then washed 3 times with distilled water, followed by Nissl staining (Beyotime, C0117) for 10 min, and washing twice with 70% ethanol. The morphological alterations of the Nissl bodies were observed under the microscope.
Western blotting
The cells were treated with 200 μL protein lysate (containing 1 mmol/L PMSF) and placed on ice for 20 min followed by 12000 r/min centrifugation at 4°C for 15 min. The supernatant was collected. After measuring the proteins, their concentrations were adjusted to the same value with BCA. A total of 20 μg of protein sample was loaded in 12% SDS-PAGE gel and then electrophoretically (90 V, 60 min) transferred onto membranes. The membranes were blocked in 5% skim milk at room temperature for 1 h and then incubated with primary antibody (dilution at 1:1500) at 4°C overnight followed by washing three times for 10 min each with TBST. The membranes were then incubated with the secondary antibody (dilution at 1:8000) at room temperature for 1 h and washed thrice with TBST for 10 min each. The relative expressions of the proteins were observed and analyzed using ImageJ software.
Quantitative real-time polymerase chain reaction
The TRIzol reagent was used to isolate total RNA. The RNA concentration was evaluated by a spectrophotometer (A260/280). Reverse transcription was performed using a reverse transcription kit (TAKARA). cDNA was obtained as a template to amplify the target genes and the internal reference GAPDH gene (see Table 1 for the primer sequence list). Each reaction was repeated thrice. The following reaction conditions were maintained: pre-denaturation, 95°C for 10 min; and thermal cycle (45 cycles), 95°C for 12 s, 60°C for 35 s, and 72°C for 30 s. The amplification and the dissolution were checked to confirm the validation of the amplification. The relative expression levels of the genes were calculated by using 2-ΔΔCT method. ΔΔCT = (average CT of the target gene in the test samples - average CT of the reference gene in the test samples) - (average CT of the target gene in the control samples - average CT of the reference gene in the control samples).
Table 1.
Primer sequences
| Gene | Forward primer (5’→3’) | Reverse primer (5’→3’) | Base length (bp) |
|---|---|---|---|
| GAPDH | AGGGCTACTTTTAACTCTGGT | CCCCACTTGATTTTGGAGGGA | 206 |
| Bax | CTGGAAGATGGCTCTGTCTG | GAGCACGACGAGGACAATAAC | 188 |
| Caspase-3 | ATGATATTCCCCCCACTT | CAAAACCATAATCCCAGA | 178 |
Immunohistochemistry (IHC)
Briefly, the dissected dorsal root ganglion tissues were fixed in 4% paraformaldehyde at 4°C for 6 h, put into 30% sucrose at 4°C overnight, and embedded in a paraffin block. The tissue blocks were then cut into slides and deparaffinized for IHC according to the IHC kit protocol. The relative protein expressions of Ngb, c-Fos, and GAP-43 were observed under an inverted microscope.
Statistical analysis
The statistical analyses were conducted with SPSS21.0. Analysis of variance was chosen to compare the differences among multiple groups. Pairwise comparison between two groups was analyzed using the least significant difference t test. Dunnett’s test was performed when the variance of two groups was not equal. P < 0.05 was considered significant. All data are shown as mean ± standard deviation (mean ± SD).
Results
Effects of IHHP on ultrastructure changes of cells in the four groups
As shown in Figure 1, the transmission electron microscope showed the intact nuclei, abundant organelles, smooth endoplasmic reticulum, tubular-shaped mitochondria, complete membranes, and undamaged axon structures in the glial cells and neurons from the Sham and IHHP groups. In contrast, all of these normal structures were heavily destroyed in the glial cells and neurons from the I/R group, representing as unclear or incomplete cell boundaries and damaged or dissolved organelles. Moreover, the number of mitochondria was significantly less, while a large number of autophagolysosomes and autophagosomes were observed. It was found that less damage occurred in the nucleus and cell body. Restored normal organelles and mitochondria as well as decreased autophagolysosomes were apparent in cells from the IHHP+I/R group.
Figure 1.
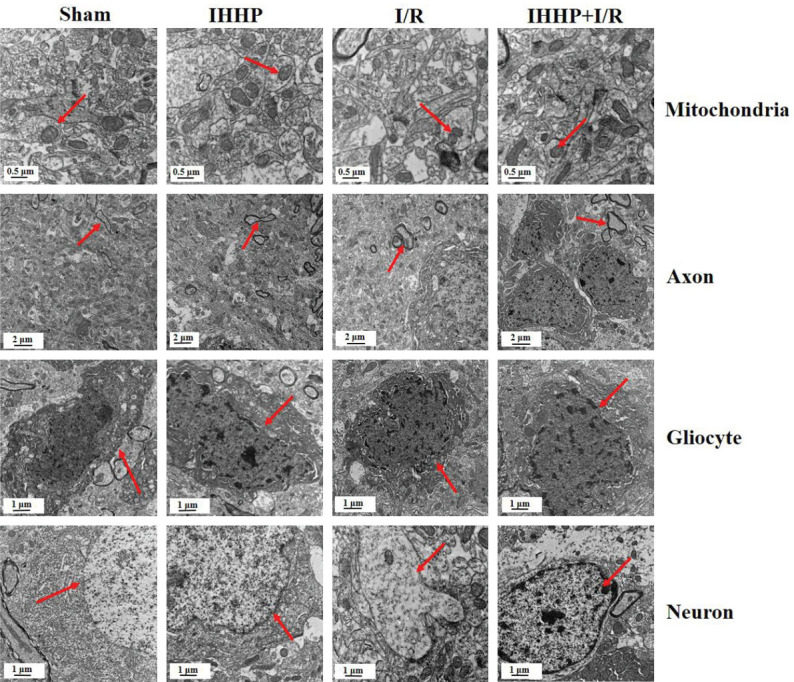
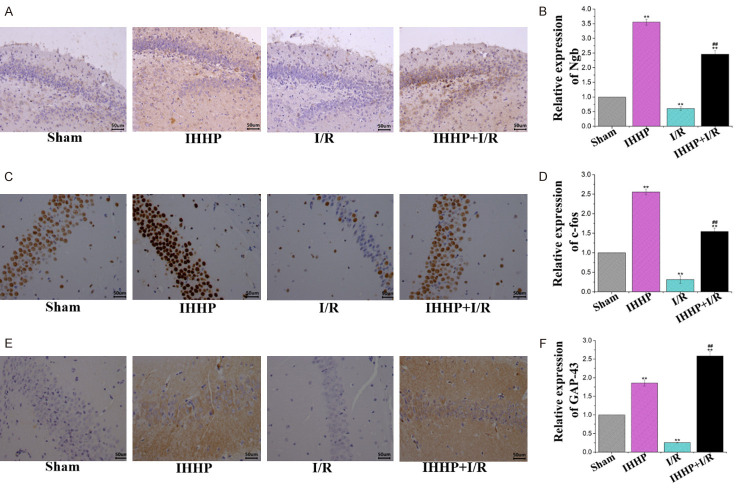
Changes in neural ultrastructures using transmission electron microscopy following IHHP and cerebral ischemia/reperfusion. The red arrows indicate mitochondria, axon, gliocyte and neuron, respectively. In Sham and Intermittent hypobaric hypoxia preconditioning (IHHP) groups, glial cells and neurons were intact, organelles were rich and complete, and mitochondria and axons were structurally intact. In the ischemia/reperfusion (I/R) group, the structures of glial cells and neurons were severely damaged, and the number of mitochondria was significantly reduced. The damage of organelles and mitochondria was decreased in the IHHP+I/R group.
Changes in Nissl bodies and apoptosis-related genes in the four groups
As shown in Figure 2A, Nissl bodies were stained purple blue and nuclei were stained pale blue in the Sham group. Intense staining was obvious in cells with intact morphology in the IHHP group. However, poor Nissl staining was accompanied with significantly reduced pyramidal cells in the ischemia/reperfusion group. The Nissl staining was moderate following IHHP and cerebral ischemia/reperfusion compared to that in the Sham and IHHP groups. The arrangement of the pyramidal cells and cell morphology were significantly improved following IHHP and cerebral ischemia/reperfusion compared to that only underwent the ischemia/reperfusion treatment. As shown in Figure 2B, no significant difference in neuron density was observed between the Sham and IHHP groups (P > 0.05). However, ischemia/reperfusion significantly reduced the density of the surviving neurons (P < 0.05). As expected, this density was restored following IHHP treatment.
Figure 2.
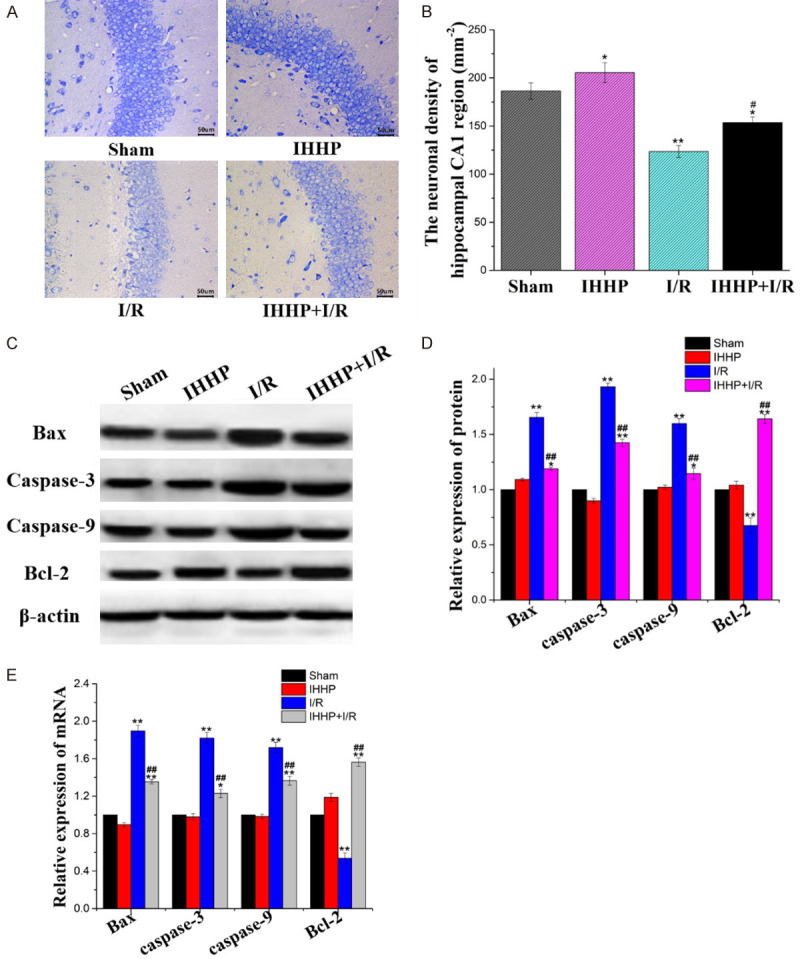
Alleviation of cerebral ischemia/reperfusion injury in rats with intermittent low-pressure hypoxic preconditioning. A. Nissl staining was used to investigate the protective effect of low-pressure hypoxic preconditioning on cerebral ischemia/reperfusion on rat neurons. B. Neuronal density. C, D. The relative expression of Bax, caspase-3, caspase-9, and Bcl-2 were determined by WB. E. The relative expression of Bax, caspase-3, caspase-9, and Bcl-2 were detected by PCR. (*P < 0.05, **P < 0.01 vs. Sham group; #P < 0.05, ##P < 0.01 vs. Ischemia/reperfusion group).
Furthermore, the expressions of the apoptosis-related genes were assessed in all the groups. As shown in Figure 2C-E, the relative expressions of Bax, caspase-3, and caspase-9 in both the protein and mRNA levels displayed no significant difference between the Sham and IHHP groups (P > 0.05). These expressions were elevated in both the I/R and IHHP+I/R groups compared to the Sham and IHHP groups (P < 0.05). However, cells from the IHHP+I/R group demonstrated downregulated expressions compared to those from the I/R group (P < 0.05). Similarly, no statistical difference in Bcl-2 expression was found between the Sham and IHHP groups (P > 0.05). The transcription level was significantly reduced in both the I/R and the IHHP+I/R groups (P < 0.05). The anti-apoptosis ability was stronger following IHHP and cerebral ischemia/reperfusion compared to that of the cells that underwent only the ischemia/reperfusion treatment (P < 0.05).
Relative expressions of Ngb, c-Fos, and GAP-43 in the four groups
As shown in Figure 3A-F, the cells in both the IHHP and the IHHP+I/R groups displayed higher expressions of Ngb, c-fos and GAP-43 protein than those in the Sham group. However, these proteins were very poorly virtualized in the I/R group. Meanwhile, significantly elevated expression levels of these proteins were observed following IHHP and cerebral ischemia/reperfusion treatment compared to those of the cells that underwent only the ischemia/reperfusion treatment (P < 0.05).
Figure 3.
Influence of intermittent hypoxic preconditioning on expression of neuroprotection-related factors. A, B. Relative expression of Ngb, mainly expressed in the perikaryon, cytoplasm, and mitochondria. C, D. Relative expression of c-fos, mainly expressed in the nucleus. E, F. Relative expression of GAP-43 in the major cellular organelles. (*P < 0.05, **P < 0.01 vs. Sham group; #P < 0.05, ##P < 0.01 vs. Ischemia/reperfusion group).
Discussion
Ischemic brain injury involves pathological events [21,22]. Our results showed that the number of glial cells, neurons, and synapses reduced following ischemia/reperfusion treatment compared to those in the sham group. Moreover, the neuronal cell and organelle morphologies were severely damaged and accompanied with considerable dissolution of enzyme bodies and autophagosomes. However, the number of neurons, synapses, and glial cells in the intermittent hypobaric hypoxia preconditioning and ischemia/reperfusion treated group were not significantly decreased, and the cell morphology was not damaged. These results indicate that IHHP can protect rat brain cerebral ischemia/reperfusion-induced neuronal apoptosis and alleviate microstructural damage. The number of mitochondria in the ischemia/reperfusion group was significantly decreased, and the cell structures were broken, expanded, and deformed. However, the number and cell structure of the mitochondria were normal following IHHP treatment, which indicated that impaired mitochondrial function contributes to the occurrence of ischemic-induced brain injury [23]. Furthermore, the results revealed that the expressions of pro-apoptotic factors (Bax, procaspase-3, and procaspase-9) increased, while that of the anti-apoptotic factor BCL-2 decreased. However, these expressions exhibited the opposite trend following IHHP treatment, indicating that IHHP can inhibit the apoptosis of nerve cells and promote cell proliferation. All of the observed results provide evidence that intermittent low-pressure hypoxia pretreatment may maintain the integrity of mitochondria by inhibiting neuronal apoptosis caused by ischemic hypoxia injury. Studies have found that inhibiting the NF-κB signaling pathway can alleviate the nerve damage caused by cerebral ischemia/reperfusion [24]. Regulator of calcineurin 1 (RCAN1) inhibits the NF-κB signaling pathway to reduce nerve cell apoptosis, thereby exerting neuroprotective effects [25]. However, the role of RCAN1 in the mechanism of intermittent hypobaric hypoxia preconditioning in inhibiting neuronal apoptosis caused by ischemia and hypoxia injury, is still unclear.
Nissl bodies are large basophilic clumps and granules in the cell body or dendrites of neurons. They can function as structural proteins and enzymes in the synthesis of renewed organelles, neurotransmitters, and neuromodulators of peptides. Nissl bodies are particularly abundant in functional neurons [26,27]. This study found that the staining color of the Nissl bodies from the IHHP and IHHP+I/R groups was more intense compared to those from the ischemia/reperfusion group. The result demonstrates that the nerve damage in the ischemia/reperfusion group was more serious, causing a decrease in the amount of Nissl bodies, their disintegration, or even their disappearance. However, the IHHP treatment increased the neuron survival rate and motivated vigorous metabolic function, leading to the accumulation and activities of the Nissl bodies in the cells following I/R injury.
Furthermore, the results demonstrated significantly downregulated expression of the Ngb protein following ischemia/reperfusion treatment compared to that in the IHHP and IHHP+I/R groups. Ngb plays an important role in adaptive protection in cerebral ischemia and hypoxia [28], which can elevate oxygen distribution to the nerve cells and upgrade their survival rate and functioning. Moreover, the results of this study showed a significantly decreased expression of the c-Fos protein in the ischemia/reperfusion group. However, this expression was up-regulated in the IHHP+I/R group. Evaluation of c-Fos immunoreactivity is a common method to detect the histologic location of neuron activity [29]. The upregulated expression of this protein in neurons represents enhanced neuron activation [30]. Therefore, the results revealed that IHHP enhanced the activity of neurons by promoting c-Fos expression. Our next experiment also showed that the expression of GAP-43 protein was significantly down-regulated in the ischemia/reperfusion group, but it was up-regulated following IHHP treatment. GAP-43 is an intrinsic determinant of synaptic plasticity and is maintained at high levels during the neuronal development and regeneration processes [31]. This result suggests that IHHP might promote nerve repair by upregulating GAP-43 expression. Together, these results illustrate that IHHP might improve neuronal regeneration and repair ability, and subsequently prevent neuronal death from ischemia/reperfusion injury in rats.
In summary, our current study demonstrated a remarkable neuroprotective effect of IHHP on I/R injury by promoting neuronal regeneration and repair ability. Moreover, IHHP stimulated axonal regeneration to protect neurons against I/R injury.
Conclusion
IHHP can improve neurological regeneration and repair ability, and subsequently attenuate the neurological deficit induced by cerebral ischemia reperfusion. The current study gives theoretical evidence for the future application of IHHP in clinical practice.
Acknowledgements
This work was mainly supported by the Qinghai Fundamental Scientific and Technological Research Plan (2018-ZJ-720), Natural Science Foundation of China (81860762), and the Qinghai Fundamental Scientific and Technological Research Plan (2018-ZJ-721).
Disclosure of conflict of interest
None.
References
- 1.Long FY, Shi MQ, Zhou HJ, Liu DL, Sang N, Du JR. Klotho upregulation contributes to the neuroprotection of ligustilide against cerebral ischemic injury in mice. Eur J Pharmacol. 2018;820:198–205. doi: 10.1016/j.ejphar.2017.12.019. [DOI] [PubMed] [Google Scholar]
- 2.Dirnagl U, Becker K, Meisel A. Preconditioning and tolerance against cerebral ischaemia: from experimental strategies to clinical use. Lancet Neurol. 2009;8:398–412. doi: 10.1016/S1474-4422(09)70054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang JL, Liu WW, Sun XJ. Hydrogen inhalation improves mouse neurological outcomes after cerebral ischemia/reperfusion independent of anti-necroptosis. Med Gas Res. 2018;8:1–5. doi: 10.4103/2045-9912.229596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han J, Zhang JZ, Zhong ZF, Li ZF, Pang WS, Hu J, Chen LD. Gualou Guizhi decoction promotes neurological functional recovery and neurogenesis following focal cerebral ischemia/reperfusion. Neural Regen Res. 2018;13:1408–1416. doi: 10.4103/1673-5374.235296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sundararajan S, Gamboa JL, Victor NA, Wanderi EW, Lust WD, Landreth GE. Peroxisome proliferator-activated receptor-gamma ligands reduce inflammation and infarction size in transient focal ischemia. Neuroscience. 2005;130:685–696. doi: 10.1016/j.neuroscience.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 6.Sun L, Xu YW, Han J, Liang H, Wang N, Cheng Y. 12/15-Lipoxygenase metabolites of arachidonic acid activate PPARγ: a possible neuroprotective effect in ischemic brain. J Lipid Res. 2015;56:502–514. doi: 10.1194/jlr.M053058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kitagawa K, Saitoh M, Ishizuka K, Shimizu S. Remote limb ischemic conditioning during cerebral ischemia reduces infarct size through enhanced collateral circulation in murine focal cerebral ischemia. J Stroke Cerebrovasc Dis. 2018;27:831–838. doi: 10.1016/j.jstrokecerebrovasdis.2017.09.068. [DOI] [PubMed] [Google Scholar]
- 8.Huang YJ, Yuan YJ, Liu YX, Zhang MY, Zhang JG, Wang TC, Zhang LN, Hu YY, Li L, Xian XH, Qi J, Zhang M. Nitric oxide participates in the brain ischemic tolerance induced by intermittent hypobaric hypoxia in the hippocampal CA1 subfield in rats. Neurochem Res. 2018;43:1779–1790. doi: 10.1007/s11064-018-2593-9. [DOI] [PubMed] [Google Scholar]
- 9.Chang HR, Lien CF, Jeng JR, Hsieh JC, Chang CW, Lin JH, Yang KT. Intermittent hypoxia inhibits Na+-H+ exchange-mediated acid extrusion via intracellular Na+ accumulation in cardiomyocytes. Cell Physiol Biochem. 2018;46:1252–1262. doi: 10.1159/000489076. [DOI] [PubMed] [Google Scholar]
- 10.Prabhakar NR, Peng YJ, Yuan GX, Nanduri J. Reactive oxygen radicals and gaseous transmitters in carotid body activation by intermittent hypoxia. Cell Tissue Res. 2018;372:427–431. doi: 10.1007/s00441-018-2807-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li N, Guan Y, Tian YM, Ma HJ, Zhang XJ, Zhang Y, Wang S. Chronic intermittent hypobaric hypoxia ameliorates renal vascular hypertension through up-regulating NOS in nucleus tractus solitarii. Neurosci Bull. 2019;35:79–90. doi: 10.1007/s12264-018-00330-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu Q, Yu KX, Ma QS, Liu YN. Effects of intermittent hypobaric hypoxia preconditioning on the expression of neuroglobin and Bcl-2 in the rat hippocampal CA1 area following ischemia-reperfusion. Genet Mol Res. 2015;14:10799–10807. doi: 10.4238/2015.September.9.18. [DOI] [PubMed] [Google Scholar]
- 13.Sun Y, Jin K, Peel A, Mao X, Xie L, Greenberg DA. Neuroglobin protects the brain from experimental stroke in vivo. Proc Natl Acad Sci U S A. 2003;100:3497–3500. doi: 10.1073/pnas.0637726100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun YJ, Jin K, Peel A, Mao XO, Xie L, Greenberg DA. Neuroglobin is up-regulated by and protects neurons from hypoxic-ischemic injury. Proc Natl Acad Sci U S A. 2001;98:15306–15311. doi: 10.1073/pnas.251466698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fordel E, Thijs L, Moens L, Dewilde S. Neuroglobin and cytoglobin expression in mice: evidence for a correlation with reactive oxygen species scavenging. FEBS J. 2007;274:1312–1317. doi: 10.1111/j.1742-4658.2007.05679.x. [DOI] [PubMed] [Google Scholar]
- 16.Duong TT, Antao S, Ellis NA, Myers SJ, Witting PK. Supplementation with a synthetic polyphenol limits oxidative stress and enhances neuronal cell viability in response to hypoxia-re-oxygenation injury. Brain Res. 2008;1219:8–18. doi: 10.1016/j.brainres.2008.04.044. [DOI] [PubMed] [Google Scholar]
- 17.Khan AA, Wang Y, Sun Y, Mao X, Xie L, Miles E, Graboski J, Chen S, Ellerby LM, Jin K, Greenberg DA. Neuroglobin-overexpressing transgenic mice are resistant to cerebral and myocardial ischemia. Proc Natl Acad Sci U S A. 2006;103:17944–17948. doi: 10.1073/pnas.0607497103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sigonre AV, Paijmans JLA, Hofreiter M, Fago A, Weber RE, Springer MS, Campbell KL. Emergence of a chimeric globin pseudogene and increased hemoglobin oxygen affinity underlie the evolution of aquatic specializations in sirenia. Mol Biol Evol. 2019;36:1134–1147. doi: 10.1093/molbev/msz044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coppola VJ, Bingman VP. c-Fos revealed lower hippocampal participation in older homing pigeons when challenged with a spatial memory task. Neurobiol Aging. 2020;87:98–107. doi: 10.1016/j.neurobiolaging.2019.11.019. [DOI] [PubMed] [Google Scholar]
- 20.Zhang L, Wang ZJ, Li B, Xia ZW, Wang X, Xiu YC, Zhang Z, Chen CJ, Song H, Li WH, Yu M, Zhang ML, Wang K, Guo XL, Ren LQ, Wang TY. The inhibition of miR-17-5p promotes cortical neuron neurite growth via STAT3/GAP-43 pathway. Mol Biol Rep. 2020;47:1795–1802. doi: 10.1007/s11033-020-05273-1. [DOI] [PubMed] [Google Scholar]
- 21.Jia LJ, Wang JW, Cao HM, Zhang XY, Rong WF, Xu ZF. Activation of PGC-1α and mitochondrial biogenesis protects against prenatal hypoxic-ischemic brain injury. Neuroscience. 2020;432:63–72. doi: 10.1016/j.neuroscience.2020.02.035. [DOI] [PubMed] [Google Scholar]
- 22.Li ZG, Shui SF, Han XW, Yan L. NLRP10 ablation protects against ischemia/reperfusion-associated brain injury by suppression of neuroinflammation. Exp Cell Res. 2020;389:111912. doi: 10.1016/j.yexcr.2020.111912. [DOI] [PubMed] [Google Scholar]
- 23.Nichols M, Pavlov EV, Robertson GS. Tamoxifen-induced knockdown of the mitochondrial calcium uniporter in Thy1-expressing neurons protects mice from hypoxic/ischemic brain injury. Cell Death Dis. 2018;9:606. doi: 10.1038/s41419-018-0607-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao JM, Chen NN, Li N, Xu F, Wang W, Lei YY, Shi JS, Gong QH. Neuroprotective effects of trilobatin, a novel naturally occurring sirt3 agonist from lithocarpus polystachyus Rehd. , mitigate cerebral ischemia/reperfusion injury: involvement of TLR4/NF-κB and Nrf2/Keap-1 signaling. Antioxid Redox Signal. 2020;33:117–143. doi: 10.1089/ars.2019.7825. [DOI] [PubMed] [Google Scholar]
- 25.Yun Y, Zhang Y, Zhang C, Huang L, Tan S, Wang P, Vilariño-Gúell C, Song W, Sun X. Regulator of calcineurin 1 is a novel RNA-binding protein to regulate neuronal apoptosis. Mol Psychiatry. 2019 doi: 10.1038/s41380-019-0487-0. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 26.Dziewulska D, Gogol A, Gogol P, Rafalowska J. Enlargement of the Nissl substance as a manifestation of early damage to spinal cord motoneurons in amyotrophic lateral sclerosis. Clin Neuropathol. 2013;32:480–485. doi: 10.5414/NP300623. [DOI] [PubMed] [Google Scholar]
- 27.Toma VA, Farcas AD, Parvu M, Silaghi DR, Roman I. CA3 hippocampal field: cellular changes and its relation with blood nitro-oxidative stress reveal a balancing function of CA3 area in rats exposed to repetead restraint stress. Brain Res Bull. 2016;130:10–17. doi: 10.1016/j.brainresbull.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 28.Ding CY, Kang DZ, Chen PQ, Wang ZL, Lin YX, Wang DL, Lin ZY, Gu JJ. Early stage neuroglobin level as a predictor of delayed cerebral ischemia in patients with aneurysmal subarachnoid hemorrhage. Brain Behav. 2020;10:e01547. doi: 10.1002/brb3.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tóth Z, Mihály A, Mátyás A, Péva BK. Non-competitive antagonists of NMDA and AMPA receptors decrease seizure-induced c-fos protein expression in the cerebellum and protect against seizure symptoms in adult rats. Acta Histochem. 2018;120:236–241. doi: 10.1016/j.acthis.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 30.Mayer U, Watanabe S, Bischof HJ. Hippocampal activation of immediate early genes Zenk and c-Fos in zebra finches (Taeniopygia guttata) during learning and recall of a spatial memory task. Neurobiol Learn Mem. 2010;93:322–329. doi: 10.1016/j.nlm.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 31.Wang ZJ, Yuan WQ, Li B, Chen XM, Zhang YJ, Chen CJ, Yu M, Xiu YC, Li WH, Cao JG, Wang X, Tao W, Guo XL, Feng SQ, Wang TY. PEITC promotes neurite growth in primary sensory neurons via the miR-17-5p/STAT3/GAP-43 axis. J Drug Target. 2019;27:82–93. doi: 10.1080/1061186X.2018.1486405. [DOI] [PubMed] [Google Scholar]