Abstract
Whether tenofovir disoproxil fumarate (TDF) is superior to entecavir in lowering the risk of hepatocellular carcinoma (HCC) development remains controversial. This retrospective study compared the incidences of HCC, cirrhotic events, and mortality between patients treated with entecavir and TDF. The study enrolled 1560 chronic hepatitis B (CHB) patients with cirrhosis from 2008 through 2018. All patients received entecavir or TDF monotherapy for at least 12 months before enrollment. Patients who had HCC or liver transplantation at initial treatment or within the first year of entecavir or TDF therapy were excluded. In the entire cohort, the cumulative incidence rates of HCC at 3, 5, and 10 years were 9.5%, 15.2%, and 25.4%, respectively. The entecavir group had a higher cumulative incidence of HCC than the TDF group (P = 0.001). A Cox regression analysis showed that entecavir group, old age, male sex, hepatic decompensation, diabetes mellitus, lower albumin levels, and platelet count were independent predictors of HCC. TDF treatment was significantly associated with a lower risk of HCC compared to entecavir treatment after adjustment with propensity score matching or inverse probability of treatment weighting in all patients. However, this association was not observed in patients with compensated cirrhosis at entry or patients enrolled after 2011, including after adjustment with propensity score matching or inverse probability of treatment weighting. No significant differences were observed in cirrhotic events and mortality or liver transplantation between the entecavir and TDF groups. In conclusion, the incidences of HCC did not differ significantly between patients with compensated cirrhosis or those enrolled over the same period treated with entecavir or TDF.
Keywords: Entecavir, tenofovir disoproxil fumarate, hepatitis B, cirrhosis, hepatocellular carcinoma
Introduction
Chronic hepatitis B (CHB) is a global health concern and results in cirrhosis, hepatocellular carcinoma (HCC), and death [1]. The goal of antiviral therapy for CHB is to prevent the progression to cirrhosis and its related complications, HCC and death [2-4]. The current treatment guidelines recommend entecavir, tenofovir disoproxil fumarate (TDF), and tenofovir alafenamide (TAF) as the first-line nucleos(t)ide analogues (NAs) for the treatment of CHB [2,3]. These oral agents have potent inhibitory activity against HBV replication, a high genetic barrier to antiviral resistance, and a favorable safety profile. Long-term treatment with entecavir or TDF results in the regression of fibrosis or cirrhosis, as well as reduced rates of cirrhotic complications, HCC, and mortality [5-8].
Two meta-analyses revealed that TDF results in a higher rate of viral suppression at one year of treatment than entecavir [9,10]. However, prospective, randomized controlled trials comparing entecavir versus TDF demonstrated that these drugs have similar rates of viral suppression at three years of treatment [11,12]. Nonetheless, no prospective, head-to-head study has compared the efficacy of entecavir and TDF in reducing the negative outcomes in the long term, such as HCC and mortality.
Two retrospective studies demonstrated a similar risk of HCC in patients treated with entecavir or TDF [13,14]. Choi et al. first reported that TDF treatment was significantly associated with a lower risk of HCC but not a lower risk of all-cause mortality or transplantation compared with entecavir treatment. That study was conducted with a nationwide cohort study in South Korea, and the result was validated in a hospital cohort [15]. A recent territory-wide retrospective study from Hong Kong also revealed a significantly lower risk of HCC in patients receiving TDF therapy than those receiving entecavir therapy [16]. However, three subsequent studies from South Korea and one study from an international consortium of CHB reported similar effect of TDF and entecavir in reducing the risk of HCC [17-20].
The results among these studies are conflicting. However, there are similar modes of HBV transmission and similar clinical practices for CHB treatment among Asian countries. Therefore, we aimed to investigate the risks of HCC and mortality or transplantation in a multicenter, retrospective cohort of CHB patients with cirrhosis treated with entecavir or TDF in Taiwan.
Materials and methods
Patients
This study retrospectively enrolled a consecutive cohort of 941 CHB patients with cirrhosis who received entecavir treatment between 2008 and 2018, as well as 351 CHB patients with cirrhosis who received TDF treatment between 2011 and 2018. The patients were enrolled from Kaohsiung Chang Gung Memorial Hospital (n = 946) and China Medical University Hospital (n = 346). This study also enrolled another consecutive cohort of 268 CHB patients with cirrhosis who received entecavir (n = 52) or TDF (n = 216) treatment between 2011 and 2018 from Chia-Yi Christian Hospital. In Taiwan, the costs of entecavir and TDF have been reimbursed for HBV treatment by Taiwan’s National Health Plan since 2008 and 2011, respectively.
Inclusion criteria
(1) All patients were more than 18 years of age and HBsAg had been positive for more than 6 months prior to NA treatment. (2) All patients had received entecavir or TDF monotherapy for at least 12 months before enrollment. (3) All patients fulfilled the diagnosis of cirrhosis according to either histology (n = 210) or repeated ultrasounds suggestive of cirrhosis and clinical features, such as splenomegaly, gastroesophageal varices, ascites or thrombocytopenia.
Exclusion criteria
(1) Patients had evidence of alcoholic liver disease, autoimmune hepatitis, or coinfection with hepatitis C virus, hepatitis D virus or human immunodeficiency virus. (2) Patients had HCC or liver transplantation at baseline or within the first year of NA treatment.
The study was conducted in accordance with the 1975 Declaration of Helsinki. All patients had signed informed consent before enrollment, and the study was approved by the Research Ethics Committees of Chang Gung Memorial Hospital (202000445B0), China Medical University Hospital (CMUH102-REC1-113), and Chia-Yi Christian Hospital (CYCH-IRB No: 101055).
Methods
During NA therapy, all patients were followed up every 1 to 3 months. Serum alanine aminotransferase (ALT) and HBV DNA levels were measured at baseline, every 3 to 6 months during treatment, and in the event of biochemical breakthrough. All patients were followed until discontinuation of entecavir or TDF therapy or the last visit. HCC surveillance was implemented by abdominal ultrasonography and serum alpha-fetoprotein (AFP) every 3 months. HCC was diagnosed according to the guidelines of the American Association for the Study of Liver Diseases (AASLD) [21].
Definitions
The APRI index was computed by using the aspartate aminotransferase (AST)-to-platelet ratio [22]. The FIB-4 index was calculated by using the following equation: age [years] × AST [U/L]/((platelet [109/L]) × (ALT [U/L])1/2) [23]. Biochemical response (BR) and virological response (VR) were defined as an ALT level of < 40 U/L and an HBV DNA level of < 20 IU/mL during NA therapy, respectively.
Diabetes mellitus (DM) was diagnosed according to the American Association of Clinical Endocrinologists and American College of Endocrinology guidelines [24]. Patients were also considered diabetic according to their medical history or if they had received insulin treatment or oral hypoglycemic agents. Hypertension was considered diagnosed according a previous diagnosis according to the medical history or having received anti-hypertensive drugs. Cirrhotic events were defined as new developments of ascites, variceal bleeding, or hepatic encephalopathy in patients without hepatic decompensation at the initiation of NA treatment.
Serology
Serum hepatitis B virus (HBV) DNA was quantified using the COBAS AmpliPrep-COBAS TaqMan HBV test with a lower detection limit of 20 IU/mL.
Statistical analysis
Continuous variables are presented as the median ± interquartile range and were compared between groups using the Mann-Whitney U test. Categorical variables were analyzed with the chi-squared test as appropriate. Kaplan-Meier analysis with the log-rank test was used to compare the cumulative incidences of HCC, cirrhotic events, and mortality among groups. Factors associated with HCC, cirrhotic events, or mortality were identified by Cox proportional hazards regression analyses.
Variables with a p value of < 0.25 in the univariate analysis were subjected to stepwise multivariate analysis. Cox proportional hazards regression models with the forward method were used to determine independent factors, and variables with p values < 0.05 were retained in the models. Missing data were assumed to be missing at random and were replaced with substituted values by multiple imputation [25]. All statistical tests were two-sided, and a p value of < 0.05 was considered statistically significant.
We used the propensity score (PS)-matching method to reduce the differences in clinical parameters between the entecavir and TDF groups by creating a ratio of 1:1. The variables included age, sex, DM, hypertension, decompensation, NA experience, baseline AST, ALT, total bilirubin, INR, albumin, platelet, estimated glomerular filtration rate (eGFR), FIB-4, APRI, HBV DNA, HBeAg status, and AFP in the entecavir and TDF groups. Pairs (ETV and TDF groups) on the propensity score logit were matched to within a range of 0.2 SD [26,27]. For the Inverse Probability of Treatment Weighting (IPTW) method, we used the average treatment effect for treatment weighting by assigning a weight of 1 to the TDF-treated subjects and PS/(1-PS) of the entecavir-treated subjects [28].
Results
Characteristics of all patients in the entecavir and TDF groups at baseline
In the entire cohort (n = 1560), the median treatment duration was 260 weeks (range: 52-732 weeks). The median treatment duration in the entecavir and TDF groups were 312 weeks (range: 52-732 weeks) and 209 weeks (range: 52-422 weeks), respectively. Table 1 compares the characteristics of all patients treated with entecavir (n = 993) or TDF (n = 567). Patients in the entecavir group had higher percentages of hepatic decompensation, NA naïve status, DM, and hypertension. They also had lower albumin levels, platelet count, and eGFR, as well as higher values of INR, Child-Pugh score, and FIB-4 than those in the TDF group.
Table 1.
Baseline characteristics of patients treated with entecavir or TDF
| Variables | Entecavir n = 993 | TDF n = 567 | p value |
|---|---|---|---|
| Age (year) | 55.4 ± 11.7 | 54.5 ± 12.9 | 0.190 |
| Sex, male | 721 (72.6%) | 428 (75.5%) | 0.215 |
| HBeAg-positive status | 209 (21.0%) | 130 (22.9%) | 0.377 |
| Decompensation status | 182 (18.3%) | 58 (10.2%) | < 0.001 |
| NA-naïve | 875 (88.1%) | 478 (84.3%) | 0.033 |
| Diabetes mellitus, yes | 232 (23.4%) | 89 (15.7%) | < 0.001 |
| Hypertension, yes | 280 (28.2%) | 131 (23.1%) | 0.028 |
| HBV DNA, log10 IU/mL | 5.42 ± 1.49 | 5.37 ± 1.44 | 0.534 |
| AST, U/L | 117.4 ± 214.3 | 113.1 ± 262.9 | 0.727 |
| ALT, U/L | 139.1 ± 268.5 | 151.7 ± 386.1 | 0.448 |
| Total bilirubin, mg/dL | 2.04 ± 3.84 | 1.57 ± 2.88 | 0.011 |
| Albumin, g/dL | 3.91 ± 0.65 | 4.07 ± 0.57 | < 0.001 |
| INR | 1.20 ± 0.28 | 1.17 ± 0.24 | 0.018 |
| eGFR, mL/min/1.73 m2 | 85.4 ± 29.9 | 98.0 ± 52.2 | < 0.001 |
| Platelet, ×103/μL | 131.1 ± 55.7 | 150.4 ± 57.1 | < 0.001 |
| AFP, ng/mL | 32.0 ± 118.4 | 31.6 ± 113.8 | 0.952 |
| Child-Pugh score | 5.89 ± 1.60 | 5.49 ± 1.19 | < 0.001 |
| FIB-4 | 5.13 ± 5.43 | 3.69 ± 3.84 | < 0.001 |
| APRI | 3.17 ± 6.42 | 2.59 ± 6.55 | 0.090 |
AFP, alpha-fetoprotein; APRI, AST to platelet ratio index; AST, aspartate aminotransferase; ALT, alanine aminotransferase; eGFR, estimated glomerular filtration rate; FIB-4, fibrosis index based on four factors; HBV, hepatitis B virus; HBeAg, hepatitis B e antigen; INR, international normalized ratio; NA, nucleos(t)ide analogue; TDF, Tenofovir disoproxil fumarate.
Incidence and predictors of HCC for all patients
In the entire cohort, 244 subjects developed HCC during 7704.25 person-years of follow-up. The incidence of HCC was 3.2 (95% confidence interval (CI): 3.1-4.1) per 100 person-years. The cumulative incidences of HCC at 3, 5, and 10 years were 9.5%, 15.2%, and 25.4%, respectively. Of the 993 patients in the entecavir group, 196 developed HCC during 5448.96 person-years of follow-up. The incidence of HCC was 3.60 (95% CI: 3.1-4.2) per 100 person-years. The cumulative incidences at 3, 5, and 8 years were 10.5%, 17.3%, and 25.3%, respectively.
Among the 567 patients in the TDF group, 48 developed HCC during 2255.29 person-years of follow-up. The incidence of HCC was 2.2 (95% CI: 1.6-2.8) per 100 person-years. The cumulative incidences at 3, 5, and 8 years were 7.6%, 10.9%, and 12.8%, respectively. The entecavir group had a higher annual incidence rate of HCC (P < 0.001) and a higher cumulative incidence of HCC than the TDF group (P = 0.001) (Figure 1). A Cox regression analysis revealed that entecavir treatment, old age, male sex, hepatic decompensation, DM, lower albumin levels, and platelet count were independent risk factors of HCC for all patients (Table 2).
Figure 1.
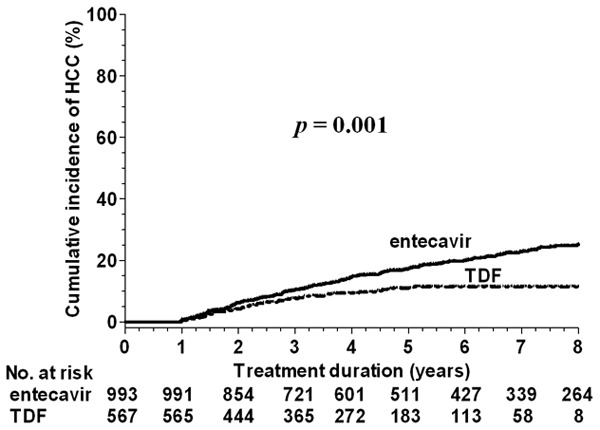
Comparison of HCC incidence between entecavir and TDF for all patients. Abbreviations: HCC, hepatocellular carcinoma; TDF, tenofovir disoproxil fumarate.
Table 2.
Univariate and multivariate analyses of factors associated with hepatocellular carcinoma in all patients
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
|
|
|
|||
| Hazard ratio (95% CI) | p value | Hazard ratio (95% CI) | p value | |
| Age (year) | 1.036 (1.025-1.046) | 0.006 | 1.038 (1.027-1.050) | < 0.001 |
| Sex, male vs. female | 1.254 (0.925-1.700) | 0.146 | 1.590 (1.166-2.167) | 0.003 |
| HBeAg, yes vs. no | 1.045 (0.775-1.408) | 0.773 | ||
| Decompensation, yes vs. no | 2.287 (1.709-3.059) | < 0.001 | 1.605 (1.094-2.34) | 0.015 |
| NA-naïve, yes vs. no | 0.928 (0.656-1.312) | 0.672 | ||
| TDF vs. entecavir | 0.585 (0.425-0.806) | 0.001 | 0.672 (0.485-0.930) | 0.017 |
| Diabetes mellitus, yes vs. no | 1.589 (1.201-2.104) | 0.001 | 1.340 (1.010-1.777) | 0.042 |
| Hypertension, yes vs. no | 1.634 (1.259-2.122) | < 0.001 | ||
| HBV DNA, per log10 IU/mL | 1.021 (0.939-1.110) | 0.629 | ||
| AST, per U/L | 1.000 (0.999-1.000) | 0.647 | ||
| ALT, per U/L | 1.000 (0.999-1.000) | 0.256 | ||
| Total bilirubin, per mg/dL | 1.005 (0.971-1.040) | 0.786 | ||
| Albumin, per g/L | 0.553 (0.459-0.666) | < 0.001 | 0.755 (0.592-0.962) | 0.023 |
| INR, per ratio | 1.432 (0.969-2.116) | 0.071 | ||
| eGFR, mL/min/1.73 m2 | 0.991 (0.987-0.996) | < 0.001 | ||
| Platelet, per 103/μL | 0.993 (0.991-0.996) | < 0.001 | 0.996 (0.994-0.999) | 0.008 |
| AFP at baseline, per ng/mL | 1.000 (0.999-1.001) | 0.994 | ||
| Child-Pugh score | 1.164 (1.087-1.248) | < 0.001 | ||
| FIB-4 | 1.051 (1.035-1.068) | < 0.001 | ||
| APRI | 1.006 (0.989-1.023) | 0.514 | ||
AFP, alpha-fetoprotein; APRI, AST to platelet ratio index; AST, aspartate aminotransferase; ALT, alanine aminotransferase; CI, confidence interval; eGFR, estimated glomerular filtration rate; FIB-4, fibrosis index based on four factors; HBV, hepatitis B virus; HBeAg, hepatitis B e antigen; INR, international normalized ratio; NA, nucleos(t)ide analogue; TDF, Tenofovir disoproxil fumarate.
We also analyzed the roles of on-treatment factors, including VR, BR, FIB-4, and AFP at 12 months of treatment in cases of HCC development in 1404 patients who had all data available at month 12. BR, FIB-4, and AFP at 12 months of treatment could predict HCC development. In patients with available data for serum ALT, HBV DNA, FIB-4, and AFP measurements at month 12, TDF-treated patients had a lower risk of HCC than entecavir-treated patients after adjusting for other factors at month 12 (Table S1).
In the 1553 patients with available ALT levels at month 12, the entecavir group had a higher rate of BR than the TDF group (780/990 (78.8%) vs. 397/562 (70.5%), P < 0.001). In the 1432 patients with available HBV DNA levels at month 12, there was no significant difference in VR between the entecavir and TDF groups (820/930 (88.2%) vs. 430/502 (85.7%), P = 0.173). In the entecavir group, the annual incidence rates of HCC were 4.9% and 2.65% within the first 4 years and 5-8 years of therapy, respectively. In the TDF group, the annual incidence rates of HCC were 3.1% and 0.93% within the first 4 years and 5-8 years of therapy, respectively. There was no significant difference in the HCC incidence within the first 4 years and 5-8 years in the entecavir group (P = 0.66). However, a significant difference was noted in the HCC incidence within the first 4 years and 5-8 years in the TDF group (P = 0.022).
Subgroup analyses of HCC incidence in the entecavir versus TDF group
Among the 1353 NA-naïve patients, a Cox regression analysis revealed that entecavir treatment, old age, male sex, hepatic decompensation, DM, and lower platelet count were independent predictors of HCC (Table S2). Among the 1320 patients with compensated cirrhosis at baseline, entecavir treatment, old age, male sex, and lower albumin levels and eGFR values were independent predictors of HCC (Table S3). Among the 1153 patients without hepatic decompensation and prior NA experience, a Cox regression analysis identified entecavir treatment, old age, male sex, DM, and lower albumin levels as independent predictors of HCC (Table S4).
TDF has been reimbursed by Taiwan’s National Health Plan since 2011. Thus, we conducted a subgroup analysis of the patients treated with entecavir or TDF between 2011 and 2018. Totals of 595 and 567 patients received entecavir and TDF treatment for median durations of 231 and 209 weeks, respectively. A Cox regression analysis showed that old age, male sex, DM, and lower albumin levels were independent predictors of HCC (Table S5). Entecavir treatment was not a significant factor for predicting HCC development in this subgroup.
Comparison of HCC incidences in the entecavir and TDF groups in the entire cohort and different subgroups using PS-matching and IPTW methods
Table S6 shows the baseline characteristics of all patients according to PS matching and IPTW methods. The PS-matching method yielded 545 and 545 patients in the entecavir and TDF groups, respectively. There were no significant differences in clinical characteristics between the two groups according to either method (Table S6). Univariate analysis showed that there was no significant difference in HCC development between entecavir and TDF therapy (Figure 2A and 2B). However, multivariate Cox regression analyses showed that old age, hepatic decompensation, DM, lower platelet count, and entecavir treatment were independent factors for HCC development after adjustment with PS matching or IPTW (Table 3). TDF-treated patients had a lower risk of HCC than entecavir-treated patients (hazard ratios [HRs] of 0.660 and 0.729 according to the PS-matching and IPTW methods, respectively). Male sex and lower albumin levels were significant predictors according to the IPTW analysis.
Figure 2.
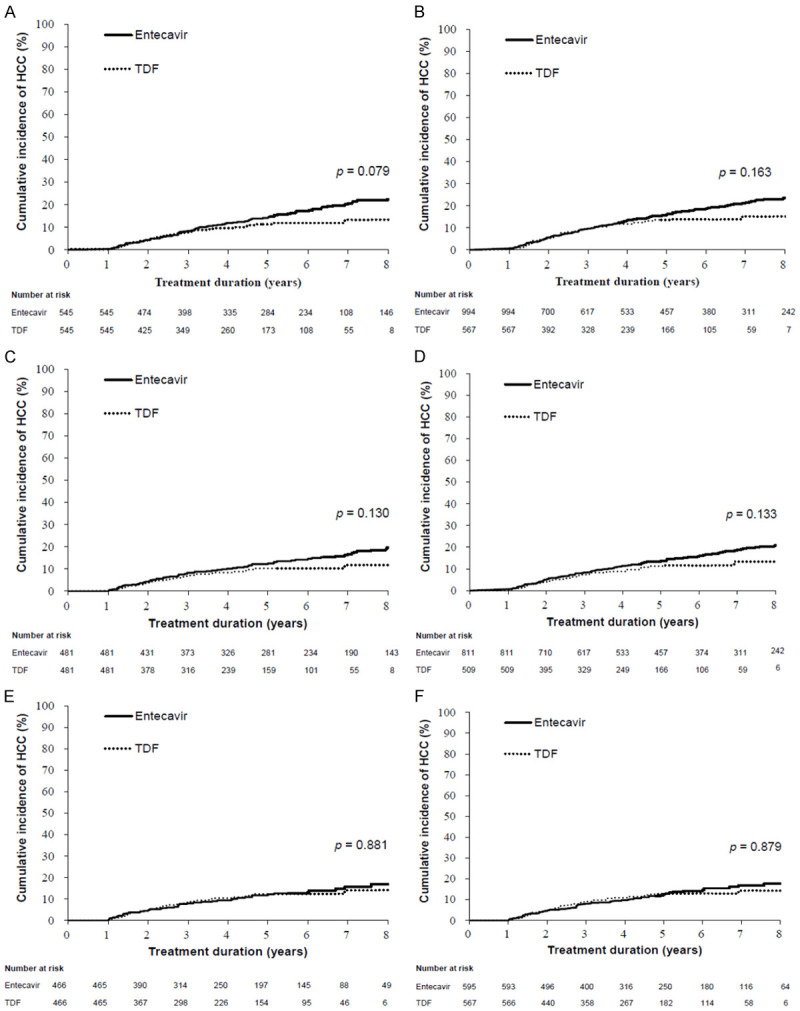
Comparison of HCC incidence between entecavir and TDF according to the PS-matching or IPTW method. All patients (A) and (B), compensated cirrhotic patients (C) and (D), and patients enrolled after 2011 (E) and (F). Abbreviations: IPTW, inverse probability of treatment weighting; HCC, hepatocellular carcinoma; TDF, tenofovir disoproxil fumarate; PS, propensity score.
Table 3.
Summary of multivariate analyses of factors associated with hepatocellular carcinoma using propensity score matching or inverse probability of treatment weighting in patients who received TDF versus entecavir treatment in all patients and different subgroups
| Variables | Propensity Score Matching | Inverse Probability of Treatment Weighting | ||
|---|---|---|---|---|
|
|
|
|||
| Hazard ratio (95% CI) | p value | Hazard ratio (95% CI) | p value | |
| All patients | ||||
| Age (year) | 1.040 (1.025-1.055) | < 0.001 | 1.038 (1.027-1.049) | < 0.001 |
| Sex, male vs. female | 1.479 (0.965-2.266) | 0.073 | 1.715 (1.250-2.354) | < 0.001 |
| Decompensation, yes vs. no | 1.983 (1.223-3.214) | 0.006 | 1.585 (1.082-2.320) | 0.018 |
| Diabetes mellitus, yes vs. no | 1.630 (1.089-2.438) | 0.018 | 1.416 (1.068-1.877) | 0.016 |
| Albumin, per g/L | NS | NS | 0.710 (0.556-0.907) | 0.006 |
| Platelet, per 103/μL | 0.996 (0.992-0.999) | 0.018 | 0.997 (0.994-1.000) | 0.020 |
| TDF vs. entecavir | 0.660 (0.461-0.945) | 0.023 | 0.729 (0.541-0.983) | 0.038 |
| Patients with compensated cirrhosis | ||||
| Age (year) | 1.027 (1.011-1.044) | < 0.001 | 1.030 (1.017-1.043) | < 0.001 |
| Sex, male vs. female | 1.628 (1.013-2.617) | 0.044 | 1.450 (1.010-2.082) | 0.044 |
| Diabetes mellitus, yes vs. no | 1.573 (1.013-2.442) | 0.044 | 1.362 (0.975-1.903) | 0.070 |
| Albumin, per g/L | 0.602 (0.410-0.884) | 0.009 | 0.660 (0.494-0.883) | 0.005 |
| eGFR, mL/min/1.73 m2 | 0.992 (0.985-1.000) | 0.040 | 0.994 (0.989-0.999) | 0.028 |
| TDF vs. entecavir | NS | 0.720 (0.507-1.022) | 0.066 | |
| Patients enrolled after 2011 | ||||
| Age (year) | 1.032 (1.014-1.050) | < 0.001 | 1.030 (1.013-1.048) | < 0.001 |
| Sex, male vs. female | 1.698 (1.017-2.836) | 0.043 | 1.736 (1.055-2.856) | 0.030 |
| Diabetes mellitus, yes vs. no | 1.583 (0.998-2.512) | 0.051 | 1.737 (1.124-2.686) | 0.013 |
| Albumin, per g/L | 0.588 (0.377-0.918) | 0.020 | 0.600 (0.411-0.877) | 0.008 |
| eGFR, mL/min/1.73 m2 | 0.992 (0.985-1.000) | 0.045 | 0.991 (0.983-0.998) | 0.011 |
| TDF vs. entecavir | NS | NS | ||
CI, confidence interval; eGFR, estimated glomerular filtration rate; NS, no significant difference in univariate analysis; TDF, Tenofovir disoproxil fumarate.
Table S7 shows the baseline characteristics of patients with compensated cirrhosis at baseline (n = 1320) according to the PS-matching and IPTW methods. The PS-matching method yielded 481 and 481 patients in the entecavir and TDF groups, respectively. There were no significant differences in clinical characteristics between the two groups according to either method (Table S7). Multivariate Cox regression analyses revealed that old age, male sex, and lower albumin and eGFR levels were independent factors for HCC development after adjustment with PS matching or IPTW (Table 3). DM was a significant predictor according to the PS matching analysis. There were no significant differences in HCC development between patients treated with entecavir and TDF according to the PS-matching or IPTW method (Figure 2C and 2D).
Table S8 shows the baseline characteristics of patients who were enrolled after 2011 (n = 1162) according to PS-matching and IPTW methods. The PS-matching method yielded 466 and 466 patients in the entecavir and TDF groups, respectively. There were no significant differences in clinical characteristics between the two groups according to either method (Table S8). Multivariate Cox regression analyses revealed that old age, male sex, and lower albumin and eGFR levels were independent factors for HCC development after adjustment with PS matching or IPTW (Table 3). DM was a significant predictor according to the IPTW analysis. There were no significant differences in HCC development between patients treated with entecavir and TDF according to the PS-matching or IPTW method (Figure 2E and 2F).
Incidences and predictors of cirrhotic events
Among the 1320 patients with compensated cirrhosis at baseline, 66 experienced cirrhotic events during treatment, of which 38, 30, and 7 developed ascites, variceal bleeding, and hepatic encephalopathy, respectively. The cumulative incidences of cirrhotic events at 3, 5, and 10 years were 3.2%, 6.3%, and 7.8%, respectively. A multivariate Cox regression analysis revealed that lower albumin levels and platelet count were independent predictors for cirrhotic events (Table 4). There were no significant differences in cirrhotic events between patients in the entecavir and TDF groups (P = 0.115).
Table 4.
Univariate and multivariate analyses of factors associated with hepatic evens (new ascites, varices bleeding and hepatic encephalopathy) in patients with compensated cirrhosis at baseline
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
|
|
|
|||
| Hazard ratio (95% CI) | p value | Hazard ratio (95% CI) | p value | |
| Age (year) | 1.012 (0.992-1.033) | 0.236 | ||
| Sex, male vs. female | 1.193 (0.671-2.122) | 0.548 | ||
| HBeAg, yes vs. no | 0.861 (0.470-1.580) | 0.629 | ||
| NA-naïve, yes vs. no | 1.895 (0.761-4.718) | 0.169 | ||
| TDF vs. entecavir | 0.627 (0.350-1.121) | 0.115 | ||
| Diabetes mellitus, yes vs. no | 1.509 (0.878-2.594) | 0.137 | ||
| Hypertension, yes vs. no | 1.041 (0.611-1.775) | 0.881 | ||
| HBV DNA, per log10 IU/mL | 0.893 (0.757-1.054) | 0.180 | ||
| AST, per U/L | 1.000 (0.998-1.002) | 0.706 | ||
| ALT, per U/L | 0.998 (0.995-1.001) | 0.141 | ||
| Total bilirubin, per mg/dL | 1.057 (0.949-1.176) | 0.315 | ||
| Albumin, per g/L | 0.265 (0.176-0.400) | < 0.001 | 0.335 (0.217-0.517) | < 0.001 |
| INR, per ratio | 1.999 (1.040-3.843) | 0.038 | ||
| eGFR, mL/min/1.73 m2 | 0.995 (0.986-1.003) | 0.244 | ||
| Platelet, per 103/μL | 0.983 (0.978-0.989) | < 0.001 | 0.986 (0.980-0.991) | < 0.001 |
| AFP at baseline, per ng/mL | 1.001 (0.997-1.004) | 0.674 | ||
| Child-Pugh score | 1.891 (1.550-2.308) | < 0.001 | ||
| FIB-4 | 1.086 (1.053-1.120) | < 0.001 | ||
| APRI | 1.023 (0.972-1.076) | 0.389 | ||
AFP, alpha-fetoprotein; APRI, AST to platelet ratio index; AST, aspartate aminotransferase; ALT, alanine aminotransferase; CI, confidence interval; eGFR, estimated glomerular filtration rate; FIB-4, fibrosis index based on four factors; HBV, hepatitis B virus; HBeAg, hepatitis B e antigen; INR, international normalized ratio; NA, nucleos(t)ide analogue; TDF, Tenofovir disoproxil fumarate.
Incidences and predictors of all-cause and liver-related mortalities
In the entire cohort, 131 subjects developed all-cause mortality during treatment, including 20 patients who underwent liver transplantation. The cumulative incidences of all-cause mortality at 3, 5, and 10 years were 2.4%, 6.3%, and 18.2%, respectively. A multivariate Cox regression analysis revealed that old age, hepatic decompensation, and lower baseline levels of ALT, albumin, and platelet count were independent predictors for all-cause mortality (Table 5).
Table 5.
Univariate and multivariate analyses of factors associated with all-cause mortality or liver transplantation in all patients
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
|
|
|
|||
| Hazard ratio (95% CI) | p value | Hazard ratio (95% CI) | p value | |
| Age (year) | 1.033 (1.019-1.048) | < 0.001 | 1.027 (1.012-1.043) | < 0.001 |
| Sex, male vs. female | 1.121 (0.746-1.687) | 0.582 | ||
| HBeAg, yes vs. no | 0.737 (0.469-1.156) | 0.184 | ||
| Decompensation, yes vs. no | 3.538 (2.458-5.093) | < 0.001 | 2.170 (1.310-3.597) | 0.003 |
| NA-naïve, yes vs. no | 1.339 (0.782-2.294) | 0.288 | ||
| TDF vs. entecavir | 0.822 (0.527-1.280) | 0.385 | ||
| Diabetes mellitus, yes vs. no | 1.688 (1.163-2.450) | 0.006 | ||
| Hypertension, yes vs. no | 1.171 (0.807-1.700) | 0.407 | ||
| HBV DNA, per log10 IU/mL | 0.937 (0.836-1.050) | 0.265 | ||
| AST, per U/L | 0.999 (0.997-1.000) | 0.085 | ||
| ALT, per U/L | 0.997 (0.996-0.999) | 0.008 | 0.997 (0.995-0.999) | 0.001 |
| Total bilirubin, per mg/dL | 1.017 (0.977-1.060) | 0.408 | ||
| Albumin, per g/L | 0.400 (0.313-0.511) | < 0.001 | 0.600 (0.432-0.823) | 0.002 |
| INR, per ratio | 2.068 (1.332-3.213) | 0.001 | ||
| eGFR, mL/min/1.73 m2 | 0.995 (0.989-1.001) | 0.117 | ||
| Platelet, per 103/μL | 0.989 (0.986-0.993) | < 0.001 | 0.994 (0.990-0.997) | 0.001 |
| AFP at baseline, per ng/mL | 1.000 (0.999-1.002) | 0.559 | ||
| Child-Pugh score | 1.290 (1.191-1.397) | < 0.001 | ||
| FIB-4 | 1.062 (1.041-1.083) | < 0.001 | ||
| APRI | 0.998 (0.970-1.026) | 0.865 | ||
AFP, alpha-fetoprotein; APRI, AST to platelet ratio index; AST, aspartate aminotransferase; ALT, alanine aminotransferase; CI, confidence interval; eGFR, estimated glomerular filtration rate; FIB-4, fibrosis index based on four factors; HBV, hepatitis B virus; HBeAg, hepatitis B e antigen; INR, international normalized ratio; NA, nucleos(t)ide analogue; TDF, Tenofovir disoproxil fumarate.
Among the 131 deaths, 98 were liver related. The cumulative incidences of liver-related mortality at 3, 5, and 10 years were 1.9%, 5.3%, and 12.4%, respectively. A Cox regression analysis showed that hepatic decompensation (HR: 2.408, 95% CI: 1.352-4.290, P = 0.003), lower baseline ALT (HR: 0.996, 95% CI: 0.993-0.998, P = 0.002), lower baseline albumin levels (HR: 0.567, 95% CI: 0.386-0.832, P = 0.004), and lower baseline platelet count (HR: 0.991, 95% CI: 0.986-0.995, P < 0.001) were independent predictors for liver-related mortality. There were no significant differences in all-cause (P = 0.384) or liver-related mortality (P = 0.107) between the entecavir and TDF groups.
Discussion
This multicenter, retrospective cohort study examined 1560 predominantly treatment-naïve cirrhotic patients and demonstrated that TDF treatment was associated with a lower risk of HCC than entecavir treatment (HR 0.672, 95% CI: 0.485-0.930). A lower risk of HCC was similarly observed in patients treated with TDF in the PS-matched cohort (HR 0.660, 95% CI: 0.461-0.945) and the IPTW-adjusted cohort (HR 0.729, 95% CI: 0.541-0.983). The multivariate analyses of patient subgroups revealed that TDF treatment was significantly associated with a lower risk of HCC in the treatment-naïve cohort, compensated cirrhotic cohort, and the treatment-naïve compensated cirrhotic cohort, but not in the cohort enrolled after 2011. Moreover, TDF treatment was not associated with a lower risk of HCC in patients with compensated cirrhosis or patients enrolled after 2011 after adjustment with PS matching or IPTW. TDF treatment was not associated with a lower risk of decompensation events in patients with compensated cirrhosis or a lower risk of all-cause or liver-related mortality or transplantation in the entire cohort.
In the present cohort, the risk of HCC was 3.2 (TDF vs. entecavir: 2.2 vs. 3.6) per 100 person-years, and the cumulative incidences of HCC at 3, 5, and 10 years were 9.5%, 15.2%, and 25.4%, respectively (3, 5, and 8 years: TDF vs. entecavir: 7.6%, 10.9%, and 12.8% vs. 10.5%, 17.3%, and 25.3%). A previous multicenter retrospective study examined cirrhotic patients (90% Child A) receiving entecavir therapy for a median duration of 4 years from Taiwan. The study reported an average annual HCC risk of 2.2% with a cumulative incidence of 11.3% at 5 years [7]. Choi et al. reported annual risks of 2.08 vs. 2.76 and 2.12 vs. 3.62 per 100 person-years for TDF vs. entecavir in a PS-matched Korean nationwide cohort and hospital cohort of cirrhotic patients, respectively [15]. Our study cohort comprised 87% treatment-naïve patients and 84.8% compensated cirrhotic patients who received entecavir or TDF therapy for median durations of 312 and 209 weeks, respectively. Therefore, our observation of the annual HCC risk in a similar population of patients was consistent with the two previous studies [7,15].
Notably, three Korean studies with groups comprising 31.4%, 34.7%, and 40% cirrhotic patients did not find significant differences in HCC incidence between entecavir and TDF in either noncirrhotic or cirrhotic patients [17-19]. These studies excluded patients with decompensated cirrhosis [17,18] or simultaneously enrolled patients treated with entecavir or TDF during the defined time interval after 2011 (2012 to 2014; 2011 to 2014 for entecavir vs. 2013 to 2015 for TDF) [17,19]. Yip et al. observed an even greater effect of HCC prevention for TDF in comparison with entecavir (HR 0.39, P = 0.0016) in a large-scale population study from Hong Kong, which included only 13.2% cirrhotic patients [16]. However, the small number of cirrhotic patients precluded further comparison of the HCC risk between entecavir and TDF treatment among the cirrhotic subgroup. They speculated that the low percentage of cirrhotic patients might have accounted for the observed lower HR of HCC risk for TDF in their study. This was in consideration of the hypothesis that NA may not reduce the risk of HCC in cirrhotic patients as efficiently as in noncirrhotic patients. However, an alternative interpretation appears to arise from our observation that TDF treatment was associated with a lower risk of HCC in the entire cohort despite after adjustment with PS matching or IPTW, but was not associated with a lower risk of HCC in patients with compensated cirrhosis or patients enrolled after 2011 after adjustment with PS matching or IPTW. We posit that the apparent association of TDF treatment with a lower HCC risk might have resulted from the inclusion of patients with decompensated cirrhosis [15,16], particularly patients who had received entecavir treatment before 2011, which were the two categories of patients enrolled in previous studies that revealed a lower risk of HCC for TDF [15,16]. After excluding these patients from analysis, a significant association between TDF treatment and a lower risk of HCC could no longer be demonstrated.
Another interesting observation of this study was that the annual incidence rates of HCC during the first 4 years and during year 5 to 8 were not significantly different in patients treated with entecavir, but they were significantly different in those treated with TDF. It took less time for TDF to exert its full effect of preventing HCC occurrence than entecavir. Hepatocarcinogenesis is a multi-step process that may involve the stepwise disruption of key growth regulatory pathways leading to the development of cancer [29]. A point of no return may exist along the path of cancer development where therapeutic intervention may no longer be able to revert the carcinogenic process. NAs act by inhibiting HBV replication, thereby resolving hepatic necroinflammation, ameliorating angiogenesis, and regressing hepatic fibrosis, thus delaying or preventing the occurrence of HCC [30]. The entecavir cohort preferentially enrolled patients with more advanced cirrhosis, particularly those enrolled before 2011 (Tables S9 and S10). This group may represent patients who are less amenable to HCC prevention by NA therapy.
There are several possible explanations for why TDF treatment was associated with a lower risk of HCC in cirrhotic patients than entecavir treatment. First, the baseline risk of HCC might not be comparable between the two treatment cohorts. Because the cost of entecavir started being reimbursed three years earlier than TDF in Taiwan, patients with more advanced cirrhosis might have been channeled toward entecavir treatment (Group 1 vs. Group 2 and Group 1 vs. Group 3 in Tables S9 and S10). Moreover, patients with co-morbidities such as DM, hypertension, and chronic kidney disease might have been prioritized for entecavir treatment due to safety concerns related to kidney and bone issues, even though both drugs were available for prescription (Group 2 vs. Group 3 in Tables S9 and S10).
Together, these two factors might have imbalanced the severity of liver disease and the confounding risk of HCC among the two treatment cohorts. Indeed, patients in the entecavir group had higher proportions of decompensated cirrhosis, DM, and hypertension, as well as lower albumin levels and platelet count and higher values of INR and FIB-4 than the TDF group. All of these factors are indicative of more advanced liver disease and higher confounding risk of HCC.
To overcome these potential biases, we comprehensively adopted PS matching and IPTW methods to minimize the confounding effects of possible parameters (n = 19) on the observed HCC risk. Despite these statistical approaches, a significantly different effect on the reduction of HCC in favor of TDF treatment was still observed in the entire cohort. However, the association of TDF treatment with a lower HCC risk was no longer observed after we excluded patients with decompensated cirrhosis and, most remarkably, those who started entecavir treatment before 2011. This suggests the possible existence of residual bias due to unmatched or unmeasured confounding in patients enrolled before 2011.
The second possible explanation is that the entecavir group had a significantly higher rate of BR than the TDF group, and both groups had similar rates of VR at 12 months of treatment. This suggested that neither factor could play a role in the differential effect of TDF versus entecavir on HCC prevention. Third, early animal studies indicated that entecavir has carcinogenic potential and induces tumors in rats and mice at doses higher than those used in humans [31]. However, a large-scale prospective, randomized study with NA treatment for up to 10 years did not reveal significantly different incidences of HCC or non-HCC malignant tumors among patients treated with entecavir or the comparator NA [32]. Thus, there is no convincing evidence in support of this possibility.
Fourth, Murata et al. demonstrated that nucleotide analogues (adefovir and TDF) but not nucleoside analogues (lamivudine and entecavir) induced the expression of interferon λ3 in patients with CHB during treatment [33]. Interferon λ3 shows potent antitumor activity in murine models of cancer, including HCC [34]. Perhaps, TDF acts in a similar manner to exert some additional antitumor effect against HCC that we observed in this study. Further study is warranted to test this hypothesis.
Entecavir and TDF showed similar efficacy in preventing cirrhotic events, all-cause mortality, and liver-related mortality. Predictors of cirrhotic events in compensated patients or predictors of mortality in the entire cohort are more reflective of the remaining hepatic reserve (albumin level and hepatic decompensation) and the degree of portal hypertension (platelet count) at the time of treatment initiation. Entecavir and TDF exhibit comparable antiviral potency and therapeutic efficacy during treatment, so it is expected that both drugs would recover liver function and improve these clinical outcomes similarly. In this regard, our findings are consistent with those of previous studies [15,17-19].
A strength of this study is that we enrolled a large cohort of cirrhotic patients with detailed baseline characteristics, adequate follow-up periods (median: 5 years), and a significant number of incident events. This enabled us to investigate the incidence rates and predictors of HCC, cirrhotic events, and mortality during entecavir or TDF treatment. Most importantly, it also allowed us to compare the effectiveness for preventing such outcomes between entecavir and TDF in different patient subgroups, such as patients with compensated cirrhosis and patients enrolled over the same period.
Nevertheless, there are several limitations to note. First, this was a retrospective analysis of two treatment cohorts enrolled from two tertiary medical centers and one regional hospital, which may have introduced selection bias. Nonetheless, we used PS matching and IPTW methods to meticulously adjust the potential biases of the confounding factors to match these two cohorts. Moreover, we conducted subgroup analyses to unveil the residual confounding effect imposed by the imbalanced severity of liver disease among the two treatment cohorts. A large-scale prospective, randomized comparative trial will be the ideal approach to solve this issue.
A second limitation is that cirrhosis was diagnosed based on histology (n = 210) or ultrasonographic findings plus clinical features of portal hypertension. This was done because liver biopsy was not a routine clinical practice, and noninvasive diagnostic modalities such as FibroScan were not available during a large part of the study period. It is possible that patients with early cirrhosis might have been underdiagnosed and excluded from enrollment. The proportion of patients with at least a moderate degree of cirrhosis might have been overrepresented, and the overall annual incidence of HCC might have been overestimated in the present cohort (3.2 per 100 person-years). Moreover, this diagnostic uncertainty of the severity of liver cirrhosis might have affected the main aim of this study, which was to compare the effectiveness for preventing clinical outcomes among entecavir and TDF.
Third, despite the inclusion of a list of key baseline clinical characteristics for analysis, we lacked information on body mass index, alcohol use, smoking habit, HBV genotype, and quantitative hepatitis B surface antigen (HBsAg), which are also regarded as potential confounding factors. The inclusion of DM and hypertension as baseline parameters might have led to the partial representation of this population. Genotype and HBsAg are not routine tests in clinical practice in Taiwan. Genotypes B and C are the two major genotypes of HBV that cause CHB in Taiwan. We previously demonstrated that these genotypes accounted for 56% and 44% of infections in patients with cirrhosis, respectively. We also showed that neither genotype nor HBsAg is an independent predictor of HCC, cirrhotic events, or liver-related mortality in this population [35].
In conclusion, we have demonstrated that TDF treatment was associated with a lower risk of HCC than entecavir treatment in this retrospective, predominantly treatment-naïve cohort of cirrhotic patients. However, both treatments exhibited a similar risk of HCC in patients with compensated cirrhosis and patients who were enrolled after 2011 after adjustment with PS matching or IPTW. Moreover, TDF was not as-sociated with a lower risk of cirrhotic events, all-cause or liver-related mortality, or transplantation in the entire cohort.
Acknowledgements
The authors would like to thank Ms. Chia-Hsin Lin for statistical analysis. This study was supported by grants CMRPG8D1181 and CMRPG891481 from Chang Gung Memorial Hospital, Taiwan, and DMR-101-011 from China Medical University Hospital, Taichung, Taiwan.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Hutin Y, Nasrullah M, Easterbrook P, Nguimfack BD, Burrone E, Averhoff F, Bulterys M. Access to treatment for hepatitis B virus infection-worldwide, 2016. MMWR Morb Mortal Wkly Rep. 2018;67:773–777. doi: 10.15585/mmwr.mm6728a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, Brown RS Jr, Bzowej NH, Wong JB. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560–1599. doi: 10.1002/hep.29800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver. EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370–398. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 4.Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, Chen DS, Chen HL, Chen PJ, Chien RN, Dokmeci AK, Gane E, Hou JL, Jafri W, Jia J, Kim JH, Lai CL, Lee HC, Lim SG, Liu CJ, Locarnini S, Al Mahtab M, Mohamed R, Omata M, Park J, Piratvisuth T, Sharma BC, Sollano J, Wang FS, Wei L, Yuen MF, Zheng SS, Kao JH. Asian-pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1–98. doi: 10.1007/s12072-015-9675-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang TT, Liaw YF, Wu SS, Schiff E, Han KH, Lai CL, Safadi R, Lee SS, Halota W, Goodman Z, Chi YC, Zhang H, Hindes R, Iloeje U, Beebe S, Kreter B. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology. 2010;52:886–893. doi: 10.1002/hep.23785. [DOI] [PubMed] [Google Scholar]
- 6.Marcellin P, Gane E, Buti M, Afdhal N, Sievert W, Jacobson IM, Washington MK, Germanidis G, Flaherty JF, Schall RA, Bornstein JD, Kitrinos KM, Subramanian GM, McHutchison JG, Heathcote EJ. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381:468–475. doi: 10.1016/S0140-6736(12)61425-1. [DOI] [PubMed] [Google Scholar]
- 7.Su TH, Hu TH, Chen CY, Huang YH, Chuang WL, Lin CC, Wang CC, Su WW, Chen MY, Peng CY, Chien RN, Huang YW, Wang HY, Lin CL, Yang SS, Chen TM, Mo LR, Hsu SJ, Tseng KC, Hsieh TY, Suk FM, Hu CT, Bair MJ, Liang CC, Lei YC, Tseng TC, Chen CL, Kao JH. Four-year entecavir therapy reduces hepatocellular carcinoma, cirrhotic events and mortality in chronic hepatitis B patients. Liver Int. 2016;36:1755–1764. doi: 10.1111/liv.13253. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen MH, Yang HI, Le A, Henry L, Nguyen N, Lee MH, Zhang J, Wong C, Wong C, Trinh H. Reduced incidence of hepatocellular carcinoma in cirrhotic and noncirrhotic patients with chronic hepatitis B treated with tenofovir-a propensity score-matched study. J Infect Dis. 2019;219:10–18. doi: 10.1093/infdis/jiy391. [DOI] [PubMed] [Google Scholar]
- 9.Woo G, Tomlinson G, Nishikawa Y, Kowgier M, Sherman M, Wong DK, Pham B, Ungar WJ, Einarson TR, Heathcote EJ, Krahn M. Tenofovir and entecavir are the most effective antiviral agents for chronic hepatitis B: a systematic review and Bayesian meta-analyses. Gastroenterology. 2010;139:1218–1229. doi: 10.1053/j.gastro.2010.06.042. [DOI] [PubMed] [Google Scholar]
- 10.Zuo SR, Zuo XC, Wang CJ, Ma YT, Zhang HY, Li ZJ, Song LY, Deng ZZ, Liu SK. A meta-analysis comparing the efficacy of entecavir and tenofovir for the treatment of chronic hepatitis B infection. J Clin Pharmacol. 2015;55:288–297. doi: 10.1002/jcph.409. [DOI] [PubMed] [Google Scholar]
- 11.Sriprayoon T, Mahidol C, Ungtrakul T, Chun-On P, Soonklang K, Pongpun W, Laohapand C, Dechma J, Pothijaroen C, Auewarakul C, Tanwandee T. Efficacy and safety of entecavir versus tenofovir treatment in chronic hepatitis B patients: a randomized controlled trial. Hepatol Res. 2017;47:E161–E168. doi: 10.1111/hepr.12743. [DOI] [PubMed] [Google Scholar]
- 12.Cai D, Pan C, Yu W, Dang S, Li J, Wu S, Jiang N, Wang M, Zhang Z, Lin F, Xin S, Yang Y, Shen B, Ren H. Comparison of the long-term efficacy of tenofovir and entecavir in nucleos(t)ide analogue-naive HBeAg-positive patients with chronic hepatitis B: a large, multicentre, randomized controlled trials. Medicine (Baltimore) 2019;98:e13983. doi: 10.1097/MD.0000000000013983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu JH, Jin YJ, Lee JW, Lee DH. Remaining hepatocellular carcinoma risk in chronic hepatitis B patients receiving entecavir/tenofovir in South Korea. Hepatol Res. 2018;48:862–871. doi: 10.1111/hepr.13194. [DOI] [PubMed] [Google Scholar]
- 14.Kim BG, Park NH, Lee SB, Lee H, Lee BU, Park JH, Jung SW, Jeong ID, Bang SJ, Shin JW. Mortality, liver transplantation and hepatic complications in patients with treatment-naive chronic hepatitis B treated with entecavir vs. tenofovir. J Viral Hepat. 2018;25:1565–1575. doi: 10.1111/jvh.12971. [DOI] [PubMed] [Google Scholar]
- 15.Choi J, Kim HJ, Lee J, Cho S, Ko MJ, Lim YS. Risk of hepatocellular carcinoma in patients treated with entecavir vs. tenofovir for chronic hepatitis B: a Korean nationwide cohort study. JAMA Oncol. 2019;5:30–36. doi: 10.1001/jamaoncol.2018.4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yip TC, Wong VW, Chan HL, Tse YK, Lui GC, Wong GL. Tenofovir is associated with lower risk of hepatocellular carcinoma than entecavir in patients with chronic HBV infection in China. Gastroenterology. 2020;158:215–225. e216. doi: 10.1053/j.gastro.2019.09.025. [DOI] [PubMed] [Google Scholar]
- 17.Kim SU, Seo YS, Lee HA, Kim MN, Lee YR, Lee HW, Park JY, Kim DY, Ahn SH, Han KH, Hwang SG, Rim KS, Um SH, Tak WY, Kweon YO, Kim BK, Park SY. A multicenter study of entecavir vs. tenofovir on prognosis of treatment-naive chronic hepatitis B in South Korea. J Hepatol. 2019;71:456–464. doi: 10.1016/j.jhep.2019.03.028. [DOI] [PubMed] [Google Scholar]
- 18.Lee SW, Kwon JH, Lee HL, Yoo SH, Nam HC, Sung PS, Nam SW, Bae SH, Choi JY, Yoon SK, Han NI, Jang JW. Comparison of tenofovir and entecavir on the risk of hepatocellular carcinoma and mortality in treatment-naive patients with chronic hepatitis B in Korea: a large-scale, propensity score analysis. Gut. 2012;69:1301–1308. doi: 10.1136/gutjnl-2019-318947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oh H, Yoon EL, Jun DW, Ahn SB, Lee HY, Jeong JY, Kim HS, Jeong SW, Kim SE, Shim JJ, Sohn JH, Cho YK Long-Term Safety of Entecavir and Tenofovir in Patients With Treatment-Naive Chronic Hepatitis B Virus (CHB) Infection (SAINT) Study. No difference in incidence of hepatocellular carcinoma in patients with chronic hepatitis B virus infection treated with entecavir vs. tenofovir. Clin Gastroenterol Hepatol. 2020;18:2793–2802. e6. doi: 10.1016/j.cgh.2020.02.046. [DOI] [PubMed] [Google Scholar]
- 20.Hsu YC, Wong GL, Chen CH, Peng CY, Yeh ML, Cheung KS, Toyoda H, Huang CF, Trinh H, Xie Q, Enomoto M, Liu L, Yasuda S, Tanaka Y, Kozuka R, Tsai PC, Huang YT, Wong C, Huang R, Jang TY, Hoang J, Yang HI, Li J, Lee DH, Takahashi H, Zhang JQ, Ogawa E, Zhao C, Liu C, Furusyo N, Eguchi Y, Wong C, Wu C, Kumada T, Yuen MF, Yu ML, Nguyen MH. Tenofovir versus entecavir for hepatocellular carcinoma prevention in an international consortium of chronic Hepatitis B. Am J Gastroenterol. 2020;115:271–280. doi: 10.14309/ajg.0000000000000428. [DOI] [PubMed] [Google Scholar]
- 21.Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatology. 2018;68:723–750. doi: 10.1002/hep.29913. [DOI] [PubMed] [Google Scholar]
- 22.Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 23.Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, M SS, Torriani FJ, Dieterich DT, Thomas DL, Messinger D, Nelson M. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 24.Handelsman Y, Bloomgarden ZT, Grunberger G, Umpierrez G, Zimmerman RS, Bailey TS, Blonde L, Bray GA, Cohen AJ, Dagogo-Jack S, Davidson JA, Einhorn D, Ganda OP, Garber AJ, Garvey WT, Henry RR, Hirsch IB, Horton ES, Hurley DL, Jellinger PS, Jovanovic L, Lebovitz HE, LeRoith D, Levy P, McGill JB, Mechanick JI, Mestman JH, Moghissi ES, Orzeck EA, Pessah-Pollack R, Rosenblit PD, Vinik AI, Wyne K, Zangeneh F. American association of clinical endocrinologists and American college of endocrinology-clinical practice guidelines for developing a diabetes mellitus comprehensive care plan-2015. Endocr Pract. 2015;21(Suppl 1):1–87. doi: 10.4158/EP15672.GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rubin DB, Schenker N. Multiple imputation in health-care databases: an overview and some applications. Stat Med. 1991;10:585–598. doi: 10.1002/sim.4780100410. [DOI] [PubMed] [Google Scholar]
- 26.D’Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 27.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10:150–161. doi: 10.1002/pst.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lunceford JK, Davidian M. Stratification and weighting via the propensity score in estimation of causal treatment effects: a comparative study. Stat Med. 2004;23:2937–2960. doi: 10.1002/sim.1903. [DOI] [PubMed] [Google Scholar]
- 29.Dhanasekaran R, Nault JC, Roberts LR, Zucman-Rossi J. Genomic medicine and implications for hepatocellular carcinoma prevention and therapy. Gastroenterology. 2019;156:492–509. doi: 10.1053/j.gastro.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Papatheodoridis GV, Chan HL, Hansen BE, Janssen HL, Lampertico P. Risk of hepatocellular carcinoma in chronic hepatitis B: assessment and modification with current antiviral therapy. J Hepatol. 2015;62:956–967. doi: 10.1016/j.jhep.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 31.US Food and Drug Administration. NDA: pharmacology/Toxicity Review and Evaluation: NDA No.21-797: submitted Sep 30 2004. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2005/21797_BARACLUDE_pharmr.PDF. Assessed June 07, 2020.
- 32.Hou JL, Zhao W, Lee C, Hann HW, Peng CY, Tanwandee T, Morozov V, Klinker H, Sollano JD, Streinu-Cercel A, Cheinquer H, Xie Q, Wang YM, Wei L, Jia JD, Gong G, Han KH, Cao W, Cheng M, Tang X, Tan D, Ren H, Duan Z, Tang H, Gao Z, Chen S, Lin S, Sheng J, Chen C, Shang J, Han T, Ji Y, Niu J, Sun J, Chen Y, Cooney EL, Lim SG. Outcomes of long-term treatment of chronic HBV infection with entecavir or other agents from a randomized trial in 24 countries. Clin Gastroenterol Hepatol. 2020;18:457–467. e421. doi: 10.1016/j.cgh.2019.07.010. [DOI] [PubMed] [Google Scholar]
- 33.Murata K, Asano M, Matsumoto A, Sugiyama M, Nishida N, Tanaka E, Inoue T, Sakamoto M, Enomoto N, Shirasaki T, Honda M, Kaneko S, Gatanaga H, Oka S, Kawamura YI, Dohi T, Shuno Y, Yano H, Mizokami M. Induction of IFN-lambda3 as an additional effect of nucleotide, not nucleoside, analogues: a new potential target for HBV infection. Gut. 2018;67:362–371. doi: 10.1136/gutjnl-2016-312653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abushahba W, Balan M, Castaneda I, Yuan Y, Reuhl K, Raveche E, de la Torre A, Lasfar A, Kotenko SV. Antitumor activity of type I and type III interferons in BNL hepatoma model. Cancer Immunol Immunother. 2010;59:1059–1071. doi: 10.1007/s00262-010-0831-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiang HH, Lee CM, Hu TH, Hung CH, Wang JH, Lu SN, Lai HC, Su WP, Lin CH, Peng CY, Chen CH. A combination of the on-treatment FIB-4 and alpha-foetoprotein predicts clinical outcomes in cirrhotic patients receiving entecavir. Liver Int. 2018;38:1997–2005. doi: 10.1111/liv.13889. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


