Abstract
Patients with Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) have poor prognosis, and the efficacy of chemotherapy plus tyrosine kinase inhibitors (TKIs) followed by mismatched donor stem cell infusion (microtransplantation, MST) has not been determined. We retrospectively summarized 45 patients including 11 undergoing MST with TKIs, 17 receiving allogeneic transplant and 17 undergoing chemotherapy with TKIs. Improved 4-year overall survival rate was observed in the MST group (91%) compared with either transplant group (31%, P = .005) or chemotherapy group (36%, P = .013). The MST group also had higher 2-year and 4-year leukemia-free survival rates (91% and 72%, respectively) compared with either transplant group (33%, P = .005 and 33%, P = .021, respectively) or chemotherapy group (41%, P = .017 and 31%, P = .023, respectively). 2-year and 4-year cumulative incidences of hematologic relapse were lower in the MST group (9% and 28%, respectively) compared with those in the chemotherapy group (56%, P = .025 and 67%, P = .034, respectively). In patients undergoing MST, donor microchimerism was detected (1.07 × 10-5 to 6.6 × 10-4 copies from 9 to 1499 days) in 7 patients, and donor/patient-derived HLA*0201/2402+WT1+CD8+ T cells were found from 0.05% to 0.67% in 6 patients. MST may provide a favorable treatment for patients with Ph+ ALL.
Keywords: Philadelphia chromosome-positive acute lymphoblastic leukemia, microtransplantation, chemotherapy, allogeneic stem cell transplantation, microchimerism
Introduction
Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) accounts for approximately 30% of cases in adult ALL and 5% in childhood ALL, which presents with an aggressive clinical course and poor prognosis [1]. Most patients with Ph-positive ALL relapse with a 5-year overall survival (OS) less than 10% in the pre-tyrosine kinase inhibitor (TKI) era [2]. Despite the combination of TKIs with multiagent chemotherapy has substantially improved the overall outcome, 5-year OS is approximately 40% at best [3]. Although allogeneic stem cell transplantation (allo-SCT) provides potentially curative opportunity for patients in complete remission (CR), its superiority over TKIs plus chemotherapy is still controversial, and concerns for transplant-related toxicities and low quality of life continuously exist [4-7].
We have previously devised a strategy termed microtransplantation (MST), which uses chemotherapy combined with granulocyte colony-stimulating factor (G-CSF) mobilized peripheral blood mononuclear cell (GPBMC) infusion from a human leukocyte antigen (HLA)-mismatched donor, and have successfully applied into patients with acute myeloid leukemia (AML), myelodysplastic syndrome and lymphoma [8-10]. MST contains standard dose of chemotherapy or targeted therapy instead of immunosuppressive conditioning, which preserves recipient’s immune function. GPBMCs from the mismatched donor are infused following chemotherapy [11]. The safety and improved overall outcome from MST in these diseases were validated by multiple studies from our center and others [12-16]. However, MST for patients with Ph+ ALL has not been reported, and the outcome comparing MST with TKIs plus chemotherapy or allogeneic transplant is unknown. In this study, we retrospectively summarized 45 patients with Ph+ ALL who achieved CR in our center, aiming to compare the efficacy of MST, allo-SCT and chemotherapy as post-remission treatment.
Materials and methods
Patients and donors
From May 1, 2004 to September 30, 2019, patients aged 11-68 years with Ph+ ALL in CR status were included. Patients undergoing allo-SCT were enrolled from 2004 following the approval of imatinib in China. Enrollment of patients receiving TKIs plus hyper-CVAD-based chemotherapy was started from 2011, while the hyper-CVAD-based MST protocol was initiated from 2013. Ph positivity was determined at diagnosis by standard karyotype and/or fluorescence in situ hybridization analysis and/or BCR-ABL fusion transcript detection with quantitative reverse-transcription polymerase chain reaction (qRT-PCR). All patients were evaluated for eligibility of allo-SCT when getting CR. Patients who had a matched/haploidentical donor and agreed to allo-SCT proceeded to modified reduced-intensity conditioning (RIC) allo-SCT. Myeloablative conditioning (MAC) was not considered due to the noninferior outcome of our RIC regimen compared with MAC [17]. Others that refused allo-SCT or had no suitable transplant donor received MST with TKIs or chemotherapy with TKIs according to personal choice. Finally, 17 patients were treated with chemotherapy plus TKIs (the chemotherapy group), and 11 patients who had a mismatched related donor received MST plus TKIs (the MST group) as post-remission consolidation. Seventeen patients received RIC allo-SCT in remission (the SCT group), including 9 with a haploidentical donor, 4 with a matched unrelated donor and 4 with a matched sibling donor. Among all patients, only one was younger than 15 and underwent allo-SCT. The study protocol was approved by the Human Ethics Committee of The Fifth Medical Center, Chinese PLA General Hospital and was in accordance with the Declaration of Helsinki. All patients and their donors provided written informed consent before the study.
Chemotherapy
For patients who received chemotherapy with TKIs, a regimen with modified hyper-CVAD was administered as consolidation/maintenance after remission. Briefly, patients received 8 alternating courses of fractionated cyclophosphamide 300 mg/m2 every 12 hours on days 1-3, vincristine 2 mg on day 1, doxorubicin 50 mg/m2 on day 4 and dexamethasone 40 mg/d on days 1-4 (1, 3, 5, 7 courses), and high-dose methotrexate 2 g/m2 and cytarabine 2 g/m2 every 12 hours on days 1-3 (2, 4, 6, 8 courses). Cytarabine reduced to 1 g/m2 for age ≥ 60 years. An additional VMCD regimen consisting of vincristine 2 mg on day 1, mitoxantrone 12 mg/m2 on days 1 to 2, cyclophosphamide 450 mg/m2 on days 2 and 5 and dexamethasone 7.5 mg/m2 on days 1 to 7 was administered between the 5th and 6th courses in 12 out of 17 patients. All patients received first or second generation TKIs according to personal choice. Imatinib, dasatinib or nilotinib was given at an initial dose of 400 mg, 100 mg and 600 mg daily respectively at diagnosis, stopped when neutrophils < 0.5 × 109/L and/or platelets < 50 × 109/L, and resumed until neutrophils ≥ 1 × 109/L and platelets ≥ 50 × 109/L from the induction to maintenance phase. TKIs continued until relapse/death in transplant-free patients. Supportive care including infection prophylaxis and G-CSF was administered based on the standard practice guidelines.
MST
The chemotherapy and TKI schedules were the same as that in chemotherapy group. Ten out of 11 patients received an additional VMCD regimen between the 5th and 6th courses. GPBMCs were infused 24 hours after each completion of cytarabine. None of the patients received any prophylaxis of graft-versus-host disease (GVHD).
Transplantation
Conditioning regimens were described previously [17]. Briefly, this included 30 mg/m2 fludarabine daily for 5 consecutive days together with 5-8 mg/kg rabbit anti-thymocyte globulin (SangStat, Lyon, France), 80 mg/kg cyclophosphamide and 6-9 g/m2 cytarabine. Patients undergoing haploidentical SCT were given additional 2 Gy total body irradiation with a dose rate of 5 cGy/min. TKI therapy continued until the initiation of conditioning, and discontinued after transplantation until hematologic or cytogenetic relapse. The protocol of GVHD prophylaxis was also described previously [17].
Central nervous system leukemia (CNSL)
For patients receiving chemotherapy or MST, CNSL prophylaxis was given by intrathecal injection of methotrexate 15 mg, cytarabine 50 mg, and dexamethasone 5 mg during each consolidation course. Intrathecal injection was given at least 6 times for those who were scheduled for allo-SCT. Patients with cytologic CNSL evidence received intrathecal injections twice a week until microscopic clearance of cerebrospinal fluid.
Mobilization and apheresis of donor PBMCs
For related donors, apheresis of PBMCs was administered with a CS-3000S cell separator (Baxter) following subcutaneous injections of 5 ug/kg G-CSF twice a day for 5 consecutive days. Donor cells were equally divided and cryopreserved in liquid nitrogen, but fresh cells were infused in the first course. GPBMCs from unrelated donors for transplant were aquired from the China Marrow Donor Program. In the MST group, median numbers of mononuclear, CD34 and CD3 cells infused per course were 1.99 × 108/kg (range, 1-4.03 × 108/kg), 2.31 × 106/kg (range, 0.57-3.77 × 106/kg), and 0.93 × 108/kg (range, 0.21-1.23 × 108/kg), respectively. In the SCT group, median numbers of mononuclear, CD34 and CD3 cells infused were 8.8 × 108/kg (range, 3-21.66 × 108/kg), 8.44 × 106/kg (range, 3.08-23.38 × 106/kg), and 2.55 × 108/kg (range, 0.86-8.68 × 108/kg), respectively.
Minimal residual disease (MRD) assessment
Quantitative RT-PCR assays were performed to monitor BCR-ABL transcript changes in the bone marrow. The BCR-ABL mRNA amount was expressed relative to the ABL mRNA amount.
Chimerism and microchimerism assessment
In the SCT group, donor chimerism was monitored in all patients using short tandem repeat-polymerase chain reaction assay with 10-2 sensitivity as previously described [18]. In the MST group, donor microchimerisms (donor cells ≤ 1%) were monitored in 8 patients using a more sensitive real-time PCR method with 10-5 sensitivity as described previously [19]. Briefly, pretransplant host and donor DNA samples were screened by 32 Indel-primers and positive controls (actin). Donor or pretransplant host informative-specific primers and probes were chosen for quantification assay. Quantitative PCR were performed using ABI7500 real-time PCR system. The ratio of donor microchimerism was then determined by the ΔΔct method according to the published protocol, using the actin primer/probe set to normalize for the actual DNA amount. ΔΔct was calculated as follows: ΔΔct = ((ctmarker_post - ctactin_post) - (ctmarker_pre - ctactin_pre)).
Analysis of WT1+CD8+ T cells
Seven of 11 patients in the MST group and 4 of 17 patients in the SCT group who and/or whose donor had HLA-A*02:01 and/or HLA-A*24:02 were monitored for circulating WT1+HLA-A*02:01+CD8+ and/or WT1+HLA-A*24:02+CD8+ T cells as previously described [8]. A proportion of pentamer-positive T cells among total CD8+ T cells ≥ 0.05% was considered significant.
Response criteria and outcome evaluation
CR was defined as < 5% blasts in the bone marrow with the absence of blasts in the peripheral blood and extramedullary disease. A neutrophil count > 1.0 × 109/L and platelet count > 100 × 109/L were also required. Complete molecular response (CMR) was defined as the absence of a detectable BCR-ABL transcript with 0.01% sensitivity. Patients who died within the first month after start of the study were considered early death. Hematologic relapse was defined as the recurrence of lymphoblasts in the bone marrow (> 5%) or peripheral blood or any extramedullary site. The recovery time of neutrophil was defined as the first of 3 consecutive days when the neutrophil count was 0.5 × 109/L. Platelet recovery time was defined as the first of 3 consecutive days when the platelet count was 30 × 109/L.
OS was calculated from enrollment to death or the last follow-up until May 1st, 2020. Leukemia-free survival (LFS) was defined as the period from enrollment until relapse or death in remission. Nonrelapse mortality (NRM) was calculated as the time of death while in CR. Acute and chronic GVHD were defined based on published criteria [20,21]. Adverse events were graded according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. Grade III-IV acute GVHD and Grade 3-4 non-hematologic toxicities were considered as severe adverse events.
Statistical analysis
Categorical and continuous variables related to clinical characteristics, MRD results or post-SCT disease status among groups were compared by the Chi-square test and analysis of variance, respectively. Survival analyses were performed by the Kaplan-Meier method. The log-rank test was used to compare survival rates. Univariate analyses were performed using the log-rank test to identify prognostic variables for OS, LFS and relapse. Variables with a P value < 0.1, as determined by univariate analyses, were considered for entry into multivariate analyses according to Cox proportional hazards regression models. GraphPad Prism v8.0 (GraphPad Software Inc., San Diego, CA) software was used for preparing plots of microchimerism and WT1+CD8+ T cells. Two-sided P < .05 was considered statistically significant. Statistical analyses were performed by Statistical Packages for the Social Sciences v25.0 (SPSS Inc., Chicago, IL) software.
Results
Patient characteristics
A total of 45 Ph+ ALL patients in CR were enrolled in the study. Seventeen (37.78%) patients were enrolled in the SCT group, 11 (24.44%) patients were enrolled in the MST group and 17 (37.78%) patients were enrolled in the chemotherapy group. No patient proceeded to allo-SCT after getting remission in MST or chemotherapy group, and only one patient proceeded to salvage SCT after relapse in the chemotherapy group. Characteristics of patients are shown in Table 1. Baseline characteristics were much the same among groups, except for the median white blood cell (WBC) count between the SCT and MST group and number of patients with WBC ≥ 30 × 109/L between the MST and chemotherapy group. All patients received first or second generation TKIs at initial treatment with no significant difference among groups. The donor had 0-2 (related, n = 3) or 5 (related, n = 8) matched HLA-loci with the patient in the MST group, while the donor had 5-8 (related, n = 9) or 10 (related, n = 4 and unrelated, n = 4) matched HLA-loci with the patient in the SCT group.
Table 1.
Characteristics of patients
| Parameter | Total (n = 45) | SCT (n = 17) | MST (n = 11) | Chemo (n = 17) | P |
|---|---|---|---|---|---|
| Median age (range), years | 42 (11-68) | 41 (11-55) | 47 (20-68) | 43 (21-65) | .158 |
| Age > 50 y, n (%) | 12 (27) | 2 (12) | 4 (36) | 6 (35) | .226 |
| Males, n (%) | 29 (64) | 8 (47) | 8 (73) | 13 (76) | .162 |
| Median WBC (range), × 109/L | 33.3 (1.36-318.3) | 74.6 (4.42-183.1) | 12.3 (1.36-125) | 51 (1.7-318.3) | .010a |
| WBC ≥ 30 × 109/L, n (%) | 24 (53) | 10 (59) | 2 (18) | 12 (71) | .021b |
| Extramedullary leukemia, n (%) | 5 (11) | 3 (18) | 2 (18) | 0 (0) | .161 |
| Chromosome type, n (%) | .522 | ||||
| Ph alone | 36 (80) | 12 (71) | 10 (91) | 14 (82) | |
| Ph with other | 9 (20) | 5 (29) | 1 (9) | 3 (18) | |
| BCR-ABL transcript, n (%) | .535 | ||||
| P190 | 30 (67) | 13 (76) | 7 (64) | 10 (59) | |
| P210/P230c | 15 (33) | 4 (24) | 4 (36) | 7 (41) | |
| CMR at enrollment, n (%) | 28 (62) | 8 (47) | 7 (64) | 13 (76) | .208 |
| TKI type at enrollment, n (%) | .214 | ||||
| 1st generation | 30 (67) | 12 (71) | 5 (45) | 13 (76) | |
| 2nd generationd | 15 (33) | 5 (29) | 6 (55) | 4 (24) | |
| Median time from diagnosis to enrollment (range), months | 4.1 (0.9-80.2) | 5.6 (2.3-23.7) | 3.4 (2.1-11.6) | 3.5 (0.9-80.2) | .724 |
| Donors, n | .000 | ||||
| 10 loci matched related | 4 | 4 | 0 | 0 | |
| 5-9 loci matched related | 17 | 9 | 8 | 0 | |
| 0-4 loci matched related | 3 | 0 | 3 | 0 | |
| Matched unrelated | 4 | 4 | 0 | 0 | |
| No donor | 17 | 0 | 0 | 17 |
SCT vs. MST (P = .019); SCT vs. Chemo (P = .995); MST vs. Chemo (P = .079).
SCT vs. MST (P = .054); SCT vs. Chemo (P = .473); MST vs. Chemo (P = .007).
One patient in the MST group conserved the P230 transcript.
Fourteen patients were given dasatinib, and one patient was given nilotinib in the MST group.
Abbreviations: SCT, stem cell transplantation; MST, microtransplantation; Chemo, Chemotherapy; WBC, white blood cell; CMR, complete molecular response; TKI, tyrosine kinase inhibitor.
Count recovery and adverse events
Median times to neutrophil and platelet count recovery were 12 days vs. 12 days and 13.5 days vs. 13 days, respectively, between the MST and chemotherapy groups with no significant differences (Table 2). Patients in the SCT group experienced faster hematopoietic recovery than the other two groups within expectation. Overall rates of severe adverse events associated with treatment were higher in the SCT group than those in the MST or chemotherapy group. The most frequent events were organ failure (n = 6, 35%) followed by III-IV GVHD (n = 2, 12%) and severe infections (n = 2, 12%) in the SCT group. Four patients (24%) presented with severe infections in the chemotherapy group. No any severe adverse event including acute GVHD occurred in the MST group.
Table 2.
Outcomes of patients
| Parameter | Total (n = 45) | SCT (n = 17) | P (SCT vs. Chemo) | MST (n = 11) | P (SCT vs. MST) | Chemo (n = 17) | P (MST vs. Chemo) |
|---|---|---|---|---|---|---|---|
| Median time of ANC recovery (range), days | 12 (8-18) | 9 (8-14) | < .001 | 12 (10-17) | < .001 | 12 (10-18) | .462 |
| Median time of platelet recovery (range), days | 13 (6-18) | 10 (6-15) | < .001 | 13.5 (7-18) | < .001 | 13 (10-18) | .969 |
| Severe adverse event, n (%) | < .001 | < .001 | .132 | ||||
| Severe infections | 6 (13) | 2 (12) | 0 (0) | 4 (24) | |||
| III-IV aGVHD | 2 (4) | 2 (12) | 0 (0) | 0 (0) | |||
| Organ failure | 6 (13) | 6 (35) | 0 (0) | 0 (0) | |||
| Early death, n (%) | 1 (2) | 1 (6) | .476 | 0 (0) | .804 | 0 (0) | .804 |
| Total death, n (%) | 24 (53) | 11 (65) | .486 | 4 (36) | .142 | 9 (53) | .390 |
| Causes of death, n (%) | .067 | .062 | < .999 | ||||
| Relapse | 13 (27) | 2 (12) | 4 (36) | 7 (41) | |||
| Infections | 4 (9) | 3 (18) | 0 (0) | 1 (6) | |||
| GVHD | 2 (4) | 2 (12) | 0 (0) | 0 (0) | |||
| Organ failure | 5 (11) | 4 (24) | 0 (0) | 1 (6) | |||
| OS, No. of Events (%) | 24 (47) | 11 (31) | .253 | 4 (47) | .046 | 9 (24) | .022 |
| 2-year OS | 14 (69) | 10 (39) | .018 | 1 (91) | .012 | 3 (80) | .492 |
| 4-year OS | 20 (56) | 11 (31) | .159 | 1 (91) | .005 | 8 (36) | .013 |
| LFS, No. of Events (%) | 27 (40) | 11 (33) | .580 | 6 (41) | .111 | 10 (31) | .060 |
| 2-year LFS | 21 (53) | 11 (33) | .428 | 1 (91) | .005 | 9 (41) | .017 |
| 4-year LFS | 24 (47) | 11 (33) | .580 | 3 (72) | .021 | 10 (31) | .023 |
| Hematologic relapse, No. of Events (%) | 18 (40) | 3 (20) | .148 | 6 (59) | .585 | 9 (67) | .083 |
| 2-year relapse | 12 (27) | 3 (20) | .226 | 1 (9) | .429 | 8 (56) | .025 |
| 4-year relapse | 15 (33) | 3 (20) | .148 | 3 (28) | .847 | 9 (67) | .034 |
| NRM, No. of Events (%) | 9 (20) | 8 (47) | .008 | 0 (0) | .008 | 1 (6) | .421 |
| 1-year NRM | 8 (18) | 7 (41) | .019 | 0 (0) | .018 | 1 (6) | .421 |
| 2-year NRM | 9 (20) | 8 (47) | .008 | 0 (0) | .008 | 1 (6) | .421 |
Abbreviations: SCT, stem cell transplantation; MST, microtransplantation; Chemo, Chemotherapy; ANC, absolute neutrophil count; aGVHD, acute graft-versus-host disease; OS, overall survival; LFS, leukemia-free survival; NRM, non-relapse mortality.
OS and LFS
The median follow-up was 27.7 months (range, 0.8-191.2 months). At the last follow-up time, 21 patients survived and 24 patients died (deaths due to disease progression [n = 13], organ failure after relapse [n = 1], infection after relapse [n = 1] and deaths while patients were in CR [n = 9]). Early death occurred in one patient in the SCT group (Table 2). Median OS times were 12.1, 66.9 and 27.7 months in the SCT, MST and chemotherapy groups, respectively. The 2-year OS rate was lower in the SCT group (39%) than that in the MST or chemotherapy group (91%, P = .012, 80%, P = .018, respectively). The MST group showed improved 4-year OS rate (91%) compared with either SCT group (31%, P = .005) or chemotherapy group (36%, P = .013), while the chemotherapy group showed comparable 4-year OS rate with the SCT group (P = .159) (Figure 1A).
Figure 1.
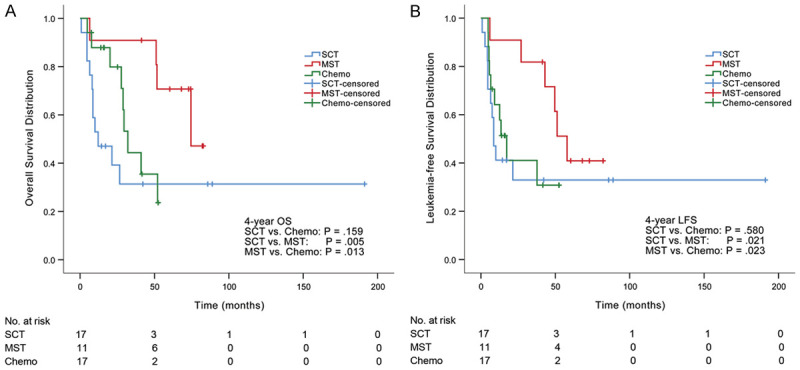
OS and LFS in three groups. A. The MST group showed improved 4-year OS rate (91%) compared with either SCT group (31%) or chemotherapy group (36%). B. The MST group had significantly higher 4-year LFS (72%) compared with either SCT group (33%) or chemotherapy group (31%).
Median LFS times were 8.6, 51.2 and 13.5 months in SCT, MST and chemotherapy groups, respectively. The MST group had significantly higher 2-year and 4-year LFS rates (91% and 72%, respectively) compared with either SCT group (33%, P = .005 and 33%, P = .021, respectively) or chemotherapy group (41%, P = .017 and 31%, P = .023, respectively) (Figure 1B). There was no significant difference in 2-year or 4-year LFS rate between SCT and chemotherapy groups (P = .428 and P = .580, respectively).
Relapse and NRM
The median hematologic relapse time was 10.9 months (range, 2.7-57.9 months). 2-year and 4-year cumulative incidences of hematologic relapse were lower in the MST group (9% and 28%, respectively) compared with those in the chemotherapy group (56%, P = .025 and 67%, P = .034, respectively) (Figure 2A). Only one patient (9%) underwent relapse within 2 years in the MST group compared with 8 patients (56%) in the chemotherapy group. In the MST group, all 5 patients (45%) who had no hematologic relapse maintained cytogenetic remission until the last follow-up, and 2 out of 6 patients who underwent hematologic relapse transformed from imatinib to dasatinib. In the chemotherapy group, 1 out of 8 patients who had no hematologic relapse underwent cytogenetic relapse, and 5 out of 9 patients who experienced hematologic relapse transformed from imatinib to dasatinib. No significant difference was observed in 2-year or 4-year cumulative incidence of hematologic relapse between MST and SCT groups (P = .429 and P = .847, respectively). In the SCT group, 3 patients (20%) experienced hematologic relapse including 2 proceeding to the original TKI plus reinduction chemotherapy and 1 transforming from imatinib to dasatinib; among 14 patients (82%) in hematologic remission, 3 (21%) underwent cytogenetic relapse and returned to CMR after continuing the original TKI.
Figure 2.
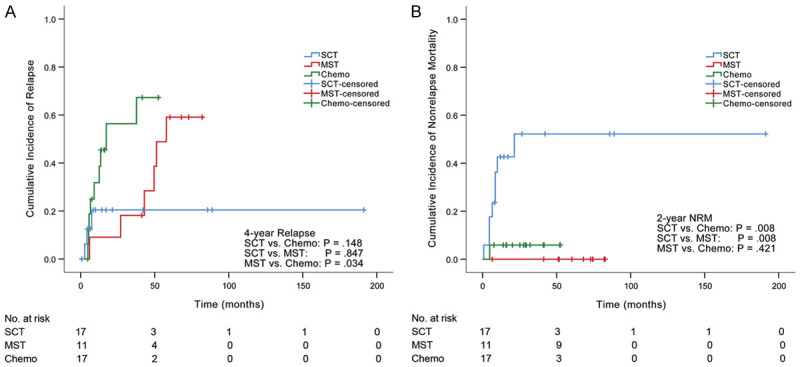
Relapse and NRM in three groups. A. The 4-year cumulative incidence of hematologic relapse was lower in the MST group (28%) compared with that in the chemotherapy group (67%). B. The 2-year cumulative NRM was significantly higher in the SCT group (47%) compared with that in either MST group (0%) or chemotherapy group (6%).
The overall NRM was 20%. The MST group had comparable 1-year or 2-year cumulative NRM (0% and 0%, respectively) compared with the chemotherapy group (6%, P = .421 and 6%, P = .421, respectively). In contrast, the 1-year and 2-year cumulative NRM were significantly higher in the SCT group (41% and 47%, respectively) compared with either MST group (P = .018 and P = .008, respectively) or chemotherapy group (P = .019 and P = .008, respectively) (Figure 2B).
GVHD
In the SCT group, I-II acute GVHD occurred in seven patients (41%), while two patients (12%) presented with III-IV acute GVHD (Figure 3). Two patients (12%) experienced limited chronic GVHD. No any acute or chronic GVHD occurred in the MST group.
Figure 3.
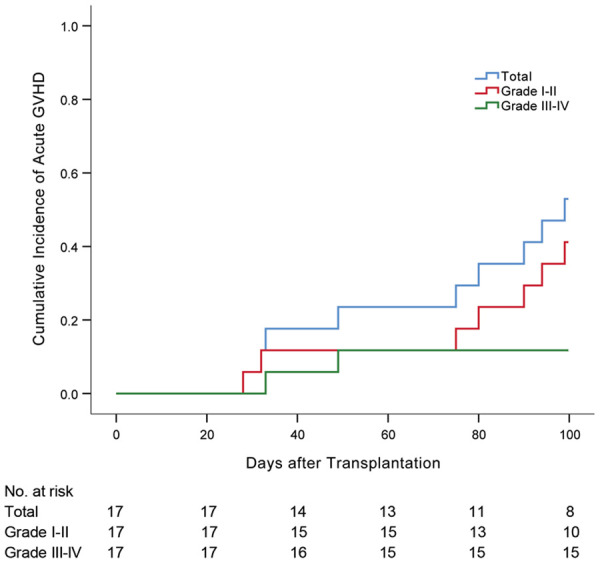
Cumulative incidence of acute graft-versus-host disease (GVHD) in the SCT group. Nine patients (53%) experienced acute GVHD including seven (41%) with Grade I-II and two (12%) with Grade III-IV.
MRD changes
The BCR-ABL transcripts were consecutively monitored post enrollment in 43 patients. Fifteen patients had positive MRD at enrollment including 7 from the SCT group, 4 from the MST group and 4 from the chemotherapy group. After 3 months of treatment, all 7 patients (100%) achieved CMR in the SCT group while 2 patients (50%) achieved CMR in both MST and chemotherapy groups. The proportions of patients getting CMR within 6 months were 100%, 75% and 50% in SCT, MST and chemotherapy groups, respectively (Table 3). On the other side, 28 patients were in CMR at enrollment including 8 from the SCT group, 7 from the MST group and 13 from the chemotherapy group. Three of 8 patients (38%) and 2 of 13 patients (15%) experienced molecular/hematologic relapse in the SCT and chemotherapy group, respectively, while no patient underwent any relapse in the MST group within 3 months.
Table 3.
Changes of minimal residual disease after enrollment (n = 43)a
| Parameter | Total (n = 43) | SCT (n = 15) | MST (n = 11) | Chemo (n = 17) | P |
|---|---|---|---|---|---|
| From CR to CMR, n (%) | 15 (35) | 7 (47) | 4 (36) | 4 (24) | |
| Within 3 months | 11 (73) | 7 (100) | 2 (50) | 2 (50) | .118 |
| Within 6 months | 12 (80) | 7 (100) | 3 (75) | 2 (50) | .123 |
| From CMR to Relapseb, n (%) | 28 (65)c | 8 (53) | 7 (64) | 13 (76) | |
| Within 3 months | 5 (18) | 3 (38) | 0 (0) | 2 (15) | .167 |
| Within 6 months | 8 (29) | 3 (38) | 2 (29) | 3 (23) | .865 |
BCR-ABL quantification was not monitored in 2 patients enrolled before 2008 in the SCT group.
Relapse included hematologic and molecular relapse.
Fifteen patients in sustained CMR until the last follow-up were included.
Abbreviations: SCT, stem cell transplantation; MST, microtransplantation; Chemo, Chemotherapy; CR, complete remission; CMR, complete molecular response.
Chimerism and microchimerism
All patients from the SCT group achieved full donor chimerism (FDC). The median time of establishment of full donor chimerism was 32 days (6-200 days) post transplantation. Three patients who experienced hematologic relapse underwent drop of donor chimerism as low as 0%, 66% and 95%, respectively. Two patients presented with molecular relapse before the achievement of FDC. There is no significant difference of time-to-FDC between relapsed (14-200 days, n = 6) and nonrelapsed (6-103 days, n = 11) cohorts (median time 46.5 vs. 16 days, P = .242). In the MST group, eight accessible patients were monitored for microchimerism/chimerism at totally 53 timepoints (2-9 timepoints for each patient). Thirty-five out of 53 timepoints (66%) had detectable donor chimerism, including 2 timepoints with donor chimerism over 1% (4.877% and 1.07%) from 2 patients on the first and fourth day respectively after donor cell infusion. Microchimerism was detected in 7 out of 8 accessible patients ranging from 1.07 × 10-5 to 6.6 × 10-4 copies from 9 to 1499 days after MST (Figure 4A).
Figure 4.

Monitoring of microchimerisms and HLA*0201/2402+WT1+CD8+ T cells. A. Microchimerism was detected in 7 out of 8 patients ranging from 1.07 × 10-5 to 6.6 × 10-4 from 9 to 1499 days after MST. B. Seven patients were monitored for HLA*0201/2402+WT1+CD8+ T cells after MST, and 6 patients had significant T cell response. Frequencies varied between 0.05% and 0.67% from 297 to 1743 days after the first donor cell infusion. C. Four patients were monitored for HLA*0201/2402+WT1+CD8+ T cells after SCT, and 2 patients had significant T cell response with frequencies between 0.06% and 0.52% from 116 to 1265 days post transplantation. In (B and C), the black dot represents data from patient/donor pairs with double-positive HLA*02:01 or HLA*24:02, the red dot represents data with donor-only HLA*02:01 or HLA*24:02, and the blue dot represents data with patient-only HLA*02:01 or HLA*24:02.
WT1+CD8+ T cell assessment
In the MST group, 8 out of 11 patient/donor pairs were either HLA-A*02:01 positive or HLA-A*24:02 positive. Among these 8 patients, WT1+CD8+ T cells were not monitored in one patient with positive HLA-A*02:01 due to lack of samples. WT1+CD8+ T cell responses were monitored at 23 timepoints from the other 7 patients (1-6 timepoints for each patient), and 6 of them had significant WT1+CD8+ T cell responses (positive at 14 timepoints). WT1+CD8+ T cell frequencies varied between 0.05% and 0.67% from 297 to 1743 days after MST (Figure 4B). Among these patients, two donor/recipient pairs were both HLA-A*02:01 positive; two pairs were donor-only HLA-A*02:01 positive; one pair was donor-only HLA-A*02:01 positive and patient-only HLA-A*24:02 positive, whereas one pair was patient-only HLA-A*02:01 positive and donor-only HLA-A*24:02 positive. In the SCT group, WT1+CD8+ T cells were monitored at 9 timepoints from 4 out of 17 patients (1-4 timepoints for each patient) who or whose donor were either HLA-A*02:01 positive or HLA-A*24:02 positive, and 2 patients had significant WT1+CD8+ T cell responses (positive at 5 timepoints) with frequencies between 0.06% and 0.52% from 116 to 1265 days after SCT. Among these patients, one patient/donor pair was both HLA-A*02:01 positive, and one pair was donor-only HLA-A*24:02 positive (Figure 4C).
Univariate and multivariate analyses
Patient characteristics (age, gender, WBC, chromosome type, BCR-ABL transcript, CMR at enrollment, TKI type at enrollment), time from diagnosis to enrollment and treatment group were analyzed to identify potential factors influencing OS, LFS and relapse (Table 4). On multivariable analysis, MST treatment was identified as the sole independent variable predicting 4-year OS.
Table 4.
Univariate and multivariate analyses for factors influencing OS, LFS and relapse (n = 45)
| Variables | 4-year OS | 4-year LFS | 4-year Relapse | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||||
|
|
|
|
|
|
|
|||||||
| % | P | HR (95% CI) | P | % | P | HR (95% CI) | P | % | P | HR (95% CI) | P | |
| Age | ||||||||||||
| > 50 years | 75.0 | .264 | - | - | 66.7 | .215 | - | - | 16.7 | .206 | - | - |
| ≤ 50 years | 48.5 | - | - | 39.4 | - | - | 39.4 | - | - | |||
| Gender | ||||||||||||
| Male | 51.7 | .754 | - | - | 41.4 | .717 | - | - | 37.9 | .635 | - | - |
| Female | 62.5 | - | - | 56.3 | - | - | 25.0 | - | - | |||
| WBC | ||||||||||||
| ≥ 30 × 109/L | 45.8 | .112 | - | - | 37.5 | .088 | 1.465 (0.580-3.700) | .419 | 41.7 | .088 | 2.039 (0.604-6.880) | .251 |
| < 30 × 109/L | 66.7 | - | - | 57.1 | 1 | - | 23.8 | 1 | - | |||
| Chromosome type | ||||||||||||
| Ph alone | 61.1 | .101 | - | - | 50.0 | .198 | - | - | 30.6 | .227 | - | - |
| Ph with other | 33.3 | - | - | 33.3 | - | - | 44.4 | - | - | |||
| BCR-ABL transcript | ||||||||||||
| P190 | 50.0 | .333 | - | - | 43.3 | .480 | - | - | 36.7 | .440 | - | - |
| P210/P230 | 66.7 | - | - | 53.3 | - | - | 26.7 | - | - | |||
| CMR at enrollment | ||||||||||||
| Yes | 60.7 | .482 | - | - | 53.6 | .250 | - | - | 28.6 | .330 | - | - |
| No | 47.1 | - | - | 35.3 | - | - | 41.2 | - | - | |||
| TKI type at enrollment | ||||||||||||
| 1st generation | 46.7 | .181 | - | - | 36.7 | .080 | 2.049 (0.740-5.674) | .167 | 36.7 | .341 | - | - |
| 2nd generation | 73.3 | - | - | 66.7 | 1 | - | 26.7 | - | - | |||
| Time from diagnosis to enrollment | ||||||||||||
| ≥ 4 months | 45.5 | .099 | 1.483 (0.568-3.868) | .421 | 36.4 | .168 | - | - | 27.3 | .771 | - | - |
| < 4 months | 65.2 | 1 | 56.5 | - | - | 39.1 | - | - | ||||
| Treatment | ||||||||||||
| SCT | 35.3 | .005 | 1 | 35.3 | .048 | 1 | - | 17.6 | .085 | 1 | - | |
| MST | 90.9 | 0.080 (0.010-0.643) | .018 | 72.7 | 0.329 (0.078-1.396) | .132 | 27.3 | 1.126 (0.194-6.538) | .895 | |||
| Chemo | 52.9 | 0.602 (0.229-1.581) | .303 | 41.2 | 0.820 (0.345-1.949) | .654 | 52.9 | 2.750 (0.738-10.248) | .132 | |||
Abbreviations: OS, overall survival; LFS, leukemia-free survival; WBC, white blood cell; CMR, complete molecular response; TKI, tyrosine kinase inhibitor; SCT, stem cell transplantation; MST, microtransplantation; Chemo, Chemotherapy.
Discussion
This study reports outcomes comparing the efficacy of SCT, MST and chemotherapy protocols for patients with Ph+ ALL. Patients undergoing MST showed favorable 4-year OS, 2-year LFS and 4-year LFS compared with those receiving either SCT or chemotherapy. Patients in the MST group also displayed lower relapse rate than those in the chemotherapy group, and manifested satisfactory tolerance with lower NRM compared with those receiving allo-SCT. In addition, donor microchimerisms and specific HLA*02:01/24:02+WT1+CD8+ T cells were detected in patients post MST, implying the persistence of donor components and potential immune reaction.
Outcome of standard chemotherapy for patients with Ph+ ALL has been poor. 3-year OS rates are less than 20% without allo-SCT and only 30-40% with allo-SCT [2,22]. Although allo-SCT in first CR is the standard intervention in patients with Ph+ ALL, optimal use of TKIs with chemotherapy has significantly improved efficacy with less treatment-related mortality, which shows benefit especially for older patients who account for the major population of Ph+ ALL. We also incorporated TKIs into this study, and OS and LFS rates were 47% and 40%, respectively at the time of last follow-up, which are comparable with previous reports [23].
Previous studies have confirmed survival benefits of MST in acute myeloid leukemia [12]. In this study, we first applied MST to treat patients with Ph+ ALL, despite the fact that the sample of patients enrolled was small. 4-year OS was 91% and 4-year LFS was 72%, which showed significant benefit over chemotherapy of this study and other similar studies [24,25]. Only 3 patients (28%) relapsed within 4 years in the MST group compared with 9 patients (67%) in the chemotherapy group, implying the enhanced anti-leukemic effect through programmed allogeneic PBMC infusion. Although MST did not show superiority in count recovery as reported previously in AML patients [18], none of patients died from treatment-related toxicities and no GVHD was observed in the MST group, which further confirmed the safety and chemotherapy-supporting role of GPBMC infusion. With these features, MST may shed a light on the treatment of elderly and/or frail patients with Ph+ ALL who are ineligible for allogeneic transplant or intensive chemotherapy, which is similar to the application of MST in elderly patients with AML [12].
In previous studies we utilized the real-time quantitative PCR technology detecting the sex-determining region of the Y chromosome to determine the persistence of minimal donor components in the recipient’s circulation (microchimerism), however, this method is restricted to male-to-female pairs which narrows its application in general populations. In this study, we applied an alternative approach based on real-time quantitative PCR using the TaqMan technology, which showed increased sensitivity of 10-4-10-5 independent of donor and recipient gender [19,26,27]. The highest level of donor microchimerism reached 6.6 × 10-4 copies and the persistence was as long as 1499 days post MST. Although no convincing relationship has been found between detectable microchimerism and clinical outcome due to limited patient number and discordant detection timepoints among patients, these results were consistent with our previous microchimerism study in AML patients with the longest persistence of 1020 days post MST [8], indicating the possibility of long-term existence of donor components in the recipient, even though no strong immunosuppressive conditioning was involved.
WT1+CD8+ T cells had been monitored with a HLA-A*02:01 pentamer in our previous MST study, and only 38.6% of patients were HLA-A*02:01 positive [8]. In this study, we utilized two pentamers targeting HLA-A*02:01 and/or HLA-A*24:02 which further expanded the percentage of detectable population over 60%. It has been reported that the emergence of WT1+CD8+ T cells was considered to be the indirect evidence for graft-versus-leukemia (GVL) and recipient-versus-leukemia (RVL) effects following donor lymphocyte infusion [28,29]. In the MST group, we monitored WT1+CD8+ T cells in 7 subjects who or whose donor conserved HLA-A*02:01 and/or HLA-A*24:02 locus. Two patient/donor pairs presented with significant WT1+CD8+ T cell response but were unable to distinguish the cell origin due to the possession of the same HLA-A locus (A*02:01) between the donor and recipient. Significant WT1+CD8+ T cell response was also detected in 2 pairs with patient-only HLA-A*02:01 or HLA-A*24:02 as long as 644 and 580 days post MST, respectively, indicating RVL effect. Interestingly, 3 out of 4 pairs who had donor-only A*02:01 or A*24:02 also preserved significant WT1+CD8+ T cell response even 518, 644, and 858 days, respectively after donor cell infusion, implying long-term GVL effect. In contrast, 2 out of 4 accessible patient/donor pairs in the SCT group presented with significant WT1+CD8+ T cell response after transplantation, only indicating GVL effect. These results revealed again that MST induced specific anti-leukemic activity through both GVL and RVL effects, which was in accordance with our previous study [8,30].
Although allogeneic SCT in first remission was considered the first choice for Ph+ ALL patients in the pre-TKI era, the introduction of TKIs has questioned SCT as standard of care. Patients with Ph+ ALL who underwent Hyper-CVAD chemotherapy plus TKIs and achieved CMR at 3 months have excellent long-term outcomes with a 4-year OS rate of 66% even without SCT [31]. However, 4-year OS rates were approximately 50% and 40% in patients undergoing matched sibling and matched unrelated SCT, respectively, associated with increased death in remission compared with patients receiving chemotherapy [32]. In this study, patients in the SCT group also experienced significantly higher incidence of transplant-associated adverse events followed by increased NRM. The reason may lie in that over half of patients (9 out of 17) underwent haploidentical SCT which may lead to higher treatment-related mortality compared with those who received matched related SCT [17,33]. It is also notable that the SCT group had equivalent 4-year relapse rate compared with the chemotherapy group, which may result from the MRD-triggered strategy instead of prophylactic use of TKIs post transplant. In the SCT group of this study, TKIs were discontinued to alleviate transplant-related toxicities until MRD existed, which is distinct from common recommendations for 1-year TKI duration post transplant [34]. However, even though several non-randomized studies validated the priority of prophylactic TKI strategy post transplant, a multicenter randomized controlled trial showed similar survival and relapse between two strategies, making this issue still controversial [35]. The efficacy of post-transplantation cyclophosphamide (PTCy)-based haploidentical SCT has not been determined in large cohort of Ph+ ALL patients, however, this regimen in high-risk ALL population has been reported by several studies [36,37]. In addition, other alternative haploidentical SCT regimens for Ph+ ALL have also been investigated with promising results [38-40]. Although the efficacy of our RIC regimen has been reported in a multicenter study [17], its use in the Ph+ ALL population still needs to be determined in a larger cohort.
In summary, MST showed improved outcomes compared with chemotherapy and allo-SCT in Ph+ ALL patients, which is worthy to be further evaluated in a larger population. Future studies may explore the combination of MST with novel immunotherapy like chimeric antigen receptor-modified T cells or novel agents including next-generation TKIs and blinatumomab, which may further challenge the application of SCT.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 81800150 to Bo Cai, No. 81670110 to Kaixun Hu and No. 31500732 to Yi Wang), the capital characteristic clinical research of PR China (No. Z161100000516235 to Jianhui Qiao), Translational Research Grant of NCRCH (No. 2020ZKZB02 to Mei Guo) and the Foundation for Young Scientists of Chinese PLA General Hospital (No. QNF19043 to Bo Cai, No. QNF19041 to Yi Wang and No. QNC19034 to Kaixun Hu).
Disclosure of conflict of interest
None.
Abbreviations
- Ph+ ALL
Philadelphia chromosome-positive acute lymphoblastic leukemia
- OS
overall survival
- TKI
tyrosine kinase inhibitor
- allo-SCT
allogeneic stem cell transplantation
- CR
complete remission
- MST
microtransplantation
- G-CSF
granulocyte colony-stimulating factor
- GPBMC
G-CSF mobilized peripheral blood mononuclear cell
- HLA
human leukocyte antigen
- AML
acute myeloid leukemia
- qRT-PCR
quantitative reverse-transcription polymerase chain reaction
- RIC
reduced-intensity conditioning
- MAC
myeloablative conditioning
- GVHD
graft-versus-host disease
- CNSL
central nervous system leukemia
- MRD
minmal residual disease
- CMR
complete molecular response
- LFS
leukemia-free survival
- NRM
nonrelapse mortality
- CTCAE
Common Terminology Criteria for Adverse Events
- FDC
full donor chimerism
- GVL
graft-versus-leukemia
- RVL
recipient-versus-leukemia
- PTCy
post-transplantation cyclophosphamide
References
- 1.Lee HJ, Thompson JE, Wang ES, Wetzler M. Philadelphia chromosome-positive acute lymphoblastic leukemia: current treatment and future perspectives. Cancer. 2011;117:1583–1594. doi: 10.1002/cncr.25690. [DOI] [PubMed] [Google Scholar]
- 2.Kantarjian HM, O’Brien S, Smith TL, Cortes J, Giles FJ, Beran M, Pierce S, Huh Y, Andreeff M, Koller C, Ha CS, Keating MJ, Murphy S, Freireich EJ. Results of treatment with hyper-CVAD, a dose-intensive regimen, in adult acute lymphocytic leukemia. J. Clin. Oncol. 2000;18:547–561. doi: 10.1200/JCO.2000.18.3.547. [DOI] [PubMed] [Google Scholar]
- 3.Daver N, Thomas D, Ravandi F, Cortes J, Garris R, Jabbour E, Garcia-Manero G, Borthakur G, Kadia T, Rytting M, Konopleva M, Kantarjian H, O’Brien S. Final report of a phase II study of imatinib mesylate with hyper-CVAD for the front-line treatment of adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Haematologica. 2015;100:653–661. doi: 10.3324/haematol.2014.118588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El Fakih R, Jabbour E, Ravandi F, Hassanein M, Anjum F, Ahmed S, Kantarjian H. Current paradigms in the management of Philadelphia chromosome positive acute lymphoblastic leukemia in adults. Am J Hematol. 2018;93:286–295. doi: 10.1002/ajh.24926. [DOI] [PubMed] [Google Scholar]
- 5.Schultz KR, Carroll A, Heerema NA, Bowman WP, Aledo A, Slayton WB, Sather H, Devidas M, Zheng HW, Davies SM, Gaynon PS, Trigg M, Rutledge R, Jorstad D, Winick N, Borowitz MJ, Hunger SP, Carroll WL, Camitta B Children’s Oncology Group. Long-term follow-up of imatinib in pediatric Philadelphia chromosome-positive acute lymphoblastic leukemia: Children’s oncology group study AALL0031. Leukemia. 2014;28:1467–1471. doi: 10.1038/leu.2014.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ravandi F, Othus M, O’Brien SM, Forman SJ, Ha CS, Wong JYC, Tallman MS, Paietta E, Racevskis J, Uy GL, Horowitz M, Takebe N, Little R, Borate U, Kebriaei P, Kingsbury L, Kantarjian HM, Radich JP, Erba HP, Appelbaum FR. US intergroup study of chemotherapy plus dasatinib and allogeneic stem cell transplant in philadelphia chromosome positive ALL. Blood Adv. 2016;1:250–259. doi: 10.1182/bloodadvances.2016001495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang J, Jiang Q, Xu LP, Zhang XH, Chen H, Qin YZ, Ruan GR, Jiang H, Jia JS, Zhao T, Liu KY, Jiang B, Huang XJ. Allogeneic stem cell transplantation versus tyrosine kinase inhibitors combined with chemotherapy in patients with philadelphia chromosome-positive acute lymphoblastic leukemia. Biol Blood Marrow Transplant. 2018;24:741–750. doi: 10.1016/j.bbmt.2017.12.777. [DOI] [PubMed] [Google Scholar]
- 8.Guo M, Hu KX, Liu GX, Yu CL, Qiao JH, Sun QY, Qiao JX, Dong Z, Sun WJ, Sun XD, Zuo HL, Man QH, Liu ZQ, Liu TQ, Zhao HX, Huang YJ, Wei L, Liu B, Wang J, Shen XL, Ai HS. HLA-mismatched stem-cell microtransplantation as postremission therapy for acute myeloid leukemia: long-term follow-up. J. Clin. Oncol. 2012;30:4084–4090. doi: 10.1200/JCO.2012.42.0281. [DOI] [PubMed] [Google Scholar]
- 9.Hu KX, Sun QY, Guo M, Qiao JX, Yu CL, Qiao JH, Dong Z, Sun WJ, Zuo HL, Huang YJ, Cai B, Ai HS. A study of human leukocyte antigen mismatched cellular therapy (stem cell microtransplantation) in high-risk myelodysplastic syndrome or transformed acute myelogenous leukemia. Stem Cells Transl Med. 2016;5:524–529. doi: 10.5966/sctm.2015-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao HX, Sun WJ, Li J, Hu HL, Ai HS. Programmed haploidentical hematopoietic stem cell infusion combined with systemic chemotherapy improves the outcomes of patients with refractory or relapsed lymphoma. Leuk Lymphoma. 2015;56:270–273. doi: 10.3109/10428194.2014.914196. [DOI] [PubMed] [Google Scholar]
- 11.Cai B, Guo M, Ai H. Microtransplantation: clinical applications and mechanisms. Curr Opin Hematol. 2018;25:417–424. doi: 10.1097/MOH.0000000000000470. [DOI] [PubMed] [Google Scholar]
- 12.Guo M, Chao NJ, Li JY, Rizzieri DA, Sun QY, Mohrbacher A, Krakow EF, Sun WJ, Shen XL, Zhan XR, Wu DP, Liu L, Wang J, Zhou M, Yang LH, Bao YY, Dong Z, Cai B, Hu KX, Yu CL, Qiao JH, Zuo HL, Huang YJ, Sung AD, Qiao JX, Liu ZQ, Liu TQ, Yao B, Zhao HX, Qian SX, Liu WW, Fores R, Duarte RF, Ai HS Microtransplantation Interest Group. HLA-mismatched microtransplant in older patients newly diagnosed with acute myeloid leukemia: results from the microtransplantation interest group. JAMA Oncol. 2018;4:54–62. doi: 10.1001/jamaoncol.2017.2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sung AD, Jauhari S, Siamakpour-Reihani S, Rao AV, Staats J, Chan C, Meyer E, Gadi VK, Nixon AB, Lyu J, Xie J, Bohannon L, Li Z, Hourigan CS, Dillon LW, Wong HY, Shelby R, Diehl L, de Castro C, LeBlanc T, Brander D, Erba H, Galal A, Stefanovic A, Chao N, Rizzieri DA. Microtransplantation in older patients with AML: a pilot study of safety, efficacy and immunologic effects. Am J Hematol. 2020;95:662–671. doi: 10.1002/ajh.25781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu Y, Zhao H, Zhang X, Wu Y, Xie Y, Li Y, Lian Y, Huang J, Li J, Chen Y, Qian S. Decitabine before low-dose cytarabine-based chemotherapy combined with human leukocyte antigen-mismatched stem cell microtransplantation improved outcomes in elderly patients with newly diagnosed acute myeloid leukemia. Biol Blood Marrow Transplant. 2017;23:830–835. doi: 10.1016/j.bbmt.2017.01.085. [DOI] [PubMed] [Google Scholar]
- 15.Liu L, Zhang X, Qiu H, Tang X, Han Y, Fu C, Jin Z, Zhu M, Miao M, Wu D. HLA-mismatched stem cell microtransplantation compared to matched-sibling donor transplantation for intermediate/high-risk acute myeloid leukemia. Ann Hematol. 2019;98:1249–1257. doi: 10.1007/s00277-018-3583-3. [DOI] [PubMed] [Google Scholar]
- 16.Hu KX, Du X, Guo M, Yu CL, Qiao JH, Sun QY, Zuo HL, Cai B, Huang YJ, Ai HS, Dong Z, Wang Y. Comparative study of micro-transplantation from HLA fully mismatched unrelated and partly matched related donors in acute myeloid leukemia. Am J Hematol. 2020;95:630–636. doi: 10.1002/ajh.25780. [DOI] [PubMed] [Google Scholar]
- 17.Yu CL, Zheng D, Qiao ZH, Wang JM, Huang H, Liang YM, Wu DP, Chen BA, Bai H, Shi BF, Sun WJ, Qiao JX, Guo M, Qiao JH, Sun QY, Hu KX, Huang YJ, Zuo HL, Huang XJ, Ai HS. The long-term outcome of reduced-intensity allogeneic stem cell transplantation from a matched related or unrelated donor, or haploidentical family donor in patients with leukemia: a retrospective analysis of data from the China RIC cooperative group. Ann Hematol. 2017;96:279–288. doi: 10.1007/s00277-016-2864-y. [DOI] [PubMed] [Google Scholar]
- 18.Guo M, Hu KX, Yu CL, Sun QY, Qiao JH, Wang DH, Liu GX, Sun WJ, Wei L, Sun XD, Huang YJ, Qiao JX, Dong Z, Ai HS. Infusion of HLA-mismatched peripheral blood stem cells improves the outcome of chemotherapy for acute myeloid leukemia in elderly patients. Blood. 2011;117:936–941. doi: 10.1182/blood-2010-06-288506. [DOI] [PubMed] [Google Scholar]
- 19.Alizadeh M, Bernard M, Danic B, Dauriac C, Birebent B, Lapart C, Lamy T, Le Prise PY, Beauplet A, Bories D, Semana G, Quelvennec E. Quantitative assessment of hematopoietic chimerism after bone marrow transplantation by real-time quantitative polymerase chain reaction. Blood. 2002;99:4618–4625. doi: 10.1182/blood.v99.12.4618. [DOI] [PubMed] [Google Scholar]
- 20.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, Thomas ED. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 21.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, Martin P, Chien J, Przepiorka D, Couriel D, Cowen EW, Dinndorf P, Farrell A, Hartzman R, Henslee-Downey J, Jacobsohn D, McDonald G, Mittleman B, Rizzo JD, Robinson M, Schubert M, Schultz K, Shulman H, Turner M, Vogelsang G, Flowers ME. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Dombret H, Gabert J, Boiron JM, Rigal-Huguet F, Blaise D, Thomas X, Delannoy A, Buzyn A, Bilhou-Nabera C, Cayuela JM, Fenaux P, Bourhis JH, Fegueux N, Charrin C, Boucheix C, Lhéritier V, Espérou H, MacIntyre E, Vernant JP, Fière D Groupe d’Etude et de Traitement de la Leucémie Aiguë Lymphoblastique de l’Adulte (GET-LALA Group) Outcome of treatment in adults with Philadelphia chromosome-positive acute lymphoblastic leukemia--results of the prospective multicenter LALA-94 trial. Blood. 2002;100:2357–2366. doi: 10.1182/blood-2002-03-0704. [DOI] [PubMed] [Google Scholar]
- 23.Chalandon Y, Thomas X, Hayette S, Cayuela JM, Abbal C, Huguet F, Raffoux E, Leguay T, Rousselot P, Lepretre S, Escoffre-Barbe M, Maury S, Berthon C, Tavernier E, Lambert JF, Lafage-Pochitaloff M, Lhéritier V, Chevret S, Ifrah N, Dombret H Group for Research on Adult Acute Lymphoblastic Leukemia (GRAALL) Randomized study of reduced-intensity chemotherapy combined with imatinib in adults with Ph-positive acute lymphoblastic leukemia. Blood. 2015;125:3711–3719. doi: 10.1182/blood-2015-02-627935. [DOI] [PubMed] [Google Scholar]
- 24.Chiaretti S, Vitale A, Vignetti M, Piciocchi A, Fazi P, Elia L, Falini B, Ronco F, Ferrara F, De Fabritiis P, Luppi M, La Nasa G, Tedeschi A, Califano C, Fanin R, Dore F, Mandelli F, Meloni G, Foa R. A sequential approach with imatinib, chemotherapy and transplant for adult Ph+ acute lymphoblastic leukemia: final results of the GIMEMA LAL 0904 study. Haematologica. 2016;101:1544–1552. doi: 10.3324/haematol.2016.144535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foà R, Vitale A, Vignetti M, Meloni G, Guarini A, De Propris MS, Elia L, Paoloni F, Fazi P, Cimino G, Nobile F, Ferrara F, Castagnola C, Sica S, Leoni P, Zuffa E, Fozza C, Luppi M, Candoni A, Iacobucci I, Soverini S, Mandelli F, Martinelli G, Baccarani M GIMEMA Acute Leukemia Working Party. Dasatinib as first-line treatment for adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 2011;118:6521–6528. doi: 10.1182/blood-2011-05-351403. [DOI] [PubMed] [Google Scholar]
- 26.Kishikawa H, Kinoshita T, Yonemoto S, Kawamura M, Nakazawa S, Ueda N, Hirai T, Nishimura K, Hashimoto M, Ichikawa Y. Early microchimerism in peripheral blood following kidney transplantation. Transplant Proc. 2014;46:388–390. doi: 10.1016/j.transproceed.2013.12.036. [DOI] [PubMed] [Google Scholar]
- 27.Kim SY, Jeong MH, Park N, Ra E, Park H, Seo SH, Kim JY, Seong MW, Park SS. Chimerism monitoring after allogeneic hematopoietic stem cell transplantation using quantitative real-time PCR of biallelic insertion/deletion polymorphisms. J Mol Diagn. 2014;16:679–688. doi: 10.1016/j.jmoldx.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 28.Medina DJ, Gharibo M, Savage P, Cohler A, Kuriyan M, Balsara B, Anand M, Schaar D, Krimmel T, Saggiomo K, Manago J, Talty L, Dudek L, Grospe S, Rubin A, Strair RK. A pilot study of allogeneic cellular therapy for patients with advanced hematologic malignancies. Leuk Res. 2008;32:1842–1848. doi: 10.1016/j.leukres.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 29.Rezvani K, Yong AS, Mielke S, Savani BN, Musse L, Superata J, Jafarpour B, Boss C, Barrett AJ. Leukemia-associated antigen-specific T-cell responses following combined PR1 and WT1 peptide vaccination in patients with myeloid malignancies. Blood. 2008;111:236–242. doi: 10.1182/blood-2007-08-108241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu KX, Ai HS, Guo M, Yu CL, Qiao JH, Sun QY, Dong Z, Cai B, Sun WJ, Wang Y, Zhan X, Liu X. Donor selection in HLA-mismatched hematopoietic stem cell microtransplantation for acute myeloid leukemia. Stem Cells Dev. 2020;29:648–654. doi: 10.1089/scd.2019.0295. [DOI] [PubMed] [Google Scholar]
- 31.Short NJ, Jabbour E, Sasaki K, Patel K, O’Brien SM, Cortes JE, Garris R, Issa GC, Garcia-Manero G, Luthra R, Thomas D, Kantarjian H, Ravandi F. Impact of complete molecular response on survival in patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 2016;128:504–507. doi: 10.1182/blood-2016-03-707562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fielding AK, Rowe JM, Richards SM, Buck G, Moorman AV, Durrant IJ, Marks DI, McMillan AK, Litzow MR, Lazarus HM, Foroni L, Dewald G, Franklin IM, Luger SM, Paietta E, Wiernik PH, Tallman MS, Goldstone AH. Prospective outcome data on 267 unselected adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia confirms superiority of allogeneic transplantation over chemotherapy in the pre-imatinib era: results from the International ALL Trial MRC UKALLXII/ECOG2993. Blood. 2009;113:4489–4496. doi: 10.1182/blood-2009-01-199380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang B, Yu R, Cai L, Bin G, Chen H, Zhang H, He P, Lu X. Haploidentical versus matched donor stem cell transplantation for patients with hematological malignancies: a systemic review and meta-analysis. Bone Marrow Transplant. 2019;54:99–122. doi: 10.1038/s41409-018-0239-9. [DOI] [PubMed] [Google Scholar]
- 34.Chen H, Liu KY, Xu LP, Liu DH, Chen YH, Zhao XY, Han W, Zhang XH, Wang Y, Zhang YY, Qin YZ, Liu YR, Huang XJ. Administration of imatinib after allogeneic hematopoietic stem cell transplantation may improve disease-free survival for patients with Philadelphia chromosome-positive acute lymphobla stic leukemia. J Hematol Oncol. 2012;5:29. doi: 10.1186/1756-8722-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pfeifer H, Wassmann B, Bethge W, Dengler J, Bornhäuser M, Stadler M, Beelen D, Vucinic V, Burmeister T, Stelljes M, Faul C, Dreger P, Kiani A, Schäfer-Eckart K, Schwerdtfeger R, Lange E, Kubuschok B, Horst HA, Gramatzki M, Brück P, Serve H, Hoelzer D, Gökbuget N, Ottmann OG GMALL Study Group. Randomized comparison of prophylactic and minimal residual disease-triggered imatinib after allogeneic stem cell transplantation for BCR-ABL1-positive acute lymphoblastic leukemia. Leukemia. 2013;27:1254–1262. doi: 10.1038/leu.2012.352. [DOI] [PubMed] [Google Scholar]
- 36.Srour SA, Milton DR, Bashey A, Karduss-Urueta A, Al Malki MM, Romee R, Solomon S, Nademanee A, Brown S, Slade M, Perez R, Rondon G, Forman SJ, Champlin RE, Kebriaei P, Ciurea SO. Haploidentical transplantation with post-transplantation cyclophosphamide for high-risk acute lymphoblastic leukemia. Biol Blood Marrow Transplant. 2017;23:318–324. doi: 10.1016/j.bbmt.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bazarbachi A, Labopin M, Angelucci E, Gülbas Z, Ozdogu H, Arat M, de Rosa L, Pastano R, Pioltelli P, Montserrat R, Martino M, Ciceri F, Koç Y, Socié G, Blaise D, Herrera C, Chalandon Y, Bernasconi P, Marotta G, Castagna L, McDonald A, Visani G, Carluccio P, Vitek A, Simand C, Afanasyev B, Rösler W, Diez-Martin JL, Nagler A, Brissot E, Giebel S, Mohty M. Haploidentical transplantation with post-transplantation cyclophosphamide for T cell acute lymphoblastic leukemia: a report from the european society for blood and marrow transplantation acute leukemia working party. Biol Blood Marrow Transplant. 2020;26:936–942. doi: 10.1016/j.bbmt.2020.01.003. [DOI] [PubMed] [Google Scholar]
- 38.Gao L, Zhang C, Gao L, Liu Y, Su Y, Wang S, Li B, Yang T, Yuan Z, Zhang X. Favorable outcome of haploidentical hematopoietic stem cell transplantation in Philadelphia chromosome-positive acute lymphoblastic leukemia: a multicenter study in Southwest China. J Hematol Oncol. 2015;8:90. doi: 10.1186/s13045-015-0186-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gu B, Wu X, Chen G, Ma X, Jin Z, Tang X, Han Y, Fu C, Qiu H, Sun A, Wu D. Haploidentical allogeneic hematopoietic stem cell transplantation compared to matched unrelated transplantation for Philadelphia chromosome-positive acute lymphoblastic leukemia. Leuk Res. 2017;59:41–46. doi: 10.1016/j.leukres.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 40.Chen H, Liu KY, Xu LP, Chen YH, Han W, Zhang XH, Wang Y, Qin YZ, Liu YR, Huang XJ. Haploidentical hematopoietic stem cell transplantation without in vitro T cell depletion for the treatment of philadelphia chromosome-positive acute lymphoblastic leukemia. Biol Blood Marrow Transplant. 2015;21:1110–1116. doi: 10.1016/j.bbmt.2015.02.009. [DOI] [PubMed] [Google Scholar]


