Abstract
Recent advances suggest the fallopian tube as the main anatomic site for high-grade ovarian or pelvic serous carcinoma (O/PSC). Many studies on the biologic role of tubal secretory cells in O/PSC development has been performed in the last decade. However, the role of tubal ciliated cells in this regard has rarely been explored. The purpose of this study was to determine if the change of the tubal ciliated cells is associated with serous neoplasia within the female pelvis. This study included 3 groups (low-risk or benign control, high-risk, and O/PSC) of patients and they were age-matched. Age of patients ranged from 20 to 85 and the age-associated data was stratified by 10-year intervals. The number of tubal ciliated cells was determined by microscopy and by tubulin immunohistochemical staining. The data was then professionally analyzed. The results showed that the absolute number of tubal ciliated cells decreased significantly with age within each age group. A reduction in ciliated cell counts within the tubal segments remained a significant risk factor for the development of serous cancers within the female pelvis after age adjustment. A dramatic decrease of tubal ciliated cells was identified in patients with high-risk and with O/PSC compared to those in the benign control or low-risk group (P < 0.001). Further, within the tubal fimbria, the number of ciliated cells reduction was more prominent in the high-risk group when compared to those of O/PSC patients. Our findings suggest that a decreased number of ciliated cells within women’s fallopian tubes represents another histologic hallmark for early serous carcinogenesis. There is a relationship between loss of tubal ciliated cells and aging, the presence of high-risk factors for tubal-ovarian cancer, and co-existing O/PSCs. This represents an initial study identifying the role of tubal ciliated cells in the development of high-grade serous carcinoma in women’s pelvis.
Keywords: Fallopian tube, oviduct, pathogenesis, carcinogenesis, tubal ciliated cells, tubal-ovarian high-grade serous carcinoma, pelvic serous carcinoma, extra-uterine serous cancers, ovarian cancer
Introduction
Ovarian cancer is one of the most lethal malignancies, with over 20,000 new cases and about 14,000 associated deaths every year [1]. Epithelial ovarian cancer is the most prevalent subtype, accounting for about 80% to 90% of cases. Among epithelial ovarian cancers, serous carcinomas (OSCs) are the most common subtypes (~70%), followed by mucinous, endometrioid, and clear cell carcinomas [2,3]. Histologically, OSCs can be classified into two groups: high-grade serous carcinomas (HGSCs) and low-grade serous carcinomas (LGSCs). HGSCs account for about 90% of OSCs and account for approximately 70% of all ovarian cancer-related deaths. These cancers typically present as metastasis, involving pelvic and upper abdominal organs. Unclear etiology and lack of effective early detection methods for HGSC remain the major obstacles to curing this disease [2].
The concept of HGSC includes primary sites of the ovary, fallopian tube, and peritoneal cavity. By general convention, they are usually lumped into the ovarian or pelvic serous carcinoma (O/PSC) category. Formerly, O/PSCs were thought to originate from ovarian surface epithelial cells [4]. Nevertheless, recent advances suggest that the precancerous lesions of O/PSC might commonly originate from the fallopian tube, rather than from the ovary or peritoneal surface, therefore a term of tubal-ovarian cancer has been proposed [2,5-12]. Within the fallopian tubal mucosa, there are two major morphologically cell types, ciliated and secretory cells. It is commonly believed that the secretory cells of the fallopian tube serve as the cells of origin for the majority of O/PSCs [2,5,6,13,14]. However, debates about the cell of origin remain.
Detailed histopathological examination of the resected ovaries and fallopian tubes in BRCA mutation carriers has led to the research focus of ovarian serous carcinogenesis on the fallopian tube. Precancerous or precursor lesions including secretory cell expansion (SCE), secretory cell outgrowth (SCOUT), p53 signatures, serous tubal intraepithelial carcinoma (STIC), and serous tubal intraepithelial lesions (STIL) are almost all present in the distal portion of the fallopian tube, but not in the ovary. Most of the above precursor lesions mainly consist of tubal secretory cells rather than ciliated cells. Findings from morphological and molecular studies including those genetically engineered mouse models suggest that tubal secretory cells from the fimbriated end are likely to be the cell of origin for HGSC [2,11,15-27]. The above studies identified multistep accumulation of molecular and genetic alterations in morphologically recognizable pre-neoplastic and neoplastic lesions. A stepwise progression model of tubal serous carcinogenesis suggests that HGSC develops from tubal epithelia to precursor lesions to carcinoma with the sequence of SCE -> SCOUT -> p53 signature -> STIC -> HGSC [25]. However, this pathogenesis model does not explain why many disseminated HGSC cases do not exhibit precursor lesions within the fallopian tube, and instead present mainly in the ovary and peritoneal cavity. More recently, a new concept of early serous proliferations (ESP) in the fallopian tube with “precursor escape” was proposed [28]. ESPs consist of low-proliferative tubal cell groups and some of them with aberrant p53 expression phenotype. Although the concept of precursor escape provided a rationale to explain the phenomenon of no serous precursor lesions found in the majority of advanced stages of HGSCs, it remains to be accepted due to lacking solid scientific evidence to support. All the above studies seem to focus on the tubal secretory cells and the tubal ciliated cells have been bypassed for a long time albeit they are similarly located in the tubal mucosae.
The physical and functional alterations of tubal ciliated cells in the relationship of the development of O/PSC are largely unknown. Recently, we used single-cell sequencing approaching to address the roles of tubal epithelial cells in the process of O/PSC development, we surprisingly found that the tubal ciliated cells have stem-cell-like nature and many other unexpected findings (manuscript in preparation), further indicating there are many undiscovered biologic functions and mechanisms of the tubal ciliated cells. This study aimed to determine if the O/PSC precursor model could be further scrutinized through the study of physiological changes of the number of tubal ciliated cells in the fallopian tube. The current study addressed the following questions: 1) What are the overall change of tubal ciliated cell numbers within the different tubal segments and their relationship with patients’ age? 2) What is the extent of the number of ciliated cellular changes in patients with a high-risk compared to those in normal or low-risk controls as well as to patients with O/PSC? 3) Whether these cellular changes are independent of the aging process? and 4) If the changed number of tubal ciliated cells observed within the fallopian tube may represent an early biomarker for the risk of O/PSC development.
Materials and methods
Case collection
A total of 240 cases of fallopian tubes, which were surgically removed from 2007 to 2015 were identified from pathology files of University of Arizona Medical Center in Tucson, Arizona. The study was approved by the institutional review board. Cases were divided into three groups of patients: low-risk (n=120), high-risk (n=60), and patients with O/PSC (n=60). Low-risk patients served as the control group and consisted of those patients post hysterectomies and salpingectomies performed for benign disease (leiomyomata, adenomyosis, ovarian benign cysts including endometriosis, or uterine prolapse, etc). Controls were further divided into age groups to determine the normal distribution of tubal ciliated and secretory cells. High-risk patients were those with either BRCA1/2 mutations (n=32), history of breast cancer (n=20), or first-degree family history of ovarian cancer (n=8). Typically, these patients underwent prophylactic bilateral salpingo-oophorectomy prior to noticeable disease development. The median and mean interval between previous breast cancer and prophylactic bilateral salpingo-oophorectomy were 78 and 85 months, respectively. The 60 O/PSC patients represented FIGO stage 2 (n=6), stage 3 (n=49) and stage 4 (n=5). Based on clinicopathologic findings, the primary sites for the O/PSC cases included ovary (n=40), unilateral fallopian tube (n=14), and peritoneum (n=6). Fallopian tubal samples from patients with O/PSC were either intact or with only serosal involvement by high-grade serous carcinoma. To avoid potential compounding factors, samples were excluded if tumors including serous tubal intraepithelial carcinoma involving tubal lumen or extensive tubal mucosa. The age of patients was matched among the three groups. The clinical data related to three groups of patients are summarized in Table S1.
Tissue handling
For benign controls, at least two representative sections of the fallopian tube, one from the ampulla (proximal) and the other from the fimbria, were submitted. Fallopian tubes from benign control cases were processed by embedding all fimbriated ends similar to cancer patients with additional representative 2 cross-sections of the ampulla as described previously [5]. For patients with high-risk or O/PSC, the fallopian tube was examined by using the SEE-FIM protocol [29]. Among all sections, 2 sections (one from fimbria and one from ampulla) from each case of the high-risk group were examined under the microscope. The tissues were fixed in 10% buffered formalin and processed routinely for paraffin embedding. Five-micron sections for immunohistochemistry (IHC) were cut and placed on Super Plus slides (Fisher Scientific, Pittsburgh, PA) followed by a section of each specimen that was stained with hematoxylin and eosin and examined microscopically to confirm the diagnosis.
Counting the number of ciliated cells in tubal mucosa
The ciliated cells of the fallopian tubal mucosa were readily identifiable under the light microscopy when cilia are present on the apical part of the cells, while the epithelial cells without cilia were assumed as secretory cells. Nonetheless, cilia sometimes could not be visible due to different plane orientations since tissue sections are random. The number of ciliated cells within the tubal fimbria and ampulla epithelia were counted in the fallopian tube in each case and evaluated by 2 methods: light microscopy and IHC with tubulin (a marker for ciliated cells) and PAX8 (a marker for secretory cells) as described previously [23,30]. After a defined area was selected under the microscope, the absolute number of ciliated cells was derived from 3 different high power fields (400× magnification). Microscopically, the number of tubal epithelial cells ranged from 275 to 500 with an average of 410 epithelial cells in each high power field. For the cases stained with tubulin, the number of ciliated cells was counted based on the cells with positive cilia on the cell apical border and the counting method was the same as the routine microscope for HE slides. The percentage of the ciliated cells was calculated based on the total number of cells counted. The authors performing slide reviews were blinded as to the group status of the tubal sections.
Immunohistochemical analysis
For IHC analyses, 4-μm-thick sections were cut from formalin-fixed paraffin-embedded (FFPE) tissue blocks, deparaffinized in xylene, and then rehydrated through sequential washes of alcohol and distilled water.
Tubulin and Pax-8 were detected using ready-to-use monoclonal antibodies against tubulin and Pax-8 as previously described [23,30]. PAX8 has been considered as a Mullerian epithelial biomarker identifying tubal secretory, but not ciliated cells [5,24,31]. Alpha-tubulin identifies cellular surface cilia and is an appropriate marker to identify tubal ciliated cells [5,14,32]. The slides were heated at 120°C for 5 min in 0.01 M citrate buffer (pH 6.0) before immunostaining. These slides were then incubated with the above-mentioned antibodies for 2 hours at room temperature. Antibody binding was visualized via the EnVision+ Dual-link system and diaminobenzidine as a chromogen (Dako, CA, USA). The slides were counterstained with methyl green or hematoxylin and mounted.
Benign tubal ligated sections from subjects without known risks for cancer were served as positive controls for the antibodies studied in this project. Negative controls were performed by replacing the primary antibody with nonimmune IgG. All slides were reviewed independently by 2 professional pathologists (YiW or WL and WZ). The percentage of positive cells that showed dark brown nuclear or cilia staining was recorded. Only moderate or strong intensity of the IHC staining was considered as positive, while weak stains were considered as negative.
Data evaluation and statistical analysis
Two methods were used to evaluate the number of ciliated cells in the fallopian tubal mucosa. With the IHC stains or light microscopy methodology, an average number of the ciliated cells as well as the percentage of the ciliated cells within the tubal mucosa for each tubal segment was used for statistical analysis. The following parameters were calculated for the cases studied: 1) The number of ciliated cells and its distribution (fimbria vs ampulla) was calculated by both tubulin stains and microscopy counting. The cellular numbers were arranged according to age organized in 10-year intervals; 2) Comparisons of ciliated cells in ampulla and fimbria within the fallopian tubes among the three groups; 3) Comparisons of the percentage of positive hormone receptor expressions among the control and study groups; and 4) Evaluation if high-risk and/or O/PSC associations are independent of age for the number of ciliated cell changes.
The data were analyzed by standard contingency table methods, nonparametric Mann-Whitney U-tests, and Spearman correlation analysis using GraphPad Prism 7.0 for Windows (GraphPad Software, San Diego, CA, USA) and Stat View (SAS Institute, Cary, NC, USA) computer package programs. All P values were two-sided, and a value of P < 0.05 was considered statistically significant. To adjust for age differences and the varying numbers of sections or microscopic fields examined for each case, the data were calculated on the assumption that the number of ciliated cells in each case follows a Poisson distribution, which is commonly used to model count data, with an offset term used to account for the microscopic fields examined. For comparisons with patients’ age, the age data were stratified into 10-year intervals ranging from 20-29 to older than 80.
Results
As age increased, the number of ciliated cells decreased
A total of 240 patients’ fallopian tube specimens, divided into 10 groups based on age (ie 20-29, 30-39, … and > 80) were studied. These included benign or low risk (n=120), high-risk (n=60) and O/PSC (n=60).
Overall, there was a significantly decreased number of ciliated cells in the fallopian tube with increasing age in the cases studied. Compared with the age 20-29 group, which contained an average of 258 ciliated cells/HPF in fimbria and 260/HPF in the ampulla, the average number of ciliated cells decreased 95% and 82% with an average number of ciliated cells of 15/HPF in fimbria and 48/HPF in the ampulla, respectively at the age group of > 80 years. There was a clear trend that the number of ciliated cells decreases as a function of age in both fimbria and ampulla segments (P < 0.001) and the significant reduction of the ciliated cells starting from age of 30 years old (Figure 1A and 1B). Compared with the ampulla region, the number of ciliated cells in fimbria further decreased significantly starting from the age of 40 years old. The detailed data are summarized in Table S2 and visualized as a bar graph in Figure 1A-C.
Figure 1.
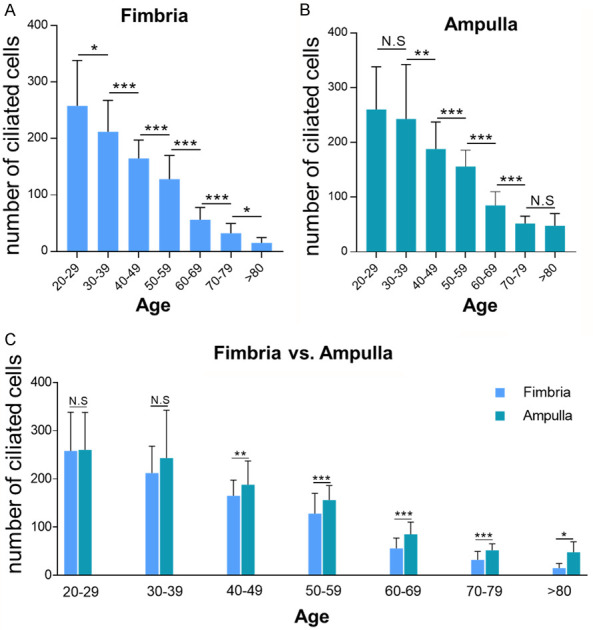
Ciliated cells distribution trend among different age groups, calculated by microscopic direct counting (H&E). A clear trend of reduction of the number of tubal ciliated cells was present in the aging process. A significant reduction started at age 30s in both fimbria and ampulla segments. Compared with ampulla region, further reduction of the tubal ciliated cells was detected in the fimbria. A. The number of ciliated cells in tubal fimbria region; B. The number of ciliated cells in tubal ampulla region; C. Comparisons of ciliated cells distribution between ampulla and fimbria. Statistically significant differences were determined using the Mann-Whitney U test. N.S, no significance; *P < 0.05; **P < 0.01; ***P < 0.001.
The number of ciliated cells was significantly decreased in tubal segments of patients with high-risk or ovarian/pelvic serous carcinoma
Among 120 patients with benign gynecologic diseases or with low risk for ovarian cancers, we selected 60 high-risk patients and additional 60 patients with O/PSCs matched for age to the low-risk group for the comparisons of the overall number of ciliated cells in the fallopian tube by using both morphologic and IHC methods. With tubulin stains, the average number of ciliated cells in the fimbria region in low-risk tubes was 236/HPF (Table S3). This cellular number decreased significantly with an average of 118/HPF in the high-risk group and 156/HPF in the O/PSC group, respectively (P < 0.001), which was translated to 50% and 34% reduction of the ciliated cells, respectively in tubal fimbria (Table S3). A similar trend of reduction of the tubal ciliated cells was found in the ampulla segment in the high-risk and the O/PSC groups, compared with that of the low-risk group (P < 0.001) (Figure 2A, 2B). Although the number of ciliated cells/HPF was slightly higher in the ampulla region than that in the fimbria, it did not reach to a statistical significance in all 3 patient groups (Figure 2C). We also compared the number of ciliated cells between the high-risk and the O/PSC groups. Interestingly, there was a further 25% reduction, from an average of 156/HPF of the O/PSC group to 118/HPF of the high-risk group, of ciliated cells in the tubal fimbria (P < 0.001) (Figure 3A). However, there was no statistical difference of the number of ciliated cells in the ampulla region between the high-risk and O/PSC groups (P=0.18) (Figure 2B). The detailed data are summarized in Table S3.
Figure 2.
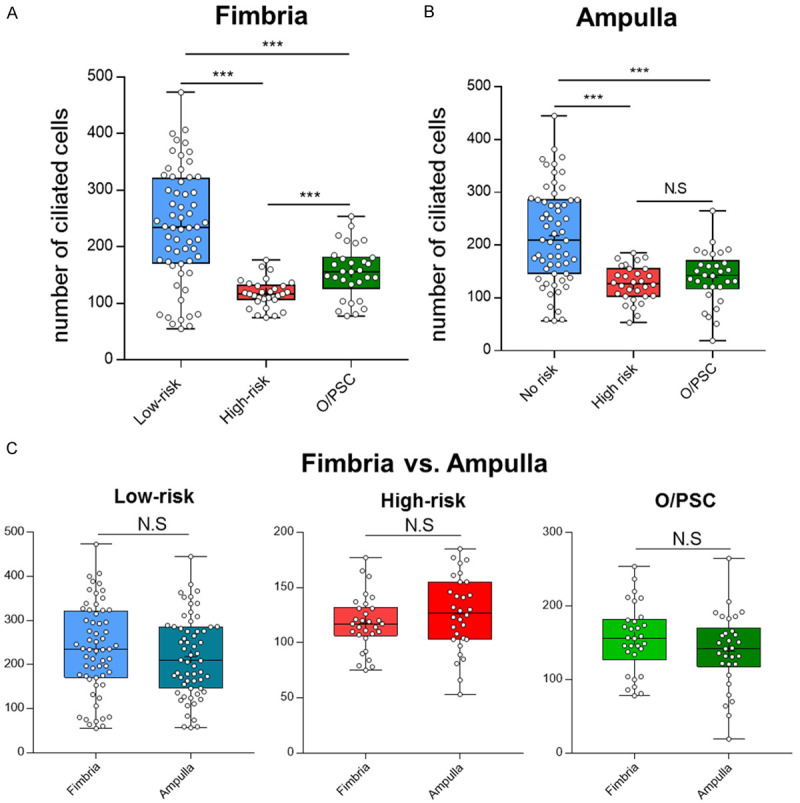
Comparisons of the number of ciliated cells calculated by tubulin staining method among control (low-risk) and study groups (high-risk or O/PSC). Compared with the control group, the number of tubal ciliated cells was significantly reduced in both study groups in both tubal segments (A, B). There was no statistical significant difference detected when the number of tubal ciliated cells was compared between fimbria and ampulla in the same group (C). Statistically significant differences were determined using the Mann-Whitney U test. N.S, no significance; ***P < 0.001.
Figure 3.
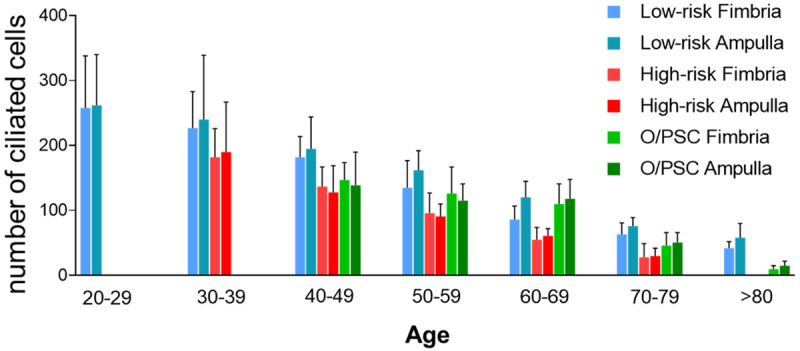
Overview of ciliated cells distribution in fimbria and ampulla with age and among patients with low-risk, high-risk, and ovarian/pelvic serous carcinoma (O/PSC). A clear trend of tubal ciliated cell reduction was observed in the aging process.
When the data were arranged based on age distribution within each group, we found that there was also a significantly decreased number of ciliated cells in the fallopian tube with increasing age in all three groups. Compared with the low-risk group, the number of ciliated cells in high-risk and O/PSC groups were further reduced (P < 0.001). The detailed data are summarized in Table S4 and the corresponding bar graph in Figure 3.
The number of tubal ciliated cells counted by microscopy was comparable to the number of ciliated cells detected by tubulin staining
To evaluate whether tubulin IHC stain can accurately reflect the distribution of ciliated cells, we examined the relationship between the number of tubal ciliated cells calculated with microscopic direct counting (H&E) in tubal tissues and the number calculated with IHC (tubulin) method from 120 matched tissues. A robustly positive correlation was observed. (Fimbria: r=0.68, P < 0.0001; Ampulla: r=0.72, P < 0.0001; Spearman correlation analysis) (Figure 4).
Figure 4.
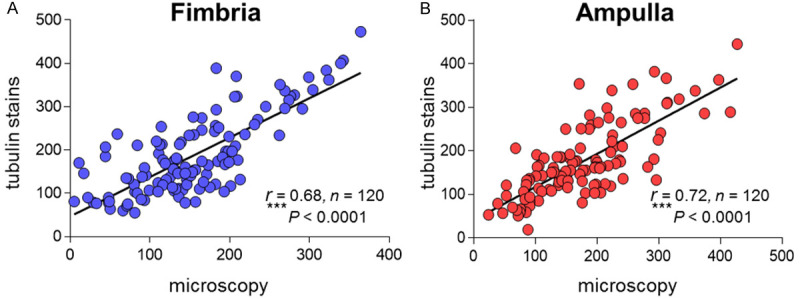
Correlation of the number of tubal ciliated cells between the method of microscopic direct counting (HE) and the method with tubulin staining (IHC) from matched tubal tissue sections. A Robust correlation between the two methods was present. A. Ciliated cells distribution in tubal ampulla region; B. Ciliated cells distribution in tubal Ampulla region; Spearman rank correlation (r) was used for the correlation analysis; ***P < 0.001.
Interestingly, Pax-8 stained cells partially overlapped with ciliated cells, while tubulin illustrated the tubal ciliated cells only. This was the reason we did not consider Pax-8 negative cells as ciliated cells. Representative pictures of the tubal ciliated cells identified by morphology and tubulin IHC stains are illustrated in Figures 5, 6.
Figure 5.
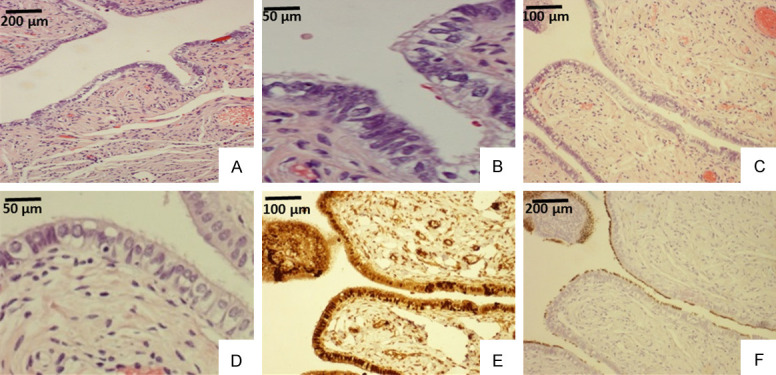
Example of tubal ciliated cells detected by microscopy and tubulin staining in a patient of 45 years old. Tubal ampulla segment (A, B) showed ciliated cells, which are easily visible in a high power (upper right corner of B). Tubal fimbria region (C, D) showed cilia on the apical cellular border under a high power view (D). The same fimbria region (C) stained with PAX8 for tubal secretory cells (E) and tubulin for ciliated cells (F). Apparently, PAX8 stained both secretory and ciliated cells (E) as the ciliated cells were illustrated by tubulin stain (F). Original magnifications: (A, C, E, and F) 100×; (B and D), 400×.
Figure 6.
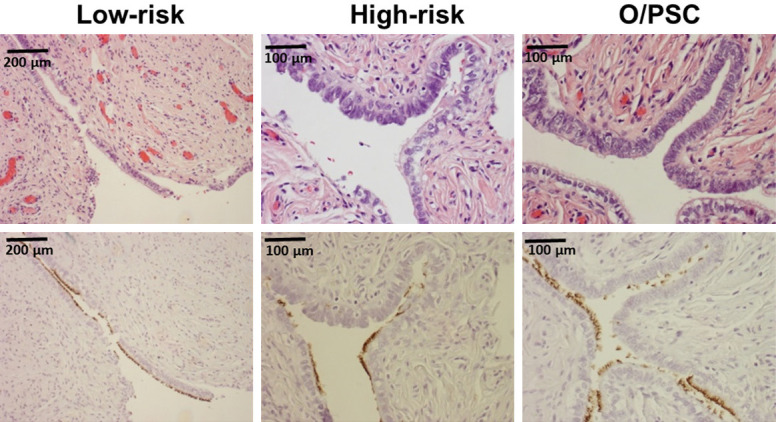
Morphologic and immunohistochemical identification of tubal ciliated cells in tubal fimbria. One representative section of tubal fimbria from patients in an age group of 40s was presented. Top panel shows morphologic picture of tubal fimbria, while bottom panel shows corresponding tubulin stains. The number of ciliated cells (tubulin+) was 180, 128, and 141 for low-risk, high-risk, and O/PSC patients, respectively. Original magnifications: left 100×, middle 200×, right 200×.
Decreased number of ciliated cells was significantly associated with age, the high-risk factors, and the presence of ovary/pelvic serous carcinoma
As we demonstrated above, decreased number of tubal ciliated cells was associated with age and more strikingly with high-risk and O/PSC patients. Therefore, we explored whether the decreased number of ciliated cells in high-risk or O/PSC patients are independent of age. We addressed this question in a regression model that adjusted for age, as well as the number of cross-sections examined for the number of ciliated cells by linear regression analysis. The three groups (low-risk or control, high-risk, and O/PSC) of patients were divided according to 10-year intervals and the average number of ciliated cells for the intervals was compared. There were still significant differences in the decreased number of ciliated cells as a function of increasing age in both case and control groups (Table S4 and the corresponding bar graph in Figure 3). We observed a significant correlation with a determination coefficient factor of 0.171 when we combined the benign controls and the studied cases for comparison. The coefficient factor of 0.171 indicated that approximately 17.1% of decreased tubal ciliated cells could be attributed to age. We also observed that both high-risk and O/PSC groups had more reduced number of ciliated cells than low-risk or benign controls (P < 0.001), an average decrease of 0.90 logs counted for high-risk patients vs low-risk controls and 0.83 logs for patients with O/PSC vs benign controls. Therefore, age, high-risk factors, and O/PSC all represented independent risk factors for the reduction of the ciliated cells within the tubal epithelia. Patients with a high-risk showed the strongest association with a decreased number of tubal ciliated cells, while age was the weakest, nevertheless, all these observations were statistically significant. Compared to the ampulla segment, we noticed that the number of ciliated cells in the fimbriated end was slightly higher (Figure 1C; Table S2).
Discussion
Most of the available studies regarding cell of origin of tubal-ovarian high-grade serous carcinoma or O/PSCs do not take into account the potential contributions of tubal ciliated cells. Ciliated cells of the fallopian tube are more abundant in the fimbria section (50%), progressively decrease in number toward the uterus (30%), and are mostly located at the apex of epithelial papillae [33]. The main function of ciliated cells in the fallopian tube is to transport the ovum from the ovary toward the uterus and help to remove genotoxic stress after ovulation [34,35]. Tubal ciliated cells are generally considered as terminally differentiated cells from either tubal secretory cells or stem-like cells within the fallopian tube and induced by estrogen stimulation [36-38]. Tubal ciliated cells in vitro may reversely differentiate into non-ciliated cells (morphologically show no difference from the secretory cells) [39]. It is not clear how this happens and whether these ciliated cells have the potential to initiate serous neoplasia after becoming non-ciliated cells. We believe tubal ciliated cells influenced by genetic and environmental factors are involved in the onset of ovarian serous tumorigenesis. The reduction of tubal ciliated cells may be due to reduced capacity for removing follicular fluid-induced genotoxicity within the fallopian tube [40]. Thus, it is not surprising that there is a reduction of ciliated cells with age, in particular over the age 40s. One early study about the role of tubal epithelia in supporting early embryo development showed that tubal motile cilia is lost in vitro when estradiol is withdrawn [41]. But overall, there is a limited understanding of the tubal ciliated cells on the aspects of cellular differentiation and neoplasia. As the tubal mucosa is mainly composed of ciliated and secretory cells, people may have a general impression that the increased density of tubal secretory cells reflects the decreased number of ciliated cells. In reality, however, such a relationship has never been systemically studied.
This study examined the global change of tubal ciliated cells and its relationship with the aging process by counting the number of ciliated cells in patients with low-risk (benign group), high-risk, and O/PSC. Although the study is descriptive in nature, it explores a novel approach to evaluate the risk for ovarian or pelvic serous carcinogenesis. This represents an early or initial study to describe the changes in the number of tubal ciliated cells in relation to age as well as the risk factors associated with O/PSC. For patients in the low-risk (control group) for tubal-ovarian or pelvic serous cancers, the number of tubal ciliated cells decreased with age, starting at age 30s and decreasing by 94% after age 80. It appears that the reduction of ciliated cells is consistent with the increment of tubal secretory cells with aging as we demonstrated earlier [2,21]. However, it is unclear if the reduction of tubal ciliated cells and increased the number of secretory cells are independent or reciprocal. Our observations of loss of tubal ciliated cells with aging may go along with the well-known epidemiologic findings that O/PSC increases with age and shows a peak incidence starting from menopause [19,42,43]. A more dramatic decrease of tubal ciliated cells is observed in patients with high-risk factors, including germline mutations of BRCA1/2 genes or family history of tubal-ovarian or pelvic serous cancers. This decreased number of tubal ciliated cells is also closely associated with age in both high-risk and O/PSC groups, indicating that a decreased number of tubal ciliated cells is linked to “ovarian” or pelvic serous neoplasia.
Since a decreased number of tubal ciliated cells was associated with age and more strikingly associated with patients having high-risk factors as well as O/PSCs, we further examined if the decreased number of ciliated cells in patients with high-risks or O/PSCs are independent of age in the study. By using linear regression analysis, we discovered that all three factors namely age, high-risk status, and patients with O/PSC, are independent risks for the decreased number of tubal ciliated cells when both the cases and controls were normalized.Approximately 10% to 17% of decreased ciliated cells in the fallopian tube is accredited to age, while 83% to 90% attributed to O/PSC and high-risk status, respectively. Patients with germline BRCA mutations showed the sturdiest association with the decreased number of ciliated cells in the fallopian tube, consistent with its known risks for O/PSC development [19,44-46]. While there is still a connotation found of reduction of ciliated cells as well in the O/PSC group, this group is not restricted to cases with serous neoplasia. Thus, diverse tumor microenvironmental changes exist which could impact the number of neighboring ciliated cells by other genetic or environmental mechanisms. From these findings, we wonder that patients with high-risk factors and/or with O/PSCs have an unidentified mechanism to cause the reduction of tubal ciliated cells in addition to the aging process and such changes are unlikely to be influenced by hormone level changes since the study is age-matched.
It is believed that the fallopian tube provides a cellular source for the majority of O/PSC, while tubal fimbria is considered as the anatomical location of the cell of origin of the O/PSC [47]. Our current study examined the number of ciliated cells and their distributions in both tubal fimbria and ampulla segments. The study showed that there is no significant difference between the two tubal segments for the number of ciliated cells. However, the number of tubal ciliated cells are significantly fewer in high-risk group than that in O/PSC patients within the tubal fimbria (Figure 2A). It is unclear how to explain this phenomenon. However, it suggests that patients with BRCA mutations may have some undiscovered mechanisms to cause more of the reduction of tubal ciliated cells. Such findings are also consistent with that patients with germline mutations of BRCA genes, and occurrence of HGSCs at an earlier age than those who have sporadic ovarian cancers [48], as well as with the well-accepted concept that tubal fimbria as the main anatomic site for ovarian or pelvic serous neoplasia [49,50].
The functional role of cilia in human carcinogenesis is unclear. There are no studies on the role of multiple motile cilia for the tubal ciliated cells in the process of ovarian carcinogenesis. Through literature search, however, we found a few studies on the biologic function of primary cilia of the fallopian tube in the process of cancer development [51,52]. Egeberg et al. suggested that defects of primary cilium in ovarian tumorigenesis may be related to the deregulation of cilia signaling pathways such as Hedgehog, platelet-derived growth factor, and aurora A kinase signaling [51]. Some other earlier studies showed that primary cilia may play a critical role in tumorigenesis and cancer progression by functioning as a tumor suppressor organelle that regulates cell cycle/proliferation, differentiation, polarity, and migration [53,54]. On the other hand, loss of tubal ciliated cells may also reduce the capacity of removing follicular fluid-induced genotoxicity within the fallopian tube [55]. In one of our studies about ovarian serous carcinogenesis, we also notice that gradual motile cilia loss from serous cystadenoma to a serous borderline tumor and finally complete loss of cilia in low-grade serous carcinoma [5]. More recently, we have studied tubal epithelial cells from BRCA1 mutation carriers and benign controls without a known history of BRCA1 mutation by using single-cell sequencing technology. We found that tubal ciliated cells express SOX2 biomarker, which is known to be one of the stem cell markers, while secretory cells do not (manuscript in preparation). It would be interesting to study the biologic function of tubal ciliated cells and their motile cilia in the process of serous carcinogenesis.
Tubal mucosa consists of both secretory and ciliated cells, arranged in a recurring pattern of alternating each other in the normal fallopian tube of reproductive-aged women. PAX-8 is a member of the pair-box (PAX) family of transcription factor genes. Studies have shown that PAX8 is a biomarker of tubal secretory cells and is used to distinguish gynecologic cancers from non-gynecologic malignancies [56]. Therefore, PAX-8 has been widely used in the clinic. In this study, we used PAX-8 to distinguish tubal secretory cells from tubal ciliated cells. However, not infrequently we have found that not only secretory cells, but also some of the ciliated cells are positively stained by PAX-8 (Figure 5). This is the reason for us to use tubulin highlighting the ciliated cells in the study. Although morphologically tubal ciliated cells are easily distinguished from secretory cells because of the presence of multi-motile cilia on the cellular apex, these two cell types may be interchangeable, which is supported by ciliation changes of the tubal epithelia in the menstrual cycle [57]. That can explain why some of the ciliated cells are positive for PAX-8 expression in the current study.Studies to identify regulatory factors for the transitions between ciliated and secretory cells are needed to help us uncover the role and functions of these tubal epithelial cells.
In summary, our findings offer a novel perspective on the initial mechanisms involved in the development of tubal-ovarian high-grade serous carcinoma or O/PSC, which can facilitate further experimental researches of the tubal epithelial cells including the ciliated cell clearance in the process of serous carcinogenesis. Tubal ciliated cells are morphologically identifiable under microscope. Therefore, counting the number of tubal ciliated cells may represent a practical approach to be used to study serous carcinogenesis in its initial stages. Identification of tubal ciliated cells can be easily carried out by microscopy or by IHC stain with tubulin. Single-cell sequencing study illustrating the relationship between secretory and ciliated cells are ongoing in our laboratory. Molecular mechanism studies of tubal ciliated cells and the relationship between tubal secretory and ciliated cells may aid to develop optimal strategies of ovarian cancer prevention and possibly early intervention.
Acknowledgements
This work was partially supported by grants from the Department of Science and Technology of Henan province (162102310174); Natural Science Foundation of Henan province (ZC20180062). National Natural Science Foundation of China (81972441). The project was also supported in part by Mark and Jane Gibson Endowment Fund, UTSW Medical Center.
Disclosure of conflict of interest
None.
Abbreviations
- HGSC
high-grade serous carcinoma
- O/PSC
ovarian or pelvic serous carcinoma
- CC
ciliated cells
- IHC
immunohistochemistry
Supporting Information
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Li J, Fadare O, Xiang L, Kong B, Zheng W. Ovarian serous carcinoma: recent concepts on its origin and carcinogenesis. J Hematol Oncol. 2012;5:8. doi: 10.1186/1756-8722-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delair D, Soslow RA. Key features of extrauterine pelvic serous tumours (fallopian tube, ovary, and peritoneum) Histopathology. 2012;61:329–339. doi: 10.1111/j.1365-2559.2011.04167.x. [DOI] [PubMed] [Google Scholar]
- 4.Zhang W, Wei L, Li L, Yang B, Kong B, Yao G, Zheng W. Ovarian serous carcinogenesis from tubal secretory cells. Histol Histopathol. 2015;30:1295–302. doi: 10.14670/HH-11-645. [DOI] [PubMed] [Google Scholar]
- 5.Li J, Abushahin N, Pang S, Xiang L, Chambers SK, Fadare O, Kong B, Zheng W. Tubal origin of ‘ovarian’ low-grade serous carcinoma. Mod Pathol. 2011;24:1488–1499. doi: 10.1038/modpathol.2011.106. [DOI] [PubMed] [Google Scholar]
- 6.Quick CM, Ning G, Bijron J, Laury A, Wei TS, Chen EY, Vargas SO, Betensky RA, McKeon FD, Xian W, Crum CP. PAX2-null secretory cell outgrowths in the oviduct and their relationship to pelvic serous cancer. Mod Pathol. 2012;25:449–455. doi: 10.1038/modpathol.2011.175. [DOI] [PubMed] [Google Scholar]
- 7.Carlson JW, Miron A, Jarboe EA, Parast MM, Hirsch MS, Lee Y, Muto MG, Kindelberger D, Crum CP. Serous tubal intraepithelial carcinoma: its potential role in primary peritoneal serous carcinoma and serous cancer prevention. J. Clin. Oncol. 2008;26:4160–4165. doi: 10.1200/JCO.2008.16.4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crum CP, Drapkin R, Miron A, Ince TA, Muto M, Kindelberger DW, Lee Y. The distal fallopian tube: a new model for pelvic serous carcinogenesis. Curr Opin Obstet Gynecol. 2007;19:3–9. doi: 10.1097/GCO.0b013e328011a21f. [DOI] [PubMed] [Google Scholar]
- 9.Lee Y, Miron A, Drapkin R, Nucci MR, Medeiros F, Saleemuddin A, Garber J, Birch C, Mou H, Gordon RW, Cramer DW, McKeon FD, Crum CP. A candidate precursor to serous carcinoma that originates in the distal fallopian tube. J Pathol. 2007;211:26–35. doi: 10.1002/path.2091. [DOI] [PubMed] [Google Scholar]
- 10.Callahan MJ, Crum CP, Medeiros F, Kindelberger DW, Elvin JA, Garber JE, Feltmate CM, Berkowitz RS, Muto MG. Primary fallopian tube malignancies in BRCA-positive women undergoing surgery for ovarian cancer risk reduction. J. Clin. Oncol. 2007;25:3985–3990. doi: 10.1200/JCO.2007.12.2622. [DOI] [PubMed] [Google Scholar]
- 11.Crum CP. Intercepting pelvic cancer in the distal fallopian tube: theories and realities. Mol Oncol. 2009;3:165–170. doi: 10.1016/j.molonc.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soong TR, Howitt BE, Horowitz N, Nucci MR, Crum CP. The fallopian tube, “precursor escape” and narrowing the knowledge gap to the origins of high-grade serous carcinoma. Gynecol Oncol. 2019;152:426–433. doi: 10.1016/j.ygyno.2018.11.033. [DOI] [PubMed] [Google Scholar]
- 13.Chen EY, Mehra K, Mehrad M, Ning G, Miron A, Mutter GL, Monte N, Quade BJ, McKeon FD, Yassin Y, Xian W, Crum CP. Secretory cell outgrowth, PAX2 and serous carcinogenesis in the Fallopian tube. J Pathol. 2010;222:110–116. doi: 10.1002/path.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levanon K, Ng V, Piao HY, Zhang Y, Chang MC, Roh MH, Kindelberger DW, Hirsch MS, Crum CP, Marto JA, Drapkin R. Primary ex vivo cultures of human fallopian tube epithelium as a model for serous ovarian carcinogenesis. Oncogene. 2010;29:1103–1113. doi: 10.1038/onc.2009.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piek JM, Torrenga B, Hermsen B, Verheijen RH, Zweemer RP, Gille JJ, Kenemans P, van Diest PJ, Menko FH. Histopathological characteristics of BRCA1- and BRCA2-associated intraperitoneal cancer: a clinic-based study. Fam Cancer. 2003;2:73–78. doi: 10.1023/a:1025700807451. [DOI] [PubMed] [Google Scholar]
- 16.Carcangiu ML, Radice P, Manoukian S, Spatti G, Gobbo M, Pensotti V, Crucianelli R, Pasini B. Atypical epithelial proliferation in fallopian tubes in prophylactic salpingo-oophorectomy specimens from BRCA1 and BRCA2 germline mutation carriers. Int J Gynecol Pathol. 2004;23:35–40. doi: 10.1097/01.pgp.0000101082.35393.84. [DOI] [PubMed] [Google Scholar]
- 17.Cass I, Holschneider C, Datta N, Barbuto D, Walts AE, Karlan BY. BRCA-mutation-associated fallopian tube carcinoma: a distinct clinical phenotype? Obstet Gynecol. 2005;106:1327–1334. doi: 10.1097/01.AOG.0000187892.78392.3f. [DOI] [PubMed] [Google Scholar]
- 18.Levanon K, Crum C, Drapkin R. New insights into the pathogenesis of serous ovarian cancer and its clinical impact. J. Clin. Oncol. 2008;26:5284–5293. doi: 10.1200/JCO.2008.18.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crum CP, McKeon FD, Xian W. BRCA, the oviduct, and the space and time continuum of pelvic serous carcinogenesis. Int J Gynecol Cancer. 2012;22(Suppl 1):S29–34. doi: 10.1097/IGC.0b013e31824d7269. [DOI] [PubMed] [Google Scholar]
- 20.Folkins AK, Saleemuddin A, Garrett LA, Garber JE, Muto MG, Tworoger SS, Crum CP. Epidemiologic correlates of ovarian cortical inclusion cysts (CICs) support a dual precursor pathway to pelvic epithelial cancer. Gynecol Oncol. 2009;115:108–111. doi: 10.1016/j.ygyno.2009.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li J, Ning Y, Abushahin N, Yuan Z, Wang Y, Wang Y, Yuan B, Cragun JM, Chambers SK, Hatch K, Kong B, Zheng W. Secretory cell expansion with aging: risk for pelvic serous carcinogenesis. Gynecol Oncol. 2013;131:555–560. doi: 10.1016/j.ygyno.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 22.Perets R, Wyant GA, Muto KW, Bijron JG, Poole BB, Chin KT, Chen JY, Ohman AW, Stepule CD, Kwak S, Karst AM, Hirsch MS, Setlur SR, Crum CP, Dinulescu DM, Drapkin R. Transformation of the fallopian tube secretory epithelium leads to high-grade serous ovarian cancer in Brca;Tp53;Pten models. Cancer Cell. 2013;24:751–765. doi: 10.1016/j.ccr.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Li L, Wang Y, Tang SN, Zheng W. Fallopian tube secretory cell expansion: a sensitive biomarker for ovarian serous carcinogenesis. Am J Transl Res. 2015;7:2082–2090. [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y, Wang Y, Li D, Li L, Zhang W, Yao G, Jiang Z, Zheng W. IMP3 signatures of fallopian tube: a risk for pelvic serous cancers. J Hematol Oncol. 2014;7:49. doi: 10.1186/s13045-014-0049-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang W, Wei L, Li L, Yang B, Kong B, Yao G, Zheng W. Ovarian serous carcinogenesis from tubal secretory cells. Histol Histopathol. 2015;30:1295–1302. doi: 10.14670/HH-11-645. [DOI] [PubMed] [Google Scholar]
- 26.Dobroff AS, D’Angelo S, Eckhardt BL, Ferrara F, Staquicini DI, Cardo-Vila M, Staquicini FI, Nunes DN, Kim K, Driessen WHP, Hajitou A, Lomo LC, Barry M, Krishnamurthy S, Sahin A, Woodward WA, Prossnitz ER, Anderson RL, Dias-Neto E, Brown-Glaberman UA, Royce ME, Ueno NT, Cristofanilli M, Hortobagyi GN, Marchio S, Gelovani JG, Sidman RL, Arap W, Pasqualini R. Towards a transcriptome-based theranostic platform for unfavorable breast cancer phenotypes. Proc Natl Acad Sci U S A. 2016;113:12780–12785. doi: 10.1073/pnas.1615288113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sowamber R, Nelson O, Dodds L, DeCastro V, Paudel I, Milea A, Considine M, Cope L, Pinto A, Schlumbrecht M, Slomovitz B, Shaw PA, George SHL. Integrative transcriptome analyses of the human fallopian tube: fimbria and ampulla-site of origin of serous carcinoma of the ovary. Cancers (Basel) 2020;12:1090. doi: 10.3390/cancers12051090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soong TR, Howitt BE, Miron A, Horowitz NS, Campbell F, Feltmate CM, Muto MG, Berkowitz RS, Nucci MR, Xian W, Crum CP. Evidence for lineage continuity between early serous proliferations (ESPs) in the Fallopian tube and disseminated high-grade serous carcinomas. J Pathol. 2018;246:344–351. doi: 10.1002/path.5145. [DOI] [PubMed] [Google Scholar]
- 29.Medeiros F, Muto MG, Lee Y, Elvin JA, Callahan MJ, Feltmate C, Garber JE, Cramer DW, Crum CP. The tubal fimbria is a preferred site for early adenocarcinoma in women with familial ovarian cancer syndrome. Am J Surg Pathol. 2006;30:230–236. doi: 10.1097/01.pas.0000180854.28831.77. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Li L, Wang Y, Yuan Z, Zhang W, Hatch KD, Zheng W. IMP3 as a cytoplasmic biomarker for early serous tubal carcinogenesis. J Exp Clin Cancer Res. 2014;33:60. doi: 10.1186/s13046-014-0060-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiang L, Li J, Wang LJ, Zheng WX, Kong BH. Origin of ovarian epithelial inclusions and its relationship with the development of low-grade serous carcinoma. Zhonghua Fu Chan Ke Za Zhi. 2011;46:729–735. [PubMed] [Google Scholar]
- 32.Wen J, Shi JL, Shen DH, Chen YX, Song QJ. Morphologic changes of fallopian tubal epithelium in ovarian serous tumors. Zhonghua Bing Li Xue Za Zhi. 2012;41:433–437. doi: 10.3760/cma.j.issn.0529-5807.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 33.Lyons R, Saridogan E, Djahanbakhch O. The reproductive significance of human Fallopian tube cilia. Hum Reprod Update. 2006;12:363–372. doi: 10.1093/humupd/dml012. [DOI] [PubMed] [Google Scholar]
- 34.King SM, Hilliard TS, Wu LY, Jaffe RC, Fazleabas AT, Burdette JE. The impact of ovulation on fallopian tube epithelial cells: evaluating three hypotheses connecting ovulation and serous ovarian cancer. Endocr Relat Cancer. 2011;18:627–642. doi: 10.1530/ERC-11-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lyons R, Djahanbakhch O, Mahmood T, Saridogan E, Sattar S, Sheaff M, Naftalin A, Chenoy R. Fallopian tube ciliary beat frequency in relation to the stage of menstrual cycle and anatomical site. Hum Reprod. 2002;17:584–588. doi: 10.1093/humrep/17.3.584. [DOI] [PubMed] [Google Scholar]
- 36.Alwosaibai K, Abedini A, Al-Hujaily EM, Tang Y, Garson K, Collins O, Vanderhyden BC. PAX2 maintains the differentiation of mouse oviductal epithelium and inhibits the transition to a stem cell-like state. Oncotarget. 2017;8:76881. doi: 10.18632/oncotarget.20173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Comer M, Leese H, Southgate J. Induction of a differentiated ciliated cell phenotype in primary cultures of Fallopian tube epithelium. Hum Reprod (Oxford, England) 1998;13:3114–3120. doi: 10.1093/humrep/13.11.3114. [DOI] [PubMed] [Google Scholar]
- 38.Paik DY, Janzen DM, Schafenacker AM, Velasco VS, Shung MS, Cheng D, Huang J, Witte ON, Memarzadeh S. Stem-like epithelial cells are concentrated in the distal end of the fallopian tube: a site for injury and serous cancer initiation. Stem Cells. 2012;30:2487–2497. doi: 10.1002/stem.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levanon K, Ng V, Piao HY, Zhang Y, Chang MC, Roh MH, Kindelberger DW, Hirsch MS, Crum CP, Marto JA. Primary ex vivo cultures of human fallopian tube epithelium as a model for serous ovarian carcinogenesis. Oncogene. 2010;29:1103–1113. doi: 10.1038/onc.2009.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coan M, Rampioni Vinciguerra GL, Cesaratto L, Gardenal E, Bianchet R, Dassi E, Vecchione A, Baldassarre G, Spizzo R, Nicoloso MS. Exploring the role of Fallopian ciliated cells in the pathogenesis of high-grade serous ovarian cancer. Int J Mol Sci. 2018;19:2512. doi: 10.3390/ijms19092512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Comer MT, Leese HJ, Southgate J. Induction of a differentiated ciliated cell phenotype in primary cultures of Fallopian tube epithelium. Hum Reprod. 1998;13:3114–3120. doi: 10.1093/humrep/13.11.3114. [DOI] [PubMed] [Google Scholar]
- 42.Goodman MT, Shvetsov YB. Incidence of ovarian, peritoneal, and fallopian tube carcinomas in the United States, 1995-2004. Cancer Epidemiol Biomarkers Prev. 2009;18:132–139. doi: 10.1158/1055-9965.EPI-08-0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crum CP, McKeon FD, Xian W. The oviduct and ovarian cancer: causality, clinical implications, and “targeted prevention”. Clin Obstet Gynecol. 2012;55:24–35. doi: 10.1097/GRF.0b013e31824b1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.George SH, Shaw P. BRCA and early events in the development of serous ovarian cancer. Front Oncol. 2014;4:5. doi: 10.3389/fonc.2014.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kobayashi H, Iwai K, Niiro E, Morioka S, Yamada Y, Ogawa K, Kawahara N. The conceptual advances of carcinogenic sequence model in high-grade serous ovarian cancer. Biomed Rep. 2017;7:209–213. doi: 10.3892/br.2017.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kyo S, Ishikawa N, Nakamura K, Nakayama K. The fallopian tube as origin of ovarian cancer: change of diagnostic and preventive strategies. Cancer Med. 2020;9:421–431. doi: 10.1002/cam4.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singh N, Gilks CB, Wilkinson N, McCluggage WG. The secondary Mullerian system, field effect, BRCA, and tubal fimbria: our evolving understanding of the origin of tubo-ovarian high-grade serous carcinoma and why assignment of primary site matters. Pathology. 2015;47:423–431. doi: 10.1097/PAT.0000000000000291. [DOI] [PubMed] [Google Scholar]
- 48.Conner JR, Meserve E, Pizer E, Garber J, Roh M, Urban N, Drescher C, Quade BJ, Muto M, Howitt BE, Pearlman MD, Berkowitz RS, Horowitz N, Crum CP, Feltmate C. Outcome of unexpected adnexal neoplasia discovered during risk reduction salpingo-oophorectomy in women with germ-line BRCA1 or BRCA2 mutations. Gynecol Oncol. 2014;132:280–286. doi: 10.1016/j.ygyno.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mehra K, Mehrad M, Ning G, Drapkin R, McKeon FD, Xian W, Crum CP. STICS, SCOUTs and p53 signatures; a new language for pelvic serous carcinogenesis. Front Biosci (Elite Ed) 2011;3:625–634. doi: 10.2741/e275. [DOI] [PubMed] [Google Scholar]
- 50.Semmel DR, Folkins AK, Hirsch MS, Nucci MR, Crum CP. Intercepting early pelvic serous carcinoma by routine pathological examination of the fimbria. Mod Pathol. 2009;22:985–988. doi: 10.1038/modpathol.2009.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Egeberg DL, Lethan M, Manguso R, Schneider L, Awan A, Jorgensen TS, Byskov AG, Pedersen LB, Christensen ST. Primary cilia and aberrant cell signaling in epithelial ovarian cancer. Cilia. 2012;1:15. doi: 10.1186/2046-2530-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abdelhamed ZA, Ryan TA, Fuller M, Coulson-Gilmer C, Abdelmottaleb DI, Wang TL, Kaun JC, Wang P, Hutson R, Wilkinson N, Bell SM, Johnson CA. Characterization of primary cilia in normal fallopian tube epithelium and serous tubal intraepithelial carcinoma. Int J Gynecol Cancer. 2018;28:1535–1544. doi: 10.1097/IGC.0000000000001321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mans DA, Voest EE, Giles RH. All along the watchtower: is the cilium a tumor suppressor organelle? Biochim Biophys Acta. 2008;1786:114–125. doi: 10.1016/j.bbcan.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 54.Plotnikova OV, Golemis EA, Pugacheva EN. Cell cycle-dependent ciliogenesis and cancer. Cancer Res. 2008;68:2058–2061. doi: 10.1158/0008-5472.CAN-07-5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Coan M, Rampioni Vinciguerra GL, Cesaratto L, Gardenal E, Bianchet R, Dassi E, Vecchione A, Baldassarre G, Spizzo R, Nicoloso MS. Exploring the role of fallopian ciliated cells in the pathogenesis of high-grade serous ovarian cancer. Int J Mol Sci. 2018;19:2512. doi: 10.3390/ijms19092512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Y, Wang Y, Li J, Yuan Z, Yuan B, Zhang T, Cragun JM, Kong B, Zheng W. PAX8: a sensitive and specific marker to identify cancer cells of ovarian origin for patients prior to neoadjuvant chemotherapy. J Hematol Oncol. 2013;6:60. doi: 10.1186/1756-8722-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ascher E, Madelenat P, Rose D. Tubal physiology: structures and functions. J Gynecol Obstet Biol Reprod (Paris) 1986;15:717–729. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


