Abstract
We retrospectively analyzed preoperative serum CA153, carcinoembryonic antigen (CEA) and white blood cells (WBC) in 121 breast cancer patients who underwent breast-conserving therapy and sentinel lymph node biopsy (SLNB) between June 2017 and April 2019 in our institution. The receiver operating characteristic curve (ROC curve) was used to determine the optional cut-offs of these biomarkers for predicting SLN metastasis. The relationship between the parameters to SLN metastasis of breast cancer was assessed by univariate analysis and multivariate logistic regression models. We finally enrolled 121 breast cancer patients who all underwent SLNB, of whom 56 were confirmed as positive SLN by histopathology. ROC curve analysis calculated an ideal CA153 cutoff value of 7.85 U/ml in prediction of SLN metastasis, with a sensitivity of 73.2%, and specificity of 67.7%. The ideal cutoff value for CEA to predict SLN metastasis was 1.66 ng/ml (sensitivity 67.9%, specificity 73.8%). CA153 combined with CEA showed specificity 78.6% and specificity 76.9%. CA153 and CEA combined with WBC presented sensitivity 80.4% and specificity 78.5%. In the multivariate logistic regression analysis, CA153 (odds ratio (OR): 1.165, 95% confidence interval (CI) 1.061-1.279, P<0.001) and CEA (OR: 3.440, 95% CI: 1.859-6.366, P<0.001) were independent predictive factors of SLN metastasis in patients with breast cancer.
Keywords: Breast cancer, CA153, CEA, WBC, SLN metastasis
Introduction
Breast cancer is the most common cancer among women in the world [1]. Sentinel lymph nodes (SLN) are the first lymph nodes to be reached by primary tumor metastasis. SLN metastasis is related to the stage and prognosis of breast cancer, and is also an important factor in the selection of surgical methods [2]. Sentinel lymph node biopsy (SLNB) which has become the standard of nodal stage care in early breast cancer patients, is an important method for clinical evaluation of axillary lymph node metastasis [3]. SLNB can exempt some patients from axillary lymph node dissection and reduce the complications of axillary lymph node dissection, for example limb lymph edema and nerve injuries. However, SLNB also has some deficiencies. First, it is an invasive procedure that can lead to vital complications, such as shoulder dysfunction, nerve damage, and lymphedema, which can significantly affect the patient’s quality of life [4,5]. Moreover, SLNB showed a false negative rate of 5.5-16.7% [6]. Third, SLNB is related to some factors, such as dye injection sites and level of experience of the operator. Therefore, it is valuable to develop a preoperative noninvasive method for predicting SLN metastasis in breast cancer.
Serum markers are used as risk factors for metastasis, prognosis, and therapeutic effectiveness in malignant tumors [6-8]. Serum markers are easier to obtain and less invasive than biopsy or surgical tissue samples. Cancer antigen 15-3 (CA153) and carcinoembryonic antigen (CEA) are both tumor-associated antigens. They are the most used and recommended tumor markers in breast cancer [9,10]. CA153 and CEA have been evaluated in the diagnosis and prognosis of patients with breast cancer [9,11,12]. They are also studied as tumor markers for predicting lymph node metastasis of gastric cancer [8]. Recently, Li et al. reported the value of CA153 and CEA in predicting the survival of different breast cancer molecular subtypes [13]. Nonetheless, little is known about the association of tumor markers (CA153 and CEA) in predicting SLN metastasis of breast cancer. Moreover, studies [14-16] have shown that the white blood cell (WBC) count is a predictor of cancer mortality and postmenopausal women with higher WBC counts have a higher risk of breast and other cancers. The relationship between WBC count and sentinel lymph nodes in breast cancer is unknown.
In this study, we aim to explore single or combinations of preoperative CA153, CEA, and WBC for the prediction of breast cancer SLN metastasis.
Methods
This is a retrospective study approved by the Institutional Ethical Board of West China Hospital of Sichuan University and the requirement for informed consent was waived.
Patients and data collection
We retrospectively reviewed consecutive 211 breast cancer patients who underwent breast-conserving therapy and SLNB between June 2017 and April 2019 in our institution. All the operations were performed by experienced surgeons and postoperative pathological results were determined by two experienced pathologists in our center. Our inclusion criteria were (1) histologically confirmed invasive breast cancer; (2) definite SLN state after operation; (3) no radiotherapy or chemotherapy before operation; (4) serum CA153, CEA and WBC count were tested within 2 weeks before surgery. Exclusion criteria included the following: (1) stage III and IV breast cancer; (2) incomplete clinicopathologic data; (3) combination with other tumors. Pathologic diagnosis is the gold standard for the diagnosis of breast cancer and SLN state. Eventually, we enrolled a total of 121 clinical stage I and II breast cancer patients in this study.
We collected patients’ clinical and histopathologic data which included age, tumor size, CA153 level, CEA level, WBC count, estrogen receptor [ER], progesterone receptor [PR], human epidermal growth factor receptor-2 [HER2], proliferation marker Ki-67 and SLD statue (SLN with macrometastasis or micrometastasis was considered positive).
Statistical analysis
Characteristics of the patients were expressed with frequency or descriptive analysis. Continuous variables were presented as means ± standard deviation and were analyzed using the Student t test. The receiver operating characteristic (ROC) curves were plotted and the areas under the curve (AUC) were calculated to determine the suitable cut-off value for predicting the SLN metastatic. We calculated the sensitivity and specificity of CA153, CEA and WBC according to the cut-off value. Univariate and multivariate logistic regression were performed to identify independent factors for SLN metastasis. The P value <0.05 was deemed significant for all analyses. The statistical analysis was performed using the SPSS version 25.0 (IBM, Armonk, NY, USA).
Results
Patients’ characteristics
We enrolled a total of 121 female patients with breast cancer, including 56 SLN positive and 65 SLN negative. These patients all underwent breast-conserving therapy and SLNB. The average age at surgery was 47.9 years old (range: 23-91 years old), of whom, 70 (57.9%) were >45 years old. According to the 2013 St. Gallen guidelines [17], of these patients, twenty-two (18.2%) patients were pathologically diagnosed with luminal A, forty-nine (40.5%) were luminal B, thirty-three (27.3%) were HER2-enriched, and seventeen (14.0%) were basal-like breast cancer. Clinicopathological characteristics of the 121 patients are shown (Table 1). No significant differences were found in age, proliferation index (Ki-67) and molecular subtypes between the two groups.
Table 1.
Patient characteristics
| Characteristic | Patients (n=121) |
|---|---|
| Agea (years) | 47.9±12.8 (23-91) |
| Tumor sizea (cm) | 2.1±1.1 (0.5-5.0) |
| Molecular subtype | |
| Luminal A | 22 |
| Luminal B | 49 |
| HER2 positive | 33 |
| Triple negative | 17 |
| Maximum tumor diameter (cm) | |
| ≤2 | 78 |
| >2 but ≤5 | 42 |
| >5 | 1 |
| CA153a (ng/ml) | 9.92±5.29 (3.33-26.29) |
| CEAa (U/ml) | 1.78±1.55 (0.35-10.33) |
| WBCa (109/L) | 5.89±1.65 (3.07-11.65) |
CA153 cancer antigen 15-3, CEA carcinoembryonic antigen, WBC white blood cell.
Parameters are presented as median, Standard Deviation, and range.
ROC analysis
ROC analysis predicted SLN metastasis of breast cancer (Figure 1 and Table 2). We calculated AUC and cut-off value. The AUC (Table 2) of CA153; CEA; WB; CA153 and CEA; Combination of WBC, CA153 and CEA was 0.725 (CI 0.634-0.816, P<0.001), 0.741 (CI 0.652-0.831, P<0.001), 0.53 (CI 0.505-0.711, P=0.042), 0.789 (CI 0.704-0.874, P<0.001), 0.806 (CI 0.725-0.888, P<0.001) respectively. The cut-off value of CA153 was 7.85 U/ml which generated a sensitivity of 73.2%, specificity of 67.7%. The CEA cut-off value of 1.66 ng/ml generated a sensitivity of 67.9%, specificity of 73.8% while WBC was 7.51 109/L showed a sensitivity of 30.4%, a specificity of 95.4%. The combination of CEA and CA153 resulted in a sensitivity of 78.6% and specificity of 76.9%. The combination of all three markers resulted in a sensitivity of 80.4% and specificity of 78.5% for prediction of SLN metastasis.
Figure 1.
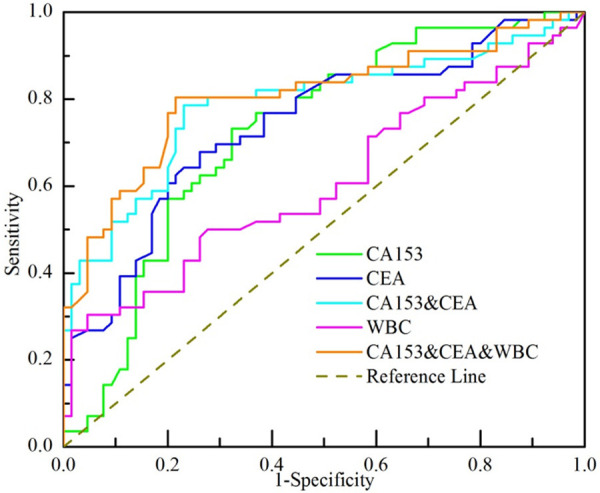
Receiver operating characteristic (ROC) curve analysis of different markers to predict the SLN metastatic status.
Table 2.
Area under the curve
| Preoperative Factor | Area | Std. Errora | P | 95% CI | |
|---|---|---|---|---|---|
|
| |||||
| Lower Bound | Lower Bound | ||||
| CA153 | 0.725 | 0.046 | <0.001 | 0.634 | 0.816 |
| CEA | 0.741 | 0.046 | <0.001 | 0.652 | 0.831 |
| CA153 and CEA | 0.789 | 0.043 | <0.001 | 0.704 | 0.874 |
| WBC | 0.608 | 0.053 | 0.042 | 0.505 | 0.711 |
| CA153 and CEA and WBC | 0.806 | 0.041 | <0.001 | 0.725 | 0.888 |
Under the nonparametric assumption.
Univariate and multivariate analysis the correlation of preoperative factors with SLN metastasis
Univariate and multivariate analysis for SLN metastasis prediction in breast cancer patients was presented (Table 3). In the univariate analysis, CA153, CEA, WBC, tumor size and hormone receptor state were significant predictors for SLN metastasis. In the multivariate logistic regression analysis, CA153 (OR: 1.165, 95% CI: 1.061-1.279, P<0.001), CEA (OR: 3.440, 95% CI: 1.859-6.366, P<0.001) and WBC (OR: 1.475, 95% CI: 1.077-2.022, P<0.001), these three factors remained independent predictors.
Table 3.
Univariate and multivariate analysis of SLN multivariate patients
| Preoperative Factors | Univariate | Multivariate | P | OR | 95% CI for OR | |
|---|---|---|---|---|---|---|
|
|
|
|||||
| p | Wald | Lower | Upper | |||
| Age | 0.188 | NA | NA | NA | NA | NA |
| CA153 | 0.000 | 10.289 | 0.001 | 1.165 | 1.061 | 1.279 |
| CEA | 0.000 | 15.482 | <0.001 | 3.440 | 1.859 | 6.366 |
| Tumor size | 0.030 | 0.533 | 0.465 | 1.181 | 0.756 | 2.022 |
| WBC | 0.009 | 5.855 | 0.016 | 1.475 | 1.077 | 2.022 |
| HER2 | 0.221 | NA | NA | NA | NA | NA |
| ER | 0.032 | NA | NA | NA | NA | NA |
| PR | 0.010 | NA | NA | NA | NA | NA |
| Ki-67 | 0.442 | 0.001 | 0.977 | 0.957 | 0.046 | 19.771 |
| Molecular subtypes | 0.768 | 0.087 | 0.768 | 1.083 | 0.637 | 1.842 |
NA, not available.
Discussion
Breast cancer is the most common malignant tumor and the second leading cause of cancer-related mortality in women [1,18]. Early breast cancer is considered a curable disease while metastatic breast cancer is incurable with currently practicable therapeutic regimens. Currently, SLNB has been used for some breast cancer patients as a routine medical service. It protects patients from many of the side effects of axillary lymph node dissection. However, SLNB still can cause lymphedema, upper limb pain, numbness and dye allergy in a small number of patients [20]. Whether SLNB can be avoided or replaced by other more suitable methods is a great challenge. In this study, we systematically assessed the ability of the role of CA153, CEA, and WBC alone and in combination for predicting SLN metastasis in breast cancer.
The collection of serum specimens is relatively noninvasive, easily-accessible and cost-effective in patient care. Serum biomarkers play an important role in many malignances. CA153, a specific solute transmembrane glycoprotein [21], has been used as serum biomarker for breast cancer since 1980s. CEA, a tumor-associated antigen, is widely used as a serum tumor marker for breast cancer and other malignancy. Both of them are FDA-approved for monitoring breast cancer [22]. Previously many studies [23-25] suggested CA153 and CEA can be applied for early detection of breast cancer metastasis. Moreover, CEA is inferior to CA153. Our study showed CA153 and CEA for the prediction of SLN metastasis were similar and the sensitivity and specificity of CA153 were 73.2% and 67.7%, respectively, and CEA had 67.9% and 73.8%, respectively. When the two tumor markers were combined, the sensitivity and specificity were slightly improved. Wang et al. [25] found that the sensitivity of CA153 in the diagnosis of metastatic breast cancer was 44.5%, and that of CEA was 56.7%. Petra et al. [23] showed that the sensitivity of CA153 and CEA alone in the detection of metastatic breast cancer was 55.6% and 40.6% respectively, and the sensitivity could be increased to 66.3% when the two were combined. This is consistent with our findings.
Previous studies [14-16] have linked higher WBC count to an increased risk of breast cancer and other cancers in postmenopausal women. However, little is known about the value of WBC in predicting SLN metastasis. Routine blood sampling is the most common test for admission, so we also include WBC count. Our study suggested that WBC has statistical significance and it has a surprising sensitivity of up to 95.4%, but the specificity is low. The reason for this result needs further exploration.
There are some limitations to our study. This was a single-center retrospective study. Moreover, we collected onlydata from patients who underwent SLNB in our center; small sample size may cause selection bias which can influence the representativeness. Therefore, further prospective multi-center studies with larger sample sizes are warranted to better understand the relationship between multiple clinical factors and sentinel lymph node metastasis in breast cancer.
In conclusion, our study determined that preoperative serum CA153, CEA, and WBC can be a favorable, cost-effective method for predicting SLN metastasis in breast cancer.
Acknowledgements
We acknowledge the whole staff of the Department of Breast Surgery, West China Hospital, who generously provided assistance in the collection of data throughout the duration of the study. This study has received funding by Key Research and Development Project of Sichuan Province (2017SZ0065).
Disclosure of conflict of interest
None.
References
- 1.DeSantis CE, Ma J, Gaudet MM, Newman LA, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Breast cancer statistics, 2019. CA Cancer J Clin. 2019;69:438–451. doi: 10.3322/caac.21583. [DOI] [PubMed] [Google Scholar]
- 2.Lyman GH, Somerfield MR, Bosserman LD, Perkins CL, Weaver DL, Giuliano AE. Sentinel lymph node biopsy for patients with early-stage breast cancer: american society of clinical oncology clinical practice guideline update. J. Clin. Oncol. 2017;35:561–564. doi: 10.1200/JCO.2016.71.0947. [DOI] [PubMed] [Google Scholar]
- 3.Esposito E, Di Micco R, Gentilini OD. Sentinel node biopsy in early breast cancer. A review on recent and ongoing randomized trials. Breast. 2017;36:14–19. doi: 10.1016/j.breast.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Manca G, Rubello D, Tardelli E, Giammarile F, Mazzarri S, Boni G, Chondrogiannis S, Marzola MC, Chiacchio S, Ghilli M, Roncella M, Volterrani D, Colletti PM. Sentinel lymph node biopsy in breast cancer: indications, contraindications, and controversies. Clin Nucl Med. 2016;41:126–33. doi: 10.1097/RLU.0000000000000985. [DOI] [PubMed] [Google Scholar]
- 5.Canavese G, Bruzzi P, Catturich A, Tomei D, Carli F, Garrone E, Spinaci S, Lacopo F, Tinterri C, Dozin B. Sentinel lymph node biopsy versus axillary dissection in node-negative early-stage breast cancer: 15-year follow-up update of a randomized clinical trial. Ann Surg Oncol. 2016;23:2494–500. doi: 10.1245/s10434-016-5177-4. [DOI] [PubMed] [Google Scholar]
- 6.Ni XG, Bai XF, Mao YL, Shao YF, Wu JX, Shan Y, Wang CF, Wang J, Tian YT, Liu Q, Xu DK, Zhao P. The clinical value of serum CEA, CA19-9, and CA242 in the diagnosis and prognosis of pancreatic cancer. Eur J Surg Oncol. 2005;31:164–9. doi: 10.1016/j.ejso.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 7.Wang K, Jiang X, Ren Y, Ma Z, Cheng X, Li F, Xiao J, Yu Z, Jiao Z. The significance of preoperative serum carcinoembryonic antigen levels in the prediction of lymph node metastasis and prognosis in locally advanced gastric cancer: a retrospective analysis. BMC Gastroenterol. 2020;20:100. doi: 10.1186/s12876-020-01255-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li F, Li S, Wei L, Liang X, Zhang H, Liu J. The correlation between pre-operative serum tumor markers and lymph node metastasis in gastric cancer patients undergoing curative treatment. Biomarkers. 2013;18:632–7. doi: 10.3109/1354750X.2013.840800. [DOI] [PubMed] [Google Scholar]
- 9.Li X, Dai D, Chen B, Tang H, Xie X, Wei W. Clinicopathological and prognostic significance of cancer antigen 15-3 and carcinoembryonic antigen in breast cancer: a meta-analysis including 12,993 patients. Dis Markers. 2018;2018:9863092. doi: 10.1155/2018/9863092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu SG, He ZY, Zhou J, Sun JY, Li FY, Lin Q, Guo L, Lin HX. Serum levels of CEA and CA153 in different molecular subtypes and prognostic value in Chinese breast cancer. Breast. 2014;23:88–93. doi: 10.1016/j.breast.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Dai D, Chen B, Tang H, Wang B, Zhao Z, Xie X, Wei W. Nomograms for predicting the prognostic value of pre-therapeutic CA153 and CEA serum levels in TNBC patients. PLoS One. 2016;11:e0161902. doi: 10.1371/journal.pone.0161902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang W, Xu X, Tian B, Wang Y, Du L, Sun T, Shi Y, Zhao X, Jing J. The diagnostic value of serum tumor markers CEA, CA19-9, CA125, CA153, and TPS in metastatic breast cancer. Clin Chim Acta. 2017;470:51–55. doi: 10.1016/j.cca.2017.04.023. [DOI] [PubMed] [Google Scholar]
- 13.Li J, Liu L, Feng Z, Wang X, Huang Y, Dai H, Zhang L, Song F, Wang D, Zhang P, Ma B, Li H, Zheng H, Song F, Chen K. Tumor markers CA153, CA125, CEA and breast cancer survival by molecular subtype: a cohort study. Breast Cancer. 2020;27:621–630. doi: 10.1007/s12282-020-01058-3. [DOI] [PubMed] [Google Scholar]
- 14.Shankar A, Mitchell P, Rochtchina E, Wang JJ. The association between circulating white blood cell count, triglyceride level and cardiovascular and all-cause mortality: population-based cohort study. Atherosclerosis. 2007;192:177–83. doi: 10.1016/j.atherosclerosis.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 15.Erlinger TP, Muntner P, Helzlsouer KJ. WBC count and the risk of cancer mortality in a national sample of U. S. adults: results from the Second National Health and Nutrition Examination Survey mortality study. Cancer Epidemiol Biomarkers Prev. 2004;13:1052–6. [PubMed] [Google Scholar]
- 16.Rubin R. White blood cells might provide clues to breast cancer risk. JAMA. 2020;323:1123. doi: 10.1001/jama.2020.2457. [DOI] [PubMed] [Google Scholar]
- 17.Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thürlimann B, Senn HJ Panel members. Personalizing the treatment of women with early breast cancer: highlights of the St gallen international expert consensus on the primary therapy of early breast cancer 2013. Ann Oncol. 2013;24:2206–23. doi: 10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 19.Giuliano AE, Ballman KV, McCall L, Beitsch PD, Brennan MB, Kelemen PR, Ollila DW, Hansen NM, Whitworth PW, Blumencranz PW, Leitch AM, Saha S, Hunt KK, Morrow M. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: the ACOSOG Z0011 (Alliance) randomized clinical trial. JAMA. 2017;318:918–926. doi: 10.1001/jama.2017.11470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Killelea BK, Long JB, Dang W, Mougalian SS, Evans SB, Gross CP, Wang SY. Associations between sentinel lymph node biopsy and complications for patients with ductal carcinoma in situ. Ann Surg Oncol. 2018;25:1521–1529. doi: 10.1245/s10434-018-6410-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Darwish IA, Wani TA, Khalil NY, Blake DA. Novel automated flow-based immunosensor for real-time measurement of the breast cancer biomarker CA153 in serum. Talanta. 2012;97:499–504. doi: 10.1016/j.talanta.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 22.Uehara M, Kinoshita T, Hojo T, Akashi-Tanaka S, Iwamoto E, Fukutomi T. Long-term prognostic study of carcinoembryonic antigen (CEA) and carbohydrate antigen 15-3 (CA 15-3) in breast cancer. Int J Clin Oncol. 2008;13:447–51. doi: 10.1007/s10147-008-0773-3. [DOI] [PubMed] [Google Scholar]
- 23.Stieber P, Nagel D, Blankenburg I, Heinemann V, Untch M, Bauerfeind I, Di Gioia D. Diagnostic efficacy of CA 15-3 and CEA in the early detection of metastatic breast cancer-A retrospective analysis of kinetics on 743 breast cancer patients. Clin Chim Acta. 2015;448:228–31. doi: 10.1016/j.cca.2015.06.022. [DOI] [PubMed] [Google Scholar]
- 24.Maccio G, Goussot V, Berriolo-Riedinger A, Riedinger JM. Clinical value of CEA for detection of distant metastases in newly diagnosed breast cancer: comparison with CA 15-3. Ann Biol Clin (Paris) 2017;75:431–441. doi: 10.1684/abc.2017.1258. [DOI] [PubMed] [Google Scholar]
- 25.Wang W, Xu X, Tian B, Wang Y, Du L, Sun T, Shi Y, Zhao X, Jing J. The diagnostic value of serum tumor markers CEA, CA19-9, CA125, CA153, and TPS in metastatic breast cancer. Clin Chim Acta. 2017;470:51–55. doi: 10.1016/j.cca.2017.04.023. [DOI] [PubMed] [Google Scholar]


