Sideroblastic anemias (SA) are a group of hematological disorders that are characterized by inadequate levels of hemoglobin and ringed sideroblasts in the bone marrow.1 They can be acquired or congenital. Acquired SA are part of the myelodysplastic syndromes with onset in adulthood. 1 Congenital SA show both early onset in infancy or childhood and in adulthood, and they are the result of germline mutations (inherited or de novo).2 Several genes have been associated with SA, and these are involved in mitochondrial pathways, such as for heme synthesis, iron-sulfur cluster biogenesis, and mitochondrial metabolism. 1 Point mutations in the ALAS2, SLC25A38, GLRX5, and HSPA9 genes have been reported for the nonsyndromic SA. Point mutations in the ABCB7, PUS1, YARS2, LARS2, TRNT1, SLC19A2, and MT-ATP6 genes, and deletion of mitochondrial DNA and the NDUFB11 gene are causal for the syndromic forms of SA.
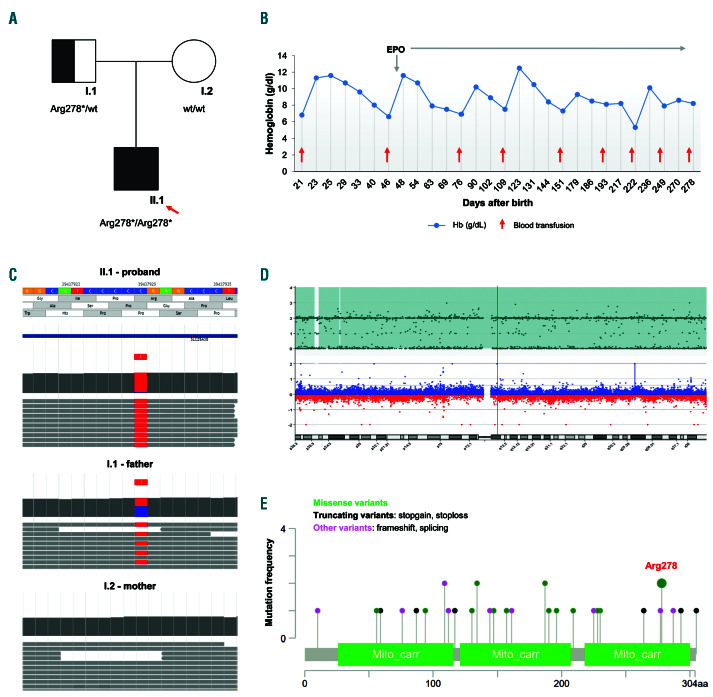
We report here on a case of an infant with microcytic anemia and paternal isodisomy at the SLC25A38 locus (Figure 1A). We revealed uniparental isodisomy (UPD) in the proband, which carried a homozygous recessive mutation, with only one parent heterozygous for the same variant (excluding a de novo mutation), and in the absence of parental consanguineity. UPD is characterized by the presence of two identical copies of a complete or partial chromosome from one of the parents. This results in homozygosity for an autosomal recessive gene, and thus the clinical expression of a recessive disease.3 To date, UPD has never been described as a pathogenic mechanism of SA.
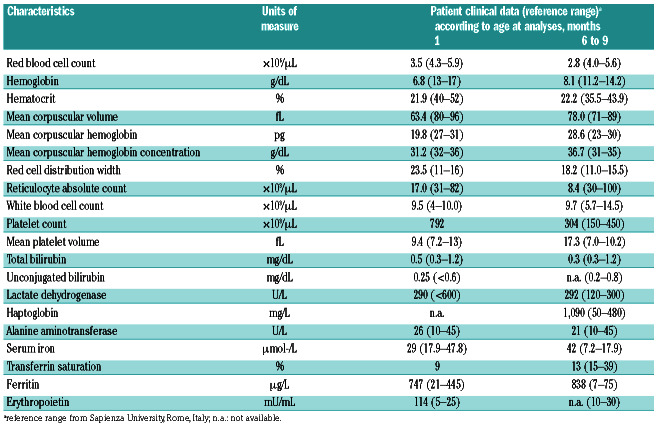
The patient considered here was a newborn male of nonconsanguineous parents from Italy (Figure 1A). At 21 days old, he was admitted to the pediatric Emergency Department due to deep asthenia. He showed mucocutaneous pallor, hypospadia, and micropenis, without any jaundice, fever, or other clinical signs and symptoms. Peripheral blood tests showed severe hypochromic and microcytic anemia, normal white blood cells and platelet counts, and reticulocytopenia (Table 1). A peripheral blood smear showed marked anisopoichylocytosis, with some dacrocytes, microcytes, and spherocytes. His ferritin level was high (747 mg/L), with serum iron and transferrin in the normal range (Table 1); his bilirubin, haptoglobin, and lactate dehydrogenase levels were normal; and his serum erythropoietin (EPO) level was high (114 mUI/mL) (Table 1). Bone marrow smear evaluation was not performed. He received one blood transfusion (10 cm3/kg), and reached a hemoglobin (Hb) of 11.3 g/dL at 23 days old. After the first transfusion, he showed a slow and progressive reduction in Hb (Figure 1B).
The first suspected condition was the sequelae of fetal maternal hemolytic diseases, but the immune-hematological tests were negative. Due to the early onset of the condition, the patient was suspected of having a hereditary anemia. Accordingly, a first level of investigation was performed on the parents. Both parents showed normal red blood cell parameters without abnormal hemoglobins. Thus, a second level of investigation was performed, as genetic testing, using a targeted next-generation sequencing (t-NGS) 86-gene custom panel for hereditary anemias. This panel is an updated version of a similar previously published one.4 The genetic analysis of the proband revealed the presence of the rare nonsense variant c.832C>T, p.Arg278* (rs147431446, alternative allele frequency <0.0001 in GnomAD_exome database; NM_017875.4; predicted as pathogenic by the ACMG/AMP 2015 guideline) as homozygous (Figure 1A, C). The assumed mechanism of the mutation p.Arg278 *is non-expression of the protein due to nonsense mediated decay. Analysis of the inheritance pattern was carried out for both parents. According to the recessive inhertiance pattern, the father was a heterozygous carrier of the variant. However, no mutations in the SLC25A38 gene were identified for the mother (Figure 1A, C). We also confirmed the t-NGS data by Sanger sequencing analysis on DNA from the proband and parents. Interestingly, the proband was homozygous for all of the variants located on chromosome 3. In particular, beyond the SLC25A38 nonsense variant, two additional single nucleotide variants (SNV) showed a Mendelian violation of inheritance: rs1049296, a coding variant in the TF gene (NM_001063.3:c.1765C>T, p.Pro589Ser); and rs16861582, an intronic variant in the CP gene (NM_000096.3:c.2662-12T>C). These variants resulted in the homozygous state for the proband and the heterozygous state for the father, but none of these were identified in the mother (Figure 1C). Thus, a third level of investigation was performed, using single nucleotide polymorphism (SNP) array in the proband and parents, to define either a heterozygous deletion or UPD. No genomic deletions or rearrangements at or around the SLC25A38 locus were identified in the proband or parents at 3p22.1. The SNP array identified large regions of homozygosity that involved the whole paternal chromosome 3, which included the SLC25A38 locus: 3p26. 3p25. 3(54290_9540289), 3p25. 3p12. 1 (11549397_ 84682450), 3q11. 2q12.3 (94565518_101899846), and 3q12. 3q29 (102540948_197959451) (Figure 1D).
Table 1.
Clinical characteristics of the proband affected by sideroblastic anemia type II.
Figure 1.
Description of a patient with sideroblastic anemia type II, and characterization of uniparental isodisomy. (A) Genetic pedigree of the family. Square: male; circle: female; solid symbols: affected person; arrow: proband. According to uniparental isodisomy, only the father carried the variant c.832C>T, p.Arg278 *in the SLC25A38 gene. (B) Hemoglobin levels of the patient after blood transfusions and erythropoietin (EPO) supplementation. (C) Alignment track of next-generation sequencing analysis of the proband, mother, and father, showing presence of the c.832C>T variant as homozygous in the proband (mutated allele [T] frequency, 97% [184/190 reads]; wild-type [wt] allele (C) frequency, 3% [6/190 reads]), as heterozygous in the father, and absent in the mother. (D) Agilent CytoGenomic view of the single nucleotide polymorphism (SNP) data. Top panel: number of uncut alleles. Bottom panel: comparative genomic hybridization (CGH) data (log2 ratios). Data from GenetiSure Postnatal Research CGH+SNP 2x400 Array (Agilent), which show large regions of homozygosity on chromosome 3. (E) Diagram of the SLC25A38 protein structure and the pathogenic variants, as obtained from the Human Gene Mutation Database Professional (updated in June 2020). Circle colors define the mutation types (for different mutation types at a single position, the color defines the most frequent) (https://www.cbioportal.org/mutation_mapper).
Currently, only one case of paternal UPD for whole chromosome 3 has been described with no apparent disease phenotype, suggesting the absence of paternal imprinted genes on this chromosome.5
Homozygous or compound heterozygous mutations in the SLC25A38 gene are causal for SA type II.1 A recent retrospective multicenter European study of a cohort of patients with childhood-onset congenital SA showed that in SA, SLC25A38 is the second most commonly mutated gene after ALAS2.6,7 Different recessive loss-offunction mutations (i.e., nonsense, frameshift, splicing, missense) have been reported for the SLC25A38 along the entire gene (Figure 1E).1,6-12 These variants impair the transport of glycine in the mitochondria. Indeed, SLC25A38 is a mitochondrial carrier that is expressed in erythroblasts.8 It is located on the mitochodrial inner membrane and imports glycine into the erythroid mitochondria. The glycine condenses with succinylCoA to form δ-aminolevulinic acid (ALA), a substrate for heme that it is then exported into the cytosol.8,13 Thus, mutations in SLC25A38 result in alterations to heme synthesis and the hematological phenotype characterized by hypochromic microcytic anemia, and iron depositions around the nucleus in the mitochondria of erythroblasts in the bone marrow.1 Patients with SLC25A38 mutations show neonatal microcytic anemia and high ferritin levels (higher than those of patients with mutated ALAS2), as described for the patient here.7 Of note, patients who carry biallelic pathogenic variants in SLC25A38 gene show iron overload not only in the liver, but also in the myocardium,6 and the follow-up of this patient will include such evaluation. The same amino acid, arginine 278, was mutated in glycine in a patient previously described (Figure 1E).9 Comparison of our case and others with nonsense mutations with the patients with biallelic missense mutations reveals no differences. Most of the patients with SLC25A38 mutations present a severe or moderate microcytic anemia, with early onset, transfusion dependency and severe or moderate hepatic iron overload. All patients with a mutated SLC25A38 gene require transfusion at a mean rate of 13.3 transfusions/year (range: 0.3–20/year), with chelation therapy and vitamin B6 supplementation.6 Accordingly, the patient considered here continued to receive erythrocyte transfusions from the age of 6 months, with a median interval of 30 days. His median pre-transfusion Hb was 7.3 g/dL, and this transfusion regimen maintained his Hb at ~8.5 g/dL. He also received vitamin supplementation at a standard dose, without high-dose pyridoxine. Pharmacological supplementation with glycine and folate might improve his heme synthesis. In yeast and zebrafish models, exogenous glycine in combination with folate ameliorated the heme levels.14 This combined supplementation was also administered to three patients with SLC25A38 mutations, although without any improvements. 14 So, to date, the only therapeutic strategy here is chronic blood transfusions and iron chelation. The potentially curative therapy remains hematopoietic stemcell transplantation.7
Due to religious beliefs, families can sometimes refuse permission for blood transfusions, therefore, prior to obtaining the results of the genetic testing, we started the patient on EPO therapy. Initially, this resulted in an apparent lengthening of the transfusion interval (Figure 1B), but later he showed a reduction in Hb. Thus, the treatment with EPO was stopped. To date, no cases of patients with SA being treated with EPO have been described in the literature. Indeed, EPO is not expected to be not effective in this disease, as down-regulation of SLC25A38 is described in these patients, which is similar to patients with myelodysplastic syndrome with ring sideroblasts.15 In our patient, we noted a similar picture, with an apparent initial response to EPO, with progressive lengthening of the transfusion interval. Unfortunately, EPO treatment did not improve his Hb.
Currently, the patient is 9 months old, and his psychophysical development is normal. He does not require iron chelation at present. For his sexual phenotype, he has just undergone subcutaneous substitutive testosterone therapy. Testosterone stimulates erythropoiesis, and we believe that this patient will benefit from its administration, for both his anemia and his hypogonadism, although over a short period.
According to the literature, the clinical features in this patient confirm that SA represent a severe transfusiondependent disease with no valid options for treatment. From a diagnostic perspective, this case highlights the importance of the evaluation of the possible occurrence of UPD for patients with SA due to SLC25A38 mutations, and of an assessment of the inheritance pattern of the identified variants. Indeed, the incidence of UPD is higher for rare autosomal recessive diseases compared to common autosomal recessive diseases with higher carrier frequencies.3 Nevertheless, in the presence of a normal karyotype, the recurrence risk of a rare autosomal recessive disease caused by UPD of a whole chromosome is negligible since it is a rare, generally sporadic event.3
Supplementary Material
Acknowledgments
The authors thank the CEINGE Service Facility platforms of the Sequencing Core and Oligo Synthesis.
Funding Statement
Funding: this work was supported by an EHA Junior Research Grant to Immacolata Andolfo (3978026), and by a Bando Star Linea 1 - Junior Principal Ivestigator Grants - COINOR, Università degli Studi di Napoli ‘Federico II’ to Roberta Russo. The authors also thank the parents of the patient for granting their permission for the case to be communicated to the scientific community.
References
- 1.Ducamp S, Fleming MD. The molecular genetics of sideroblastic anemia. Blood. 2019;133(1):59-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu G, Guo S, Anderson GJ, Camaschella C, Han B, Nie G. Heterozygous missense mutations in the GLRX5 gene cause sideroblastic anemia in a Chinese patient. Blood. 2014;124(17):2750-2751. [DOI] [PubMed] [Google Scholar]
- 3.Niida Y, Ozaki M, Shimizu M, Ueno K, Tanaka T. Classification of uniparental isodisomy patterns that cause autosomal recessive disorders: proposed mechanisms of different proportions and parental origin in each pattern. Cytogenet Genome Res. 2018;154(3):137-146. [DOI] [PubMed] [Google Scholar]
- 4.Russo R, Andolfo I, Manna F, et al. Multi-gene panel testing improves diagnosis and management of patients with hereditary anemias. Am J Hematol. 2018;93(5):672-682. [DOI] [PubMed] [Google Scholar]
- 5.Xiao P, Liu P, Weber JL, Papasian CJ, Recker RR, Deng HW. Paternal uniparental isodisomy of the entire chromosome 3 revealed in a person with no apparent phenotypic disorders. Hum Mutat. 2006; 27(2):133-137. [DOI] [PubMed] [Google Scholar]
- 6.Fouquet C, Le Rouzic MA, Leblanc T, et al. Genotype/ phenotype correlations of childhood-onset congenital sideroblastic anaemia in a European cohort. Br J Haematol. 2019;187(4):530-542. [DOI] [PubMed] [Google Scholar]
- 7.Cazzola M, Malcovati L. Diagnosis and treatment of sideroblastic anemias: from defective heme synthesis to abnormal RNA splicing. Hematology Am Soc Hematol Educ Program. 2015;2015:19-25. [DOI] [PubMed] [Google Scholar]
- 8.Guernsey DL, Jiang H, Campagna DR, et al. Mutations in mitochondrial carrier family gene SLC25A38 cause nonsyndromic autosomal recessive congenital sideroblastic anemia. Nat Genet. 2009; 41(6):651-653. [DOI] [PubMed] [Google Scholar]
- 9.Kannengiesser C, Sanchez M, Sweeney M, et al. Missense SLC25A38 variations play an important role in autosomal recessive inherited sideroblastic anemia. Haematologica. 2011;96(6):808-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong WS, Wong HF, Cheng CK, et al. Congenital sideroblastic anaemia with a novel frameshift mutation in SLC25A38. J Clin Pathol. 2015;68(3):249-251. [DOI] [PubMed] [Google Scholar]
- 11.Le Rouzic MA, Fouquet C, Leblanc T, et al. Non-syndromic childhood onset congenital sideroblastic anemia: a report of 13 patients identified with an ALAS2 or SLC25A38 mutation. Blood Cells Mol Dis. 2017;66:11-18. [DOI] [PubMed] [Google Scholar]
- 12.Shefer Averbuch N, Steinberg-Shemer O, Dgany O, et al. Targeted next generation sequencing for the diagnosis of patients with rare congenital anemias. Eur J Haematol. 2018;101(3):297-304. [DOI] [PubMed] [Google Scholar]
- 13.Furuyama K, Kaneko K. Iron metabolism in erythroid cells and patients with congenital sideroblastic anemia. Int J Hematol. 2018; 107(1):44-54. [DOI] [PubMed] [Google Scholar]
- 14.LeBlanc MA, Bettle A, Berman JN, et al. Study of glycine and folic acid supplementation to ameliorate transfusion dependence in congenital SLC25A38 mutated sideroblastic anemia. Pediatr Blood Cancer. 2016;63(7):1307-1309. [DOI] [PubMed] [Google Scholar]
- 15.Del Rey M, Benito R, Fontanillo C, et al. Deregulation of genes related to iron and mitochondrial metabolism in refractory anemia with ring sideroblasts. PLoS One. 2015;10(5):e0126555. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.