Key Points
Question
Among surgically naive children with obstructive sleep apnea, do the patterns of collapse during drug-induced sleep endoscopy differ in children with obesity or children with Down syndrome (DS) compared with children without obesity and DS?
Findings
In this prospective cohort study of 317 surgically naive children with obstructive sleep apnea, children with obesity or DS demonstrated proportionally greater tonsillar obstruction than children without obesity or DS. Children with DS also demonstrated worse arytenoid obstruction.
Meaning
Arytenoid obstruction in children with DS may contribute to the higher rate of failure of adenotonsillectomy in children with DS. Routine drug-induced sleep endoscopy should be considered in surgically naive children with DS to help inform the surgical plan.
Abstract
Importance
Persistent obstructive sleep apnea after adenotonsillectomy is common in children with Down syndrome or obesity. Drug-induced sleep endoscopy could help to identify anatomic differences in these patients that might affect surgical decision-making.
Objective
To assess drug-induced sleep endoscopy findings in surgically naive children with obstructive sleep apnea with obesity or Down syndrome and compare these findings with children without obesity or Down syndrome.
Design, Setting, and Participants
This cross-sectional analysis of data from a prospective cohort study of patients enrolled between May 1, 2015, and December 31, 2019, was conducted at an academic tertiary care children’s hospital and included a consecutive sample of surgically naive children (age 2-18 years) who underwent drug-induced sleep endoscopy at the time of adenotonsillectomy for sleep-disordered breathing. Indications for sleep endoscopy included severe sleep apnea, age older than 7 years, obesity, African American race, and Down syndrome.
Exposures
Drug-induced sleep endoscopy.
Main Outcomes and Measures
Sleep endoscopy findings were scored according to the Sleep Endoscopy Rating Scale. Ratings at 6 anatomic levels for children with obesity and those with Down syndrome were compared with controls without obesity or Down syndrome using several measures of effect size (Cohen d, Cramer V, and η2).
Results
A total of 317 children (158 girls [50%]; 219 [69%] White, 20 [6%] Black, and 103 [34%] Hispanic; mean [95% CI] age, 9.6 [9.2-10.0] years) were included, of whom 115 (36%) were controls without obesity or Down syndrome, 179 (56%) had obesity without Down syndrome, and 23 (7%) had Down syndrome. The mean apnea-hypopnea index was 16 (95% CI, 13-19), and the mean minimum O2 saturation was 83% (95% CI, 81%-85%). Compared with controls without obesity or Down syndrome, children with Down syndrome demonstrated greater overall obstruction (mean sleep endoscopy rating scale total score of 5.6 vs 4.8; Cohen d, 0.46), and greater tonsillar (percentage of complete obstruction: 65% vs 54%), tongue base (percentage of complete obstruction: 26% vs 12%), and arytenoid obstruction (percentage of at least partial obstruction, 35% vs 6%). Children with obesity had greater tonsillar (percentage of complete obstruction, 74% vs 54%) and less base of tongue obstruction (percentage of complete obstruction, 2% vs 12%) compared with controls.
Conclusions and Relevance
In this cohort study, surgically naive children with obesity with obstructive sleep apnea had predominantly tonsillar obstruction, whereas children with Down syndrome demonstrated greater obstruction of the tonsils, tongue base, and arytenoids compared with controls. Routine drug-induced sleep endoscopy should be considered in surgically naive children with Down syndrome to help inform the surgical plan.
This cohort study examines drug-induced sleep endoscopy findings in surgically naive children with obesity and children with Down syndrome.
Introduction
Obstructive sleep apnea (OSA) in the pediatric population has an estimated prevalence of 2% to 6%.1,2,3 Untreated OSA can lead to long-term health consequences in children, including hypertension, cardiac arrhythmias, developmental delay, behavioral disturbances, failure to thrive, and excessive daytime sleepiness.4,5 Although the first-line treatment for OSA in children is adenotonsillectomy (AT),6 children with obesity or Down syndrome (DS) have a high prevalence of OSA (13%-50% and 30%-66%, respectively) and lower rates of success after AT (30%-60% and 16%-33%, respectively) compared with children without obesity or DS (60%-80%).7,8,9 This has been attributed to greater pharyngeal collapsibility and multilevel obstruction.10
Drug-induced sleep endoscopy (DISE) is a diagnostic technique used to evaluate the location and severity of obstruction during sedated sleep. Drug-induced sleep endoscopy findings have been shown to be associated with the severity of baseline disease and outcome of AT.11,12,13,14 There is a paucity of literature regarding the patterns of obstruction observed in children with obesity and DS.9,10,15 The aim of this study was to assess the DISE findings observed in children with obesity or DS and compare these findings with children without obesity or DS. Specifically, we hypothesized that, compared with children without obesity or DS, the degree of obstruction observed at 1 or more locations in the upper airway is significantly different in children with obesity or DS.
Methods
Study Population
This was a cross-sectional analysis of baseline data collected from consecutive patients enrolled in a prospective cohort study of AT outcomes in pediatric patients with OSA. The patients were enrolled between June 1, 2015, and December 31, 2019, at the tertiary care Doernbecher Children’s Hospital in Portland, Oregon, who were considered high risk for residual OSA after AT. The study included surgically naive patients aged 2 to 18 years who were candidates for AT for OSA or sleep-disordered breathing and who also satisfied 1 or more of the following criteria that have been previously reported as risk factors for persistent OSA after AT: obesity (body mass index [calculated as weight in kilograms divided by height in meters squared] >95th percentile or z score >1.65 for age), African American race, DS, severe OSA (apnea hypopnea index [AHI] score >10), and age older than 7 years.
Patients with craniofacial anomalies, genetic abnormalities other than Trisomy 21, neuromuscular disorders (including cerebral palsy and hypotonia), subglottic or tracheal stenosis, tracheostomy dependence, or severe cardiopulmonary disease requiring supplemental oxygen at night were excluded. This study was approved by the Oregon Health and Science University institutional review board, and written informed consent was obtained from the caregivers of all study participants.
Data Sources
Baseline data collected as part of the prospective cohort study were entered into a Redcap research database and included in the cross-sectional analysis. Variables included demographics (age and sex), comorbidities, body mass index z scores, overnight attended polysomnography data (AHI score, minimum oxygen saturation level, and oxygen desaturation index score, if available), and DISE ratings.
Drug-Induced Sleep Endoscopy
Drug-induced sleep endoscopy was performed before AT, and ratings were noted at that time by one of 5 attending pediatric otolaryngologists at our institution (C.J.M., H.A.M., E.F.K., L.Q.D., and D.J.L.). Raters were not masked to the patients’ comorbidities or OSA severity. Patients were sedated using a combination of dexmedetomidine (1-2 mcg/kg bolus over 5-10 minutes followed by 0.5-1 mcg/kg/h infusion) and ketamine (1 mg/kg boluses up to 40 mg, as needed). Topical decongestant was not applied to the nasal cavities before endoscopic evaluation. Once adequate sedation was achieved, endoscopy was performed using a flexible 2.8-mm bronchoscope advanced transnasally. The DISE findings were scored according to the previously validated sleep endoscopy rating scale (SERS) score,13 which assesses the degree of obstruction at 6 locations in the upper airway: the nasal airway, nasopharynx (adenoids), velum, oropharynx (including tonsils), tongue base (including the epiglottis), and arytenoids. The degree of obstruction is scored on a 3-point rating scale as none (0), partial (+1), or complete (+2) at each anatomic site. The scores at each anatomic site are then summed into a SERS total score (range, 0-12). Multisite obstruction was defined as complete obstruction (+2) at more than 1 anatomic site.
Analysis
This was an exploratory analysis, so there was no specified a priori hypothesis and no power calculation was performed before data analysis. The cohort was stratified into children with DS, children without DS with obesity, and controls without DS or obesity. Baseline characteristics among these 3 groups were compared by calculating the η2 effect size measure for continuous variables and Cramer V coefficient for categorical variables. η2 is defined as the proportion of variance of a dependent variable explained by the categorical variable of interest.16 Cramer V quantifies the strength of the association among categorical variables. It ranges from 0 (no association) to 1 (perfect association) with the following interpretation: less than 0.1 is no association, 0.1 to 0.2 is a weak association, 0.2 to 0.4 is a moderate association, and greater than 0.4 is a strong association.17 Separate analyses were performed comparing children with obesity and children with DS with controls.
DISE Comparisons
Mean SERS total scores and 95% CIs were calculated for each subgroup. To assess the relative contribution of the tonsils and adenoids to the overall obstruction, the SERS scores for the nasopharynx (adenoids) and oropharynx (tonsils) were summed to yield a composite score for adenotonsillar obstruction (ATO). The SERS scores at all nonadenotonsillar sites were summed to yield a score for nonadenotonsillar obstruction (NATO). The mean composite scores of children with obesity or DS were then compared with children without obesity or DS in a pairwise fashion by calculating the Cohen d statistic. This statistic reflects the effect size for independent sample t tests, with the following interpretation: 0.2 indicates a small effect, 0.5 a medium effect, and 0.8 a large effect.18
Because SERS ratings are ordinal ratings of the severity of obstruction and not continuous variables, the distribution of ratings at each anatomic level was also presented. In the comparisons among subgroups (those with obesity vs those without obesity or DS and DS vs those without obesity or DS), the strength of association between comorbidities and the degree of obstruction at each anatomic level was quantified with a Cramer V coefficient. All analysis was performed using Stata, version 15.1 (StataCorp).
Results
There were 317 patients included in the study with a mean age of 9.6 (95% CI, 9.2-10.0) years. Of these, 115 (36%) did not have obesity or DS, 179 (56%) had obesity but not DS, and 23 (7%) were children with DS (Table 1). Among the children with DS, 5 (22%) had obesity. There were no meaningful differences in age distribution across the 3 subgroups. With respect to race and ethnicity distribution, there were some differences across the subgroups. The group of children with obesity had a large proportion of Hispanic individuals compared with children without obesity (42% vs 21% respectively), while children with DS demonstrated large proportions of both non-Black minority individuals (39%) and Hispanic individuals (36%). Baseline polysomnography data were available in 129 patients (40%). The mean obstructive AHI score was 16.1 (95% CI, 12.8-19.4), and the mean minimum O2 saturation was 83.2 (95% CI, 81.4-85.0). The mean SERS total score was 4.9 (95% CI, 4.7-5.0). There were no meaningful differences in obstructive AHI score, oxygen nadir, or oxygen desaturation index score among the 3 subgroups. There were no complications associated with DISE in any of the patients.
Table 1. Patient Characteristics.
| Characteristic | Overall | Without obesity or Down syndrome | With obesity and without Down syndrome | Down syndrome | Effect size |
|---|---|---|---|---|---|
| No. (%) | 317 | 115 (36) | 179 (56) | 23 (7) | NA |
| Demographic | |||||
| Age, mean (95% CI), y | 9.6 (9.2-10.0) | 9.6 (9.0-10.3) | 9.6 (9.1-10.1) | 8.8 (7.0-10.6) | 0.003 |
| <5 | 35 (11) | 14 (12) | 16 (9) | 5 (22) | 0.09 |
| 5-9.9 | 160 (50) | 52 (45) | 96 (54) | 12 (52) | NA |
| 10-18 | 122 (38) | 49 (43) | 67 (37) | 6 (26) | NA |
| Race/ethnicity | |||||
| Whitea | 219 (69) | 91 (79) | 114 (64) | 14 (61) | 0.16 |
| Black | 20 (6) | 12 (10) | 8 (4) | 0 (0) | 0.14 |
| Other | 42 (13) | 16 (14) | 17 (9) | 9 (39) | 0.22 |
| Hispanic | 103 (34) | 23 (21) | 72 (42) | 8 (36) | 0.21 |
| Female | 158 (50) | 62 (54) | 84 (47) | 12 (52) | 0.07 |
| Polysomnography (95% CI) | |||||
| oAHI | 16.1 (12.8-19.4) | 14.6 (8.6-20.5) | 17.6 (13.1-22.0) | 12.4 (2.9-21.8) | 0.009 |
| SpO2 nadir, % | 83.2 (81.4-85.0) | 85.0 (82.4-87.5) | 81.8 (79.0-84.5) | 86.0 (82.5-89.5) | 0.02 |
| ODI | 14.1 (10.6-17.6) | 13.8 (5.9-21.8) | 14.4 (10.5-18.4) | 12.9 (0.5-25.2) | 0.0006 |
Abbreviations: NA, not applicable; oAHI, obstructive apnea hypopnea index; ODI, oxygen desaturation index; SpO2, oxygen saturation.
Race/ethnicity categories are not mutually exclusive, with some individuals identifying as multiracial, so column percentages may not total 100%. Summary statistics are presented as mean (95% CI) or No. (%) for continuous or categorical variables, respectively. Effect size was calculated by Cramer V for categorical variables and η2for continuous variables.
Children With Obesity vs Controls Without Obesity or DS
There was no difference in mean SERS total score between children without and with obesity (4.8; 95% CI, 4.4-5.1; vs 4.8;95% CI, 4.6-5.1, respectively) (Table 2). Multisite obstruction was seen more in children with obesity vs those without (48% vs 37%, respectively), although this difference represented a weak association (Cramer V, 0.10). Children with obesity did demonstrate greater AT obstruction compared with those without (ATO score, 2.7; 95% CI, 2.6-2.9; vs 2.2;95% CI, 2.0-2.4; Cohen d, 0.47). In contrast, at non-AT sites, children with obesity demonstrated less obstruction than those without (NATO score, 2.1; 95% CI, 1.9-2.2; vs 2.5; 95% CI, 2.3-2.8, respectively; Cohen d, 0.40). When we examined the distribution of SERS obstructive ratings at individual anatomic sites, children with obesity had greater obstruction of the tonsils than those without obesity (74% vs 54% complete obstruction, respectively; Cramer V, 0.25) and less obstruction at the base of tongue (77% vs 51% no obstruction, respectively; Cramer V, 0.30) (Table 2 and Figure).
Table 2. Comparison of DISE Findings in Children With Obesity vs Children Without Obesity or Down Syndromea.
| Characteristic | Without obesity or Down syndrome (n = 115) | With obesity (n = 179) | Effect size | ||||
|---|---|---|---|---|---|---|---|
| SERS total score (95% CI) | 4.8 (4.4-5.1) | 4.8 (4.6-5.1) | 0.03 | ||||
| Multisite obstruction, No. (%) | 43 (37) | 86 (48) | 0.10 | ||||
| ATO score (95% CI) | 2.2 (2.0-2.4) | 2.7 (2.6-2.9) | 0.47 | ||||
| NATO score (95% CI) | 2.5 (2.3-2.8) | 2.1 (1.9-2.2) | 0.40 | ||||
| Distribution of SERS scores by anatomic site | |||||||
| Anatomic site, No. (%) | None | Partial | Complete | None | Partial | Complete | Effect size |
| Nasal airway | 23 (20) | 67 (58) | 25 (22) | 45 (25) | 103 (57) | 31 (17) | 0.07 |
| Nasopharynx | 46 (40) | 41 (36) | 28 (24) | 59 (33) | 55 (30) | 65 (36) | 0.12 |
| Velopharynx | 40 (35) | 50 (44) | 24 (21) | 68 (38) | 65 (37) | 45 (25) | 0.08 |
| Oropharynx | 16 (14) | 37 (32) | 61 (54) | 6 (3) | 40 (22) | 132 (74) | 0.25 |
| Base of tongue | 58 (51) | 42 (37) | 14 (12) | 136 (77) | 36 (20) | 4 (2) | 0.30 |
| Arytenoids, No. (%) | 107 (94) | 7 (6) | 0 (0) | 167 (95) | 8 (5) | 1 (1) | 0.06 |
Abbreviations: ATO, adenotonsillar obstruction, sum of SERS scores at nasopharynx (adenoids) and oropharynx (tonsils); DISE, drug-induced sleep endoscopy; NATO, nonadenotonsillar obstruction, sum of SERS scores at nonadenotonsillar sites; SERS, Sleep Endoscopy Rating Scale.
Summary statistics presented as mean (95% CI) or No. (%) for continuous or categorical variables, respectively. Effect size calculated by Cramer V for categorical variables and Cohen d for continuous variables.
Figure. Sleep Endoscopy Rating Scale (SERS) Ratings by Anatomic Location in Children Without Obesity and Down Syndrome (DS) vs Children With Obesity or DS.
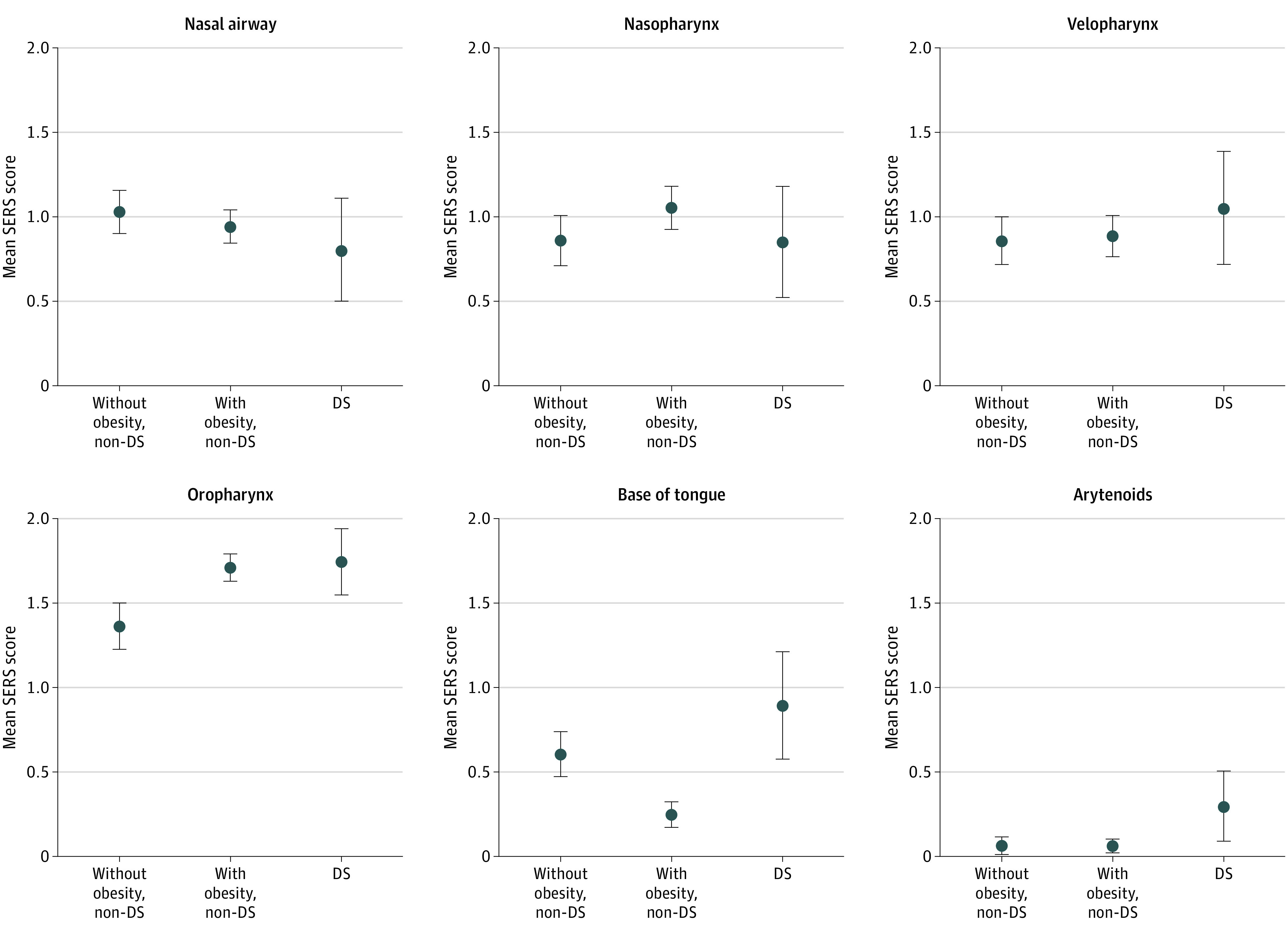
Error bars indicate 95% CI.
Children With DS vs Controls Without Obesity or DS
Children with DS demonstrated worse overall obstruction compared with controls without DS (mean SERS total score, 5.6; 95% CI, 4.9-6.2; vs 4.8; 95% CI, 4.4-5.1, respectively; Cohen d, 0.46). The rate of multisite obstruction was overall similar (43% vs 37%, respectively; Cramer V = 0.05); however, children with DS did demonstrate greater obstruction at non-AT sites than children without DS (NATO score, 3.2; 95% CI, 2.7-3.6; vs 2.5; 95% CI, 2.3-2.8, respectively; Cohen d, 0.50) (Table 3). However, when we examined the distribution of SERS ratings at individual anatomic sites, children with DS showed less nasal obstruction than children without DS (61% vs 80% with at least partial obstruction, respectively; Cramer V, 0.17) and greater obstruction of the oropharynx (100% vs 86% with at least partial obstruction; Cramer V, 0.17), base of tongue (74% vs 49% with at least partial obstruction; Cramer V, 0.20), and arytenoids (35% vs 6% with partial obstruction; Cramer V, 0.34) (Table 3 and Figure).
Table 3. Comparison of DISE Findings in Children With DS vs Children Without DS or Obesitya.
| Characteristic | Without DS or obesity (n = 115) | DS (n = 23) | Effect size | ||||
|---|---|---|---|---|---|---|---|
| SERS total score (95% CI) | 4.8 (4.4-5.1) | 5.6 (4.9-6.2) | 0.46 | ||||
| Multisite obstruction, No. (%) | 43 (37) | 10 (43) | 0.05 | ||||
| ATO score (95% CI) | 2.2 (2.0-2.4) | 2.4 (2.0-2.8) | 0.14 | ||||
| NATO score (95% CI) | 2.5 (2.3-2.8) | 3.2 (2.7-3.6) | 0.50 | ||||
| Distribution of SERS scores by anatomic site | |||||||
| Anatomic site | None | Partial | Complete | None | Partial | Complete | Effect size |
| Nasal airway | 23 (20) | 67 (58) | 25 (22) | 9 (39) | 11 (48) | 3 (13) | 0.17 |
| Nasopharynx | 46 (40) | 41 (36) | 28 (24) | 10 (43) | 9 (39) | 4 (17) | 0.06 |
| Velopharynx | 40 (35) | 50 (44) | 24 (21) | 5 (22) | 11 (47) | 7 (30) | 0.12 |
| Oropharynx | 16 (14) | 37 (32) | 61 (54) | 0 (0) | 8 (35) | 15 (65) | 0.17 |
| Base of tongue | 58 (51) | 42 (37) | 14 (12) | 6 (26) | 11 (48) | 6 (26) | 0.20 |
| Arytenoids | 107 (94) | 7 (6) | 0 (0) | 15 (65) | 8 (35) | 0 (0) | 0.34 |
Abbreviations: ATO, adenotonsillar obstruction, sum of SERS scores at nasopharynx (adenoids) and oropharynx (tonsils); DISE, drug-induced sleep endoscopy; DS, Down syndrome; NATO, nonadenotonsillar obstruction, sum of SERS scores at nonadenotonsillar sites; SERS, Sleep Endoscopy Rating Scale.
Summary statistics presented as mean (95% CI) or No. (%) for continuous or categorical variables, respectively. Effect size calculated by Cramer V for categorical variables and Cohen d for continuous variables.
Discussion
Children with obesity or DS are known to have a greater risk of OSA than those without obesity or DS,19,20 and they are at greater risk for persistent OSA after AT.21,22 It is frequently assumed that the cause for these elevated risks is associated with anatomic or physiologic predisposition to multisite obstruction that may not be adequately addressed by AT alone. However, the mechanism of obstruction in these high-risk populations has not been well studied. Compared with children without obesity or DS, our results demonstrated worse obstruction at the level of the oropharynx but less obstruction at the base of tongue in children with obesity and worse obstruction at the oropharynx, base of tongue, and arytenoids in children with DS.
It is interesting that children with obesity and DS were noted to have overall worse oropharyngeal obstruction due to tonsillar hypertrophy and that children with obesity had less base of tongue obstruction than children without obesity, with no differences noted at other anatomic sites. This may represent particularly hypertrophic tonsillar tissue in patients with obesity. The senior author (D.J.L.) has anecdotally noted that many patients with obesity tend to have large palatine tonsils that can extend caudally into the hypopharynx during DISE, preventing glossoptosis and thus decreasing the apparent degree of tongue base obstruction compared with children without obesity. The observation that large palatine tonsils can sometimes act as airway stents has been suggested in a recent case report.23 The association between obesity and tonsillar hypertrophy in children with OSA is unclear, with conflicting findings reported in different studies.24,25,26 However, these studies are based on awake clinic assessments of tonsil size, which have not shown a strong association with OSA severity or response to tonsillectomy.11,27 Our findings suggest that children with obesity may generally have proportionally greater tonsillar hypertrophy and obstruction than children without obesity.
With respect to children with DS, we noted a greater SERS total score reflecting overall obstruction compared with children without DS and, specifically, greater obstruction at the oropharynx, base of tongue, and arytenoids. Based on these differences, it was somewhat surprising that we did not observe any meaningful differences in proportions with multisite obstruction, but this may be because of the definition of multisite obstruction that only considered complete obstruction and not partial obstruction at more than 1 site. Nevertheless, these findings suggest a greater predisposition to obstruction across multiple different sites in the pharynx of children with DS compared with controls.
Our findings in children with DS differ somewhat from the observations of Maris et al,10 who noted relatively infrequent base of tongue obstruction (24%) and a high rate of tonsillar obstruction (66%). However, our finding of worse arytenoid obstruction compared with controls is consistent with previous reports suggesting a high prevalence of occult laryngomalacia in children with DS.28 Another study by Fung et al21 noted that patients with DS had less adenoid obstruction and greater collapse of the tongue base and hypopharynx in children with DS compared with controls. These findings are somewhat different than what we observed, but it is possible that some of these differences may be attributed to differences in demographic characteristics and sedation protocol.21
Taken together, these observations may explain the higher rate of failure of AT in children with DS and suggest that routine pre-AT DISE should be considered in this population. The DISE findings could alter the surgical plan and possibly avoid the need for a second procedure in the event of AT failure. However, the same argument cannot be applied to our findings in children with obesity, which are somewhat counterintuitive. The findings of greater tonsillar obstruction in children with obesity with lesser obstruction at non-AT sites suggest that children with obesity should generally respond with high rates of success with AT, but this is not what has been observed in multiple previous studies.29,30 It is possible that, in this population, pre-AT DISE findings may not accurately reflect changes in the dynamics of airway obstruction after AT. Further study is needed to determine whether and how pre-AT DISE observations of obstructive patterns change after AT.
Limitations
This study does have important limitations. First, the study population included a relatively small sample size of patients with DS, potentially limiting the precision of our results. However, even with this limited sample size, we were able to detect some important differences in DISE findings. In particular, our findings of greater mean overall and non-AT obstruction and greater arytenoid obstruction demonstrated moderate effect size differences compared with children without DS. Second, the controls without obesity or DS were drawn from a selected sample with 1 or more risk factors for AT failure. While this could affect the generalizability of our findings, it would likely create a conservative bias, because even the controls were at greater risk for AT failure, presumably because of multilevel obstruction or obstruction at non-AT anatomic sites. Third, raters were not masked to the patients’ comorbidities or OSA severity, which could have affected their scoring of DISE findings. Nevertheless, while these limitations could affect the external validity of our findings with respect to comparisons with the general pediatric population, our observations of the patterns of obstruction during DISE in surgically naive children with obesity and DS can potentially be used to help inform OSA treatment strategies and lay the foundation for future comparative outcome studies.
Based on our findings, we recommend routine pre-AT DISE for children with DS based on the possibility that other findings, such as arytenoid prolapse, could alter the surgical plan, suggesting, for example, the need for supraglottoplasty in addition to AT. However, we would not recommend routine pre-AT DISE for children with obesity undergoing AT for OSA, given that our observed DISE findings do not appear likely to alter the recommended first-line treatment of AT in this population.
Conclusions
In this study, we observed greater AT obstruction with less base of tongue obstruction in children with obesity compared with controls. In children with DS, we observed more non-AT obstruction, particularly at the base of tongue and arytenoids, compared with controls. Further study is needed to confirm these exploratory findings. We suggest that routine DISE should be considered for surgical planning purposes in surgically naive children with DS.
References
- 1.Bixler EO, Vgontzas AN, Lin HM, et al. Sleep disordered breathing in children in a general population sample: prevalence and risk factors. Sleep. 2009;32(6):731-736. doi: 10.1093/sleep/32.6.731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li AM, So HK, Au CT, et al. Epidemiology of obstructive sleep apnoea syndrome in Chinese children: a two-phase community study. Thorax. 2010;65(11):991-997. doi: 10.1136/thx.2010.134858 [DOI] [PubMed] [Google Scholar]
- 3.Lumeng JC, Chervin RD. Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):242-252. doi: 10.1513/pats.200708-135MG [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brouillette RT, Fernbach SK, Hunt CE. Obstructive sleep apnea in infants and children. J Pediatr. 1982;100(1):31-40. doi: 10.1016/S0022-3476(82)80231-X [DOI] [PubMed] [Google Scholar]
- 5.Park JG, Ramar K, Olson EJ. Updates on definition, consequences, and management of obstructive sleep apnea. Mayo Clin Proc. 2011;86(6):549-554. doi: 10.4065/mcp.2010.0810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitchell RB, Archer SM, Ishman SL, et al. Clinical practice guideline: tonsillectomy in children (update)—executive summary. Otolaryngol Head Neck Surg. 2019;160(2):187-205. doi: 10.1177/0194599818807917 [DOI] [PubMed] [Google Scholar]
- 7.Maris M, Verhulst S, Wojciechowski M, Van de Heyning P, Boudewyns A. Prevalence of obstructive sleep apnea in children with Down syndrome. Sleep. 2016;39(3):699-704. doi: 10.5665/sleep.5554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scheffler P, Wolter NE, Narang I, et al. Surgery for obstructive sleep apnea in obese children: literature review and meta-analysis. Otolaryngol Head Neck Surg. 2019;160(6):985-992. doi: 10.1177/0194599819829415 [DOI] [PubMed] [Google Scholar]
- 9.Best J, Mutchnick S, Ida J, Billings KR. Trends in management of obstructive sleep apnea in pediatric patients with Down syndrome. Int J Pediatr Otorhinolaryngol. 2018;110:1-5. doi: 10.1016/j.ijporl.2018.04.008 [DOI] [PubMed] [Google Scholar]
- 10.Maris M, Verhulst S, Saldien V, Van de Heyning P, Wojciechowski M, Boudewyns A. Drug-induced sedation endoscopy in surgically naive children with Down syndrome and obstructive sleep apnea. Sleep Med. 2016;24:63-70. doi: 10.1016/j.sleep.2016.06.018 [DOI] [PubMed] [Google Scholar]
- 11.Lam DJ, Krane NA, Mitchell RB. Relationship between drug-induced sleep endoscopy findings, tonsil size, and polysomnographic outcomes of adenotonsillectomy in children. Otolaryngol Head Neck Surg. 2019;161(3):507-513. doi: 10.1177/0194599819860777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dahl JP, Miller C, Purcell PL, et al. Airway obstruction during drug-induced sleep endoscopy correlates with apnea-hypopnea index and oxygen nadir in children. Otolaryngol Head Neck Surg. 2016;155(4):676-680. doi: 10.1177/0194599816653113 [DOI] [PubMed] [Google Scholar]
- 13.Lam DJ, Weaver EM, Macarthur CJ, et al. Assessment of pediatric obstructive sleep apnea using a drug-induced sleep endoscopy rating scale. Laryngoscope. 2016;126(6):1492-1498. doi: 10.1002/lary.25842 [DOI] [PubMed] [Google Scholar]
- 14.Alsufyani N, Isaac A, Witmans M, Major P, El-Hakim H. Predictors of failure of DISE-directed adenotonsillectomy in children with sleep disordered breathing. J Otolaryngol Head Neck Surg. 2017;46(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akkina SR, Ma CC, Kirkham EM, Horn DL, Chen ML, Parikh SR. Does drug induced sleep endoscopy-directed surgery improve polysomnography measures in children with Down syndrome and obstructive sleep apnea? Acta Otolaryngol. 2018;138(11):1009-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olejnik S, Algina J. Generalized eta and omega squared statistics: measures of effect size for some common research designs. Psychol Methods. 2003;8(4):434-447. [DOI] [PubMed] [Google Scholar]
- 17.Rea LM, Parker RA. Designing and Conducting Survey Research: a Comprehensive Guide. Jossey-Bass, 2014. [Google Scholar]
- 18.Cohen J Statistical Power Analysis for the Behavioral Sciences. 2nd ed L. Erlbaum Associates, 1988. [Google Scholar]
- 19.Shott SR Down syndrome: common otolaryngologic manifestations. Am J Med Genet C Semin Med Genet. 2006;142C(3):131-140. [DOI] [PubMed] [Google Scholar]
- 20.Arens R, Muzumdar H. Childhood obesity and obstructive sleep apnea syndrome. J Appl Physiol (1985). 2010;108(2):436-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fung E, Witmans M, Ghosh M, Cave D, El-Hakim H. Upper airway findings in children with Down syndrome on sleep nasopharyngoscopy: case-control study. J Otolaryngol Head Neck Surg. 2012;41(2):138-144. [PubMed] [Google Scholar]
- 22.Mitchell RB, Boss EF. Pediatric obstructive sleep apnea in obese and normal-weight children: impact of adenotonsillectomy on quality-of-life and behavior. Dev Neuropsychol. 2009;34(5):650-661. [DOI] [PubMed] [Google Scholar]
- 23.Zalzal HG, Coutras S. Palatine tonsil stenting of the airway as determined by drug-induced sleep endoscopy. Case Rep Otolaryngol. 2018;2018:2614143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daar G, Sarı K, Gencer ZK, Ede H, Aydın R, Saydam L. The relation between childhood obesity and adenotonsillar hypertrophy. Eur Arch Otorhinolaryngol. 2016;273(2):505-509. [DOI] [PubMed] [Google Scholar]
- 25.Dayyat E, Kheirandish-Gozal L, Sans Capdevila O, Maarafeya MMA, Gozal D. Obstructive sleep apnea in children: relative contributions of body mass index and adenotonsillar hypertrophy. Chest. 2009;136(1):137-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scott B, Johnson RF, Mitchell Md RB, Obstructive Sleep Apnea MD. Obstructive sleep apnea: differences between normal-weight, overweight, obese, and morbidly obese children. Otolaryngol Head Neck Surg. 2016;154(5):936-943. [DOI] [PubMed] [Google Scholar]
- 27.Nolan J, Brietzke SE. Systematic review of pediatric tonsil size and polysomnogram-measured obstructive sleep apnea severity. Otolaryngol Head Neck Surg. 2011;144(6):844-850. [DOI] [PubMed] [Google Scholar]
- 28.Mitchell RB, Call E, Kelly J. Diagnosis and therapy for airway obstruction in children with Down syndrome. Arch Otolaryngol Head Neck Surg. 2003;129(6):642-645. [DOI] [PubMed] [Google Scholar]
- 29.Mitchell RB, Kelly J. Outcome of adenotonsillectomy for severe obstructive sleep apnea in children. Int J Pediatr Otorhinolaryngol. 2004;68(11):1375-1379. doi: 10.1016/j.ijporl.2004.04.026 [DOI] [PubMed] [Google Scholar]
- 30.Marcus CL, Moore RH, Rosen CL, et al. ; Childhood Adenotonsillectomy Trial (CHAT) . A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med. 2013;368(25):2366-2376. doi: 10.1056/NEJMoa1215881 [DOI] [PMC free article] [PubMed] [Google Scholar]


