Abstract
Background
Brain tumor patients are at high risk of impaired medical decision-making capacity (MDC), which can be ethically challenging because it limits their ability to give informed consent to medical treatments or participation in research. The European Association of Neuro-Oncology Palliative Care Multidisciplinary Task Force performed a systematic review to identify relevant evidence with respect to MDC that could be used to give recommendations on how to cope with reduced MDC in brain tumor patients.
Methods
A literature search in several electronic databases was conducted up to September 2019, including studies with brain tumor and other neurological patients. Information related to the following topics was extracted: tools to measure MDC, consent to treatment or research, predictive patient- and treatment-related factors, surrogate decision making, and interventions to improve MDC.
Results
A total of 138 articles were deemed eligible. Several structured capacity-assessment instruments are available to aid clinical decision making. These instruments revealed a high incidence of impaired MDC both in brain tumors and other neurological diseases for treatment- and research-related decisions. Incapacity appeared to be mostly determined by the level of cognitive impairment. Surrogate decision making should be considered in case a patient lacks capacity, ensuring that the patient’s “best interests” and wishes are guaranteed. Several methods are available that may help to enhance patients’ consent capacity.
Conclusions
Clinical recommendations on how to detect and manage reduced MDC in brain tumor patients were formulated, reflecting among others the timing of MDC assessments, methods to enhance patients’ consent capacity, and alternative procedures, including surrogate consent.
Keywords: brain metastases, capacity, consent, glioma, neurodegenerative disease
Medical decision-making capacity (MDC) is a higher-order functional ability referring to the cognitive and emotional ability of a person to make informed decisions related to his or her treatment and care.1 Medical decision making involving individuals, particularly with compromised neurocognitive functions, can be ethically challenging because the lack of capacity may limit their ability to give free and informed consent to medical treatments or research. Determining whether a patient is capable of making his or her own decisions is inherent to every physician-patient interaction, and requires a balance between respecting the autonomy of patients and protecting patients with impaired MDC.2 Furthermore, it is a fundamental principle of ethics and human rights’ safeguards that a person should be judged to lack MDC only when all attempts to empower his or her capacity have been unsuccesful.1,3
About half of patients suffering from brain tumors were found to have impaired capacity to give consent shortly after diagnosis,1,4,5 indicating that this is a crucial issue in clinical practice. Moreover, neurocognitive functioning worsens over time,6 which may subsequently result in further deterioration of MDC. Thus, MDC is not static in the disease trajectory and may also differ between situations (eg, hospitalized patients vs patients visiting outpatient clinics). The reduced MDC of brain tumor patients has relevant implications in different disease stages: It may influence the capacity to consent to medical treatment or clinical trial enrollment, as well as the process of end-of-life (EOL) decision making. In addition, a higher standard of MDC is needed when consenting to a clinical trial compared to initiating standard treatments such as antiepileptic drugs.
According to existing medical literature and ethics, to make valid treatment or research decisions, the individual must be able to 1) understand the risks and benefits; 2) appreciate the personal consequences of his or her choice; 3) make a rational choice concerning treatment or research; and 4) express a choice,7,8 and in some countries 5) act on a decision.9 In brain tumor patients, several factors may affect these core capacities of understanding, appreciation, reasoning, and expression of choice, of which impaired neurocognitive function is the main determinant.1,4,5,10–13 Impaired neurocognitive functioning has been reported in up to 91% of brain metastases patients14 and in up to 63% of primary brain tumor patients preoperatively,15 suggesting that brain tumor patients are prone to impaired MDC.
Although brain tumor patients are at high risk of impaired MDC, explicit assessment of MDC seems to be neglected in clinical practice and research, which is emphasized by the limited number of studies focusing on MDC. To provide guidance on how to approach adult brain tumor patients with reduced MDC, the European Association for Neuro-Oncology Palliative Care Multidisciplinary Task Force explored evidence from studies performed in brain tumor patients and stroke, multiple sclerosis (MS), dementia, Huntington disease, Parkinson disease, and other neurodegenerative diseases, that may be generalized to the brain tumor patient population.
The overall aim of this systematic review was to identify all relevant evidence with respect to MDC that could be used to give recommendations on how to cope with reduced MDC in brain tumor patients. More specifically, we aimed to identify how (1) MDC could be assessed, (2) which patients are prone to impaired MDC, (3) how we could obtain valid informed consent to treatment and research trial enrollment, and (4) what the role of surrogate consent is in medical decision making.
Methods
Search Strategy
A literature search in the electronic databases PubMed/Medline, Embase, PsycINFO, Emcare, Cochrane Library, and Web of Science was conducted covering January 2000 to September 23, 2019. A combination of search terms and synonyms for “medical capacity,” “brain tumors,” and “neurological diseases” was used (see Supplemental File 1 for the full search string used in PubMed). All identified abstracts were screened independently by 2 reviewers (A.P. and L.D.), and full texts of potentially relevant articles were evaluated according to predefined inclusion and exclusion criteria. Disagreement was resolved in consensus.
Data Extraction
For each eligible article, information on population characteristics was extracted, as well as information related to at least one of the predefined topics: tools to measure MDC, consent to treatment, consent to research, predictive patient- and treatment-related factors, views of surrogate decision makers, and interventions to improve MDC.
Definition of Medical Decision-Making Capacity
The percentages of patients with impaired MDC as presented in this review are based on the tools and cutoffs as defined in each specific study, and these definitions may vary (see Supplemental Table 1).
Results
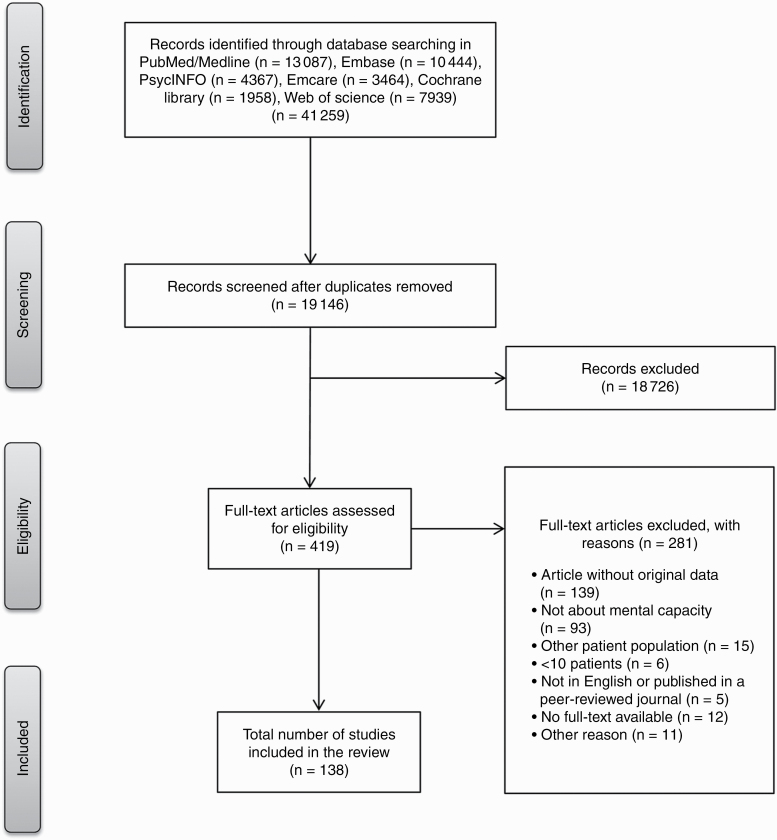
A total of 19 146 unique abstracts were identified. Of these, 419 abstracts were selected for full-text screening, of which 138 were deemed eligible. See Figure 1 for an overview of the selection process. Table 1 provides an overview of the most important study characteristics and outcomes in brain tumor patients only (ie, 9 studies) and Supplemental Table 1 for all included studies (n = 136 studies including brain tumor, stroke, MS, dementia, Huntington disease, Parkinson disease, and other neurodegenerative diseases).
Fig. 1.
Flowchart of the article selection procedure.
Table 1.
Overview of Most Important Study Characteristics and Outcomes of Included Studies in Brain Tumor Patients
| Article | No. and type of participants | Consent for research or treatment | Percentage of patients capacitated/incapacitated patients | Type of instruments and score | Factors predictive of incapacity | Surrogate decision makers | |
|---|---|---|---|---|---|---|---|
| 1 | Gerstenecker et al, 201510 | 41 patients with brain metastases and 41 matched cognitively healthy controls | Treatment | 25/41 (61%) had intact reasoning (score > 1.5 SD below control group mean) | CCTI | Cognitive functioning (impaired memory and processing speed) associated with impaired reasoning | |
| Reasoning (range, 0-6): 4.6 in intact patients, 1.3 in impaired patients | |||||||
| 2 | Gerstenecker et al, 201511 | 41 patients with brain metastases and 41 matched cognitively healthy controls | Treatment | 22/41 (54%) had intact understanding (score > 1.5 SD below control group mean) | CCTI | Cognition associated with understanding (particularly memory and language) | |
| Understanding (range, 0-82): 34.1 in intact patients, 19.4 in impaired patients | |||||||
| 3 | Kerrigan et al, 201216 | 247 patients with brain tumors | Treatment | 3.6% had certificate of incapacity | |||
| 4 | Kerrigan et al, 201412 | 100 brain tumor patients (mostly primary) | Treatment | 75/100 (75%) had capacity determined by one assessor blinded to outcome of discussion | MacCAT-T | Patients with glioblastoma, male sex, and more cognitive impairments more often deemed incapacitated | |
| Patients with and without capacity differed significantly on scores on understanding, appreciation, and reasoning (scores not reported). | < 4/7 in semantic verbal fluency subset of ACE-R predictive of incapacity | ||||||
| 5 | Marson et al, 20104 | 26 glioma patients and 22 healthy controls | Research | No impairment in capacity defined as > 1.5 SD below control group mean | CCRI | KPS, steroid use, and cognitive functioning associated with understanding, steroid use, and cognition with reasoning, and only cognition with reasoning. | |
| Percentage of patients with impaired capacity | Mean scores for patients/controls | ||||||
| Expressing choice: 0% | Expressing choice (range, 0-2): 1.96/2.0 (NS) | ||||||
| Appreciation: 31% | Appreciation (range, 0-4): 2.5/3.4a | ||||||
| Reasoning: 23% | Reasoning (range, 0-6): 3.2/4.6a | ||||||
| Understanding: 38% | Understanding (range, 0-91): 50.3/65.4a | ||||||
| 6 | Martin et al, 201513 | 26 primary brain tumor and 45 brain metastases patients | Treatment | Fully capable defined as > 1.5 SD compared to control group mean, marginally capable 1.5-2.5 SD below control group mean, incapable < 2.5 SD. | CCTI | KPS associated with understanding and reasoning. Patients with higher KPS had better capacity | |
| 24/71 (34%) of patients deemed fully capable. | Mean scores per KPS group: 90-100/70-80/50-60 | ||||||
| Percentage of patients fully capable based on KPS: | Understanding (range, 0-78): 28.5/20.9/14.7a | No difference between types of brain tumors | |||||
| KPS 90-100: 46% | Reasoning (range, 0-12): 3.7/2.8/1.0 (NS) | ||||||
| KPS 70-80: 23% | Appreciation (range, 0-8): 3.4/2.8/2.8a | ||||||
| KPS 50-60: 0% | |||||||
| Percentage of patients fully capable per aspect per KPS group 90-100/70-80/50-60: | |||||||
| Understanding: 62/31/17% | |||||||
| Reasoning: 72/46/0% | |||||||
| Appreciation: 87/69/67% | |||||||
| 7 | Sizoo et al, 201217 | 101 glioma patients | EOL treatment | 20% incompetent last months before death, 52% incompetent last weeks before that, 85% incompetent last days | Incompetence based on judgment of treating physician | Surrogate decision-makers need support. Recommended: HCPs help surrogates understand disease trajectory, undertake ACP, and HCPs act as empathic guides. A key worker should be available for surrogates to address questions to, and carer support programs should be set up. | |
| 8 | Triebel et al, 20095 | 26 glioma patients and 22 healthy controls | Treatment | Fully capable defined as > 1.5 SD compared to control group mean, marginally capable 1.5-2.5 SD, incapable < 2.5 SD compared to control group mean (for appreciation, reasoning, and understanding). Capable in choice defined as 2 (maximum), incapable as 0. | CCTI | KPS and several domains of cognitive functioning associated with capacity. | |
| Percentage of patients/controls capable | Mean scores for patients/controls | ||||||
| Expressing choice: 96.2%/100% | Expressing choice (range, 0-2): 1.8/2.0 (NS) | ||||||
| Appreciation: 76.9%/90.9% | Appreciation (range, 0-4): 3.0/3.6 (NS) | ||||||
| Reasoning: 65.4%/90.9% | Reasoning (range, 0-6): 3.3/4.9a | ||||||
| Understanding: 46.2%/90.9% | Understanding (range, 0-40): 24.2/33.0a | ||||||
| 9 | Triebel et al, 20151 | 41 patients with brain metastases (shortly after diagnosis), and 41 matched controls | Treatment | Fully capable defined as > 1.5 SD compared to control group mean, marginally capable 1.5-2.5 SD, and incapable < 2.5 SD compared to control group mean (for appreciation, reasoning, and understanding). Capable in choice defined as 2 (maximum), incapable as 0. | CCTI | ||
| 25/41 (61%) patients had some deficit in capacity (on any aspect). | Mean scores for patients/controls | ||||||
| Percentage of patients with incapacity: | Expressing choice (range, 0-2): 1.98/1.95 (NS) | ||||||
| Expressing choice: 2.4% | Appreciation (range, 0-4): 3.34/3.66 (NS) | ||||||
| Appreciation: 17% | Reasoning (range, 0-8): 3.32/4.76a | ||||||
| Reasoning: 39% | Understanding (range, 0-41): 26.76/33.61a | ||||||
| Understanding: 46% | If complexity of consent standard increased, so did number of patients with impaired capacity. |
Abbreviations: ACE-R, Addenbrook’s Cognitive Evaluation–Revised; ACP, advanced care planning; CCTI, capacity to consent to treatment instrument; EOL, end of life; HCP, health care professional; MacCAT-T, MacArthur Competence Assessment Tool–Treatment; NS, not significant.
“Range” as reported for the tools represents the possible range of scores, unless otherwise stated.
Scores on a capacity instrument that are not statistically different between patients and controls are reported as “(NS).”
aStatistically significant between any of the groups.
Tools to Measure Medical Capacity
Although the assessment of MDC is usually an implicit clinical judgment made by the treating physician, structured capacity assessment instruments may aid clinical decision making. Instruments to assess capacity have been tested in neurological populations including brain tumors,1,4,5,10–13 dementia,3,18-46 Parkinson disease,18,47,48 neuropsychiatric disorders,3,23,46 stroke,49 and MS.50,51 These instruments usually seek to test 4 abilities underpinning capacity: understanding, appreciation, reasoning, and choice. This can be achieved by tailoring the instrument to real-life (clinical) decisions facing the patient,3,12,17,33,36,40 but has more often been studied using hypothetical clinical vignettes,1,4,5,10,11,13,18,20–23,25,27–32,34,35,37–39,41,42,45,47,48,50–54 The MacArthur Competence Assessment Tool for Treatment is the most frequently studied test in either its standard form (MacCAT-T3,12,20,21,26,34–36,42,46,52) or modified for clinical research (MacCAT-CR24–35,27–31,39,41,54). Both MacCAT tools are semistructured interviews that measure capacity to consent to medical treatment or participation in research, tailored to a specific situation.55,56 The Capacity to Consent to Treatment Instrument (CCTI) is also relatively well studied.1,4,5,10,11,13,18,20,22,32,34,37,38,48,51 The CCTI is a standardized psychometric instrument that consists of 2 clinical vignettes that present hypothetical medical problems and simulates an informed consent dialogue between the physician and the patient.57
The agreement between different instruments administered to the same patient has rarely been reported, but one study showed that different instruments do not consistently match one another in determining capacity.20 No instrument is currently considered the gold standard. Instead, the consensus of experienced clinicians is frequently taken as a reference standard for determining capacity,19,24,26,27,29,31,32,39,41,46,54 but the (implicit) criteria clinicians use to determine capacity are unclear. Moreover, one study showed that 48% of incapacitated brain tumor patients identified with a validated instrument were not recognized as incapacitated by their treating physicians, emphasizing the difficulty in assessing MDC.12 Factors contributing to this difficulty include patient-related characteristics (eg, cognitive impairment), differing opinions (eg, between patient and physician), and familial and legal situations.58
Among brain tumor patients, the most frequently studied tool is the CCTI.1,5,10,11,13 Capacity in these patients may vary inversely with verbal fluency, verbal memory, and/or executive function.4,5,10,11 Assessment of verbal fluency has been suggested as a promising way to quickly identify brain tumor patients who may require a more detailed capacity assessment: The MacCAT-T to test capacity preoperatively in 100 brain tumor patients showed that impaired verbal fluency was associated with incapacity, with 96% sensitivity and 63% specificity.
Capacity assessment instruments may aid in determining the level of MDC in patients, but should not be used alone.1,4,5,10,13,24,38,59 Instead, a combination of instruments with implicit clinical judgement is proposed.33,48 Limitations of instruments include a reliance on hypothetical situations1,5,10,13,20,22,23,32,34–38,42,46,48,50–52 rather than a real informed consent design,3,36 low interrater agreement,33,35 requirement for training in their interpretation of the interview (ie, subjective), and sparse data regarding their validity in populations such as brain tumor patients. Future research is important to establish whether bedside tests such as verbal fluency can screen for patients who require a detailed assessment of capacity with a validated instrument.
Consent to Treatment
Decisions with respect to treatment must be made during the entire disease course, from diagnosis until EOL. In patients with brain tumors, the EOL phase is typically defined as the last 3 months of life.60 Patients with various neurological diseases, including brain tumors, may already have relevant reduction in MDC early after diagnosis, with a further decline over time, as shown by assessments (eg, MacCAT, CCTI, and the Hopement Capacity Assessment Interview [HCAI])1,13,20,22,36–38,42,53 or by physicians’ estimation.32 Physicians tend to overestimate patients’ MDC for treatment-related decisions.17,32,34,46
The percentage of incapacitated patients varied widely between studies, ranging from 26% to 91% for making treatment decisions.3,26,32,33,36,46,61 In studies with primary brain tumor or brain metastases patients, more than half were reported to be already compromised in MDC early in the disease course, either in all consent standards or with respect to reasoning and understanding only.1,5,10,13,17 Patients suffering from mild cognitive impairment or mild to moderate dementia,22,34,36–38,42,53 Parkinson disease with cognitive impairment,47,48 or progressive MS50,51 often show signs of impaired reasoning, understanding, and appreciation, although they may still be able to express a treatment choice.22,34,36–38,42,47,48,51,53
Important medical decisions are likely to be made in most patients approaching EOL, and comprise decisions relating to symptom relief, withdrawal of or refraining from medication (eg, antibiotics, dexamethasone, antitumor treatment), questions regarding tube feeding and artificial fluid intake, resuscitation,17,47,62–67 admission to the emergency department or intensive care unit, or surgery treatment (eg, placing a pacemaker, surgery for hip fracture). If patients’ autonomy is not preserved until the EOL phase, advanced care planning (ACP) should be initiated early in the disease course. It is recommended that ACP be actively addressed by treating physicians early in the disease course, despite their reservations to do so.17,47,62,64,66–68 Many patients and their relatives may have fewer reservations to discuss ACP early in the disease course.47,62,67 To know patients’ preferences and implement them accordingly, it is suggested to involve proxies in patients’ ACP.1,5,62,64,67
Consent to Research
According to the Declaration of Helsinki, an approval from a research ethics committee (REC) is needed for every scientific study.69 Special precautions are needed for research in patients with impaired neurocognitive functioning, but RECs often differ in their interpretation on the consent procedure.
The available data on the capacity to consent to research in brain tumor patients are scarce. In patients with neurodegenerative diseases, between 26% and 84% were deemed incapacitated to consent to research.19,24,25,27–29,31,39–41,70,71 Patients, including glioma patients, were particularly impaired in understanding (37%-47%),4,25,28,29,41,54 reasoning (23%-35%),4,25,28,29 and appreciation (7%-81%),4,25,28,29 and performed significantly worse on MDC assessments than controls.4,24,25,29,39,41 The capacity to consent to participation in a clinical trial was related to the risk of harms of the study. Indeed, the capacity to give consent decreased with the complexity of the trial.1,54 Fifty-nine percent27,31 of dementia patients were incapable of consenting to a clinical drug trial, whereas this was 84% for a high-risk neurosurgical trial,27 as measured with hypothetical vignettes. Capacity also clearly affected trial participation, as larger impairments in capacity resulted in less willingness to participate,30 limiting the generalizability of results.
In many situations brain tumor patients may not be able to provide self-consent because of focal neurological deficits such as dysphasia, visual or motor deficits, or loss of consciousness. The exact percentage of patients ineligible for participation in clinical studies because of impaired capacity is currently unknown, mostly likely because it has not been measured and thus reported. When patients are not able to provide self-consent, REC-approved surrogate consent is an important alternative, referring to the “proxy” or “surrogate” choosing what the patient might have wished.72 In stroke studies, a substantial number of patients (range, 6%-77%) were not able to provide self-consent,49,73–76 but percentages of proxy consent for research also varied considerably between countries.
Associations Between Patient- and Treatment-Related Factors and Capacity
Between 25% and 66% of brain tumor patients were reported as incapacitated,1,10–13 without differences between primary or metastatic brain tumor patients.13 In general, patients with neurological disorders scored significantly lower on most aspects of capacity when compared to controls.22,23,26,37,38,45,51,71,77 In stroke, a large proportion of patients was incapable of giving consent themselves, mostly because of neurological deficits.49,73–75 Both in brain tumors and neurodegenerative diseases, the percentage of incapacitated patients increased with disease severity. Parkinson disease patients with dementia scored significantly lower on all capacity domains than patients without dementia48 or cognitive deficits.54 Similarly, there was no difference in capacity between patients with probable dementia and controls,52 but differences became apparent when patients with dementia were compared to controls.22,23,26,37,45,71 During the last months of life, 20% of brain tumor patients were incapacitated, which increased to 85% in the last days, mostly because of decreased consciousness.17
Although the percentage of incapacitated patients varied widely between studies, ranging from 26% to 91% for medical or research decisions,19,24,26–29,31–33,39-41,46,61,70,71 incapacity appeared to be mostly determined by the level of cognitive impairment rather than the underlying pathology: Cognitive dysfunction was associated with incapacity in patients with brain tumors,1,4,5,10–13 MS,50,51 Parkinson disease,18,47,48,54 and dementia.18,19,23,31,34,36,39,40,42,45,52 Although there was no linear association, Mini-Mental State Examination scores below 18 to 2026,28,40 were found to be helpful in discriminating patients without capacity. The most commonly affected neurocognitive domains that were associated with decreased capacity in brain tumor patients were memory,10,11 processing speed,10 language,11 and semantic verbal fluency.12 Other characteristics that were predictive for incapacity in brain tumors were glioblastoma histology,12 male sex,12 steroid use,4 and KPS.4,5,13 Of interest, even in glioma patients with a KPS of 90% to 100%, impaired capacity (defined as a score 2.5 SD below the mean of the control group on any of the capacity aspects) was greater than 50%.13 In patients with dementia, for example, impaired capacity was further associated with not being aware of the disease,26 higher age,39 higher caregiver burden,17 race,17 functional status,42 and educational level.19,39
Brain tumor patients had most frequently impaired understanding (38%-54%),1,4,5,11 followed by impaired reasoning (23%-39%)1,4,5,10 and impaired appreciation (17%-31%).1,4,5 Impairments in these 3 domains were also found in dementia patients,18,21,22,25,29,32,34,37,38 Parkinson disease,18,48,54 and those with MS,51 although in some studies reasoning was more affected than understanding. Decline in capacity over time is mostly attributable to reduced reasoning22,35 and understanding,22,37 which seem the most essential aspects for providing consent, but also appreciation.22
Surrogate Decision Making
If patients are no longer capable of making decisions, surrogate decision making is a frequently used alternative if allowed by law. Several studies have focused on the role of proxies in decision making in dementia and stroke patients, and these results may be transferable to brain tumor patients.
Surrogate consent may play a role in treatment decisions and decisions to participate in clinical research. It has previously been shown that there is poor agreement between the patients’ preferences and the proxies’ and physicians’ perception of those preferences.21 This is particularly important in EOL decisions, for which surrogate preferences play a sensitive role,21,23,53,62–66,72,78 because relatives and health care professionals (HCPs) typically not reliably predict patients’ preferences,21,53,79 and stakeholders involved might also disagree with each other.69 Therefore, early discussion of the patient’s preferences is crucial when considering implementation of proxy (surrogate) treatment decision making, particularly in palliative and EOL care, because it is more likely that the patient’s MDC is still intact early in the disease trajectory.27,64,66,68,79,80 Good communication between HCPs and proxies is also key for proxies to obtain relevant information needed to make a decision.67,81,82 Additionally, family consensus is warranted. HCPs should have a role, and skills, in mediating these discussions.79,81 Even if proxies know the patient’s preferences, it should be recognized that different family members may know different expressions of these preferences because these can change with disease progression, different phases of care, fluctuating capacity, and the environment.64,78,83 Many surrogates have to grow into their role as the main decision maker and learn how to cope with the accompanying stress, particularly when overriding the patient’s wish.84
Although surrogate consent seems an effective method to enroll patients with stroke into clinical trials, surrogates are less likely to agree to the patient’s participation in research than to treatment.72 Indeed, the agreement between patients and surrogates to participate in research is only moderate for clinical trials (49%-74%).72 Patients and surrogates both felt that the most important reasons to participate in research were the prospect of direct benefits25,71,85 and altruism.25 Decisions by surrogates were mostly driven by the “best interest” for the patient, instead of “substituted judgment” (ie, choosing as the patient would choose if he or she still had capacity).71,85 Apart from patient-related barriers in recruitment for trials, other barriers are mainly HCP related or study related.86 For example, lack of time for recruitment, paternalism of the HCP, and low public awareness of research were found to be important barriers.86 Nevertheless, the use of surrogate consent increases the generalizability and value of trials and can accelerate the trial recruitment process, potentially resulting in cost savings and faster time scales for implementation of findings.75 It is necessary though that proxies understand what clinical research entails. The degree of leeway taken by the proxy in terms of decision making on behalf of the patient needs further research.71,87
Factors valued by the proxy should also be considered, because decisions made on behalf of the patient can reflect these values.84,88 It should also be acknowledged that not everyone wishes to discuss their dying and some patients are content to rely on others’ decision making. Patients with mild to moderate cognitive impairments can be involved in decision making, and they should be supported in this process.82,85 Proxies should understand what their responsibilities are as decision makers, which should include how to assess the patient’s decision-making capability.31,85
The process of surrogate decision making to obtain consent from patients should be considered with a range of methods available for gaining consent,71,85,86,88 but legal frameworks must be taken into account.78 Different countries may have different legal systems and requirements for appointing proxies,89 sometimes resulting in unwieldly and time-consuming processes to gain consent. There is a need for training and support in advocacy and this should include HCPs, patients, caregivers and, when there is no family member, public guardians must be considered.27,67,82 There may also be a role for an “empathic guide” as a primary contact for the proxy.90
Interventions to Improve Capacity/Solutions for Incapacity
A small number of studies in neurological patients have suggested that patients with cognitive deficits may, with appropriate support and enabling approach, be involved in the decision-making process. One study in patients with early Alzheimer disease showed that a memory and organizational aid might improve patient capacity to give informed consent for research.41 In patients with advanced dementia, a specific decision aid about feeding options reduced decisional conflict for surrogates.63 However, other studies in similar populations indicated that linguistically adapted vignettes did not improve MDC,45 nor did an enhanced consent procedure using multimedia.77
In clinical practice, common approaches in patients presenting with cognitive impairment are mainly aimed to ensure that patients’ “best interests” are the focus of the decision-making process, for example, by using surrogate decision makers. However, using proxy consent can be time consuming, for example, because of the involvement of a court in appointing a legal proxy.89 In addition, clinicians appear to hold ambivalent attitudes toward involvement of an independent advocacy service because they frequently feel that this is useful in only a minority of ethically complicated decisions82 and because of time constraints. Implementation of strategies aimed to support the decision-making process itself may help to protect the rights of individuals with impaired MDC and may reduce the use of surrogate decision makers. In a study including MS patients with cognitive impairment, patients accurately understood 60% of the information from an informed consent protocol. However, repetition and cueing significantly enhanced patients’ ability to provide consent to “normal” levels.49 In general, simplification of language (ie, suitable for a low level of reading skills), use of absolute terms (ie, conveying true differences, not proportions), the reduction of information load, use of multiple modalities to convey information, and verification of comprehension of the information presented may all help ensure that obtained consent is valid and support the ability of patients to make informed decisions.1,3,91–93 User testing, a method to develop patient information, could be considered as a valid tool in evaluating the comprehensibility of patient information forms for clinical studies.94 To facilitate inclusion of patients in low-risk clinical trials, a hospital-based informed consent framework could also be used.95
Discussion
Although the determination of a patient’s capacity depends on the method of assessment,20,46 as well as the definition of impaired capacity, the results of this review show a high incidence of impairments in MDC in patients affected by brain tumors as well as other neurological diseases. Patients with brain tumors are particularly vulnerable to impaired capacity because of the early and progressive decline in cognitive abilities. This, in combination with their often short life expectancy, hampers their ability to participate in treatment decisions, research participation, and planning of EOL treatment. Because this systematic review confirmed that the literature on MDC in brain tumor patients is scarce, evidence from studies performed in patient populations with similar characteristics, for example, dementia, were used to provide guidance on how to approach brain tumor patients with reduced MDC.
Respect for patients’ autonomy and their right for self-determination in medical decisions is a fundamental ethical duty as described in the Declaration of Helsinki. Although the need for protection for decisionally impaired individuals has received recognition in ethical debates and most European countries’ law jurisdictions, there is still a lack of clear guidelines about assessment of MDC, protection of patients’ rights, and how to offer strategies to enhance consent procedures for medical treatment or research.
In clinical practice, a judgment about the patient’s ability to make a decision is usually implicitly made by the treating physician. Such judgments on the patient’s capacity are always relative to a specific decision, at a particular time and context. However, depending on the tools that are used, capacity comprises a spectrum, ranging from having insufficient capacity, to partial, adequate, or full capacity. Although the prevalence of incapacity is high in brain tumor patients,1,4,5,10–13,16,17 in only a small percentage (3.6%) of patients is their incapacity formally registered.16 This underlines that assessment of MDC is a complex issue and needs to be better defined. Even though a combination of validated tools (eg, MacCAT, CCTI, HCAI) with clinical assessment is recommended,33,48 use of these instruments is time consuming and may be the reason why they are underused. Integrating MDC into routine service provision may be facilitated by using the US National Implementation Research Network framework and toolkit.96 While certain clinical factors such as performance status and age were associated with the level of capacity, many studies reported the strongest correlation between capacity to consent to treatment or research and neurocognitive deficits, both in brain tumors and neurological patients. Routine evaluation of neurocognitive functioning might thus help to identify those patients with impaired decisional capacity and to develop personalized strategies to empower residual capacity. Other aspects like educational level and socioeconomic status should also be considered.
One of the most difficult aspects of evaluating capacity is to determine the exact level of MDC. Many studies recognize that the level of capacity may change during the course of disease both in neurological and brain tumor patients. Patients with cognitive impairments may maintain the capacity to make certain basic decisions, for example, to appoint a surrogate decision maker, but not concerning more complex treatment decisions or participation in (high-risk) clinical trials. These aspects are a matter of debate, particularly in the ethical and legal literature, and there is no clear consensus on how to address this in daily clinical practice in different countries.
In clinical practice, when a patient is unable to make decisions himself or herself, most European countries’ legislations promote strategies of individual autonomy protection applying the ethical standards of respect for previously expressed wishes, substituted judgments, or best interests. In the everyday clinical decision-making process, the most-used strategy consists of involving the patients’ proxies in the decision-making process. However, proxy decisions present a number of issues, including potential conflicts of interest and strong evidence of poor agreement between patients’ wishes and surrogates.93 Moreover, decisions by surrogates are mostly based on “best interest” for the patient, instead of “substituted judgment.” 71,84,85 The timing of surrogate decisions may also be of impact; not in all situations is there time to gather additional information about the patient’s wishes, consult with others, and deliberate about the benefits and burdens of options as well as seeking ways to enhance the patient’s own decision-making capacity. To protect the autonomy of patients with impaired MDC, several interventions to enhance consent procedures have been proposed, with controversial outcomes.3 The value of cognitive rehabilitation in brain tumor patients has been demonstrated in randomized trials97 but the impact on patients’ capacity should be evaluated in future studies. Moreover, whether cognitive rehabilitation is beneficial for patients with limited survival, such as glioblastoma patients or those with brain metastases, is questionable. Because patients may be capable of making certain decisions but not others (eg, basic treatment vs research-related decisions), those interventions should be explored that are relevant for the individual patient. Targeting specific aspects of capacity may also be valuable to increase the number of patients included in clinical trials. This will increase the generalizability of results, because those patients with preserved capacity are not representative of the whole population.73
Decisional capacity becomes particularly impaired during the EOL phase of patients with brain tumors.17 Because EOL treatment decisions in patients unable to express their preferences are among the most challenging ethical issues, with a great impact both on the patient’s family and HCPs involved in the decision, these should be discussed earlier in the disease trajectory. With the aim to protect patients’ autonomy, the use of timely advanced directives and ACP are considered methods to obtain patients’ treatment preferences in people who are expected to lose their MDC. ACP has been defined as “a process of discussion about goals of care and means of setting on record preferences for care of patients who may lose capacity or communicating ability in the future.” 98 However, more research is needed to determine the effectiveness of ACP in brain tumor patients. Currently, a disease-specific ACP program has been developed for glioblastoma patients, and the best time to introduce such a program in the disease trajectory has been studied, as well as barriers and facilitators for participation in such a program and implementation in clinical practice.99 Whether this program will have an impact on patient- and care-related outcomes remains to be investigated, after which inclusion in guidelines may follow. Early palliative care integration and implementation of simultaneous models of care may also help to improve the quality of care at EOL and to facilitate ACP and the shared decision-making process in brain tumors. In Germany a multicenter randomized trial is investigating the effect of early palliative care in glioblastoma patients, and ACP plays a prominent role (DRKS00016066).
In conclusion, there is an urgent need for recommendations for the detection and management of brain tumor patients with reduced MDC in different settings of care, such as research enrollment and particularly in the EOL decision-making process, preserving the patient’s right for self-determination. Because the level of evidence of the already limited number of available studies is generally low, the formulation of clinical recommendations was hampered. Therefore, we used the available results of studies in patients with brain tumors and other neurological diseases, together with expert opinion (experts in various fields, such as palliative care in neuro-oncology and other neurological diseases, and bioethics), to formulate preliminary recommendations for the management of brain tumor patients with impaired MDC (Table 2), because this is a major issue in brain tumor patients and should be addressed immediately. Further research into this topic is strongly encouraged.
Table 2.
Preliminary Clinical Recommendations to Enhance Medical Decision Capacity in Brain Tumor Patients
| Clinical recommendations | |
|---|---|
| 1 | Health care professionals should try to involve patients in the decision-making process whenever possible. |
| 2 | Careful evaluation of medical decision-making capacity should be performed in brain tumor patients on a case-by-case basis, with multiple evaluations over time, particularly at the time a decision needs to be made. |
| 3 | Clinicians should be aware that they generally tend to overestimate patient’s medical decision-making capacity in different settings, which may affect the procedure for consent to treatment and research. |
| 4 | Screening of neurocognitive functioning (eg, bedside tests) could help in identifying those patients with impaired decisional capacity, for whom subsequent assessment with a validated capacity instrument should be initiated. |
| 5 | Health care professionals should try to enhance patients’ consent capacity by offering cues reducing memory or attention demands. |
| 6 | Patient information forms should contain simple language, use absolute terms, refrain from lengthy and irrelevant texts, and make use of pictures or figures when possible. |
| 7 | Physicians should address advanced care planning soon after diagnosis of a brain tumor. Because surrogate decisions are often necessary with progressive disease, relatives or other trusted individuals should be involved in the process of patients’ advanced care planning. |
| 8 | Surrogate decision making should be considered in case the patient lacks capacity, ensuring that the patient’s “best interests” and wishes are guaranteed. |
| 9 | If no surrogate is available for decisions in clinical practice, health care professionals should make the decision based on the patient’s “best interests.” |
Supplementary Material
Acknowledgments
All authors substantially contributed to the design and concept of this article. A.P. and L.D. performed the systematic literature search. Each author prepared a part of the article and the first and last author combined their input. Next, all authors critically revised the article for important intellectual content. All authors gave final approval for the version to be published.
Conflict of interest statement. Dr Grant reports personal fees from UCB, and other from UCB, outside the submitted work; Dr Le Rhun reports grants and personal fees from Mundipharma, grants from Amgen, personal fees and nonfinancial support from Abbvie, and personal fees from Daiichi Sankyo and Tocagen, outside the submitted work; Kathy Oliver, for and on behalf of the International Brain Tumour Alliance (IBTA) as chair and codirector, reports grants to the IBTA from Bristol-Myers Squibb, Novocure, Pfizer, Bayer, Novartis, Northwest Biotherapeutics, MagForce, Medac, Photonamic, Apogenix, AbbVie, Elekta, Roche, Lilly, and VBL Therapeutics, outside the submitted work; Dr Rudà reports personal fees from UCB and Novocure, outside the submitted work; Dr Weller reports grants and personal fees from Abbvie, grants from Adastra, personal fees from BMS, grants and personal fees from MSD, grants and personal fees from Merck (EMD), grants from Novocure, personal fees from Celgene, personal fees from Orbus, and personal fees from Tocagen, outside the submitted work. All other authors declare no conflicts of interest.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Triebel KL, Gerstenecker A, Meneses K, et al. Capacity of patients with brain metastases to make treatment decisions. Psychooncology. 2015;24(11):1448–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Appelbaum PS. Clinical practice. Assessment of patients’ competence to consent to treatment. N Engl J Med. 2007;357(18):1834–1840. [DOI] [PubMed] [Google Scholar]
- 3. Wong JG, Clare CH, Holland AJ, Watson PC, Gunn M.. The capacity of people with a ‘mental disability’ to make a health care decision. Psychol Med. 2000;30(2):295–306. [DOI] [PubMed] [Google Scholar]
- 4. Marson DC, Martin RC, Triebel KL, Nabors LB.. Capacity to consent to research participation in adults with malignant glioma. J Clin Oncol. 2010;28(24):3844–3850. [DOI] [PubMed] [Google Scholar]
- 5. Triebel KL, Martin RC, Nabors LB, Marson DC.. Medical decision-making capacity in patients with malignant glioma. Neurology. 2009;73(24):2086–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bosma I, Vos MJ, Heimans JJ, et al. The course of neurocognitive functioning in high-grade glioma patients. Neuro Oncol. 2007;9(1):53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Appelbaum PS, Grisso T. The MacArthur Treatment Competence Study. I: mental illness and competence to consent to treatment. Law Hum Behav. 1995;19(2):105–126. [DOI] [PubMed] [Google Scholar]
- 8. Appelbaum PS, Roth LH. Competency to consent to research: a psychiatric overview. Arch Gen Psychiatry. 1982;39(8):951–958. [DOI] [PubMed] [Google Scholar]
- 9.Scottish Government. Adults with incapacity: guide to assessing capacity.Edinburgh, UK: The Scottish Government; https://www.gov.scot/publications/adults-incapacity-scotland-act-2000-communication-assessing-capacity-guide-social-work-health-care-staff/pages/2/. Accessed May 20, 2020. [Google Scholar]
- 10. Gerstenecker A, Duff K, Meneses K, Fiveash JB, Nabors LB, Triebel KL.. Cognitive predictors of reasoning through treatment decisions in patients with newly diagnosed brain metastases. J Int Neuropsychol Soc. 2015;21(6):412–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gerstenecker A, Meneses K, Duff K, Fiveash JB, Marson DC, Triebel KL.. Cognitive predictors of understanding treatment decisions in patients with newly diagnosed brain metastasis. Cancer. 2015;121(12):2013–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kerrigan S, Erridge S, Liaquat I, Graham C, Grant R.. Mental incapacity in patients undergoing neuro-oncologic treatment: a cross-sectional study. Neurology. 2014;83(6):537–541. [DOI] [PubMed] [Google Scholar]
- 13. Martin RC, Gerstenecker A, Nabors LB, Marson DC, Triebel KL.. Impairment of medical decisional capacity in relation to Karnofsky Performance Status in adults with malignant brain tumor. Neurooncol Pract. 2015;2(1):13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Meyers CA, Smith JA, Bezjak A, et al. Neurocognitive function and progression in patients with brain metastases treated with whole-brain radiation and motexafin gadolinium: results of a randomized phase III trial. J Clin Oncol. 2004;22(1):157–165. [DOI] [PubMed] [Google Scholar]
- 15. van Kessel E, Baumfalk AE, van Zandvoort MJE, Robe PA, Snijders TJ.. Tumor-related neurocognitive dysfunction in patients with diffuse glioma: a systematic review of neurocognitive functioning prior to anti-tumor treatment. J Neurooncol. 2017;134(1):9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kerrigan S, Dengu F, Erridge S, Grant R, Whittle IR.. Recognition of mental incapacity when consenting patients with intracranial tumours for surgery: how well are we doing? Br J Neurosurg. 2012;26(1):28–31. [DOI] [PubMed] [Google Scholar]
- 17. Sizoo EM, Pasman HR, Buttolo J, et al. Decision-making in the end-of-life phase of high-grade glioma patients. Eur J Cancer. 2012;48(2):226–232. [DOI] [PubMed] [Google Scholar]
- 18. Griffith HR, Dymek MP, Atchison P, Harrell L, Marson DC.. Medical decision-making in neurodegenerative disease: mild AD and PD with cognitive impairment. Neurology. 2005;65(3):483–485. [DOI] [PubMed] [Google Scholar]
- 19. Guarino PD, Vertrees JE, Asthana S, et al. Measuring informed consent capacity in an Alzheimer’s disease clinical trial. Alzheimers Dement (N Y). 2016;2(4):258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gurrera RJ, Karel MJ, Azar AR, Moye J.. Agreement between instruments for rating treatment decisional capacity. Am J Geriatr Psychiatry. 2007;15(2):168–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hamann J, Bronner K, Margull J, et al. Patient participation in medical and social decisions in Alzheimer’s disease. J Am Geriatr Soc. 2011;59(11):2045–2052. [DOI] [PubMed] [Google Scholar]
- 22. Huthwaite JS, Martin RC, Griffith HR, Anderson B, Harrell LE, Marson DC.. Declining medical decision-making capacity in mild AD: a two-year longitudinal study. Behav Sci Law. 2006;24(4):453–463. [DOI] [PubMed] [Google Scholar]
- 23. Karel MJ, Gurrera RJ, Hicken B, Moye J.. Reasoning in the capacity to make medical decisions: the consideration of values. J Clin Ethics. 2010;21(1):58–71. [PMC free article] [PubMed] [Google Scholar]
- 24. Karlawish J, Kim SY, Knopman D, van Dyck CH, James BD, Marson D.. Interpreting the clinical significance of capacity scores for informed consent in Alzheimer disease clinical trials. Am J Geriatr Psychiatry. 2008;16(7):568–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Karlawish JH, Casarett DJ, James BD. Alzheimer’s disease patients’ and caregivers’ capacity, competency, and reasons to enroll in an early-phase Alzheimer’s disease clinical trial. J Am Geriatr Soc. 2002;50(12):2019–2024. [DOI] [PubMed] [Google Scholar]
- 26. Karlawish JH, Casarett DJ, James BD, Xie SX, Kim SYH.. The ability of persons with Alzheimer disease (AD) to make a decision about taking an AD treatment. Neurology. 2005;64(9):1514–1519. [DOI] [PubMed] [Google Scholar]
- 27. Kim SY, Appelbaum PS, Kim HM, et al. Variability of judgments of capacity: experience of capacity evaluators in a study of research consent capacity. Psychosomatics. 2011;52(4):346–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim SY, Caine ED. Utility and limits of the Mini Mental State Examination in evaluating consent capacity in Alzheimer’s disease. Psychiatr Serv. 2002;53(10):1322–1324. [DOI] [PubMed] [Google Scholar]
- 29. Kim SY, Caine ED, Currier GW, Leibovici A, Ryan JM.. Assessing the competence of persons with Alzheimer’s disease in providing informed consent for participation in research. Am J Psychiatry. 2001;158(5):712–717. [DOI] [PubMed] [Google Scholar]
- 30. Kim SY, Cox C, Caine ED. Impaired decision-making ability in subjects with Alzheimer’s disease and willingness to participate in research. Am J Psychiatry. 2002;159(5):797–802. [DOI] [PubMed] [Google Scholar]
- 31. Kim SY, Karlawish JH, Kim HM, Wall IF, Bozoki AC, Appelbaum PS.. Preservation of the capacity to appoint a proxy decision maker: implications for dementia research. Arch Gen Psychiatry. 2011;68(2):214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Marson DC, Earnst KS, Jamil F, Bartolucci A, Harrell LE.. Consistency of physicians’ legal standard and personal judgments of competency in patients with Alzheimer’s disease. J Am Geriatr Soc. 2000;48(8):911–918. [DOI] [PubMed] [Google Scholar]
- 33. Mitoku K, Shimanouchi S. The decision-making and communication capacities of older adults with dementia: a population-based study. Open Nurs J. 2014;8:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Moye J, Karel MJ, Azar AR, Gurrera RJ.. Capacity to consent to treatment: empirical comparison of three instruments in older adults with and without dementia. Gerontologist. 2004;44(2):166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Moye J, Karel MJ, Gurrera RJ, Azar AR.. Neuropsychological predictors of decision-making capacity over 9 months in mild-to-moderate dementia. J Gen Intern Med. 2006;21(1):78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mueller T, Haberstroh J, Knebel M, et al. Assessing capacity to consent to treatment with cholinesterase inhibitors in dementia using a specific and standardized version of the MacArthur Competence Assessment Tool (MacCAT-T). Int Psychogeriatr. 2017;29(2):333–343. [DOI] [PubMed] [Google Scholar]
- 37. Okonkwo OC, Griffith HR, Copeland JN, et al. Medical decision-making capacity in mild cognitive impairment: a 3-year longitudinal study. Neurology. 2008;71(19):1474–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Okonkwo O, Griffith HR, Belue K, et al. Medical decision-making capacity in patients with mild cognitive impairment. Neurology. 2007;69(15):1528–1535. [DOI] [PubMed] [Google Scholar]
- 39. Palmer BW, Harmell AL, Pinto LL, et al. Determinants of capacity to consent to research on Alzheimer’s disease. Clin Gerontol. 2017;40(1):24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pucci E, Belardinelli N, Borsetti G, Rodriguez D, Signorino M.. Information and competency for consent to pharmacologic clinical trials in Alzheimer disease: an empirical analysis in patients and family caregivers. Alzheimer Dis Assoc Disord. 2001;15(3):146–154. [DOI] [PubMed] [Google Scholar]
- 41. Rubright J, Sankar P, Casarett DJ, Gur R, Xie SX, Karlawish J.. A memory and organizational aid improves Alzheimer disease research consent capacity: results of a randomized, controlled trial. Am J Geriatr Psychiatry. 2010;18(12):1124–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Santos RL, Barroso de Sousa MF, Simões Neto JP, et al. MacArthur competence assessment tool for treatment in Alzheimer disease: cross-cultural adaptation. Arq Neuropsiquiatr. 2017;75(1):36–43. [DOI] [PubMed] [Google Scholar]
- 43. Stocking CB, Hougham GW, Danner DD, Patterson MB, Whitehouse PJ, Sachs GA.. Variable judgments of decisional capacity in cognitively impaired research subjects. J Am Geriatr Soc. 2008;56(10):1893–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Stormoen S, Almkvist O, Eriksdotter M, Sundström E, Tallberg IM.. Cognitive predictors of medical decision-making capacity in mild cognitive impairment and Alzheimer’s disease. Int J Geriatr Psychiatry. 2014;29(12):1304–1311. [DOI] [PubMed] [Google Scholar]
- 45. Thalén L, Heimann Mühlenbock K, Almkvist O, Eriksdotter M, Sundström E, Tallberg IM.. Do adapted vignettes improve medical decision-making capacity for individuals with Alzheimer’s disease? Scand J Psychol. 2017;58(6):497–503. [DOI] [PubMed] [Google Scholar]
- 46. Vollmann J, Bauer A, Danker-Hopfe H, Helmchen H.. Competence of mentally ill patients: a comparative empirical study. Psychol Med. 2003;33(8):1463–1471. [DOI] [PubMed] [Google Scholar]
- 47. Abu Snineh M, Camicioli R, Miyasaki JM. Decisional capacity for advanced care directives in Parkinson’s disease with cognitive concerns. Parkinsonism Relat Disord. 2017;39:77–79. [DOI] [PubMed] [Google Scholar]
- 48. Martin RC, Okonkwo OC, Hill J, et al. Medical decision-making capacity in cognitively impaired Parkinson’s disease patients without dementia. Mov Disord. 2008;23(13):1867–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dani KA, McCormick MT, Muir KW. Brain lesion volume and capacity for consent in stroke trials: potential regulatory barriers to the use of surrogate markers. Stroke. 2008;39(8):2336–2340. [DOI] [PubMed] [Google Scholar]
- 50. Basso MR, Candilis PJ, Johnson J, Ghormley C, Combs DR, Ward T.. Capacity to make medical treatment decisions in multiple sclerosis: a potentially remediable deficit. J Clin Exp Neuropsychol. 2010;32(10):1050–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gerstenecker A, Lowry K, Myers T, et al. Medical decision-making capacity and its cognitive predictors in progressive MS: preliminary evidence. J Neurol Sci. 2017;380:38–43. [DOI] [PubMed] [Google Scholar]
- 52. Gurrera RJ, Karel MJ, Azar AR, Moye J.. Neuropsychological performance within-person variability is associated with reduced treatment consent capacity. Am J Geriatr Psychiatry. 2014;22(11):1200–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Horton-Deutsch S, Twigg P, Evans R. Health care decision-making of persons with dementia. Dementia. 2007;6(1):105–120. [Google Scholar]
- 54. Karlawish J, Cary M, Moelter ST, et al. Cognitive impairment and PD patients’ capacity to consent to research. Neurology. 2013;81(9):801–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Appelbaum PS, Grisso T. MacArthur Competence Assessment Tool for Clinical Research (MacCAT-CR). Sarasota, FL: Professional Resource Press; 2001. [Google Scholar]
- 56. Grisso T, Appelbaum PS. Assessing Competence to Consent to Treatment. New York, Oxford: Oxford University Press; 1998. [Google Scholar]
- 57. Marson DC, Ingram KK, Cody HA, Harrell LE.. Assessing the competency of patients with Alzheimer’s disease under different legal standards. A prototype instrument. Arch Neurol. 1995;52(10):949–954. [DOI] [PubMed] [Google Scholar]
- 58. Iseli LM, Wangmo T, Hermann H, Trachsel M, Elger BS. Evaluating decision-making capacity challenges faced by clinicians in Switzerland. Geropsych 2018;31(2):67–75. [Google Scholar]
- 59. Moelter ST, Weintraub D, Mace L, et al. Research consent capacity varies with executive function and memory in Parkinson’s disease. Mov Disord. 2016;31(3):414–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pace A, Dirven L, Koekkoek JAF, et al. ; European Association of Neuro-Oncology Palliative Care Task Force European Association for Neuro-Oncology (EANO) guidelines for palliative care in adults with glioma. Lancet Oncol. 2017;18(6):e330–e340. [DOI] [PubMed] [Google Scholar]
- 61. Gambina G, Bonazzi A, Valbusa V, et al. Awareness of cognitive deficits and clinical competence in mild to moderate Alzheimer’s disease: their relevance in clinical practice. Neurol Sci. 2014;35(3):385–390. [DOI] [PubMed] [Google Scholar]
- 62. Caron CD, Griffith J, Arcand M. Decision making at the end of life in dementia: how family caregivers perceive their interactions with health care providers in long-term-care setting. J Appl Gerontol. 2005;24(3):231–247. [Google Scholar]
- 63. Hanson LC, Carey TS, Caprio AJ, et al. Improving decision-making for feeding options in advanced dementia: a randomized, controlled trial. J Am Geriatr Soc. 2011;59(11):2009–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lamahewa K, Mathew R, Iliffe S, et al. A qualitative study exploring the difficulties influencing decision making at the end of life for people with dementia. Health Expect. 2018;21(1):118–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Teno JM, Mitchell SL, Kuo SK, et al. Decision-making and outcomes of feeding tube insertion: a five-state study. J Am Geriatr Soc. 2011;59(5):881–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Black BS, Fogarty LA, Phillips H, et al. Surrogate decision-makers’ understanding of dementia patients’ prior wishes for end-of-life care. J Aging Health. 2009;21(4):627–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Forbes S, Bern-Klug M, Gessert C. End-of-life decision making for nursing home residents with dementia. J Nurs Scholarsh. 2000;32(3):251–258. [DOI] [PubMed] [Google Scholar]
- 68. Rurup ML, Onwuteaka-Philipsen BD, Pasman HR, Ribbe MW, van der Wal G.. Attitudes of physicians, nurses and relatives towards end-of-life decisions concerning nursing home patients with dementia. Patient Educ Couns. 2006;61(3):372–380. [DOI] [PubMed] [Google Scholar]
- 69. World Medical Association. WMA Declaration of Helsinki—ethical principles for medical research involving human subjects. July 9, 2018. https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/. Accessed May 21, 2020.
- 70. Goodman C, Baron NL, Machen I, et al. Culture, consent, costs and care homes: enabling older people with dementia to participate in research. Aging Ment Health. 2011;15(4):475–481. [DOI] [PubMed] [Google Scholar]
- 71. Karlawish J, Kim SY, Knopman D, van Dyck CH, James BD, Marson D.. The views of Alzheimer disease patients and their study partners on proxy consent for clinical trial enrollment. Am J Geriatr Psychiatry. 2008;16(3):240–247. [DOI] [PubMed] [Google Scholar]
- 72. Bryant J, Skolarus LE, Smith B, Adelman EE, Meurer WJ.. The accuracy of surrogate decision makers: informed consent in hypothetical acute stroke scenarios. BMC Emerg Med. 2013;13:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Chen DT, Case LD, Brott TG, et al. ; ISGS Investigators Impact of restricting enrollment in stroke genetics research to adults able to provide informed consent. Stroke. 2008;39(3):831–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Demarquay G, Derex L, Nighoghossian N, et al. Ethical issues of informed consent in acute stroke. Analysis of the modalities of consent in 56 patients enrolled in urgent therapeutic trials. Cerebrovasc Dis. 2005;19(2):65–68. [DOI] [PubMed] [Google Scholar]
- 75. Flaherty ML, Karlawish J, Khoury JC, Kleindorfer D, Woo D, Broderick JP.. How important is surrogate consent for stroke research? Neurology. 2008;71(20):1566–1571. [DOI] [PubMed] [Google Scholar]
- 76. Jackson C, Crossland L, Dennis M, Wardlaw J, Sudlow C.. Assessing the impact of the requirement for explicit consent in a hospital-based stroke study. QJM. 2008;101(4):281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Palmer BW, Harmell AL, Dunn LB, et al. Multimedia aided consent for Alzheimer’s disease research. Clin Gerontol. 2018;41(1):20–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ayalon L, Bachner YG, Dwolatzky T, Heinik J.. Preferences for end-of-life treatment: concordance between older adults with dementia or mild cognitive impairment and their spouses. Int Psychogeriatr. 2012;24(11):1798–1804. [DOI] [PubMed] [Google Scholar]
- 79. Fetherstonhaugh D, McAuliffe L, Bauer M, Shanley C.. Decision-making on behalf of people living with dementia: how do surrogate decision-makers decide? J Med Ethics. 2017;43(1):35–40. [DOI] [PubMed] [Google Scholar]
- 80. Caron CD Griffith J, Arcand M. End-of-life decision making in dementia: the perspective of family caregivers. Dementia. 2005;4(1):113–136. [Google Scholar]
- 81. de Boer ME, Depla M, Wojtkowiak J, et al. Life-and-death decision-making in the acute phase after a severe stroke: interviews with relatives. Palliat Med. 2015;29(5):451–457. [DOI] [PubMed] [Google Scholar]
- 82. Luke L, Redley M, Clare I, Holland A.. Hospital clinicians’ attitudes towards a statutory advocacy service for patients lacking mental capacity: implications for implementation. J Health Serv Res Policy. 2008;13(2):73–78. [DOI] [PubMed] [Google Scholar]
- 83. St-Amant O, Ward-Griffin C, DeForge RT, et al. Making care decisions in home-based dementia care: why context matters. Can J Aging. 2012;31(4):423–434. [DOI] [PubMed] [Google Scholar]
- 84. Carter G, McLaughlin D, Kernohan WG, et al. The experiences and preparedness of family carers for best interest decision-making of a relative living with advanced dementia: a qualitative study. J Adv Nurs. 2018;74(7):1595–1604. [DOI] [PubMed] [Google Scholar]
- 85. Black BS, Wechsler M, Fogarty L. Decision making for participation in dementia research. Am J Geriatr Psychiatry. 2013;21(4):355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Boxall L, Hemsley A, White N. Exploring recruitment issues in stroke research: a qualitative study of nurse researchers’ experiences. Nurse Res. 2016;23(5):8–14. [DOI] [PubMed] [Google Scholar]
- 87. Kim SY, Kim HM, Ryan KA, et al. How important is ‘accuracy’ of surrogate decision-making for research participation? PLoS One. 2013;8(1):e54790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Overton E, Appelbaum PS, Reyes Fisher S, Dohan D, Weiss Roberts L, Dunn LB.. Alternative decision-makers’ perspectives on assent and dissent for dementia research. Am J Geriatr Psychiatry. 2013;21(4):346–354. [DOI] [PubMed] [Google Scholar]
- 89. Gainotti S, Fusari Imperatori S, Spila-Alegiani S, et al. How are the interests of incapacitated research participants protected through legislation? An Italian study on legal agency for dementia patients. PLoS One. 2010;5(6):e11150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Shanley C, Fetherstonhaugh D, McAuliffe L, Bauer M, Beattie E.. Providing support to surrogate decision-makers for people living with dementia: healthcare professional, organisational and community responsibilities. Health Soc Care Community. 2017;25(5):1563–1570. [DOI] [PubMed] [Google Scholar]
- 91. Bilodeau G, Witteman H, Légaré F, et al. Reducing complexity of patient decision aids for community-based older adults with dementia and their caregivers: multiple case study of decision boxes. BMJ Open. 2019;9(5):e027727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Reen GK, Silber E, Langdon DW. Best methods of communicating clinical trial data to improve understanding of treatments for patients with multiple sclerosis. Value Health. 2018;21(7):762–766. [DOI] [PubMed] [Google Scholar]
- 93. Reinert C, Kremmler L, Burock S, et al. Quantitative and qualitative analysis of study-related patient information sheets in randomised neuro-oncology phase III-trials. Eur J Cancer. 2014;50(1):150–158. [DOI] [PubMed] [Google Scholar]
- 94. Ponzio M, Uccelli MM, Lionetti S, et al. User testing as a method for evaluating subjects’ understanding of informed consent in clinical trials in multiple sclerosis. Mult Scler Relat Disord. 2018;25:108–111. [DOI] [PubMed] [Google Scholar]
- 95. Holden TR, Keller S, Kim A, et al. Procedural framework to facilitate hospital-based informed consent for dementia research. J Am Geriatr Soc. 2018;66(12):2243–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Brémault-Phillips S, Pike A, Charles L, et al. Facilitating implementation of the Decision-Making Capacity Assessment (DMCA) Model: senior leadership perspectives on the use of the National Implementation Research Network (NIRN) Model and frameworks. BMC Res Notes. 2018;11(1):607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Coomans MB, van der Linden SD, Gehring K, Taphoorn MJB.. Treatment of cognitive deficits in brain tumour patients: current status and future directions. Curr Opin Oncol. 2019;31(6):540–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Fritz L, Dirven L, Reijneveld JC, et al. Advance care planning in glioblastoma patients. Cancers (Basel). 2016;8(11):102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Fritz L, Zwinkels H, Koekkoek JAF, et al. Advance care planning in glioblastoma patients: development of a disease-specific ACP program. Support Care Cancer. 2020;28(3):1315–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.