Abstract
Background
Despite putative benefits associated with proton radiotherapy in the setting of CNS tumors, numerous barriers limit treatment accessibility. Given these challenges, we explored the association of proton use with variations in treatment timing.
Methods
Pediatric and adult patients with histologically confirmed CNS tumors were identified from the National Cancer Database (2004-2015). Univariable and multivariable regression models were constructed to assess factors impacting radiation timing. Multivariable Cox regression was used to evaluate the effect of treatment delay on survival.
Results
A total of 76 157 patients received photon or proton radiotherapy. Compared to photons, time to proton administration was longer in multiple pediatric (embryonal, ependymal, nonependymal glial, and other) and adult (ependymal, nonependymal glial, meningeal, other) tumor histologies. On adjusted analysis, proton radiotherapy was associated with longer delays in radiotherapy administration in pediatric embryonal tumors (+3.00 weeks, P = .024) and in all adult tumors (embryonal [+1.36 weeks, P = .018], ependymal [+3.15 weeks, P < .001], germ cell [+2.65 weeks, P = .024], glial [+2.15 weeks, P < .001], meningeal [+5.05 weeks, P < .001], and other [+3.06 weeks, P < .001]). In patients with high-risk tumors receiving protons, delays in adjuvant radiotherapy were independently associated with poorer survival (continuous [weeks], adjusted hazard ratio = 1.09, 95% CI = 1.02-1.16).
Conclusions
Proton radiotherapy is associated with later radiation initiation in pediatric and adult patients with CNS tumors. In patients with high-risk CNS malignancies receiving protons, delayed adjuvant radiotherapy is associated with poorer survival. Further studies are needed to understand this discrepancy to maximize the potential of proton radiotherapy for CNS malignancies.
Keywords: central nervous system, particle therapy, proton therapy, radiation therapy, treatment planning
Radiotherapy is an important treatment modality for many primary CNS tumors in pediatric and adult patients. Although postoperative radiotherapy following surgical resection is a standard of care in high-grade glial tumors (World Health Organization grades III and IV), this treatment remains controversial in lower-grade glial tumors because of concerns for radiation-induced cognitive deficits and secondary malignancies.1,2 Proton radiotherapy may mitigate these risks—in pediatric CNS tumors, dosimetric comparisons have suggested lower radiotherapy dose to normal tissues adjacent to the target, potentially reducing treatment-related adverse effects.3,4 Even in patients with glioblastoma multiforme, high-dose adjuvant particle therapy may improve outcomes independent of surgical resection extent.5
Despite its potential, implementation of proton radiotherapy can be challenging. As of early 2019, there are only 92 clinically operational proton centers in the world; furthermore, the majority of these are located in either the United States (n = 31) or Japan (n = 20), making global access to proton radiotherapy challenging.6 Even within the United States, only 20 states have clinically operational proton centers, with the majority located in metropolitan regions.6 In addition to geographic hurdles, insurance approval for proton radiotherapy may be difficult to obtain and can result in substantial delays in initiation of proton radiotherapy.7 Particularly in neurosurgical patients, unpredictability in postoperative care can introduce uncertainty and undermine recommended treatment guidelines. These hurdles pose significant barriers to treatment for the majority of pediatric and adult CNS tumors with proton radiotherapy.
To better understand nationwide use of proton radiotherapy in pediatric and adult patients with CNS tumors and how proton usage affects treatment timing, we conducted an analysis of a large, hospital-based cancer registry of more than 75 000 patients with CNS malignancies treated with either photon or proton radiotherapy.
Methods
This study was exempt from review by the Stanford University School of Medicine institutional review board because of the deidentified nature of the analyzed data. Patients were queried from the National Cancer Database (NCDB), a nationwide hospital database sourced from the registries of more than 1500 Commission on Cancer–accredited facilities.8 As previously described, cases are queried using histology codes listed in the International Classification of Diseases for Oncology, third edition.9,10 The data span more than 70% of cancer cases nationwide and encompasses more than 34 million historical records. Data quality is ensured by a series of more than 600 electronic automated edit checks to identify missing and inconsistent data fields.8
Cohort Selection
A total of 76 157 pediatric (age 0-17 years) and adult (age 18+ years) patients diagnosed with primary CNS malignancies from 2004 to 2015 who received radiotherapy following biopsy and/or surgical resection were included in this study (Table 1). Separate subset analyses were performed to understand the temporal impact of proton radiotherapy in patients receiving definitive radiotherapy and in those receiving adjunctive radiotherapy after surgical resection. Patients with tumor primary sites in either the brain, spinal cord, cranial nerves, cerebral and spinal meninges, intracranial glands (pituitary, craniopharyngeal duct, and pineal), and other unspecified CNS locations were included. Histology groupings, defined according to the World Health Organization guidelines, stratified tumors into embryonal, ependymal, germ cell, glial (nonependymal), meningeal, and other tumors11 (a list of unique histology/behavior codes is in Supplementary Table 1). Exclusion criteria included diagnosis dates preceding the facility reference date, lack of pathological diagnosis confirmation, and invalid treatment dates. Although the NCDB records only treatments provided prior to disease recurrence or relapse, we also excluded patients receiving radiation more than 365 days after their initial biopsy or surgery to minimize risk of misclassification. CNS tumor histologies without at least one patient receiving proton radiotherapy were excluded. For subset analyses, high-risk tumors were defined as grade IV embryonal, grade IV nonependymal glial tumors, grade III meningiomas, and grade III ependymomas.
Table 1.
Cohort Characteristics. Characteristics of patients With CNS Malignancies Receiving Either Photon or Proton Radiotherapy. P Values from Chi-Square Test
| Characteristic | Photon (n = 74 787) | Proton (n = 1 370) | P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Year of diagnosis (median, IQR) | 2010 | 2007-2013 | 2013 | 2011-2014 | < .001 |
| Age, mean, SD, y | 54.71 | 17.71 | 29.86 | 22.85 | < .001 |
| Sex (n, %) | .552 | ||||
| Male | 42 607 | 57 | 769 | 56.1 | |
| Female | 32 180 | 43 | 601 | 43.9 | |
| Race (n, %) | < .001 | ||||
| White | 66 155 | 88.5 | 1155 | 84.3 | |
| Black | 53 16 | 7.1 | 71 | 5.2 | |
| Other | 3316 | 4.4 | 144 | 10.5 | |
| Charlson Comorbidity score (n, %) | < .001 | ||||
| 0 | 57 722 | 77.2 | 1207 | 88.1 | |
| 1 | 10 960 | 14.7 | 118 | 8.6 | |
| 2+ | 6105 | 8.2 | 45 | 3.3 | |
| Insurance status (n, %) | < .001 | ||||
| No insurance | 3060 | 4.1 | 29 | 2.1 | |
| Medicaid | 6683 | 8.9 | 207 | 15.1 | |
| Medicare | 22 221 | 29.7 | 135 | 9.9 | |
| Private insurance | 40 530 | 54.2 | 945 | 69 | |
| Unknown | 2293 | 3.1 | 54 | 3.9 | |
| Income (n, %), $ | < .001 | ||||
| < 38 000 | 1201 | 1.6 | 14 | 1 | |
| 38 000-47 999 | 10 686 | 14.3 | 126 | 9.2 | |
| 48 000-62 999 | 16 379 | 21.9 | 226 | 16.5 | |
| > 63 000 | 20 460 | 27.4 | 362 | 26.4 | |
| Unknown | 26 061 | 34.8 | 642 | 46.9 | |
| Region (n, %)a | < .001 | ||||
| Mountain | 3123 | 4.2 | 5 | 0.4 | |
| North Atlantic | 13 978 | 18.7 | 115 | 8.4 | |
| North Central | 17 038 | 22.8 | 97 | 7.1 | |
| Pacific | 7355 | 9.8 | 188 | 13.7 | |
| South Atlantic | 12 146 | 16.2 | 27 | 2 | |
| South Central | 7961 | 10.6 | 50 | 3.6 | |
| Unknown | 13 186 | 17.6 | 888 | 64.8 | |
| Tumor histology (n, %) | < .001 | ||||
| Embryonal | 2536 | 3.4 | 327 | 23.9 | |
| Ependymal | 2161 | 2.9 | 198 | 14.5 | |
| Germ cell | 367 | 0.5 | 33 | 2.4 | |
| Glial | 61 318 | 82 | 526 | 38.4 | |
| Meningeal | 4376 | 5.9 | 128 | 9.3 | |
| Other | 4029 | 5.4 | 158 | 11.5 | |
| WHO grade (n, %) | < .001 | ||||
| I | 2167 | 2.9 | 93 | 6.8 | |
| II | 6781 | 9.1 | 255 | 18.6 | |
| III | 9808 | 13.1 | 265 | 19.3 | |
| IV | 49 259 | 65.9 | 463 | 33.8 | |
| Unknown | 6772 | 9.1 | 294 | 21.5 | |
| Resection extent (n, %) | < .001 | ||||
| Biopsy | 9270 | 12.4 | 224 | 16.4 | |
| GTR | 14 175 | 19 | 494 | 36.1 | |
| STR | 13 594 | 18.2 | 307 | 22.4 | |
| Other/Unknown | 37 748 | 50.5 | 345 | 25.2 | |
| Academic facility (n, %)a | < .001 | ||||
| Yes | 12 146 | 19.7 | 27 | 5.6 | |
| No | 25 634 | 41.6 | 182 | 37.8 | |
| Unknown | 23 821 | 38.7 | 273 | 56.6 | |
| Time to treatment initiation, d | |||||
| Pediatric (median, IQR) | |||||
| Embryonal | 30 | 27 to 40 | 33 | 28 to 69 | < .001 |
| Ependymal | 41 | 32 to 60 | 48 | 38 to 67 | < .001 |
| Germ cell | 104 | 36 to 139 | 122 | 111 to 157 | .482 |
| Glial | 30 | 22 to 42 | 42 | 29 to 69 | < .001 |
| Meningeal | 57 | 45 to 94 | 59 | 36 to 63 | .584 |
| Other | 52 | 32 to 95 | 80 | 56 to 125 | < .001 |
| Adult (median, IQR) | |||||
| Embryonal | 41 | 31 to 55 | 43 | 32 to 57 | .770 |
| Ependymal | 54 | 37 to 80 | 74 | 50 to 112 | < .001 |
| Germ cell | 56 | 30 to 127 | 100 | 45 to 146 | .083 |
| Glial | 31 | 24 to 40 | 45 | 34 to 66 | < .001 |
| Meningeal | 64 | 43 to 104 | 99 | 69 to 149 | < .001 |
| Other | 68 | 39 to 119 | 97 | 70 to 136 | < .001 |
Abbreviations: GTR, gross total resection; IQR, interquartile range; STR, subtotal resection; WHO, World Health Organization.
Bold P values indicate statistical significance (p < 0.05).
aFacility type available only for patients age 40 or older.
Statistical Analyses
Wilcoxon rank-sum statistics and Pearson chi-square testing were used to compare continuous and categorical variables, respectively. Nonparametric Spearman correlation was used to evaluate trends over time. Histology-specific trends over time were evaluated by fitting univariable linear regressions to proton usage rates over time; hypothesis testing was conducted to assess whether the best-fit slope was significantly different from zero. Multiple linear regression was used to determine the independent effect of proton use on time to radiotherapy initiation in the context of each tumor histology. Interaction terms between tumor histology and radiotherapy modality were included in multiple regression models. The impact of delays in proton radiotherapy initiation on survival was evaluated using multivariable Cox regression. An a priori–defined univariable P value threshold of 0.2 was used to determine covariates included in the multivariable Cox model. Survival was measured from time of radiation initiation. Additional sensitivity analyses were conducted to evaluate survival starting from date of surgery. All statistical tests were 2-sided and significance threshold was established at an α of .05. All statistical and graphical analyses were conducted in R (Version 3.4.3) and GraphPad Prism 7.
Results
Cohort Characteristics
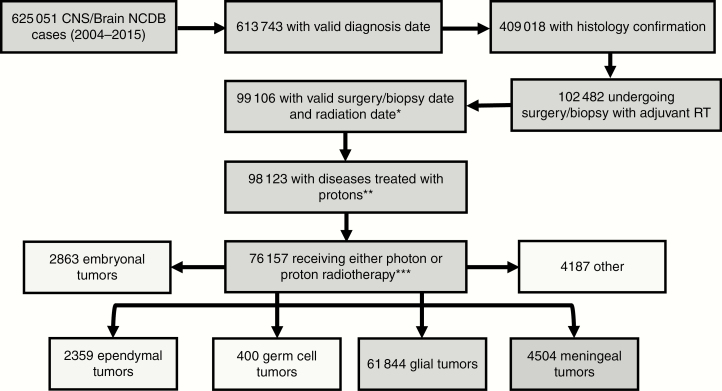
We identified 76 157 patients diagnosed between 2004 and 2015 with primary CNS neoplasms (2863 embryonal, 2359 ependymal, 400 germ cell, 61 844 glial, 4504 meningeal, 4187 other) (Figure 1, additional characteristics described in Table 1). The majority of patients were adults (N = 71 966 vs 4161 pediatric cases).
Figure 1.
Cohort selection. Inclusion criteria were established prior to analysis and cases were classified according to World Health Organization guidelines. Patients receiving radiation therapy (RT) more than 365 days following biopsy/surgery were excluded (*). Tumor histologies with no patients receiving proton radiotherapy were excluded (retaining only histology groups in which at least one patient received proton radiotherapy) (**). NCDB, National Cancer Database.
Treatment Patterns and Trends in Proton Use
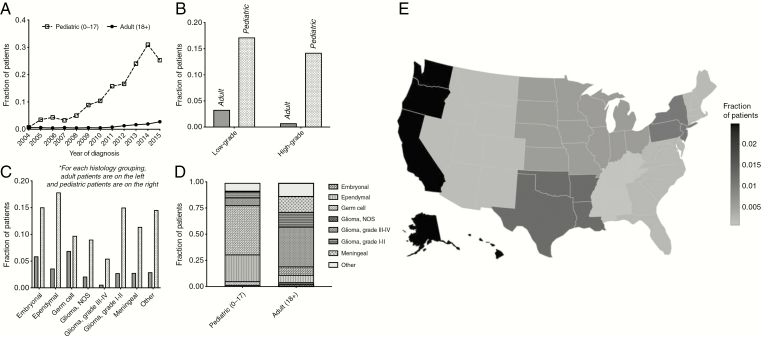
Proton usage in pediatric and adult CNS malignancies increased between 2004 and 2015. The fraction of pediatric patients receiving protons has increased from 0.71% to 25.30% (Spearman ρ = 0.972; P < .001) and the fraction of adult patients receiving protons has increased from 0.61% to 2.76% Spearman ρ = 0. 699; P = .014) (Figure 2A). This increase was particularly pronounced in pediatric patients with embryonal, ependymal, and germ cell tumors (Supplementary Figure 1). Pediatric and adult patients with grade III and IV tumors were less likely to receive proton radiotherapy than those with lower-grade tumors (grade I and II) (Figure 2B). Across all tumor histologies, pediatric patients were more likely to receive protons than adult patients (Figure 2C). In the subset of pediatric patients receiving proton radiotherapy, most had tumors of either embryonal or ependymal origin (such as ependymoma, medulloblastoma, and pineoblastoma). In contrast, most adult patients receiving proton radiotherapy had nonependymal glial tumors (Figure 2D). In adult patients age at least 40 years (facility location restricted for patients younger than 40), geographic distribution of proton radiotherapy usage was primarily on the West Coast (2.49%). Patients treated at facilities in the mountain region (Arizona, Colorado, Idaho, Montana, New Mexico, Nevada, Utah, and Wyoming) (0.16%) were least likely to received proton radiotherapy (Fig. 2E).
Figure 2.
Characteristics of patients receiving proton radiotherapy. A, Historical trends in fraction of biopsy/surgery + radiotherapy patients receiving proton-based therapy. B, Comparison of high-grade and low-grade tumors in adult and pediatric tumors. C, Cross-histology comparison of proton-therapy administration rates (colored bars reflect pediatric patients). D, Histological breakdown of proton-receiving pediatric and adult patients. E, Color reflects the fraction of patients receiving biopsy/surgery and adjuvant radiotherapy who were administered a proton-based modality. Patients in the Atlantic Northeast, Central South, and Western United States were most likely to receive proton radiotherapy. (Geographic data available only for patients older than 39 years).
Proton Radiotherapy and Treatment Timing
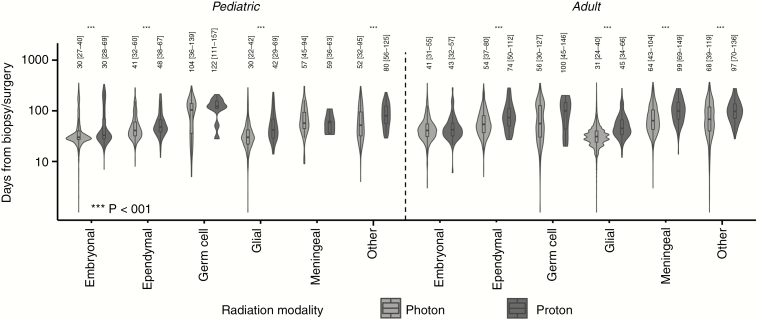
When assessing patients receiving either definitive or adjuvant radiotherapy, those treated with proton radiotherapy experienced longer delays in time to radiation initiation than patients receiving photon radiotherapy. In 4 of 6 pediatric histologies (embryonal, ependymal, nonependymal glial, and other), patients treated with proton radiotherapy experienced longer latency periods between biopsy/surgery and initiation of radiation (Figure 3). Similarly, in 4 of 6 adult histologies (ependymal, nonependymal glial, meningeal, and other), patients receiving protons experienced longer delays in radiation initiation (Figure 3).
Figure 3.
Postoperative delays associated with proton radiotherapy. Patients treated with proton-based radiotherapy modalities experience longer postoperative delays than patients receiving photon-based modalities. Median (interquartile range) indicated.
In the subgroup receiving radiotherapy after biopsy only, pediatric patients with embryonal and glial tumors and adult patients with glial and meningeal tumors receiving proton radiotherapy experienced longer times to radiation initiation following biopsy compared to patients receiving photon radiotherapy (median [pediatric embryonal] 41 days vs 29 days, median [pediatric glial] 40.5 days vs 26 days; median [adult glial] 49 days vs 31 days, median [adult meningeal] 95 days vs 59 days). In the adjuvant radiotherapy setting, pediatric patients with tumors classified as embryonal, ependymal, and glial, and adult patients with ependymal, glial, and meningeal tumors also encountered longer delays in radiation when pursing protons compared to photons (median [pediatric embryonal] 33 days vs 31 days, P = .003; median [pediatric ependymal] 46.5 days vs 40 days, P = .027; median [pediatric glial] 45 days vs 30 days, P < .001; median [adult ependymal] 71 days vs 56 days, P = .012; median [adult glial] 45 days vs 32 days, P < .001; median [adult meningeal] 95.5 days vs 64 days, P < .001).
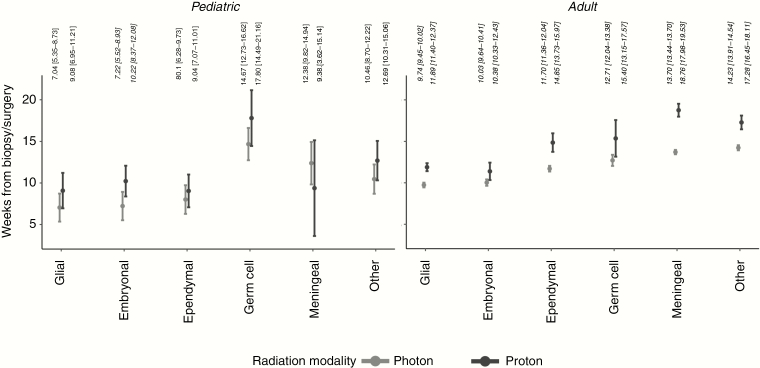
After accounting for differences in available covariates (age, sex, race, insurance, median income of region, tumor grade, comorbidity burden, and resection extent) and including an interaction term between proton use and tumor histology, proton radiotherapy was associated with significant increase in time to radiotherapy initiation in all histology categories in adult patients and in embryonal tumors in pediatric patients (marginal effects depicted in Figure 4). Effect sizes of other covariates included are shown in Supplementary Table 2.
Figure 4.
Marginal effects of proton radiotherapy in multiple regression model. The marginal effects of proton and photon radiotherapy use after covariate adjustment in the setting of various pediatric and adult tumor histologies. Estimate (95% CI) indicated; significant differences indicated in bold italics.
Association of Delayed Adjuvant Proton Radiotherapy With Survival
In the subgroup of patients receiving proton radiotherapy after tumor resection, we sought to evaluate the impact of delays in treatment delivery on overall survival. The impact of treatment delay was evaluated for all tumor grades and for a subset of high-risk tumors (grade IV embryonal and nonependymal glial tumors, and grade III meningiomas and ependymomas). Delays in proton radiotherapy after surgery were not independently associated with survival in the entire cohort (continuous [weeks], adjusted hazard ratio [aHR] = 1.01, 95% CI = 0.97-1.05). However, in patients with high-risk tumors receiving proton radiotherapy, delayed adjuvant treatment was associated with worse survival, even after adjusting for available demographic and treatment-associated covariates (continuous [weeks], aHR = 1.09, 95% CI = 1.02-1.16, Table 2). Sensitivity analyses evaluating survival from date of surgery also demonstrated worse survival with delayed adjuvant radiotherapy (continuous [weeks], aHR = 1.08, 95% CI = 1.01-1.15). In this high-risk population, when assessing both photon- and proton-treated patients and adjusting for proton usage in addition to the covariates included in Table 2, there was a trend toward delays in adjuvant radiotherapy initiation being associated with poorer prognosis (aHR = 1.01, 95% CI = 1.00-1.02).
Table 2.
Multivariable Cox Regression Model. Univariable and Multivariable Cox Model Evaluating Association of Available Covariates With Overall Survival
| Characteristic | Univariable | Multivariable | |||
|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | P | |
| Time to proton initiation (continuous, wk) | 1.042 | 1.001-1.085 | 1.079 | 1.013-1.149 | .018 |
| Age (continuous, y) | 1.035 | 1.022-1.049 | 1.014 | 0.986-1.044 | .322 |
| Sex | |||||
| Male (reference) | – | – | – | – | – |
| Female | 0.871 | 0.439-1.730 | – | – | – |
| Race | |||||
| White (reference) | – | – | – | – | – |
| Black | 1.318 | 0.314-5.528 | – | – | – |
| Other | 0.542 | 0.165-1.778 | – | – | – |
| Insurance | |||||
| Private insurance (reference) | – | – | – | – | – |
| No Insurance | – | – | – | – | – |
| Medicaid | 0.899 | 0.343-2.361 | 0.558 | 0.157-1.991 | .369 |
| Medicare | 3.428 | 1.393-8.435 | 0.477 | 0.132-1.723 | .258 |
| Unknown | – | – | – | – | – |
| Income, $ | |||||
| < 38 000 (reference) | – | – | – | – | – |
| 38 000-47 999 | 0.698 | 0.242-2.014 | 1.556 | 0.425-5.691 | .504 |
| 48 000-62 999 | 0.487 | 0.177-1.342 | 0.483 | 0.148-1.572 | .227 |
| > 63 000 | 0.247 | 0.091-0.667 | 0.310 | 0.083-1.155 | .081 |
| Tumor histology | |||||
| Glial (reference) | – | – | – | – | – |
| Embryonal | 0.064 | 0.027-0.154 | 0.090 | 0.026-0.311 | < .001 |
| Ependymal | 0.095 | 0.032-0.285 | 0.146 | 0.038-0.554 | .005 |
| Germ cell | – | – | – | – | – |
| Meningeal | 0.39 | 0.088-1.727 | 0.151 | 0.025-0.895 | .037 |
| Other | 2.903 | 0.375-22.441 | 3.889 | 0.319-47.358 | .287 |
| Facility typea | |||||
| Academic center (reference) | – | – | – | – | – |
| Nonacademic center | 0.286 | 0.055-1.477 | 1.098 | 0.174-6.929 | .921 |
| Unknown | 0.121 | 0.028-0.512 | 2.030 | 0.215-19.195 | .537 |
| Charlson Comorbidity score | |||||
| 0 (ref) | – | – | – | – | – |
| 1 | 4.22 | 1.617-11.016 | 3.762 | 0.676-20.921 | .130 |
| 2+ | 2.929 | 0.882-9.729 | 5.102 | 1.299-20.043 | .020 |
| Resection extent | |||||
| GTR (reference) | – | – | – | – | – |
| STR | – | – | – | – | – |
| Other/Unknown | 0.974 | 0.234-4.060 | – | – | – |
Abbreviations: GTR, gross total resection; HR, hazard ratio; IQR, interquartile range; STR, subtotal resection; WHO, World Health Organization.
aFacility type available only for patients age 40 or older.
Assessment of Proton Radiotherapy Treatment Delays in the Context of Existing Clinical Trials
The putative impact of delayed proton radiotherapy was evaluated in the context of radiotherapy timing guidelines established by ongoing and completed clinical trials in CNS malignancies. In a trial evaluating proton radiotherapy in the context of pediatric and young adult (age 3-25 years) medulloblastoma and pineoblastoma (NCT01063114), patients were required to initiate treatment within 35 days of surgery. Applying this threshold to the cohort of patients with medulloblastoma or pineoblastoma in our cohort, proton radiotherapy was independently associated with a greater risk of delayed therapy beyond 35 days (aOR = 1.40; 95% CI = 1.01-1.93).
In a trial evaluating proton radiotherapy in adult patients with grade II or III gliomas, inclusion criteria required radiotherapy to be initiated within 30 days of baseline testing (NCT03180502). In the analogous subset of adult patients with grade II and grade III gliomas, proton radiotherapy was independently associated with an increased risk of delaying therapy beyond 30 days (aOR = 3.49; 95% CI = 2.35-5.44).
Discussion
Our results suggest that, across a broad range of histologies and both in the definitive and adjuvant treatment settings, patients receiving proton radiotherapy for CNS tumors encounter significantly longer delays in treatment compared to those receiving photon radiotherapy. In patients with high-risk malignancies receiving proton radiotherapy, this delay portended worse survival. Given the increasing use of proton for treating CNS malignancies, describing and understanding the effect of proton radiotherapy on treatment timing may encourage future efforts to reduce treatment delays.
Proton radiotherapy is characterized by unique dose deposition patterns that could potentially reduce radiation-induced adverse events.12 The detrimental effects of radiation damage on the CNS are well documented; acute neurological injuries include edema and headache, whereas late-onset neurological injuries include lasting phenotypic changes, such as white-matter necrosis and vascular damage.13 Dosimetric comparison of traditional fractionated photon-based modalities with particle-based modalities, including protons, suggests decreased exit dose distal to the radiation target, increasing sparing of normal tissue.14 In CNS malignancies, investigators have suggested new delivery methods, such as intensity modulated proton therapy, allow for more conformal treatment plans than photon-based methods such as intensity modulated radiotherapy and volumetric modulated arc therapy.15,16 Particularly for pediatric patients, in whom radiation to the CNS has been associated with a broad range of secondary pathologies including stroke, hemorrhage, and neurological deficits, reduction of radiation-induced adverse events would constitute a significant milestone.17–21 However, additional prospective, longitudinal studies are needed to better understand the clinical consequences of proton radiotherapy and how to optimally integrate it with neurosurgery.
Research on proton radiotherapy has primarily focused on effectiveness in the context of controlled clinical trials. Although prior studies have investigated the factors associated with proton usage in a variety of tumors, the effect of proton radiotherapy on radiation timing has not been studied before. Tumor repopulation prior to and during radiation therapy in cancer histologies ranging from head and neck squamous cell carcinoma to lung and breast cancer necessitates prompt initiation of therapy.22–24 The clinical impact of radiotherapy timing in pediatric and adult CNS malignancies is less well understood. Although some studies have claimed that a shorter interval between neurosurgery and radiation is associated with improved outcomes,25–27 other studies have suggested there may be benefit with delay to allow for maximum postoperative healing prior to radiation.28–30 In pediatric patients with embryonal tumors, immediate radiotherapy following surgical resection has been shown to be associated with improved outcomes compared to delayed radiotherapy following postoperative chemotherapy.31 Additional investigations are needed to better understand how radiation timing affects outcome, and tumor repopulation remains a concern for highly proliferative neoplasms, such as glioblastoma and medulloblastoma.25,31–33 In the subset of adult patients with high-risk tumors, our study found increased delay in administration of protons to be negatively associated with survival. Although these analyses were meant to be hypothesis-generating and additional prospective evidence is necessary, these results suggest that delayed administration of protons could be detrimental in patients with highly undifferentiated tumors most at risk of tumor regrowth following surgery.
Clinical trials studying proton and photon radiotherapy in CNS malignancies often standardize treatment timing. Completed (NCT00002875 [photon; 4 weeks], NCT00006461 [photon; 6 weeks], and NCT00031590 [photon; 4 weeks]) and ongoing (NCT02724579 [photon; 5 weeks]) clinical trials evaluating therapies for childhood medulloblastoma require trial participants to receive radiotherapy within a defined time period following surgery. In a trial sponsored by Massachusetts General Hospital investigating the efficacy of proton radiotherapy in medulloblastoma and pineoblastoma, patients are required to initiate proton radiotherapy within 5 weeks of definitive surgery (NCT01063114). Using thresholds extracted from completed and ongoing clinical trial eligibility criteria, we investigated whether proton radiotherapy was associated with delayed radiation. In patients with medulloblastoma or pineoblastoma, receipt of proton radiotherapy was independently associated with radiotherapy delay using a threshold of 5 weeks after biopsy/surgery (NCT02724579).
In adults, the impact of radiotherapy delay on clinical outcomes is more controversial. Prior studies of glioblastoma have suggested delays ranging from 28 to 37 days in radiation initiation is potentially associated with poor survival.25,32,33 In contrast, other investigations suggest either less or no impact of delayed radiotherapy on survival.29,34 However, there remains a paucity of prospective, randomized studies providing definitive evidence on the clinical impact of delayed radiation. Currently, clinical trials evaluating particle and photon radiotherapy in various gliomas require initiation of radiotherapy within defined periods following baseline testing (NCT03180502 [proton; 4 weeks], NCT01165671 [proton and carbon; 12 weeks], and NCT03587038 [photon; 7 weeks]). Using a threshold of 4 weeks, adult patients receiving proton radiotherapy for grade II or III gliomas were more likely to experience clinically meaningful delays in radiation compared to those receiving photon therapy.
Although the significant delays associated with delivery of proton radiotherapy are concerning, the mechanisms for its occurrence remain unclear. Logistical challenges could pose significant hurdles for patients seeking novel therapies; Ning et al emphasize that, despite many patients eventually being approved for proton radiotherapy, the insurance approval and appeal process is both time- and resource-intensive.7 As previously mentioned, the scarcity of clinically operational proton centers remains a major barrier to timely delivery of care.6 Lastly, it is possible that the process of seeking out secondary opinions and clinical trials using novel therapies could contribute to delay of treatment initiation. Regardless of the etiology of this delay, efficient and predictable transfer of patients from neurosurgery to adjunctive therapies remains important for ensuring optimal patient care.
Our study includes the standard limitations of any retrospective database analysis. Though inclusion criteria established prior to initiation of the study were instituted to ensure optimal data quality, misclassification and miscoding of variables remain a concern in large patient registries combining information from diverse sources. Although statistical methods were used to control for available covariates, selection bias may still be present because of variables not included in the data set, particularly because of the novel nature of proton radiotherapy. More broadly, despite the NCDB covering more than 70% of newly diagnosed cancer cases in the United States, the NCDB was not designed to be a population-based database because only Commission on Cancer–accredited facilities can contribute data.35 Finally, clinically significant variables, such as local disease control and treatment-induced adverse events, were not available for analyses. In histologies such as medulloblastoma, for which patients receiving photon therapy may be at higher risk of disease progression and poor outcomes, further studies with granular clinical data are necessary. Outcome analyses in our study were restricted to overall survival assessment, and additional studies are necessary to define causal relationships between treatment timing and survival. Despite these limitations, the strengths of our study lie in the size of our cohort that spans multiple regions and institutions. Though controlled, randomized trials are necessary to definitively evaluate the clinical benefit of proton radiotherapy, epidemiological studies of proton usage offer unique opportunities to interrogate the current landscape of proton radiotherapy usage.
Conclusion
Across multiple pediatric and adult tumor subtypes, use of protons was associated with longer delays prior to initiation of radiotherapy. In patients with high-risk CNS tumors, delayed administration of adjuvant proton radiotherapy was associated with poorer survival. Although the clinical implications of delays in the timely administration of therapy are concerning, describing and understanding the mechanisms driving this delay could help optimize future use of proton radiotherapy.
Supplementary material
Supplementary material is available online at Neuro-Oncology Practice (http://nop.oxfordjournals.org/).
Funding
None.
Conflict of interest statement. None declared.
References
- 1. Douw L, Klein M, Fagel SS, et al. Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: long-term follow-up. Lancet Neurol. 2009;8(9):810–818. [DOI] [PubMed] [Google Scholar]
- 2. Schmiegelow K, Levinsen MF, Attarbaschi A, et al. Second malignant neoplasms after treatment of childhood acute lymphoblastic leukemia. J Clin Oncol. 2013;31(19):2469–2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boehling NS, Grosshans DR, Bluett JB, et al. Dosimetric comparison of three-dimensional conformal proton radiotherapy, intensity-modulated proton therapy, and intensity-modulated radiotherapy for treatment of pediatric craniopharyngiomas. Int J Radiat Oncol Biol Phys. 2012;82(2):643–652. [DOI] [PubMed] [Google Scholar]
- 4. Merchant TE, Hua CH, Shukla H, Ying X, Nill S, Oelfke U. Proton versus photon radiotherapy for common pediatric brain tumors: comparison of models of dose characteristics and their relationship to cognitive function. Pediatr Blood Cancer. 2008;51(1):110–117. [DOI] [PubMed] [Google Scholar]
- 5. Matsuda M, Yamamoto T, Ishikawa E, et al. Prognostic factors in glioblastoma multiforme patients receiving high-dose particle radiotherapy or conventional radiotherapy. Br J Radiol. 2011;84(Spec No 1):S54–S60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Facilities in Operation. Particle therapy co-operative group2019. https://www.ptcog.ch/index.php/facilities-in-operation. Accessed March 14, 2019.
- 7. Ning MS, Gomez DR, Shah AK, et al. The insurance approval process for proton radiation therapy: a significant barrier to patient care. Int J Radiat Oncol Biol Phys. 2019;104(4):724–733. [DOI] [PubMed] [Google Scholar]
- 8. Boffa DJ, Rosen JE, Mallin K, et al. Using the national cancer database for outcomes research: a review. JAMA Oncol. 2017;3(12):1722–1728. [DOI] [PubMed] [Google Scholar]
- 9. Jin MC, Harris JP, Sabolch AN, et al. Proton radiotherapy and treatment delay in head and neck squamous cell carcinoma [published online ahead of print December 14, 2019]. Laryngoscope. doi:10.1002/lary.28458. [DOI] [PubMed] [Google Scholar]
- 10. Jin MC, Prolo LM, Wu A, et al. Patterns of care and age-specific impact of extent of resection and adjuvant radiotherapy in pediatric pineoblastoma. Neurosurgery. 2020;86(5):E426–E435. [DOI] [PubMed] [Google Scholar]
- 11. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 12. Combs SE, Laperriere N, Brada M. Clinical controversies: proton radiation therapy for brain and skull base tumors. Semin Radiat Oncol. 2013;23(2):120–126. [DOI] [PubMed] [Google Scholar]
- 13. Greene-Schloesser D, Robbins ME, Peiffer AM, Shaw EG, Wheeler KT, Chan MD. Radiation-induced brain injury: a review. Front Oncol. 2012;2:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vogel J, Carmona R, Ainsley CG, Lustig RA. The promise of proton therapy for central nervous system malignancies. Neurosurgery. 2019;84(5):1000–1010. [DOI] [PubMed] [Google Scholar]
- 15. Eekers DBP, Roelofs E, Cubillos-Mesías M, et al. Intensity-modulated proton therapy decreases dose to organs at risk in low-grade glioma patients: results of a multicentric in silico ROCOCO trial. Acta Oncol. 2019;58(1):57–65. [DOI] [PubMed] [Google Scholar]
- 16. Munck Af Rosenschöld P, Engelholm S, Ohlhues L, Law I, Vogelius I, Aage S. Photon and proton therapy planning comparison for malignant glioma based on CT, FDG-PET, DTI-MRI and fiber tracking. Acta Oncol. 2011;50(6):777–783. [DOI] [PubMed] [Google Scholar]
- 17. Bojaxhiu B, Ahlhelm F, Walser M, et al. Radiation necrosis and white matter lesions in pediatric patients with brain tumors treated with pencil beam scanning proton therapy. Int J Radiat Oncol Biol Phys. 2018;100(4):987–996. [DOI] [PubMed] [Google Scholar]
- 18. Campen CJ, Kranick SM, Kasner SE, et al. Cranial irradiation increases risk of stroke in pediatric brain tumor survivors. Stroke. 2012;43(11):3035–3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chawla S, Korones DN, Milano MT, et al. Spurious progression in pediatric brain tumors. J Neurooncol. 2012;107(3):651–657. [DOI] [PubMed] [Google Scholar]
- 20. Hua C, Bass JK, Khan R, Kun LE, Merchant TE. Hearing loss after radiotherapy for pediatric brain tumors: effect of cochlear dose. Int J Radiat Oncol Biol Phys. 2008;72(3):892–899. [DOI] [PubMed] [Google Scholar]
- 21. Kralik SF, Mereniuk TR, Grignon L, et al. Radiation-induced cerebral microbleeds in pediatric patients with brain tumors treated with proton radiation therapy. Int J Radiat Oncol Biol Phys. 2018;102(5):1465–1471. [DOI] [PubMed] [Google Scholar]
- 22. Bese NS, Sut PA, Ober A. The effect of treatment interruptions in the postoperative irradiation of breast cancer. Oncology. 2005;69(3):214–223. [DOI] [PubMed] [Google Scholar]
- 23. De Ruysscher D, Lueza B, Le Péchoux C, et al; RTT-SCLC Collaborative Group. Impact of thoracic radiotherapy timing in limited-stage small-cell lung cancer: usefulness of the individual patient data meta-analysis. Ann Oncol. 2016;27(10):1818–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Harris JP, Chen MM, Orosco RK, Sirjani D, Divi V, Hara W. Association of survival with shorter time to radiation therapy after surgery for US patients with head and neck cancer. JAMA Otolaryngol Head Neck Surg. 2018;144(4):349–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Irwin C, Hunn M, Purdie G, Hamilton D. Delay in radiotherapy shortens survival in patients with high grade glioma. J Neurooncol. 2007;85(3):339–343. [DOI] [PubMed] [Google Scholar]
- 26. Kann BH, Park HS, Lester-Coll NH, et al. Postoperative radiotherapy patterns of care and survival implications for medulloblastoma in young children. JAMA Oncol. 2016;2(12):1574–1581. [DOI] [PubMed] [Google Scholar]
- 27. Lannering B, Rutkowski S, Doz F, et al. Hyperfractionated versus conventional radiotherapy followed by chemotherapy in standard-risk medulloblastoma: results from the randomized multicenter HIT-SIOP PNET 4 trial. J Clin Oncol. 2012;30(26):3187–3193. [DOI] [PubMed] [Google Scholar]
- 28. Adeberg S, Bostel T, Harrabi S, et al. Impact of delays in initiating postoperative chemoradiation while determining the MGMT promoter-methylation statuses of patients with primary glioblastoma. BMC Cancer. 2015;15:558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Blumenthal DT, Won M, Mehta MP, et al. Short delay in initiation of radiotherapy may not affect outcome of patients with glioblastoma: a secondary analysis from the radiation therapy oncology group database. J Clin Oncol. 2009;27(5):733–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Randolph DM II, McTyre ER, Paulsson AK, et al. Impact of timing of radiotherapy in patients with newly diagnosed glioblastoma. Clin Neurol Neurosurg. 2016;151:73–78. [DOI] [PubMed] [Google Scholar]
- 31. Kortmann RD, Kühl J, Timmermann B, et al. Postoperative neoadjuvant chemotherapy before radiotherapy as compared to immediate radiotherapy followed by maintenance chemotherapy in the treatment of medulloblastoma in childhood: results of the German prospective randomized trial HIT ‘91. Int J Radiat Oncol Biol Phys. 2000;46(2):269–279. [DOI] [PubMed] [Google Scholar]
- 32. Do V, Gebski V, Barton MB. The effect of waiting for radiotherapy for grade III/IV gliomas. Radiother Oncol. 2000;57(2):131–136. [DOI] [PubMed] [Google Scholar]
- 33. Gliński B, Urbański J, Hetnał M, et al. Prognostic value of the interval from surgery to initiation of radiation therapy in correlation with some histo-clinical parameters in patients with malignant supratentorial gliomas. Contemp Oncol (Pozn). 2012;16(1):34–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang TJ, Jani A, Estrada JP, et al. Timing of adjuvant radiotherapy in glioblastoma patients: a single-institution experience with more than 400 patients. Neurosurgery. 2016;78(5):676–682. [DOI] [PubMed] [Google Scholar]
- 35. Palma DA. National Cancer Data Base: an important research tool, but not population-based. J Clin Oncol. 2017;35(5):571. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.