Abstract
Background/aims
Weight-bearing jump tests measure lower extremity muscle power, velocity, and force, and may be more strongly related to physical performance than grip strength. However, these relationships are not well described in older adults.
Methods
Participants were 1242 older men (mean age 84 ± 4 years) in the Osteoporotic Fractures in Men (MrOS) Study. Jump peak power (Watts/kg body weight), force (Newton/kg body weight) at peak power, and velocity (m/s) at peak power were measured by jump tests on a force plate. Grip strength (kg/kg body weight) was assessed by hand-held dynamometry. Physical performance included 400 m walk time (s), 6 m usual gait speed (m/s), and 5-repeated chair stands speed (#/s).
Results
In adjusted Pearson correlations, power/kg and velocity moderately correlated with all performance measures (range r = 0.41–0.51; all p < 0.001), while correlations for force/kg and grip strength/kg were weaker (range r = 0.20–0.33; all p < 0.001). Grip strength/kg moderately correlated with power/kg (r = 0.44; p < 0.001) but not velocity or force/kg. In adjusted linear regression with standardized βs, 1 SD lower power/kg was associated with worse: 400 m walk time (β = 0.47), gait speed (β = 0.42), and chair stands speed (β = 0.43) (all p < 0.05). Associations with velocity were similar (400 m walk time: β = 0.42; gait speed: β = 0.38; chair stands speed: β = 0.37; all p < 0.05). Force/kg and grip strength/kg were more weakly associated with performance (range β = 0.18–0.28; all p < 0.05).
Conclusions/discussion
Jump power and velocity had stronger associations with physical performance than jump force or grip strength. This suggests lower extremity power and velocity may be more strongly related to physical performance than lower extremity force or upper extremity strength in older men.
Keywords: Epidemiology, Muscle, Countermovement, Power, Physical function
Introduction
Weight-bearing power tests (i.e., jump tests) may more closely approximate the ability of older adults to perform activities of daily living (e.g., walking and chair rising) than non-weight-bearing tests. However, power has often been assessed in a seated position with power rigs [1–4] or leg press [5, 6]. Past studies utilizing jump tests are limited, since they have traditionally reported power only for jumps with a flight phase (i.e., participant able to lift the feet off the ground) [4, 7–17]. The novel force plate jump testing methodology which we developed [18] also calculates power from jumps without a flight phase.
While both muscle power, the ability of a muscle to exert force quickly [7, 19], and strength are lower at older ages compared to younger ages, larger magnitudes of age-related decline have been described for power than for strength [8, 18, 20–23]. This suggests that power rather than strength may be an earlier indicator of age-related muscle function loss. Inconsistent relationships for both muscle power and strength with standard physical performance tests (i.e., usual gait speed, 400 m walk time, 6 min walking distance, chair stand speed, the Short Physical Performance Battery score, and stair climb time) have been found [1–6, 9–13, 24], possibly due to variability in power test protocols [25], particularly lack of task-based muscle function assessments such as the jump test.
The extent to which muscle power and its components (force and velocity), and strength may be differentially associated with multiple physical performance measures is unclear, though critical for selecting the most appropriate muscle function tests to predict late-life functional decline and disability. We examined associations of our novel jump test measures (jump peak power, and velocity and force at peak power) and grip strength with physical performance (400 m walk time, 6 m usual gait speed, and 5-repeated chair stands speed) in older men. We hypothesized that jump peak power and velocity would be more strongly related to physical performance than jump force and grip strength.
Methods
Participants
The Osteoporotic Fractures in Men (MrOS) Study (http://mrosdata.sfcc-cpmc.net) is a multicenter, longitudinal cohort study designed to evaluate healthy aging, with a focus on risk factors for osteoporosis and fractures. Baseline visits occurred between March 2000 and April 2002 at six U.S. sites (N = 5994; aged 73.7 ± 5.9 years) [26, 27]. Initial eligibility criteria included age ≥ 65 years; ability to walk without assistance or walking aid; ability to provide self-reported data and informed consent; residence near a clinical site; and absence of bilateral hip replacement or any severe disease/condition that would result in imminent death. Of 5994 at baseline, 3570 did not have a follow-up visit in 2014–2016 (2822 deaths, 386 prior terminations, 362 refusals), and 583 completed questionnaires only and did not have an in-person clinic visit. The remaining 1841 who completed a 2014–2016 clinic visit were included in current analyses.
Jump test
Advanced Mechanical Technology Inc. (AMTI) Accu-Power force plates (Netforce Acquisition Software Version 3.05.01) collected force signals from jump trials at a 1000 Hz sampling rate. As previously described [18], 24.8% (N = 456/1841) participants were initially excluded from attempting jump tests for reasons related to health (unable to walk or stand with/without an aid, self-reported severe pain, or selected surgeries in the past 6 months: spinal or lower extremity surgery, or knee or hip replacement) or other safety/logistical reasons (refusal, examiner deemed test unsafe, could not perform without orthotics, shortened clinic visit). An additional 6.4% (N = 117/1841) who attempted but did not complete jump tests were unable or refused practice tests mostly for balance-related issues, had severe pain during practice tests, or technical issues [18].
Three calf rises were completed as a warm-up before a practice jump. Three countermovement jumps (4–5 maximum if ≥ 1/3 jumps had data quality or technical problems) were performed on the force plate. Jump instructions were to jump as quickly and as high as possible without pausing between bending the knees and jumping, land smoothly, stand up straight, and remain still. Participants were not instructed on use of arms during jumps for a more free-living movement. Pain intensity (scale: 0–10; “0” = none, “10” = severe pain) and pain location were reported after jump tests; 4.6% (N = 58/1268) with jump tests reported post-jump pain with only two participants stopping further testing due to pain. No serious adverse safety events occurred.
The University of Pittsburgh Reading Center and Southern Denmark University Processing Center (SDUPC) reviewed force plate data. SDUPC batch analyzed valid trials with custom-designed software [7, 8, 14, 15, 18]. Calculation of analytical variables has been previously described [18]. Briefly, the vertical velocity of the body center of mass was obtained by time integration of the instantaneous acceleration. Either the trial with the highest jump height, due to instructions to jump as high as possible, or highest peak power if all jump trials/participant were without flight, was selected for analyses, with 2.1% (N = 26/1268) excluded for technical and data processing problems with all trials. Body weight recorded during each test was used to standardize peak power and force at peak power. Analytic variables from selected trials were peak power (Watts/kg body weight), velocity (m/s) at peak power, and force (Newton/kg body weight) at peak power. Jump measures in a small subset had high reproducibility and reliability [18]. Participants with jump measures (N = 1242) were more likely than those without jump tests (N = 573) to complete grip strength (98% vs. 84%; p < 0.05) and all physical performance measures (400 m walk time: 95% vs. 43%; gait speed: 99% vs. 87%; chair stands speed: 96% vs. 54%; all p < 0.05) (Table 1).
Table 1.
Percent completion of grip strength and physical performance tests by jump test completion
| % | Total with jump measures (N = 1242) | Total without jump tests (N = 573) |
|---|---|---|
| Grip strength | 1222 (98.4) | 481 (83.9) |
| 400 m walk time | 1174 (94.5) | 248 (43.3) |
| 6 m usual gait speed | 1240 (99.8) | 497 (86.7) |
| Chair stands speed | 1191 (95.9) | 309 (53.9) |
All completion rates p < 0.05 comparing participants with jump measures vs. without jump tests
Grip strength/kg
Jamar dynamometers (Sammons Preston Rolyan, Boling-brook, IL, USA) [28] were used to measure grip strength for two trials of both hands. Maximum grip strength was normalized to body weight (kg/kg body weight). After exclusions for recent hand pain/arthritis symptoms (N = 46/1841; 2.5%), hand surgery in the past 3 months (N = 1/1841; 0.05%), refusal (N = 39/1841; 2.1%), unable (N = 25/1841; 1.4%), or missing (N = 1/1841; 0.05%), grip strength data were available in 93.9% (N = 1729/1841).
400 m walk time
Time to walk 400 m (10 laps on 20 m course) at the participant’s usual pace without overexertion was recorded (N = 1447/1841; 78.6%) [29]. Exclusions were for inability to attempt the test due to use of a walking aid other than a single straight cane (N = 86/1841; 4.7%), safety (N = 71/1841; 3.9%), shortened clinic visit (N = 50/1841; 2.7%), course obstruction/unavailability (N = 2/1841; 0.1%), refusal (N = 79/1841; 4.3%), or others (N = 13/1841; 0.7%). Participants could rest for ≤ 60 s at any time during testing (without leaning on surfaces or sitting). Testing was stopped (N = 93/1841; 5.0%) for distress (e.g., labored breathing, confusion, unresponsiveness), pain, resting > 60 s, leaning on a surface twice during rest, requesting an assistive device other than a single straight cane, or requesting to stop.
Gait speed
Time to walk 6 m at the participant’s usual pace was recorded [27]. Gait speed was calculated from the fastest time of two trials (m/s) (N = 1763/1841; 95.8%). Exclusion was for inability to attempt the test (N = 78/1841; 4.2%).
Chair stands speed
Ability to rise once from a standard chair, and time to complete five-repeated stands, without using arms were recorded (N = 1524/1841; 82.8%). Exclusions were for missing data (N = 1/1841; 0.05%), inability to attempt the test (N = 106/1841; 5.8%), or refusal (N = 21/1841; 1.1%). Men who attempted but did not complete the initial chair stand (N = 160/1841; 8.7%) or five-repeated stands (N = 29/1841; 1.5%) were included in analyses with a value of 0 stands/second.
Covariates
Age, race, smoking status (current/past/never) and alcohol consumption (number of drinks/day) [30], and any hip/joint pain in the past year and ≥ 1 fall in the past year were obtained from self-administered questionnaires. Body Mass Index (BMI) was calculated from weight (balance beam or digital scales) and height (Harpenden stadiometers; Dyved UK). Physical activity (PA) total energy expenditure was collected from accelerometry (SenseWear armband; Body Media, Inc., Pittsburgh, PA; N = 1088) [31]. Average systolic and diastolic blood pressures (SBP; DBP) were measured with BP Tru automated blood pressure monitors (Coquitlam, British Columbia, Canada) [32]. Total hip bone mineral density (BMD) was assessed by dual-energy X-ray absorptiometry (Hologic, Inc, Waltham, MA, USA) [33]. Brief global cognitive testing was performed using the Teng Modified Mini-Mental State (3MS) Examination [34] and executive function was measured using Trails B [35] completion time. Comorbidities included diabetes and hypertension (self-report physician diagnosis and/or medication use), and self-reported history of congestive heart failure (CHF), myocardial infarction (MI), stroke, and Parkinson’s Disease. Total number of medications was calculated from current prescription medications brought to the clinic visit [36].
Statistical analyses
Descriptive statistics included two-sided t tests and Chi-square tests of proportions to compare characteristics at the 2014–2016 clinic visit in men with jump measures (N = 1242) to those without jump tests (N = 573). Those with jump measures were stratified by gait speed to compare gait speed ≥ 1.0 m/s (N = 994/1242) vs. < 1.0 m/s (N = 246/1242) [37]. To determine if participants with jump measures and impaired function were comparable to those who did not complete jump tests, we compared gait speed groups ≥ 1.0 m/s and < 1.0 m/s vs. without jump tests. The percent completing grip strength and physical performance measures were compared to percent completing jump tests. Partial Pearson correlations were calculated between jump measures, grip strength, and lower extremity physical performance, and adjusted for age, race, site, and height. Correlations with jump velocity were additionally weight-adjusted, since velocity was not weight-corrected. Separate stepwise multivariable-adjusted linear regression models were built to evaluate associations per standard deviation (SD) of jump measures and grip strength with physical performance. Covariates with p < 0.10 in any model (age, race, site, height, falls history, SBP, DBP, hip/joint pain, BMD, Trails B, diabetes, hypertension, CHF, MI, stroke, Parkinson’s Disease, and total number of medications) were retained in all the final models. Standardized β coefficients were reported to compare results across all models. As a sensitivity analysis for the subset with the accelerometry measure, PA was added to final models. Analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
Results
All men with jump measures (N = 1242), as well as those with gait speed ≥ 1.0 m/s (N = 994), were younger, had lower BMI, higher PA, higher DBP, higher SPB (gait speed ≥ 1.0 m/s only), less hip/joint pain, better cognitive/executive function, lower prevalence of history of falls, fewer comorbidities, fewer medications, higher grip strength, and better physical performance vs. without jump tests (N = 573) (Table 2). Compared to men with jump measures and gait speed < 1.0 m/s, those with gait speed ≥ 1.0 m/s were younger, had lower BMI, higher PA, higher DBP, higher BMD, better cognitive/executive function, fewer comorbidities, fewer medications, and higher power/kg, velocity and force/kg. Those with jump measures and gait speed < 1.0 m/s were younger (85 ± 4 vs. 86 ± 5 years), had lower prevalence of history of falls (36% vs. 51%) and slower gait speed (0.88 ± 0.10 vs. 0.92 ± 0.26 m/s) vs. without jump tests.
Table 2.
Characteristics by jump test completion and gait speed
| Mean ± standard deviation or % | Total with jump measures | Total without jump tests (N = 573) | ||
|---|---|---|---|---|
| All (N = 1242) | Gait speed ≥ 1.0 m/s (N = 994) | Gait speed < 1.0 m/s (N = 246) | ||
| Demographics | ||||
| Age (years) | 84 ± 4* | 83 ± 4*,† | 85 ± 4* | 86 ± 5 |
| White race (%) | 90 | 90 | 90 | 93 |
| Anthropometry | ||||
| Height (cm) | 172.5 ± 6.6* | 172.6 ± 6.6* | 171.9 ± 6.5 | 171.7 ± 7.4 |
| Weight (kg) | 79.2 ± 12.1* | 78.8 ± 12.0*,† | 80.7 ± 12.5 | 81.3 ± 713.6 |
| Body Mass Index (kg/m2) | 27 ± 4* | 26 ± 3*,† | 27 ± 4 | 28 ± 4 |
| Lifestyle characteristics | ||||
| Current smoker (%) | 1 | 2 | 0 | 1 |
| ≥1 alcohol drink/week (%) | 52 | 54* | 42 | 42 |
| Physical activity (kcal/day) | 2177 ± 365* | 2200 ± 371*,† | 2080 ± 320 | 2076 ± 386 |
| Clinical measures | ||||
| Systolic blood pressure (mmHg) | 128 ± 18 | 128 ± 18*,† | 126 ± 18 | 126 ± 21 |
| Diastolic blood pressure (mmHg) | 72 ± 11* | 72 ± 11*,† | 70 ± 11 | 71 ± 11 |
| Bone mineral density (g/cm2) | 0.93 ± 0.15 | 0.94 ± 0.14† | 0.91 ± 0.16 | 0.92 ± 0.16 |
| Hip/joint pain (%) | 27* | 26* | 32 | 38 |
| Teng 3MS (score) | 92.6 ± 6.8* | 93.1 ± 6.4*,† | 90.9 ± 8.1 | 89.9 ± 9.0 |
| Trails B (seconds to complete) | 136 ± 67* | 128 ± 63*,† | 166 ± 74 | 163 ± 77 |
| Falls history | ||||
| ≥1 fall in past year (%) | 34* | 33* | 36* | 51 |
| Comorbidity | ||||
| Diabetes (%) | 15* | 13*,† | 22 | 22 |
| Hypertension (%) | 53 | 51*,† | 60 | 57 |
| Congestive heart failure (%) | 7* | 6*,† | 11 | 13 |
| Myocardial infarction (%) | 12* | 11*,† | 15 | 19 |
| Stroke (%) | 4* | 4* | 5 | 8 |
| Parkinson’s disease (%) | 1* | 1* | 2 | 3 |
| Medications | ||||
| Total medications (#) | 8.8 ± 5.4* | 8.6 ± 4.8*,† | 9.3 ± 4.4 | 9.9 ± 4.9 |
| Muscle function | ||||
| Peak power (W/kg body wt) | 20.8 ± 5.3 | 21.8 ± 5.1† | 16.5 ± 4.1 | – |
| Velocity at peak power (m/s) | 1.2 ± 0.3 | 1.3 ± 0.2† | 1.0 ± 0.2 | – |
| Force at peak power (N/kg body wt) | 16.7 ± 1.9 | 16.9 ± 1.9† | 15.8 ± 1.7 | – |
| Grip strength (kg/kg body wt) | 0.5 ± 0.1* | 0.5 ± 0.1*,† | 0.4 ± 0.1 | 0.4 ± 0.1 |
| Physical performance | ||||
| 400 m walk time (s) | 396 ± 85* | 374 ± 59*,† | 499 ± 112 | 488 ± 153 |
| 6 m usual gait speed (m/s) | 1.18 ± 0.22* | 1.25 ± 0.17*,† | 0.88 ± 0.10* | 0.92 ± 0.26 |
| Chair stands speed (#/s) | 0.42 ± 0.14* | 0.44 ± 0.13*,† | 0.30 ± 0.14 | 0.25 ± 0.19 |
p < 0.05 for all with jump measures, gait speed ≥ 1.0 m/s and gait speed < 1.0 m/s vs. total without jump tests (N = 26 excluded for data quality);
p < 0.05 gait speed ≥ 1.0 m/s vs. gait speed < 1.0 m/s (N = 2 missing gait speed)
Jump power/kg was moderately correlated with grip strength/kg (partial r = 0.44; p < 0.001) and all physical performance measures (range partial r = 0.44–0.51; all p < 0.001) (Table 3). Jump velocity was also moderately correlated with all physical performance measures (range partial r = 0.41–0.45; p < 0.001), but only weakly correlated with grip strength/kg (partial r = 0.34; p < 0.001). All correlations of jump force/kg and grip strength/kg with physical performance (range partial r = 0.20–0.33; all p < 0.001) were weaker, as was the correlation of these strength measures to each other (partial r = 0.24; p < 0.001). In adjusted linear regression with standardized βs, 1 SD lower jump power/kg was associated with longer 400 m walk time (β = 0.47), slower gait speed (β = 0.42), and slower chair stands speed (β = 0.43) (Fig. 1; all p < 0.05) after adjustment. Jump velocity results were similar (400 m walk time: β = 0.42; gait speed: β = 0.38; chair stands speed: β = 0.37; all p < 0.05), while jump force/kg was more weakly associated with physical performance (range β = 0.18–0.24; all p < 0.05). Similarly, grip strength was more weakly associated with physical performance (range β = 0.23–0.28; all p < 0.05) vs. jump power/kg and velocity. Both jump force and grip strength had β magnitudes typically 50% lower compared to jump power/kg and velocity in associations to performance. Results were not attenuated when PA was added to the final models (data not shown).
Table 3.
Correlations between jump measures, grip strength, and physical performance
| Velocity (m/s) | Force (N/kg body wt) | Grip strength (kg/kg body wt) | 400 m walk time (s) | 6 m usual gait speed (m/s) | Chair stands speed (#/s) | |
|---|---|---|---|---|---|---|
| Power (W/kg body wt) | 0.87 | 0.56 | 0.44 | − 0.51 | 0.44 | 0.47 |
| Velocity (m/s) | 0.10 | 0.34 | − 0.45 | 0.41 | 0.43 | |
| Force (N/kg body wt) | 0.24 | − 0.29 | 0.20 | 0.27 | ||
| Grip strength (kg/kg body wt) | − 0.33 | 0.28 | 0.26 |
r < 0.4 weak, r ≥ 0.4 to ≤ 0.6 moderate, r > 0.6 strong
p < 0.001 for all correlations adjusted for age, race, site and height, and weight (velocity only since this measure was not weight-corrected)
Fig. 1.
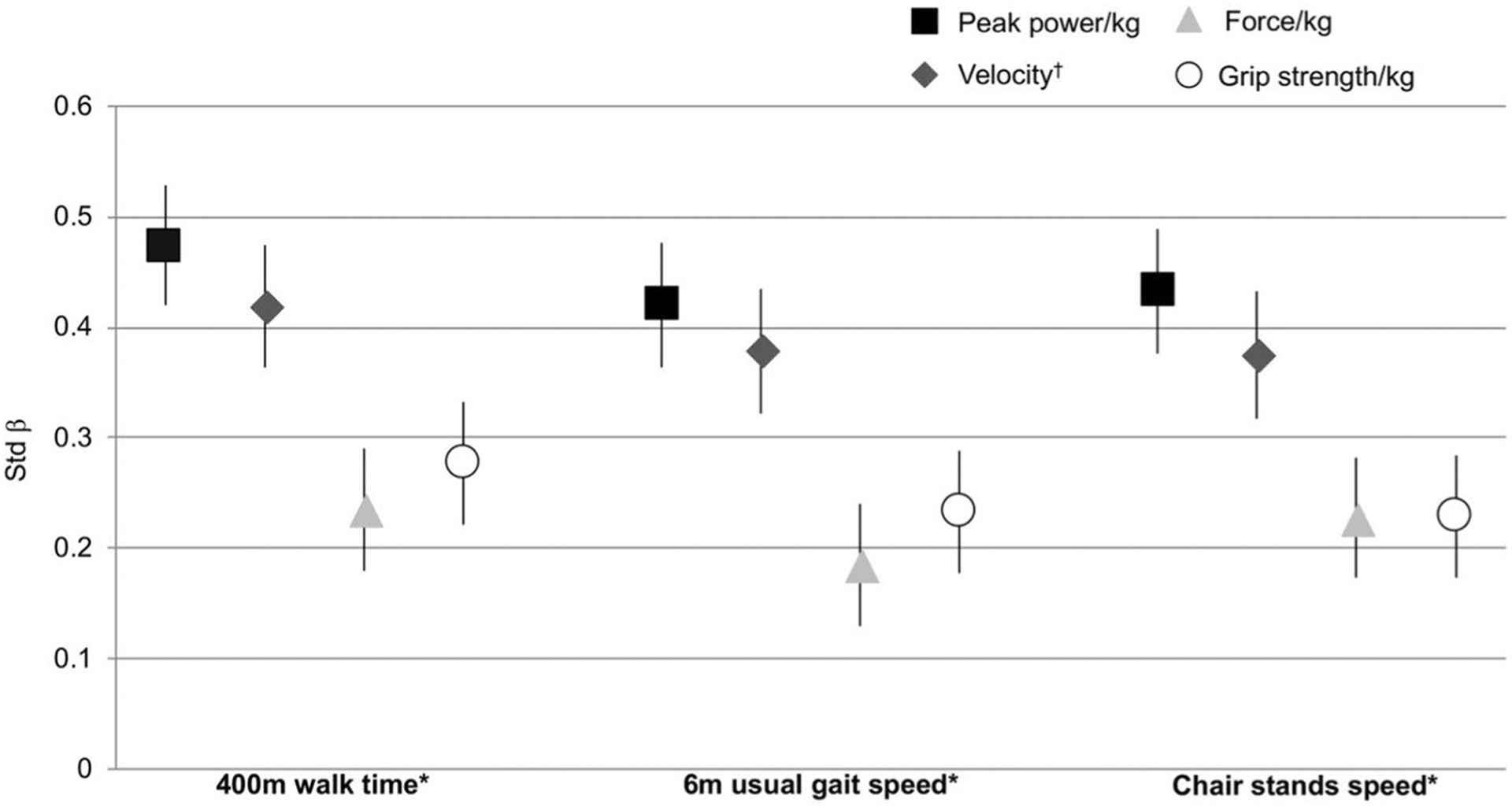
Associations of jump measures and grip strength with physical performance. *p < 0.05 for all jump measures and grip strength/kg, adjusted for age, race, site, height, falls history, SBP, DBP, hip/joint pain, BMD, Trails B, diabetes, hypertension, CHF, MI, stroke, Parkinson’s Disease, and total number of medications; †additionally adjusted for weight
Discussion
Jump power/kg and velocity at peak power had magnitudes of associations with physical performance that were approximately twofold higher compared to jump force/kg and grip strength/kg. To our knowledge, this study is the first to compare jump power, as well as its distinct velocity and force components, to grip strength and multiple physical performance measures in a large multicenter epidemiologic study. Previous studies relating jump tests to physical performance have not included the oldest adults or participants unable to lift their feet off the ground, who were included with our novel methods [9, 10, 12, 13]. These studies potentially excluded individuals with worse physical function and likely resulted in samples with narrower ranges of physical performance and not representative of the population with mobility limitation. Our study had a wide range of function; 20% with jump tests had gait speed < 1.0 m/s and 28% had ≥ 1 jump without flight [18], suggesting that our more inclusive methodology may be more appropriate for individuals with poorer function and/or risk for future functional decline. Functional, task-based power methods (e.g., jump tests) may more closely approximate the ability to perform activities of daily living compared to traditional strength measures, because the velocity of movement is incorporated. Weight-bearing tests, such as jump tests, should be considered in studies of muscle function, especially for individuals excluded from seated tests (e.g., those with certain joint conditions, such as arthritis) and studies focused on performance-based outcomes.
Power and the velocity component for generating movement may be more important factors necessary for preventing functional loss at the oldest ages compared to force or strength. However, the relationships of power, velocity, and force with physical performance have not been previously included in one single study. In an earlier small study in men (N = 37, mean age: 80 ± 1 years), velocity measured with seated leg press had only a slightly higher association with 400 m walk gait speed (standardized β = 0.42, p = 0.005) than strength (standardized β = 0.36, p = 0.02), but the relationship of power to physical performance was not reported [38]. Two past studies of men and women aged 72 ± 5 years (range 69–81 years) [13] and 65 ± 17 years (range 27–96 years) [9] reported unstandardized estimates and showed that power/kg and velocity were only weakly correlated with walking (6 min distance [13] and usual gait speed [9]); though moderately correlated with chair stands [9]. Our study had a similar mean and range for gait speed as this past study, though included only men at older ages (77–101 years) with a wider range of function [18]. We compared standardized estimates across various physical performance measures, since power, velocity, force, and grip strength each have distinct measurement units. Another study aged 76 ± 3 years (range 71–87 years) that included jump tests and compared standardized estimates showed that lower jump power/kg had three times higher association with slower gait speed and two times higher association with chair stands time vs. jump force/kg [12]. We found estimates of jump power/kg and force/kg to physical performance with similar magnitudes as the previous studies, suggesting that the velocity component of power, rather than the force component, is likely more crucial for movement, and, therefore, physical function, in the oldest adults. The jump test may more closely estimate physical performance than seated muscle power tests or grip strength due to other neuromuscular factors (e.g., balance) that are required to complete this task-based, weight-bearing measure [39, 40].
Muscle power may be an earlier predictor of age-related muscle function loss vs. strength alone. The age-related decrease in area of Type II muscle fibers responsible for generating short, quick bursts of movement [41] may explain larger declines in muscle power and velocity compared to force. Past studies showing substantially lower power at older ages vs. younger ages than strength [8, 18, 20–23] have rarely included adults > 80 years. However, in our previously published study of MrOS men aged 77–101 years, for each 5-year increase in age, power/kg was 10% lower and velocity was approximately 7% lower, whereas force/kg was 3% lower [18]. Differences in power and velocity were even higher in magnitude in those > 90 vs. ≤ 80 years old (power: 30% lower; velocity: 24% lower; force/kg: 9% lower) [18]. As jump power and velocity may have larger age-related declines than jump force, these may be more strongly related to physical performance than strength.
Strengths
Unlike many previous large epidemiologic studies in older adults, we had multiple measures of both muscle function and physical performance. Only a few studies measured both lower extremity muscle power and upper extremity grip strength in association with physical performance [1, 4, 9]. However, these studies had power tests in the seated position [1, 4] or did not calculate or compare both power and force to physical performance [1, 4, 9]. The novel force plate methodology developed for this study allowed inclusion of individuals unable to jump (i.e., unable to lift the feet off the ground). Among the oldest old ranging from 77 to 101 years, this method allowed us to include those who performed jump tests despite poor function (gait speed < 1.0 m/s), though had similar health (e.g., diabetes, hypertension, and myocardial infarction) and physical function (e.g., grip strength, 400 m walk time, and chair stands speed) to those without jump tests. MrOS also collected data on many covariates to adjust for independent associations of jump test measures and strength with physical performance.
Limitations
The cross-sectional study design does not allow us to establish temporal relationships or examine longitudinal decline in muscle function associated with performance-based measures. Our conservative approach for safety may have excluded some participants able to jump, largely for balance issues as evidenced by 51% of excluded men with falls in the past year vs. 34% with jump tests. Excluding these frail men limits generalizability. Additionally, our community-dwelling, largely white population of men limits generalizability. The time intensive data processing of jump trials due to custom-designed engineering algorithms limited feasibility for many large studies and immediate clinical application. Other muscle power measures were not collected and, therefore, not compared to jump power.
Conclusions
Jump test measures of lower extremity power/kg and velocity at peak power had two times higher association with multiple physical performance measures than jump force/kg or grip strength/kg. Weight-bearing power and velocity may be stronger predictors of poor physical performance than strength in the oldest adults. Functional power from weight-bearing tasks should be evaluated in diverse populations to determine if similar relationships are observed in a wider age range of older adults, women, or other race/ethnic groups. Future longitudinal studies should determine whether jump power and velocity are stronger predictors of physical performance decline and disability, such as loss of mobility, than jump force or traditional power/strength measures. If results are confirmed, interventions aimed at improving power and velocity may result in improved physical performance.
Acknowledgements
The authors are grateful for the contributions of Tue Skallgaard (Department of Sports Science and Clinical Bio-mechanics, University of Southern Denmark, Odense, Denmark) for providing engineering expertise for the development of the custom jump analysis software. Results were presented to The 21st International Association of Gerontology and Geriatrics World Congress of Gerontology and Geriatrics, July 26, 2017, San Francisco, CA, USA. The Osteoporotic Fractures in Men (MrOS) Research Group: Administrative Center (Oregon Health & Sciences University): E. Orwoll (Principal Investigator), J. Lapidus (co-Investigator), C. Nielson (co-Investigator), L. Marshall (co-Investigator), C. Pedersen (Project Director), M. Abrahamson, Y. Wang, J. Wiedrick, N. Fino, E. Hooker, J. Nava; Coordinating Center (California Pacific Medical Center Research Institute and University of California, San Francisco): S.R. Cummings (Principal Investigator), D.C. Bauer (co-Investigator), D.M. Black (co-Investigator), P.M. Cawthon (co-Investigator), K.L. Stone (co-Investigator), R. Collins (Project Director), B. Black, T. Blackwell, A. Burghardt, L. Concepcion, S. Ewing, S.L. Harrison, L.Y. Lui, S. Majumdar, C. Navy, N. Parimi, S. Patel, K. Peters, A. Schafer, C. Schambach, A. Schwartz, A. Yu; University of Alabama, Birmingham: J. Shikany (Principal Investigator), C. Lewis (co-Investigator), M. Kilgore (co-Investigator), P. Johnson (Project Director), M. Young (Study Coordinator), N. Webb, S. Felder, C. Collier, K. Hardy; University of Minnesota: K. Ensrud (Principal Investigator), H. Fink (co-Investigator), S. Diem (co-Investigator), J. Schousboe (co-Investigator), B. Taylor (co-Investigator), L. Langsetmo (co-Investigator), S. Potter (Project Director), N. Nelson (Clinic Coordinator), P. Van Coevering (Program Director), K. Jacobson, A. Kats, S. Luthi, K. Moen, E. Penland-Miller, T. Vo; Stanford University: M. Stefanick (Principal Investigator), A. Hoffman (co-Investigator), N. Ellsworth, K. Kent; University of Pittsburgh: J. Cauley (Principal Investigator), J. Zmuda (co-Investigator), E. Strotmeyer (co-Investigator), D. Cusick (Project Director), C. Newman, A. Flaugh, S. Happe; University of California, San Diego: D. Kado (Principal Investigator), E Barrett-Connor (co-Investigator), L Claravall (Project Director), M.L. Carrion-Petersen, P Miller, M. Stephens, and J Smith.
Funding The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute on Aging (NIA), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Center for Advancing Translational Sciences (NCATS), and NIH Roadmap for Medical Research under the following grant numbers: U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, U01 AG042143, U01 AG042145, U01 AG042168, U01 AR066160, and UL1 TR000128. Additional support was provided by the Department of Epidemiology, University of Pittsburgh, NIH National Institute on Aging T32-AG000181 (Newman AB), Department of Sports Science and Clinical Biomechanics and the Center for Active and Healthy Ageing, University of Southern Denmark (Caserotti P), and in part, by NIH, National Institute on Aging, Intramural Research Program (Harris TB).
Footnotes
Conflict of interest We have no conflicts of interest to declare.
Human and animal rights statement All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (University of Pittsburgh Institutional Review Board, Committee D, IRB # REN18050217/IRB980305) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent Informed consent was obtained from all individual participants included in the study.
References
- 1.Hicks GE, Shardell M, Alley DE et al. (2012) Absolute strength and loss of strength as predictors of mobility decline in older adults: the InCHIANTI study. J Gerontol A Biol Sci Med Sci 67:66–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bean JF, Leveille SG, Kiely DK et al. (2003) A comparison of leg power and leg strength within the InCHIANTI study: which influences mobility more? J Gerontol A Biol Sci Med Sci 58:728–733 [DOI] [PubMed] [Google Scholar]
- 3.Marsh AP, Miller ME, Saikin AM et al. (2006) Lower extremity strength and power are associated with 400-meter walk time in older adults: the InCHIANTI study. J Gerontol A Biol Sci Med Sci 61:1186–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lauretani F, Russo CR, Bandinelli S et al. (1985) Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol 2003:1851–1860 [DOI] [PubMed] [Google Scholar]
- 5.Cuoco A, Callahan DM, Sayers S et al. (2004) Impact of muscle power and force on gait speed in disabled older men and women. J Gerontol A Biol Sci Med Sci 59:1200–1206 [DOI] [PubMed] [Google Scholar]
- 6.Puthoff ML, Nielsen DH (2007) Relationships among impairments in lower-extremity strength and power, functional limitations, and disability in older adults. Phys Ther 87:1334–1347 [DOI] [PubMed] [Google Scholar]
- 7.Caserotti P, Aagaard P, Simonsen EB et al. (2001) Contraction-specific differences in maximal muscle power during stretch-shortening cycle movements in elderly males and females. Eur J Appl Physiol 84:206–212 [DOI] [PubMed] [Google Scholar]
- 8.Caserotti P, Aagaard P, Larsen JB et al. (2008) Explosive heavy-resistance training in old and very old adults: changes in rapid muscle force, strength and power. Scand J Med Sci Sports 18:773–782 [DOI] [PubMed] [Google Scholar]
- 9.Siglinsky E, Krueger D, Ward RE et al. (2015) Effect of age and sex on jumping mechanography and other measures of muscle mass and function. J Musculoskelet Neuronal Interact 15:301–308 [PMC free article] [PubMed] [Google Scholar]
- 10.Hong N, Kim CO, Youm Y et al. (2018) Low peak jump power is associated with elevated odds of dysmobility syndrome in community-dwelling elderly individuals: the Korean Urban Rural Elderly (KURE) study. Osteoporos Int 29:1427–1436 [DOI] [PubMed] [Google Scholar]
- 11.Thompson BJ, Whitson M, Sobolewski EJ et al. (2018) Effects of age, joint angle, and test modality on strength production and functional outcomes. Int J Sports Med 39:124–132 [DOI] [PubMed] [Google Scholar]
- 12.Hannam K, Hartley A, Clark EM et al. (2017) Feasibility and acceptability of using jumping mechanography to detect early components of sarcopenia in community-dwelling older women. J Musculoskelet Neuronal Interact 17:246–257 [PMC free article] [PubMed] [Google Scholar]
- 13.Maden-Wilkinson TM, McPhee JS, Jones DA et al. (2015) Age-related loss of muscle mass, strength, and power and their association with mobility in recreationally-active older adults in the United Kingdom. J Aging Phys Act 23:352–360 [DOI] [PubMed] [Google Scholar]
- 14.Caserotti P, Aagaard P, Puggaard L (2008) Changes in power and force generation during coupled eccentric-concentric versus concentric muscle contraction with training and aging. Eur J Appl Physiol 103:151–161 [DOI] [PubMed] [Google Scholar]
- 15.Holsgaard Larsen A, Caserotti P, Puggaard L et al. (2007) Reproducibility and relationship of single-joint strength vs multi-joint strength and power in aging individuals. Scand J Med Sci Sports 17:43–53 [DOI] [PubMed] [Google Scholar]
- 16.Rittweger J, Schiessl H, Felsenberg D et al. (2004) Reproducibility of the jumping mechanography as a test of mechanical power output in physically competent adult and elderly subjects. J Am Geriatr Soc 52:128–131 [DOI] [PubMed] [Google Scholar]
- 17.De Vito G, Bernardi M, Forte R et al. (1998) Determinants of maximal instantaneous muscle power in women aged 50–75 years. Eur J Appl Physiol Occup Physiol 78:59–64 [DOI] [PubMed] [Google Scholar]
- 18.Strotmeyer ES, Winger ME, Cauley JA et al. (2018) Normative values of muscle power using force plate jump tests in men aged 77–101 years: the osteoporotic fractures in Men (MrOS) Study. J Nutr Health Aging 22:1167–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bean JF, Kiely DK, Herman S et al. (2002) The relationship between leg power and physical performance in mobility-limited older people. J Am Geriatr Soc 50:461–467 [DOI] [PubMed] [Google Scholar]
- 20.Dietzel R, Gast U, Heine T et al. (2013) Cross-sectional assessment of neuromuscular function using mechanography in women and men aged 20–85 years. J Musculoskelet Neuronal Interact 13:312–319 [PubMed] [Google Scholar]
- 21.Stephenson ML, Smith DT, Heinbaugh EM et al. (2015) Total and lower extremity lean mass percentage positively correlates with jump performance. J Strength Cond Res 29:2167–2175 [DOI] [PubMed] [Google Scholar]
- 22.Zengin A, Pye SR, Cook MJ et al. (2017) Associations of muscle force, power, cross-sectional muscle area and bone geometry in older UK men. J Cachexia Sarcopenia Muscle 8:598–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dionyssiotis Y, Galanos A, Michas G et al. (2010) Assessment of musculoskeletal system in women with jumping mechanography. Int J Womens Health 1:113–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bean JF, Kiely DK, LaRose S et al. (2007) Is stair climb power a clinically relevant measure of leg power impairments in at-risk older adults? Arch Phys Med Rehabil 88:604–609 [DOI] [PubMed] [Google Scholar]
- 25.Alcazar J, Guadalupe-Grau A, Garcia-Garcia FJ et al. (2018) Skeletal muscle power measurement in older people: a systematic review of testing protocols and adverse events. J Gerontol A Biol Sci Med Sci 73:914–924 [DOI] [PubMed] [Google Scholar]
- 26.Blank JB, Cawthon PM, Carrion-Petersen ML et al. (2005) Overview of recruitment for the osteoporotic fractures in men study (MrOS). Contemp Clin Trials 26:557–568 [DOI] [PubMed] [Google Scholar]
- 27.Orwoll E, Blank JB, Barrett-Connor E et al. (2005) Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study—a large observational study of the determinants of fracture in older men. Contemp Clin Trials 26:569–585 [DOI] [PubMed] [Google Scholar]
- 28.Harkonen R, Harju R, Alaranta H (1993) Accuracy of the Jamar dynamometer. J Hand Ther 6:259–262 [DOI] [PubMed] [Google Scholar]
- 29.Lange-Maia BS, Newman AB, Strotmeyer ES et al. (2015) Performance on fast- and usual-paced 400-m walk tests in older adults: are they comparable? Aging Clin Exp Res 27:309–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ewing JA (1984) Detecting alcoholism. The CAGE questionnaire. JAMA 252:1905–1907 [DOI] [PubMed] [Google Scholar]
- 31.Mackey DC, Manini TM, Schoeller DA et al. (2011) Validation of an armband to measure daily energy expenditure in older adults. J Gerontol A Biol Sci Med Sci 66:1108–1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Myers MG, Godwin M, Dawes M et al. (2011) Conventional versus automated measurement of blood pressure in primary care patients with systolic hypertension: randomised parallel design controlled trial. BMJ 342:d286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cummings SR, Bates D, Black DM (2002) Clinical use of bone densitometry: scientific review. JAMA 288:1889–1897 [DOI] [PubMed] [Google Scholar]
- 34.Teng EL, Chui HC (1987) The modified mini-mental state (3MS) examination. J Clin Psychiatry 48:314–318 [PubMed] [Google Scholar]
- 35.Reitan RM, Wolfson D (2004) The Trail Making Test as an initial screening procedure for neuropsychological impairment in older children. Arch Clin Neuropsychol 19:281–288 [DOI] [PubMed] [Google Scholar]
- 36.Pahor M, Chrischilles EA, Guralnik JM et al. (1994) Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol 10:405–411 [DOI] [PubMed] [Google Scholar]
- 37.Studenski S, Perera S, Wallace D et al. (2003) Physical performance measures in the clinical setting. J Am Geriatr Soc 51:314–322 [DOI] [PubMed] [Google Scholar]
- 38.Sayers SP, Guralnik JM, Thombs LA et al. (2005) Effect of leg muscle contraction velocity on functional performance in older men and women. J Am Geriatr Soc 53:467–471 [DOI] [PubMed] [Google Scholar]
- 39.Edwards MH, Buehring B (2015) Novel approaches to the diagnosis of sarcopenia. J Clin Densitom 18:472–477 [DOI] [PubMed] [Google Scholar]
- 40.Zbinden-Foncea H, Valenzuela T, Espildora F et al. (2014) Muscular power as a function of load in elderly women. Comput Methods Biomech Biomed Eng 17:92–93 [DOI] [PubMed] [Google Scholar]
- 41.Canepari M, Pellegrino MA, D’Antona G et al. (2010) Single muscle fiber properties in aging and disuse. Scand J Med Sci Sports 20:10–19 [DOI] [PubMed] [Google Scholar]


