Abstract
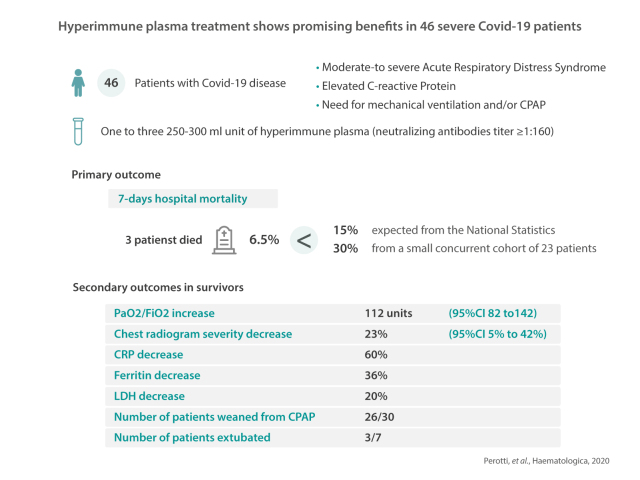
Hyperimmune plasma from patients convalescing from COVID-19 is a potential treatment for severe COVID-19 in other patients. We conducted a multicenter, one-arm, proof-of-concept interventional study. Patients with COVID-19 with moderate-to-severe acute respiratory distress syndrome, elevated C-reactive protein level and need for mechanical ventilation and/or continuous positive airway pressure were enrolled. One to three units (each of 250-300 mL) of hyperimmune plasma (neutralizing antibody titer ≥1:160) were administered. The primary outcome measure was 7-day hospital mortality. Secondary study outcomes were PaO2/FiO2, changes in laboratory and radiological parameters, weaning from mechanical ventilation and safety of the intervention. The study enrolled 46 patients between March 25 and April 21, 2020. The mean age of the patients was 63 years and 61% were male. Thirty of the patients were on continuous positive airway pressure and seven were intubated. The mean PaO2/FiO2 was 128 (standard deviation [SD] 47). Bilateral infiltrates on chest X-ray were present in 36 patients (84%). The mean duration of symptoms and ARDS was 14 (SD 7) and 6 (SD 3) days, respectively. Three patients (6.5%) died within 7 days as compared to an expected 15% according to national statistics and 30% in a small concurrent cohort of 23 patients. The upper one-sided 90% confidence interval (CI) was 13.9%, allowing rejection of the null hypothesis of a 15% mortality. The PaO2/FiO2 increased by 112 units (95% CI: 82-142) in survivors and the severity of the chest X-ray findings decreased in 23% (95% CI: 5%-42%). C-reactive protein, ferritin and lactate dehydrogenase levels decreased by 60%, 36% and 20%, respectively. Weaning from continuous positive airway pressure was achieved in 26/30 patients and it was possible to extubate three of the seven patients who had been intubated. Five serious adverse events occurred in four patients (2 likely and 2 possibly treatment-related). In conclusion, hyperimmune plasma showed promising benefits in COVID-19. Although these benefits need to be confirmed in a randomized controlled trial, this proof-of-concept study could open the way to future developments including hyperimmune plasma banking, standardized pharmaceutical products and monoclonal antibodies.
Introduction
At the end of 2019, a new coronavirus strain was reported in the Chinese province of Wuhan and was named 2019-nCoV or SARS-CoV-2.1-3 The rapid spread of infection by this virus and its resultant disease, COVID-19, in western countries almost overcame the capacity of health systems to respond, leading to high numbers of hospitalized people and deaths. There has been an inevitable lag between the onset of the pandemic and the availability of an effective therapy, and, as of today, no treatment has been convincingly shown to be effective.4-7 Previous data on the use of convalescent plasma during the SARS and MERS epidemics suggest that antibodies could be passively transferred to patients by administering specific antibodies contained in the plasma from recovered/convalescent subjects.8-16 A meta-analysis on the use of hyperimmune immunoglobulins in severe acute respiratory infections of viral etiology, published in 2014, concluded that the technique is effective and safe, although well-designed clinical trials were advocated.17 At the time of designing this study, there were very few studies in the literature demonstrating the feasibility and efficacy of hyperimmune plasma in the SARS-CoV-2 pandemic, and all of them reported small case series. Shen et al. described five severely ill patients who showed an improvement in a variety of signs and symptoms of COVID-19 after the infusion of hyperimmune plasma.18 In the same journal, Roback and Guarner discussed the need for larger studies. 19 Duan and colleagues presented a study of ten severely ill COVID-19 patients;20 the primary endpoint was safety. They demonstrated that all patients tolerated plasma transfusion without severe adverse events and had improvements in clinical symptoms and laboratory values from day 3 after infusion.
On this background, we designed and conducted a proof-of-concept, interventional, multicenter study to determine the potential efficacy and safety of infusions of hyperimmune plasma, obtained from convalescent donors, in COVID-19 patients with respiratory failure and lung infiltration at chest radiogram, hospitalized in the participating Centers.
Methods
Design
This was a proof-of-concept, one-arm, multicenter interventional study on the short-term (7 days) efficacy and safety of the infusion of hyperimmune plasma in COVID-19 patients with moderately to severely compromised respiratory function, according to the Berlin score. It was hoped that the evidence collected would help either to plan an informed large clinical trial or to dismiss the proposed treatment if irrelevant. The primary endpoint of the study was 7-day mortality; secondary endpoints, all evaluated at 7 days, were changes in respiratory function (PaO2/FiO2 ratio), laboratory values (C-reactive protein, ferritin, lactate dehydrogenase, viral load) and radiological signs, as well as weaning from mechanical ventilation (continuous positive airway pressure [CPAP] and/or naso-tracheal intubation).
Setting and population
The study was conducted in two university hospitals and one general hospital in northern Italy and was registered at clinicaltrials. gov as NCT 04321421. It was approved by the local ethical committee on March 17, 2020 (n. 20200027967). Patients were enrolled between March 25, 2020 and April 21, 2020. Follow-up was closed on April 28, 2020.
Eligibility criteria are summarized in Online Supplementary Table S1. Data were entered into a database in REDCap hosted at the Fondazione IRCCS Policlinico San Matteo (Pavia, Italy) and monitored remotely for missing data. The schedule of assessments is summarized in Online Supplementary Table S2. Mortality data from a control cohort of 23 consecutive patients from the Pavia COVID Registry, observed between March 10, 2020 and March 24, 2020 and satisfying the same entry criteria, were retrieved for comparison. These patients were observed for 7 days for comparison with the trial cohort.
Selection of convalescent donors and hyperimmune plasma
Male adults or females with no previous pregnancy who had recovered from COVID-19 and had two consecutive negative naso-pharyngeal swabs performed in the 7 to 30 days before potential recruitment as donors were identified from the hospital records; their suitability was assessed according to current Italian guidelines and transfusion law.21,22 Their plasma was collected using latest-generation cell separators (Trima Accel –Terumo BCT and Amicus –Fresenius Kabi). A plasma volume of about 660 mL was collected during each procedure and immediately divided equally into two bags using a sterile tubing welder. Plasma pathogen reduction was performed with the INTERCEPT processing system (Cerus Europe BV) or the Mirasol PRT System (Terumo BCT, Lakewood, CO, USA). The collected units were stored at a controlled temperature ranging from -40°C to -25°C.23 (see the Online Supplementary Material for further details).
Plasma infusion
Plasma was delivered ready-for-use by the Immunohematology Service to the COVID Units and was administered to the patients over 30 to 60 min, under supervision of the treating physician.
SARS-CoV2 RNA detection
Total nucleic acids (DNA/RNA) were extracted from 200 mL of respiratory specimens. Clinical samples were pretreated with 1:1 ATL lysis buffer and the nucleic acids were extracted using the QIAsymphony® instrument with a QIAsymphony® DSP Virus/Pathogen Midi Kit (Complex 400 protocol) according to the manufacturer’s instructions (QIAGEN, Hilden, Germany). Specific real-time reverse transcriptase-polymerase chain reactions targeting RNA-dependent RNA polymerase and E genes were used to detect the presence of SARS-CoV-2, in accordance with World Health Organization guidelines24 and the protocol published by Corman et al.25
SARS-CoV-2 microneutralization assay
The titer of neutralizing antibodies against SARS-CoV-2 was determined using the following protocol.26,27 Briefly, 50 mL of a serum sample from each patient, starting from 1:10 in a serial 4- fold dilution series, were added to two wells of a flat-bottomed tissue culture microtiter plate (COSTAR, Corning Incorporated, NY, USA), mixed with an equal volume of 50 TCID50 of a SARSCoV- 2 strain isolated from a symptomatic patient. The plates were incubated at 33°C in 5% CO2. The SARS-CoV-2 strain was previously titrated to calculate the 50TCID50 to be used in the test. All dilutions were made in Eagle minimum essential medium with addition of 1% penicillin, streptomycin and glutamine and 5 mL/mL of trypsin. After 1 h of incubation at 33°C in 5% CO2, VERO E6 cells (VERO C1008 [Vero 76, clone E6, Vero E6]; ATCC® CRL-1586™] were added to each well. After another 48 h of incubation at 33°C in 5% CO2, the wells were stained with Gram crystal violet solution (Merck KGaA, 64271 Damstadt, Germany) plus 5% formaldehyde 40% m/v (Carlo ErbaSpA, Arese, MIlan, Italy) for 30 min. The microtiter plates were then washed in running water. Wells were scored to evaluate the degree of cytopathic effect compared to that of the virus control. Blue staining of wells indicates the presence of neutralizing antibodies. The neutralizing titer was the maximum dilution with reduction of 90% of the cytopathic effect. A positive titer was defined as one equal or greater than 1/10. Positive and negative controls were included in all test runs.
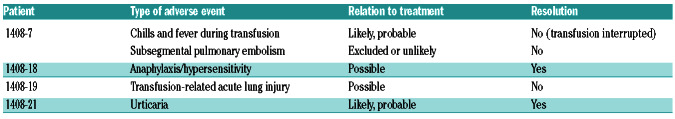
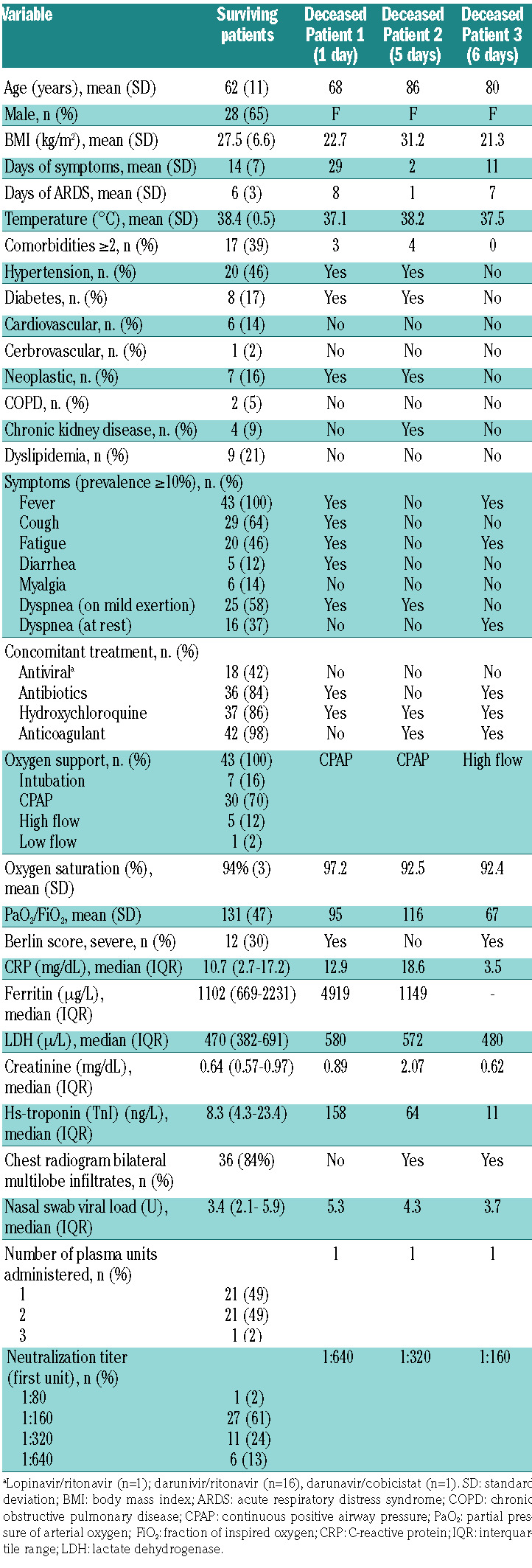
Table 1.
Description of medical history, baseline symptoms and laboratory findings.
Sample size
The first case of COVID-19 was diagnosed in Italy on February 20, 2020. Considering the hospitalization and mortality data retrieved from the Italian National Institute of Health on March 16,28,29 for benchmarking purposes, we used a conservative estimate of mortality of about 15% (i.e., a null hypothesis, H0, of survival of 85%) in patients treated according to the standard of care, corresponding to the general COVID-related mortality in Italy. We expected the mortality to decrease to 5% (survival 95%; H1) with the proposed hyperimmune plasma infusion. This being a proof-of-concept study, we used a one-sided type I error of 10%. According to the one-stage Fleming design, 43 patients would give a power of more than 80% to reject H0. If we were to observe at least 40 successes, the H0 hypothesis could be rejected and we would consider proceeding with a future, larger trial. Three additional patients were enrolled to allow for possible drop-outs.
Statistical analysis
All continuous variables are summarized using the mean and standard deviation (SD) or the median and interquartile range (IQR). Frequencies and percentages are reported for all categorical measures. All enrolled patients who received a plasma infusion constitute the analysis population.
Primary endpoint
The observed mortality rate was computed as the number of deaths over the full analysis population. The one-sided exact binomial confidence interval (CI), at the 90% level (by design) is presented. The clinical and laboratory findings at baseline are described in aggregate for patients surviving 7 days and listed individually for patients who died. No formal tests were performed.
Secondary endpoints
To assess changes in PaO2/FiO2 ratio, lactate dehydrogenase, Creactive protein and ferritin levels, and viral load over time we fitted repeated measures linear models (with Huber-White clustered robust standard errors to account for intra-patient correlation) or bootstrapped median regression models (depending on the distribution). The coefficients comparing day 7 to day 1 together with their 95% CI are presented to describe the changes at the end of the study.
Table 2.
List of adverse events and relation to treatment for each patient.
Results
Study cohort
We enrolled 46 patients from the three centers participating in this proof-of-concept study. The patients’ mean age was 63 years (SD 12) and 28 were male (61%). Their mean oxygen saturation was 94% (SD 3) and their mean PaO2/FiO2 ratio was 128 (SD 47). Fourteen (33%) had a severe Berlin score, 30 patients (70%) were on CPAP and seven (16%) were intubated. Nineteen (41%) had two or more comorbidities and 36 (84%) had bilateral multilobe infiltrates as shown by chest X-ray. Patients had been symptomatic for a mean of 14 days (SD 7) and had had acute respiratory distress syndrome for a mean of 6 days (SD 3). More than 80% of patients were treated with antibiotics, hydroxychloroquine and anticoagulants (Table 1).
Plasma infusion and safety
Twenty-four patients received one unit of plasma, 21 received two units and one patient received three units. The plasma units administered at the first infusion had a neutralizing antibody titer of 1:160 or 1:320 in 85% of patients and of 1:320 in 12%; one patient only received plasma with a 1:80 titer (Table 1). At the second infusion, the titers were 1:80 in two patients, 1:160 in 11 patients, 1:320 in seven patients and 1:640 in one patient. The third infusion performed in a single patient had a titer of 1:320.
The plasma infusion was well tolerated in 42/46 patients. It was interrupted in one case.
Five serious adverse events occurred in four patients. In one case the transfusion had to be interrupted. In two cases the relation to treatment was considered as likely and in two as possible (Table 2). Three adverse events did not resolve spontaneously and were treated accordingly.
Primary endpoint
Three patients of the 46 (6.5%) died within 7 days (on days 1, 4 and 6); the upper one-sided 90% CI was 13.9% and 40 of the first 43 patients enrolled survived, allowing us to reject the null hypothesis of a 15% mortality. The main characteristics of the three patients who died are listed in Table 2. Two had important comorbidities, including diabetes, hypertension and cancer, while the third had an extremely low PaO2/FiO2 ratio of 67 at the time of the plasma infusion. Among survivors, the severity of the condition at baseline was confirmed by the low oxygen saturation (mean 94%) and PaO2/FiO2 (mean 131). More than 89% of patients showed bilateral multilobe infiltrates on chest X-rays and all had markedly elevated laboratory biomarkers (Table 1).
In a concurrent cohort of 23 patients from the Pavia COVID Registry, observed between March 10, 2020 and March 24, 2020 who met the same entry criteria as used for this study and who were followed up for 7 days (Online Supplementary Table S3), the observed mortality was 30% (two-sided 80% CI: 18%-46%, or equivalently, the lower 90% one-sided limit was 18%, which is higher than the upper limit, reported above, of 13.9% for the treated cohort)
Secondary endpoints
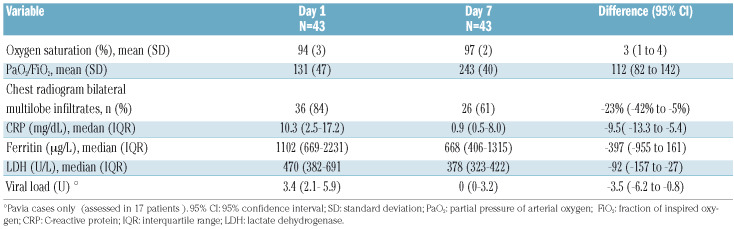
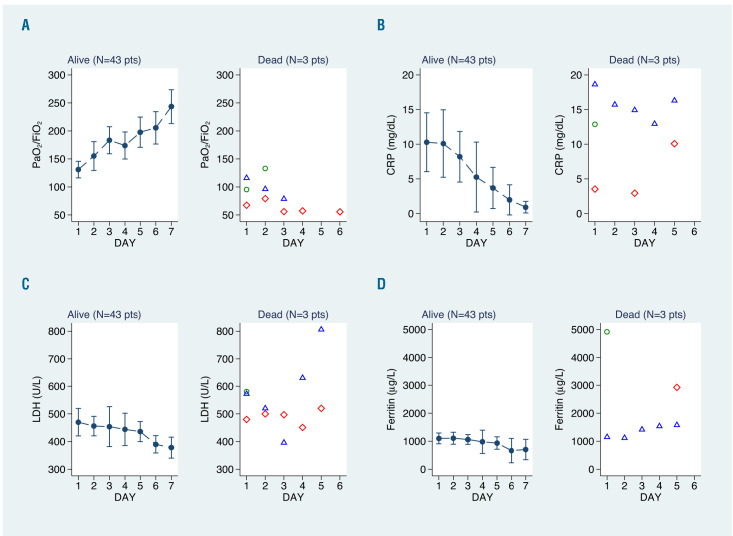
At 7 days after plasma infusion the PaO2/FiO2 increased by 112 units (95% CI: 82-142) in survivors and bilateral multilobe infiltrates on the chest X-ray had disappeared in 23% of patients (95% CI: 5%-42%). C-reactive protein, ferritin and lactate dehydrogenase levels all decreased, by 90%, 36% and 20%, respectively (Table 3, Figure 1). Conversely, no or little improvement was documented in the three patients who died (Figure 1).
Overall, 30 patients were on CPAP and seven were intubated. Weaning from CPAP was achieved in 26 patients over a median time of 2 days (IQR 0-3) and three of the intubated patients were extubated after a median of 2 days (IQR 1-5). Two of 16 patients who were in the Intensive Care Unit were discharged from the Unit within the 7 days following the infusion (both on day 3).
Two patients were put on extracorporeal membrane oxygenation 1 and 6 days after the plasma infusion. No patient was discharged from the hospital within the 7- day study observation period.
Discussion
This proof-of-concept study showed that the infusion of highly specific hyperimmune plasma in COVID-19 patients with severe respiratory failure reduces short-term mortality by 2.5 times, from an expected 15% (i.e., about 1 in 6 patients) at the time of study design to 6% (i.e., about 1 in 15 patients). Compared with the mortality rate in a concurrent series of 23 patients, satisfying the same entry criteria and observed in the period March 10 to March 24, 2020, the decrease in deaths was even more dramatic (5- fold, from 30% to 6%). Only three patients died during the 7-day study period. Of these three patients, two had important comorbidities while the third had an extremely low PaO2/FiO2 ratio at the time of the plasma infusion.
Patients had been symptomatic for 2 weeks at the time of plasma infusion. Most had been on treatment with antibiotics, hydroxychloroquine and anticoagulants. Among survivors, the PaO2/FiO2 ratio increased 2-fold, mirrored by decreases in the levels of biomarkers: C-reactive protein by 90%, ferritin by 36%, and lacate dehydrogenase by 20%. The bilateral multilobe infiltrates on chest radiograms disappeared in one-third of the study population. The viral load was reduced to null.
Serious adverse events occurred in four patients; in two cases, they were likely related to the transfusion. Of note, one adverse event was potentially transfusion-related acute lung injury (TRALI), the features of which are similar to those of COVID-19 and thus may be underdiagnosed. Importantly TRALI may be triggered by transfused antibodies. 30 Indeed TRALI was reported in 11 cases by Joyner et al. in a large, safety study of 5,000 COVID-19 patients treated with convalescent plasma.31 In an attempt to minimize the risk of TRALI and other transfusion-related serious adverse events, we excluded women who had had previous pregnancies from donating plasma for this study. Overall, both our data and, on a larger scale, those from Joyner et al.31 and Duan et al.20 confirm that transfusion of convalescent plasma appears to be safe in hospitalized patients with COVID-19.
Table 3.
Changes from baseline in functional, laboratory and radiological parameters in survivors.
Figure 1.
Changes of respiratory function and laboratory parameters over time from day 1 to day 7 for survivors and patients who died. (A) Whisker plots of the mean and 95% confidence interval (95% CI) of the PaO2/FiO2 values. (B-D) Whisker plots of the median and 95% CI values for C-reactive protein (B), lactate dehydrogenase (C) and ferritin (D). Estimates and 95% CI values were obtained from linear (A) and quantile (B-D) regression models for repeated measures. PaO2: partial pressure of arterial oxygen; FiO2: fraction of inspired oxygen; CRP: C-reactive protein; LDH: lactate dehydrogenase
Although hyperimmune plasma was used for the treatment of severe cases in the 2002-2004 SARS outbreak,8-14,17 few data are available from the COVID-19 epidemics. Our results are consistent with those of preliminary experiences from China. As recently described in JAMA,18,19 five critically ill patients at Shenzhen Third People’s Hospital (Shenzhen, China) were treated with convalescent plasma with a neutralizing titer of 1:80 to 1:480, 10 to 20 days after admission. The patients’ clinical conditions and laboratory findings improved. As a result, three patients were discharged; the other two patients were still hospitalized after 1 month. In a second study, performed in three hospitals in Wuhan (China),20 the outcomes of ten patients with severe disease, who were treated with convalescent plasma with high neutralizing titers (≥1:640) at a median of 16 days after the onset of symptoms, were determined. Following improvements in clinical and laboratory parameters, three patients were discharged, and the other seven were ready for discharge. In contrast, among a group of historical controls, similar for baseline characteristics, only one patient improved, six were stable and three died. A third report of compassionate use of hyperimmune plasma gave encouraging results as well.32
In contrast, in a fourth retrospective study, administration of convalescent plasma to six patients led to suboptimal results.33 Prior determination of neutralizing antibody response had not been performed.
Indeed, both the Food and Drug Administration34 and the European Commission35 strongly recommend that SARSCoV- 2 neutralizing antibody titers be measured in the donated plasma. While the Food and Drug Administration recommends a minimum neutralizing antibody titer of 1:160, indicating that a titer of 1:80 might be acceptable in some cases, the European Commission considers titers of 1:320 or more to be optimal, although lower thresholds could be considered. In our study, of the 68 units of hyperimmune plasma administered, only three (4%) had a titer below 1:160; 58 (84%) had a titer between 1:160 and 1:320 and seven had a titer of 1:640, largely consistent with the international recommendations. Of note, the promising efficacy of using neutralizing antibodies was described in a recent study that reported on a human monoclonal antibody neutralizing SARS-CoV-2 (and SARS-CoV) in cell cultures. 36
A strong and novel point of our study is that we titrated the plasma from patients who had recovered from COVID-19 by quantifying COVID-19-specific neutralizing antibodies. For this purpose, we developed a new rapid microneutralization test based on evaluation of a 90% reduction of cytopathic effect in 48 h with Gram crystal violet staining. We introduced this staining to increase the readout of our neutralization test compared to that of other microneutralization test assays, such as the plaque reduction neutralization test, which require the overlay of cells and a longer time for the result.37 The plaque reduction neutralization test is a gold standard for the detection of neutralizing antibodies and has a high sensitivity and specificity.
Recently, Li and colleagues presented the results of the first randomized clinical trial of convalescent plasma therapy for patients with COVID-19 conducted in China.38 The study, which enrolled 103 patients, showed more favorable outcomes (measured as time to clinical improvement within 28 days, 28-day mortality and time to discharge) for patients who received convalescent plasma compared to the outcomes of patients in the control group. However, the differences were not statistically significant, the trial having been terminated early and as such, was underpowered. 38
Our study has some limitations. First of all, it lacked a randomized control arm; it was, however, designed as a proof-of-concept study to verify the potential efficacy and safety of the administration of hyperimmune plasma in severely compromised COVID-19 patients and to inform the design of a rigorous, randomized controlled trial. Despite the lack of a randomized control arm, mortality was shown to be decreased by the treatment, both when compared to the mortality of hospitalized patients in Italy at the time of designing the study and when compared to the mortality in our concurrent, similar observational cohort. Interestingly, the 8.5% absolute mortality risk reduction observed in our study was very similar to that in the recent randomized trial from China.38 Indeed, in a subgroup analysis including 23 cases and 22 controls with severe COVID-19, the authors observed a 9.1% reduction in the mortality rate in the group of patients treated with convalescent plasma.
A second limitation is that the study was designed at the very beginning of the pandemic in Italy. The patients were included under the pressure of a medical emergency in order to provide them with a potentially effective treatment in the very short term, given the high mortality.39 For this reason, some information was not planned to be collected, such as, but not only, the levels of D-dimer or other markers of inflammation, and long-term outcome.
In conclusion, we were able to show a promising benefit of hyperimmune plasma in COVID-19 patients, both through a reduction of mortality, an improvement in respiratory function and decreases in inflammatory indices. This was a proof-of-concept study, thus these findings should not be over-interpreted and efficacy cannot be advocated yet. Nevertheless, the results pave the way for future developments including the rigorous demonstration of hyperimmune plasma efficacy in a randomized clinical trial, and possibly, the need for hyperimmune plasma banking to anticipate a potential second wave of the pandemic, the development of standardized pharmaceutical products made from the purified antibody fraction (concentrated COVID-19 H-Ig) and last, but not least, the production of monoclonal antibodies on a large scale.
Supplementary Material
Graphical Abstract.
Acknowledgments
The authors would like to thank Valeria Scotti, librarian at Fondazione IRCCS Policlinico San Matteo for her help with the references. We also thank all the donors who, after experiencing a difficult time being victims of the COVID-19 disease, are freely giving their convalescent plasma for the benefit of all.
COVID-19 Plasma Task Force
Angelo Corsico,1 Federica Melazzini,1,2 Marco Lenti,1,2 Cristina Mortellaro,1 Edoardo Vecchio Nepita,1 Gianluca Viarengo,1 Giorgio Iotti,1 Luciano Perotti,1 Marco Maurelli,1 Margherita Sambo,1 Mariangela Delliponti,1 Raffaella Di Martino,1 Roberta Maserati,1 Valentina Zuccaro1 and Gennaro Mascaro.3
1Fondazione IRCCS Policlinico San Matteo, Pavia; 2University of Pavia, Pavia and 3Ospedale Maggiore della Carità, Novara, Italy.
References
- 1.Zhai P, Ding Y, Wu X, Long J, Zhong Y, Li Y. The epidemiology, diagnosis and treatment of COVID-19. Int J Antimicrob Agents. 2020;55(5):105955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peeri NC, Shrestha N, Rahman MS, et al. The SARS, MERS and novel coronavirus (COVID-19) epidemics, the newest and biggest global health threats: what lessons have we learned? Int J Epidemiol. 2020:49(3);717-726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xie M, Chen Q. Insight into 2019 novel coronavirus - an updated interim review and lessons from SARS-CoV and MERS-CoV. Int J Infect Dis. 2020;94:119-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang L, Liu Y. Potential interventions for novel coronavirus in China: a systematic review. J Med Virol. 2020;92(5):479-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou M, Zhang X, Qu J. Coronavirus disease 2019 (COVID-19): a clinical update. Front Med. 2020;14(2):126-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rome BN, Avorn J. Drug evaluation during the Covid-19 pandemic. N Engl J Med. 2020;382(24):2282-2284. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. Published March 2020. https://www.who.int/publicationsdetail/clinical-management-of-severe-acuterespiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected [Accessed April 24, 2020]. [Google Scholar]
- 8.Wong HK, Lee CK. Pivotal role of convalescent plasma in managing emerging infectious diseases. Vox Sang. 2020;115(7):545-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luke TC, Kilbane EM, Jackson JL, Hoffman SL. Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? Ann Intern Med. 2006;145(8):599-609. [DOI] [PubMed] [Google Scholar]
- 10.Zhou B, Zhong N, Guan Y. Treatment with convalescent plasma for influenza A (H5N1) infection. N Engl J Med. 2007;357(14):1450-1451. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization. Use of convalescent whole blood or plasma collected from patients recovered from Ebola virus disease for transfusion, as an empirical treatment during outbreaks. Interim guidance for national health authorities and blood transfusion services. Published September 2004. http://apps.who.int/iris/bitstream/10665/135591/1/WHO_HIS_SDS_2014.8_eng.pdf?ua=1 [Accessed April 24, 2020]. [Google Scholar]
- 12.Marano G, Vaglio S, Pupella S, et al. Convalescent plasma: new evidence for an old therapeutic tool? Blood Transfus. 2016;14(2):152-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hung IF, To KK, Lee CK, et al. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin Infect Dis. 2011;52(4):447-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keller MA, Stiehm ER. Passive immunity in prevention and treatment of infectious diseases. Clin Microbiol Rev. 2000;13(4):602-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bloch EM, Shoham S, Casadevall A, et al. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Invest. 2020;130(6):2757-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casadevall A, Pirofski LA. The convalescent sera option for containing COVID-19. J Clin Invest. 2020;130(4):1545-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mair-Jenkins J, Saavedra-Campos M, Baillie JK, et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2015;211(1):80-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen C, Wang Z, Zhao F, et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323(16): 1582-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roback JD, Guarner J. Convalescent plasma to treat COVID-19: possibilities and challenges. JAMA. 2020;323(16):1561-1562. [DOI] [PubMed] [Google Scholar]
- 20.Duan K, Liu B, Li C, et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A. 2020;117(17):9490-9496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Istituto Superiore di Sanità. Centro Nazionale Sangue. https://www.centronazionalesangue.it/sites/default/files/GU%20SG%20n.300%20del%2028-12-2015_SO_069.pdf [Accessed May 11, 2020]. [Google Scholar]
- 22.Franchini M, Marano G, Velati C, Pati I, Pupella S, Liumbruno GM. Operational protocol for donation of anti-COVID-19 convalescent plasma in Italy. Vox Sang. 2020. Apr 23. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perotti C, Del Fante C, Baldanti F, et al. Plasma from donors recovered from new Corona virus 2019 as therapy for critical patients with COVID-19 (COVID-19 PLASMA study). A multicentre study protocol. Int Emerg Med. 2020. May 28. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization. Diagnostic detection of 2019-nCoV by real-time RTPCR. Published January 2020. https://www.who.int/docs/defaultsource/coronaviruse/protocol-v2-1.pdf [Accessed April 24, 2020]. [Google Scholar]
- 25.Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019- nCoV) by real-time RT-PCR. Euro Surveill. 2020;25(3):2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Percivalle E, Cassaniti I, Sarasini A, et al. West Nile or Usutu virus? A three-year follow- up of humoral and cellular response in a group of asymptomatic blood donors. Viruses. 2020;12(2):157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Percivalle E, Cambiè G, Cassaniti I, et al. Prevalence of SARS-CoV-2 specific neutralising antibodies in blood donors from the Lodi Red Zone in Lombardy, Italy, as at 06 April 2020. Euro Surveill. 2020;25(24): 2001031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Torresi M. Statistiche coronavirus Lombardia. https://statistichecoronavirus.it/regioni-coronavirus-italia/lombardia/ [Accessed May 11, 2020]. [Google Scholar]
- 29.Rosini U. COVID-19 Italia - Monitoraggio situazione- Dati Regionali. https://github.com/pcm-dpc/COVID-19/blob/master/dati-regioni/dpc-covid19-ita-regioni-20200316.csv [Accessed May 11, 2020]. [Google Scholar]
- 30.Semple JW, Rebetz J, Kapur R. Transfusionassociated circulatory overload and transfusion- related acute lung injury. Blood. 2019;133(17):1840-1853. [DOI] [PubMed] [Google Scholar]
- 31.Joyner M, Wright RS, Fairweather DL, et al. Early safety indicators of COVID-19 convalescent plasma in 5,000 patients. medRxiv 2020; doi:https://doi.org/10.1101/2020.05.12.20099879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang B, Liu S, Tan T, et al. Treatment with convalescent plasma for critically ill patients with SARS-CoV-2 infection. Chest. 2020;158(1):e9-e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeng QL, Yu ZJ, Gou JJ, et al. Effect of convalescent plasma therapy on viral shedding and survival in COVID-19 patients. J Infect Dis. 2020;222(1):38-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Food and Drug Administration. Center for Biologics Evaluation and Research. Investigational COVID-19 convalescent plasma. Guidance for industry. Published May 2020. https://www.fda.gov/media/136798/download [Accessed May 11, 2020]. [Google Scholar]
- 35.European Commission Directorate-General For Health And Food Safety. An EU programme of COVID-19 convalescent plasma collection and transfusion. Guidance on collection, testing, processing, storage, distribution and monitored use. Published April 2020. https://ec.europa.eu/health/sites/health/files/blood_tissues_organs/docs/guidance_plasma_covid19_en.pdf [Accessed May 11, 2020]. [Google Scholar]
- 36.Wang C, Li W, Drabek D, et al. A human monoclonal antibody blocking SARS-CoV-2 infection. Nat Commun. 2020;11(1):2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perera RA, Mok CK, Tsang OT, et al. Serological assays for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), March 2020. Euro Surveill 2020;25(16): 2000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li L, Zhang W, Hu Y, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA. 2020;324(5)1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Characteristics of SARS-CoV-2 patients dying in Italy. Report based on available data on June 18th, 2020. https://www.epicentro.iss.it/en/coronavirus/bollettino/Report-COVID-2019_18_june_2020.pdf [Accessed June 26, 2020]. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.