Abstract
BACKGROUND
A type 2b immunoglobulin G4 (IgG4)-related sclerosing cholangitis (SC) without autoimmune pancreatitis is a rare condition with IgG4-SC. While the variety of the imaging modalities have tested its usefulness in diagnosing the IgG4-SC, however, the usage of ultrasonography for the assessment of the response to steroidal therapy on the changes of bile duct wall thickness have not been reported in the condition. Therefore, the information of our recent case and reported cases have been summarized.
CASE SUMMARY
We report the case of an 82-year-old Japanese man diagnosed with isolated IgG4-related SC based on the increase of serum IgG4, narrowing of the bile duct, its wall thickness, no complication of autoimmune pancreatitis, and IgG4 positive inflammatory cell infiltration to the wall with the fibrotic changes. The cholangiogram revealed type 2b according to the classification. Corticosteroid treatment showed a favorable effect, with the smooth decrease in serum IgG4 and the improvement of the bile duct wall thickness.
CONCLUSION
As isolated type 2b, IgG4-SC is rare, the images, histological findings, and clinical course of our case will be helpful for physicians to diagnose and treat the new cases appropriately.
Keywords: Immunoglobulin G4-related sclerosing cholangitis, Type 2b, Corticosteroid, Autoimmune pancreatitis, Ultrasonography, Imaging, Case report
Core Tip: Immunoglobulin G4-associated sclerosing cholangitis (IgG4-SC) is a biliary tract lesion of the systemic IgG4-related disease. It is often complicated with autoimmune pancreatitis, and if not related, it is diagnosed as an isolated IgG4-SC. The corticosteroid treatment shows the favorable response by improving the infiltration of inflammatory cells, wall thickness, and decrease the serum IgG4. With a variety of the types of IgG4-SC determined by cholangiogram, differential diagnosis from the malignancy and primary sclerosing cholangitis is essential. In the present study, we report a rare elderly and asymptomatic case of type 2b, isolated IgG4-SC who was differentially diagnosed from primary sclerosing cholangitis by liver biopsy. The administration of corticosteroid improved the inflammation, normalized the serum IgG4 Level, and improved the wall thickness of the bile duct. As isolated type 2b, IgG4-SC is rare, the images, histological findings, and clinical course of our case will be helpful for physicians to diagnose and treat the new cases appropriately.
INTRODUCTION
Immunoglobulin G4-related sclerosing cholangitis (IgG4-SC) is a biliary tract lesion of the systemic IgG4-reltaed disease involving the peri-orbital tissues, salivary glands, meninges, lymph nodes, thyroid glands, lungs, aorta, retroperitoneum, kidney, pancreas, biliary tree, gall bladder, liver, and prostate gland[1-3]. IgG4-related disease is characterized by an increase in the serum IgG4 Level, infiltration of the lymphoplasmacytes, and IgG4-bearing plasma cells, positive response to corticosteroid therapy, and involvement of multiple organs showing inflammation with diffuse or localized swelling[2,3]. IgG4-SC shows above-listed characteristics and reveals cholangitis due to the thickening of the bile duct wall and fibrosis caused by the infiltration of IgG4-positive plasma cells in the wall and often complicated with autoimmune pancreatitis[1,2,4-7]. According to the diagnostic criteria[5,8] and the images of the cholangiogram[9], diagnosis and the classification of the types can be done and when not complicated with the autoimmune pancreatitis, it is diagnosed with isolated IgG4-SC[10]. Computed tomography (CT), magnetic resonance imaging, magnetic resonance cholangiopancreatography (MRCP), abdominal ultrasonography (US), endoscopic ultrasonography (EUS), and endoscopic retrograde cholangio-pancreatography (ERCP) are the imaging modalities useful for the diagnosis[11]. However, as the imaging studies reveal thickening and stenosis of the biliary duct wall, differentiating the condition from the malignancy and other sclerosing cholangitis, including bile duct cancer and primary sclerosing cholangitis (PSC) are essential[12-14]. For this differentiation, histological analyses are important. The determination of a dense lymphoplasmacytic infiltrate rich in IgG4-positive cells with fibroinflammatory changes are characteristic of this disease entity, and no detection of malignant cells[15] or onion-skin fibrosis in the liver tissue which is the characteristic findings of PSC are helpful for the differentiation[16]. Response to corticosteroid (CS) treatment, with improvement of symptoms, decrease of serum IgG4 and hepatobiliary enzymes, bile duct wall thickness, and bile duct stenosis are diagnostic option[8]. Here, we report the case of an elderly and asymptomatic patient with type 2b isolated IgG4-SC. He was differentially diagnosed from PSC by liver biopsy, and the administration of CS improved the inflammation, normalized the serum IgG4 Level, and improved the wall thickening of the bile duct. A discussion of the case presented here is intended to promote wider recognition and better understanding of this pathology.
CASE PRESENTATION
Chief complaints
An 80-year-old Japanese man was referred to our hospital for further exploration of the increase in biliary enzymes, immunoglobulins, bile duct wall thickening, and bile duct dilation.
History of past illness
He had a history of cerebral infarction and antihypertensive treatment, but no history or symptoms of inflammatory bowel disease and family history were remarkable.
Physical examination
He showed no remarkable signs and symptoms, and physical examination upon admission revealed no jaundice or lymphadenopathy and no abnormal findings on his abdomen.
Laboratory and imaging examinations
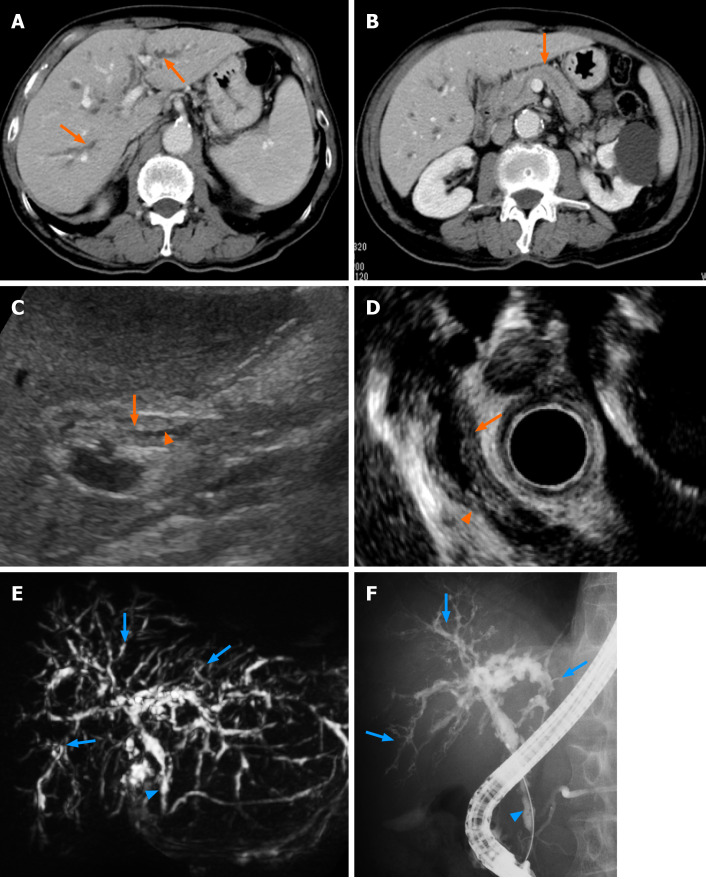
Laboratory results on the day of admission revealed an increase in the serum levels of aspartate aminotransferase (75 IU/L), alanine aminotransferase (77 IU/L), alkaline phosphatase (1060 IU/L), γ-glutamyl transpeptidase (1160 IU/L), and immunoglobulin G (1850 mg/dL) and its subtype 4 (255 mg/dL). No increase in bilirubin and tumor markers was seen (Table 1). CT scans revealed intrahepatic bile duct dilatation, bile duct wall thickening with a mild enhance effect were noted (Figure 1A). No changes in the pancreatic ducts or parenchymal structure were seen (Figure 1B). Abdominal US revealed the wall thickening of the common bile duct with narrowing of its diameter (Figure 1C). EUS also revealed the thickened bile duct wall and narrowing of its diameter with no significant irregularity of the epithelial surface (Figure 1D). The changes of the bile duct were confirmed with a MRCP showing the stenotic lesions in relatively in the proximal side including the lower common bile duct (Figure 1E). ERCP revealed multiple stenoses with long stricter in the lower bile duct and only a partial dilatation of the bile duct (Figure 1F). There were no abnormal findings at the pancreatic duct (Figure 1E and F). Following the ERCP, a biopsy of the lower bile duct was performed, showing the long stenotic lesion (Figure 1E and F, blue arrowheads) and the endoscopic nasobiliary drainage tube was placed for drainage.
Table 1.
Results of laboratory investigation
|
Hematology
|
Biochemistry
|
||||
| WBC | 6260 × 106/μL | TP | 7.9 g/dL | IgG | 1850 mg/dL |
| Neutro | 49.3% | Alb | 3.6 g/dL | IgA | 457 mg/dL |
| Lymp | 34.0% | BUN | 18 mg/dL | IgM | 42 mg/dL |
| Eos. | 9.4% | Cre | 1.1 mg/dL | IgG4 | 255 mg/dL |
| Bas. | 0.5% | T-Bil | 0.9 mg/dL | CEA | 1.9 ng/mL |
| Mon. | 6.8% | D-Bil | 0.2 mg/dL | CA19-9 | 59 IU/mL |
| RBC | 453 × 104 /μL | AST | 75 IU/L | ||
| Hb | 13.7 g/dL | ALT | 77 IU/L | ||
| Ht. | 40.5% | ALP | 1060 IU/L | ||
| Plt. | 11.7 × 104 /μL | LDH | 222 IU/L | ||
| γ-GTP | 1160 IU/L | ||||
| ChE | 167 IU/L | ||||
| Na | 140 mEq/L | ||||
| K | 4.2 mEq/L | ||||
| Cl | 106 mEq/L | ||||
| Coagulation | P | 3.7 mg/dL | |||
| PT% | 113% | Ca | 9.4 mg/dL | ||
| PT-INR | 0.93 | CRP | 3.9 mg/dL | ||
| APTT | 28.4 s | FBS | 90 mg/dL | ||
| HbA1c | 5.7% | ||||
| TG | 90 mg/dL | ||||
| HDL-C | 66 mg/dL | ||||
| LDL-C | 79 mg/dL | ||||
WBC: White blood cell; RBC: Red blood cell; Hb: Hemoglobinopathy; Plt: Platelets; PT: Prothrombin time; APTT: Activated partial thromboplastin time; TP: Total Protein; BUN: Blood Urea Nitrogen; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; ALP: Alkaline phosphatase; LDH: Lactate dehydrogenase; GTP: Guanosine triphosphate; CRP: C-reactive protein; FBS: Fasting blood sugar; HbA1c: Hemoglobin A1c; TG: Triglyceride; HDL-C: High-density lipoproteincholesterol; LDL-C: Low-density lipoprotein cholesterol; IgG: Immunoglobulin G; CA19-9: Carbohydrate antigen 19-9.
Figure 1.
Contrast-enhanced computed tomography showed stricture and mild dilatation of intrahepatic bile ducts and its wall thickening with an enhance effect (A, orange arrows). No significant swelling of the pancreas and the dilatation of main pancreatic duct were observed (B, orange arrow); C: Abdominal ultrasonography and D: Endoscopic ultrasonography showed thickening of the bile duct wall (C and D, orange arrows) and stenosis of the bile duct (C and D, orange arrowheads) in the lower bile duct. Magnetic resonance cholangiopancreatography and endoscopic retrograde cholangiopancreatography revealed stenosis of the lower bile duct (E and F, blue arrowheads) and intrahepatic bile ducts (E and F, blue arrows).
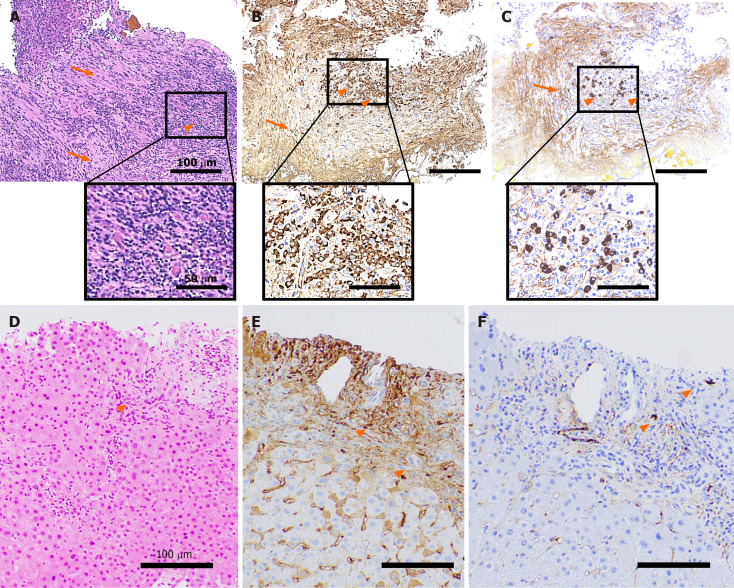
Histological analyses revealed the infiltration of inflammatory cells and severe storiform fibrosis in the bile duct tissue (Figure 2A). These inflammatory cells included the IgG (Figure 2B) and IgG4 (Figure 2C) positively-stained plasma cells (more than 10 cells per high power field) and the IgG4/IgG-positive cell ratio was approximately 30% (Figure 2B and C). To differentially diagnose PSC, liver biopsy was performed. The tissue showed mild infiltration of inflammatory cells (Figure 2D) partly stained with IgG (Figure 2E) and IgG4 (Figure 2F), but no onion-skin fibrosis was seen.
Figure 2.
Histopathological findings. A tissue sample was collected from the stenotic lower bile duct and stained with hematoxylin and eosin staining (A), IgG (B), IgG4 (C). Marked infiltration of the inflammatory cells (A-C, orange arrowheads) and storiform fibrosis (A-C, orange arrows) were observed. An increase in the number of IgG- (B) and IgG4-positive cells (C) was noted. Liver tissue showed infiltration of inflammatory cells (D: hematoxylin-eosin staining; E: IgG; F: IgG4, orange arrowheads) partly positive for IgG (E) and IgG4 (F). The scale bars represent 100 µm and 50 µm in the insets.
FINAL DIAGNOSIS
On the basis of these findings, a diagnosis of IgG4-related sclerosing cholangitis of the subtypes of type 2b was made.
TREATMENT
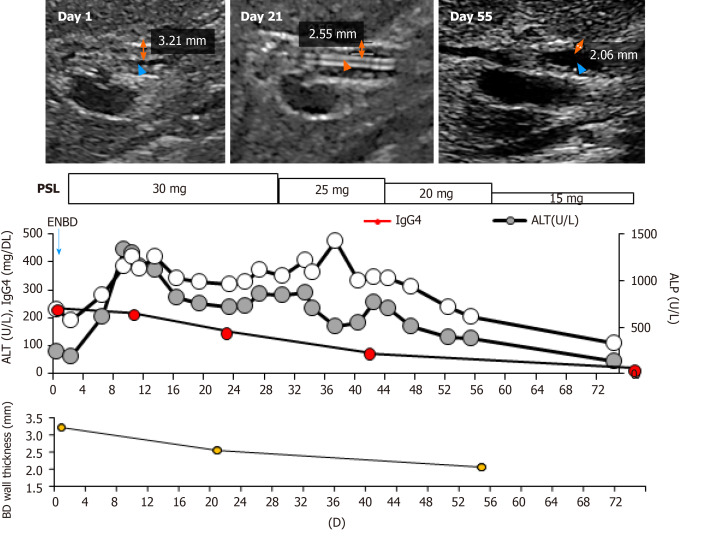
Following the diagnosis, oral CS therapy at a dose of 0.6 mg/kg/day, amounting to 30 mg/day, was initiated (Figure 3). The daily dose was tapered to 25 mg after 4 wk of 30 mg administration, then to 20 mg for the next 2 wk, and tapered by 5 mg every 2 wk thereafter.
Figure 3.
Clinical course. The orange two-direction arrows indicate the wall thickness determined by abdominal ultrasonography. Orange arrowheads indicate the bile duct. The blue arrowhead indicates the endoscopic nasobiliary drainage tube. BD: Bile duct; PSL: Prednisolone; ENBD: Endoscopic nasobiliary drainage; IgG4: Immunoglobulin G4; ALT: Alanine aminotransferase; ALP: Alkaline phosphatase.
OUTCOME AND FOLLOW-UP
The bile duct wall thickness improved by day 21 and the endoscopic nasobiliary drainage tube was removed. The thickening further recovered by day 55. The serum IgG4 concentrations gradually decreased to 81.1 mg/dL within 8 wk and the hepatobiliary enzymes also returned to the normal range (Figure 3). No relapse of the disease has been observed since then.
DISCUSSION
SC is classified as either PSC, IgG4-SC, or secondary SC. Among them, IgG4-SC is considered to be a biliary lesion of a systemic IgG4-related disease[1,2,8]. It is characterized by an increase in serum levels of IgG4, infiltration of IgG4 inflammatory cells in the bile duct wall which induce fibrosis and obstructive phlebitis[1]. Various clinical diagnosis criteria including the HISORt criteria[5] and the one established in Japan[8] have been used to date. The HISORt criteria were originally established for acute interstitial pneumonia (AIP), with which most of the IgG4-SC cases are complicated. Approximately 10% of IgG4-SC cases that are not associated with AIP are described as “isolated IgG4-SC”[4,11]. For these cases, the Japanese clinical diagnostic criteria 2012 for IgG4-SC[8] are useful for its diagnoses and determination of the therapeutic options[11]. As our case showed no complications of AIP, the case was diagnosed according to the Japanese criteria[8].
For the IgG-SC, the male dominance has been reported and the age distribution of patients revealed that patients in their 60s have the highest risk of developing the disease[4], and our patient was older than that. Although jaundice is the most frequently-encountered symptom (35% of cases)[4] followed by pruritus, abdominal pain, and cholangitis, no specific symptoms have been reported. Remarkably, 28% of the cases were diagnosed without symptoms, like in our case. Serum biochemical assays reflect the activity of the disease, showing the increase of hepatobiliary enzymes, serum IgG, and serum IgG4[4,5,8].
Based on these characteristics, the diagnostic criteria include factors of biliary tract imaging with the segmental narrowing due to the wall thickening, increase of serum IgG4 (> 135 mg/dL), coexisting of AIP or other IgG4-related diseases, characteristic histological findings, and response to CS[8]. For the imaging analyses, abdominal US, contrast-enhanced CT, and magnetic resonance imaging /MRCP have been reported to be useful in detecting wall thickening of the bile duct, dilatation and stricture of the bile duct, and pancreatic involvement. In addition to these findings, EUS is useful in detecting the smooth epithelial surface reflecting the subepithelial damage by the inflammatory cells compared to that of PSC. ERCP is most useful in diagnosing IgG4-SC with its subtyping[11]. Based on the cholangiogram, the IgG4-SC is classified into: Type 1 which shows stenosis only in the lower bile duct; Type 2, which shows diffusely-distributed stenosis throughout the intra- and extrahepatic bile duct, and can be further sub-classified into 2a, characterized by intrahepatic bile duct with prestenotic dilatation and 2b, characterized by stricture of the intrahepatic bile ducts without prestenotic dilation and fewer bile duct branches[1,8,9,11]; Type 3 which shows stenosis in the hilar hepatic lesions and lower bile duct; and Type 4 which only shows stricture of the bile duct in the hilar hepatic lesions[1,8,9,11]. The frequencies of the various types are 64% (Type 1), 5% (Type 2a), 8% (Type 2b), 10% (Type 3), and 10% (Type 4). The representative characteristics of the imaging modalities and the reports focusing on the usefulness of the modalities have been summarized in Table 2 and Table 3[10,15,17-24]. The histological characteristics include marked infiltration of inflammatory cells and fibrosis associated with the bile duct wall thickening. An increase of IgG4-positive cells in the tissues is characteristic, but not specific, and a careful consideration of the histological findings and clinical information are essential for diagnosis[11]. The histological diagnostic criteria include: Marked inflammation of the lymphoplasmaticytic cells and fibrosis; > 10 IgG4-positive cells per high power field; storiform fibrosis; and obliterative phlebitis. The identification of any three of these factors confirms the diagnosis[8]. Our case exhibited all of these characteristics except for the phlebitis. In addition, our case showed IgG4-positive cells in the liver, which is rarely found in the liver biopsy samples[11]. Based on the available information, our patient was diagnosed with the isolated Type 2b IgG4-SC, a rare type[4] which needs to be differentiated from PSC. For the differential diagnosis between PSC and type 2b IgG4-SC, age at onset, which is typically more advanced in IgG4-SC; elevation of serum IgG4; coexistence of other IgG4-related diseases; longer strictures and stricture in the lower bile duct; inflammation of full layers of bile duct mucosa; abundant IgG4-positive plasma cell infiltration with no periductal onion-skin fibrosis; and response to CS with good prognoses are the key points[11]. Compared to the short strictures of the bile duct encountered in PSC, the stricture is relatively longer in IgG4-SC, and dilation after the stricture with stricture of the lower bile duct are often seen in IgG4-SC. Our case showed an increase in IgG4, long strictures in the lower bile duct, inflammation in the bile duct mucosa, IgG4-positive cell inflammation in the liver with no periductal onion-skin fibrosis, and response to CS (Figure 2) As such, it was successfully differentially diagnosed from PSC.
Table 2.
Representative findings of the imaging modalities for immunoglobulin G4-related sclerosing cholangitis
|
Modality
|
Findings
|
Miscellaneous
|
Ref.
|
| US | Wall thickening of the bile duct, dilatation of intrahepatic bile duct | Not specific, low sensitivity | |
| EUS | High sensitivity | Combined with fine needle aspiration | [17-20] |
| IDUS | High sensitivity and specificity, high-resolution, images of the duct wall | May differentiate from cholangiocarcinoma | |
| CT | Wall thickness, dilatation, the thickened segment shows progressive homogeneous contrast enhancement, with more enhancement seen in the delayed phase | Combined with contrast enhancement for differential diagnosis | |
| MRI/MRCP | Bile duct wall thickening with iso-hypointense signal on T2-weighted image | Assessment of biliary system | |
| PET | Uptake of FDG in bile duct wall | ||
| ERCP | Useful for the classification of the types | Useful for the situations in which an intervention, like stent placement, and biopsy is needed |
US: Transabdominal ultrasonography; EUS: Endoscopic ultrasonography; IDUS: Intraductal ultrasonography; CT: Computed tomography; MRI: Magnetic resonance imaging; MRCP: Magnetic resonance cholangiopancreatography; PET: Positron emission tomography; ERCP: Endoscopic retrograde cholangiopancreatogr.
Table 3.
Usefulness of the imaging modality for immunoglobulin G4-related sclerosing cholangitis
|
Ref.
|
Modality
|
Use
|
Usefulness
|
| Kobori et al[20] | US | Diagnosis follow up | Diagnosis, improvement after PSL |
| Matsumoto et al[15] | EUS | Diagnosis | Differential diagnosis from the cholangiocarcinoma |
| Swensson et al[18] | CT, MRI | Diagnosis | Differential diagnosis from the cholangiocarcinoma |
| Zhang et al[21] | Laboratory exams | Follow up | Response to PSL |
| Ohno et al[22] | Laparoscopy andIDUS | Diagnosis follow up | Symmetric circumferentially thickened wall (IDUS), discoloration with red lobular markings and multiple small depressed lesions |
| Naitoh et al[19] | IDUS, Histology | Diagnosis | Differential diagnosis from the cholangiocarcinoma |
| Graham et al[10] | Histology, radiologic features | Diagnosis | Differential diagnosis from the cholangiocarcinoma |
| Horiguchi et al[23] | IDUS, ERCP, laparoscopy | Diagnosis | Differential diagnosis from the cholangiocarcinoma |
| Shimizu et al[24] | EUS, IDUS, CT, MRCP | Diagnosis follow up | Diagnosis |
| Our case | US, EUS, ERCP, MRCP | Diagnosis, follow up | Diagnosis of isolated, type 2 IgG4-SC and response to PSL |
US: Transabdominal ultrasonography; EUS: Endoscopic ultrasonography; CT: Computed tomography; MRI: Magnetic resonance imaging; IDUS; Intraductal ultrasonography; ERCP: Endoscopic retrograde cholangiopancreatography; PSL: Prednisolone.
As the inflammation is induced via an autoimmune mechanism, the response to CS therapy is also one of the diagnostic criteria for the disease. The validity and safety of endoscopic biliary stenting for the associated biliary stenosis during CS therapy and successful removal one month after the steroid initiation have been reported[25]. On the other hand, Miyazawa et al[26] reported that IgG4-SC-related obstructive jaundice without infection can be treated safely with CS monotherapy without drainage. These reports suggest the sensitivity of this disease to CS among the variety of other cholangitis and cholestatic liver diseases[27]. Oral administration of prednisolone at 0.6 mg/kg/day is recommended for the induction of remission, followed by the gradual reduction to a maintenance dose of 5 mg/day over 2-3 mo, with which our case showed a good clinical course with the doses, and a decrease in serum IgG4, biliary enzymes, and wall thickness of the bile duct (Figure 3). The improvement of the wall thickness was able to be evaluated by the transabdominal US reflecting the response to prednisolone (PSL). As the transabdominal US is non-invasive and the response to PSL is one of the key phenomena differentially diagnosing from the cholangiocarcinoma, the findings presented here demonstrated the usefulness of US[20] (Table 3).
CONCLUSION
In conclusion, we reported the rare case of isolated IgG4-SC of Type 2b in an elderly patient, successfully treated with CS. Our summary demonstrated the usefulness of ultrasonography for the assessment of the response to the therapy.
Footnotes
Informed consent statement: Written informed consent was obtained from the patient for publication of this case report and the accompanying images.
Conflict-of-interest statement: The authors declare that they have no current financial arrangement or affiliation with any organization that may have a direct influence on their work.
CARE Checklist (2016) statement: CARE Checklist (2016) of information was included and the form was attached.
Manuscript source: Invited manuscript
Peer-review started: September 5, 2020
First decision: September 30, 2020
Article in press: October 26, 2020
Specialty type: Medicine, research and experimental
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fan RY, Liu QC S-Editor: Zhang L L-Editor: A P-Editor: Xing YX
Contributor Information
Yuto Tanaka, Division of Gastroenterology and Hepatology, Graduate School of Medical and Dental Sciences, Niigata University, Niigata 9518510, Japan.
Kenya Kamimura, Division of Gastroenterology and Hepatology, Graduate School of Medical and Dental Sciences, Niigata University, Niigata 9518510, Japan. kenya-k@med.niigata-u.ac.jp.
Ryota Nakamura, Division of Gastroenterology and Hepatology, Graduate School of Medical and Dental Sciences, Niigata University, Niigata 9518510, Japan.
Marina Ohkoshi-Yamada, Division of Gastroenterology and Hepatology, Graduate School of Medical and Dental Sciences, Niigata University, Niigata 9518510, Japan.
Yohei Koseki, Division of Gastroenterology and Hepatology, Graduate School of Medical and Dental Sciences, Niigata University, Niigata 9518510, Japan.
Takeshi Mizusawa, Division of Gastroenterology and Hepatology, Graduate School of Medical and Dental Sciences, Niigata University, Niigata 9518510, Japan.
Satoshi Ikarashi, Division of Gastroenterology and Hepatology, Graduate School of Medical and Dental Sciences, Niigata University, Niigata 9518510, Japan.
Kazunao Hayashi, Division of Gastroenterology and Hepatology, Graduate School of Medical and Dental Sciences, Niigata University, Niigata 9518510, Japan.
Hiroki Sato, Division of Gastroenterology and Hepatology, Graduate School of Medical and Dental Sciences, Niigata University, Niigata 9518510, Japan.
Akira Sakamaki, Division of Gastroenterology and Hepatology, Graduate School of Medical and Dental Sciences, Niigata University, Niigata 9518510, Japan.
Junji Yokoyama, Division of Gastroenterology and Hepatology, Graduate School of Medical and Dental Sciences, Niigata University, Niigata 9518510, Japan.
Shuji Terai, Division of Gastroenterology and Hepatology, Graduate School of Medical and Dental Sciences, Niigata University, Niigata 9518510, Japan.
References
- 1.Nakazawa T, Naitoh I, Hayashi K, Okumura F, Miyabe K, Yoshida M, Yamashita H, Ohara H, Joh T. Diagnostic criteria for IgG4-related sclerosing cholangitis based on cholangiographic classification. J Gastroenterol. 2012;47:79–87. doi: 10.1007/s00535-011-0465-z. [DOI] [PubMed] [Google Scholar]
- 2.Kamisawa T, Zen Y, Pillai S, Stone JH. IgG4-related disease. Lancet. 2015;385:1460–1471. doi: 10.1016/S0140-6736(14)60720-0. [DOI] [PubMed] [Google Scholar]
- 3.Inoue D, Yoshida K, Yoneda N, Ozaki K, Matsubara T, Nagai K, Okumura K, Toshima F, Toyama J, Minami T, Matsui O, Gabata T, Zen Y. IgG4-related disease: dataset of 235 consecutive patients. Medicine (Baltimore) 2015;94:e680. doi: 10.1097/MD.0000000000000680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanaka A, Tazuma S, Okazaki K, Nakazawa T, Inui K, Chiba T, Takikawa H. Clinical Features, Response to Treatment, and Outcomes of IgG4-Related Sclerosing Cholangitis. Clin Gastroenterol Hepatol 2017; 15: 920-926. :e3. doi: 10.1016/j.cgh.2016.12.038. [DOI] [PubMed] [Google Scholar]
- 5.Ghazale A, Chari ST, Zhang L, Smyrk TC, Takahashi N, Levy MJ, Topazian MD, Clain JE, Pearson RK, Petersen BT, Vege SS, Lindor K, Farnell MB. Immunoglobulin G4-associated cholangitis: clinical profile and response to therapy. Gastroenterology. 2008;134:706–715. doi: 10.1053/j.gastro.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 6.Tanaka A, Tazuma S, Okazaki K, Tsubouchi H, Inui K, Takikawa H. Nationwide survey for primary sclerosing cholangitis and IgG4-related sclerosing cholangitis in Japan. J Hepatobiliary Pancreat Sci. 2014;21:43–50. doi: 10.1002/jhbp.50. [DOI] [PubMed] [Google Scholar]
- 7.Masamune A, Kikuta K, Hamada S, Tsuji I, Takeyama Y, Shimosegawa T, Okazaki K Collaborators. Nationwide epidemiological survey of autoimmune pancreatitis in Japan in 2016. J Gastroenterol. 2020;55:462–470. doi: 10.1007/s00535-019-01658-7. [DOI] [PubMed] [Google Scholar]
- 8.Ohara H, Okazaki K, Tsubouchi H, Inui K, Kawa S, Kamisawa T, Tazuma S, Uchida K, Hirano K, Yoshida H, Nishino T, Ko SB, Mizuno N, Hamano H, Kanno A, Notohara K, Hasebe O, Nakazawa T, Nakanuma Y, Takikawa H Research Committee of IgG4-related Diseases; Research Committee of Intractable Diseases of Liver and Biliary Tract; Ministry of Health; Labor and Welfare; Japan; Japan Biliary Association. Clinical diagnostic criteria of IgG4-related sclerosing cholangitis 2012. J Hepatobiliary Pancreat Sci. 2012;19:536–542. doi: 10.1007/s00534-012-0521-y. [DOI] [PubMed] [Google Scholar]
- 9.Nakazawa T, Ohara H, Sano H, Ando T, Joh T. Schematic classification of sclerosing cholangitis with autoimmune pancreatitis by cholangiography. Pancreas. 2006;32:229. doi: 10.1097/01.mpa.0000202941.85955.07. [DOI] [PubMed] [Google Scholar]
- 10.Graham RP, Smyrk TC, Chari ST, Takahashi N, Zhang L. Isolated IgG4-related sclerosing cholangitis: a report of 9 cases. Hum Pathol. 2014;45:1722–1729. doi: 10.1016/j.humpath.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Kamisawa T, Nakazawa T, Tazuma S, Zen Y, Tanaka A, Ohara H, Muraki T, Inui K, Inoue D, Nishino T, Naitoh I, Itoi T, Notohara K, Kanno A, Kubota K, Hirano K, Isayama H, Shimizu K, Tsuyuguchi T, Shimosegawa T, Kawa S, Chiba T, Okazaki K, Takikawa H, Kimura W, Unno M, Yoshida M. Clinical practice guidelines for IgG4-related sclerosing cholangitis. J Hepatobiliary Pancreat Sci. 2019;26:9–42. doi: 10.1002/jhbp.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huggett MT, Culver EL, Kumar M, Hurst JM, Rodriguez-Justo M, Chapman MH, Johnson GJ, Pereira SP, Chapman RW, Webster GJM, Barnes E. Type 1 autoimmune pancreatitis and IgG4-related sclerosing cholangitis is associated with extrapancreatic organ failure, malignancy, and mortality in a prospective UK cohort. Am J Gastroenterol. 2014;109:1675–1683. doi: 10.1038/ajg.2014.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zen Y, Kawakami H, Kim JH. IgG4-related sclerosing cholangitis: all we need to know. J Gastroenterol. 2016;51:295–312. doi: 10.1007/s00535-016-1163-7. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka A. IgG4-Related Sclerosing Cholangitis and Primary Sclerosing Cholangitis. Gut Liver. 2019;13:300–307. doi: 10.5009/gnl18085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsumoto R, Miura S, Kanno A, Ikeda M, Sano T, Tanaka Y, Nabeshima T, Hongou S, Takikawa T, Hamada S, Kume K, Kikuta K, Masamune A. IgG4-related Sclerosing Cholangitis Mimicking Cholangiocarcinoma Diagnosed by Endoscopic Ultrasound-guided Fine-needle Aspiration. Intern Med. 2020;59:945–950. doi: 10.2169/internalmedicine.3905-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chapman MH, Thorburn D, Hirschfield GM, Webster GGJ, Rushbrook SM, Alexander G, Collier J, Dyson JK, Jones DE, Patanwala I, Thain C, Walmsley M, Pereira SP. British Society of Gastroenterology and UK-PSC guidelines for the diagnosis and management of primary sclerosing cholangitis. Gut. 2019;68:1356–1378. doi: 10.1136/gutjnl-2018-317993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madhusudhan KS, Das P, Gunjan D, Srivastava DN, Garg PK. IgG4-Related Sclerosing Cholangitis: A Clinical and Imaging Review. AJR Am J Roentgenol. 2019;213:1221–1231. doi: 10.2214/AJR.19.21519. [DOI] [PubMed] [Google Scholar]
- 18.Swensson J, Tirkes T, Tann M, Cui E, Sandrasegaran K. Differentiating IgG4-related sclerosing cholangiopathy from cholangiocarcinoma using CT and MRI: experience from a tertiary referring center. Abdom Radiol (NY) 2019;44:2111–2115. doi: 10.1007/s00261-019-01944-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naitoh I, Nakazawa T, Ohara H, Ando T, Hayashi K, Tanaka H, Okumura F, Takahashi S, Joh T. Endoscopic transpapillary intraductal ultrasonography and biopsy in the diagnosis of IgG4-related sclerosing cholangitis. J Gastroenterol. 2009;44:1147–1155. doi: 10.1007/s00535-009-0108-9. [DOI] [PubMed] [Google Scholar]
- 20.Kobori I, Suda T, Nakamoto A, Saito H, Okawa O, Sudo R, Gyotoku Y, Katayama Y, Tamano M. Two cases of immunoglobulin G4-related sclerosing cholangitis in which transabdominal ultrasonography was useful in diagnosis and follow-up observation. J Med Ultrason (2001) 2016;43:271–277. doi: 10.1007/s10396-015-0676-7. [DOI] [PubMed] [Google Scholar]
- 21.Zhang J, Guo X, Li H, Shao X, Deng J, Liang Z, Zhang X, Feng J, Lin H, Qi X. A good response to steroid therapy in IgG4-related sclerosing cholangitis: a case report. Clin Exp Hepatol. 2018;4:205–209. doi: 10.5114/ceh.2018.78126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohno Y, Kumagi T, Imamura Y, Kuroda T, Koizumi M, Watanabe T, Yoshida O, Tokumoto Y, Takeshita E, Abe M, Harada K, Hiasa Y. Usefulness of laparoscopy and intraductal ultrasonography in a patient with isolated immunoglobulin G4-related sclerosing cholangitis. Clin J Gastroenterol. 2018;11:62–68. doi: 10.1007/s12328-017-0787-3. [DOI] [PubMed] [Google Scholar]
- 23.Horiguchi S, Ikeda F, Shiraha H, Yamamoto N, Sakakihara I, Noma Y, Tsutsumi K, Kato H, Hagihara H, Yasunaka T, Nakamura S, Kobashi H, Kawamoto H, Yamamoto K. Diagnostic usefulness of precise examinations with intraductal ultrasonography, peroral cholangioscopy and laparoscopy of immunoglobulin G4-related sclerosing cholangitis. Dig Endosc. 2012;24:370–373. doi: 10.1111/j.1443-1661.2012.01300.x. [DOI] [PubMed] [Google Scholar]
- 24.Shimizu S, Naitoh I, Nakazawa T, Hayashi K, Miyabe K, Kondo H, Nishi Y, Yoshida M, Umemura S, Hori Y, Kato A, Ohara H, Kuno T, Takahashi S, Joh T. IgG4-related Sclerosing Cholangitis with No Biliary Stricture but Severe Thickening of the Bile Duct Wall. Intern Med. 2016;55:1575–1579. doi: 10.2169/internalmedicine.55.6302. [DOI] [PubMed] [Google Scholar]
- 25.Kuraishi Y, Muraki T, Ashihara N, Ozawa M, Nakamura A, Watanabe T, Ito T, Hamano H, Kawa S. Validity and safety of endoscopic biliary stenting for biliary stricture associated with IgG4-related pancreatobiliary disease during steroid therapy. Endosc Int Open. 2019;7:E1410–E1418. doi: 10.1055/a-0966-8494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyazawa M, Takatori H, Kawaguchi K, Kitamura K, Arai K, Matsuda K, Urabe T, Inamura K, Komura T, Mizuno H, Fuchizaki U, Yamashita T, Sakai Y, Yamashita T, Mizukoshi E, Honda M, Kaneko S. Management of biliary stricture in patients with IgG4-related sclerosing cholangitis. PLoS One. 2020;15:e0232089. doi: 10.1371/journal.pone.0232089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yokoda RT, Rodriguez EA. Review: Pathogenesis of cholestatic liver diseases. World J Hepatol. 2020;12:423–435. doi: 10.4254/wjh.v12.i8.423. [DOI] [PMC free article] [PubMed] [Google Scholar]