Abstract
The relationship between metabolites and multiple myeloma (MM) is becoming a research focus in the field. In this study, we performed metabolic profiling of multiple myeloma and identified potential metabolites associated with clinical characteristics, therapeutic efficacy, and prognosis of the disease. Fifty-five patients with newly-diagnosed multiple myeloma and thirty-seven healthy controls from August 2016 to October 2017 were randomly collected. The serum metabolic profiling was investigated by gas chromatography-mass spectrometry (GC-MS) technique and underwent statistical analysis. Twenty-seven metabolites were found to be significantly different between healthy controls and multiple myeloma patients. Eleven metabolites were significantly elevated, while sixteen metabolites were decreased in the multiple myeloma population. Metabolic changes were also observed in patients with renal impairment and bone destruction. Levels of urea were significantly decreased after treatment while levels of hypotaurine showed significant increase in the good-effect group (P<0.05), but not in the no-good-effect group (P>0.05). In multivariate statistical analyses, high cysteine and high hypotaurine are independent risk factors for poor treatment outcome. After adjustment for critical clinical characteristics, patients with high levels of glycolic acid and xylitol were found to be less likely to experience disease progression. Multiple myeloma demonstrates different metabolic characteristics compared with the healthy population. Among multiple myeloma patients, renal impairment and bone destruction showed additional metabolic characteristics. Cysteine and hypotaurine have value in predicting the treatment outcome, while glycolic acid and xylitol may be important prognostic factors for multiple myeloma.
Keywords: Multiple myeloma, gas chromatography-mass spectrometry, metabolic biomarkers, treatment outcome, prognostic factors
Introduction
Multiple myeloma (MM) is a malignancy of clonal proliferation of malignant plasma cells in bone marrow and abnormal monoclonal immunoglobulin [1]. The clinical and biological heterogeneities of this malignancy lead to variable responses to therapy and to variable outcomes [2]. With a rising number of patients and more complicated clinical scenarios, it is important to explore MM in depth, especially in the aspects of novel diagnostic tools, response to treatment and prognostic factors [3]. In order to ameliorate the patient’s condition, new breakthroughs should aim at not only improving survival but also developing better tools to evaluate the prognosis and to monitor treatment efficacy [4].
Metabolic profiling is a useful tool to study biomarkers of the disease [5,6]. Particularly in the cancer setting [7,8], metabolomics has been applied to develop novel early diagnostic biomarkers in renal cancer [9], colorectal cancer [10], pancreatic cancer [11], ovarian cancer [12] and oral cancer [13]. Many analytical techniques including gas/liquid chromatography-mass spectrometry (GC/LC-MS) and nuclear magnetic resonance (NMR) have been used to identify metabolite structures and to measure the relative and absolute concentrations of those molecules [14,15].
In this study, we investigated the metabolic profiles of healthy controls and multiple myeloma patients, using untargeted gas chromatography-mass spectrometry (GC-MS) technique [16]. The aim of our study was to provide preliminary analysis on metabolic characteristics of MM patients and to identify potential metabolites associated with clinical characteristics, therapeutic efficacy, overall survival and progress-free survival.
Materials and methods
Chemicals and reagents
Derivatization reagents [Pyridine, methoxyamine hydrochloride and N-methyl-N-(trimethylsilyl)-trifluoroacetamide] were obtained from Sigma-Aldrich. The internal standard (IS, 2,4-dichlorobenzoic acid) and other reference standards for compound identification were obtained from Sigma-Aldrich, Alfa Aesar or JK Chemical Ltd. Ultrapure water was from Milli-Q system (Millipore, USA). An N-alkane mixture of C7-C40 for the Kovat retention index calculation was purchased from ANPEL Laboratory Technologies (Shanghai, China).
Patient selection and sample collection
Fifty-five patients in the First Affiliated Hospital of Soochow University with newly-diagnosed multiple myeloma from August 2016 to October 2017 were enrolled in this study. All of them were also included in a phase 4 clinical trial (NCT02577783) in which patients were randomized to receive induction with PDD regimen (doxorubicin hydrochloride iposome, bortizomib and dexamethasone) or PAD regimen (bortizomib, dexamethasone and doxorubicin). None of these patients had significant metabolic disorders (like diabetes). Thirty-seven healthy controls were selected randomly during the same period of time in our center. The responses to treatment were divided into 6 subcategories based on the International Myeloma Working Group uniform response criteria: complete response (CR); very good partial response (VGPR); partial response (PR); minor response (MR); stable disease (SD); and progressive disease (PD). CR and VGPR are considered as the good-effect group, while PR, MR, SD and PD are thought to be the no-good-effect group. The patient characteristics are summarized in Table 1.
Table 1.
Characteristics of patients
| Characteristics | MM |
|---|---|
| Sex (man/female) | 34/21 |
| Age [M, (range)] | 60 (42-73) |
| Ig type | |
| IgG | 30 |
| IgA | 9 |
| IgD | 0 |
| IgM | 1 |
| Light chain | 12 |
| κ | 3 |
| λ | 9 |
| Oligosecret | 2 |
| Double clone | 1 |
| DS stage | |
| I | 2 |
| II | 5 |
| III | 46 |
| No data | 2 |
| ISS stage | |
| I | 16 |
| II | 19 |
| III | 20 |
| R-ISS stage | |
| I | 16 |
| II | 30 |
| III | 9 |
| Plasmacyte [%, M (range)] | 24 (0-72.5) |
| M.protein.quantify [g/L, M (range)] | 22.72 (0-85.17) |
| Serum.β2.MG [mg/L, M (range)] | 3.87 (0.88-31.23) |
| IgH (positive/negative) | 11/44 |
| Abonormal.light.chain (abonormal/normal) | May-50 |
| Karyotype (abonormal/normal) | 11/44 |
| FISH (abonormal/normal) | 15/40 |
| disease state | |
| ACR, VGPR | 37 |
| BPR, MR, SD and PD | 18 |
| Overall Survival [month, M (range)] | 23.33 (6.40-34.40) |
| Progression-free Survival [month, M (range)] | 22.53 (5.87-34.27) |
Characteristics of patients with multiple myeloma (N=55).
CR, complete response; VGPR, very good partial response;
PR, partial response; MR, minor response; SD, stable disease; PD, progressive disease.
All peripheral venous serum samples were collected with yellow tubes with inert separation gel and coagulant in the morning after initial diagnosis and 4 courses of chemotherapy. The coagulant can quickly activate the blood coagulation mechanism and accelerate the blood coagulation process. After the collection, the tubes were inverted 5-8 times and stood still for 20-30 mins. Then, the serum was transferred in test tubes and was stored at -80°C until analysis.
Discovery set and validation set
To minimize the confounding factors in metabolomics, a matched control set composed of 37 serum samples from healthy subjects was introduced with t test and chi-square test. The following factors were matched in 55 patients: age, and sex (Page=0.284, Psex=0.637). Metabolic profiling from serum samples were obtained by gas chromatography-mass spectrometry. Seventeen patients with MM and seventeen healthy controls were randomly selected to set up the discovery set (Page=0.119, Psex=0.473), the rest (38 patients and 20 controls) were included in the validation set (Page=0.127, Psex=0.969).
Sample preparations
Before analysis, the serum samples were thawed at room temperature. 100 μL of serum sample were transferred into 2 mL Eppendorf tubes. Then 400 μL acetonitrile and 80 μL of 2,4-dichlorobenzoic acid (0.2 mg/mL) internal standard were added and vortexed for 2 min. After this, centrifugation was performed for 15 min (13000 rpm) at 4°C. Then 470 μL of supernatant was dried by vacuum in a speed vacuum concentrator (Labconco, USA). Thereafter, the dried samples were resolved with methoxyamine pyridine solution (15 mg/mL, 50 uL) and vortexed for 1 min. Subsequently, the sample was oximated in a 70°C water bath for 1 h and cooled to room temperature, followed by silylation reaction with 50 μL N-methyl-N-trimethyl-silyl trifluoroacetamide in a water bath at 70°C for 1 h. After the derivatization, the solution was centrifuged (13000 r/min, 15 min, 4°C) and 150 μL supernatant was used for subsequent GC-MS analysis.
The quality control (QC) sample was prepared by equally mixing serum samples from all subjects including patients and controls to evaluate the stability of the GC-MS analytical system.
Gas chromatography-mass spectrometry
Serum metabolic profiling was obtained by Agilent 7890/5975C GC-MS (Agilent Technologies). One microliter derivatized sample was injected into a DB-5 fused silica capillary column (30 mm × 0.25 mm × 0.25 μm, J&W Scientific) in a split mode (ratio 10:1). The carrier gas (99.9% helium) was operated with a constant flow rate of 1.1 ml/min. The initial column temperature of 80°C was maintained for 5 minutes and then increased to 170°C at 5°C per minute intervals and to 300°C at 10°C per minute intervals. The temperatures of inlet ion source and the electron ionization source were 280°C and 230°C, respectively. Mass spectra were acquired in the full scan mode with m/z 30-600.
Identification of the metabolites in the plasma was conducted using the commercial libraries (NIST 11.0, Mainlib) or the available commercial authentic standards. Additionally, the Kovat retention index of the plasma metabolites based on n-alkanes standards mixture of C7-C40 was used to differentiate the metabolites with similar mass spectra.
Statistical analysis
The differential variables were selected based on P value from the 2-tailed t test (P<0.05) and false discovery rate (FDR) <0.05. Logistic regression, Kaplan-Meier analysis, Cox regression, and leave-one-out cross-validation were used to evaluate the association of the potential metabolites with the multiple myeloma treatment outcome, overall survival, and progress-free survival. The models were compared with AUC.
Results
The metabolic deregulation in MM
The serum metabolic profiling was investigated by a GC-MS technique. To evaluate the stability of the analytical system during running samples, seven QC samples were inserted into the analytical sequence. The reproducibility was assessed by the distributions of relative standard deviation (RSD) of metabolites in all QC samples. 70.21% and 93.61% of the peaks were lower than 15% and 30%, respectively, which indicated that the serum metabolic profiles acquired in GC-MS were stable and reproducible (Figure S1). Typical total ion chromatograms of samples of serum metabolic profiling based on GC-MS is shown in Figure S2. Forty-seven compounds were identified, including amino acids, organic acids, carbohydrates, and fatty acids.
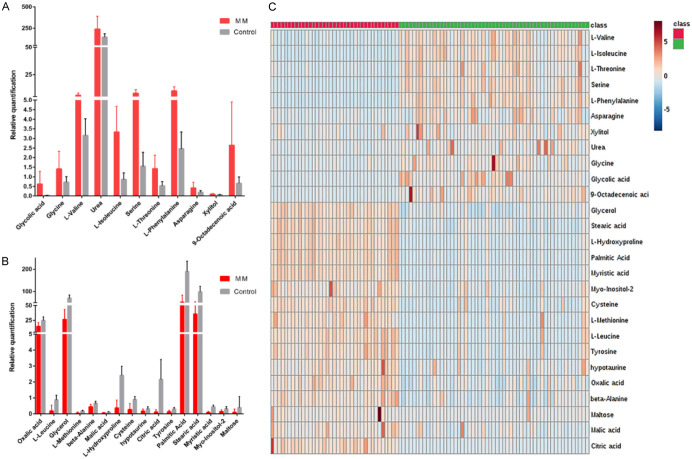
Serum metabolic profiles of healthy individuals and multiple myeloma patients at the time of diagnosis were compared to identify the potential metabolic biomarkers in the discovery set. Student’s t test was performed to find significantly altered metabolites. Among 47 metabolites, 29 metabolites that differentiated MM patients from healthy controls were identified in the discovery set (Table S1). We further assessed these 29 metabolites in the validation set. It turned out that 27 of the 29 metabolites showed differences between MM patients and healthy controls (Table S2). Glycolic acid, glycine, L-valine, urea, L-isoleucine, serine, L-threonine, L-phenylalanine, asparagine, xylitol and 9-Octadecenoic acid were significantly increased (Figure 1A), while oxalic acid, L-Leucine, glycerol, L-methionine, beta-alanine, malic acid, L-hydroxyproline, cysteine, hypotaurine, citric acid, tyrosine, palmitic acid, stearic acid, myristic acid, myo-Inositol-2, maltose were decreased in the MM population (Figure 1B). Significant metabolic changes were observed between the healthy group and the newly diagnosed MM patients (Figure 1C).
Figure 1.
The changed trend of 27 metabolic biomarkers between the healthy group and the newly diagnosed MM patients. A: 11 metabolic biomarkers in the MM population were increased compared with the control group. B: 16 metabolic biomarkers were decreased compared with the control group. C: 27 metabolic changes between healthy group and the newly diagnosed MM patients heat map.
The changes of metabolites in 31 paired samples from the same multiple myeloma patients before and after treatment were compared with the 2-tailed t test. In the good-effect group, hypotaurine increased significantly after treatment and there was a trend towards levels of healthy controls (P=0.015). Urea significantly declined after treatment in the good-effect group (P=0.05), and there was an upward trend compared with the healthy control group. However, such changes were not observed in the no-good-effect group (P>0.05) (Figure 2A, 2B).
Figure 2.
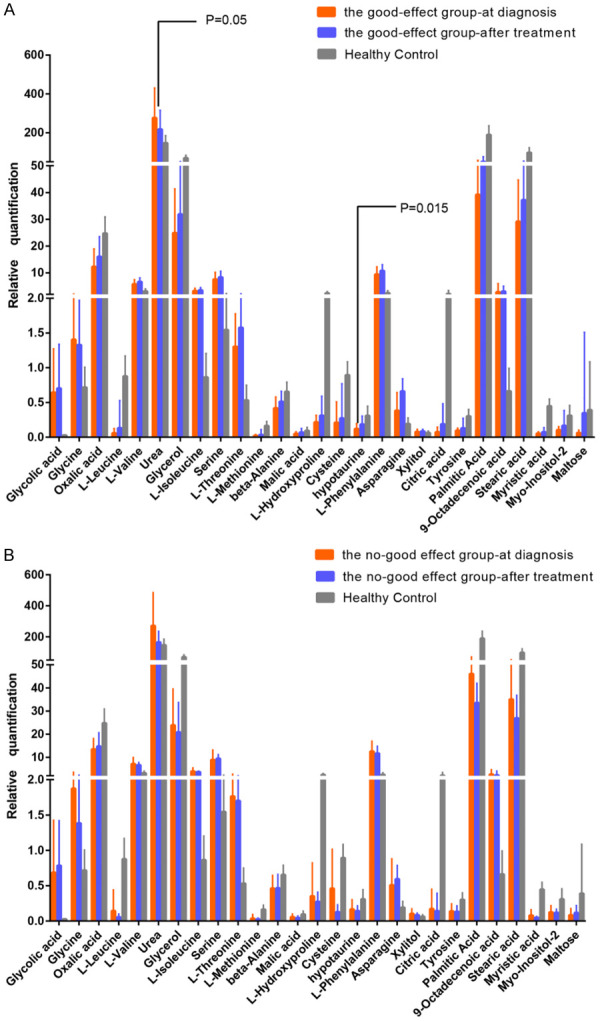
The changed tendency of two special metabolic substances in 31 paired samples from same multiple myeloma patients after treatment and comparion of healthy group. A: The changes of metabolites in patients with good treatment effect and health controls were illustrated. Levels of urea were significantly decreased after treatment while levels of hypotaurine showed significant increase. B: The changes of metabolites in patients without good treatment effect and health controls are illustrated. (CR and VGPR are considered as the good-effect group, while PR, MR, SD and PD are thought to be the no-good-effect group).
The association between metabolites and clinical characteristics among the patients with MM
We analyzed the association of the clinical characteristics with biomarkers (Mann-Whitney U test). In our data, significant metabolic changes were observed in patients with renal impairment and bone destruction. Urea levels were obviously increased (P=0.047), while oxalic acid levels were significantly decreased (P=0.029) in renal impairment patients. In patients with bone destruction, glycolic acid (P=0.015), serine, malic acid (P=0.025) and L-phenylalanine (P=0.032) levels increased significantly compare to those without bone destruction (Table S3).
Potential metabolites associated with treatment outcome
We first performed univariate analyses with Logistic regression to predict the treatment outcome of multiple myeloma with 27 metabolites and the following clinical characteristics at diagnosis: sex, age, abnormal karyotype, deletion of 13q14, 1q21 amplification/gain, Rb1 deletion, IgH rearrangement, abnormal light chain, renal function (serum creatinine levels), destructive bone lesions on imaging, M protein, total protein, plasma cell percentage, hemoglobin levels, platelet levels, serum calcium levels, serum beta 2 microglobulin levels, serum albumin levels, serum lactate dehydrogenase (LDH) levels, serum C-reaction protein (CRP) levels and 24-hour urine protein levels. Our data showed that age, presence of destructive bone lesions on imaging, serum calcium levels, serum albumin levels and CRP levels, which were included in the subsequent multivariate models (Logistic regression), were associated with treatment outcome of multiple myeloma. Regarding metabolites, we divided all biomarkers into low and high groups on the basis of the Youden index. The univariate analyses of biomarkers showed high levels of beta-alanine (cutoff =0.388, OR 4.25, 95 CI 1.05-17.2, P=0.043), cysteine (cutoff =0.102, OR 4.8, 95 CI 1.4-16.46, P=0.013) and hypotaurine (cutoff =0.120, OR 6.46, 95 CI 1.76-23.71, P=0.005) were associated with poor treatment outcome of multiple myeloma. In multivariate analyses, high beta-alanine is a risk factor of poor treatment outcome, but it is not independent (OR 3.71, 95 CI 0.66-20.84, P=0.136). Both high cysteine and high hypotaurine are independent risk factors for poor treatment outcome (cysteine OR 11.84, 95 CI 1.91-73.59, P=0.008; hypotaurine OR 7.43, 95 CI 1.38-40.06, P=0.02) (Table S4). Comparison of ROC curves (Figure 3A) for the clinical characteristics alone with those for the clinical characteristics combined with cysteine (Figure 3B) and/or hypotaurine (Figure 3C) to predict treatment outcome showed that a model including both cysteine and hypotaurine (Figure 3D) had a significantly larger AUC than a model with clinical characteristics alone (P=0.09872). According to the leave-one-out cross validation, the prediction accuracy of the model with both cysteine and hypotaurine was 0.71. Therefore, the model with cysteine and hypotaurine was superior to the model with clinical factors alone in predicting treatment outcome of multiple myeloma.
Figure 3.
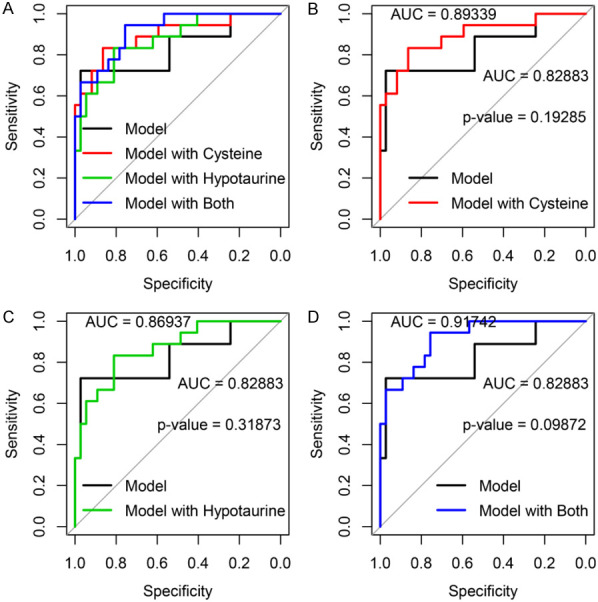
Models consisting of clinical characteristics alone or the clinical characteristics combined with cysteine, hypotaurine, or both in predicting MM. The models consisting of clinical characteristics combined with cysteine, hypotaurine, or both, were compared to the models consisting of clinical characteristics alone (A). The models with cysteine (B) or hypotaurine (C) had larger AUC compared with clinical characteristics alone, but the difference was not significant (B, P=0.19285; C, P=0.31873). The model with clinical characteristics alone was inferior to model with cysteine and hypotaurine (D, P=0.09872). All AUC comparisons were based on Delong’s test.
Potential metabolites associated with OS and PFS
We analyzed the association of all these biomarkers with overall survival (OS) based on COX regression. In univariate analyses, no biomarkers had significant effect on OS. We next investigated the likely relationships between the biomarkers and disease progression-free survival with COX regression. Patients were divided into low and high levels of biomarkers groups. The cutoff points were obtained by using a visual assessment of the functional form of the association of each biomarker with patients’ outcome (i.e., a plot of Martingale residuals from a null Cox model against each biomarker). According to Kaplan-Meier analyses, low levels of glycolic acid (cutoff =0.2, P=0.0032, Figure 4A), L-valine (cutoff =6, P=0.0249, Figure 4B), serine (cutoff =6, P=0.0295, Figure 4C), malic acid (cutoff =0.03, P=0.0262, Figure 4D), L-phenylalanine (cutoff =7.5, P=0.0224, Figure 4E), and xylitol (cutoff =0.075, P=0.0147, Figure 4F) were associated with disease progression.
Figure 4.
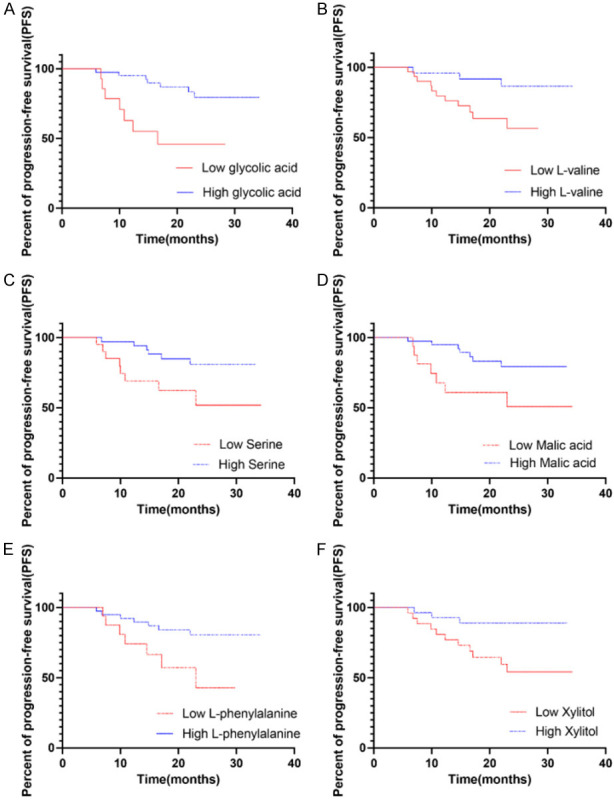
Kaplan Meier curves for progression free survival (PFS) of different metabolites. The cumulative incidence of progression-free survival between patients divided into low and high based the level of glycolic acid, L-valine, serine, malic acid, L-phenylalanine and xylitol are compared. Low levels of glycolic acid (cutoff =0.2, P=0.0032), L-valine (cutoff =6, P=0.0249), serine (cutoff =6, P=0.0295), malic acid (cutoff =0.03, P=0.0262), L-phenylalanine (cutoff =7.5, P=0.0224), and xylitol (cutoff =0.075, P=0.0147) were associated with disease progression.
In univariate analyses, the following clinical characteristics were related to disease progression: plasma cell percentage and serum LDH levels. After adjustment for these significant clinical characteristics, patients with high levels of glycolic acid, and xylitol were less likely to experience disease progression (glycolic acid HR 0.2521, 95 CI 0.08322-0.7639, P=0.0148; xylitol HR 0.2789, 95 CI 0.07571-1.028, P=0.055), which can be considered as independent protective factors. High levels of L-valine, serine, malic acid and L-phenylalanine were also protective factors, but they were not independent. (L-valine HR 0.3761, 95 CI 0.09801-1.443, P=0.154; serine HR 0.5193, 95 CI 0.1618-1.667, P=0.2708, malic acid HR 0.456, 95 CI 0.1424-1.461, P=0.1862, L-phenylalanine HR 0.4368, 95 CI 0.1321-1.445, P=0.175) (Tables 2, S5).
Table 2.
Effect of potential risk factors on PFS
| Variable | Multivariate | Multivariate | Multivariate | Multivariate | Multivariate | Multivariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
|
||||||||
| Hazard Ratio (95% CI) | P value | Hazard Ratio (95% CI) | P value | Hazard Ratio (95% CI) | P value | Hazard Ratio (95% CI) | P value | Hazard Ratio (95% CI) | P value | Hazard Ratio (95% CI) | P value | Hazard Ratio (95% CI) | P value | |
| Plasmacyte percentage (Continuous) | 1.024 (0.9984, 1.049) | 0.0669 | 1.0305 (1.00389, 1.0579) | 0.0244 | 1.0211 (0.9951, 1.048) | 0.113 | 1.0184 (0.9928, 1.045) | 0.1612 | 1.016 (0.9879, 1.044) | 0.2736 | 1.0138 (0.9863, 1.042) | 0.329 | 1.0229 (0.99786, 1.049) | 0.0734 |
| ALDH (Continuous) | 1.006 (1.0004, 1.012) | 0.0352 | 1.0050 (0.99954, 1.0105) | 0.0724 | 1.0046 (0.99873, 1.010) | 0.125 | 1.0052 (0.9998, 1.011) | 0.0606 | 1.006 (1.0001, 1.012) | 0.0446 | 1.0062 (1.0005, 1.012) | 0.034 | 1.0045 (0.99884, 1.010) | 0.12 |
| Glycolic acid (High vs. Low cutoff =0.2) | 0.2521 (0.08322, 0.7639) | 0.0148 | ||||||||||||
| L-Valine (High vs. Low cutoff =6) | 0.3761 (0.09801, 1.443) | 0.154 | ||||||||||||
| Serine (High vs. Low cutoff =6) | 0.5193 (0.1618, 1.667) | 0.2708 | ||||||||||||
| Malic acid (High vs. Low cutoff =0.03) | 0.456 (0.1424, 1.461) | 0.1862 | ||||||||||||
| L-Phenylalanine (High vs. Low cutoff =0.102) | 0.4368 (0.1321, 1.445) | 0.175 | ||||||||||||
| Xylitol (High vs. Low cutoff =0.075) | 0.2789 (0.07571, 1.028) | 0.055 | ||||||||||||
The models consisting of clinical characteristics combined with all single significant biomarkers (selected by P<0.1) on PFS.
LDH, serum lactate dehydrogenase levels.
Discussion
Metabolic changes constitue a general hallmark for most cancers [17]. In our study, all serum metabolites were extracted by GC-MS to create a metabolic fingerprint of MM. We found 27 metabolites to be significantly different between healthy controls and MM patients among which 11 metabolites increased and 16 metabolites decreased in the MM patients compared with healthy controls. However, experimental results show great variability among different technologies. Yasuyuki et al. [18] noted that levels of saturated and n-6 polyunsaturated fatty acids increased significantly in MM patients compared to the control group with time-of-flight secondary ion mass spectrometry (TOF-SIMS). Leonor et al. [19] showed that metabolic profiles of multiple myeloma patients at diagnosis exhibited higher levels of isoleucine, arginine, acetate, phenylalanine, and tyrosine, and decreased levels of 3-hydroxybutyrate, lysine, glutamine, and some lipids compared with the control set by 1H-NMR.
It is well known that urea is associated with renal impairment [20,21]. In our study, higher levels of urea were correlated with renal dysfunction. In the previous report, oxalic acid could react with calcium in vivo to form insoluble calcium oxalate and has been clearly linked with acute renal impairment [22]. In our data, the levels of oxalic acid were low in newly diagnosis MM patients, especially in renal impairment group, which suggests that oxalic acid may not be directly related to renal impairment in MM patients.
Phenylketonuria (PKU), a rare disease resulting from deficiency of phenylalanine hy-droxylase, is often complicated by progressive bone impairment, which suggests that phenylalanine may affect bone metabolism [23]. However, the exact relationship between phenylalanine and bone impairment is not clear. It is reported that high variations of phenylalanine levels were associated with osteoporosis in children and young people with PKU [24]. In our data, patients with bone destruction had high levels of phenylalanine. Expression of nuclear factor of activated T cells (NFAT) 2 plays an important role in multinucleated cell formation, which is essential for osteoclastogenesis [25]. It was observed that L-serine showed NFAT2-inducing activity. In our study, MM patients with bone destruction tended to have high L-serine levels, which is consistent with the observation above.
Our data showed that age, presence of destructive bone lesions on imaging, serum calcium levels, serum albumin levels and CRP levels were associated with treatment outcome of multiple myeloma. We also found cysteine and hypotaurine were associated with predicting treatment effects. Cysteine is a semi-essential amino acid required in the manufacture of amino acid taurine and hypotaurine [26]. Cysteine is a precursor for hypotaurine synthesis, where they may share the same signaling pathway. Levels of hypotaurine were decreased at diagnosis in MM patients. After treatment, levels of hypotaurine increased significantly in patients with good outcome, but was still very low in patients with ineffective treatment, which suggests that low levels of hypotaurine may have a certain relationship with the occurrence of MM. Hypotaurine, as an antioxidant, has also been reported to play an important role in the hepatoprotective effect against oxidative stress-mediated liver injuries [27,28]. Additionally, it has been reported that hypotaurine can quench oxidants released by human neutrophils and inhibit lipid peroxidation due to its antioxidant activity [29]. Oxidative stress is associated with ROS and ROS is known to be induced to a level that triggers apoptosis of cancer cells by chemotherapy agents [30,31]. The mechanism of hypotaurine in MM patients is unclear.
Glycolic acid is the smallest alpha-hydroxy acid (AHA). Glycolic acid is a known inhibitor of tyrosinase [32]. MM implicates JAK1 and JAK2 genes in its pathogenesis, which is similar to pathogenesis in myelofibrosis [33]. It was found that JAK1 and JAK2 were overexpressed in 27% and 57% of MM patients respectively. The success of JAK inhibitors in myelofibrosis has prompted preclinical experiments in other hematologic cancers, specifically MM, owing to similarities in their pathogenesis [34]. Success in preclinical data using JAK inhibitors for the treatment of MM has further prompted early-phase studies. In our study, we found high levels of glycolic acid were associated with longer PFS. Whether the increase levels of glycolic acid are related to JAK signaling pathway and the prognosis of multiple myeloma patients is not clear, and needs to be investigated in the future.
In our study, high levels of xylitol prolonged PFS. Little is known about the effect of xylitol against cancer cells. A higher concentration of xylitol is required to inhibit the growth of normal cells, suggesting that xylitol is more cytotoxic for cancer cells [35]. More studies confirmed this inhibitory effect of xylitol with a variety of cell lines [36,37]. Moreover, xylitol induced cell morphological changes and autophagy in lung cancer cells [35]. These results indicate that xylitol could be a candidate for a novel anti-cancer agent.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Nos. 81730003, 81773361 and 81974001), the Jiangsu “333” Talent Project (BRA2015497), the Jiangsu Social Development Program (BE2018651), the Jiangsu Summit Six Top Talent Person project, and Jiangsu Medical Junior Talent Person award (QNRC2016707). National Science and Technology Major Project (2017ZX09304021). National Key R&D Program of China (2019YFC0840604, 2017YFA0104502), Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), Jiangsu Medical Outstanding Talents Project (JCRCA2016002), Jiangsu Provincial Key Medical Center (YXZXA2016002). All the samples were from Jiangsu Biobank of Clinical Resources. All subjects (55 patients and 37 healthy controls) provided written informed consent before the start of this study in accordance with the Declaration of Helsinki and the approval from the Faculty Hospital Ethics Committee at the First Affiliated Hospital of Soochow University (Registration number approved by ethics committee for this study: 2015054).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364:1046–1060. doi: 10.1056/NEJMra1011442. [DOI] [PubMed] [Google Scholar]
- 2.Chng WJ, Dispenzieri A, Chim CS, Fonseca R, Goldschmidt H, Lentzsch S, Munshi N, Palumbo A, Miguel JS, Sonneveld P, Cavo M, Usmani S, Durie BG, Avet-Loiseau H. IMWG consensus on risk stratification in multiple myeloma. Leukemia. 2014;28:269–277. doi: 10.1038/leu.2013.247. [DOI] [PubMed] [Google Scholar]
- 3.Munker R, Monohan G. Progress in multiple myeloma. Indian J Med Res. 2019;149:693–694. doi: 10.4103/ijmr.IJMR_770_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steiner N, Müller U, Hajek R, Sevcikova S, Borjan B, Jöhrer K, Göbel G, Pircher A, Gunsilius E. The metabolomic plasma profile of myeloma patients is considerably different from healthy subjects and reveals potential new therapeutic targets. PLoS One. 2018;13:e0202045. doi: 10.1371/journal.pone.0202045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao P, Xu G. Mass-spectrometry-based microbial metabolomics: recent developments and applications. Anal Bioanal Chem. 2015;407:669–680. doi: 10.1007/s00216-014-8127-7. [DOI] [PubMed] [Google Scholar]
- 6.Kumar B, Prakash A, Ruhela RK, Medhi B. Potential of metabolomics in preclinical and clinical drug development. Pharmacol Rep. 2014;66:956–963. doi: 10.1016/j.pharep.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 7.Goveia J, Pircher A, Conradi LC, Kalucka J, Lagani V, Dewerchin M, Eelen G, DeBerardinis RJ, Wilson ID, Carmeliet P. Meta-analysis of clinical metabolic profiling studies in cancer: challenges and opportunities. EMBO Mol Med. 2016;8:1134–1142. doi: 10.15252/emmm.201606798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheung PK, Ma MH, Tse HF, Yeung KF, Tsang HF, Chu MKM, Kan CM, Cho WCS, Ng LBW, Chan LWC, Wong SCC. The applications of metabolomics in the molecular diagnostics of cancer. Expert Rev Mol Diagn. 2019;19:785–793. doi: 10.1080/14737159.2019.1656530. [DOI] [PubMed] [Google Scholar]
- 9.Wang Z, Liu X, Liu X, Sun H, Guo Z, Zheng G, Zhang Y, Sun W. UPLC-MS based urine untargeted metabolomic analyses to differentiate bladder cancer from renal cell carcinoma. BMC Cancer. 2019;19:1195. doi: 10.1186/s12885-019-6354-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim DJ, Yang J, Seo H, Lee WH, Ho Lee D, Kym S, Park YS, Kim JG, Jang IJ, Kim YK, Cho JY. Colorectal cancer diagnostic model utilizing metagenomic and metabolomic data of stool microbial extracellular vesicles. Sci Rep. 2020;10:2860. doi: 10.1038/s41598-020-59529-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaiser RA, Pessia A, Ateeb Z, Davanian H, Fernández Moro C, Alkharaan H, Healy K, Ghazi S, Arnelo U, Valente R, Velagapudi V, Sällberg Chen M, Del Chiaro M. Integrated targeted metabolomic and lipidomic analysis: a novel approach to classifying early cystic precursors to invasive pancreatic cancer. Sci Rep. 2019;9:10208. doi: 10.1038/s41598-019-46634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Plewa S, Horała A, Dereziński P, Nowak-Markwitz E, Matysiak J, Kokot ZJ. Wide spectrum targeted metabolomics identifies potential ovarian cancer biomarkers. Life Sci. 2019;222:235–244. doi: 10.1016/j.lfs.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Chen X, Yu D. Metabolomics study of oral cancers. Metabolomics. 2019;15:22. doi: 10.1007/s11306-019-1483-8. [DOI] [PubMed] [Google Scholar]
- 14.Yan M, Xu G. Current and future perspectives of functional metabolomics in disease studies-a review. Anal Chim Acta. 2018;1037:41–54. doi: 10.1016/j.aca.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 15.MacIntyre DA, Jiménez B, Lewintre EJ, Martín CR, Schäfer H, Ballesteros CG, Mayans JR, Spraul M, García-Conde J, Pineda-Lucena A. Serum metabolome analysis by 1H-NMR reveals differences between chronic lymphocytic leukaemia molecular subgroups. Leukemia. 2010;24:788–797. doi: 10.1038/leu.2009.295. [DOI] [PubMed] [Google Scholar]
- 16.Commisso M, Strazzer P, Toffali K, Stocchero M, Guzzo F. Untargeted metabolomics: an emerging approach to determine the composition of herbal products. Comput Struct Biotechnol J. 2013;4:e201301007. doi: 10.5936/csbj.201301007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel S, Ahmed S. Emerging field of metabolomics: big promise for cancer biomarker identification and drug discovery. J Pharm Biomed Anal. 2015;107:63–74. doi: 10.1016/j.jpba.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 18.Nagata Y, Ishizaki I, Waki M, Ide Y, Hossen MA, Ohnishi K, Miyayama T, Setou M. Palmitic acid, verified by lipid profiling using secondary ion mass spectrometry, demonstrates anti-multiple myeloma activity. Leuk Res. 2015;39:638–645. doi: 10.1016/j.leukres.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 19.Puchades-Carrasco L, Lecumberri R, Martínez-López J, Lahuerta JJ, Mateos MV, Prósper F, San-Miguel JF, Pineda-Lucena A. Multiple myeloma patients have a specific serum metabolomic profile that changes after achieving complete remission. Clin Cancer Res. 2013;19:4770–4779. doi: 10.1158/1078-0432.CCR-12-2917. [DOI] [PubMed] [Google Scholar]
- 20.Zittema D, Casteleijn NF, Bakker SJ, Boesten LS, Duit AA, Franssen CF, Gaillard CA, Gansevoort RT. Urine concentrating capacity, vasopressin and copeptin in ADPKD and IgA nephropathy patients with renal impairment. PLoS One. 2017;12:e0169263. doi: 10.1371/journal.pone.0169263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al-Ani A, Al-Jalham K, Ibrahim T, Majzoub A, Al-Rayashi M, Hayati A, Mubarak W, Al-Rayahi J, Khairy AT. Factors determining renal impairment in unilateral ureteral colic secondary to calcular disease: a prospective study. Int Urol Nephrol. 2015;47:1085–1090. doi: 10.1007/s11255-015-0986-0. [DOI] [PubMed] [Google Scholar]
- 22.Meimaridou E, Jacobson J, Seddon AM, Noronha-Dutra AA, Robertson WG, Hothersall JS. Crystal and microparticle effects on MDCK cell superoxide production: oxalate-specific mitochondrial membrane potential changes. Free Radic Biol Med. 2005;38:1553–1564. doi: 10.1016/j.freeradbiomed.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 23.Roato I, Porta F, Mussa A, D’Amico L, Fiore L, Garelli D, Spada M, Ferracini R. Bone impairment in phenylketonuria is characterized by circulating osteoclast precursors and activated T cell increase. PLoS One. 2010;5:e14167. doi: 10.1371/journal.pone.0014167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barat P, Barthe N, Redonnet-Vernhet I, Parrot F. The impact of the control of serum phenylalanine levels on osteopenia in patients with phenylketonuria. Eur J Pediatr. 2002;161:687–688. doi: 10.1007/s00431-002-1091-9. [DOI] [PubMed] [Google Scholar]
- 25.Ogawa T, Ishida-Kitagawa N, Tanaka A, Matsumoto T, Hirouchi T, Akimaru M, Tanihara M, Yogo K, Takeya T. A novel role of L-serine (L-Ser) for the expression of nuclear factor of activated T cells (NFAT)2 in receptor activator of nuclear factor kappa B ligand (RANKL)-induced osteoclastogenesis in vitro. J Bone Miner Metab. 2006;24:373–379. doi: 10.1007/s00774-006-0705-0. [DOI] [PubMed] [Google Scholar]
- 26.Ding S, Fang J, Liu G, Veeramuthu D, Naif Abdullah AD, Yin Y. The impact of different levels of cysteine on the plasma metabolomics and intestinal microflora of sows from late pregnancy to lactation. Food Funct. 2019;10:691–702. doi: 10.1039/c8fo01838c. [DOI] [PubMed] [Google Scholar]
- 27.Wan QL, Fu X, Meng X, Luo Z, Dai W, Yang J, Wang C, Wang H, Zhou Q. Hypotaurine promotes longevity and stress tolerance via the stress response factors DAF-16/FOXO and SKN-1/NRF2 in Caenorhabditis elegans. Food Funct. 2020;11:347–357. doi: 10.1039/c9fo02000d. [DOI] [PubMed] [Google Scholar]
- 28.Sakuragawa T, Hishiki T, Ueno Y, Ikeda S, Soga T, Yachie-Kinoshita A, Kajimura M, Suematsu M. Hypotaurine is an energy-saving hepatoprotective compound against ischemia-reperfusion injury of the rat liver. J Clin Biochem Nutr. 2010;46:126–134. doi: 10.3164/jcbn.09-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Green TR, Fellman JH, Eicher AL, Pratt KL. Antioxidant role and subcellular location of hypotaurine and taurine in human neutrophils. Biochim Biophys Acta. 1991;1073:91–97. doi: 10.1016/0304-4165(91)90187-l. [DOI] [PubMed] [Google Scholar]
- 30.Zou Z, Chang H, Li H, Wang S. Induction of reactive oxygen species: an emerging approach for cancer therapy. Apoptosis. 2017;22:1321–1335. doi: 10.1007/s10495-017-1424-9. [DOI] [PubMed] [Google Scholar]
- 31.Martinez-Outschoorn U, Sotgia F, Lisanti MP. Tumor microenvironment and metabolic synergy in breast cancers: critical importance of mitochondrial fuels and function. Semin Oncol. 2014;41:195–216. doi: 10.1053/j.seminoncol.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 32.Ma D, Tu ZC, Wang H, Zhang L, He N, McClements DJ. Mechanism and kinetics of tyrosinase inhibition by glycolic acid: a study using conventional spectroscopy methods and hydrogen/deuterium exchange coupling with mass spectrometry. Food Funct. 2017;8:122–131. doi: 10.1039/c6fo01384h. [DOI] [PubMed] [Google Scholar]
- 33.Plosker GL. Ruxolitinib: a review of its use in patients with myelofibrosis. Drugs. 2015;75:297–308. doi: 10.1007/s40265-015-0351-8. [DOI] [PubMed] [Google Scholar]
- 34.Ghermezi M, Spektor TM, Berenson JR. The role of JAK inhibitors in multiple myeloma. Clin Adv Hematol Oncol. 2019;17:500–505. [PubMed] [Google Scholar]
- 35.Park E, Park MH, Na HS, Chung J. Xylitol induces cell death in lung cancer A549 cells by autophagy. Biotechnol Lett. 2015;37:983–990. doi: 10.1007/s10529-014-1757-1. [DOI] [PubMed] [Google Scholar]
- 36.Han SJ, Jeong SY, Nam YJ, Yang KH, Lim HS, Chung J. Xylitol inhibits inflammatory cytokine expression induced by lipopolysaccharide from Porphyromonas gingivalis. Clin Diagn Lab Immunol. 2005;12:1285–1291. doi: 10.1128/CDLI.12.11.1285-1291.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park E, Na HS, Kim SM, Wallet S, Cha S, Chung J. Xylitol, an anticaries agent, exhibits potent inhibition of inflammatory responses in human THP-1-derived macrophages infected with Porphyromonas gingivalis. J Periodontol. 2014;85:e212–223. doi: 10.1902/jop.2014.130455. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.