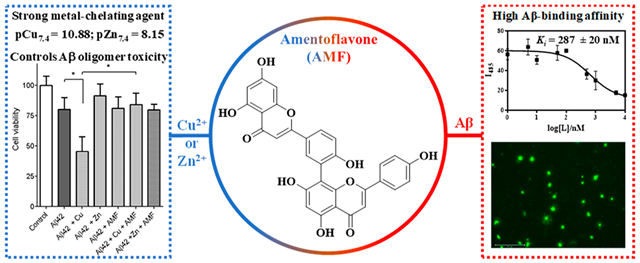
Abstract
Alzheimer’s disease (AD) is the most common neurodegenerative disorder, yet the cause and progression of this disorder are not completely understood. While the main hallmark of AD is the deposition of amyloid plaques consisting of the β-amyloid (Aβ) peptide, transition metal ions are also known to play a significant role in disease pathology by expediting the formation of neurotoxic soluble β-amyloid (Aβ) oligomers, reactive oxygen species (ROS), and oxidative stress. Thus, bifunctional metal chelators that can control these deleterious properties are highly desirable. Herein, we show that amentoflavone (AMF), a natural biflavonoid compound, exhibits good metal-chelating properties, especially for chelating Cu2+ with very high affinity (pCu7.4 = 10.44). In addition, AMF binds to Aβ fibrils with a high affinity (Ki = 287 ± 20 nM), as revealed by a competition thioflavin T (ThT) assay, and specifically labels the amyloid plaques ex vivo in the brain sections of transgenic AD mice, as confirmed via immunostaining with an Aβ antibody. The effect of AMF on Aβ42 aggregation and disaggregation of Aβ42 fibrils was also investigated and revealed that AMF can control the formation of neurotoxic soluble Aβ42 oligomers, both in the absence and presence of metal ions, as confirmed via cell toxicity studies. Furthermore, an ascorbate consumption assay shows that AMF exhibits potent antioxidant properties and can chelate Cu2+ and significantly diminish the Cu2+-ascorbate redox cycling and reactive oxygen species (ROS) formation. Overall, these studies strongly suggest that AMF acts as a bifunctional chelator that can interact with various Aβ aggregates and reduce their neurotoxicity and can also bind Cu2+ and mediate its deleterious redox properties. Thus AMF has the potential to be a lead compound for further therapeutic agent development for AD.
Keywords: Alzheimer’s disease, amyloid plaques, biflavonoids, metal-Aβ adducts, Aβ oligomers, oxidative stress
Graphical Abstract
INTRODUCTION
Alzheimer’s disease (AD) is affecting a large fraction of the senior population all over the world, and more than five million people are affected alone in the United States.1 Currently, there is no treatment for AD, and the unambiguous diagnosis of the disease is done by postmortem analysis of the brain. The brains of AD patients are characterized by the deposition of amyloid plaques and formation of soluble oligomers of the beta-amyloid (Aβ) peptide.2–5 One widely employed therapeutic target has been the effective clearance of the amyloid plaques and soluble Aβ oligomers from the brain as a potential solution for AD treatment.
In addition, both in vitro and in vivo studies have shown evidence for the interaction of metal ions with Aβ aggregates, and postmortem examination of AD brains showed that copper, zinc, and iron are found in high concentrations in the amyloid plaques.6,7 These metal ions are believed to play a key role in the amyloid aggregation process8–16 as well as in the formation of reactive oxygen species (ROS) leading to oxidative stress.17–22 Our studies have also recently suggested that while Cu can stabilize neurotoxic soluble Aβ oligomers,23 Zn promotes the formation of amorphous and nontoxic Aβ aggregates.24
Given the mounting evidence supporting the role of transition metal ions in the pathophysiology of AD, use of metal-chelating chemical agents is emerging as a promising treatment strategy.3,4,25–28 Bifunctional metal chelators can interact with the Aβ species, and the metal ions are proposed to potentially be more effective therapeutics for AD.29–36 These chelators should be able to cross the blood-brain barrier (BBB) and have to be minimally neurotoxic to be a valid method to control the onset of AD.30 However, we have previously shown that, for the Aβ42 peptide, in contrast to the Aβ40 peptide, the previously employed strategy of inhibiting Aβ aggregation and promoting amyloid fibril disaggregation may not be optimal for the development of potential AD therapeutics, due to formation of neurotoxic soluble Aβ42 oligomers.37 Thus, the development of metal chelating compounds that do not lead to formation of toxic Aβ42 oligomers should be promoted.33,38 A strategy has been the search for such bifunctional chelators among naturally occurring compounds, and flavonoid compounds have been shown to interact with amyloidogenic peptides and arrest or redirect aggregation pathways.39–46 For example, the green tea extracts (−)-epigallocatechin-3-gallate (EGCG) and myricetin inhibit metal-induced Aβ40 aggregation by forming EGCG-metal-Aβ40 complexes (Scheme 1).39,47 We and others have previously found that the naturally occurring biflavonoids including amentoflavone (AMF) potently attenuate Aβ aggregation and cytotoxicity;48–50 however, the metal-chelating ability of AMF and its effect on Aβ aggregation in the presence of metal ions has not been investigated to date. Herein we report a detailed investigation of metal-binding ability of AMF via spectroscopic methods and determine its metal complex stability constants, which suggest that AMF exhibits high binding affinity for Cu2+ versus a moderate binding affinity for Zn2+. Furthermore, a Cu2+-induced ascorbate consumption assay shows that AMF can significantly diminish the Cu-mediated formation of hydroxyl radicals. The effect of AMF on Aβ aggregation in the absence and presence of metal ions was also studied, to reveal that AMF reduced the formation of Cu-stabilized neurotoxic soluble Aβ42 oligomers. Overall, these studies strongly suggest that AMF acts as a bifunctional chelator that can interact with various Aβ aggregates and reduce their neurotoxicity, and can also bind Cu2+ and mediate its deleterious redox properties. Thus, AMF has the potential to be a lead compound for further therapeutic agent development for AD.
Scheme 1.
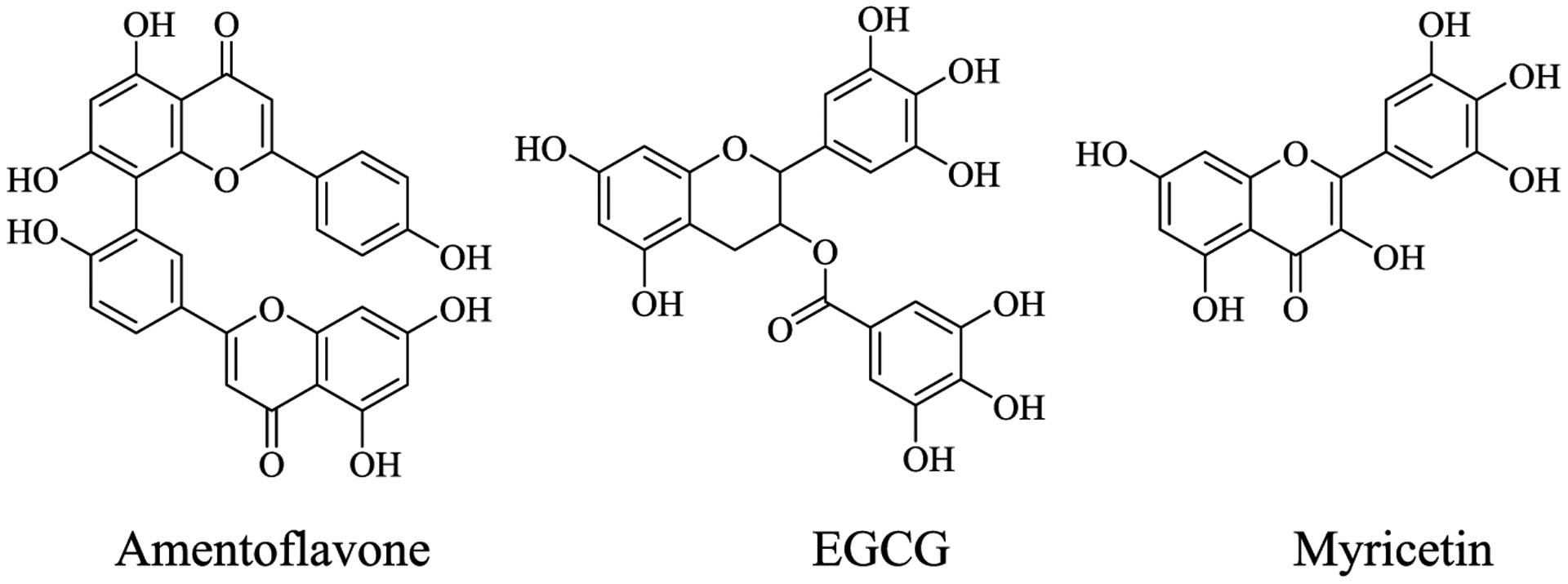
Chemical Structures of Amentoflavone (AMF), EGCG, and Myricetin
RESULTS AND DISCUSSION
Metal-Chelating Properties of AMF.
The UV-vis spectra of AMF reveal absorption bands at 275 and 360 nm (Figure S1).51 In order to determine if AMF can interact with Cu2+, various stoichiometric ratios of Cu2+ were added to the AMF solution. The spectral changes suggest that AMF can bind to more than one equivalent of Cu2+ (Figure 1). Job’s plot analysis was performed to determine the AMF:Cu2+ stoichiometry in solution. The break in Job’s plot at 0.7 mole fraction of Cu suggests that AMF can bind at least 2 equiv of Cu (Figure 1), which is in line with the proposed multiple metal ion binding sites consisting of the several ketone and phenolate groups on the adjacent rings of AMF (Figure S3).51
Figure 1.
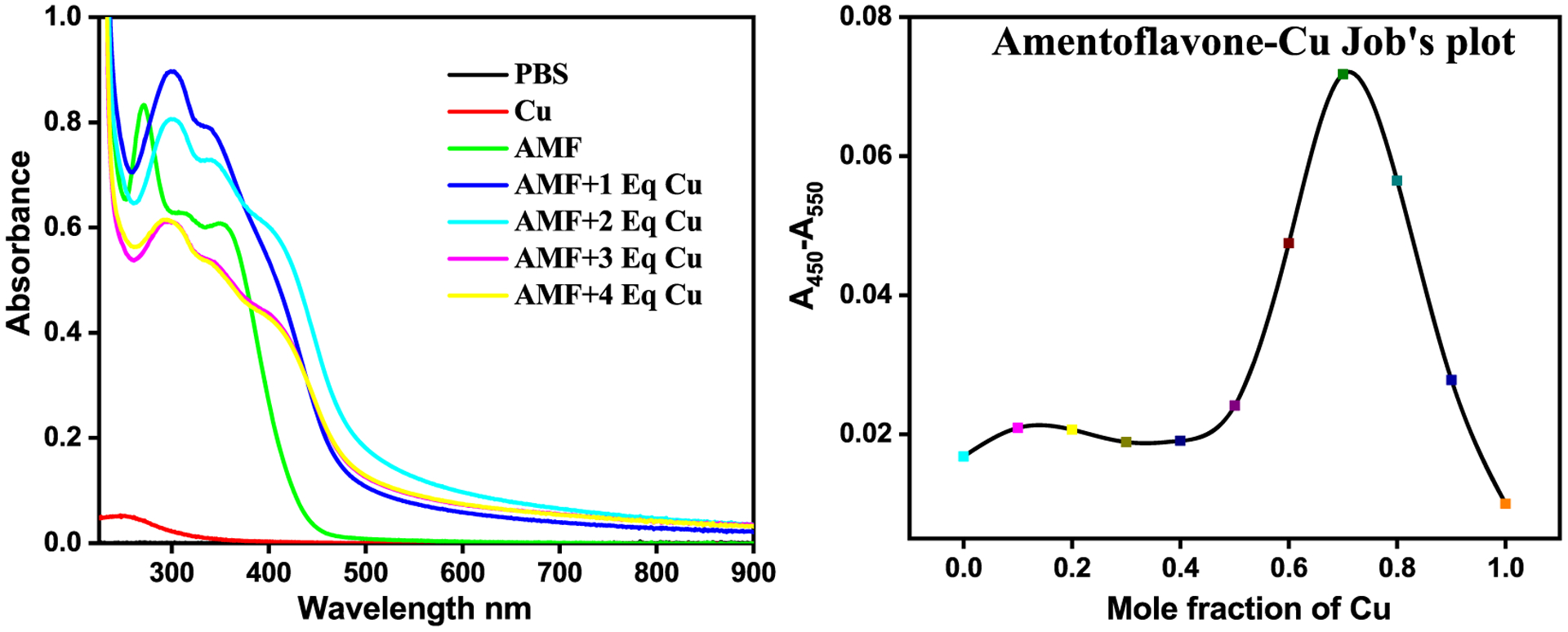
UV-vis characterization of Cu2+ binding to AMF. Left: Spectral changes upon addition of 0–4 equiv of CuCl2 to AMF (25 μM in PBS). Right: Job’s plot for AMF and Cu2+ in EtOH-PBS (1:10).
Since AMF contains phenol and ketone groups that can undergo protonation and deprotonation, the acidity constants (pKa) of AMF were determined using UV-vis spectrophotometric titrations. The UV-vis titrations from pH 3.0 to 11.0 reveal several changes in the spectra (Figure 2), and the best fit to the data was obtained with five pKa values: 1.42, 4.57, 7.61, 8.71, and 10.96. Based on previously reported acidity constants for various phenols, we assigned the lowest pKa value to the protonation of one keto group, the next two pKa values to the deprotonation of the o-carbonyl-phenol groups, for which the corresponding conjugate base is stabilized by conjugation similar to acetylacetone (Hacac),52 and the highest two pKa values are likely due to deprotonation of phenol groups in AMF.
Figure 2.
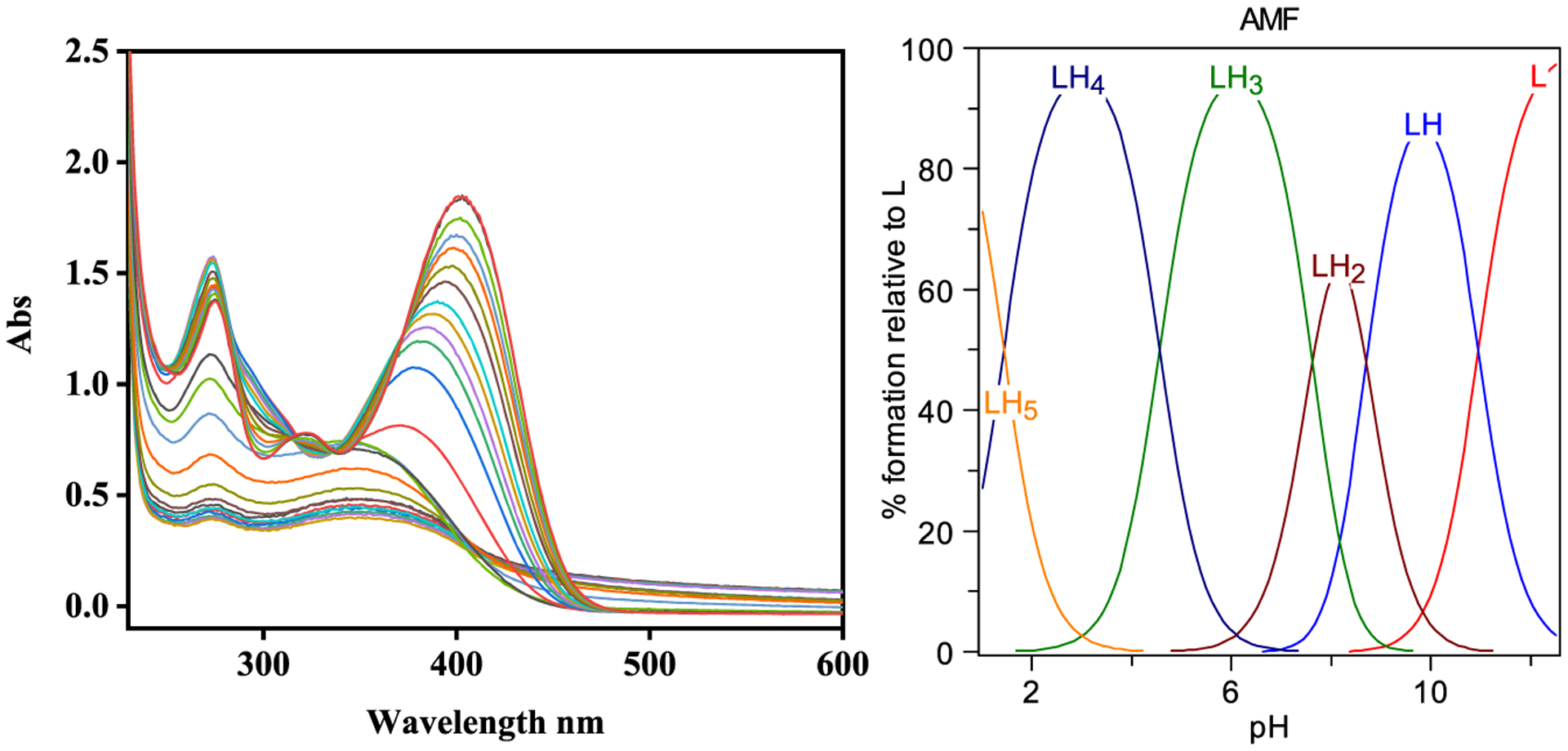
Variable pH (pH 3–11) UV spectra of AMF ([AMF] = 25 μM; 25 °C, I = 0.1 M NaCl) and species distribution plot, corresponding to the following five pKa values: 1.42, 4.57, 7.61, 8.71, and 10.96. Neutral AMF corresponds to the LH4 form.
Metal Complex Stability Constants Determination.
We then performed spectropotentiometric titrations of AMF in the presence of Cu2+ and Zn2+ ions to determine the corresponding metal complex stability constants. In the presence of Cu2+, the absorption band at 350 nm decreases regularly in the pH 3–8 range (Figure 3). At higher pH values (pH 8–12), a species with a broad absorption band at 400 nm slowly increases. The data was fitted with the HYPSPEC program using a 2:1 Cu:AMF stoichiometry and the presence of only two species was observed: Cu2LH and Cu2L, which matches the stoichiometry suggested by the Job’s plot analysis. The obtained stability constant of 32.53 for the Cu2(AMF) complex formation suggests that AMF has a very strong affinity for Cu (Table 1). In the presence of Zn2+, the complex formation was observed from pH 3 to 9, but at higher pH some free ligand was observed based on spectral similarity (Figure 4). The data fitting and stability constants value of 24.96 of Zn2(AMF) formation (Table 1) suggest a reasonable strong affinity for Zn albeit much lower than that for Cu. Since the stability constants are a measure of the overall stability of a metal-ligand complex and do not reflect the metal-binding affinity of ligands at a specific pH value,37,53 a better comparison between different metal complexes can be obtained by comparing the concentration of unchelated metal ions pM, where pM = −log[Munchelated]. Using the acidity and stability constants obtained from the data fits, pM values were obtained for the AMF: Zn system at pH 7.4 and for the AMF:Cu system at pH 6.6 and 7.4 (Table 2).53 The higher pM values obtained for the AMF:Cu system (10.44 at pH 7.4 and 9.62 at pH 6.6) suggests that AMF is a surprisingly strong chelator for Cu2+, similarly to commonly used Cu chelators such as DTPA.54
Figure 3.
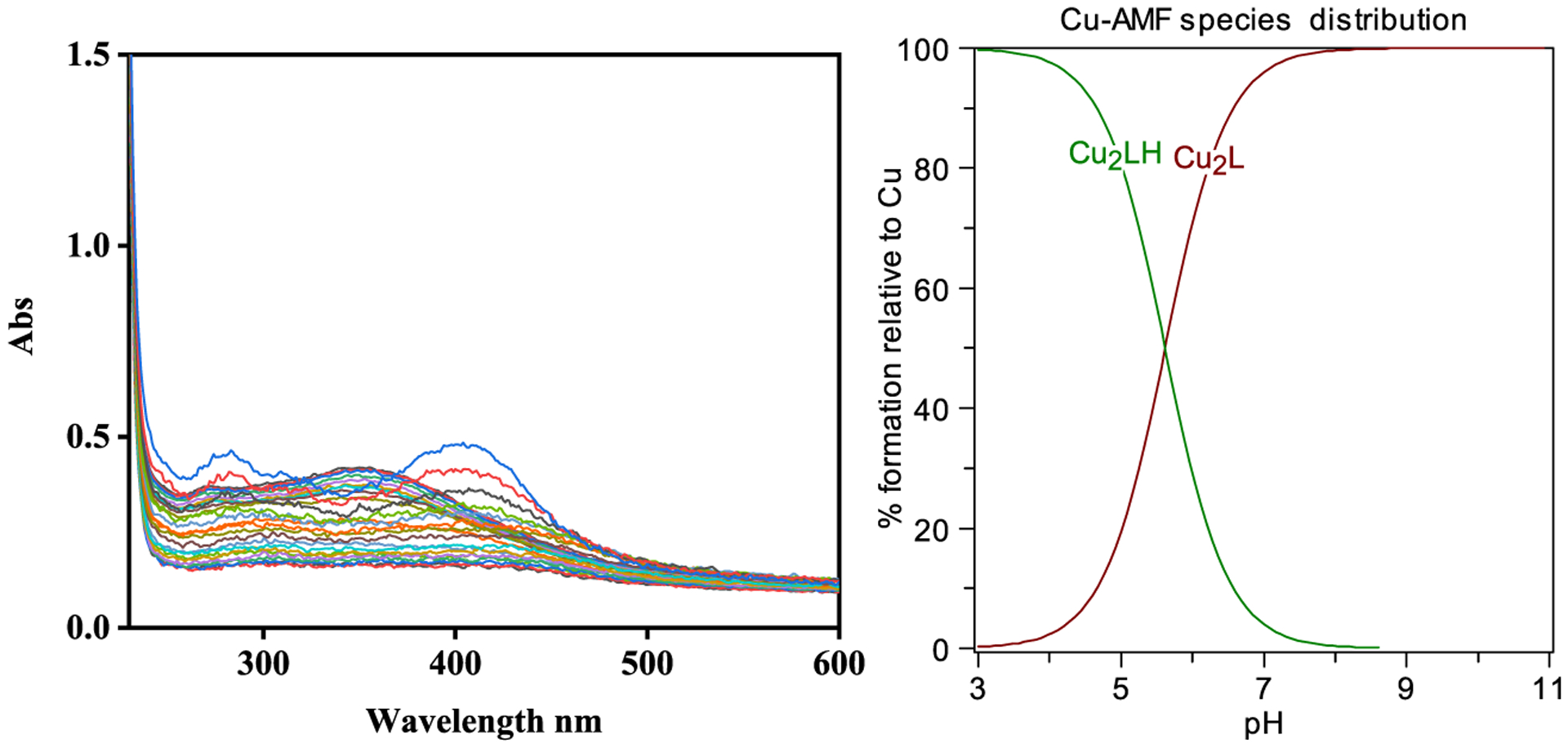
Variable pH (pH 3–11) UV-vis spectra of AMF and Cu2+ spectropotentiometric titration ([AMF] = 25 μM; [Cu2+] = 50 μM, 25 °C, I = 0.1 M NaCl) and species distribution plot (L represents the dideprotonated AMF).
Table 1.
Stability Constants for AMF:Cu2+ and AMF:Zn2+ Complexes
| metal ion | 2M2+ + L = [M2L] | 2M2+ + LH = [M2LH]+ |
|---|---|---|
| Cu2+ | 32.5(2) | 5.6(2) |
| Zn2+ | 25.11(2) | 4.46(3) |
Figure 4.
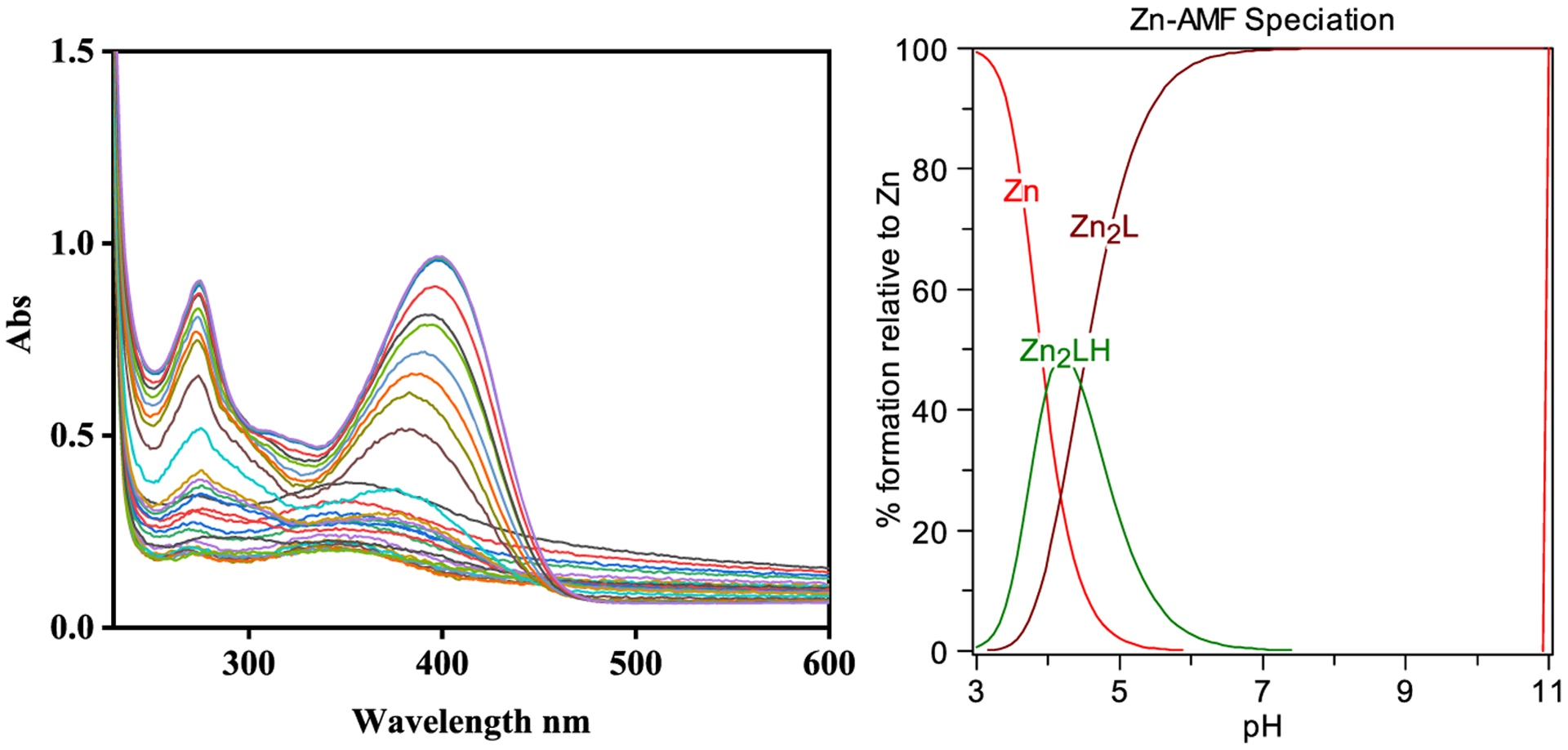
Variable pH (pH 3–11) UV-vis spectra of AMF and Zn2+ spectropotentiometric titration ([AMF] = 25 μM; [Zn2+] = 50 μM, 25 °C, I = 0.1 M NaCl) and species distribution plot (L represents the dideprotonated AMF).
Table 2.
Calculated pM Values (pM = −log[M]free; M = Zn2+, Cu2+) for a Solution Containing a 2:1 Metal:AMF Mixture ([M2+]tot = 25 μM; [AMF]tot = 50 μM) at a Given pH
| pZn | pCu | |
|---|---|---|
| pH 7.4 | pH 6.6 | pH 7.4 |
| 8.15 | 9.62 | 10.44 |
Binding Affinity of AMF for Aβ Fibrils.
Naturally occurring flavonoids have previously been shown to interact with amyloidogenic peptides and control the aggregation process.39,55,56 However, the direct determination of the binding affinities toward Aβ fibrils using fluorescence assays has not been employed for such natural products. Therefore, we have performed Thioflavin T (ThT) competition assays using Aβ fibrils, and AMF (0–10 μM) was added to solutions containing Aβ fibrils and ThT and a decrease in the ThT fluorescence intensity was recorded within a few minutes. We consider that AMF should not affect nature of the Aβ fibrils in such a short period of time (see below), and thus, the main reason for the reduction in fluorescence intensity is that AMF replaces the ThT molecules that bind to the amyloid fibrils. Fitting of the data using a one-site competitive binding model suggests a Ki = 287 ± 20 nM affinity of AMF toward Aβ fibrils (Figure 5), a value that is very similar to the IC50 value reported recently for the inhibition of Aβ42 fibrillization.50
Figure 5.
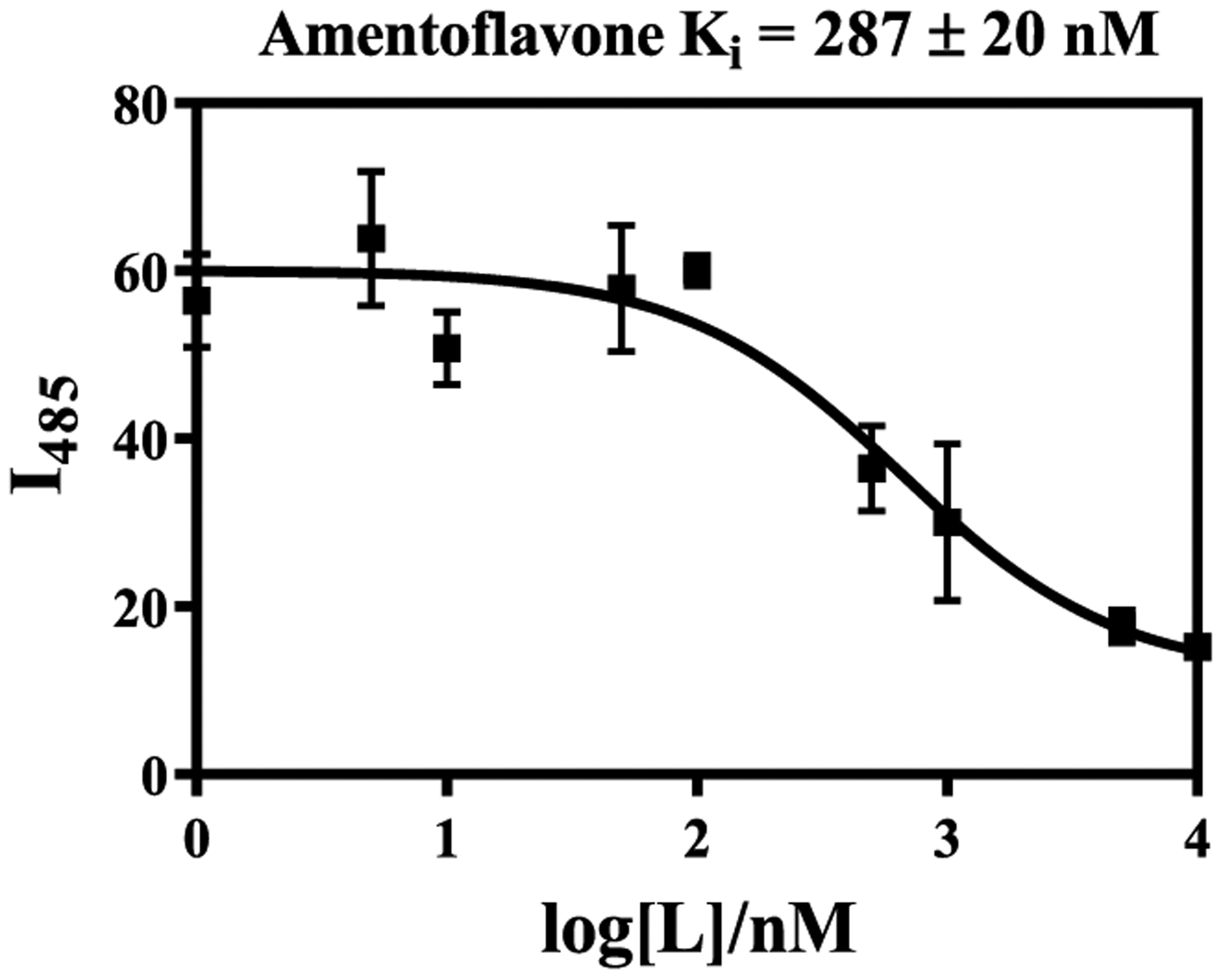
ThT fluorescence competition assays for AMF ([Aβ] = 2 μM, [ThT] = 1 μM). The error bars represent the standard deviation from three independent experiments.
Molecular Docking Studies.
In order to better understand the interaction of AMF with the Aβ aggregates, we performed a series of docking studies using the Schrödinger program Glide57 and different Aβ aggregate structures from the RCSB database: the Aβ42 tetramers (PDB ID: 6RHY) were used as a working model for the toxic Aβ oligomers associated with cell membrane disruption in AD58 as well as the Aβ40 fibrils (PDB ID: 2M4J) and the Aβ42 fibrils (PDB ID: 5OQV) structures. In order to select the best poses for each aggregate structures, the conformations of the AMF-Aβ species adducts were ranked by the docking score and the Glide e-model energy. For the AMF-Aβ42 tetramer adducts, the structures that have the best docking scores show that AMF mainly interacts through hydrogen bonds with the phenol groups with the hydrophilic amino acid residues such as Asp-1, His-6, and Asp-7 located at the N-terminus and outside of the hydrophobic core (Figure 6a). Importantly, several reports have shown that when Cu2+ ions bind to the Aβ peptides, the N-terminus amino acids such as Asp-1, His-6, His-13, or His-14 can strongly chelate the Cu2+ ions to form Cu2+-Aβ species,59 suggesting that AMF may have the ability to modulate the Cu2+-mediated stabilization of soluble Aβ oligomers (vide infra). While the other evaluated poses show interactions of AMF with other residues of the such as Glu-3, Asp-7, Tyr-10, Gln-15, and Glu-22 (Figures 6a and S6), all these residues are hydrophilic and could interact with metal ions such as Cu2+, and therefore, AMF could modulate the Cu2+-Aβ oligomers interactions.51 When docked onto the Aβ40 fibril structure, AMF is positioned near the KLVFF hydrophobic core and interacts via π−π interactions with the Phe-19 and Phe-20 residues and via hydrogen bonds with Gln-15, Leu-17, and Lys-28. Interestingly, the KLVFF core is also proposed to be the binding site for Thioflavin T,60 thus suggesting AMF can replace ThT efficiently and in line with the results obtained from the ThT fluorescence competition assays (Figures 6b and S5b).51 When docked onto the Aβ42 fibril structure, AMF interacts with the Cu-binding amino acid residues such as Glu-3 and His-6 (Figure 6c), further indicating that AMF may have the capability to modulate the interaction between Cu2+ and the Aβ42 fibrils (vide infra). Although the binding sites for AMF are slightly different for the Aβ40 vs the Aβ42 fibrils, in both cases the AMF selectively binds to the groove located on the terminus of the axis of fibril growth, which may explain why AMF can mitigate the growth of the Aβ fibrils. Moreover, comparing among these AMF-Aβ adducts, the best docking score and Glide e-model energy are comparable when AMF binds to the Aβ oligomers and fibrils, suggesting that AMF can interact similarly with these types of Aβ aggregates (Table S1).51
Figure 6.
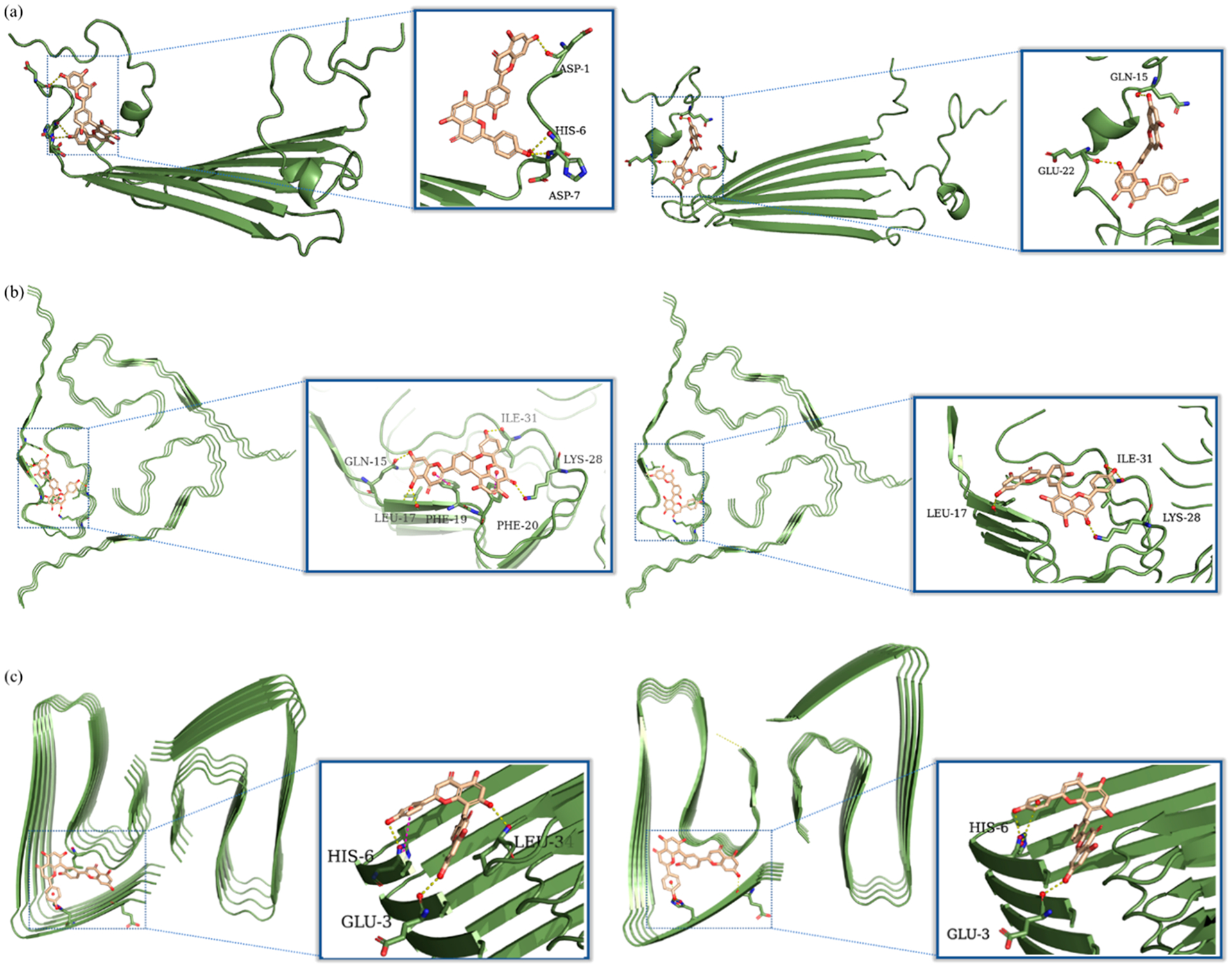
Calculated binding modes of AMF to various Aβ aggregates: (a) Molecular docking poses of AMF interacting with the Aβ42 tetramer (PDB ID: 6RHY). The left figure represents the pose that has the lowest docking score (−4.483), while the right figure represents the pose that has the lowest Glide e-model energy (−65.51 kcal/mol). (b) Molecular docking poses of AMF interacting with the Aβ40 fibrils (PDB ID: 2M4J). The left figure represents the pose that has the lowest docking score (−5.198), while the right figure represents the pose that has the lowest Glide e-model energy (−67.03 kcal/mol). (c) Molecular docking poses of AMF interacting with the Aβ42 fibrils (PDB ID: 5OQV). The left figure represents the pose that has the lowest docking score (−6.557), while the right figure represents the pose that has the lowest Glide e-model energy (−72.98 kcal/mol). The predicted hydrogen bonds and π−π interactions are highlighted with yellow and magenta dashed lines.
Fluorescence Staining of 5xFAD Mouse Brain Sections.
To further probe the ability of AMF to interact with various Aβ aggregates, we took advantage of the intrinsic fluorescence properties of AMF (Figure S2) to stain the native amyloid plaques present in the brain sections of 7 month old 5xFAD transgenic mice. These brain sections were first stained with AMF, followed by immunostaining with the HJ3.4 antibody that can bind to a wide range of Aβ species.61 Excitingly, AMF exhibits strong fluorescent intensity on the stained 5xFAD mouse brain sections that shows good colocalization with the immunofluorescence of HJ3.4 (Pearson’s correlation coefficient = 0.65, Figure 7), suggesting that AMF can bind to the amyloid plaques, especially to the dense core of the amyloid plaques when compared to the HJ3.4 antibody. Moreover, we have also stained the 5xFAD brain sections with the Cu2+-AMF and Zn2+-AMF complexes, followed by immunostaining with the HJ3.4 antibody. For the Zn2+-AMF adduct, a low fluorescence intensity is observed, indicating that either this complex exhibits quenched fluorescence or that it cannot specifically bind to the amyloid plaques (Figure S4).51 However, staining of the 5xFAD brain sections with the Cu2+-AMF adduct shows very good colocalization with HJ3.4 (Pearson’s correlation coefficient = 0.83, Figure 7), indicating that Cu2+-AMF can selectively bind to the amyloid plaques ex vivo. It is important to note that the absolute fluorescence intensity of the Cu2+-AMF amyloid plaque staining was significantly reduced vs staining with AMF alone, likely due to the fluorescence quenching ability of the Cu2+ ions. Also, no appreciable autofluorescence from the amyloid plaques was observed, either in the FITC or the Texas Red channel (Figure S5).
Figure 7.
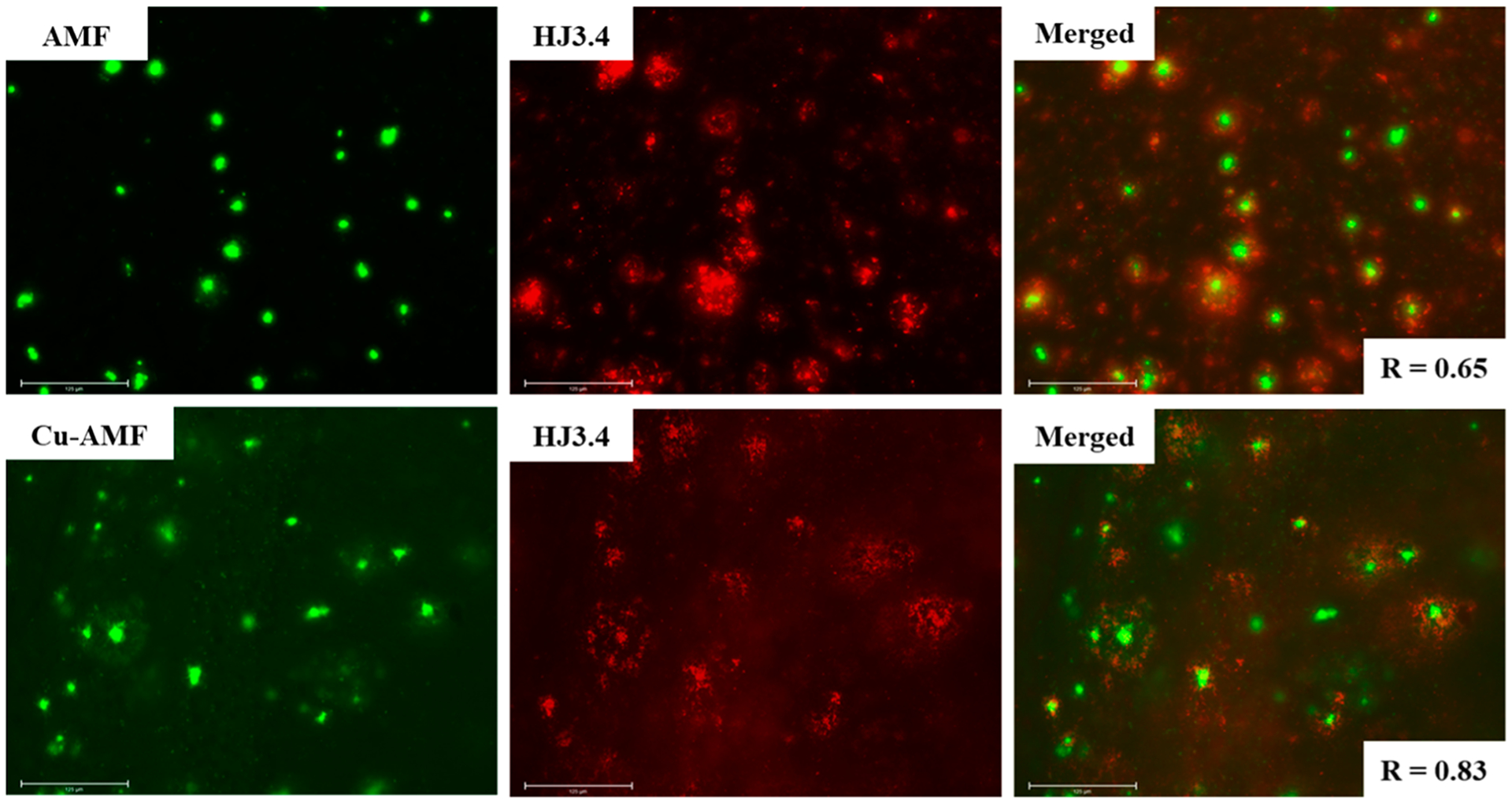
Fluorescence microscopy images of 5xFAD mice brain sections coincubated with AMF and Cu-AMF (left panels), HJ3.4 antibody (middle panels), and merged images (right panels, along with the Parson’s correlation coefficients R). Concentrations used: [AMF] = [Cu2+] = 25 μM, [HJ3.4] = 1 μg/mL (scale bar: 125 μm).
The Effect of AMF on Aβ42 Aggregation.
We then investigated the effect of AMF on Aβ aggregation, both in the presence and absence of metal ions. For this purpose, we have used the Aβ42 peptide, which was also shown to form neurotoxic soluble Aβ oligomers.62–66 To study the inhibitory effect of AMF on Aβ aggregation, freshly prepared monomeric Aβ42 solutions were treated with metal ions, AMF, or both.37 Interestingly, the ThT fluorescence assays reveal that AMF dramatically reduced the fibrillization of Aβ42, both in presence and absence of Cu2+ or Zn2+ (Figure 8).67
Figure 8.
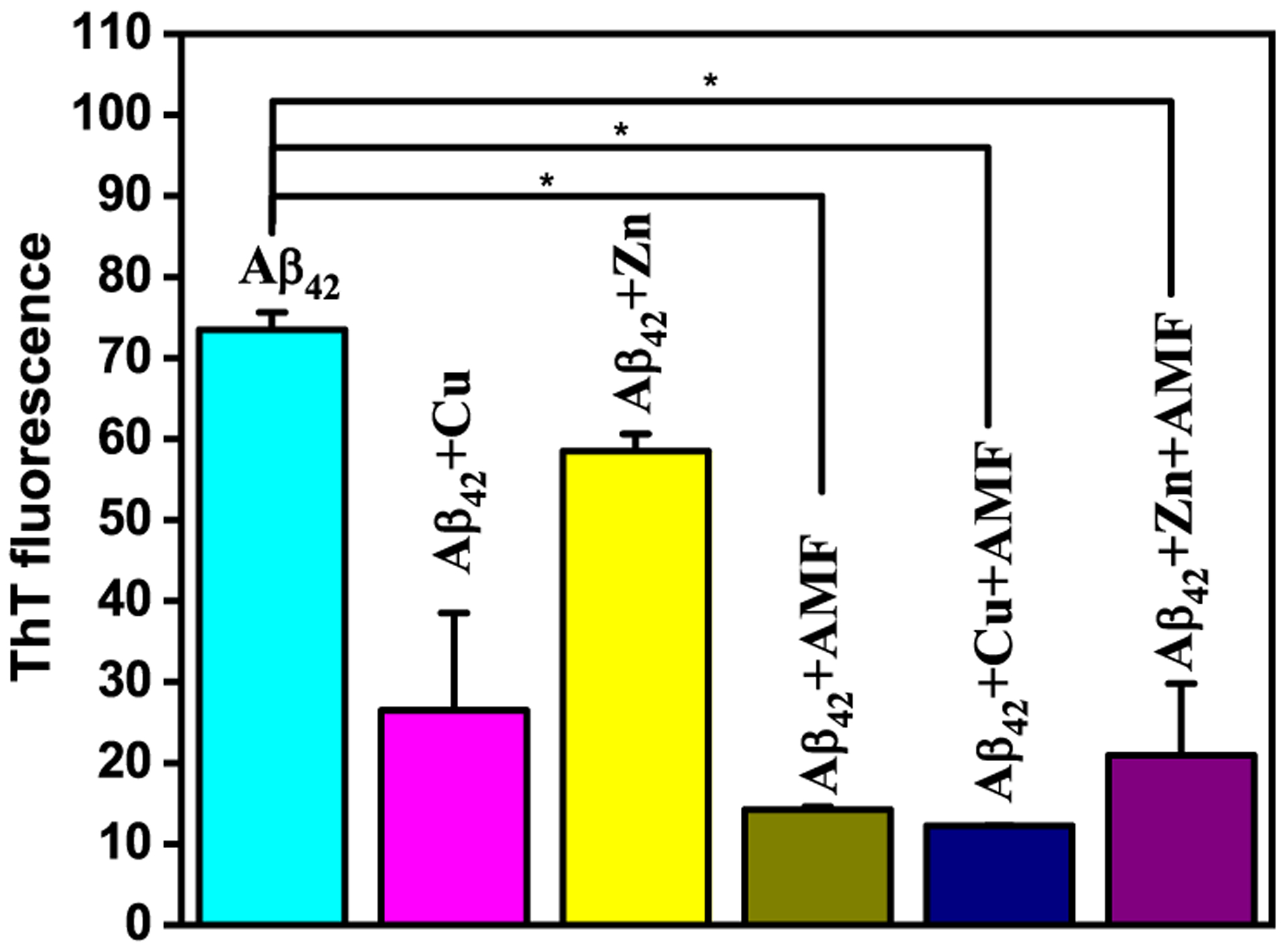
ThT fluorescence of inhibition of Aβ fibrillization, measured upon incubation at 37 °C for 24 h. Samples are as indicated on top of the lanes (conditions: PBS, [Aβ] = 25 μM; [M2+] = 25 μM; [AMF] = 25 μM). For the ThT fluorescence plate reader measurements, the samples were diluted 10-fold and 10 μM ThT was added to each well. The error bars represent the standard deviation from two independent experiments, and the statistical analysis was evaluated according to one-way ANOVA (*p < 0.05).
To further study the effect of AMF on Aβ42 aggregation, native gel electrophoresis/Western blot analysis and transmission electron microscopy (TEM) were used as these techniques provide a more in-depth analysis of the various size Aβ aggregates formed. Similar to previous reports, Aβ42 forms fibrils within 24 h of incubation, while the presence of Cu2+ generates a range of soluble Aβ42 oligomers, and the presence of Zn2+ leads to amorphous Aβ42 aggregates (Figures 9a–c and S7).24,37 Interestingly, the presence of AMF arrests the Aβ42 aggregation process and only small amorphous aggregates were observed by TEM, both in the absence and presence of Cu2+ (Figure 9d and e), while in the presence of Zn2+ and AMF a range of amorphous and fibrillar aggregates is formed (Figure 9f). The native gel/Western blot further supports that AMF dramatically reduces the amount of large Aβ42 aggregates, while in the presence of Cu2+ the amount of soluble Aβ42 oligomers is also reduced, and in the presence of AMF and Zn2+ a wide range of various-size aggregates is formed (Figure 9).
Figure 9.
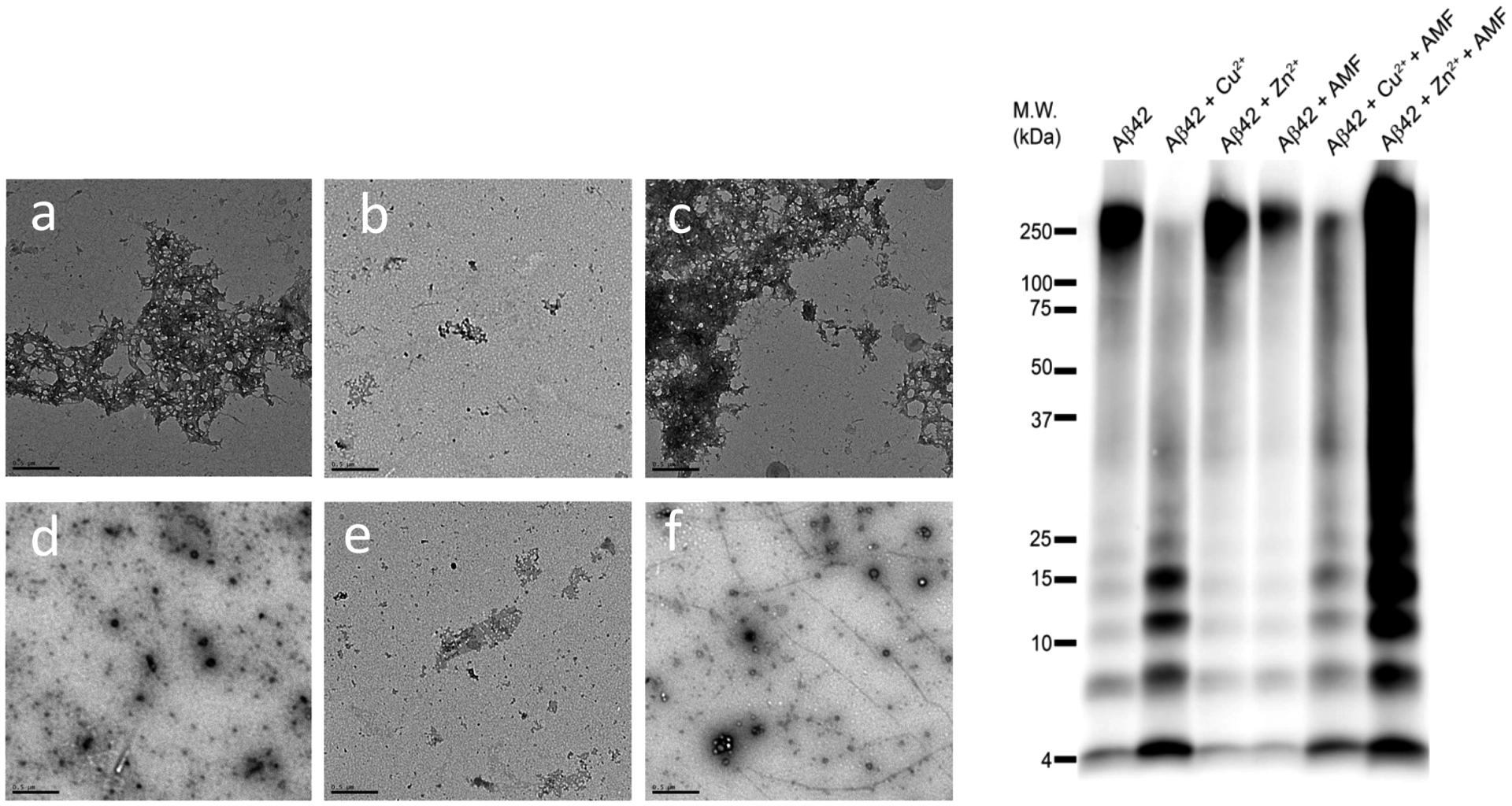
TEM images and native gel electrophoresis/Western blot for the inhibition of Aβ42 aggregation by AMF, in the presence or absence of metal ions ([Aβ42] = [M2+] = 25 μM, [AMF] = 25 μM, 37 °C, 24 h; scale bar = 500 nm). Samples are (a) Aβ42, (b) Aβ42 + Cu2+, (c) Aβ42 + Zn2+, (d) Aβ42 + AMF, (e) Aβ42 + Cu2+ + AMF, and (f) Aβ42 + Zn2+ + AMF.
The Effect of AMF on Disaggregation of Aβ Fibrils.
Inspired by the inhibition of Aβ42 aggregation results, we then investigated the ability of AMF to disaggregate the preformed Aβ42 fibrils, in the presence or absence of metal ions. The Aβ42 fibrils (prepared by incubating for 24 h at 37 °C) were incubated with AMF for an additional 24 h at 37 °C, and the extent of disaggregation in these samples was evaluated by ThT fluorescence, TEM, and native gel/Western blotting. The ThT fluorescence results were similar to those obtained in the inhibition of Aβ42 aggregation experiments and show that AMF is efficient at reducing the amount of Aβ42 fibrils, both in the absence and presence of Cu2+ ions (Figure 10).
Figure 10.
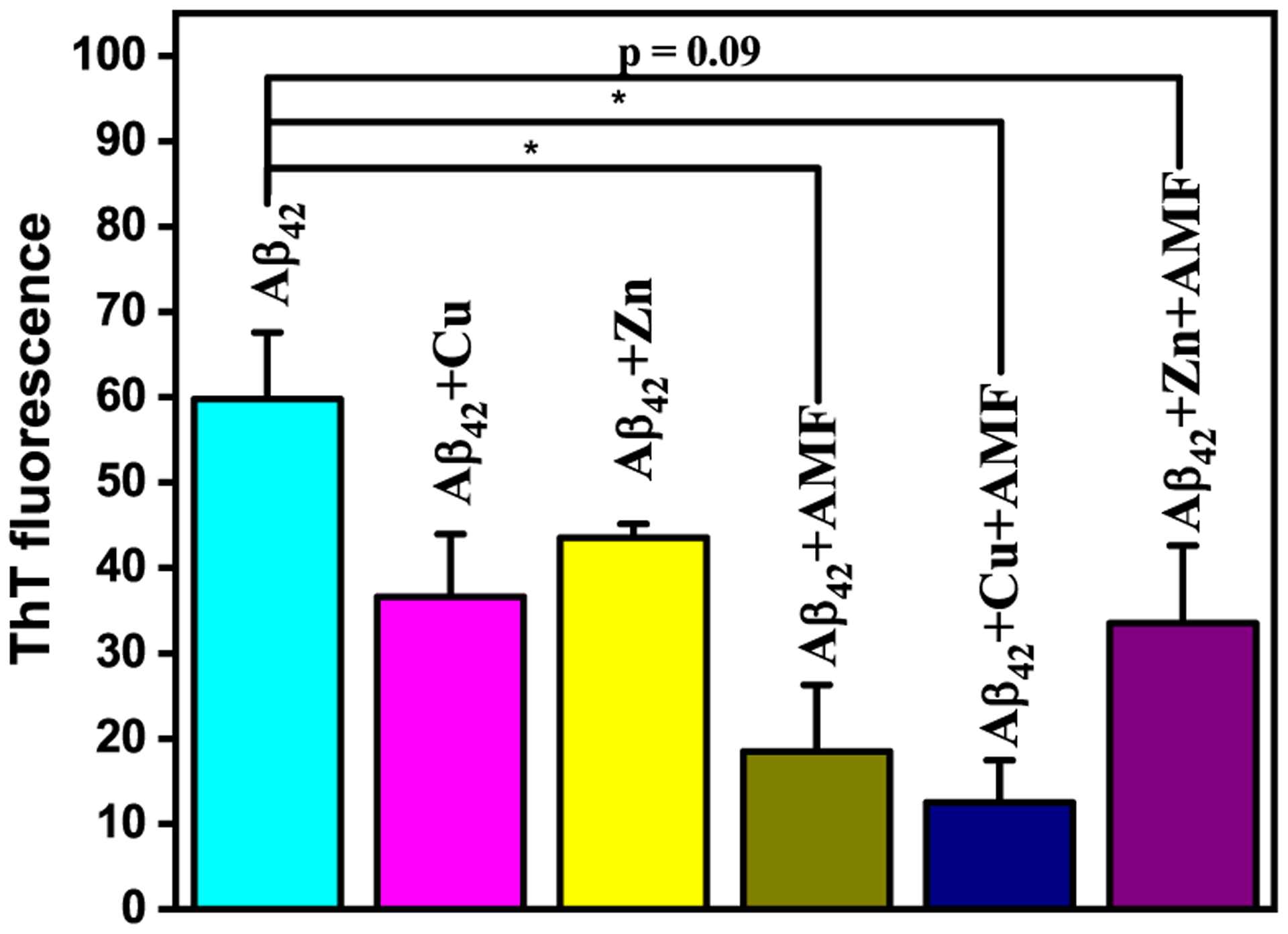
ThT fluorescence for the disaggregation of Aβ fibrils by AMF, measured upon incubation at 37 °C for 24 h. Samples are as indicated on top of the lanes ([Aβ] = 25 μM; [M2+] = 25 μM; [AMF] = 25 μM). The error bars represent the standard deviation from two independent experiments, and the statistical analysis was evaluated according to one-way ANOVA (*p < 0.05).
The TEM and native gel/Western blot analyses also yield similar results to those obtained from the inhibition of Aβ42 aggregation studies. While the 48 h incubation gives mature Aβ42 fibrils, the presence of Cu2+ stabilizes a range of soluble Aβ42 oligomers and the presence of Zn2+ leads to wide range of amorphous aggregates (Figure 11a–c). The addition of AMF disaggregates the Aβ42 fibrils efficiently, and only a reduced amount of amorphous smaller aggregates is observed (Figure 11d). Excitingly, the addition of AMF to the Cu2+-incubated Aβ42 aggregates shows the reduction in the amount of both large aggregates and smaller Aβ42 oligomers (Figure 11e), while the amount of Zn2+-incubated Aβ42 amorphous aggregates also decreases to some extent upon the addition of AMF (Figure 11f). Overall, both the inhibition of Aβ42 and disaggregation of Aβ42 fibrils studies strongly suggest that AMF is a good agent for inhibition of Aβ aggregation as well as for disaggregating the preformed Aβ aggregates. Moreover, in the presence of Cu2+ ions, the amount of soluble Aβ42 oligomers is also reduced by AMF, which is a desirable property targeted in the development of Aβ-binding and metal-chelating bifunctional compounds.37
Figure 11.
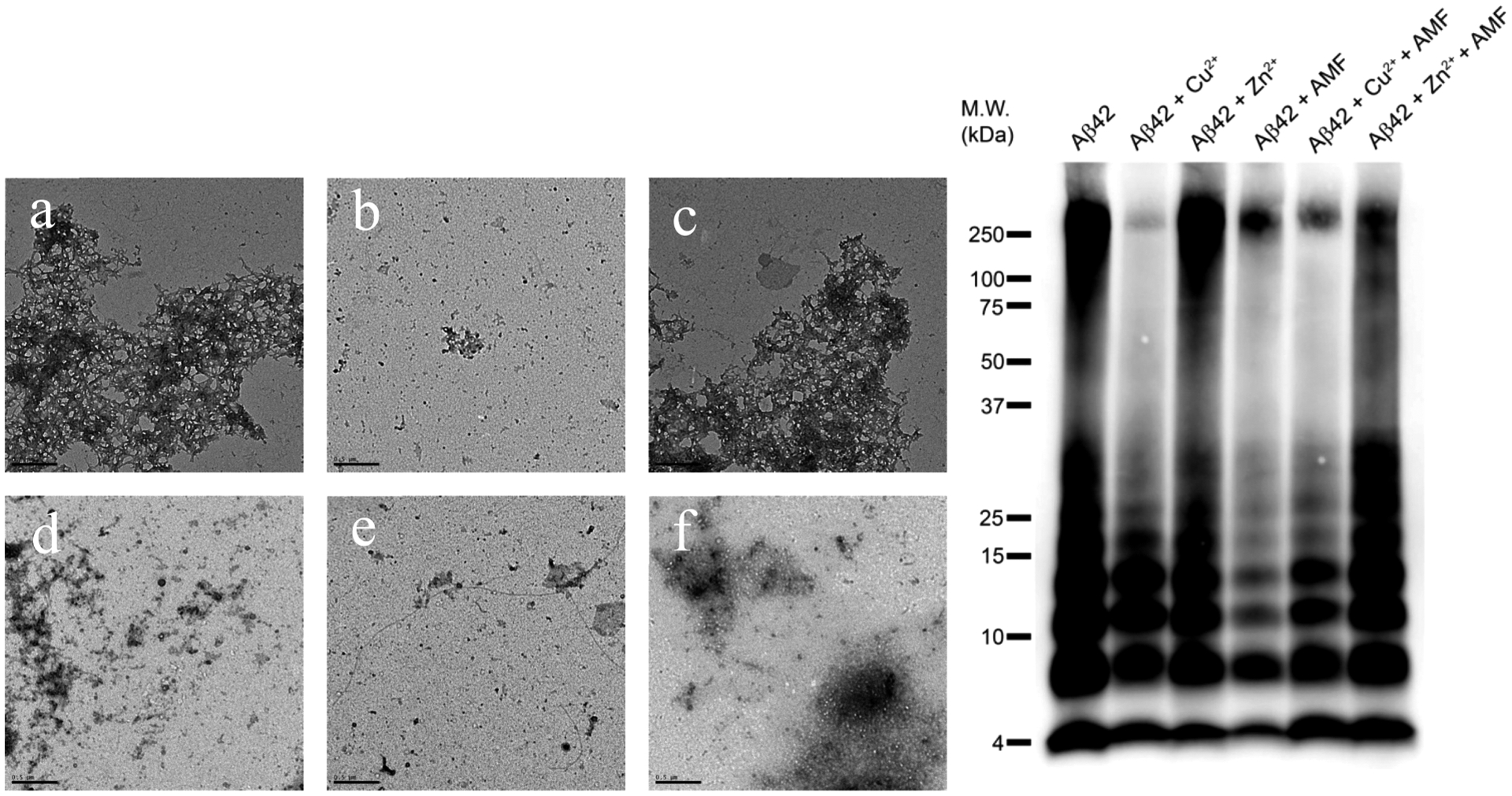
TEM images and native gel electrophoresis/Western blot for the disaggregation of Aβ42 fibrils by AMF in the presence or absence of metal ions (conditions used: [Aβ42] = [M2+] = 25 μM, [AMF] = 25 μM, 37 °C, 24 h for fibrilization with or without metal ions, followed by AMF addition and a further 24 h incubation for disaggregation, scale bar = 500 nm). Samples are (a) Aβ42, (b) Aβ42 + Cu2+, (c) Aβ42 + Zn2+, (d) Aβ42 + AMF, (e) Aβ42 + Cu2+ + AMF, and (f) Aβ42 + Zn2+ + AMF.
Cu2+-Induced Ascorbate Consumption Assays.
It has been shown previously that Aβ42 species can bind Cu2+ and promote its reduction to Cu+, which can react with dioxygen to generate reactive oxygen species (ROS) and lead to oxidative stress in vivo.68,69 Herein, we have employed an ascorbate consumption assay to evaluate the ability of AMF to suppress the Cu2+-ascorbate redox cycling. In the absence of any other additives, the consumption of ascorbate in the presence of Cu2+ is rapid (Figure 12a, black).51 However, if Cu2+ is premixed with 2 equiv of AMF, the ascorbate consumption is dramatically reduced (Figure 12a, red),51 indicating that the AMF can bind tightly to Cu2+ and mitigate its reduction to Cu2+, thus acting as an antioxidant and arrest the redox cycling. Furthermore, when 2 equiv AMF was added into the Cu2+-ascorbate solution, the consumption of ascorbate is rapidly reduced significantly (Figure 12a, blue),51 showing that AMF can alleviate the Cu2+-ascorbate redox cycling even when it was not premixed with Cu2+.
Figure 12.
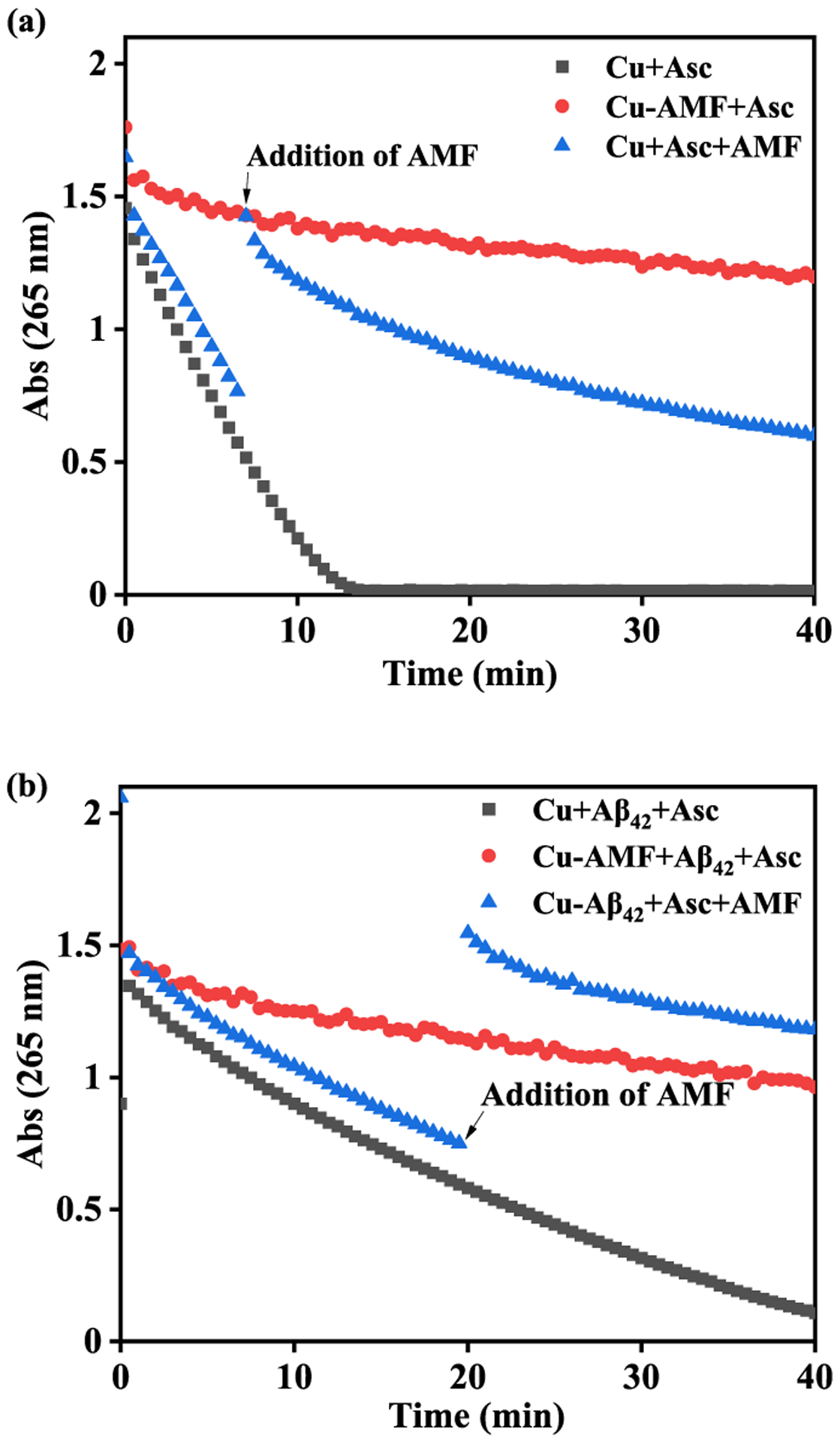
Kinetics of ascorbate consumption monitored by UV-vis spectroscopy at 265 nm. (a) Cu2+ + ascorbate (black), Cu2+-AMF + ascorbate (red), Cu2+ + ascorbate + AMF (blue); (b) Cu2+-Aβ42 + ascorbate (black), Cu2+-AMF + Aβ42 + ascorbate (red), Cu2+-Aβ42 + ascorbate + AMF (blue); [Cu2+] = 10 μM, [Aβ42] = 12 μM, [AMF] = 24 μM, [ascorbate] = 100 μM. The large increase in absorbance observed for the blue curves in (a) and (b) likely results from the addition of AMF, which has an absorption band around 265 nm (Figure S1).
We have also investigated ascorbate consumption in the presence of monomeric Aβ42, mimicking the conditions that are more relevant to AD. Compared with the Cu2+-Aβ42 species that rapidly lead to consumption of ascorbate through redox cycling (Figure 12b, black),51 the presence of the premixed AMF-Cu2+ complex leads to a significant inhibition of the ascorbate consumption (Figure 12b, red),51 indicating that Aβ42 cannot outcompete AMF for binding to the Cu2+ ions. Moreover, when AMF added to the Cu2+-Aβ42-ascorbate solution, it was able efficiently reduce the consumption of ascorbate via the Cu2+-ascorbate redox cycling even in the presence of Aβ42 species (Figure 12b, blue),51 suggesting that AMF can outcompete Aβ42 for binding to the Cu2+ ions. Overall, these results strongly suggest that AMF has potent antioxidant properties and can efficiently chelate the Cu2+ ions to prevent the reduction by ascorbate, even with the presence of Aβ42 species, and thus inhibit the formation of ROS during the Cu2+-ascorbate redox cycling.
Cell Toxicity Studies.
Since it was recently shown that the Cu2+ ions can promote the formation of neurotoxic soluble Aβ oligomers,24,70 42 we have investigated whether AMF can modulate the formation of such Cu2+-stabilized soluble Aβ42 oligomers. First, the Neuro2A cells were treated with the Aβ42 fibrils that lead to some neurotoxicity (80 ± 5% cell viability) compared with the DMSO control, while the Cu2+-stabilized Aβ42 oligomers showed a significantly increased cytotoxicity (60 ± 2% cell viability) vs the Aβ42 fibrils (Figure 13, top).24 The presence of Zn2+ did not significantly affect the Aβ42 cytotoxicity. Excitingly, the addition of AMF significantly attenuates the cytotoxicity of the Cu2+-stabilized Aβ42 oligomers (85 ± 5% cell viability), further suggesting that AMF can mitigate the interaction of Cu2+ with the Aβ42 species. Next, we have examined the cytotoxicity of the preformed Aβ42 aggregates (Figure 13, bottom). While the Aβ42 aggregates formed in the presence of Cu2+ ions dramatically increased the cytotoxicity compared to the Aβ42 fibrils alone (45 ± 8% versus 79 ± 8% cell viability, respectively), the addition of AMF significantly reduced cytotoxicity of the Cu2+-containing Aβ42 aggregates (83 ± 7% cell viability). To the best of our knowledge, these results suggest for the first time that AMF can act as a bifunctional chelator and mitigate the neurotoxicity of Cu2+-Aβ42 species in a cellular assay.
Figure 13.
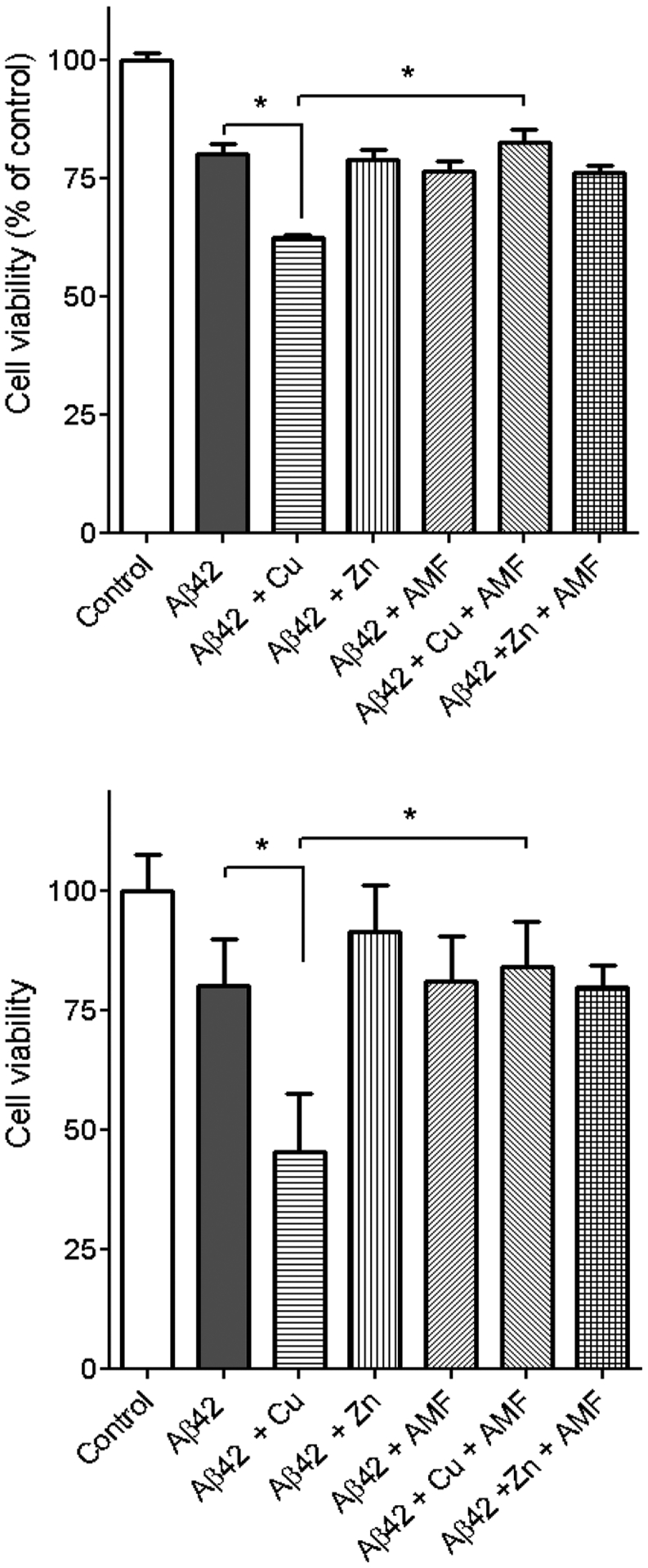
Cell viability results (% relative to the DMSO control) upon incubation of Neuro2A cells with Aβ42 in the presence or absence of metal ions and AMF, under inhibition of Aβ42 aggregation (top) and disaggregation of Aβ42 fibrils (bottom) conditions. The error bars represent the standard deviation from five independent experiments, and the statistical analysis was evaluated according to one-way ANOVA (*p < 0.05).
CONCLUSIONS
In summary, herein we report that amentoflavone (AMF), a naturally occurring biflavonoid compound, exhibits good metal-chelating properties, especially for binding Cu2+ with a very high affinity (pCu7.4 = 10.44), as shown via spectropotentiometric titrations. In addition, AMF binds to Aβ fibrils with a high affinity (Ki = 287 ± 20 nM), as revealed by a competition thioflavin T (ThT) assay, and specifically labels the amyloid plaques ex vivo in the brain sections of transgenic 5xFAD mice, as confirmed via immunostaining with an Aβ antibody. The effect of AMF on Aβ42 aggregation and disaggregation of Aβ42 fibrils was also investigated, to reveal that AMF can control the cytotoxicity of Aβ42 aggregates, especially the marked neurotoxicity of the Cu2+-stabilized soluble Aβ42 oligomers. Furthermore, a Cu2+-induced ascorbate consumption assay shows that AMF exhibits potent antioxidant properties and can chelate Cu2+ and significantly diminish its reduction and Cu2+-ascorbate redox cycling and ROS formation. Overall, these studies strongly suggest that AMF acts as a bifunctional chelator that can interact with various Aβ aggregates and reduce their neurotoxicity and can also bind Cu2+ and mediate its deleterious redox properties. Thus, AMF has the potential to be a lead compound for further therapeutic agent development for AD.
METHODS
All reagents were purchased from commercial sources and used as received unless stated otherwise. AMF was obtained from Sigma-Aldrich. All solutions and buffers were prepared using metal-free Millipore water that was treated with Chelex overnight and filtered through a 0.22 μm nylon filter. UV-visible spectra were recorded on a Varian Cary 50 Bio spectrophotometer and are reported as λmax, nm (ε, M−1 cm−1). The monoclonal anti-Aβ antibody HJ3.4 was obtained from Prof. David Holtzman (Department of Neurology, Washington University School of Medicine). The primary antibodies were labeled with the CF594 fluorescent dye by using the Mix-n-Stain CF 594 Antibody Labeling Kit (Sigma-Aldrich), in accordance with the protocol provided by the manufacturer. All animal studies have been approved by the Washington University Animal Studies Committee, and the handling of mice was performed in accordance with institutional regulations.
Acidity and Stability Constant Determination.
UV-vis pH titrations were employed for the determination of acidity constants of AMF and its stability constants with Cu2+ and Zn2+. For the acidity constants determinations, solutions of AMF (25 μM, 0.1 M NaCl, pH 3) were titrated with small aliquots of 0.1 M NaOH at room temperature under N2. At least 30 UV-vis spectra were collected in the pH 3–11 range. DMSO stock solutions (5 mM) were diluted in EtOH-water mixtures in which EtOH did not exceed 10% (v:v). Similarly, the metal complex stability constants were determined by titrating solutions of AMF and Cu(ClO4)2·6H2O or Zn(ClO4)2·6H2O with small aliquots of 0.1 M NaOH at room temperature. At least 30 UV-vis spectra were collected in the pH 3–11 range. The acidity and stability constants were calculated using the HypSpec program (Protonic Software, UK),71 and speciation plots of the compounds and their metal complexes were calculated using the program HySS2009 (Protonic Software, UK).72
Amyloid β Peptide Experiments.
Aβ monomeric films were prepared by dissolving commercial Aβ42 (or Aβ40 for Aβ fibril-binding studies) peptide (Keck Biotechnology Resource Laboratory, Yale University) in HFIP (1 mM) and incubating for 1 h at room temperature.73 This solution was then aliquoted out and evaporated overnight. The aliquots were vacuum centrifuged, and the resulting monomeric films stored at −80 °C. Aβ fibrils were generated by dissolving monomeric Aβ films in DMSO, diluting into the appropriate buffer, and incubating for 24 h at 37 °C with continuous agitation (final DMSO concentration was <2%). For metal-containing fibrils, the corresponding metal ions were added before the initiation of the aggregation conditions. For the inhibition studies, AMF (25 μM) was added to Aβ solutions (25 μM) in the absence or presence of metal salts (CuCl2 or ZnCl2, 25 μM) and incubated for 24 h at 37 °C with constant agitation. For the disaggregation studies, the preformed Aβ fibrils in the absence or presence of metal ions were treated with AMF and incubated for 24 h at 37 °C with constant agitation. For the preparation of soluble Aβ42 oligomers a literature protocol was followed.74,75 A monomeric film of Aβ42 was dissolved in anhydrous DMSO, followed by addition of DMEM-F12 media (1:1 v:v, without phenol red, Invitrogen). The solution (50–100 μM) was incubated at 4 °C for 24 h and then centrifuged at 10,000 g for 10 min. The supernatant was used as a solution of soluble Aβ42 oligomers.
Histological Staining of 5xFAD Mice Brain Sections.
Brain sections obtained from 7 month old transgenic 5xFAD mice were washed with PBS (3 × 5 min) and blocked with bovine serum albumin (2% BSA in PBS, pH 7.4, 10 min). Then the sections were incubated with 25 μM AMF or metal-AMF solutions and then sequentially stained with the CF594-labeled HJ3.4 antibody (1 μg/mL) for 1 h. The brain sections were treated with 2% BSA-PBS for 4 min to remove any compounds or antibodies that were nonspecifically binding to the tissue. Finally, the sections were washed with PBS (3 × 2 min) and Millipore water (2 min) and then mounted with a coverslip and Vectashield mounting medium. The stained brain sections were analyzed using an EVOS FL Auto 2 fluorescence microscope. The fluorescence images of the AMF/Cu-AMF complex staining and CF594-labeled HJ3.4 antibody immunostaining were separately imaged in the FITC and Texas Red channels, respectively. The visualization and determination of the Pearson’s correlation coefficients were performed using the imaging software Fiji (ImageJ 1.52p).
Molecular Docking.
The Schrödinger Suite was used to predict the docking posed and the binding interactions of AMF with the structure of various Aβ aggregates from the RCSB database. The Aβ aggregates used for docking are the Aβ42 tetramers (PDB ID: 6RHY),58 the Aβ40 fibrils (PDB ID: 2M4J)76 and the Aβ42 fibrils (PDB ID: 5OQV).77 The AMF molecule was prepared for docking using Ligprep, and the pH was set as 7.0 ± 2.0 using Epik. The four different protonation states of AMF were obtained from the preparation of the ligand and used for the docking studies. The three Aβ aggregate structures were processed by minimal minimization with the OPLS3 force field using the Protein Preparation Wizard program. The grid size was set to 36 Å in each direction to include all amino acid sequences. The molecular docking was performed using Glide.78 The four best poses obtained for each Aβ aggregate structure were ranked by both the docking score and Glide e-model energy, and the structures with the best docking scores and Glide e-model energies were rendered in PyMol.79
Fluorescence Measurements.
All fluorescence measurements were performed using a SpectraMax M2e plate reader (Molecular Devices). For the ThT fluorescence studies, the samples were diluted to a final concentration of 2.5 μM Aβ in PBS containing 10 μM ThT and the fluorescence measured at 485 nm (λex = 435 nm). For the Aβ fibril binding studies in a Thioflavin T (ThT) competition assay, the Aβ (1 μM) fibril solution with ThT (2 μM) was titrated with small amounts of AMF and the decrease in ThT fluorescence was measured (λex/λem = 435/485 nm). For calculating Ki values, a Kd value of 1.17 μM was used for the binding of ThT to Aβ40 fibrils, as determined previously.24,37
Transmission Electron Microscopy (TEM).
Glow-discharged grids (Formvar/Carbon 300 mesh, Electron Microscopy Sciences) were treated with Aβ samples (25 μM, 5 μL) for 5 min at room temperature. The excess solution was removed using filter paper, and the grids were rinsed twice with H2O (5 μL), stained with uranyl acetate (1% w/v, H2O, 5 μL) for 1 min, blotted with filter paper, and dried for 15 min at room temperature. Images were captured using a FEI G2 Spirit Twin microscope (60–80 kV, 6500–97 000× magnification) at the Nano Research Facility (NRF) at Washington University in St. Louis, MO.
Native Gel Electrophoresis and Western Blotting.
All gels, buffers, membranes, and other reagents were purchased from Invitrogen and used as directed except where otherwise noted. Samples were separated on 10–20% gradient Tris-tricine mini gels. The gel was transferred to a nitrocellulose membrane in an ice bath and the protocol was followed as suggested except that the membrane was blocked overnight at 4 °C. After blocking, the membrane was incubated in a solution (1:2000 dilutions) of 6E10 anti-Aβ primary antibody (Covance) for 3 h. Invitrogen’s Western Breeze Chem-iluminescent kit was used to visualize the bands. An alkaline-phosphatase antimouse secondary antibody was used, and the protein bands were imaged using a FUJIFILM Luminescent Image Analyzer LAS-1000CH.
Cu2+-Induced Ascorbate Consumption Assays.
A 10 mM stock solution of sodium ascorbate was prepared in 10 mM PBS buffer, and a 10 mM stock solution of CuSO4 was prepared in Millipore water. A 4 mM AMF stock solution was prepared in DMSO. Monomeric Aβ42 was dissolved in PBS buffer to make a 100 μM stock solution. Final concentrations in the assay were as follows: 100 μM ascorbate, 10 μM CuSO4, 12 μM Aβ42, and 24 μM AMF. For the assays without the Aβ peptide, three different conditions were employed as follows: (1) ascorbate was first added into the PBS buffer following by the addition of CuSO4 solution; (2) the AMF solution was premixed with the CuSO4 solution for 30 min, and then this was added to the ascorbate solution; (3) ascorbate was first added into the PBS buffer followed by the addition of CuSO4 solution, and then when the absorbance at 265 nm reached half of that at the starting point, AMF was added to the solution. The solutions were monitored at 265 nm for 40 min, and spectra were collect every 30 s. The same conditions were used for the assays in the presence of 12 μM Aβ42.
Cytotoxicity Studies.
Cytotoxicity studies were performed according to our published protocol using the Alamar Blue assay.24,37 Mouse neuroblastoma Neuro2A (N2A) cells were purchased from the American Type Culture Collection. Cells were grown in Dulbecco’s modified Eagle’s medium (DMEM; Gibco) supplemented with 10% fetal bovine serum and antibiotics at 37 °C in a humidified 5% CO2 incubator. N2A cells were suspended in the medium and seeded into a 96-well plate (2.5 × 104 cells/well). At 24 h later, cells were washed with DMEM/N2 medium (Gibco) and treated with Aβ42 species, AMF, and metal ions in a final volume of 100 μL. After an additional incubation of 40 h, Alamar blue (Resazurin; Sigma-Aldrich) was added to each well at a final concentration of 30 μg/mL and incubated at 37 °C for 90 min, the absorbance was read at 570 nm, and the cell viability was calculated.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by research funding from the NIH (R01GM114588 to L.M.M.) and the Alzheimer’s Association (NIRG 12-259199 to L.M.M.).
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acschemneuro.0c00376.
Absorption and fluorescence spectra of AMF, additional brain section fluorescence images, and additional docking studies data (PDF)
The authors declare no competing financial interest.
Contributor Information
Liang Sun, Department of Chemistry, University of Illinois at Urbana-Champaign, Urbana, Illinois 61801, United States.
Anuj K. Sharma, Department of Chemistry, Central University of Rajasthan, Ajmer 305801, Rajasthan, India
Byung-Hee Han, Department of Pharmacology, A.T. Still University of Health Sciences, Kirksville, Missouri 63501, United States.
Liviu M. Mirica, Department of Chemistry, University of Illinois at Urbana-Champaign, Urbana, Illinois 61801, United States; Hope Center for Neurological Disorders, Washington University School of Medicine, St. Louis, Missouri 63110, United States.
REFERENCES
- (1).Alzheimer’s Association (2020) Alzheimer’s disease facts and figures. Alzheimer’s Dementia 16, 391–460. [Google Scholar]
- (2).Hardy J, and Selkoe DJ (2002) The Amyloid Hypothesis of Alzheimer’s Disease: Progress and Problems on the Road to Therapeutics. Science 297 (5580), 353–356. [DOI] [PubMed] [Google Scholar]
- (3).DeToma AS, Salamekh S, Ramamoorthy A, and Lim MH (2012) Misfolded proteins in Alzheimer’s disease and type II diabetes. Chem. Soc. Rev 41 (2), 608–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Kepp KP (2012) Bioinorganic chemistry of Alzheimer’s disease. Chem. Rev 112 (10), 5193–5239. [DOI] [PubMed] [Google Scholar]
- (5).Jakob-Roetne R, and Jacobsen H (2009) Alzheimer’s Disease: From Pathology to Therapeutic Approaches. Angew. Chem., Int. Ed 48 (17), 3030–3059. [DOI] [PubMed] [Google Scholar]
- (6).Bush AI, Pettingell WH, Multhaup G, Paradis MD, Vonsattel JP, Gusella JF, Beyreuther K, Masters CL, and Tanzi RE (1994) Rapid Induction of Alzheimer a-Beta Amyloid Formation by Zinc. Science 265 (5177), 1464–1467. [DOI] [PubMed] [Google Scholar]
- (7).Atwood CS, Moir RD, Huang XD, Scarpa RC, Bacarra NME, Romano DM, Hartshorn MK, Tanzi RE, and Bush AI (1998) Dramatic aggregation of Alzheimer A beta by Cu(II) is induced by conditions representing physiological acidosis. J. Biol. Chem 273 (21), 12817–12826. [DOI] [PubMed] [Google Scholar]
- (8).Rana M, and Sharma AK (2019) Cu and Zn interactions with Abeta peptides: consequence of coordination on aggregation and formation of neurotoxic soluble Abeta oligomers. Metallomics 11 (1), 64–84. [DOI] [PubMed] [Google Scholar]
- (9).Lovell MA, Robertson JD, Teesdale WJ, Campbell JL, and Markesbery WR (1998) Copper, iron and zinc in Alzheimer’s disease senile plaques. J. Neurol. Sci 158 (1), 47–52. [DOI] [PubMed] [Google Scholar]
- (10).Faller P (2009) Copper and Zinc Binding to Amyloid-β: Coordination, Dynamics, Aggregation, Reactivity and Metal-Ion Transfer. ChemBioChem 10 (18), 2837–2845. [DOI] [PubMed] [Google Scholar]
- (11).Zatta P, Drago D, Bolognin S, and Sensi SL (2009) Alzheimer’s disease, metal ions and metal homeostatic therapy. Trends Pharmacol. Sci 30 (7), 346–355. [DOI] [PubMed] [Google Scholar]
- (12).Bagheri S, Squitti R, Haertle T, Siotto M, and Saboury AA (2018) Role of Copper in the Onset of Alzheimer’s Disease Compared to Other Metals. Front. Aging Neurosci 9, 446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Faller P, and Hureau C (2009) Bioinorganic chemistry of copper and zinc ions coordinated to amyloid-β peptide. Dalton Trans. 38 (7), 1080–1094. [DOI] [PubMed] [Google Scholar]
- (14).Kepp KP (2017) Alzheimer’s disease: How metal ions define β-amyloid function. Coord. Chem. Rev 351, 127–159. [Google Scholar]
- (15).Atrian-Blasco E, Gonzalez P, Santoro A, Alies B, Faller P, and Hureau C (2018) Cu and Zn coordination to amyloid peptides: From fascinating chemistry to debated pathological relevance. Coord. Chem. Rev 371, 38–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Abeyawardhane DL, Fernandez RD, Murgas CJ, Heitger DR, Forney AK, Crozier MK, and Lucas HR (2018) Iron Redox Chemistry Promotes Antiparallel Oligomerization of alpha-Synuclein. J. Am. Chem. Soc 140 (15), 5028–5032. [DOI] [PubMed] [Google Scholar]
- (17).Zhu X, Su B, Wang X, Smith M, and Perry G (2007) Causes of oxidative stress in Alzheimer disease. Cell. Mol. Life Sci 64 (17), 2202–2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Crichton RR, Dexter DT, and Ward RJ (2008) Metal based neurodegenerative diseases—From molecular mechanisms to therapeutic strategies. Coord. Chem. Rev 252 (10–11), 1189–1199. [Google Scholar]
- (19).Cheignon C, Tomas M, Bonnefont-Rousselot D, Faller P, Hureau C, and Collin F (2018) Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 14, 450–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Tonnies E, and Trushina E (2017) Oxidative Stress, Synaptic Dysfunction, and Alzheimer’s Disease. J. Alzheimer’s Dis 57 (4), 1105–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Hureau C, and Faller P (2009) Aβ-mediated ROS production by Cu ions: structural insights, mechanisms and relevance to Alzheimer’s disease. Biochimie 91 (10), 1212–1217. [DOI] [PubMed] [Google Scholar]
- (22).Cheignon C, Jones M, Atrian-Blasco E, Kieffer I, Faller P, Collin F, and Hureau C (2017) Identification of key structural features of the elusive Cu-Abeta complex that generates ROS in Alzheimer’s disease. Chem. Sci 8 (7), 5107–5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Zhang Y, Rempel DL, Zhang J, Sharma AK, Mirica LM, and Gross ML (2013) Pulsed hydrogen-deuterium exchange mass spectrometry probes conformational changes in amyloid beta (Aβ) peptide aggregation. Proc. Natl. Acad. Sci. U. S. A 110, 14604–14609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Sharma AK, Pavlova ST, Kim J, Kim J, and Mirica LM (2013) The effect of Cu2+ and Zn2+ on the Aβ42 peptide aggregation and cellular toxicity. Metallomics 5 (11), 1529–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Que EL, Domaille DW, and Chang CJ (2008) Metals in neurobiology: probing their chemistry and biology with molecular imaging. Chem. Rev 108 (5), 1517–1549. [DOI] [PubMed] [Google Scholar]
- (26).Esmieu C, Guettas D, Conte-Daban A, Sabater L, Faller P, and Hureau C (2019) Copper-Targeting Approaches in Alzheimer’s Disease: How To Improve the Fallouts Obtained from in Vitro Studies. Inorg. Chem 58 (20), 13509–13527. [DOI] [PubMed] [Google Scholar]
- (27).Faller P, Hureau C, and Berthoumieu O (2013) Role of metal ions in the self-assembly of the Alzheimer’s amyloid-beta peptide. Inorg. Chem 52 (21), 12193–206. [DOI] [PubMed] [Google Scholar]
- (28).Eskici G. z., and Axelsen PH (2012) Copper and Oxidative Stress in the Pathogenesis of Alzheimer’s Disease. Biochemistry 51 (32), 6289–6311. [DOI] [PubMed] [Google Scholar]
- (29).Braymer JJ, DeToma AS, Choi J-S, Ko KS, and Lim MH (2011) Recent Development of Bifunctional Small Molecules to Study Metal-Amyloid-β Species in Alzheimer’s Disease. Int. J. Alzheimer’s Dis 2011, 623051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Rodríguez-Rodríguez C, Telpoukhovskaia M, and Orvig C (2012) The art of building multifunctional metal-binding agents from basic molecular scaffolds for the potential application in neurodegenerative diseases. Coord. Chem. Rev 256 (19), 2308–2332. [Google Scholar]
- (31).Savelieff MG, Nam G, Kang J, Lee HJ, Lee M, and Lim MH (2019) Development of Multifunctional Molecules as Potential Therapeutic Candidates for Alzheimer’s Disease, Parkinson’s Disease, and Amyotrophic Lateral Sclerosis in the Last Decade. Chem. Rev 119 (2), 1221–1322. [DOI] [PubMed] [Google Scholar]
- (32).Jones MR, Mathieu E, Dyrager C, Faissner S, Vaillancourt Z, Korshavn KJ, Lim MH, Ramamoorthy A, Wee Yong V, Tsutsui S, Stys PK, and Storr T (2017) Multi-target-directed phenol-triazole ligands as therapeutic agents for Alzheimer’s disease. Chem. Sci 8 (8), 5636–5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Cho H-J, Sharma AK, Zhang Y, Gross ML, and Mirica LM (2020) A Multifunctional Chemical Agent as an Attenuator of Amyloid Burden and Neuroinflammation in Alzheimer’s Disease. ACS Chem. Neurosci 11 (10), 1471–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Sharma AK, Schultz JW, Prior JT, Rath NP, and Mirica LM (2017) Coordination Chemistry of Bifunctional Chemical Agents Designed for Applications in Cu-64 PET Imaging for Alzheimer’s Disease. Inorg. Chem 56 (22), 13801–13814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Beck MW, Derrick JS, Kerr RA, Oh SB, Cho WJ, Lee SJC, Ji Y, Han J, Tehrani ZA, Suh N, Kim S, Larsen SD, Kim KS, Lee JY, Ruotolo BT, and Lim MH (2016) Structure-mechanism-based engineering of chemical regulators targeting distinct pathological factors in Alzheimer’s disease. Nat. Commun 7, 13115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Johnston HM, Pota K, Barnett MM, Kinsinger O, Braden P, Schwartz TM, Hoffer E, Sadagopan N, Nguyen N, Yu Y, Gonzalez P, Tircso G, Wu H, Akkaraju G, Chumley MJ, and Green KN (2019) Enhancement of the Antioxidant Activity and Neurotherapeutic Features through Pyridol Addition to Tetraazamacrocyclic Molecules. Inorg. Chem 58 (24), 16771–16784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Sharma AK, Pavlova ST, Kim J, Finkelstein D, Hawco NJ, Rath NP, Kim J, and Mirica LM (2012) Bifunctional Compounds for Controlling Metal-mediated Aggregation of the Aβ42 Peptide. J. Am. Chem. Soc 134 (15), 6625–6636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Sharma AK, Kim J, Prior JT, Hawco NJ, Rath NP, Kim J, and Mirica LM (2014) Small Bifunctional Chelators That Do Not Disaggregate Amyloid β Fibrils Exhibit Reduced Cellular Toxicity. Inorg. Chem 53 (21), 11367–11376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).DeToma AS, Choi J-S, Braymer JJ, and Lim MH (2011) Myricetin: A Naturally Occurring Regulator of Metal-Induced Amyloid-β Aggregation and Neurotoxicity. ChemBioChem 12 (8), 1198–1201. [DOI] [PubMed] [Google Scholar]
- (40).Porat Y, Abramowitz A, and Gazit E (2006) Inhibition of Amyloid Fibril Formation by Polyphenols: Structural Similarity and Aromatic Interactions as a Common Inhibition Mechanism. Chem. Biol. Drug Des 67 (1), 27–37. [DOI] [PubMed] [Google Scholar]
- (41).Bieschke J, Russ J, Friedrich RP, Ehrnhoefer DE, Wobst H, Neugebauer K, and Wanker EE (2010) EGCG remodels mature α-synuclein and amyloid-β fibrils and reduces cellular toxicity. Proc. Natl. Acad. Sci. U. S. A 107 (17), 7710–7715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Ehrnhoefer DE, Bieschke J, Boeddrich A, Herbst M, Masino L, Lurz R, Engemann S, Pastore A, and Wanker EE (2008) EGCG redirects amyloidogenic polypeptides into unstructured, off-pathway oligomers. Nat. Struct. Mol. Biol 15 (6), 558–566. [DOI] [PubMed] [Google Scholar]
- (43).Lemkul JA, and Bevan DR (2012) Morin Inhibits the Early Stages of Amyloid β-Peptide Aggregation by Altering Tertiary and Quaternary Interactions to Produce “Off-Pathway” Structures. Biochemistry 51 (30), 5990–6009. [DOI] [PubMed] [Google Scholar]
- (44).Sinha S, Du Z, Maiti P, Klärner F-G, Schrader T, Wang C, and Bitan G (2012) Comparison of Three Amyloid Assembly Inhibitors: The Sugar scyllo-Inositol, the Polyphenol Epigallocatechin Gallate, and the Molecular Tweezer CLR01. ACS Chem. Neurosci 3 (6), 451–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Yang F, Lim GP, Begum AN, Ubeda OJ, Simmons MR, Ambegaokar SS, Chen P, Kayed R, Glabe CG, Frautschy SA, and Cole GM (2005) Curcumin inhibits formation of amyloid β oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J. Biol. Chem 280 (7), 5892–5901. [DOI] [PubMed] [Google Scholar]
- (46).Nam G, Ji Y, Lee HJ, Kang J, Yi Y, Kim M, Lin Y, Lee YH, and Lim MH (2019) Orobol: An Isoflavone Exhibiting Regulatory Multifunctionality against Four Pathological Features of Alzheimer’s Disease. ACS Chem. Neurosci 10 (8), 3386–3390. [DOI] [PubMed] [Google Scholar]
- (47).Hyung S-J, DeToma AS, Brender JR, Lee S, Vivekanandan S, Kochi A, Choi J-S, Ramamoorthy A, Ruotolo BT, and Lim MH (2013) Insights into antiamyloidogenic properties of the green tea extract (−)-epigallocatechin-3-gallate toward metal-associated amyloid-β species. Proc. Natl. Acad. Sci. U. S. A 110 (10), 3743–3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Kang SS, Lee JY, Choi YK, Song SS, Kim JS, Jeon SJ, Han YN, Son KH, and Han BH (2005) Neuroprotective effects of naturally occurring biflavonoids. Bioorg. Med. Chem. Lett 15 (15), 3588–3591. [DOI] [PubMed] [Google Scholar]
- (49).Thapa A, Woo E-R, Chi EY, Sharoar MG, Jin H-G, Shin SY, and Park I-S (2011) Biflavonoids Are Superior to Monoflavonoids in Inhibiting Amyloid-β Toxicity and Fibrillogenesis via Accumulation of Nontoxic Oligomer-like Structures. Biochemistry 50 (13), 2445–2455. [DOI] [PubMed] [Google Scholar]
- (50).Choi EY, Kang SS, Lee SK, and Han BH (2020) Polyphenolic Biflavonoids Inhibit Amyloid-Beta Fibrillation and Disaggregate Preformed Amyloid-Beta Fibrils. Biomol. Ther 28 (2), 145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51). See the Supporting Information.
- (52).Perrin DD (1972)Dissociation constants of organic bases in aqueous solution, Butterworths, London. [Google Scholar]
- (53).Storr T, Merkel M, Song-Zhao GX, Scott LE, Green DE, Bowen ML, Thompson KH, Patrick BO, Schugar HJ, and Orvig C (2007) Synthesis, characterization, and metal coordinating ability of multifunctional carbohydrate-containing compounds for Alzheimer’s therapy. J. Am. Chem. Soc 129 (23), 7453–7463. [DOI] [PubMed] [Google Scholar]
- (54).Martell AE, and Smith RM (1976) Critical Stability Constants, p 1, Vol. IV, Plenum, New York. [Google Scholar]
- (55).Hyung SJ, DeToma AS, Brender JR, Lee S, Vivekanandan S, Kochi A, Choi JS, Ramamoorthy A, Ruotolo BT, and Lim MH (2013) Insights into antiamyloidogenic properties of the green tea extract (−)-epigallocatechin-3-gallate toward metal-associated amyloid-beta species. Proc. Natl. Acad. Sci. U. S. A 110 (10), 3743–3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Bieschke J, Russ J, Friedrich RP, Ehrnhoefer DE, Wobst H, Neugebauer K, and Wanker EE (2010) EGCG remodels mature alpha-synuclein and amyloid-beta fibrils and reduces cellular toxicity. Proc. Natl. Acad. Sci. U. S. A 107 (17), 7710–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Friesner RA, Banks JL, Murphy RB, Halgren TA, Klicic JJ, Mainz DT, Repasky MP, Knoll EH, Shelley M, Perry JK, Shaw DE, Francis P, and Shenkin PS (2004) Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J. Med. Chem 47 (7), 1739–49. [DOI] [PubMed] [Google Scholar]
- (58).Ciudad S, Puig E, Botzanowski T, Meigooni M, Arango AS, Do J, Mayzel M, Bayoumi M, Chaignepain S, Maglia G, Cianferani S, Orekhov V, Tajkhorshid E, Bardiaux B, and Carulla N (2020) Abeta(1–42) tetramer and octamer structures reveal edge conductivity pores as a mechanism for membrane damage. Nat. Commun 11 (1), 3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Atrian-Blasco E, Gonzalez P, Santoro A, Alies B, Faller P, and Hureau C (2018) Cu and Zn coordination to amyloid peptides: From fascinating chemistry to debated pathological relevance. Coord. Chem. Rev 371, 38–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Biancalana M, and Koide S (2010) Molecular mechanism of Thioflavin-T binding to amyloid fibrils. Biochim. Biophys. Acta, Proteins Proteomics 1804 (7), 1405–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Fagan AM, and Holtzman DM (2010) Cerebrospinal fluid biomarkers of Alzheimer’s disease. Biomarkers Med. 4 (1), 51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Lee SJC, Nam E, Lee HJ, Savelieff MG, and Lim MH (2017) Towards an understanding of amyloid-beta oligomers: characterization, toxicity mechanisms, and inhibitors. Chem. Soc. Rev 46 (2), 310–323. [DOI] [PubMed] [Google Scholar]
- (63).Gong YS, Chang L, Viola KL, Lacor PN, Lambert MP, Finch CE, Krafft GA, and Klein WL (2003) Alzheimer’s disease-affected brain: Presence of oligomeric A beta ligands (ADDLs) suggests a molecular basis for reversible memory loss. Proc. Natl. Acad. Sci. U. S. A 100 (18), 10417–10422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Walsh DM, and Selkoe DJ (2007) Aβ Oligomers - A Decade of Discovery. J. Neurochem 101 (5), 1172–1184. [DOI] [PubMed] [Google Scholar]
- (65).Haass C, and Selkoe DJ (2007) Soluble Protein Oligomers in Neurodegeneration: Lessons from the Alzheimer’s Amyloid b-Peptide. Nat. Rev. Mol. Cell Biol 8 (2), 101–112. [DOI] [PubMed] [Google Scholar]
- (66).Benilova I, Karran E, and De Strooper B (2012) The Toxic Aβ Oligomer and Alzheimer’s Disease: An Emperor in Need of Clothes. Nat. Neurosci 15, 349–357. [DOI] [PubMed] [Google Scholar]
- (67).LeVine H (1999) 3rd, Quantification of beta-sheet amyloid fibril structures with thioflavin T. Methods Enzymol. 309, 274–84. [DOI] [PubMed] [Google Scholar]
- (68).Conte-Daban A, Beyler M, Tripier R, and Hureau C (2018) Kinetics Are Crucial When Targeting Copper Ions to Fight Alzheimer’s Disease: An Illustration with Azamacrocyclic Ligands. Chem. - Eur. J 24 (33), 8447–8452. [DOI] [PubMed] [Google Scholar]
- (69).Gu M, Bode DC, and Viles JH (2018) Copper Redox Cycling Inhibits Aβ Fibre Formation and Promotes Fibre Fragmentation, while Generating a Dityrosine Aβ Dimer. Sci. Rep 8 (1), 16190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Hindo SS, Mancino AM, Braymer JJ, Liu YH, Vivekanandan S, Ramamoorthy A, and Lim MH (2009) Small Molecule Modulators of Copper-Induced A beta Aggregation. J. Am. Chem. Soc 131 (46), 16663–16664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Gans P, Sabatini A, and Vacca A (1999) Determination of equilibrium constants from spectrophotometric data obtained from solutions of known pH: The program pHab. Ann. Chim 89, 45–49. [Google Scholar]
- (72).Alderighi L (1999) Hyperquad simulation and speciation (HySS): A utility program for the investigaion of equilibria involving soluble and partially soluble species. Coord. Chem. Rev 184, 311. [Google Scholar]
- (73).Klein WL (2002) Aβ toxicity in Alzheimer’s disease: globular oligomers (ADDLs) as new vaccine and drug targets. Neurochem. Int 41 (5), 345–352. [DOI] [PubMed] [Google Scholar]
- (74).Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, Wals P, Zhang C, Finch CE, Krafft GA, and Klein WL (1998) Diffusible, nonfibrillar ligands derived from A beta(1–42) are potent central nervous system neurotoxins. Proc. Natl. Acad. Sci. U. S. A 95 (11), 6448–6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Klein WL (2002) ADDLs & protofibrils - the missing links? Neurobiol. Aging 23 (2), 231–233. [DOI] [PubMed] [Google Scholar]
- (76).Lu JX, Qiang W, Yau WM, Schwieters CD, Meredith SC, and Tycko R (2013) Molecular structure of beta-amyloid fibrils in Alzheimer’s disease brain tissue. Cell 154 (6), 1257–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Gremer L, Scholzel D, Schenk C, Reinartz E, Labahn J, Ravelli RBG, Tusche M, Lopez-Iglesias C, Hoyer W, Heise H, Willbold D, and Schroder GF (2017) Fibril structure of amyloid-beta(1–42) by cryo-electron microscopy. Science 358 (6359), 116–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Glide, Schrödinger, LLC, New York, 2020. Available from: https://www.schrodinger.com/glide/ (accessed on 2020-01-20). [Google Scholar]
- (79).PyMOL Molecular Graphics System, ver. 2.3., Schrodinger, LLC, New York, 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


