Abstract
We assessed the association between severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and Kawasaki disease (KD)-like multisystem inflammatory syndrome in a retrospective case–control study in France. RT-PCR and serological tests revealed SARS-CoV-2 infection in 17/23 cases vs 11/102 controls (matched odds ratio: 26.4; 95% confidence interval: 6.0–116.9), indicating strong association between SARS-CoV-2 infection and KD-like illness. Clinicians should keep a high level of suspicion for KD-like illness during the COVID-19 pandemic.
Keywords: Kawasaki-like disease, PIMS-TS, MIS-C, SARS-CoV-2, COVID-19
Multisystem inflammatory syndrome in children and adolescents (MIS-C) emerged during the coronavirus disease (COVID-19) pandemic, leading to a first alert by the United Kingdom National Health Service on 25 April 2020 [1]. Since then, several case studies in regions with high rates of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) community transmission have reported MIS-C cases [2], with a substantial proportion of patients meeting the American Heart Association criteria for Kawasaki disease (KD) [3-5]. We investigated this potential association in the Paris metropolitan area (Île-de-France), France, using a matched case–control design.
Definition of cases and controls and matching
Our retrospective case–control study using patient data from two previous studies [3,6] covered the period from 14 April to 26 May 2020.
Cases with KD-like illness were children and adolescents (≤ 18 years) fulfilling the American Heart Association criteria for complete (fever > 4 days and ≥ 4 principal criteria) or incomplete (fever > 4 days and 2 or 3 principal criteria, and without characteristics suggestive of another diagnosis) KD [7], admitted to the general paediatric department of Necker-Enfants malades referral hospital [3]. Each case was matched randomly to a maximum of five controls sampled without replacement from a prospective multicentre cross-sectional study conducted in 27 primary care paediatric private practices in the Paris area during the same period [6]. In brief, controls were children (≤ 15 years), symptomatic or not, visiting one of the paediatricians from the Association Clinique et Thérapeutique Infantile du Val de Marne (ACTIV) network. Cases and controls were matched by ZIP code (exact matching of the first two numbers) and age (± 2 years).
Laboratory investigations
For each child/adolescent, nasopharyngeal swabs were obtained to test for SARS-CoV-2 using reverse transcription-PCR (RT-PCR). Cases were tested using the SARS-CoV-2 R-GENE test (bioMerieux, Marcy-l’Etoile, France); controls were tested with the Allplex 2019-nCoV assay (Seegene, Seoul, South Korea). Positivity for RT-PCR was considered consistent with recent or ongoing SARS-CoV-2 infection.
All children/adolescents were also tested for antibodies against SARS-CoV-2. Cases were tested using the Architect SARS-CoV-2 assay (Abbott Core Laboratory, Illinois, United States (US)), a chemiluminescent immunoassay for the quantitative detection of IgG antibodies in serum. Controls were tested using the COVID-19 BSS test (Biosynex, Strasbourg, France) [8], a rapid chromatographic immunoassay for the qualitative detection of IgG antibodies on fingerstick whole-blood specimens. These tests were both evaluated by the French National Reference Centre for respiratory viruses at the Institut Pasteur, Paris and were among those approved by the French national health authority (Haute Autorité de Santé), Saint-Denis. Positive serology was defined as the detection of IgG against SARS-CoV-2 and was deemed consistent with recent infection with SARS-CoV-2 [9].
Analyses
We described baseline characteristics of cases and controls and performed conditional logistic regressions to calculate matched odds ratios (ORs) to assess the association between SARS-CoV-2 infection and KD-like illness. We tested the association in the following subgroups: (i) overall positive (positive RT-PCR and/or serology), (ii) RT-PCR positive (independently of the serology result) and (iii) serology positive (independently of the RT-PCR result). We conducted two sensitivity analyses: (i) matching by ZIP code, age and sex, and (ii) matching by ZIP code, age and exact week of inclusion. Statistical analyses were conducted using Stata/SE 15 (StataCorp, College Station, US).
Ethical statement
For cases, the study protocol was approved by the Necker Hospital Institutional Review Board (No. 20200618174239) and by an ethical committee (CPP Ouest IV, No. DC-2017–2987). For controls, the study protocol was approved by an ethical committee (CPP IDF IX, Number 08–022) and was registered at ClinicalTrials.gov (NCT04318431). All parents provided written informed consent. This study was conducted in accordance with the Helsinki Declaration.
Association between SARS-CoV-2 infection and Kawasaki-like multisystem inflammatory syndrome
Among 30 eligible cases (i.e. all cases with KD-like illness hospitalised in our general paediatric department) and 605 controls already described elsewhere [3,6], the matching process could not identify controls for seven cases; these were therefore excluded from the case–control study. For the remaining 23 cases, the matching process allowed for pairing one control for one case, two controls for two cases, three controls for one case, four controls for one case and five controls for 18 cases, respectively. Thus, analyses relied on data for 23 cases with KD-like illness (mean age 6.8; range: 0.3–16.6 years) and 102 controls (mean age 5.8; range: 0.05–16.0 years), slightly less than half of whom were females (Figure and Table ).
Figure.
Flowchart of study participant selection
KD: Kawasaki disease; RT-PCR: reverse transcription-PCR; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2.
a Each case was matched to a maximum of five controls, with matching on ZIP code and age. Among 30 eligible cases with KD-like illness, seven could not be matched with any control and were excluded.
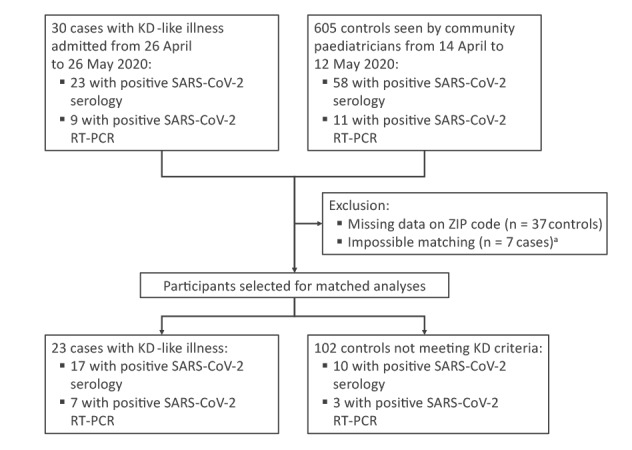
Table. Characteristics of cases with Kawasaki disease-like illness and controls (n = 125).
| Characteristics | Cases (n = 23) |
Controls (n = 102) |
Matched OR (95% CI)a |
||
|---|---|---|---|---|---|
| Number | % | Number | % | ||
| Sex, females | 11 | 48 | 48 | 47 | NA |
| Age, mean in years (SD) | 6.8 (4.8) | NA | 5.8 (4.1) | NA | Matching criterion |
| Name of Department within Paris metropolitan area (first two digits of ZIP code) | Matching criterion | ||||
| Paris (75) | 4 | 17 | 16 | 16 | NA |
| Seine-et-Marne (77) | 2 | 9 | 10 | 10 | |
| Yvelines (78) | 1 | 4 | 2 | 2 | |
| Essonne (91) | 2 | 9 | 7 | 7 | |
| Hauts-de-Seine (92) | 4 | 17 | 20 | 20 | |
| Seine-Saint-Denis (93) | 6 | 26 | 27 | 26 | |
| Val-de-Marne (94) | 4 | 17 | 20 | 20 | |
| Overall evidence of SARS-CoV-2 infection i.e. positive RT-PCR and/or serology | |||||
| Positive | 17 | 74 | 11 | 11 | 26.4 (6.0–116.9) |
| Negative | 6 | 26 | 91 | 89 | Reference |
| SARS-CoV-2 RT-PCR testing independently of the serology result | |||||
| Positive | 7 | 30 | 3 | 3 | 13.9 (2.8–68.6) |
| Negative | 16 | 70 | 99 | 97 | Reference |
| SARS-CoV-2 serology testing independently of the RT-PCR result | |||||
| Positive | 17 | 74 | 10 | 10 | 27.7 (6.3–122.7) |
| Negative | 6 | 26 | 92 | 90 | Reference |
CI: confidence interval; NA: not applicable; OR: odds ratio; RT-PCR: reverse transcription-PCR; SD: standard deviation.
a Conditional logistic regression with patients matched on ZIP code and age.
Overall, 17 of 23 (74%) cases and 11 of 102 (11%) controls tested positive for SARS-CoV-2 by RT-PCR and/or serology (matched OR: 26.4; 95% confidence interval (CI): 6.0–116.9). The association remained significant when limiting the assessment to RT-PCR results and serological results separately (matched OR: 13.9; 95% CI: 2.8–68.6 and 27.7; 95% CI: 6.3–122.7, respectively; Table ). The association was robust in sensitivity analyses with matching on ZIP code, age and sex (21 cases vs 88 controls; matched OR: 21.6; 95% CI: 4.8–97.1), as well as on ZIP code, age and week of inclusion (12 cases vs 38 controls; matched OR: 24.3; 95% CI: 3.0–198.5).
Discussion and conclusions
During the COVID-19 pandemic, an outbreak of MIS-C with features of myocarditis, toxic shock syndrome and KD was reported by several teams [2], including ours [3]. An association between SARS-CoV-2 infection and this KD-like illness has been hypothesised, based on the 2 to 4 weeks delay between the peak of SARS-CoV-2 infections and that of KD-like illness cases in the populations studied [2,3]. However, because these studies lacked control groups without KD-like illness, they could not formally test this hypothesis. To date, a causal link between viral infections and the occurrence of KD remains probable but unproven. Several viral pathogens, such as Epstein-Barr virus [10], cytomegalovirus [11], parvovirus B19 [12] and bocavirus [13], have been suggested as possibly involved in the pathogenesis of KD, but the association with coronaviruses remains debated because of conflicting results [14]. Further to the evidence from observational case series, this study provides strong evidence of an association between infection with SARS-CoV-2 and the occurrence of KD-like illness observed during the COVID-19 pandemic, with 25-fold higher odds of exposure to SARS-CoV-2 in patients with KD-like illness.
This study has several strengths. First, we focused on patients fulfilling criteria for KD [7], as MIS-C definitions can potentially bring clinical heterogeneity [15,16]. Second, cases and controls were included over the same period and geographical area, which reduces confounding due to spatiotemporal variations of exposure to SARS-CoV-2. Lastly, we conducted our research in the Paris metropolitan area, a region heavily affected by COVID-19, which enabled us to include enough cases.
This study also has limitations. First, despite matching by ZIP code, age, sex and week of inclusion, we cannot exclude residual confounding due to socioeconomic and ethnic factors. Indeed, some populations might have higher exposure to SARS-CoV-2 infection or particular genetic predispositions [3]. Unfortunately, these data were not available in controls. Second, Necker-Enfants malades hospital hosts a referral centre for paediatric cardiovascular disease and a large intensive care facility; thus, we may have included the most severe cases of KD-like illness. This potential selection bias may have led to an overestimation of the odds ratio. Third, serology and RT-PCR assays used in cases and controls were different, and we could not exclude measurement bias. Fourth, this study was conducted during the national lockdown and relied on a small sample of children/adolescents recruited in a limited geographical area. Our findings should be validated in larger international studies and in periods of more active community transmission of the virus.
In conclusion, we provide evidence of a strong association between SARS-CoV-2 infection and KD-like illness during the COVID-19 pandemic. Clinicians should be aware of this association and keep a high level of suspicion for KD-like illness, notably in children with clinical or laboratory evidence of recent infection with SARS-CoV-2.
Acknowledgements
We are grateful to the investigators of the COVILLE/ACTIV study: Akou’ou M.H, Auvrignon A, Belaroussi N, Benani M, Cambier Nappo E, Chartier Albrech C, Coicadan L, Condor R, D’Acremont G, D’Ovidio N, De Brito B, Deberdt P, Delatour A, Gorde-Grosjean S, Louvel M, Michot-Cottias A-S, Ravilly S, Seror E, Turberg-Romain C. We thank the staff from all the units who participated in the study in Necker hospital and Gras-Leguen C (CHU Nantes, France) who helped with the ethical and regulatory considerations of this study. We also thank Poirault C, Abadie V, Chalouhi C, Taylor M, Le Gouez M, and Taupin P for their help to the Necker study.
Funding: There was no specific funding for the study that enrolled cases in Necker Hospital. For the ACTIV study that enrolled controls, funding was received from the French Ministry of Health (DGOS PHRC regional IDF 2020 no. AOR20095).
Data sharing agreement: Data are available upon reasonable request.
Conflict of interest: ML has received travel grants to scientific meeting from Biomerieux and Abbott. All other authors report no potential conflicts.
Authors’ contributions: Study conception: SA, MC, JFC, RC, CL, JT. Data collection: RC, CL, CJ, MLV, EV, FB, NO, JC, AE, JT. Data analysis and interpretation: SA, MC, JFC, RC, CL, MLV, JLC, CJ, SB, JT. Drafting the manuscript: SA, MC, JFC, JT. Revising the manuscript for important intellectual content: All authors. Approved the final version submitted: All authors. Study supervision: MC, RC.
References
- 1.Paediatric Intensive Care Society (PICS). PICS Statement: increased number of reported cases of novel presentation of multisystem inflammatory disease 2020 (updated April 27, 2020). Geneva: PICS; 2020. Available from: https://picsociety.uk/wp-content/uploads/2020/04/PICS-statement-re-novel-KD-C19-presentation-v2-27042020.pdf
- 2. Abrams JY, Godfred-Cato SE, Oster ME, Chow EJ, Koumans EH, Bryant B, et al. Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with SARS-CoV-2: A Systematic Review. J Pediatr. 2020. 10.1016/j.jpeds.2020.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Toubiana J, Poirault C, Corsia A, Bajolle F, Fourgeaud J, Angoulvant F, et al. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ. 2020;369:m2094. 10.1136/bmj.m2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Whittaker E, Bamford A, Kenny J, Kaforou M, Jones CE, Shah P, et al. Clinical Characteristics of 58 Children With a Pediatric Inflammatory Multisystem Syndrome Temporally Associated With SARS-CoV-2. JAMA. 2020;324(3):259-69. 10.1001/jama.2020.10369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moraleda C, Serna-Pascual M, Soriano-Arandes A, Simó S, Epalza C, Santos M, et al. Multi-Inflammatory Syndrome in Children related to SARS-CoV-2 in Spain. Clin Infect Dis. 2020;ciaa1042. 10.1093/cid/ciaa1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen R, Jung C, Ouldali N, Sellam A, Batard C, Cahn-Sellem F, et al. Assessment of spread of SARS-CoV-2 by RT-PCR and concomitant serology in children in a region heavily affected by COVID-19 pandemic. medRxiv. 2020:2020.06.12.20129221.
- 7. McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, et al. American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Cardiovascular Surgery and Anesthesia; and Council on Epidemiology and Prevention Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease: A Scientific Statement for Health Professionals From the American Heart Association. Circulation. 2017;135(17):e927-99. 10.1161/CIR.0000000000000484 [DOI] [PubMed] [Google Scholar]
- 8. Bryan A, Pepper G, Wener MH, Fink SL, Morishima C, Chaudhary A, et al. Performance Characteristics of the Abbott Architect SARS-CoV-2 IgG Assay and Seroprevalence in Boise, Idaho. J Clin Microbiol. 2020;58(8):e00941-20. 10.1128/JCM.00941-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sethuraman N, Jeremiah SS, Ryo A. Interpreting Diagnostic Tests for SARS-CoV-2. JAMA. 2020;323(22):2249-51. 10.1001/jama.2020.8259 [DOI] [PubMed] [Google Scholar]
- 10. Kikuta H, Sakiyama Y, Matsumoto S, Hamada I, Yazaki M, Iwaki T, et al. Detection of Epstein-Barr virus DNA in cardiac and aortic tissues from chronic, active Epstein-Barr virus infection associated with Kawasaki disease-like coronary artery aneurysms. J Pediatr. 1993;123(1):90-2. 10.1016/S0022-3476(05)81546-X [DOI] [PubMed] [Google Scholar]
- 11. Catalano-Pons C, Quartier P, Leruez-Ville M, Kaguelidou F, Gendrel D, Lenoir G, et al. Primary cytomegalovirus infection, atypical Kawasaki disease, and coronary aneurysms in 2 infants. Clin Infect Dis. 2005;41(5):e53-6. 10.1086/432578 [DOI] [PubMed] [Google Scholar]
- 12. Nigro G, Zerbini M, Krzysztofiak A, Gentilomi G, Porcaro MA, Mango T, et al. Active or recent parvovirus B19 infection in children with Kawasaki disease. Lancet. 1994;343(8908):1260-1. 10.1016/S0140-6736(94)92154-7 [DOI] [PubMed] [Google Scholar]
- 13. Bajolle F, Meritet JF, Rozenberg F, Chalumeau M, Bonnet D, Gendrel D, et al. Markers of a recent bocavirus infection in children with Kawasaki disease: "a year prospective study". Pathol Biol (Paris). 2014;62(6):365-8. 10.1016/j.patbio.2014.06.002 [DOI] [PubMed] [Google Scholar]
- 14. McCrindle BW, Manlhiot C. SARS-CoV-2-Related Inflammatory Multisystem Syndrome in Children: Different or Shared Etiology and Pathophysiology as Kawasaki Disease? JAMA. 2020;324(3):246-8. 10.1001/jama.2020.10370 [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention (CDC). Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with Coronavirus Disease 2019 (COVID-19) 2020 (updated 14 May 2020). Atlanta: CDC; 2020. Available from: https://emergency.cdc.gov/han/2020/han00432.asp
- 16.World Health Organization (WHO). Multisystem inflammatory syndrome in children and adolescents temporally related to COVID-19 2020 (updated 27 Aug 2020). Geneva: WHO; 2020. Available from: https://www.who.int/news-room/commentaries/detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19


