Polymorphonuclear neutrophils (PMN) are key actors in the pathophysiology of sickle cell disease (SCD), but signaling pathways underlying their activation and sustained inflammation are not well documented. We thus investigated the protein profile of neutrophils from SCD patients (SS genotype) using a proteomic approach. Unexpectedly, SCD neutrophils exhibit a high expression of interferon signaling proteins (ISP) belonging to the type 1 interferon (IFN-1) response pathway. We also showed that SCD patients at steady state displayed a higher level of plasmatic IFNα. Overall, we reported a dramatic high-level expression of ISP in neutrophils from SS patients suggesting an abnormal activation that could be important in developing new anti-inflammatory therapies.
SCD is a hemoglobinopathy leading to major red blood cell (RBC) dysfunction, but other cell types (vascular endothelium, leukocytes, platelets)1-3 also represent key actors in the pathophysiology of the disease. Important studies have highlighted the role of PMN, both during the vaso-occlusive crisis (VOC) and the associated long-term morbidity and mortality.4 In SCD, patients have an increased leukocyte count at steady state, and exhibit neutrophil activation, rendering them more susceptible to inflammatory stimuli.5 Moreover recent data have demonstrated the presence of different sub-phenotypes of PMN especially in cancer and inflammation6,7 as well as in a preclinical model of SCD.8 Despite these advances, signaling pathways underlying sustained inflammation in SCD remain elusive. In addition, a fine understanding of PMN activation profile is necessary to better decipher the inflammatory paradigm in SCD and develop tailored therapies. In the present study, we investigated for the first time the proteomic profile of PMN in SCD at basal state by a label-free global proteomic approach.
We performed a proteomic comparative study of purified neutrophils from four SS patients (SS1-4) at basal state and four AA blood type healthy donors (AA1-4). All patients included in this study were homozygous (SS genotype), aged 2 to 18 years (mean age 9.7 years), and without any associated co-morbidity. They were free from any infections and exhibited C-reactive protein level < 10 mg/L at the day of the inclusion. The controls were voluntary blood donors, all healthy and of AA genotype.
Figure 1.
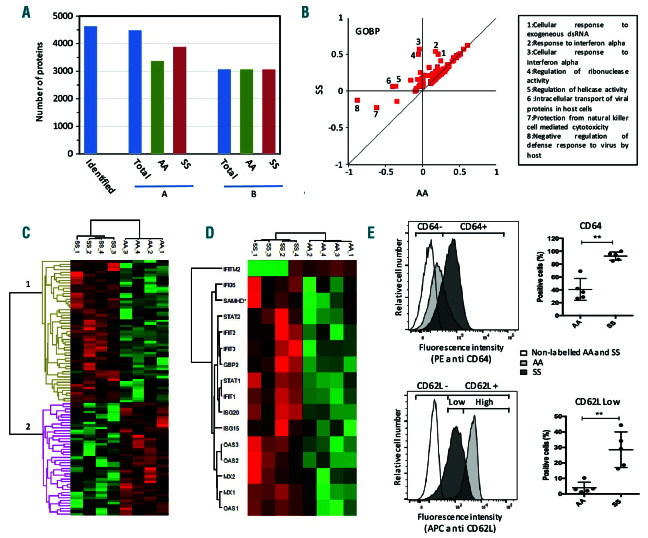
Proteomic analysis of neutrophils from healthy donors (AA) and SS genotype patients (SS) at steady state. (A) Overall proteomics results: number of proteins identified and quantified in at least one sample (A) or in at least three samples and in at least one group (B). (B) 2D enrichment analysis of the proteins expressed in at least 75% of the samples and in at least one group. Protein annotation databases from GOBP. Annotations out of the diagonal corresponding to differential expression in SS or AA samples are indicated. (C) Cluster analysis of proteins differentially expressed in SS and AA neutrophils. Proteins with significantly different expression values (P<0.05 and fold change>0.3) were selected, their LFQ values were z score transformed and analyzed by Euclidian clustering. Clusters 1 and 2 correspond to proteins upregulated and downregulated in SS neutrophils, respectively. (D) Cluster analysis of differentially expressed proteins with the “cellular response to type I interferon” GOBP annotation. (E) Characterization of neutrophils from SS patients by flow cytometry analysis, typical result: over-expression of the Fc fragment of IgG/CD64 and under-expression of L-selectin/CD62L. Data are presented as mean ± standard devaition (SD). Mann-Whitney test was used to compare SS and AA; *P<0.05 compared with AA; **P<0.01 compared with AA.
Figure 2.
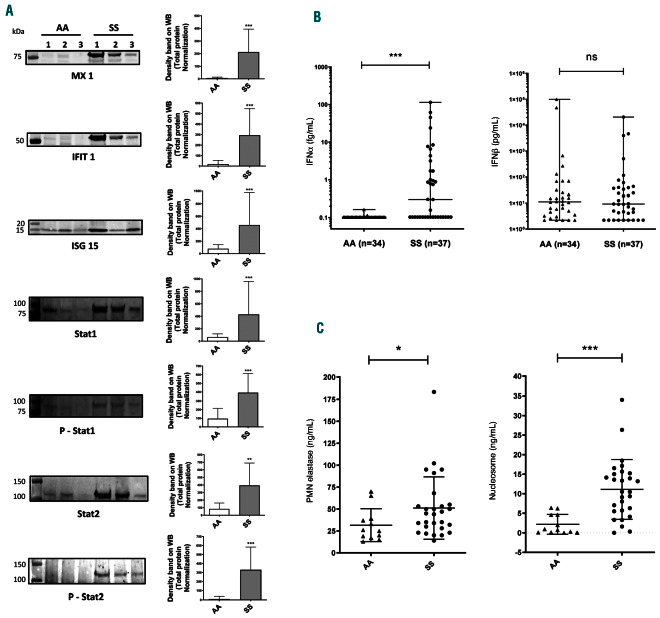
Activation of IFN-1 pathway in SS gentype neutrophils. (A) Representative images of Western blots for MX1, IFIT-1, ISG15, STAT 1, Phospho-STAT 1, STAT 2 and Phospho-STAT 2 expressed in neutrophils from healthy volunteers (AA, n=10) and SS patients (SS, n=10). The Western blots are represented as the ratio of the density of the specific band on the total protein in each sample. (B) Level of IFNα and IFNb in plasma from healthy donors (AA, n=34) and SS patients at basal state (SS, n=37) using a digital-ELISA assay (SIMOA). (C) Level of neutrophil elastase and nucleosome in plasma from healthy donors (AA, n=12) and SS patients at basal state (SS, n=28) using ELISA assay. Data are presented as mean ± standard devaitaion (SD). Mann-Whitney test was used to compare SS and AA; *P<0.05 compared with AA; **P<0.01 compared with AA; ***P<0.001.
After mass spectrometry analysis, 4,634 proteins were identified and 4,487 of them could be reliably quantified. Restricting the analysis to proteins quantified in at least 75% of the samples and in at least one group (AA and/or SS) led to the comparison of 3,069 proteins (Figure 1A). To identify biological pathways modified in neutrophils from SS patients, we first performed a 2D annotation enrichment test9 using GO, KEGG and keywords annotation databases. This analysis revealed the presence of many neutrophil membrane and secreted proteins involved in immune response (Figure 1B). Next, we analyzed the SS and the AA proteomes and found 101 proteins significantly differentially expressed using a SS/AA ratio >1.3 or <0.7. Sixty-eight proteins were overexpressed (cluster 1, Figure 1C), and 33 proteins were down-regulated in the SS group compared to the AA group (cluster 2, Figure 1C). Prior to further investigating a new biological pathway, we aimed to confirm the already known surface markers of sickle cell neutrophils. Indeed, several studies in mouse models or in patients have shown an activated and aged phenotype of PMN in SCD.8,10 By using our proteomics data, we found two proteins described as markers of activation (Fc fragment of IgG, high affinity Ia, receptor/CD64) and ageing (L-Selectin/CD62L) of neutrophils respectively overexpressed (5.8-fold) and underexpressed (0.6-fold) in SS patients compared to the AA group (Online Supplementary Table S1 and Online Supplementary Proteomic File). These data were confirmed by cytometry analysis of freshly isolated neutrophils from five SS patients and five controls (Figure 1E). We therefore concluded that the proteomic analysis could be a relevant tool for exploring neutrophil abnormalities in SCD.
A Fisher exact test performed using proteins down-regulated in SS neutrophils did not evidence any common biological pathway (data not shown). In contrast, analyses of the upregulated proteins revealed a major involvement of the type1 interferon (IFN-I) response (Online Supplementary Table S1 and Online Supplementary Proteomic File). Importantly, major ISP including IFIT1, IFIT2, IFIT3, ISG15, ISG20, GBP2, IFI35, MX1 and MX2 were increased 3- to 84-fold in the proteome of SS neutrophils compared to the one of AA neutrophils (Figure 1D). Moreover, we found a significant overexpression of STAT1 and STAT2 in the neutrophil proteome of the SS group consistent with an activation of the IFN-related JAK/STAT signaling pathways. In order to confirm the proteomic data, we assessed the overexpression of the main ISP using Western blotting experiments of purified neutrophils from 10 other SS patients at steady state and 10 other AA controls. In agreement with the proteomic data, we found a significant increase of ISP expression including MX1, ISG15 and IFIT1 as well as the STAT1 and STAT2 proteins, in neutrophil lysates from SS patients compared to controls (Figure 2A). The nuclear translocation of STAT1 and STAT2 are activated by JAK and TYK2-mediated phosphorylation of the Y701 and Y689, respectively, that stimulates the IFN-1 responses.11,12 We showed that both Y701 of STAT1 and Y689 of STAT2 were highly phosphorylated in SS compared to AA neutrophils (Figure 2A). These findings confirmed the strong activation of the IFN-1 signaling pathway in SS neutrophils via the JAK/STAT1/2 pathway.
In order to investigate further and to assess whether the IFN-I response was due to either IFNα or IFNb, we measured the level of both cytokines in the plasma of 34 healthy AA donors and 37 SS patients at steady state, using the novel digital-ELISA technology. Interestingly, we found a significantly increased level of IFNα in the plasma from half of the SS patients compared to AA controls (Mann-Whitney test, P<0.001), even though no difference was observed for IFNb (Figure 2B). Although the specific role of the different types of IFN-1 is not fully understood, it appears that IFNα, in contrast to IFNb is mainly involved in autoimmune and auto-inflammatory diseases.13 It is noteworthy that 20 SS patients exhibited an increase of IFNα from 10- to 1,000-fold compared to healthy individuals although 17 of the 37 plasma samples had normal levels of IFNα. Clinical and biological investigations of these 37 SS patients did not show any correlation between the plasmatic level of IFNα and biologic markers including leucocyte, neutrophils, reticulocytes and platelets counts, hemoglobin (Hb) level, Hb haplotypes or age (Online Supplementary Table S2), and plasmatic cytokine concentration (including CX3CL-1, Rantes, MCP-1, MCP-3, TNFα, IL1b, IL10, IL18, and IL6). Since it is known that neutrophil extracellular trap formation (NETosis) plays a role in the pathogenesis of SCD, we next investigated the NETosis by measuring the neutrophil elastase and nucleosome, in the plasma from 28 patients investigated for IFNα and IFNb. As expected, we found a significant high level of both markers of NETosis (Figure 2C) in SS patients compared to AA controls, but no correlation with the IFNα level (data not shown).
Finally, we analyzed the clinical data from the patients, and found no significant difference between the “high IFN” and “low IFN” group of patients and the number of acute events (including number of VOC per year, acute chest syndrome, stroke, cerebral vasculopathy, acute splenic sequestration nor splenectomy). It is noteworthy that no patient has been treated with hydroxycarbamide and none of them has followed a transfusion program.
Moreover, it is interesting to note that of the four SS plasma samples used for the neutrophil proteomic analysis one had low IFNα level, while the other three exhibited 7- to 60-fold increased levels compared to controls, although all four neutrophil samples expressed high level of ISP. Therefore, it is highly probable that plasma IFNα has a transient secretion while the downstream activation of the signaling pathway is persistent. Altogether, our data indicated that SS patients may have inappropriate transient high IFNα secretions (i.e., outside of any acute and infectious events), responsible for the activation of the IFN-1 signaling pathway in neutrophils. Although the mechanism of this activation remains to be elucidated, some recent data described a clear relationship between INF-1 responses and red blood cell alloimmunization in murine models.14,15 Since alloimmunization represents a detrimental issue in SCD, our data highlight the importance of testing the link between ISP and alloimmunization in SS patients.
In conclusion, we showed for the first time by quantitative proteomic analyses of purified neutrophils a particular immune and inflammatory signature in SCD. Our findings provide evidence of a dysfunction of the IFNα signaling pathway that could play an important role in the pathogenesis of SCD. Future studies using a cohort of patients are needed to determine the relationship between IFNα activation and clinical complications and to establish if ISP may represent therapeutic targets to decrease inflammation in SCD.
Supplementary Material
Acknowledgments
we thank Dr Marie-Helène Odièvre for her contribution to patient’s recruitment. We are indebted to Wassim El Nemer for helpful comments and for reading the manuscript.
Funding Statement
Funding: We thank the patients and their families for their participation in the study and all members of the Sickle Cell Disease Center from the Robert Debré Hospital for the management of blood samples. This work was supported by a grant from l’Association Recherche et Transfusion, the Institut National de la Transfusion Sanguine, and the Laboratory of Excellence GR-Ex, reference ANR-11-LABX-0051; GR-Ex is funded by the program “Investissements d’avenir” of the French National Research Agency, reference ANR-11-IDEX-0005-02. The Orbitrap Fusion mass spectrometer was acquired with funds from the FEDER through the "Operational Programme for Competitiveness and Employment 2007-2013" and from the "Canceropole Ile de France".
References
- 1.Kaul DK, Finnegan E, Barabino GA. Sickle red cell-endothelium interactions. Microcirculation. 2009;16(1):97-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Proenca-Ferreira R, Brugnerotto AF, Garrido VT, et al. Endothelial activation by platelets from sickle cell anemia patients. PLoS One. 2014;9(2):e89012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koehl B, Nivoit P, El Nemer W, et al. The endothelin B receptor plays a crucial role in the adhesion of neutrophils to the endothelium in sickle cell disease. Haematologica. 2017;102(7):1161-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med. 1994;330(23):1639-1644. [DOI] [PubMed] [Google Scholar]
- 5.Lum AF, Wun T, Staunton D, Simon SI. Inflammatory potential of neutrophils detected in sickle cell disease. Am J Hematol. 2004;76(2):126-133. [DOI] [PubMed] [Google Scholar]
- 6.Yang P, Li Y, Xie Y, Liu Y. Different faces for different places: heterogeneity of neutrophil phenotype and function. J Immunol Res. 2019;2019:8016254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang X, Qiu L, Li Z, Wang XY, Yi H. Understanding the multifaceted role of neutrophils in cancer and autoimmune diseases. Front Immunol. 2018;9:2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang D, Xu C, Manwani D, Frenette PS. Neutrophils, platelets, and inflammatory pathways at the nexus of sickle cell disease pathophysiology. Blood. 2016;127(7):801-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geiger T, Wehner A, Schaab C, Cox J, Mann M. Comparative proteomic analysis of eleven common cell lines reveals ubiquitous but varying expression of most proteins. Mol Cell Proteomics. 2012;11(3):M111.014050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fadlon E, Vordermeier S, Pearson TC, et al. Blood polymorphonuclear leukocytes from the majority of sickle cell patients in the crisis phase of the disease show enhanced adhesion to vascular endothelium and increased expression of CD64. Blood. 1998;91(1):266-274. [PubMed] [Google Scholar]
- 11.Bancerek J, Poss ZC, Steinparzer I, et al. CDK8 kinase phosphorylates transcription factor STAT1 to selectively regulate the interferon response. Immunity. 2013;38(2):250-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiesauer I, Gaumannmuller C, Steinparzer I, Strobl B, Kovarik P. Promoter occupancy of STAT1 in interferon responses is regulated by processive transcription. Mol Cell Biol. 2015;35(4):716-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crow MK, Ronnblom L. Type I interferons in host defence and inflammatory diseases. Lupus Sci Med. 2019;6(1):e000336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu D, Gibb DR, Escamilla-Rivera V, et al. Type 1 IFN signaling critically regulates influenza-induced alloimmunization to transfused KEL RBCs in a murine model. Transfusion. 2019;59(10):3243-3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gibb DR, Liu J, Natarajan P, et al. Type I IFN is necessary and sufficient for inflammation-induced red blood cell alloimmunization in mice. J Immunol. 2017;199(3):1041-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.