Abstract
Cell migration is a highly coordinated process that involves not only integrin-mediated adhesion but also de-adhesion. We previously found that a cryptic de-adhesive site within fibronectin molecule, termed FNIII14, weakens cell adhesion to the extracellular matrix by inactivating β1-integrins. Surprisingly, eukaryotic translation elongation factor-1A (eEF1A), an essential factor during protein biosynthesis, was identified as a membrane receptor that mediates the de-adhesive effect of FNIII14. Here, we demonstrate that FNIII14-mediated de-adhesion causes enhanced migration and invasion in two types of highly invasive/metastatic cancer cells, resulting in the initiation of metastasis. Both in vitro migration and invasion of highly invasive human melanoma cell line, Mum2B, were inhibited by a matrix metalloproteinase (MMP)-2/9 inhibitor or a function-blocking antibody against FNIII14 (anti-FNIII14 Ab), suggesting that MMP-mediated exposure of the cryptic de-adhesive site FNIII14 was responsible for Mum2B cell migration and invasion. The MMP-induced FNIII14 exposure was also shown to be functional in the migration and invasion of highly metastatic mouse breast cancer cell line 4T1. Overexpression and knockdown experiments of eEF1A in Mum2B cells revealed that the migration and invasion were dependent on the membrane levels of eEF1A. In vivo experiments using tumor xenograft mouse models derived from Mum2B and 4T1 cell lines showed that the anti-FNIII14 Ab has a significant anti-metastatic effect. Thus, these results provide novel insights into the regulation of cancer cell migration and invasion and suggest promising targets for anti-metastasis strategies.
Keywords: Integrin, fibronectin, extracellular matrix, eukaryotic translation elongation factor 1A, de-adhesion, metastasis
Introduction
Cancer metastasis, which is a typical feature of malignant tumors, is the most serious and life-threatening event in cancer patients. Therefore, understanding the cellular and molecular mechanisms involved in metastasis could provide an effective therapeutic strategy to prevent this lethal process. Metastasis begins with the invasive migration of tumor cells away from the primary tumor mass and spreads to the surrounding stroma. During the process, adhesive interaction of cells with the extracellular matrix (ECM) scaffold and proteolytic degradation of the ECM components are indispensable for efficient cell migration and invasion [1-3]. From these perspectives, adhesion receptors and matrix-degrading enzymes have been targeted for the development of anti-metastatic drugs [4,5]; however, cancer therapeutics that target them have not yet been established.
Cell adhesion to the ECM, which is mediated mainly by β1 subfamily of integrins [6], provides a pivotal driving force necessary for migration and invasion of tumor cells [7-9]. In contrast to membrane receptors for humoral factors including cytokines, adhesion receptor integrins are unique in altering the binding affinity of their ligands, ECM proteins. Integrin exists mainly as two different structural states: an inactive conformation lacking in ligand-binding ability and an active one with high affinity [10]. This affinity conversion of integrin is reversible, allowing cells to migrate through the ECM architecture [11]. Cell migration is a coordinated process involving dynamic changes in the activation status of integrins. At the leading edge of a migrating cell, nascent adhesions and their stabilization are induced by an active form of integrins, while disassembly of adhesion occurs in response to β1-integrin inactivation at the rear side. These dynamic changes in the integrin-mediated adhesive interaction are followed by retraction of the actin cytoskeleton, resulting in cell movement to the side of the leading edge [12,13]. In addition to such affinity changes of integrins, proteinase-dependent ECM remodeling plays an additional role in the invasive migration of tumor cells; matrix metalloproteinases (MMPs) produced by tumor cells allow active dissemination of tumor cells by destroying the ECM barrier that prevents them from spreading [3,14,15].
The ECM components not only provide a scaffold for cell adhesion and migration, but also serve cells with various signals for cell regulation. Some of these signals are derived from biologically active cryptic sites within the ECM protein molecules that can be exposed by proteolytic modification and/or structural unfolding of these molecules based on cell adhesion and intermolecular interaction [16]. We previously found that fibronectin, which is one of the most abundant and ubiquitous ECM proteins, harbors a cryptic functional site termed FNIII14-corresponding to the amino acid sequence YTIYVIAL within the 14th fibronectin type III repeat-which opposes cell adhesion to the ECM [17]. A 22-mer fibronectin peptide containing this de-adhesive site FNIII14 can change β1-integrin conformation from the active form to the inactive one to induce functional inactivation [18], thereby affecting various cellular behaviors [19-21]. This cryptic de-adhesive site FNIII14 can be disclosed by proteolytic cleavage of fibronectin with inflammatory proteinases, such as MMPs and thrombin [22,23]. Surprisingly, we previously found that a minor part of eukaryotic translation elongation factor 1A (eEF1A) is expressed on the cell surface, and functions as a membrane receptor mediating the de-adhesive effect of FNIII14 [24]. It has been established that eEF1A serves as a key player during the protein biosynthesis on the ribosome [25]. Besides this canonical role, our finding suggests that eEF1A also contributes to cell regulation as a membrane receptor for the de-adhesion effect of FNIII14.
Here, we demonstrate that functional exposure of the de-adhesive site FNIII14 buried within the fibronectin molecule and its binding with the membrane-type eEF1A induces β1-integrin inactivation in melanoma and breast cancer cells, which stimulates cell migration/invasion and consequently causes metastasis.
Materials and methods
Materials and antibodies
Synthetic peptide FNIII14 (amino acid sequence: TEATITGLEPGTEYTIYVIALC) was prepared by Operon Biotechnologies (currently Eurofins Genomics, Ebersberg, Germany). The MMP-2/MMP-9 inhibitor II, (2R)-[(4-biphenylylsulfonyl)-amino]-N-hydroxy-3-phenylpropionamide (BiPS, #444249), was purchased from Merck-Calbiochem (Darmstadt, Germany); normal rabbit IgG (#17312) was from Immuno-Biological Laboratories (Gunma, Japan); anti-eEF1A monoclonal antibody (#05-235, CBP-KK1) was from Merk-Upstate; monoclonal antibody (#D050-3, AG89) that recognizes the active conformation of β1-integrin and anti-hemagglutinin (HA)-tag antibody (#561) were from Medical & Biological Laboratories (Aichi, Japan); anti-β-actin antibody (#A2066) was from Merk-Sigma-Aldrich; and anti-MMP-9 (#3852) was from Cell Signaling Technology (Danvers, MA). A function-blocking antibody directed to the de-adhesive site FNIII14 (anti-FNIII14 Ab) was generated as described previously [24].
Cell lines and cell culture
Human melanoma cell lines Mum2B (highly invasive) and Mum2C (poorly invasive) [26,27] were gifts from Prof. Vito Quaranta (Vanderbilt University, Nashville, TN). Mouse breast cancer cell line 4T1 (CRL-2539) and human breast cancer cell line MCF-7 (HTB-22) were purchased from ATCC (Manassas, VA). Cells were cultured in RPMI-1640 (Mum2B, Mum2C, 4T1) or DMEM (MCF-7) supplemented with penicillin, streptomycin, L-glutamine, and 10% fetal bovine serum (FBS). Cells were maintained at 37°C in a 5% CO2.
In vitro cell migration assay
Cell migration on the fibronectin substrate was evaluated using scratch wound healing, as described previously [28]. After scratching of the confluent cell monolayer, medium was replaced with serum-free culture medium (100 μL) containing dimethyl sulfoxide (DMSO), normal rabbit IgG, anti-FNIII14 Ab, or BiPS. The progress of cell migration was photographed at 0 and 6 hours after scratching. Migration distance was quantified with Image J software (http://Imagej.Nih.Gov/Ij/).
In vitro invasion assay
Matrigel invasion assays were performed in Transwells (BD Falcon Cell Culture Inserts; BD Biosciences, San Jose, CA) with an 8.0-μm pore size, as described previously [29]. Cells (3.0 × 104) suspended in serum-free culture medium containing DMSO, BiPS, normal rabbit IgG or anti-FNIII14 Ab were added to the top chamber. Culture medium supplemented with 5% FBS was added to the bottom chamber. After 6-hour (Mum2B) or 24-hour (Mum2C, 4T1, MCF-7) culture, the numbers of cells invaded were counted in 4 fields at 100 × magnification under microscope.
Flow cytometry
Mum2B cells were treated with MnCl2 (1 mM) in the presence or absence of peptide FNIII14 (50 μg/mL) at 4°C for 45 min, and then the activation status of β1-integrins on the cells was evaluated by flow cytometric analysis (BD FACSAria; BD Biosciences) using a monoclonal antibody (clone AG89) that recognizes the active conformation of β1-integrin and an FITC-conjugated secondary antibody.
Adhesion assay
Adhesion assay was performed in the presence of normal rabbit IgG (12.5 μg/mL) or anti-FNIII14 Ab (12.5 μg IgG/mL), as described previously [24].
Immunoblot analysis
Cells were lysed in Laemmli’s buffer containing protease inhibitor cocktail (#25955-24; Nacalai Tesque, Kyoto, Japan). Cell lysates (10 μg protein) were separated by SDS-PAGE, transferred onto PVDF membranes, and blocked with 5% BSA in T-TBS. Membranes were incubated in primary antibodies specific to eEF1A, HA-tag, β-actin or MMP-9 overnight at 4°C, followed by incubation with an HRP-linked secondary antibody.
Detection of membrane-type eEF1A by affinity labeling method
Membrane-type eEF1A was detected as a 50-kDa band by affinity-labeling technique using biotinylated peptide FNIII14 and immunoblot analysis with HRP-linked streptavidin (#3999; Cell Signaling Technology), as described previously [24].
Gelatin zymography
Gelatin zymography was performed using the culture supernatants from equal numbers of cells (4.0 × 103), according to the method reported by Toth et al. [30]. The gelatinolytic bands were quantified with image J software.
eEF1A knockdown and overexpression
Reverse transfection was performed for transient knockdown and overexpression of human eEF1A (GenBank Accession No. NM_001958). For eEF1A knockdown, cells were transfected with Sigma ultra siPerfect negative control (Merck-Sigma-Aldrich) or eEF1A siRNA using Lipofectamin RNAiMAX (Thermo Fisher Scientific, Waltham, MA) according to the manufacturer’s instructions. The following eEF1A siRNAs were used: sense, 5’-AGGAGAAGACCCACAUCAATT-3’; antisense, 5’-UUGAUGUGGGUCUUCUCCUTG-3’ (Hs_EEF1A2_6 FlexiTube siRNA; Qiagen). For eEF1A overexpression, human eEF1A cDNA was amplified by PCR using following primers: forward, 5’-CCAGCCCCTCACACTCCCAG-3’; reverse, 5’-TACCTCCGCATTTGTAGATGA-3’. Cells were transfected with empty pCMV vector or pCMV vector encoding a HA-tagged eEF1A using Opti-MEM medium (Thermo Fisher Scientific) and FuGENE HD transfection reagent (Promega, Madison, WI) according to the manufacturer’s instructions. These cells were used for subsequent experiments 48 hours after transfection.
In vivo metastasis assay
KSN/SLC mice (6 weeks old) and BALB/c mice (5 weeks old) were obtained from Japan SLC (Shizuoka, Japan). All animals were cared for in accordance with the guidelines set out by Tokyo University of Science. Animal protocols were approved by the Animal Care Committee of Tokyo University of Science.
For the evaluation of melanoma metastasis, Mum2B cells (2 × 106 cells/mouse) suspended in serum-free RPMI-1640 containing fibronectin (1 μg/mL) was subcutaneously injected into the right hind footpads of 7-week-old male KSN/SLC mice. Immediately after the implantation, 3 mice each was randomly assigned to 2 groups, control group received normal rabbit IgG (100 μg/mouse) or treated group received anti-FNIII14 Ab (100 μg IgG/mouse). The antibodies dissolved in PBS were injected intraperitoneally to the mice on days 10, 15, 20 and 25 after the implantation. Tumor sizes at the implanted site were measured once every five days after implantation. On day 30 after the implantation, the mice were sacrificed. The lungs and subiliac lymph nodes were excised and subjected to the evaluation of metastasis by detecting human genomic DNA in these tissues, as follows. Purified genomic DNA from the lungs and subiliac lymph nodes was isolated with the GenElute Mammalian Genomic DNA Miniprep Kit (Merck-Sigma-Ardrich) accordance to the manufacturer’s instructions. The following primers were used to amplify the human protein tyrosine phosphatase receptor type C genomic DNA (hPTPRC): forward, 5’-ATTTATTTTGTCCTTCTCCCA-3’; reverse, 5’-GTTAACAACTTTTGTGTGCC-3’ [31]. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was also amplified with the following primers: forward, 5’-CCCATGTTCGTCATGGGTGT-3’; reverse, 5’-TGGTCATGAGTCCTTCCACGATA-3’. PCR products were electrophoresed on a 2% agarose gel. The gel was stained with a 0.5 μg/mL ethidium bromide solution. Mum2B cells cultured and corresponding tissues excised from untreated mice were used as positive and negative controls, respectively.
For the evaluation of breast cancer metastasis, 4T1 cells (1 × 106 cells/mouse) suspended in PBS containing fibronectin (1 μg/mL) was subcutaneously injected into the right hind footpads of 6-week-old female BALB/c mice. Immediately after the implantation, 5 mice each was randomly assigned to 2 groups, control group received normal rabbit IgG (100 μg/mouse) or treated group received anti-FNIII14 Ab (100 μg IgG/mouse). The antibodies dissolved in PBS were injected intraperitoneally to the mice on days 8, 12, 15, and 18 after the implantation. On day 21, the tumor-bearing legs were resected under anesthesia. On day 35 after the implantation, the mice were sacrificed, and lung tissues were collected. The lungs were fixed in Bouin’s solution, and then metastatic nodules on the lung surface were counted under a dissecting microscope.
Statistical analysis
Statistical analyses were performed with SAS system (SAS Institute, Cary, NC). An unpaired Student’s t-test was used to compare differences between 2 groups. To compare more than 2 groups, Tukey’s test was used for all pairwise comparisons or for comparisons between the control and each treatment group, respectively. All statistical tests were two-sided, and P < 0.05 was considered statistically significant.
Results
Exposure of the cryptic de-adhesive site FNIII14 in response to fibronectin cleavage by MMP-9 stimulates melanoma migration and invasion
A number of reports showing that de-adhesion is necessary for efficient migration and invasion [32-35] prompted us to verify a possible role of the cryptic de-adhesive site FNIII14 in tumor cell migration and invasion. The migratory and invasive capabilities of two kinds of human melanoma cell lines with different invasive capacities, that is, highly invasive Mum2B and poorly invasive Mum2C, were examined in vitro. In these experiments, fibronectin, which is abundantly and ubiquitously distributed in mammalian tissues, was used as a scaffold substrate for cell adhesion. As expected, the migration and invasion of Mum2B and Mum2C cells evaluated by in vitro experiments were positively correlated with their invasive potential, which has been confirmed by in vivo experiments using a xenograft model [36]. Both the migratory and invasive capabilities of highly invasive Mum2B cells were much higher than those of low invasive Mum2C cells (Figure 1A and 1B).
Figure 1.
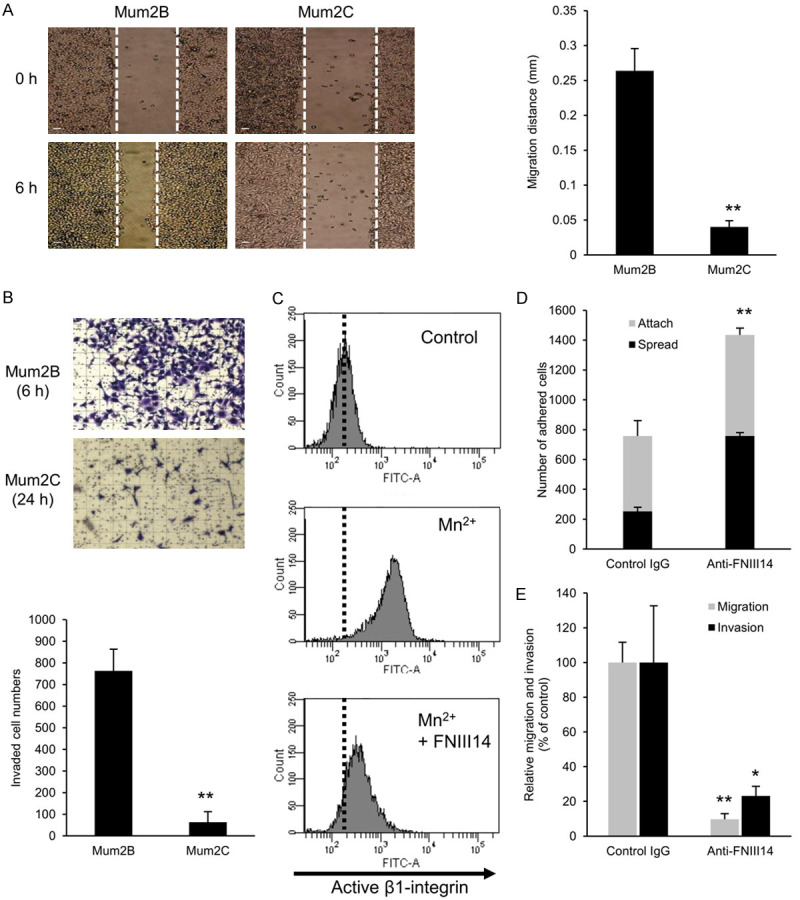
Cryptic anti-adhesive site FNIII14 stimulates melanoma migration and invasion. A. Comparison of in vitro migratory capability between Mum2B and Mum2C cells. An in vitro migration assay was performed as described in Materials and Methods. Representative phase contrast images (left, scale bar = 100 μm) and migration distances (right) are shown at 0 and 6 hours after scratching. B. Comparison of in vitro invasive capability between Mum2B and Mum2C cells. An in vitro invasion assay was performed as described in Materials and Methods. Representative phase contrast images (top, grid = 100 μm squares) and the numbers of cells invaded (bottom) are shown at 6-hour (Mum2B) or 24-hour (Mum2C) culture. C. Inactivation of β1-integrin by de-adhesive peptide FNIII14 in Mum2B cells. Mum2B cells were incubated with MnCl2 (1 mM) in the presence or absence of peptide FNIII14 (50 μg/mL). Control is untreated cells. Activation status of β1-integrin was evaluated by flow cytometry using an anti-β1-integrin monoclonal antibody, AG89, which recognizes the active β1-integrin conformation-specific epitope. Representative data of three individual experiments is shown. D. Detection of the de-adhesive site FNIII14 in exposed state within the fibronectin substrate. Mum2B cells were subjected to adhesion assay in the presence or absence of normal rabbit IgG (Control IgG, 12.5 μg/mL) or anti-FNIII14 Ab (Ani-FNIII14, 12.5 μg IgG/mL). E. Suppression of Mum2B cell migration and invasion by anti-FNIII14 Ab. The in vitro cell migration and invasion assays were performed in the presence of normal rabbit IgG (Control IgG, 50 μg/mL) or anti-FNIII14 Ab (Anti-FNIII14, 50 μg IgG/mL). Values in the picture are mean ± S.D. of three individual experiments containing three replicates/condition. *P < 0.05, **P < 0.01 by Student’s t-test.
It was of interest to examine whether the de-adhesive site FNIII14 contributes to cell migration and invasion by inactivating β1-integrins. Before our investigation, we first confirmed whether the de-adhesive site FNIII14 actually inactivates β1-integrins of Mum2B cells. As has been observed using various cell types, exogenous addition of peptide FNIII14 was shown to inactivate β1-integrin of Mum2B cells, as demonstrated by flow cytometric analysis using a monoclonal antibody that recognizes specifically active conformation of β1-integrin (Figure 1C). Therefore, we next examined whether the de-adhesive site FNIII14 buried within the fibronectin molecule could be spontaneously exposed and affect cell adhesion to the ECM. When cell adhesion to the fibronectin substrate was examined in the presence or absence of the function-blocking antibody directed to FNIII14 (anti-FNIII14 Ab) [24], the number of cells attached and spread on the fibronectin substrate was significantly increased by the addition of anti-FNIII14 Ab (Figure 1D), implying that the de-adhesive site FNIII14 was exposed spontaneously on a portion of the fibronectin molecule coated on the culture plate and functioned to weaken cell adhesion. Based on these results, we then examined whether the de-adhesive site FNIII14 actually influences migration and invasion of Mum2B cells. To clarify this, in vitro migration and invasion assays were performed in the presence or absence of anti-FNIII14 Ab. With the addition of anti-FNIII14 Ab, both the migration and invasion of Mum2B cells were reduced to an extent similar to as those of Mum2C cells (Figure 1E). Thus, the migration and invasion of Mum2B cells was dependent on the degree of exposure of the de-adhesive site FNIII14 from the fibronectin molecule as a scaffold for cell adhesion.
The cryptic de-adhesive site FNIII14 can be exposed by processing with MMPs [22,23]. Gelatin zymography showed that high gelatinolytic activity was detected in Mum2B cells at molecular masses of 82-kDa and 92-kDa, which corresponded to human pro- and active-MMP-9, respectively (Figure 2A). In contrast, only low gelatinolytic activity was detected in Mum2C cells (Figure 2A). Reflecting on these observations, the high migratory and invasive capabilities of Mum2B cells were suppressed by the addition of BiPS, a specific inhibitor for MMP-2/9 (Figure 2B), suggesting the involvement of MMP-induced proteolytic exposure of FNIII14 from the fibronectin matrix in the active migration and invasion of Mum2B cells.
Figure 2.
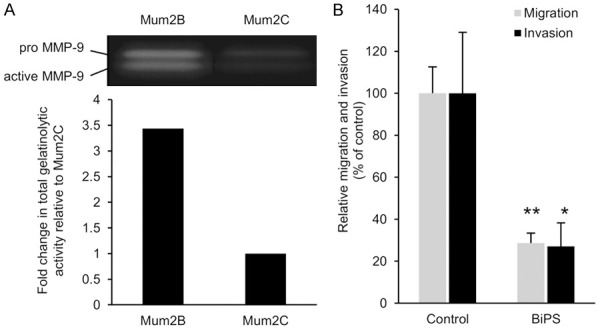
Involvement of MMP-9 in Mum2B cell migration and invasion. (A) Comparison of gelatinolytic activity between Mum2B and Mum2C cells. Gelatin zymography of culture supernatants of the equal number of these cells were performed as described in Materials and Methods. Gel image (top) and the densitometric data (bottom, as total of pro- and active-MMP-9) are shown. Data shown are representative of three individual experiments. (B) Suppression of Mum2B cell migration and invasion by BiPS, a MMP-2/9 inhibitor. The in vitro cell migration and invasion assays were performed in the presence of DMSO (Control) or BiPS (50 μM). Values in (B) are mean ± S.D. of three individual experiments containing three replicates/condition. *P < 0.05, **P < 0.01 by Student’s t-test.
Fibronectin containing the de-adhesive site FNIII14 is widely distributed in various cancer stroma, and is closely involved in cancer development [14]. It is interesting to ascertain if the exposure of FNIII14 also plays a role in the migration and invasion of other types of cancer cells. Two breast cancer cell lines with different metastatic potentials, a lower metastatic human cell line MCF-7 [37] and a highly metastatic mouse cell line 4T1 [38], were used. As with the melanoma cell lines, both the migratory and invasive capabilities of highly metastatic 4T1 cells were much higher than those of lower metastatic MCF-7 cells (Figure 3A and 3B), and any of the active migration and invasion of 4T1 cells were significantly suppressed by the anti-FNIII14 Ab (Figure 3C). Furthermore, results of the effect of the MMP-2/9 inhibitor (Figure 3D) revealed that proteolytic exposure of the de-adhesive site FNIII14 by the MMP self-secreted (Figure 3E) was also involved in 4T1 cell migration and invasion.
Figure 3.
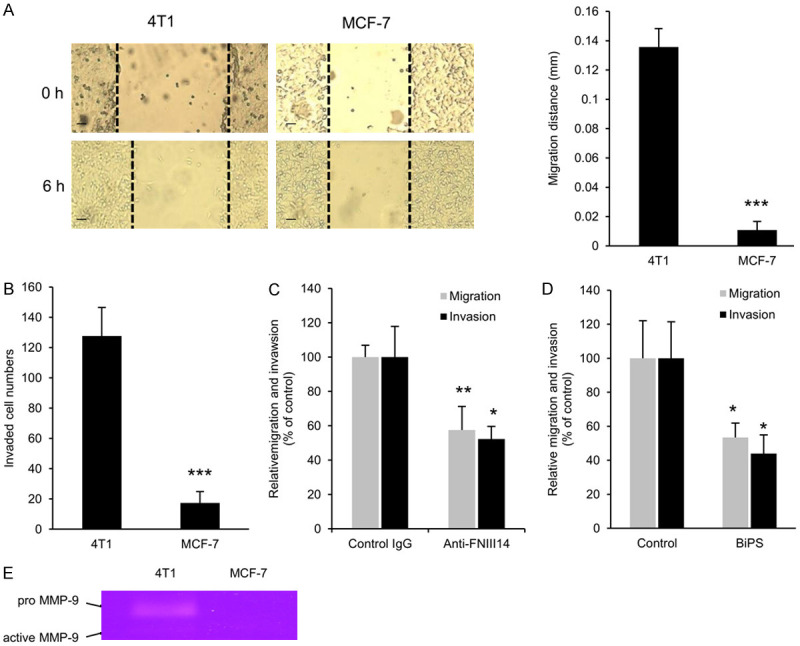
Involvement of cryptic anti-adhesive site FNIII14 and MMP-9 in 4T1 cell migration and invasion. A. Comparison of in vitro migratory capability between 4T1 and MCF-7 cells. An in vitro migration assay was performed as described in Materials and Methods. Representative phase contrast images (left, scale bar = 100 μm) and migration distances (right) are shown at 0 and 6 hours after scratching. B. Comparison of in vitro invasive capability between 4T1 and MCF-7 cells. An in vitro invasion assay was performed as described in Materials and Methods. The numbers of cells invaded are shown at 24-hour culture. C. Suppression of 4T1 cell migration and invasion by anti-FNIII14 Ab. The in vitro cell migration and invasion assays were performed in the presence of normal rabbit IgG (Control IgG, 50 μg/mL) or anti-FNIII14 Ab (Anti-FNIII14, 50 μg IgG/mL). D. Suppression of 4T1 cell migration and invasion by BiPS, a MMP-2/9 inhibitor. The in vitro cell migration and invasion assays were performed in the presence of DMSO (Control) or BiPS (50 μM). E. Comparison of gelatinolytic activity between 4T1 and MCF-7 cells. Gelatin zymography of culture supernatants of the equal number of these cells were performed as described in Materials and Methods. Gel image shown is representative of three individual experiments. Values in the picture are mean ± S.D. of three individual experiments containing three replicates/condition. *P < 0.05, **P < 0.01, ***P < 0.01 by Student’s t-test.
Taken together with the effects of the MMP-2/9 inhibitor and anti-FNII14 Ab, it is thus assumed that MMP-induced exposure of the de-adhesive site FNIII14 is responsible for active migration and invasion of at least melanoma and breast cancer cell lines used in this study.
Binding of the exposed FNIII14 with membrane-type eEF1A causes enhanced melanoma cell migration and invasion
Of primary importance in FNIII14-associated migration and invasion is that the de-adhesive effect of FNIII14 is mediated by the membrane-type eEF1A [24]. We first analyzed the membrane expression of eEF1A of the four cancer cell lines used in this study. Membrane eEF1A was detected as a 50-kDa membrane protein (p50) with specific binding affinity for FNIII14 [39], as evaluated by the affinity-labeling technique. As can be seen in Figure 4A, there was no remarkable difference in p50 level between highly invasive Mum2B cells and poorly invasive Mum2C cells. The results indicate that the difference in migration/invasion ability of these cells was not depended on the membrane expression of the de-adhesion receptor eEF1A, but on the exposure of its ligand FNIII14. In breast cancer cells, a considerable amount of p50 was detected in 4T1 cells, but hardly in MCF-7 cells (Figure 4B), implying that the low migration/invasion capacity of MCF-7 was attributed not only to the low production of MMP (Figure 3E), but also to the low expression of membrane eEF1A.
Figure 4.
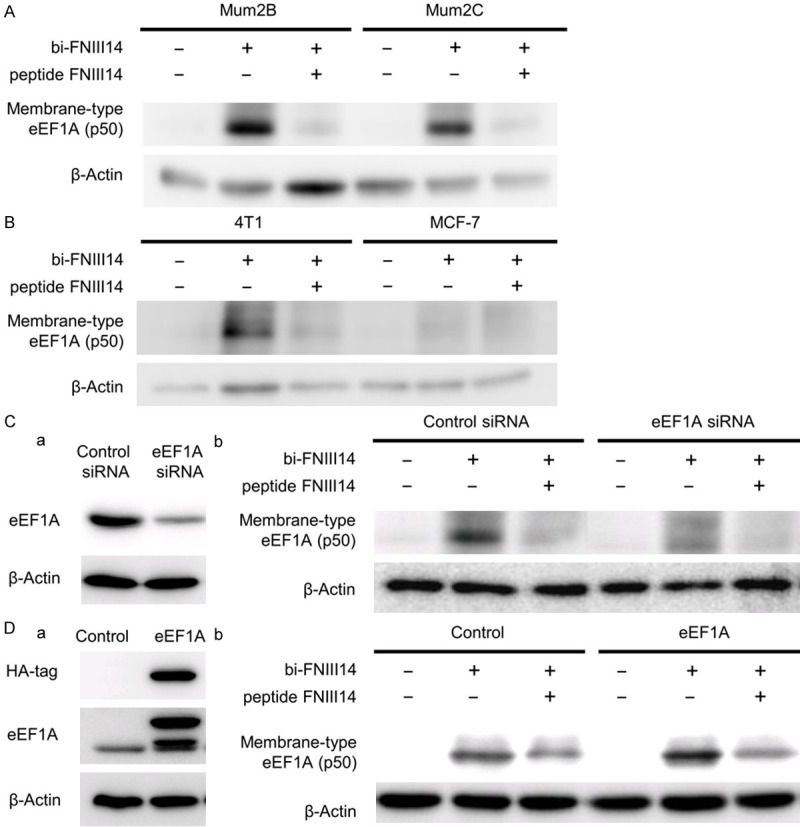
Up- and down-regulation of membrane-type eEF1A expression by genetic control. (A and B) Membrane-type eEF1A expression of Mum2B and Mum2C (A), and 4T1 and MCF-7 (B). To detect membrane-type eEF1A as p50, cells were subjected to affinity-labeling analysis using biotinylated peptide FNIII14 (bi-FNIII14, 1 μg/mL) in the presence or absence of a 10-fold molar excess of unlabeled peptide FNIII14. (C) Down-regulation of total expression of eEF1A and consequent decrease in membrane-type eEF1A. Mum2B cells transfected with control siRNA or eEF1A siRNA were subjected to immunoblot analysis (a) using the antibodies to eEF1A and β-actin, and affinity-labeling analysis (b) using biotinylated peptide FNIII14 (bi-FNIII14, 1 μg/mL) in the presence or absence of a 10-fold molar excess of unlabeled peptide FNIII14. (D) Up-regulation of total expression of eEF1A and consequent increase in membrane-type eEF1A. Mum2B cells transfected with empty vector (Control) or HA-tagged eEF1A vector (eEF1A) were subjected to immunoblot analysis (a) using the antibodies to HA-tag, eEF1A and β-actin, and affinity-labeling analysis (b) as in (Cb). β-actin is a loading control. Data in the picture are representative of three individual experiments.
Next, we examined the effects of genetic control of eEF1A expression level on melanoma cell migration and invasion using Mum2B cells. When Mum2B cells were transfected with eEF1A siRNA, the expression of membrane-type eEF1A was significantly decreased (Figure 4Cb), in parallel to the decrease in cellular eEF1A levels (Figure 4Ca). In contrast, up-regulation of cellular eEF1A levels by enforced transfection of eEF1A-expressing vector (Figure 4Da) caused a significant increase in the membrane-type eEF1A (Figure 4Db).
These melanoma cells with down- or up-regulated expression of membrane-type eEF1A were then examined for their in vitro abilities of cell migration and invasion. As shown in Figure 5A, the migration of Mum2B cells was reduced in parallel to the decreased level of membrane-type eEF1A in response to siRNA-based knockdown of eEF1A. Similarly, the invasion of Mum2B cells was reduced concomitant with the decreased level of membrane-type eEF1A (Figure 5A). Conversely, both the migration and invasion of Mum2B cells were promoted in parallel with the increase in the membrane-type eEF1A (Figure 5B), in response to forced expression of eEF1A. Moreover, an enhanced invasion of Mum2B cells accompanied by an increase in membrane-type eEF1A was inhibited both by anti-FNIII14 Ab (Figure 5C) and BiPS (Figure 5D) to levels similar to those of Mum2B cells without transfection. Changes in membrane expression levels of eEF1A had no effect on the expression and gelatinolytic activity of MMP-9, as demonstrated by gelatin zymography and immunoblot analysis (Figure 5E and 5F). These results suggested that the migratory and invasive capabilities were positively correlated with eEF1A membrane levels, in which the MMP-9-dependent proteolytic exposure of the de-adhesive site FNIII14 was also required for the promotion of cell migration and invasion.
Figure 5.
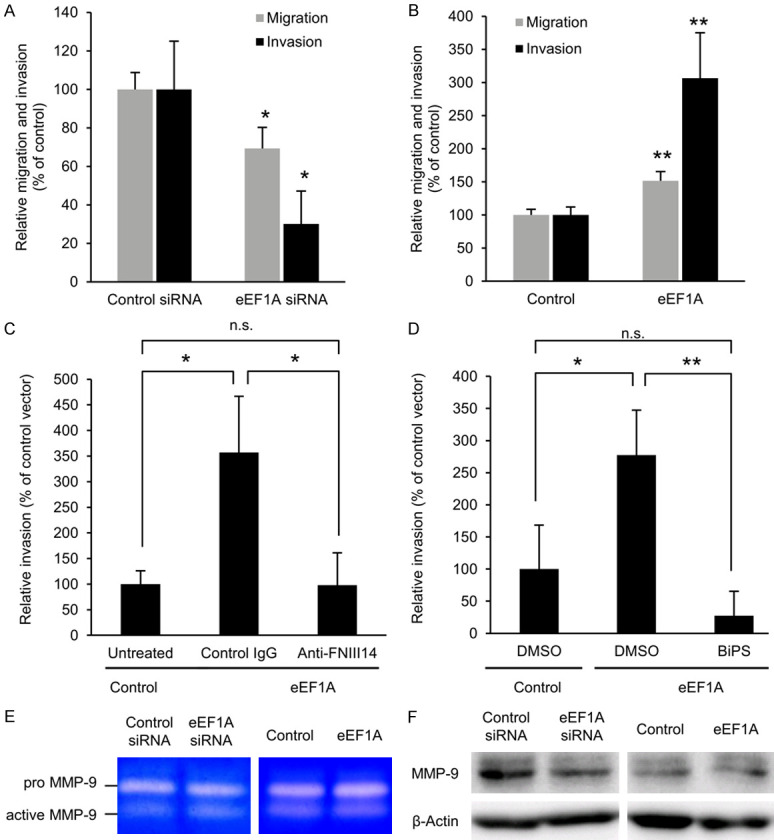
Mum2B cell migration and invasion are dependent on the expression of membrane-type eEF1A and proteolytic exposure of FNIII14. (A) Suppression of Mum2B cell migration and invasion by decreased expression of membrane-type eEF1A. Mum2B cells transfected with control siRNA or eEF1A siRNA were subjected to the migration or invasion assay. (B) Promotion of Mum2B cell migration and invasion by increased expression of membrane-type eEF1A. Mum2B cells transfected with empty vector (Control) or HA-tagged eEF1A vector (eEF1A) were subjected to migration or invasion assay. (C) Promotion of Mum2B cell invasion by increased expression of membrane-type eEF1A is abrogated by anti-FNIII14 Ab. Mum2B cells transfected with empty vector (Control) or HA-tagged eEF1A vector (eEF1A) were subjected to the invasion assay in the presence of normal rabbit IgG (Control IgG, 12.5 μg/mL) or anti-FNIII14 Ab (Anti-FNIII14, 12.5 μg IgG/mL). (D) Promotion of Mum2B cell invasion by increased expression of membrane-type eEF1A is abrogated by MMP-2/9 inhibitor. Mum2B cells transfected with empty vector (Control) or HA-tagged eEF1A vector (eEF1A) were subjected to the invasion assay in the presence of DMSO (vehicle) or BiPS (5 mM). (E) Knockdown and overexpression of eEF1A have no effects on gelatinolytic activity. Gelatin zymography of pro- and active-MMP-9 was performed by using culture supernatant of Mum2B cells transfected with control siRNA or eEF1A siRNA, or with empty vector (Control) or HA-tagged eEF1A vector (eEF1A). Representative gel images are shown. (F) Knockdown and overexpression of eEF1A have no effects on expression of MMP-9. Immunoblot analysis with the antibody to MMP-9 or β-actin was performed by using the lysate of Mum2B cells transfected with control siRNA or eEF1A siRNA, or with empty vector (Control) or HA-tagged eEF1A vector (eEF1A). β-actin is a loading control. Representative membrane images are shown. Values in the picture are mean ± S.D. of three individual experiments. *P < 0.05, **P < 0.01 by Student’s t-test (A, B) or Turkey’s test (C, D).
De-adhesive site FNIII14 as a target for prevention of cancer metastasis
As mentioned above, the in vitro migration and invasion of highly invasive Mum2B melanoma cells and highly metastatic 4T1 breast cancer cells could be significantly suppressed by masking the de-adhesive site FNIII14 with anti-FNIII14 Ab. Thereafter, we investigated whether masking the de-adhesive site FNIII14 could successfully inhibit the aggressive behaviors in vivo in a xenograft mouse model.
Mum2B cells were subcutaneously injected into the right hind footpads of male KSN/SLC nude mice, and normal rabbit IgG or anti-FNIII14 Ab was injected intraperitoneally on days 10, 15, 20, and 25 after the implantation of tumor cells. On day 30 after tumor cell implantation, the lungs and subiliac lymph nodes were excised from the mice to detect Mum2B cell metastasis by PCR analysis of a tumor-derived gene [40,41]. In this experiment, PCR amplification of hPTPRC derived from Mum2B cells allowed us to detect melanoma micrometastasis in the lungs and subiliac lymph nodes. The lungs from 2 of 3 mice and subiliac lymph nodes from 3 of 3 mice treated with normal rabbit IgG were clearly positive for human-derived gene hPTPRC (Figure 6A). The detection of hPTPRC evidenced the metastases of Mum2B cells in these tissues. In contrast, hPTPRC was not detected in either the lungs or subiliac lymph nodes from any mice treated with anti-FNIII14 Ab (Figure 6A), indicating that both hematogenous metastasis to the lungs and lymphatic metastasis to the subiliac lymph nodes could be prevented by masking the de-adhesive site FNIII14 with the blocking antibody. Since anti-FNIII14 Ab had no significant effect on the size of the primary tumors grown in the footpads (Figure 6B), it is unlikely that prevention of metastasis by anti-FNIII14 Ab was due to inhibition of primary tumor growth. In addition, none of the mice showed significant body weight loss or gross evidence of tissue or organ damage.
Figure 6.
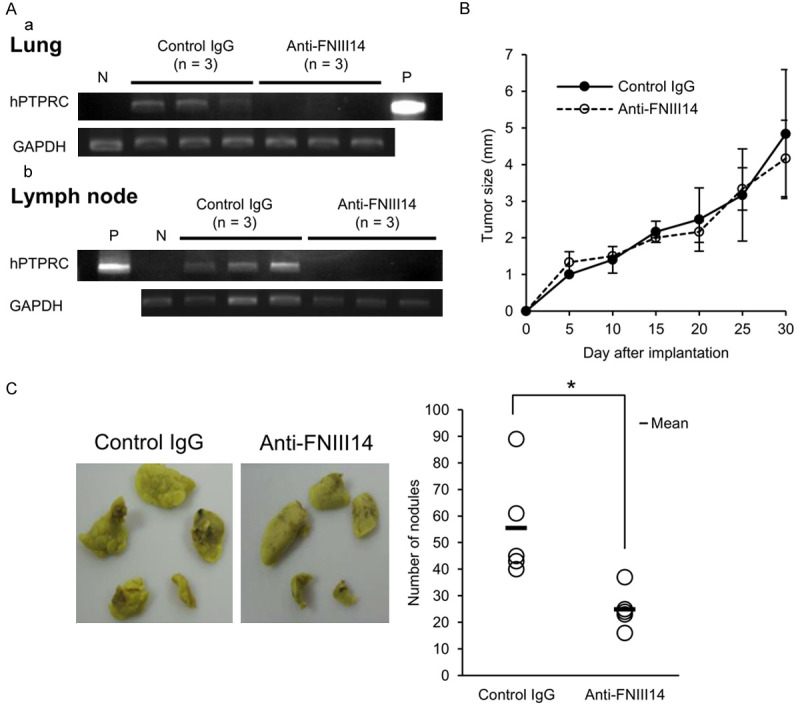
Suppression of melanoma and breast cancer metastasizes by anti-FNIII14 Ab. (A and B) Mum2B cells were subcutaneously injected into the right hind footpads of male KSN/SLC nude mice, and normal rabbit IgG (Control IgG, 100 µg/mouse, n = 3) or anti-FNIII14 Ab (Anti-FNIII14, 100 µg IgG/mouse, n = 3) was injected intraperitoneally on days 10, 15, 20, and 25 after the implantation. Metastasis of Mum2B cells to the lungs and subiliac lymph nodes was detected by PCR analysis of hPTPRC genomic DNA (A), as described in Materials and Methods. GAPDH was used as an internal control. P, positive control (cultured Mum2B cells); N, negative control (tissues from untreated mice). Tumor sizes at the implanted site were measured once every five days after implantation (statistically not significance) (B). (C) 4T1 cells were subcutaneously injected into the right hind footpads of female BALB/c mice, and normal rabbit IgG (Control IgG, 100 µg/mouse, n = 5) or anti-FNIII14 Ab (Anti-FNIII14, 100 µg IgG/mouse, n = 5) was injected intraperitoneally on days 8, 12, 15, and 18 after the implantation. Representative photographs of mice lung from both control IgG- and anti-FNIII14 Ab-treated groups, fixed with Bouins’ solution (left). Metastatic nodules on the lung surface were counted (right). Values in (B) are mean ± S.D. of three mice in each group. Data in the picture are representative of two individual experiments. *P < 0.05 by Student’s t-test (C).
To investigate further the effect of anti-FNIII14 Ab on tumor metastasis, we used another model widely known for spontaneous lung metastasis: That is, inoculation of 4T1 cells into the footpad causes lung metastasis [42]. 4T1 cell suspension was subcutaneously injected into the right hind footpads of BALB/c mice, and normal rabbit IgG or anti-FNIII14 Ab was injected intraperitoneally to the mice, as described in Materials and Methods. On day 35 after the implantation, lung tissues were collected and metastatic nodules on the lung surface were counted. As shown in Figure 6C, the implantation of 4T1 cells resulted in lung metastasis, which was clearly suppressed by administration of anti-FNIII14 Ab. Administration of anti-FNIII14Ab had little effect on mouse body weight.
Discussion
De-adhesion in the trailing edge of cells is considered to be required for efficient migration and invasion. Using an integrative tension sensor that enables integrin tension mapping by fluorescence imaging, Zhao and collaborators recently succeeded in the calibration and mapping of integrin tension in migrating keratinocytes [43]. Their study revealed that high-level integrin tension is narrowly generated at the cell rear margin during cell migration. They demonstrated that migrating keratocytes concentrate mechanical force at the cell rear to mediate rear de-adhesion. However, the molecular process that generates the mechanical force to break the integrin-mediated adhesion at the cell rear remains unclear.
On the other hand, Ramos and colleagues have shown that the ribosomal S6 kinase (RSK) family (RSK1-4) plays an important role in cancer cell migration and invasion by controlling cell adhesion through effects on cell adhesion molecules including β1-integrins [35]. In particular, RSK2 promotes cell motility and invasion by reducing ECM-binding affinity of β1-integrin through the phosphorylation of filamin A, a protein component of the focal adhesion [44]. Their proposal is in good agreement with our conclusion that β1-integrin inactivation is responsible for the induction of migration, invasion, and metastasis in cancer cells, though the molecular mechanisms differ. Thus, functional inactivation of β1-integrin with conformational change appears to play a pivotal role in cell migration and invasion. Whether it is RSK-filamin A or membrane-type eEF1A-FNIII14 that functions as a driver of cancer cell migration and invasion based on β1-integrin inactivation may depend on the expression levels of key protein components that constitute each system. In both highly invasive/metastatic Mum2B and 4T1 cells, inactivation of β1-integrin depended on the membrane level of eEF1A and exposure level of FNIII14, and the latter was further dependent on the expression of MMP-9 and its proteolytic activity. At least in our study, an active form of MMP-9 was detected in highly invasive/metastatic Mum2B and 4T1 cells but not in lower invasive/metastatic Mum2C and MCF-7 cells. Therefore, the expression of MMP-9, which induces FNIII14 exposure, and/or the membrane expression of eEF1A, which mediates de-adhesion by FNIII14, may be important determinants of the invasion/metastatic potential of these types of cancer cells. However, to generalize the importance of the FNIII14-membrane eEF1A system in β1-integrin inactivation for these cancer cells, it should be confirmed again in different cell lines derived from each cancer. Alternatively, it is also of interest to clarify whether the RSK2-filamin A system functions cooperatively with the membrane-type eEF1A-FNIII14 system to inactivate β1-integrin, resulting in enhanced cancer cell invasion and metastasis.
Our in vitro study showed that anti-FNIII14 Ab as well as MMP-2/9 inhibitor strongly inhibited both the migration and invasion of Mum2B and 4T1 cells. The results indicate that preventing exposure of the FNIII14 by MMP-9 inhibition and/or dysfunction of the FNIII14 site itself may contribute to the development of anti-metastatic drugs. However, despite evidence showing the involvement of MMPs in cancer metastasis, many MMP inhibitors have failed in clinical trials in various cancers. Insufficient knowledge about the biological complexity of metastasis and the lack of inhibitor specificity is a major cause of failure [4]; therefore, it would be practically difficult to prevent exposure of the FNIII14 site using MMP inhibitors. In contrast, because the FNIII14 function-blocking antibody effectively suppressed the metastases of Mum2B and 4T1 cells in xenograft mice, dysfunction of the de-adhesive site FNIII14 may be a more reliable strategy for the prevention of metastasis. It should be examined whether the findings demonstrated in this study are confirmed also in other melanoma and breast cancer cell lines, and other types of cancer cells.
Our study also shows that in addition to the de-adhesive site FNIII14, its membrane receptor eEF1A could be an attractive target to enable FNIII14 dysfunction. Human eEF1A has two isoforms, eEF1A1 and eEF1A2, sharing 96% sequence identity [45]. Interestingly, the isoform eEF1A2 was reported to function as a putative oncoprotein in various cancers [46-51]. The eEF1A isoform associated with cancer migration and invasion must be identified. It is also important to investigate the expression level of the eEF1A isoform in normal cells and various tumor cells with different metastatic potential.
In conclusion, the present study provides new insights into the molecular basis of cancer cell migration and invasion/metastasis and a promising target for anti-metastatic therapy.
Acknowledgements
We would like to thank Prof. Vito Quaranta (Vanderbilt University, Nashville, TN) for kindly providing human melanoma cell lines, Mum2B and Mum2C. We also express our gratitude to Dr. Naohiko Koshikwa (Kanagawa Cancer Center Research Institute, Kanagawa, Japan) for his support in this study. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure of conflict of interest
None.
References
- 1.Jiang WG, Sanders AJ, Katoh M, Ungefroren H, Gieseler F, Prince M, Thompson SK, Zollo M, Spano D, Dhawan P, Sliva D, Subbarayan PR, Sarkar M, Honoki K, Fujii H, Georgakilas AG, Amedei A, Niccolai E, Amin A, Ashraf SS, Ye L, Helferich WG, Yang X, Boosani CS, Guha G, Ciriolo MR, Aquilano K, Chen S, Azmi AS, Keith WN, Bilsland A, Bhakta D, Halicka D, Nowsheen S, Pantano F, Santini D. Tissue invasion and metastasis: molecular, biological and clinical perspectives. Semin Cancer Biol. 2015;35(Suppl):S244–S275. doi: 10.1016/j.semcancer.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Liotta LA. Tumor invasion and metastases--role of the extracellular matrix: rhoads memorial award lecture. Cancer Res. 1986;46:1–7. [PubMed] [Google Scholar]
- 3.Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–374. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- 4.Vandenbroucke RE, Libert C. Is there new hope for therapeutic matrix metalloproteinase inhibition? Nat Rev Drug Discov. 2014;13:904–927. doi: 10.1038/nrd4390. [DOI] [PubMed] [Google Scholar]
- 5.Danen EHJ. Integrin signaling as a cancer drug target. ISRN Cell Biology. 2013;2013:1–14. [Google Scholar]
- 6.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 7.Mierke CT, Frey B, Fellner M, Herrmann M, Fabry B. Integrin α5β1 facilitates cancer cell invasion through enhanced contractile forces. J Cell Sci. 2011;124:369–383. doi: 10.1242/jcs.071985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer. 2002;2:91–100. doi: 10.1038/nrc727. [DOI] [PubMed] [Google Scholar]
- 9.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ye F, Kim C, Ginsberg MH. Reconstruction of integrin activation. Blood. 2012;119:26–33. doi: 10.1182/blood-2011-04-292128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sugimori T, Griffith DL, Arnaout MA. Emerging paradigms of integrin ligand binding and activation. Kidney Int. 1997;51:1454–1462. doi: 10.1038/ki.1997.199. [DOI] [PubMed] [Google Scholar]
- 12.Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 13.Chen WT. Mechanism of retraction of the trailing edge during fibroblast movement. J Cell Biol. 1981;90:187–200. doi: 10.1083/jcb.90.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nabeshima K, Inoue T, Shimao Y, Sameshima T. Matrix metalloproteinases in tumor invasion: role for cell migration. Pathol Int. 2002;52:255–264. doi: 10.1046/j.1440-1827.2002.01343.x. [DOI] [PubMed] [Google Scholar]
- 16.Davis GE, Bayless KJ, Davis MJ, Meininger GA. Regulation of tissue injury responses by the exposure of matricryptic sites within extracellular matrix molecules. Am J Pathol. 2000;156:1489–1498. doi: 10.1016/S0002-9440(10)65020-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fukai F, Takahashi H, Habu Y, Kubushiro N, Katayama T. Fibronectin harbors anticell adhesive activity. Biochem Biophys Res Commun. 1996;220:394–398. doi: 10.1006/bbrc.1996.0416. [DOI] [PubMed] [Google Scholar]
- 18.Kato R, Ishikawa T, Kamiya S, Oguma F, Ueki M, Goto S, Nakamura H, Katayama T, Fukai F. A new type of antimetastatic peptide derived from fibronectin. Clin Cancer Res. 2002;8:2455–2462. [PubMed] [Google Scholar]
- 19.Fujita M, Yamamoto T, Iyoda T, Fujisawa T, Sasada M, Nagai R, Kudo C, Otsuka K, Kamiya S, Kodama H, Fukai F. Aggressive progression in glioblastoma cells through potentiated activation of integrin α5β1 by the Tenascin-C-derived peptide TNIIIA2. Mol Cancer Ther. 2019;18:1649–1658. doi: 10.1158/1535-7163.MCT-18-1251. [DOI] [PubMed] [Google Scholar]
- 20.Fujita M, Ito-Fujita Y, Iyoda T, Sasada M, Okada Y, Ishibashi K, Osawa T, Kodama H, Fukai F, Suzuki H. Peptide TNIIIA2 derived from Tenascin-C contributes to malignant progression in colitis-associated colorectal cancer via β1-integrin activation in fibroblasts. Int J Mol Sci. 2019;20:2752. doi: 10.3390/ijms20112752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sasada M, Iyoda T, Asayama T, Suenaga Y, Sakai S, Kase N, Kodama H, Yokoi S, Isohama Y, Fukai F. Inactivation of beta1 integrin induces proteasomal degradation of Myc oncoproteins. Oncotarget. 2019;10:4960–4972. doi: 10.18632/oncotarget.27131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watanabe K, Takahashi H, Habu Y, Kamiya-Kubushiro N, Kamiya S, Nakamura H, Yajima H, Ishii T, Katayama T, Miyazaki K, Fukai F. Interaction with heparin and matrix metalloproteinase 2 cleavage expose a cryptic anti-adhesive site of fibronectin. Biochemistry. 2000;39:7138–7144. doi: 10.1021/bi992670r. [DOI] [PubMed] [Google Scholar]
- 23.Saito Y, Owaki T, Fukai F. Cell regulation through membrane rafts/caveolae. In: Ohshima H, editor. Electrical phenomena at interfaces and biointerfaces. New Jersey: John Wiley & Sons; 2012. pp. 767–781. [Google Scholar]
- 24.Itagaki K, Naito T, Iwakiri R, Haga M, Miura S, Saito Y, Owaki T, Kamiya S, Iyoda T, Yajima H, Iwashita S, Ejiri S, Fukai F. Eukaryotic translation elongation factor 1A induces anoikis by triggering cell detachment. J Biol Chem. 2012;287:16037–16046. doi: 10.1074/jbc.M111.308122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ejiri S. Moonlighting functions of polypeptide elongation factor 1: from actin bundling to zinc finger protein R1-associated nuclear localization. Biosci Biotechnol Biochem. 2002;66:1–21. doi: 10.1271/bbb.66.1. [DOI] [PubMed] [Google Scholar]
- 26.Seftor EA, Meltzer PS, Kirschmann DA, Pe’er J, Maniotis AJ, Trent JM, Folberg R, Hendrix MJ. Molecular determinants of human uveal melanoma invasion and metastasis. Clin Exp Metastasis. 2002;19:233–246. doi: 10.1023/a:1015591624171. [DOI] [PubMed] [Google Scholar]
- 27.Folberg R, Kadkol SS, Frenkel S, Valyi-Nagy K, Jager MJ, Pe’er J, Maniotis AJ. Authenticating cell lines in ophthalmic research laboratories. Invest Ophthalmol Vis Sci. 2008;49:4697–4701. doi: 10.1167/iovs.08-2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fujita M, Yamamoto T, Iyoda T, Fujisawa T, Nagai R, Kudo C, Sasada M, Kodama H, Fukai F. Autocrine production of PDGF stimulated by the Tenascin-C-derived peptide TNIIIA2 induces hyper-proliferation in glioblastoma cells. Int J Mol Sci. 2019;20:3183. doi: 10.3390/ijms20133183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suzuki H, Sasada M, Kamiya S, Ito Y, Watanabe H, Okada Y, Ishibashi K, Iyoda T, Yanaka A, Fukai F. The promoting effect of the extracellular matrix peptide TNIIIA2 derived from Tenascin-C in colon cancer cell infiltration. Int J Mol Sci. 2017;18:181. doi: 10.3390/ijms18010181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toth M, Sohail A, Fridman R. Assessment of gelatinases (MMP-2 and MMP-9) by gelatin zymography. Methods Mol Biol. 2012;878:121–135. doi: 10.1007/978-1-61779-854-2_8. [DOI] [PubMed] [Google Scholar]
- 31.Nicholas RS, Partridge J, Donn RP, Hawkins C, Boggild MD. The role of the PTPRC (CD45) mutation in the development of multiple sclerosis in the North West region of the United Kingdom. J Neurol Neurosurg Psychiatry. 2003;74:944–945. doi: 10.1136/jnnp.74.7.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greenwood JA, Murphy-Ullrich JE. Signaling of de-adhesion in cellular regulation and motility. Microsc Res Tech. 1998;43:420–432. doi: 10.1002/(SICI)1097-0029(19981201)43:5<420::AID-JEMT8>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 33.Murphy-Ullrich JE. The de-adhesive activity of matricellular proteins: is intermediate cell adhesion an adaptive state? J Clin Invest. 2001;107:785–790. doi: 10.1172/JCI12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Franco SJ, Huttenlocher A. Regulating cell migration: calpains make the cut. J Cell Sci. 2005;118:3829–3838. doi: 10.1242/jcs.02562. [DOI] [PubMed] [Google Scholar]
- 35.Sulzmaier FJ, Ramos JW. RSK isoforms in cancer cell invasion and metastasis. Cancer Res. 2013;73:6099–6105. doi: 10.1158/0008-5472.CAN-13-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Triozzi PL, Aldrich W, Singh A. Effects of interleukin-1 receptor antagonist on tumor stroma in experimental uveal melanoma. Invest Ophthalmol Vis Sci. 2011;52:5529–5535. doi: 10.1167/iovs.10-6331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ziegler E, Hansen MT, Haase M, Emons G, Gründker C. Generation of MCF-7 cells with aggressive metastatic potential in vitro and in vivo. Breast Cancer Res Treat. 2014;148:269–277. doi: 10.1007/s10549-014-3159-4. [DOI] [PubMed] [Google Scholar]
- 38.Bausero MA, Bharti A, Page DT, Perez KD, Eng JW, Ordonez SL, Asea EE, Jantschitsch C, Kindas-Muegge I, Ciocca D, Asea A. Silencing the hsp25 gene eliminates migration capability of the highly metastatic murine 4T1 breast adenocarcinoma cell. Tumour Biol. 2006;27:17–26. doi: 10.1159/000090152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miura S, Kamiya S, Saito Y, Wada S, Hayashi R, Taira J, Kodama H, Yajima H, Ueki M, Fukai F. Antiadhesive sites present in the fibronectin type III-like repeats of human plasma fibronectin. Biol Pharm Bull. 2007;30:891–897. doi: 10.1248/bpb.30.891. [DOI] [PubMed] [Google Scholar]
- 40.Nakanishi H, Kodera Y, Tatematsu M. Molecular method to quantitatively detect micrometastases and its clinical significance in gastrointestinal malignancies. Adv Clin Chem. 2004;38:87–110. doi: 10.1016/s0065-2423(04)38003-0. [DOI] [PubMed] [Google Scholar]
- 41.Giese T, Engstner M, Mansmann U, Hartschuh W, Arden B. Quantification of melanoma micrometastases in sentinel lymph nodes using real-time RT-PCR. J Invest Dermatol. 2005;124:633–637. doi: 10.1111/j.0022-202X.2005.23633.x. [DOI] [PubMed] [Google Scholar]
- 42.Kyutoku M, Taniyama Y, Katsuragi N, Shimizu H, Kunugiza Y, Iekushi K, Koibuchi N, Sanada F, Oshita Y, Morishita R. Role of periostin in cancer progression and metastasis: inhibition of breast cancer progression and metastasis by anti-periostin antibody in a murine model. Int J Mol Med. 2011;28:181–186. doi: 10.3892/ijmm.2011.712. [DOI] [PubMed] [Google Scholar]
- 43.Zhao Y, Wang Y, Sarkar A, Wang X. Keratocytes generate high integrin tension at the trailing edge to mediate rear De-adhesion during rapid cell migration. iScience. 2018;9:502–512. doi: 10.1016/j.isci.2018.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gawecka JE, Young-Robbins SS, Sulzmaier FJ, Caliva MJ, Heikkilä MM, Matter ML, Ramos JW. RSK2 protein suppresses integrin activation and fibronectin matrix assembly and promotes cell migration. J Biol Chem. 2012;287:43424–43437. doi: 10.1074/jbc.M112.423046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Knudsen SM, Frydenberg J, Clark BF, Leffers H. Tissue-dependent variation in the expression of elongation factor-1 alpha isoforms: isolation and characterisation of a cDNA encoding a novel variant of human elongation-factor 1 alpha. Eur J Biochem. 1993;215:549–554. doi: 10.1111/j.1432-1033.1993.tb18064.x. [DOI] [PubMed] [Google Scholar]
- 46.Tomlinson VA, Newbery HJ, Wray NR, Jackson J, Larionov A, Miller WR, Dixon JM, Abbott CM. Translation elongation factor eEF1A2 is a potential oncoprotein that is overexpressed in two-thirds of breast tumours. BMC Cancer. 2005;5:113. doi: 10.1186/1471-2407-5-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee MH, Surh YJ. eEF1A2 as a putative oncogene. Ann N Y Acad Sci. 2009;1171:87–93. doi: 10.1111/j.1749-6632.2009.04909.x. [DOI] [PubMed] [Google Scholar]
- 48.Anand N, Murthy S, Amann G, Wernick M, Porter LA, Cukier IH, Collins C, Gray JW, Diebold J, Demetrick DJ, Lee JM. Protein elongation factor EEF1A2 is a putative oncogene in ovarian cancer. Nat Genet. 2002;31:301–305. doi: 10.1038/ng904. [DOI] [PubMed] [Google Scholar]
- 49.Yang S, Lu M, Chen Y, Meng D, Sun R, Yun D, Zhao Z, Lu D, Li Y. Overexpression of eukaryotic elongation factor 1 alpha-2 is associated with poorer prognosis in patients with gastric cancer. J Cancer Res Clin Oncol. 2015;141:1265–1275. doi: 10.1007/s00432-014-1897-7. [DOI] [PubMed] [Google Scholar]
- 50.Sharma S, Tammela J, Wang X, Arnouk H, Driscoll D, Mhawech-Fauceglia P, Lele S, Kazim AL, Odunsi K. Characterization of a putative ovarian oncogene, elongation factor 1alpha, isolated by panning a synthetic phage display single-chain variable fragment library with cultured human ovarian cancer cells. Clin Cancer Res. 2007;13:5889–5896. doi: 10.1158/1078-0432.CCR-07-0703. [DOI] [PubMed] [Google Scholar]
- 51.Kawamura M, Endo C, Sakurada A, Hoshi F, Notsuda H, Kondo T. The prognostic significance of eukaryotic elongation factor 1 alpha-2 in non-small cell lung cancer. Anticancer Res. 2014;34:651–658. [PubMed] [Google Scholar]


