Abstract
Cytokines are one of the first immunotherapeutics utilized in trials of human cancers with significant success. However, due to their significant toxicity and often lack of efficacy, cytokines have given their spotlight to other cancer immunotherapeutics such as immune checkpoint inhibitors. Nevertheless, only a subset of cancer patients respond to checkpoint inhibitors. Therefore, developing a novel cytokine-based immunotherapy is still necessary. Among an array of cytokine candidates, IL-27 is a unique one that exhibits clear anti-tumor activity with low toxicity. Systemically delivered IL-27 by adeno-associated virus (AAV-IL-27) is very well tolerized by mice and exhibits potent anti-tumor activity in a variety of tumor models. AAV-IL-27 exerts its anti-tumor activity through directly stimulation of immune effector cells and systemic depletion of Tregs, and is particularly suitable for delivery in combination with checkpoint inhibitors or vaccines. Additionally, AAV-IL-27 can also be delivered locally to tumors to exert its unique actions. In this review, we summarize the evidence that support these points and propose AAV-delivered IL-27 as a potential immunotherapeutic for cancer.
Keywords: IL-27, recombinant adeno-associated virus, cancer immunotherapy, regulatory T cells
Introduction
Cytokines are one of the first immunotherapeutics approved for the treatment of human cancer. For instance, IFN-α was the first cytokine approved for the treatment of hairy cell leukemia, and IL-2 was approved for the treatment of metastatic renal cell cancer and advanced melanoma. Other cytokines, such as IL-12, IL-15, IL-21 and GM-CSF, are undergoing clinical evaluation [1]. Despite efforts to develop systemic therapy for cancer, cytokines as monotherapy have not fulfilled the initial excitement due to a number of reasons. First, compared to other therapeutics such as checkpoint inhibitors, systemically delivered cytokines often show less efficacy or no efficacy. Second, soluble cytokines normally act over short distances in a paracrine or autocrine fashion. Therefore, large quantities must be administered to achieve a sufficient concentration of cytokines within the tumor. However, large quantities of cytokines are often associated with severe toxicities. Third, due to toxicity, often lower doses of cytokines have to be used in clinical trials. Therefore, there is a failure to achieve adequate concentrations of cytokines at immune cells in the tumor bed. Fourth, the cytokines often elicit immune checkpoints; for example, IL-2 is known to stimulate the survival of regulatory T cells (Tregs) [2]. It is hoped that with novel combination approaches, cytokines will ultimately play a major role in cancer immunotherapy. Alternatively, novel low toxic cytokines with anti-tumor activity should be explored for cancer immunotherapy.
Although the role of IL-27 in tumor immunity has been appreciated for more than 10 years, developing IL-27 into a therapeutic to enhance tumor immunity has not been well achieved. Our recent studies [3,4] suggest that developing adeno-associated viral vectors delivering IL-27 (AAV-IL-27) as a therapeutic is feasible. First, AAV-IL-27 significantly inhibited the growth of a broad-spectrum of tumor types in mice with low or no toxicity. Second, AAV-IL-27 treatment resulted in dramatic reduction of Tregs without causing autoimmunity. Third, we have found that AAV-IL-27 therapy shows strong synergy with PD-1 antibody or cancer vaccines in inhibiting tumor growth. Fourth, IL-27 can also be delivered locally to exert its unique actions in tumor microenvironment. Based on these observations, we propose that AAV-delivered IL-27 is a potential immunotherapeutic for cancer.
IL-27 biology and its multi-faceted roles in tumor immunity and autoimmunity
IL-27 is a member of the IL-12 cytokine family that consists of an IL-12 p40-related protein subunit, EBV-induced gene 3 (EBI3), and a p35-related subunit, p28 [5]. IL-27 is produced by activated antigen presenting cells (APCs) such as dendritic cells (DCs) and macrophages [6-8]. It signals through a heterodimeric receptor (IL-27R) composed of the WSX-1 (IL-27Rα) and the gp130 subunits in a variety of cell types, including T lymphocytes [9]. IL-27R signaling enhances the recruitment of several JAK family kinases and activates STAT family transcription factors 1 and 3 [10,11]. Previous studies have revealed that IL-27 differentially regulates T cell subsets: First, IL-27 inhibits Th2 and Th17 responses by blocking the expression of transcription factors GATA-3 (Th2) and RoRγτ (Th17) [12,13]; second, IL-27 is a potent inducer of IL-10 production by T cells [14-16]; and third, IL-27 can induce PD-L1 expression in T cells which restrain T cell effector functions by interaction with PD-1 on T cells [17]. These functional activities suggest that IL-27 is an immunosuppressive cytokine that inhibits autoimmunity. Indeed, the anti-inflammatory effect of IL-27 has been verified in numerous autoimmune disease models [18-20]. Although it has been shown that IL-27 inhibits Th1 responses under certain circumstances [21], the majority of studies, including those by us, suggests that IL-27 enhances Th1/Tc1 responses by activating Stat1-T-bet axis and promotes T cell expression of T-bet, Eomes, IL-12Rβ2, granzyme B and Perforin [22-24]. These properties of IL-27 suggest that it is an anti-tumor cytokine. Indeed, both endogenous [25-28] and exogenous [29-42] IL-27 have been shown to enhance tumor immunity. A variety of mechanisms have been proposed, including directly inhibiting cancer cell growth, proliferation and migration [36,39,40]; inhibiting tumor angiogenesis [34], enhancing NK activity [35,37] and most importantly, enhancing tumor-specific T cell responses [29-31,33,41]. Our previous studies [42,43] have revealed that IL-27 programs CD8+ T effector cells into a unique T effector stem cell (TSEC) phenotype, which enhances T cell survival in the TME. The unique functions of IL-27 suggest that developing an IL-27-based cancer immunotherapy may lead to enhancing tumor immunity while attenuating autoimmune inflammations that is often associated with immunotherapy.
Systemic delivery of IL-27 using AAV inhibits tumor growth with low or no toxicity
Recombinant adeno-associated viral vectors (rAAV) are quickly establishing themselves as highly versatile gene delivery agents for gene therapy. The relatively low immunogenicity and toxic effects of rAAV makes them arguably the gene therapy vector of choice for human clinical trials [44]. In our recent study [3], we used adeno-associated viral vectors to systemically deliver IL-27 (AAV-IL-27) to treat mice with tumors. We found that one single administration of AAV-IL-27 i.m. resulted in a high level and persistent production of IL-27 in blood. AAV-IL-27 therapy significantly inhibited the growth of a broad-spectrum of tumor types in mice. Consistent with tumor inhibition, we observed significantly increased infiltration of T and NK cells in tumors from AAV-IL-27 treated mice. Thus, AAV-IL-27 monotherapy alone can significantly inhibit tumor growth and induce anti-tumor immunity.
A critical problem for systemic delivery of cytokines for cancer therapy is toxicity [1]. For instance, IL-2 is one of the first effective immunotherapy for human cancer but it is also known to be very toxic [45]. IL-12 exhibits potent anti-tumor activity [46] via promoting Th1/Tc1 response [46,47] and enhancing T cell trafficking to tumors through induction of chemokines [48]. However, systemically delivered IL-12 causes fatal toxicity [49,50]. IL-27 is considered to be a cytokine with low toxicity [51]. Indeed, our recent study [3] suggests that IL-27 has amazingly low toxicity since persistent, high concentrations of IL-27 in blood were very well tolerized by mice, and at two months after treatment, we did not find inflammatory lesions in the heart, lung, liver, pancreas, kidney and colon in AAV-IL-27-treated WT mice. In fact, we compared mice tolerability to different cytokines systemically delivered by AAV. As shown in Figure 1, groups of mice were treated with equal doses (2 × 1011 DRP/mouse) of either AAV-IL-27, or AAV-IL-2 or AAV-GM-CSF or AAV-ctrl. Mice receiving AAV-IL-27 and AAV-ctrl survived in good health throughout the observation period, while none of the mice receiving AAV-IL-2 or AAV-GM-CSF survived for more than 10 days. Thus, unlike any other cytokines in use, AAV-IL-27 has amazingly low toxicity despite high concentrations in blood.
Figure 1.
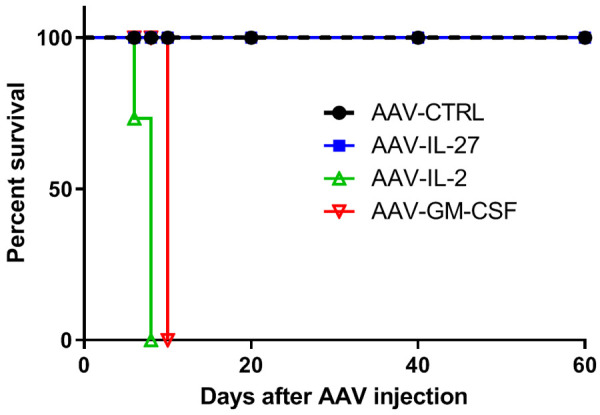
Mice tolerize AAV-IL-27 therapy extremely well. BALB/c mice were injected with AAV-IL-27, or AAV-IL-2, or AAV-GM-CSF or AAV-ctrl i.m. at a dose of 2 × 1011 DRP/mouse. Mice survival was monitored thereafter.
AAV-IL-27 therapy causes systemic depletion of Tregs without causing significant autoimmune adverse events
In our recent study [3], we found that AAV-IL-27 treatment had a potent effect in depletion of Tregs in peripheral lymphoid organs and more pronouncedly in tumors. A previous study [52] has revealed that in IL-27 transgenic mice, Tregs were found to be depleted, and inhibition of IL-2 production by T cells was considered to be the mechanism responsible for Treg depletion [52]. However, in this study, we found that reduction of IL-2 does not fully explain AAV-IL-27-mediated Treg depletion because we found that AAV-IL-27-mediated inhibition of IL-2 production was not dramatic, especially in peripheral lymphoid organs. Experiments involving mixed bone marrow chimera and gene deficient mice clearly suggested that AAV-IL-27-mediated Treg-depletion depended on IL-27R and Stat1 signaling in Tregs. Since AAV-IL-27 therapy down-regulated CD25 expression in Tregs, we consider that inhibition of IL-2 signaling in Tregs by IL-27 is the major mechanism for AAV-IL-27-mediated Treg depletion (Figure 2). Mechanistically, IL-27-mediated activation of Stat1 is known to induce the expression of Socs3 [53]. Socs3 can inhibit IL-2-induced activation of Jak1 [54], leading to compromised Stat5 activation and subsequent CD25 expression [55]. Although this model requires further testing, expression of Socs3 in Treg cells has been shown to inhibit Treg proliferation, survival and effector functions [56]. Heightened IL-2 signaling can prevent AAV-IL-27-mediated Treg depletion [3] and suggests that this mechanism is active when IL-2 signaling is low.
Figure 2.
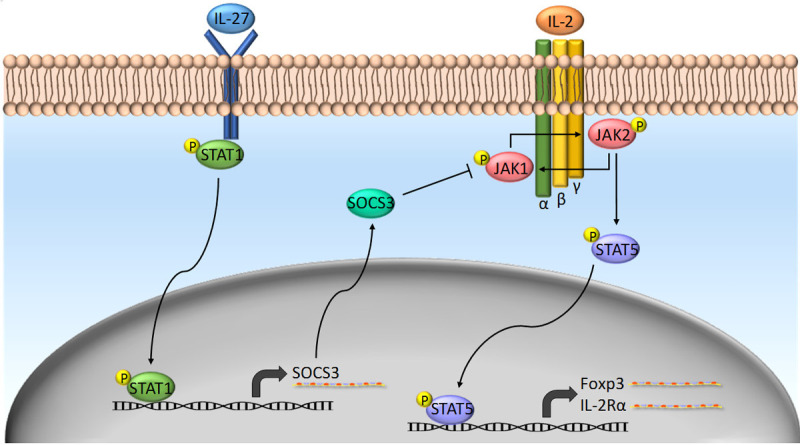
Possible molecular mechanisms of AAV-IL-27-mediated depletion of Tregs: IL-27 signaling in Tregs inhibits their response to IL-2. Socs3 is a possible key link between IL-27 and IL-2 pathways.
Immune-related adverse events (IrAEs) is a new term coined to describe adverse events associated with immunotherapy [57]. In clinical trials, the most common IrAEs include skin rashes/lesions, colitis (diarrhea), hepatitis, and kidney defects. However, we did not find any symptoms of IrAEs in AAV-IL-27 treated mice that rejected tumors. We consider the following mechanisms important in preventing autoimmunity in AAV-IL-27-treated mice. First, AAV-IL-27-mediated Treg depletion is incomplete in lymphoid organs and the residual Tregs may be sufficient to prevent the development of autoimmunity. Second, AAV-IL-27 can directly inhibit autoimmune Th2 and Th17 responses via blocking the expression of transcription factors GATA-3 (Th2) and RoRγτ (Th17) [12,13], and induce a number of inhibitory pathways in T cells [14], which may prevent the development of autoimmunity. In this context, we recently showed that AAV-IL-27 therapy could block the development of both autoimmune colitis [58] and encephalomyelitis [59]. Third, we have found that AAV-IL-27 therapy induced IL-10-producing T cells, which played a role in preventing adverse events. IL-10 is a major cytokine that defines a major subset of Tregs and plays a critical role in Treg function. By promoting IL-10 production in effector T cells, AAV-IL-27 therapy may have bypassed the need for Tregs in protection against autoimmune adverse events.
Induction of immune checkpoints by AAV-IL-27 therapy
IL-27 is known to have “Yin” and “Yang” aspects in induction of anti-tumor immunity [43]. Our recent study [3] on AAV-IL-27 is consistent with previous studies on induction of IL-10 and PD-L1 in T cells. First, IL-27 is known to induce T cell production of IL-10 [14-16], a potent anti-inflammatory cytokine that is normally thought to inhibit T cell responses. We found that AAV-IL-27 therapy mainly induced IL-10 production in CD8+ T cells and conventional CD4+ T cells. Although Treg cell-production of IL-10 is most eminent [60], Tregs are largely depleted by AAV-IL-27 therapy in the TME. Our previous studies [42,61] suggest that IL-10 production by effector T cells enhances their survival and persistence. Other studies have shown that IL-10 producing CTLs are more highly activated and cytotoxic than IL-10 deficient CTLs [27,62,63]. Thus, AAV-IL-27-induced T effector cell production of IL-10 may not be a limitation, but rather important for anti-tumor activity and control of autoimmunity (toxicity). This point has been addressed by our recent study [3]. Second, IL-27 is known to induce T cell expression of PD-L1 [17]. PD-L1-PD-1 interaction among T cells may inhibit T cell effector functions thereby limiting IL-27-mediated anti-tumor efficacy. However, our recent studies [3,4] suggest that this same activity renders tumors more susceptible to anti-PD-1 therapy, which is currently a major form of checkpoint blockade cancer immunotherapy. Thus, the limitation of IL-27-induced PD-L1 expression in T cells can serve as an opportunity rather than obstacles, as outlined in the following section.
How should AAV-IL-27 be used as a therapeutic?
Based on our recent studies [3,4], we propose that AAV-IL-27 can be used in the following four ways to induce best cancer immunotherapy, i.e. combination with checkpoint inhibitors; combination with vaccines; combination with T cell adoptive transfer and AAV-IL-27 local therapy.
AAV-IL-27 combination with checkpoint inhibitors
Cancer immunotherapies based on blockade of immune checkpoints [64-66] have achieved significant success. However, majorities of patients with advanced cancer are not sensitive to this type of immunotherapy. Although the factors that are responsible for cancer resistance to immunotherapy are not fully understood, the following factors are considered to be important. First, lack of pre-existing T cell infiltration in TME is considered to be the most important factor for anti-PD-1 resistance [67]. Second, although not well-established in human cancer, regulatory T cells (Tregs) in the TME has been shown to contribute to anti-PD-1 resistance in mouse models [68]. Third, although not absolute, tumor expression of PD-L1 has been considered to be another important factor. We here propose a new approach of cancer immunotherapy by administration of AAV-IL-27 and anti-PD-1/PD-L1 for cancer immunotherapy. The new approach addresses all restraining factors mentioned above, as diagrammed in Figure 3. First, AAV-IL-27 therapy can enhance T cell infiltration in the TME by induction of chemokines and chemokine receptors, and optimally stimulating T cells into multi-functional Th1/Tc1 effector stem cells [42,43]. Second, through massive reduction of Tregs by AAV-IL-27, we seek to remove active immune suppression. Third, AAV-IL-27 therapy overcomes anti-PD-1 resistance by inducing PD-L1 expression in T cells. Finally, this approach has the potential to simultaneously inhibit autoimmunity and is thereby less toxic.
Figure 3.
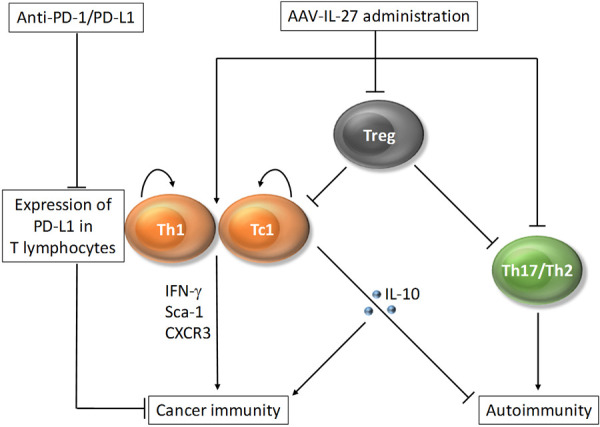
A new approach for cancer immunotherapy: AAV-IL-27 enhances cancer immunity by induction of multi-functional Th1/Tc1 effector stem cells and depletion of Tregs. IL-27 also controls autoimmunity by inhibiting induction of Th17/Th2 cells and inducing IL-10 production in T cells. The limitation for induction of T cell expression of PD-L1 can be utilized for developing combination therapy.
AAV-IL-27 combination with vaccine
Tumor-induced expansion of Tregs is a significant obstacle for successful cancer immunotherapy [69]. Although vaccines can prime T cell responses to tumor antigens, it is usually accompanied by the expansion of Tregs [70], which can limit the expansion of tumor antigen-specific T cells. GM-CSF-secreting tumor vaccine is a potent inducer of anti-tumor T cell responses [71]. In our recent study [3], we found that it had a similar efficacy to AAV-IL-27 monotherapy. However, these two therapeutics together have potent synergy in inducing anti-tumor T cell responses to neoantigens in B16 tumors, leading to long term survival of mice. Therefore, the Treg-depletion ability of AAV-IL-27 and the ability of T cell priming by GM-CSF vaccine may make these two an ideal combination for cancer immunotherapy.
AAV-IL-27 combination with T cell adoptive transfer
Adoptive transfer of TIL T cells is a well-established therapy for patients with solid tumors such as melanoma [72]. In a standard protocol, a pretreatment of recipients with chemotherapy drugs is usually needed to deplete Tregs and make room for T cell homeostatic proliferation [73]. Consistent with this notion, we recently found [4] that adoptive transfer of TILs alone was insufficient to induce tumor regression in non-lymphopenic mice. However, intra-tumoral injection of AAV-IL-27 showed significant synergy with TIL therapy in the absence of pretreatment of recipient mice. This outcome is due to several mechanisms. First, IL-27-induced CXCR3 upregulation can enhance T cell trafficking into tumors. Second, as we previously demonstrated [42,43], IL-27 can directly stimulate TIL T cells, enhancing their survival ability and IFN-γ production. Third, IL-27-mediated depletion of Tregs can bypass the need of lymphodepletion prior to T cell transfer. Thus, our results suggest that intra-tumoral injection of AAV-IL-27 in combination with TIL adoptive transfer is a potential combination for cancer therapy.
AAV-IL-27 local therapy
A potential caveat of systemic delivery of IL-27 using rAAV is that there is no practical method to terminate IL-27 production when its biological activity is no longer needed. Therefore, we recently [4] tested if directly injecting AAV-IL-27 into tumors could lead to a similar anti-tumor effect while avoiding uncontrolled IL-27 production. We found that high levels of IL-27 was produced in tumors and released to peripheral blood after AAV-IL-27 intra-tumoral injection. AAV-IL-27 local therapy showed potent anti-tumor activity in mice bearing plasmacytoma J558 tumors and modest anti-tumor activity in mice bearing B16.F10 tumors. Intra-tumoral injection of AAV-IL-27 induced infiltration of immune effectors including CD8+ T cells and NK cells into tumors, caused systemic reduction of Tregs, and stimulated protective immunity. Mechanistically, we found that IL-27 induced T cell expression of CXCR3 in an IL-27R-dependent manner. Additionally, we found that AAV-IL-27 local therapy had synergistic effects with anti-PD-1 or T cell adoptive transfer therapy. Importantly, in mice whose tumors were completely rejected, IL-27 serum levels were significantly reduced or diminished. Thus, intra-tumoral injection of AAV-IL-27 is a feasible approach that can be used alone or combination with anti-PD-1 antibody or T cell adoptive transfer for the treatment of cancer.
Conclusions and future directions
Our recent studies [3,4] suggest that one single dose of AAV-IL-27 injection (either i.m. or intratumorally) resulted in highly efficient production of IL-27, as reflected by high levels of IL-27 in blood. Mice receiving AAV-IL-27 therapy remain healthy during the observation period, suggesting low toxicity of IL-27 despite high concentrations of IL-27. AAV-IL-27 therapy inhibits the growth of a variety of tumors in mice through a few mechanisms. First, IL-27 enhances T cell infiltration into tumors via chemokine/chemokine receptor induction in tumors and lymphocytes. Second, IL-27 enhances Th1/Tc1 responses and directly programs T cells into multi-functional effectors. Third, IL-27 directly acts on Treg cells to induce their systemic depletion via inhibiting IL-2 signaling. Due to these properties, AAV-IL-27 therapy dramatically enhances the efficacy of cancer vaccine and tumor sensitivity to anti-PD-1 therapy (by directly inducing PD-L1 expression in T cells). Importantly, AAV-IL-27 therapy did not cause significant toxicity and tissue damage. In fact, AAV-IL-27 therapy prevents the development of autoimmune inflammation in a variety of tissues. These properties of IL-27 suggest that AAV-delivered IL-27 may be a potential therapeutic for cancer.
In the future, a few things need to be done to pave the way for AAV-IL-27 to enter the clinic. First, it is still necessary to further determine the mechanisms of action of AAV-IL-27. These include evaluating the roles of IL-27-induced novel pathways in AAV-IL-27 monotherapy, determining the molecular mechanisms of IL-27 therapy-induced depletion of Tregs, and why autoimmunity is prevented despite Treg depletion. Second, AAV-IL-27 has its best effect in combination with anti-PD-1/PD-L1 or vaccine. It is necessary to determine if other combination therapies will also work. These will lead to the development of IL-27-based novel immunotherapies for cancer. Third, a potential caveat of using rAAV to systemically deliver IL-27 is that there is no practical method of terminating gene expression when its biological activity is no longer needed. Therefore, developing an IL-27 control-released AAV may be necessary. Finally, it is critically important to determine if the findings in mice can be recapitulated in human tumor models and human immune cells. We recently produced AAV that express human IL-27 (AAV-hIL-27) and injected AAV-hIL-27 or AAV-ctrl virus into NSG mice. Our preliminary studies are promising and show that AAV-hIL-27 can induce similar phenotypes in human immune cells to that seen in mice. These results will lay the foundation for further clinical trials in human cancer patients using AAV-IL-27.
Acknowledgements
This work is supported by a grant (R01CA229254) from the National Cancer Institute.
Disclosure of conflict of interest
None.
References
- 1.Waldmann TA. Cytokines in cancer immunotherapy. Cold Spring Harb Perspect Biol. 2018;10:a028472. doi: 10.1101/cshperspect.a028472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 3.Zhu J, Liu JQ, Shi M, Cheng X, Ding M, Zhang JC, Davis JP, Varikuti S, Satoskar AR, Lu L, Pan X, Zheng P, Liu Y, Bai XF. IL-27 gene therapy induces depletion of Tregs and enhances the efficacy of cancer immunotherapy. JCI Insight. 2018;3:e98745. doi: 10.1172/jci.insight.98745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu A, Ding M, Zhu J, Liu JQ, Pan X, Ghoshal K, Bai XF. Intra-tumoral delivery of IL-27 using adeno-associated virus stimulates anti-tumor immunity and enhances the efficacy of immunotherapy. Front Cell Dev Biol. 2020;8:210. doi: 10.3389/fcell.2020.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J, Hibbert L, Churakova T, Travis M, Vaisberg E, Blumenschein WM, Mattson JD, Wagner JL, To W, Zurawski S, McClanahan TK, Gorman DM, Bazan JF, de Waal Malefyt R, Rennick D, Kastelein RA. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity. 2002;16:779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 6.Murugaiyan G, Mittal A, Weiner HL. Identification of an IL-27/osteopontin axis in dendritic cells and its modulation by IFN-gamma limits IL-17-mediated autoimmune inflammation. Proc Natl Acad Sci U S A. 2010;107:11495–11500. doi: 10.1073/pnas.1002099107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pirhonen J, Siren J, Julkunen I, Matikainen S. IFN-alpha regulates Toll-like receptor-mediated IL-27 gene expression in human macrophages. J Leukoc Biol. 2007;82:1185–1192. doi: 10.1189/jlb.0307157. [DOI] [PubMed] [Google Scholar]
- 8.Remoli ME, Gafa V, Giacomini E, Severa M, Lande R, Coccia EM. IFN-beta modulates the response to TLR stimulation in human DC: involvement of IFN regulatory factor-1 (IRF-1) in IL-27 gene expression. Eur J Immunol. 2007;37:3499–3508. doi: 10.1002/eji.200737566. [DOI] [PubMed] [Google Scholar]
- 9.Pflanz S, Hibbert L, Mattson J, Rosales R, Vaisberg E, Bazan JF, Phillips JH, McClanahan TK, de Waal Malefyt R, Kastelein RA. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J Immunol. 2004;172:2225–2231. doi: 10.4049/jimmunol.172.4.2225. [DOI] [PubMed] [Google Scholar]
- 10.Hibbert L, Pflanz S, De Waal Malefyt R, Kastelein RA. IL-27 and IFN-alpha signal via Stat1 and Stat3 and induce T-Bet and IL-12Rbeta2 in naive T cells. J Interferon Cytokine Res. 2003;23:513–522. doi: 10.1089/10799900360708632. [DOI] [PubMed] [Google Scholar]
- 11.Hunter CA. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat Rev Immunol. 2005;5:521–531. doi: 10.1038/nri1648. [DOI] [PubMed] [Google Scholar]
- 12.Lucas S, Ghilardi N, Li J, de Sauvage FJ. IL-27 regulates IL-12 responsiveness of naive CD4+ T cells through Stat1-dependent and -independent mechanisms. Proc Natl Acad Sci U S A. 2003;100:15047–15052. doi: 10.1073/pnas.2536517100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diveu C, McGeachy MJ, Boniface K, Stumhofer JS, Sathe M, Joyce-Shaikh B, Chen Y, Tato CM, McClanahan TK, de Waal Malefyt R, Hunter CA, Cua DJ, Kastelein RA. IL-27 blocks RORc expression to inhibit lineage commitment of Th17 cells. J Immunol. 2009;182:5748–5756. doi: 10.4049/jimmunol.0801162. [DOI] [PubMed] [Google Scholar]
- 14.Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, Ernst M, Saris CJ, O’Shea JJ, Hunter CA. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol. 2007;8:1363–1371. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 15.Awasthi A, Carrier Y, Peron JP, Bettelli E, Kamanaka M, Flavell RA, Kuchroo VK, Oukka M, Weiner HL. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol. 2007;8:1380–1389. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 16.Fitzgerald DC, Ciric B, Touil T, Harle H, Grammatikopolou J, Das Sarma J, Gran B, Zhang GX, Rostami A. Suppressive effect of IL-27 on encephalitogenic Th17 cells and the effector phase of experimental autoimmune encephalomyelitis. J Immunol. 2007;179:3268–3275. doi: 10.4049/jimmunol.179.5.3268. [DOI] [PubMed] [Google Scholar]
- 17.Hirahara K, Ghoreschi K, Yang XP, Takahashi H, Laurence A, Vahedi G, Sciume G, Hall AO, Dupont CD, Francisco LM, Chen Q, Tanaka M, Kanno Y, Sun HW, Sharpe AH, Hunter CA, O’Shea JJ. Interleukin-27 priming of T cells controls IL-17 production in trans via induction of the ligand PD-L1. Immunity. 2012;36:1017–1030. doi: 10.1016/j.immuni.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, Lee J, de Sauvage FJ, Ghilardi N. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 19.Amadi-Obi A, Yu CR, Liu X, Mahdi RM, Clarke GL, Nussenblatt RB, Gery I, Lee YS, Egwuagu CE. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med. 2007;13:711–718. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- 20.Kastelein RA, Hunter CA, Cua DJ. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu Rev Immunol. 2007;25:221–242. doi: 10.1146/annurev.immunol.22.012703.104758. [DOI] [PubMed] [Google Scholar]
- 21.Villarino AV, Hunter CA. Biology of recently discovered cytokines: discerning the pro- and anti-inflammatory properties of interleukin-27. Arthritis Res Ther. 2004;6:225–233. doi: 10.1186/ar1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tamada K, Shimozaki K, Chapoval AI, Zhu G, Sica G, Flies D, Boone T, Hsu H, Fu YX, Nagata S, Ni J, Chen L. Modulation of T-cell-mediated immunity in tumor and graft-versus-host disease models through the LIGHT co-stimulatory pathway. Nat Med. 2000;6:283–289. doi: 10.1038/73136. [DOI] [PubMed] [Google Scholar]
- 23.Morishima N, Owaki T, Asakawa M, Kamiya S, Mizuguchi J, Yoshimoto T. Augmentation of effector CD8+ T cell generation with enhanced granzyme B expression by IL-27. J Immunol. 2005;175:1686–1693. doi: 10.4049/jimmunol.175.3.1686. [DOI] [PubMed] [Google Scholar]
- 24.Schneider R, Yaneva T, Beauseigle D, El-Khoury L, Arbour N. IL-27 increases the proliferation and effector functions of human naive CD8+ T lymphocytes and promotes their development into Tc1 cells. Eur J Immunol. 2011;41:47–59. doi: 10.1002/eji.201040804. [DOI] [PubMed] [Google Scholar]
- 25.Natividad KD, Junankar SR, Mohd Redzwan N, Nair R, Wirasinha RC, King C, Brink R, Swarbrick A, Batten M. Interleukin-27 signaling promotes immunity against endogenously arising murine tumors. PLoS One. 2013;8:e57469. doi: 10.1371/journal.pone.0057469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shinozaki Y, Wang S, Miyazaki Y, Miyazaki K, Yamada H, Yoshikai Y, Hara H, Yoshida H. Tumor-specific cytotoxic T cell generation and dendritic cell function are differentially regulated by interleukin 27 during development of anti-tumor immunity. Int J Cancer. 2009;124:1372–1378. doi: 10.1002/ijc.24107. [DOI] [PubMed] [Google Scholar]
- 27.Curran MA, Geiger TL, Montalvo W, Kim M, Reiner SL, Al-Shamkhani A, Sun JC, Allison JP. Systemic 4-1BB activation induces a novel T cell phenotype driven by high expression of eomesodermin. J Exp Med. 2013;210:743–755. doi: 10.1084/jem.20121190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei J, Xia S, Sun H, Zhang S, Wang J, Zhao H, Wu X, Chen X, Hao J, Zhou X, Zhu Z, Gao X, Gao JX, Wang P, Wu Z, Zhao L, Yin Z. Critical role of dendritic cell-derived IL-27 in antitumor immunity through regulating the recruitment and activation of NK and NKT cells. J Immunol. 2013;191:500–508. doi: 10.4049/jimmunol.1300328. [DOI] [PubMed] [Google Scholar]
- 29.Hisada M, Kamiya S, Fujita K, Belladonna ML, Aoki T, Koyanagi Y, Mizuguchi J, Yoshimoto T. Potent antitumor activity of interleukin-27. Cancer Res. 2004;64:1152–1156. doi: 10.1158/0008-5472.can-03-2084. [DOI] [PubMed] [Google Scholar]
- 30.Salcedo R, Stauffer JK, Lincoln E, Back TC, Hixon JA, Hahn C, Shafer-Weaver K, Malyguine A, Kastelein R, Wigginton JM. IL-27 mediates complete regression of orthotopic primary and metastatic murine neuroblastoma tumors: role for CD8+ T cells. J Immunol. 2004;173:7170–7182. doi: 10.4049/jimmunol.173.12.7170. [DOI] [PubMed] [Google Scholar]
- 31.Chiyo M, Shimozato O, Iizasa T, Fujisawa T, Tagawa M. Antitumor effects produced by transduction of dendritic cells-derived heterodimeric cytokine genes in murine colon carcinoma cells. Anticancer Res. 2004;24:3763–3767. [PubMed] [Google Scholar]
- 32.Chiyo M, Shimozato O, Yu L, Kawamura K, Iizasa T, Fujisawa T, Tagawa M. Expression of IL-27 in murine carcinoma cells produces antitumor effects and induces protective immunity in inoculated host animals. Int J Cancer. 2005;115:437–442. doi: 10.1002/ijc.20848. [DOI] [PubMed] [Google Scholar]
- 33.Salcedo R, Hixon JA, Stauffer JK, Jalah R, Brooks AD, Khan T, Dai RM, Scheetz L, Lincoln E, Back TC, Powell D, Hurwitz AA, Sayers TJ, Kastelein R, Pavlakis GN, Felber BK, Trinchieri G, Wigginton JM. Immunologic and therapeutic synergy of IL-27 and IL-2: enhancement of T cell sensitization, tumor-specific CTL reactivity and complete regression of disseminated neuroblastoma metastases in the liver and bone marrow. J Immunol. 2009;182:4328–4338. doi: 10.4049/jimmunol.0800471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shimizu M, Shimamura M, Owaki T, Asakawa M, Fujita K, Kudo M, Iwakura Y, Takeda Y, Luster AD, Mizuguchi J, Yoshimoto T. Antiangiogenic and antitumor activities of IL-27. J Immunol. 2006;176:7317–7324. doi: 10.4049/jimmunol.176.12.7317. [DOI] [PubMed] [Google Scholar]
- 35.Oniki S, Nagai H, Horikawa T, Furukawa J, Belladonna ML, Yoshimoto T, Hara I, Nishigori C. Interleukin-23 and interleukin-27 exert quite different antitumor and vaccine effects on poorly immunogenic melanoma. Cancer Res. 2006;66:6395–6404. doi: 10.1158/0008-5472.CAN-05-4087. [DOI] [PubMed] [Google Scholar]
- 36.Yoshimoto T, Morishima N, Mizoguchi I, Shimizu M, Nagai H, Oniki S, Oka M, Nishigori C, Mizuguchi J. Antiproliferative activity of IL-27 on melanoma. J Immunol. 2008;180:6527–6535. doi: 10.4049/jimmunol.180.10.6527. [DOI] [PubMed] [Google Scholar]
- 37.Matsui M, Kishida T, Nakano H, Yoshimoto K, Shin-Ya M, Shimada T, Nakai S, Imanishi J, Yoshimoto T, Hisa Y, Mazda O. Interleukin-27 activates natural killer cells and suppresses NK-resistant head and neck squamous cell carcinoma through inducing antibody-dependent cellular cytotoxicity. Cancer Res. 2009;69:2523–2530. doi: 10.1158/0008-5472.CAN-08-2793. [DOI] [PubMed] [Google Scholar]
- 38.Morishima N, Mizoguchi I, Okumura M, Chiba Y, Xu M, Shimizu M, Matsui M, Mizuguchi J, Yoshimoto T. A pivotal role for interleukin-27 in CD8+ T cell functions and generation of cytotoxic T lymphocytes. J Biomed Biotechnol. 2010;2010:605483. doi: 10.1155/2010/605483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ho MY, Leu SJ, Sun GH, Tao MH, Tang SJ, Sun KH. IL-27 directly restrains lung tumorigenicity by suppressing cyclooxygenase-2-mediated activities. J Immunol. 2009;183:6217–6226. doi: 10.4049/jimmunol.0901272. [DOI] [PubMed] [Google Scholar]
- 40.Cocco C, Giuliani N, Di Carlo E, Ognio E, Storti P, Abeltino M, Sorrentino C, Ponzoni M, Ribatti D, Airoldi I. Interleukin-27 acts as multifunctional antitumor agent in multiple myeloma. Clin Cancer Res. 2010;16:4188–4197. doi: 10.1158/1078-0432.CCR-10-0173. [DOI] [PubMed] [Google Scholar]
- 41.Zhu S, Lee DA, Li S. IL-12 and IL-27 sequential gene therapy via intramuscular electroporation delivery for eliminating distal aggressive tumors. J Immunol. 2010;184:2348–2354. doi: 10.4049/jimmunol.0902371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Z, Liu JQ, Talebian F, Wu LC, Li S, Bai XF. IL-27 enhances the survival of tumor antigen-specific CD8+ T cells and programs them into IL-10-producing, memory precursor-like effector cells. Eur J Immunol. 2013;43:468–479. doi: 10.1002/eji.201242930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li MS, Liu Z, Liu JQ, Zhu X, Liu Z, Bai XF. The Yin and Yang aspects of IL-27 in induction of cancer-specific T-cell responses and immunotherapy. Immunotherapy. 2015;7:191–200. doi: 10.2217/imt.14.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aalbers CJ, Tak PP, Vervoordeldonk MJ. Advancements in adeno-associated viral gene therapy approaches: exploring a new horizon. F1000 Med Rep. 2011;3:17. doi: 10.3410/M3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosenberg SA. IL-2: the first effective immunotherapy for human cancer. J Immunol. 2014;192:5451–5458. doi: 10.4049/jimmunol.1490019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Colombo MP, Trinchieri G. Interleukin-12 in anti-tumor immunity and immunotherapy. Cytokine Growth Factor Rev. 2002;13:155–168. doi: 10.1016/s1359-6101(01)00032-6. [DOI] [PubMed] [Google Scholar]
- 47.Del Vecchio M, Bajetta E, Canova S, Lotze MT, Wesa A, Parmiani G, Anichini A. Interleukin-12: biological properties and clinical application. Clin Cancer Res. 2007;13:4677–4685. doi: 10.1158/1078-0432.CCR-07-0776. [DOI] [PubMed] [Google Scholar]
- 48.Hu J, Sun C, Bernatchez C, Xia X, Hwu P, Dotti G, Li S. T-cell homing therapy for reducing regulatory T cells and preserving effector T-cell function in large solid tumors. Clin Cancer Res. 2018;24:2920–2934. doi: 10.1158/1078-0432.CCR-17-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Car BD, Eng VM, Schnyder B, LeHir M, Shakhov AN, Woerly G, Huang S, Aguet M, Anderson TD, Ryffel B. Role of interferon-gamma in interleukin 12-induced pathology in mice. Am J Pathol. 1995;147:1693–1707. [PMC free article] [PubMed] [Google Scholar]
- 50.Ryffel B. Interleukin-12: role of interferon-gamma in IL-12 adverse effects. Clin Immunol Immunopathol. 1997;83:18–20. doi: 10.1006/clin.1996.4306. [DOI] [PubMed] [Google Scholar]
- 51.Yoshimoto T, Chiba Y, Furusawa J, Xu M, Tsunoda R, Higuchi K, Mizoguchi I. Potential clinical application of interleukin-27 as an antitumor agent. Cancer Sci. 2015;106:1103–1110. doi: 10.1111/cas.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wojno ED, Hosken N, Stumhofer JS, O’Hara AC, Mauldin E, Fang Q, Turka LA, Levin SD, Hunter CA. A role for IL-27 in limiting T regulatory cell populations. J Immunol. 2011;187:266–273. doi: 10.4049/jimmunol.1004182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Owaki T, Asakawa M, Kamiya S, Takeda K, Fukai F, Mizuguchi J, Yoshimoto T. IL-27 suppresses CD28-mediated [correction of medicated] IL-2 production through suppressor of cytokine signaling 3. J Immunol. 2006;176:2773–2780. doi: 10.4049/jimmunol.176.5.2773. [DOI] [PubMed] [Google Scholar]
- 54.Babon JJ, Varghese LN, Nicola NA. Inhibition of IL-6 family cytokines by SOCS3. Semin Immunol. 2014;26:13–19. doi: 10.1016/j.smim.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hennighausen L, Robinson GW. Interpretation of cytokine signaling through the transcription factors STAT5A and STAT5B. Genes Dev. 2008;22:711–721. doi: 10.1101/gad.1643908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pillemer BB, Xu H, Oriss TB, Qi Z, Ray A. Deficient SOCS3 expression in CD4+CD25+FoxP3+ regulatory T cells and SOCS3-mediated suppression of Treg function. Eur J Immunol. 2007;37:2082–2089. doi: 10.1002/eji.200737193. [DOI] [PubMed] [Google Scholar]
- 57.Fecher LA, Agarwala SS, Hodi FS, Weber JS. Ipilimumab and its toxicities: a multidisciplinary approach. Oncologist. 2013;18:733–743. doi: 10.1634/theoncologist.2012-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhu X, Liu Z, Liu JQ, Zhu J, Zhang J, Davis JP, Chu J, Yu J, Zhou J, Li MS, Bai XF. Systemic delivery of IL-27 by an adeno-associated viral vector inhibits T cell-mediated colitis and induces multiple inhibitory pathways in T cells. J Leukoc Biol. 2016;100:403–411. doi: 10.1189/jlb.3A1215-540R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhu J, Liu JQ, Liu Z, Wu L, Shi M, Zhang J, Davis JP, Bai XF. Interleukin-27 gene therapy prevents the development of autoimmune encephalomyelitis but fails to attenuate established inflammation due to the expansion of CD11b(+)Gr-1(+) myeloid cells. Front Immunol. 2018;9:873. doi: 10.3389/fimmu.2018.00873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stewart CA, Metheny H, Iida N, Smith L, Hanson M, Steinhagen F, Leighty RM, Roers A, Karp CL, Muller W, Trinchieri G. Interferon-dependent IL-10 production by Tregs limits tumor Th17 inflammation. J Clin Invest. 2013;123:4859–4874. doi: 10.1172/JCI65180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang L, Liu JQ, Talebian F, Liu Z, Yu L, Bai XF. IL-10 enhances CTL-mediated tumor rejection by inhibiting highly suppressive CD4 T cells and promoting CTL persistence in a murine model of plasmacytoma. Oncoimmunology. 2015;4:e1014232. doi: 10.1080/2162402X.2015.1014232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Trandem K, Zhao J, Fleming E, Perlman S. Highly activated cytotoxic CD8 T cells express protective IL-10 at the peak of coronavirus-induced encephalitis. J Immunol. 2011;186:3642–3652. doi: 10.4049/jimmunol.1003292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mumm JB, Emmerich J, Zhang X, Chan I, Wu L, Mauze S, Blaisdell S, Basham B, Dai J, Grein J, Sheppard C, Hong K, Cutler C, Turner S, LaFace D, Kleinschek M, Judo M, Ayanoglu G, Langowski J, Gu D, Paporello B, Murphy E, Sriram V, Naravula S, Desai B, Medicherla S, Seghezzi W, McClanahan T, Cannon-Carlson S, Beebe AM, Oft M. IL-10 elicits IFNgamma-dependent tumor immune surveillance. Cancer Cell. 2011;20:781–796. doi: 10.1016/j.ccr.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 64.Phan GQ, Yang JC, Sherry R, Hwu P, Topalian SL, Schwartzentruber DJ, Restifo NP, Hawarth LR, Seipp CA, Freezer LJ, Morton KE, Marvroukakis SA, Duray P, Steinberg SM, Allison JP, Davis TA, Rosenberg SA. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen-4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 2003;100:8372–8377. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, Dronca R, Gangadhar TC, Patnaik A, Zarour H, Joshua AM, Gergich K, Elassaiss-Schaap J, Algazi A, Mateus C, Boasberg P, Tumeh PC, Chmielowski B, Ebbinghaus SW, Li XN, Kang SP, Ribas A. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, West AN, Carmona M, Kivork C, Seja E, Cherry G, Gutierrez AJ, Grogan TR, Mateus C, Tomasic G, Glaspy JA, Emerson RO, Robins H, Pierce RH, Elashoff DA, Robert C, Ribas A. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ngiow SF, Young A, Jacquelot N, Yamazaki T, Enot D, Zitvogel L, Smyth MJ. A threshold level of intratumor CD8+ T-cell PD1 expression dictates therapeutic response to Anti-PD1. Cancer Res. 2015;75:3800–3811. doi: 10.1158/0008-5472.CAN-15-1082. [DOI] [PubMed] [Google Scholar]
- 69.Colombo MP, Piconese S. Regulatory-T-cell inhibition versus depletion: the right choice in cancer immunotherapy. Nat Rev Cancer. 2007;7:880–887. doi: 10.1038/nrc2250. [DOI] [PubMed] [Google Scholar]
- 70.Zhou G, Drake CG, Levitsky HI. Amplification of tumor-specific regulatory T cells following therapeutic cancer vaccines. Blood. 2006;107:628–636. doi: 10.1182/blood-2005-07-2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, Jackson V, Hamada H, Pardoll D, Mulligan RC. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci U S A. 1993;90:3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science. 2015;348:62–68. doi: 10.1126/science.aaa4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE, Mavroukakis SA, Rogers LJ, Gracia GJ, Jones SA, Mangiameli DP, Pelletier MM, Gea-Banacloche J, Robinson MR, Berman DM, Filie AC, Abati A, Rosenberg SA. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J. Clin. Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]


