Abstract
CD8+ T cells are crucial adaptive immune effectors and express receptors (T cell receptors, TCRs) that specifically recognize and eradicate tumor cells. The diversity of the TCR repertoire is generated by specialized genetic diversification mechanisms, which lead to an extremely variable TCR repertoire that is capable of recognizing a wide range of antigens. However, the variations in CD8+ TCR diversity and their clinical implications in acute myeloid leukemia (AML) patients remain unknown. CD8+ T cells were enriched from 10 healthy donors and 31 AML patients at diagnosis and after chemotherapy, and TCRβ deep sequencing was performed to analyze CD8+ T cell clonal expansion and TCR repertoire diversity. Diminished TCR repertoire diversity and increased T cell clone expansion were noted in the bone marrow of AML patients. In relapsed patients, T cells were found to be more clonally expanded after chemotherapy than at new diagnosis. Moreover, significantly more expanded TCRβ clonotypes were noted in CD8+ PD-1+ T cells than in CD8+ PD-1- T cells regardless of the time of examination. Our systematic T cell repertoire analysis may help better characterize CD8+ T cells before and after chemotherapy in AML, which may provide insights into therapeutic strategies for hematological malignancies.
Keywords: CD8+ T cell, T cell receptor, bone marrow, programmed death-1, acute myeloid leukemia
Introduction
Acute myeloid leukemia (AML) is a progressive malignant disorder that is often associated with poor survival rates. In recent decades, little has changed in the induction chemotherapy used to treat AML. While the majority of AML patients achieve complete remission (CR) at the outset, most of them fail to sustain such responses for a long time due to chemoresistance [1]. Considerable effort has been dedicated to elucidating the drug resistance mechanisms in leukemia, but little attention has been paid to the differences in immune signaling in response to chemotherapy between responsive patients and nonresponsive patients [2]. Cancer immunotherapy has attracted considerable attention as a novel therapeutic approach because of reported successes in checkpoint blockade intervention and T cell engineering [3]. Potential new immunotherapeutic modalities hold much promise for the treatment of AML and are likely to increase in the future [4-8]; however, many significant challenges remain [9,10]. Hence, characterization of the immune and biological features of this disease is the key to optimizing the therapeutic strategy for AML patients.
CD8+ T cells are the main cytotoxic effector cells and mediate apoptosis through the T cell receptor (TCR), which recognizes tumor-expressed antigenic peptides bound to MHC class I molecules [11]. The TCR is composed of two constituent chains (αβ or γδ), which undergo a V(D)J recombination process. As the most diverse portion of the variable region, complementarity-determining region 3 (CDR3) is the primary contributor to the specificity of T cell antigen recognition [12,13]. Recent studies suggested that δ-CDR3s in γδ T cells can predict clinical responses in AML patients [14,15]. Despite the potential of the TCR as a therapeutic target, CD8+ TCR repertoire dynamics and the mechanisms by which the TCR is modulated during chemotherapy are still poorly understood. αβ T cells are the predominant subset, making up approximately 90-95% of all peripheral T cells in healthy adults [16]. Therefore, it is crucial to assess the characteristics of the CD8+ TCR repertoire and investigate its clinical significance in AML patients.
It has become increasingly clear that programmed death-1 (PD-1) is a key regulator of T cell dysfunction after exposure to antigen stimulation [17]. A recent study reported functional skewing of CD8+ T cells in newly diagnosed AML patients, which was correlated with upregulation of PD-1 expression [18]. As expected, the response to chemotherapy is associated with upregulation of costimulatory molecules and attenuation of apoptotic signaling [19]. Compared to that at the time of new diagnosis, significantly higher PD-1 expression on CD8+ T cells was noted at the time of relapse. However, treatment with PD-1 pathway blockade or the costimulatory molecule OX40 results in increased CD8+ T cell effector function [2,6,20]. These findings indicated that implicated PD-1+ CD8+ T cells in AML progression and suggested that this factor may be used to promote cytotoxicity.
The diversity of the TCR repertoire may reflect differential recognition of neoantigens. The PD-1+ CD8+ phenotype has been identified as a marker of T cell exhaustion and has been proven to display neoantigen-specific cytotoxic activity [21,22]. A diversified TCR repertoire enables the host immune system to recognize a large variety of tumor neoantigens [23]. Hence, an improved understanding of the PD-1+ CD8+ TCR repertoire would enable more effective clinical and therapeutic strategies for AML patients.
In this study, we performed RNA sequencing of the CDR3 region from TCRβ chains of αβ CD8+ T cells and examined the different clonal expansion patterns between AML and healthy donor samples. In addition, we analyzed T cell clonality and distribution in paired patient samples collected at diagnosis and after chemotherapy. We further studied the TCR repertoire dynamics of PD-1- negative and PD-1- positive CD8+ T cells. We found that TCR repertoire diversity was correlated with clinical outcomes. Collectively, these results underscored the antitumor reactivity of CD8+ T cells and the value of the CD8+ T cell repertoire as a potential prognostic marker for AML, highlighting the importance of immune intervention.
Materials and methods
Samples
This study was approved by the Institutional Review Board (IRB) of the Affiliated Hospital of Guizhou Medical University. All subjects provided their written informed consent in accordance with the Declaration of Helsinki. The clinical and demographic data for all the subjects are summarized in Table S1. Peripheral blood mononuclear cells (PBMCs) and bone marrow mononuclear cells (BMMCs) were obtained from healthy donors and AML patients at diagnosis and after chemotherapy. Complete remission (CR) was considered to be less than 5% blasts in the bone marrow (BM) by morphologic evaluation, with a neutrophil count greater than 1 × 109/L and a platelet count greater than 1 × 1011/L.
Flow cytometry
PBMCs or BMMCs were collected via density gradient centrifugation using Ficoll-Paque reagents (GE Healthcare, Sweden), cryopreserved in 90% human serum, and formulated with 10% DMSO. CD8+ T cells were purified using the Dynabeads CD8 Positive Isolation Kit (Life Technologies, Norway) according to the manufacturer’s protocol. The purity of sorted T cell samples was >90%, as determined by flow cytometry. PD-1 expression analysis of CD8+ T cells and CD8+ PD-1+ and CD8+ PD-1- T cell sorting was performed on a BD Biosciences FACS Aria II.
RNA isolation and TCRβ sequencing
CD8+ T cells were enriched using the Dynabeads CD8 Positive Isolation Kit. RNA was extracted using the Ultrapure RNA Kit (Cwbiotech, Beijing, China). For quantitating RNA samples, a Qubit RNA HS Assay Kit (Thermo Fisher Scientific, Pleasanton CA, USA) was used. The Agilent RNA 6000 Nano Kit and Agilent 2100 Bioanalyzer were used for RNA integrity assessment. For each sample, 20 ng of total RNA was reverse transcribed using SuperScript IV VILO Master Mix (Thermo Fisher Scientific), and then 25 ng cDNA was target amplified using the Oncomine TCR Beta-LR Assay Kit (Thermo Fisher Scientific). Libraries were purified, diluted 1:100 and quantified using the Ion Library Quantitation Kit (Thermo Fisher Scientific) and then diluted to 25 pM with low TE buffer. Samples were pooled on an Ion 530 chip for sequencing via the Ion S5 System, followed by analysis via the Ion Reporter. Total RNA from the Jurkat cell line was obtained from a clonotype with a T cell phenotype. T cell leukemia (Jurkat) total RNA was derived from a cell line consisting of a single T cell clonotype. For the control, running the Oncomine™ TCR Beta Assay on Jurkat total RNA detected a single clonotype.
Equal numbers of available cells in CD8+ PD-1+ (2~5 × 104 cells) and CD8+ PD-1- (2~5 × 104 cells) T cell populations from each sample were collected for RNA extraction and subsequent TCRβ sequencing.
Data processing and analysis
Repertoire quality metric analysis was performed via the Oncomine™ TCR Beta-LR Assay workflow in the Ion Reporter™ Software. The Shannon index and Gini index were used to characterize the frequency and sequence features of clonotypes. The Gini index was calculated as A/(A+B), in which, for a set of clone frequencies, A represents the difference between the total area under the line of equality and the area under the Lorenz curve, and B represents the area under the Lorenz curve. The index ranges from 0 to 1. Higher values indicate dissimilar clone sizes, while lower values indicate more similar clone sizes. Shannon entropy was calculated as - ∑R i=1 pilog2(pi), where pi indicates the frequency of the ith clone, and R indicates the total number of clones [24]. Samples with many clones of similar frequencies have high Shannon diversity. Subsequent analysis of the TCR repertoire was performed using VDJtools [25], tcRpackages [26], Treemap [27], and mothur [28].
The Mann-Whitney U-test was used to determine whether there were differences between the two groups. Analysis of covariance was initially used for the multiple group comparison. Correction for multiple tests was performed using the false discovery rate method. The Wilcoxon signed-rank test was used for matched paired comparisons.
Results
Extensive clonally expanded CD8+ T cell populations in the BM of AML patients
The overall design of this study is shown schematically in Figure 1. The distribution plot of the top 100 TCR clonotypes in BM and PB from one AML patient and one healthy donor is shown in Figure 2A. The graph demonstrates increased clonal expansion in the BM of the AML patient compared to the other groups. Figure 2B shows that the total/unique clonotype ratios were higher in the BM of AML patients than in the PB of AML patients and in the BM and PB of healthy donors. A markedly higher frequency of highly expanded clones (HECs) [29] was noted in the BM and PB of AML patients than in those of healthy donors (Figure 2C). In addition, the Shannon index and Gini index were used to evaluate the TCR repertoire diversity. As shown in Figure 2D, the Shannon index for the BM of AML patients was significantly higher than that for the PB of AML patients and the BM and PB of healthy donors; in contrast, the Gini index for the BM of AML patients showed a pronounced reduction compared with that for the other groups (Figure 2E). Collectively, these findings showed that in CD8+ T cells from the BM of AML patients, a decline in T cell repertoire diversity is closely associated with clonotypic expansion.
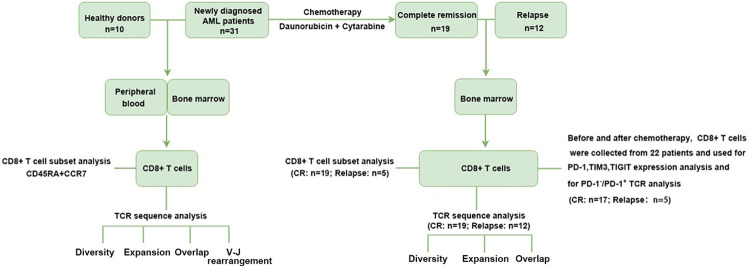
Figure 1.
Schematic illustration of the overall study design. The differences of TCR repertoire between AML patients and healthy donors were compared on BM and PB samples by evaluating several indicators, e.g., the CDR3β diversity, V-J usage, clonal expansion and sequence overlap. CD8+ T cells in BM and PB from AML patients and healthy donors were phenotypically analyzed based on the coordinated expression of CD45RA and CCR7. The dynamics of TCR repertoire, phenotypic composition, expression levels of co-inhibitory receptors including PD-1, TIM3, TIGIT, and TCR repertoire distribution in PD-1-/PD-1+ T cells were assessed in BM CD8+ T cells from AML patients before and after chemotherapy.
Figure 2.
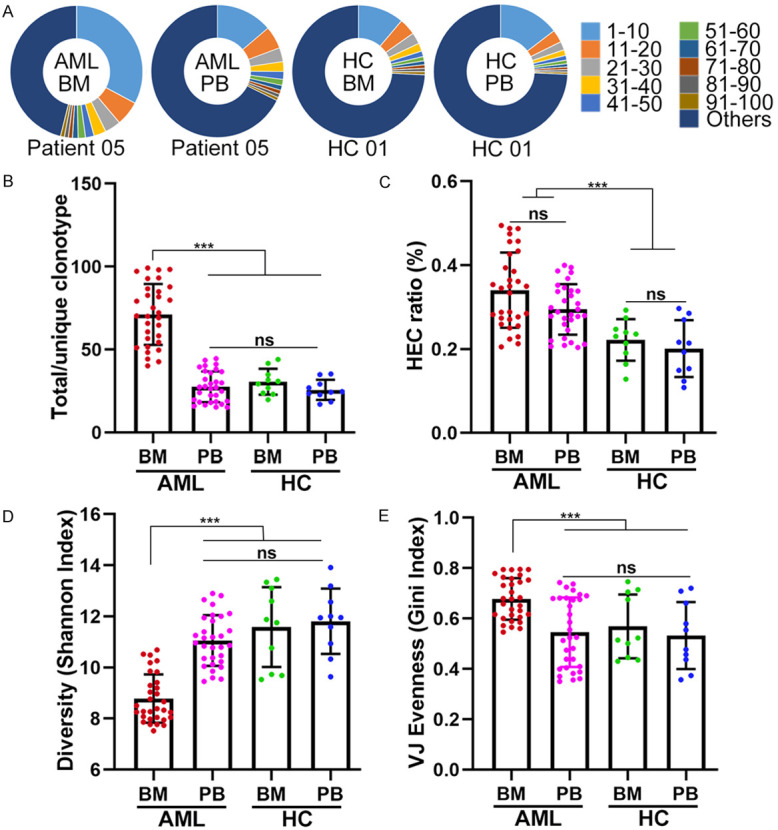
Clonal expansion and diversity of PB and BM CD8+ T cells from AML patients and healthy donors. (A) The distribution profile of the top 100 clonotypes from the BM and PB of one AML patient and one healthy donor, as depicted in a pie chart. The TCR repertoire diversity was evaluated by the total/unique clonotype ratio (B), the HEC ratio (C), the Shannon diversity index (D), and the Gini index (E) in four study groups containing BM (n = 31) and PB (n = 31) samples obtained from AML patients and BM (n = 10) and PB (n = 10) samples obtained from healthy donors. A dot is used to represent one patient or one donor sample. Analysis of covariance was initially used for the multiple group comparison. Correction for multiple tests was performed using the false discovery rate method. ns indicates not significant; *** indicates P < 0.01.
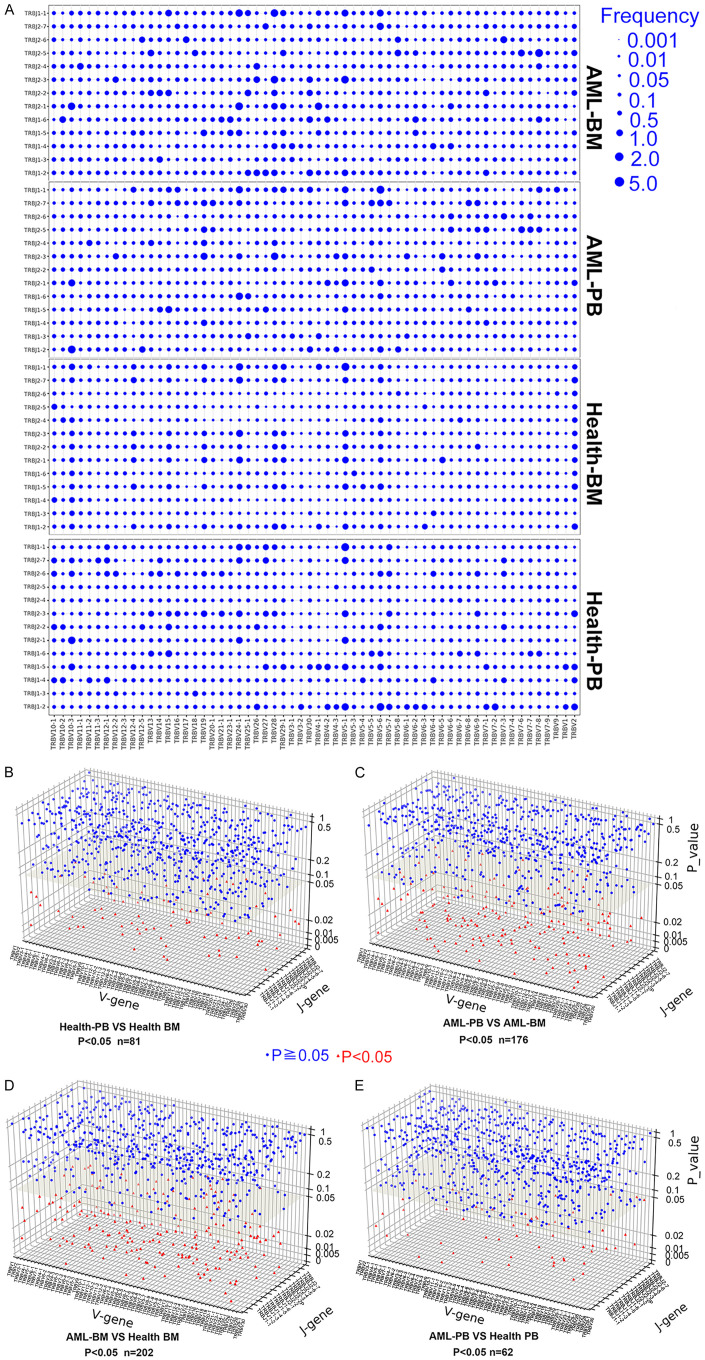
Comparison of overall usage of TCRβ V-J rearrangements in AML patients and healthy donors
We identified a total of 60 distinguishable gene transcription segments from the TCRβ V (TRBV) loci, 2 from the TCRβ D (TRBD) loci, 13 from the TCRβ J (TRBJ) loci, and 780 rearrangements in the TRBV-J region. In the graph in Figure 3A, each rearrangement event in TRBV-J is denoted by a dot, with its size indicating the average frequency of the rearrangement in the sample group. Similar overall usage profiles of the rearranged TRBV/J segments were noted between BM and PB from healthy donors (81 differentially expressed rearrangements) and between PB from AML patients and PB from healthy donors (62 differentially expressed rearrangements); however, the differences were greater between BM and PB from AML patients (176 differentially expressed rearrangements) and between BM from AML patients and BM from healthy donors (202 differentially expressed rearrangements) (Figure 3B-E). Similarly, we found comparable usage patterns of TRBV gene segments between PB from healthy donors and BM from healthy donors and between PB from healthy donors and PB from AML patients but found relatively different usage patterns between BM from healthy donors and BM from AML patients and between PB from AML patients and BM from AML patients (Figure S1). These data suggest that CD8+ T cells in the BM of AML patients exhibit expression of specific TRBV-J rearrangements, indicating that they may recognize bone marrow-specific antigens.
Figure 3.
Usage patterns of TCRβ V-J rearrangements in PB and BM CD8+ T cells from AML patients and healthy donors. (A) Dot plots depicting the mean frequency distribution of TCRβ V-J gene rearrangements for CD8+ T cells from the four groups. The variations in TCRβ V-J rearrangements for normal PB vs. healthy BM (B), AML PB vs. AML BM (C), AML BM vs. healthy BM (D), and AML PB vs. healthy PB (E) were investigated. The Mann-Whitney U-test was used to determine whether there were differences between the two groups. Blue circle, P < 0.05; red triangle, P ≥ 0.05.
Comparison of identical clonotypes in AML patients and healthy donors
We analyzed the CDR3 amino acid sequences of CD8+ T cells in PB and BM from 31 AML patients and 10 healthy donors to identify the presence of shared clones (i.e., shared CDR3 sequences) in the top 1,000 or 5,000 clones between any two sample pairs (Figure 4A). Through intragroup analysis of the CDR3 sequences between different sample pairs, we found a higher ratio of identical T cell clones between sample pairs in the bone marrow of the AML patients (AML BM VS BM, n = 465) when compared to sample pairs in other groups (AML PB VS PB, n = 465 or healthy donor BM VS BM/PB VS PB, n = 45) (Figure 4B). Intergroup analysis indicated that there was no difference in the percentage of identical T cell clones between sample pairs from different groups (AML patient BM vs. PB, n = 930; healthy donor BM vs. PB, n = 90; AML patient BM or PB vs. healthy donor BM or PB, n = 310) (Figure 4C). By analyzing identical T cell clones in the PB and BM of AML patients or healthy donors (i.e., comparison between BM and PB from the same individual, n = 31 or n = 10), we found that the percentage of identical T cell clones between PB and BM from the same individual was significantly higher than that between peripheral blood and bone marrow among different individuals. Moreover, the percentage of identical T cell clones between PB and paired BM from the healthy donors was significantly higher than that in PB and paired BM from the AML patients (Figure 4D). These results suggested that the clonal expansion of BM CD8+ T cells in AML patients was highly specific.
Figure 4.
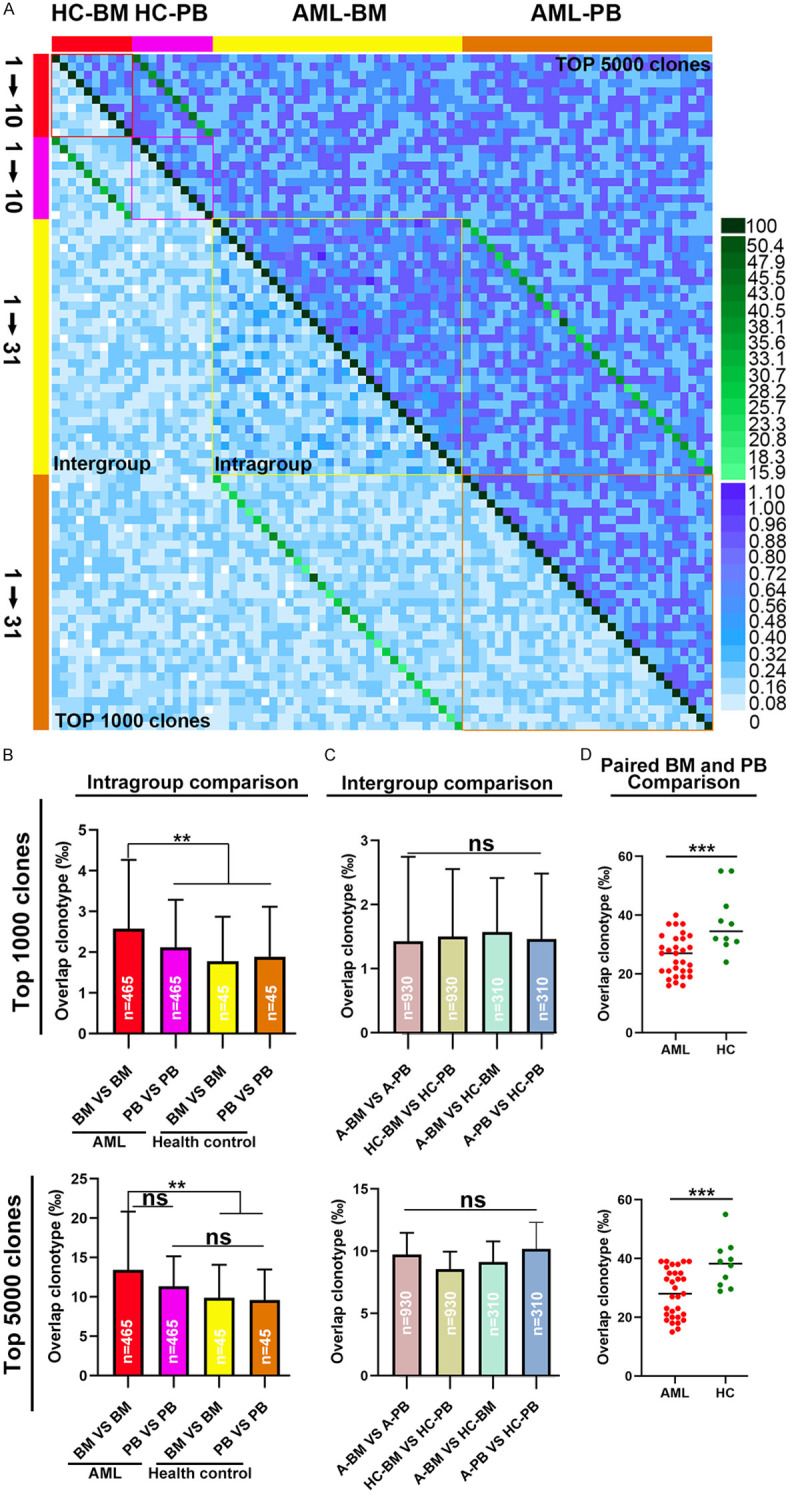
Metrics for TCR β repertoire overlap in PB and BM CD8+ T cells from AML patients and healthy donors. (A) heat map was plotted to assess the similarity of the top 1000 most abundant CDR3 amino acid sequences or the top 5000 most abundant CDR3 amino acid sequences between any two samples. (B) Based on the top 1000 or top 5000 most abundant CDR3 amino acid sequences, repertoire overlap analysis was performed within each group of samples and (C) between groups of samples. (D) Based on the top 1000 or top 5000 most abundant CDR3 amino acid sequences, overlaps between BM and PB from the same individual in AML patients and healthy donors were determined. A dot is used to represent a patient sample. The Mann-Whitney U-test was used to determine whether there were differences between the two groups. Analysis of covariance was initially used for the multiple group comparison. Correction for multiple tests was performed using the false discovery rate method. ns indicates not significant; **P < 0.05; ***P < 0.01.
TCRβ repertoire variety and stability among CR patients and relapsed patients
Figure 5A shows BM CD8+ T cell clonal expansion in one relapsed patient and one patient with sustained CR at new diagnosis and after chemotherapy. After discontinuation of chemotherapy, markedly expanded T cell clones were noted in the BM from the relapsed patient but not that from the patient who achieved CR. Looking more deeply into the clonal diversity of the TCRβ repertoires, we found considerably less diversity in the relapse post chemotherapy group than in the other groups (Figure 5B). We further calculated the V gene usage of CD8+ T cells in this relapse patient, and the results showed that compared to that at new diagnosis, the preferential usage pattern of some V gene segments changed dramatically after relapse, and the number of different clones with the same V gene usage was also remarkably different (Figure S2). We further analyzed the Shannon and Gini indexes of BM T cells in all 31 patients at new diagnosis and after chemotherapy. Compared to that at new diagnosis, the Shannon entropy of the relapsed patients showed a marked reduction, whereas the Gini index increased significantly. No difference was observed in the Shannon and Gini indexes in patients with CR (Figure 5C and 5D). By assessing the ratio of identical clones among the top 1,000 T clones in the BM of patients who achieved CR or in patients who experienced relapse at new diagnosis and after treatment, we found that the ratio of identical T clones in the relapsed patients before and after treatment was significantly lower than that in the CR group. However, when we increased the number of clones assessed to 5,000, no difference was observed between the relapse group and the CR group in terms of the ratio of identical T clones at new diagnosis and after chemotherapy (Figure 5E). These results showed that compared to those of the AML patients who were in CR after chemotherapy, some of the CD8+ T cells of the patients who relapsed after treatment exhibited massive clonal expansion and reduced clonal diversity, with a significant change in the T cell composition among the top 1,000 clones.
Figure 5.
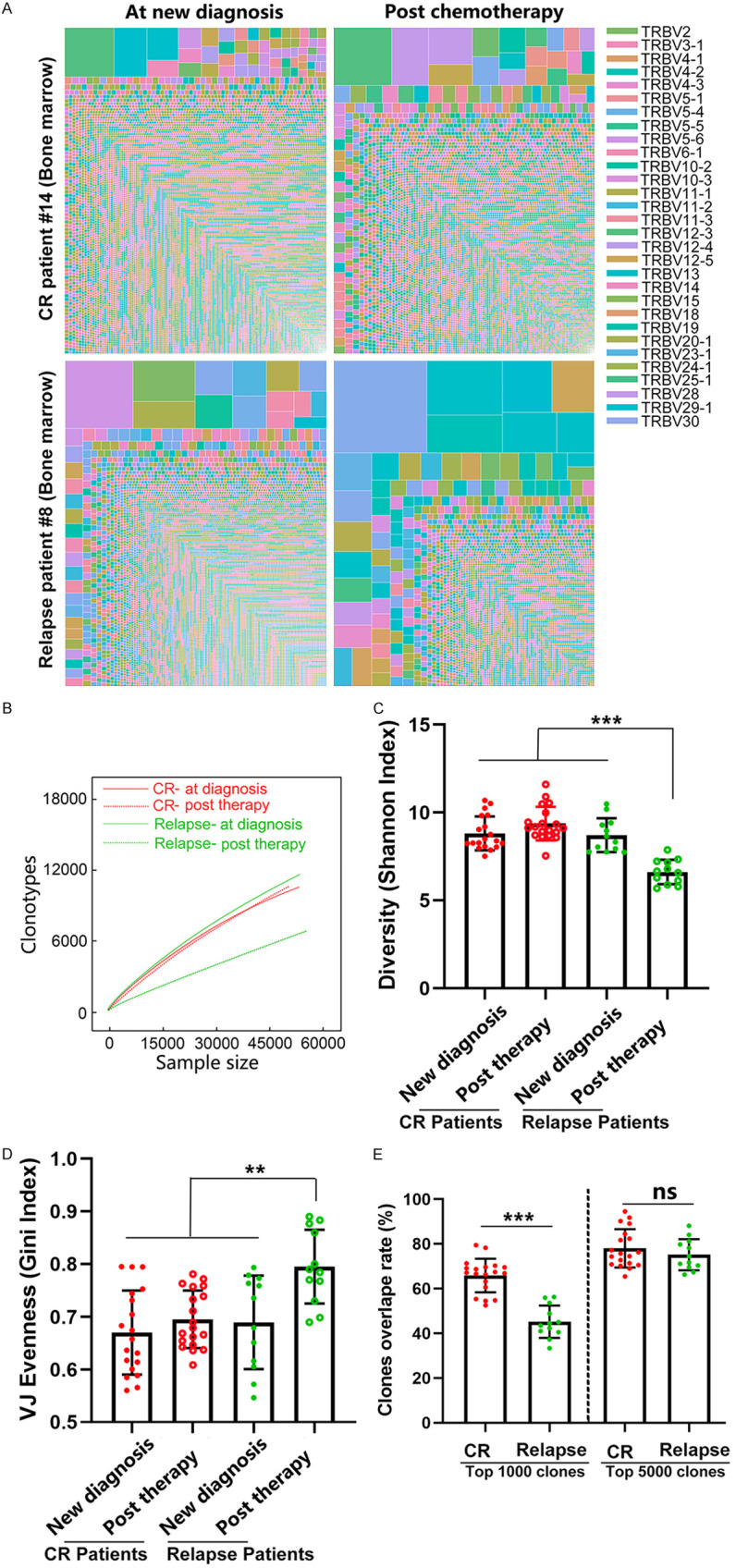
A high degree of clonal expansion in BM CD8+ T cells from relapsed AML patients. (A) Treemap showing the clone repertoires in one patient who relapsed and one patient who remained in remission at new diagnosis and after chemotherapy. Each amino acid sequence was perceived as a separate clonotype, which was represented by a square, and the frequency was indicated by its area; different V genes are represented by different colors. (B) Rarefaction analysis of repertoires from one patient who relapsed and one patient who remained in remission at new diagnosis and after chemotherapy. The number of unique clonotypes in a subsample is plotted against its size (number of TCR cDNA molecules). The diversities of the T cell receptor repertoire in 12 patients who relapsed and 19 patients who had CR after chemotherapy were characterized by computing the Shannon index (C) and the Gini index (D). A dot is used to represent a patient sample. (E) Repertoire overlaps were determined at new diagnosis and after chemotherapy in BM from the same individual based on the top 1000 or top 5000 clonotypes. CR patients: patients who were newly diagnosed with AML and remained in complete remission after chemotherapy. Relapsed patients: patients who were newly diagnosed with AML and relapsed after chemotherapy. The Mann-Whitney U-test was used to determine whether there were differences between the two groups. Analysis of covariance was initially used for the multiple group comparison. Correction for multiple tests was performed using the false discovery rate method. ns indicates not significant; **P < 0.05; ***P < 0.01.
TCR repertoire distribution of CD8+ T cells based on PD-1 expression
Four maturation states of CD8+ T cells were distinguished using CD45RA and CCR7. We found a significantly increased percentage of effector memory T cells (TEM, CD45RA-CCR7-), and CD45RA+ effector memory T cells (TEMRA, CD45RA+CCR7-) and a reduced percentage of naive T cells (T naive, CD45RA+CCR7+) and central memory T cells (TCM, CD45RA-CCR7+), in both peripheral blood and bone marrow of AML patients compared to those of HCs. Meanwhile, the percentage of TEM and TEMRA in bone marrow was higher than in peripheral blood of AML patients (Figure S3A). Following chemotherapy, the percentages of the four subsets had no changes compared to pre-treatment levels (Figure S3B). Several previous reports have suggested the functional involvement of co-inhibitors in AML progression [29-35]. Compared to patients at the time of diagnosis, PD-1-, TIGIT- or TIM3-expressing CD8+ T cells displayed a marked increase in BM of relapsed patients and a concomitant decrease in BM of patients who achieved CR (Figures 6A and S4).
Figure 6.
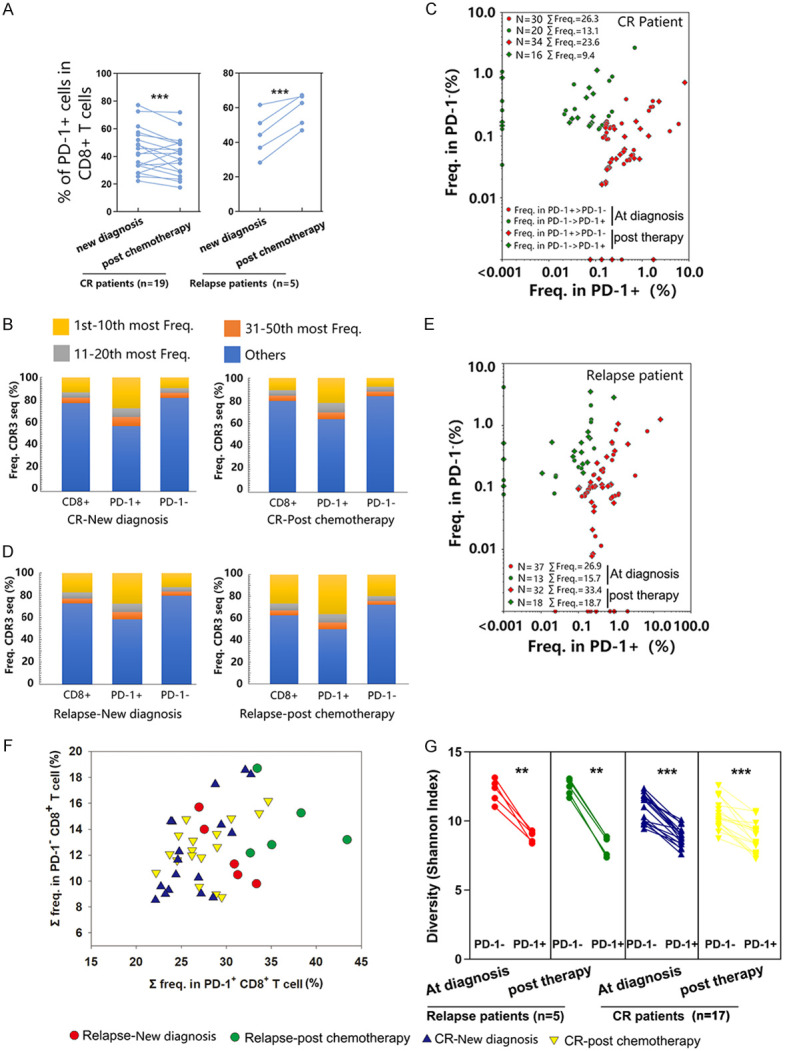
TCR repertoire distribution of CD8+ T cells based on PD-1 expression in CR and relapsed AML patients. (A) Expression of PD-1 was determined by flow cytometry. Samples from 5 patients who relapsed and 19 patients who had CR after chemotherapy were collected for flow cytometry analysis. (B-F) The clonotype distribution of CD8+ T cells was associated with PD-1 expression. CD8+ T cells were sorted from one patient who had CR (B and C) and one relapsed patient (D and E) based on the expression of PD-1. mRNA extraction and deep sequencing of TCRβ CDR3 were performed. (B and D) Analysis of TCRβ repertoire clonal frequency in CD8+ (as shown above), CD8+ PD-1+ (4 × 104 cells), and CD8+ PD-1- (4 × 104 cells) T cells. The abundances of the top 1 to 10, 11 to 20, and 21 to 50 clonotypes, as well as the remaining clonotypes, are shown. (C and E) The distribution of the top 50 most frequent clonotypes among CD8+ T cells was compared between the CD8+ PD-1+ populations and CD8+ PD-1- populations. Each dot represents a clonotype from a newly diagnosed sample; each rhombus represents a clonotype from a sample post chemotherapy. Red represents the frequency of clonotypes in CD8+ PD-1+ > CD8+ PD-1-; green represents the frequency of clonotypes in CD8+ PD-1- > CD8+ PD-1+. The total frequency (Σ freq.) of the clonotypes in each population is cumulative. (F) The top 50 clonotypes in CD8+ T cells were frequently distributed in the CD8+ PD-1+ and CD8+ PD-1- populations. Red dots represent samples collected at new diagnosis from patients who relapsed post chemotherapy; green dots represent samples collected after treatment from patients who relapsed post chemotherapy; blue triangles represent samples collected at new diagnosis from patients with sustained CR post chemotherapy; yellow triangles represent samples collected after treatment from patients with sustained CR post chemotherapy; CD8+ PD-1- and CD8+ PD-1+ T cell from 5 patients who relapsed and 17 patients who had CR after chemotherapy were collected for TCRβ CDR3 sequencing. (G) Shannon index comparison between CD8+ PD-1+ and CD8+ PD-1- populations. The Wilcoxon signed-rank test was used for matched paired comparisons. **P < 0.05; ***P < 0.01.
To determine whether CD8+ PD-1+ cells exhibited more clonal expansion, TCRβ deep sequencing of CD8+ PD-1+ and CD8+ PD-1- T cells was performed. Figure 5B-E depicts the TCR repertoire distribution in 4 samples from one patient who relapsed and one patient who achieved sustained remission at new diagnosis and after chemotherapy. In all the samples analyzed, CD8+ PD-1+ T cells were found to be more oligoclonal than CD8+ PD-1- T cells. The cumulative frequency of the top 50 clonotypes was 42.9%, 35.9%, 41.5%, and 50% of the total PD-1+ T cells at the time of CR diagnosis, CR post chemotherapy, relapse diagnosis, and relapse post chemotherapy, respectively, but only 18.2%, 15.8%, 20.4%, and 29.6% of the total PD-1- frequency at the time of CR diagnosis, CR post chemotherapy, relapse diagnosis, and relapse post chemotherapy, respectively (Figure 6B and 6D). We further analyzed the distribution of the top 50 clonotypes among CD8+ PD-1+ cells and CD8+ PD-1- T cells in bulk CD8+ T cells based on previous results for these two patients. Both at diagnosis and after chemotherapy, the clones with a higher frequency and the overall frequency of the top 50 clonotypes in the CD8+ PD-1+ group were higher than those in the CD8+ PD-1- group (Figure 6C and 6E; Tables S2, S3, S4 and S5). Moreover, the top 50 prevalent clonotypes in the CD8+ PD-1+ group were far less frequent in the PD-1- group (Figure S5). Analysis of all samples from the 5 relapsed patients and 17 CR patients also revealed that the top 50 clonotypes in each CD8+ population were more frequent in the CD8+ PD-1+ group than in the CD8+ PD-1- group (Figure 6F). There were significant reductions in the Shannon index in the CD8+ PD-1+ group compared to the CD8+ PD-1- group, indicating lower TCR repertoire diversity in the CD8+ PD-1+ group (Figure 6G).
Discussion
CD8+ T cells are cytotoxic effector cells of the immune system that are involved in cell-mediated immunity. Cytotoxic T lymphocyte (CTL) reactivity depends mainly on the hypervariable TCR heterodimer CDR3, which is composed of TCRα and TCRβ chains and is responsible for recognition of cell surface MHC-peptide complexes [36]. Currently, our understanding of TCR repertoires in CD8+ T cells and the effect of chemotherapy on TCR repertoires in AML is still lacking. We performed a comprehensive analysis of TCR repertoires in PB and BM CD8+ T cells from 10 healthy donors and 31 AML patients at diagnosis and after chemotherapy. The results reveal (i) diminished TCR repertoire diversity and increased clonal expansion in AML BM but not PB; (ii) a higher identical clone ratio in AML BM; (iii) higher T cell clonal expansion post chemotherapy in relapsed patients compared to patients at new diagnosis; and (iv) greater expansion and lower diversity of TCRβ clonotypes in CD8+ PD-1+ T cells than in CD8+ PD-1- T cells.
In the present study, we observed greater clonal expansion of CD8+ T cells in BM but not in PB of AML patients compared to healthy donors. Zhang et al. reported higher clonal expansion of both T cells and B cells in the PB of AML patients [15]. In contrast to their findings, we did not find clonal expansion of CD8+ T cells in the PB of AML patients. This may be due to the different methods used for obtaining TCR sequences and the heterogeneity of the samples. We obtained approximately 3,280,000 unique CD8+ TCRβ CDR3 sequences from 82 samples by using the target amplification method; in comparison, Zhang et al. obtained 225,000 CDR3 sequences for the TCR α, β, γ, and δ chains and 1,210,000 CDR3 sequences for the B cell immunoglobulin (Ig) heavy and light chains from unselected bulk tumor RNA-seq data from 369 samples. Based on the data characteristics, we were able to better assess CD8+ T cell clones, whereas Zhang et al. could analyze the overall T cell (CD4+ T cells and CD8+ T cells) clone characteristics based on a larger sample size. At the same time, we found that compared with healthy donors, AML patients had a lower rate of identical CD8+ T cell clones between the BM and their own PB. In addition, compared to those at the time of diagnosis, the Top 1000 CD8+ T cell clones in the BM of patients who relapsed after chemotherapy showed greater changes. Originating from myeloid hematopoietic progenitors, AML cells can grow and differentiate within the BM microenvironment [37]. Additionally, the BM has been proven to be a preferential site for T cells specific for blood-borne or non-small-cell lung cancer-associated antigens [38,39]. These features may support our notion that CD8+ T cell clones in the BM of AML patients have special characteristics.
Several studies reported increased levels of PD-1 as well as multiple co-expressed inhibitor receptors (IRs) on CD8+ T cells of AML patients at diagnosis [19,40]. Longitudinal observation of changes in the co-inhibitor receptors expression pattern from our study and others indicated that PD-1 and TIM3, together with several other co-inhibitory receptors, are increased in BM CD8+ T cells in non-responders and reduced in responders post chemotherapy [2,33]. Our analysis of TCRβ sequences showed that TCRβ clonotypes in CD8+ PD-1+ populations were more oligoclonal than those in CD8+ PD-1- T populations. These observations highlight the importance of further evaluating TCR clonotypic frequency and unique phenotypic traits of CD8+ T cells in AML to identify patient-specific repertoires of tumor-reactive CD8+ lymphocytes. For therapies that require isolation of T cells expressing inhibitory receptors [41,42], T cell exhaustion and dysfunction pose a major challenge. However, the present study and other recently published works suggested that CD8+ PD-1+ cells underwent extensive clonal expansion [21], produced IFN-γ and lysed tumors in vitro [19]. This adds to evidence that the process of T cell dysfunction associated with co-expressed inhibitory receptors is reversible and that the enrichment of tumor-reactive cells for patient treatment is reproducible.
Our study has several limitations. First, high disease heterogeneity and a small sample size may have limited our ability to uncover specific CDR3 amino sequences as biomarkers for AML characteristics or determine the exact correlation between the baseline TCR repertoire diversity and prognosis. Second, isolation of the patient-specific repertoire of tumor-reactive CD8+ T cells was not performed, so the anti-leukemia effect was not evaluated. AML is characterized by reduced immunogenicity and has one of the lowest mutation rates compared to other cancer types [43]; nevertheless, it is the quality of mutations, not the mutational burden, that may be of more significance in enhancing immune functions. Several other studies have shown that NPM1 mutation-related TCRs correlate with enhanced anticancer effects and improved clinical outcomes [44,45]. Thus, isolation and recognition of antigens derived from common AML mutations such as those in NPM1, DNMT3A, and FLT3, identification of TCRs that can recognize these antigens, and analysis of related TCRs in patients may be the logical starting point for the implementation of immunotherapy in AML. Third, owing to the limited sample availability, a comprehensive comparison between patients at new diagnosis and patients after chemotherapy was difficult. Extensive analysis of the TCR repertoire differences in relapsed patients before and after chemotherapy would provide valuable information, and further studies should be carried out on this topic.
Conclusions
Our findings provide the first objective data characterizing the signatures of the CD8+ TCR repertoire at diagnosis and after chemotherapy in patients with AML. Sequence analysis of CDR3 has shown that CD8+ T cells in the BM of AML patients display specific TCRβ clonal expansion, and more expansion is found in patients who relapse after chemotherapy and among PD-1+ T cells. The antitumor reactivity of individual TCRs can be harnessed in the future to develop tailored T cell therapies for AML patients, and understanding immune responses to chemotherapy is thus crucial for the development and use of immunotherapies to treat patients with AML.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Grant Nos. 81660616 and 81960032), The Social Development Research Project of Guiczhou Province (Grant No. [2015]3036) and National Center for clinical medicine of hematological diseases (2020ZKPB03). We thank Drs. Wenqiang Zhang and Feng Ye for offering their insights and advice on this project.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Ferrara F, Schiffer CA. Acute myeloid leukaemia in adults. Lancet. 2013;381:484–495. doi: 10.1016/S0140-6736(12)61727-9. [DOI] [PubMed] [Google Scholar]
- 2.Knaus HA, Berglund S, Hackl H, Blackford AL, Zeidner JF, Montiel-Esparza R, Mukhopadhyay R, Vanura K, Blazar BR, Karp JE, Luznik L, Gojo I. Signatures of CD8+ T cell dysfunction in AML patients and their reversibility with response to chemotherapy. JCI Insight. 2018;3:e120974. doi: 10.1172/jci.insight.120974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khalil DN, Smith EL, Brentjens RJ, Wolchok JD. The future of cancer treatment: immunomodulation, CARs and combination immunotherapy. Nat Rev Clin Oncol. 2016;13:394. doi: 10.1038/nrclinonc.2016.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kenderian SS, Ruella M, Shestova O, Klichinsky M, Aikawa V, Morrissette JJ, Scholler J, Song D, Porter DL, Carroll M, June CH, Gill S. CD33-specific chimeric antigen receptor T cells exhibit potent preclinical activity against human acute myeloid leukemia. Leukemia. 2015;29:1637–1647. doi: 10.1038/leu.2015.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casucci M, Nicolis di Robilant B, Falcone L, Camisa B, Norelli M, Genovese P, Gentner B, Gullotta F, Ponzoni M, Bernardi M, Marcatti M, Saudemont A, Bordignon C, Savoldo B, Ciceri F, Naldini L, Dotti G, Bonini C, Bondanza A. CD44v6-targeted T cells mediate potent antitumor effects against acute myeloid leukemia and multiple myeloma. Blood. 2013;122:3461–3472. doi: 10.1182/blood-2013-04-493361. [DOI] [PubMed] [Google Scholar]
- 6.Daver N, Garcia-Manero G, Basu S, Boddu PC, Alfayez M, Cortes JE, Konopleva M, Ravandi-Kashani F, Jabbour E, Kadia T, Nogueras-Gonzalez GM, Ning J, Pemmaraju N, DiNardo CD, Andreeff M, Pierce SA, Gordon T, Kornblau SM, Flores W, Alhamal Z, Bueso-Ramos C, Jorgensen JL, Patel KP, Blando J, Allison JP, Sharma P, Kantarjian H. Efficacy, safety, and biomarkers of response to azacitidine and nivolumab in relapsed/refractory acute myeloid leukemia: a nonrandomized, open-label, phase ii study. Cancer Discov. 2019;9:370–383. doi: 10.1158/2159-8290.CD-18-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gill S, Tasian SK, Ruella M, Shestova O, Li Y, Porter DL, Carroll M, Danet-Desnoyers G, Scholler J, Grupp SA, June CH, Kalos M. Preclinical targeting of human acute myeloid leukemia and myeloablation using chimeric antigen receptor-modified T cells. Blood. 2014;123:2343–2354. doi: 10.1182/blood-2013-09-529537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tawara I, Kageyama S, Miyahara Y, Fujiwara H, Nishida T, Akatsuka Y, Ikeda H, Tanimoto K, Terakura S, Murata M, Inaguma Y, Masuya M, Inoue N, Kidokoro T, Okamoto S, Tomura D, Chono H, Nukaya I, Mineno J, Naoe T, Emi N, Yasukawa M, Katayama N, Shiku H. Safety and persistence of WT1-specific T-cell receptor gene-transduced lymphocytes in patients with AML and MDS. Blood. 2017;130:1985–1994. doi: 10.1182/blood-2017-06-791202. [DOI] [PubMed] [Google Scholar]
- 9.Perna F, Berman SH, Soni RK, Mansilla-Soto J, Eyquem J, Hamieh M, Hendrickson RC, Brennan CW, Sadelain M. Integrating proteomics and transcriptomics for systematic combinatorial chimeric antigen receptor therapy of AML. Cancer Cell. 2017;32:506–519. e505. doi: 10.1016/j.ccell.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim MY, Yu KR, Kenderian SS, Ruella M, Chen S, Shin TH, Aljanahi AA, Schreeder D, Klichinsky M, Shestova O, Kozlowski MS, Cummins KD, Shan X, Shestov M, Bagg A, Morrissette JJD, Sekhri P, Lazzarotto CR, Calvo KR, Kuhns DB, Donahue RE, Behbehani GK, Tsai SQ, Dunbar CE, Gill S. Genetic inactivation of CD33 in hematopoietic stem cells to enable CAR T cell immunotherapy for acute myeloid leukemia. Cell. 2018;173:1439–1453. e1419. doi: 10.1016/j.cell.2018.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reiser J, Banerjee A. Effector, memory, and dysfunctional CD8(+) T cell fates in the antitumor immune response. J Immunol Res. 2016;2016:8941260. doi: 10.1155/2016/8941260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cole DK, Miles KM, Madura F, Holland CJ, Schauenburg AJ, Godkin AJ, Bulek AM, Fuller A, Akpovwa HJ, Pymm PG, Liddy N, Sami M, Li Y, Rizkallah PJ, Jakobsen BK, Sewell AK. T-cell receptor (TCR)-peptide specificity overrides affinity-enhancing TCR-major histocompatibility complex interactions. J Biol Chem. 2014;289:628–638. doi: 10.1074/jbc.M113.522110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fozza C, Barraqueddu F, Corda G, Contini S, Virdis P, Dore F, Bonfigli S, Longinotti M. Study of the T-cell receptor repertoire by CDR3 spectratyping. J Immunol Methods. 2017;440:1–11. doi: 10.1016/j.jim.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Jin Z, Luo Q, Lu S, Wang X, He Z, Lai J, Chen S, Yang L, Wu X, Li Y. Oligoclonal expansion of TCR Vdelta T cells may be a potential immune biomarker for clinical outcome of acute myeloid leukemia. J Hematol Oncol. 2016;9:126. doi: 10.1186/s13045-016-0353-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang J, Hu X, Wang J, Sahu AD, Cohen D, Song L, Ouyang Z, Fan J, Wang B, Fu J, Gu S, Sade-Feldman M, Hacohen N, Li W, Ying X, Li B, Liu XS. Immune receptor repertoires in pediatric and adult acute myeloid leukemia. Genome Med. 2019;11:73. doi: 10.1186/s13073-019-0681-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morath A, Schamel WW. alphabeta and gammadelta T cell receptors: similar but different. J Leukoc Biol. 2020;107:1045–1055. doi: 10.1002/JLB.2MR1219-233R. [DOI] [PubMed] [Google Scholar]
- 17.Han J, Duan J, Bai H, Wang Y, Wan R, Wang X, Chen S, Tian Y, Wang D, Fei K, Yao Z, Wang S, Lu Z, Wang Z, Wang J. TCR repertoire diversity of peripheral PD-1+ CD8+ T cells predicts clinical outcomes after immunotherapy in patients with non-small cell lung cancer. Cancer Immunol Res. 2020;8:146–154. doi: 10.1158/2326-6066.CIR-19-0398. [DOI] [PubMed] [Google Scholar]
- 18.Sharpe AH, Pauken KE. The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol. 2018;18:153–167. doi: 10.1038/nri.2017.108. [DOI] [PubMed] [Google Scholar]
- 19.Jia B, Wang L, Claxton DF, Ehmann WC, Rybka WB, Mineishi S, Rizvi S, Shike H, Bayerl M, Schell TD, Hohl RJ, Zheng H. Bone marrow CD8 T cells express high frequency of PD-1 and exhibit reduced anti-leukemia response in newly diagnosed AML patients. Blood Cancer J. 2018;8:34. doi: 10.1038/s41408-018-0069-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laszlo GS, Gudgeon CJ, Harrington KH, Walter RB. T-cell ligands modulate the cytolytic activity of the CD33/CD3 BiTE antibody construct, AMG 330. Blood Cancer J. 2015;5:e340. doi: 10.1038/bcj.2015.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gros A, Robbins PF, Yao X, Li YF, Turcotte S, Tran E, Wunderlich JR, Mixon A, Farid S, Dudley ME, Hanada K, Almeida JR, Darko S, Douek DC, Yang JC, Rosenberg SA. PD-1 identifies the patient-specific CD8(+) tumor-reactive repertoire infiltrating human tumors. J Clin Invest. 2014;124:2246–2259. doi: 10.1172/JCI73639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han J, Duan J, Bai H, Wang Y, Wan R, Wang X, Chen S, Tian Y, Wang D, Fei K, Yao Z, Wang S, Lu Z, Wang Z, Wang J. TCR repertoire diversity of peripheral PD-1(+)CD8(+) T cells predicts clinical outcomes after immunotherapy in patients with non-small cell lung cancer. Cancer Immunol Res. 2020;8:146–154. doi: 10.1158/2326-6066.CIR-19-0398. [DOI] [PubMed] [Google Scholar]
- 23.Glanville J, Huang H, Nau A, Hatton O, Wagar LE, Rubelt F, Ji X, Han A, Krams SM, Pettus C, Haas N, Arlehamn CSL, Sette A, Boyd SD, Scriba TJ, Martinez OM, Davis MM. Identifying specificity groups in the T cell receptor repertoire. Nature. 2017;547:94–98. doi: 10.1038/nature22976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang L, Cham J, Paciorek A, Trager J, Sheikh N, Fong L. 3D: diversity, dynamics, differential testing - a proposed pipeline for analysis of next-generation sequencing T cell repertoire data. BMC Bioinformatics. 2017;18:129. doi: 10.1186/s12859-017-1544-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shugay M, Bagaev DV, Zvyagin IV, Vroomans RM, Crawford JC, Dolton G, Komech EA, Sycheva AL, Koneva AE, Egorov ES, Eliseev AV, Van Dyk E, Dash P, Attaf M, Rius C, Ladell K, McLaren JE, Matthews KK, Clemens EB, Douek DC, Luciani F, van Baarle D, Kedzierska K, Kesmir C, Thomas PG, Price DA, Sewell AK, Chudakov DM. VDJdb: a curated database of T-cell receptor sequences with known antigen specificity. Nucleic Acids Res. 2018;46:D419–D427. doi: 10.1093/nar/gkx760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nazarov VI, Pogorelyy MV, Komech EA, Zvyagin IV, Bolotin DA, Shugay M, Chudakov DM, Lebedev YB, Mamedov IZ. tcR: an R package for T cell receptor repertoire advanced data analysis. BMC Bioinformatics. 2015;16:175. doi: 10.1186/s12859-015-0613-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Di Lorenzo B, Ravens S, Silva-Santos B. High-throughput analysis of the human thymic Vdelta1(+) T cell receptor repertoire. Sci Data. 2019;6:115. doi: 10.1038/s41597-019-0118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, Weber CF. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han Y, Liu X, Wang Y, Wu X, Guan Y, Li H, Chen X, Zhou B, Yuan Q, Ou Y, Wu R, Huang W, Wang Y, Zhang M, Zhang Y, Zhu D, Zhu H, Yang L, Yi X, Huang C, Huang J. Identification of characteristic TRB V usage in HBV-associated HCC by using differential expression profiling analysis. Oncoimmunology. 2015;4:e1021537. doi: 10.1080/2162402X.2015.1021537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Casucci M, Di Robilant BN, Falcone L, Camisa B, Norelli M, Genovese P, Gentner B, Gullotta F, Ponzoni M, Bernardi M, Marcatti M, Saudemont A, Bordignon C, Savoldo B, Ciceri F, Naldini L, Dotti G, Bonini C, Bondanza A. CD44v6-targeted T cells mediate potent antitumor effects against acute myeloid leukemia and multiple myeloma. Blood. 2013;122:3461–3472. doi: 10.1182/blood-2013-04-493361. [DOI] [PubMed] [Google Scholar]
- 31.Reiser J, Banerjee A. Effector, memory, and dysfunctional CD8+ T cell fates in the antitumor immune response. J Immunol Res. 2016;2016:8941260. doi: 10.1155/2016/8941260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gill S, Tasian SK, Ruella M, Shestova O, Li Y, Porter DL, Carroll M, Danet-Desnoyers G, Scholler J, Grupp SA, June CH, Kalos M. Preclinical targeting of human acute myeloid leukemia and myeloablation using chimeric antigen receptor-modified T cells. Blood. 2014;123:2343–2354. doi: 10.1182/blood-2013-09-529537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dama P, Tang M, Fulton N, Kline J, Liu H. Gal9/Tim-3 expression level is higher in AML patients who fail chemotherapy. J Immunother Cancer. 2019;7:175. doi: 10.1186/s40425-019-0611-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kong Y, Zhang J, Claxton DF, Ehmann WC, Rybka WB, Zhu L, Zeng H, Schell TD, Zheng H. PD-1(hi)TIM-3(+) T cells associate with and predict leukemia relapse in AML patients post allogeneic stem cell transplantation. Blood Cancer J. 2015;5:e330. doi: 10.1038/bcj.2015.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kong Y, Zhu L, Schell TD, Zhang J, Claxton DF, Ehmann WC, Rybka WB, George MR, Zeng H, Zheng H. T-cell immunoglobulin and ITIM domain (TIGIT) associates with CD8+ T-cell exhaustion and Poor Clinical Outcome in AML patients. Clin Cancer Res. 2016;22:3057–3066. doi: 10.1158/1078-0432.CCR-15-2626. [DOI] [PubMed] [Google Scholar]
- 36.Chen G, Yang X, Ko A, Sun X, Gao M, Zhang Y, Shi A, Mariuzza RA, Weng NP. Sequence and structural analyses reveal distinct and highly diverse human CD8(+) TCR repertoires to immunodominant viral antigens. Cell Rep. 2017;19:569–583. doi: 10.1016/j.celrep.2017.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shafat MS, Gnaneswaran B, Bowles KM, Rushworth SA. The bone marrow microenvironment - home of the leukemic blasts. Blood Rev. 2017;31:277–286. doi: 10.1016/j.blre.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 38.Feuerer M, Beckhove P, Garbi N, Mahnke Y, Limmer A, Hommel M, Hammerling GJ, Kyewski B, Hamann A, Umansky V, Schirrmacher V. Bone marrow as a priming site for T-cell responses to blood-borne antigen. Nat Med. 2003;9:1151–1157. doi: 10.1038/nm914. [DOI] [PubMed] [Google Scholar]
- 39.Safi S, Yamauchi Y, Stamova S, Rathinasamy A, Op den Winkel J, Junger S, Bucur M, Umansky L, Warth A, Herpel E, Eichhorn M, Winter H, Hoffmann H, Beckhove P. Bone marrow expands the repertoire of functional T cells targeting tumor-associated antigens in patients with resectable non-small-cell lung cancer. Oncoimmunology. 2019;8:e1671762. doi: 10.1080/2162402X.2019.1671762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williams P, Basu S, Garcia-Manero G, Hourigan CS, Oetjen KA, Cortes JE, Ravandi F, Jabbour EJ, Al-Hamal Z, Konopleva M, Ning J, Xiao L, Hidalgo Lopez J, Kornblau SM, Andreeff M, Flores W, Bueso-Ramos C, Blando J, Galera P, Calvo KR, Al-Atrash G, Allison JP, Kantarjian HM, Sharma P, Daver NG. The distribution of T-cell subsets and the expression of immune checkpoint receptors and ligands in patients with newly diagnosed and relapsed acute myeloid leukemia. Cancer. 2018;125:1470–1481. doi: 10.1002/cncr.31896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Farhood B, Najafi M, Mortezaee K. CD8(+) cytotoxic T lymphocytes in cancer immunotherapy: a review. J Cell Physiol. 2019;234:8509–8521. doi: 10.1002/jcp.27782. [DOI] [PubMed] [Google Scholar]
- 42.Durgeau A, Virk Y, Corgnac S, Mami-Chouaib F. Recent advances in targeting CD8 T-Cell immunity for more effective cancer immunotherapy. Front Immunol. 2018;9:14. doi: 10.3389/fimmu.2018.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cancer Genome Atlas Research Network. Ley TJ, Miller C, Ding L, Raphael BJ, Mungall AJ, Robertson A, Hoadley K, Triche TJ Jr, Laird PW, Baty JD, Fulton LL, Fulton R, Heath SE, Kalicki-Veizer J, Kandoth C, Klco JM, Koboldt DC, Kanchi KL, Kulkarni S, Lamprecht TL, Larson DE, Lin L, Lu C, McLellan MD, McMichael JF, Payton J, Schmidt H, Spencer DH, Tomasson MH, Wallis JW, Wartman LD, Watson MA, Welch J, Wendl MC, Ally A, Balasundaram M, Birol I, Butterfield Y, Chiu R, Chu A, Chuah E, Chun HJ, Corbett R, Dhalla N, Guin R, He A, Hirst C, Hirst M, Holt RA, Jones S, Karsan A, Lee D, Li HI, Marra MA, Mayo M, Moore RA, Mungall K, Parker J, Pleasance E, Plettner P, Schein J, Stoll D, Swanson L, Tam A, Thiessen N, Varhol R, Wye N, Zhao Y, Gabriel S, Getz G, Sougnez C, Zou L, Leiserson MD, Vandin F, Wu HT, Applebaum F, Baylin SB, Akbani R, Broom BM, Chen K, Motter TC, Nguyen K, Weinstein JN, Zhang N, Ferguson ML, Adams C, Black A, Bowen J, Gastier-Foster J, Grossman T, Lichtenberg T, Wise L, Davidsen T, Demchok JA, Shaw KR, Sheth M, Sofia HJ, Yang L, Downing JR, Eley G. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013;368:2059–2074. doi: 10.1056/NEJMoa1301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van der Lee DI, Reijmers RM, Honders MW, Hagedoorn RS, de Jong RC, Kester MG, van der Steen DM, de Ru AH, Kweekel C, Bijen HM, Jedema I, Veelken H, van Veelen PA, Heemskerk MH, Falkenburg JHF, Griffioen M. Mutated nucleophosmin 1 as immunotherapy target in acute myeloid leukemia. J Clin Invest. 2019;129:774–785. doi: 10.1172/JCI97482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Greiner J, Ono Y, Hofmann S, Schmitt A, Mehring E, Gotz M, Guillaume P, Dohner K, Mytilineos J, Dohner H, Schmitt M. Mutated regions of nucleophosmin 1 elicit both CD4(+) and CD8(+) T-cell responses in patients with acute myeloid leukemia. Blood. 2012;120:1282–1289. doi: 10.1182/blood-2011-11-394395. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.