Key Points
Question
What implications do state crisis standards of care (CSC) guidelines have for patients with cancer?
Findings
In this cross-sectional analysis of CSC guidelines, 55% deprioritized some patients with cancer, and 26% categorically excluded some patients with cancer during scarce health care resource allocation. The presence of an in-state National Cancer Institute–designated Comprehensive Cancer Center was associated with guideline availability, palliative care provisions, and lower odds of cancer-related exclusions.
Meaning
These data suggest that equitable state-level CSC considerations for patients with cancer benefit from the input of oncology stakeholders.
Abstract
Importance
State crisis standards of care (CSC) guidelines in the US allocate scarce health care resources among patients. Anecdotal reports suggest that guidelines may disproportionately allocate resources away from patients with cancer, but no comprehensive evaluation has been performed.
Objective
To examine the implications of US state CSC guidelines for patients with cancer, including allocation methods, cancer-related categorical exclusions and deprioritizations, and provisions for blood products and palliative care.
Design, Setting, and Participants
This cross-sectional population-based analysis examined state-endorsed CSC guidelines published before May 20, 2020, that included health care resource allocation recommendations.
Main Outcomes and Measures
Guideline publication before or within 120 days after the first documented US case of coronavirus disease 2019 (COVID-19), inclusion of cancer-related categorical exclusions and/or deprioritizations, provisions for blood products and/or palliative care, and associations between these outcomes and state-based cancer demographics.
Results
Thirty-one states had health care resource allocation guidelines that met inclusion criteria, of which 17 had been published or updated since the first US case of COVID-19. States whose available hospital bed capacity was predicted to exceed 100% at 6 months (χ2 = 3.82; P = .05) or that had a National Cancer Institute–designated Comprehensive Cancer Center (CCC; χ2 = 6.21; P = .01) were more likely to have publicly available guidelines. The most frequent primary methods of prioritization were the Sequential Organ Failure Assessment score (27 states [87%]) and deprioritizing persons with worse long-term prognoses (22 states [71%]). Seventeen states’ (55%) allocation methods included cancer-related deprioritizations, and 8 states (26%) included cancer-related categorical exclusions. The presence of an in-state CCC was associated with lower likelihood of cancer-related categorical exclusions (multivariable odds ratio, 0.06 [95% CI, 0.004-0.87]). Guidelines with disability rights statements were associated with specific provisions to allocate blood products (multivariable odds ratio, 7.44 [95% CI, 1.28-43.24). Both the presence of an in-state CCC and having an oncologist and/or palliative care specialist on the state CSC task force were associated with the inclusion of palliative care provisions.
Conclusions and Relevance
Among states with CSC guidelines, most deprioritized some patients with cancer during resource allocation, and one-fourth categorically excluded them. The presence of an in-state CCC was associated with guideline availability, palliative care provisions, and lower odds of cancer-related exclusions. These data suggest that equitable state-level CSC considerations for patients with cancer benefit from the input of oncology stakeholders.
This cross-sectional study examines the implication of US state crisis standards of care guideline recommendations for patients with cancer, including allocation methods, cancer-related categorical exclusions and deprioritizations, and provisions for blood products and palliative care.
Introduction
A diagnosis of cancer is accompanied by many burdens, including increased risks of infection, social stigma, financial struggles, anxiety, and even death.1,2,3,4 Under normal medical and social standards, many patients with cancer successfully undergo treatment, receiving acute care and blood transfusions for complications while using palliative mechanisms to manage their disease. With the spread of severe acute respiratory syndrome coronavirus 2, however, normal standards have been upended by new care delivery strategies targeting reductions in viral transmission and the conservation of health care resources. One such measure, which may further increase the vulnerability of those with cancer, is resource allocation through crisis standards of care (CSC).5,6
Crisis standards of care outline system frameworks for catastrophic disaster responses.7 One such crisis occurs when health care demand outstrips supply. When this occurs, the primary goal of medical care shifts from the promotion of well-being for the individual to the promotion of collective well-being.8 This shift is fundamental, as it requires the medical system to identify which patients should receive potentially life-saving treatments first, last, or not at all. Crisis standards require society to make explicit choices between potentially conflicting goals such as maximizing life-years saved—which may deprioritize and/or exclude vulnerable populations such as those with cancer—and treating all patients equally, which may allocate resources to those less likely to survive.8,9 After the 2009 H1N1 influenza pandemic, there was considerable development of state government CSC guidelines, including formal recommendations for the allocation of scarce health care resources.7 Despite their potential effect on patients with cancer, CSC guidelines have, to our knowledge, received relatively little attention from the oncology community until recently.
Soon after the coronavirus disease 2019 (COVID-19) pandemic reached the United States, modeling projections suggested that hospital bed capacity and the demand for critical care resources (eg, ventilators and intensive care unit beds) would outpace availability, prompting reexamination of CSC guidelines.10,11 Concerns have since been raised by clinicians, bioethicists, and patient groups about some states’ lack of guidelines and how others exclude or allocate care away from persons with cancer or other vulnerable populations.12,13 Despite these concerns, examinations of CSC guideline–based allocation remain sparse, and none have focused on implications for oncology. A recent systematic review evaluated CSC state guideline concordance with Institute of Medicine recommendations but did not characterize specific allocation frameworks or the equity of their allocation.14 Another study evaluated a survey-based sample of institutional CSC guidelines, finding significant heterogeneity in the principles used to direct allocation and in provisions separating triage decisions from clinical care.15 This study, however, did not capture state CSC guidance, which is enforceable and has the potential to affect larger populations.
Despite significant attention in the lay press and medical literature to the possibility of those with disabilities and/or worse long-term prognosis being treated unfairly by some state CSCs,12,13 little has been reported about how individual state CSC guidelines prioritize patients to receive scarce health care resources. The extent to which these guidelines exclude persons with cancer from access to scarce resources, or systematically give them less preferential access to these resources, remains unexplored. To investigate these questions, we identified all publicly available US state government CSC guidelines, abstracted their allocation frameworks and provisions for patients with cancer, and compared them with relevant state-level demographic data to identify factors associated with cancer-related allocation decisions.
Methods
Search Strategy
Two study investigators (among A.H., J.M.M., M.C., S.K., and E.M.) independently searched for CSC guidelines on 2 separate dates between May 4 and May 20, 2020. May 20 was the data cutoff date, which provided states with 120 days from January 21, 2020 (the date of the first confirmed US case of COVID-19), to publish guidelines10; guidelines published both before and after January 21 were included, including CSC guidelines that were not created specifically to address COVID-19. Reflecting the Institute of Medicine CSC recommendations for transparency,16 the goal was to identify guidelines that were publicly available, readily accessible, and state endorsed at the time of the search. The study was deemed exempt by the Dana-Farber Cancer institute institutional review board because it was determined to not be human subjects research.
Our search methods were designed to exclude guidelines that were found via search engine query but could not be confirmed through state government website publication. This led to a strategy of public search engine queries followed by review of states’ websites. Public search engine queries were performed on Google (http://www.google.com) using the full name of the state plus the nonexact terms “crisis standards of care,” “COVID triage,” and/or “pandemic triage.” The first 10 links available on the first results page were reviewed for state guidelines. Then, state departments of public health and emergency preparedness health care professional and COVID resource web pages were reviewed manually. If no CSC guideline could be identified at this point, or if guidelines found through search engine queries did not link to or were also not identified on state web pages to confirm state endorsement, they were not included. This method was explicitly chosen to minimize inclusion of guidelines that were not actively endorsed by states at that time (for example, an old version on a university or hospital website that had since been revised and/or revoked by the state). Further details of the research approach and search strategy are available in the eAppendix in the Supplement.
CSC Structured Guideline Abstraction
Abstraction categories were developed based on a 2009 Institute of Medicine letter describing key elements of CSCs,16 recommendations from a 2014 CHEST task force on the provisions of care during pandemics,17 and the American Society of Clinical Oncology’s recent “Recommendations for the Oncology Community During the COVID-19 Pandemic.”9 Final categories included underlying ethical values and principles, health care scarce resource coverage, task force membership, allocation goals and methods, and disability rights. Categories were subdivided into quantitative fields (prespecified dichotomous or categorical abstractions) and qualitative fields (free text descriptions and/or exemplar quotes). The final structure, reviewer instructions, and further details are available in eTable 1 and the eAppendix in the Supplement. Crisis standards of care guidelines were abstracted independently by 2 study investigators (among A.H., J.M.M., M.C., S.K., and E.M.). All abstractions were reviewed for differences, which were adjudicated by a third investigator (G.A.A.). Interrater reliability between the 2 reviewers (before adjudication) was performed on dichotomous and categorical fields. The mean κ value for all categories was 0.88 (95% CI, 0.85-0.91).
Additional Data
Data on state-level hospital capacity, population, number of oncologists, and number of cancer centers were obtained from the Medicare Physician Compare data set; Surveillance, Epidemiology, and End Results Program State Cancer Profiles; US Census Bureau American Community Survey; and the Harvard Global Health Initiative COVID-19 hospital capacity projection data.18 Details regarding these data sources can be found in the eAppendix in the Supplement.
Statistical Analysis
Dichotomous and categorical fields were reported with descriptive statistics with frequencies, percentages, medians with interquartile ranges, and means with SDs. The Pearson χ2 test or the Fisher exact test was performed for dichotomous variables, as appropriate. Cancer-related exclusions and deprioritizations, blood product triage, and palliative care triage were assessed using 4 multivariable logistic regression models with the following state-based demographic characteristics and guideline-based variables: cancer prevalence per capita, active oncologists per 10 000 persons, presence or absence of a National Cancer Institute Comprehensive Cancer Center (NCI CCC), presence or absence of an oncologist or a palliative care specialist on the CSC task force, and presence or absence of a disability rights statement(s) in the CSC guideline. A multivariable logistic regression model was constructed to assess for factors associated with guideline availability. This model included cancer prevalence per capita, active oncologists per 10 000 persons, presence or absence of an in-state NCI CCC, and 6-month hospital capacity projections for each state to exceed 100% of potentially available hospital and intensive care unit beds. Analyses were performed using STATA SE, version 15.1 (StataCorp); P ≤ .05 was considered significant, and 2-sided hypothesis tests were used.
Results
Guideline Availability
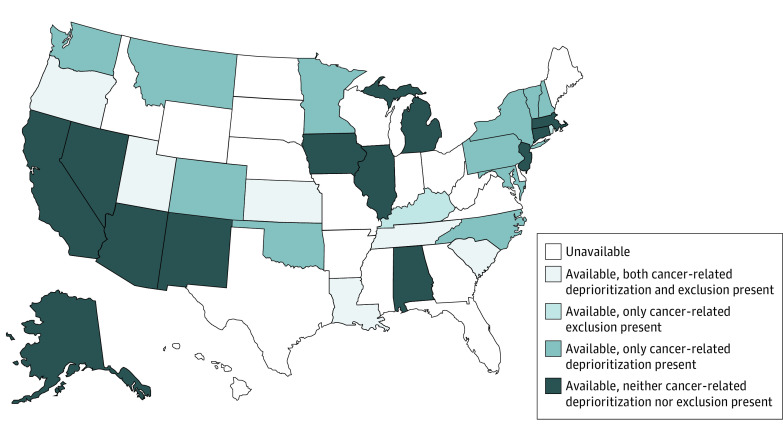
Of the 50 states reviewed, our search strategy resulted in 31 states with CSC guidelines that included recommendations for scarce resource allocation (Figure 1; eTable 2 in the Supplement). Thirty-seven states’ guidelines were identified through search engine queries. Thirty-three states’ guidelines were confirmed on state websites (Florida, Indiana, Ohio, and Texas guidelines could not be confirmed), of which 31 had allocation recommendations and were included in the analysis (Mississippi and Missouri guidelines had none). A PRISMA diagram of search results is shown in the eFigure in the Supplement, and a link to guidelines is in the eAppendix in the Supplement. Of states with available guidelines, 17 (55%) published or updated their guidelines since the first confirmed US case of COVID-19; guidelines were published a mean of 930 days (95% CI, 433-1423 days) and a median of 82 days (interquartile range, 44-1541 days) before the 120-day data cutoff date. Three states had an oncologist or a palliative care specialist specifically listed on the guideline task force, 18 did not, and 10 did not list authors. States whose potentially available hospital bed capacity would exceed 100% at 6 months (χ2 = 3.82; P = .05) and states with an NCI CCC (χ2 = 6.21; P = .01) were both more likely to have publicly available guidelines. No factors were associated with guideline availability in multivariable analysis.
Figure 1. Availability of Crisis Standards of Care Guidelines and Presence of Cancer-Related Allocation Provisions by State.
Allocation Methods, Goals, and Scope
Table 1 details guideline characteristics. The primary goals of allocation were most often to maximize the number of lives saved (31 states [100%]) and maximize the number of life-years saved (22 states [71%]). Three states (10%) incorporated additional primary goals. The Sequential Organ Failure Assessment score19 was recommended by 27 state guidelines (87%) during adult critical care resource allocation, and the Pediatric Logistic Organ Dysfunction score20 was recommended by 10 (32%) for pediatric allocation. Twenty-two states (71%) incorporated life-limiting comorbidities and/or prognosis to maximize life-years during primary allocation, while 3 (10%) did so using age categories. Guidelines for 26 states (84%) recommended use of a triage team or officer to separate allocation decisions from frontline clinicians, and 13 (42%) allowed at least 1 type of appeal for allocation decisions.
Table 1. State Crisis Standards of Care Guideline Abstraction Outline.
| Category and characteristic | No. (%) (N = 31) |
|---|---|
| Health care resource allocated | |
| Ventilators | 28 (90) |
| Renal replacement therapy | 14 (45) |
| Intravenous fluids | 17 (55) |
| Blood products | 17 (55) |
| Mental or behavioral health care | 16 (52) |
| Medications | 22 (71) |
| Staffing | 28 (90) |
| Palliative care | 29 (94) |
| Primary allocation goal | |
| Lives saved | 31 (100) |
| Life-years saved | 22 (71) |
| Other goals | 3 (10) |
| Primary allocation method | |
| SOFA (or SOFA family of scores) | 27 (87) |
| PELOD (or PELOD family of scores) | 10 (32) |
| Life-years through comorbidities or prognosis | 22 (71) |
| Life-years through age | 3 (10) |
| Secondary allocation goal | |
| Lives saved | 5 (16) |
| Life-years saved | 7 (23) |
| Lottery | 8 (26) |
| First-come, first-served | 5 (16) |
| Youngest first | 5 (16) |
| Reciprocity | 5 (16) |
| Instrumental value | 10 (32) |
| Included in allocation process | |
| Use of triage team and/or officer | 26 (84) |
| Allocation appeals process | 13 (42) |
| Disability rights | |
| Disability rights statement | 20 (65) |
| Any categorical exclusions | 9 (29) |
| Standardized ventilator reassessment | 20 (65) |
| Cancer-related categorical exclusion | 8 (26) |
| Cancer-related deprioritization | 17 (55) |
Abbreviations: PELOD, Pediatric Logistic Organ Dysfunction; SOFA, Sequential Organ Failure Assessment.
Disabilities and Cancer-Related Provisions
Of the 31 states with reviewable CSCs, 20 states’ (65%) guidelines included explicit statements regarding the rights of persons with disabilities. Guidelines from 24 states (77%) deprioritized or categorically excluded at least some patients with comorbid conditions, 19 (61%) of which included cancer (Figure 1). Of states with published guidelines, 17 (55%) had provisions that deprioritized at least some patients with cancer within resource allocation and 8 (26%) categorically excluded at least some patients with cancer from allocation; visualization of cancer care–related characteristics of interest are shown in Figure 2. Measures of association are in Table 2 and multivariable analyses are in Table 3. The presence of an NCI CCC in a state was associated with a lower likelihood of cancer-related categorical exclusions (multivariable odds ratio, 0.06 [95% CI, 0.004-0.87]). The inclusion of a disability rights statement was associated with the presence of specific provisions to allocate blood products (multivariable odds ratio, 7.44 [95% CI, 1.28-43.24]). Both the presence of an NCI CCC and the inclusion of an oncologist and/or a palliative care specialist were associated with the inclusion of palliative care. We identified no statistically significant associations for cancer-related deprioritizations. There were also no associations between these outcomes and whether the guideline had been updated since the pandemic or not.
Figure 2. Frequency of Cancer Care Provisions in State Crisis Standards of Care Guidelines.
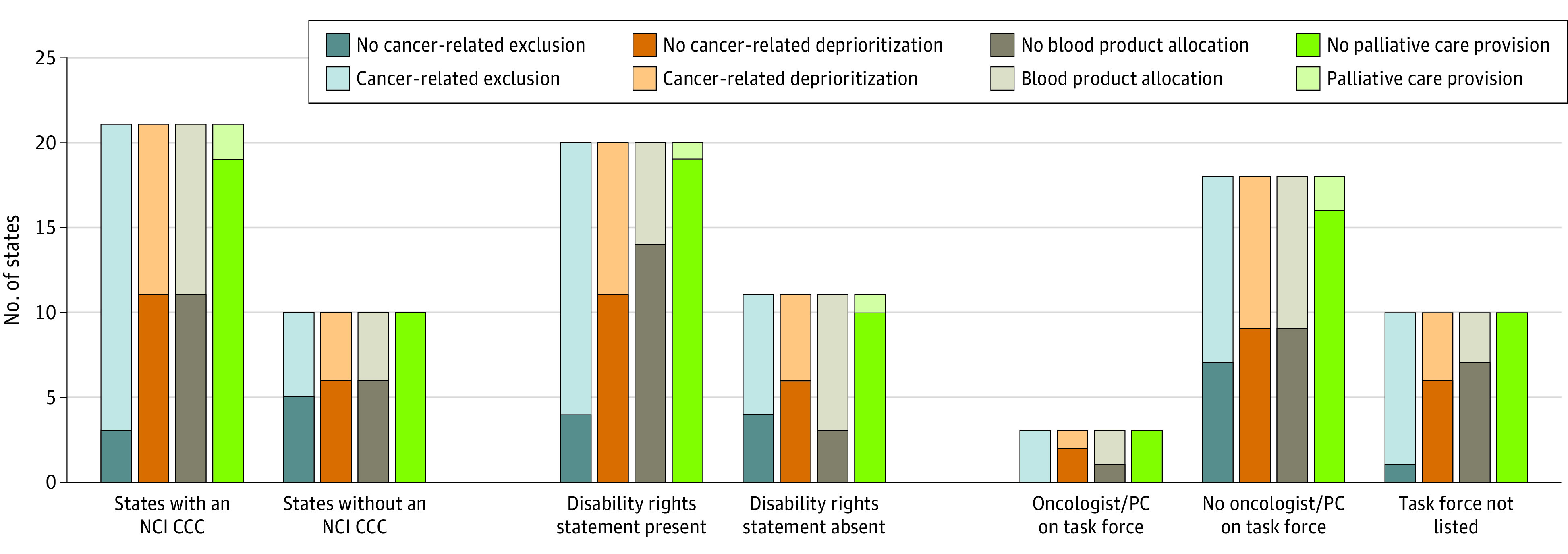
NCI CCC indicates National Cancer Institute Comprehensive Cancer Center; and PC, palliative care clinician.
Table 2. Tests of Association for Cancer-Related Provisions.
| Cancer-related provision (No. with provision) | Value | P value |
|---|---|---|
| Cancer-related exclusion (n = 8) | ||
| Presence of NCI CCCa | χ2 = 4.51 | .03 |
| Disability rights statementa | χ2 = 0.99 | .32 |
| Oncologist or PC clinician on task forceb | NA | .19 |
| Cancer-related deprioritization (n = 17) | ||
| Presence of NCI CCCa | χ2 = 0.16 | .69 |
| Disability rights statementa | χ2 = 0.01 | .98 |
| Oncologist or PC clinician on task forceb | NA | .52 |
| Blood products (n = 17) | ||
| Presence of NCI CCCa | χ2 = 0.16 | .12 |
| Disability rights statementa | χ2 = 5.23 | .02 |
| Oncologist or PC clinician on task forceb | NA | .54 |
| Palliative care (n = 29) | ||
| Presence of NCI CCCb | NA | .47 |
| Disability rights statementb | NA | .66 |
| Oncologist or PC clinician on task forceb | NA | .73 |
Abbreviations: NA, not applicable; NCI CCC, National Cancer Institute Comprehensive Cancer Center; PC, palliative care.
χ2 test.
The Fisher exact test was used because of low observations per cell.
Table 3. Multivariable Logistic Regression Models of Cancer-Related Provisionsa.
| Model | Odds ratio (95% CI) | P value |
|---|---|---|
| Cancer-related exclusions | ||
| Presence of NCI CCC | 0.06 (0.004-0.87) | .04 |
| Disability rights statement | 0.41 (0.05-3.14) | .39 |
| Oncologist or PC clinician on task force | 0.97 (0.94-1.00) | .06 |
| Cancer prevalence per capita | 0.93 (0.25-3.55) | .92 |
| Oncologists per 10 000 persons | 0.34 (0.001-169.92) | .73 |
| Blood products | ||
| Presence of NCI CCC | 1.09 (0.16-7.64) | .93 |
| Disability rights statement | 7.44 (1.28-43.24) | .03 |
| Oncologist or PC clinician on task force | 1.01 (0.99-1.03) | .30 |
| Cancer prevalence per capita | 1.24 (0.43-3.56) | .69 |
| Oncologists per 10 000 persons | 0.14 (0.002-9.88) | .36 |
| Cancer-related deprioritization | ||
| Presence of NCI CCC | 0.88 (0.15-5.2) | .89 |
| Disability rights statement | 0.99 (0.21-4.63) | .99 |
| Oncologist or PC clinician on task force | 1.00 (0.99-1.02) | .71 |
| Cancer prevalence per capita | 1.66 (0.63-4.37) | .31 |
| Oncologists per 10 000 persons | 1.24 (0.02-63.96) | .92 |
| Palliative care | ||
| Presence of NCI CCC | 1 [Reference] | <.001 |
| Disability rights statement | 0.74 (0.21-4.63) | .88 |
| Oncologist or PC clinician on task force | 1 [Reference] | <.001 |
| Cancer prevalence per capita | 1.14 (0.63-4.37) | .92 |
| Oncologists per 10 000 persons | 0.01 (0.02-63.96) | .45 |
Abbreviations: NCI CCC, National Cancer Institute Comprehensive Cancer Center; PC, palliative care.
Each model includes all 5 variables listed.
Discussion
In this cancer-focused analysis of state CSC guidelines, most states with publicly available guidelines gave lower priority to some patients with cancer during resource allocation and one-fourth categorically excluded patients with cancer from consideration for allocation. The presence of an NCI-designated CCC was associated with guideline publication and the inclusion of patients with cancer in prioritization. These data suggest that equitable CSC considerations for patients with cancer would likely benefit from the input of state-level oncology stakeholders such as CCCs.
We recognize that creating guidelines for scarce health care resource allocation is a necessary but unenviable task that many states have confronted with candor, inclusiveness, and an honest effort to respect all persons in their jurisdiction. That so many states have published and updated guidelines since the COVID-19 pandemic began is a testament to the resolve of their departments of public health. We commend the states with publicly available guidelines and hope the information herein will advance oncology crisis planning across the US in a constructive and transparent manner.
Unless a pure lottery is instituted, deprioritization of some patient groups is an obligate part of crisis allocation. Bioethicists largely agree that scarce health care resource allocation should aim to save the most lives and prioritize those with characteristics that portend better immediate-term survival15,21,22; our analysis demonstrates the predominance of this principle in state CSC guidelines. How this outcome is specifically accomplished, and what other allocation goals should be incorporated, is less certain. This uncertainty is reflected in the variety of approaches used to incorporate saving the most life-years and the number of secondary allocation goals such as prioritizing the young and/or health care workers. We also found that some approaches have the potential to disproportionately affect patients with cancer.
Despite these varying goals, there is increasing agreement that absolute deprioritizations (ie, categorically excluding persons from the allocation process for nonacute conditions such as cancer or intellectual disability) devalue individuals and are unacceptably discriminatory.13 For example, if a patient’s diagnosis affects quality of life but not survival, summary exclusion from access to scarce health care resources may be unfairly discriminatory and not aligned with the goal of saving the most lives. This is especially true when the scarce health care resource is lifesaving, as is the case with ventilators, where exclusion is tantamount to death. Yet, we found 9 states with such exclusions 4 months after the first US case of COVID-19. The association between the presence of an NCI-designated CCC and absence of cancer-related exclusions is also provocative, suggesting that the effect of these institutions may go beyond cancer investigation and treatment to outreach and even advocacy.23
Our data also suggest that the uncertainty inherent in contemporary oncology-related prognostication extends to CSC allocation methods. If one agrees that prioritization beyond immediate-term survival should be included, deprioritizing those with a poor chance of short-term survival owing to a life-limiting illness may seem reasonable24; however, the process by which this occurs in CSC guidelines is heterogeneous and risks unfairly singling out patients with cancer. Although some states are very specific in their deprioritization statements, others use deprioritization language that is subject to interpretation and relies on allocation decision-makers to have accurate information about individuals’ cancer-related prognosis: “solid organ or hematopoietic malignancy with poor prognosis for recovery” (Vermont); others deprioritize for long-term cancer-related prognosis: “malignancy with a <10 year expected survival” (Montana); and others deprioritize based on the type of treatment received: “cancer being treated only with palliative interventions” (North Carolina). Although most of these statements center on patients with metastatic disease, this category constitutes hundreds of thousands of persons25 with complex, heterogeneous, and evolving prognoses.26
The variety of cancer-related deprioritization statements may account for why NCI-designated CCCs were not associated with the presence or absence of deprioritizations, despite their being associated with exclusions. The fairness of deprioritization statements depends on the ever-changing field of cancer-related prognosis. As such, if cancer-related prognoses are considered during allocation, the American Society of Clinical Oncology has suggested that they be obtained through consultation with an oncologist who has disease-specific expertise.9 Rather than listing particular oncologic deprioritization criteria, this approach seems prudent. Moreover, a guideline development process that includes key oncologic stakeholders, such as patients and clinicians, is essential.
All states with NCI-designated CCCs included provisions for palliative care, which further supports their potential influence on state health care policy; indeed, the presence of an NCI-designated CCC is a possible proxy of cancer-related health care resources and quality of care.27 In contrast, almost half of available guidelines did not include provisions for the distribution of scarce blood products. Blood products are also essential for a variety of nononcologic conditions, which may be why the presence of an in-state CCC was not associated with their inclusion. As the maintenance of blood counts is vital for patients with cancer undergoing treatment, our analysis suggests a critical gap in CSC guidelines for many states.
Limitations
Our methods have some limitations. First, the goals of our search strategy—availability, accessibility, and state endorsement—and the cross-sectional nature of the search may have excluded some state guidelines from inclusion at the time of abstraction and certainly excluded guidelines revised or published after May 20, 2020. Second, while we had high levels of agreement between reviewers, the abstraction process requires simplifying complex text and nuanced concepts into categorical or dichotomous measures, which can introduce bias. Third, there are undoubtedly additional factors, both associated with cancer care and not associated with oncology, that were not captured in our analysis of state-level cancer care–related demographic characteristics. However, we attempted to use the most relevant and cohesive data sources available to our outcome of interest. Fourth, the use of a cancer-focused approach was purposeful but cannot account for possible competing factors that may have led to guideline allocation decisions.
Conclusions
Our analysis demonstrated that most states with available CSC guidelines deprioritized at least some patients with cancer during scarce health care resource allocation, and one-fourth categorically excluded patients with cancer. Having at least 1 NCI-designated CCC was associated with the presence of publicly available guidelines and inclusion of patients with cancer in allocation processes. Predictions of a second wave of COVID-19 infection, the recent US Food and Drug Administration emergency-use authorization of remdesivir,28 and ongoing shortages of personal protective equipment29 make the need for equitable CSC guidelines paramount. As such guidelines evolve to include allocation of additional scarce resources, oncology populations need to be carefully considered given their baseline vulnerability.
eAppendix. Methods
eTable 1. CSC Guideline Abstraction Outline
eTable 2. CSC Guideline Versions Dates and Cancer-Related Categorizations
eFigure. CSC Guideline Screening and Selection Process
eReferences
References
- 1.Persoskie A, Ferrer RA, Klein WM. Association of cancer worry and perceived risk with doctor avoidance: an analysis of information avoidance in a nationally representative US sample. J Behav Med. 2014;37(5):977-987. doi: 10.1007/s10865-013-9537-2 [DOI] [PubMed] [Google Scholar]
- 2.Surveillance, Epidemiology, and End Results Program. SEER cancer statistics review (CSR) 1975-2016. Updated April 9, 2020. Accessed May 15, 2020. https://seer.cancer.gov/csr/1975_2016/
- 3.Williamson TJ, Choi AK, Kim JC, et al. . A longitudinal investigation of internalized stigma, constrained disclosure, and quality of life across 12 weeks in lung cancer patients on active oncologic treatment. J Thorac Oncol. 2018;13(9):1284-1293. doi: 10.1016/j.jtho.2018.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brand JS, Colzani E, Johansson ALV, et al. . Infection-related hospitalizations in breast cancer patients: risk and impact on prognosis. J Infect. 2016;72(6):650-658. doi: 10.1016/j.jinf.2016.04.003 [DOI] [PubMed] [Google Scholar]
- 5.Mehta AK, Smith TJ. Palliative care for patients with cancer in the COVID-19 era. JAMA Oncol. 2020. doi: 10.1001/jamaoncol.2020.1938 [DOI] [PubMed] [Google Scholar]
- 6.Lou E, Beg S, Bergsland E, et al. . Modifying practices in GI oncology in the face of COVID-19: recommendations from expert oncologists on minimizing patient risk. JCO Oncol Pract. 2020;16(7):383-388. doi: 10.1200/OP.20.00239 [DOI] [PubMed] [Google Scholar]
- 7.Institute of Medicine Crisis Standards of Care: A Systems Framework for Catastrophic Disaster Response. The National Academies Press; 2012. [PubMed] [Google Scholar]
- 8.Crisis Standards of Care Committee Crisis standards of care: planning guidance for the COVID-19 pandemic. Executive Office of Health and Human Services: The Commonwealth of Massachusetts; 2020.
- 9.Marron JM, Joffe S, Jagsi R, Spence RA, Hlubocky FJ. Ethics and resource scarcity: ASCO recommendations for the oncology community during the COVID-19 pandemic. J Clin Oncol. 2020;38(19):2201-2205. doi: 10.1200/JCO.20.00960 [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention First travel-related case of 2019 novel coronavirus detected in United States. Published January 21, 2020. Accessed May 18, 2020. https://www.cdc.gov/media/releases/2020/p0121-novel-coronavirus-travel-case.html
- 11.Baker M, Fink S At the top of the Covid-19 curve, how do hospitals decide who gets treatment? New York Times March 31, 2020. Accessed April 20, 2020. https://www.nytimes.com/2020/03/31/us/coronavirus-covid-triage-rationing-ventilators.html
- 12.Bebinger M. After uproar, Mass. revises guidelines on who gets an ICU bed or ventilator amid COVID-19 surge. CommonHealth April 22, 2020. Accessed April 30, 2020. https://www.wbur.org/commonhealth/2020/04/20/mass-guidelines-ventilator-covid-coronavirus
- 13.Mello MM, Persad G, White DB. Respecting disability rights—toward improved crisis standards of care. N Engl J Med. 2020;383(5):e26. doi: 10.1056/NEJMp2011997 [DOI] [PubMed] [Google Scholar]
- 14.Romney D, Fox H, Carlson S, Bachmann D, O’Mathuna D, Kman N. Allocation of scarce resources in a pandemic: a systematic review of US state crisis standards of care documents. Disaster Med Public Health Prep. 2020;1-7. doi: 10.1017/dmp.2020.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antommaria AHM, Gibb TS, McGuire AL, et al. . Ventilator triage policies during the COVID-19 pandemic at U.S. hospitals associated with members of the Association of Bioethics Program Directors. Ann Intern Med. 2020;173(3):188-194. doi: 10.7326/M20-1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Institute of Medicine Guidance for Establishing Crisis Standards of Care for Use in Disaster Situations: A Letter Report. The National Academies Press; 2009. [PubMed] [Google Scholar]
- 17.Dries D, Reed MJ, Kissoon N, et al. ; Task Force for Mass Critical Care . Special populations: care of the critically ill and injured during pandemics and disasters: CHEST consensus statement. Chest. 2014;146(4)(suppl):e75S-e86S. doi: 10.1378/chest.14-0737 [DOI] [PubMed] [Google Scholar]
- 18.Jha AK, Tsai T, Figueroa J, Jacobson B, Friedhoff S Pandemics explained: US hospital capacity. Harvard Global Health Institute. Published March 17, 2020. Accessed May 10, 2020. https://globalepidemics.org/our-data/hospital-capacity/
- 19.Vincent JL, Moreno R, Takala J, et al. ; Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine . The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22(7):707-710. doi: 10.1007/BF01709751 [DOI] [PubMed] [Google Scholar]
- 20.Leteurtre S, Duhamel A, Grandbastien B, Lacroix J, Leclerc F. Paediatric Logistic Organ Dysfunction (PELOD) score. Lancet. 2006;367(9514):897. doi: 10.1016/S0140-6736(06)68371-2 [DOI] [PubMed] [Google Scholar]
- 21.Truog RD, Mitchell C, Daley GQ. The toughest triage—allocating ventilators in a pandemic. N Engl J Med. 2020;382(21):1973-1975. doi: 10.1056/NEJMp2005689 [DOI] [PubMed] [Google Scholar]
- 22.Maves RC, Downar J, Dichter JR, et al. ; ACCP Task Force for Mass Critical Care . Triage of scarce critical care resources in COVID-19: an implementation guide for regional allocation: an expert panel report of the Task Force for Mass Critical Care and the American College of Chest Physicians. Chest. 2020;158(1):212-225. doi: 10.1016/j.chest.2020.03.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Cancer Institute NCI-designated cancer centers. Published 2020. Accessed May 23, 2020. https://www.cancer.gov/research/infrastructure/cancer-centers
- 24.Sprung CL, Joynt GM, Christian MD, Truog RD, Rello J, Nates JL. Adult ICU triage during the coronavirus disease 2019 pandemic: who will live and who will die? recommendations to improve survival. Crit Care Med. Published online May 6, 2020. doi: 10.1097/CCM.0000000000004410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mariotto AB, Etzioni R, Hurlbert M, Penberthy L, Mayer M. Estimation of the number of women living with metastatic breast cancer in the United States. Cancer Epidemiol Biomarkers Prev. 2017;26(6):809-815. doi: 10.1158/1055-9965.EPI-16-0889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hui D Prognostication of survival in patients with advanced cancer: predicting the unpredictable? Cancer Control. 2015;22(4):489-497. doi: 10.1177/107327481502200415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolfson JA, Sun CL, Wyatt LP, Hurria A, Bhatia S. Impact of care at comprehensive cancer centers on outcome: results from a population-based study. Cancer. 2015;121(21):3885-3893. doi: 10.1002/cncr.29576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.U.S. Food and Drug Administration. Coronavirus (COVID-19) update: FDA issues emergency use authorization for potential COVID-19 treatment. Published May 1, 2020. Accessed May 29, 2020. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-emergency-use-authorization-potential-covid-19-treatment
- 29.O’Sullivan ED PPE guidance for Covid-19: be honest about resource shortages. BMJ. 2020;369:m1507. doi: 10.1136/bmj.m1507 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Methods
eTable 1. CSC Guideline Abstraction Outline
eTable 2. CSC Guideline Versions Dates and Cancer-Related Categorizations
eFigure. CSC Guideline Screening and Selection Process
eReferences