Abstract
BACKGROUND
Dipeptidyl peptidase-4 (DPP4) is commonly targeted to achieve glycemic control and has potent anti-inflammatory and immunoregulatory effects. Recent structural analyses indicated a potential tight interaction between DPP4 and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), raising a promising hypothesis that DPP4 inhibitor (DPP4i) drugs might be an optimal strategy for treating coronavirus disease 2019 (COVID-19) among patients with diabetes. However, there has been no direct clinical evidence illuminating the associations between DPP4i use and COVID-19 outcomes.
AIM
To illuminate the associations between DPP4i usage and the adverse outcomes of COVID-19.
METHODS
We conducted a multicenter, retrospective analysis including 2563 patients with type 2 diabetes who were hospitalized due to COVID-19 at 16 hospitals in Hubei Province, China. After excluding ineligible individuals, 142 patients who received DPP4i drugs and 1115 patients who received non-DPP4i oral anti-diabetic drugs were included in the subsequent analysis. We performed a strict propensity score matching (PSM) analysis where age, sex, comorbidities, number of oral hypoglycemic agents, heart rate, blood pressure, pulse oxygen saturation (SpO2) < 95%, CT diagnosed bilateral lung lesions, laboratory indicators, and proportion of insulin usage were matched. Finally, 111 participants treated with DPP4i drugs were successfully matched to 333 non-DPP4i users. Then, a linear logistic model and mixed-effect Cox model were applied to analyze the associations between in-hospital DPP4i use and adverse outcomes of COVID-19.
RESULTS
After rigorous matching and further adjustments for imbalanced variables in the linear logistic model and Cox adjusted model, we found that there was no significant association between in-hospital DPP4i use (DPP4i group) and 28-d all-cause mortality (adjusted hazard ratio = 0.44, 95%CI: 0.09-2.11, P = 0.31). Likewise, the incidences and risks of secondary outcomes, including septic shock, acute respiratory distress syndrome, or acute organ (kidney, liver, and cardiac) injuries, were also comparable between the DPP4i and non-DPP4i groups. The performance of DPP4i agents in achieving glucose control (e.g., the median level of fasting blood glucose and random blood glucose) and inflammatory regulation was approximately equivalent in the DPP4i and non-DPP4i groups. Furthermore, we did not observe substantial side effects such as uncontrolled glycemia or acidosis due to DPP4i application relative to the use of non-DPP4i agents in the study cohort.
CONCLUSION
Our findings demonstrated that DPP4i use is not significantly associated with poor outcomes of COVID-19 or other adverse effects of anti-diabetic treatment. The data support the continuation of DPP4i agents for diabetes management in the setting of COVID-19.
Keywords: COVID-19, SARS-CoV-2, DPP4 inhibitors, Diabetes, Glucose control, Adverse effects
Core Tip: Our study illuminated that there were no significant associations between in-hospital use of dipeptidyl peptidase-4 (DPP4) inhibitor (DPP4i) and 28-d all-cause mortality or multiorgan injury. In addition, the glucose control effects, inflammatory response, and associated adverse events were also comparable between patients in the DPP4i and non-DPP4i groups. These data provided direct clinical evidence to support the continuation of DPP4i therapy in diabetic patients with coronavirus disease 2019 (COVID-19). Moreover, this work paves the way for prospective studies and randomized controlled clinical trials on DPP4 inhibitor therapy for COVID-19.
INTRODUCTION
The coronavirus disease 2019 (COVID-19) pandemic triggered by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has caused hundreds of thousands of deaths. Unfortunately, the catastrophe is currently still spreading rapidly worldwide with no effective drugs in sight. Diabetes is one of the most important comorbidities linked to the remarkably elevated severity and mortality of COVID-19[1]. Our most recent study demonstrated that glucose control is the key strategy to avoid the poor outcomes of COVID-19 among patients with diabetes[2]. However, the optimal method for managing glycemia is still a major concern for diabetic patients infected by SARS-CoV-2.
Dipeptidyl peptidase 4 (DPP4) is commonly targeted to lower glucose levels and has key roles in inflammatory and immune regulation[3,4]. Because of the general efficacy of DPP4 inhibitor (DPP4i; gliptins) drugs in controlling glycemia and their well-tolerated risk of hypoglycemia, as shown in more than 10 years of clinical experience, these prescriptions have sharply increased in the United States from 0.4% of hypoglycemia treatment in 2005 to approximately 21% in 2016[5]. Aside from its role in glucose regulation, DPP4i drugs exhibit favorable anti-inflammatory and immunoregulatory effects by suppressing immune cell activation, cytokine induction, and peptide hormone imbalance in patients with diabetes[4]. In the setting of COVID-19, while the commonly applied antidiabetic drugs, e.g., metformin and sodium-glucose-cotransporter 2 inhibitors, may have side effects such as acidosis, DPP4 inhibitors are empirically recommended as well-tolerated glucose-lowering agents to be continued for treating patients with diabetes. Furthermore, as the well-established Middle East respiratory syndrome coronavirus (MERS-CoV) cellular receptor[6], DPP4 was predicted to have a tight interaction with SARS-CoV-2 spike glycoprotein in a recent molecular modeling study[7]. Indeed, DPP4 antibodies could directly inhibit coronavirus infection in primary human bronchial epithelial cells[6]. These observations sparked intense debate about whether DPP4 inhibitors might be promising therapeutic agents against COVID-19 among patients with diabetes[8-10]. However, there has been no direct clinical evidence supporting the associations between DPP4i usage and COVID-19 prognosis so far.
Here, to investigate the potential impacts of DPP4i drugs on COVID-19 among patients with diabetes, we performed a multicenter retrospective cohort analysis of 2563 inpatients with type 2 diabetes who were infected with SARS-CoV-2. The associations of in-hospital DPP4i use with COVID-19-related mortality, multiorgan injury, glucose control, and inflammatory response were compared with the corresponding associations with other oral glucose-lowering drugs following rigorous matching and adjustments for confounders.
MATERIALS AND METHODS
Study population and data collection
This multicenter retrospective cohort study included 2563 COVID-19 patients with type 2 diabetes admitted to 16 hospitals in Hubei, China between December 30, 2019 and April 23, 2020. The study design was approved by the central ethics boards and was accepted or approved by each collaborating hospital. The requirement for informed consent was waived by the ethics boards of the hospitals.
Patients with COVID-19 were diagnosed based on chest computed tomography (CT) manifestations or reverse transcription-polymerase chain reaction (RT-PCR) according to the New Coronavirus Pneumonia Prevention and Control Program (5th edition) published by the National Health Commission of China and WHO interim guidance[11,12]. The status of coexisting type 2 diabetes was determined based on the admitting diagnosis and disease history. The exclusion criteria included incomplete medical records (e.g., transfer to any other hospital), age < 18 or ≥ 85 years, pregnancy, acute lethal organ injury (e.g., acute myocardial infarction, severe acute pancreatitis, or acute stroke), death from surgery and a surgical complication, decompensated or end-stage chronic organ dysfunction (e.g., decompensated liver cirrhosis, estimated glomerular filtration rate (eGFR) < 30 mL/min per 1.73 m2, or chronic renal insufficiency above stage III), and prehospital confusion or mental disorders. Patients not taking oral hypoglycemic drugs during the observation period were also excluded.
The medical data of the included patients were collected, including demographic information, physical examination results, laboratory findings, radiographic data from CT, history of comorbidities, medication records, and clinical outcomes during hospitalization. We extracted information on the use of glucose-lowering drugs from the patients’ medication records. The laboratory findings included fasting blood glucose (FBG) and glucose (GLU) reflecting glycemic control, leukocyte count, neutrophil count, neutrophil percentage, red blood cell count, C-reactive protein (CRP), procalcitonin, D-dimer, and serum biochemical markers representing liver injury, kidney injury, and cardiac dysfunction. All the information was collected by anonymizing the personal identifying information (e.g., name and ID) by giving each participant a new study ID through a coding system. A team of professional physicians carefully interpreted and double-checked all data to guarantee the accuracy of the data.
Definition
The onset of COVID-19 was defined as the day when the first symptom was noticed. Patients with type 2 diabetes who received DPP4i during hospitalization were classified as a DPP4i group (142 participants). Patients with type 2 diabetes who never received DPP4i but orally took other anti-diabetic drugs during hospitalization were classified as a non-DPP4i group (1115 participants).
The primary endpoint was 28-d all-cause death. The secondary endpoints were defined as acute respiratory distress syndrome (ARDS), septic shock, acute cardiac injury, acute kidney injury, and acute liver injury. ARDS and septic shock were defined according to the WHO interim guideline “clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected”[13]. Acute cardiac injury was defined when the serum level of cardiac troponin I (cTNI), cardiac troponin T (cTNT), or high sensitivity cardiac troponin I (hs-cTNI) was above the upper limit of normal (ULN). Acute kidney injury was defined as an elevation in serum creatinine level equal to or above 26.5 μmol/L within 48 h[14]. Acute liver injury was defined when acutely increased levels of serum alanine aminotransferase (ALT) or alkaline phosphatase (ALP) above three times the upper limit of normal (ULN) were observed[15]. The increase or decrease of biochemical indicators was defined as exceeding or below their normal range, respectively, according to the laboratory standard of each hospital.
The adverse effects during hospitalization included hyperglycemia, hypoglycemia, metabolic acidosis, and lactic acidosis. Hyperglycemia was defined as FBG ≥ 6.1 mmol/L or 2-h postprandial blood glucose (2hPBG) ≥ 7.8 mmol/L or GLU ≥ 11.1 mmol/L[16]. Hypoglycemia was defined when FBG < 3 mmol/L[17]. Metabolic acidosis was defined as a blood pH < 7.35 and a decrease in plasma bicarbonate concentration (HCO3−) < 22 mmol/L, and lactic acidosis was defined when blood pH < 7.35 and the arterial lactate level > 5 mmol/L[18].
Propensity score-matched analysis and sensitivity analysis
Variables that were expected to be potential confounders for DPP4i use and for the association of in-hospital DPP4i use with COVID-19 outcomes were addressed using the propensity score matching (PSM) model. These variables included age, sex, heart rate, blood pressure, pulse oxygen saturation (SpO2) < 95%, CT-diagnosed bilateral lung lesions, incidence of increased leukocyte count, neutrophil count, CRP, procalcitonin, ALT, D-dimer, and low density lipoprotein cholesterol (LDL-c), decreased lymphocyte count and eGFR, comorbidities (chronic obstructive pulmonary disease, cerebrovascular diseases, chronic liver disease, and chronic renal diseases), number of oral hypoglycemic agents, and proportion of insulin usage. A nonparametric missing value imputation based on the missForest procedure in R was adopted to explain the missing laboratory data. A random forest model based on the other variables in the data set was applied to predict the missing values and to estimate the internally cross-validated errors. According to the propensity scores, the DPP4i and non-DPP4i users were paired at a ratio of 1:3 through exact matching with a caliper size of 0.05. The balance between covariates was evaluated by estimating standardized differences before and after matching, and those with absolute values less than 0.1 were regarded as successfully balanced. A sensitivity analysis was performed to assess the robustness of our findings.
Statistical analysis
The statistical methods of this study were reviewed by Dr. Jing Chen from Department of Mathematics and Physics, Wuhan Institute of Technology. Statistical analyses were conducted with R-3.6.3 (R Foundation for Statistical Computing, Vienna, Austria) and SPSS Statistics (version 23.0, IBM, Armonk, NY, United Stated). Continuous variables are presented as medians and interquartile ranges (IQRs), and categorical variables are presented as frequencies and percentages (%). Statistical differences between two groups were analyzed using t tests (normally distributed data) or the Kruskal-Wallis rank sum test (non-normally distributed data) for continuous variables, and comparisons of categorical variables were performed by Fisher’s exact test or the χ2 test.
The association between DPP4i use and the risk of COVID-19 outcomes was analyzed using a logistic regression model and a mixed-effect Cox model. The corresponding adjusted odds ratio (OR) and hazard ratio (HR) were calculated after adjusting for the incidence of increased CRP between the DPP4i and non-DPP4i groups. For the mixed-effect Cox model, we modeled site as a random effect, and the proportional hazard assumptions were verified using correlation testing based on Schoenfeld residuals. Considering the possibility of type 1 error due to multiple comparisons, the findings from the analyses of the secondary endpoints should be interpreted as exploratory.
RESULTS
Clinical characteristics of DPP4i group vs non-DPP4i group
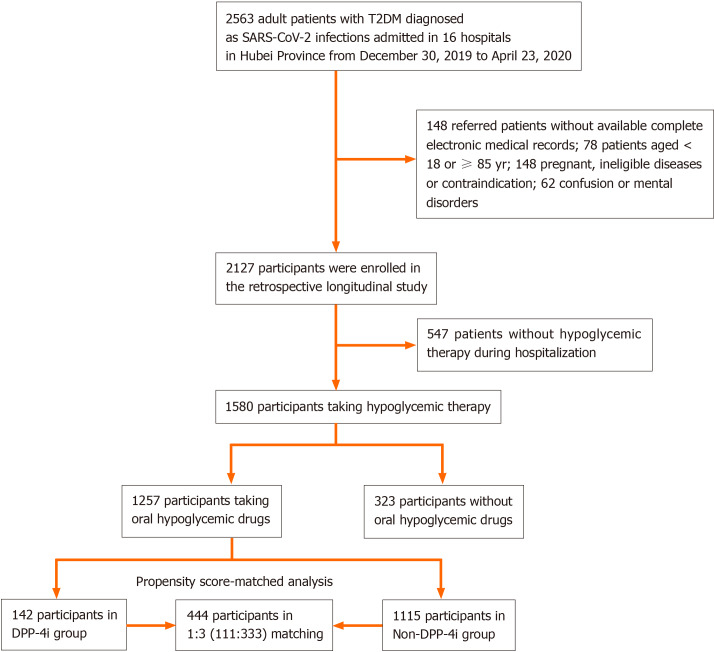
This study included a total of 2563 participants with type 2 diabetes who were admitted to hospitals due to COVID-19. After excluding 148 patients without complete electronic medical records, 78 aged ≥ 85 or < 18 years, 148 pregnant patients or patients with ineligible diseases or contraindications, 62 with confusion or mental disorders, 547 without hypoglycemic therapy, and 323 not taking oral hypoglycemic agents during hospitalization, 1257 patients [aged 64 years (IQR, 56-69); 52.03% male] were included for the retrospective longitudinal analysis (Figure 1). The 142 participants [aged 63 years (IQR, 55-67); 46.48% male] who received DPP4i drugs were classified into the DPP4i group, while the remaining 1115 [aged 64 years (IQR, 56.5-69); 52.74% male] were included in the non-DPP4i group (Table 1). All of the included participants were followed for a total of 28 d after admission. Compared to that in the non-DPP4i group, a higher percentage of participants in the DPP4i group took three or more types of anti-diabetic agents (36.62% in the DPP4i group vs 12.11% in the non-DPP4i group for 3 types; 13.38% vs 1.08% for ≥ 4 types). Compared to that in the non-DPP4i group, the incidence of elevated ALT was lower but the incidence of elevated LDL-c was higher in the DPP4i group at the time of admission (Table 1).
Figure 1.
Flow chart of study inclusion. T2D: Type 2 diabetes; DPP4i: Dipeptidyl peptidase-4 inhibitors.
Table 1.
Characteristics of patients with type 2 diabetes in dipeptidyl peptidase-4 inhibitor and non-dipeptidyl peptidase-4 inhibitor groups before and after propensity score matching
|
|
Unmatched
|
Matched (1:3)3
|
||||
| Parameter | DPP4i4 (n = 142) | Non-DPP4i5 (n = 1115) | SD | DPP4i4 (n = 111) | Non-DPP4i5 (n = 333) | SD |
| Clinical characteristics on admission | ||||||
| Age, median (IQR) | 63 (55-67) | 64 (56.5-69) | -0.107 | 63 (55.5-67) | 64 (56-69) | -0.068 |
| Male gender, n (%) | 66 (46.48) | 588 (52.74) | -0.125 | 57 (51.35) | 159 (47.75) | 0.072 |
| Female gender, n (%) | 76 (53.52) | 527 (47.26) | 0.125 | 54 (48.65) | 174 (52.25) | -0.072 |
| Heart rate, median (IQR) | 82 (75-91) | 85 (78-98) | -0.192 | 82.5 (75-97.25) | 82 (76-96) | 0.032 |
| Respiratory rate, median (IQR) | 20 (19-21) | 20 (19-21) | 0.030 | 20 (19-20) | 20 (19-21) | 0.014 |
| DBP, median (IQR) | 80 (73.25-90) | 80 (72-89) | 0.116 | 80 (72-91) | 80 (74-89) | 0.018 |
| SBP, median (IQR) | 133 (123-146) | 133 (121-145) | 0.070 | 133 (123-146) | 135 (122-148) | -0.021 |
| SpO2, median (IQR) | 97 (95-98) | 97 (95-98) | 0.018 | 97 (95-98) | 97 (95-98) | 0.049 |
| Comorbidities on admission | ||||||
| Heart failure, n (%) | 0 (0.00) | 3 (0.27) | -0.073 | 0 (0.00) | 0 (0.00) | 0 |
| Coronary heart disease, n (%) | 16 (11.27) | 150 (13.45) | -0.066 | 13 (11.71) | 44 (13.21) | -0.045 |
| Cerebrovascular diseases, n (%) | 5 (3.52) | 41 (3.68) | -0.008 | 4 (3.60) | 16 (4.80) | -0.060 |
| Chronic liver disease, n (%) | 0 (0.00) | 25 (2.24) | -0.214 | 0 (0.00) | 0 (0.00) | 0 |
| Chronic renal diseases, n (%) | 2 (1.41) | 23 (2.06) | -0.050 | 2 (1.80) | 3 (0.90) | 0.078 |
| Chronic obstructive pulmonary disease, n (%) | 1 (0.70) | 9 (0.81) | -0.012 | 1 (0.90) | 3 (0.90) | 0 |
| Background anti-diabetic drugs 1 | ||||||
| 1 type, n (%) | 24 (16.90) | 526 (47.17) | -0.686 | 24 (21.62) | 82 (24.62) | -0.071 |
| 2 types, n (%) | 47 (33.10) | 439 (39.37) | -0.131 | 47 (42.34) | 133 (39.94) | 0.049 |
| 3 types, n (%) | 52 (36.62) | 135 (12.11) | 0.596 | 39 (35.14) | 113 (33.93) | 0.025 |
| ≥ 4 types, n (%) | 19 (13.38) | 12 (1.08) | 0.489 | 1 (0.90) | 5 (1.50) | -0.055 |
| Insulin, n (%) | 81 (57.04) | 607 (54.44) | 0.052 | 59 (53.15) | 184 (55.26) | -0.042 |
| Chest CT on admission | ||||||
| Bilateral lesions, n (%) | 119 (92.25) | 957 (90.71) | 0.055 | 91 (91.92) | 289 (92.33) | -0.015 |
| Laboratory examination on admission | ||||||
| Leukocyte count > 9.5 × 109/L, n (%) | 16 (11.35) | 101 (9.41) | 0.063 | 13 (11.71) | 29 (8.95) | 0.091 |
| Neutrophil count > 6.3 × 109/L, n (%) | 22 (15.60) | 166 (15.47) | 0.004 | 18 (16.22) | 53 (16.36) | -0.004 |
| Lymphocyte count < 1.1, 109/L, n (%) | 55 (39.01) | 437 (40.73) | -0.035 | 42 (37.84) | 130 (40.12) | -0.047 |
| Red blood count < 3.5 (for female), 4.0 (for male) × 1012/L, n (%) | 61 (43.26) | 480 (44.73) | -0.030 | 48 (43.24) | 156 (48.15) | -0.099 |
| C-reactive protein increase > ULN2, n (%) | 36 (49.32) | 298 (50.85) | -0.031 | 26 (44.07) | 98 (56.32) | -0.247 |
| Procalcitonin level increase > ULN2, n (%) | 54 (46.96) | 399 (44.19) | 0.056 | 39 (43.82) | 132 (48.18) | -0.087 |
| ALT increase > 40 U/L, n (%) | 22 (15.83) | 234 (21.89) | -0.155 | 16 (14.68) | 53 (16.46) | -0.049 |
| eGFR, median (IQR) | 104.20 (90.60-119.76) | 101.18 (84.33-119.66) | 0.088 | 103.78 (90.77-116.18) | 101.33 (83.55-117.81) | 0.028 |
| D-dimer > ULN2, n (%) | 62 (49.60) | 526 (53.08) | -0.070 | 51 (52.04) | 148 (50.00) | 0.041 |
| LDL-c > 3.4 mmol/L, n (%) | 25 (22.73) | 135 (15.96) | 0.172 | 16 (19.51) | 51 (19.54) | -0.001 |
Background anti-diabetic drugs were defined as the number of types of combined oral hypoglycemic agents and the proportion of patients using insulin.
Upper limit of normal was defined according to criteria in each hospital and normal ranges of tests in each hospital.
In the propensity score matched model, age, gender, heart rate, blood pressure, SpO2 < 95%, computed tomography-diagnosed bilateral lung lesions, incidence of increased neutrophil count, leukocyte count, C-reactive protein, procalcitonin, alanine transaminase, D-dimer, low density lipoprotein cholesterol and decreased lymphocyte count, estimated glomerular filtration rate, comorbidities (chronic obstructive pulmonary disease, cerebrovascular diseases, chronic liver disease, and chronic renal diseases), the numbers of oral hypoglycemic agents, and the proportion of insulin usage were matched.
Patients with type 2 diabetes who received dipeptidyl peptidase-4 inhibitors (DPP4i) throughout the whole 28-d follow-up period were enrolled in the DPP4i cohort.
Patients with type 2 diabetes who never received DPP4i but took other anti-diabetic drugs throughout the observation time during hospitalization were classified as the non-DPP4i group. Data are n (%) or medians (IQR).
DPP4i: Dipeptidyl peptidase-4 inhibitors; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; SpO2: Pulse oxygen saturation; ALT: Alanine transaminase; eGFR: Estimated glomerular filtration rate; LDL-c: Low density lipoprotein cholesterol; IQR: Interquartile range; SD: Standardized difference; ULN: Upper limit of normal; CT: Computed tomography.
In-hospital use of DPP4i is not significantly associated with primary or secondary outcomes of COVID-19
To demonstrate the associations between in-hospital DPP4i use and COVID-19-related mortality, we performed PSM analysis and successfully matched 111 participants in the DPP4i group with 333 non-DPP4i users at a ratio of 1:3. The clinical characteristics of the DPP4i group vs the non-DPP4i group on admission after PSM are presented in Table 1.
In the logistic regression model, we did not observe any significant difference between patients taking DPP4i drugs and those taking other oral hypoglycemic drugs regarding the incidence or risk of 28-d all-cause mortality (1.8% vs 3.3%; OR = 0.58 95%CI: 0.12–2.68, P = 0.48). The incidences and risks of the secondary outcomes and the occurrences of septic shock, ARDS, or acute organ (kidney, liver, and cardiac) injury were comparable between the DPP4i and non-DPP4i groups (Table 2).
Table 2.
Associations of in-hospital use of dipeptidyl peptidase-4 inhibitors with coronavirus disease 2019 outcomes in propensity score matched analysis followed by logistic regression and mixed-effect Cox model analyses [n = 444, n (%)]
|
|
Logistic regression model
|
Mixed-effect Cox model
|
||||
|
Parameter
|
DPP4i (n = 111)
|
Non-DPP4i (n = 333)
|
Adjusted1 OR (95%CI)
|
P
value3
|
Adjusted2 HR (95%CI)
|
P
value3
|
| Primarily outcome | ||||||
| All-cause mortality | 2 (1.8) | 11 (3.3) | 0.58 (0.12,2.68) | 0.48 | 0.44 (0.09,2.11) | 0.31 |
| Secondary outcome | ||||||
| Septic shock | 1 (0.9) | 4 (1.2) | 0.78 (0.09,7.05) | 0.82 | 0.66 (0.07,6.13) | 0.71 |
| Acute respiratory distress syndrome | 17 (15.3) | 51 (15.3) | 1.06 (0.58,1.95) | 0.85 | 1.02 (0.59,1.77) | 0.95 |
| Acute kidney injury | 1 (0.9) | 3 (0.9) | 1.03 (0.11,10.03) | 0.98 | 1.04 (0.11,10.07) | 0.97 |
| Acute liver injury | 10 (9.0) | 20 (6.0) | 1.6 (0.72,3.54) | 0.25 | 1.62 (0.74,3.53) | 0.23 |
| Acute cardiac injury | 11 (9.9) | 24 (7.2) | 1.53 (0.71,3.28) | 0.28 | 1.35 (0.65,2.79) | 0.42 |
| Any | 30 (27) | 84 (25.2) | 1.17 (0.71,1.94) | 0.54 | 1.06 (0.69,1.62) | 0.79 |
Logistic regression model adjusted the incidence of increased C-reactive protein between DPP4i and non-DPP4i groups.
Cox proportional hazard model using the hospital site as a random effect and adjusting the incidence of increased C-reactive protein.
P values were calculated based on logistic regression model and mixed-effect Cox model, respectively.
Data are n (%). Measures of associations are adjusted odds ratios and hazard ratios. DPP4i: Dipeptidyl peptidase-4 inhibitors; OR: Odds ratio; HR: Hazard ratio; CI: Confidence interval.
After further adjusting for imbalanced variables (the incidence of elevated CRP) in the mixed-effect Cox model using site as a random effect, the results remained nonsignificant for the association of DPP4i use with 28-d all-cause mortality in patients with COVID-19 (adjusted HR = 0.44, 95%CI: 0.09-2.11, P = 0.31) or with the abovementioned secondary outcomes (Table 2). The insignificant association between the in-hospital use of DPP4i and 28-d mortality due to COVID-19 was further validated in the sensitivity analyses (adjusted HR = 0.45, 95%CI: 0.10-2.08, P = 0.31 in the first sensitivity analysis with caliper size at 0.04; adjusted HR = 0.35, 95%CI: 0.07-1.71, P = 0.19 in the second sensitivity analysis without matching procalcitonin).
DPP4i shows comparable glycemic control efficacy to non-DPP4i oral anti-diabetic agents
Our recent study demonstrated that patients with well-controlled glucose levels had a significantly reduced risk of COVID-19-related mortality compared to those with poorly controlled glucose levels among patients with coexisting diabetes[2]. We thus further analyzed whether DPP4i treatment had a better performance in maintaining glucose control than non-DPP4i treatment. However, the median, maximum, and minimum FBG and GLU were all approximately equivalent between the patients who received DPP4i drugs and those who received non-DPP4i drugs during hospitalization (Table 3). Although the relative duration of FBG above 7.0 mmol/L was slightly longer in the DPP4i group than in the non-DPP4i group, there was no statistically significant difference (Table 3). Likewise, the glucose control-related adverse outcomes, including hyperglycemia requiring treatment, hypoglycemia, acidosis, lactic acid elevation, and the probability of discontinuing the current regimen, were all comparable among patients with diabetes in the DPP4i and non-DPP4i groups (Table 4).
Table 3.
Differences of glycemic control efficacy in dipeptidyl peptidase-4 inhibitors group vs non-dipeptidyl peptidase-4 inhibitors group (n = 444)
|
Parameter
|
DPP4i (n = 111)
|
Non-DPP4i (n = 333)
|
P
value4
|
| Glycemic control efficacy during hospitalization | |||
| Median of FBG, median (IQR) | 8.01 (6.75-10.31) | 8.35 (6.48-11.12) | 0.84 |
| Median of GLU, median (IQR) | 10.55 (8.70-12.58) | 10.70 (8.90-13.45) | 0.90 |
| Max FBG, median (IQR) | 11.18 (7.82-13.92) | 10.50 (7.33-15.48) | 0.95 |
| Max GLU, median (IQR) | 14.90 (12.40-19.10) | 16.30 (13.30-20.55) | 0.30 |
| Min FBG, median (IQR) | 6.67 (5.30-7.98) | 6.74 (5.39-8.76) | 0.54 |
| Min GLU, median (IQR) | 7.10 (5.00-9.30) | 7.10 (5.65-8.90) | 0.87 |
| FBG > 7 mmol/L, day/total days1 (%) | 9.09 (4.26-16.67) | 6.52 (2.67-13.64) | 0.05 |
| GLU > 11.1 mmol/L, day/total days2 (%) | 9.09 (5.56-23.75) | 10.00 (4.08-25.40) | 0.81 |
| Titrating time3, median (IQR) | 4.00 (1.00-9.00) | 3.00 (1.00-11.00) | 0.71 |
The day/total days of FBG > 7 mmol/L was defined as the days with FBG > 7 mmol/L as a percentage of the entire observation period.
The day/total days of GLU > 11.1 mmol/L was defined as the days with GLU > 11.1 mmol/L as a percentage of the entire observation period.
Titrating time was defined as the number of days that FBG met the normal range for the first time since admission.
P values were calculated based on Kruskal-Wallis rank sum test.
Data are n (%) or medians (IQR). DPP4i: Dipeptidyl peptidase-4 inhibitors; FBG: Fasting blood glucose; GLU: Random blood glucose; Max: Maximum; Min: Minimum; IQR: Interquartile range.
Table 4.
Incidences and adjusted odds ratios of glycemic control efficacy and side effects in dipeptidyl peptidase-4 inhibitors group compared to non-dipeptidyl peptidase-4 inhibitors group [n = 444, n (%)]
|
Parameter
|
DPP4i (n = 111)
|
Non-DPP4i (n = 333)
|
Adjusted5 OR (95%CI)
|
P
value6
|
| FBG < 7 mmol/L | 47 (42.3) | 150 (45.0) | 0.91 (0.59,1.4) | 0.67 |
| GLU < 11.1 mmol/L | 29 (26.1) | 102 (30.6) | 0.78 (0.48,1.27) | 0.31 |
| Hyperglycemia requiring treatment1 | 16 (14.4) | 69 (20.7) | 0.65 (0.36,1.17) | 0.15 |
| Hypoglycemia2 | 1 (0.9) | 1 (0.3) | 2.96 (0.18,47.95) | 0.44 |
| Metabolic acidosis | 3 (2.7) | 8 (2.4) | 1.21 (0.31,4.66) | 0.79 |
| Lactic acidosis | 1 (0.9) | 5 (1.5) | 0.62 (0.07,5.42) | 0.67 |
| Elevation of lactic acid3 | 12 (10.8) | 29 (8.7) | 1.33 (0.65,2.72) | 0.44 |
| Probability to discontinue current regimen4 | 16 (14.4) | 75 (22.5) | 0.58 (0.32,1.04) | 0.07 |
Hyperglycemia requiring treatment was defined as the proportion of patients with elevated blood glucose and temporarily treated with insulin.
Hypoglycemia was defined when fasting blood glucose < 3 mmol/L.
The elevation of lactic acid was identified as > 2.2 mmol/L.
Probability to discontinue current regimen was defined as proportion of patients who added insulin treatment on the basis of existing hypoglycemic regimens.
Logistic regression model adjusted the incidence of increased C-reactive protein between DPP4i versus non-DPP4i groups.
P values were calculated based on logistic regression model.
Data are n (%). Measures of associations are adjusted odds ratios. DPP4i: Dipeptidyl peptidase-4 inhibitors; FBG: Fasting blood glucose; GLU: Random blood glucose; OR: Odds ratio; CI: Confidence interval.
No association between DPP4i usage and inflammation among patients with diabetes
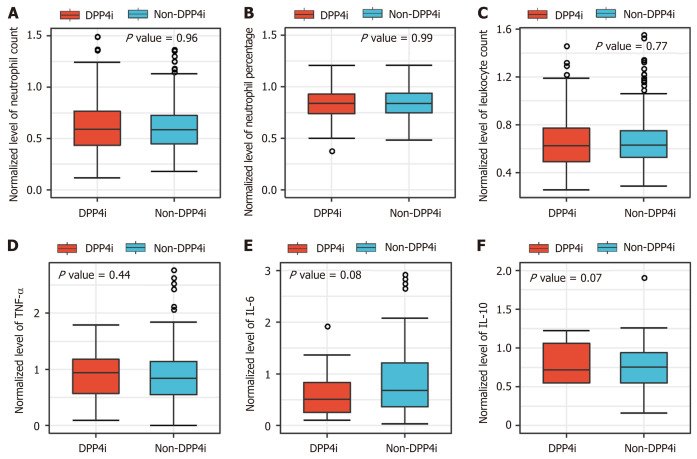
It has been reported previously that DPP4i might exert a comprehensive anti-inflammatory effect by blocking inflammatory cell activation and cytokine production[4]. However, the box plot demonstrates that there were no significant differences in neutrophil count, leukocyte count, or cytokine concentrations (e.g., tumor necrosis factor-α [TNF-α] and interleukin-6 [IL-6]) between the DPP4i and non-DPP4i groups among patients with COVID-19 with coexisting type 2 diabetes (Figure 2).
Figure 2.
Box plots showing the median levels of inflammatory response indicators in dipeptidyl peptidase-4 inhibitor and non-dipeptidyl peptidase-4 inhibitor groups (n = 444). A-F: Box chart analysis of median levels of neutrophil count, neutrophil percentage, leukocyte count, tumor necrosis factor-α, interleukin (IL)-6, and IL-10 between the dipeptidyl peptidase-4 inhibitor (DPP4i) and non-DPP4i groups. The median levels of those parameters of each participant during the follow-up duration were applied and were normalized according to their upper limits of normal range of each hospital. DPP4i: Dipeptidyl peptidase-4 inhibitors; TNF-α: Tumor necrosis factor-α; IL-6: Interleukin-6; IL-10: Interleukin-10.
DISCUSSION
DPP4i drugs are a well-established therapy with outstanding abilities to modulate glycemia and hyperactive inflammation[4,19]. In addition, a recent docking-based structural analysis speculated a tight interaction between DPP4 and SARS-CoV-2[7]. These insights raised a hypothesis about whether DPP4i drugs might be promising therapeutic agents for COVID-19 among patients with hyperglycemia[8-10]. Here, we retrospectively enrolled 2563 patients with type 2 diabetes who were infected with SARS-CoV-2 from 16 hospitals in Hubei Province, China to investigate the possible associations of DPP4i use with COVID-19 mortality and adverse outcomes. Notably, in-hospital DPP4i use was not associated with 28-d all-cause mortality or with multiorgan injury related to COVID-19. The effects of DPP4i drugs on glucose control and inflammatory regulation were comparable to those of other oral anti-diabetic drugs among the enrolled COVID-19 patients with pre-existing type 2 diabetes. Furthermore, we did not observe substantial side effects such as uncontrolled glycemia or acidosis due to DPP4i application compared to those due to non-DPP4i agents in the study cohort. These findings support the continuous use of DPP4i drugs among inpatients infected with SARS-CoV-2, which is consistent with the recently published practical recommendation for the management of diabetes in patients with COVID-19[8].
Although the effects of DPP4 are not yet fully understood, this protein has been reported to play a major role in reducing insulin secretion and increasing glucose levels by degrading incretins (e.g., glucagon-like peptide and glucose-dependent insulinotropic polypeptide)[3]. DPP4 is also involved in modulating pro-inflammatory cytokines, chemokines, and immune cell composition[4]. Furthermore, DPP4 was identified as a functional receptor for the spike protein of MERS-CoV for host-cell entry. Administration of neutralizing monoclonal antibodies directed against DPP4 binding to the MERS-CoV spike protein could protect mice against MERS-CoV infection[6]. The extensive function and ubiquitous expression of DPP4 in multiple tissues, including the respiratory tract, raise the prospect of DPP4-targeted treatment to inhibit SARS-CoV-2 infection[20]. However, there has been no direct clinical evidence illuminating the associations between the use of DPP4i and COVID-19 outcomes. In our study, after rigorous matching and adjustments, the clinical evidence did not support that in-hospital DPP4i use led to any meaningful improvements or risks in terms of COVID-19-related complications or mortality. We also did not observe any significant associations of DPP4i usage with substantial adverse outcomes compared to the use of non-DPP4i anti-diabetic agents among patients with COVID-19 and type 2 diabetes.
DPP4 mediates glycemia and inflammation both through catalytic and noncatalytic capacities, but DPP4 inhibitors mainly selectively inhibit the catalytic activity of the soluble moiety of DPP4[3]. Extensive preclinical and clinical studies have demonstrated that DPP4i drugs could reduce DPP4 activity by more than 50% within 24 h[21]. However, the major effects of DPP4i agents on the inflammatory response lack consistent clinical evidence. While a pilot study showed that sitagliptin slightly reduced the level of CXCL10 in HIV subjects[22], clinical data from another larger-scale study suggested that sitagliptin and vildagliptin did not significantly improve plasma inflammatory biomarker levels, such as IL-6, CRP, TNF, or CD4 and CD8, among patients with diabetes[23-25]. Letko et al[26] showed that a chimeric SARS-CoV-2 spike protein was capable of entering cells expressing ACE2 but not DPP4, which might explain the negligible influence of DPP4i agents on SARS-CoV-2 infection or COVID-19 progression observed in our study.
The safety of DPP4i agents is not a major concern since they have been clinically applied for diabetes management for more than 13 years. A population-based cohort study, including 22435 subjects treated with DPP4i agents and 188614 participants taking other noninsulin glucose-lowering agents, found no increased risk of infection or pneumonia with DPP4i drugs[27]. Furthermore, large-scale, randomized, double-blind clinical trials indicated that sitagliptin, saxagliptin, alogliptin, and linagliptin did not lead to increased risks of infections, immunity, inflammatory disorders, cardiovascular events, hypoglycemia, body weight gain, or mortality among individuals with diabetes and those at risk for cardiovascular or renal diseases[28-31]. However, the function and adverse effects of DPP4i drugs in diabetic patients infected by SARS-CoV-2 are largely unknown. In the setting of COVID-19, our data showed that in-hospital DPP4i use was not correlated with the clinical severity of COVID-19 or the adverse effects of anti-diabetic agents and thus might be considered a relatively safe option for managing patients infected by SARS-CoV-2 with pre-existing type 2 diabetes. Our study thus paved the way for large-scale prospective studies and randomized clinical trials to further clarify the safety and efficiency of DPP4i agents when treating individuals with diabetes and COVID-19.
There are several limitations in our study. First, the data in our study were collected from hospitalized patients, and thus, the associations of DPP4i drugs with COVID-19-related adverse outcomes might not be fully applicable for outpatients with diabetes or for ethnically or geographically diverse populations. Second, the prehospitalization data regarding the use of hypoglycemic agents were not completely retrieved because of the retrospective nature of the study and the urgent circumstance of the COVID-19 epidemic. Third, the sample size of DPP4i users was relatively small in our study, and thus, the associations of different DPP4 inhibitors (gliptins) could not be comparatively evaluated. Thus, large-scale prospective cohort studies and randomized clinical trials with ethnically and geographically diverse cohorts are needed to better understand the effects of DPP4i agents on mortality, glycemic control, and side effects among COVID-19 individuals.
CONCLUSION
In conclusion, among hospitalized patients with COVID-19 and pre-existing type 2 diabetes, DPP4i use is not significantly associated with increased risks of 28-d all-cause mortality or multiorgan injury related to COVID-19. DPP4i agents do not show adverse effects such as uncontrolled glycemia or inflammatory responses compared to non-DPP4i oral glucose-lowering agents among patients with COVID-19 and diabetes.
ARTICLE HIGHLIGHTS
Research background
Diabetes is one of the most important comorbidities linked to the remarkably increased severity and mortality of coronavirus disease 2019 (COVID-19). Our most recent retrospective study based on one of the largest scale patient cohorts has demonstrated that glucose control is the key point to diminish COVID-19 mortality for patients with co-existing diabetes. Dipeptidyl peptidase 4 (DPP4) is commonly targeted for glucose lowering and has outstanding roles in inflammatory and immune regulation. As the well-established Middle East respiratory syndrome coronavirus cellular receptor, DPP4 was predicted to have a tight interaction with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike glycoprotein in the recent molecular modeling study.
Research motivation
The latest observations sparked intense debates about whether DPP4 inhibitors might be promising therapeutic agents against COVID-19 among patients with diabetes. However, there is no direct clinical evidence supporting the associations between DPP4i usage and COVID-19 prognosis so far.
Research objectives
To investigate the potential impacts of DPP4i on COVID-19 among patients with diabetes, and to clarify whether continuation of DPP4i for diabetes management among patients in the setting of COVID-19 is an appropriate option.
Research methods
We enrolled 15451 confirmed COVID-19 cases, including 2563 inpatients with type 2 diabetes from 16 hospitals in Hubei Province, between December 30, 2019 and April 23, 2020. After excluding those ineligible individuals, 142 patients receiving DPP4i and 1115 cases receiving non-DPP4i oral anti-diabetic were included in the subsequent analysis. Then we performed a strict propensity score matching (PSM) analysis where age, sex, comorbidities, number of oral hypoglycemic agents, heart rate, blood pressure, pulse oxygen saturation (SpO2) < 95%, CT diagnosed bilateral lung lesions, laboratory indicators, and proportion of insulin usage were matched. Finally, 111 participants treated with DPP4i were successfully matched with 333 non-DPP4i users. Then logistics linear model and mixed-effect Cox model were applied to analyze the associations between in-hospital use of DPP4i and adverse outcomes of COVID-19.
Research results
We found that there was no significant association between patients taking DPP4i vs other oral hypoglycemic drugs regarding the incidence or risk of 28-d all-cause death (adjusted hazard ratio = 0.44, 95%CI: 0.09-2.11, P = 0.31). Likewise, the incidences and risks of secondary outcomes including septic shock, ARDS, or acute organ (kidney, liver, and cardiac) injuries were also comparable between the DPP4i and non-DPP4i groups. And the performances of DPP4i on glucose control (e.g., the median level of fasting blood glucose and random blood glucose) and inflammatory regulation were all kept approximately equivalent in the DPP4i vs non-DPP4i group. Furthermore, we did not observe substantial side effects on uncontrolled glycaemia or acidosis due to DPP4i application compared to non-DPP4i agents in the study cohort.
Research conclusions
These data demonstrated that there are no significant correlations between DPP4i treatment and adverse clinical outcomes among COVID-19 patients with diabetes, and DPP4i does not show any other significant adverse effects on anti-diabetic treatment. Thus, DPP4i might be a relative safe option for managing pro-existing type 2 diabetes patients infected by SARS-CoV-2, which may provide certain reference value for clinical decision-making. Our study also paves the way for perspective studies and randomized controlled clinical trials on DPP4i therapy for COVID-19.
Research perspectives
Large-scale prospective cohort studies and randomized clinical trials with ethnically and geographically diverse cohorts are needed to better understand the effects of DPP4i agents on mortality, glycemic control, and side effects among COVID-19 individuals.
Footnotes
Institutional review board statement: The study was reviewed and approved by the Science and Research Office of Renmin Hospital and Zhongnan Hospital of Wuhan University (Wuhan).
Informed consent statement: Informed consent was waived by the ethics boards of the involved hospitals.
Conflict-of-interest statement: There are no conflicts of interest to report.
STROBE statement: The authors have read the STROBE Statement-checklist of items, and the manuscript was prepared and revised according to the STROBE Statement-checklist of items.
Manuscript source: Unsolicited manuscript
Peer-review started: July 6, 2020
First decision: August 8, 2020
Article in press: November 26, 2020
Specialty type: Medical informatics
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Bansal A, Singh R S-Editor: Ma YJ L-Editor: Wang TQ P-Editor: Zhang YL
Contributor Information
Jiang-Hua Zhou, Department of Cardiology, Renmin Hospital of Wuhan University, Basic Medical School, Wuhan University, Wuhan 430071, Hubei Province, China.
Bin Wu, Department of Cardiology, Renmin Hospital of Wuhan University, Basic Medical School, Wuhan University, Wuhan 430071, Hubei Province, China.
Wen-Xin Wang, Department of Cardiology, Renmin Hospital of Wuhan University, Basic Medical School, Wuhan University, Wuhan 430071, Hubei Province, China.
Fang Lei, Basic Medical School, Institute of Model Animal, Wuhan University, Wuhan 430071, Hubei Province, China.
Xu Cheng, Department of Cardiology, Renmin Hospital of Wuhan University, Basic Medical School, Wuhan University, Wuhan 430071, Hubei Province, China.
Juan-Juan Qin, Department of Cardiology, Renmin Hospital of Wuhan University, Basic Medical School, Wuhan University, Wuhan 430071, Hubei Province, China.
Jing-Jing Cai, Department of Cardiology, the 3rd Xiangya Hospital, Central South University, Changsha 410013, Hunan Province, China.
Xiao-Jing Zhang, Basic Medical School, Institute of Model Animal, Wuhan University, Wuhan 430071, Hubei Province, China.
Feng Zhou, Medical Science Research Center, Zhongnan Hospital of Wuhan University, Institute of Model Animal, Wuhan University, Wuhan 430072, Hubei Province, China.
Ye-Mao Liu, Department of Cardiology, Renmin Hospital of Wuhan University, Basic Medical School, Wuhan University, Wuhan 430071, Hubei Province, China.
Hao-Miao Li, Department of Cardiology, Renmin Hospital of Wuhan University, Basic Medical School, Wuhan University, Wuhan 430071, Hubei Province, China.
Li-Hua Zhu, Department of Cardiology, Renmin Hospital of Wuhan University; Institute of Model Animal of Wuhan University, Wuhan 430071, Hubei Province, China.
Zhi-Gang She, Department of Cardiology, Renmin Hospital of Wuhan University; Institute of Model Animal of Wuhan University, Wuhan 430071, Hubei Province, China.
Xin Zhang, Institute of Model Animal, Wuhan University, Department of Gastroenterology, Wuhan Third Hospital, Tongren Hospital of Wuhan University, Wuhan 430072, Hubei Province, China.
Juan Yang, Department of Cardiology, Renmin Hospital of Wuhan University; Institute of Model Animal of Wuhan University, Wuhan 430071, Hubei Province, China.
Hong-Liang Li, Department of Cardiology, Renmin Hospital of Wuhan University; Institute of Model Animal of Wuhan University, Wuhan 430071, Hubei Province, China. lihl@whu.edu.cn.
Data sharing statement
No additional data are available.
References
- 1.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu L, She ZG, Cheng X, Qin JJ, Zhang XJ, Cai J, Lei F, Wang H, Xie J, Wang W, Li H, Zhang P, Song X, Chen X, Xiang M, Zhang C, Bai L, Xiang D, Chen MM, Liu Y, Yan Y, Liu M, Mao W, Zou J, Liu L, Chen G, Luo P, Xiao B, Zhang C, Zhang Z, Lu Z, Wang J, Lu H, Xia X, Wang D, Liao X, Peng G, Ye P, Yang J, Yuan Y, Huang X, Guo J, Zhang BH, Li H. Association of Blood Glucose Control and Outcomes in Patients with COVID-19 and Pre-existing Type 2 Diabetes. Cell Metab 2020; 31: 1068-1077. :e3. doi: 10.1016/j.cmet.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tahrani AA, Barnett AH, Bailey CJ. Pharmacology and therapeutic implications of current drugs for type 2 diabetes mellitus. Nat Rev Endocrinol. 2016;12:566–592. doi: 10.1038/nrendo.2016.86. [DOI] [PubMed] [Google Scholar]
- 4.Klemann C, Wagner L, Stephan M, von Hörsten S. Cut to the chase: a review of CD26/dipeptidyl peptidase-4's (DPP4) entanglement in the immune system. Clin Exp Immunol. 2016;185:1–21. doi: 10.1111/cei.12781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montvida O, Shaw J, Atherton JJ, Stringer F, Paul SK. Long-term Trends in Antidiabetes Drug Usage in the U.S.: Real-world Evidence in Patients Newly Diagnosed With Type 2 Diabetes. Diabetes Care. 2018;41:69–78. doi: 10.2337/dc17-1414. [DOI] [PubMed] [Google Scholar]
- 6.Raj VS, Mou H, Smits SL, Dekkers DH, Müller MA, Dijkman R, Muth D, Demmers JA, Zaki A, Fouchier RA, Thiel V, Drosten C, Rottier PJ, Osterhaus AD, Bosch BJ, Haagmans BL. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature. 2013;495:251–254. doi: 10.1038/nature12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vankadari N, Wilce JA. Emerging WuHan (COVID-19) coronavirus: glycan shield and structure prediction of spike glycoprotein and its interaction with human CD26. Emerg Microbes Infect. 2020;9:601–604. doi: 10.1080/22221751.2020.1739565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bornstein SR, Rubino F, Khunti K, Mingrone G, Hopkins D, Birkenfeld AL, Boehm B, Amiel S, Holt RI, Skyler JS, DeVries JH, Renard E, Eckel RH, Zimmet P, Alberti KG, Vidal J, Geloneze B, Chan JC, Ji L, Ludwig B. Practical recommendations for the management of diabetes in patients with COVID-19. Lancet Diabetes Endocrinol. 2020;8:546–550. doi: 10.1016/S2213-8587(20)30152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bassendine MF, Bridge SH, McCaughan GW, Gorrell MD. COVID-19 and comorbidities: A role for dipeptidyl peptidase 4 (DPP4) in disease severity? J Diabetes. 2020;12:649–658. doi: 10.1111/1753-0407.13052. [DOI] [PubMed] [Google Scholar]
- 10.Strollo R, Pozzilli P. DPP4 inhibition: Preventing SARS-CoV-2 infection and/or progression of COVID-19? Diabetes Metab Res Rev. 2020:e3330. doi: 10.1002/dmrr.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.NHCo China. New Coronavirus Pneumonia Prevention and Control Program. 2020. [Cited 29 June 2020] Available from: http://www.nhc.gov.cn/jkj/s3577/202002/a5d6f7b8c48c451c87dba14889b30147.shtml .
- 12.World Health Organization. Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases Interim guidance. 2020. [Cited 29 June 2020] Available from: https://www.who.int/publications/i/item/Laboratory-testing-for-2019-novel-coronavirus-in-suspected-human-cases-20200117 .
- 13.World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected: interim guidance, 28 January 2020. [Cited 29 June 2020] Available from: https://apps.who.int/iris/handle/10665/330893 .
- 14.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179–c184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 15.Marrone G, Vaccaro FG, Biolato M, Miele L, Liguori A, Araneo C, Ponziani FR, Mores N, Gasbarrini A, Grieco A. Drug-induced liver injury 2017: the diagnosis is not easy but always to keep in mind. Eur Rev Med Pharmacol Sci. 2017;21:122–134. [PubMed] [Google Scholar]
- 16.American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019;42:S13–S28. doi: 10.2337/dc19-S002. [DOI] [PubMed] [Google Scholar]
- 17.Freeman J. Management of hypoglycemia in older adults with type 2 diabetes. Postgrad Med. 2019;131:241–250. doi: 10.1080/00325481.2019.1578590. [DOI] [PubMed] [Google Scholar]
- 18.Donnan K, Segar L. SGLT2 inhibitors and metformin: Dual antihyperglycemic therapy and the risk of metabolic acidosis in type 2 diabetes. Eur J Pharmacol. 2019;846:23–29. doi: 10.1016/j.ejphar.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Min SH, Yoon JH, Hahn S, Cho YM. Comparison between SGLT2 inhibitors and DPP4 inhibitors added to insulin therapy in type 2 diabetes: a systematic review with indirect comparison meta-analysis. Diabetes Metab Res Rev. 2017;33 doi: 10.1002/dmrr.2818. [DOI] [PubMed] [Google Scholar]
- 20.Qi F, Qian S, Zhang S, Zhang Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem Biophys Res Commun. 2020;526:135–140. doi: 10.1016/j.bbrc.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bergman AJ, Stevens C, Zhou Y, Yi B, Laethem M, De Smet M, Snyder K, Hilliard D, Tanaka W, Zeng W, Tanen M, Wang AQ, Chen L, Winchell G, Davies MJ, Ramael S, Wagner JA, Herman GA. Pharmacokinetic and pharmacodynamic properties of multiple oral doses of sitagliptin, a dipeptidyl peptidase-IV inhibitor: a double-blind, randomized, placebo-controlled study in healthy male volunteers. Clin Ther. 2006;28:55–72. doi: 10.1016/j.clinthera.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 22.Goodwin SR, Reeds DN, Royal M, Struthers H, Laciny E, Yarasheski KE. Dipeptidyl peptidase IV inhibition does not adversely affect immune or virological status in HIV infected men and women: a pilot safety study. J Clin Endocrinol Metab. 2013;98:743–751. doi: 10.1210/jc.2012-3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dubé MP, Chan ES, Lake JE, Williams B, Kinslow J, Landay A, Coombs RW, Floris-Moore M, Ribaudo HJ, Yarasheski KE. A Randomized, Double-blinded, Placebo-controlled Trial of Sitagliptin for Reducing Inflammation and Immune Activation in Treated and Suppressed Human Immunodeficiency Virus Infection. Clin Infect Dis. 2019;69:1165–1172. doi: 10.1093/cid/ciy1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sromova L, Busek P, Posova H, Potockova J, Skrha P, Andel M, Sedo A. The effect of dipeptidyl peptidase-IV inhibition on circulating T cell subpopulations in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2016;118:183–192. doi: 10.1016/j.diabres.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 25.van Poppel PC, Gresnigt MS, Smits P, Netea MG, Tack CJ. The dipeptidyl peptidase-4 inhibitor vildagliptin does not affect ex vivo cytokine response and lymphocyte function in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2014;103:395–401. doi: 10.1016/j.diabres.2013.12.039. [DOI] [PubMed] [Google Scholar]
- 26.Letko M, Marzi A, Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol. 2020;5:562–569. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wvan der Zanden R, de Vries F, Lalmohamed A, Driessen JH, de Boer A, Rohde G, Neef C, den Heijer C. Use of Dipeptidyl-Peptidase-4 Inhibitors and the Risk of Pneumonia: A Population-Based Cohort Study. PLoS One. 2015;10:e0139367. doi: 10.1371/journal.pone.0139367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, Ohman P, Frederich R, Wiviott SD, Hoffman EB, Cavender MA, Udell JA, Desai NR, Mosenzon O, McGuire DK, Ray KK, Leiter LA, Raz I SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–1326. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 29.Heller SR, Bergenstal RM, White WB, Kupfer S, Bakris GL, Cushman WC, Mehta CR, Nissen SE, Wilson CA, Zannad F, Liu Y, Gourlie NM, Cannon CP EXAMINE Investigators. Relationship of glycated haemoglobin and reported hypoglycaemia to cardiovascular outcomes in patients with type 2 diabetes and recent acute coronary syndrome events: The EXAMINE trial. Diabetes Obes Metab. 2017;19:664–671. doi: 10.1111/dom.12871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, Garg J, Josse R, Kaufman KD, Koglin J, Korn S, Lachin JM, McGuire DK, Pencina MJ, Standl E, Stein PP, Suryawanshi S, Van de Werf F, Peterson ED, Holman RR TECOS Study Group. Effect of Sitagliptin on Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2015;373:232–242. doi: 10.1056/NEJMoa1501352. [DOI] [PubMed] [Google Scholar]
- 31.Rosenstock J, Kahn SE, Johansen OE, Zinman B, Espeland MA, Woerle HJ, Pfarr E, Keller A, Mattheus M, Baanstra D, Meinicke T, George JT, von Eynatten M, McGuire DK, Marx N CAROLINA Investigators. Effect of Linagliptin vs Glimepiride on Major Adverse Cardiovascular Outcomes in Patients With Type 2 Diabetes: The CAROLINA Randomized Clinical Trial. JAMA. : 2019. doi: 10.1001/jama.2019.13772. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.