Abstract
It is unknown whether the route of administration impacts dendritic cell (DC)-based immunotherapy for pancreatic ductal adenocarcinoma (PDAC). We compared the effect of intraperitoneal (i.p.), subcutaneous (s.c.), and intratumoral (i.t.) administration of DC vaccine on induction of antitumor responses in a KPC mouse model of PDAC. Histological analysis and flow cytometry were used to evaluate tumor progression and antitumor immunity after different routes of DC vaccination. Using a flank mouse model of PDAC, we found that the i.t. route of DC vaccination had no significant effect on tumor growth rates compared with i.p. and s.c. routes (i.p. 6.66 ± 2.58% vs s.c. 6.79 ± 1.36% vs i.t. 8.57 ± 2.36%; P = 0.33). However, in an orthotopic PDAC model, i.p. injection of DC vaccine effectively suppressed tumor growth, inhibited tumor progression, and increased antitumor immunity compared with s.c. vaccination (tumor weight: i.p. 71.60 ± 15.55 mg vs control 200.40 ± 53.04 mg; P = 0.048; s.c. 151.40 ± 41.64 mg vs control 200.40 ± 53.04 mg; P = 0.49). Our study suggests that immunization via an i.p. route results in superior antitumor immune response and tumor suppression when compared with other routes.
Keywords: Pancreatic cancer, dendritic cell, route of administration
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a devastating malignant disease and it is the 3rd most frequent tumor-related cause of death in the U.S. [1]. Surgical resection is the only curative treatment option [2]. Unresectable tumors constitute up to 80% of PDAC at the time of diagnosis and are associated with a 5-year overall survival of less than 5% [3,4]. Systemic chemotherapies and molecularly targeted therapies have offered little or no survival benefit [5]. One of the major reasons for the poor prognosis is the lack of effective treatments in preventing and controlling relapse. Immunotherapy for patients with PDAC aims to activate the power and specificity of immune system for treatment of PDAC, inducing long-lasting protection against recurrent disease [6]. Currently, it is been actively explored in clinical trials for treatment of advanced/unresectable PDAC [6,7].
Dendritic cells (DCs) are the most potent professional antigen-presenting cells in the immune system [8]. DCs stimulate the activation of both B and T lymphocytes and upregulate co-stimulatory molecules, such as cytokines, to generate cytotoxic T lymphocytes (CTLs) immunity [8]. As such, DC-based vaccine has become a promising immunotherapy for advanced cancers and has been employed in clinical studies for PDAC therapy. However, its clinical efficacy is still limited, underlining the necessity to further explore the potential of DC vaccines. Thus, there is a need to optimize different parameters such as DC maturation and activation status, route, dose, and frequency of administration [9].
Despite the tremendous developments made in the past decade, a standardized method for DC vaccine delivery has not yet been established for preclinical or clinical applications. The delivery route clearly directs the distribution of the DC vaccine upon injection and consequently may induce different immunologic responses [10-14]. Our group previously showed that immunization with apoptotic PDAC cells pulsed DC vaccine via an intraperitoneal (i.p.) route induce an effective antitumor T cell response in a mouse model of PDAC [15-18]. However, it is unknown whether the route of administration influences the efficacy of DC-based immunotherapy for PDAC. Therefore, the aim of this study was to evaluate i.p., intratumor (i.t.), and subcutaneous (s.c.) DC vaccine administration to determine the impact of different routes of vaccination on the induction of antitumor immunologic responses in a mouse model of PDAC.
Materials and methods
All studies were approved by the institutional animal care and use committee of Northwestern University and performed in accordance with National Institutes of Health guidelines.
Cell lines
LSL-KrasG12D/+; LSL-Trp53R172H/+; Pdx-1-Cre (KPC) cells were derived from a spontaneous tumor in a 6-month-old KPC mouse and used for the cellular studies and growing both subcutaneous and orthotopic tumors in mice. Cells were cultured on collagen-coated plastic for < 12 passages. KPC cell was cultured in complete RPMI 1640 medium containing 10% FBS, 100 μg/mL streptomycin, 100 U/mL penicillin, and 2 mM L-glutamine.
Mice
All the animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the Northwestern University. 6-8 weeks old female C57BL/6 mice (Charles River, Wilmington, MA) were used for deriving bone marrow-derived dendritic cells (BMDC). 8-10 weeks old female C57BL/6 mice (Charles River, Wilmington, MA) were used for establishing both subcutaneous and orthotopic pancreatic cancer models.
Generation of mature DCs
DCs were derived from bone marrow progenitor cells as previously described [19]. Briefly, bone marrow cells were harvested from the femurs of 6-8 weeks old C57BL/6 female mice and cultured in complete RPMI1640 containing mouse recombinant GM-CSF (10 ng/ml) and IL-4 (1 ng/ml) (both Invivogen, San Diego, CA) for 8 days in petri dish. Medium was refreshed on day 3 and day 6. On day 8, immature DCs were harvested by collecting non-adherent cells and subsequently were pulsed by incubation with KPC tumor cell lysates in the presence of 100 ng/ml IFN-γ and 250 ng/ml LPS-E. coli 0111:B4 (both from Invivogen, San Diego, CA). KPC lysates were generated by collecting and resuspending KPC tumor cells at 1 × 106 cells/ml in PBS, followed by irradiation with UV for 20 minutes (0.75 J/cm2) and 24 h incubation.
Flank KPC tumor implantation
Female C57BL/6 mice aged 8-10 weeks were used for establishing flank PDAC models. Viable KPC cells (5 × 105, < 12 passages) suspended in 100 μl PBS were directly injected into the flank of female C57BL/6 mice (aged 8-10 weeks) for tumor induction. Cohorts of mice were randomized into different treatment groups (5 mice per group) at 7 days following tumor inoculation. 3 × 106 DCs in a volume of 10 μl were weekly injected via the i.t., s.c. or i.p. routes for 3 weeks. Tumor size was assessed two times a week using micro caliper and was expressed as tumor volume, calculated by the following formula: tumor volume (mm3) = (major axis) * (minor axis)2/2. Tumor growth rate (TGR) was expressed as the percentage change in tumor volume days (%/day): TGR = 100 × (exp(TG)-1), where the growth rate (TG) = 3 × log(Dt/D0)/time (days). On day 22 after tumor challenge, all mice were euthanized.
Orthotopic KPC tumor implantation
Female C57BL/6 mice aged 8-10 weeks were used for establishing orthotopic PDAC models. 5 × 104 viable KPC cells (< 12 passages) suspended in a 3:1 PBS to Matrigel (Sigma-Aldrich, St Louis, MO) solution were directly inoculated into the pancreas for orthotopic tumor growth. Cohorts of mice were randomized into different treatment groups (5 mice per group) at 7 days following tumor inoculation. 3 × 106 DCs in a volume of 50 μl were weekly injected via the s.c. or i.p. routes for 3 weeks.
Flow cytometry analysis
After tumor was dissected, spleens were harvested and homogenized to single cell suspension. Then splenocytes were stained with anti-mouse CD3e-Alexa 488 (clone: 145-2C11), CD45-V450 (clone: 30-F11), or CD8-APC (clone: 53-6.7) antibodies (BD Biosciences, San Jose, CA) after neutralization of unspecific binding with FcR blocker (BD Biosciences, San Jose, CA). Data was collected on a BD LSRFortessaTM cell analyzer (BD Biosciences, San Jose, CA) and analyzed using the FlowJo software (TreeStar Inc, Ashland, OR).
Enzyme-linked immunosorbent assay (ELISA)
IFN-γ expression was detected by ELISA. The mice serum collected after 2 days of the last treatment. The concentrations of IFN-γ in serum were determined using mouse IFN-γ kit (R&D bioscience, Minneapolis, MN) according to the manufacturer’s protocols. The absorbance was measured at 450 nm with a microplate reader.
Histology analysis
Tissues were fixed in 10% formalin and embedded in paraffin. 5 μm sections of pancreatic tumor tissues and lymph nodes were selected for histological analysis. The H&E and Masson’s Trichrome stains were conducted according to manufacturer’s instructions. Whole-tissue slide scans were performed on TissueFAXS system. The histological quantification was done by the investigator who was blinded to the groups. Six 20× images were randomly collected per sample. Image analysis was performed using Image J software (Version 1.5a, https://imagej.nih.gov/ij/).
Immunohistochemistry (IHC)
Tissues were fixed in 10% formalin and embedded in paraffin. Then 5 μm sections were deparaffinized in xylene, rehydrated in graded ethanol, and subjected to antigen retrieval by steam heating in Citra antigen retrieval solution (Vector, Burlingame, CA). After blocking for 1 h at room temperature in blocking buffer (5% goat serum, 2.5% BSA in 1× PBS), slides were incubated overnight in a humidified chamber at 4°C with anti-mouse CK19 (kindly provided by the Developmental Studies Hybridoma Bank), rabbit monoclonal anti-Ki67 (Clone SP6, Invitrogen), Anti-Granzyme B (GrB) antibody (ab4059, Abcam, UK), and rat monoclonal anti-mouse CD8 (Clone 4SM15, Invitrogen, Carlsbad, CA). Immunostaining was detected using 3,3’-diaminobenzidine (DAB) (Vector, Burlingame, CA). Quantification was performed using ImageJ software at a high field magnification.
Statistical analysis
The values are reported as mean and the standard error of the mean (SEM). Statistical significance was either assessed via an unpaired two-tailed Student’s t-test or one-way ANOVA with Bonferroni correction. The overall survival was assessed using the Kaplan-Meier method, and the survival difference between groups was compared using the log-rank test. P < 0.05 was considered significant. Statistical analysis was performed using GraphPad Prism software version 7.0 (La Jolla, CA).
Results
Effects of different routes of administration on tumor growth in flank KPC tumor
To investigate the impacts of different immunization routes on the tumor growth, we utilized a flank PDAC model in which KPC cells were introduced subcutaneously. DC vaccines were injected i.p., i.t., or s.c. into KPC-tumor bearing mice, the size of growing KPC tumors was measured every 3-4 days using micro calipers. The representative photomicrographs of isolated flank tumors at day 22 and the tumor growth curves of volume are shown in Figure 1A, 1C-F. There was no significant difference in tumor growth rates by vaccination routes (P = 0.3327, Figure 1B). Taken together, these results suggested that the tumor location is important for pre-clinical testing of PDAC therapies.
Figure 1.
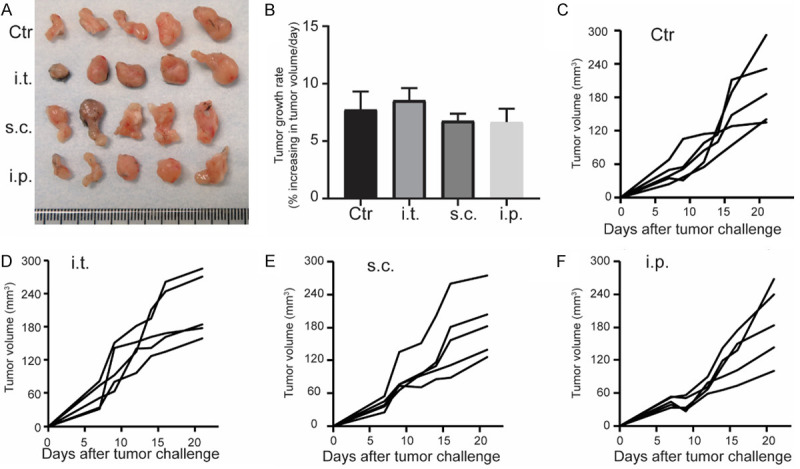
Tumor growth in subcutanouse KPC tumor after different adminstration delivery of DC vaccines. Representative photomicrographs of isolated flank tumors at day 22 (A) and the tumor growth rates (B). The size of growing KPC tumors was measured every 3-4 days using microcalipers (C-F). Each group consisted of five mice. n = 5.
Effects of different routes of administration on tumor growth in orthotopic KPC tumor
There was a slight reduction of tumor growth rate using i.p. (6.66 ± 2.58%) and s.c. routes (6.79 ± 1.36%) compared with i.t. injection (8.57 ± 2.36%) (Figure 1B) in the subcutaneous PDAC mice. We further evaluated the effect of i.p. versus s.c. administration of DC vaccines on tumor growth in orthotopic KPC-tumor bearing mice. The representative photomicrographs of isolated tumors at day 22 and the tumor weight were shown in Figure 2A and 2B. The i.p. injection group showed significantly lower tumor weight (71.6 ± 15.5 mg) than control group (199.8 ± 53.4 mg) (P = 0.0499) while there was no significant difference between s.c. injection group (151.4 ± 41.6 mg) and control group (n = 5 per group, P = 0.4949). These results suggest that i.p. delivery of DC vaccine could induce superior tumor inhibition response compared with s.c. vaccination.
Figure 2.
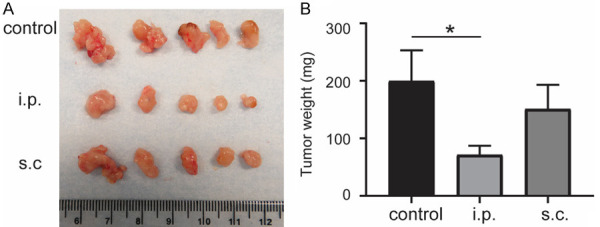
Tumor growth in orthotopic KPC tumor after different adminstration delivery of DC vaccines. Representative photomicrographs of isolated orthotopic tumors (A) and the tumor weight (B) at day 22. *P < 0.05. Data are expressed as the mean ± SEM. n = 5.
Routes of DC vaccine administration on tumor progression in orthotopic KPC tumor
To further compare the effects of i.p and s.c. injections on tumor progression, we conducted histological analysis to evaluate the KPC tumor progression after treatment. The H&E staining slices showed the absence of normal-looking tissue and the presence of desmoplastic reaction within the tumors of all groups (Figure 3A-C). Masson-trichrome staining was performed to evaluate the formation of fibrosis. We found that the i.p. injection diminished intratumoral fibrosis compared with control mice (P = 0.0465) while there was no significant difference between s.c. injection group and control group (P = 0.4041) (Figure 3D-G).
Figure 3.
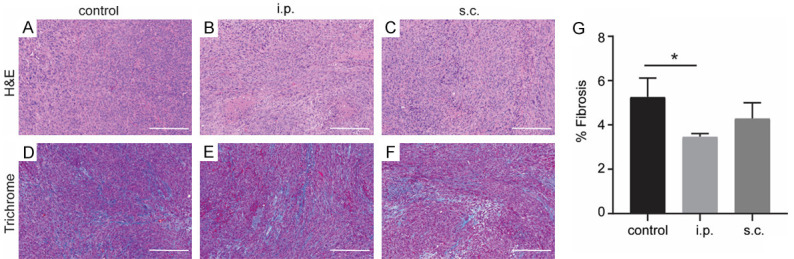
Histological analysis of tumor tissue in orthotopic KPC mice after different routes of DC vaccines. H&E staining of KPC tumor from control group (A), i.p. vaccination group (B), and s.c. vaccination group (C). Masson trichrome staining of KPC tumor from control group (D), i.p. vaccination group (E), and s.c. vaccination group (F). (G) Quantification are shown (n = 5). Scale bars = 200 μm. *P < 0.05. Data are expressed as the mean ± SEM.
CK19 (ductal marker) staining showed ductal differentiation in pancreatic tumor cells (Figure 4A-D). However, no significant changes of CK19 expression between different groups. Importantly, i.p. vaccination treated KPC tumors had significantly decreased staining with the proliferation marker Ki67 compared with the control group (P = 0.0450) (Figure 4E-H). In addition, the staining of apoptosis marker, cleaved-caspase 3 (C-cas 3, was significantly increased in i.p. vaccination treated KPC tumors compared with control group (P = 0.0002) (Figure 4I-L).
Figure 4.
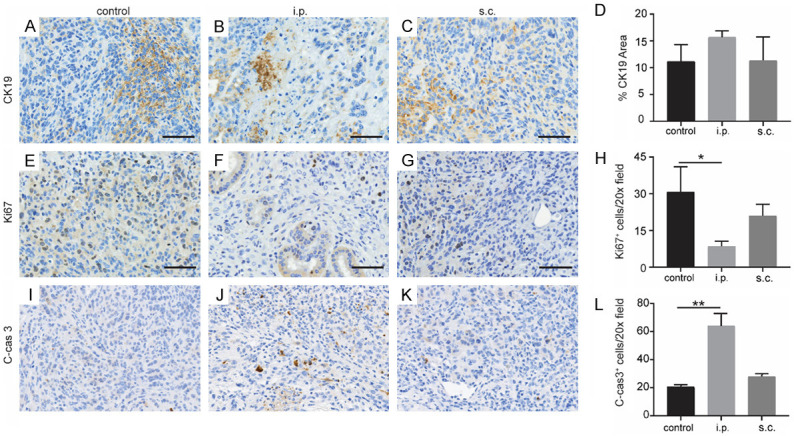
Tumor progression after different DC vaccination in KPC orthotopic mice. Representative IHC images of CK19 staining in KPC tumor from control group (A), i.p. vaccination group (B), and s.c. vaccination group (C). (D) Quantification of CK19 is shown (n = 5). Representative staining of Ki67 of tumor from control group (E), i.p. vaccination group (F), and s.c. vaccination group (G). (H) Quantification of Ki67 is shown. Representative staining of C-cas 3 of tumor from control group (I), i.p. vaccination group (J), and s.c. vaccination group (K). (L) Quantification of C-cas 3 is shown. n = 5. Scale bars = 50 μm. *P < 0.05, **P < 0.001. Data are expressed as the mean ± SEM.
Routes of DC vaccine injection on tumor antigen-specific responses in orthotopic KPC tumor
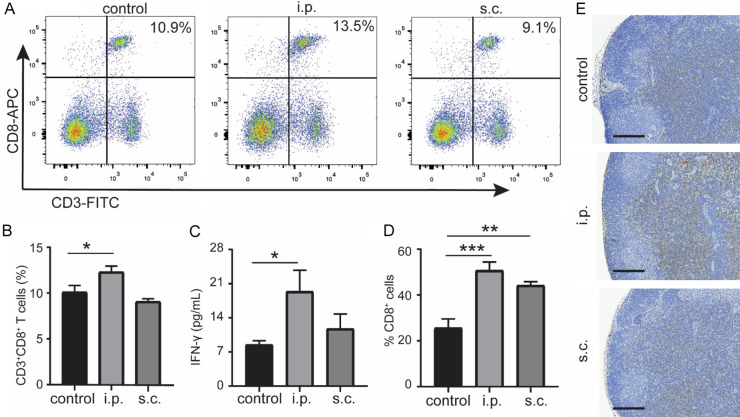
To investigate whether the different vaccination routes resulted in differences of immunologic responses, the intratumoral CD8+ cells and GrB+ cells were analyzed in this study, however, we found only slightly increased expression of CD8+ T cells in KPC tumor following different routes of DC vaccination without significance (Figure 5A-D). Remarkably, significantly increased expression of GrB, which is associated with T cell mediated tumor killing function, was observed in i.p. vaccination group compared with the control group (P = 0.043) (Figure 5E-H). However, no significant increase of GrB was found between the s.c. vaccination group and the control group (P = 0.21).
Figure 5.
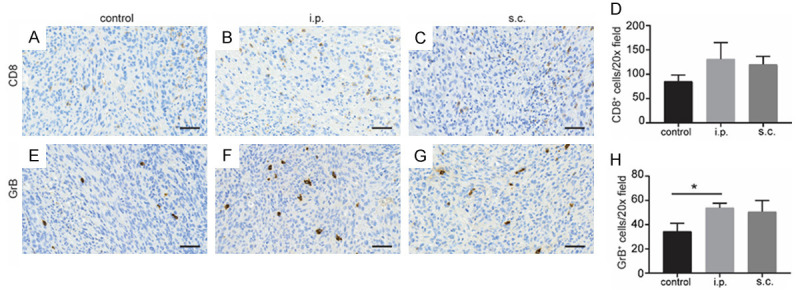
Tumor-infiltrating cells in KPC orthotopic mice. Representative anti-CD8 IHC of tumor tissues from control group (A), i.p. vaccination group (B), and s.c. vaccination group (C). (D) Quantification of CD8 staining was shown. Representative staining of GrB of tumor from control group (E), i.p. vaccination group (F), and s.c. vaccination group (G). (H) Quantification of GrB staining was shown. n = 4. Scale bars = 50 μm. *P < 0.05. Data are expressed as the mean ± SEM.
To further evaluate the systemic antitumor immune response, CD3+CD8+ T cells in the splenocytes were gated on CD45+ events. We found that CD3+CD8+ T cells were significantly increased in the spleen of i.p. administration group compared with the control group (P = 0.02), whereas no difference between s.c. vaccination group and control group (P = 0.41) was observed (Figure 6A, 6B). Furthermore, measurement of cytokines associated with the inflammatory response in mouse blood showed a significantly increased serum level of IFN-γ in the i.p. injection group compared with control group (P = 0.04), while no significance between the s.c. group and control group (P = 0.67) was noted (Figure 6C). Additionally, the tumor draining lymph node (LN) from vaccination groups displayed increased CD8 staining compared with control group, while the i.p. group increased more significant (Figure 6D, 6E).
Figure 6.
Tumor-specific responses after DC vaccination. Representative scatter plots (A) and ratio (B) of CD8+ T cells in the spleen of tumor-bearing mice as determined by flow cytometry. Measurement of IFN-γ in serum from treated mice on day 2 after the last treatment (C) (n = 5). Percentage (D) and representative images (E) of CD8 immunostaining of LN from different groups (n = 4). *P < 0.05, **P < 0.01, ***P < 0.001. Data are expressed as the mean ± SEM.
Discussion
In this study, we compared i.p. with s.c. and i.t. injection of DC vaccine with regard to the antigen-specific immune responses induced in a flank KPC mouse model of PDAC. We did not observe any significant influence of the route of immunization on the tumor growth in flank KPC mouse model. The results showed that i.p. and s.c. immunization slightly induced reduction of tumor volume compared with i.t. immunization (growth rate, 8.57 ± 1.05% for i.t. group, 6.79 ± 0.61% for s.c. group, and 6.66 ± 1.15% for i.p. group). However, in the orthotopic KPC mouse model, i.p. injection of DC vaccine significantly inhibited tumor growth compared with s.c. vaccination. Furthermore, pathological results revealed that the stromal fibrosis was significantly decreased in the i.p. vaccination treated orthotopic KPC mouse model. Additionally, we also observed that the i.p. injection of DC vaccine induced superior immunologic response, as shown by increased intratumoral GrB positive staining, serum level of IFN-γ, and spleen ratio of CD3+CD8+ T cells.
Several injection routes, including s.c [20], i.t. [21], intravenous (i.v.) [22], or intranodal (i.d.) [23], have been used in clinical setting, but the therapeutic responses were not durable. Our observation that i.t. vaccination does not result in inhibition of tumor growth in a flank KPC model, which is in inconsistent with previous studies that intratumoral DC vaccination induces a strong tumor-specific immune response [24,25]. This is possibly due to the different vaccination approaches or adjuvant was used in these studies. We observed that s.c. immunization can slightly inhibit delay tumor growth in a flank KPC mouse model. These results are in line with previous studies in a flank tumor model in which s.c. administration can elicit more potent antitumor response compared with other injection routes [26,27]. However, it is unclear whether vaccination routes have the impact on the induction of antitumor response in the orthotopic PADC mouse model while all these studies were conducted in the flank PDAC models.
Different administration routes of DC vaccines result in activation of T cells in different lymphoid organs, such as i.v. injected DC mainly access the spleen, s.c. delivered DC migrate to peripheral LN draining the injection area, whereas i.p. injected DC most probably enter the intraperitoneal LN [19,28-30]. The migration of DC vaccine to the tumor draining LNs is pivotal for generation of antitumor immunity. Regarding PDAC localization, i.p. injection of DC vaccine may be more effective for PDAC treatment because it could efficiently deliver DC vaccines to intraperitoneal LNs. In previous studies, we found that DC vaccination via the i.p. route induced potent anti-tumor effect in both orthotopic and transgenic mouse model of PDAC [16,18]. A previous clinical study revealed that the i.p. injection route induced some beneficial effects in patients with peritoneal carcinomatosis and mesotheliomas when compared with s.c. vaccination [31]. In accordance, we showed that i.p. vaccination resulted in superior antitumor response when compared with s.c. vaccination in an orthotopic PDAC mouse model. In short, the results of our study and other studies, suggest that the i.p. injection route has significant advantage over s.c. vaccination for abdominal tumors. Moreover, our results suggest that the tumor location is important for pre-clinical testing of PDAC therapies.
Our study had limitations. First, the intranodal delivery route was not included in this study. One reason is that the LN of mouse is too small for intranodal vaccination. The other is that injection of DCs directly into a lymph node may lead to a partial destruction of the LN architecture [32]. Second, the number of DC migration to the draining LNs was not compared among different administration routes. While our previous study has shown that i.p. injection improves the delivery of DC vaccine to spleen compare with footpad injection [19], DC migration to intraperitoneal LNs by different routes requires further investigation.
In conclusion, this study demonstrates that i.p. injected DC vaccine may induce more potent antitumor responses when compared with s.c. and i.t. routes of DC vaccine injections in KPC mouse model of PDAC.
Acknowledgements
This study was supported by the National Cancer Institute (grants R01CA209886, R01CA241532); 2019 Harold E. Eisenberg Foundation Scholar Award; the Fishel Fellowship Award at the Robert H. Lurie Comprehensive Cancer Center; and SIR Foundation Pilot Grant (PR-0000000012).
Disclosure of conflict of interest
None.
References
- 1.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 2.Warshaw AL, Fernandez-del Castillo C. Pancreatic carcinoma. N Engl J Med. 1992;326:455–465. doi: 10.1056/NEJM199202133260706. [DOI] [PubMed] [Google Scholar]
- 3.Sohal DPS, Mangu PB, Khorana AA, Shah MA, Philip PA, O’Reilly EM, Uronis HE, Ramanathan RK, Crane CH, Engebretson A, Ruggiero JT, Copur MS, Lau M, Urba S, Laheru D. Metastatic pancreatic cancer: american society of clinical oncology clinical practice guideline. J. Clin. Oncol. 2016;34:2784–2796. doi: 10.1200/JCO.2016.67.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perez K, Clancy TE, Mancias JD, Rosenthal MH, Wolpin BM. When, what, and why of perioperative treatment of potentially curable pancreatic adenocarcinoma. J. Clin. Oncol. 2017;35:485–489. doi: 10.1200/JCO.2016.70.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barton MK. Germline mutations in pancreatic cancer become better defined. CA Cancer J Clin. 2016;66:93–94. doi: 10.3322/caac.21300. [DOI] [PubMed] [Google Scholar]
- 6.Kunk PR, Bauer TW, Slingluff CL, Rahma OE. From bench to bedside a comprehensive review of pancreatic cancer immunotherapy. J Immunother Cancer. 2016;4:14. doi: 10.1186/s40425-016-0119-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foley K, Kim V, Jaffee E, Zheng L. Current progress in immunotherapy for pancreatic cancer. Cancer Lett. 2016;381:244–251. doi: 10.1016/j.canlet.2015.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banchereau J, Steinman R. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 9.Figdor CG, de Vries IJM, Lesterhuis WJ, Melief CJ. Dendritic cell immunotherapy: mapping the way. Nat Med. 2004;10:475–480. doi: 10.1038/nm1039. [DOI] [PubMed] [Google Scholar]
- 10.Eggert AA, Schreurs MW, Boerman OC, Oyen WJ, de Boer AJ, Punt CJ, Figdor CG, Adema GJ. Biodistribution and vaccine efficiency of murine dendritic cells are dependent on the route of administration. Cancer Res. 1999;59:3340–3345. [PubMed] [Google Scholar]
- 11.Mullins DW, Sheasley SL, Ream RM, Bullock TN, Fu YX, Engelhard VH. Route of immunization with peptide-pulsed dendritic cells controls the distribution of memory and effector T cells in lymphoid tissues and determines the pattern of regional tumor control. J Exp Med. 2003;198:1023–1034. doi: 10.1084/jem.20021348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dudda JC, Simon JC, Martin S. Dendritic cell immunization route determines CD8+ T cell trafficking to inflamed skin: role for tissue microenvironment and dendritic cells in establishment of T cell-homing subsets. J Immunol. 2004;172:857–863. doi: 10.4049/jimmunol.172.2.857. [DOI] [PubMed] [Google Scholar]
- 13.Fong L, Brockstedt D, Benike C, Wu L, Engleman EG. Dendritic cells injected via different routes induce immunity in cancer patients. J Immunol. 2001;166:4254–4259. doi: 10.4049/jimmunol.166.6.4254. [DOI] [PubMed] [Google Scholar]
- 14.Pinyopich A, Ditta GS, Savidge B, Liljegren SJ, Baumann E, Wisman E, Yanofsky MF. Assessing the redundancy of MADS-box genes during carpel and ovule development. Nature. 2003;424:85–88. doi: 10.1038/nature01741. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Z, Li W, Procissi D, Li K, Sheu AY, Gordon AC, Guo Y, Khazaie K, Huan Y, Han G, Larson AC. Antigen-loaded dendritic cell migration: MR imaging in a pancreatic carcinoma model. Radiology. 2015;274:192–200. doi: 10.1148/radiol.14132172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pan L, Shang N, Shangguan J, Figini M, Xing W, Wang B, Sun C, Yang J, Zhang Y, Hu S, Ma Q, Wang J, Velichko Y, Yaghmai V, Benson AB 3rd, Zhang Z. Magnetic resonance imaging monitoring therapeutic response to dendritic cell vaccine in murine orthotopic pancreatic cancer models. Am J Cancer Res. 2019;9:562–573. [PMC free article] [PubMed] [Google Scholar]
- 17.Shangguan A, Shang N, Figini M, Pan L, Yang J, Ma Q, Hu S, Eresen A, Sun C, Wang B, Velichko Y, Yaghmai V, Zhang Z. Prophylactic dendritic cell vaccination controls pancreatic cancer growth in a mouse model. Cytotherapy. 2020;22:6–15. doi: 10.1016/j.jcyt.2019.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Yang J, Hu S, Shangguan J, Eresen A, Li Y, Pan L, Ma Q, Velichko Y, Wang J, Hu C, Yaghmai V, Zhang Z. Dendritic cell immunotherapy induces anti-tumor effect in a transgenic mouse model of pancreatic ductal adenocarcinoma. Am J Cancer Res. 2019;9:2456–2468. [PMC free article] [PubMed] [Google Scholar]
- 19.Wang B, Sun C, Wang S, Shang N, Shangguan J, Figini M, Pan L, Zhou K, Ma Q, Procissi D, Velichko Y, Yaghmai V, Li G, Zhang Z. Mouse dendritic cell migration in abdominal lymph nodes by intraperitoneal administration. Am J Transl Res. 2018;10:2859–2867. [PMC free article] [PubMed] [Google Scholar]
- 20.Baek S, Kim YM, Kim SB, Kim CS, Kwon SW, Kim Y, Kim H, Lee H. Therapeutic DC vaccination with IL-2 as a consolidation therapy for ovarian cancer patients: a phase I/II trial. Cell Mol Immunol. 2015;12:87–95. doi: 10.1038/cmi.2014.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Subbiah V, Murthy R, Hong DS, Prins RM, Hosing C, Hendricks K, Kolli D, Noffsinger L, Brown R, McGuire M, Fu S, Piha-Paul S, Naing A, Conley AP, Benjamin RS, Kaur I, Bosch ML. Cytokines produced by dendritic cells administered intratumorally correlate with clinical outcome in patients with diverse cancers. Clin Cancer Res. 2018;24:3845–3856. doi: 10.1158/1078-0432.CCR-17-2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morse MA, Deng Y, Coleman D, Hull S, Kitrell-Fisher E, Nair S, Schlom J, Ryback ME, Lyerly HK. A phase I study of active immunotherapy with carcinoembryonic antigen peptide (CAP-1)-pulsed, autologous human cultured dendritic cells in patients with metastatic malignancies expressing carcinoembryonic antigen. Clin Cancer Res. 1999;5:1331–1338. [PubMed] [Google Scholar]
- 23.Bol KF, Aarntzen EH, Pots JM, Olde Nordkamp MA, van de Rakt MW, Scharenborg NM, de Boer AJ, van Oorschot TG, Croockewit SA, Blokx WA, Oyen WJ, Boerman OC, Mus RD, van Rossum MM, van der Graaf CA, Punt CJ, Adema GJ, Figdor CG, de Vries IJ, Schreibelt G. Prophylactic vaccines are potent activators of monocyte-derived dendritic cells and drive effective anti-tumor responses in melanoma patients at the cost of toxicity. Cancer Immunol Immunother. 2016;65:327–339. doi: 10.1007/s00262-016-1796-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ehtesham M, Kabos P, Gutierrez MAR, Samoto K, Black KL, Yu JS. Intratumoral dendritic cell vaccination elicits potent tumoricidal immunity against malignant glioma in rats. J Immunol. 2003;26:107–116. doi: 10.1097/00002371-200303000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Song W, Levy R. Therapeutic vaccination against murine lymphoma by intratumoral injection of naive dendritic cells. Cancer Res. 2005;65:5958–5964. doi: 10.1158/0008-5472.CAN-05-0406. [DOI] [PubMed] [Google Scholar]
- 26.Edele F, Dudda JC, Bachtanian E, Jakob T, Pircher H, Martin SF. Efficiency of dendritic cell vaccination against B16 melanoma depends on the immunization route. PLoS One. 2014;9:e105266. doi: 10.1371/journal.pone.0105266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okada N, Tsujino M, Hagiwara Y, Tada A, Tamura Y, Mori K, Saito T, Nakagawa S, Mayumi T, Fujita T, Yamamoto A. Administration route-dependent vaccine efficiency of murine dendritic cells pulsed with antigens. Br J Cancer. 2001;84:1564–1570. doi: 10.1054/bjoc.2001.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Austyn JM, Kupiec-Weglinski JW, Hankins DF, Morris PJ. Migration patterns of dendritic cells in the mouse. Homing to T cell-dependent areas of spleen, and binding within marginal zone. J Exp Med. 1988;167:646–651. doi: 10.1084/jem.167.2.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ludewig B, Ehl S, Karrer U, Odermatt B, Hengartner H, Zinkernagel RM. Dendritic cells efficiently induce protective antiviral immunity. J Virol. 1998;72:3812–3818. doi: 10.1128/jvi.72.5.3812-3818.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lappin MB, Weiss JM, Delattre V, Mai B, Dittmar H, Maier C, Manke K, Grabbe S, Martin S, Simon JC. Analysis of mouse dendritic cell migration in vivo upon subcutaneous and intravenous injection. Immunology. 1999;98:181–188. doi: 10.1046/j.1365-2567.1999.00850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Freedman RS, Vadhan-Raj S, Butts C, Savary C, Melichar B, Verschraegen C, Kavanagh JJ, Hicks ME, Levy LB, Folloder JK, Garcia ME. Pilot study of Flt3 ligand comparing intraperitoneal with subcutaneous routes on hematologic and immunologic responses in patients with peritoneal carcinomatosis and mesotheliomas. Clin Cancer Res. 2003;9:5228–5237. [PubMed] [Google Scholar]
- 32.de Vries IJM, Krooshoop DJEB, Scharenborg NM, Lesterhuis WJ, Diepstra JHS, van Muijen GNP, Strijk SP, Ruers TJ, Boerman OC, Oyen WJG, Adema GJ, Punt CJA, Figdor CG. Effective migration of antigen-pulsed dendritic cells to lymph nodes in melanoma patients is determined by their maturation state. Cancer Res. 2003;63:12–17. [PubMed] [Google Scholar]