Abstract
The potential effects of a 24 or 72‐h delay in post‐mortem inspection (PMI) of ungulates on public health and monitoring of animal health and welfare was evaluated. The assessment used a survey of meat inspectors, expert opinion, literature search and a stochastic model for Salmonella detection sensitivity. Disease detection sensitivity at a delayed PMI is expected to reduce detection sensitivity to a variable extent, depending on the hazard and on the signs/lesions and organs involved. No reduction is expected for Trichinella detection in meat from susceptible animal species and any decrease in detection of transmissible spongiform encephalopathies (TSEs) will not exceed the current tolerance for fallen stock. A 24‐h delay in PMI could result in a small reduction in sensitivity of detection for tuberculosis, echinococcosis and cysticercosis. A greater reduction is expected for the detection of pyaemia and Rift valley fever. For the detection of Salmonella, the median model estimates are a reduction of sensitivity of 66.5% (90% probability interval (PI) 0.08–99.75%) after 24‐h delay and 94% (90% PI 0.83–100%) after 72‐h delay of PMI. Laboratory testing for tuberculosis following a sampling delay of 24–72 h could result in no, or a moderate, decrease in detection depending on the method of confirmation used (PCR, culture, histopathology). For chemical contaminants, a delay in meat inspection of 24 or 72 h is expected to have no impact on the effectiveness of detection of persistent organic pollutants and metals. However, for certain pharmacologically active substances, there will be a reduced effectiveness to detect some of these substances due to potential degradation in the available matrices (tissues and organs) and the non‐availability of specific preferred matrices of choice.
Keywords: chemical residues, contaminants, delay, lesions, meat inspection, post‐mortem, ungulates
Summary
Following a request from the European Commission, the EFSA Panel on Biological Hazards (BIOHAZ) was asked to assess the impact on effectiveness of post‐mortem inspection (PMI) in terms of any change in the sensitivity of detection of a list of diseases/conditions when carried out after up to 24 h or up to 72 h after slaughter, or arrival in the game‐handling establishment, in comparison to when it is carried out immediately after slaughter or arrival in the game handling establishment. The expertise required for the diseases/conditions specified in the terms of reference was covered by three EFSA Panels.
The EFSA Panel on Animal Health and Welfare (AHAW) evaluated the effect of delayed PMI on the sensitivity of detection of animal diseases of domestic and wild ungulates listed according to Article 5 of Regulation (EC) No 2016/429 (Animal Health Law (AHL)) and cysticercosis, and on the sensitivity of detection of septicaemia, pyaemia, toxaemia or viraemia. The BIOHAZ Panel evaluated the effect of delayed PMI on the sensitivity of detecting transmissible spongiform encephalopathies (TSEs) and Trichinella, and on the use of Salmonella detection as a process hygiene criterion (PHC). The EFSA Panel on Contaminants in the Food Chain (CONTAM) evaluated the effects of delayed PMI on the sensitivity of detecting chemical residues and contaminants in light of Council Directive 96/23/EC which lays down the requirements for official control for these substances at farm level and PMI at slaughterhouse level.
Almost all animal diseases that affect ungulates and are listed according to the AHL are transmissible. They provoke, in most cases, acute forms of disease with clinical signs, and should therefore be detected mainly at the farm or at ante‐mortem inspection (AMI) at the slaughterhouse; thus, any possible decrease in the sensitivity of the delayed PMI relative to the current procedure would not be relevant in practice for the clinical forms of these diseases. For suspect animals detected in the frame of official active surveillance programmes (as for some diseases listed under AHL), there should not be any delay at PMI, and thus, these do not fall within the remit of this assessment. Diseases where target organs and related lesions are not screened/observed at slaughterhouse, in particular those inducing lesions in the brain, e.g. rabies, also fall outside the remit of this assessment.
Nevertheless, for certain diseases, subclinical or asymptomatic presentations that could be missed at AMI have been described, and therefore, animals affected by such conditions could be sent to the abattoir and culled as part of the routine slaughter process. Thus, the targets of this assessment are subclinically infected animals that enter the abattoir, pass the AMI, are slaughtered and may present detectable lesions at PMI.
The lesions associated with each disease evaluated in this opinion were retrieved from the scientific literature and/or based on expert knowledge. There is, however, very little data on the frequency, distribution and severity of the lesions in subclinical/asymptomatic cases, which results in some uncertainty.
Information about the lesions related to each disease was screened by the WG experts to select those for which detection might be affected by a delay of 24 or 72 h. Information on the possible decrease in the sensitivity of PMI for detecting these lesions resulting from delayed PMI was collected through a survey undertaken by 18 meat inspectors from six EU MSs. This information was used as evidence in a set of Expert Knowledge Elicitation workshops conducted with the participation of the EFSA WG, during which the mean number of carcasses from infected animals assessed as diseased in a PMI carried out immediately after slaughter (current procedure) per 100 inspections that would still be detectable after 24 or 72 h for each disease was elicited.
According to the results from the present assessment, the sensitivity of disease detection at a delayed PMI is expected to decrease, with the magnitude of this decrease depending on the type of lesions and organs involved. This reduction in sensitivity is highly variable and depends on the type of lesions, for example, from more than 80 carcasses out of 100 still detected at both 24‐ or 72‐h delay for tuberculosis (with greater than 95% certainty), to values between 36 and 97 after a 72‐h delay for Rift Valley Fever (RVF) (with a 90% certainty).
At 24‐h delayed PMI, the only diseases for which there was less than 95% certainty of being able to detect at least 50 out of 100 affected carcasses with the current procedure are foot and mouth disease in wild boars and surra.
At 72‐h delayed PMI, diseases for which there was less than 95% certainty of being able to detect at least 50 out of 100 affected carcasses with the current procedure are RVF, surra, foot and mouth disease (FMD), West Nile fever, African Swine Fever and African horse sickness.
For diseases for which the diagnosis at the slaughterhouse is most important, i.e. tuberculosis, echinococcosis, cysticercosis and pyaemia, the estimated mean number of affected carcasses, out of 100 detected with the current procedure, that would be still detectable with a 90% certainty is (i) after a 24‐h PMI delay, between 83 and 100 (median = 95) for tuberculosis, echinococcosis and cysticercosis, and between 53 and 99 (median = 86) for pyaemia and (ii) after a 72‐h PMI delay, between 83 and 98 (median = 92) for tuberculosis, between 72 and 99 (median = 92) for cysticercosis and echinococcosis and between 47 and 94 (median = 76) for pyaemia.
For chronic type lesions, including those due to tuberculosis, the assessment concluded that a 24‐ or 72‐h delay for PMI would not lead to a decrease in their detectability.
Concerning tuberculosis, the overall effect of a delay in the PMI on the ability to confirm tuberculosis in infected animals is a combination of the effect of such delay on the ability to detect compatible lesions during the PMI, and on the performance of the laboratory tests used to confirm the infection. There is considerable uncertainty about the impact of delayed inspection and testing due to the lack of available data. Nevertheless, a 24‐h delay could result in the confirmation of between 73 and 100 animals out of 100 confirmed with the current procedure depending on the diagnostic test used. A 72‐h delay could result in the confirmation of between 74 (direct polymerase chain reaction (PCR)), 65 (culture) and 61 (histopathology) and affected 100 animals out of every 100 animals confirmed using the current procedure.
The sources of uncertainty that could explain the width of the elicited distribution of estimates relating to this first assessment question were (i) uncertainty on the severity, distribution and type of lesions that may be observed in subclinical and/or asymptomatic infected animals and (ii) the lack of data on the effect that the delay could have on their detection during a routine PMI.
The current primary testing requirements for TSEs do not include animals slaughtered for human consumption. Should the testing of healthy slaughter animals be introduced or reintroduced for any species, delays in PMI of 24 or 72 h could potentially reduce diagnostic sensitivity of the testing programme but any decrease in TSE detection will not exceed the tolerance already in place for surveillance testing in fallen stock. The analytical sensitivity and specificity of TSE tests have been shown to be unaffected by delays of this length.
For the detection of Trichinella, the panel did not find any evidence that would suggest a decrease in sensitivity during cold storage and it is almost certain (99–100%) that there is no decrease in sensitivity of detection after a delay of PMI of 24 or 72 h.
The effect of delayed PMI on the sensitivity of Salmonella detection as a process hygiene criterion has been estimated using a stochastic model. Factors included were the initial concentration of Salmonella cells on the carcass after dressing before chilling, viability and culturability of Salmonella, detachment of Salmonella by the used sampling method, and selectivity and capacity of enrichment media on Salmonella detection. Model input variables have been elicited by expert knowledge elicitation (EKE), informed by data from the literature.
The median estimate for the reduction in sensitivity of Salmonella detection with a 24‐h delay of PMI after slaughtering is 66.5%. The 90% probability interval for this reduction in sensitivity ranges from 0.08% (5th percentile) to 99.75% (95th percentile). The median estimate for the reduction in sensitivity of Salmonella detection with a 72‐h delay of PMI after slaughtering is 94%. The 90% probability interval for this reduction in sensitivity ranges from 0.83% (5th percentile) to 100% (95th percentile). The high uncertainty on the above estimates originates mainly from the uncertainty in the initial Salmonella concentration among carcasses. In general, the lower the initial Salmonella counts, the higher the estimated reduction in the sensitivity of detection.
The CONTAM Panel evaluated the effects of delayed meat inspection on chemical residues and contaminants in light of the Council Directive 96/23/EC which lays down the requirements for official control for these substances at farm level and post‐mortem meat inspection at slaughterhouse level. The national residue control plans (NRCPs) implement these demands. The objective of the NRCPs is to detect illegal treatment of food producing animals, controlling compliance with withdrawal periods and the MRLs for veterinary medicinal products, and the maximum levels for certain contaminants. The CONTAM Panel emphasised the fact that substances to be analysed at PMI cover a broad range with different physico‐chemical properties and biological behaviour. This is especially true for the groups of persistent organic pollutants (POPs) and metals on the one hand and pharmacologically active substances on the other hand.
The CONTAM Panel concluded that due to their stability, poor microbial and chemical degradation and persistence, there is no impact of a delayed meat inspection of 24 or 72 h on the effectiveness to detect POPs and metals.
The fate of legally and illegally administered pharmacologically active substances in the animal's body depends on the mode of application, formula, biological half‐life, elimination rate and withdrawal period, while the possibility of their detection is related not only to the sensitivity of the analytical method applied, but especially to the matrices chosen for analysis and their proper handling between collection and analysis. The CONTAM Panel concluded that due to potential degradation in the available matrices and the non‐availability of specific preferred matrices of choice, the effectiveness to detect certain pharmacologically active substances may be reduced when meat inspection is delayed by 24 or 72 h. In general, there is very little information on the potential post‐mortem degradation in organs and tissues.
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
The scope of this mandate is to evaluate certain aspects of meat inspection in order to assess the fitness of the meat for human consumption and to monitor food‐borne zoonotic infections (public health) without jeopardising the timely detection of certain animal diseases at slaughter.
Ante‐mortem and post‐mortem inspections (together called “meat inspection”) are essential official controls to monitor animal and public health at slaughter through the verification of human and animal health requirements of animals and to evaluate if the meat can be declared fit for human consumption and not causing any concern on the transmission of animal diseases.
Revised meat inspection procedures for all species apply from 14 December 2019. As regards ungulates, the revision took into account the Scientific Opinions of the BIOHAZ Panel of the European Food Safety Authority on public health hazards to be covered by inspection of meat in:
swine, published on 3 October 2011,
bovine animals; domestic sheep and goats, farmed game and domestic solipeds, all published on 27 June 2013.
According to the above Scientific Opinions, the main biological and chemical hazards to public health to be addressed in meat inspection are summarised in Table 1.
Table 1.
The main biological and chemical hazards to public health to be addressed in meat inspection
| Species | Biological hazards | Chemical hazards |
|---|---|---|
| Swine | Salmonella, Yersinia enterocolitica, Toxoplasma gondii and Trichinella | Dioxins, dioxin‐like polychlorinated biphenyls and the antibiotic chloramphenicol |
| Cattle | Verotoxin‐producing Escherichia coli (VTEC), Salmonella | Dioxins, dioxin‐like polychlorinated biphenyls |
| Sheep and goats | VTEC, Toxoplasma | Dioxins, dioxin‐like polychlorinated biphenyls |
| Solipeds | Trichinella | Phenylbutazone*, chemical elements (cadmium) |
| Farmed game (deer) | Toxoplasma | None |
| Farmed game (wild boar) | Salmonella, Toxoplasma * | None |
| Farmed game (reindeer, ostriches, rabbits) | None* | None |
For some biological hazards, available evidence was considered insufficient to rank related tasks.
The Opinions also stress the importance of meat inspection in the monitoring of animal diseases, such as tuberculosis, brucellosis, enzootic bovine leukosis and glanders (solipeds), and the (early) detection of the animal diseases listed in Article 5(1)(a) and according to Article 5(1)(b)1 of Regulation (EU) 2016/429 of the European Parliament and of the Council (the Animal Health Law). The listing of diseases under Article 5(1)(b) of the Animal Health Law resulting in Regulation (EC) 2018/1629 involved also a large number of EFSA scientific opinions that are relevant for this mandate.
The practical arrangements for the revised meat inspection have been laid down in Commission Implementing Regulation (EU) 2019/6272. In summary:
Ante‐mortem inspection (AMI) must take place within 24 h of arrival in the slaughterhouse and less than 24 h before slaughter. By way of derogation, AMI may take place at the holding of provenance within 3 days before slaughter, except for small producers of farmed game where AMI can be carried out up to 28 days before slaughter.
AMI includes a verification of food business operators’ obligation to ensure that animals are clean, and an inspection in order to verify if there are any signs that animal health and welfare are compromised (presence of abnormalities or disease that make the meat unfit for human consumption or that adversely affect animal health; use of prohibited or unauthorised substances; misuse of veterinary medicinal products or the presence of residues or contaminants).
AMI is not required for wild game, but a trained person must be present during hunting.3
Post‐mortem inspection (PMI) must be carried out without undue delay after slaughter or as soon as possible after arrival of wild game carcasses at a game‐handling establishment. However, wild game might be stored in refrigerated collection centres before transport to a game‐handling establishment, without a defined time limit. In low capacity slaughterhouses or game‐handling establishments, a delay of a maximum of 24 h can be allowed by the competent authorities under certain conditions.
PMI comprises checking of all external surfaces, including those of the body cavities of carcasses and offal and pay particular attention, including additional examinations, to the detection of zoonoses or relevant animal diseases. In case of wild game, the viscera and head (except in case of Trichinella susceptible species), may not accompany the carcasses to the game‐handling establishment (and therefore might not undergo PMI).
Species‐ and age‐specific arrangements for the visual inspection, incisions and palpation of carcasses during PMI are laid down in the Articles 18 to 23 and 27–28 of the Regulation. When there are indications of possible risks to public or animal health, additional incisions and palpations must be carried out (Article 24).
Additional official controls on specific hazards are included in PMI:
TSE testing in ruminants
Cysticercus in domestic bovine animals and Suidae
Trichinella in Suidae and solipeds
Glanders in solipeds
Tuberculosis and brucellosis in all ungulates
Salmonella (process hygiene criterion on carcasses) in all ungulates
Testing for chemical residues and contaminants in all ungulates
For practical reasons, requests have been made for the possibility to delay PMI in order to consider:
Carrying out PMI of carcasses and offal of animals slaughtered on the day before when AMI has been carried out on the animals slaughtered that day;
Carrying out PMI on wild game in game‐handling establishment after the weekend on carcasses arriving on Friday evening or Saturday to the game handling establishment.
When delaying PMI, the obligation remains that the meat must be chilled immediately after slaughter to ensure a temperature throughout the meat of not more than 3°C for offal4 and 7°C for other meat (carcasses5) along a chilling curve that ensures a continuous decrease of the temperature. In case of large wild game (e.g. red deer, wild boar, etc.), chilling must begin within a reasonable period of time after killing and achieve a temperature throughout the meat of not more than 7°C.
Terms of Reference
EFSA is asked to deliver a scientific opinion on the evaluation of the public and animal health risks in case of a delayed PMI of ungulates in any slaughterhouse or game‐handling establishment. More specifically, considering implementation of AMI and PMI according to Implementing Regulation (EU) 2019/6276, EFSA is asked to assess the effectiveness of PMI (in terms of its sensitivity in detecting the diseases/conditions listed below) when carried in both the following delays:
-
a)
up to 24 h after slaughter or arrival in the game‐handling establishment, or
-
b)
up to 72 h after slaughter or arrival in the game‐handling establishment,
in comparison to when it is carried out immediately after slaughter or arrival in the game handling establishment.
The effectiveness should be evaluated considering the implications for public and animal health of any changes suggested to current meat inspection methods, and in particular:
timely detection of animal diseases listed according to Article 57 of Regulation (EU) 2016/429 in all ungulates;
detection of generalised conditions such as septicaemia, pyaemia, toxaemia or viraemia in all ungulates;
detection of transmissible spongiform encephalopathies (TSEs) in cattle, sheep, goats and cervids;
detection of cysticercosis in domestic bovine animals and Suidae;
detection of Trichinella in Suidae and solipeds;
detection of glanders in solipeds;
detection of tuberculoid lesions in all ungulates;
detection of Brucella in all ungulates;
detection of Salmonella spp. (process hygiene criterion on carcasses) in all ungulates;
detection of chemical residues and contaminants in all ungulates.
When comparing the effectiveness, inspection or sampling of carcasses and offal must be considered. Differentiation per species should be considered if relevant.
1.2. Interpretation of the Terms of Reference
Ante‐ and post‐mortem meat inspections are undertaken to ensure safe meat for human consumption and to monitor animal health and welfare. Zoonotic diseases and poor hygienic conditions of slaughtered animals may generate meat that could cause human diseases, while undetected animal diseases may spread via meat and offal. Animal welfare is essential for meat quality and because good animal welfare is demanded by consumers. Thus, European‐wide uniform controls have been laid down for AMI and PMI practices including location and time when this examination must be performed. AMI must be performed within 24 h of arrival in the slaughterhouse and less than 24 h before slaughter and PMI should be performed immediately after slaughter. This opinion focuses on the reduction in the sensitivity of detecting diseases or conditions in ungulates as specified in the TORs after a 24‐h and 72‐h delay of PMI as compared to PMI immediately after slaughter. Public health risk due to a delayed PMI is not assessed in this opinion.
In addition, EFSA has been asked to assess the potential reduction in the sensitivity of detecting Salmonella as a Process Hygiene Criterion (PHC), which is only defined for domestic ungulates and, therefore, is not assessed for wild game.
Only animals that are categorised with no expected food safety risk, according to the Food Chain Information (FCI) and AMI, that can progress to slaughter and be subjected to routine PMI, are the target of the assessment of this opinion.
As far as bovine tuberculosis is concerned, the assessment performed in this opinion is exclusively focused on animals presenting lesions compatible with tuberculosis and detected through passive surveillance (PMI), since animals subjected to post‐mortem laboratory tests for confirmation of an ante‐mortem suspicion of tuberculosis should be slaughtered in conditions that require and allow immediate PMI and sampling even in the absence of macroscopic lesions. This also applies to all animal diseases or zoonoses concerned by this assessment which have been the subject of an ante‐mortem suspicion.
It is assumed that all parts of the slaughter carcass, including intestines and offal (the intestine and offal cannot be further processed directly after slaughter, if PMI is delayed), are stored under refrigeration temperature as required by legislation and separated from meat declared fit for human consumption, as well as separated from meat and offal declared unfit for human consumption, with +7°C max. for carcasses and +3°C max. for offal (Regulation (EC) No 853/20048, Annex III, Section I, Chapter VII). They must all be available for PMI.
Cross‐contamination between carcasses after slaughter is described as one of the risk factors for carcass contamination with pathogens (Borcher and Arinder, 2002). Next to direct contact between carcasses, sources of contamination such as walls, floors, food contact surfaces, knives, operators and the generation of aerosols and dust in the abattoir are responsible for cross‐contamination (Whyte et al., 2011). Infectious agents may be present on the surfaces or in the interior of organs or the carcasses of slaughtered animals. Such carriage of infectious agents is not necessarily accompanied by clinical illness or pathological alterations visible at slaughter. Surfaces of slaughter carcasses under hygienic conditions contain 104–105 microorganisms per cm2. Bacteria can be transferred from the skin, hides, intestinal tract and abattoir surroundings and chill areas during slaughter and processing (Grau, 1986; O'Brien et al., 2005). As well as spoilage organisms, these microorganisms may include animal pathogens if, for example, the animal is infected at the time of slaughter (Whyte et al., 2011). An extended storage of 24–72 h of carcasses before PMI will extend the time for a possible cross‐contamination between carcasses through aerosols and dust. It is expected that carcasses and offal not yet declared fit or not fit for human consumption might be stored together and thus, may increase the chance of cross‐contamination with human and animal pathogens. The public health risk of storage for 24 or 72 h before PMI has not been assessed in this opinion. It is also assumed that Good Hygiene Practices (GHP) and Good Manufacturing Practices (GMP) are respected.
‘Wild game’ ungulates comprise wild ungulates that are hunted for human consumption and are considered to be wild game under the applicable law in the Member State concerned, including mammals living in enclosed territory under conditions of freedom similar to those of wild game (Regulation (EC) No 853/2004). This includes a variety of wild ruminants and wild boar. ‘Farmed game’ means farmed ratites and farmed land mammals other than ‘domestic ungulates’ (i.e. domestic bovine (including Bubalus and Bison species), porcine, ovine and caprine animals, domestic solipeds). Whereas PMI of farmed game is carried out according to equivalent domestic animal species, specific requirements exist for wild game.
To address the TOR, the following assessment questions (AQ) have been formulated:
AQ 1. Is the sensitivity of detection of animal diseases listed according to Article 5 of Regulation (EC) No 2016/429 and cysticercosis in domestic bovine animals and Suidae and the sensitivity in detection of conditions such as septicaemia, pyaemia, toxaemia or viraemia reduced if the PMI is delayed by 24 or 72 h after slaughter or arrival in the game‐handling establishment?
As far as bovine tuberculosis is concerned, is the performance of the laboratory diagnostic tests for confirmation of tuberculosis in animals with detectable suspect lesions reduced if the PMI is delayed by 24 or 72 h after slaughter or arrival in the game‐handling establishment?
AQ 2. Is the detection of transmissible spongiform encephalopathies (TSEs) in cattle, sheep, goats and cervids, Trichinella in Suidae and solipeds reduced if the PMI is delayed by 24 or 72 h after slaughter or arrival in the game‐handling establishment?
AQ 3. What is the percentage of reduction (%) in sensitivity of Salmonella detection as a process hygiene criterion if the PMI is delayed by 24 or 72 h after slaughter?
AQ 4. Is the sensitivity of detecting of chemical residues and contaminants in ungulates reduced if the PMI is delayed by 24 or 72 h after slaughter/arrival in the game‐handling establishment?
1.3. General aspects of meat inspection
1.3.1. Meat inspection in domestic ungulates and farmed game
Meat inspection is a multistep procedure that includes the Food Chain Information (FCI) analysis, AMI, PMI, and, where foreseen by the regulation or when relevant, laboratory testing. In ungulates, it is performed for each slaughtered animal/carcass. As a result of the inspection process, an animal can be accepted for slaughter or not, and the meat of a slaughtered animal can be declared fit or unfit for human consumption.
1.3.1.1. Analysis of food chain information
FCI is shared between farms or holding establishments and slaughterhouses and includes data on individual animal identification and movements, epidemiological data, relevant reports about previous AMI and PMI of animals from the same farm, holding establishment etc. (full list is provided in Section 3, Annex II of Regulation (EC) 853/2004). FCI is received by the slaughterhouse operators not less than 24 h before the arrival of animals or, at the latest, on arrival of animals at the slaughterhouse. The official veterinarian shall verify the results of the checks and evaluations of FCI provided by the slaughterhouse and shall take those checks and evaluations into account when carrying out AMI and PMI (Regulation (EU) 2019/627). FCI serves to help categorise the animals according to their expected food safety risk before arrival at the slaughterhouse or at least before slaughter (i.e. non‐suspect/low risk and suspect/or high‐risk animals/batches of animals), so that slaughter procedures and/or decisions on fitness for human consumption can be adapted accordingly (Buncic, 2006).
1.3.1.2. Ante‐mortem inspection of domestic ungulates
AMI is a visual examination carried out by the official veterinarian – or under his/her supervision or responsibility – to evaluate the health and welfare of the animals, and to prevent animals having, or suspected of having, a disease/condition that may adversely affect human or animal health from entering the slaughter line. This visual examination does not allow the identification of diseased animals with mild clinical signs. Particular attention is given to the detection of zoonotic diseases and animal diseases for which animal health rules are laid down in Regulation (EU) 2016/429. AMI serves also to detect signs of use of prohibited or unauthorised substances, misuse of veterinary medicinal products or the presence of chemical residues or contaminants as well as to assess animal cleanliness (to avoid unacceptable risk of meat contamination). All ungulates are subjected to AMI before slaughter within 24 h of arrival of animals at the slaughterhouse and less than 24 h before slaughter, while the official veterinarian may require an additional AMI at any other time (Regulation (EU) 2019/627). AMI is conducted by visual observation of animals, preferably in motion, at the holding of provenance (in accordance with Article 5 of Delegated Regulation (EU) 2019/6249, during arrival to slaughterhouse (i.e. unloading) or in the lairage. Additionally, in the case of animals suspected of carrying a disease, more detailed ante‐mortem examination is conducted in a detention pen. The official veterinarian makes a decision based on the FCI analysis and AMI whether the individual animal can proceed to slaughter and will provide meat suitable for human consumption. The initial FCI‐based risk categorisation of animals should be re‐evaluated in the light of any relevant ante‐mortem findings. Then, animals are sorted into three broad categories: (1) animals that can progress to slaughter and be subjected to routine PMI; (2) animals that must be removed from the food chain; and (3) animals that require to be processed separately from the routine slaughter chain and/or require a more detailed PMI.
1.3.1.3. Consideration about disease detection at slaughterhouse
According to Section 1.2, the animals affected by acute forms of diseases of public health interest or transmissible to animals (e.g. acute forms of haemorrhagic diseases) and/or notifiable diseases, like most of those listed according to article 5 of Regulation (EU) No 2016/429, which are diagnosed at farm or at AMI because of detectable clinical signs, are excluded from PMI or, if this is done, it is for diagnostic purposes and to confirm disease suspicion. In any case, these animals do not enter the food chain and they will not be slaughtered to produce meat for human consumption (see Regulation (EU) 2017/62510). For these diseases, an assessment of the effect of a delayed PMI would not be needed. The assessment is still conducted in the present opinion, but this consideration has to be kept in mind. The same would apply for diseases where target organs and related lesions are not screened or observed at slaughterhouse, in particular those inducing lesions in the brain, e.g. rabies. Instead, the main target of the assessment would be those ungulates with subclinical disease that enter the abattoir, that do not present any signs of disease at AMI, then are slaughtered and may present detectable lesions at PMI. Unfortunately, in general, for most of these diseases, the extent of the lesions in subclinical or asymptomatic forms is not described in the literature.
A third possible situation involves the animals that present chronic lesions (e.g. nodules, abscesses etc.); these are the animals most readily detectable at PMI, and in general, the sensitivity of PMI would not change at 24 or 72 h, because this type of lesions will be still present and visible.
Separate consideration should be given to laboratory tests carried out at the slaughterhouse, and if and how these would be affected by a delayed PMI. The situations where sampling for laboratory tests can be done in slaughterhouses are:
laboratory tests mandated by regulation: e.g. tuberculosis (TB) or trichinella;
laboratory tests done on request by the veterinary service due to suspicion of disease (e.g. ASF, horses imported from endemic areas of glanders etc.);
laboratory tests performed on the request of a veterinary inspector for ruling out a disease of PH or AH relevance that is suspected at ante‐mortem: in this case sampling would mostly happen immediately after slaughter.
A summary of the possible situations faced at a slaughterhouse is presented in Figure 1.
Figure 1.
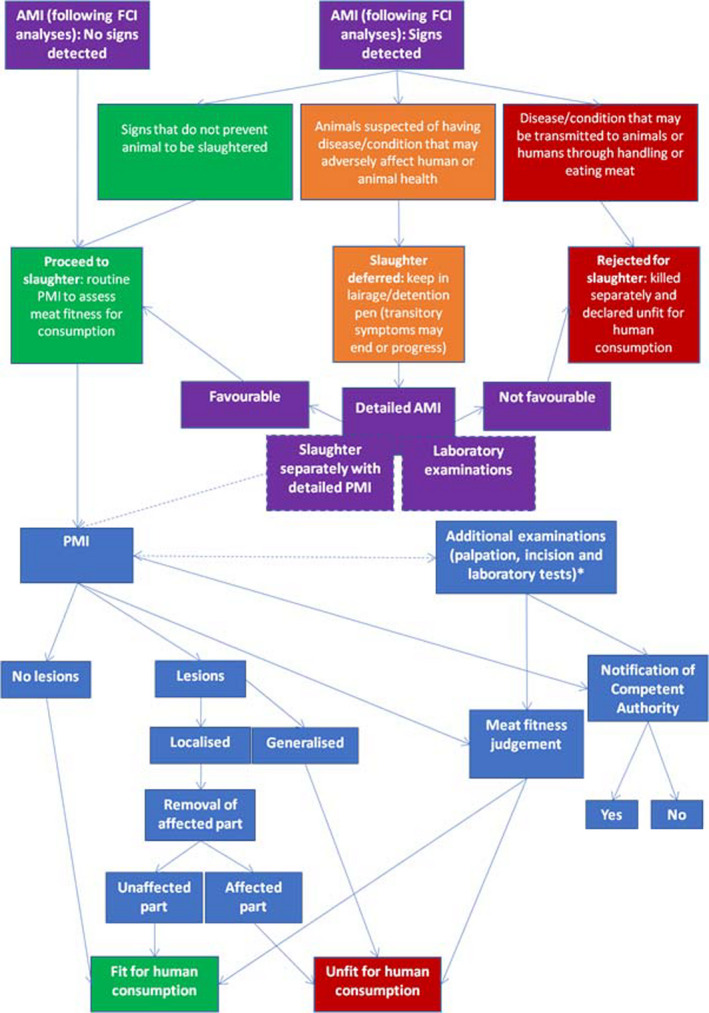
Post‐mortem inspection of domestic and farmed game ungulates
-
*: Shall be carried out if needed to:a) reach a definitive diagnosis of a suspected hazard; orb) detect the presence of:i) an animal disease for which animal health rules are laid down in Regulation (EU) 2016/429;ii) chemical residues or contaminants as referred to in Directive 96/23/EC 11 and Decision 97/747/EC 12, especially:— chemical residues in excess of the levels laid down in Regulations (EU) No 37/201013 and (EC) No 396/200514;— contaminants exceeding the maximum levels laid down in Regulations (EC) No 1881/200615 and (EC) No 124/200916; or— residues of substances that are prohibited or unauthorised in accordance with Regulation (EU) No 37/2010 or Directive 96/22/EC 17;iii) non‐compliance with the microbiological criteria referred to in Article 3(1)(b) of Regulation (EC) No 2073/200518 or the possible presence of other microbiological hazards that would make the fresh meat unfit for human consumption;iv) other factors that might require the fresh meat to be declared unfit for human consumption or restrictions to be placed on its use.
Animals affected by subclinical diseases may not show abnormalities at ante‐mortem inspection and thus are not detected. Therefore, in practice, a proportion of diseased animals pass AMI as healthy, meaning they are subjected to routine PMI.
1.3.1.4. Post‐mortem inspection domestic and farmed game ungulates
PMI primarily serves to identify abnormal carcasses/organs, to determine if the findings are acute or chronic and whether the findings refer to localised or generalised conditions with an ultimate aim to determine fitness for consumption of the meat and edible offal (Buncic, 2006; Ninios et al., 2014). Except in special cases, e.g. in the presence of highly pathognomonic lesions or when laboratory analyses (e.g. bovine tuberculosis) are requested and performed, PMI does not lead to a diagnosis of a specific animal disease or a specific risk for public health.
Appropriate facilities and sufficient space are required to perform PMI. These include access to carcasses and offal at the inspection points, a proper identification system for carcasses and associated offal, hand and equipment (gauntlets, knives, steels, hooks etc.) sanitation units, separate areas for retained meat etc. In addition, an efficient recording system and adequate time to perform inspection are required.
The official veterinarian has to ensure that ‘carcasses of domestic solipeds, bovine animals over eight months old and domestic swine more than five weeks old’ to be submitted for PMI are ‘split lengthways into half carcasses down the spinal column’. If the inspection necessitates it, the official veterinarian may also require ‘any head or any carcass to be split lengthways. However, to take account of particular eating habits, technological developments or specific sanitary situations, the official veterinarian may authorise the submission for post‐mortem inspection of carcasses of domestic solipeds, bovine animals more than eight months old and domestic swine more than five weeks old that are not split in half’ (Regulation (EU) 2019/627).
According to Commission Implementing Regulation (EU) 2019/627, ‘carcasses and accompanying offals’ of domestic ungulates shall be subjected to PMI ‘without delay after slaughter’. ‘The competent authorities shall: (a) check all external surfaces, including those of body cavities of carcasses, as well as offal’, and ‘b) pay particular attention to the detection of zoonotic diseases and animal diseases for which animal health rules are laid down in Regulation (EU) 2016/429’.
PMI of domestic ungulates is conducted macroscopically (i.e. visual inspection, palpation and/or incision) at multiple inspection points at the slaughter line (Table 2).
Table 2.
Post‐mortem inspection practices of domestic ungulates and game(a)
| Meat inspection practices | Cattle | Small ruminants | Solipeds | Pigs | Large wild game | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Regulation (EU) 2019/627 | Youngb | Other | Youngc | Other | ||||||||||
| M | Ad | M | Ad | M | Ad | M | Ad | M | Ad | M | Ad | M | ‘where appropriate’e | |
| HEAD | V | V | Vf | Vf | V | V | V+P+I | |||||||
| Mouth | V | V | Vf | Pf | Vf | Pf | V | V | V+P+I | |||||
| Throat | V | V | Vf | Pf | Vf | Pf | V | V | V+P+I | |||||
| Fauces | V | V | P | V | V | V+P+I | ||||||||
| Retropharyngeal lymph nodes | P | I | I | Vf | Pf | P+I | V+P+I | |||||||
| Submaxillary lymph nodes | I | P+I | I | V+P+I | ||||||||||
| Parotid lymph nodes | I | Vf | Pf | Vf | Pf | P+I | V+P+I | |||||||
| Masseters | I | V+P+I | ||||||||||||
| Tongue | P | P | Vf | Pf | Vf | Pf | V | P | V | V+P+I | ||||
| LUNGS | V+P | If | V+P | If | V | P+I | V+P | I | V | P+If | V | P+If | V+P+I | |
| Trachea | V | If | V | If | V | I | V | I | V | If | V | If | V+P+I | |
| Main branches of bronchi | If | If | If | If | V+P+I | |||||||||
| Mediastinal lymph nodes | P | If | I | V | I | P | I | V | P+I | P | V+P+I | |||
| Bronchial lymph nodes | P | If | I | V | I | P | I | V | P+I | P | V+P+I | |||
| OESOPHAGUS | V | V | V | I | V | I | V | V | V+P+I | |||||
| HEART | V | I | V+I | V | I | V | I | V | I | V | I | V+P+I | ||
| Pericardium | V | V | V | V | V | V | V+P+I | |||||||
| DIAPHRAGM | V | V | V | V | V | V | V+P+I | |||||||
| LIVER | V | V | P+I | V | P+I | V+P+I | V | P+I | V | P | V+P+I | |||
| Hepatic lymph nodes | V | V | P | V | P | V+P | V | P+I | V | P | V+P+I | |||
| Pancreatic lymph nodes | V | V | P | V | P | V | V | P+I | V | P | V+P+I | |||
| GASTROINTESTINAL TRACT | V | V | V | V | V | V | V+P+I | |||||||
| Mesentery | V | V | V | V | V | V | V+P+I | |||||||
| Gastric lymph nodes | V | I | V+P | I | V | V | V | I | V | P+I | V+P+I | |||
| Mesenteric lymph nodes | V | I | V+P | I | V | V | V | I | V | P+I | V+P+I | |||
| SPLEEN | V | P | V | P | V | P | V | P | V | P | V | P | V+P+I | |
| KIDNEYS | V | I | V | I | V | I | V | I | V | P+If | V | I | V+P+I | |
| Renal lymph nodes | I | I | I | I | I | I | V+P+I | |||||||
| GENITAL ORGANS | V | V | V | V | Vj | V+P+I | ||||||||
| UDDER | V | P+If | V | V | V | V+P+I | ||||||||
| Supramammary lymph nodes | V | P+If | V | V | I | V | I | V+P+I | ||||||
| DRESSED CARCASS (external surfaces) | V | V | V | V | V | V | V | V+P+I | ||||||
| Pleura | V | V | V | V | V | V | V | V+P+I | ||||||
| Peritoneum | V | V | V | V | V | V | V | V+P+I | ||||||
| Umbilical region | Vh | (P+I)(h) | V | P+I | Vh | (P+I)h | Vh | (P+I)h | V | V+P+I | ||||
| Joints | Vh | (P+I)h | V | P+I | Vh | (P+I)h | Vh | (P+I)h | V | V+P+I | ||||
| Muscles and lymph nodes of shoulders | Vi | |||||||||||||
A: additional; I: incision M: mandatory; P: palpation V: visual inspection.
PMI of farmed game is carried out according to equivalent domestic animal species. Specific requirements exist for wild game.
< 8 months or < 20 months if reared without access to pasture during their whole life in an officially tuberculosis‐free MS or region.
Sheep < 12 months or no permanent incisor erupted, goats < 6 months.
When there are indications of a possible risk to human health, animal health or animal welfare, based on (a) the checks and analysis of documents (i.e. FCI) (b) the findings of ante‐mortem inspection; (c) the results of the verifications of compliance with animal welfare rules; (d) the findings of post‐mortem inspection (mandatory procedures) (e) additional epidemiological data or other data from the holding of provenance of the animals.
When hunter and trained person detect no abnormities, the carcass (eviscerated, skin on) is delivered to a game‐handling establishment, where veterinary post‐mortem inspection is conducted. To this end, the skinned carcass is inspected and the information on the accompanying certificate is taken into account. If no trained person is available, the eviscerated carcass is accompanied by all viscera except stomach and intestines. If abnormalities have been detected, the eviscerated carcass plus all viscera are presented to veterinary inspectors.
Not necessary if excluded from human consumption.
Through the entire kidney in grey horses.
Young animals (not specified how young).
In grey horses, examination for melanosis and melanomata beneath the scapular cartilage after loosening the attachment of one shoulder.
Relates only to testes (‘orchitis’).
1.3.1.5. Post‐mortem inspection of wild game (wild ungulates)
PMI of large wild game must be carried out as soon as possible after arrival of wild game carcasses at a game‐handling establishment. It should be noted that carcasses of large wild game are not always immediately transported to approved game‐handling establishments but may be stored in refrigerated collection centres from which they are picked up by refrigerated trucks serving the game‐handling establishments. Thus, the time from killing and evisceration to arrival of the carcass at the game‐handling establishment is variable. Data from Austrian game‐handling establishments indicate that typically (median) time from killing to PMI was 5 days for red deer (range 2–16 days), 4 days for wild boar (range 2–14 days; Staubmann, 2017) or 8 days for various animal species (range 3–14 days; Paulsen, 2005). Carcasses arrive in eviscerated, skin‐on condition. Since full PMI requires inspection of the skinned body (‘all surfaces’), the earliest possible point for inspection is during skinning. It is questionable if game‐handling establishments process carcasses according to first‐in‐first‐out principle; it will be more common to process carcasses species‐wise.
An initial examination of hunted wild game intended for human consumption is carried out as soon as possible after killing by specially qualified hunters or gamekeepers (‘trained persons’) (Regulation (EC) No 853/2004; Annex III, Section IV, Chapter II). ‘After killing, large wild game must have their stomach and intestines removed as soon as possible and, if necessary, be bled’. ‘The trained person must carry out an examination of the body, and of any viscera removed, to identify any characteristics that may indicate that the meat presents a health risk’. Trained persons ‘attach to the animal body (carcass) a numbered declaration’ containing not only information on traceability but also specifying, where applicable, that ‘no abnormal behaviour was observed before killing’, and that there was ‘no suspicion of environmental contamination’, such as lead or radioactive contamination. In this case, there is no requirement that the head and the viscera accompany the body, ‘except in the case of species susceptible to Trichinellosis’ (Suidae, solipeds), ‘whose head (except for tusks) and diaphragm must accompany the body’ (point 4(a) of Chapter II of Section IV of Annex III to Regulation (EC) No 853/2004).
In other circumstances (i.e. no trained person available), ‘the head (except for tusks, antlers and horns) and all viscera except for the stomach and intestines must accompany the body. The trained person who carried out the examination must inform the competent authority of the abnormal characteristics, abnormal behaviour or suspicion of environmental contamination that prevented […] a declaration’. In the latter case, ‘the viscera must accompany the body’ and ‘must be identifiable as belonging to a given animal’.
‘Meat of large wild game may be placed on the market only if the body is transported to a game‐handling establishment as soon as possible after the examination’ detailed above (Chapter II of Section IV of Annex III to Regulation (EC) No 853/2004)).
According to Commission Implementing Regulation (EU) 2019/627, for ‘large wild game, the official veterinarian at the game‐handling establishment shall examine and take into account the declaration accompanying the body of the animal, as issued by a trained person in accordance with point 4(a) of Chapter II of Section IV of Annex III to Regulation (EC) No 853/2004’. Thus, PMI of hunted game usually does not include examination of stomach, intestines and other offal by official veterinarians. PMI procedures should follow the requirements of Article 12 of Commission Implementing Regulation (EU) 2019/627. Article 13 of this Regulation allows, with some additional requirements, delayed PMI ‘by a maximum period of 24 h from slaughter or arrival in the game‐handling establishment’ for plants processing ‘fewer than 1,000 livestock units per year. This is different from previous Regulation (EC) No 854/2004, which required that ‘wild game is to be inspected as soon as possible after admission to the game‐handling establishment’, without setting a time limit for ‘immediate’ or delayed PMI. The course of PMI (Article 28) remains essentially the same as in the previous Regulation (EC) No 854/2004 and differs from that of domestic animals as it is not organ‐, but symptom‐oriented.
Figure 2.
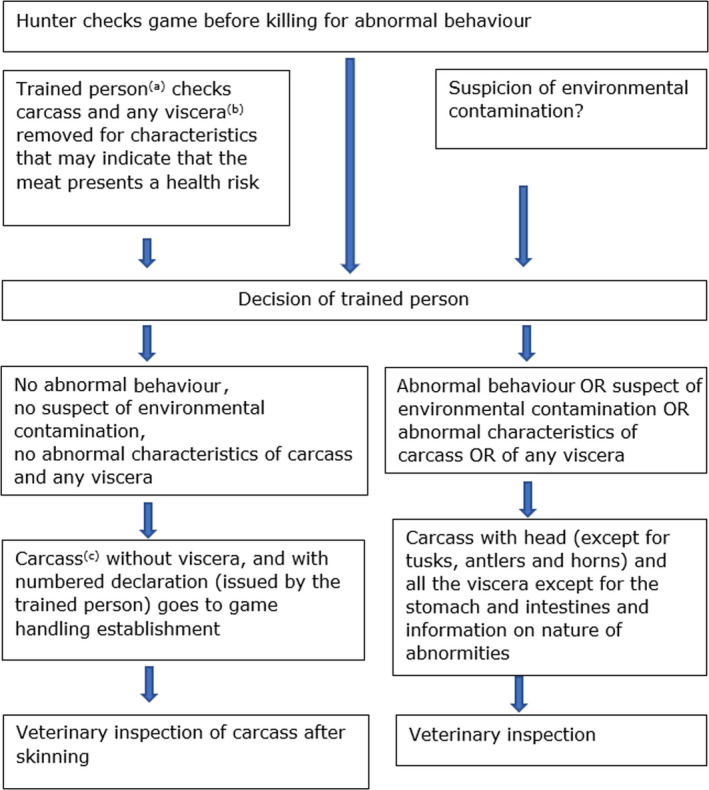
Examinations carried out by hunters and post‐mortem inspection of large game
Summarises the PMI for hunted large game.
-
(a): if no trained person is available, in a particular case, the head (except for tusks, antlers and horns) and all the viscera except for the stomach and the intestines must accompany the body. The viscera must be identifiable as belonging to a specific animal. (Note: this assumes that hunters during evisceration already perform some examination of stomach and intestines)(b):‘Viscera’ means the organs of the thoracic, abdominal and pelvic cavities, as well as the trachea and oesophagus.(c): Head can be removed before, except in species susceptible for Trichinella.
1.3.1.6. Laboratory tests for tuberculosis and trichinellosis
PMI is followed, when needed, by sampling for laboratory testing. The laboratory tests are used to reach a definitive diagnosis, to detect the presence of animal diseases, residues of pharmacologically active substances or contaminants, to check compliance with microbiological criteria and to investigate if other factors make the meat unfit for human consumption. Some of the tests commonly carried out include testing for the presence of oedema, acetonaemia, unusual odour (e.g. boar taint), imperfect bleeding, tuberculosis, residues of antimicrobials, as well as microbiological tests, Trichinella testing, TSE testing etc. (Buncic, 2006; Ninios et al., 2014).
In the case of animal tuberculosis, post‐mortem laboratory tests should be carried out:
when macroscopic lesions compatible with the disease are observed during PMI (i.e. not detected through active on‐farm surveillance) and,
in animals suspected to be infected, even in the absence of macroscopic lesions, due to positive results in the ante‐mortem tests implemented on farm (i.e. active surveillance).
As mentioned in point 1.2, the assessment performed in this opinion is exclusively focused on animals presenting lesions compatible with tuberculosis and detected through passive surveillance (PMI).
According to Annex B of Council Directive 64/432/EEC19, the presence of members of the Mycobacterium tuberculosis complex (MTBC), causative agents of animal tuberculosis, in tissues collected from suspected animals can be demonstrated by examination of stained smears or immunoperoxidase techniques and confirmed by cultivation of the organism on primary isolation medium. A polymerase chain reaction (PCR) test may also be employed for the detection of the MTBC. The tests referred to in accordance with Directive 64/432/EEC are valid until 21/04/2021. After that, the Regulation 2016/429 (Animal Health Law) and Commission Delegated Regulation (EU) 2020/68920 will apply. The tests listed in Annexes III section 2 and IV part II to the Commission Delegated Regulation (EU) 2020/689 supplementing Regulation (EU) 2016/429 of the European Parliament and the Council laying down rules for surveillance, eradication programmes and disease freedom for certain listed and emerging diseases are, by virtue of its Article 6(2), only for the purposes of granting and maintaining of disease‐free status. That is why the tests listed there are only for live animals. For diagnostic tests for other purposes, including these for surveillance, Article 6(1) of Regulation (EU) 2020/689 applies. For post‐mortem investigations, the tests are a part when granting and maintaining the MTBC‐free status (sections 1 and 2 of chapter 2 of Part II of Annex IV to Regulation (EU) 2020/689).
Regulation (EU) 2015/137521, Article 2, laying down‐specific rules on official controls for Trichinella in meat, requires that carcasses of domestic swine shall be sampled in slaughterhouses as part of PMI. All breeding sows and boars are to be tested, and for fattening pigs, it depends on the type of holding whether a 10% sample or all animals are tested. Carcasses of horses, wild boar and other farmed and wild animal species susceptible to Trichinella infestation shall be systematically sampled in slaughterhouses or game‐handling establishments as part of the post‐mortem examination. Sample locations (striated muscle) and sample sizes, as well as a reference method (‘Magnetic stirrer method for pooled sample digestion’; Annex 1, Chapter 1) and alternative methods are detailed in said regulation. Most methods are based on a simulated gastric digestion of the sample, after which the larvae are collected by sedimentation or by filtration; one method uses latex agglutination. Another proprietary method relies on digestion at alkaline pH with subsequent antigen detection. The latter two methods are applicable to domestic swine only.
1.3.1.7. Process Hygiene Criterion for Salmonella
In accordance with the Commission Regulation (EC) No 2073/2005, carcases of pigs, cattle, sheep, goats and horses, after dressing but before chilling, are tested for Salmonella presence/absence by an EN/ISO 6579 analytical method. An abrasive sponge sampling method must be used. Carcass areas most likely to be contaminated are sampled using an abrasive sponge, and the total sampling area must cover a minimum of 400 cm2. Five carcases must be sampled at random during each sampling session. Hence, during 10 consecutive sampling sessions, 50 samples are derived (n). The maximum number of samples in which it is permissible for Salmonella to be detected (c) is three for pigs and two for cattle, sheep, goats and horses. In the case of unsatisfactory results (i.e. when the presence of Salmonella is detected in more than c/n samples), improvements in slaughter hygiene and review of process controls, origin of animals and of the biosecurity measures in the farms of origin, are actions to be taken.
2. Data and methodologies
Various methods have been used in the assessment based on different legislative requirements and on available scientific and expert information. When data based on scientific literature were scarce, questionnaires, modelling and experts’ opinion have been used to assess the different TORs and to calculate possible uncertainties.
2.1. Data and Methodology related to diseases listed according to Article 5 of Regulation (EU) 2016/429 and cysticercosis
2.1.1. Data
Data used in the assessment originated mainly from three sources. First, a list of post‐mortem changes associated with the listed diseases was generated using scientific literature and the expertise of the working group members. In this respect, it is important to note that the lesions described in the literature or known to the experts most often correspond to those observed in animals that had died as a result of the disease in question or that were slaughtered following the appearance of symptoms sufficiently pronounced to be easily detected on clinical examination. However, the lesions observable in infected but asymptomatic or mildly symptomatic slaughtered animals are never well known or specified.
Subsequently, information relevant to PMI and post‐mortem laboratory tests was gathered through two ad hoc surveys designed for this scientific opinion and submitted to meat inspectors and/or reference laboratories of EU Member States (see Sections 2.1.2.4 and 2.1.2.5).
2.1.2. Methodology
To assess a possible decrease in sensitivity of detection at PMI for the diseases listed under article 5 of Regulation (EU) 2016/429, in the absence of information in the literature, it was agreed to estimate this output by expert knowledge elicitation (EKE) based on different sources of evidence: the clinical and post‐mortem signs of the diseases as derived from the literature, the opinion from meat inspectors from EU about the chance of detecting the typical post‐mortem lesions of each disease and the discussion of the results obtained by EKE with the members of the WG.
To prepare evidence to support the EKE, the steps below were followed:
Development of a disease map where all clinical and post‐mortem signs of the disease were compiled;
Selection of the type of lesions subjected to possible changes if PMI is delayed;
Building a table for disease mapping in order to connect animal species–organs–lesions to related disease;
Design and distribution of a questionnaire on detectability of the lesions to meat inspectors in the EU and to National Reference Laboratories (NRL) for bovine tuberculosis (bTB);
Summary of results and preparation of an evidence dossier;
Performing EKE to provide a judgement about the number of carcasses out of 100 where the disease would be still detectable at 24 or 72 h delayed PMI;
Interpretation of the results.
2.1.2.1. Step 1: Disease map
In order to identify the main lesions associated with the target diseases, which were in turn grouped into categories for major pathological changes, the first step was to create a table of the diseases, with information about the clinical forms (acute, subacute, chronic or latent forms) and clinical signs, as well as potential lesions that could be observed on the respective diseased animals.
The epidemiological situation in Europe and the existence of regulated surveillance have also been taken into account for each disease.
As described in Section 1.3.1.3 for most of these diseases listed according to article 5 of AHL, the diagnosis is mainly done at farm, not at the slaughterhouse, in particular for acute or subacute clinical forms.
Considering the above, the following information is indicated for each disease/condition in the disease map:
The susceptible animal species.
Whether there is any surveillance programme in place in the EU.
The signs associated with the disease that could be detected at AMI.
The lesions associated with the disease that could be detected at PMI.
The probability of detecting the disease during the PMI as normally carried out.
Whether carcass swabbing and/or laboratory tests are normally carried out.
2.1.2.2. Step 2: Selection of lesions subjected to possible changes at delayed PMI
Each disease/condition assessed is manifested through a range of lesions in offal and/or dressed carcasses of slaughtered animals. Only lesions that are practically detectable during routine PMI (i.e. macroscopic lesions) are considered and, when present, in organs that are supposed to be inspected by procedures laid down in Regulation (EU) 2019/627 (Table 2).
Acute inflammatory lesions (fibrin etc.) are considered prone to changes, mostly due to drying during cold storage of meat/organs for 24 or 72 h and thus might become less, or even non‐detectable, at delayed meat inspection. Chronic lesions (abscesses, granulomas etc.), on the other hand, are considered not to be significantly affected during prolonged storage, so remain detectable even after storage of 24 or 72 h.
2.1.2.3. Step 3: Map of lesions to diseases
In order to facilitate the EKE about the potential loss in sensitivity of the delayed PMI (24 or 72 h) in the detection of specific lesions in specific organs for each specific disease, a complementary table was built.
The objective here was to align for each disease the list of organs or tissues and the associated lesions with the terms used in standard meat inspection practice (and as used in EU regulations). This terminology formed the basis of the first survey submitted to the meat inspectors (see below). The data on diseased organs and tissues and the corresponding lesions collected from the literature and included in the ‘disease map’ for each disease were thus transcribed into a supplementary table in order to align with the organ/tissue terms listed in previous Table 2 and the short list of potential lesions as mentioned in Section 2.1.2.2.
2.1.2.4. Step 4 a: Questionnaire to meat inspectors in Europe
Eighteen meat inspectors, from Austria, Denmark, Belgium, France, Serbia, Spain and Sweden, were asked to provide a numerical answer to the following question:
‘During routine PMI (considering the minimum requirements by legislation) of ungulates (bovine, small ruminants, equine, suids, game ungulates) assuming that you find a lesion (the one selected according to Section 2.1.2.2 ) at PMI immediately after slaughter, how many carcasses out of 100 with that lesion, will still be detected after 24‐ or 72‐h of refrigerated storage?’
including, next to each answer, how certain they were about their answer, by giving a number between 1 and 10 (10 = very sure; 1 = very uncertain).
In addition, they were asked to confirm whether, during routine PMI (considering minimum requirements by legislation) of ungulates (bovine, small ruminants, equine, suids), chronic lesions remain detectable, if the inspection is done with a delay of 24 or 72 h.
The purpose of this questionnaire was to explore the opinion of meat inspectors working in slaughterhouses and to provide a further source of evidence for the EKE.
2.1.2.5. Step 4 b: Questionnaire to NRLs for sampling for bovine tuberculosis diagnosis
A delay in the collection of samples could have an impact on the performance of the diagnostic tests used to confirm tuberculosis (i.e. decrease in their sensitivity) due to changes occurring in clinical samples – either from animals with lesions or suspected animals (positive in tests) – stored for 24 or 72 h under refrigeration. The sensitivity of bacteriological isolation could be impaired due to a decrease in the viability of the MTBC bacteria present in the sample and/or an increased contamination of the sample by other more rapidly growing microorganisms that could then overgrow the MTBC when the sample is inoculated on culture media. Performance of histopathology may decrease due to the degradation of cellular structures, particularly in the case of early lesions. Finally, the sensitivity of a direct PCR test for detection of MTBC could be affected due to the degradation of the bacterial DNA in the sample.
In order to assess the specific effect of the delay on the performance of the laboratory diagnostic tests for confirmation of tuberculosis in animals presenting macroscopic lesions compatible with the disease, a survey asking for the possible effect of a delay on PMI and sampling for bovine tuberculosis diagnosis was submitted to the NRLs of the 27 EU Member States including the UK and to the European Reference Laboratory (EURL) for bovine tuberculosis. Participants were asked to estimate the possible effect of a delay in the sensitivity of three laboratory tests currently used for confirmation of tuberculosis in animals according to the present EU Regulation (Council Directive 64/432/EEC ‐ Annex):
Bacteriological culture (i.e. in vitro isolation of an MTBC member in solid or liquid culture media).
Direct‐PCR (i.e. direct detection of DNA of a MTBC member through PCR).
Histopathology (i.e. detection of characteristic histological lesions – caseous necrosis, mineralisation, epithelioid cells – with or without evidencing the presence of acid‐fast bacilli using stained smears or immunoperoxidase techniques).
The question posed was:
‘How likely is that the sensitivity of each diagnostic method decreases if the PMI and sample collection was performed after storing the carcass for 24‐ or 72‐h, at refrigeration, compared to immediate testing at PMI, on a scale of 1‐100% using the following Table 3 for guidance (EFSA Scientific Committee, 2018 )?’
Table 3.
Approximate probability scale adopted for harmonised use in EFSA (EFSA Scientific Committee, 2018)
| Probability term | Subjective probability range | Additional options | |
|---|---|---|---|
| Almost certain | 99–100% | More likely than not: > 50% |
Unable to give any probability: range is 0–100% Report as ‘inconclusive’, ‘cannot conclude’, or ‘unknown’ |
| Extremely likely | 95–99% | ||
| Very likely | 90–95% | ||
| Likely | 66–90% | ||
| About as likely as not | 33–66% | ||
| Unlikely | 10–33% | ||
| Very unlikely | 5–10% | ||
| Extremely unlikely | 1–5% | ||
| Almost impossible | 0–1% | ||
2.1.2.6. Steps 5 and 6: evidence dossier and EKE
In order to translate the judgements regarding the impact of the delayed PMI on the detection of specific lesions in specific organs received from the meat inspectors (see Section 2.1.2.4) into the effect that such delayed PMI would have on the detection of the target diseases/conditions, three expert knowledge elicitation (EKE) exercises were conducted within the working group. These exercises had as their objective to elicit judgements from the members of the working group on the effect of the delayed PMI on the detection of the target diseases/conditions given the available evidence (the responses obtained in the survey from the meat inspectors) and their expert knowledge. These EKEs were carried out according to the Sheffield method (EFSA, 2014; Oakley and O'Hagan, 2016) with certain modifications to fit the workflow of the opinion.
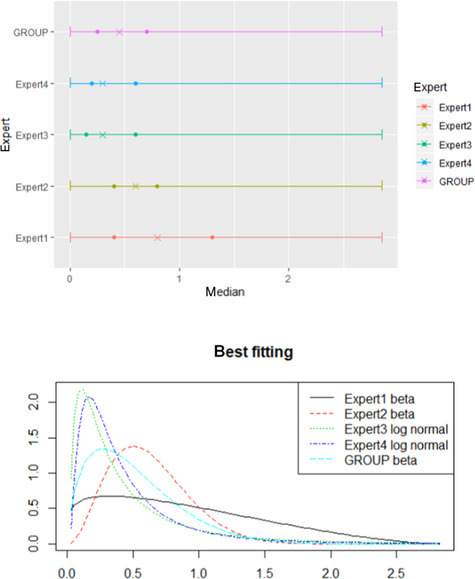
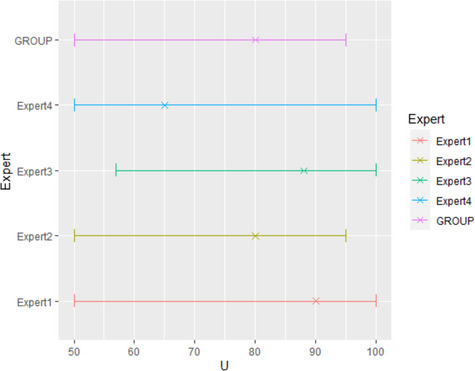
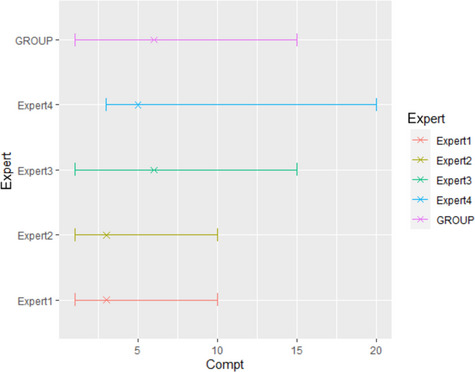
Results from the survey from all meat inspectors regarding the impact of the delayed PMI (24 or 72 h) on the detection of specific lesions in specific organs were compiled and summaries of the responses received were used as evidence to inform the judgements to be made during the EKE. Briefly, the responses on lesions on organs associated with each disease were identified and displayed together as dot plots that also showed the 50% central part of the distribution as boxplots. The size of the dot for each response was proportional to the certainty stated by the respondent. In addition, tables showing the minimum, maximum, mean and median values along with the first and third quartile for each organ–lesion pair relevant for each disease were also produced (see Appendix A).
These summaries of all replies in relation to organs and lesions relevant for each disease were compiled as evidence dossiers that also included the parameter of interest that would be elicited for each disease/condition and delay, defined as ‘the mean number of carcasses from infected animals assessed as diseased in a PMI carried out immediately after slaughter (current procedure) per 100 that would still be detectable after 24‐ or 72 h’. Experts participating in the EKE were reminded that assessing a carcass as ‘diseased’ meant the detection of lesions during (visual) inspection compatible with the presence of a disease (not necessarily that the disease in question would have been identified). Since judgements on the ability to detect a carcass from an animal with a given disease were made based on the presence of specific lesions, for certain diseases/conditions in which the same set of organs and lesions were identified in the mapping exercise (diseases affecting the reproductive tract, i.e. brucellosis, contagious equine metritis, dourine, Japanese encephalitis, Q fever, infectious bovine rhinotracheitis/infectious pustular vulvovaginitis and trichomonosis; diseases affecting the respiratory tract, i.e. contagious bovine and caprine pleuropneumonia; toxaemia and viraemia; cysticercosis and echinococcosis), single respective distributions were elicited.
The experts received the evidence dossier with the information on the diseases that would be assessed in each of the three EKE exercises at least four working days in advance, along with information on probabilistic judgements available at http://www.tonyohagan.co.uk/shelf/ecourse.html, that was briefly reviewed at the beginning of the first EKE exercise. Since the EKE exercises were based on the quartile method (EFSA, 2014), experts were asked to provide one day before the session their individual judgements on the median and first and third quartile for the distribution of the parameter of interest (i.e. two judgements per disease/condition, one for 24 and another for 72 h) since the lower and upper plausible limits were set at 0 and 100 based on the replies of the meat inspectors.
During the EKE exercises, four members of the working group reviewed their individual judgements for the parameter of interest for each disease and each delay period (24 and 72 h) considered, and agreed upon a consensus distribution with the support of a facilitator. Each consensus distribution was then scrutinised and subjected to variations, when considered necessary, until the group felt it adequately represented their knowledge on the parameter of interest.
In the case of bovine tuberculosis and given that laboratory tests were considered a critical part in the routine overall assessment for the presence of the disease in inspected animals, a separate EKE session to elicit the probability distribution reflecting the knowledge on the effect of the delay in the performance of the diagnostic tests used currently in the EU (culture, direct PCR and histopathology) was also conducted. In this case, the experts were asked to consider all infected animals with macroscopic lesions that would be sampled and would yield a positive result in a laboratory test for bovine tuberculosis if samples were collected immediately after slaughter. The parameter of interest about which they were asked was ‘the mean number per 100 of those (already identified as diseased) animals that would still yield a positive result (in each laboratory test) if samples were collected after 24‐ or 72 h’. The EKE exercise followed the same process described above.
The overall assessment on the effect of a delay in the PMI on the detection of tuberculosis in infected animals will be the combination of the effect of such a delay on the detection of macroscopic lesions compatible with the disease (step 1) and on the performance of the diagnostic tests on samples collected after the delay (step 2). Logically, the overall decrease in the ability to detect and confirm tuberculosis‐infected animals must be equal to or lower than the result of direct multiplication of the effect in step 1 and step 2 (see Appendix B for detailed explanation). Therefore, the result of multiplying the effects of the two steps is a lower bound for the overall effect.
The distribution for this overall effect (representing the uncertainty of the outcome in the worst‐case scenario) could be estimated by multiplying the individual distributions estimated for the effects in steps 1 and 2 (elicited separately in the EKEs) if the uncertainties affecting these two effects can be considered independent. However, the working group agreed that the assumption of independence might not be true in this case. If the true frequency of detecting lesions after the 24‐ or 72‐h delay in step 1 was in the upper end of the elicited distribution, this would be likely related to the size of the lesions (larger and easier to detect), which would also result in a higher frequency of positive results in the laboratory diagnostic tests (and vice versa, with lower chances of detecting carcasses with lesions leading to lower chances of laboratory confirmation of the infection). Therefore, to take into account the possible effect of some dependence between the effect of the delay in steps 1 and 2, the overall effect was estimated under three scenarios:
Scenario 1: Assuming perfect independence between the effects (i.e. as the product of both distributions)
Scenario 2: Assuming an almost perfect positive correlation between the effects (i.e. incorporating the correlation in the multiplication of the effects)
Scenario 3: Assuming no effect of the PMI delay on the detection of the lesions (i.e. considering only the effect of the delay on the laboratory tests) as an upper bound
Scenarios 1 and 2 are alternative estimates of a lower bound for the detection and confirmation of tuberculosis when the effects of the delay on the detection of lesions at the PMI and on the laboratory testing are combined, while Scenario 3 represents an upper bound. When considered together, the three scenarios represent the maximum range of uncertainty about the combined effects of the delay.
Additional details can be found in Appendix B.
2.2. Data and methodology related to Trichinella
The impact of delayed PMI on the sensitivity of Trichinella detection takes into account digestion methods. It is assumed that the sample is not taken at slaughter, but (in compliance with existing law) taken at (delayed) PMI. The following effects on the detection of trichinae will be considered:
changes in the food matrix affecting the efficacy of the pepsin‐HCl digestion: Drying of the sample might reduce efficacy of digestion and, thus, larvae may not be released from the meat
larvae might disintegrate during storage of the carcass and thus, be fully digested during pepsin‐HCl digestion
changes in trichinae affecting their resistance to pepsin‐HCl digestion: larvae might become less resistant to pepsin‐HCl digestion.
In order to obtain data on the above‐mentioned effects, a literature search was conducted in SCOPUS; in addition, informational material from the EURL for Parasites (Trichinella,Echinococcus,Anisakis) was considered. The SCOPUS search (trichin* AND (surviv* OR storag* OR digest*)) in the title, abstract or keywords retrieved 933 records, from which the abstracts were screened for relevance.
In addition, a non‐systematic literature search was conducted by screening the references listed in the reference sections of the relevant articles retrieved from the systematic literature search and by considering textbooks and information material from the EURL.
2.3. Data and methodology related to TSEs
The performance of commercial tests ussed for the statutory screening of carcases for TSE has been formally and extensively evaluated as described in a number of previous EFSA opinions (EFSA 2004, 2005a, 2005b, 2007a, 2007b; EFSA BIOHAZ Panel, 2009, 2012). These included the assessment of the effects, if any, of autolysis on both test sensitivity and specificity. The assessment in this opinion is based on these previous evaluations, and their associated references.
2.4. Data and methodology to assess the effect of delayed inspection on the probability of laboratory detection of Salmonella spp.
The impact of delayed PMI on the sensitivity of Salmonella detection was assessed in the context of a process hygiene criterion (PHC), not of the animal disease.22 For this assessment, the moment of sampling (before chilling) could be changed to the moment of (delayed) PMI. As it reads now, the PHC applies to any carcass after dressing but before chilling. Therefore, if the criterion is extended to encompass the delayed PMI, then the sensitivity of laboratory detection of Salmonella will be assessed for the time span from slaughtering until the inspection, assuming that carcasses are stored under chill conditions immediately after slaughtering, with maximum permissible temperature of carcasses being 7°C.
The effect of delayed inspection on the probability of laboratory detection of Salmonella was assessed per sample, without assessing the performance of the regulated sampling plan,23 i.e. n = 50, c = 2 (for cattle, sheep, goat and horses) and n = 50, c = 3 for pig carcasses.
The probability of Salmonella detection (i.e. the probability of finding Salmonella when it is present on a swabbed area of a carcass) is highly dependent on several factors that affect:
the levels of the organism expected on carcasses at the time of sampling, based on the available evidence;
the viability and culturability of Salmonella as affected by the cold shock caused by chilling and possible reduction of carcasses surface aw;
the ability of the sampling method (swabbing) to detach Salmonella from carcasses surfaces, depending on the attachment strength of Salmonella on the carcass and the distribution of Salmonella over the carcass as the location, e.g. in crevices, due to the surface roughness of the carcass and the removal of surface moisture.
the selectivity and capacity of the enrichment media to support growth of low and possibly injured Salmonella cells, given the growth initiation potential of Salmonella at the beginning of enrichment and the levels, the composition and the fitness of competing background meat microbiota.
In the above context, the possible injury of Salmonella due to cold stress may induce a lag time and thus, delay in growth initiation.
In principle, it is assumed that detection of Salmonella via a standard laboratory method requires the presence on carcass of a minimum (SLm Min) number of cells, as not all cells are viable, culturable (non‐irreversibly injured) and detachable by swabbing, at the time of sampling. SLm Min practically represents the required number of Salmonella cells present on a sampled area of carcass to ensure that at least one cell (=Limit of Detection according to the ISO 6579‐1 method (Anonymous, 2007)) will be detected.
Figure 3 outlines the methodology in a conceptual graph.
Figure 3.
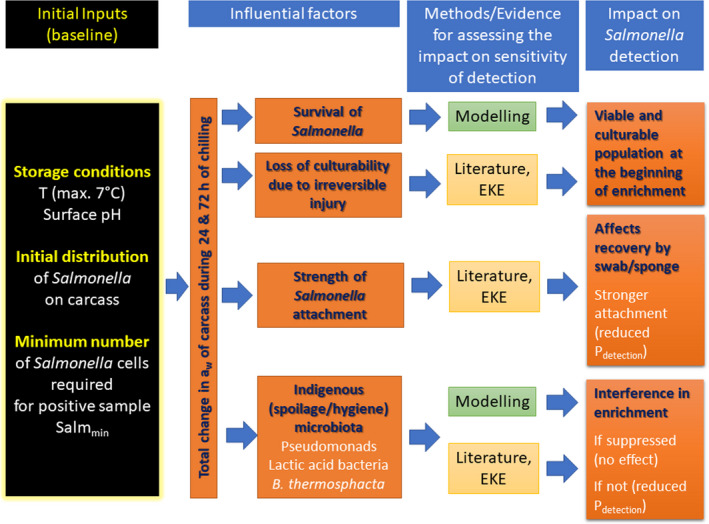
Conceptual graph of methodology to be undertaken for assessing the factors affecting the sensitivity of Salmonella detection after 24 or 72 h of carcass chilled storage
Approach to answer the assessment question:
What is the percentage of reduction (%) in sensitivity of Salmonella detection as a process hygiene criterion if the PMI is delayed by 24 or 72 h after slaughter?
To assess the impact of delayed PMI on the sensitivity of Salmonella detection, a stochastic model was applied to estimate the reduction after a delay of 24 and 72 h that is further described below.
The reference condition of the assessment is 100 positive carcasses, immediately after slaughtering (time 0). By definition, these carcasses contain sufficient culturable Salmonella cells on the swabbed area that are successfully transferred to the enrichment medium. The enrichment media have the ability to support the growth of Salmonella, including the recovery of cells that may be stressed, and allow the subsequent isolation of Salmonella on selective plates.
The impact of chilling and subsequent chilled carcass storage (≤ 7°C) for 24 or 72 h on Salmonella detection was assessed by evaluating the effect of storage conditions on the factors listed above and consequently, the resulting population of Salmonella added to the enrichment medium compared to that residing on the carcass. The reduction in sensitivity of Salmonella detection (%) is estimated by subtracting the number of carcasses (out of the 100 originally positive) that remain positive after 24 or 72 h.
The quantification of the impact of the aforementioned critical factors on Salmonella detection is implemented via a stochastic model that takes into account the variability and uncertainty in the initial population (time 0), the uncertainty of the minimum Salmonella levels required on carcass for a positive sample (SLmMin), the uncertainty about aw of carcass surface (e.g. due to surface dehydration) and the uncertainty in the contribution of each of the above factors on the levels and recovery of Salmonella, after 24 or 72 h of chilled storage.
Modelling assumptions and methodology
Two distributions of Salmonella population are taken into consideration for each of the assessment time points, i.e. 0, 24 and 72 h of chilled storage (Table 4): (i) the initial distribution of Salmonella load on carcasses and (ii) the distribution of Salmonella on the same carcasses after 24 or 72 h. If the delayed PMI has no impact on sensitivity of Salmonella detection, the two distributions will theoretically overlap completely. Conversely, if delaying PMI negatively impacts Salmonella detection, the distribution of Salmonella after storage will be shifted to the left (thus, partially overlapping), depending on the Log10 reduction (i.e. loss of viability) due to chilled storage, the loss of culturability of Salmonella while on carcass (e.g. due to permanent injury), the reduced detachment of Salmonella from carcass surface by swabbing (e.g. due to morphological changes of carcass surface during chilling and embedding of Salmonella on niches below the surface) and the reduced ability of Salmonella to outcompete the indigenous microbiota during enrichment. The fitness of Salmonella in the enrichment medium is thought to depend on the selectivity of the medium, the capacity of Salmonella to restore growth, the relative levels of the pathogen and meat microbiota at the beginning of enrichment and the strain/serovar‐dependent ‘fitness’ (Singer et al., 2009). The combined effect of these factors on Salmonella levels and recovery after delayed PMI, consistent with the preceding description of each factor's contribution to Salmonella detection, is introduced in the model based on the following concept (bold abbreviations in parenthesis are the labels of model variables):
Survival/inactivation during storage (SR: Log10 units): the Log10 reduction (loss of viability) of Salmonella after 24 or 72 h of chill storage at ≤ 7°C surface temperature, due to a drop of surface aw. It is predicted by using the non‐thermal inactivation model of Pin et al. (2011), in response to pH (fixed at an average value of 5.8), temperature (T), at 7°C (max. allowed by the Regulation (EC) 852/200424) and an average aw value described by a probability distribution reflecting the uncertainty of the mean (Table 4).
Irreversible injury (physiological state = Phys: 0–1): it represents the proportion of the surviving Salmonella population after chilled storage that may be irreversibly injured and thus, not culturable. Given that the above non‐thermal inactivation is based on broth data, the injury of cells, manifested as lack of culturability but not loss of viability is often underestimated, and thus the predicted reductions in Salmonella populations are lower than those actually occurring on carcass (Chang et al., 2003). This additional injury could be attributed to the combined outcome of cold and osmotic stress (sudden aw downshifts), experienced by cells due to the surface roughness of the carcass, that cannot be quantitatively accounted for by the predicted model (i.e. uncertainty of the model).
Sponging efficacy (SpEff: 0–1): it determines the proportion of surviving and non‐irreversibly injured cells that can be detached by swabbing. It is scored from 0 to 1 and the value reflects the reduced swabbing efficacy compared to time 0. For instance, a value of 0.6 suggests that the swabbing efficacy after 24 or 72 h is reduced by 40% compared to time 0.
Fitness of Salmonella in the enrichment (Compt: 0 to 1): it refers to the probability of the surviving population, that is not irreversibly injured and is detached by swabbing, to be outcompeted by indigenous meat microbiota. Such population is not capable of growing in the enrichment medium at levels sufficient to be isolated on a selective plate. Thus, the value used as model input for these variables is the complementary percentage, i.e. 1‐Compt.
Table 4.
Probability distribution of the stochastic model input variables for assessing the reduction in sensitivity of Salmonella detection
| Model variable | Element addressed | Probability distribution | Justification or source of input values |
|---|---|---|---|
| Average (μ0) of Log No: Initial population on time 0 (i.e. immediately after slaughtering) | Uncertainty | Form of the distribution elicited by EKE | μ0 elicited by EKE |
| Standard deviation of Log No (σ) (independent of μ0 ) | Uncertainty | BetaPert (min, most likely, max) | Input values discusseda and agreed in the WG |
| Log No | Variability | Normal (μ0, σ) | Input values discussed and agreed in the WG |
| SLm Min: minimum number of Salmonella cells required on carcass for a positive sample |
Uncertainty This is defined for time 0 and is considered not to be affected by subsequent storage |
Form of the distribution elicited by EKE | Input values elicited by EKE |
| Water activity a w of carcass surface after 24 or 72 h of chilled (7°C) storage: aw used as input variable in the non‐thermal inactivation model of Salmonella for estimating the reduction of viable and culturable population on carcass (SR of equation 1) | Uncertainty | BetaPert (min, most likely, max) | Input values discussed and agreed in the WG |
| Phys: Proportion of surviving cells (Log No‐SR) that are irreversibly injured | Uncertainty | Form of the distribution elicited by EKE | Input values elicited by EKE |
| SpEff: Proportion of surviving, non‐irreversibly injured cells [(No‐10^SR)*(1‐phys)], that are detached from carcass by swabbing | Uncertainty | BetaPert (min, most likely, max) | Input values discussed and agreed in the WG |
| Compt: Proportion of SpEff that is outcompeted by indigenous meat microbiota | Uncertainty | BetaPert (min, most likely, max) | Input values discussed and agreed in the WG |
Discussions are based on literature and contribute to a consensus among experts.
The serovar is thought to greatly affect the performance of the enrichment and colony isolation methods and is reflected in the parameter values of the probability distributions of the model, described below.
The model allows a quantitative description of the impact of the aforementioned factors on the sensitivity in Salmonella detection, by assigning numerical values (based on literature and expert knowledge) to the contribution of each factor on the change in levels and recovery of the original Salmonella levels on carcass. The uncertainty associated with each factor is described in the form of probability distributions (Table 4). The model variables account for the average conditions on ungulate carcass surface, without considering the variability in carcass properties. As such, the probability distributions describe the uncertainty about the mean (i.e. the central tendency) of each input variable. Having assigned input values and the associated uncertainty distributions to each of the above factors for 24‐ and 72‐h PMI‐delay intervals, the model is run with Monte Carlo simulation and estimates the uncertainty range of reduction in sensitivity of Salmonella detection, per time point.
Due to uncertainty in the parameters of initial Salmonella distribution (i.e. mean Log No and standard deviation), the initial percentage of positive carcasses in Monte Carlo iterations may be slightly lower than 100%.
The calculation of the mean Salmonella population (Log Nt) after each time interval (24‐ or 72‐h) of chilled storage is provided by the following formula, using the aforementioned model variables:
Based on Log Nt, the updated number of viable and culturable Salmonella retrieved by swabbing and exceeding SLm Min enables the calculation of the number of carcasses that remain positive after 24 or 72 h of chilled storage. This outcome is dependent on the relative change of the distribution of viable and culturable Salmonella (transferred to the swab) compared to the same SLm Min pre‐ and post‐chilling. The difference between the initial and final (after 24 or 72 h) percentage (%) of positive carcasses shows the reduction in sensitivity of Salmonella detection due to delayed PMI.
To assess the Salmonella detection limit and the mean values of Salmonella counts on carcass of ungulates, a literature search was done in PUBMED using the terms ‘Salmonella AND carcass AND detection limit’. A total number of 23 articles were retrieved which were screened by title and abstract. From 19 articles, the whole text was screened. Additionally, a literature search was performed in SCOPUS using the search terms ‘carcass AND Salmonella AND detection limit AND pork’ giving a total number of 661 articles which were screened by title. From 31 articles, the abstract was screened and the whole text was screened from 14 articles. The same procedure was performed for search terms ‘carcass AND Salmonella AND detection limit AND beef’ retrieving a total number of 867 articles which were screened by title. Leading to 38 articles from which the abstract was screened and remaining eight articles for whole text screening.
Another literature search was performed in SCOPUS with search terms ‘Salmonella AND slaughter AND detection limit AND MPN’ that retrieved 11 articles which were screened by title and abstract yielding three articles for full text screening and with terms ‘Salmonella AND slaughter AND detection limit AND quantification AND swabs’ which retrieved nine articles, screened by title and abstract giving one article for full text screen.
2.5. Data and methodologies related to chemical residues and contaminants
2.5.1. Selection of relevant compounds
The CONTAM Panel decided to focus in the current assessment on those chemical hazards that had been identified in the previous assessments on meat inspection (EFSA BIOHAZ, CONTAM and AHAW Panels, 2011; EFSA BIOHAZ Panel, 2013a,b,c,d) as high and medium potential concern for the different animal species relevant for this assessment; namely:
Stilbenes
Thyrostats
Gonadal (sex) steroids
Resorcylic acid lactones
β‐agonists
Pharmacologically active substances for which no maximum levels could be fixed25: chloramphenicol, nitrofurans and nitroimidazoles
Phenylbutazone
Contaminants: cadmium, mercury, lead, dioxins and dioxin‐like polychlorinated biphenyls (DL‐PCBs), non‐dioxin‐like PCBs (NDL‐PCBs), Polybrominated diphenyl ethers (PBDEs), mycotoxins (ochratoxin A (OTA)).
In addition, the CONTAM Panel decided to include perfluoralkyl substances (PFASs) in this assessment. Recent risk assessments have shown the presence of these compounds in food of animal origin at relevant concentrations and identified a concern for public health (EFSA CONTAM Panel, 2018a, 2020a).
For those substances that have been addressed previously by the CONTAM Panel, the working group checked which information is available in the opinions that is relevant for the current assessment. This information concerns inter alia physico‐chemical properties, toxicokinetic data, such as half‐life in the living animal and stability in different matrices. For the substances that have not been addressed in previous CONTAM opinions, a literature search was conducted to identify relevant information to address the terms of reference. For groups 1–4 and the nitroimidazoles, the CONTAM Panel used the Community Reference Laboratories (CRL) Guidance paper from 2007 (CRL, 2007) to identify the single substances to be selected in the literature search for each of those classes. The CONTAM Panel noted that this document has been updated and a new version of the document has become available in September 2020. However, for the identification of the single substances, the document available at that time (CRL, 2007) was used. For the β‐agonists (group 5), the national residue control plan (NRCP) evaluation (EURLs for Pharmacologically Active Substances, 2019) was used. In the report, the compounds in this group are divided into three categories: absolute minimum requirements, recommended and optional. The Panel did not select the substances mentioned as optional. Table 5 gives an overview of all substances included in the literature search. In addition, EFSA contacted the responsible EURLs to request information on the chemical stability of the respective substances.
Table 5.
Overview of the individual compounds per substance group included in the literature search
| Substance group | Substances included |
|---|---|
| Stilbenes | diethylstilbestrol (DES), dienestrol (DE) and hexestrol (HEX) |
| Thyrostats | thiouracil, methylthiouracil, propylthiouracil and tapazol® |
| Steroids | boldenone, 17β‐19‐nortestosterone, ethinylestradiol, 17β‐oestradiol, 17β‐testosterone, methyltestosterone, 17β‐trenbolone, stanozolol, megestrol, melengestrol, chlormadinone, medroxy‐progesterone |
| Resorcylic acid lactones including zeranol | zeranol, zearalanone |
| β‐agonists |
Absolute minimum requirements: clenbuterol, brombuterol, isoxsuprine, ractopamine, salbutamol, zilpaterol Recommended: chlorbrombuterol, cimaterol, cimbuterol, mabuterol, mapenterol, tulobuterol, terbutaline, salmeterol, ritodrine, clenproperol, clenpenterol, clencyclohexerol |
| Nitroimidazoles | dimetridazole, metronidazole, ronidazole and the hydroxy‐metabolites (2‐hydroxymethyl‐1‐methyl‐5‐nitroimidazole (HMMNI) and 1‐(2‐hydroxyethyl)‐2‐hydroxymethyl‐5‐nitroimidazole (MNZOH)) |
2.5.2. Collection and selection of evidence
After the identification of the relevant substances, a comprehensive literature search was performed in November 2019 in Web of Science26 and PubMed.27 The search strings are presented in Table 6.
Table 6.
Search strings used for the literature search performed by EFSA
| Substance groups | Search string |
|---|---|
| Nitroimidazoles except metronidazole |
(1) (substance) AND (2) (half‐life) OR (persisten*) OR (toxicokinetic*) OR (stability) OR (depletion) OR (storage) AND (3) (matrix) OR (sample) OR (analytical) OR (urine) OR (hair) OR (tissue) OR (plasma) OR (serum) OR (kidney) OR (liver) OR (muscle) |
| Metronidazole |
(1) AND (2) AND (3) AND (4) (pig) OR (piglet*) OR (swine) OR (farmed game) OR (boar) OR (deer) OR (rabbit*) OR (ostrich) OR (reindeer) |
| Stilbenes, gonadal (sex) steroids, resorcylic acid lactones |
(1) AND (2) AND (3) AND (5) (bovine) OR (calf) OR (cow) OR (sheep) OR (goat) OR (cattle) OR (heifer) OR (steer) |
| Thyrostats |
(1) AND (2) AND (3) AND (4) (thyroid) is added to (2) |
| β‐agonists |
(1) AND (2) AND (3) AND (4) (eye) is added to (2) |
In all cases, no language restriction was applied and the β was excluded from the name of the compounds in the search strings. After merging the obtained papers of the two libraries per class, a check for duplicates was performed.
The number of papers obtained in the two databases and the total number obtained per class are mentioned in Table 7.
Table 7.
Number of papers obtained for the identified substances and their representative keywords in Pubmed and Web of Science
| Substance groups | Pubmed | Web of Science | Merged and after duplicate check |
|---|---|---|---|
| Stilbenes | 27 | 102 | 101 |
| Thyrostats | 19 | 62 | 61 |
| Steroids | 419 | 560 | 764 |
| Resorcylic acid lactones including zeranol | 9 | 10 | 13 |
| β‐agonists | 77 | 248 | 192 |
| Nitroimidazoles | 122 | 112 | 178 |
The references obtained from the literature search were imported and saved using a software package (EndNote28). The references obtained were screened based on title and abstract using Distiller SR to identify the relevant literature.
Additionally, relevant scientific evaluations by national or international bodies and reviews were considered for the current risk assessment.
2.5.3. Appraisal of evidence
The information retrieved was screened and evaluated by relevant domain experts from the working group on delayed meat inspection and used for the present assessment. Limitations in the information used are documented in this Scientific Opinion.
Selection of the scientific papers for inclusion or exclusion was based on consideration of the extent to which the study was relevant to the assessment or on general study quality considerations (e.g. sufficient details on the methodology, performance and outcome of the study), irrespective of the results.
3. Assessment
3.1. Assessment of detection at delayed PMI of animal diseases listed according to article 5 of Regulation (EU) 2016/429
3.1.1. Animal diseases listed according to article 5 of Regulation (EU) 2016/429
The animal diseases listed according to Article 5 of Regulation (EU) 2016/429 including Regulation (EU) 2018/162929 that affect ungulates, which will be object of the present assessment are:
Foot and mouth disease (FMD)
Classical swine fever (CSF)
African swine fever (ASF)
African horse sickness (AHS)
Infection with rinderpest virus
Infection with Rift Valley fever virus (RVFV)
Infection with Brucella abortus,B. melitensis and B. suis
Infection with Mycobacterium tuberculosis complex (M. bovis,M. caprae and M. tuberculosis)
Infection with rabies virus
Infection with bluetongue virus (serotypes 1–24) (BTV)
Infestation with Echinococcus multilocularis
Infection with epizootic haemorrhagic disease virus (cattle, deer) (EHDV)
Anthrax
Surra (Trypanosoma evansi)
Paratuberculosis
Japanese encephalitis
West Nile fever (WNF)
Q fever
Infection with lumpy skin disease virus (LSDV)
Infection with Mycoplasma mycoides subsp. mycoides SC (Contagious bovine pleuropneumonia – CBPP) –
Infectious bovine rhinotracheitis (IBR)/infectious pustular vulvovaginitis (IPV)
Bovine viral diarrhoea (BVD)
Bovine genital campylobacteriosis
Trichomonosis
Enzootic bovine leukosis (EBL) – subclinical
Sheep pox and goat pox
Infection with peste des petits ruminants virus (PPRV)
Contagious caprine pleuropneumonia (CCPP)
Ovine epididymitis (Brucella ovis)
Infection with Burkholderia mallei (Glanders) –
Infection with equine arteritis virus (EAV)
Equine infectious anaemia (EIA)
Dourine
Venezuelan equine encephalomyelitis (VEE)
Contagious equine metritis (CEM)
Equine encephalomyelitis (Eastern and Western – EEE, WEE)
Infection with Aujeszky's disease virus (ADV) –
Infection with porcine reproductive and respiratory syndrome virus (PPRSV)
3.1.2. Disease map
The table of the target diseases (disease map) as described in Section 2.1.2.1 is reported on the Knowledge Junction community of EFSA on Zenodo at https://doi.org/10.5281/zenodo.4247447. The sources of literature used for compiling the disease map are the EFSA opinions on listed and categorised diseases under AHL,30 the related OIE manual chapters (OIE, 2019) and disease cards (OIE, 2020), the publication by Coetzer and Tustin (2005) and Animal Disease Information provided by the Center for Food Security and Public Health.31
The main outcome of the disease map is to have a summary table where the main lesions of each disease can be extracted and used for the compilation of the questionnaire.
3.1.3. Selection of lesions subjected to changes at delayed PMI
Acute and chronic inflammation
Acute inflammation is the initial response to an injurious agent and lasts from a few hours to a few days (Ackermann, 2017). Usually, one or more of the following gross signs are present: redness, heat, swelling, pain and loss of function (Cheville, 2006; Ackermann, 2017). Acutely inflamed tissue appears red due to vasodilation of arterioles and capillaries which results in increased blood flow (hyperaemia) to the site of injury (Cheville, 2006; Ackermann, 2017). Swelling is caused by increased vascular permeability, which leads to leakage of fluid and plasma proteins into the extravascular space (Cheville, 2006; Ackermann, 2017). Another typical sign of acute inflammation is the presence of fibrin. Fibrin is formed when fibrinogen leaks into the perivascular tissue and polymerises into fibrin (Ackermann, 2017). Macroscopically, fibrin is seen as a pale, thick, stringy, elastic white‐grey to yellow meshwork on tissue surfaces (Cheville, 2006; Ackermann, 2017). These surfaces include the serous membranes of the body cavities such as the pericardium and pleura, where deposits of fibrin give the surface a dry appearance (Schoen, 2005; Ackermann, 2017; Jensen et al., 2017). In solid organs, the presence of fibrin will be seen as swelling and dryness (Jensen et al., 2017).
A chronic inflammatory response is of longer duration, usually weeks, months or years. It is characterised by cellular infiltration and reparatory changes, including fibrosis and/or the formation of granulation tissue (Ackermann, 2017). Grossly, the presence of fibrous connective tissue gives the tissue a firm texture and a grey to white colour, whereas areas with granulation tissue often appear red and haemorrhagic (Ackermann, 2017).
Post‐mortem, the main observation of acute inflammation differentiating it from chronic lesions is the swelling, redness and exudation of fibrin.
There are observations that cooling will affect both the colour (decline of redness) and dryness (increased dryness during cooling) of meat, i.e. cooling will cause an increased dryness due to evaporation and due to a lower solubility of oxygen, and a decline in redness will be the result of cooling. Moreover, both reactions are dependent on the method used for the cooling process and the actual temperature (2, 4 or 6°C). It is assumed that the same changes occur at storage temperatures of 3 and 7°C.
In conclusion, chilling for longer periods (24 and 72 h) affects the colour (decline of redness in case of hyperaemia/haemorrhage due to lower solubility of oxygen), dryness (increased dryness during cooling) and swelling, due to evaporation and reduced moisture content. Therefore, at delayed meat inspection the impact of enhanced dryness with respect to disclosure of fibrin and necrosis in acute inflammatory lesions and the reduced redness and swelling in these processes are likely to hamper detection of acute lesions after cooling.
Other lesions investigated in the questionnaire for the meat inspectors were fibrin, necrosis, hyperaemic/haemorrhages, erosions and oedema (or enlargement for lymph nodes, liver and spleen, and thickening for intestine mucosa).
3.1.4. Lesion map
From the disease map, the list of organs to be considered at PMI and the selected lesions as in section above, a lesion map was built by connecting animal species with organs with lesions and corresponding disease. This facilitated the construction of the questionnaire where, for each organ and the five types of lesions, the meat inspectors had to provide a numerical answer about how many carcasses out of 100 with the given lesion, will be still detected after 24 or 72 h of refrigerated storage.
The lesion map also serves to connect the results from the questionnaire to each disease and create the main source of evidence to perform the EKE (see Section 3.1.7)
The lesion map is visible on the Knowledge Junction community of EFSA on Zenodo at https://doi.org/10.5281/zenodo.4247447.
3.1.5. Questionnaire from meat inspectors
A total of 18 meat inspectors/experts in meat inspection from six Member States and Serbia were contacted and replied to the survey, although for certain combinations of organs and lesions, the number of replies was lower. The range of values provided for the number of organs with a given lesion still detectable after 24 or 72 h out of 100 typically encompassed the whole 0–100 possible range. Most inspectors predicted a moderate effect of the delay with high certainty: median values for the number of carcasses with a given lesion in a given organ that would be still detectable after 24 and 72 h were always equal to or above 70 and 50, respectively. Overall, the probability of detecting necrosis and haemorrhages was less affected by the delay than erosions, fibrin and oedema and, as expected, a greater impact was observed for a delay of 72 h compared to 24 h. The results are provided in form of boxplot, an example for lesions in head and mouth is reported in Figure 4, the results for all organs are reported in Appendix A.
Figure 4.
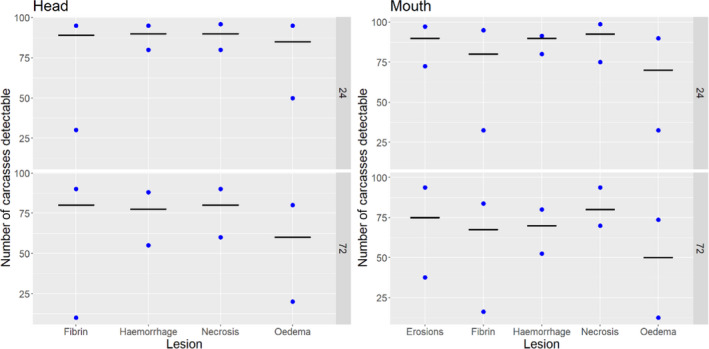
First and third quartiles (dots) and median (line) of the distributions of the responses provided by 18 experts in meat inspection about the number of organs out of 100 with a given lesion detected at PMI conducted immediately after slaughter and still detectable after 24 or 72 h (top and bottom of each graph)
Certainties of the responses were also very variable and ranged between 1 and 10, although mean values were always above 7 for all types of lesions considered.
On the question related to chronic lesions, the majority (15–18 out of total 18 respondents) of the meat inspectors agreed with the assumption that chronic lesions would remain detectable if the PMI was carried out with a delay of 24 or 72 h after slaughter with a mean certainty above 7.5 on a scale from 1 to 10, with the exception of the thickening of intestine mucosa (Table 8).
Table 8.
Number of inspectors that were in agreement/disagreement and certainty (on a scale of 1–10) with the following statementw ‘It is generally assumed that during routine PMI (considering minimum requirements by legislation) of ungulates (bovines, small ruminants, equines, suids), chronic lesions remain detectable, if the inspection is done with a delay of 24‐ or 72‐h after slaughtering an animal and the carcass and organs are kept refrigerated during this time, compared to when the meat inspection is done just after slaughtering’
| Lesion | Responses in agreement | Responses in disagreement | ||
|---|---|---|---|---|
| Number | Mean certainty | Number | Mean certainty | |
| Abscesses | 18 | 8.6 | 0 | – |
| Adenomegaly | 18 | 8.3 | 0 | – |
| Arthritis | 18 | 8.1 | 0 | – |
| Bursitis | 17 | 7.9 | 1 | 7.0 |
| Nodular lesions | 16 | 8.5 | 2 | 8.5 |
| Orchitis | 16 | 7.6 | 1 | 9.0 |
| Splenomegaly | 16 | 8.4 | 1 | 8.0 |
| Thickening of the intestinal mucosa | 13 | 6.9 | 4 | 6.5 |
| Tuberculous lesions | 15 | 8.5 | 3 | 6.3 |
In order to use this information as evidence in the EKE exercises, the responses for organs and lesions relevant to each disease were combined (see Appendix C for the evidence presented for each disease).
3.1.6. Results from questionnaire to NRLs for bovine tuberculosis
The NRLs from 19 countries (five non‐OTF32, three with OTF regions and 11 OTF countries) replied to the survey (plus the EURL for bovine tuberculosis). Of these, six countries did not answer any question due to a perceived lack of experience on the subject. The remaining 13 countries plus the EURL answered one or more questions. Between 11 and 14 answers were available for each of the tests and time delays evaluated. The NRLs that declared themselves competent to answer the survey were sent the summarised results and given the opportunity to update their answers. All NRLs agreed with their answers. The results of the survey are summarised in Table 9 and Figure 5.
Table 9.
Number of NRLs that considered each possible likelihood of decrease in sensitivity for each MTBC diagnostic test (grouped results)
| Test | Delay | Effect (Likelihood of observing a decrease in the sensitivity) | Responses | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0–1% | 1–5% | 5–10% | 10–33% | 33–66% | 66–90% | 90–95% | |||
| Culture | 24 h | 6 | 3 | 3 | 1 | 0 | 0 | 0 | 13 |
| 72 h | 1 | 4 | 0 | 3 | 2 | 1 | 0 | 11 | |
| PCR | 24 h | 7 | 3 | 3 | 0 | 0 | 0 | 0 | 13 |
| 72 h | 5 | 2 | 3 | 1 | 0 | 0 | 0 | 11 | |
| HP * | 24 h | 7 | 5 | 1 | 1 | 0 | 0 | 0 | 14 |
| 72 h | 1 | 2 | 3 | 2 | 0 | 2 | 2 | 12 | |
Histopathology.
Figure 5.
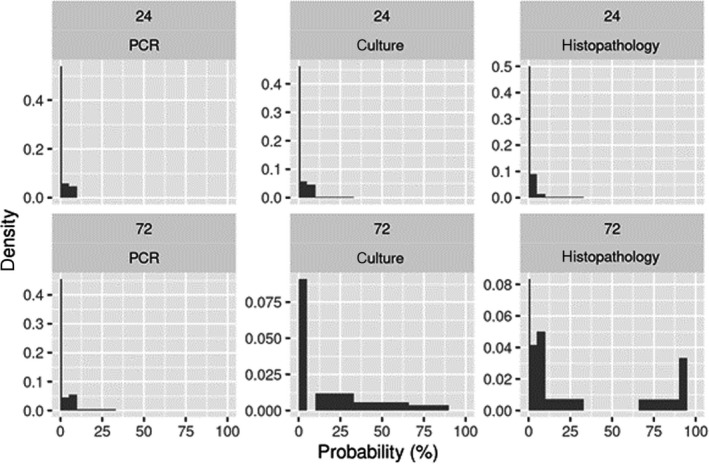
Distribution of NRL and EURL responses on the probability of observing a decrease in the sensitivity of each diagnostic test after a delay of 24 h (first row) or 72 h (second row)
First column: PCR, second column: culture; third column: histopathology.
For a 24‐h delay, between 9 and 12 out of the 13–14 respondents considered that observing a reduction in the sensitivity of post‐mortem laboratory methods for the detection of the MTBC was between almost impossible (0–1% likelihood) to extremely unlikely (1–5% likelihood) for all three tests considered. Between two and four laboratories considered that the likelihood of decrease was higher (very unlikely – 5–10% – or unlikely – 10–33%, Table 9, Figure 5). Still, overall, the likelihood of a decrease was considered from almost impossible to very low irrespective of the test considered.
When the 72‐h delay scenario is considered, responses were more variable. In the case of the culture method, five out of 11 laboratories considered that the likelihood of a decrease was still either almost impossible or extremely unlikely and the remaining six laboratories considered that this likelihood was unlikely (10–33%, n = 3), about as likely as not (33–66%, n = 2) or likely (66–90%, n = 1). In the case of the PCR method, the likelihood of observing a decrease in the sensitivity after 72 h was very similar to what was considered for the 24‐h scenario, with 11/12 laboratories reporting a likelihood below 10% and one of 10–33% (unlikely). In the case of histopathology, responses were more variable, with between 1–3 laboratories considering likelihoods ranging from almost impossible to extremely likely (Figure 5, Table 9).
3.1.7. Sensitivity of delayed PMI for detection of diseases listed under article 5 of AHL
The effect of the delay in the PMI on the probability of detecting the target diseases was elicited in three different Expert Knowledge Elicitation (EKE) exercises as ‘the number of carcasses from infected animals assessed as diseased in a PMI carried out immediately after slaughter (current procedure) per 100 that would still be detectable after 24‐ or 72‐h’. For those diseases leading to the occurrence of similar non‐pathognomonic lesions in the same organs (i.e. reproductive diseases: brucellosis, CEM, dourine, Japanese encephalitis, Q fever, IBR/IPV and trichomonosis; respiratory diseases: contagious bovine pleuropneumonia and contagious caprine pleuropneumonia), a single distribution representing the uncertainty about the parameter of interest for all of them was elicited.
Even though individual judgements on the median and quartile values of the quantity of interest elicited prior to the EKE exercises varied between experts, a consensus distribution was always reached after discussion (details on the EKE exercises are provided in Appendix B). Evidence and reasoning leading to these consensus distributions is summarised in the annex. The 5th, 25th, 50th, 75th and 95th percentiles of the consensus distribution for the target diseases are shown in Figure 6.
Figure 6.
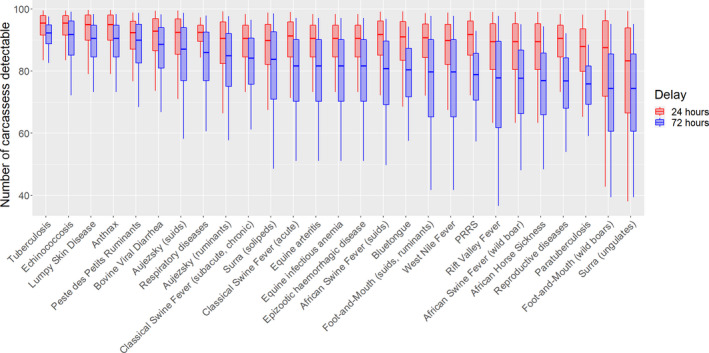
Boxplots of 5th, 25th, 50th, 75th and 95th percentiles of the consensus distribution about mean number of carcasses with a given target disease still detectable at 24‐h or 72‐h delayed PMI 33
3.1.8. Sensitivity of the laboratory tests for confirmation of tuberculosis infection
The effect of the delay of the PMI on the performance of tuberculosis diagnostic test was elicited in a separate EKE exercise that was based on the information extracted from the survey submitted to the NRLs of the EU and the EURL for Bovine Tuberculosis. The parameter of interest in this case was the mean number of carcasses out of 100 with visible granulomatous lesions detected in a PMI conducted immediately after slaughter that would be positive in a laboratory test if submitted immediately to the laboratory and that would still be positive in that same laboratory test if the sample was stored for 24 or 72 h under refrigeration. The elicited distributions for the parameters of interest revealed a limited effect on the ability of the tests to confirm infection in animals with lesions if the delay was restricted to 24‐h: the experts were 95% certain that at least 87 out of every 100 carcasses confirmed under the current procedure would be still confirmed regardless of the technique (Figure 7). However, the effect of a 72‐h delay was estimated to be higher particularly in the case of culture and histopathology, so that this number could be as low as 73 (histopathology) or 79 (culture) out of every 100 carcasses confirmed following the current procedure.
Figure 7.
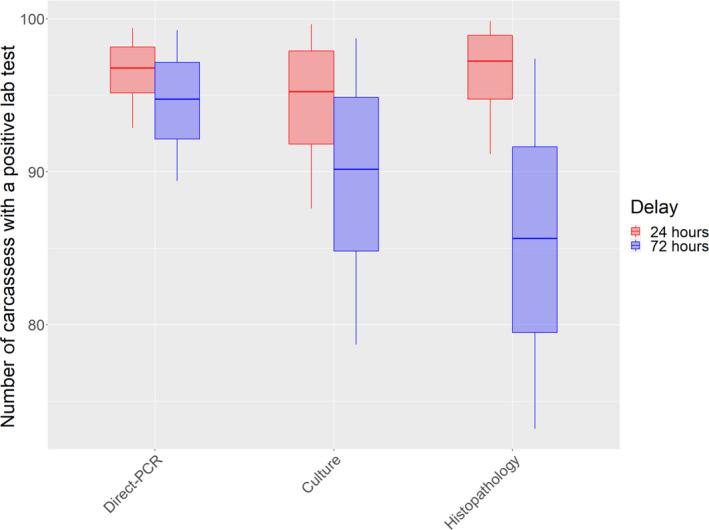
Boxplots of 5th, 25th, 50th, 75th and 95th percentiles of the consensus distribution about mean number of carcasses out of 100 with visible granulomatous lesions detected in a PMI conducted immediately after slaughter that would be positive in a laboratory test (direct PCR, culture, histopathology) if submitted immediately to the laboratory that would be still positive in that same laboratory test if the sample was stored for 24 or 72 h under refrigeration
3.1.9. Effect of the delay on the overall ability to detect and confirm tuberculosis infection
The final step was to estimate the overall effect of a 24‐ or 72‐h delay on the efficacy of the PMI and laboratory tests to confirm bovine tuberculosis infection in comparison with the current procedure. This was done by combining the effect of the delay on the probability of detecting a tuberculosis‐like lesion compared with the current procedure (shown in Figure 6) and on the ability of laboratory tests to confirm the infection in detected carcasses (shown in Figure 7). This combination was repeated for three possible scenarios (complete independence between the two effects – scenario 1, complete correlation between the two effects – scenario 2 and no effect of the delay on the probability of detecting the lesion as the upper bound of the quantity of interest – scenario 3) as explained before in section 2.1.2.6.
The results for the three scenarios for each test and time delay are shown in Figure 8. Scenarios 1 and 2 represent alternative estimates of a lower bound (worst case, i.e. large impact of delayed PMI after 24 and 72 h) for the combined effect of delayed inspection on lesions and laboratory tests. The distributions are wider for scenario 2 than for scenario 1, reflecting the increased uncertainty expected for the combined effect if respective decreases in detection of lesions and laboratory tests are positively correlated. However, the difference between these scenarios is minor when compared to the difference between them and the distribution for scenario 3, which represents an upper bound for the combined effect of delayed inspection on lesions and laboratory tests. In other words, the difference between the lower and upper bound estimates is a larger source of uncertainty than uncertainty about the degree of dependence affecting the lower bound.
Figure 8.
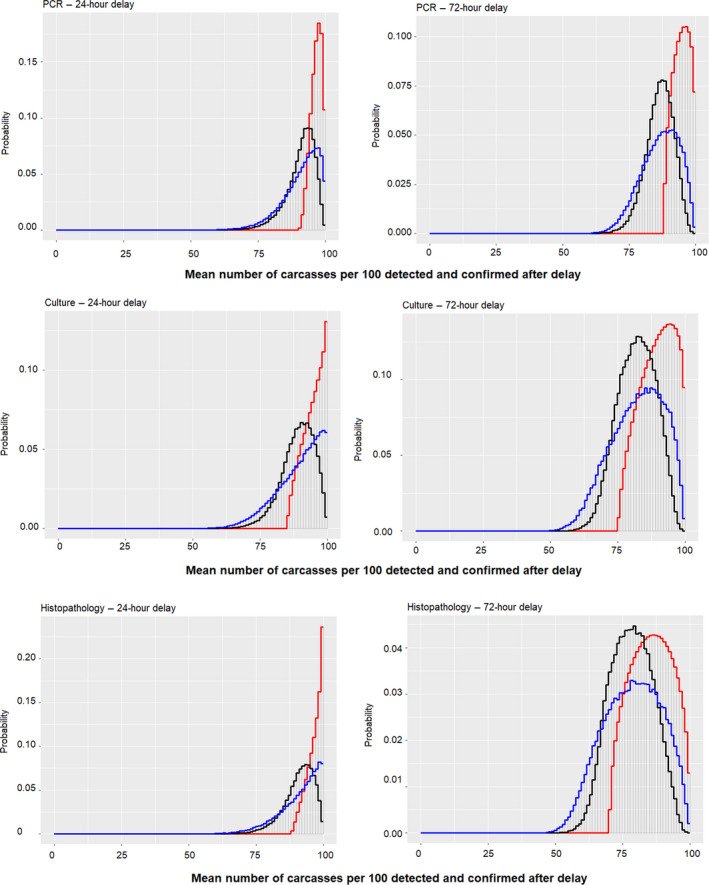
Probability density functions of the estimates of the overall effect of a 24‐ or 72‐h delay on the efficacy of the PMI and laboratory tests to confirm bovine tuberculosis infection in comparison with the current procedure
- Black, blue and red lines represent scenario 1 (complete independence), scenario 2 (complete correlation) and scenario 3 (upper bound). The curve shows the overall judgement about the range and likelihood of values for the mean number of carcasses per 100 still detected and confirmed after 24 and 72 h using direct‐PCR, culture or histopathology as the laboratory confirmatory tests.
The proportion of affected carcasses that would be still detectable considering both effects is necessarily lower than when each effect is considered separately (Figures 8). The median of the distributions of the average number of carcasses out of 100 detected and confirmed under the current procedure that would be still detected and confirmed after a 24‐h delay varied depending on the scenario and the laboratory test considered (Table 10): for direct‐PCR for the median estimates ranged between 91.8 and 92.8 assuming complete independence and complete correlation (scenarios 1 and 2 representing lower bound for the quantity of interest), respectively (90% probability interval: 80–97 and 77–99); in the case of culture, median values ranged between 89.7 and 90.9 (90% PI: 78–97 and 73–99), while for histopathology median estimates ranged between 91.7 and 92.7 (90% PI: 80–99 and 76–99).
Table 10.
Median, lower and upper bound (5th and 95th percentile) of the distributions of the average number of carcasses out of 100 detected and after a 24‐h and 72‐h delay varied depending on the scenario and the laboratory test
| Laboratory test | Delay | Scenario | Median | Lower bound (5th percentile)(a) | Higher bound (95th percentile)(b) |
|---|---|---|---|---|---|
| Direct‐PCR | 24 h | 1 | 91.8 | 80 | 97 |
| Direct‐PCR | 24 h | 2 | 92.8 | 77 | 99 |
| Culture | 24 h | 1 | 89.7 | 78 | 97 |
| Culture | 24 h | 2 | 90.9 | 73 | 99 |
| Histopathology | 24 h | 1 | 91.7 | 80 | 99 |
| Histopathology | 24 h | 2 | 92.7 | 76 | 99 |
| Direct‐PCR | 72 h | 1 | 86.9 | 77 | 94 |
| Direct‐PCR | 72 h | 2 | 87.3 | 74 | 97 |
| Culture | 72 h | 1 | 82.2 | 70 | 93 |
| Culture | 72 h | 2 | 83.1 | 65 | 96 |
| Histopathology | 72 h | 1 | 78.1 | 65 | 91 |
| Histopathology | 72 h | 2 | 78.9 | 61 | 95 |
Lowest 5th percentile in the lower bound scenarios (scenarios 1 and 2).
95th percentile in the upper bound scenario (scenario 3).
When the 72‐h delay is considered, this overall effect is more pronounced: in this case the median estimates decreased to 86.9 and 87.3 for direct‐PCR (90% PI: 77–94 and 74–97), 82.2 and 83.1 for culture (90% PI: 70–93 and 65–96) and 78.1 and 78.9 for histopathology (90% CI: 65–91 and 61–95).
In order to assess the overall uncertainty of the combined effect, it is necessary to combine the estimates obtained above along with any additional uncertainties affecting them. Based on the values obtained for the lower and upper bound scenarios, a ≥ 90% probability interval for the effect that the 24‐ or 72‐h delay could have on the ability to detect and confirm tuberculosis infection in diseased animals relative to the current procedure is presented in Table 11. Further details are presented in Appendix C.
Table 11.
The ≥ 90% probability interval for the number of MTBC‐infected animals that would be detected and confirmed every 100 relative to the current procedure considering a 24‐ or 72‐h delay in the PMI and three possible diagnostic tests.(c)
| Delay | Laboratory test | Lowest 5th percentilea | Highest 95th percentileb |
|---|---|---|---|
| 24 h | Direct‐PCR | 77 | 99 |
| 24 h | Culture | 73 | 100 |
| 24 h | Histopathology | 76 | 100 |
| 72 h | Direct‐PCR | 74 | 99 |
| 72 h | Culture | 65 | 99 |
| 72 h | Histopathology | 61 | 97 |
Lowest 5th percentile in the lower bound scenarios (scenarios 1 and 2).
95th percentile in the upper bound scenario (scenario 3).
There is at least 90% probability that the true value lies in the range between the lowest 5th percentile and highest 95th percentile).
Therefore, the decrease in the ability of the PMI and laboratory tests to confirm an infection relative to the current procedures could be affected, particularly in the case of a 72‐h delay, and when confirmation of the infection is performed using culture or histopathology as indicated by the lower bounds when this delay/laboratory tests are considered.
3.1.10. Uncertainty analysis
The uncertainty about the effect that a delayed PMI can have on the timely detection of animal diseases listed according to Article 5 of Regulation (EU) 2016/429 in ungulates and for the generalised conditions such as toxaemia, viraemia, septicaemia and pyaemia is related to:
the wide range of type of lesions and the degree of extension of those lesions that can occur in infected animals, as well as to
the lack of scientific literature on the specific effect that 24 or 72 h under refrigeration may have on the probability of detecting such lesions during the routine abattoir inspection.
As mentioned previously, the details of the lesions associated with each of the diseases evaluated in this opinion were retrieved from the scientific literature (and expert knowledge) under the assumption that the animals of interest for this assessment would be those with no or only mild clinical signs, since otherwise they would have been detected during AMI (and thus should not be subjected to a delayed PMI). There is, however, very little data on the severity of the lesions in subclinical/asymptomatic cases, thus resulting in an additional source of uncertainty.
Furthermore, the probability of detecting such lesions during the routine inspection at abattoir after 24 or 72 h under refrigeration is also very difficult to determine since delayed inspection is not currently practiced routinely. Evidence on the possible effect of this delay was generated through a questionnaire filled in by the members of the working group, with experience on PMI, as well as by other professionals involved in meat inspection from several Member States. The variability in the responses obtained was very different depending on the specific lesion (e.g. necrosis, haemorrhage, oedema, erosion or fibrin for acute lesions) and the organ, suggesting a different level of uncertainty of what the true effect could be.
These two sources of uncertainty were considered simultaneously during the EKE exercises conducted within the working group, and led to wider/narrower distributions for the quantity of interest depending on the disease (related to the variability on the expected lesions when such disease was present, to the effect of the delay on such lesions).
In the case of bovine tuberculosis, the specific effect of the delay on the ability of laboratory tests to confirm the disease was also uncertain due to the very limited data (mostly limited to culture). The responses from the NRLs and the EURL for bovine tuberculosis in the survey, asking about the possible effect that such delays would have also demonstrated greater uncertainty for certain techniques (notably, histopathology after 72 h) than for others (direct‐PCR). This was also considered in the distribution elicited during the EKE session.
Finally, the combination of the effect of the delay on the post‐mortem detection of macroscopic lesions compatible with tuberculosis and on the performance of the laboratory tests led to further uncertainty due to the need to consider different scenarios representing lower and upper bounds for the overall effect. This has been addressed by generating a probability interval that considers both limits and that therefore contains the true value of the quantity of interest with a high probability.
3.2. Assessment of detection at delayed PMI of cysticercosis
Beef and pork tapeworms – Taenia saginata and T. solium, respectively – are two of the three species causing taeniasis in humans (T. asiatica is the third one but has not been reported in Europe). Both have an obligate two‐host life cycle. The adult tapeworms live in the small intestine of humans who are the only final hosts while bovines and porcines, respectively, act as the intermediate hosts. Proglottids of the mature tapeworm, which are filled with eggs, detach and are shed with faeces of a human carrier. Bovines and porcines, respectively, acquire the infection by ingesting the eggs, through feed or water contaminated with human faeces. Upon ingestion by a susceptible animal host, the oncospheres migrate from the intestines primarily to the muscle tissues (but may also travel to different organs) where they develop into the infective cysticerci (metacestode). Humans become infected by eating raw or undercooked meat containing viable cysticerci. The cysticerci then attach to the intestinal wall where the parasites develop into the adult tapeworms. A particular characteristic of the pork tapeworm is that humans can also be infected by accidentally ingesting tapeworm eggs from human faeces, and then develop cysticercosis.
Metacestodes when young are seen as very small cysts (1 mm) with no scolex visible and surrounded by a delicate connective‐tissue capsule. When mature, they are transparent fluid‐filled oval cysts, about 5–10 × 5 mm in size, with the scolex seen inside as a white dot. At a later stage, the capsule of the cyst becomes thickened, opaque and greyish‐white in colour. Cysticerci remain viable for several months and sometimes even several years, after which they start to die, degenerate and eventually calcify. Degenerating cysts vary in appearance – degeneration from caseation to complete calcification can be seen (Gracey et al., 1999; WHO/FAO/OIE, 2005; OIE, 2018).
As infected animals are usually asymptomatic, diagnosis of cysticercosis primarily relies on PMI for cystic lesions (cysticerci) in predilection sites for T. saginata/solium cysticercus.
T. saginata cysticerci are seen usually in masseters and heart of bovines, but can be present in tongue, diaphragm, oesophagus and other muscles and, occasionally, in liver, lungs, kidney, fat etc. Incisions of masseter and heart muscle are the most useful inspection procedures for detection of bovine cysticercosis (WHO/FAO/OIE, 2005; Scandrett et al., 2009); visual inspection of all cut surfaces of muscles on dressed carcass as well as of other organs rarely detects the parasite.
Viable cysticerci, which are usually present in small numbers, are difficult to detect in bovines. Cysticerci are more easily detected once they are not viable but, at that time, it is harder to differentiate them from other lesions such as abscesses or granulomas caused by injections, or from Sarcocystis infection (Ogunremi et al., 2004). The vast majority of bovine cysticerci detected during PMI are degenerated or calcified (Dupuy et al., 2014; Jansen et al., 2017).
Whether or not cysticerci will be detected is dependent on the skills and diligence of the meat inspector. The sensitivity of routine meat inspection in detection of bovine cysticercosis is generally considered low, i.e. 10–30% (Dorny et al., 2000; WHO/FAO/OIE, 2005; Eichenberger et al., 2011, 2013). Furthermore, it has recently been estimated that the sensitivity may be as low as 0.76% (Jansen et al., 2017). Low sensitivity is associated with the fact that the vast majority of bovine cysticercosis cases are mild infections (Dorny and Praet, 2007).
T. solium cysticerci are seen primarily in heart, tongue, masseters and diaphragm, as well as in other muscles (like intercostal and abdominal) of Suidae; occasionally they are present in brain and liver. Cysts once degenerated can have a gaseous or calcified manifestation and are more easily detected. The sensitivity of meat inspection for T. solium cysticerci detection is also related to the degree of infection; for mild infections, it is less than 25% (Dorny et al., 2004; WHO/FAO/OIE, 2005).
According to the Regulation (EU) 2019/627, ‘the post‐mortem inspection procedures described in Articles 18, 19 and 23 shall be the minimum requirements for the examination for cysticercosis in bovine animals and Suidae (domestic swine, farmed game and wild game). In the case of bovine animals referred to in Article 19, the competent authorities may decide that incision of the masseters at PMI is not compulsory if:
-
a)
a specific serological test is used;
-
b)
the animals have been raised on a holding of provenance officially certified to be free of cysticercosis; or,
-
c)
a prevalence of the source population or in a well‐defined sub‐population below one in a million has been demonstrated with 95% certainty or no cases have been detected in all slaughtered animals in the past five years (or two years where supported and justified by the competent authorities’ risk analysis) based on data from reporting carried out in accordance with Article 9(1) of Directive 2003/99/EC’.34
Change in detection after 24 or 72 h
Studies that directly investigated detectability of cysticerci in beef/pork after a 24‐ or 72‐h delay are lacking. However, several studies investigated freezing time/temperature combinations needed to inactivate cysticerci or their survival at refrigeration temperatures. Namely, Hilwig et al. (1978) assessed viability of T. saginata cysticerci in beef kept at different temperatures ranging from −5°C to −30°C for periods ranging from 72 h to 360 h; Fan et al. (1998) assessed survival of T. solium cysticerci in pig carcasses kept at +4°C for different periods, ranging from 1 to 5 days and from 31 to 36 days, while Sotelo (1986) assessed viability of T. solium cysticerci in large pieces of pork kept at different temperatures ranging from –24°C to +11°C for periods of 1–15 days. Based on these studies, it was concluded that every assessment of viability/non‐viability of cysticerci was related to their detection in meat first, which indirectly proved that cysticerci are detectable even after longer time periods than 24/72 h.
The mean number of carcasses out of 100 with cysticercosis detected when PMI was carried out immediately after slaughter that would be still detectable after 24 or 72 h according to the EKE exercise was very high, with a very high confidence (95% certainty) that this number would be above 82 and 72 for 24 and 72 h, respectively (Figure 9).
Figure 9.
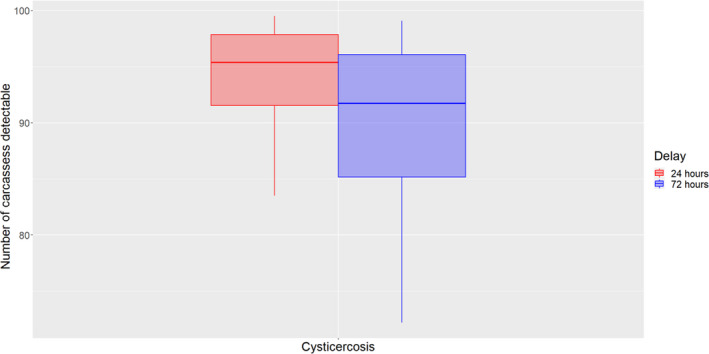
Boxplots of 5th, 25th, 50th, 75th and 95th percentiles of the consensus distribution about mean number of carcasses with cysticercosis still detectable at 24‐ or 72‐h delayed PMI
In conclusion, a delay of 24 or 72 h in PMI will not significantly affect the detection of T. saginata or T. solium cysticerci. This is primarily because the expected decrease in sensitivity was mainly associated with carcasses that contain mostly, or only, viable cysts (a minority compared to carcasses containing mostly, or only, degenerated cysts) which are already difficult to detect even if PMI is performed without delay.
Uncertainties about the effect of a delayed PMI on the detection of cysticercosis are described together with the other animal diseases in Section 3.1.10.
3.3. Assessment of detection at delayed PMI of generalised conditions
At PMI, the suspicion of acute pyaemia, viraemia, toxaemia and septicaemia is based on the presence of hyperaemia, haemorrhages (seen as red‐coloration) and exudation of fibrin and/or necrosis (seen as dry areas) on various serosal surfaces and within different organs. By storage of organs and carcasses at low temperatures (cooling), it is well documented, that both the coloration (decline of redness due to a lower solubility of oxygen) and dryness (increased dryness due to evaporation) will be changed. Therefore, it is expected that cold storage of organs and carcasses with acute lesions due to pyaemia, viraemia, toxaemia and septicaemia will hamper the possibility for detection of these lesions as they will not be as conspicuous compared to inspection of these lesions immediately after slaughter. Moreover, it is expected that post‐mortem delay for 72 h will hamper the disclosure of acute lesions due to pyaemia, viraemia, toxaemia and septicaemia to a greater degree compared to inspection after 24 h. Finally, it must be noted that acute lesions of pyaemia, viraemia, toxaemia and septicaemia all require total condemnation.
The results of the EKE for the lesions of pyaemia, toxaemia and viraemia (dealt with together because they express the same types of lesions, i.e. hyperaemia, haemorrhage, fibrin exudation and necrosis. Toxaemia and viraemia, compared to pyaemia, will not result in the formation of purulent material) are reported in Figure 10. By definition, septicaemia and sepsis are terms used to describe the clinical manifestations (i.e. ante‐mortem manifestations) of the presence of bacteria, viruses and/or toxins within the systemic circulation. Therefore, these terms are post‐mortem encompassed within the terms pyaemia, viraemia and toxaemia.
Figure 10.
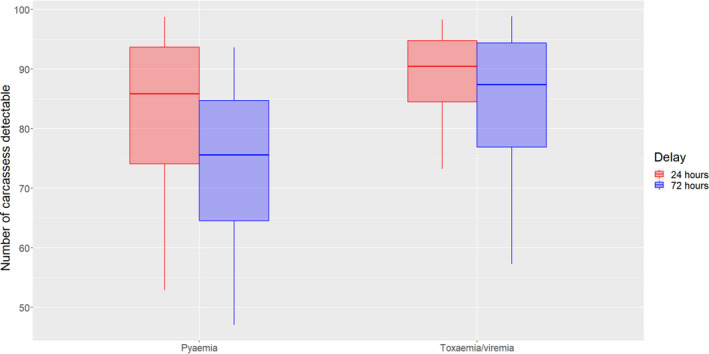
Boxplots of 5th, 25th, 50th, 75th and 95th percentiles of the consensus distribution about mean number of carcasses with pyaemia or toxaemia/viraemia still detectable at 24‐h or 72‐h delayed PMI
In conclusion, the ability to detect the presence of acute pyaemia or toxaemia/viraemia (and septicaemia) will decrease if PMI is delayed by 24 or 72 h. This decline is most prominent for both pyaemia and toxaemia/viraemia after 72 h. The uncertainty associated with this assessment is explained in Section 3.1.10.
3.4. Assessment of detection at delayed PMI of TSEs
The transmissible spongiform encephalopathies, also known as prion diseases, are a family of fatal neurodegenerative diseases caused by abnormal protease‐resistant isoforms (PrPSc) of the normal host‐encoded prion protein (PrP). The pathognomonic feature of these diseases is the accumulation of the PrPSc in specific neuroanatomical locations within the central nervous system and, in some instances, in lymphoreticular tissues. These diseases have incubation periods ranging from 18 months to several years depending on the host species, the strain of the agent and the specific genetics of the affected individual, which can influence incubation time, tissue distribution of infectivity and clinical presentation. Animal prion diseases in ungulates include bovine spongiform encephalopathy (BSE) in cattle and goats, scrapie in sheep and goats, chronic wasting disease (CWD) in cervids, and more recently camel prion disease (CPD), some of them with multiple variants/strains. Active surveillance for these diseases has identified previously unknown prion disease variants in a range of species, and these are widely referred to as ‘atypical’ TSE.
For any of these forms of TSE, clinically affected animals may present with a range of non‐specific signs such as weight loss/poor condition and reduced milk yields, drooling and/or difficulty in swallowing, and may also show nervousness, an abnormal gait/ataxia, pruritus and/or tremors. Circling may also be a feature of the disease, particularly in small ruminants, and cattle with atypical BSE may exhibit depression, dullness, unresponsiveness and difficulty in rising. None of these clinical signs, individually or in combination, are considered pathognomonic of these diseases, although they should be sufficient to trigger clinical suspicion of TSE on the farm or at AMI. Disease then needs to be confirmed by post‐mortem testing.
However, the clinical signs associated with prion diseases occur very late in an otherwise asymptomatic incubation period that could last for years and are not a sensitive or reliable way to detect infected animals, and there is currently no live animal test. Both the detection of infection and/or disease and the confirmation of clinical suspicion require the post‐mortem demonstration of abnormal prion protein in brain and/or lymphoid tissues using immunodetection methods. Even with these methods, there is still a ‘latent period’ post exposure in which there are no methods currently available to detect such recently infected animals (Arnold et al., 2007; EFSA BIOHAZ Panel, 2014, 2017).
3.4.1. TSE testing requirements
TSE testing requirements for all species have changed over time and are stipulated in the consolidated Commission Regulation (EC) 999/2001.35
3.4.1.1. Cattle
At the height of the BSE epidemic, there was a food safely requirement to test all healthy slaughter (HS) cattle over the age of 30 months. Following any positive screening test, the whole carcass (plus the one before and two after on the line) were removed from the food chain and disposed of as Category 1 Animal By‐Products (ABP). Over time the age threshold for testing increased, and since 2013, most MS have been allowed to discontinue the testing of HS cattle.
The testing of any HS cattle is now undertaken in only two MS, and solely for the purpose of disease surveillance. When HS testing is undertaken, carcasses must not be released from the slaughterhouse until a negative test result has been received. All MS are obliged to test for TSE in animals that are clinically suspect (including at ante‐mortem examination), dead in transit, culled in the context of disease eradication schemes or are fallen stock or emergency slaughtered animals of different ages (for more details, see 2018 EUSR TSE (EFSA, 2019)). There is no specified timeframe for this testing, and samples may be batched for logistical reasons, therefore, a sample may not be tested for several days after the death of the animal.
BSE prevalence is now very low. In 2018 (EFSA, 2019), a total of 1,181,997 cattle were tested, with a total of four cases detected in two MS (1 C‐BSE, 1 L‐BSE and 2 H‐BSE).
3.4.1.2. Small ruminants
The testing of annual samples of the adult sheep and goat populations in each MS is required for disease surveillance purposes. The sheep and goat populations are considered separately. The sample size is based on the size of the adult standing population, varies with the surveillance stream and ranges from 0 tested in the SHC (slaughtered for human consumption) category if the national population is less than 750,000, up to 10,000 animals if the standing population is larger than 750,000. These samples can be all SHC, or replaced with up to 5,000 animals not slaughtered for human consumption (NSHC) at the discretion of the MS. In the case of animals NSHC, the number ranges from 0 up to 100 animals if the total population is less than 40,000, and up to 10,000 in MS with an adult population above 750,000. There is no requirement for systematic testing of SHC animals from a food safety perspective. However, the carcass of any SHC animal that is tested is held until the results are available, and any ‘test positives’ are removed from the food chain and disposed of as category 1 ABP.
In 2018 (EFSA, 2019), a total of 325,386 sheep and 138,128 goats were tested, resulting in the detection of a total of 934 cases of scrapie in sheep and 523 in goats.
3.4.1.3. Cervids
Historically, testing for cervid TSE within the EU has been confined to passive surveillance, with the exception of one period during which a survey was conducted in certain MS during the BSE epidemic (EFSA BIOHAZ Panel, 2010). Following the identification of TSE in reindeer, moose and red deer in Norway (Benestad et al., 2016; Pirisinu et al., 2018; Vikøren et al., 2019) a mandatory 3‐year active surveillance programme was proposed (EFSA BIOHAZ Panel, 2017) that has been implemented in Estonia, Finland, Latvia, Lithuania, Poland and Sweden as of 2018. The selection of animals for this surveillance programme is focussed on the high‐risk groups for two main management systems, i.e.
from the farmed or captive population, and in order of priority (surveillance value) animals which are fallen/culled, clinical/sick, slaughtered farmed cervids which have been declared unfit for human consumption and finally slaughtered farmed cervids considered fit for human consumption,
From the wild/semi-domesticated population, and in order of priority (surveillance value) animals which are fallen/culled, road‐ or predator‐injured or killed, clinical/sick, wild hunted cervids and slaughtered semi‐domesticated cervids which have been declared unfit for human consumption, and finally hunted wild game and slaughtered semi‐domesticated cervids considered fit for human consumption.
There is no current requirement for the testing of SHC animals from a food safety perspective.
In 2018, 5,110 animals were tested in the context of the mandatory cervid surveillance programme, and one positive moose (a fallen wild animal) was identified in Finland. The voluntary testing of a further 3,075 animals in other MS did not result in any other positive animals being identified (EFSA, 2019). The majority (67%) of the animals tested came from the lowest priority (SHC) population. In 2019, a further three positive cases have been detected, all wild moose from Sweden (two clinically abnormal animals that were culled, and one apparently healthy hunter‐killed animal) (EFSA BIOHAZ Panel, 2019).
3.4.2. TSE testing protocols
Primary screening for TSE is usually conducted using ELISA‐based ‘rapid tests’ regardless of the species or tissue being tested. Such testing will report a ‘presence/absence’ of abnormal PrPSc, but subsequent confirmation of disease, and its classification, is delivered by confirmatory testing which is usually immunohistochemistry or Western Blotting, although a second ELISA with different antibodies is acceptable (OIE manual).36
A key feature of the disease‐specific protein PrPSc is that it is proteinase‐K resistant, a feature that is exploited by many testing protocols. This also means that it is at least partially resistant to other proteases and persists even when tissue is markedly autolysed. Studies looking at test performance in known positive autolysed samples show no reduction in analytical sensitivity or specificity even with advanced autolysis (Chaplin et al., 2002; Monleón et al., 2003; EFSA, 2004; Wear et al., 2005; EFSA BIOHAZ Panel, 2012; Sarasa et al., 2013). However, such studies have not been carried out for goat scrapie, for atypical forms of disease (in which the PrPSc has been shown to be more sensitive to protease digestion (Everest et al., 2006; Klingeborn et al., 2006)), or for the newly identified European cervid TSE. It is hypothetically possible that there are unknown forms of disease in which the abnormal protein is more protease‐sensitive and are therefore not detected by any current testing strategy.
Due to the very precise and restricted neuroanatomical location of the abnormal protein in the early stages of the incubation period, accurate subsampling of the brainstem, at the level of the obex, is required to ensure that tissues from the appropriate neuroanatomical areas are available for both the screening test and the confirmatory test (Arnold et al., 2007; Ryder et al., 2009; EFSA BIOHAZ Panel, 2017, 2018, https://science.vla.gov.uk/tse-lab-net/documents/tse-oie-rl-samp.pdf). This is compromised if poor tissue preservation prevents anatomical orientation of the sample, or if tissue integrity is completely lost. Test specificity remains unaltered, so a positive result is valid, but a negative result is less reliable if the neuroanatomical origin of the tissue presented to the test cannot be confirmed. This aspect of testing has the most substantial effect on the overall sensitivity of any TSE testing protocol. For example, the EFSA (2015) report on negligible scrapie risk (Denmark) states – ‘the existing laboratory data on the sensitivity of the screening diagnostic tests from past EU test evaluations are not representative of the sensitivity under field conditions, and may result in an overestimation of the surveillance sensitivity’.
Current sampling protocols are based on the neuroanatomical areas known to be the first to accumulate detectable PrPSc in classical forms of disease in cattle and small ruminants, and in the majority of cases of CWD. Atypical and newly identified forms of disease also involve these areas, although the neuropathogenesis of these alternative disease presentations is yet to be established. In animals in which disease pathogenesis includes an initial peripheral lymphoid stage, such animals would be detected earlier in the incubation period if lymphoid tissue is sampled than would be possible if only brainstem was sampled for testing. However, lymphoid testing alone would fail to identify those animals with no detectable involvement of the lymphoid system. Greater overall diagnostic sensitivity would be achievable by the dual sampling of brainstem with lymph node, as recommended for the current cervid surveillance.
In summary, the current testing requirements for TSE are driven by disease surveillance, rather than food safety, as a direct objective, and focus primarily on animals not slaughtered for human consumption. Delays of several days before the sampling and testing of fallen stock are accepted. Should the testing of HS be maintained, introduced or reintroduced for any species, delays in PMI of 24 or 72 h could potentially reduce the overall diagnostic sensitivity of the testing programme through the effects autolysis may have on tissue integrity and the ability to identify and sample specific neuroanatomical areas with accuracy, but will not exceed the tolerance already in place for fallen stock.
Uncertainties about the effect of a delayed PMI on the detection of TSEs are summarised in Appendices D.1 and E.
3.5. Assessment of detection at delayed PMI of Trichinella
The genus Trichinella consists of several species and genotypes, with a total of 12 variants (Pozio et al., 2009). In Europe, the presence of the species T. spiralis,T. britovi,T. pseudospiralis and T. nativa has been documented (Pozio and Zarlenga, 2013). The resistance to freezing varies between species (Martinez et al., 2001), but also depends on whether the infection had occurred recently or some longer time ago.
The muscle larvae are able to adapt to low temperatures by production of heat‐shock proteins (Martinez et al., 2001). The larvae show enhanced tolerance to low storage temperatures when still embedded in the meat matrix, in part attributable to the nurse cell and/or the collagen capsule in encapsulated species (Pozio et al., 1989; Sacchi et al., 2001).
3.5.1. Factors that could influence the sensitivity of Trichinella examination when meat inspection (PMI) is delayed for 24 or 72 h
Items to consider with respect to delayed meat inspection are:
are Trichinella larvae impaired in a way that would affect their detectability?
is the efficacy of digestion of the meat matrix or the sedimentation/filtration process impaired after a prolonged storage of the sample before PMI?
is the diagnostic system (microscopic examination) impaired?
The assessment must not only take into account scientific studies but should also consider that in small‐scale slaughterhouses, samples for Trichinella testing may be sent to a laboratory designated by the competent authority, which is already a one‐day delay at least.
3.5.1.1. Evidence for survival of Trichinella muscle larvae during meat storage (post‐mortem conditions)
A number of studies deal with the inactivation of Trichinella larvae at sub‐zero or cooking temperatures (see, e.g. ICMSF, 1996; Pozio, 2018; Franssen et al., 2019), and not all references specify which species or genotypes were actually studied.
The ability of Trichinella muscle larvae to maintain their integrity and infectivity in muscle tissues for a prolonged period after death of the host is a major facilitator for the transmission of the parasite (Pozio, 2016; Rossi et al., 2019).
Data on survival of Trichinella muscle larvae (either in meat matrix or in liquid media) at temperatures in the range of 0–8°C are compiled in Table 12. Riva et al. (2012) studied the recovery and morphology of larvae in infected mice that had been buried in winter (2–11°C) or stored in the lab (8°C) (Figure 11). They found no changes in the structure of the nurse cells and of the glycogen content of T. spiralis larvae during a 6‐week storage. The numbers of recovered larvae (vital and dead) are given in Figure 11. The percentage of vital larvae was 98.5% at day 0, and after 7 days storage in winter or at 8°C, it was 97.8 and 100%, respectively.
Table 12.
Data on numbers of vital Trichinae after cold storage
| Species | Matrix | Temperature | Vitalityb after | Vitality after | Reference |
|---|---|---|---|---|---|
| T. nativa | Liquid medium | 4°C | 2, 4, 8 h: Unaffected |
5 days: 100% 9 days: 99% |
Martinez et al. (2001) |
| T. spiralis | Liquid medium | 4°C | 2,4,8 h Unaffected |
5 days: 97% 9 days: 95% |
Martinez et al. (2001) |
| T. nelsoni | Liquid medium | 4°C | 2,4,8 h Unaffected |
5 days: 90% 9 days: 80% |
Martinez et al. (2001) |
| T. nativa | Water | 37°C | 72 h: 70% | Theodoropoulos et al. (2003) | |
| T. spiralis | Water | 37°C | 72 h: 70% | Theodoropoulos et al. (2003) | |
| T. nelsoni | Water | 37°C | 72 h: 70% | Theodoropoulos et al. (2003) | |
| T. spiralis | Horsea | 5°C | 1 days: 81.4% |
2 days: 86.1% 7 days: 60.5% |
Hill et al. (2007) |
| T. spiralis | Piga | 4°C (buried) | 7 days: 99.99% | Jovic et al. (2001) |
Experimental infection.
Motility.
Figure 11.
Number of larvae recovered from experimentally infected mice buried in winter or stored at 8°C (Lab.)
- X‐axis: weeks of storage, y‐axis: number of larvae recovered (vital and dead); redrawn from data in Riva et al. (2012).
Von Köller et al. (2001) studied the survival of encapsulated Trichinella larvae (nine different genotypes) in meat from rat and red fox. Samples were stored at room temperature and 100% humidity, and survival (infectivity) ranged from 1 to 4 weeks, with T. nelsonii being most resistant, and non‐encapsulated species were the least resistant. Survival time was also dependent on the time from infection of the animal to killing the animal: the longer this period, the longer was the survival of Trichinella encapsulated in meat stored at room temperature.
Similarly, larvae of T. papuae remained infectious in the muscles of an infected pig for up to 9, and some even 14 days, at storage temperatures up to 38°C (Owen and Reid, 2007). Admittedly, such experiments simulate putrefaction rather than carcass or meat storage. Obviously, these experimental settings described above are more targeted towards to the role of wildlife as a reservoir for the parasite. The lack of recent studies on the survival of Trichinella species prevalent in Europe in meat from suids can be explained by the fact that the introduction and persistence in holdings is very unlikely when the holdings implement appropriate biosecurity measures compliant with those required to obtain official recognition of ‘controlled housing conditions’ according to Regulation (EU) 2015/1375.
Since the authors tested for vital larvae, there is no indication on what numbers of inactivated, but intact larvae were present in the samples. The results (no or only moderate reduction in infectivity) presented in this section are thus likely to overestimate the effects of delayed sampling on detection of the parasite. Given the similarity of the composition of muscle tissue amongst mammals, it is conceivable to assume that a 24‐ or 72‐h delay in sampling would not reduce numbers of structurally intact larvae.
3.5.1.2. Evidence on detectability of Trichinella larvae from stored reference material/proficiency samples
This subsection deals with the resistance of Trichinella muscle larvae to HCl‐pepsin digestion and maintenance of the structural integrity of the larvae. Digestion of the larvae would cause an underestimation.
Since EU legislation does not distinguish between dead and vital larvae, and artificial digestion as prescribed in EU legislation would recover both motile and non‐viable larvae, studies testing for loss of infectivity will give an overestimate for the decrease in sensitivity, as was asked for in the scope of this mandate.
The mode of Trichinella testing is specified in Regulation (EU) 2015/1375 (for the principle, see Gajadhar et al., 2019). The sensitivity of the digestion method is dependent on the sample size: for 1 g sample, it is 3–5 larvae/g under practical conditions (Nöckler et al., 2000). This regulation gives no maximum limit for the time between slaughter and testing the sample. Samples should be analysed as soon as possible or stored at 2–8°C to slow down decomposition (Gajadhar et al., 2019). It has been reported that freezing samples can impair the detection of larvae by the digestion method (Gajadhar et al., 2019). Non‐encapsulated larvae (e.g. T. pseudospiralis) are more susceptible to environmental conditions than encapsulated ones (e.g. T. spiralis).
Current practice in preparing and delivering Trichinella samples for proficiency tests gives an indication of how long larvae embedded in meat stored at temperatures of 0–4°C can be detected quantitatively. For instance, the EURL for Parasites (Trichinella,Echinococcus,Anisakis) specifies for the artificially inoculated samples stored at +4 and +15°C that ‘naked larvae’ remain viable up to 5 days, while encapsulated larvae remain viable up to 20 days from the date of preparation (PT: ‘Magnetic stirrer method for pooled sample digestion according to the Regulation (EU) 2015/1375’).37 In the proficiency tests organised by the EURL, sample stability is checked and guaranteed after storage at room temperature, 3 and 5 days after packaging.38 It should be noted that this refers to the more vulnerable ‘naked’ larvae, whereas encapsulated larvae should be more resistant. Even when considering the time needed to send a sample to a designated laboratory and to have the sample actually tested would be 2 days, a delay of 3 days in sampling would result in 5 days from slaughter to sample testing, which is the period tolerated in proficiency tests. The EURL for Parasites (2006) allows for Trichinella testing of indicator species, that muscle samples can be stored ‘at room temperature if they are delivered to the laboratory and processed in a short period of time (within 2 days); for a period of time between 1 and 3 weeks, muscle samples can be refrigerated at +4°C’. It is assumed that such storage would not impair sensitivity of the analysis. However, for this testing for epidemiological purposes, larger sample sizes are recommended (Nöckler et al., 2000; EURL for Parasites, 2006). For samples for proficiency tests, storage at 5 +/− 3°C and a maximum transport time to the laboratory of 48 h is recommended (Gajadhar et al., 2019). This should ensure that sensitivity for samples with 3–5 larvae/g is not negatively affected. The EURL for Parasites (undated) specifies in its Guideline QAS.1166800938 for reference samples a storage period of 7–10 days for fresh meat from infested animals, and that proficiency samples should be delivered within 48 h to the laboratory, and after arrival, it should be analysed within 24 h; at 4 +/− 2°C.
It was concluded that a 24‐ to 72‐h delay in testing a meat sample for Trichinella would mimic the scenario for proficiency samples with a number of larvae at the limit of detection, where 72‐h cold storage from sample preparation to testing are tolerated. The proficiency samples are prepared by spiking meat with free or encapsulated larvae, which are more susceptible to environmental conditions; naturally contaminated meat samples should provide more protection.
These data are based on T. spiralis s.s. and not necessarily applicable to the other three species prevalent in Europe.
3.5.1.3. Evidence on the effect of drying of the meat sample and thus, incomplete digestion of the sample (no or not all larvae are released)
Larvae remain embedded in muscle fragments, which are retained by the 180 μm mesh and do not pass the sieve. Larvae are released, but incomplete digested meat particles passing the sieve will mask the larvae in the petri dish and will lead to an underestimation.
Mincing of the meat has to be adjusted to facilitate digestion (Gamble et al., 2000; Gajadhar et al., 2019). Too intense mincing could result in a loss of parasites (Riehn et al., 2013; Rossi and Pozio, 2008). Mincing should be done until no visible meat particles remain (EURL Community Reference Laboratory for Parasites, undated). The digestion is considered satisfactory when the undigested residue does not exceed 5% of the sample weight (Regulation (EU) 2015/1375).
There is no clear indication on what degree of dryness can be the result of a 24‐ or 72‐h delay in PMI and sampling for Trichinella. However, the size and diameter of the muscles to be sampled (diaphragm pillar in pigs; if not present, rib part or breastbone part of the diaphragm, jaw muscles, tongue, abdominal muscles; in wild boar: the foreleg, tongue or diaphragm; in horses: lingual or jaw muscle; if lacking, then a larger sized specimen is to be taken from a pillar of the diaphragm) will allow to take a specimen which is not dried off.
The following uncertainties exist in this assessment:
Data on survival/structural integrity of Trichinella muscle larvae in chilled (0–7°C) meat from suids and solipeds are not available for all four Trichinella species prevalent in Europe;
some tissues may take longer to digest (e.g. also dried tissues when sample are taken 24‐ or 72‐h post slaughter) but the EU reference method foresees that duration of digestion can be extended, and specifies that at least 95% of the samples must be digested in order to consider the procedure ‘satisfactory’.
Uncertainties about the effect of a delayed PMI on the detection of Trichinella are summarised in Appendices D.2 and E.
In conclusion, the panel did not find any evidence that would point to a decrease in sensitivity during cold storage and is almost certain (99–100%) that there is no decrease in the sensitivity of Trichinella detection in meat from susceptible animal species after a delay of PMI of 24 or 72 h.
3.6. Effect of delayed inspection on the probability of laboratory detection of Salmonella spp
The impact of extended cold storage on the listed factors affecting Salmonella detection is discussed based on literature data and along with an EKE process, used to derive the input values for the uncertainty distribution of the variables of the stochastic model.
3.6.1. Defining model inputs for the Salmonella distribution on carcass pre‐chilling
Several studies have investigated Salmonella counts on carcasses of pork, beef and lamb using either most probable numbers (MPNs) or direct counting methods. Zhou et al. (2018) defined at different steps during pig slaughter the MPN of Salmonella. Highest values were identified after exsanguination: 2.50 ± 0.94 MPN/m2 and after splitting with 2.24 ± 0.72 MPN/ m2. Similarly, low numbers were obtained after dehairing, flaming, washing and after chilling with 1.39 ± 0.42, 1.36 ± 0.31, 1.49 ± 0.34 and 1.38 ± 0.30, respectively. Martinez‐Chavez et al. (2015) tested beef carcasses by sponge swabbing an area of 300 cm2 on 142 beef carcasses. Twenty‐six were identified positive for Salmonella with a mean value of 1.3 +/‐ 0.9 Log10 MPN/300 cm2 and a distribution from 0.3 to 3.4 Log10 MPN/300 cm2. Approximately one‐fifth had counts higher than 2 Log10 MPN/300 cm2. After chilling, approximately 1% tested positive for Salmonella. Other studies that analysed Salmonella counts on beef carcasses obtained less than 1 Log10 colony‐forming unit (CFU)/cm2 (Khen et al., 2014), 0.31 MPN/cm2 (Fegan et al., 2005), 1.6 × 10° CFU/100 cm2 (Brichta‐Harhay et al., 2007) or 0.5–2.2 CFU/100 cm2 (Bosilevac et al., 2009).
Based on the available literature, data and discussions in the working group during the EKE process, the following distributions were elicited for the uncertainty of the distribution of mean Salmonella Log10 counts (Log10 CFU/100 cm2) on carcasses, before chilling (i.e. time 0, Log10 No of Table 4 in Section 2.4). For the purpose of the EKE, the experts selected a hypothetical minimum average of 0 Log10 CFU/100 cm2 even though the actual minimum count is lower in one publication (Zhou et al., 2018).
The distribution that described the consensus of the elicited expert estimates (GROUP) was a beta distribution with the parameters alpha = 1.74 and beta = 7.97 (Figure 12).
Figure 12.
Elicited uncertainty distributions for the mean Salmonella Log10 concentration on carcass (μ0) just after slaughter and before chilling, expressed in log10 CFU/100 cm2
- GROUP beta = Beta (1.74, 7.97).
3.6.1.1. Defining the inputs of the parameter SLmMin
As detailed in Section 2.4, according to ISO 6579‐1:2017 (Anonymous, 2007), one viable cell of Salmonella added in the pre‐enrichment medium (Buffered Peptone Water) is enough to give a positive sample. This is the theoretical limit of detection of the laboratory method. Nonetheless, the likelihood of recovering one viable cell added to BPW is highly dependent on additional factors related to the levels of viable and culturable Salmonella cells and their recovery by swabbing (i.e. overall sample preparation). Due to scarcity of data in this area, an EKE was applied to elicit the opinion of experts about the average value of this parameter for Salmonella on ungulate carcasses. Based on the EKE process, a consensus was reached that the uncertainty of the average SLm Min can be described by a normal distribution with average 4.67 cells and standard deviation 1.59, as shown in Figure 13.
Figure 13.
Elicited uncertainty distributions for the minimum required Salmonella cells on sampled carcass area to give a positive sample (SLmMin)
- Consensus distribution = normal (4.67, 1.59); truncated at 1.
3.6.1.2. Survival, reduction (‘SR’ parameter) and rate of lack of culturability (‘phys’ parameter) of Salmonella on carcass
During delayed PMI, carcasses are stored at a maximum of 7°C (based on Regulation (EC) 853/2004), which does not support the growth of Salmonella, at least not within the time frame of 72 h considered by the current assessment. The levels of the organism are affected by the combined effect of low temperature and reduction of surface aw down to levels of 0.93–0.95, due to free water loss (Kinsella et al., 2006; Reid et al., 2017). Chilling is known to induce cold‐shock in Salmonella (Mackey and Derrick, 1986; Ricke et al., 2018), thereby affecting their ability to survive or recover in culture media. A drop in aw may further induce decline of Salmonella and/or injury. In the current assessment, the uncertainty of the average aw considered by the stochastic model was described by a Pert distribution with minimum, most likely and maximum values of 0.94, 0.95 and 0.97 for 24 h and 0.93, 0.95 and 0.97, for 72 h post‐chill, respectively.
The majority of the evidence available in the scientific literature on the levels and/or presence of Salmonella on carcass after or during slaughter clearly suggest that during chilling Salmonella detection decreases. Some studies identifying no change or even an apparent increase in the level of Salmonella after chilling suggest that these observations are most likely due to contamination during chilled storage (Lenahan et al., 2010). Mannion et al. (2012) studied pig carcasses during slaughter and detected 10.2% of carcasses that tested positive for Salmonella pre‐wash, 3.9% post‐wash and only 1.8% that were positive post‐chill. Narvaez‐Bravo et al. (2013) showed a reduction from 49% before evisceration to 6% after chilling of beef carcasses for the detection of Salmonella.
The non‐thermal inactivation model of Pin et al. (2011), based on broth‐data and laboratory challenge studies with pork products (available in Combase), estimates a reduction of Salmonella of less than 0.5 Log10 unit during storage for up to 72 h at 7°C, at an average carcass surface pH of 5.8 and an uncertainty distribution of the average aw as detailed above. The reduction is rather limited and possibly does not encompass the additional injury caused by the combined effect of cold shock and drop of aw. For example, King et al. (2016) demonstrated that dynamic changes in temperature and aw during carcass chilling caused Escherichia coli O157:H7 to enter in the so‐called viable but non‐culturable state for at least 42 h, with markedly slow recovery up to 72 h. As for Salmonella, in the study of Chang et al. (2003), who inoculated pork carcasses with or without skin with faecal slurries bearing low (3.2 Log10 CFU/cm2) or high (5.4 Log10 CFU/cm2) Salmonella levels, it was observed that both blast and conventional chilling caused reduction in Salmonella concentrations of 0.7–1 and 1.1–1.3 Log10 units for low and high inoculum, respectively. Blast chilling, on average, showed slightly higher Log10 reductions (i.e. up to 0.2) than conventional chilling. Considering that in such challenge studies, the attachment and/or entrapment of inoculated organism on carcass surface is not as strong as in naturally contaminated samples, the observed reductions may be attributed mainly to loss in viability or culturability of the organisms. Given the discrepancy between the predictions of modelling by Pin et al. (2011) and the literature data on reductions in levels and detection capability for detecting Salmonella, an EKE process was performed to assess the uncertainty of an average reduction in culturability of Salmonella (i.e. additionally to the Log10 reductions predicted by the model), due to cold shock and reduction in water activity after 24 h and 72 h. To deliver the extreme values of the uncertainty distribution of the average level of injury, the experts took also into account the potentially low aw of certain carcass surface microenvironments (e.g. even lower than 0.94 after 24 h or 0.93 after 72 h, respectively, on crevices, hairy vs non‐hairy areas) and for which, there are no experimental data available in the literature for the possible levels of aw. The uncertainty distribution of the average loss of Salmonella culturability during chilling was by consensus described via a Beta distribution with parameters alpha = 1.30 and beta = 0.80 after 24 h (Figure 14) and alpha = 2.10 and beta = 1.32 after 72 h (Figure 15).
Figure 14.
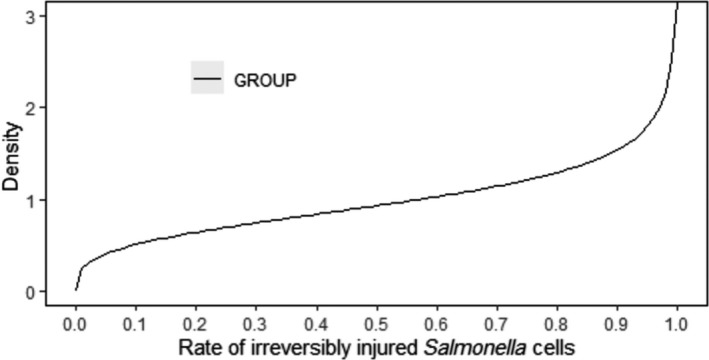
Elicited uncertainty distributions for the average rate of irreversibly injured Salmonella cells after 24 h of chilling (‘phys’ model parameter)
- GROUP beta = beta (1.30, 0.80).
Figure 15.
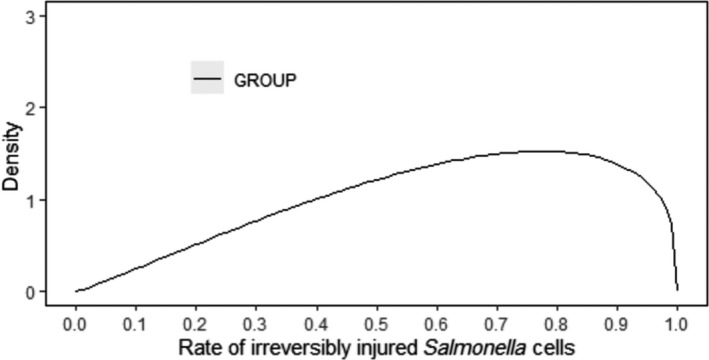
Elicited uncertainty distributions for the average rate of irreversibly injured Salmonella cells after 72 h of chilling (‘phys’ model parameter)
- GROUP beta = beta (2.10, 1.32).
3.6.1.3. Defining % reduction in swabbing/sponging efficacy (‘SpEff’ parameter) post‐chilling
Chilling is known to cause morphological changes on carcass surfaces. Such changes increase the depth and/or the number of crevices (Yu et al., 2001), which trap and protect Salmonella. This results practically in a lower transfer rate of Salmonella from carcass to swab after chilling. Swabbing (non‐destructive) is less effective than excision (destructive method) to recover (detach) and enable detection/enumeration of Salmonella on carcasses after chilling and based on the available evidence increasing storage of carcasses for up to 72 h is expected to reduce further the efficiency of the swab method to detect Salmonella. Other studies showed that the swab method was more sensitive to the negative effect of chilling than destructive methods (Vanantwerpen et al., 2016). Chang et al. (2003) analysed chilling effects of blast chilling and conventional chilling of pork carcasses on the survival of Salmonella Typhimurium. Experimental studies were carried out inoculating high (5 Log10 CFU/cm2) and low (3 Log10 CFU/cm2) numbers of cultures of S. Typhimurium on carcasses overnight, and conventional and blast chilling procedure was applied for 24 h and 21 h, respectively. Contaminated skin samples and off‐skin samples were excised (by a destructive method) and analysed for Salmonella by direct streaking and enrichment. At both high and low inoculation levels, Salmonella were reduced by about 1 log10 for both chilling methods.
Differences between sponging, swabbing and destructive methods were analysed by other authors on pig and beef carcasses using the detection of coliforms, total aerobic counts and Escherichia coli. Even though this cannot be directly compared with Salmonella detection, results show a high variability in enumeration after chilling (Palumbo et al., 1999; Gill and Jones, 2000).
Based on the above data and relevant discussions in the working group during the EKE process, a consensus was reached that the average detachment efficiency 24‐h post‐chill is reduced by 0–40% (with most likely value 10%) compared to pre‐chill. This range was introduced in the model. For 72‐h post‐chill, a total average reduction of 5–50%, with most likely value 20%, compared to pre‐chill stage, was elicited and used in the probability distributions describing the uncertainty of this parameter as model input.
3.6.1.4. Impact of indigenous microbiota on Salmonella growth during enrichment (‘Compt’ parameter)
Indigenous (i.e. commensal, spoilage) bacteria on a carcass surface include psychrotrophic species, e.g. pseudomonads, that may grow at the maximum permissible temperature of 7°C, and thus increase their levels compared to those of Salmonella after 24 or 72 h of delayed inspection. The inhibitory effect of pseudomonads growth at levels close to the stationary phase of growth (approx. 8–9 Log10 CFU/g) on enteric pathogens has been demonstrated against Escherichia coli, on fish burger at 8°C (Speranza et al., 2010) and against Salmonella on ground pork from 4 to 24°C (Møller et al., 2013), but whether this holds true also for Salmonella at the optimal temperatures of 37 or 42°C, during enrichment, is not yet demonstrated. Particularly in Møller et al. (2013), it was demonstrated that the growth and maintenance of pseudomonads at high levels and at temperatures as high as 24°C did not prevent Salmonella from reaching similar levels, albeit more slowly due to the lower growth rate. No such microbial interaction models (i.e. based on Jameson effect or Lotka Volterra model) are available for the combination of Salmonella with lactic acid bacteria or Brochothrix thermosphacta.
The growth of pseudomonads and B. thermosphacta, naturally present on the carcass surfaces may be predicted by available predictive models, such as those in Combase. In particular, based on Combase models, growth of pseudomonads, and B. thermosphacta for up to 72 h at 7°C, pH 5.8 and aw 0.95–0.97 ranges from 0 to 0.46 and 2 to 3 Log10 units growth, respectively. Under proper hygiene conditions, the initial levels of most of these organisms are expected to be lower than 2 Log10 CFU/cm2 and thus, the limited Log10 increase predicted for B. thermosphacta, combined with the high selectivity of enrichment media are not expected to constrain the growth of Salmonella at detectable levels. Furthermore, most of these models are either broth‐based, or they do not take into account the concomitant reduction of surface aw during prolonged storage of carcasses under chill conditions. Thus, the predicted growth of meat microbiota is rather overestimated. This is further supported by the findings of Reid et al. (2017), who reported an average increase in Log10 numbers of pseudomonads and B. thermosphacta of 0.2 and 0.99, respectively, during chilling of beef carcasses.
Based on the above and the discussions that took place during the EKE process, the variable ‘Compt’ was considered to have limited to negligible impact on the reduction in sensitivity of Salmonella detection. The uncertainty distribution of the average rate (Compt) of Salmonella cells outcompeted by meat microbiota during enrichment was described by a Pert distribution with minimum, most likely and maximum values of 1, 3 and 10% for 24‐h post‐chill and 1, 6 and 15% for 72‐h post‐chill, respectively.
3.6.1.5. Serovar/Strain effect
It is widely recognised that there is strain variability in the survival and growth characteristics of microorganisms. Some serovars (Heidelberg, Senftenberg) tend to be more resistant to chilling temperatures and thus may have a higher ability to survive and be less injured during cold storage than other serovars (Pathania et al., 2010). If more than one serovar (strains) are on the carcass and have different viability, this will not affect the outcome of detection after chilling, because the strain with the highest fitness and/or which survives best will produce a positive test result (Gorski, 2012).
These strain‐specific differences were accounted for within the model variable ‘physiological state’.
3.6.2. Outputs of Monte Carlo simulation
The output of the stochastic model simulation (10.000 iterations, 1 simulation) based on the aforementioned uncertainty distributions of the input values was a probability distribution, describing the uncertainty about the reduction in sensitivity of Salmonella detection.
3.6.2.1. Simulation after a 24‐h delay of PMI
Figure 16 shows pre‐ and post‐chill distribution of Salmonella levels, clearly illustrating the shift of the post‐chill distribution to the left of the original one. Each datapoint of the left (red) distribution is derived from a sampled (Log No) value from the original (blue) and the relative change described by equation 1.
Figure 16.
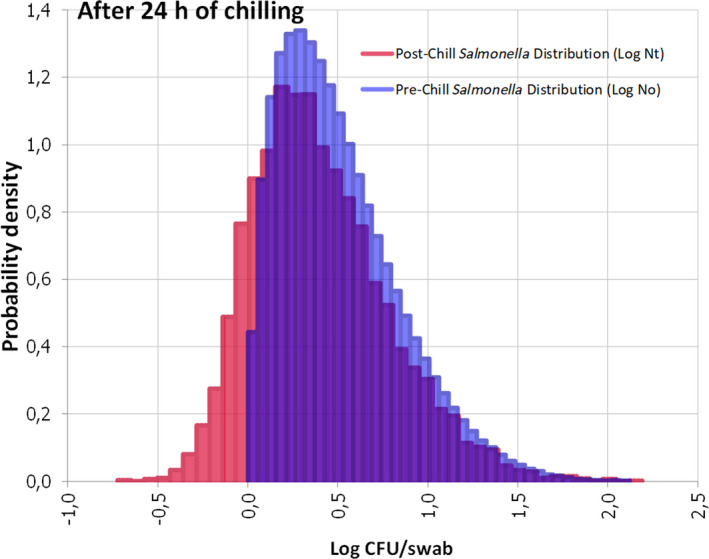
Pre‐ (blue = Log No) and post‐ (red = Log Nt) chill distribution of Salmonella levels at 24 h in samples where Salmonella is detected pre‐chill
Considering the uncertainty distribution of SLm Min, the number of carcasses with Salmonella (according to the post‐chill distribution) above each sampled SLm Min value is estimated both at pre‐ and post‐chill level. Due to uncertainty also in the initial Salmonella distribution (i.e. mean Log No and standard deviation), the initial percentage of positive carcasses in some Monte Carlo iterations may be slightly lower than 100% and in some cases (e.g. due to the extreme left part of the Log No distribution) detection could also be 0%. Taking the above together, the resulting probability distribution of the reduction in sensitivity is shown in Figure 17. This distribution describes the uncertainty of the WG about this parameter. The extremes of the distribution at 0 and 100% represent, respectively, the cases at the tail of the distribution, either with markedly higher pre‐chill levels of Salmonella than the SLm Min, that remain detectable also post‐chilling (i.e. not impacted by chilling), or the markedly lower pre‐chill levels than the SLm Min , that correspond to 0% detection both pre‐ and post‐chill (Figure 16).
Figure 17.
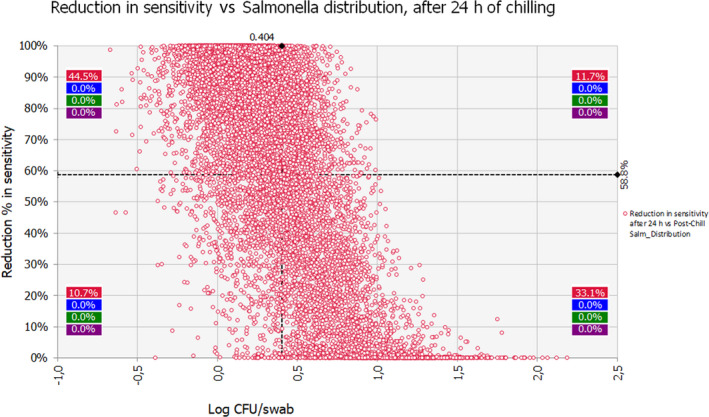
Scatter plot of post‐chill viable and culturable Salmonella levels (Log Nt) detached from carcass and reduction (%) in Salmonella sensitivity
- Each dot represents a combination of a value from the post‐chill distribution of Salmonella concentration and the corresponding value from the uncertainty distribution of the % reduction in probability of Salmonella detection.
According to the sensitivity analysis (Figure 18), the variance of the uncertainty distribution of the model output is mainly (73.1%) due to the uncertainty about the average initial Salmonella contamination (explaining 58.6% of the variance) and the uncertainty in Slmmin (explaining 13.5% of the variance).
Figure 18.
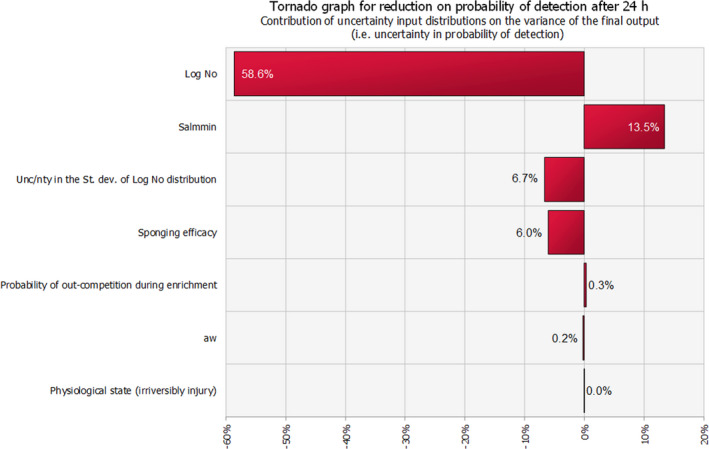
Contribution of uncertainty input distributions on the variance of the final output (i.e. uncertainty in probability of detection)
3.6.2.2. Simulation after a 72‐h delay of PMI
The model inputs were further updated via EKE for the 72 h of total post‐mortem chill duration as follows:
The minimum level of expected aw in the corresponding uncertainty distribution was further reduced to 0.93.
The probability distributions describing the uncertainty of the physiological state due to the additional injury of Salmonella caused by extra chilled storage, was considered (again) a beta distribution with alpha 2.10 and beta 1.32.
The elicited parameter values of the probability distributions describing the uncertainty for the sponging efficacy (PerU) were lower than those after 24 h. It was suggested that the minimum, most likely and maximum sponging efficacy is expected to be reduced by another 10%, 10% and 5%, respectively, from 24 to 72 h.
The most likely and maximum percentage of viable and culturable Salmonella cells outcompeted by background microbiota were reduced by 3 and 5%, respectively, more than the corresponding percentages for 24‐h.
Figure 19 shows pre‐ and post‐chill distribution of Salmonella levels after 72‐h.
Figure 19.
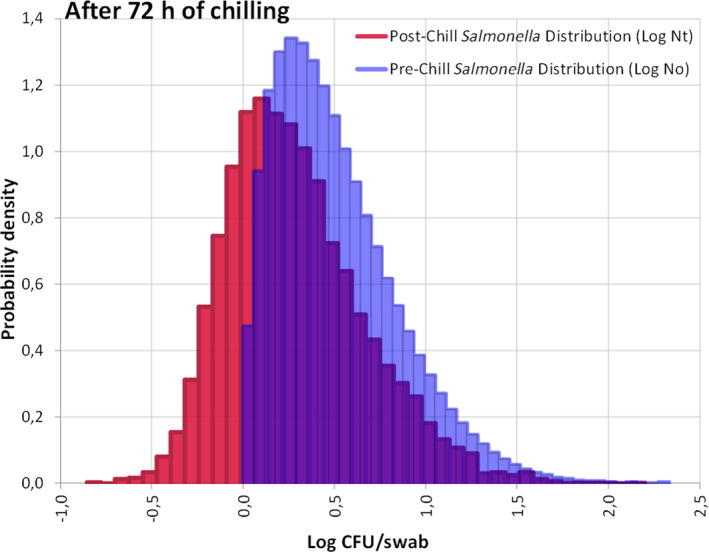
Pre‐ (blue = Log No) and post‐ (red = Log Nt) chill distribution of Salmonella levels at 72 h
The sensitivity analysis of the model output for 72‐h derived a similar result as that for 24‐h.
Table 13 shows the cumulative probabilities of reduction in sensitivity of Salmonella detection, for selected percentiles of its probability distribution (shown in Table 13), after 24 h and 72 h. These probability distributions represent the uncertainty about the reduction in sensitivity, and therefore, the cumulative probabilities for each predefined percentage of reduction can be interpreted as the probability of observing from no reduction up to this percentage.
Table 13.
Cumulative probabilities of reduction in sensitivity of Salmonella detection after 24 and 72 h of chilled storage
| Percentage of reduction (%) | After 24 h | After 72 h | ||
|---|---|---|---|---|
| Cumulative probability | Probability of greater reduction | Cumulative probability | Probability of greater reduction | |
| 10 | 0.15 | 0.85 | 0.09 | 0.91 |
| 20 | 0.2 | 0.8 | 0.12 | 0.88 |
| 30 | 0.25 | 0.75 | 0.14 | 0.86 |
| 40 | 0.31 | 0.69 | 0.17 | 0.83 |
| 50 | 0.37 | 0.63 | 0.20 | 0.8 |
| 60 | 0.44 | 0.56 | 0.23 | 0.77 |
| 70 | 0.53 | 0.47 | 0.27 | 0.73 |
| 80 | 0.63 | 0.37 | 0.33 | 0.67 |
| 90 | 0.75 | 0.25 | 0.43 | 0.57 |
For example, the probability to observe a reduction of up to 10% (after 24 h) is 0.15. Consequently, the probability of observing a reduction in sensitivity of more than 10% is 0.85. Similarly, it can be seen from the table that the probability of observing a reduction from 0 to 80% (after 24 h) is 0.63, while there is 0.37 probability of observing a reduction higher than 80%. Additionally, it can be seen that for 24 h, the 1st quartile (25th percentile) of the probability distribution of the reduction in sensitivity is 30% and the 3rd quantile (75th percentile) is 90%, meaning that there is a 0.5 probability that the reduction in sensitivity will be between 30% and 90%. Similar considerations can be made for 72 h, using the respective cumulative probabilities from the table. A comparative illustration of cumulative probabilities of percentage (%) of reduction in probability of Salmonella detection in 24 (RED) and 72 (BLUE) h, post chilling is shown in Figure 20. The median values of these distributions are 66.5% for 24 h and 94% for 72 h.
Figure 20.
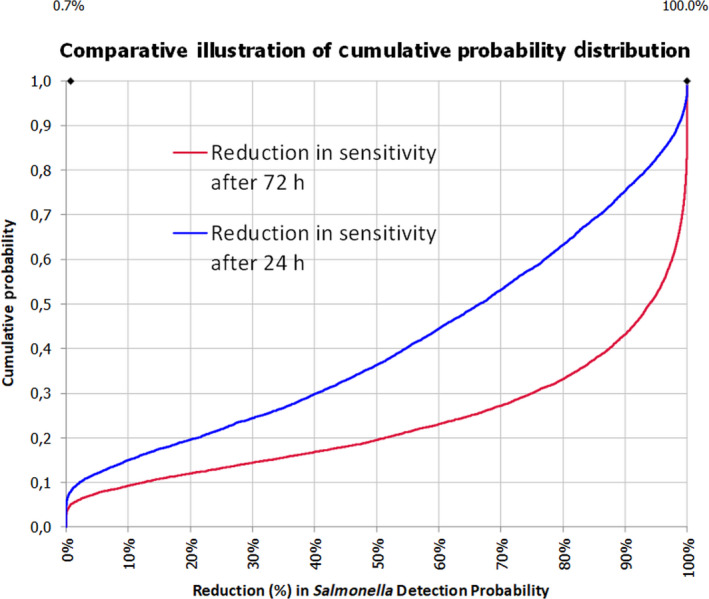
Comparative illustration of cumulative probabilities of percentage (%) of reduction in probability of Salmonella detection after 24 (RED) and 72 (BLUE) h, post chilling
Uncertainties about the effect of a delayed PMI on the detection of Salmonella are summarised in the Appendices D.3–D.5.
The developed model estimates the percentage of reduction (%) in sensitivity of Salmonella detection as applied for the process hygiene criterion, as follows:
The median estimate for the reduction in sensitivity of Salmonella detection with a 24‐h delay of PMI after slaughtering is 66.5%. The 90% probability interval for this reduction in sensitivity ranges from 0.08% (5th percentile) to 99.75% (95th percentile).
The median estimate for the reduction in sensitivity of Salmonella detection with a 72‐h delay of PMI after slaughtering is 94%. The 90% probability interval for this reduction in sensitivity ranges from 0.83% (5th percentile) to 100% (95th percentile).
The high uncertainty on the above estimates originates mainly from the uncertainty in the initial Salmonella concentration on carcasses. In general, as illustrated in Figure 17, the lower the initial Salmonella counts, the higher the estimated reduction in the sensitivity of detection.
3.7. Effect of delayed meat inspection on the detection of chemical residues and contaminants
3.7.1. Introduction
Council Directive 96/23/EC requires Member States to adopt and implement a national residue control plan (NRCP) for specific groups of residues of pharmacologically active substances and other compounds, such as chemical contaminants and pesticide residues. Pharmacologically active substances do not only cover authorised veterinary medicinal products that are legally administered and have prescribed withdrawal periods, but also prohibited substances, such as growth promoters that may be illegally applied to food producing animals, and substances that are not authorised to be used as veterinary medicinal products. The group of contaminants comprises organochlorine compounds including polychlorinated biphenyls (PCBs), as well as metals, which can, due to exposure from environmental sources, contaminate animals and thereof derived food products. Certain mycotoxins are also included due to potential transfer from feed. The NRCPs are not a tool to assess the background exposure for the consumers but aim at detecting illegal treatment of food‐producing animals, controlling compliance with withdrawal periods and the maximum residue limits (MRLs) for veterinary medicinal products, and the maximum levels (MLs) for contaminants.
The Directive 96/23/EC lays down sampling levels and sampling frequencies on farms and in slaughterhouses, as well as the group of substances to be monitored for each category of live animals or animal products. Thus, these requirements are an important tool for sampling at PMI. As from January 2023, official controls will be performed under Regulation (EU) 2017/625.
Considering that the mandate received from the European Commission (see Section 1.1) did not specify the chemical hazards to be included in the current opinion, the CONTAM Panel decided to focus on those chemical hazards that had been identified in the previous assessments on meat inspection as high and medium potential concern for the different animal species relevant for this assessment (Table 14) (EFSA BIOHAZ, CONTAM and AHAW Panel, 2011; EFSA BIOHAZ Panel, 2013a, 2013b, 2013c, 2013d). In addition to the substances listed in Table 14, a number of other substances are detected in the framework of the NRCP (e.g. corticosteroids). The assessment of these substances is beyond the scope of the current Opinion.
Table 14.
Overview of substances that should be addressed by meat inspection at EU level and that were identified as high and medium potential concern in the previous assessments on meat inspection (EFSA BIOHAZ, CONTAM and AHAW Panel, 2011; EFSA BIOHAZ Panel, 2013a,b,c,d)
| Species | High potential concern | Medium potential concern |
|---|---|---|
| Farmed game | / |
|
| Swine |
|
|
| Cattle |
|
|
| Sheep and goats |
|
|
| Solipeds |
|
/ |
DL‐PCBs: dioxin‐like polychlorinated biphenyls; NDL‐PCBs: non dioxin‐like polychlorinated biphenyls; OTA: ochratoxin A; PBDEs: Polybrominated diphenyl ethers.
In the previous assessments, the main risks for public health that should be addressed by meat inspection at EU level were identified and ranked. The ranking into categories of potential concern was performed by a multi‐step approach. As a first step, the CONTAM Panel considered substances listed in Council Directive 96/23/EC and evaluated the outcome of the NRCPs for the period 2005–2010. For swine, the period covered only the years 2005–2009. The CONTAM Panel noted that the percentage of non‐compliant samples is of a low order of magnitude. While the number of non‐compliant samples for pharmacologically active substances is generally well below 1%, the respective rate for metals occasionally exceeds 1%. Potentially high exposure of consumers to pharmacologically active substances takes place only incidentally, as a result of non‐compliance with the withdrawal period or illegal application of prohibited substances.
Independently from the occurrence data reported from the NRCPs, other criteria used for the identification and ranking of chemical substances of potential concern included the identification of substances that were found in other testing programmes, that bioaccumulate in the food chain, and the likelihood that a substance under consideration will occur in ungulate carcasses. Taking into account these criteria, the individual compounds were then ranked by the CONTAM Panel into four categories denoted as of high, medium, low and negligible potential concern. As mentioned earlier, substances identified as of high and medium potential concern are subject of this opinion.
In addition, the CONTAM Panel decided to include perfluoroalkyl substances (PFASs) in this assessment. Recent risk assessments have shown the presence of these compounds in food of animal origin at relevant concentrations and identified a concern for public health (EFSA CONTAM Panel, 2018a, 2020a). The CONTAM Panel included these compounds in the current assessment because in the future these substances could be included in the NRCP due to the identified health concern.
Chemical residues and contaminants are analysed in PMI samples with validated analytical methods. The ability of the analytical method to detect a chemical residue or contaminant in PMI samples depends on the sensitivity of the method and the concentration of the substance in the sample. The sensitivity of these methods is characterised by the limit of detection (LOD)/limit of quantification (LOQ) or decision limit (CCα)39/detection capability (CCβ).40 In the present assessment, this parameter (i.e. method sensitivity) is considered as fixed, meaning that the sensitivity of the analytical method is not influenced by a possible delay of the PMI. On the other hand, the concentration of the substance at the moment of analysis is considered variable and depends on the concentration present at slaughter, possible degradation that may occur between slaughter and the sampling during delayed PMI and possible degradation that may occur between sampling and analysis in the laboratory. Post‐mortem degradation has been described to occur in different matrices and for different substances even when matrices are cooled (Nouws and Laurensen, 1990; Sanders et al., 1991). Potential degradation that may occur between sampling and analysis in the laboratory is not changed by a possible delay of the PMI. The concentration at slaughter is not changed when a PMI is delayed. However, when post‐mortem degradation takes place between slaughter and sampling, the concentration at slaughter may still have an influence on the level at the time of analysis. In addition, delaying a PMI has an influence on the matrix that can be sampled; urine is e.g. a matrix of choice for several pharmacologically active substances, but this cannot be sampled when PMI is delayed for 24 or 72 h. Also plasma cannot be sampled when PMI is delayed for 24 or 72 h. Consequently, the effectiveness may be influenced when the alternative matrix contains lower concentrations and/or analytical methods for this matrix are less sensitive. Based on this analysis, the CONTAM Panel predominantly concentrated in the present opinion on the assessment of the stability of the relevant substances in the carcass and the influence of changing the sampled matrix, if applicable, on the effectiveness to detect the substance.
The CONTAM Panel noted the limited information available regarding the stability of chemical residues and contaminants in carcasses; while depletion studies were identified for most of the pharmacologically active substances. These studies are performed in living animals to get knowledge on how quickly a substance is metabolised and excreted, which is especially important to define the withdrawal period between last application of a veterinary drug and slaughter of the respective animal. After slaughter, enzymes can still catalyse reactions which may have an influence on the concentration of pharmacologically active substances, and thus on the likelihood to detect non‐compliance with legal limits or to discover illegal application of banned compounds which is an important part of PMI. However, this depends on the matrix, time of sample collection, temperature of storage and the properties of the compound in question. Despite this shortcoming, the CONTAM Panel decided to take the depletion studies, if available, into account as these may give supporting evidence regarding the fate of the compounds after slaughter.
The following discusses the impact of delayed meat inspection on the detection of the identified chemical hazards. The CONTAM Panel emphasises that the selection of the compounds addressed in this opinion does not claim for completeness as e.g. other pharmacologically active compounds may have gained increasing importance in the past decade. However, the assessed compounds cover a wide range of endogenous and exogenous applied substances with different physico‐chemical properties and biological behaviour. Thus, they can serve as reliable examples, to assess the effectiveness if PMI is delayed for 24 or 72 h.
3.7.2. Persistent organic pollutants
Persistent organic pollutants (POPs) are defined as ‘organic compounds that, to a varying degree, resist photolytic, biological and chemical degradation. POPs are often halogenated and characterised by low water solubility and high lipid solubility, leading to their bioaccumulation in fatty tissues. They are also semi‐volatile, enabling them to move long distances in the atmosphere before deposition occurs’.41 Classified POPs of interest within this remit are dioxins, PCBs, polybrominated diphenyl ethers (PBDEs) and per‐ and polyfluoroalkyl‐substances (PFASs).
Commission Regulation (EC) No 1881/2006 laid down MLs of dioxins and PCBs in meat and offal from ungulates.
Dioxins and dioxin‐like PCBs (DL‐PCBs) have been identified as chemical hazards of high potential concern, and non‐dioxin‐like PCBs (NDL‐PCBs) as compounds of medium potential concern in swine, cattle and small ruminants in previous assessments on meat inspection (EFSA BIOHAZ, CONTAM, AHAW Panel, 2011; EFSA BIOHAZ Panel, 2013a,c). PBDEs were identified as medium potential concern substances in swine (EFSA BIOHAZ, CONTAM, AHAW Panel, 2011). The EFSA CONTAM Panel has assessed the PFASs in two recent scientific opinions (EFSA CONTAM Panel, 2018a, 2020a), and applied a mixture approach for perfluorooctanesulfonic acid (PFOS), perfluorooctanoic acid (PFOA), perfluorononanoic acid (PFNA) and perfluorohexanesulfonic acid (PFHxS).
No specific literature was identified that would allow drawing conclusions on the fate of these POPs in carcasses of ungulates after slaughter. Therefore, the CONTAM Panel evaluated the reviews on the sources, characteristics and environmental fate of dioxins, PCBs, PBDEs and PFASs summarised in earlier scientific EFSA opinions (EFSA CONTAM Panel, 2011a,b, 2012a, 2018a,b), and EFSA reports (EFSA, 2010a,b, 2012). The following is an excerpt of these reviews:
The term ‘dioxin’ is commonly used to refer to the two groups of polychlorinated dibenzo‐p‐dioxins (PCDDs) and polychlorinated dibenzofurans (PCDFs). Depending on the number of chlorine atoms and the substitution pattern, a total of 210 different PCDDs/PCDFs, termed congeners, can be distinguished. Dioxins have never been produced on an industrial scale and have no technological use. They are formed unintentionally in a number of industrial and thermal processes, and thus are typical environmental contaminants that have found a global distribution. Dioxins are highly resistant to acids and bases, possess a low vapour pressure and are thermally stable below 600°C. They are poorly soluble in water but highly soluble in lipids. Once released into the environment, dioxins adhere to soil and sediment particles. Although it was shown that lower chlorinated dioxins can be degraded by aerobic bacteria, and higher chlorinated dioxins are known to be reductively dechlorinated in anaerobic sediments, dioxins are only poorly degradable in the environment.
Due to their lipophilic properties and poor degradation, the congeners with 2,3,7,8‐positions chlorinated are stored in fatty tissues and accumulate in the food chain. Because of the lipophilic properties and the high accumulation potential, products of animal origin are of special importance.
PCBs are a group of organochlorine substances that consists of 209 different compounds, also termed ‘congeners’. In contrast to dioxins, PCBs were widely used in numerous open and closed applications, generally in the form of complex technical mixtures. They were produced with an estimated total world production of 1.2–1.5 million tonnes between 1929 and the end of the 1970s, when their production was abandoned in the majority of countries due to their high persistence in the environment and biota. As a result of their widespread use, leakages, improper disposal practices and persistence, PCBs (like dioxins) also have found a global distribution in the environment. Some of the congeners are poorly degraded and, due to their lipophilic properties, they are bioaccumulated in the food chain and stored in fatty tissues.
Based on structural characteristics and toxicological effects, PCBs can be divided into two groups. One group consists of 12 congeners that easily can adopt a coplanar structure and show toxicological properties similar to dioxins. This group is therefore called ‘dioxin‐like PCBs’ (DL‐PCBs). The other PCBs do not show dioxin‐like toxicity and have a different toxicological profile. This group is termed ‘non dioxin‐like PCBs’ (NDL‐PCBs).
Because of the toxicological similarity between dioxins and DL‐PCBs, these two groups are generally considered together in risk assessment. Dioxins and DL‐PCBs are not generated or released into the environment as single compounds but as complex mixtures with varying composition dependent on the respective source. However, since they have different toxic potencies, an estimation of the toxic potential cannot be performed by summing up the concentrations of the analysed congeners. In order to compare the toxicity of a mixture of dioxins and/or DL‐PCB congeners, the concept of toxic equivalents (TEQs) based on different toxic equivalency factors (TEFs) for the relevant congeners was introduced. The TEQs are also the basis for the legal limits established in the EU for dioxins and DL‐PCBs in food and feed.
Within the group of NDL‐PCBs, six congeners (28, 52, 101, 138, 153, 180) are considered as indicators of the different PCB patterns in various sample types, not based on their toxicology which is lower compared to the DL‐PCBs. The sum of these six NDL‐PCBs are the basis for the legal limits established in the EU for NDL‐PCBs. Due to the lower toxicological properties compared to DL‐PCBs, NDL‐PCBs were ranked by the CONTAM Panel (EFSA BIOHAZ, CONTAM, AHAW Panel, 2011; EFSA BIOHAZ Panel, 2013a,c) as medium potential concern.
PBDEs are additive flame retardants which are applied in plastics, textiles, electronic castings and circuitry. As mentioned before, PBDEs were considered by the CONTAM Panel in its previous opinion as medium potential concern in swine. PBDEs are ubiquitously present in the environment and likewise in biota, and in food and feed. There are 209 possible compounds, referred to as congeners. Eight congeners were considered by the CONTAM Panel (2011a) to be of primary interest: BDE‐28, ‐47, ‐99, ‐100, ‐153, ‐154, ‐183 and ‐209. These congeners are therefore generally analysed, especially in samples of animal origin. The PBDEs have low vapour pressure, are poorly soluble in water but highly soluble in lipids. They are resistant to microbial degradation, bioaccumulate in the food chain and are stored in fatty tissues. Based on the physico‐chemical and reactivity properties, PBDEs are often generalised as persistent even though individual PBDE congeners show differences in degrees of persistency, due to their structures.
PFASs are organofluorine compounds consisting of a hydrophobic alkyl chain of varying length (typically C4–C16) and a hydrophilic end‐group. The chemical resistance, surface tension lowering properties and the ability to create stable foams have made PFASs extremely versatile. Since the 1940s, PFASs have been produced and used in numerous commercial and industrial applications, including textile, carpet and leather treatment (water and dirt proofing), surfactants, firefighting foams, metal plating and paper grease‐proofing treatments.
PFOS and PFOA are the best studied compounds, and are often considered as indicator compounds for the group of PFASs. PFOS (eight perfluorinated carbons) and PFOA (seven perfluorinated carbons) both have an anionic end‐group and belong to, respectively, the perfluoroalkyl sulfonic acids (PFSAs) and the perfluoroalkyl carboxylic acids (PFCAs). Many PFASs are considered to be potential precursors of PFSAs and PFCAs, and these precursors are usually not environmentally persistent, but may be transformed in the environment among others through biodegradation to inter alia PFOS and PFOA. Due to the strong covalent C‐F bond, PFOS and PFOA are highly resistant to physical and microbiological degradation, and are thus extremely persistent in the environment and biota. The widespread use of PFOS, PFOA and their precursors, in combination with their persistence, has resulted in a widespread contamination of the environment. PFOS and PFOA can be released to the environment at various stages of production, through product use and as a result of disposal of the products at the end of their life. They are ubiquitous and due to bioaccumulation are found in a variety of compartments, including wildlife, farm animals and humans.
Conclusion on POPs
The CONTAM Panel evaluated the physico‐chemical properties of dioxins, PCBs, PBDEs, and PFASs, and concluded that based on their stability, poor degradation and persistence, a delayed meat inspection of 24 or 72 h would not result in a change of concentration of these POPs in carcasses. Therefore, the CONTAM Panel concluded that there is no impact of a delayed PMI of 24 or 72 h on the effectiveness to detect POPs.
3.7.3. Metals
Among the chemical elements, metals traditionally have gained attention as contaminants in animal tissues, as they may accumulate in certain organs, particularly in kidneys and liver over the lifespan of an animal. Exposure of animals is commonly related to contaminated feed materials and soil ingestion.
Commission Regulation (EC) No 1881/2006 laid down MLs of lead and cadmium in meat and offal from ungulates and Regulation (EC) No 396/2005 laid down MRLs of mercury (Hg) in food of animal origin. In the remit of this opinion, cadmium, lead and mercury are of special importance. These three metals were identified in earlier opinions on meat inspection as compounds of medium potential concern in swine, cattle, sheep and goats, and cadmium as compound of medium potential concern in farmed game (EFSA BIOHAZ, CONTAM, AHAW Panel, 2011; EFSA BIOHAZ Panel, 2013a,b,c). The metals are predominantly stored in kidney and to a lesser extent in liver, which are thus both the target tissues in PMI for detection of chemical elements. As no specific literature could be identified on the fate and stability of metals in carcasses after slaughter, the working group evaluated the relevant information published in the CONTAM Panel opinions on the respective metals (EFSA CONTAM Panel, 2009, 2010, 2012b).
Conclusion on metals
Due to the poor water solubility, resistance against microbial degradation and high persistence, the CONTAM Panel concluded that a delayed meat inspection of 24 or 72 h would not result in a concentration change of the metals cadmium, lead and mercury in carcasses or tissues taken from these carcasses. Therefore, the CONTAM Panel concluded that there is no impact of a delayed PMI of 24 or 72 h on the effectiveness to detect cadmium, lead and mercury.
3.7.4. Ochratoxin A
Ochratoxin A (OTA) is a mycotoxin produced by various fungi of the genus Aspergillus and Penicillium e.g. A. ochraceus,A. carbonarius and P. verrucosum. OTA can be present in feed used for food‐producing animals and may accumulate in the meat and organs of some monogastric farm animals, such as pigs, pre‐ruminant calves and rabbits. The CONTAM Panel identified OTA as medium potential concern substance in swine in the previous assessment (EFSA BIOHAZ, CONTAM, AHAW Panel, 2011).
Ochratoxin is recommended to be included in the NRCP as a minimum requirement and the matrices to be used are kidney, blood and edible offal (EURLs, 2019). No MLs for OTA in food of animal origin are laid down in the EU legislation.
EFSA assessed the risk related to the presence of OTA in food in 2006 and 2020 (EFSA 2006; EFSA CONTAM Panel, 2020b). The current assessment is based on these opinions and Appendix E summarises the relevant information available in these opinions.
In 2020, the CONTAM Panel concluded ‘OTA is a cumulative mycotoxin with relatively rapid absorption and distribution but slow elimination and excretion, which is mostly due to its high extent of binding to plasma proteins in particular albumin and its low rate of metabolism. Both faecal and urinary excretion are important for the plasma clearance of OTA. Plasma half‐life range from several days in rodents and pigs to several weeks in humans and non‐human primates.’ As shown in Appendix E, the plasma half‐life in pigs is 6 days.
Because of its high bioavailability and long half‐life in some monogastric farm animals, such as pigs and preruminant calves, OTA may accumulate in the meat and organs of these animals exposed via the feed (Battacone et al., 2010; Duarte et al., 2011). Consequently, OTA may be found in serum, kidney, liver and muscle, with the highest concentrations normally determined in the kidney (Battacone et al., 2010).
Whether OTA is detectable after a delayed PMI depends on the concentration at slaughter and post‐mortem degradation. There is large uncertainty regarding these parameters, and therefore, the assessment of the impact of a delayed PMI of 24 or 72 h on the effectiveness to detect OTA is inconclusive.
3.7.5. Stilbenes
Stilbenes (i.e. diethylstilbestrol (DES), dienestrol (DE) and hexestrol (HEX)) are synthetic nonsteroidal oestrogens that were especially used between the 1940s and 1970s in human medicine, e.g. to help maintain pregnancy and in the treatment of prostate cancer, but were removed from these uses due to their carcinogenic and genotoxic properties. As significant improvements in weight gain and feed conversion ratios following the administration of DES to farm animals were observed, this compound became a widely used livestock growth stimulant in the 1970s, especially in animals with low endogenous sexual hormone levels, such as steers and veal calves. Although not authorised for use in food producing animals in the EU since the 1980s, stilbenes were occasionally detected in samples analysed in the frame of the NRCPs and thus are still mandatory compounds to be analysed. The stilbenes were ranked by the CONTAM Panel as of medium potential concern in cattle (EFSA BIOHAZ Panel, 2013a). Within the NRCP, the matrices of choice are urine followed by liver (EURL, 2020). Stilbenes are most stable in urine (Sterk, 2019, personal communication). Muscle is not the matrix of choice as the concentrations of residues are very low. For official control purposes, the minimum method performance requirement (MMPR)42 is 0.5 μg/kg for DES and 1 μg/kg for DE and HEX in urine. For liver, the MMPR for all three substances is 1 μg/kg (EURL, 2020). The CONTAM Panel noted that it is possible to take a urine sample at slaughter but not when PMI is delayed for 24 or 72 h, while liver can be sampled at all three time intervals, under the condition that the liver is kept with the carcass after slaughter.
Stilbenes can be administered orally, by intramuscular (i.m.) injection or subcutaneous (s.c.) implantation to farm animals. The route of application determines the rate of absorption and excretion. After oral administration, DES is readily absorbed and distributed in the whole organism. The kinetics of a single oral dose of radiolabelled DES (10 mg) in cattle follows a biphasic depletion curve, attributed to hepatic clearance. An initial steeper slope represents a biological half‐life of 17 h, while the half‐life for the later phase is 5.5 days. In contrast, pellets of 24–36 mg DES implanted s.c. in cattle or steers resulted in a systematic release of about 56–74 μg of DES per day. The half‐life was estimated to be 80–90 days. The fraction of the stilbenes eliminated in urine is in the conjugated form as a glucuronide, while the fraction appearing in the faeces is in the free form (IARC, 2012).
Aschbacher (1976) investigated the distribution of 14C at various time intervals after a single oral dose of 14C‐DES was administered to steers. After 24, 48 and 72 h from dosing to sacrifice, 21.7%, 52.8% and 72.75% of 14C were measured in faeces. The respective recoveries in urine were 7.98%, 14.65 and 22.30%, indicating that faeces and urine accounted for the greatest amount of 14C eliminated by ruminants after oral 14C‐DES gavage. In contrast, the 14C recoveries in organs and carcasses after 24, 48 and 72 h from dosing to sacrifice were determined as 0.96%, 0.29% and 0.26%.
Wozniak (1998) investigated the stability of DES in frozen spiked meat samples depending on storage at 2, 4 and 6 months at −20°C. DES showed reductions during this time by 14.0–21.7%.
In summary, the CONTAM Panel noted that it is not possible to take a urine sample when PMI is delayed for 24 or 72 h. Considering that the preferred matrix, urine, cannot be used anymore for analysis, the liver has to be sampled for control purposes in case of delayed PMI. No information regarding the stability in liver post‐mortem is available. However, in spiked meat samples stored at −20°C reductions are observed. This shows that degradation is occurring even if the specific experiment was using a different matrix, temperature and times. Therefore, a delayed PMI of 24 or 72 h could reduce effectiveness to detect residues of illegal stilbene application if the concentration drops below the required level of sensitivity. However, the extent of degradation in the liver cannot be predicted based on the available information.
3.7.6. Thyrostats
Thyrostats are pharmacologically active substances that affect the functioning of the thyroid glands through the inhibition of the production of the hormones triiodothyronine (T3) and thyroxine (T4). Synthetic thyrostats include thiouracil, methylthiouracil (MTU), propylthiouracil, methimazole (tapazol®, methylmercaptoimidazole) and mercaptobenzimidazole (MBI). In livestock, the administration of thyrostats results in a considerable live weight gain, mainly caused by increased water retention in edible tissue and forced augmented filling of the gastrointestinal tract (Vanden Bussche et al., 2012). Due to their adverse health effects, administration of thyrostats to livestock for fattening purposes was banned in the EU by Council Directive 81/602/EEC43 since 1981. An evaluation of the outcome of the NRCP for cattle, sheep and goats indicates that in the group of thyrostats (thiouracil, MTU, propylthiouracil, phenylthiouracil and methimazole), the non‐compliant samples predominately relate to thiouracil. In this respect, it should be mentioned that several Member States claimed that the presence of thiouracil was not due to illegal treatment but was caused by feed containing cruciferous plants (EFSA BIOHAZ Panel, 2013a). Pinel et al. (2006) demonstrated a relationship between a diet based on cruciferous vegetables and the occurrence of thiouracil in urine of bovines. Vanden Bussche et al. (2011) showed that upon hydrolysis thiouracil could be detected in samples of rapeseed, rapeseed cake, broccoli and cauliflower.
The matrices of choice for the determination of thyrostats in the frame of the NRCPs are urine and thyroid gland, of which the analysis in urine generally predominates. The MMPR is 10 μg/kg for thiouracil, MTU, propylthiouracil and methimazole (EURL, 2020). The CONTAM Panel noted that it is possible to take a urine sample at slaughter but not when PMI is delayed for 24 or 72 h, while thyroid gland can be sampled at all three time intervals under the condition that the thyroid gland is kept with the carcass after slaughter.
Heeremans et al. (1998) investigated the elimination of MTU in cows after oral ingestion of a single dose of 4 g. Maximum MTU levels were found 4–8 h after ingestion in urine, plasma and milk. The concentration of MTU in urine was 50–100 times that in plasma and 250–500 times that in milk. The MTU levels showed a very rapid and parallel decline as a function of time in the first 80 h after treatment. The half‐life of MTU in urine and plasma was estimated as 5.7 and 4.8 days after single ingestion, respectively.
In a further experiment, the authors studied the elimination of MTU in cows after ingestion of daily doses of 5 g for several weeks. After 2, 3 or 4 weeks of MTU application, the levels in urine and plasma were found to be two to three times higher than the maximum concentration observed after ingestion of a single dose. After withdrawal of MTU, the data indicated that there are at least two phases in the disappearance of MTU from plasma or urine. In the first 4–5 days after stopping MTU treatment, the MTU levels in plasma and urine declined rapidly, and then the elimination rate decreased by a factor of 20 so that, after a withdrawal period of more than 60 days, still distinct levels of MTU (≥ 10 μg/L) were found in urine or plasma. During that period the residue levels in urine were approximately five times higher than those in the corresponding plasma samples which explain the choice of urine as the preferred matrix as compared to plasma for analysis of thyrostats. The half‐life of MTU in urine and plasma was estimated to be 10 days for the first phase and 12 days for the second phase (Heeremans et al., 1998).
Vanden Bussche et al. (2012) investigated the stability of thyrostats (thiouracil, methylthiouracil, ethylthiouracil, propylthiouracil, phenylthiouracil, methimazole and mercaptobenzimidazole) in urine. Spiked urine samples were stored at −70°C and each sample underwent one, two, three or four freeze–thaw cycles with a defrosting period of 3 h during 4 consecutive days. A significant decrease in thyrostat concentration (up to 97%) was observed with increasing number of freeze–thaw cycles. The losses were more pronounced in bovine than in porcine urine. In addition, a matrix effect of urine was observed, since the concentration in water treated in the same way as a control remained stable. In a further experiment, bovine urine was spiked with thyrostats and kept at room temperature. Each hour, one aliquot was analysed and a concentration decrease of 6–12.2% per h was observed. The effect of daylight, boiling and salt addition was not found to be significant. Addition of copper (II) caused losses of more 50–80%. In contrast, the analytes remained stable at pH 1–3, except thiouracil for which losses up to 35% were determined. Thus, the stability of thyrostats in urine can be increased by adjusting the pH to 1 upon sampling and addition of ethylenediaminetetraacetic acid (EDTA) to inhibit the interaction between copper ions and thyrostats.
In summary, the CONTAM Panel noted that it is not possible to take a urine sample when PMI is delayed for 24 or 72 h. Thyrostats are not stable in urine; however, a stabilisation protocol is used to circumvent this issue. No information regarding the stability in the other matrix of choice, the thyroid gland, was identified. Considering that the preferred matrix, urine, cannot be used anymore for analysis, the thyroid gland has to be sampled for control purposes in case of delayed PMI. Given that the extent of degradation in the thyroid gland cannot be predicted based on the available information, the effectiveness of a delayed PMI of 24 or 72 h could be reduced if the concentration drops below the required level of sensitivity. Overall, the Panel cannot conclude on the effectiveness of the PMI when delayed for 24 or 72 h.
3.7.7. Gonadal (sex) steroids
A broad range of endogenous and synthetic steroids derived from oestrogens, androgens and gestagens are available and have been illegally used as growth‐promoting agents in food‐producing animals. Gonadal (sex) steroids are given to animals typically as implants (generally at the base of the ear) or as injections, but may also be administered via the skin as ‘pour‐on’ applications, and for synthetic steroids even orally. Anabolic steroids influence the growth rate and feed conversion efficiency in cattle, with animals responding by increased growth rate and feed conversion efficiency. Anabolic steroids are widely available on the black market, so there is the possibility for illicit use in livestock production (EFSA BIOHAZ Panel, 2013a).
All use of steroids as growth‐promoting agents in food‐producing animals is banned in the EU by Council Directive 81/602/EEC since 1981.
The NRCPs request to analyse ungulate samples on the farm and/or at slaughter for synthetic androgens, such as α‐boldenone, ß‐boldenone, 17α‐methyltestosterone, 17α‐nortestosterone, 17ß‐nortestosterone, 16ß‐hydroxy stanozolol, 17α‐trenbolone and 17ß‐trenbolone. Besides the synthetic oestrogen 17α‐ethinylestradiol, the NRCPs also cover the synthetic gestagens medroxyprogesterone acetate, chlormadinone acetate, megestrol acetate, melengestrol acetate and the natural steroids 17ß‐estradiol, 17ß‐testosterone and progesterone. Within the NRCPs, the matrices of choice for synthetic androgens are urine, followed by liver. For natural steroids and their esters, the matrix of choice is serum and for synthetic gestagens kidney fat (EURL, 2020). To detect illegal administration, the natural steroids are measured in serum/plasma with LC‐MS/MS techniques and compared to their established physiological levels. Due to the rapid blood post‐mortem changes, blood sampling should be performed in living animals. In addition, changes in hormone concentrations upon storage at room temperature (e.g. free‐17ß‐estradiol is reported to increase) make it difficult the comparison with the established physiological levels. Muscle is only used for imports and aquaculture products. The CONTAM Panel noted that it is possible to take a urine and plasma sample at slaughter but not when PMI is delayed for 24 or 72 h, while liver and kidney fat can be sampled at all three time intervals under the condition that the liver and kidney are kept with the carcass after slaughter. The MMPRs differ among the substances, the matrices used and the sex and age of the animal in case of natural steroids. They range from 0.5 to 2 μg/kg for synthetic androgens, from 0.1 to 30 μg/kg for natural steroids and from 1 to 5 μg/kg for synthetic gestagens (EURL, 2020). Hair can be used to detect the illegal administration of steroid esters (Duffy et al., 2009a,b; Stolker et al., 2009; Nielen et al., 2011; Gray et al., 2013; EURL, 2020). While the esters are relatively rapidly hydrolysed, making it difficult to differentiate them from endogenous steroids when analysing urine, the esters can be detected intact in hair for several weeks after treatment. The concentrations of steroid esters in hair are related to the distance of sampling from the point of injection, to the time post‐treatment and to the dose given to the animal (Duffy et al., 2009a). Due to the need to remove possible external contamination, the clean‐up of hair samples is quite complicated. Moreover, it is questionable whether the remaining amount of hair on the head, provided the head is stored when the PMI is delayed for 24 or 72 h, is sufficient to detect the often‐small ester concentrations. Therefore, the use of hair as a routine analysis to detect illegal steroid application was not further considered in this opinion.
Because of the potential occurrence of some of these substances endogenously in cattle and pigs, particularly substances such as α and ß‐boldenone, epinandrolone (especially in pregnant cows) and the natural hormones, it is often difficult to differentiate between endogenous occurrence and illegal treatment, and to establish a reliable estimate for the level of abuse of anabolic steroids in EU livestock production.
The EFSA CONTAM Panel ranked these substances as of medium potential concern in cattle, sheep and goats (EFSA BIOHAZ Panel, 2013a,c).
While there are numerous publications on the release of endogenous and extraneous steroids and their esters, especially from administered implants, and their distribution in livestock, representative data on the fate of these compounds in animal tissues after slaughter are lacking. In general, compounds, applied as esters have an elimination rate that may be delayed by 40–50% compared to the free compound. There are also differences in the type of implanted steroids, kind of esters, time and length of application before slaughter, and in animal species. In addition, it was shown that the elimination rate of a single substance could change when two compounds are administered together. For example, the elimination of 17ß‐estradiol in urine and faeces is significantly delayed and shows a changed elimination pattern when it is administered together with trenbolone‐acetate (Hoffmann and Karg, 1976).
Rattenberger et al. (1987) investigated the elimination of 19‐nortestosterone (19‐NT) in female calves, given 100 mg 19‐NT‐laurate or decanoate i.m. or subcutaneous (s.c.). 19‐NT could be detected up to 65 days after s.c. treatment. While the half‐life in urine of the decanoate ester was around 9 days, the laurate ester revealed a half‐life of 14–16 days. Residues of 1.0, 8.8 and 15.8 mg 19‐NT‐laurate were determined in the injection sites (sample weight: 277, 263 and 186 g, respectively) of three out of four calves on day 73 after the application of the ester.
Fahmi et al. (1985) studied the influence of some sample‐handling factors on progesterone and testosterone analysis in goats. Blood was collected from 23 does and 8 bucks and divided into three sodium fluoride‐potassium oxalate, one heparin and one EDTA tubes and also into a tube without anticoagulant. Plasma from a sodium fluoride‐potassium oxalate tube was separated immediately from the blood cells by centrifugation. Serum or plasma was also separated after storage for 24 h with sodium fluoride‐potassium oxalate, heparin or EDTA anticoagulant at 22°C or with sodium fluoride‐potassium oxalate at 5°C. A significant decline in progesterone levels occurred in samples stored at 22°C with each anticoagulant used and in the serum sample. Samples stored at 5°C for 24 h with sodium fluoride‐potassium oxalate anticoagulant contained concentrations of progesterone which did not differ significantly from those in samples where plasma was removed immediately. Testosterone levels did not change with the anticoagulant used or vary with the storage temperature when sodium fluoride‐potassium oxalate tubes were stored at 5°C and 22°C for 24 h.
Reimers et al. (1991) studied the effects of haemolysis and storage on determination of several hormones in blood samples inter alia from cattle. They reported that storage of bovine blood at 2–4°C and 20–22°C for 18–22 h caused progesterone concentrations to decrease (p < 0.05); the effect was not enhanced or diminished by haemolysis.
Rattenberger (1991) examined serum and plasma separated from blood at different times after sampling (up to 48 h) of a male calf as well as of a bull for testosterone concentrations. The initial testosterone concentrations were 0.25 μg/L and 3.0 μg/L, respectively. An 11% and 15% decrease of serum testosterone concentration in the separation time between 7 and 48 h after blood sampling was observed. In plasma, the testosterone concentrations remained constant over the whole experiment, however, showed to be significantly lower (average of 17%) as in serum. The authors concluded that for a reliable testosterone determination serum should be separated from blood within 2–7 h after sampling, as there is a tendency of decreasing levels after that time period.
Guan et al. (2005) developed an analytical method for the detection of the anabolic steroids testosterone, normethandrolone, nandrolone, boldenone, methandrostenolone, tetrahydrogestrinone, trenbolone and stanozolol in equine plasma. As part of the method validation, they also investigated the stability of these compounds in spiked plasma stored at various temperatures. To assess the stability of the eight anabolic steroids at ambient temperature (25°C), the plasma samples were allowed to remain on the benchtop for 2, 4, 6 and 24 h, spiked with internal standard, extracted and analysed. For assessment of the stability at other temperatures, spiked plasma samples were stored at 4°C for 13 days, and at−20° and −70°C for 34 days, thawed, brought to ambient temperature, spiked with the internal standard, extracted and analysed at different time intervals. The results of the stability tests showed that the concentrations of the eight anabolic steroids were stabile under all storage conditions investigated.
Within their single‐laboratory validation of a method to detect trenbolone residues in bovine liver and muscle, MacNeil et al. (2003) prepared pools of liver and meat, respectively, with incurred trenbolone residues and stored these at −20°C. In order to investigate the analyte stability, duplicate test portions from the liver pools were analysed at 0, 7, 12 and 27 weeks and from the muscle pools at 0, 6, 12 and 28 weeks of storage, respectively. The authors report that the data did not demonstrate any significant loss of trenbolone over a period of up to 28 weeks during storage at –20°C.
Summary
For natural sex steroids, the matrix of choice is serum. Research has shown that the serum should be separated from blood within 2–7 h after sampling, which should be performed in living animals. Such a procedure is not possible when PMI is delayed for 24 or 72 h. An alternative matrix is muscle; however, muscle is not the matrix of choice due to lower concentrations of the analytes. Therefore, the effectiveness of the PMI to detect illegal use of natural steroids will be reduced when delayed for 24 or 72 h.
For synthetic gestagens, the matrix of choice is kidney fat. The sampling of this matrix is not affected by a delay of the PMI. No information regarding the stability in kidney fat post‐mortem is available. However, enzymatic activity in fat tissue is considered lower compared to liver or muscle tissue. This information provides an indication that no substantial degradation is expected in kidney fat. Therefore, a delayed PMI of 24 or 72 h is not expected to reduce the effectiveness to detect residues of synthetic gestagens application. However, in the absence of stability data in kidney fat of the synthetic gestagens included in the NRCPs, there is uncertainty regarding this conclusion.
For synthetic androgens, the CONTAM Panel noted that it is not possible to take a urine sample when PMI is delayed for 24 or 72 h. Considering that the preferred matrix, urine, cannot be used anymore for analysis, the liver has to be sampled for control purposes in case of delayed PMI. In spiked liver and meat samples stored at −20°C, no reductions of the synthetic androgen trenbolone were observed. However, it is unclear whether a reduction would take place at a higher temperature and this adds to the uncertainty of the assessment. Given that the extent of degradation in the liver cannot be predicted based on the available information, the effectiveness of a delayed PMI of 24 or 72 h could be reduced if the concentration drops below the required level of sensitivity. Overall, the Panel cannot conclude on the effectiveness of the PMI when delayed for 24 or 72 h.
3.7.8. Resorcylic acid lactones
Resorcylic acid lactones are a class of mycotoxins with oestrogenic activity. Compounds that are included in this class are zearalenone (ZEN), zearalanone, zeranol (α‐zearalanol; α‐ZAL), taleranol (β‐zearalanol; β‐ZAL), α‐zearalenol (α‐ZEL) and β‐zearalenol (β‐ZEL).
α‐ZAL is a non‐steroidal oestrogenic growth promoter that is not authorised for use in the EU (Launay et al., 2004). Administration is by s.c. implant in the ear of cattle. Taleranol is the stereoisomer of α‐ZAL. α‐ZAL is metabolised in ZEN and β‐ZAL and all three compounds are excreted both in free and as glucuronide and sulfate conjugates. However, the presence of these compounds can also be the consequence of exposure of the animals to ZEN in feed which is metabolised into α‐ZAL. Several studies have demonstrated (Erasmuson et al., 1994; Kennedy et al., 1995, 1998; Miles et al., 1996) that α‐ZAL can be present in urine and bile from sheep and cattle following exposure to the mycotoxins ZEN and α‐ZEL present in feed. Therefore, simultaneous determination of zeranol, taleranol and the mycotoxins ZEN, α‐ZEL and β‐ZEL was recommended (Kennedy et al., 1998).
The matrices of choice in the NRCP are urine followed by liver (EURL, 2020). Resorcylic acid lactones are most stable in urine (Sterk, 2019, personal communication) The MMPR is 2 μg/kg, except for zeranol in urine and muscle for which the MMPR is 1 μg/kg (EURL, 2020). When both α‐ZAL and ZEN are present, the presence of α‐ZAL is considered as a result of mycotoxin contamination (EURL, 2020). Samples are considered compliant when there is finding of the mycotoxins ZEN and α‐ZEL and ß‐ZEL in addition to α‐ZAL, β‐ZAL and/or zearalanone. The CONTAM Panel noted that it is possible to take a urine sample at slaughter but not when PMI is delayed for 24 or 72 h, while liver can be sampled at all three time intervals under the condition that the liver is kept with the carcass after slaughter. An alternative matrix for α‐ZAL residue testing is bile (Kennedy et al., 1998; Lega et al., 2017).
Depletion studies show that an implant with α‐ZAL gives a peak of the residue levels in liver, kidney, muscle, fat and bile after approximately 5 days followed by a slow decrease until 65 days post‐implantation. Highest concentrations are reported in the bile, followed by liver, kidney, muscle and perirenal fat (FAO/WHO, 1988).
No information was identified regarding the stability of α‐ZAL and its metabolites in samples stored before analysis.
In summary, the CONTAM Panel noted that it is not possible to take a urine sample when PMI is delayed for 24 or 72 h. No information regarding the stability in the other matrix of choice, the liver, nor the alternative matrix, bile, was identified. Considering that the preferred matrix, urine, cannot be used anymore for analysis, the liver or bile has to be sampled for control purposes in case of delayed PMI. Given that the extent of degradation in the liver or bile cannot be predicted based on the available information, the effectiveness of a delayed PMI of 24 or 72 h could be reduced if the concentration drops below the required level of sensitivity. Overall, the Panel cannot conclude on the effectiveness of the PMI when delayed for 24 or 72 h.
3.7.9. β‐agonists
β‐agonists were originally used for smooth muscle relaxation i.e. as bronchodilators in the treatment of pulmonary diseases. They act as agonists to the β‐adrenoreceptors. To tackle the illegal use of β‐agonists as growth promoters (‘repartitioning agents’), Directive 96/22/EC prohibited their use in food‐producing animals. The only exception is clenbuterol, which can be applied under strict veterinary control as tocolytic agent in calving cows and to treat pulmonary diseases in horses. This prohibition is applied to domestic production and is extended to the imports of meat of animals that have been treated with β‐agonists for the purpose of growth promotion. Besides the legal application, some of these substances are illegally administered as growth promoters e.g. clenbuterol, salbutamol and zilpaterol in calves and ractopamine in swine.
There are two main classes of β‐agonists: anilinic β‐agonists (e.g. clenbuterol) and the phenolic β‐agonists (e.g. salbutamol) (O'Keeffe et al., 1999). These classes have a different stability (Polzer, 2020, personal communication).
The EURL guidance (EURL, 2020) indicates that urine, liver and retina are the matrices of choice and particularly the retina since higher residue concentrations can be found for a much longer period. The analysis of complete eyes is the second choice compared to the analysis of retina. In addition, the lung can be used for sampling at the slaughterhouse (EURLs, 2019). The MMPRs differ among the substances and the matrices sampled. In retina, the MMPR ranges from 1 to 5 μg/kg and in liver and urine from 0.1 to 1 μg/kg (EURL, 2020). The CONTAM Panel noted that it is possible to take a urine sample at slaughter but not when PMI is delayed for 24 or 72 h, while retina and liver can be sampled at all three time intervals under the condition that the head and liver are kept with the carcass after slaughter. Hair is also a recommendable matrix for the measurement of residues of β‐agonist application. However, the concentrations found depend on the substance and the colour of the hair. Anilinic ß‐agonists are detected at higher concentrations in hair than in urine, while phenolic ß‐agonists are detected at higher concentrations in urine than in hair. Therefore, it is recommended to analyse hair and urine together (Radeck, 2010; EURL, 2020). Considering that it is not possible to take a urine sample when PMI is delayed for 24 or 72 h, and the limited information which can be obtained by a stand‐alone hair analysis, the use of hair as a matrix was not further considered in this opinion.
3.7.9.1. Clenbuterol
Clenbuterol is well absorbed after oral administration and is widely distributed to the tissues. After treatment, the highest concentrations of residues are found in the liver and kidney compared to the muscle and fat. The residues also deplete the slowest from the liver and kidney (CVMP, 2000).
However, some research papers have shown that the highest residue concentrations of clenbuterol are found in the retina compared to other tissues (including the liver) and that the release from the retina is slow. Meyer and Rinke (1991) reported a half‐life for clenbuterol in eyes of 3–7 days in veal calves. Sauer et al. (1995) did not report a half‐life for clenbuterol in eyes but concluded that concentrations would still be detectable for several weeks after cessation of the treatment.
Pinheiro et al. (2009) studied the stability of clenbuterol during storage of bovine urine and liver samples. Incurred samples were stored at 4, −20 and −60°C. Clenbuterol remained stable at 4°C for at least 12 weeks in urine samples and up to 20 weeks in liver samples. Clenbuterol was also stable for 20 weeks at −20 and −60°C in combination with six consecutive freezing and thawing cycles.
3.7.9.2. Ractopamine
Ractopamine hydrochloride is legally used as a feed additive in pigs and cattle to enhance growth performance and carcass leanness in several non‐EU countries (e.g. USA, Canada, Mexico).
As for clenbuterol, ractopamine has a high affinity for retinal tissue. After 8 days of treatment withdrawal, concentrations up to 90 μg/kg were detected in the retina of pigs treated with 18 μg/kg body weight (bw) per day during 28 days (Vulic et al., 2012).
In 2009, EFSA's Panel on Additives and Products or Substances used in Animal Feed (FEEDAP Panel) performed a safety evaluation of ractopamine. The Panel concluded that ractopamine hydrochloride is rapidly absorbed, distributed and eliminated and 95% of the amount ingested is excreted during the first 3 days. The main route of excretion is via the faeces (about 90% and 55% for pigs and cattle, respectively), with lower amount being excreted in the urine (10% and 45%, respectively) under the form of glucuronides. Significant biliary excretion occurs indicating first‐pass metabolism (EFSA FEEDAP Panel, 2009).
3.7.9.3. Zilpaterol
Zilpaterol hydrochloride is legally used as a veterinary drug in cattle to enhance growth performance and carcass leanness in several non‐EU countries (e.g. USA, Canada, Mexico). It is mixed in the feed at a concentration of 7.5 mg/kg (90% dry matter) to result in an exposure of 0.15 mg/kg bw per day (EFSA, 2016). Withdrawal periods range from 2 to 4 days (FAO/WHO, 2013).
Zilpaterol is readily absorbed and together with its metabolites, the major ones being deisopropylzilpaterol and hydroxy‐zilpaterol, eliminated primarily in the urine (80% in cattle, 85% in swine and 50% in rats). The parent compound is the main compound excreted in the urine (FAO/WHO, 2013).
EFSA performed an assessment of zilpaterol in 2016 and concluded that a withdrawal period of more than 3 days is needed (EFSA et al., 2016).
Radioactive residues were detected in the liver of cattle treated with a single oral dose of 0.2 mg/kg bw [14C]zilpaterol after 8 days. Residues were detected in the kidney, fat or muscle after 1 or 2 days, but not after 8 days (Tulliez, 1992 in FAO/WHO, 2013).
Stachel et al. (2003) reported the detection of zilpaterol in liver, kidney and muscle up to 10 days after cessation of the treatment (0.15 mg/kg bw per day during 25 days) in cattle. However, the detected concentrations were low (≤ 0.03 μg/kg). In pigs, zilpaterol was detected in the liver and kidney up to 4 days after cessation of the treatment and in muscle up to 5 days. The authors reported high concentrations in the retina of pigs (8,062 μg/kg) and cattle (2,448 μg/kg) after a discontinuation of the treatment of 5 and 10 days, respectively, and concluded that retina is the recommended target matrix for the control of this drug which is prohibited in the EU.
3.7.9.4. Summary on β‐agonists
The CONTAM Panel noted that it is not possible to take a urine sample when PMI is delayed for 24 or 72 h. However, the preferred matrix of choice for PMI at slaughterhouse is retina because high residue concentrations can be found for a longer period. The sampling of this matrix is not affected by a delay of the PMI under the condition that the head is kept after slaughter. Therefore, the effectiveness to detect residues of β‐agonist application during a delayed PMI of 24 or 72 h is not reduced when retina is sampled.
In addition, the liver is a matrix of choice. Clenbuterol is stable at 4, ‐20 and ‐60°C for up to 20 weeks in incurred liver samples. However, the stability of anilinic and phenolic β‐agonists is different, and therefore, the assessment of the impact of a delayed PMI of 24 or 72 h on the effectiveness to detect residues of β‐agonist application when liver is sampled is inconclusive.
No information regarding the stability in the lung could be identified. Due to the large uncertainty regarding stability of residues of β‐agonists, the assessment of the impact of a delayed PMI of 24 or 72 h on the effectiveness to detect residues of β‐agonist application when lung is sampled is inconclusive.
3.7.10. Chloramphenicol
Chloramphenicol is a broad‐spectrum antimicrobial agent that is not authorised for use in food‐producing animals in the European Union (EU). EFSA assessed the risk related to the presence of chloramphenicol in food and feed in 2014 (EFSA CONTAM Panel, 2014). The current assessment is based on that opinion. Appendix E summarises the relevant information available in EFSA CONTAM Panel (2014).
In the context of the NRCPs the relevant matrices for chloramphenicol to be taken from ungulates at the slaughterhouse are muscle and urine (EURL, 2020). The MMPR is the reference point for action (RPA) of 0.3 μg/kg. However, from November 2022, an RPA of 0.15 μg/kg will be in force (Regulation (EU) 2019/187144) The CONTAM Panel noted that it is possible to take a urine sample at slaughter but not when PMI is delayed for 24 or 72 h, while muscle can be sampled at all three time intervals.
In general, chloramphenicol is widely distributed in tissues following different routes of exposure (i.e. oral, intravascular (i.v.), i.m., s.c. or intramammary injection) and half‐lives in blood/serum/plasma and tissues like liver, kidney and muscle are typically below 24 h. Information on the metabolites formed by ruminants and in particular the ruminal flora, is limited. No information was available on farmed game, but instead data on domestic species were used. In pigs, residues of the parent drug and its main metabolites (chloramphenicol base and chloramphenicol glucuronide) are slowly depleted and have been detected in the μg/kg range several days after cessation of treatment in muscle, liver and fat.
Chloramphenicol is rapidly metabolised due to oxidation catalysed by cytochrome P450 (CYP) and phase II glucuronidation. Due to the extensive post‐mortem metabolism, a decrease of the chloramphenicol concentration in spiked liver and kidney is observed with time. Therefore, the liver and kidney are considered to be unsuitable matrices for the monitoring of chloramphenicol residues (Sanders et al., 1988). However, these matrices can be used when piperonyl butoxide, a CYP inhibitor, is used or when the samples were cut into cubes and cooled at ‐20°C just after slaughter (Parker and Shaw, 1988; Sanders et al., 1988; Cooper et al., 1998).
In summary, the CONTAM Panel noted that it is not possible to take a urine sample when PMI is delayed for 24 or 72 h. Considering that urine cannot be used anymore for analysis, the muscle has to be sampled for control purposes in case of delayed PMI. No information regarding the stability in muscle post‐mortem is available. Due to the large uncertainty regarding stability of residues of CAP in muscle, the assessment of the impact of a delayed PMI of 24 or 72 h on the effectiveness to detect residues of CAP application when muscle is sampled is inconclusive.
3.7.11. Nitrofurans
Nitrofurans (i.e. furazolidone, furaltadone, nitrofurantoin, nitrofurazone and nifursol) are broad‐spectrum antibiotics that are not authorised for use in food‐producing animals in the EU. EFSA assessed the risk related to the presence of nitrofurans and their metabolites in food in 2015 (EFSA CONTAM Panel, 2015). The current assessment is based on that opinion. Appendix E summarises the relevant information available in EFSA CONTAM Panel (2015).
In the context of the NRCPs, the relevant matrix for nitrofurans to be taken from ungulates at the slaughterhouse is muscle (EURL, 2020). The MMPR is the RPA of 1 μg/kg. However, from November 2022, an RPA of 0.5 μg/kg will be in force (Regulation (EU) 2019/1871).
Nitrofurans contain a nitrofuran ring, which is coupled to a side chain. The side chains differ for the various nitrofurans, being 3‐amino‐2‐oxazolidinone (AOZ) for furazolidone, 3‐amino‐5‐methylmorpholino‐2‐oxazolidinone (AMOZ) for furaltadone, 1‐aminohydantoin (AHD) for nitrofurantoin, semicarbazide (SEM) for nitrofurazone and 3,5‐dinitrosalicylic acid hydrazide (DNSH) for nifursol. Nitrofurans have short half‐lives in animals and therefore the parent compounds do not occur generally as residues in foods of animal origin. Reactive metabolites are able to bind covalently to tissue macromolecules, including proteins, have relatively long half‐lives in food‐producing animals, and are persisting for several weeks in edible tissues (Vroomen Louis et al., 1986; Hoogenboom et al., 1991, 1992; Vass et al., 2008). Under acidic conditions, side chains can be released from these protein‐bound metabolites, namely AOZ, AMOZ, AHD, SEM and DNSH in the case of furazolidone, furaltadone, nitrofurantoin, nitrofurazone and nifursol, respectively. These side chains are therefore used as marker metabolites for the detection of illicit use of nitrofurans in food‐producing animals.
In summary, the relevant matrix for sampling at the slaughterhouse is muscle. The sampling of this matrix is not affected by a delay of the PMI. Protein‐bound metabolites of nitrofurans and therefore releasable marker metabolites persist for several weeks in edible tissues. Therefore, the CONTAM Panel concluded that there is no impact of a delayed PMI of 24 or 72 h on the effectiveness to detect nitrofurans.
3.7.12. Nitroimidazoles
Nitroimidazoles are used for the prevention and treatment of infections with protozoa (e.g. histomoniasis (blackhead) in turkeys and trichomoniasis in pigeons) and treatment of swine dysentery. They are not authorised for use in food‐producing animals in the EU. Examples of nitroimidazoles illegally used for treatment of food‐producing animals are dimetridazole, metronidazole, ronidazole and ipronidazole. Only limited information on ungulates was available and the text below describes the identified information including those for poultry.
For nitroimidazoles, the matrices of choice to be taken from ungulates at the slaughterhouse are plasma/serum, muscle and retina. The MMPR is 1 μg/kg for all matrices, except retina for which no MMPR has been defined (EURL, 2020). The CONTAM Panel noted that it is possible to take a plasma or serum sample at slaughter but not when PMI is delayed for 24 or 72 h, while retina and muscle can be sampled at all three time intervals; the retina only under the condition that the head is kept with the carcass after slaughter.
Dimetridazole is metabolised into 2‐hydroxymethyl‐1‐methyl‐5‐nitroimidazole (HMMNI), the predominant metabolite found in urine of pigs and turkeys (CVMP, 1996a). For metronidazole, the main metabolite is 1‐(2‐hydroxyethyl)‐2‐hydroxymethyl‐5‐nitroimidazole (MNZOH) (Pan et al., 2017). The hydroxy metabolites of ronidazole and ipronidazole are HMMNI and 1‐methyl‐2‐(2’‐hydroxyisopropyl)‐5‐nitroimidazole (IPZOH). Polzer et al. (2004) have shown that HMMNI and IPZOH are the relevant target analytes for residue control in turkeys treated with dimetridazole and ipronidazole, while the parent compounds are the most relevant analyte for residue control in turkeys treated with metronidazole or ronidazole.
Storage conditions influence the stability of nitroimidazole residues in incurred muscle samples from turkeys. For ronidazole‐treated turkeys, the mean concentration of ronidazole in the muscle samples was reduced by 74% when stored at 4°C for 24 h instead of direct freezing. For ipronidazole‐treated turkeys, the reduction of IPZOH was 32%. In addition, the analytes are not homogeneously distributed in muscle samples and it is recommended to use lyophilisation for an effective residue control (Polzer and Gowik, 2005).
In swine, residues of dimetridazole deplete rapidly during 24 h to levels below 1 μg/kg (Newkirk et al., 1990). These results were confirmed in a study by Carignan et al. (1988). The concentration of HMMNI was measured in plasma, muscle, kidney and liver of pigs exposed to medicated feed (125 mg/kg). A withdrawal period of 25 h resulted in a concentration of 0.85 μg/kg in muscle and no detectable level in kidney and liver. After 49 h, the concentration in the muscle was below 0.5 μg/kg. In the liver, even after 2 h, the concentration was below 1 μg/kg.
In pigeons, the half‐lives of dimetridazole and HMMNI following i.v. administration were 3.9 h and 12.14 h, respectively. Following oral administration, the half‐lives of dimetridazole and HMMNI were 3.09 and 10.5 h in non‐fed pigeons, and 3.78 and 10.57 h in pigeons that received feed 1 h before dimetridazole was administered (Inghelbrecht et al., 1996).
EMEA reported that dimetridazole and HMMNI were detected in skin/fat of pigs until 9 days and in turkeys until 12 days after treatment at the therapeutic dosage regimen (CVMP, 1996a).
In a depletion study in pigs and broilers, metronidazole was the predominant residue in liver, kidney and muscle and was detected up to 14 days after withdrawal of the treatment (decision limit (CCα)39 ranged from 0.5 to 1 μg/kg and detection capability (CCβ)40 from 1 to 5 μg/kg in the tissues of pigs and broilers). The elimination half‐lives for metronidazole for liver, kidney and muscle ranged from 1.9 to 2.3 days in pigs and broilers, and for MNZ‐OH from 1.5 to 1.9 days (Pan et al., 2017). The elimination half‐life of metronidazole in plasma following i.v. administration in turkeys was 3–6 h, and 4–7 h for the hydroxy‐metabolite. Following oral administration, similar elimination half‐lives (3–6 h) were obtained for both compounds (Switala et al., 2016).
Ronidzaole is extensively metabolised and its metabolites are rapidly eliminated (CVMP, 1996b). A residue depletion study in turkeys has shown that residues of ronidazole were not detectable anymore (LOD = 20–40 μg/kg) after 5 days of withdrawal. In pigs, the residues were not detectable (LOD = 2 μg/kg) after 2 days of withdrawal (FAO/WHO, 1994). In pigeons, the half‐life of ronidazole was 1–2 h (with previous feeding) and 15–29 h (without previous feeding) following oral administration (Herman et al., 1989).
In summary, the CONTAM Panel noted that it is not possible to take a serum/plasma sample when PMI is delayed for 24 or 72 h. Consequently, the muscle or retina has to be sampled for control purposes in case of delayed PMI. It should be noted that the latter can only be sampled when the head is kept after slaughter.
No information regarding the post‐mortem stability of nitroimidazoles in retina sampled from ungulates is available. Due to the large uncertainty regarding stability of residues of nitroimidazoles in retina samples from ungulates, the assessment of the impact of a delayed PMI of 24 or 72 h on the effectiveness to detect residues of nitroimidazole application when retina is sampled is inconclusive.
No information regarding the post‐mortem stability of nitroimidazoles in muscle tissue sampled from ungulates is available. In poultry, a degradation of nitroimidazoles takes place in muscle when stored at 4°C for 24 h. In addition, studies in pigs show that nitroimidazoles can be rapidly metabolised. This information indicates that degradation may occur post‐mortem although the specific experiments were conducted under different circumstances than applicable under delayed PMI of ungulates. The CONTAM Panel concluded that a delayed PMI of 24 or 72 h is expected to reduce the effectiveness to detect residues of nitroimidazole application if the concentration drops below the required level of sensitivity. However, the extent of degradation cannot be predicted based on the available information and there is uncertainty due to the limitations (i.e. information from poultry and depletion studies) of the used information.
3.7.13. Phenylbutazone
Phenylbutazone is a non‐steroidal anti‐inflammatory drug (NSAID) that is not authorised for use in food‐producing animals in the EU following an evaluation by the Committee for Medicinal Products for Veterinary Use (CVMP) in 1997.
The matrices of choice to be taken from ungulates at the slaughterhouse are muscle, followed by kidney, liver and plasma. The MMPR is 5 μg/kg (EURL, 2020). The CONTAM Panel noted that it is possible to take a plasma sample at slaughter but not when PMI is delayed for 24 or 72 h, while muscle, kidney and liver can be sampled at all three time intervals; the kidney and liver only under the condition that they are kept with the carcass after slaughter.
Phenylbutazone is analysed together with other NSAIDs using multiresidue methods (e.g. Van Hoof et al., 2004; Gentili et al., 2012). Since several NSAIDs have an MRL in muscle, this matrix is most frequently sampled for testing of phenylbutazone residues in official control. For analysis of phenylbutazone only, plasma is a suitable matrix given the higher concentration compared to muscle (Polzer, 2020, personal communication).
Phenylbutazone is rapidly and almost completely absorbed following oral exposure. The elimination half‐life in most laboratory animals, pigs and horses is around 5–8 h. Longer elimination half‐lives have been reported for goats (15 h) and cattle (37–66 h). Phenylbutazone binds strongly to plasma proteins (CVMP, 1997). The CVMP indicated that there is evidence of rapid phenylbutazone degradation in muscle tissue homogenates.
Lees and Toutain (2013) reviewed available data on pharmacokinetics and depletion of phenylbutazone and concluded that, inter alia in horses, muscle concentrations are systematically lower than plasma concentrations by at least one order of magnitude. Given the strong binding to proteins, the general distribution is limited.
The principal metabolites are oxyphenbutazone and γ‐hydroxyphenylbutazone and the concentrations of these metabolites in urine are higher than in plasma (Lees and Toutain, 2013).
In summary, there is evidence of rapid phenylbutazone degradation in muscle tissue homogenates. However, this degradation may be due to the activation of enzymes during the homogenisation. No information regarding the stability in kidney and liver was identified. Due to the large uncertainty regarding the stability of residues of phenylbutazone in muscle, kidney and liver samples, the assessment of the impact of a delayed PMI of 24 or 72 h on the effectiveness to detect residues of phenylbutazone application when these matrices are sampled is inconclusive.
3.7.14. Uncertainty analysis
The evaluation of the inherent uncertainties in this assessment for chemical residues and contaminants has been performed following the guidance on uncertainty analysis in scientific assessments (EFSA Scientific Committee, 2018). The uncertainties were assessed for all substances under evaluation and the identified uncertainties are presented in Appendix F.
No uncertainties were identified for POPs, metals, natural steroids and nitrofurans. For the other substances, the extent of the potential degradation that may occur after slaughter and before sampling was considered to be an important source of uncertainty. Degradation of the residues may result in a non‐detectable concentration while a non‐compliant result would have been reported if sampling would have been done immediately after slaughter. This depends also on the concentration at the moment of slaughter; when a high concentration of residues is present at slaughter, the residues may still be detected despite some degradation, while a low concentration of residues at slaughter may not be detectable anymore due to degradation.
4. Conclusions
To address the different parts of the ToR, the conclusions have been formulated as answers to the following assessment questions:
Assessment question 1: Is the sensitivity of detection of animal diseases listed according to Article 5 of Regulation (EC) No 2016/429, of cysticercosis in domestic bovine animals and Suidae, and of conditions such as septicaemia, pyaemia, toxaemia or viraemia reduced if the PMI is delayed by 24 or 72 h after slaughter/arrival in the game‐handling establishment?
Additionally, in the case of bovine tuberculosis, is the performance of the laboratory diagnostic tests for confirmation of tuberculosis in animals with detectable suspect lesions reduced if the PMI is delayed by 24 or 72 h after slaughter or arrival in the game‐handling establishment?
-
•
The main targets of this assessment were ungulates subclinically infected with the diseases listed according to Article 5 of Regulation (EU) 2016/429 including Regulation (EU) 2018/1629 that enter the slaughterhouse, pass the AMI, are then slaughtered and may present detectable lesions at PMI. In particular, the following cases were excluded from the assessment:
-
o
Disease cases with acute or subacute forms with detectable clinical signs, which are mainly detected and/or diagnosed at farm level and not at the slaughterhouse;
-
o
Diseases whose target organs and related lesions are not screened/observed at the slaughterhouse, in particular those inducing lesions in the brain, e.g. rabies;
-
o
Suspect animals detected in the frame of official active surveillance programmes (as for some diseases listed under according to article 5 of Regulation (EC) 2016/429), for which there should not be any delay in PMI.
-
o
For certain diseases listed according to article 5 of Regulation (EC) 2016/429, subclinical/asymptomatic forms have been described that might not be detected at AMI and therefore animals could be sent to the slaughterhouse where accurate PMI is required to detect infection (e.g. bovine tuberculosis).
-
•
According to the results of the present assessment, the ability to detect the diseases listed under article 5 of the AHL Regulation according to article 5 of Regulation (EC) 2016/429, if PMI is delayed, is expected to decrease. This reduction in sensitivity is highly variable and depends on the type of lesions; for example more than 80 affected carcasses out of 100 would still be detectable after either a 24‐ or 72‐h delay for tuberculosis (with certainty greater than 95% but only between 36 and 97 after a 72‐h delay for RVF (with 90% certainty).
-
•
At 24‐h delayed PMI, the only diseases for which there was less than 95% certainty of being able to detect at least 50 out of 100 affected carcasses with the current procedure, are FMD in wild boars and surra.
-
•
At 72‐h delayed PMI, diseases for which there was less than 95% certainty of being able to detect at least 50 affected carcasses are RVF, surra, FMD, West Nile fever, African Swine Fever and African horse sickness.
-
•
For diseases for which the diagnosis at the slaughterhouse is most important, i.e. tuberculosis, echinococcosis, cysticercosis and pyaemia, the estimated mean number of affected carcasses, out of 100 detected with the current procedure, that would be still detectable with a 90% certainty is:
-
o
After a 24‐h delay in the PMI, between 83 and 100 (median = 95) for tuberculosis, echinococcosis and cysticercosis, and between 53 and 99 (median = 86) for pyaemia.
-
o
After a 72‐h delay in the PMI, between 83 and 98 (median = 92) for tuberculosis, between 72 and 99 (median = 92) for cysticercosis and echinococcosis and between 47 and 94 (median = 76) for pyaemia.
-
o
-
•
For chronic type lesions, including those due to tuberculosis, a 24‐ or 72‐h delay in PMI does not lead to a reduction in detectability.
-
•
Concerning tuberculosis, the overall effect of a delay in the PMI on the ability to confirm infection in animals is the result of a combination of the effect of such delay on the ability to detect compatible lesions during the PMI, and on the performance of the laboratory tests used to confirm the infection. There is considerable uncertainty about the impact of delayed inspection and testing due to the lack of available data. Nevertheless, it was concluded that a 24‐h delay could result in the confirmation of between 73 and 100 animals out of 100 confirmed with the current procedure, depending on the diagnostic test used. A 72‐h delay could result in the confirmation of between 74 (direct PCR), 65 (culture) and 61 (histopathology) and 100 affected animals out of every 100 animals confirmed using the current procedure.
-
•
The sources of uncertainty that could explain the width of the elicited distribution of estimates related to this first assessment question were:
-
o
Uncertainty on the severity, distribution and type of lesions that may be observed in subclinical and/or asymptomatic infected animals, and
-
o
the lack of data on the effect that the delay could have on their detection during routine PMI.
-
o
Assessment question 2: Is the detection of transmissible spongiform encephalopathies (TSEs) in cattle, sheep, goats and cervids and Trichinella in Suidae and solipeds reduced if the PMI is delayed by 24 or 72 h after slaughter/arrival in the game‐handling establishment?
Delays in PMI of 24 or 72 h could potentially reduce diagnostic sensitivity of the testing programme if autolysis was to compromise sampling accuracy. However, this would not exceed the tolerance already in place for fallen stock surveillance sampling. The analytical sensitivity and specificity of TSE laboratory tests have been shown to be unaffected by delays of this duration.
For the detection of Trichinella the panel did not find any evidence that would suggest a decrease in sensitivity during cold storage and it is almost certain (99–100%) that there is no decrease in sensitivity of detection after a delay of PMI of 24 or 72 h.
Assessment question 3: What is the percentage of reduction (%) in sensitivity of Salmonella detection as a process hygiene criterion if the PMI is delayed by 24 or 72 h after slaughter?
The developed model estimates the percentage of reduction (%) in sensitivity of Salmonella detection as applied for the process hygiene criterion, as follows:
The median estimate for the reduction in sensitivity of Salmonella detection with a 24‐h delay of PMI after slaughtering is 66.5%. The 90% probability interval for this reduction in sensitivity ranges from 0.08% (5th percentile) to 99.75% (95th percentile).
The median estimate for the reduction in sensitivity of Salmonella detection with a 72‐h delay of PMI after slaughtering is 94%. The 90% probability interval for this reduction in sensitivity ranges from 0.83% (5th percentile) to 100% (95th percentile).
The high uncertainty on the above estimates originates mainly from the uncertainty in the initial Salmonella concentration on carcasses. In general, the lower the initial Salmonella counts, the higher the estimated reduction in the sensitivity of detection.
Assessment question 4: Is the sensitivity of detecting of chemical residues and contaminants in ungulates reduced if the PMI is delayed by 24 or 72 h after slaughter/arrival in the game‐handling establishment?
In general, there is little information on the potential post‐mortem degradation of chemical residues and contaminants in organs and tissues.
As regards POPs and metals, the CONTAM Panel concluded that there is no impact of a delayed meat inspection of 24 or 72 h on the effectiveness to detect these substances due to their stability, poor microbial and chemical degradation, and persistence.
As regards pharmacologically active substances, the CONTAM Panel concluded that due to potential degradation in the available matrices and the non‐availability of specific preferred matrices of choice, the effectiveness to detect these substances may be reduced when a delayed meat inspection of 24 or 72 h is performed.
5. Recommendations
There are large data gaps regarding how the PMI detection of pathological and morphological changes might alter following 24‐h or 72‐h chilled storage. If a delayed PMI regime is established, Member States should keep track of any changes in the number of recorded lesions.
Due to the lack of information regarding the frequency, pattern, severity and post‐mortem stability of the lesions in subclinical or asymptomatic cases, and, consequently, the high uncertainty about the reduction of detectability of certain disease lesions after a 24‐h delay of PMI, and an even greater discrepancy after a 72‐h delay, there is a need for studies addressing these issues for diseases listed according to article 5 of Regulation (EC) 2016/429. Such studies could help identify situations where it might be advisable to limit the use of delayed PMI.
Additional training of official veterinarians and meat inspectors might be necessary to provide efficient PMI when performed at delay of 24 or 72 h after slaughter.
An extended storage period of 24–72 h of carcasses before PMI will increase the time for possible cross‐contamination between carcasses, aerosols and dust. Thus, it is expected that carcasses and offal not yet declared fit or not fit for human consumption might be stored together and thus, increase the risk of cross‐contamination by zoonotic and animal pathogens. This would need to be considered if such a delay was implemented.
There is a need for studies on the post‐mortem stability, particularly for pharmacologically active substances in different matrices other than urine and plasma.
Abbreviations
- 19‐NT
19‐nortestosterone
- ABP
Animal By‐Products
- ADV
Aujeszky's disease virus
- AH
Animal health
- AHD
1‐aminohydantoin
- AHS
African horse sickness
- AMI
Ante‐mortem inspection
- AMOZ
3‐amino‐5-methylmorpholino‐2-oxazolidinone
- AOZ
3‐amino-2‐oxazolidinone
- AQ
Assessment question
- ASF
African swine fever
- BSE
Bovine spongiform encephalopathy
- BTV
Bluetongue virus
- BVD
Bovine viral diarrhoea
- CBPP
Contagious bovine pleuropneumonia
- CCPP
Contagious caprine pleuropneumonia
- CCα
decision limit
- CCβ
detection capability
- CEM
Contagious equine metritis
- CONTAM
Panel on Contaminants in the Food Chain
- CRL
Community Reference Laboratories
- CSF
Classical swine fever
- CVMP
Committee for Veterinary Medicinal Products
- CWD
Chronic wasting disease
- CYP
cytochrome P450
- DE
dienestrol
- DES
diethylstilbestrol
- DL‐PCBs
dioxin‐like polychlorinated biphenyls
- DNSH
3,5‐dinitrosalicylic acid hydrazide
- EAV
Equine arteritis virus
- EBL
Enzootic bovine leukosis
- EDTA
ethylenediaminetetraacetic acid
- EEE
Eastern equine encephalomyelitis
- EHDV
Epizootic haemorrhagic disease virus
- EIA
Equine infectious anaemia
- EKE
Expert knowledge elicitation
- EURL
European Union Reference Laboratory
- FCI
Food Chain Information
- FMD
Foot and mouth disease
- GHP
Good Hygiene Practices
- GMP
Good Manufacturing Practices
- HCl
Hydrochloric acid
- HEX
hexestrol
- HMMNI
2‐hydroxymethyl‐1-methyl‐5-nitroimidazole
- HS
Healthy slaughter
- i.m.
intramuscular
- i.v.
intravascular
- IARC
International Agency for Research on Cancer
- IBR
Infectious bovine rhinotracheitis
- IPV
Infectious pustular vulvovaginitis
- IPZOH
1‐methyl‐2-(2’‐hydroxyisopropyl)‐5-nitroimidazole
- LOD
limit of detection
- LOQ
limit of quantification
- LSDV
Lumpy skin disease virus
- MBI
mercaptobenzimidazole
- ML
maximum level
- MNZOH
1‐(2-hydroxyethyl)‐2-hydroxymethyl‐5-nitroimidazole
- MPNs
Most probable numbers
- MRL
maximum residue limit
- MMPR
minimum method performance requirement
- MS
Member State
- MTBC
Mycobacterium tuberculosis complex
- MTU
methylthiouracil
- NDL‐PCBs
non dioxin‐like PCBs
- NRCP
national residue control plan
- NRLs
National Reference Laboratories
- NSAID
non‐steroidal anti‐inflammatory drug
- NSHC
Not slaughtered for human consumption
- OTA
Ochratoxin A
- PBDEs
Polybrominated diphenyl ethers
- PCBs
polychlorinated biphenyls
- PCDDs
polychlorinated dibenzo‐p-dioxins
- PCDFs
polychlorinated dibenzofurans
- PCR
Polymerase chain reaction
- PFASs
perfluoroalkyl substances
- PFCAs
perfluoroalkyl carboxylic acids
- PFHxS
Perfluorohexanesulfonic acid
- PFNA
Perfluorononanoic acid
- PFOA
Perfluorooctanoic acid
- PFOS
Perfluorooctanesulfonic acid
- PFSAs
perfluoroalkyl sulfonic acids
- PH
Public Health
- PHC
Process hygiene criterion
- PMI
Post‐mortem inspection
- POPs
persistent organic pollutants
- PPRSV
Porcine reproductive and respiratory syndrome virus
- PPRV
Peste des petits ruminants virus
- PrP
Prion protein
- PrPSc
Abnormal protease‐resistant isoforms of prion protein
- RVFV
Rift Valley fever virus
- s.c.
subcutaneous
- SEM
semicarbazide
- SHC
Slaughtered for human consumption
- T3
triiodothyronine
- T4
thyroxine
- TB
Tuberculosis
- TEFs
toxic equivalency factors
- TEQs
toxic equivalents
- TOR
Terms of Reference
- TSE
Transmissible spongiform encephalopathy
- VEE
Venezuelan equine encephalomyelitis
- WEE
Western equine encephalomyelitis
- WNF
West Nile fever
- ZEN
zearalenone
- α‐ZAL
Zeranolaaa α‐zearalanol
- α‐ZEL
α‐zearalenol
- β‐ZAL
Taleranolaaa β‐zearalanol
- β‐ZEL
β‐zearalenol
Appendix A – Results of the questionnaire to meat inspectors
1.
First and third quartiles (dots) and median (line) of the distributions of the responses provided by 18 experts in meat inspection about the number of organs out of 100 with a given lesion detected at PMI conducted immediately after slaughter and still detectable after 24 or 72 h (top and bottom of each graph).
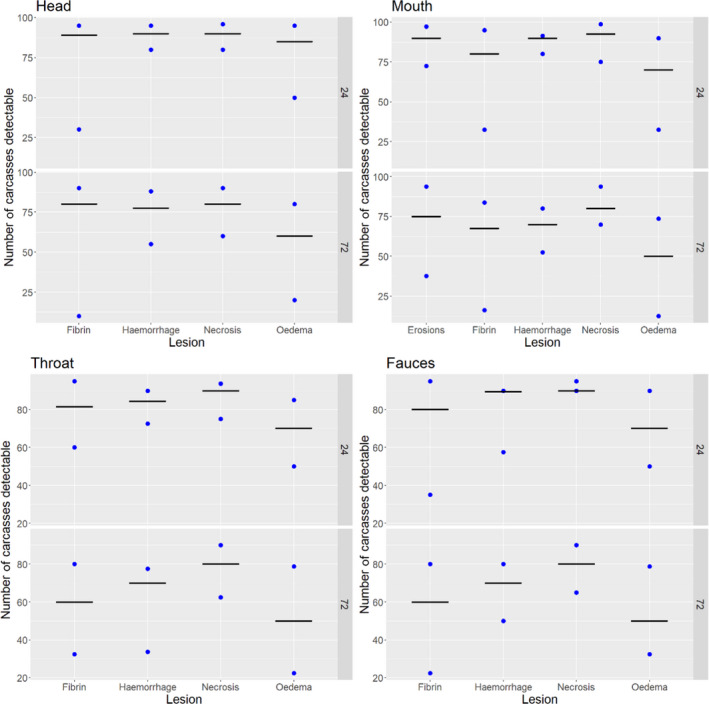
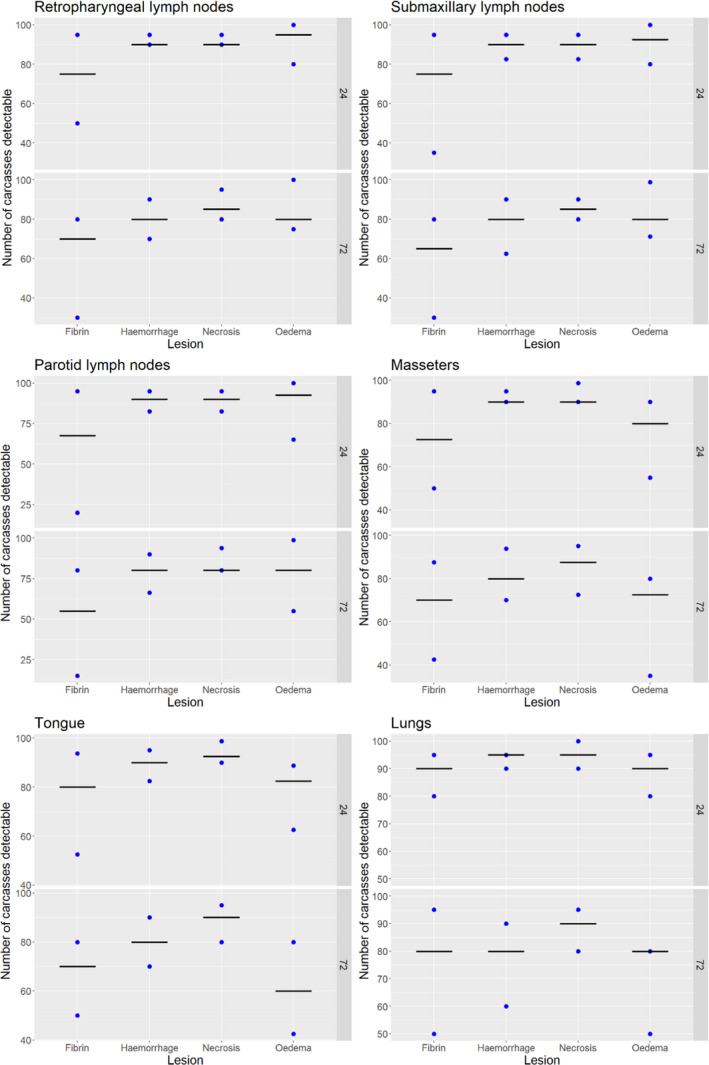
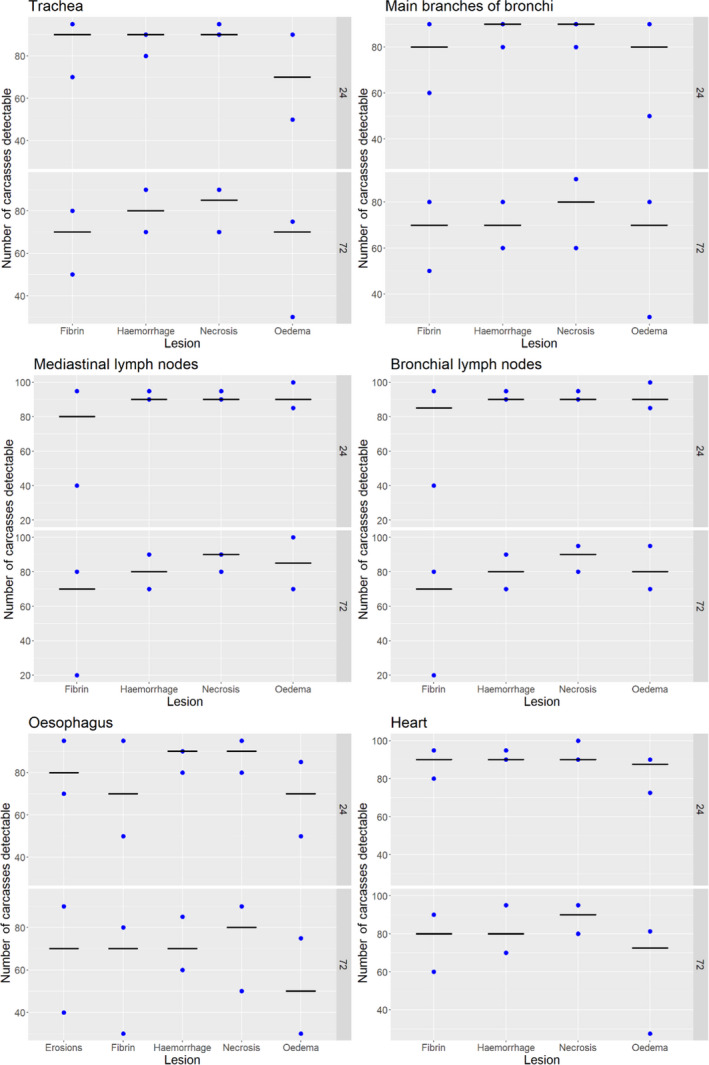
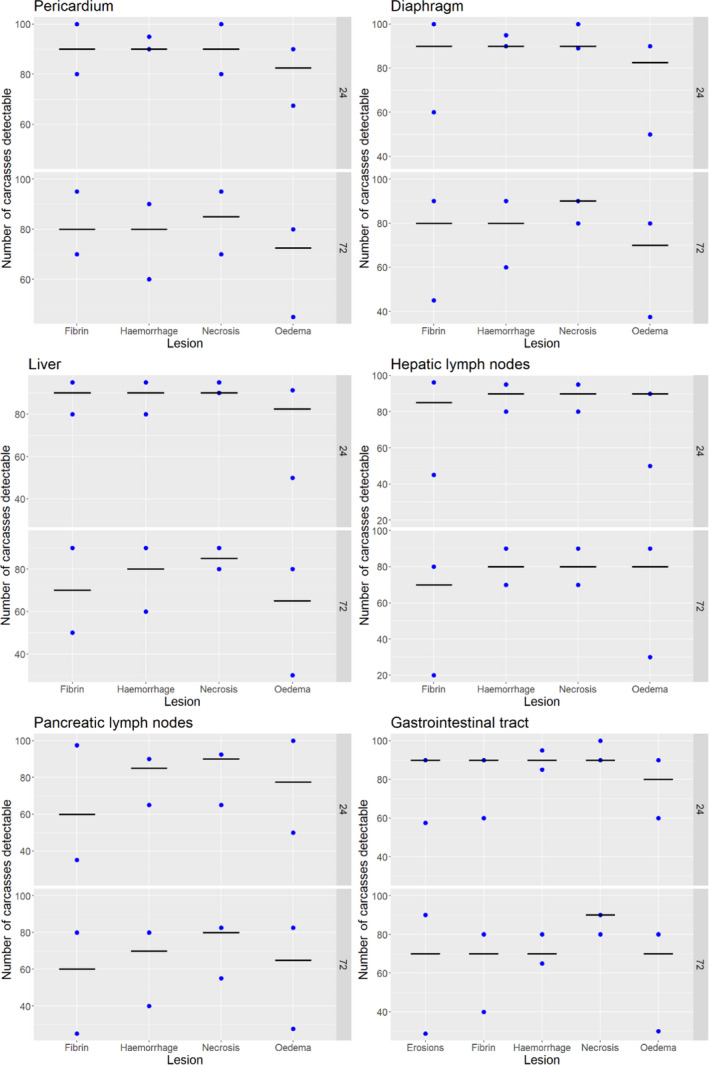
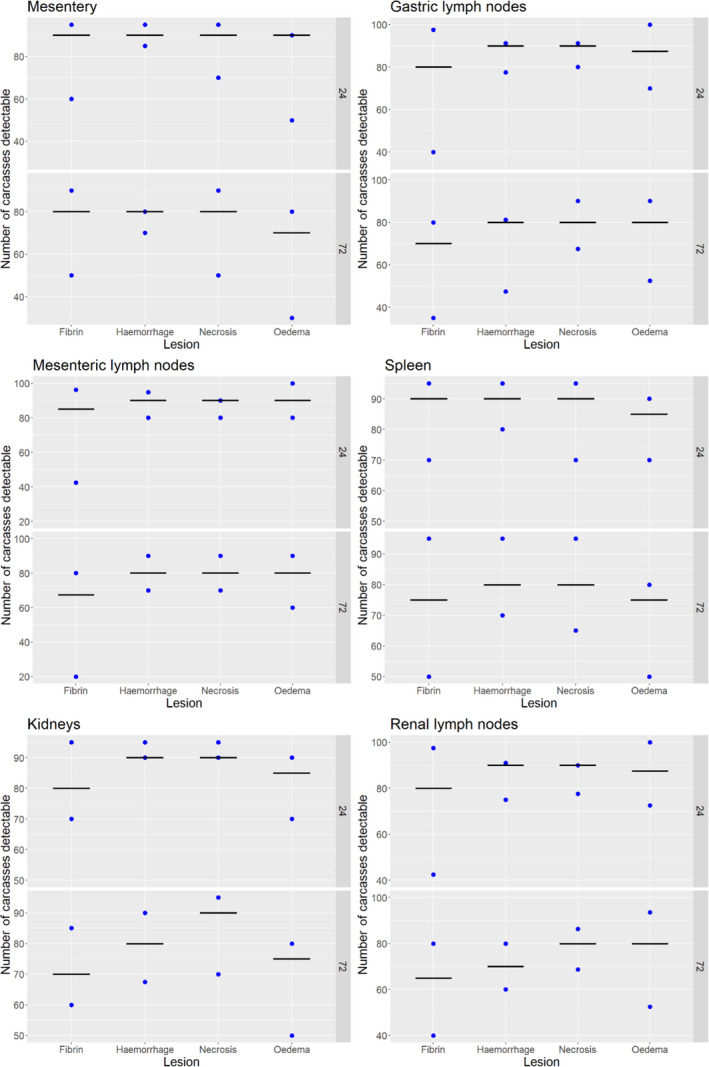
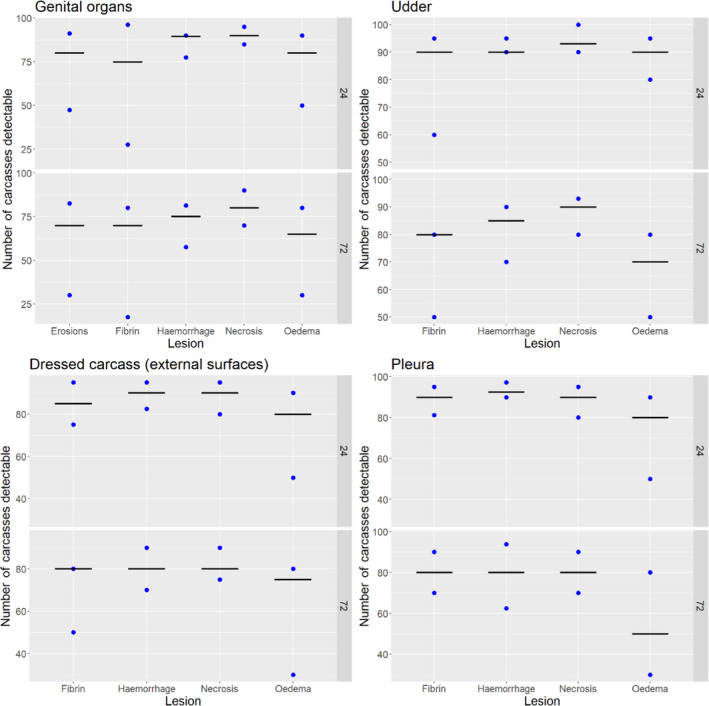
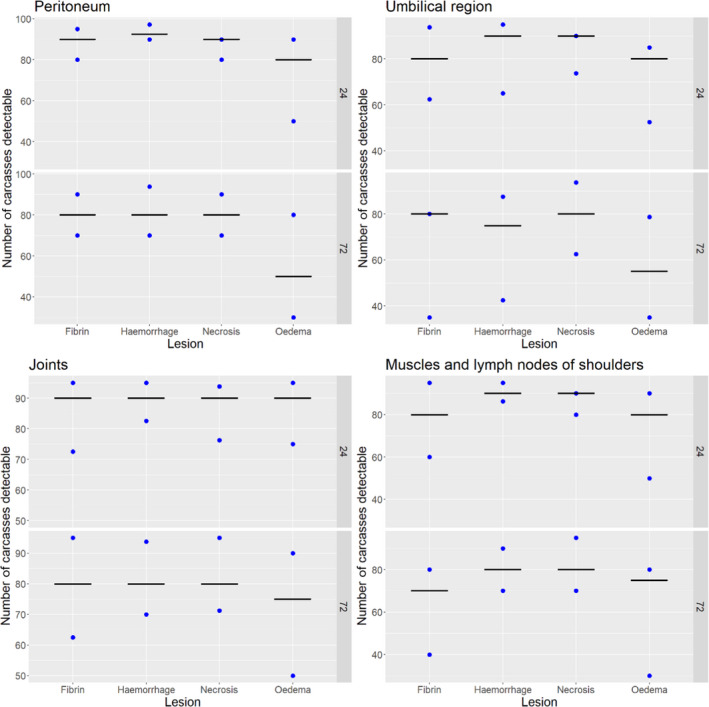
Appendix B – Expert Knowledge Elicitation (EKE) workshops
1.
As described in Section 2.1.2.6, several EKE exercises were conducted to elicit the impact of a delayed PMI on the detection of specific lesions in specific organs and on the performance of the tuberculosis laboratory diagnostic tests. All members of the AHAW subworking group participated in the EKE workshops as experts except one member that acted as facilitator. The EKE workshops followed the Sheffield method and were held on the four sessions that spanned between 3.5 and 5 h each.
| Session | 1st EKE | 2nd EKE | 3rd EKE | 4th EKE |
|---|---|---|---|---|
| Date | May 14 | May 20 | June 1 | June 12 |
| Topic | Impact of the delay on the detection of animals with: | Impact of the delay on the detection of:
|
Impact of the delay on the detection of animals with:
|
Impact of the delay on the performance of laboratory tests for confirmation of a tuberculosis infection:
|
| Parameter of interest | Mean number of carcasses from infected animals assessed as diseased in a PMI carried out immediately after slaughter (current procedure) per 100 that would still be detectable after 24 or 72 h | Mean number of carcasses with lesions detected in a PMI conducted after slaughter and positive in a test if analysed immediately per 100 that would be still positive in that test if the sample was stored for 24 or 72 h | ||
Brucellosis, contagious equine metritis, dourine, Japanese encephalitis, Q fever, IBR/IPV, trichomoniasis.
Contagious bovine pleuropneumonia and contagious caprine pleuropneumonia.
All ungulates except solipeds.
Experts received the evidence dossier with the information described in Section 2.1 at least four working days in advance of each session. The evidence dossier contained the definition of the parameter of interest in addition to the topics that would be evaluated in each of the sessions. In addition, prior to the first EKE, the experts received some introductory information on probabilistic judgement.
The conduct of the EKE sessions was slightly different in the first EKE compared to the following ones: ahead of that first session the experts were required to provide only the median of the distribution of the parameter of interest at least 24 h ahead of the workshop. This was done due to time constraints and to simplify the process since experts had received limited introductory information. During the initial session, the information provided on probabilistic judgement was first briefly reviewed, allowing the experts to ask questions if something was not clear. Then, the evidence available for each disease/condition assessed was briefly reviewed. Experts agreed on setting the lower and upper plausible limits to 0 and 100 based on the available evidence. Then, a plot of the individually elicited medians was shown to the group. Experts were asked to justify their individual judgements, and after some discussion, a consensus median was established. Then, experts were asked individually to make their judgements for the first (Q1) and third (Q3) quartiles, which were then provided to the facilitator, who showed the group the distributions resulting from the values provided (0 and 100 as lower and upper plausible limits, consensus median and individual Q1 and Q3). A group discussion followed, in which each of the experts was given the opportunity to justify their individual judgements, until there was agreement on a consensus distribution representing the knowledge available to the group. This consensus distribution was plotted and examined by the experts, and subjected to variations until all experts considered it adequately represented their collective belief about the parameter of interest according to the Rational Impartial Observer (RIO) concept1, explained at the beginning of the session.
Before the 2nd and 3rd EKE sessions, and once the evidence dossiers had been received, the experts were asked to provide the first and third quartiles in addition to the median ahead of the workshop. Then, during the meeting and after reviewing the available evidence for each disease, the experts were directly shown a plot of their individual distributions (based on their individual Q1, median, Q3 and agreed lower and upper plausible limits) which was followed by a group discussion until a consensus distribution was agreed upon, which was then scrutinised as described above.
Discussions during the three EKE sessions focused on detection of lesions at the abattoir and related aetiology (diseases) were typically based on the following points:
Experts providing higher values for the parameter of interest stressed that for lesions to be detected at time 0 they would typically have to be fairly evident and therefore remain detectable at 24 or 72 h, since PMI has a limited sensitivity for the detection of mild pathological changes (even if conducted immediately after slaughter).
Certain experts had direct experience on the effect of cold storage in certain organs and/or on the range of lesions expected for several of the diseases under evaluation, and therefore, their opinions on the likelihood of detecting these diseases after 24 or 72 h were typically very influential during the discussion to arrive at the consensus distribution.
The difficulties of predicting for an animal affected by any of the listed diseases the likely range of lesions that would be present at the PMI (among those listed as possible for that disease) was often brought up. This was discussed along with the fact that these animals should not have severe clinical signs since otherwise they should have been detected at the ante‐mortem inspection (and should be culled immediately, so that no delay on the PMI would be expected). This often resulted in an increase in the uncertainty of the elicited consensus distribution (typically achieved by decreasing the values of the consensus Q1).
The evidence included the opinion of some meat inspectors of which very few of these considered that the mean number of animals with a given lesion that would be still detectable at 24 or 72 h out of 100 would be very low (or zero) for almost all lesions. These were considered non‐representative outliers by the experts, since they were typically very few (1–3 out of 18 meat inspectors) while the remaining answers were much more consistent (i.e. spanned a much higher and limited range of possible values).
Detection at the PMI of diseases in which multiple organs could be involved and/or chronic lesions could be present were typically considered less affected by the delay than those affecting only a more limited range of tissues and/or in which only acute lesions were described. The effect of the delay was judged potentially more important in the case of lesions involving haemorrhages and fibrin, since it was the opinion of the experts that these would be harder to detect, particularly after 72 h (which was in agreement with the evidence available). Because of this, consensus distributions elicited for diseases leading only (or mostly) to the presence of haemorrhages and fibrin in a limited range of organs were typically lower.
As expected, and in agreement with the available evidence, the impact of the delay on the mean number of carcasses that would be still detectable after a delay in the PMI was always judged to be higher at 72 h than at 24 h, although this difference was limited for diseases in which chronic lesions were expected (e.g. tuberculosis, echinococcosis, lumpy skin disease). In addition, the consensus distribution elicited for 72 h was also typically wider than for 24 h, reflecting a larger uncertainty on the possible effect of the cold storage on the range of possible lesions present, and on the impact of such an effect on the likelihood of the lesion being detected after a longer delay.
Ahead of the fourth and last EKE session, that was focused on the impact of the delay on the performance of laboratory tests for confirmation of tuberculosis, experts were asked to provide their individual judgements for the lower and upper plausible limits, Q1, Q3 and median values for the parameter of interest. This was done because in the opinion of the working group the evidence did not support setting the lower and upper plausible limits at 0 and 100 as was done in the first three EKEs. During the EKE, experts were shown their individual distributions for each test and delay considered, and after the facilitated discussion, the group arrived at a consensus distribution for all tests. Discussion for the culture and PCR was brief since all experts judged that the impact of the delay on the parameter of interest should be low (also in agreement with the evidence available), while a longer discussion was held in the case of histopathology in the case of a 72‐h delay due to the more limited direct experience of the experts with this technique and the wider range of responses provided in the survey filled in by the National Reference Laboratories/ EU Reference Laboratory. Furthermore, the subjectivity associated with the interpretation of this test (i.e. judging a lesion as due to tuberculosis infection given the wide range of possible pathological changes that could be present, as opposed to obtaining a positive PCR/bacteriology result) further increased the uncertainty of the experts regarding the possible impact of the delay. After the discussion, there was agreement that the chance of observing a decrease in the parameter of interest was higher for histopathology than for the other techniques, though the elicited plausible interval was still well above 60.
Appendix C – Overall assessment of the uncertainty on the effect of a 24‐h or 72‐h delay on the detection and confirmation of tuberculosis in ungulates
1.
Tuberculosis is the only disease among those listed under Article 5 of AHL in which laboratory tests are carried out routinely in case of a suspicion of the infection during the PMI. Therefore, in order to quantify the overall effect of the delay on the sensitivity for detecting this disease, it was necessary to consider both the effect of the delay on the detection of the lesions associated with tuberculosis in infected animals, and the effect of such delay on the confirmation of the infection through laboratory tests when lesions were detected and submitted to the laboratory. The target effect, fD, was defined as the proportion of animals with lesions detected in a PMI conducted immediately after slaughter (time 0) and confirmed as infected through laboratory tests conducted according to the current procedure (i.e. without any delay) that would be still detected and confirmed through laboratory tests if the PMI was carried out after a 24‐h or 7‐h delay (Table C.1), or:
Table C.1.
Fractions (ratios of numbers of carcasses having identified characteristics). The group of carcasses counted in each numerator is a subgroup of the carcasses counted in the corresponding denominator so that the corresponding fraction lies between 0 and 1
| Fraction | Characteristics of carcasses counted in the denominator | Additional characteristics of carcasses counted in the numerator |
|---|---|---|
| fA | Lesions would be detected if PMI at time 0 (no delay) | Lesions would still be detected if PMI at time 24 h/72 h |
| fA* | Lesions would be detected if PMI at time 0 (no delay) and lab test would be positive if carried out without delay and still be positive if carried out at t 24/72 h | Lesions would still be detected if PMI at time t 24/72 h |
| fB | Lesions would be detected if PMI at time 0 (no delay) and lab test would be positive if carried out without delay | Lab test would still be positive if carried out at t 24/72 h |
| fD | Lesions would be detected if PMI at time 0 (no delay) and lab test would be positive if carried out without delay | Lesions would still be detected if PMI at time t 24/72 h and the lab test would still be positive if carried out at t 24/72 h |
Using the process described in section 2.1.2.6, two parameters of interest were generated and available to the working group (in the form of elicited distributions reflecting the uncertainty about their true value):
fA: mean number of carcasses from infected animals assessed as diseased in a PMI carried out immediately after slaughter (current procedure) that would still be detectable after 24 or 72 h (Table C.1), namely
fB: mean number of carcasses with lesions detected in a PMI conducted after slaughter and positive in a test if analysed immediately that would be still positive in that test if the sample was stored for 24 or 72 h (Table C.1), namely
Because fA and fB are not referring to the same subpopulations fD cannot be obtained by simply combining (multiplying) their values. However, fD could be estimated as the product of fB and a third parameter, fA*,
with fA* defined as the proportion of carcasses with lesions detected in a PMI conducted immediately after slaughter and confirmed as infected through laboratory tests conducted both without any delay and also if the samples were stored for 24 or 72 hours in which lesions would be still detected if the PMI was carried out after a 24 or 72 hour delay (Table C.1), or:
fA* is unknown, but is only different from fA in that in the denominator animals, in addition to have lesions that would be detected if the PMI was carried out at time 0 (with no delay), would be confirmed as infected both if the laboratory test was conducted according to the current procedure (without further delay) and also if carcasses were stored for 24 or 72 hour before taking samples for laboratory testing. Therefore, the denominator of fA* is a subset of the one of fA in which a higher bacterial load (that would be a better target for the laboratory tests immediately and also after 24/72 h of cold storage of the samples) was present. Based on this definition, the working group made the assumption that fA* ≥ fA. The probability of this assumption to be correct, that is, P(fA* ≥ fA), was estimated as > 99% by the WG experts since animals with lesions and confirmed as infected through lab tests after 24/72 h would typically be those in a more advanced stage of the infection (hence the higher bacterial load) that would therefore be expected to have more easily detectable lesions (compared with the whole population of infected animals with lesions detected at time 0). Because of this, the product fA × fB was considered to represent a lower bound for fD.
In order to estimate the lower bound for fD, the possible dependency between the uncertainties about fA and fB had to be taken into account: the working group considered that it was likely that the uncertainties about the true values of fA and fB (and therefore the distributions representing such uncertainties) were not independent, since higher values for fA (i.e. most of the animals with lesions detected at time 0 would in fact be still detected after a 24/72‐h delay) would probably lead to higher values for fB (since if most lesions are still detectable after the delay, they are probably quite evident, and thus, a higher bacterial load would be typically expected). However, the degree of such dependency was not known. Therefore, to assess its possible impact, the lower bound for fD was estimated under two possible scenarios: complete independence (by simply multiplying the two distributions) and complete positive rank‐correlation (incorporating the correlation in the multiplication of the effects, so that the value of fA increased as fB increased and vice versa).
Then, an upper bound for the parameter fD was considered in a third scenario, in which fA* = 1 (and therefore fD = fB).
The 5th and 95th percentiles of the distributions obtained for each of the three scenarios were finally used to obtain a probability interval expressing the WG's assessment of overall uncertainty about the true value of the parameter fD. The lowest 5th percentile of those obtained for scenarios 1 (independence) and 2 (correlation) was used as the lower bound of this probability interval, while the 95th percentile coming from scenario 3 was used as the upper bound. Therefore, this interval contains ≥ 90% of the distribution of the parameter of interest, since the values used to estimate the lower bound from which the 5th percentile was extracted very likely (with P(fA* ≥ fA) > 99% as explained above) lead to an underestimation of the true value of the 5th percentile of fD, while the 95th percentile coming from the upper bound is likely an overestimation of the 95th percentile of fD (since it is almost certain that fA < 1).
Appendix D – Uncertainty assessment for TSE, Trichinella and Salmonella
D.1. Uncertainty table TSE
| Factor, parameter or model feature affected by the uncertainty | One sentence description of the cause of uncertainty affecting this factor, parameter or model feature (one row per cause of uncertainty) | One sentence description of how this source of uncertainty might lead to incorrect answer, or why that might be possible |
|---|---|---|
| Structural integrity of the brain/brainstem at the time of sampling | The analytical sensitivity and specificity of PrPSc detection methods are unaffected by autolysis, but if structural integrity is lost due to autolysis following delayed sampling, it may not be possible to take the correct anatomical areas, or to ensure that the correct areas have been included in the sample. False negatives may occur if the wrong area is sampled. An inaccurately sampled positive case may have insufficient anatomically correct tissue to enable the confirmatory diagnostic step to be performed | It is almost certain that autolysis would be limited in the controlled environment of chilled storage for 24 or 72 h after slaughter and would not exceed the tolerance already in place for fallen stock |
D.2. Uncertainty table Trichinella
| Factor, parameter or model feature affected by the uncertainty | One sentence description of the cause of uncertainty affecting this factor, parameter or model feature (one row per cause of uncertainty) | One sentence description of how this source of uncertainty might lead to incorrect answer, or why that might be possible |
|---|---|---|
| Digestibility of sample | Since the diaphragm is a thin muscle, it could dry and this could result in incomplete digestion and thus, incomplete release of larvae (still, it must be considered that the meat samples are minced prior to digestion) | This might lead to an underestimation of the hazard |
| Resistance of larvae to digestion | Larvae might be impaired and thus, be digested (not very probable) | This might lead to an underestimation of the hazard |
D.3. Uncertainty table Salmonella
| Factor, parameter or model feature affected by the uncertainty | One sentence description of the cause of uncertainty affecting this factor, parameter or model feature (one row per cause of uncertainty) | One sentence description of how this source of uncertainty might lead to incorrect answer, or why that might be possible |
|---|---|---|
| Uncertainty sources about the modelling approach | Assumption that variability of initial concentration of Salmonella can be represented by the Normal distribution | Depending on the actual shape of the distribution, if not normal, an overall under‐ or overestimation of the reduction in probability of Salmonella detection after 24 h and/or 72 h could have occurred |
| Assumption that Pert distribution (with minimum, most likely and maximum values as distribution parameters) represents uncertainty for sd, independently of the average of initial concentration of Salmonella | Depending on the actual shape of the distribution, if not Pert, an overall under‐ or overestimation of the reduction in probability of Salmonella detection after 24 h and/or 72 h could have occurred | |
|
Uncertainty about water activity. We assume aw input in the model is constant throughout chilling, which may not be realistic, as aw may be reduced during chilled storage Possible existence of microenvironments, where Salmonella cells reside, with lower aw than that reported, which cannot be assessed due to limitations in the spatial resolution of the aw measurement |
This could have caused higher reduction of Salmonella after 24 and 72 h of chilled storage, which may have led to an underestimation of the reduction in sensitivity of Salmonella detection This could have caused higher reduction of Salmonella after 24 and 72 h of chilled storage, which may have led to an underestimation of the reduction in sensitivity of Salmonella detection. Nonetheless, it is expected to have minor contribution to the overall uncertainty and impact on the outcome, because even though the extent of this phenomenon is unknown, it may be relatively limited, since it accounts for a limited portion of the sampled area |
|
| Model inputs were based on information from the literature, which sometimes was related to other Enterobacteriaceae, assuming those were representative also for Salmonella | An assumption was made that some data on sponging efficacy for other Enterobacteriaceae may be used to approximate the relevant model input for Salmonella | An overall under‐ or overestimation of the reduction in probability of Salmonella detection after 24 h and/or 72 h could have occurred |
| Uncertainty about model structure | The impact of this source of uncertainty on the outcome cannot be predicted | |
| Unquantified dependencies between the uncertainties | The impact of this source of uncertainty on the outcome cannot be predicted |
D.4. Elicitation record Salmonella
D.4.1. Effectiveness of PMI up to 24‐h delayed after slaughter
| Attendance and role | Expert | Role |
| Federica Barrucci | Facilitator | |
| Marios Georgiadis | Facilitator | |
| Katrin Bote | Secretary | |
| Michaela Hempen | Secretary | |
| BLAGOJEVIC Bojan | Expert | |
| SKANDAMIS PANAGIOTIS | Expert | |
| PAULSEN Peter | Expert | |
| HILBERT Friederike | Expert | |
| Purpose of elicitation |
The purpose of the elicitation is to agree on distributions of input parameters for the Salmonella stochastic model, used to assess the effectiveness of Post‐mortem inspection PMI (in terms of its sensitivity in detecting the diseases/conditions) when carried up to 24 h after slaughter or arrival in the game‐handling establishment, in comparison to when it is carried out immediately after slaughter or arrival in the game‐handling establishment Input parameters were assessed by WG experts in order to provide minimum, maximum and most probable values. These values were used to create triangular distributions representing uncertainty related to the corresponding parameters. Finally, model was run using as input variables the derived triangular distributions in order to identify the most relevant input parameters. Only the 3 most relevant parameters were subsequently elicited with EKE |
|
| Input variable | Approach | |
| Mean of initial distribution of Salmonella | EKE | |
| Standard deviation of the initial distribution of Salmonella | WG judgement | |
| LoD/SLm Min | EKE | |
| Aw | WG judgement | |
| pH | Fixed value | |
| T | Fixed value | |
| Physiology | EKE | |
| Sponge efficacy | WG judgement | |
| Outcompetition | WG judgement | |
| This record | Participants are aware that this elicitation will be conducted using the Sheffield Elicitation Framework, and that this document, including attachments, will form a record of the session | |
| Orientation and training |
The experts received a document (Scientific documentation) explaining the purpose of the elicitation and gave a brief explanation of the task that experts would be asked to perform The facilitator gave a presentation in which the task of judging probabilities was explained in more detail |
|
| Evidence | The experts received a document (Scientific documentation) presenting the available evidence | |
| Method | Quartile | |
| Results | PARAMETER: Average Salmonella concentration on carcass just after slaughter in log10 CFU/100 cm2 | |
|
QUESTION: What is the average value of concentration on carcass just after slaughter? Provide your estimate in Log10 CFU/100 cm2 Please consider an average situation, make an average over different animal species and over the different sampling areas | ||
| Experts participating: BLAGOJEVIC Bojan, SKANDAMIS PANAGIOTIS, HILBERT Friederike, PAULSEN Peter | ||
|
RANGE lower and upper bound were directly derived from uncertainty distribution in preliminary quantitative assessment model, since min and max were already thoroughly discussed. The range is (0;2.85) Rationale: | ||
| MEDIAN 1st round experts provided their elicitation. Median estimates were projected together with range and discussed with experts. A 2nd round was run during which an expert modified his/her elicitation. A final consensus was not reached | ||
| QUARTILES 1st round experts provided their elicitation. Quartiles estimates were projected together with range and median and were discussed with experts. In group discussion, a consensus on quantile and median values was reached. Distributions were constructed based on each expert's input separately. Visualisation of the individual parameter distribution supported the discussion. Those distributions were discussed and finally a unique distribution was agreed upon by all experts (GROUP beta in the graph below) | ||
|
ELICITED DISTRIBUTION Beta(1.735962, 7.965873)
| ||
| PARAMETER:SLm Min, the minimal number of CFU needed to guarantee a positive testing of the samples | ||
| QUESTION: which is the minimal number of CFU needed to guarantee a positive testing of the samples? | ||
| Experts participating: BLAGOJEVIC Bojan, SKANDAMIS PANAGIOTIS, HILBERT Friederike | ||
| RANGE lower and upper bound were elicited: (1; 10) | ||
|
MEDIAN 1st round experts provided their elicitation. Median estimates were projected together with range and discussed with experts In group discussion, a consensus on median values was reached. Distributions were constructed based on each expert's input separately. Those distributions were discussed, and finally, a unique distribution was agreed upon by all experts |
||
| ||
| QUARTILES Quantile were not elicited | ||
ELICITED DISTRIBUTION Normal with Mean = 4.67 SD = 1.59
| ||
| PARAMETER: Physiological status | ||
| QUESTION: Consider 100 cells, how many will be injured due to cold storage for 24 h at a temperature up to 7°C | ||
| Experts participating: BLAGOJEVIC Bojan, SKANDAMIS PANAGIOTIS, HILBERT Friederike | ||
| RANGE lower and upper bound were discussed. The range agreed upon was (25;90). | ||
| MEDIAN 1st round experts provided their elicitation. Median estimates were projected together with range and discussed with experts. No consensus was reached | ||
|
QUARTILE 1st round experts provided their elicitation. Quantiles estimates were projected together with range and median and were discussed with experts. In group discussion, a consensus on quantile and median values was reached. Visualisation of the individual parameter distribution supports the discussion. Distributions were constructed based on each expert's input separately. Those distributions were discussed and finally a unique distribution was agreed upon by all experts (GROUP beta in the graph below) Rationale: | ||
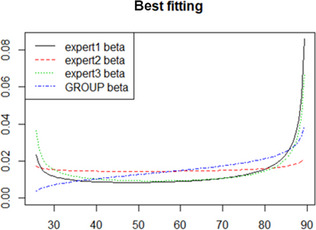
| ||
| ELICITED DISTRIBUTION Beta(1.3014260, 0.7998150) | ||
D.4.2. Effectiveness of PMI up to 72 h delayed after slaughter
| Attendance and role | Expert | Role |
| Federica Barrucci | Facilitator | |
| Michaela Hempen | Secretary | |
| BLAGOJEVIC Bojan | Expert | |
| SKANDAMIS PANAGIOTIS | Expert | |
| PAULSEN Peter | Expert | |
| HILBERT Friederike | Expert | |
| Purpose of elicitation | The purpose of the elicitation is to agree on distributions of input parameters for the Salmonella stochastic model, used to assess the effectiveness of Post‐mortem inspection PMI (in terms of its sensitivity in detecting the diseases/conditions) when carried up to 72 h after slaughter or arrival in the game‐handling establishment, in comparison to when it is carried out immediately after slaughter or arrival in the game‐handling establishment | |
| This record | Participants are aware that this elicitation will be conducted using the Sheffield Elicitation Framework, and that this document, including attachments, will form a record of the session | |
| Orientation and training |
The experts received a document (Elicitation record) explaining the purpose of the elicitation and gave a brief explanation of the task that experts would be asked to perform It was proposed to use the same the model as for 24 h storage. Only few variables need to be updated when considering 72 h of chilling: |
|
| Input variable | Potentially change/does not change under 72 h chilling condition | |
| Mean of initial distribution of Salmonella | No change | |
| Standard deviation of the initial distribution of Salmonella | No change | |
| LoD/SLm Min | No change | |
| Aw | Change | |
| pH | Fixed value | |
| T | Fixed value | |
| Physiology | Change | |
| Sponge efficacy | Change | |
| Outcompetition | Change | |
|
Therefore, it was proposed to perform elicitation of uncertainty distribution for the following parameters:
Starting from values elicited under 24 h cold storage condition, experts were asked to update their estimates considering the 72 h of cold storage condition. Due to time constraints, experts were asked to provide their estimate via email. This short cut was possible due to the fact that these parameters were discussed in detail during elicitation under 24 h cold storage condition | ||
| Evidence | The experts received a document (Elicitation record) presenting definition of parameters, instruction and values elicited under 24 h cold storage condition | |
| Method | Quartile/min‐max‐most probable value | |
Results
| PARAMETER: Physiological status. | |
| PARAMETER DESCRIPTION: It represents the proportion of the surviving population after chilled storage that may be irreversibly injured and, thus, is not culturable. This injury is additional to the one accounted for by the predicted model and could be attributed to the combined outcome of cold and osmotic stress (sudden aw downshifts), experienced by cells while residing on the carcass micro‐environments (niches) | |
|
Method: 72 h chilling is obtained extending the cold storage beyond 24 h, therefore uncertainty distributions of the physiological status parameter after 72 h of chilling are correlated to its respective distribution elicited under 24 h of cold storage condition Starting from consensus value for the 5 quantiles elicited under 24 h cold storage condition, update of estimates of the quantile of the uncertainty distribution was conducted for the parameter physiological status under the 72 h of cold storage condition Experts were asked to provide their individual estimates for 72 h cold storage condition via email | |
|
EVIDENCE Graphical representation of individual experts’ judgement on quantiles of the uncertainty distribution for 24 h of cold storage was provided together with representation of consensus value (GROUP). Best‐fitting distributions for 24 h of cold storage based on each expert's input separately and for the consensus values were additionally provided | |
| CONSENSUS QUANTILE | Elicitation for 24 h of cold storage |
| Lower bound | 25 |
| 1st quartile | 50 |
| Median | 70 |
| 3rd quartile | 80 |
| Upper bound | 90 |
| |
| QUESTION: Consider 100 cells, how many will be injured due to cold storage for 72 h at a temperature up to 7°C? | |
| RANGE: 1st round experts provided their elicitation via email. Range, median and quantiles estimates were projected and discussed with experts. A discussion was run during which experts modified their elicitation of range. A final consensus was reached: lower bound = 30, upper bound = 99 | |
|
MEDIAN AND QUANTILE: Median and quantiles estimates were projected and were discussed with experts. Visualisation of the individual parameter distribution supports the discussion. There was among expert agreements on using the linear pooling distribution. Several distributions were fitted to the linear pooling distribution, in order to obtain a distribution to enter into the model. Since the fitting was poor, experts finally agree on consensus value for quantiles and median values Median = 75; 1st quantile = 60; 3rd quantile = 85 | |
|
ELICITED DISTRIBUTION Beta(2.104995, 1.3199485) Experts’ and consensus quantiles and fitted distributions: | |
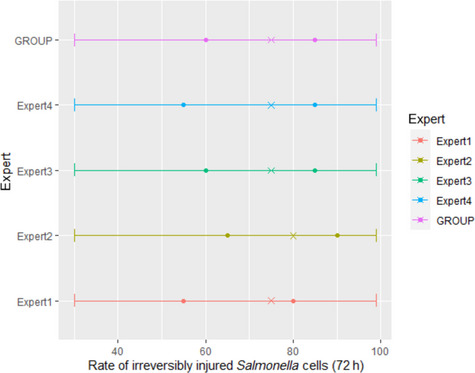
| |
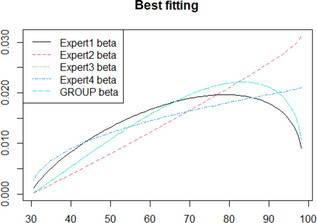
| |
| PARAMETER: Sponging efficacy (SpEff: 0 to 1) | |
| PARAMETER DESCRIPTION: it determines the proportion of surviving and non‐irreversibly injured cells that can be detached by swabbing. It is scored from 0 to 1 and the value reflects the reduced swabbing efficacy compared to time 0. For instance, a value of 0.6 suggests that the swabbing efficacy after 72 h is reduced by 40% compared to time 0 | |
|
Method: 72 h chilling is obtained extending the cold storage beyond 24 h, therefore uncertainty distributions of the sponging efficacy parameter after 72 h of chilling are related to its respective distribution elicited under 24 h of cold storage condition Starting from values for min, max and most probable value elicited under 24 h cold storage condition, estimates of these 3 points of the uncertainty distribution for sponging efficacy were updated under the 72 h of cold storage condition | |
| EVIDENCE Pert distribution parameters elicited under 24 h of cold storage condition. | |
| QUANTILE | Elicitation for 24 h of cold storage |
| Min (1% percentile) | 60 |
| Max (99% percentile) | 100 |
| Most probable value | 90 |
| QUESTION: Consider 100 surviving and non‐irreversibly injured cells, how many will not be detached by swabbing due to cold storage for 72 h at a temperature up to 7°C? | |
|
RESULTS Individuals's judgements and consensus values: | |
| Consensus values and distribution: | |
| QUANTILE | Elicitation for 72 h of cold storage |
| Min (1% percentile) | 50 |
| Max (99% percentile) | 95 |
| Most probable value | 80 |
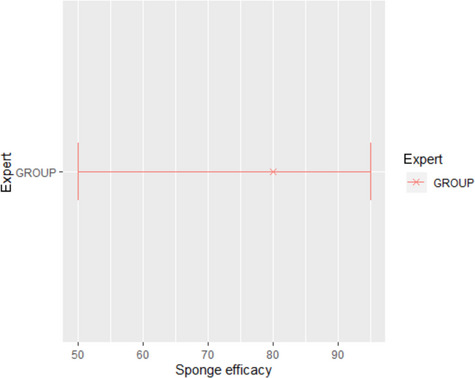
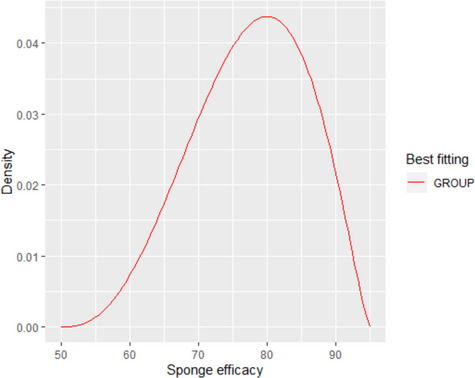
| |
| PARAMETER: Fitness of Salmonella in the enrichment (Compt: 0 to 1) | |
| PARAMETER DESCRIPTION: it refers to the probability of the surviving population, that is not irreversibly injured and is detached by swabbing, to be outcompeted by indigenous meat microbiota. Such population is not capable of growing in the enrichment medium at levels sufficient to be isolated on a selective plate | |
|
Methods: 72 h chilling is obtained extending the cold storage beyond 24 h, therefore uncertainty distributions of the outcompeted proportion parameter after 72 h of chilling are correlated to its respective distribution elicited under 24 h of cold storage condition Starting from values for min, max and most probable value elicited under 24 h cold storage condition, estimates of these 3 points of the uncertainty distribution for the probability to be outcompeted were updated under the 72 h of cold storage condition | |
| EVIDENCE Pert distribution parameters elicited under 24 h of cold storage condition | |
| QUANTILE | Elicitation for 24 h of cold storage |
| Min (1% percentile) | 1 |
| Max (99% percentile) | 10 |
| Most probable value | 3 |
| QUESTION: Consider 100 cells, that are not irreversibly injured and are detached by swabbing, how many will be outcompeted by indigenous meat microbiota due to cold storage for 72 h at a temperature up to 7°C? | |
|
RESULTS Individuals’ judgements
| |
| Consensus values and distribution: | |
| QUANTILE | Elicitation for 72 h of cold storage |
| Min (1% percentile) | 1 |
| Max (99% percentile) | 15 |
| Most probable value | 6 |
| |
D.5. Model output for two different average initial contamination levels of Salmonella (without uncertainty): variability included
Merged cumulative probability outputs of the model for average (without uncertainty) Log No = 1 Log CFU/100 cm2 and 0 Log CFU/100 cm2. Variability is still considered in these simulations. It is the uncertainty about the average Log No that was eliminated.
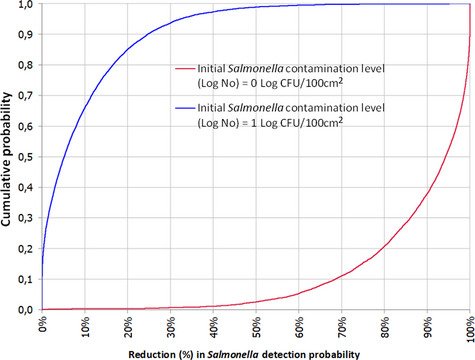
Appendix E – Summary of studies reporting half‐lives of ochratoxin A, chloramphenicol, nitrofurans and zeranol that were used in previous CONTAM opinions
1.
| Compound | Half‐life | Stability | |||
|---|---|---|---|---|---|
| Study | Animals | Treatment details | Time | ||
| OTA | Hagelberg et al. (1989) a | Wistar rats | Oral or i.v. administration of 50 µg/kg bw | Plasma: 5 days | |
| Hult et al. (1979) b | Pigs | i.v. administration of 50 µg of toxin per kg bw | Plasma: 6 days | ||
| Stander et al. (2001) | Monkeys | i.v. injection, 0,8, 1,5 and 2 mg/kg bw | Plasma: 19–21 days, average total body clearance 0.22 mL/h per kg bw | ||
| Han et al. (2013) | Male SD rats | gavage of a single dose of 0.2 mg OTA/kg bw | Plasma: 76 h (± 3 days) | ||
| Studer‐Rohr et al. (2000) | A male adult volunteer (empty stomach) | Single oral dose (0.02 nmol/kg bw) of tritium‐labelled OTA |
First 6 days: about 20 h After 6 days: 35 days |
||
| Mantle (2008) | Rats (Fischer, F1 hybrids from SD and Fisher Dark Agouti) | gavage or via feed. Doses ranging from 50 to 30,000 µg/kg bw |
Fischer rats: 8–10 days Dark Agouti rats: 2–3 days |
||
| Chloramphenicol | Nouws and Ziv (1978) | Dairy cows | Single i.m. dose of 43 mg/kg bw |
Muscle: 14 h Kidney: 22 h |
|
| Nouws and Ziv (1979) | Cows, sheep and goat | i.m. injections, 36 and 50 mg/kg bw | Blood: 88–643 min, depending on the preparation and plasma levels | ||
| Nouws and Ziv (1982) | Dairy cows | Intramammary infusion 5, 12.5, 25 g | Serum levels: 146, 213 and 285 min | ||
| Nouws et al. (1986) | Dairy cows and ruminant calves | Single i.m. injection, 50 mg/kg bw | Plasma and milk: 10 h | ||
| Burrows et al. (1984) | Calves | 25 mg/kg bw | Plasma:450 min to 3 days and 150 min, depending on age | ||
| Sanders et al. (1988) | Bullocks | i.v. injection 40 mg/kg bw and after two weeks either i.m. or s.c. treatment with 90 mg/kg, which was repeated after 48h. same treatment after another 3 weeks | Plasma: 4 h | ||
| Gassner and Wuethrich (1994) | Female beef‐type calves | 4 oral doses, 25 mg/kg bw at 12 h intervals | Plasma: 4.5 h | ||
| Etuk and Onyeyili (2005a, 2005b) | Sokoto red goats | Single i.v. dose of 25 g/kg bw |
Plasma: 0.13–3.6 h Tissues (liver, kidney, lung, heart, spleen and bone marrow): 1–4 h Tissues (muscle and brain): 24 and 21 h, respectively. Levels in muscle were not detectable after 11 days |
||
| Dagorn et al. (1990) | Sheep | i.v., i.m. or s.c. single dose of 30 mg/kg bw | Plasma: 1.7 (i.v.), 2.7 (i.m.) and 17.9 h (s.c.) | ||
| Rao and Clarenburg (1977) | 7‐week‐old pigs | i.v. administration of 22 mg/kg bw | Plasma: 55 min | ||
| Mercer et al. (1978) | 12‐ to 16‐week old crossbred pigs | i.v. administration of 22 mg/kg bw |
Plasma: 2.6 ± 1 h Kidney: 1.25 h Fat: 5.89 h Most major organs: 2–5 h |
||
| Buonpane et al. (1988) | Foals | oral administration of 50 mg/kg bw | Serum: 1.44 h | ||
| Gronwall et al. (1986) | Adult horses | Intragastrical administration of 50 mg/kg bw | Serum: 1.8 h | ||
| Nitrofurans | Cooper et al. (2005) | 8‐week‐old‐piglets | Fed for 10 days with feed medicated with furazolidone, furaltadone, nitrofurantoin or nitrofurazone at a dose of 400 mg/kg feed |
No parent compounds could be detected AOZ (in the case of furazolidone): Liver and kidney: 7 days Muscle: 12 days AHD (in the case of nitrofurantoin): Muscle: 15 days |
Cooper and Kennedy ( 2007 ): storage of pig liver and muscle at −20°C for 8 months did not cause a significant reduction of the concentrations of marker metabolites (AOZ, AMOZ, AHD and SEM) |
| Nouws and Laurensen (1990) and McCracken et al. (1995) | Nitrofuran parent compounds have a short in vivo half‐life due to extensive metabolism, primarily a reduction of the nitro‐group, such that they do not occur generally as residues in foods of animal origin. Therefore, monitoring of nitrofuran residues in livestock based on the identification of the parent compounds is not appropriate | ||||
| Vroomen Louis et al. (1986), Hoogenboom et al. (1991, 1992) and Vass et al. (2008) | The nitro reduction results in the formation of reactive metabolites able to bind covalently to tissue macromolecules, including proteins, which, in food‐producing animals, have relatively long half‐lives, persisting for several weeks in edible tissues | ||||
| Hoogenboom et al. (1991), Hoogenboom and Polman (1993), Horne et al. (1996) and Leitner et al. (2001) | As nitrofuran parent compounds do not persist as residues in animal tissues and do not occur at concentrations comparable to those of the marker metabolites (as protein‐bound adducts), the marker metabolites AOZ, AMOZ, AHD, SEM and DNSH are appropriate for identifying the illicit use of nitrofurans | ||||
| Compound | Study | Animals | Treatment details | Residues found in tissues (ng/g) | |
| Zeranol | Jansky (1983) in Paris et al. (2006) | Male calves |
Témoins, 1 à 3 implantations of Ralgo® (1 to 3 × 65 days) 6 implantations of Ralgo® (6 × 65 days) |
Liver: 0.102; 0.315; 1.209 Kidney: 0.014; 0.104; 0.219 Muscle: 0.105; 0.040; 0.115 Adipose tissue: 0.010; 0.039; 0.218 |
|
| O'Keeffe (1984) in Paris et al. (2006) | Steers | Témoins, Ralgo® |
Liver: 0.140; 0.350 Kidney: 0.028; 0.076 Muscle: 0.001; 0.014 Adipose tissue: 0.064; 0.060 |
||
| Dixon and Russell (1986) in Paris et al. (2006) | Cows | Ralgo® (70 days after implantation) |
Liver: 0.300 Kidney: 0.160 Muscle: 0.130 Adipose tissue: 0.180 |
||
| Dixon et al. (1986) in Paris et al. (2006) | Steers |
Témoins Ralgo® (7 days after implantation) Ralgo® (30 days after implantation) Ralgo® (70 days after implantation) Ralgo® (120 days after implantation) |
Liver: 0.100; 0.470; 0.810; 0.200; 0.100 Kidney: 0.100; no result; no result; 0.130; 0.084 Muscle: 0.280; 0.290; 0.280; 0.730; 0.280 Adipose tissue: 0.075; 0.077; 0.110; 0.073; 0.066 |
||
| Paris et al. (2006) | Residues of zeranol and trenbolone do not occur in animal tissues under normal conditions but can be measured following the use of GPHs containing these compounds. It should be noted that the mycotoxin zearalenone can be present in various feed materials can be converted into zeranol. This fact might complicate the monitoring of undesirable residues in animal tissues | ||||
| Gajecka (2013) | In prepubertal female beagle dogs, orally administered with ZEN for 42 days, the presence of ZEN and α‐ and β‐ZEL was observed in plasma throughout the experiment. The highest relative α‐ZEL concentration (58–74% of ZEN) was observed on the last 5 days in the high‐dose group | ||||
| EFSA CONTAM Panel (2011c) | Limited data on food of animal origin did not show ZEN or its phase I metabolites above the detection limits. This led the CONTAM Panel to state that it seemed reasonable to conclude that the possible contribution of ZEN residues in animal products is negligible for the total ZEN exposure of the consumer | ||||
AHD: 1‐aminohydantoin, AMOZ: 3‐amino‐5‐methylmorpholino‐2‐oxazolidinone; AOZ: 3‐amino‐2‐oxazolidinone; DNSH: 3,5‐dinitrosalicylic acid hydrazide; i.m.: intramuscular, i.v.: intravenous, OTA: ochratoxin A; SD: Sprague Dawley; s.c.: subcutaneous; SEM: semicarbazide; ZEN: zearalenone; α‐ZEL: α‐zearalenol; β‐ZEL: β‐zearalenol.
EFSA (2006) stated that the data is mentioned in Dietrich et al, 2005, but the original data and study design is found in Hagelberg et al. (1989).
EFSA (2006) stated that the data is mentioned in Dietrich et al, 2005, but the original data and study design is found in Hult et al. (1979).
Appendix F – Uncertainty analysis chemical residues and contaminants
1.
| Group of substancesa | Factor, parameter or model feature contributing to the uncertainty | One sentence description of the cause of uncertainty affecting this factor, parameter or model feature (one row per cause of uncertainty) | One sentence description of how this source of uncertainty might lead to incorrect answer, or why that might be possible |
|---|---|---|---|
| OTA | Degradation | It is unclear to what extent degradation in the carcass after slaughter and before sampling during PMI will happen. The carcasses are chilled which may slow down the degradation. Degradation of OTA may result in a non‐detectable concentration while the concentration would have been detectable if sampling was done immediately after slaughter | This extent of degradation cannot be predicted based on the available information and therefore the impact of this factor on the effectiveness of a delayed PMI for 24 or 72 h cannot be assessed |
| The concentration at the moment of slaughter |
If the concentration is high, the compound may still be detectable even if degradation occurs If the concentration is close to the LOD of the analytical method, degradation may lead to a non‐detectable concentration |
The concentration at the moment of slaughter is not known and is a source of uncertainty. Therefore, the impact of this factor on the effectiveness of a delayed PMI for 24 or 72 h cannot be assessed | |
| Stilbenes | Degradation |
It is unclear to what extent degradation in the liver after slaughter and before sampling during PMI will happen. The carcasses are chilled which may slow down the degradation. Degradation of stilbenes may result in a non‐detectable concentration while a non‐compliant result would have been reported if sampling was done immediately after slaughter Information was used from spiked frozen meat samples which showed a reduction by 14–22% after storage of at least 2 months. This information provides evidence of degradation although the evidence originates from a different matrix, different time interval and different applied temperature |
A degradation can be expected and therefore the effectiveness of a delayed PMI of 24 or 72 h could be reduced if the concentration drops below the required level of sensitivity. However, the extent of degradation cannot be predicted based on the available information |
| The concentration at the moment of slaughter |
If the concentration is high, the compound may be still detectable even if degradation occurs If the concentration is close to the CCα/CCβ of the analytical method used, degradation may lead to a non‐detectable concentration This concentration depends on the route of application, the dose applied and the time of administration. This information is unknown since it is an illegal application |
The concentration at the moment of slaughter is not known and is a source of uncertainty. Therefore, the impact of this factor on the effectiveness of a delayed PMI for 24 or 72 h cannot be assessed | |
| Thyrostats | Degradation |
It is unclear to what extent degradation in the thyroid gland after slaughter and before sampling during PMI will happen. The carcasses are chilled which may slow down the degradation. Degradation of thyrostats may result in a non‐detectable concentration while a non‐compliant result would have been reported if sampling was done immediately after slaughter No information is available regarding the stability in thyroid gland. Thyrostats are not stable in urine and a stabilisation protocol is implemented |
Given that the extent of degradation in the thyroid gland cannot be predicted based on the available information, the effectiveness of a delayed PMI of 24 or 72 h could be reduced if the concentration drops below the required level of sensitivity |
| The concentration at the moment of slaughter |
If the concentration is high, the compound may be still detectable even if degradation occurs If the concentration is close to the CCα/CCβ of the analytical method used, degradation may lead to a non‐detectable concentration This concentration depends on the route of application, the dose applied and the time of administration. This information is unknown since it is an illegal application |
The concentration at the moment of slaughter is not known and is a source of uncertainty. Therefore, the impact of this factor on the effectiveness of a delayed PMI for 24 or 72 h cannot be assessed | |
| Synthetic gestagens | Degradation |
It is unclear to what extent degradation in the kidney fat after slaughter and before sampling during PMI will happen. The carcasses are chilled which may slow down the degradation Enzymatic activity in fat tissue is considered lower compared to liver or muscle tissue |
In kidney fat, no substantial degradation is expected. Therefore, a delayed PMI of 24 or 72 h is not expected to reduce the effectiveness to detect residues of synthetic gestagens application. However, in the absence of stability data on synthetic gestagens in kidney fat, there is uncertainty regarding this conclusion |
| Synthetic androgens | Degradation |
It is unclear to what extent degradation in the liver after slaughter and before sampling during PMI will happen. The carcasses are chilled which may slow down the degradation. Degradation of synthetic androgens may result in a non‐detectable concentration while a non‐compliant result would have been reported if sampling was done immediately after slaughter Information was used from spiked frozen liver and meat samples which showed no reduction after storage at ‐20°C during 27 weeks. This information indicates that no degradation may occur although the evidence originates from a different time interval and different applied temperature |
Given that the extent of degradation in the liver cannot be predicted based on the available information, the effectiveness of a delayed PMI of 24 or 72 h could be reduced if the concentration drops below the required level of sensitivity |
| The concentration at the moment of slaughter |
If the concentration is high, the compound may be still detectable even if degradation occurs If the concentration is close to the CCα/CCβ of the analytical method used, degradation may lead to a non‐detectable concentration This concentration depends on the route of application, the dose applied and the time of administration. This information is unknown since it is an illegal application |
The concentration at the moment of slaughter is not known and is a source of uncertainty. Therefore, the impact of this factor on the effectiveness of a delayed PMI for 24 or 72 h cannot be assessed | |
| RAL | Degradation |
It is unclear to what extent degradation in the liver and bile after slaughter and before sampling during PMI will happen. The carcasses are chilled which may slow down the degradation. Degradation of RAL may result in a non‐detectable concentration while a non‐compliant result would have been reported if sampling was done immediately after slaughter No information is available regarding the stability in liver and bile |
Given that the extent of degradation in the liver and bile cannot be predicted based on the available information, the effectiveness of a delayed PMI of 24 or 72 h could be reduced if the concentration drops below the required level of sensitivity |
| The concentration at the moment of slaughter |
If the concentration is high, even if degradation occurs the compound may be still detectable If the concentration is close to the CCα/CCβ of the analytical method used, degradation may lead to a non‐detectable concentration This concentration depends on the route of application, the dose applied and the time of administration. This information is unknown since it is an illegal application |
The concentration at the moment of slaughter is not known and is a source of uncertainty. Therefore, the impact of this factor on the effectiveness of a delayed PMI for 24 or 72 h cannot be assessed | |
| β‐agonists | Degradation |
In retina, high residue concentrations can be found for a longer period In liver samples, clenbuterol is stable at 4, ‐20 and ‐60°C for up to 20 weeks. However, information on the stability of other β‐agonists in liver samples was not identified For the lung, no information regarding the stability was identified |
For the retina, the stability has been demonstrated for several substances within the group of β‐agonists and is therefore not considered as a source of uncertainty For the liver, information is available for clenbuterol only. However, it is uncertain whether the same conclusion can be drawn for the other β‐agonists. Consequently, the extent of degradation cannot be predicted based on the available information and therefore the impact of this factor on the effectiveness of a delayed PMI for 24 or 72 h cannot be assessed for β‐agonists other than clenbuterol For the lung, the extent of degradation cannot be predicted based on the available information and therefore the impact of this factor on the effectiveness of a delayed PMI for 24 or 72 h cannot be assessed |
| The concentration at the moment of slaughter |
If the concentration is high, the compound may be still detectable even if degradation occurs If the concentration is close to the CCα/CCβ of the analytical method used, degradation may lead to a non‐detectable concentration This concentration depends on the route of application, the dose applied and the time of administration. This information is unknown since it is an illegal application |
The concentration at the moment of slaughter is not known and is a source of uncertainty. Therefore, the impact of this factor on the effectiveness of a delayed PMI for 24 or 72 h cannot be assessed | |
| Chloramphenicol | Degradation |
It is unclear to what extent degradation in the muscle after slaughter and before sampling during PMI will happen. The carcasses are chilled which may slow down the degradation. Degradation of chloramphenicol may result in a non‐detectable concentration while a non‐compliant result would have been reported if sampling was done immediately after slaughter Information was used from liver and kidney. This information indicates that a degradation may occur although the evidence originates from a different matrix, temperature and duration |
A degradation can be expected and therefore the effectiveness of a delayed PMI of 24 or 72 h could be reduced if the concentration drops below the required level of sensitivity. However, the extent of degradation cannot be predicted based on the available information. |
| The concentration at the moment of slaughter |
If the concentration is high, the compound may be still detectable even if degradation occurs If the concentration is close to the CCα/CCβ of the analytical method used, degradation may lead to a non‐detectable concentration This concentration depends on the route of application, the dose applied and the time of administration. This information is unknown since it is an illegal application |
The concentration at the moment of slaughter is not known and is a source of uncertainty. Therefore, the impact of this factor on the effectiveness of a delayed PMI for 24 or 72 h cannot be assessed | |
| Nitroimidazoles | Degradation |
It is unclear to what extent degradation in the retina and muscle after slaughter and before sampling during PMI will happen. The carcasses are chilled which may slow down the degradation. Degradation of nitroimidazole residues may result in a non‐detectable concentration while a non‐compliant result would have been reported if sampling was done immediately after slaughter No relevant information was identified regarding the stability in retina samples from ungulates For muscle, information was used from muscle samples from poultry which showed a reduction up to 12% when stored for 7 days at 4°C. This information provides evidence of degradation although the evidence originates from poultry and not from ungulates. In addition, depletion studies show that nitroimidazoles are rapidly metabolised. However, EFSA had no access to the full study reports |
A degradation can be expected when muscle is used and therefore the effectiveness of a delayed PMI of 24 or 72 h could be reduced if the concentration drops below the required level of sensitivity. However, the extent of degradation cannot be predicted based on the available information For the retina, the extent of degradation cannot be predicted based on the available information and therefore the impact of this factor on the effectiveness of a delayed PMI for 24 or 72 h cannot be assessed |
| The concentration at the moment of slaughter |
If the concentration is high, the compound may be still detectable even if degradation occurs If the concentration is close to the CCα/CCβ of the analytical method used, degradation may lead to a non‐detectable concentration This concentration depends on the route of application, the dose applied and the time of administration. This information is unknown since it is an illegal application |
The concentration at the moment of slaughter is not known and is a source of uncertainty. Therefore, the impact of this factor on the effectiveness of a delayed PMI for 24 or 72 h cannot be assessed | |
| Phenylbutazone | Degradation |
It is unclear to what extent degradation in the muscle, liver and kidney after slaughter and before sampling during PMI will happen. The carcasses are chilled which may slow down the degradation. Degradation of phenylbutazone may result in a non‐detectable concentration while a non‐compliant result would have been reported if sampling was done immediately after slaughter There is evidence of rapid phenylbutazone degradation in muscle tissue homogenates. However, this degradation may be due to the activation of enzymes during the homogenisation. No information regarding the stability in kidney and liver was identified |
The extent of degradation in muscle, liver and kidney cannot be predicted based on the available information and therefore the impact of this factor on the effectiveness of a delayed PMI for 24 or 72 h cannot be assessed. |
| The concentration at the moment of slaughter |
If the concentration is high, the compound may be still detectable even if degradation occurs If the concentration is close to the CCα/CCβ of the analytical method used, degradation may lead to a non‐detectable concentration This concentration depends on the route of application, the dose applied and the time of administration. This information is unknown since it is an illegal application |
The concentration at the moment of slaughter is not known and is a source of uncertainty. Therefore, the impact of this factor on the effectiveness of a delayed PMI for 24 or 72 h cannot be assessed |
CCα: decision limit; CCβ: detection capability; OTA: ochratoxin A; PMI: post‐mortem inspection; RAL: resorcylic acid lactones.
No uncertainties were identified for persistent organic pollutants, metals, natural steroids and nitrofurans
EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , Koutsoumanis K, Allende A, Alvarez‐Ordóñez A, Bolton D, Bover‐Cid S, Chemaly M, Davies R, De Cesare A, Herman L, Lindqvist R, Nauta M, Peixe L, Ru G, Simmons M, Skandamis P, Suffredini E, Sánchez JÁ, Blagojevic B, Fürst P, Garin‐Bastuji B, Jensen HE, Paulsen P, Baert K, Barrucci F, Broglia A, Georgiadis M, Hempen M and Hilbert F, 2020. Scientific Opinion on the evaluation of public and animal health risks in case of a delayed post‐mortem inspection in ungulates. EFSA Journal 2020;18(12):6307, 125 pp. 10.2903/j.efsa.2020.6307
Requestor: European Commission
Question number: EFSA‐Q‐2019‐00124
Panel members: Ana Allende, Avelino Alvarez‐Ordóñez, Declan Bolton, Sara Bover‐Cid, Marianne Chemaly, Robert Davies, Alessandra De Cesare, Lieve Herman, Friederike Hilbert, Konstantinos Koutsoumanis, Roland Lindqvist, Maarten Nauta, Luisa Peixe, Giuseppe Ru, Marion Simmons, Panagiotis Skandamis and Elisabetta Suffredini.
Acknowledgments: The BIOHAZ Panel wishes to thank the following for the support provided to this scientific output: the AHAW Panel: Julio Alvarez, Dominique Joseph Bicout, Paolo Calistri, Klaus Depner, Julian AshleyDrewe, Bruno Garin‐Bastuji, Jose Luis Gonzales Rojas, Christian Gortazar Schmidt, Virginie Michel, Miguel Angel Miranda Chueca, Søren Saxmose Nielsen, Helen Clare Roberts, Liisa Helena Sihvonen, Hans Spoolder, Karl Stahl, Antonio Velarde, Arvo Viltrop and Christoph Winckler; the CONTAM Panel: Margherita Bignami, Laurent Bodin, James Kevin Chipman, Jesus del Mazo, Bettina Grasl‐Kraupp, Christer Hogstrand, Laurentius (Ron) Hoogenboom, Jean‐Charles Leblanc, Carlo Stefano Nebbia, Elsa Nielsen, Evangelia Ntzani, Annette Petersen, Salomon Sand, Dieter Schrenk, Tanja Schwerdtle, Christiane Vleminckx and Heather Wallace; Andy Hart, Peter Craig; Kelly Niermans, Katrin Bote, Loran Postolovski, Erik Jergil, Asima Aganovic, Thea Ottinger, Jaime Garcia Alcorlo, Eduardo Medina, Jean‐Michel Cappelier, Vincent Hinoux, Manuel Varillas, Tina Lysgaard Hale, Gerardo Domínguez Peñafiel, Isabel Murillo, Carmen Olmos, Karel Dossche. The BIOHAZ Panel wishes to thank the hearing experts: Joachim Polzer, Saskia Sterk and Eric Verdon for the support provided to this scientific output.
Adopted: 21 October 2020
This article was originally published on the EFSA website www.efsa.europa.eu on 2 December 2020 as part of EFSA's publication procedures.
Notes
Regulation (EU) 2018/1629.
OJ L 131, 17 May 2019, p. 51.
Regulation (EC) No 853/2004, Annex III, Section IV, Chapter I.
‘Offal’ means fresh meat other than that of the carcass, including viscera and blood.
Carcass’ means the body of an animal after slaughter and dressing.
Commission Implementing Regulation (EU) 2019/627 of 15 March 2019 laying down uniform practical arrangements for the performance of official controls on products of animal origin intended for human consumption OJ L 131, 17.5.2019, p. 51–100.
Regulation (EU) 2016/429 of the European Parliament and of the Council of 9 March 2016 on transmissible animal diseases and amending and repealing certain acts in the area of animal health (‘Animal Health Law’). OJ L 84, 31.3.2016, p. 1–208 including Regulation (EU) 2018/1629 (see section 3.1.1 of this opinion).
Regulation (EC) No 853/2004 of the European Parliament and of the Council of 29 April 2004 laying down specific hygiene rules for food of animal origin. OJ L 139, 30.4.2004, p. 55–205.
Commission Delegated Regulation (EU) 2019/624 of 8 February 2019 concerning specific rules for the performance of official controls on the production of meat and for production and relaying areas of live bivalve molluscs in accordance with Regulation (EU) 2017/625 of the European Parliament and of the Council. OJ L 131, 17.5.2019, p. 1–17.
Regulation (EU) 2017/625 of the European Parliament and of the Council of 15 March 2017 on official controls and other official activities performed to ensure the application of food and feed law, rules on animal health and welfare, plant health and plant protection products, amending Regulations (EC) No 999/2001, (EC) No 396/2005, (EC) No 1069/2009, (EC) No 1107/2009, (EU) No 1151/2012, (EU) No 652/2014, (EU) 2016/429 and (EU) 2016/2031 of the European Parliament and of the Council, Council Regulations (EC) No 1/2005 and (EC) No 1099/2009 and Council Directives 98/58/EC, 1999/74/EC, 2007/43/EC, 2008/119/EC and 2008/120/EC, and repealing Regulations (EC) No 854/2004 and (EC) No 882/2004 of the European Parliament and of the Council, Council Directives 89/608/EEC, 89/662/EEC, 90/425/EEC, 91/496/EEC, 96/23/EC, 96/93/EC and 97/78/EC and Council Decision 92/438/EEC (Official Controls Regulation). OJ L 95, 7.4.2017, p. 1–142.
Council Directive 96/23/EC of 29 April 1996 on measures to monitor certain substances and residues thereof in live animals and animal products and repealing Directives 85/358/EEC and 86/469/EEC and Decisions 89/187/EEC and 91/664/EEC. OJ L 125, 23.5.1996, p. 10–32.
Commission Decision 97/747/EC of 27 October 1997 fixing the levels and frequencies of sampling provided for by Council Directive 96/23/EC for the monitoring of certain substances and residues thereof in certain animal products. OJ L 303, 6.11.1997, p. 12–15.
Commission Regulation (EU) No 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. OJ L 15, 20.1.2010, p. 1–72.
Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC. OJ L 70, 16.3.2005, p. 1–16.
Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. OJ L 364, 20.12.2006, p. 5–24.
Commission Regulation (EC) No 124/2009 of 10 February 2009 setting maximum levels for the presence of coccidiostats or histomonostats in food resulting from the unavoidable carry‐over of these substances in non‐target feed. OJ L 40, 11.2.2009, p. 7–11.
Council Directive 96/22/EC of 29 April 1996 concerning the prohibition on the use in stock farming of certain substances having a hormonal or thyrostatic action and of ß‐agonists, and repealing Directives 81/602/EEC, 88/146/EEC and 88/299/EEC. OJ L 125, 23.5.1996, p. 3–9.
Commission Regulation (EC) No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. OJ L 338, 22.12.2005, p. 1–26.
Council Directive 64/432/EEC of 26 June 1964 on animal health problems affecting intra‐Community trade in bovine animals and swine. OJ 121, 29.7.1964, p. 1977–2012.
Commission Delegated Regulation (EU) 2020/689 of 17 December 2019 supplementing Regulation (EU) 2016/429 of the European Parliament and of the Council as regards rules for surveillance, eradication programmes, and disease‐free status for certain listed and emerging diseases. OJ L 174, 3.6.2020, p. 211–340.
Commission Implementing Regulation (EU) 2015/1375 of 10 August 2015 laying down‐specific rules on official controls for Trichinella in meat. OJ L 212, 11.8.2015, p. 7–34.
Animal salmonellosis is not listed in Article 5(1)(a) and according to Article 5(1)(b) of Regulation (EU) 2016/429 of the European Parliament and of the Council (the Animal Health Law).
A third class sampling plan includes the number of tested samples (n) and the maximum number of acceptable positive samples (c), i.e., samples with Salmonella presence.
Regulation (EC) No 852/2004 of the European Parliament and of the Council of 29 April 2004 on the hygiene of foodstuffs. OJ L 139, 30.4.2004, p. 1–54.
As originally specified in Annex IV to Council Regulation (EEC) No 2377/90 of 26 June 1990. Now laid down in Annex, Table 2 of Regulation (EU) No 37/2010 of 22 December 2009.
Web of Science (WoS), formally ISI Web of Knowledge, Thomson Reuters. Available at: http://thomsonreuters.com/thomson-reuters-web-of-science/
PubMed, Entrez Global Query Cross‐Database Search System, National Center for Biotechnology Information (NCBI), National Library of Medicine (NLM), Department of the National Institutes of Health (NIH), United States Department of Health and Human Services. Available at: http://www.ncbi.nlm.nih.gov/pubmed/
EndNote X5, Thomson Reuters. Available at: http://endnote.com/
Commission Delegated Regulation (EU) 2018/1629 of 25 July 2018 amending the list of diseases set out in Annex II to Regulation (EU) 2016/429 of the European Parliament and of the Council on transmissible animal diseases and amending and repealing certain acts in the area of animal health (‘Animal Health Law’). OJ L 272, 31.10.2018, p. 11–15.
https://efsa.onlinelibrary.wiley.com/doi/toc/10.1002/(ISSN)1831‐4732.AHL
http://www.cfsph.iastate.edu/DiseaseInfo/index.php
OTF: Officially free of bovine tuberculosis according to the Council Directive 64/432/EEC.
Reproductive diseases in the graph include: brucellosis, CEM, dourine, Japanese encephalitis, Q feverIBR/IPV and trichomonosis; respiratory diseases include CBPP and CCPP.
Directive 2003/99/EC of the European Parliament and of the Council of 17 November 2003 on the monitoring of zoonoses and zoonotic agents, amending Council Decision 90/424/EEC and repealing Council Directive 92/117/EEC. OJ L 325, 12.12.2003, p. 31–40.
Regulation (EC) No 999/2001 of the European Parliament and of the Council of 22 May 2001 laying down rules for the prevention, control and eradication of certain transmissible spongiform encephalopathies. OJ L 147, 31.5.2001, p. 1–40.
https://www.oie.int/en/international‐standard‐setting/terrestrial‐manual/access‐online/
http://old.iss.it/binary/crlp/cont/MO_POPVI_01.05_Procedure_PT_Trichinella_rev_4_.pdf
https://eurlp.iss.it/wp‐content/uploads/2018/02/Technical‐report‐Trichinella‐PTs‐2016‐final.pdf
CCα is the decision limit at and above which it can be concluded with an error probability of α that a sample is non‐compliant.
CCβ is the detection capability, meaning the smallest content of the substance that may be detected, identified and/or quantified in a sample with an error probability of β.
The minimum method performance requirement (MMPR) is the minimum concentration that laboratories should be able to reliably identify. Laboratories should ensure that the CCβ for screening methods or CCα for confirmatory methods is lower than the MMPR (EURL, 2020).
Council Directive 81/602/EEC of 31 July 1981 concerning the prohibition of certain substances having a hormonal action and of any substances having a thyrostatic action. OJ L 222, 7.8.1981, p. 32–33.
Commission Regulation (EU) 2019/1871 of 7 November 2019 on reference points for action for non‐allowed pharmacologically active substances present in food of animal origin and repealing Decision 2005/34/EC. OJ L 289, 8.11.2019, p. 41–46.
References
- Ackermann MR, 2017. Inflammation and Healing In Zachary JF. and McGavin MD. (eds.). In Pathologic Basis of Veterinary Disease (6th Edition). Elsevier Health Sciences, pp. 73–131. [Google Scholar]
- Anonymous , 2017. ISO 6579–1 Microbiology of the Food Chain – Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella – Part 1: Detection of Salmonella spp. International Organization for Standardization, Geneva, Switzerland. [DOI] [PubMed] [Google Scholar]
- Arnold ME, Ryan JB, Konold T, Simmons MM, Spencer YI, Wear A, Chaplin M, Stack M, Czub S, Mueller R, Webb PR, Davis A, Spiropoulos J, Holdaway J, Hawkins SA, Austin AR and Wells GA, 2007. Estimating the temporal relationship between prpsc detection and incubation period in experimental bovine spongiform encephalopathy of cattle. Journal of General Virology, 88(Pt 11), 3198–3208. 10.1099/vir.0.82987-0 [DOI] [PubMed] [Google Scholar]
- Aschbacher PW, 1976. Diethylstilbestrol metabolism in food‐producing animals. Journal of Toxicology and Environmental Health, 45–59. [PubMed] [Google Scholar]
- Battacone G, Nudda A and Pulina G, 2010. Effects of ochratoxin a on livestock production. Toxins (Basel), 2, 1796–1824. 10.3390/toxins2071796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benestad SL, Mitchell G, Simmons M, Ytrehus B and Vikoren T, 2016. First case of chronic wasting disease in europe in a norwegian free‐ranging reindeer. Veterinary Research, 47, 88 10.1186/s13567-016-0375-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borcher E and Arinder P, 2002. Bacteriological safety issues in red meat and ready‐to‐eat meat products, as well as control measures. Meat Science, 62, 381–390. 10.1016/s0309-1740(02)00125-0 [DOI] [PubMed] [Google Scholar]
- Bosilevac JM, Arthur TM, Bono JL, Brichta‐Harhay DM, Kalchayanand N, King DA, Shackelford SD, Wheeler TL and Koohmaraie M, 2009. Prevalence and enumeration of Escherichia Coli O157:H7 and Salmonella in U.S. Abattoirs that process fewer than 1000 head of cattle per day. Journal of Food Protection, 72, 1272–1278. 10.4315/0362-028x-72.6.1272 [DOI] [PubMed] [Google Scholar]
- Brichta‐Harhay DM, Arthur TM, Bosilevac JM, Guerini MN, Kalchayanand N and Koohmaraie M, 2007. Enumeration of Salmonella and Escherichia Coli O157:H7 in ground beef, cattle carcass, hide and faecal samples using direct plating methods. Journal of Applied Microbiology, 103, 1657–1668. 10.1111/j.1365-2672.2007.03405.x [DOI] [PubMed] [Google Scholar]
- Buncic S, 2006. Integrated Food Safety and Veterinary Public Health: CABI Pub. Available online: https://books.google.de/books?id=gKYngYKNg34C
- Buonpane NA, Brown MP, Gronwall R, Stone HW and Miles N, 1988. Serum concentrations and pharmacokinetics of chloramphenicol in foals after a single oral dose. Equine Veterinary Journal, 20, 59–61. 10.1111/j.2042-3306.1988.tb01455.x [DOI] [PubMed] [Google Scholar]
- Burrows GE, Tyler RD, Craigmill AL and Barto PB, 1984. Chloramphenicol and the Neonatal Calf. American Journal of Veterinary Research, 45, 1586–1591. [PubMed] [Google Scholar]
- Carignan G, MacIntosh AI, Skakum W and Sved S, 1988. Dimetridazole Residues in Pork Tissue. Ii. Application of liquid chromatographic method to monitor elimination of drug and its major metabolite. J Assoc Off Anal Chem, 71, 1146–1149. [PubMed] [Google Scholar]
- Chang VP, Mills EW and Cutter CN, 2003. Reduction of bacteria on pork carcasses associated with chilling method. Journal of Food Protection, 66, 1019–1024. 10.4315/0362-028x-66.6.1019 [DOI] [PubMed] [Google Scholar]
- Chaplin MJ, Barlow N, Ryder S, Simmons MM, Spencer Y, Hughes R and Stack MJ, 2002. Evaluation of the effects of controlled autolysis on the immunodetection of Prp(Sc) by immunoblotting and immunohistochemistry from natural cases of scrapie and Bse. Research in Veterinary Science, 72, 37–43. 10.1053/rvsc.2001.0518 [DOI] [PubMed] [Google Scholar]
- Cheville NF, 2006. Introduction to Veterinary Pathology (Third Edition): Ames. Blackwell Publishing, Iowa: pp. 61–65. [Google Scholar]
- Coetzer JAW and Tustin RC, 2005. Infectious Diseases of Livestock, 2nd Edition ed. Oxford University Press. 1950 pp. [Google Scholar]
- Cooper AD, Tarbin JA, Farrington WH and Shearer G, 1998. Aspects of extraction, spiking and distribution in the determination of incurred residues of chloramphenicol in animal tissues. Food Additives & Contaminants, 15, 637–644. 10.1080/02652039809374692 [DOI] [PubMed] [Google Scholar]
- Cooper KM, Mulder PP, van Rhijn JA, Kovacsics L, McCracken RJ, Young PB and Kennedy DG, 2005. Depletion of four nitrofuran antibiotics and their tissue‐bound metabolites in porcine tissues and determination using Lc‐Ms/Ms and Hplc‐Uv. Food Additives & Contaminants, 22, 406–414. 10.1080/02652030512331385218 [DOI] [PubMed] [Google Scholar]
- Cooper KM and Kennedy DG, 2007. Stability studies of the metabolites of nitrofuran antibiotics during storage and cooking. Food Additives and Contaminants, 24, 935–942. [DOI] [PubMed] [Google Scholar]
- CRL (Community Reference Laboratories), 2007. Crl Guidance Paper (7 December 2008). CRLs View on State of the Art Analytical Methods for National Residue Control Plans. Available online: https://www.bvl.bund.de/SharedDocs/Downloads/07_Untersuchungen/EURL_Empfehlungen_Konzentrationsauswahl_Methodenvalierungen_EN.html?nn=11011448
- CVMP (Committee for Veterinary Medicinal Products). 1996a. Dimetridazole (3). Summary Report. Available online: https://www.ema.europa.eu/en/documents/mrl-report/dimetridazole-summary-report-3-committee-veterinary-medicinal-products_en.pdf
- CVMP (Committee for Veterinary Medicinal Products). 1996b. Ronidazole (1). Summary Report. Available online: https://www.ema.europa.eu/en/documents/mrl-report/ronidazole-summary-report-1-committee-veterinary-medicinal-products_en.pdf
- CVMP (Committee for Veterinary Medicinal Products). 1997. Phenylbutazone. Summary Report. Emea/Mrl/297/97-Final.
- CVMP (Committee for Veterinary Medicinal Products), 2000. Clenbuterol. Summary Report (2). Emea/Mrl/723/99-Final. Available online: https://www.ema.europa.eu/en/documents/mrl-report/clenbuterol-summary-report-2-committee-veterinary-medicinal-products_en.pdf
- Dagorn M, Guillot P and Sanders P, 1990. Pharmacokinetics of chloramphenicol in sheep after intravenous, intramuscular and subcutaneous administration. Veterinary Quarterly, 12, 166–174. 10.1080/01652176.1990.9694262 [DOI] [PubMed] [Google Scholar]
- Dixon SN and Russell KL, 1986. Radioimmunoassay of the Anabolic Agent Zeranol. Iv. The Determination of Zeranol Concentrations in the Edible Tissues of Cattle Implanted with Zeranol (Ralgro). Journal of Veterinary Pharmacology and Therapeutics, 9, 94–100. 10.1111/j.1365-2885.1986.tb00017.x [DOI] [PubMed] [Google Scholar]
- Dixon SN, Russell KL, Heitzman RJ and Mallinson CB, 1986. Radioimmunoassay of the Anabolic Agent Zeranol. V. Residues of Zeranol in the Edible Tissues, Urine, Faeces and Bile of Steers Treated with Ralgro. Journal of Veterinary Pharmacology and Therapeutics, 9, 353–358. 10.1111/j.1365-2885.1986.tb00055.x [DOI] [PubMed] [Google Scholar]
- Dorny P and Praet N, 2007. Taenia Saginata in Europe. Veterinary Parasitology, 149, 22–24. 10.1016/j.vetpar.2007.07.004 [DOI] [PubMed] [Google Scholar]
- Dorny P, Vercammen F, Brandt J, Vansteenkiste W, Berkvens D and Geerts S, 2000. Sero‐epidemiological study of taenia saginata cysticercosis in belgian cattle. Veterinary Parasitology, 88, 43–49. 10.1016/s0304-4017(99)00196-x [DOI] [PubMed] [Google Scholar]
- Dorny P, Phiri IK, Vercruysse J, Gabriel S, Willingham AL 3rd, Brandt J, Victor B, Speybroeck N and Berkvens D, 2004. A bayesian approach for estimating values for prevalence and diagnostic test characteristics of porcine cysticercosis. International Journal for Parasitology, 34, 569–576. 10.1016/j.ijpara.2003.11.014 [DOI] [PubMed] [Google Scholar]
- Duarte SC, Lino CM and Pena A, 2011. Ochratoxin a in feed of food‐producing animals: an undesirable mycotoxin with health and performance effects. Veterinary Microbiology, 154, 1–13. 10.1016/j.vetmic.2011.05.006 [DOI] [PubMed] [Google Scholar]
- Duffy E, Mooney MH, Elliott CT and O'Keeffe M, 2009a. Studies on the persistence of estradiol benzoate and nortestosterone decanoate in hair of cattle following treatment with growth promoters, determined by ultra‐high‐performance liquid chromatography‐tandem mass spectrometry. Journal of Chromatography A, 1216, 8090–8095. 10.1016/j.chroma.2009.04.053 [DOI] [PubMed] [Google Scholar]
- Duffy E, Rambaud L, Le Bizec B and O'Keeffe M, 2009b. Determination of hormonal growth promoters in bovine hair: comparison of liquid chromatography‐mass spectrometry and gas chromatography‐mass spectrometry methods for estradiol benzoate and nortestosterone decanoate. Analytica Chimica Acta, 637, 165–172. 10.1016/j.aca.2008.09.070 [DOI] [PubMed] [Google Scholar]
- Dupuy C, Morlot C, Gilot‐Fromont E, Mas M, Grandmontagne C, Gilli‐Dunoyer P, Gay E and Callait‐Cardinal MP, 2014. Prevalence of Taenia Saginata Cysticercosis in French Cattle in 2010. Veterinary Parasitology, 203, 65–72. 10.1016/j.vetpar.2014.02.054 [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2004. EFSA Scientific Report (2004) 18, 1‐13 on the Evaluation of Seven New Rapid post mortem BSE Tests. EFSA Journal 2004;2(11):18r, 13 pp. 10.2903/j.efsa.2004.18r [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2005a. EFSA Scientific Report (2005) 31, 1‐17 on the Evaluation of Rapid post mortem TSE Tests intended for Small Ruminants. EFSA Journal 2005;3(6):31r, 17 pp. 10.2903/j.efsa.2005.31r [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2005b. EFSA Scientific Report (2005) 49, 1‐16 on the Evaluation of Rapidpost mortem TSE Tests intended for Small Ruminants. EFSA Journal 2005;3(10):49r, 16 pp. 10.2903/j.efsa.2005.49r [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2006. Opinion of the Scientific Panel on Contaminants in the Food Chain [Contam] Related to Ochratoxin a in Food. EFSA Journal 2006;4(6):365, 56 pp. 10.2903/j.efsa.2006.365 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2007a. Scientific Opinion of the Panel on Biological Hazards on a request from the European Commission on a protocol for the evaluation of rapid post mortem tests to detect TSE in small ruminants. EFSA Journal 2007;5(6):509, 31 pp. 10.2903/j.efsa.2007.509 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2007b. Scientific Opinion of the Panel on Biological Hazards on a request from the European Commission on a protocol for the evaluation of new rapid BSE post mortem tests. EFSA Journal 2007;5(6):508, 20 pp. 10.2903/j.efsa.2007.508 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2010a. Results of the Monitoring of Dioxin Levels in Food and Feed. EFSA Journal 2010;8(3):1385, 36 pp. 10.2903/j.efsa.2010.1385 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2010b. Results of the Monitoring of Non Dioxin‐Like Pcbs in Food and Feed. EFSA Journal 2010;8(7):1701, 35 pp. 10.2903/j.efsa.2010.1701 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2012. Update of the Monitoring of Levels of Dioxins and Pcbs in Food and Feed. EFSA Journal 2012;10(7):2832, 82 pp. 10.2903/j.efsa.2012.2832 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2014. Guidance on Expert Knowledge Elicitation in Food and Feed Safety Risk Assessment. EFSA Journal 2014;12(6):3734, 278 pp. 10.2903/j.efsa.2014.3734 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2015. Scientific Report on the Evaluation of the Application of Denmark to Be Recognised as Having a Negligible Risk of Classical Scrapie. EFSA Journal 2015;13(11):4294, 24 pp. 10.2903/j.efsa.2015.4294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2019. The European Union Summary Report on Surveillance for the Presence of Transmissible Spongiform Encephalopathies (Tse) in 2018. EFSA Journal 2019;17(12):5925, 61 pp. 10.2903/j.efsa.2019.5925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2005a. EFSA Scientific Report (2005) 48, 1‐10 on the Evaluation of Two Rapid post mortem BSE Tests. EFSA Journal 2005;3(9):48r, 10 pp. http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2005.48r/full [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2009. Scientific Opinion on ASe of approved TSE rapid tests. EFSA Journal 2009;7(12):1436, 32 pp. 10.2903/j.efsa.2009.1436 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2010. Scientific Opinion on the Results of the Eu Survey for Chronic Wasting Disease (Cwd) in Cervids. EFSA Journal 2010;8(10):2010. 10.2903/j.efsa.2010.1861 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2012. Scientific Opinion on the Evaluation of New Tse Rapid Tests Submitted in the Framework of the Commission Call for Expression of Interest 2007/S204‐247339. EFSA Journal 2012;10(5):2660, 32 pp. 10.2903/j.efsa.2012.2660 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2013a. Scientific Opinion on the Public Health Hazards to Be Covered by Inspection of Meat (Bovine Animals). EFSA Journal 2013;11(6):3266, 186 pp. 10.2903/j.efsa.2013.3266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2013b. Scientific Opinion on the Public Health Hazards to Be Covered by Inspection of Meat from Farmed Game. EFSA Journal 2013;11(6):3264, 181 pp. 10.2903/j.efsa.2013.3264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2013c. Scientific Opinion on the Public Health Hazards to Be Covered by Inspection of Meat from Sheep and Goats. EFSA Journal 2013;11(6):3265, 186 pp. 10.2903/j.efsa.2013.3265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biologicial Hazards), 2013d. Scientific Opinion on the Public Health Hazards to Be Covered by Inspection of Meat (Solipeds). EFSA Journal 2013;11(6):3263, 162 pp. 10.2903/j.efsa.2013.3263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2014. Scientific Opinion on the Scrapie Situation in the Eu after 10 Years of Monitoring and Control in Sheep and Goats. EFSA Journal 2014;12(7):3781, 155 pp. 10.2903/j.efsa.2014.3781 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel, EFSA CONTAM Panel and EFSA AHAW Panel (Panels on Biological Hazards on Contaminants in the Food Chain and on Animal Health and Welfare) , 2011. Scientific Opinion on the Public Health Hazards to be Covered by Inspection of Meat (Swine). EFSA Journal 2011;9(10):2351, 198 pp. 10.2903/j.efsa.2011.2351 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), Ricci A, Allende A, Bolton D, Chemaly M, Davies R, Fernandez Escamez PS, Girones R, Herman L, Koutsoumanis K, Lindqvist R, Norrung B, Robertson L, Sanaa M, Skandamis P, Snary E, Speybroeck N, Ter Kuile B, Threlfall J, Wahlstrom H, Benestad S, Gavier‐Widen D, Miller MW, Ru G, Telling GC, Tryland M, Ortiz Pelaez A and Simmons M, 2017. Chronic Wasting Disease (Cwd) in Cervids. EFSA Journal 2017;15(1):4667, 62 pp. 10.2903/j.efsa.2017.4667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), Ricci A, Allende A, Bolton D, Chemaly M, Davies R, Fernandez Escamez PS, Girones R, Herman L, Koutsoumanis K, Lindqvist R, Norrung B, Robertson L, Ru G, Sanaa M, Skandamis P, Snary E, Speybroeck N, Kuile BT, Threlfall J, Wahlstrom H, Benestad S, Gavier‐Widen D, Miller MW, Telling GC, Tryland M, Latronico F, Ortiz‐Pelaez A, Stella P and Simmons M, 2018. Scientific Opinion on Chronic Wasting Disease (Ii). EFSA Journal 2018;15(1):5132, 59 pp. 10.2903/j.efsa.2018.5132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), Koutsoumanis K, Allende A, Alvarez‐Ordóñez A, Bolton D, Bover‐Cid S, Chemaly M, Davies R, De Cesare A, Herman L, Hilbert F, Lindqvist R, Nauta M, Peixe L, Ru C, Skandamis P, Suffredini E, Andreoletti O, Benestad SL, Comoy N, Nonno R, da Silva Felicio T, Ortiz‐Pelaez A and Simmons MM, 2019. Update on Chronic Wasting Disease (Cwd) Iii. EFSA Journal 2019;17(11):5863, 63 pp. 10.2903/j.efsa.2019.5863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2009. Cadmium in Food ‐ Scientific Opinion of the Panel on Contaminants in the Food Chain. EFSA Journal 2009;7(3):980, 139 pp. 10.2903/j.efsa.2009.980 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2010. Scientific Opinion on Lead in Food. EFSA Journal 2010;8(4):1570, 151 pp. 10.2903/j.efsa.2010.1570 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2011a. Scientific Opinion on Polybrominated Diphenyl Ethers (Pbdes) in Food. EFSA Journal 2011;9(5):2156, 274 pp. 10.2903/j.efsa.2011.2156 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2011b. Scientific Opinion on the Risk to Public Health Related to the Presence of High Levels of Dioxins and Dioxin‐Like Pcbs in Liver from Sheep and Deer. EFSA Journal 2011;9(7):2297, 71 pp. 10.2903/j.efsa.2011.2297 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2011c. Scientific Opinion on the Risks for Public Health Related to the Presence of Zearalenone in Food. EFSA Journal2011;9(6):2197, 124 pp. 10.2903/j.efsa.2011.2197 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2012a. Scientific Opinion on the Presence of Dioxins (Pcdd/Fs) and Dioxin‐Like Pcbs (Dl‐Pcbs) in Commercially Available Foods for Infants and Young Children. EFSA Journal 2012;10(12):2983, 29 pp. 10.2903/j.efsa.2012.2983 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2012b. Scientific Opinion on the Risk for Public Health Related to the Presence of Mercury and Methylmercury in Food. EFSA Journal 2012;10(12):2985, 241 pp. 10.2903/j.efsa.2012.2985 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2014. Scientific Opinion on Chloramphenicol in Food and Feed. EFSA Journal 2014;12(11):3907, 146 pp. 10.2903/j.efsa.2014.3907 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2015. Scientific Opinion on Nitrofurans and Their Metabolites in Food. EFSA Journal 2015;13(6):4140, 217 pp. 10.2903/j.efsa.2015.4140 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), Knutsen HK, Alexander J, Barregard L, Bignami M, Bruschweiler B, Ceccatelli S, Cottrill B, Dinovi M, Edler L, Grasl‐Kraupp B, Hogstrand C, Hoogenboom LR, Nebbia CS, Oswald IP, Petersen A, Rose M, Roudot AC, Vleminckx C, Vollmer G, Wallace H, Bodin L, Cravedi JP, Halldorsson TI, Haug LS, Johansson N, van Loveren H, Gergelova P, Mackay K, Levorato S, van Manen M and Schwerdtle T, 2018a. Risk to Human Health Related to the Presence of Perfluorooctane Sulfonic Acid and Perfluorooctanoic Acid in Food. EFSA Journal 2018;16(12):5194, 284 pp. 10.2903/j.efsa.2018.5194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), Knutsen HK, Alexander J, Barregard L, Bignami M, Bruschweiler B, Ceccatelli S, Cottrill B, Dinovi M, Edler L, Grasl‐Kraupp B, Hogstrand C, Nebbia CS, Oswald IP, Petersen A, Rose M, Roudot AC, Schwerdtle T, Vleminckx C, Vollmer G, Wallace H, Furst P, Hakansson H, Halldorsson T, Lundebye AT, Pohjanvirta R, Rylander L, Smith A, van Loveren H, Waalkens‐Berendsen I, Zeilmaker M, Binaglia M, Gomez Ruiz JA, Horvath Z, Christoph E, Ciccolallo L, Ramos Bordajandi L, Steinkellner H and Hoogenboom LR, 2018b. Risk for Animal and Human Health Related to the Presence of Dioxins and Dioxin‐Like Pcbs in Feed and Food. EFSA Journal 2018;16(11):5333, 331 pp. 10.2903/j.efsa.2018.5333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), Schrenk D, Bignami M, Bodin L, Chipman JK, del Mazo J, Grasl‐Kraupp B, Hogstrand C, Hoogenboom LR, Leblanc JC, Nebbia CS, Nielsen E, Ntzani E, Petersen A, Sand S, Vleminckx C, Wallace H, Barregard L, Ceccatelli S, Cravedi J‐P, Halldorsson TI, Haug LS, Johansson N, Knutsen HK, Rose M, Roudot A‐C, Van Loveren H, Vollmer G, Mackay K, Riolo F and Schwerdtle T, 2020a. Scientific Opinion on the risk to human health related to the presence of perfluoroalkyl substances in food. EFSA Journal 2020;18(9):6223, 391 pp. 10.2903/j.efsa.2020.6223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), Schrenk D, Bodin L, Kevin Chipman JK, del Mazo J, Grasl‐Kraupp B, Hogstrand C, Hoogenboom L, Leblanc JC, Stefano Nebbia C, Nielsen E, Ntzani E, Petersen A, Sand S, Schwerdtle T, Vleminckx C, Wallace H, Alexander J, Dall'Asta C, Mally A, Metzler M, Binaglia M, Horváth Z, Steinkellner H and Bignami M, 2020b. Risk Assessment of Ochratoxin a in Food. EFSA Journal 2020;18(5):6113, 150 pp. 10.2903/j.efsa.2020.6113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2009. Safety Evaluation of Ractopamine. EFSA Journal 2009;7(4):1041, 52 pp. 10.2903/j.efsa.2009.1041 [DOI] [Google Scholar]
- EFSA Scientific Committee , Benford D, Halldorsson T, John Jeger M, Katrine Knutsen H, More S, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Schlatter JR, Silano V, Solecki R, Turck D, Younes M, Craig P, Hart A, Von Goetz N, Koutsoumanis K, Mortensen A, Ossendorp B, Martino L, Merten C, Mosbach‐Schulz O and Hardy A, 2018. Guidance on Uncertainty Analysis in Scientific Assessments. EFSA Journal 2018;16(1):5123, 39 pp. 10.2903/j.efsa.2018.5123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), Arcella D, Baert K, Binaglia M, Gervelmeyer A, Innocenti ML, Ribo O, Steinkellner H and Verhagen H, 2016. Review of Proposed Mrls, Safety Evaluation of Products Obtained from Animals Treated with Zilpaterol and Evaluation of the Effects of Zilpaterol on Animal Health and Welfare. EFSA Journal 2016;14(9):4579, 15 pp. 10.2903/j.efsa.2016.4579 [DOI] [Google Scholar]
- Eichenberger RM, Stephan R and Deplazes P, 2011. Increased Sensitivity for the Diagnosis of Taenia Saginata Cysticercus Infection by Additional Heart Examination Compared to the Eu‐Approved Routine Meat Inspection. Food Control, 22, 989–992. 10.1016/j.foodcont.2010.11.033 [DOI] [Google Scholar]
- Eichenberger RM, Lewis F, Gabriel S, Dorny P, Torgerson PR and Deplazes P, 2013. Multi‐Test Analysis and Model‐Based Estimation of the Prevalence of Taenia Saginata Cysticercus Infection in Naturally Infected Dairy Cows in the Absence of a ‘Gold Standard’ Reference Test. International Journal for Parasitology, 43, 853–859. 10.1016/j.ijpara.2013.05.011 [DOI] [PubMed] [Google Scholar]
- Erasmuson AF, Scahill BG and West DM, 1994. Natural Zeranol (Alpha‐Zearalanol) in the Urine of Pasture‐Fed Animals. Journal of Agricultural and Food Chemistry, 42, 2721–2725. [Google Scholar]
- Etuk EU and Onyeyili PA, 2005a. Pharmacokinetics of Chloramphenicol in Healthy and Water‐Deprived Goats. International Journal of Pharmacology, 1, 244–248. 10.3923/ijp.2005.244.248 [DOI] [Google Scholar]
- Etuk EU and Onyeyili PA, 2005b. The Tissue Kinetics and Residues of Chloramphenicol in Sokoto Red Goats. International Journal of Pharmacology, 2, 24–27. 10.3923/ijp.2006.24.27 [DOI] [Google Scholar]
- EURL (European Union Reference Laboratories), 2020. EURL guidance on minimum method performance requirements (MMPRs) for specific pharmacologically active substances in specific animal matrices. September 2020, 8 pp. Available online: https://sitesv2.anses.fr/en/system/files/EURL_MMPR_guidance%20paper_final.pdf
- EURL for Parasites , 2006. Guideline for the Identification and Development of Sampling Methods and Design of Suitable Protocols for Monitoring of Trichinella Infection in Indicator Species. Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/biosafety_food-borne-disease_trich_guideline_sampling-methods.pdf [PubMed]
- EURLs (European Union Reference Laboratories) for Pharmacologically Active Substances, 2019. Joint Report: National Residue Control Plan (Nrcp) Evaluation 2019. 518 pp. Available Online: http://iispv.ro/documente/cat_view/17-documente-interne/150-pncr-2019
- Everest SJ, Thorne L, Barnicle DA, Edwards JC, Elliott H, Jackman R and Hope J, 2006. Atypical Prion Protein in Sheep Brain Collected During the British Scrapie‐Surveillance Programme. Journal of General Virology, 87(Pt 2), 471–477. 10.1099/vir.0.81539-0 [DOI] [PubMed] [Google Scholar]
- Fahmi HA, Williamson NB, Tibary A and Hegstad RL, 1985. The Influence of Some Sample Handling Factors on Progesterone and Testosterone Analysis in Goats. Theriogenology, 24, 227–233. 10.1016/0093-691x(85)90187-6 [DOI] [PubMed] [Google Scholar]
- Fan PC, Ma YX, Kuo CH and Chung WC, 1998. Survival of Taenia Solium Cysticerci in Carcasses of Pigs Kept at 4 C. The Journal of Parasitology, 84 10.2307/3284553 [DOI] [PubMed] [Google Scholar]
- FAO/WHO , 1988. Zeranol. Residues of Some Veterinary Drugs in Animals and Foods. Fao Food and Nutrition Paper 41/1. Monographs prepared by the Thirty‐second Meeting of the Joint FAO/WHO Expert Committee on Food Additives: pp. 38–47. [PubMed]
- FAO/WHO , 1994. Ronidazole. Residue Evaluation of Certain Veterinary Drugs. Joint FAO/WHO Expert Committee on Food Additives (JECFA), 42nd meeting 1994. FAO JECFA Monograph 41. pp. 53–65.
- FAO/WHO , 2013. Zilpaterol Hydrochloride. Residue Evaluation of Certain Veterinary Drugs. Joint FAO/WHO Expert Committee on Food Additives (JECFA), 78th meeting 2013. FAO JECFA Monograph 15: pp. 133‐159.
- Fegan N, Vanderlinde P, Higgs G and Desmarchelier P, 2005. A Study of the Prevalence and Enumeration of Salmonella Enterica in Cattle and on Carcasses During Processing. Journal of Food Protection, 68, 1147–1153. 10.4315/0362-028x-68.6.1147 [DOI] [PubMed] [Google Scholar]
- Franssen F, Gerard C, Cozma‐Petruţ A, Vieira‐Pinto M, Jambrak AR, Rowan N, Paulsen P, Rozycki M, Tysnes K, Rodriguez‐Lazaro D and Robertson L, 2019. Inactivation of Parasite Transmission Stages: Efficacy of Treatments on Food of Animal Origin. Trends in Food Science & Technology, 83, 114–128. 10.1016/j.tifs.2018.11.009 [DOI] [Google Scholar]
- Gajadhar AA, Noeckler K, Boireau P, Rossi P, Scandrett B and Gamble HR, 2019. International Commission on Trichinellosis: Recommendations for Quality Assurance in Digestion Testing Programs for Trichinella. Food Waterborne Parasitol, 16, e00059 10.1016/j.fawpar.2019.e00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajecka M, 2013. The Effect of Experimental Low Zearalenone Intoxication on Ovarian Follicles in Pre‐Pubertal Bitches. Polish Journal of Veterinary Sciences, 16, 45–54. [PubMed] [Google Scholar]
- Gamble HR, Bessonov AS, Cuperlovic K, Gajadhar AA, van Knapen F, Noeckler K, Schenone H and Zhu X, 2000. International Commission on Trichinellosis: Recommendations on Methods for the Control of Trichinella in Domestic and Wild Animals Intended for Human Consumption. Veterinary Parasitology, 93, 393–408. 10.1016/s0304-4017(00)00354-x [DOI] [PubMed] [Google Scholar]
- Gassner B and Wuethrich A, 1994. Pharmacokinetic and Toxicological Aspects of the Medication of Beef‐Type Calves with an Oral Formulation of Chloramphenicol Palmitate. Journal of Veterinary Pharmacology and Therapeutics, 17, 279–283. 10.1111/j.1365-2885.1994.tb00246.x [DOI] [PubMed] [Google Scholar]
- Gentili A, Caretti F, Bellante S, Mainero Rocca L, Curini R and Venditti A, 2012. Development and Validation of Two Multiresidue Liquid Chromatography Tandem Mass Spectrometry Methods Based on a Versatile Extraction Procedure for Isolating Non‐Steroidal Anti‐Inflammatory Drugs from Bovine Milk and Muscle Tissue. Analytical and Bioanalytical Chemistry, 404, 1375–1388. 10.1007/s00216-012-6231-0 [DOI] [PubMed] [Google Scholar]
- Gill CO and Jones T, 2000. Microbiological Sampling of Carcasses by Excision or Swabbing. Journal of Food Protection, 63, 167–173. [DOI] [PubMed] [Google Scholar]
- Gorski L, 2012. PLoS ONE, 7, e34722. 10.1371/journal.pone.0034722 Epub 2012 Apr 4. PMID: 22496847. [DOI] [PMC free article] [PubMed]
- Gracey J, Collins DS and Huey R, 1999. Meat Hygiene, 10th Edition Saunders, UK. [Google Scholar]
- Grau FD, 1986. Microbial ecology of meat and poultry In Pearson AM, Dutson TR. (eds.). CSIRO. Meat Research Laboratory, Cannon Hill, Qld, Advances in Meat Research. Volume 2/ . AVI Publishing, London. pp. 1‐47. [Google Scholar]
- Gray BP, Viljanto M, Bright J, Pearce C and Maynard S, 2013. Investigations into the Feasibility of Routine Ultra High Performance Liquid Chromatography‐Tandem Mass Spectrometry Analysis of Equine Hair Samples for Detecting the Misuse of Anabolic Steroids, Anabolic Steroid Esters and Related Compounds. Analytica Chimica Acta, 787, 163–172. 10.1016/j.aca.2013.05.058 [DOI] [PubMed] [Google Scholar]
- Gronwall R, Brown MP, Merritt AM and Stone HW, 1986. Body Fluid Concentrations and Pharmacokinetics of Chloramphenicol Given to Mares Intravenously or by Repeated Gavage. American Journal of Veterinary Research, 47, 2591–2595. [PubMed] [Google Scholar]
- Guan F, Uboh CE, Soma LR, Luo Y, Rudy J and Tobin T, 2005. Detection, Quantification and Confirmation of Anabolic Steroids in Equine Plasma by Liquid Chromatography and Tandem Mass Spectrometry. Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences, 829, 56–68. 10.1016/j.jchromb.2005.09.045 [DOI] [PubMed] [Google Scholar]
- Hagelberg S, Hult K and Fuchs R, 1989. Toxicokinetics of Ochratoxin a in Several Species and Its Plasma‐Binding Properties. Journal of Applied Toxicology, 9, 91–96. 10.1002/jat.2550090204 [DOI] [PubMed] [Google Scholar]
- Han Z, Zhao Z, Shi J, Liao Y, Zhao Z, Zhang D, Wu Y, De Saeger S and Wu A, 2013. Combinatorial Approach of Lc‐Ms/Ms and Lc‐Tof‐Ms for Uncovering in Vivo Kinetics and Biotransformation of Ochratoxin a in Rat. Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences, 925, 46–53. 10.1016/j.jchromb.2013.02.028 [DOI] [PubMed] [Google Scholar]
- Heeremans A, Ermens A, De Wasch KK, Van Peteghem C and De Brabander HF, 1998. Elimination Profile of Methylthiouracil in Cows after Oral Administration. Analyst, 123, 2629–2632. 10.1039/a805113e [DOI] [PubMed] [Google Scholar]
- Herman J, Vermeersch H, Remon JP and De Backer P, 1989. Pharmacokinetics and Bioavailability of Ronidazole from a Prolonged Release Tablet in the Homing Pigeon (Columba Livia). Journal of Veterinary Pharmacology and Therapeutics, 12, 46–49. 10.1111/j.1365-2885.1989.tb00640.x [DOI] [PubMed] [Google Scholar]
- Hill DE, Forbes L, Gajadhar AA and Gamble HR, 2007. Viability and Infectivity of Trichinella Spiralis Muscle Larvae in Frozen Horse Tissue. Veterinary Parasitology, 146, 102–106. 10.1016/j.vetpar.2007.02.001 [DOI] [PubMed] [Google Scholar]
- Hilwig Ronald W, Cramer James D and Forsyth Kerry S, 1978. Freezing Times and Temperatures Required to Kill Cysticerci of Taenia Saginata in Beef. Veterinary Parasitology, 4, 215–219. 10.1016/0304-4017(78)90048-1 [DOI] [Google Scholar]
- Hoffmann B and Karg H, 1976. Metabolic Fate of Anabolic Agents in Treated Animals and Residue Levels in Their Meat. Environmental quality and safety, 5, 181–191. [PubMed] [Google Scholar]
- Hoogenboom LAP and Polman THG, 1993. Simultaneous Detection of Protein Bound Residues of the Nitrofuran Drugs Furazolidone, Furaltadone, Nitrofurazone and Nitrofurantoin., Utrecht, Netherlands: pp. 376–381. [Google Scholar]
- Hoogenboom LAP, van Kammen M, Berghmans MCJ, Koeman JH and Kuiper HA, 1991. The Use of Pig Hepatocytes to Study the Nature of Protein‐Bound Metabolites of Furazolidone: A New Analytical Method for Their Detection. Food and Chemical Toxicology, 29, 321–328. 10.1016/0278-6915(91)90203-j [DOI] [PubMed] [Google Scholar]
- Hoogenboom LA, Berghmans MC, Polman TH, Parker R and Shaw IC, 1992. Depletion of Protein‐Bound Furazolidone Metabolites Containing the 3‐Amino‐2‐Oxazolidinone Side‐Chain from Liver, Kidney and Muscle Tissues from Pigs. Food Additives & Contaminants, 9, 623–630. 10.1080/02652039209374117 [DOI] [PubMed] [Google Scholar]
- Horne E, Cadogan A, O'Keeffe M and Hoogenboom LA, 1996. Analysis of Protein‐Bound Metabolites of Furazolidone and Furaltadone in Pig Liver by High‐Performance Liquid Chromatography and Liquid Chromatography‐Mass Spectrometry. Analyst, 121, 1463–1468. 10.1039/an9962101463 [DOI] [PubMed] [Google Scholar]
- Hult K, Hokby E, Hagglund U, Gatenbeck S, Rutqvist L and Sellyey G, 1979. Ochratoxin a in Pig Blood: Method of Analysis and Use as a Tool for Feed Studies. Applied and Environment Microbiology, 38, 772–776. 10.1128/AEM.38.5.772-776.1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC (International Agency for Research on Cancer), 2012. Diethylstilbestrol. Iarc Monographs 100a, pp. 175–218.
- ICMSF , 1996. Characteristics of Microbial Pathogens. Chapter 11: Parasites: Trichinella Spiralis Microorganisms in Foods. Vol 5 Blackie A&P, London. [Google Scholar]
- Inghelbrecht S, Vermeersch H, Ronsmans S, Remon JP, DeBacker P and Vercruysse J, 1996. Pharmacokinetics and Anti‐Trichomonal Efficacy of a Dimetridazole Tablet and Water‐Soluble Powder in Homing Pigeons (Columba Livia). Journal of Veterinary Pharmacology and Therapeutics, 19, 62–67. 10.1111/j.1365-2885.1996.tb00010.x [DOI] [PubMed] [Google Scholar]
- Jansen F, Dorny P, Berkvens D, Van Hul A, Van den Broeck N, Makay C, Praet N, Eichenbergerd RM, Deplazes P and Gabriel S, 2017. High Prevalence of Bovine Cysticercosis Found During Evaluation of Different Post‐Mortem Detection Techniques in Belgian Slaughterhouses. Veterinary Parasitology, 244, 1–6. 10.1016/j.vetpar.2017.07.009 [DOI] [PubMed] [Google Scholar]
- Jansky AM, 1983. Mise Au Point D'une Méthode Sensible D'extraction Et De Dosage Des Résidus De Zéranol. Proc. Symp. OIE sur les Anabolisants en productions animales : Santé publique, méthodes analytiques et réglementation. Edited by OIE. 15‐17 février 1983, Paris, France. E. Meissonnier (Ed), OIE, Paris, 467‐480.
- Jensen HE, Leifsson PS, Nielsen OL, Agerholm JS and Iburg T, 2017. Meat Inspection – the Pathoanatomic Basis, 1st Edition Biofolia; pp. 1–839. [Google Scholar]
- Jovic S, Djordjevic M, Kulisic Z, Pavlovic S and Radenkovic B, 2001. Infectivity of Trichinella Spiralis Larvae in Pork Buried in the Ground. Parasite, 8, S213–S215. 10.1051/parasite/200108s2213 [DOI] [PubMed] [Google Scholar]
- Kennedy DG, McEvoy JD, Blanchflower WJ, Hewitt SA, Cannavan A, McCaughey WJ and Elliott CT, 1995. Possible Naturally Occurring Zeranol in Bovine Bile in Northern Ireland. Zentralbl Veterinarmed B, 42, 509–512. 10.1111/j.1439-0450.1995.tb00742.x [DOI] [PubMed] [Google Scholar]
- Kennedy DG, Hewitt SA, McEvoy JD, Currie JW, Cannavan A, Blanchflower WJ and Elliot CT, 1998. Zeranol Is Formed from Fusarium Spp. Toxins in Cattle in Vivo. Food Additives & Contaminants, 15, 393–400. 10.1080/02652039809374658 [DOI] [PubMed] [Google Scholar]
- Khen BK, Lynch OA, Carroll J, McDowell DA and Duffy G, 2014. Prevalence and Characteristics of Salmonella in the Beef Chain in the Republic of Ireland. Zoonoses Public Health, 61, 534–536. 10.1111/zph.12099 [DOI] [PubMed] [Google Scholar]
- King T, Kocharunchitt C, Gobius K, Bowman JP and Ross T, 2016. Physiological Response of Escherichia Coli O157:H7 Sakai to Dynamic Changes in Temperature and Water Activity as Experienced During Carcass Chilling. Molecular & Cellular Proteomics: MCP, 15, 3331–3347. 10.1074/mcp.M116.063065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsella KJ, Rowe TA, Blair IS, McDowell DA and Sheridan JJ, 2006. Survival and Recovery of Salmonella Enterica Serovar Typhimurium Dt104 at Low Temperature and Water Activity in a Broth System. Foodborne Pathog Dis, 3, 375–383. 10.1089/fpd.2006.3.375 [DOI] [PubMed] [Google Scholar]
- Klingeborn M, Wik L, Simonsson M, Renstrom LH, Ottinger T and Linne T, 2006. Characterization of Proteinase K‐Resistant N‐ and C‐Terminally Truncated Prp in Nor98 Atypical Scrapie. Journal of General Virology, 87(Pt 6), 1751–1760. 10.1099/vir.0.81618-0 [DOI] [PubMed] [Google Scholar]
- von Köller J, Kapel CM, Enemark HL and Hindsbo O, 2001. Infectivity of Trichinella Spp. recovered from decaying mouse and fox muscle tissue. Parasite, 8, S209–S212. 10.1051/parasite/200108s2209 [DOI] [PubMed] [Google Scholar]
- Launay FM, Ribeiro L, Alves P, Vozikis V, Tsitsamis S, Alfredsson G, Sterk SS, Blokland M, Iitia A, Lovgren T, Tuomola M, Gordon A and Kennedy DG, 2004. Prevalence of Zeranol, Taleranol and Fusarium Spp. Toxins in Urine: Implications for the Control of Zeranol Abuse in the European Union. Food Additives & Contaminants, 21, 833–839. 10.1080/02652030400002121 [DOI] [PubMed] [Google Scholar]
- Lees P and Toutain PL, 2013. Pharmacokinetics, Pharmacodynamics, Metabolism, Toxicology and Residues of Phenylbutazone in Humans and Horses. Vet J, 196, 294–303. 10.1016/j.tvjl.2013.04.019 [DOI] [PubMed] [Google Scholar]
- Lega F, Angeletti R, Stella R, Rigoni L, Biancotto G, Giusepponi D, Moretti S, Saluti G and Galarini R, 2017. Abuse of Anabolic Agents in Beef Cattle: Could Bile Be a Possible Alternative Matrix? Food Chemistry, 229, 188–197. 10.1016/j.foodchem.2017.02.069 [DOI] [PubMed] [Google Scholar]
- Leitner A, Zöllner P and Lindner W, 2001. Determination of the Metabolites of Nitrofuran Antibiotics in Animal Tissue by High‐Performance Liquid Chromatography–Tandem Mass Spectrometry. Journal of Chromatography A, 939, 49–58. 10.1016/s0021-9673(01)01331-0 [DOI] [PubMed] [Google Scholar]
- Lenahan M, Kelly S, Fanning S and Bolton DJ, 2010. The Effect of Bovine Diet on Salmonella Survival in Synthetic Abomasal Fluid. Journal of Applied Microbiology, 109, 2060–2068. 10.1111/j.1365-2672.2010.04836.x [DOI] [PubMed] [Google Scholar]
- Mackey BM and Derrick CM, 1986. Peroxide Sensitivity of Cold‐Shocked Salmonella Typhimurium and Escherichia Coli and Its Relationship to Minimal Medium Recovery. Journal of Applied Bacteriology, 60, 501–511. 10.1111/j.1365-2672.1986.tb01089.x [DOI] [PubMed] [Google Scholar]
- MacNeil JD, Reid J, Neiser CD and Fesser AC, 2003. Single‐Laboratory Validation of a Modified Liquid Chromatographic Method with Uv Detection for Determination of Trenbolone Residues in Bovine Liver and Muscle. Journal of AOAC International, 86, 916–924. [PubMed] [Google Scholar]
- Mannion C, Fanning J, McLernon J, Lendrum L, Gutierrez M, Duggan S and Egan J, 2012. The Role of Transport, Lairage and Slaughter Processes in the Dissemination of Salmonella Spp. In Pigs in Ireland. Food Research International, 45, 871–879. 10.1016/j.foodres.2011.02.001 [DOI] [Google Scholar]
- Mantle PG, 2008. Interpretation of the Pharmacokinetics of Ochratoxin a in Blood Plasma of Rats, During and after Acute or Chronic Ingestion. Food and Chemical Toxicology, 46, 1808–1816. 10.1016/j.fct.2008.01.020 [DOI] [PubMed] [Google Scholar]
- Martinez J, Perez‐Serrano J, Bernadina WE and Rodriguez‐Caabeiro F, 2001. Stress Response to Cold in Trichinella Species. Cryobiology, 43, 293–302. 10.1006/cryo.2001.2363 [DOI] [PubMed] [Google Scholar]
- Martinez‐Chavez L, Cabrera‐Diaz E, Perez‐Montano JA, Garay‐Martinez LE, Varela‐Hernandez JJ, Castillo A, Lucia L, Avila‐Novoa MG, Cardona‐Lopez MA, Gutierrez‐Gonzalez P and Martinez‐Gonzales NE, 2015. Quantitative Distribution of Salmonella Spp. And Escherichia Coli on Beef Carcasses and Raw Beef at Retail Establishments. International Journal of Food Microbiology, 210, 149–155. 10.1016/j.ijfoodmicro.2015.06.016 [DOI] [PubMed] [Google Scholar]
- McCracken RJ, Blanchflower WJ, Rowan C, McCoy MA and Kennedy DG, 1995. Determination of Furazolidone in Porcine Tissue Using Thermospray Liquid Chromatography‐Mass Spectrometry and a Study of the Pharmacokinetics and Stability of Its Residues. Analyst, 120, 2347–2351. 10.1039/an9952002347 [DOI] [PubMed] [Google Scholar]
- Mercer HD, Heath GE, Long PE, Showalter DH and Powers TE, 1978. Drug Residues in Food Animals. I. Plasma and Tissue Kinetics of Chloramphenicol in Young Cross‐Bred Swine. Journal of Veterinary Pharmacology and Therapeutics, 1, 19–36. 10.1111/j.1365-2885.1978.tb00301.x [DOI] [Google Scholar]
- Meyer HH and Rinke LM, 1991. The Pharmacokinetics and Residues of Clenbuterol in Veal Calves. Journal of Animal Science, 69, 4538–4544. 10.2527/1991.69114538x [DOI] [PubMed] [Google Scholar]
- Miles CO, Erasmuson AF, Wilkins AL, Towers NR, Smith BL, Garthwaite I, Scahill BG and Hansen RP, 1996. Ovine Metabolism of Zearalenone to Α‐Zearalanol (Zeranol). Journal of Agricultural and Food Chemistry, 44, 3244–3250. 10.1021/jf9601325 [DOI] [Google Scholar]
- Møller CO, Ilg Y, Aabo S, Christensen BB, Dalgaard P and Hansen TB, 2013. Effect of Natural Microbiota on Growth of Salmonella Spp. In Fresh Pork–a Predictive Microbiology Approach. Food Microbiology, 34, 284–295. 10.1016/j.fm.2012.10.010 [DOI] [PubMed] [Google Scholar]
- Monleón E, Monzon M, Hortells P, Vargas A and Badiola JJ, 2003. Detection of Prp(Sc) in Samples Presenting a Very Advanced Degree of Autolysis (Bse Liquid State) by Immunocytochemistry. Journal of Histochemistry and Cytochemistry, 51, 15–18. 10.1177/002215540305100103 [DOI] [PubMed] [Google Scholar]
- Narvaez‐Bravo C, Miller MF, Jackson T, Jackson S, Rodas‐Gonzalez A, Pond K, Echeverry A and Brashears MM, 2013. Salmonella and Escherichia Coli O157:H7 Prevalence in Cattle and on Carcasses in a Vertically Integrated Feedlot and Harvest Plant in Mexico. Journal of Food Protection, 76, 786–795. 10.4315/0362-028X.JFP-12-079 [DOI] [PubMed] [Google Scholar]
- Newkirk DR, Righter HF, Schenck FJ, Okrasinski JL Jr and Barnes CJ, 1990. Gas Chromatographic Determination of Incurred Dimetridazole Residues in Swine Tissues. J Assoc Off Anal Chem, 73, 702–704. [PubMed] [Google Scholar]
- Nielen MW, Nijrolder AW, Hooijerink H and Stolker AA, 2011. Feasibility of Desorption Electrospray Ionization Mass Spectrometry for Rapid Screening of Anabolic Steroid Esters in Hair. Analytica Chimica Acta, 700, 63–69. 10.1016/j.aca.2010.08.009 [DOI] [PubMed] [Google Scholar]
- Ninios T, Lundén J, Korkeala H and Fredriksson‐Ahomaa M, 2014. Meat Inspection and Control in the Slaughterhouse: Wiley. Available online: https://books.google.de/books?id=yTNuDwAAQBAJ
- Nöckler K, Pozio E, Voigt WP and Heidrich J, 2000. Detection of Trichinella Infection in Food Animals. Veterinary Parasitology, 93, 335–350. 10.1016/s0304-4017(00)00350-2 [DOI] [PubMed] [Google Scholar]
- Nouws JF and Laurensen J, 1990. Postmortal degradation of furazolidone and furaltadone in edible tissues of calves. Veterinary Quarterly, 12, 56–59. 10.1080/01652176.1990.9694243 [DOI] [PubMed] [Google Scholar]
- Nouws JF and Ziv G, 1978. A Study of chloramphenicol distribution and residues in dairy cows. Netherlands Journal of Veterinary Science, 103, 725–735. [PubMed] [Google Scholar]
- Nouws JF and Ziv G, 1979. Serum chloramphenicol levels and the intramuscular bioavailability of several parenteral formulations of chloramphenicol in ruminants. Veterinary Quarterly, 1, 47–58. [DOI] [PubMed] [Google Scholar]
- Nouws JF and Ziv G, 1982. Pharmacological Aspects of Chloramphenicol Administration by the Intramammary Route to Lactating Dairy Cows. Veterinary Quarterly, 4, 23–31. [DOI] [PubMed] [Google Scholar]
- Nouws JF, Vree TB, Holtkamp J, Bookman M, Driessens F and Guelen PJM, 1986. Pharmacokinetic, residue and irritation aspects of chloramphenicol sodium succinate and a chloramphenicol base formulation following intramuscular administration to ruminants. Veterinary Quarterly, 8, 224–232. [DOI] [PubMed] [Google Scholar]
- Oakley JES and O'Hagan A, 2016. Shelf: The Sheffield Elicitation Framework (Version 3.0). School of Mathematics and Statistics, University of Sheffield, Uk. Available Online: Http://Tonyohagan.Co.Uk/Shelf
- O'Brien SB, Duffy G, Carney E, Sheridan JJ, Mcdowell DA and Blair IS, 2005. Prevalence and Numbers of Escherichia coliO157 on Bovine Hides at a Beef Slaughter Plant. Journal of Food Protection, 68, 660–665. [DOI] [PubMed] [Google Scholar]
- Ogunremi O, MacDonald G, Geerts S and Brandt J, 2004. Diagnosis of taenia saginata cysticercosis by immunohistochemical test on formalin‐fixed and paraffin‐embedded bovine lesions. Journal of Veterinary Diagnostic Investigation, 16, 438–441. 10.1177/104063870401600513 [DOI] [PubMed] [Google Scholar]
- OIE , 2018. Chapter 2.9.5. – Cysticercosis.
- OIE , 2019. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. World Health Organization for Animal Health, Paris, France. Available online: https://www.oie.int/standard-setting/terrestrial-manual/access-online/ [Google Scholar]
- OIE , 2020. Technical Disease Cards. World Health Organization for Animal Health, Paris: France. Available online: https://www.oie.int/animal-health-in-the-world/technical-disease-cards/ [Google Scholar]
- O'Keeffe M, 1984. Measuring Residues of Growth Promoters Used in Beef Production. Farm Fd Res, 15, 25–27. [Google Scholar]
- O'Keeffe MJ, O'Keeffe M and Glennon JD, 1999.. Supercritical Fluid Extraction (Sfe) as a Multi‐Residue Extraction Procedure for Beta‐Agonists in Bovine Liver Tissue. Analyst, 124, 1355–1360. 10.1039/a902715g [DOI] [PubMed] [Google Scholar]
- Owen IL and Reid SA, 2007. Survival of trichinella papuae muscle larvae in a pig carcass maintained under simulated natural conditions in papua new Guinea. Journal of Helminthology, 81, 429–432. 10.1017/S0022149X07850255 [DOI] [PubMed] [Google Scholar]
- Palumbo SA, Klein P, Capra J, Eblen S and Miller AJ, 1999. Comparison of excision and swabbing sampling methods to determine the microbiological quality of swine carcass surfaces. Food Microbiology, 1999, 459–464. [Google Scholar]
- Pan Y, Yi J, Zhou B, Xie S, Chen D, Tao Y, Qu W, Liu Z, Huang L and Yuan Z, 2017. Disposition and residue depletion of metronidazole in pigs and broilers. Scientific Reports, 7, 7203 10.1038/s41598-017-07443-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris A, Andre F, Antignac J‐P, Le Bizec B, Bonneau M, Briant C, Caraty A, Chillard Y, Cognie Y, Combarnous Y, Cravedi J‐P, Fabre‐Nys C, Fernandez‐Suarez A, Fostier A, Humblot P, Laudet V, Leboeuf B, Louveau I, Malpaux B, Martinat‐Botte F, Maurel MC, Pelicier‐Rubio M‐T, Picard‐Hagen N, Pinault L, Pinel G, Ponsard C, Popot M‐A, Schmidely P, Toutain P‐L and Zalko D, 2006. Hormones Et promoteurs de croissance en productions animales. De La Physiologie À L’évaluation Du Risque. INRA Prod Anim, 19, 149–240. [Google Scholar]
- Parker RM and Shaw IC, 1988. Determination of chloramphenicol in tissues‐problems with in vitro metabolism. Analyst, 113, 1875–1876. 10.1039/an9881301875 [DOI] [PubMed] [Google Scholar]
- Pathania A, McKee SR, Bilgili SF and Singh M, 2010. Antimicrobial activity of commercial marinades against multiple strains of Salmonella spp. International Journal of Food Microbiology, 139, 214–217. 10.1016/j.ijfoodmicro.2010.01.039 [DOI] [PubMed] [Google Scholar]
- Paulsen P, 2005. Control of trichinellosis: food hygiene considerations based on the haccp concept. Wiener Tierarztliche Monatsschrift, 92, 298–300 <Go to ISI>://WOS:000235672800005. [Google Scholar]
- Pin C, Avendano‐Perez G, Cosciani‐Cunico E, Gomez N, Gounadakic A, Nychas GJ, Skandamis P and Barker G, 2011. Modelling Salmonella concentration throughout the pork supply chain by considering growth and survival in fluctuating conditions of temperature, Ph and a(W). Int J Food Microbiol 145 Suppl, 1, S96–S102. 10.1016/j.ijfoodmicro.2010.09.025 [DOI] [PubMed] [Google Scholar]
- Pinel G, Mathieu S, Cesbron N, Maume D, De Brabander HF, Andre F and Le Bizec B, 2006. Evidence that urinary excretion of thiouracil in adult bovine submitted to a cruciferous diet can give erroneous indications of the possible illegal use of thyrostats in meat production. Food Additives & Contaminants, 23, 974–980. 10.1080/02652030600806370 [DOI] [PubMed] [Google Scholar]
- Pinheiro I, Jesuino B, Barbosa J, Ferreira H, Ramos F, Matos J and da Silveira MI, 2009. Clenbuterol storage stability in the bovine urine and liver samples Used for European Official Control in the Azores Islands (Portugal). Journal of Agriculture and Food Chemistry, 57, 910–914. 10.1021/jf802995e [DOI] [PubMed] [Google Scholar]
- Pirisinu L, Tran L, Chiappini B, Vanni I, Di Bari MA, Vaccari G, Vikoren T, Madslien KI, Vage J, Spraker G, Mitchell A, Balachandran T, Baron C, Casalone CM, Rolandsen KH, Roed U, Agrimi R, Nonno A and Benestad SL, 2018. Novel type of chronic wasting disease detected in moose (alces Alces), Norway. Emerging Infectious Diseases, 24, 2210–2218. 10.3201/eid2412.180702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polzer J, 2020. Message to the Working Group on Delayed Meat Inspection. Vol 20 Teleconference, May 2020. [Google Scholar]
- Polzer J and Gowik P, 2005. Nitroimidazoles in Turkey muscle—the influence of sampling conditions on measurement results in residue control. Analytica Chimica Acta, 529, 299–303. 10.1016/j.aca.2004.09.033 [DOI] [Google Scholar]
- Polzer J, Stachel C and Gowik P, 2004. Treatment of Turkeys with Nitroimidazoles. Analytica Chimica Acta, 521, 189–200. 10.1016/j.aca.2004.06.019 [DOI] [Google Scholar]
- Pozio E, 2016. Adaptation of Trichinella Spp. For Survival in Cold Climates. Food and Waterborne Parasitology, 4, 4–12. 10.1016/j.fawpar.2016.07.001 [DOI] [Google Scholar]
- Pozio E, 2018. Trichinella and Other Foodborne Nematodes In: Ortega YN. and Sterling CR. (eds.). Foodborne Parasites. Springer International Publishing; pp. 175–215. [Google Scholar]
- Pozio E and Zarlenga DS, 2013. New Pieces of the Trichinella Puzzle. International Journal for Parasitology, 43, 983–997. 10.1016/j.ijpara.2013.05.010 [DOI] [PubMed] [Google Scholar]
- Pozio E, La Rosa G, Rossi P and Fico R, 1989. Survival of trichinella muscle larvae in frozen wolf tissue in Italy. Journal of Parasitology, 75, 472–473. [PubMed] [Google Scholar]
- Pozio E, Hoberg E, La Rosa G and Zarlenga DS, 2009. Molecular taxonomy, phylogeny and biogeography of nematodes belonging to the trichinella genus. Infect Genet Evol 9, no. 4 (Jul), 606–616. 10.1016/j.meegid.2009.03.003 [DOI] [PubMed] [Google Scholar]
- Radeck W, 2010. Β‐Agonists in Hair. Presentation given at the EU‐Workshop May 2010, Berlin.
- Rao VR and Clarenburg R, 1977. Pharmacokinetics of intravenously injected chloramphenicol in baby pigs. Drug Metabolism and Disposition, 5, 253–258. [PubMed] [Google Scholar]
- Rattenberger E, 1991. Stability of testosterone in bovine blood depending on the time of separation of serum or plasma. Archiv für Lebensmittelhygiene, 42, 64–65. [Google Scholar]
- Rattenberger E, Matzke P, Funke P and Meyer HHD, 1987. Elimination of 19‐nortestosterone after treatment of calves with anabolicum, fortadex and laurabolin. Archiv für Lebensmittelhygiene, 38, 73–76. [Google Scholar]
- Reid R, Fanning S, Whyte P, Kerry J and Bolton D, 2017. The fate of salmonella typhimurium and Escherichia Coli O157 on hot boned versus conventionally chilled beef. Meat Science, 126, 50–54. 10.1016/j.meatsci.2016.12.010 [DOI] [PubMed] [Google Scholar]
- Reimers TJ, Lamb SV, Bartlett SA, Matamoros RA, Cowan RG and Engle JS, 1991. Effects of hemolysis and storage on quantification of hormones in blood samples from dogs, cattle, and horses. American Journal of Veterinary Research, 52, 1075–1080. [PubMed] [Google Scholar]
- Ricke SC, Dawoud TM, Kim SA, Park SH and Kwon YM, 2018. Salmonella cold stress response: mechanisms and occurrence in foods. Advances in Applied Microbiology, 104, 1–38. 10.1016/bs.aambs.2018.03.001 [DOI] [PubMed] [Google Scholar]
- Riehn K, Hasenclever D, Petroff D, Nockler K, Mayer‐Scholl A, Makrutzki G and Lucker E, 2013. Trichinella detection: identification and statistical evaluation of sources of error in the magnetic stirrer method for pooled sample digestion. Veterinary Parasitology, 194, 106–109. 10.1016/j.vetpar.2013.01.031 [DOI] [PubMed] [Google Scholar]
- Riva E, Steffan P, Guzman M and Fiel C, 2012. Persistence of trichinella spiralis muscle larvae in natural decaying mice. Parasitology Research, 111, 249–255. 10.1007/s00436-012-2826-9 [DOI] [PubMed] [Google Scholar]
- Rossi P and Pozio E, 2008. Guidelines for the detection of Trichinella larvae at the slaughterhouse in a quality assurance system. Ann Ist Super Sanita, 44, 195–199. https://iss-eurlp.azurewebsites.net/wp-content/uploads/2018/02/ANN_08_Rossi_revised_by_Pozio.pdf [PubMed] [Google Scholar]
- Rossi L, Interisano M, Deksne G and Pozio E, 2019. The subnivium, a haven for trichinella larvae in host carcasses. Int J Parasitol Parasites Wildl, 8, 229–233. 10.1016/j.ijppaw.2019.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder SJ, Dexter GE, Heasman L, Warner R and Moore SJ, 2009. Accumulation and dissemination of prion protein in experimental sheep scrapie in the natural host. BMC Veterinary Research, 5, 9 10.1186/1746-6148-5-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchi L, Corona S, Gajadhar AA and Pozio E, 2001. Ultrastructural characteristics of nurse cell‐larva complex of four species of trichinella in several hosts. Parasite, 8, S54–S58. 10.1051/parasite/200108s2054 [DOI] [PubMed] [Google Scholar]
- Sanders P, Guillot P and Mourot D, 1988. Pharmacokinetics of a long‐acting chloramphenicol formulation administered by intramuscular and subcutaneous routes in cattle. Journal of Veterinary Pharmacology and Therapeutics, 11, 183–190. 10.1111/j.1365-2885.1988.tb00139.x [DOI] [PubMed] [Google Scholar]
- Sanders P, Guillot P, Dagorn M and Delmas JM, 1991. Liquid chromatographic determination of chloramphenicol in calf tissues: studies of stability in muscle, liver and kidney. Journal of the Association of Official Analytical Chemists, 74, 483–486. [PubMed] [Google Scholar]
- Sarasa R, Becher D, Badiola JJ and Monzon M, 2013. A comparative study of modified confirmatory techniques and additional immuno‐based methods for non‐conclusive autolytic bovine spongiform encephalopathy cases. BMC Veterinary Research, 9, 212 10.1186/1746-6148-9-212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer MJ, Pickett RJ, Limer S and Dixon SN, 1995. Distribution and elimination of clenbuterol in tissues and fluids of calves following prolonged oral administration at a growth‐promoting dose. Journal of Veterinary Pharmacology and Therapeutics, 18, 81–86. 10.1111/j.1365-2885.1995.tb00559.x [DOI] [PubMed] [Google Scholar]
- Scandrett B, Parker S, Forbes L, Gajadhar A, Dekumyoy P, Waikagul J and Haines D, 2009. Distribution of taenia saginata cysticerci in tissues of experimentally infected cattle. Veterinary Parasitology, 164, 223–231. 10.1016/j.vetpar.2009.05.015 [DOI] [PubMed] [Google Scholar]
- Schoen FJ, 2005. In: Kumar V, Abbas AK. and Fausto N. (eds.). “The Heart”. In Robbins and Cotran Pathologic Basis of Disease (7th Edition). Elsevier Saunders, Philadelphia, Pennsylvania: 611 pp. [Google Scholar]
- Singer RS, Mayer AE, Hanson TE and Isaacson RE, 2009. Do microbial interactions and cultivation media decrease the accuracy of salmonella surveillance systems and outbreak investigations? Journal of Food Protection, 72, 707–713. 10.4315/0362-028x-72.4.707 [DOI] [PubMed] [Google Scholar]
- Sotelo J, 1986. Freezing of Infested Pork Muscle Kills Cysticerci. JAMA: The Journal of the American Medical Association, 256 10.1001/jama.1986.03380070099027 [DOI] [PubMed] [Google Scholar]
- Speranza B, Bevilacqua A, Mastromatteo M, Sinigaglia M and Corbo MR, 2010. Modelling the interactions between pseudomonas putida and Escherichia Coli O157:H7 in fish‐burgers: use of the lag‐exponential model and of a combined interaction index. Journal of Applied Microbiology, 109, 667–678. 10.1111/j.1365-2672.2010.04692.x [DOI] [PubMed] [Google Scholar]
- Stachel CS, Radeck W and Gowik P, 2003. Zilpaterol—a new focus of concern in residue analysis. Analytica Chimica Acta, 493, 63–67. 10.1016/s0003-2670(03)00863-8 [DOI] [Google Scholar]
- Stander MA, Nieuwoudt TW, Steyn PS, Shephard GS, Creppy EE and Sewram V, 2001. Toxicokinetics of Ochratoxin a in Vervet Monkeys (Cercopithecus Aethiops). Archives of Toxicology, 75, 262–269. 10.1007/s002040100227 [DOI] [PubMed] [Google Scholar]
- Staubmann M, 2017. Ph‐Werte Im Rückenmuskel Von Rot‐ Und Schwarzwildtierkörpern in Einem Wildbearbeitungsbetrieb (Diploma Thesis).” vetmeduni Vienna.
- Sterk S, 2019. Re: Request for Information Related to Delayed Meat Inspection. Message to Katleen Baert. 15 September 2019. E‐mail.
- Stolker AA, Groot MJ, Lasaroms JJ, Nijrolder AW, Blokland MH, Riedmaier I, Becker C, Meyer HH and Nielen MW, 2009. Detectability of testosterone esters and estradiol benzoate in bovine hair and plasma following pour‐on treatment. Analytical and Bioanalytical Chemistry, 395, 1075–1087. 10.1007/s00216-009-3037-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studer‐Rohr I, Schlatter J and Dietrich DR, 2000. Kinetic parameters and intraindividual fluctuations of ochratoxin a plasma levels in humans. Archives of Toxicology, 74, 499–510. 10.1007/s002040000157 [DOI] [PubMed] [Google Scholar]
- Switala M, Pozniak B, Paslawska U, Grabowski T, Motykiewicz‐Pers K and Bobrek K, 2016. Metronidazole pharmacokinetics during rapid growth in turkeys ‐ relation to changes in haemodynamics and drug metabolism. Journal of Veterinary Pharmacology and Therapeutics, 39, 373–380. 10.1111/jvp.12283 [DOI] [PubMed] [Google Scholar]
- Theodoropoulos G, Styliara M, Petrakos M and Kapel CM, 2003. Effect of fox, pig, sheep, and poultry bile on the establishment of domestic and sylvatic species of trichinella in rats. Parasitology, 126,(Pt 5), 461–464. 10.1017/s003118200300307x [DOI] [PubMed] [Google Scholar]
- Tulliez J, 1992. Metabolism and Residue Study of 14c‐Ru 42173 in the Cattle (Steer and Heifer) after Single Oral Administration. Unpublished Report of Study No. 90/01/Xl from Inra‐Laboratoire Des Xénobiotiques, 180 Chemin De Tournefeuille, 31931 Toulouse, France. Document No. V‐0238-0104. submitted to FAO by MSD Animal Health.
- Van Hoof N, De Wasch K, Poelmans S, Noppe H and De Brabander H, 2004. Multi‐residue liquid chromatography/tandem mass spectrometry method for the detection of non‐steroidal anti‐inflammatory drugs in bovine muscle: optimisation of ion trap parameters. Rapid Communications in Mass Spectrometry, 18, 2823–2829. 10.1002/rcm.1683 [DOI] [PubMed] [Google Scholar]
- Vanantwerpen G, De Zutter L, Berkvens D and Houf K, 2016. Impact of the sampling method and chilling on the Salmonella recovery from pig carcasses. International Journal of Food Microbiology, 232, 22–25. [DOI] [PubMed] [Google Scholar]
- Vanden Bussche J, Vanhaecke L, Deceuninck Y, Wille K, Bekaert K, Le Bizec B and De Brabander HF, 2011. Ultra‐High Performance Liquid Chromatography Coupled to Triple Quadrupole Mass Spectrometry Detection of Naturally Occurring Thiouracil in Urine of Untreated Livestock, Domesticated Animals and Humans. Food Addit Contam Part A Chem Anal Control Expo Risk Assess, 28, 166–172. 10.1080/19440049.2010.544681 [DOI] [PubMed]
- Vanden Bussche J, Sterk SS, De Brabander HF, Blokland MH, Deceuninck Y, Le Bizec B and Vanhaecke L, 2012. Thyreostatic drugs, stability in bovine and porcine urine. Analytical and Bioanalytical Chemistry, 403, 2973–2982. 10.1007/s00216-012-5739-7 [DOI] [PubMed] [Google Scholar]
- Vass M, Hruska K and Franek M, 2008. Nitrofuran antibiotics: a review on the application, prohibition and residual analysis. Veterinarni Medicina, 53, 469–500. [Google Scholar]
- Vikøren T, Vage J, Madslien KI, Roed KH, Rolandsen CM, Tran L, Hopp P, Veiberg V, Heum M, Moldal T, Neves CGD, Handeland K, Ytrehus B, Kolbjornsen O, Wisloff H, Terland R, Saure B, Dessen KM, Svendsen SG, Nordvik BS and Benestad SL, 2019. First detection of chronic wasting disease in a wild red deer (Cervus Elaphus) in Europe. Journal of Wildlife Diseases, 55, 970–972. 10.7589/2018-10-262 [DOI] [PubMed] [Google Scholar]
- Vroomen Louis HM, Berghmans Marcel CJ, Van Leeuwen P, Van Der Struijs Teunis DB, De Vries Peter HU and Kuiper Harry A, 1986. Kinetics of 14c‐furazolidone in piglets upon oral administration during 10 days and its interaction with tissue macro‐molecules. Food Additives and Contaminants, 3, 331–346. 10.1080/02652038609373600 [DOI] [Google Scholar]
- Vulic A, Pleadin J, Persi N, Milic D and Radeck W, 2012. Uplc‐Ms/Ms determination of ractopamine residues in retinal tissue of treated food‐producing pigs. Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences, 895–896, 102–107. 10.1016/j.jchromb.2012.03.025 [DOI] [PubMed] [Google Scholar]
- Wear A, Henderson K, Webster K and Patel I, 2005. A comparison of rapid bovine spongiform encephalopathy testing methods on autolyzed bovine brain tissue. Journal of Veterinary Diagnostic Investigation, 17, 99–102. 10.1177/104063870501700201 [DOI] [PubMed] [Google Scholar]
- WHO/FAO/OIE , 2005. Guidelines for the surveillance, prevention and control of taeniosis/cysticercosis.
- Whyte P, Fanning S, O'Brien S, O'Grady L and Solomon K, 2011. Chapter 18 Tracing pathogens in red meat and game production chains and at the abattoir. In Tracing Pathogens in the Food Chain. Woodhead Publishing Series in Food Science, Technology and Nutrition. 10.1533/9780857090508.4.393 [DOI]
- Wozniak B, 1998. Effect of freezing on anabolic hormone residues in bovine and poultry meat samples. Bulletin of the Veterinary Institute in Pulawy, 42, 173–176. [Google Scholar]
- Yu SL, Cooke P and Tu SI, 2001. Effects of chilling on sampling of bacteria attached to swine carcasses. Letters in Applied Microbiology, 32, 205–210. 10.1046/j.1472-765x.2001.00886.x [DOI] [PubMed] [Google Scholar]
- Zhou Z, Jin X, Zheng H, Li J, Meng C, Yin K, Xie X, Huang C, Lei T, Sun X, Xia Z, Zeng Y, Pan Z and Jiao X, 2018. The prevalence and load of Salmonella, and key risk points of salmonella contamination in a swine slaughterhouse in Jiangsu province, China. Food Control, 87, 153–160. 10.1016/j.foodcont.2017.12.026 [DOI] [Google Scholar]