Key Points
Question
Can pharmacogenetic results for statin myopathy risk be used clinically without the unintended harms of statin avoidance or underdosing?
Findings
In this randomized clinical trial including 408 patients, statin-naive patients whose physicians knew their SLCO1B1 genotype results at baseline did not have poorer low-density lipoprotein cholesterol reductions after 1 year, compared with patients who received usual care.
Meaning
Although these findings do not support the widespread adoption of stand-alone preemptive SLCO1B1 genotype testing, they may allay stakeholder concerns about the potential unintended harms of the clinical use of such information.
This randomized clinical trial examines the impact of delivering SLCO1B1 pharmacogenetic results to physicians on low-density lipoprotein cholesterol levels and concordance with prescribing guidelines for statin safety and effectiveness.
Abstract
Importance
Nonadherence to statin guidelines is common. The solute carrier organic anion transporter family member 1B1 (SLCO1B1) genotype is associated with simvastatin myopathy risk and is proposed for clinical implementation. The unintended harms of using pharmacogenetic information to guide pharmacotherapy remain a concern for some stakeholders.
Objective
To determine the impact of delivering SLCO1B1 pharmacogenetic results to physicians on the effectiveness of atherosclerotic cardiovascular disease (ASCVD) prevention (measured by low-density lipoprotein cholesterol [LDL-C] levels) and concordance with prescribing guidelines for statin safety and effectiveness.
Design, Setting, and Participants
This randomized clinical trial was performed from December 2015 to July 2019 at 8 primary care practices in the Veterans Affairs Boston Healthcare System. Participants included statin-naive patients with elevated ASCVD risk. Data analysis was performed from October 2019 to September 2020.
Interventions
SLCO1B1 genotyping and results reporting to primary care physicians at baseline (intervention group) vs after 1 year (control group).
Main Outcomes and Measures
The primary outcome was the 1-year change in LDL-C level. The secondary outcomes were 1-year concordance with American College of Cardiology–American Heart Association and Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for statin therapy and statin-associated muscle symptoms (SAMS).
Results
Among 408 patients (mean [SD] age, 64.1 [7.8] years; 25 women [6.1%]), 193 were randomized to the intervention group and 215 were randomized to the control group. Overall, 120 participants (29%) had a SLCO1B1 genotype indicating increased simvastatin myopathy risk. Physicians offered statin therapy to 65 participants (33.7%) in the intervention group and 69 participants (32.1%) in the control group. Compared with patients whose physicians did not know their SLCO1B1 results at baseline, patients whose physicians received the results had noninferior reductions in LDL-C at 12 months (mean [SE] change in LDL-C, −1.1 [1.2] mg/dL in the intervention group and −2.2 [1.3] mg/dL in the control group; difference, −1.1 mg/dL; 90% CI, −4.1 to 1.8 mg/dL; P < .001 for noninferiority margin of 10 mg/dL). The proportion of patients with American College of Cardiology–American Heart Association guideline-concordant statin prescriptions in the intervention group was noninferior to that in the control group (12 patients [6.2%] vs 14 patients [6.5%]; difference, −0.003; 90% CI, −0.038 to 0.032; P < .001 for noninferiority margin of 15%). All patients in both groups were concordant with CPIC guidelines for safe statin prescribing. Physicians documented 2 and 3 cases of SAMS in the intervention and control groups, respectively, none of which was associated with a CPIC guideline–discordant prescription. Among patients with a decreased or poor SLCO1B1 transporter function genotype, simvastatin was prescribed to 1 patient in the control group but none in the intervention group.
Conclusions and Relevance
Clinical testing and reporting of SLCO1B1 results for statin myopathy risk did not result in poorer ASCVD prevention in a routine primary care setting and may have been associated with physicians avoiding simvastatin prescriptions for patients at genetic risk for SAMS. Such an absence of harm should reassure stakeholders contemplating the clinical use of available pharmacogenetic results.
Trial Registration
ClinicalTrials.gov Identifier: NCT02871934
Introduction
Nearly all patients carry 1 or more genetic variants deemed actionable for their association with either the effectiveness or safety of at least 1 medication.1,2 High-quality evidence for such pharmacogenetic associations derives from decades of knowledge about candidate genes involved in pharmacokinetic pathways and from more recent developments in genome-wide association studies and large-scale phenotyping of drug response.3 However, validation of these drug-gene associations alone is insufficient to demonstrate whether the clinical use of that information is associated with improved patient outcomes. The absence of such outcomes data remains a barrier to the adoption of pharmacogenetic testing by health care practitioners, health systems, and payers. Indeed, the US Food and Drug Administration (FDA) has warned laboratories to stop marketing certain pharmacogenetic tests that it has not reviewed for safety and effectiveness, citing concerns that physicians and patients will change drug therapy on the basis of such results, potentially leading to incorrect treatment and serious health consequences.4,5
One well-described pharmacogenetic association is that between the solute carrier organic anion transporter family member 1B1 (SLCO1B1) gene and statin-associated muscle symptoms (SAMS). Statins, or 3-hydroxy-3-methylglutaryl-CoA reductase inhibitors, are cholesterol-lowering medications used by millions of patients for the primary and secondary prevention of atherosclerotic cardiovascular disease (ASCVD).6 In 2008, a genome-wide association study7 identified an association between the common nonsynonymous c.521T>C variant in SLCO1B1 (rs4149056) and severe simvastatin-related myopathy, and subsequent studies8,9,10 have reported an association between this variant and milder phenotypes of statin intolerance. The association between this genetic variant and SAMS appears strongest for simvastatin specifically; as a result, the Clinical Pharmacogenetics Implementation Consortium (CPIC) has published guidelines for simvastatin prescribing and dosing when a patient’s SLCO1B1 genotype is known.11 However, whether integrating SLCO1B1 testing into routine clinical care improves patient outcomes is unknown.12 Of particular relevance to statins is the question of whether pharmacogenetic results might influence physician and patient behavior around initiation of and adherence to therapy, given that concordance with recommended guidelines is suboptimal in many real-world clinical settings.13,14,15 In an era when patients increasingly have information about their genetic make-up, including SLCO1B1 genotype, from clinical or commercial sources, it might be more important to demonstrate that the clinical use of that information does not have the unintended consequence of worsening ASCVD prevention efforts than to demonstrate that it prevents simvastatin myopathy.
We conducted a noninferiority randomized clinical trial to test the primary hypothesis that SLCO1B1 genotyping among statin-naive primary care patients with ASCVD risk factors does not worsen 12-month reductions in low-density lipoprotein cholesterol (LDL-C). Prespecified secondary outcomes included 12-month concordance with CPIC guidelines for simvastatin use, concordance with statin guidelines for ASCVD prevention, and physician-documented SAMS.
Methods
Study Design and Oversight
The Integrating Pharmacogenetics in Clinical Care (I-PICC) Study was a pragmatic randomized clinical trial comparing the delivery of SLCO1B1 pharmacogenetic results to primary care physicians vs usual care. Detailed descriptions of the trial design, pragmatic elements, and recruitment and enrollment have been published previously.16,17 The Veterans Affairs (VA) Boston Healthcare System institutional review board approved this study. The trial protocol is provided in Supplement 1. This study follows the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Setting
The I-PICC Study enrolled physicians and patients across 8 primary care practices in the VA Boston Healthcare System in eastern Massachusetts. Patient enrollment occurred from December 2015 to July 2018, and all patients were followed up for 1 year, through July 2019.
Participants
All primary care physicians at the 8 locations were eligible to participate. Patient eligibility criteria were assignment to an enrolled physician, age 40 to 75 years, absence of prior statin prescription confirmed both by medical record review and patient telephone call, and at least 1 of the following ASCVD risk factors specified in the American College of Cardiology–American Heart Association (ACC-AHA) guidelines: prior ASCVD, diabetes, LDL-C level greater than or equal to 190 mg/dL (to convert to millimoles per liter, multiply by 0.0259), or 10-year ASCVD risk greater than or equal to 7.5%.18
Recruitment, Enrollment, and Randomization
After brief presentations at staff meetings, physicians gave written informed consent for their own participation through the electronic health record (EHR).16 Patients gave oral consent to participation by telephone call with the study staff but were not enrolled unless and until they underwent a blood draw as part of their routine clinical care. A daily semiautomated electronic query alerted study staff each time the clinical laboratory received a whole-blood specimen for a consented patient (eg, for complete blood count or hemoglobin A1c testing), at which time the study staff forwarded a laboratory order for SLCO1B1 genotyping through the EHR as a clinical alert to the enrolled physician for signature. The physician’s signature of the laboratory order enrolled the patient in the study. Upon signature of the laboratory order, the extant blood sample was sent to a reference laboratory (Boston Heart Diagnostics, Framingham, MA) for SLCO1B1 rs4149056 genotyping, and the participants were randomly allocated to having their SLCO1B1 results delivered to their physician at baseline (intervention group) or after 12 months (control group).
Intervention
The SLCO1B1 results were entered as structured data in the EHR after a median (interquartile range) turnaround time of 8 (7-9) days after enrollment of each patient in the intervention group. The results screen included genotype and standardized terms for drug transporter function phenotype (T/T, normal function; T/C, decreased function; or C/C, poor function)19 and CPIC recommendations for the use and dosing of simvastatin when SLCO1B1 genotype is known.11 A clinical alert notified the ordering physician when the results were reported in the EHR. The patient’s calculated 10-year ASCVD risk or other potential indication for statin therapy was not explicitly communicated to the physician. Because this trial endeavored to model routine medical practice, study staff members themselves did not send the SLCO1B1 results directly to patients during the observation period, but an optional SLCO1B1 results and interpretation letter template was available in the EHR for physicians to communicate results to their patients.16 For patients allocated to the control group, physicians received no further communication from the study staff after patient enrollment until the end of the 12-month observation period, at which time their SLCO1B1 results were delivered to their physicians through the EHR.
Outcomes
Primary Outcome
Data on outcomes were collected from the VA corporate data warehouse,20 EHR review, and a brief end-of-study patient telephone survey, as described elsewhere.16 The primary outcome was change in LDL-C, defined as the most recent LDL-C value on or before the enrollment date subtracted from the most recent LDL-C value 12 months after enrollment. Baseline LDL-C values were carried forward for any patient who did not undergo repeated LDL-C testing during the observation period.
Secondary Outcomes
The study had 3 prespecified secondary outcomes, as described elsewhere16: (1) concordance with CPIC guidelines for simvastatin use, determined by comparing each participant’s SLCO1B1 genotype and statin type and dose 12 months after enrollment11; (2) concordance with ACC-AHA guidelines for ASCVD prevention, determined by comparing each participant’s ASCVD risk profile with the intensity of their statin therapy 12 months after enrollment18; and (3) physician-documented SAMS during the 12-month observation period, determined from medical record review of all patient notes during the 12 months after enrollment. Additional prespecified exploratory outcomes included initiation of and changes to statin therapy during the 12-month observation period; patient continuous medication adherence to statin therapy, derived from pharmacy data and defined as proportion of days covered by medication possession greater than or equal to 80%21,22; recall of genetic testing and results; and patient-perceived necessity of and concerns about medications.23
Statistical Analysis
Statistical analysis was conducted using SAS statistical software version 9.4 (SAS Institute). Outcomes were analyzed with an intention-to-treat approach by randomization group. For the primary outcome of 12-month change in LDL-C, we used generalized estimating equations (GEEs)24 accounting for clustering by physician to derive marginal mean estimates. By use of a noninferiority design, GEEs tested the primary null hypothesis that SLCO1B1 testing resulted in poorer 12-month LDL-C reductions compared with no testing by a prespecified margin of greater than 10 mg/dL, chosen for its association with a reduction in 5-year ASCVD risk of 5%.25 We used GEEs assuming independence to test the null hypothesis that the proportion of patients in the control group whose prescriptions at 12 months met ACC-AHA guidelines for ASCVD prevention was better by a noninferiority margin of 15% compared with the intervention group. Ninety percent confidence intervals for noninferiority testing, corresponding to a 1-sided α = .05, were based on GEE estimates of the difference in outcomes between groups and their robust SEs. We estimated Fisher exact tests to test the null hypotheses that the proportion of patients with CPIC guideline concordance and with SAMS 12 months after enrollment did not differ between the 2 groups, using a superiority design. A sample size of 408 total patients enabled 80% or higher power at a 1-sided α = .05 to exclude a between-group noninferiority margin of 10 mg/dL in the primary outcome of LDL-C 12 months after enrollment and 80% or higher power at a 2-sided α = .05 to detect a between-group difference of 15% in the secondary outcome of CPIC guideline concordance. Exploratory outcomes are presented with descriptive statistics without hypothesis testing. Data analysis was performed from October 2019 to September 2020.
Results
Participant Characteristics
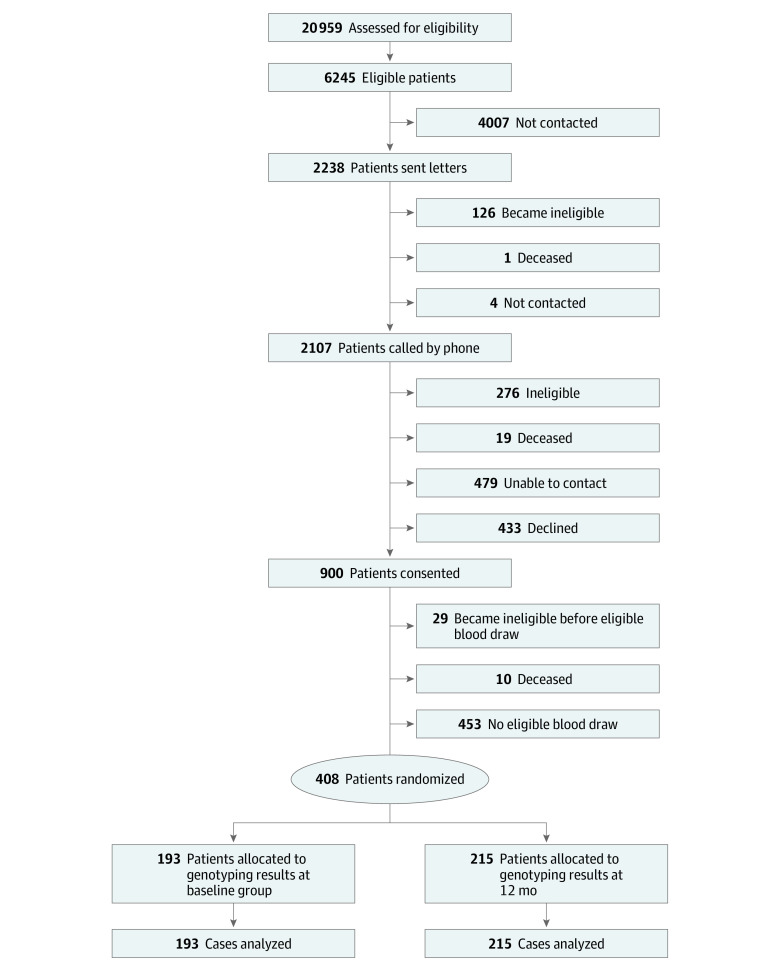
Enrollment and randomization of the prespecified sample size of 408 patients, cared for by 47 physicians, was completed on July 17, 2018 (Figure 1).17 The mean (SD) age of the participants was 64.1 (7.8) years, 25 (6.1%) were women, 56 (13.7%) were non-White, and 8 (2.0%) were of Hispanic or Latino ethnicity (Table 1). Of the patients, 193 were randomized to the intervention group (with genotyping results known at baseline), and 215 were randomized to the control group with (genotyping results unknown at baseline). Overall, 98 patients (24.0%) had diabetes and 98 (24.0%) had prior ASCVD; 223 patients (54.7%) were potentially eligible for statin therapy only because they had 10-year ASCVD risk of 7.5% or higher. Overall, 120 participants (29%) had a SLCO1B1 genotype indicating increased simvastatin myopathy risk (T/C or C/C genotype) (Table 1).
Figure 1. Patient Flowchart for the Integrating Pharmacogenetics in Clinical Care Study.
Table 1. Baseline Characteristics of Integrating Pharmacogenetics in Clinical Care Study Participants.
| Characteristic | Participants, No. (%) | |
|---|---|---|
| Genotyping results known at baseline (n = 193) | Genotyping results known at 12 mo (n = 215) | |
| Age, mean (SD), y | 64.2 (7.8) | 63.9 (7.7) |
| Women | 9 (4.7) | 16 (7.4) |
| Non-White racea | 30 (15.5) | 26 (12.1) |
| Hispanic or Latino ethnicitya | 2 (1.0) | 6 (2.8) |
| Smokers | 59 (30.6) | 78 (36.3) |
| Meeting ACC-AHA statin criteriab | ||
| ASCVD | 52 (26.9) | 46 (21.4) |
| LDL-C >190 mg/dL | 5 (2.6) | 6 (2.8) |
| Diabetes | 47 (24.4) | 51 (23.7) |
| 10-y ASCVD risk ≥7.5% | 171 (88.6) | 196 (91.2) |
| SLCO1B1 genotype | ||
| Normal function (T/T) | 148 (76.7) | 140 (65.1) |
| Decreased function (T/C) | 40 (20.7) | 70 (32.6) |
| Poor function (C/C) | 5 (2.6) | 5 (2.3) |
Abbreviations: ACC-AHA, American College of Cardiology–American Heart Association; ASCVD, atherosclerotic cardiovascular disease; LDL-C, low-density lipoprotein cholesterol.
SI conversion factor: To convert LDL-C to mmol/L, multiply by 0.0259.
Race and ethnicity were collected from administrative data to assess generalizability of enrolled cohort to overall health care system population.
Categories sum to greater than 100% because criteria are not mutually exclusive (see text).
Statin Prescriptions
During the study, physicians documented offering statin therapy to 65 participants (33.7%) in the intervention group and 69 participants (32.1%) in the control group, among whom 42 (21.8% of total) and 50 (23.3% of total) declined, respectively (Table 2). Statin therapy was prescribed at some time during the 12-month study period for 26 patients (13.5% of total) in the intervention group and 24 patients (11.1% of total) in the control group.
Table 2. Statin Prescription Outcomes of the Integrating Pharmacogenetics in Clinical Care Study.
| Outcome | Participants, No. (%) | |
|---|---|---|
| Genotyping results known at baseline (n = 193) | Genotyping results known at 12 mo (n = 215) | |
| Statin offered by physician | 65 (33.7) | 69 (32.1) |
| Statin declineda | 42 (64.6) | 50 (72.5) |
| Statin prescribeda | 26 (40.0) | 24 (34.8) |
| Statin adherenceb | 9 (45.0) | 9 (45.0) |
| Statin discontinued | 3 (11.5) | 4 (16.7) |
| ACC-AHA concordance at 12 moc | 12 (6.2) | 14 (6.5) |
| CPIC concordance at 12 mod | 193 (100.0) | 215 (100.0) |
Abbreviations: ACC-AHA, American College of Cardiology–American Heart Association; CPIC, Clinical Pharmacogenetics Implementation Consortium.
Percentages may sum to more than 100% because a patient could both initially decline statin therapy and then be prescribed statin therapy later during the observation period.
Denotes the number of participants with proportion of days covered by medication possession greater than or equal to 80% from statin initiation through the end of study enrollment; calculable denominators for each group are 20 participants.
P < .001, corresponding to 1-sided noninferiority test assuming margin of 15% favoring control.
P > .99, corresponding to 2-sided test for superiority.
Primary Outcome
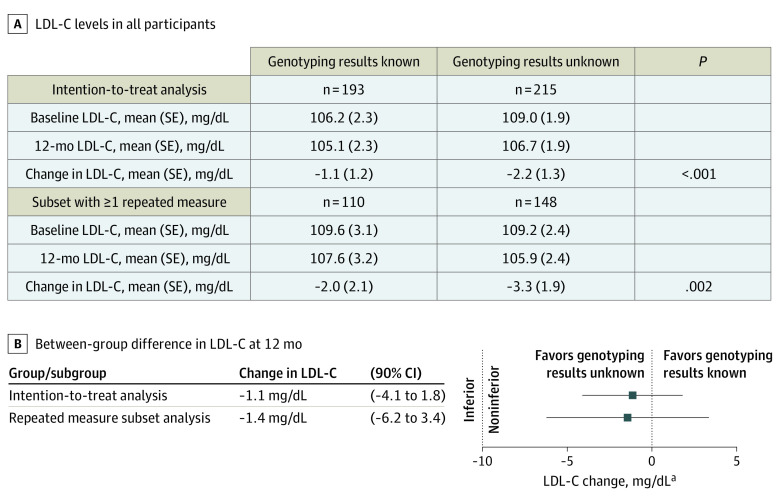
The mean (SE) LDL-C level at baseline was 106.2 (2.3) mg/dL in the intervention group and 109.0 (1.9) mg/dL in the control group (Figure 2). After 12 months of follow-up, the mean (SE) change in LDL-C was −1.1 (1.2) mg/dL in the intervention group and −2.2 (1.3) mg/dL in the control group. The between-group difference was consistent with the prespecified alternative hypothesis that SLCO1B1 testing does not worsen LDL-C levels by more than the noninferiority margin of 10 mg/dL, compared with usual care (difference, −1.1 mg/dL; 90% CI, −4.1 to 1.8 mg/dL; P < .001). Analysis among the subset of 258 patients with at least 1 repeated LDL-C measurement during the 12 months of follow-up yielded consistent results (difference, −1.4 mg/dL; 95% CI, −6.2 to 3.4 mg/dL; P = .002 (Figure 2). Eighty-one patients (42.0%) in the intervention group and 88 patients (40.9%) in the control group had end-of-study LDL-C values less than 100 mg/dL.
Figure 2. Change in Low-Density Lipoprotein Cholesterol (LDL-C) Values Among Integrating Pharmacogenetics in Clinical Care Study Participants.
SI conversion factor: To convert LDL-C to mmol/L, multiply by 0.0259.
Secondary Outcomes
Twelve months after enrollment, 12 patients (6.2%) in the intervention group and 14 patients (6.5%) in the control group had statin prescriptions that were concordant with ACC-AHA guidelines for statin therapy for ASCVD prevention (difference, −0.003; 90% CI, −0.038 to 0.032; P < .001 for noninferiority margin of 15%) (Table 2). All patients in both groups were concordant with CPIC guidelines for genotype-based safe statin dosing at 12 months (difference, 0.0; Fisher exact test P > .99) (Table 2). Physicians documented 2 (1.0%) and 3 (1.4%) possible cases of SAMS in the intervention and control groups, respectively (difference, 0.004; Fisher exact test P > .99) (eTable 1 in Supplement 2); only 1 of these was associated with simvastatin, prescribed at a dose of 20 mg for a patient with the normal T/T genotype in the intervention group. The physician of another patient in the control group with the decreased SLCO1B1 transporter function T/C genotype documented possible SAMS with an atorvastatin dose of 20 mg, before the patient or physician knew the genotype results.
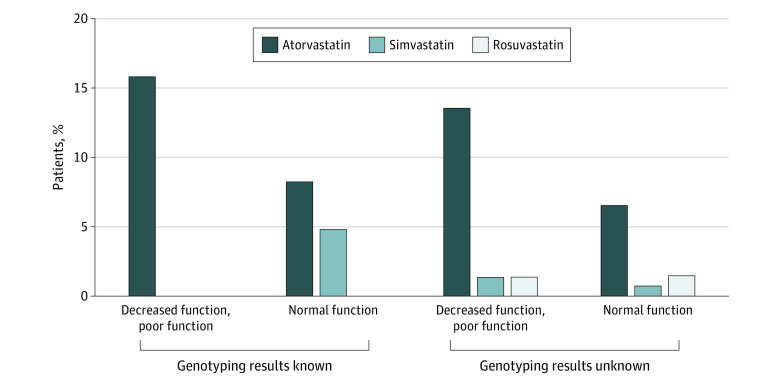
Of the 26 statin prescriptions in the intervention group, 7 were for simvastatin, and all of these were for patients with the normal transporter T/T genotype (Figure 3). Atorvastatin was the only statin prescribed to 7 patients in the intervention group with a decreased or poor transporter genotype (T/C or C/C). In contrast, approximately equal numbers of patients with the T/T genotype and with the T/C or C/C genotypes received prescriptions for atorvastatin, rosuvastatin, and simvastatin in the control group, including 1 patient with the T/C genotype who was prescribed simvastatin 20 mg (Figure 3). Among the 50 patients for whom statin therapy was initiated, statin adherence, defined as greater than or equal to 80% of days covered, was achieved by equal numbers in the 2 groups (Table 2).
Figure 3. Statin Initiations at 12 Months by Study Group and Genotype Among Integrating Pharmacogenetics in Clinical Care Study Participants With Known and Unknown Genotyping Results.
Bars represent percentages of patients with a given genotype and study group assignment who were prescribed statin therapy by 12 months. Among patients whose genotyping results were known at baseline, 45 had the genotype for decreased or poor function and 148 had the genotype for normal function. Among patients whose genotyping results were not known at baseline, 75 had the genotype for decreased or poor function and 140 had the genotype for normal function.
Exploratory Outcomes
Among the 193 patients in the intervention group, physicians entered additional documentation about the SLCO1B1 results in the EHR for 32 patients (16.6%) and documented communicating results to 30 patients (15.5%) during the 12 months after enrollment (eTable 2 in Supplement 2). In the 12-month survey, only 11 patients (6.5%) in the intervention group recalled having undergone a pharmacogenetic test for SAMS risk in the prior year, of whom only 2 correctly recalled the interpretation of their results. At 12 months, patients in the intervention and control groups did not differ in their perceived necessity of and concerns about medications (eTable 3 in Supplement 2).
Discussion
In this randomized clinical trial, preemptive SLCO1B1 genotype testing among statin-naive patients was noninferior to no testing in reducing LDL-C and achieving concordance with ASCVD prevention guidelines. No physician prescribed simvastatin to a patient known to have decreased or poor SLCO1B1 transporter function genotype. Although these results do not support a patient benefit from stand-alone preemptive SLCO1B1 genotyping, they help allay concerns about the potential unintended harms of using such pharmacogenetic results in medical practice if they are available.
Many health care systems have launched pharmacogenetic testing programs, often in the context of research studies or clinical innovation demonstration projects.26,27,28,29,30,31,32,33,34 Most of these endeavors have chosen to implement some number of well-validated drug-gene associations, such as clopidogrel-CYP2C19 and codeine-CYP2D6.35,36 Still, regulatory uncertainty remains a barrier to more widespread uptake. The FDA has expressed concern that some pharmacogenetic tests lack validity and that using the results to alter drug treatment could “lead to immediate serious health consequences for patients.”5 Although clinical laboratory, molecular pathology, and pharmacogenetics professional societies have disagreed with the agency’s assertions,37,38 empirical demonstration that the clinical use of pharmacogenetic test results does not worsen patient outcomes will help inform this debate.
In the context of statin treatment, given their demonstrated effectiveness in primary and secondary ASCVD prevention and the association of poor statin adherence with adverse ASCVD outcomes,25,39,40,41,42 pharmacogenetic testing would cause unintentional harm if it paradoxically made patients less likely to initiate and adhere to therapy with any statin, including simvastatin.16 Even without pharmacogenetic testing, physician and patient behavior around statin therapy is already highly variable, and many patients remain hesitant to adhere to recommendations.13,14,15 Change in LDL-C represents a common clinical end point for these variable physician and patient behaviors. We found that SLCO1B1 testing was not associated with a between-group difference in LDL-C reduction outside the noninferiority limit of 10 mg/dL, a surrogate outcome for a reduction in 5-year ASCVD risk of 5%.25 A previous randomized trial among 159 previously statin-intolerant patients found that SLCO1B1 genotyping and reporting, compared with end-of-study reporting, resulted in more new statin reinitiations and lower LDL-C levels 3 months after enrollment.43 Together, these findings provide some reassurance about possible unintended harms of using SLCO1B1 results.
Limitations
Pragmatic trials combine the rigor of randomization and enhanced generalizability to real-world medical practice,44 but they introduce limitations evidenced in this study. First, fewer enrollees than expected were prescribed statin therapy generally and simvastatin therapy specifically during the observation period, likely the result of patient reluctance and physician prescribing patterns that target statin therapy to a goal LDL-C less than 100 mg/dL instead of to ASCVD risk categories, particularly among patients meeting statin eligibility only because of the more recently recommended criterion of 10-year ASCVD risk greater than or equal to 7.5%. A treatment trial with a protocolized genotype-guided prescribing algorithm would have ensured higher rates of statin initiation and increased the power to demonstrate superiority of SLCO1B1 testing. Second, the absence of protocolized LDL-C measurements at baseline and follow-up introduces the potential for bias in the primary outcome, although analyses among those with at least 1 repeated LDL-C measurement yielded results similar to those for the intention-to-treat analyses. Third, by chance, randomization resulted in a lower proportion of patients with decreased or poor SLCO1B1 transporter function genotypes in the intervention group than in the control group. Stratified randomization would have prevented this imbalance, the impact of which on the trial results is unknown but expected to be minimal, because physicians and patients in the control group likely proceeded with usual care, blinded to genotype. Fourth, the pragmatic design may have limited physician and patient engagement with the pharmacogenetic results. A less pragmatic trial with dedicated study visits and a less subtle delivery of pharmacogenetic test results to physicians and patients might have resulted in greater engagement with the intervention and potentially greater clinical impact. Our observation that only 15.5% of physicians documented communicating SLCO1B1 results to intervention patients is an imperfect measurement of that engagement. Guidelines also recommend shared decision-making between patients and physicians about statin therapy, which was not measured in this trial.
Conclusions
In this practical randomized clinical trial, the clinical integration of SLCO1B1 pharmacogenetic testing for simvastatin myopathy risk did not result in poorer measures of ASCVD prevention in routine primary care settings. Such an absence of harm may reassure stakeholders contemplating the clinical use of pharmacogenetic information.
Trial Protocol
eTable 1. Evidence of Statin-Associated Muscle Symptoms (SAMS) in the Integrating Pharmacogenetics in Clinical Care (I-PICC) Study
eTable 2. Electronic Health Record (EHR) Documentation and Patient Communication Process Outcomes Among I-PICC Study Patient Participants in the Intervention (PGx+) Arm
eTable 3. Patient Beliefs About Medications and Test Results Recall at 12 Months
Data Sharing Statement
References
- 1.Van Driest SL, Shi Y, Bowton EA, et al. Clinically actionable genotypes among 10,000 patients with preemptive pharmacogenomic testing. Clin Pharmacol Ther. 2014;95(4):423-431. doi: 10.1038/clpt.2013.229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chanfreau-Coffinier C, Hull LE, Lynch JA, et al. Projected prevalence of actionable pharmacogenetic variants and level A drugs prescribed among US Veterans Health Administration pharmacy users. JAMA Netw Open. 2019;2(6):e195345. doi: 10.1001/jamanetworkopen.2019.5345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roden DM, McLeod HL, Relling MV, et al. Pharmacogenomics. Lancet. 2019;394(10197):521-532. doi: 10.1016/S0140-6736(19)31276-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.US Food and Drug Administration The FDA warns against the use of many genetic tests with unapproved claims to predict patient response to specific medications: FDA safety communication. Published October 31, 2018. Updated April 4, 2019. Accessed September 29, 2020. https://www.fda.gov/medical-devices/safety-communications/fda-warns-against-use-many-genetic-tests-unapproved-claims-predict-patient-response-specific
- 5.US Food and Drug Administration Warning letter: Inova Genomics Laboratory (MARCS-CMS 577422). Updated April 4, 2019. Accessed September 29, 2020. https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/inova-genomics-laboratory-577422-04042019
- 6.National Center for Health Statistics Health, United States, 2013: with special feature on prescription drugs. Published 2014. Accessed September 29, 2020. http://www.cdc.gov/nchs/data/hus/hus13.pdf [PubMed]
- 7.Link E, Parish S, Armitage J, et al. ; SEARCH Collaborative Group . SLCO1B1 variants and statin-induced myopathy: a genomewide study. N Engl J Med. 2008;359(8):789-799. doi: 10.1056/NEJMoa0801936 [DOI] [PubMed] [Google Scholar]
- 8.Puccetti L, Ciani F, Auteri A. Genetic involvement in statins induced myopathy: preliminary data from an observational case-control study. Atherosclerosis. 2010;211(1):28-29. doi: 10.1016/j.atherosclerosis.2010.02.026 [DOI] [PubMed] [Google Scholar]
- 9.Donnelly LA, Doney AS, Tavendale R, et al. Common nonsynonymous substitutions in SLCO1B1 predispose to statin intolerance in routinely treated individuals with type 2 diabetes: a Go-DARTS study. Clin Pharmacol Ther. 2011;89(2):210-216. doi: 10.1038/clpt.2010.255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Keyser CE, Peters BJ, Becker ML, et al. The SLCO1B1 c.521T>C polymorphism is associated with dose decrease or switching during statin therapy in the Rotterdam Study. Pharmacogenet Genomics. 2014;24(1):43-51. doi: 10.1097/FPC.0000000000000018 [DOI] [PubMed] [Google Scholar]
- 11.Ramsey LB, Johnson SG, Caudle KE, et al. The clinical pharmacogenetics implementation consortium guideline for SLCO1B1 and simvastatin-induced myopathy: 2014 update. Clin Pharmacol Ther. 2014;96(4):423-428. doi: 10.1038/clpt.2014.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vassy JL, Chun S, Advani S, Ludin SA, Smith JG, Alligood EC. Impact of SLCO1B1 pharmacogenetic testing on patient and healthcare outcomes: a systematic review. Clin Pharmacol Ther. 2019;106(2):360-373. doi: 10.1002/cpt.1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clough JD, Martin SS, Navar AM, et al. Association of primary care providers’ beliefs of statins for primary prevention and statin prescription. J Am Heart Assoc. 2019;8(3):e010241. doi: 10.1161/JAHA.118.010241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramsaran E, Preusse P, Sundaresan D, et al. Adherence to blood cholesterol treatment guidelines among physicians managing patients with atherosclerotic cardiovascular disease. Am J Cardiol. 2019;124(2):169-175. doi: 10.1016/j.amjcard.2019.04.017 [DOI] [PubMed] [Google Scholar]
- 15.Ingersgaard MV, Helms Andersen T, Norgaard O, Grabowski D, Olesen K. Reasons for nonadherence to statins: a systematic review of reviews. Patient Prefer Adherence. 2020;14:675-691. doi: 10.2147/PPA.S245365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vassy JL, Brunette CA, Majahalme N, et al. The Integrating Pharmacogenetics in Clinical Care (I-PICC) Study: protocol for a point-of-care randomized controlled trial of statin pharmacogenetics in primary care. Contemp Clin Trials. 2018;75:40-50. doi: 10.1016/j.cct.2018.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brunette CA, Miller SJ, Majahalme N, et al. Pragmatic trials in genomic medicine: the Integrating Pharmacogenetics in Clinical Care (I-PICC) study. Clin Transl Sci. 2020;13(2):381-390. doi: 10.1111/cts.12723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stone NJ, Robinson JG, Lichtenstein AH, et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25)(suppl 2):S1-S45. doi: 10.1161/01.cir.0000437738.63853.7a [DOI] [PubMed] [Google Scholar]
- 19.Caudle KE, Dunnenberger HM, Freimuth RR, et al. Standardizing terms for clinical pharmacogenetic test results: consensus terms from the Clinical Pharmacogenetics Implementation Consortium (CPIC). Genet Med. 2017;19(2):215-223. doi: 10.1038/gim.2016.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Price LE, Shea K, Gephart S. The Veterans Affairs’s Corporate Data Warehouse: uses and implications for nursing research and practice. Nurs Adm Q. 2015;39(4):311-318. doi: 10.1097/NAQ.0000000000000118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: methods, validity, and applications. J Clin Epidemiol. 1997;50(1):105-116. doi: 10.1016/S0895-4356(96)00268-5 [DOI] [PubMed] [Google Scholar]
- 22.Centers for Medicare and Medicaid Services Medicare 2019 part C and D star ratings technical notes. Published 2019. Accessed September 29, 2020. https://www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovGenIn/Downloads/2019-Part-C-and-D-Medicare-Star-Ratings-Data-v04-12-2019.zip
- 23.Horne R, Weinman J, Hankins M.. The beliefs about medicines questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medication. Psychol Health. 1999;14(1):1-24. doi: 10.1080/08870449908407311 [DOI] [Google Scholar]
- 24.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121-130. doi: 10.2307/2531248 [DOI] [PubMed] [Google Scholar]
- 25.Baigent C, Keech A, Kearney PM, et al. ; Cholesterol Treatment Trialists’ (CTT) Collaborators . Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366(9493):1267-1278. doi: 10.1016/S0140-6736(05)67394-1 [DOI] [PubMed] [Google Scholar]
- 26.Dunnenberger HM, Crews KR, Hoffman JM, et al. Preemptive clinical pharmacogenetics implementation: current programs in five US medical centers. Annu Rev Pharmacol Toxicol. 2015;55:89-106. doi: 10.1146/annurev-pharmtox-010814-124835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hinderer M, Boeker M, Wagner SA, et al. Integrating clinical decision support systems for pharmacogenomic testing into clinical routine: a scoping review of designs of user-system interactions in recent system development. BMC Med Inform Decis Mak. 2017;17(1):81. doi: 10.1186/s12911-017-0480-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luzum JA, Pakyz RE, Elsey AR, et al. ; Pharmacogenomics Research Network Translational Pharmacogenetics Program . The Pharmacogenomics Research Network Translational Pharmacogenetics Program: outcomes and metrics of pharmacogenetic implementations across diverse healthcare systems. Clin Pharmacol Ther. 2017;102(3):502-510. doi: 10.1002/cpt.630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rasmussen-Torvik LJ, Stallings SC, Gordon AS, et al. Design and anticipated outcomes of the eMERGE-PGx project: a multicenter pilot for preemptive pharmacogenomics in electronic health record systems. Clin Pharmacol Ther. 2014;96(4):482-489. doi: 10.1038/clpt.2014.137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cavallari LH, Beitelshees AL, Blake KV, et al. ; The IGNITE Pharmacogenetics Working Group . The IGNITE Pharmacogenetics Working Group: an opportunity for building evidence with pharmacogenetic implementation in a real-world setting. Clin Transl Sci. 2017;10(3):143-146. doi: 10.1111/cts.12456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van der Wouden CH, Cambon-Thomsen A, Cecchin E, et al. ; Ubiquitous Pharmacogenomics Consortium . Implementing pharmacogenomics in Europe: design and implementation strategy of the Ubiquitous Pharmacogenomics Consortium. Clin Pharmacol Ther. 2017;101(3):341-358. doi: 10.1002/cpt.602 [DOI] [PubMed] [Google Scholar]
- 32.Borobia AM, Dapia I, Tong HY, et al. Clinical implementation of pharmacogenetic testing in a hospital of the Spanish national health system: strategy and experience over 3 years. Clin Transl Sci. 2018;11(2):189-199. doi: 10.1111/cts.12526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Natasha Petry, Baye J, Aifaoui A, et al. Implementation of wide-scale pharmacogenetic testing in primary care. Pharmacogenomics. 2019;20(12):903-913. doi: 10.2217/pgs-2019-0043 [DOI] [PubMed] [Google Scholar]
- 34.Office of Public and Intergovernmental Affairs, Department of Veterans Affairs Precision health initiative brings genetic testing to Veterans. Published 2019. Updated March 12, 2019. Accessed September 29, 2020. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5216
- 35.Caudle KE, Klein TE, Hoffman JM, et al. Incorporation of pharmacogenomics into routine clinical practice: the Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline development process. Curr Drug Metab. 2014;15(2):209-217. doi: 10.2174/1389200215666140130124910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swen JJ, Nijenhuis M, van Rhenen M, et al. ; Dutch Pharmacogenetics Working Group (DPWG) of the Royal Dutch Pharmacists Association (KNMP) . Pharmacogenetic information in clinical guidelines: the European perspective. Clin Pharmacol Ther. 2018;103(5):795-801. doi: 10.1002/cpt.1049 [DOI] [PubMed] [Google Scholar]
- 37.American Clinical Laboratory Association Re: FDA actions on pharmacogenetic testing. Published September 18, 2019. Accessed September 29, 2020. https://www.acla.com/wp-content/uploads/2019/09/ACLA-Letter-to-FDA-re_-PGx-Test-Policy-Sept-18-2019.pdf
- 38.Association for Molecular Pathology AMP recommends clinical pharmacogenomic testing best practices to preserve broad access and improve patient care. Published September 4, 2019. Accessed September 29, 2020. https://www.amp.org/AMP/assets/File/pressreleases/2019/AMPPGxStatement_090319.pdf?pass=68
- 39.Alfirevic A, Neely D, Armitage J, et al. Phenotype standardization for statin-induced myotoxicity. Clin Pharmacol Ther. 2014;96(4):470-476. doi: 10.1038/clpt.2014.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mills EJ, Rachlis B, Wu P, Devereaux PJ, Arora P, Perri D. Primary prevention of cardiovascular mortality and events with statin treatments: a network meta-analysis involving more than 65,000 patients. J Am Coll Cardiol. 2008;52(22):1769-1781. doi: 10.1016/j.jacc.2008.08.039 [DOI] [PubMed] [Google Scholar]
- 41.Ho PM, Magid DJ, Shetterly SM, et al. Medication nonadherence is associated with a broad range of adverse outcomes in patients with coronary artery disease. Am Heart J. 2008;155(4):772-779. doi: 10.1016/j.ahj.2007.12.011 [DOI] [PubMed] [Google Scholar]
- 42.Rasmussen JN, Chong A, Alter DA. Relationship between adherence to evidence-based pharmacotherapy and long-term mortality after acute myocardial infarction. JAMA. 2007;297(2):177-186. doi: 10.1001/jama.297.2.177 [DOI] [PubMed] [Google Scholar]
- 43.Peyser B, Perry EP, Singh K, et al. Effects of delivering SLCO1B1 pharmacogenetic information in randomized trial and observational settings. Circ Genom Precis Med. 2018;11(9):e002228. doi: 10.1161/CIRCGEN.118.002228 [DOI] [PubMed] [Google Scholar]
- 44.Ford I, Norrie J. Pragmatic trials. N Engl J Med. 2016;375(5):454-463. doi: 10.1056/NEJMra1510059 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Evidence of Statin-Associated Muscle Symptoms (SAMS) in the Integrating Pharmacogenetics in Clinical Care (I-PICC) Study
eTable 2. Electronic Health Record (EHR) Documentation and Patient Communication Process Outcomes Among I-PICC Study Patient Participants in the Intervention (PGx+) Arm
eTable 3. Patient Beliefs About Medications and Test Results Recall at 12 Months
Data Sharing Statement